Abstract
The heat shock factor (HSF) family of transcription factors drives gene expression programs that maintain cytosolic protein homeostasis (proteostasis) in response to a vast array of physiological and exogenous stressors. The importance of HSF function has been demonstrated in numerous physiological and pathological contexts. Evidence accumulating over the last two decades has revealed that the regulatory programs driven by the HSF family can vary dramatically depending on the context in which it is activated. To broadly maintain proteostasis across these contexts, HSFs must bind and appropriately regulate the correct target genes at the correct time. Here, we discuss “the heat shock factor code”—our current understanding of how human cells use HSF paralog diversification and interplay, local concentration, post-translational modifications, and interactions with other proteins to enable the functional plasticity required for cellular resilience across a multitude of environments.
Keywords: HSF1, HSF2, HSF4, Transcriptional regulation, Post-translation modification
Introduction
All cellular life utilizes specific gene expression programs that enable the adaptation to changing environments and physiological stressors. These programs are regulated by transcription factors (TFs), which bind to DNA in a sequence-specific manner to induce or repress the expression of specific genes. TFs modulate gene expression in a highly dynamic way, in which the same TF can regulate different genes in different cell types and physiological contexts. A TF’s activity—its pattern of chromatin occupancy and effect on transcription—is a product of its cellular concentration, post-translation modifications (PTMs), subcellular localization, and interactions with other proteins. These multiple nodes are interdependent (e.g., a TF’s localization might depend on its PTMs and protein interactions), such that breaking one node may disrupt the others.1, 2, 3
Heat shock factors (HSFs) are a highly conserved family of TFs within the winged–alpha–helix family that drive gene expression programs that maintain protein homeostasis (proteostasis) in response to intrinsic and extrinsic stressors. In humans, the HSF family comprises autosome-encoded HSF1, HSF2, HSF4, HSF5, and sex chromosome-encoded HSFX and HSFY.4 HSF1 and HSF2 contain five domains with a similar overall architecture: the DNA-binding domain (DBD) in the N-terminus, oligomerization domain, which contains leucine zipper repeats 1–3 (heptad repeats HR-A/B), the regulatory domain (RD), a fourth leucine zipper repeat (HR-C), and the transactivation domain in the C-terminus.5 HSF4 lacks the HR-C domain, and this results in constitutive trimerization and DNA binding.6 HSF5, HSFX, and HSFY share a conserved DNA-binding domain, though other regions are not well conserved.7, 8
Over the years, HSF family function has been linked to many aspects of human health and disease.9, 10, 11 HSF1, the ubiquitously expressed and best-studied member of this group, has been implicated in cancer, neurodegenerative, and infectious diseases, among other pathophysiological conditions. HSF2, which is expressed in a temporal and tissue-specific manner, has been linked to cancer and development.12, 13 HSF4 is best known for its role in retinal proteostasis but has also been implicated in a subset of cancers.14 HSF5 is less explored, but recent work has demonstrated it plays a role in testis development.16, 15 Finally, little is known about HSFX and HSFY.17
Under a broad range of physiological and pathophysiological conditions, HSFs are tightly regulated through mechanisms that include their cellular protein concentration, formation of homo-oligomers or hetero-oligomers, protein–protein interactions, and PTMs, including phosphorylation, acetylation, and sumoylation. In this review, we discuss our current understanding of “the heat shock factor code”: How cells utilize extensive HSF regulation to enable the functional plasticity necessary for resilience across diverse environments (Figure 1).
Fig. 1.
The heat shock factor (HSF) code: oligomerization, local protein concentration, post-translational modifications (PTMs), and protein–protein interactions of human HSFs enable functional diversity required for cellular resilience.
An expanded family of interacting HSFs enables functional diversity
In contrast to invertebrates, which encode a single HSF essential for life, vertebrates encode an expanded family of HSF paralogs. In addition to HSF paralog diversification, several of the paralogs encode splice variants. The expansion of the HSF family in mammals enhances redundancy and robustness,18, 19 as evidenced by the dispensability of HSF1, HSF2, and HSF4 for survival in murine models.22, 20, 21 Additionally, this expansion allows for specialization, neomorphic functions, expanded protein interaction networks, and increased regulatory diversity, all of which enable more sophisticated responses to endogenous and exogenous signals. Indeed, following the discovery of HSF1 as the master transcriptional regulator of the heat shock response (HSR), many additional roles for HSF1 and its family members in diverse aspects of organismal physiology have emerged. Similarly, their misregulation has been linked to a wide range of pathological conditions, including cancer, neurodegenerative, and infectious diseases. In this section, we will discuss how HSF paralog expression, splice variants, and interplay contribute to this diverse functionality.
HSF paralogs
HSF1
HSF1 is best known for its role as the master transcriptional regulator of the HSR, a powerful adaptive gene expression program comprising molecular chaperones such as HSP70 and HSP90 that act to promote proteostasis.10 In its inactive state, monomeric HSF1 is localized to the cytoplasm and bound to HSP70.23, 24, 25 Following exposure to elevated temperature, HSP70 binds misfolded proteins, which titrate HSP70 away from HSF1. Upon its release, HSF1 localizes to the nucleus, oligomerizes, and binds specific DNA sequences to drive gene expression.29, 26, 27, 28 HSF1 oligomerization is mediated by hydrophobic intermolecular interactions in the HR-A/HR-B domains between HSF1 monomers.31, 32, 30 The HR-A/HR-B domains can also form intramolecular hydrophobic and ionic interactions with the HR-C domain, preventing HSF1 oligomerization.30 In addition to heat shock, many other stressors that disrupt normal protein folding can also activate HSF1.29, 33, 34
In normal physiology, HSF1 drives transcriptional programs important for various developmental processes. During murine embryonic development and gametogenesis, HSF1 drives a program that includes meiotic genes involved in cohesion and spindle assembly checkpoint.35 This aspect of HSF1 function is conserved, as demonstrated by the fact that the sole HSF in Caenorhabditis elegans, HSF-1, drives a transcriptional program in germ cells that supports rapid germline proliferation.36 During murine neurogenesis, HSF1 regulates the expression genes that are involved in neuronal migration and hippocampal spinogenesis.38, 37 In this context, HSF1 directly drives the expression of polysialyltransferases, ST8Sia2 and ST8Sia4, which are required for hippocampal formation, synaptic plasticity, and memory formation.38
HSF1 has also been linked to numerous disease states, including neurodegenerative diseases,39, 40 cancer,4, 26, 41, 42, 43 and infectious diseases.44 In neurons, HSF1 activity is generally important for maintaining the expression of molecular chaperones such as HSP70, which ensure proper protein folding and prevent protein aggregation. Consequently, loss of HSF1 is associated with aging and neurodegenerative diseases, such as Alzheimer’s, Huntington’s, Parkinson’s, and amyotrophic lateral sclerosis. For example, in Alzheimer’s disease, HSF1 activation protects neurons from synaptic damage induced by insoluble amyloid-β, partly by promoting HSP70 expression.45 In an Alzheimer’s disease mouse model, intranasal administration of recombinant HSP70 reduced amyloid-β accumulation, prevented amyloid plaque formation, and enhanced spatial memory.46 Similarly, in Parkinson’s disease, HSF1 drives the expression of HSP70, which prevents the loss of dopaminergic neurons and decreases the levels and cytotoxicity of α-synuclein aggregates, a hallmark of Parkinson’s disease.48, 47
In Huntington’s disease (HD), HSF1 protein levels are reduced through proteasome-dependent degradation leading to lower levels of heat shock protein (HSP) expression, including HSP70. This reduction increases the aggregation of mutant huntingtin during HD progression.49, 50 Finally, in amyotrophic lateral sclerosis, activation of a dominant active allele of HSF1 results in the reduction of insoluble and hyperphosphorylated Tar DNA-binding domain protein 43, partly due to the activity of the HSP70 co-chaperone DNAJB2.51 Taken together, HSF1 and HSF1-dependent expression of HSPs play a critical role in maintaining proper protein folding, a key process in preventing protein aggregation in neurodegenerative diseases.
A substantial body of work has established a link between HSF1 activation and tumorigenesis.4, 26, 41, 42, 43 In tumorigenesis, HSF1 activity is elevated in cancer cells as well as in the stromal cells found in the tumor microenvironment that support tumor growth.54, 55, 52, 53 Systematic loss of HSF1 reduces tumor formation and progression in multiple mouse models of malignancy.60, 56, 57, 58, 59 In cancer cells, HSF1 drives a transcriptional program comprising HSPs, but also many other genes involved in a diverse array of biological processes that act to promote cancer cell proliferation and survival.53 In the nontumorigenic cancer-associated fibroblasts of the tumor microenvironment, HSF1 drives a distinct transcriptional program to further support cancer cell proliferation in a noncell-autonomous manner.54, 61 In humans, high levels of HSF1 activation in both the cancer cells and cancer-associated fibroblasts are associated with poor prognosis and death.53, 54, 62 Mechanistically, a considerable number of studies have demonstrated direct connections between kinases in oncogenic signaling pathways and the phosphorylation and activation of HSF1. These and other factors leading to altered HSF1 PTMs will be discussed in more detail below.
Nearly all viruses modulate host cellular stress response programs that enable adaptation to the increased anabolic demands associated with the viral life cycle. In this context, viruses co-opt HSF1 to promote gene expression programs critical for viral replication and viral evolution.65, 63, 64 As one example, a genome-wide screen found that HSF1 is essential for orthopoxvirus infection and viral replication. Following infection, HSF1 drives a compact transcriptional program comprising HSPs in a manner similar to a canonical HSR.63 As another example, the nuclear matrix protein U37 of human herpesviruses 6A interacts with HSF1, inducing its phosphorylation at S326 and driving the expression of HSP90. Inhibition of HSF1 and HSP90 reduced viral protein production and viral replication.66 Finally, a series of recent studies demonstrate that HSF1, through its regulation of HSPs, contributes to a permissive host cell proteostasis environment that supports viral evolution by enhancing mutational tolerance.65, 67, 64
Beyond regulating host gene expression, HSF1 has also been shown to directly regulate the gene expression of DNA viruses. In the case of Epstein–Barr Virus (EBV) infection, HSF1 binds to the EBV nuclear antigen 1 (EBNA1) promoter and induces its expression. EBV nuclear antigen 1 is important for viral DNA replication and the transcription of other EBV genes required for maintaining viral latency. Thus, HSF1 plays a role in promoting EBV infection, and given the strong link between EBV and tumorigenesis, this further highlights the connection between HSF1 activity and cancer.68
While the examples above demonstrate that HSF1 is often co-opted to promote viral pathogenesis, there are instances where HSF1 helps thwart viral infection. In the case of human immunodeficiency virus-1, HSF1-driven HSP expression reduces both the viral number and the infectious properties of human immunodeficiency virus-1, though the underlying mechanism for these interesting findings remains to be determined.64 This highlights the complex role of HSF1 in viral infections and suggests its activation can sometimes lead to beneficial outcomes for the host.
HSF2
The overall domain architecture of HSF2 is similar to that of HSF1, with the DNA-binding and oligomerization domains being the most alike between the two factors. Given the similar structure of HSF1 and HSF2, many aspects of the chaperone titration model described for HSF1 will likely apply to HSF2.69 Indeed, in response to external stimuli, HSF2 localizes to the nucleus, oligomerizes, and binds DNA to drive gene expression.4, 17
In contrast to HSF1, HSF2 is a labile protein, highly sensitive to the rate of protein degradation.71, 70 Its expression also appears to be more variable across tissue types and in its subcellular localization.12 HSF2 does not play a major role in the HSR and cannot compensate for the loss of HSF1 in response to thermal stress. Instead, HSF2 DNA-binding is activated in response to hemin-induced differentiation in K562 cells,34 proteosome inhibitors,72, 70 and oxidative stress.74, 73 These findings suggest that HSF2 may function under conditions of moderate proteotoxic stress, in contrast to the severe proteotoxic stress caused by elevated temperatures that are required to trigger an HSF1-driven HSR.
Similar to HSF1, HSF2 also plays a role in gametogenesis and neuronal development. In male and female mice, loss of HSF2 results in gametogenesis defects. HSF2 null female mice display defects in egg production, while male mice exhibit increased apoptosis in spermatocytes.75 HSF2 is present in mature spermatozoa and bound to the HSPA1B promoter. In mature spermatozoa, chromatin is highly condensed, and transcription is ceased; thus, binding of HSF2 to HSPA1B promoter suggests a rapid and preferred expression of HSPA1B during early embryogenesis.76 Furthermore, HSF2 binds and regulates the expression of genes located in the male-specific region (MSYq) within the Y-chromosome genes critical for sperm differentiation.77
Loss of HSF2 also results in defects in brain development, as evidenced by the fact that HSF2 null mice display major defects in neuronal migration. Loss of HSF2 reduces the number of radial glia cells, which secrete the positioning protein Reelin. In addition, loss of HSF2 reduced the expression of p35, an activator of cyclin-dependent kinase 5. The loss of Reelin and cyclin-dependent kinase 5 activation both contribute to defects in neuronal migration.78
Although HSF2 has not been studied as extensively as HSF1 in cancer, its emerging role in malignancy appears to be more complex. One study reported that HSF2 suppresses prostate cancer invasion by regulating genes involved in cell adhesion and extracellular matrix biology.79 Another study identified HSF2 as a promoter of hepatocellular carcinoma proliferation.80 Additionally, a recent extensive study across breast, lung, colon, and prostate cancers demonstrated that HSF2 promotes cancer cell proliferation and tumor formation in a manner similar to HSF1.74 The cooperative interaction between HSF1 and HSF2 observed in the latter study will be discussed in more detail below.
HSF4
The mechanisms by which HSF4 is regulated are not well understood. HSF4 lacks the HR-C domain, which negatively regulates oligomerization, resulting in its constitutive binding to DNA.6 This contrasts with HSF1 and HSF2, whose DNA-binding activities are tightly regulated in response to external stimuli. Given that HSF4 is constitutively bound to DNA, its regulation likely occurs predominantly at the level of its expression.
HSF4 is primarily expressed in muscle, brain, and the retina, with the retina being the most extensively studied. In mice, loss of HSF4 resulted in cataracts, characterized by abnormal lens structures and increased proliferation and differentiation of lens fiber cells.81 Mechanistically, HSF4 drives the expression of HSP27 and gamma-crystallin in lens fiber cells, a uniquely dehydrated environment where protein concentration is extremely high, creating a particularly challenging proteostasis milieu.
Recent studies suggest that HSF4 also plays a role in tumorigenesis. Loss of HSF4 suppresses tumor development in p53-deficient and ARF-deficient mouse models, where HSF4 deficiency led to a reduction in thymoma and lymphoma formation, accompanied by increased apoptosis and senescence.20 Similar to HSF1, HSF4-deficient mouse embryonic fibroblasts isolated from these models were resistant to oncogenic transformation and exhibited reduced proliferation. However, the mechanisms underlying these effects, both in mouse embryonic fibroblasts and in tumors, remain unclear. Notably, parallels between HSF4 and HSF1 are also evident in human tumors, as high HSF4 expression in hepatocellular carcinoma is linked to poor overall survival and tumor recurrence.82
HSF Splice variants
To further expand diversification, alternative splicing generates a number of additional HSF variants. HSF1 and HSF2 are each expressed as two splice isoforms: HSF1α and HSF1β, and HSF2α and HSF2β, respectively. Neither the HSF1 nor the HSF2 isoforms have been extensively studied, and thus little is known beyond their tissue-specific expression patterns.83, 84, 85 There are two HSF4 isoforms resulting from alternative splicing between exons 8 and 9. The predominant isoform, HSF4b, is 30 amino acids longer than HSF4a and is known to activate transcription. In contrast, HSF4a represses transcription.6, 86, 87
HSF Paralog interplay
HSF paralog hetero-oligomerization adds another layer of functional complexity to HSF regulation. Several studies have demonstrated a physical interaction between HSF1 and HSF2.74, 88, 89, 90 In mice, the combined loss of HSF1 and HSF2 resulted in a more severe defect in spermatogenesis than the loss of either alone, suppressing the transcription of genes critical for this process.22 Mechanistically, chromatin immunoprecipitation and proximity ligation assay experiments revealed a functional interaction between HSF1 and HSF2 on chromatin in mouse spermatocytes under physiological temperatures or after mild hyperthermia. However, strong hyperthermia disrupted this co-occupancy, leading to increased HSF1 binding and reduced HSF2 binding.88 Similarly, a recent study demonstrated that HSF1 and HSF2 can also interact and occupy the same chromatin regions in cancer cells, cooperating to regulate gene expression critical for cell proliferation and tumor growth.74
In a different context, HSF2 plays a key role in the fetal cortex’s response to alcohol exposure, modulating radial neuronal migration through its interaction with HSF1.37 In the absence of HSF2, HSF1 cannot be activated following alcohol exposure, making HSF2 essential for HSF1 activation in this scenario. Thus, in this context, HSF2 is necessary for HSF1 activation. These findings highlight the importance of HSF1–HSF2 hetero-oligomerization, demonstrating that HSF2 can be essential for HSF1 activation in certain developmental and stress-related contexts.
HSF4 exhibits various forms of interplay with other HSF paralogs, including both direct interactions and competition for DNA binding, to regulate gene expression in different contexts. HSF4 can form a complex with HSF2, which suppresses the hypoxia-inducible factor-1 alpha (HIF-1α) expression in breast cancer.69 In another case, HSF4 competes with HSF1 for DNA binding, regulating the expression of HSPs and fibroblast growth factors (FGFs) in lens and lung tissues. In HSF4-null mice, HSP and FGF expression is increased, while HSF1-null mice show the opposite phenotype, with reduced levels of these proteins. In double HSF4/HSF1-null mice, the levels of HSPs and FGFs return to normal, suggesting that HSF4 and HSF1 compete for the expression of these genes.81 Additionally, HSF4-null mice show reduced expression of leukemia inhibitory factor (LIF), which is implicated in the development of olfactory sensory neurons, whereas HSF1-null mice exhibit increased LIF expression. In double HSF1/HSF4-null mice, LIF levels are restored to normal, further supporting the idea that HSF4 and HSF1 compete for gene regulation.91
HSF4 also appears to play roles in colorectal cancer, pancreatic cancer, and lymphoma through multiple mechanisms.14, 20, 92 However, it remains to be determined whether interactions with either HSF1 or HSF2 contribute to HSF4’s role in tumorigenesis. More work is needed to address these questions and to further explore the regulation and implications of HSF paralog interplay. Overall, the dynamic interactions between HSF paralogs add complexity to their regulation, with both competitive and cooperative mechanisms likely playing critical roles in development, stress responses, and disease.
Local concentration of HSFs
HSFs bind consensus DNA sequences known as heat shock elements, which consist of alternating inverted repeats of the sequence “nGAAn,” where “n" is any base. The number of repeats, base composition (including some variation in the conserved GAA bases), local sequence context, and chromatin accessibility of heat shock elements can all influence the binding affinity of HSF complexes. Consequently, the local concentration of HSFs will determine which sites are bound, playing a critical role in shaping the HSF regulatory program (Figure 1).
Cells regulate the total concentrations of HSF1 through mechanisms such as transcriptional regulation, protein stability, and, in cancers, DNA copy number. Although the factors regulating HSF1 transcript expression and stability have not been extensively explored, a recent study demonstrated that oncogenic NOTCH1 binds to the HSF1 promoter and directly activates its expression to drive T-cell acute lymphoblastic leukemia.58 This activation increases HSF1 protein levels, leading to enhanced transcription of HSPs. Additionally, in cardiomyocytes, miR-378 binds to the 3′ untranslated region (3′UTR) of HSF1 mRNA, repressing its expression, highlighting the role of noncoding RNAs in regulating HSF1.93
HSF1 protein levels can also be regulated through protein synthesis and proteasome-dependent degradation.94 For example, phosphorylation at S303/S307 is required for the interaction between HSF1 and the E3 ligase F-box/WD repeat-containing protein 7 (FBXW7), leading to HSF1’s proteasome-dependent degradation in a context-dependent manner. The loss of FBXW7 in melanoma induces an increase in nuclear HSF1 protein levels, whereas in HD, FBXW7 mediates HSF1 degradation.49, 50, 95
Lastly, in cancer, one of the most significant mechanisms for increasing HSF1 local concentration is through copy number amplification. The HSF1 locus is located on chromosomal segment 8q24.3, which is among the most frequently amplified regions across human cancers, including ovarian, prostate, and breast cancer.53, 62, 96 This amplification is likely the primary factor driving the elevated HSF1 mRNA and protein levels observed across multiple cancer types.62, 53
HSF2 is a labile protein, and its levels are highly sensitive to proteasome-dependent degradation. Inhibition of the proteasome by MG132 or bortezomib stabilizes HSF2 protein levels.97, 70 Interestingly, HSF2 transcription can also be regulated by proteasome inhibition, though the mechanism remains unclear.98 Several studies also show that HSF2 expression can be regulated by micro-RNAs. One study found that HSF2 expression in the testis is regulated by miR-18, which binds to the 3′UTR of HSF2 mRNA and suppresses its expression, resulting in reduced HSF2 protein levels.99 More recently, high expression of HSF2 in esophageal squamous cell carcinoma was shown to result from the downregulation of miR-202, which also binds to the 3′UTR of HSF2 and suppresses its gene expression.100
While HSF4 expression is more variable than that of HSF1 and HSF2, its regulation has not been studied as extensively. Nevertheless, HSF4 expression is significantly elevated in specific normal cell types, such as lens cells, and in a subset of cancers, including liver and pancreatic cancer.92, 82 However, further research is needed to uncover the mechanisms that drive selective HSF4 expression.
Beyond general mechanisms to regulate absolute/total cellular levels of HSFs, recent work implicates condensate formation as a biophysical mechanism to increase local HSF concentrations. This idea has been explored with HSF1 but presumably could apply to other HSF family members as well. HSF1 condensates are found at sites of active transcription comprising HSF1, mediator, RNA polymerase, and other factors.102, 101 These condensate structures are highly dynamic—they form rapidly during proteotoxic stress and dissolve following recovery. HSF1 condensates concentrate HSF1 at the site of transcription to promote the robust transcriptional activity that is characteristic of the HSR. These HSF1 condensates might enable DNA-binding specificity at the low-affinity sites where a high concentration of HSF1 is required to bind DNA.103
In summary, HSF protein concentration is regulated at multiple levels, including transcriptional and post-transcriptional control, protein stability, and biophysical mechanisms such as condensate formation. These diverse avenues of regulatory control ensure that HSF expression is tightly regulated across different cell types and contexts, enabling precise modulation of HSF activity in response to varying physiological demands.
Post-translational modifications of HSFs
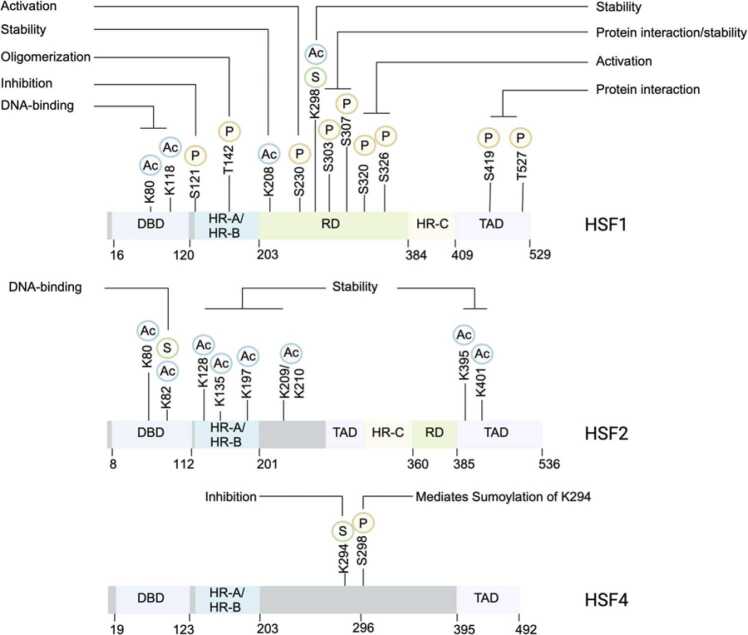
HSFs undergo extensive PTMs, including phosphorylation, acetylation, sumoylation, and ubiquitylation. HSF1, the most abundant and well-studied HSF, has numerous PTMs that have been thoroughly characterized. These modifications regulate HSF1’s activity by influencing its stability, oligomerization, and interactions with other proteins. PTMs important for the activity of HSF2 and HSF4 have also been identified (Figure 2).
Fig. 2.
The role of post-translational modifications on the function of HSFs. Post-translational modifications, including phosphorylation, acetylation, and sumoylation, are indicated on HSF1, HSF2, and HSF4. These PTMs alter HSFs DNA-binding, stability, activation, as well as protein–protein interactions. HSF, heat shock factor; PTM, post-translational modification.
Phosphorylation
Phosphorylation, the process by which kinases add phosphate groups to serine (S), threonine (T), or tyrosine (Y) residues, plays a key role in regulating many cellular processes.104 HSF1 undergoes heat-induced phosphorylation primarily at serine and threonine residues within its RD. This heat-induced phosphorylation has often been used as a marker for HSF1 activation.105, 27 The first comprehensive study of HSF1 phosphorylation was conducted by Guettouche and colleagues,27 who used mass spectrometry to analyze exogenously expressed HSF1 in HeLa cells, identifying 12 serine residues phosphorylated following heat shock: S230, S326, S419, S121, S292, S303, S307, S314, S319, S363, S344, and S444. These phosphorylation events have been reported to either activate or repress HSF1’s transcriptional activity. For instance, phosphorylation at S230, S320, S326, S419, and T142 is associated with HSF1 activation,27, 105, 106 while phosphorylation at S121, S303, S307, and S363 is associated with HSF1 repression.108, 107
Subsequent studies revealed that these heat-induced phosphorylation events can be uncoupled from HSF1 activation.109, 110, 111 For example, a study mutating 15 serine/threonine phosphorylation sites in the RD to alanine demonstrated that the HSF1 mutant remained functional—able to localize to the nucleus, bind chromatin, and drive HSP gene expression.109 This indicates that HSF1 phosphorylation is not required for its role in the HSR but instead fine-tunes its activity. While phosphorylation is dispensable for the HSR, HSF1 phosphorylation by various kinases is crucial for its ability to sense and respond to other physiological signals.
These regulatory phosphorylation events originate from a variety of signaling pathways, allowing HSF1 activity to be finely tuned in response to changing cellular states and physiological needs, highlighting both the complexity and importance of its regulation. For example, phosphorylation at S230 by calcium/calmodulin-dependent protein kinase II and at S320 by Protein Kinase A both activate HSF1.113, 112 The mammalian target of rapamycin pathway also contributes by phosphorylating S326, inducing HSF1 activation following heat shock and other proteotoxic stresses.111 Conversely, phosphorylation events that negatively regulate HSF1 include S121 by the proinflammatory mitogen activated protein kinase-activated protein kinase 2 (MAPKAP kinase 2),108 S363 by c-Jun NH2-terminal kinase,114 and S303/307 by glycogen synthase kinase-3 beta.115, 116 These diverse signaling pathways ensure that HSF1’s activity is tightly controlled, allowing it to adapt to a wide range of cellular conditions.
Aberrant signaling associated with disease has also been found to modulate HSF1 phosphorylation, which has been most extensively characterized in cancer and neurodegenerative disorders. In cancer, HER2-PI3K/AKT signaling activates mammalian target of rapamycin, which phosphorylates HSF1 at S326, promoting its activation, while simultaneously activating glycogen synthase kinase-3 beta, which phosphorylates HSF1 at S303/S307 to inhibit its activity.107, 111, 115, 117 The phosphorylation of S303/S307 is further modulated in a disease-specific manner. For instance, in HD, casein kinase 2 phosphorylates these same sites (S303/S307), priming HSF1 for proteasome-dependent degradation.49, 50
In addition to these pathways, AKT1/2 also phosphorylates HSF1 at S230, T142, and T527, each of which modulates distinct aspects of HSF1 activation. Phosphorylation of T142 within the HR-A/HR-B domain is essential for HSF1 oligomerization.118 Furthermore, the mitogen-activated protein kinase pathway, commonly activated by Rat sarcoma virus protein (RAS) mutations or loss of the tumor suppressor neurofibromatosis type 1, leads to MEK-mediated phosphorylation of HSF1 at S326.119 Finally, the loss of the tumor suppressor kinase LKB1, which inhibits AMPK activity, reduces S121 phosphorylation and activates HSF1.120
Acetylation
Acetylation is another critical PTM that regulates HSF function by modulating protein stability and DNA binding. This modification occurs when an acetyl group is added to lysine (K) residues. There are three major families of histone acetyltransferases: the general control nonderepressible-5 (GCN5)-related N-acetyltransferase family, the EP300/CBP family, and the MYST family of proteins.121 Recent evidence supports the role of EP300 in promoting HSF1 acetylation, which influences both its stability and DNA-binding ability. For example, EP300 catalyzes the acetylation of HSF1 at K208 and K298 within the RD, preventing ubiquitination and subsequent proteasome-dependent degradation, thus promoting HSF1 stability in the absence of stress.94 Adding to the complexity, EP300 also acetylates HSF1 at K118 and K80 within the DNA-binding domain, inhibiting HSF1’s ability to bind DNA.94, 122 The GCN5 acetyltransferase also selectively modifies HSF1 at K80, regulating its DNA-binding capacity through a mechanism involving the molecular chaperone p23.123
Histone acetyltransferases are counteracted by histone deacetylases, which remove acetyl groups to modulate HSF1 activity.124, 121 Thus, as with phosphorylation, acetylation can act as an on/off switch for HSF1 activity. For instance, NAD-dependent deacetylase sirtuin 1 deacetylates HSF1 at K80, reversing the acetylation performed by EP300 and restoring HSF1’s DNA-binding activity.122 In cancer, disruptions to this balance, such as elevated EP300 activity or altered sirtuin 1 function, can promote tumor progression by stabilizing HSF1 and sustaining its activity.125
HSF2 is also subject to acetylation, as observed in neural cell lines derived from Rubinstein–Taybi syndrome, a neurodevelopmental disorder characterized by brain abnormalities. In these cells, CBP/EP300 binds to HSF2 and promotes acetylation at K82 in the DNA-binding domain, corresponding to K80 in HSF1. Furthermore, CBP/EP300 acetylates additional residues in the HR-A/HR-B domain and other regions, such as K128, K135, K197, K209, K210, K395, and K401. Acetylation of these sites confers stability to HSF2 by preventing proteasome-dependent degradation. Mutations in CBP/EP300 in Rubinstein–Taybi syndrome patients disrupt HSF2 acetylation and stability, contributing to disease pathology.126
Sumoylation
Another important PTM on HSFs is sumoylation, where small ubiquitin-like modifier 1 (SUMO-1) proteins are covalently attached to lysine residues. The sumoylation process begins with the attachment of SUMO to an E1 enzyme known as SUMO-activating enzyme (SAE1/SAE2). SUMO is then transferred to the E2-conjugating enzyme (Ubc9), and finally, the E3 ligating enzyme facilitates its attachment to the target protein. Sumoylation of TFs, including HSFs, is often associated with the repression of their transcriptional activity.127
HSF1 is sumoylated at K298 within a phosphorylation-dependent SUMOylation motif (PDSM) in the RD by Ubc9, which represses HSF1’s transcriptional activity. Phosphorylation at S303 within the PDSM is required for the sumoylation of K298. During mild stress, sumoylation is sustained, maintaining repression of HSF1. However, during chronic heat shock, sumoylation is initially present but later reversed, allowing HSF1 activation when the HSR is most needed.128 Phosphorylation of S303 is known to repress HSF1 activation, so the phosphorylation-dependent sumoylation of K298 suggests that repressive phosphorylation at S303 is coupled with the repressive effect of K298 sumoylation. Additionally, phosphorylation at S303/S307 is required for proteasome-dependent degradation (as discussed in Interactome of HSFs). It is likely that the sumoylation of K298 also mediates interactions with other factors, switching HSF1 activity on and off during stress.
In contrast to HSF1, HSF2 lacks a PDSM in its RD. Instead, HSF2 is sumoylated at K82 in the DNA-binding domain, which inhibits its DNA-binding activity without affecting its trimer formation.129 Interestingly, another study demonstrated that HSF2 is a substrate for SUMO-1 and colocalizes with SUMO-1 in nuclear granules, which, in this case, enhances HSF2’s DNA-binding activity.130
HSF4 undergoes sumoylation through a mechanism similar to that of HSF1. The HSF4 transcript can be spliced to include a PDSM, which contains a SUMO consensus site at K294. The sumoylation of HSF4 at K294 is dependent on the phosphorylation of an adjacent serine residue (S298). As with HSF1, sumoylation inhibits HSF4’s transcriptional activity.87 Thus, the sumoylation of K294 in HSF4b can switch it from an activator to a repressor, suppressing HSF4b’s transcriptional activity.69, 87
Taken together, HSF1, HSF2, and HSF4 each undergo distinct PTMs at different residues, which modulate their protein interactions (Figure 2). Beyond their temporal and tissue-specific expression, these PTMs provide an additional layer of regulatory control during various developmental stages, ensuring proper transcriptional regulation.
Interactome of HSFs
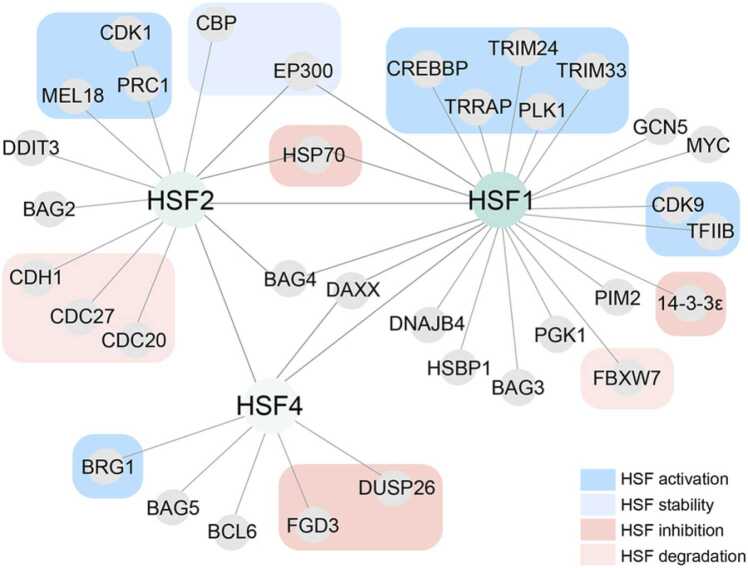
The variable interaction surfaces of HSFs, in contrast to their highly conserved DNA-binding domains, enable HSFs to form diverse protein–protein interactions that contribute to their functional specificity (Figure 3). Moreover, these interactions are dynamic, providing an additional mechanism by which HSFs achieve functional specificity, influenced by cell type, PTM status, and the expression levels of interacting proteins.
Fig. 3.
The protein–protein interaction (PPI) network of HSFs. This network illustrates key PPIs for HSF1, HSF2, and HSF4 from validated coimmunoprecipitation studies and affinity capture-mass spectrometry/affinity-captured luminescence studies derived from the BioGRID database. Nodes represent individual proteins, and edges represent interactions between proteins. HSF1, HSF2, and HSF4 are shown as central hubs, with distinct and shared interactors highlighted. HSF-interacting proteins that have been shown to promote or suppress HSF1 stability or activity are highlighted. HSF, heat shock factor.
HSF1’s activity is shaped by its interactions with various proteins, which can either repress, enhance, or modify its function in different cellular contexts. For example, HSF1 binds to the signaling protein 14-3-3ε, leading to HSF1 cytoplasmic localization and suppression of its transcriptional activity.116 Conversely, HSF1 interacts with the nuclear protein death-domain associated protein (DAXX), promoting HSF1’s transcriptional activation during stress.131 In another context, HSF1 associates with the proto-oncogene transcription factor, c-MYC, and MYC-associated factor X (MAX) on chromatin, where it recruits the histone acetyltransferase GCN5 to enhance histone acetylation and amplify c-MYC’s transcriptional activity.132 In this case, HSF1’s DNA binding is dispensable, but rather its function relies on its interaction with GCN5, which is crucial for promoting c-MYC activity and supporting tumor malignancy.
A number of studies demonstrate that HSF1’s interactions can be affected by its PTM status. For example, phosphorylation of HSF1 at S419 by Polo-like kinase facilitates its interaction with transformation/transcription domain-associated Protein (TRRAP) - a scaffold protein in HAT complexes -and p300, leading to the acetylation of histones H3 and H4. This histone acetylation is necessary for tripartite motif containing 33 and 24 (TRIM33 and TRIM24)-mediated mono-ubiquitination on HSP promoters, both modifications being essential for HSP activation.133 Additionally, phosphorylation at T527 within the transactivation domain is critical for HSF1 to interact with the key transcriptional regulators CDK9 and TFIIB, thereby facilitating the activation of gene expression.118 Phosphorylation at S303/307 is required for FBXW7-dependent ubiquitination and proteasomal degradation of HSF1, and the loss of the tumor suppressor FBXW7 in melanoma stabilizes HSF1 and promotes metastasis.95 In contrast, in HD, phosphorylation of S303/307 by CK2 alpha prime promotes FBXW7-dependent ubiquitination and degradation of HSF1.50 Collectively, these studies highlight how the PTM status of HSF1 influences its interactions and directs its function in a cell type-specific manner.
HSF1 interactions can also be regulated by PTMs on its binding partners. Notably, HSP70, the canonical chaperone regulator of HSF1, is subject to extensive PTMs, including phosphorylation, acetylation, ubiquitination, and AMPylation, all of which can modulate its binding with client proteins.134, 135, 136 A recent study demonstrates that methylation of cytoplasmic HSP70 at R469 is regulated by the arginine demethylase JMJD6. In cancer cells, JMJD6 depletion increases methylation of HSP70 and enhances its binding to HSF1, leading to inhibition of HSF1’s transcriptional activation.137 This repressive HSP70 complex in the cytoplasm is quickly reversed with increased JMJD6 levels, increasing HSF1 activity—providing a rapid regulatory mechanism rather than relying on the time-intensive and energy-intensive process of HSF1 protein synthesis. A similar mechanism operates under low ER stress, where BiP (the ER HSP70) remains AMPylated and inactive. During increased ER stress, the enzyme FicD deAMPylates and activates BiP in response to misfolded proteins.139, 138
HSF2’s functions are influenced by its protein interactions, with its role during mitosis being particularly well studied. During mitosis, most chromatin becomes compacted, halting transcription. However, certain critical sequences remain open, allowing for rapid transcription upon entry into G1—a process known as genetic bookmarking.140 HSF2 plays a key role in this bookmarking. Sumoylation of HSF2 increases during mitosis, regulated by its interaction with the polycomb protein, melanoma antigen-18 (MEL-18). MEL-18 binds to HSF2 and inhibits its sumoylation by blocking the SUMO E2 enzyme (Ubc9). As mitosis progresses, MEL-18’s interaction with HSF2 decreases, leading to enhanced HSF2 sumoylation and increased HSF2 binding activity, which promotes the bookmarking of key genes like HSP70, HSP90, HSP27, and c-FOS.76, 130, 140, 141 Additionally, HSF2 interacts with protein-regulating cytokinesis 1, a CDK substrate associated with the mitotic spindle, during prometaphase and metaphase to further facilitate the bookmarking of HSP70.142 Finally, HSF2 interacts with APC/C, an E3 ubiquitin ligase complex that regulates the cell cycle by mediating the ubiquitination and degradation of key cell-cycle regulators. In this process, APC/C, through its co-activator subunits Cdc27, and Cdh1, targets HSF2 for ubiquitination and degradation, a mechanism that is enhanced during acute proteotoxic stress.143
The HSF4 interactome has not been extensively characterized, though several studies have identified factors that regulate HSF4 activity. One study demonstrated that the transcriptionally active isoform, HSF4b, interacts with DAXX, leading to the suppression of its activity.144 Interestingly, this contrasts with HSF1, where DAXX binding promotes activation.131 Another study revealed that HSF4 interacts with extracellular signal-regulated kinase (ERK) and dual-specificity tyrosine phosphatase 26 (DUSP26). ERK1/2 binds and phosphorylates HSF4b, enhancing its DNA-binding activity. In contrast, DUSP26 binds HSF4b, modulating ERK1/2 activity, which indirectly leads to dephosphorylation and inhibition of HSF4's DNA-binding ability. The authors hypothesize that, while DUSP26 does not directly interact with ERK1/2, the association of HSF4 with both DUSP26 and ERK1/2 brings these kinases into close proximity to facilitate regulation.145
HSF4b has also been reported to recruit the SWI/SNF chromatin remodeling complex subunit, Brahma-related gene to the promoters of HSPs after the cells progress into the G1 phase of the cell cycle to enhance the transcription of HSPs, including HSP70.146 Finally, HSF4 interacts with FGD3, a member of the Rho and Rac protein family that regulates the actin cytoskeleton and cell shape and is known to inhibit cancer cell migration. In pancreatic cancer, FGD3 interacts with HSF4 and inhibits its nuclear localization, resulting in reduced pancreatic cancer growth and progression.92 While these interactions suggest important roles for HSF4, further research is needed to fully understand its broader functions and regulatory mechanisms.
In summary, the extensive network of interactions involving HSFs provides them with the functional diversity required for a wide range of cellular and developmental processes. PTMs—either on HSFs or their interacting partners themselves—can promote or inhibit specific interactions depending on the modification. This intricate regulation underscores the critical role of HSFs in maintaining cellular homeostasis and responding to environmental challenges.
Concluding remarks
Here, we have discussed how cells use HSF expression, oligomerization, PTMs, and interacting proteins to coordinate the gene expression programs required for cellular resilience across a diverse array of cellular contexts. The critical role of HSF function across a spectrum of physiological and disease states presents opportunities for therapeutic intervention. Although many exciting advances have been made, directly targeting TFs remains challenging. Therefore, focusing on the broader HSF regulatory network, including druggable enzymes responsible for HSF’s PTMs, may offer a promising direction for therapy. In the context of targeting HSFs in cancer, other indirect strategies such as leveraging the coessentiality network of HSF1 (or other HSFs), which might contain pharmacologically tractable factors particularly essential to cells dependent on HSF1147, or using chemical-genetic profiling to identify compounds whose efficacy depends on HSF1 activation status148 could be effective approaches.
Within the HSF family, HSF1 has been the focus of nearly all efforts for pharmacological inhibition and activation. A number of compounds have been reported to suppress HSF1 activity,149, 150, 151 but the only direct inhibitor identified so far seems to be direct targeted HSF1 inhibitor.152 A better understanding of the HSFs regulatory network will provide opportunities for better therapeutic strategies, where ideally, it will be possible to target HSF activity in some contexts (e.g., in cancer) while preserving its activity in other contexts (e.g., in neurons to prevent neurodegeneration diseases).
Funding and support
M.L.M. is supported by the NIH (1R01GM144617-01) and by the American Cancer Society (ABOA Impact RSG-22-086-01-TBE).
Author contributions
Milad J. Alasady and Marc L. Mendillo: Writing – review & editing, Writing – original draft.
Declarations of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Austin Klein for comments on the manuscript.
Data availability statement
No data were generated for the research described in the article.
References
- 1.Emanuele M.J., Enrico T.P., Mouery R.D., Wasserman D., Nachum S., Tzur A. Complex cartography: regulation of E2F transcription factors by cyclin F and ubiquitin. Trends Cell Biol. 2020;30:640–652. doi: 10.1016/j.tcb.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filtz T.M., Vogel W.K., Leid M. Regulation of transcription factor activity by interconnected post-translational modifications. Trends Pharmacol Sci. 2014;35:76–85. doi: 10.1016/j.tips.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothbart S.B., Strahl B.D. Interpreting the language of histone and DNA modifications. Biochim Biophys Acta. 2014;1839:627–643. doi: 10.1016/j.bbagrm.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puustinen M.C., Sistonen L. Molecular mechanisms of heat shock factors in cancer. Cells. 2020;9(5):1202–1217. doi: 10.3390/cells9051202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neudegger T., Verghese J., Hayer-Hartl M., Hartl F.U., Bracher A. Structure of human heat-shock transcription factor 1 in complex with DNA. Nat Struct Mol Biol. 2016;23(2):140–146. doi: 10.1038/nsmb.3149. [DOI] [PubMed] [Google Scholar]
- 6.Nakai A., Tanabe M., Kawazoe Y., Inazawa J., Morimoto R.I., Nagata K. HSF4, a new member of the human heat shock factor family which lacks properties of a transcriptional activator. Mol Cell Biol. 1997;17:469–481. doi: 10.1128/MCB.17.1.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tessari A., Salata E., Ferlin A., Bartoloni L., Slongo M.L., Foresta C. Characterization of HSFY, a novel AZFb gene on the Y chromosome with a possible role in human spermatogenesis. Mol Hum Reprod. 2004;10:253–258. doi: 10.1093/molehr/gah036. [DOI] [PubMed] [Google Scholar]
- 8.Widlak W., Vydra N. The role of heat shock factors in mammalian spermatogenesis. Adv Anat Embryol Cell Biol. 2017;222:45–65. doi: 10.1007/978-3-319-51409-3_3. [DOI] [PubMed] [Google Scholar]
- 9.Labbadia J., Morimoto R.I. The biology of proteostasis in aging and disease. Annu Rev Biochem. 2015;84:435–464. doi: 10.1146/annurev-biochem-060614-033955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindquist S. The heat-shock response. Ann Rev Biochem. 1986;(55):1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- 11.Roth D.M., Balch W.E. Modeling general proteostasis: proteome balance in health and disease. Curr Opin Cell Biol. 2011;2011(23):126–134. doi: 10.1016/j.ceb.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joutsen J., Pessa J.C., Jokelainen O., Sironen R., Hartikainen J.M., Sistonen L. Comprehensive analysis of human tissues reveals unique expression and localization patterns of HSF1 and HSF2. Cell Stress Chaperones. 2024;29:235–271. doi: 10.1016/j.cstres.2024.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayer M.P. Hsf1 and Hsf2 in normal, healthy human tissues: immunohistochemistry provokes new questions. Cell Stress Chaperones. 2024;29:437–439. doi: 10.1016/j.cstres.2024.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang W., Zhang X., Cheng P., et al. HSF4 promotes tumor progression of colorectal cancer by transactivating c-MET. Mol Cell Biochem. 2023;478:1141–1150. doi: 10.1007/s11010-022-04582-2. [DOI] [PubMed] [Google Scholar]
- 15.Chalmel F., Lardenois A., Evrard B., et al. Global human tissue profiling and protein network analysis reveals distinct levels of transcriptional germline-specificity and identifies target genes for male infertility. Hum Reprod. 2012;27:3233–3248. doi: 10.1093/humrep/des301. [DOI] [PubMed] [Google Scholar]
- 16.Saju J.M., Hossain M.S., Liew W.C., et al. Heat shock factor 5 is essential for spermatogenesis in zebrafish. Cell Rep. 2018;25:3252–3261. doi: 10.1016/j.celrep.2018.11.090. e3254. [DOI] [PubMed] [Google Scholar]
- 17.Gomez-Pastor R., Burchfiel E.T., Thiele D.J. Nature Reviews Molecular Cell Biology. Nature Publishing Group; 2018. Regulation of heat shock transcription factors and their roles in physiology and disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conant G.C., Wolfe K.H. Turning a hobby into a job: how duplicated genes find new functions. Nat Rev Genet. 2008;9:938–950. doi: 10.1038/nrg2482. [DOI] [PubMed] [Google Scholar]
- 19.Kondrashov A. Genetics: the rate of human mutation. Nature. 2012;488:467–468. doi: 10.1038/488467a. [DOI] [PubMed] [Google Scholar]
- 20.Jin X., Eroglu B., Cho W., Yamaguchi Y., Moskophidis D., Mivechi N.F. Inactivation of heat shock factor Hsf4 induces cellular senescence and suppresses tumorigenesis in vivo. Mol Cancer Res. 2012;10:523–534. doi: 10.1158/1541-7786.MCR-11-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura Y., Fujimoto M., Fukushima S., et al. Heat shock factor 1 is required for migration and invasion of human melanoma in vitro and in vivo. Cancer Lett. 2014;354(2):329–335. doi: 10.1016/j.canlet.2014.08.029. [DOI] [PubMed] [Google Scholar]
- 22.Wang G., Ying Z., Jin X., et al. Essential requirement for both hsf1 and hsf2 transcriptional activity in spermatogenesis and male fertility. Genesis. 2004;38:66–80. doi: 10.1002/gene.20005. [DOI] [PubMed] [Google Scholar]
- 23.Abravaya K., Myers M.P., Murphy S.P., Morimoto R.I. The human heat shock protein hsp70 interacts with HSF, the transcription factor that regulates heat shock gene expression. Genes Dev. 1992;6(7):1153–1164. doi: 10.1101/gad.6.7.1153. [DOI] [PubMed] [Google Scholar]
- 24.Kijima T., Prince T.L., Tigue M.L., et al. HSP90 inhibitors disrupt a transient HSP90-HSF1 interaction and identify a noncanonical model of HSP90-mediated HSF1 regulation. Sci Rep. 2018 doi: 10.1038/s41598-018-25404-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi Y., Mosser D.D., Morimoto R.I. Molecular chaperones as HSF1-specific transcriptional repressors. Genes Dev. 1998;12(5):654–666. doi: 10.1101/gad.12.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alasady M.J., Mendillo M.L. The multifaceted role of HSF1 in tumorigenesis. Adv Exp Med Biol. 2020;1243:69–85. doi: 10.1007/978-3-030-40204-4_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guettouche T., Boellmann F., Lane W.S., Voellmy R. Analysis of phosphorylation of human heat shock factor 1 in cells experiencing a stress. BMC Biochem. 2005;6(4) doi: 10.1186/1471-2091-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masser A.E., Kang W., Roy J., et al. Cytoplasmic protein misfolding titrates Hsp70 to activate nuclear Hsf1. Elife. 2019;8 doi: 10.7554/eLife.47791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarge K.D., Murphy S.P., Morimoto R.I. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol Cell Biol. 1993;13:1392–1407. doi: 10.1128/mcb.13.3.1392-1407.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hentze N., Le Breton L., Wiesner J., Kempf G., Mayer M.P. Molecular mechanism of thermosensory function of human heat shock transcription factor Hsf1. eLife. 2016;5 doi: 10.7554/eLife.11576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rabindran S.K., Haroun R.I., Clos J., Wisniewski J., Wu C. Regulation of heat shock factor trimer formation: role of a conserved leucine zipper. Science. 1993;259:230–234. doi: 10.1126/science.8421783. [DOI] [PubMed] [Google Scholar]
- 32.Zuo J., Baler R., Dahl G., Voellmy R. Activation of the DNA-binding ability of human heat shock transcription factor 1 may involve the transition from an intramolecular to an intermolecular triple-stranded coiled-coil structure. Mol Cell Biol. 1994;14(11):7557–7568. doi: 10.1128/mcb.14.11.7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morimoto R.I., Santoro M.G. Stress-inducible responses and heat shock proteins: new pharmacologic targets for cytoprotection. Nat Biotechnol. 1998;16:833–838. doi: 10.1038/nbt0998-833. [DOI] [PubMed] [Google Scholar]
- 34.Sistonen L., Sarge K.D., Phillips B., Abravaya K., Morimoto R.I. Activation of heat shock factor 2 during hemin-induced differentiation of human erythroleukemia cells. Mol Cell Biol. 1992;12:4104–4111. doi: 10.1128/mcb.12.9.4104-4111.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Masson F., Razak Z., Kaigo M., et al. Identification of heat shock factor 1 molecular and cellular targets during embryonic and adult female meiosis. Mol Cell Biol. 2011;31:3410–3423. doi: 10.1128/MCB.05237-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edwards S.L., Erdenebat P., Morphis A.C., et al. Insulin/IGF-1 signaling and heat stress differentially regulate HSF1 activities in germline development. Cell Rep. 2021;36 doi: 10.1016/j.celrep.2021.109623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El Fatimy R., Miozzo F., Le Mouel A., et al. Heat shock factor 2 is a stress-responsive mediator of neuronal migration defects in models of fetal alcohol syndrome. EMBO Mol Med. 2014;6:1043–1061. doi: 10.15252/emmm.201303311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uchida S., Hara K., Kobayashi A., et al. Impaired hippocampal spinogenesis and neurogenesis and altered affective behavior in mice lacking heat shock factor 1. Proc Natl Acad Sci USA. 2011;108:1681–1686. doi: 10.1073/pnas.1016424108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim H., Gomez-Pastor R. HSF1 and its role in Huntington's disease pathology. Adv Exp Med Biol. 2023;1410:35–95. doi: 10.1007/5584_2022_742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu A.Y., Minetti C.A., Remeta D.P., Breslauer K.J., Chen K.Y. HSF1, aging, and neurodegeneration. Adv Exp Med Biol. 2023;1409:23–49. doi: 10.1007/5584_2022_733. [DOI] [PubMed] [Google Scholar]
- 41.Carpenter R.L., Gokmen-Polar Y. HSF1 as a cancer biomarker and therapeutic target. Curr Cancer Drug Targets. 2019;19:515–524. doi: 10.2174/1568009618666181018162117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dai C., Sampson S.B. HSF1: guardian of proteostasis in cancer. Trends Cell Biol. 2016;26:17–28. doi: 10.1016/j.tcb.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prince T.L., Lang B.J., Guerrero-Gimenez M.E., Fernandez-Munoz J.M., Ackerman A., Calderwood S.K. HSF1: primary factor in molecular chaperone expression and a major contributor to cancer morbidity. Cells. 2020;9(4):1046–1107. doi: 10.3390/cells9041046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reyes A., Navarro A.J., Diethelm-Varela B., Kalergis A.M., Gonzalez P.A. Is there a role for HSF1 in viral infections? FEBS Open Bio. 2022;12:1112–1124. doi: 10.1002/2211-5463.13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Y., Wang B., Liu D., et al. Hsp90 chaperone inhibitor 17-AAG attenuates Abeta-induced synaptic toxicity and memory impairment. J Neurosci. 2014;34:2464–2470. doi: 10.1523/JNEUROSCI.0151-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bobkova N.V., Garbuz D.G., Nesterova I., et al. Therapeutic effect of exogenous hsp70 in mouse models of Alzheimer's disease. J Alzheimers Dis. 2014;38:425–435. doi: 10.3233/JAD-130779. [DOI] [PubMed] [Google Scholar]
- 47.Auluck P.K., Chan H.Y., Trojanowski J.Q., Lee V.M., Bonini N.M. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson's disease. Science. 2002;295:865–868. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- 48.Liangliang X., Yonghui H., Shunmei E., Shoufang G., Wei Z., Jiangying Z. Dominant-positive HSF1 decreases alpha-synuclein level and alpha-synuclein-induced toxicity. Mol Biol Rep. 2010;37:1875–1881. doi: 10.1007/s11033-009-9623-2. [DOI] [PubMed] [Google Scholar]
- 49.Gomez-Pastor R., Burchfiel E.T., Neef D.W., et al. Abnormal degradation of the neuronal stress-protective transcription factor HSF1 in Huntington’s disease. Nat Commun. 2017;13(8):14405. doi: 10.1038/ncomms14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mansky R.H., Greguske E.A., Yu D., et al. Tumor suppressor p53 regulates heat shock factor 1 protein degradation in Huntington's disease. Cell Rep. 2023;42 doi: 10.1016/j.celrep.2023.112198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen H.J., Mitchell J.C., Novoselov S., et al. The heat shock response plays an important role in TDP-43 clearance: evidence for dysfunction in amyotrophic lateral sclerosis. Brain. 2016;139:1417–1432. doi: 10.1093/brain/aww028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grunberg N., Pevsner-Fischer M., Goshen-Lago T., et al. Cancer-associated fibroblasts promote aggressive gastric cancer phenotypes via heat shock factor 1-mediated secretion of extracellular vesicles. Cancer Res. 2021;81:1639–1653. doi: 10.1158/0008-5472.CAN-20-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mendillo M.L., Santagata S., Koeva M., et al. HSF1 drives a transcriptional program distinct from heat shock to support highly malignant human cancers. Cell. 2012;150:549–562. doi: 10.1016/j.cell.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scherz-Shouval R., Santagata S., Mendillo M.L., et al. The reprogramming of tumor stroma by HSF1 is a potent enabler of malignancy. Cell. 2014;158:564–578. doi: 10.1016/j.cell.2014.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shaashua L., Ben-Shmuel A., Pevsner-Fischer M., et al. BRCA mutational status shapes the stromal microenvironment of pancreatic cancer linking clusterin expression in cancer associated fibroblasts with HSF1 signaling. Nat Commun. 2022;13:6513. doi: 10.1038/s41467-022-34081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dai C., Whitesell L., Rogers A.B., Lindquist S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell. 2007;130:1005–1018. doi: 10.1016/j.cell.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin X., Moskophidis D., Mivechi N.F. Heat shock transcription factor 1 is a key determinant of HCC development by regulating hepatic steatosis and metabolic syndrome. Cell Metab. 2011;14:91–103. doi: 10.1016/j.cmet.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kourtis N., Lazaris C., Hockemeyer K., et al. Oncogenic hijacking of the stress response machinery in T cell acute lymphoblastic leukemia. Nat Med. 2018;24:1157–1166. doi: 10.1038/s41591-018-0105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Min J.N., Huang L., Zimonjic D.B., Moskophidis D., Mivechi N.F. Selective suppression of lymphomas by functional loss of Hsf1 in a p53-deficient mouse model for spontaneous tumors. Oncogene. 2007;26:5086–5097. doi: 10.1038/sj.onc.1210317. [DOI] [PubMed] [Google Scholar]
- 60.Xi C., Hu Y., Buckhaults P., Moskophidis D., Mivechi N.F. Heat shock factor Hsf1 cooperates with ErbB2 (Her2/Neu) protein to promote mammary tumorigenesis and metastasis. J Biol Chem. 2012;287:35646–35657. doi: 10.1074/jbc.M112.377481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levi-Galibov O., Lavon H., Wassermann-Dozorets R., et al. Heat shock factor 1-dependent extracellular matrix remodeling mediates the transition from chronic intestinal inflammation to colon cancer. Nat Commun. 2020;11:6245. doi: 10.1038/s41467-020-20054-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Santagata S., Hu R., Lin N.U., et al. High levels of nuclear heat-shock factor 1 (HSF1) are associated with poor prognosis in breast cancer. Proc Natl Acad Sci USA. 2011;108:18378–18383. doi: 10.1073/pnas.1115031108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Filone C.M., Caballero I.S., Dower K., et al. The master regulator of the cellular stress response (HSF1) is critical for orthopoxvirus infection. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1003904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nekongo E.E., Ponomarenko A.I., Dewal M.B., Butty V.L., Browne E.P., Shoulders M.D. HSF1 activation can restrict HIV replication. ACS Infect Dis. 2020;6:1659–1666. doi: 10.1021/acsinfecdis.0c00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Phillips A.M., Gonzalez L.O., Nekongo E.E., et al. Host proteostasis modulates influenza evolution. Elife. 2017;6 doi: 10.7554/eLife.28652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang J.R., Arii J., Hirai M., Nishimura M., Mori Y. Human herpesvirus 6A nuclear matrix protein U37 interacts with heat shock transcription factor 1 and activates the heat shock response. J Virol. 2023;97 doi: 10.1128/jvi.00718-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Phillips A.M., Ponomarenko A.I., Chen K., et al. Destabilized adaptive influenza variants critical for innate immune system escape are potentiated by host chaperones. PLoS Biol. 2018;16 doi: 10.1371/journal.pbio.3000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang F.W., Wu X.R., Liu W.J., et al. Heat shock factor 1 upregulates transcription of Epstein-Barr Virus nuclear antigen 1 by binding to a heat shock element within the BamHI-Q promoter. Virology. 2011;421:184–191. doi: 10.1016/j.virol.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 69.Chen R., Liliental J.E., Kowalski P.E., Lu Q., Cohen S.N. Regulation of transcription of hypoxia-inducible factor-1alpha (HIF-1alpha) by heat shock factors HSF2 and HSF4. Oncogene. 2011;30:2570–2580. doi: 10.1038/onc.2010.623. [DOI] [PubMed] [Google Scholar]
- 70.Mathew A., Mathur S.K., Morimoto R.I. Heat shock response and protein degradation: regulation of HSF2 by the ubiquitin-proteasome pathway. Mol Cell Biol. 1998;18:5091–5098. doi: 10.1128/MCB.18.9.5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Santopolo S., Riccio A., Rossi A., Santoro M.G. The proteostasis guardian HSF1 directs the transcription of its paralog and interactor HSF2 during proteasome dysfunction. Cell Mol Life Sci. 2021;78:1113–1129. doi: 10.1007/s00018-020-03568-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Joutsen J., Da Silva A.J., Luoto J.C., et al. Heat shock factor 2 protects against proteotoxicity by maintaining cell-cell adhesion. Cell Rep. 2020;30:583–597. doi: 10.1016/j.celrep.2019.12.037. e586. [DOI] [PubMed] [Google Scholar]
- 73.Himanen S.V., Puustinen M.C., Da Silva A.J., Vihervaara A., Sistonen L. HSFs drive transcription of distinct genes and enhancers during oxidative stress and heat shock. Nucleic Acids Res. 2022;50:6102–6115. doi: 10.1093/nar/gkac493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smith R.S., Takagishi S.R., Amici D.R., et al. HSF2 cooperates with HSF1 to drive a transcriptional program critical for the malignant state. Sci Adv. 2022;8 doi: 10.1126/sciadv.abj6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kallio M., Chang Y., Manuel M., et al. Brain abnormalities, defective meiotic chromosome synapsis and female subfertility in HSF2 null mice. EMBO J. 2002;21:2591–2601. doi: 10.1093/emboj/21.11.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilkerson D.C., Murphy L.A., Sarge K.D. Interaction of HSF1 and HSF2 with the Hspa1b promoter in mouse epididymal spermatozoa. Biol Reprod. 2008;79:283–288. doi: 10.1095/biolreprod.107.066241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Akerfelt M., Morimoto R.I., Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. 2010;11:545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chang Y., Ostling P., Akerfelt M., et al. Role of heat-shock factor 2 in cerebral cortex formation and as a regulator of p35 expression. Genes Dev. 2006;20:836–847. doi: 10.1101/gad.366906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bjork J.K., Akerfelt M., Joutsen J., et al. Heat-shock factor 2 is a suppressor of prostate cancer invasion. Oncogene. 2016;35:1770–1784. doi: 10.1038/onc.2015.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang L.N., Ning Z.Y., Wang L., Yan X., Meng Z.Q. HSF2 regulates aerobic glycolysis by suppression of FBP1 in hepatocellular carcinoma. Am J Cancer Res. 2019;9:1607–1621. [PMC free article] [PubMed] [Google Scholar]
- 81.Fujimoto M., Izu H., Seki K., et al. HSF4 is required for normal cell growth and differentiation during mouse lens development. EMBO J. 2004;23:4297–4306. doi: 10.1038/sj.emboj.7600435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ma P., Tang W.G., Hu J.W., et al. HSP4 triggers epithelial-mesenchymal transition and promotes motility capacities of hepatocellular carcinoma cells via activating AKT. Liver Int. 2020;40:1211–1223. doi: 10.1111/liv.14410. [DOI] [PubMed] [Google Scholar]
- 83.Fiorenza M.T., Farkas T., Dissing M., Kolding D., Zimarino V. Complex expression of murine heat shock transcription factors. Nucleic Acids Res. 1995;23:467–474. doi: 10.1093/nar/23.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goodson M.L., Park-Sarge O.K., Sarge K.D. Tissue-dependent expression of heat shock factor 2 isoforms with distinct transcriptional activities. Mol Cell Biol. 1995;15:5288–5293. doi: 10.1128/MCB.15.10.5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leppa S., Pirkkala L., Saarento H., Sarge K.D., Sistonen L. Overexpression of HSF2-beta inhibits hemin-induced heat shock gene expression and erythroid differentiation in K562 cells. J Biol Chem. 1997;272:15293–15298. doi: 10.1074/jbc.272.24.15293. [DOI] [PubMed] [Google Scholar]
- 86.Tanabe M., Sasai N., Nagata K., et al. The mammalian HSF4 gene generates both an activator and a repressor of heat shock genes by alternative splicing. J Biol Chem. 1999;274:27845–27856. doi: 10.1074/jbc.274.39.27845. [DOI] [PubMed] [Google Scholar]
- 87.Hietakangas V., Anckar J., Blomster H.A., et al. PDSM, a motif for phosphorylation-dependent SUMO modification. Proc Natl Acad Sci USA. 2006;103:45–50. doi: 10.1073/pnas.0503698102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Korfanty J., Stokowy T., Widlak P., et al. Crosstalk between HSF1 and HSF2 during the heat shock response in mouse testes. Int J Biochem Cell Biol. 2014;57:76–83. doi: 10.1016/j.biocel.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 89.Ostling P., Bjork J.K., Roos-Mattjus P., Mezger V., Sistonen L. Heat shock factor 2 (HSF2) contributes to inducible expression of hsp genes through interplay with HSF1. J Biol Chem. 2007;282:7077–7086. doi: 10.1074/jbc.M607556200. [DOI] [PubMed] [Google Scholar]
- 90.Sandqvist A., Bjork J.K., Akerfelt M., et al. Heterotrimerization of heat-shock factors 1 and 2 provides a transcriptional switch in response to distinct stimuli. Mol Biol Cell. 2009;20:1340–1347. doi: 10.1091/mbc.e08-08-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Takaki E., Fujimoto M., Sugahara K., et al. Maintenance of olfactory neurogenesis requires HSF1, a major heat shock transcription factor in mice. J Biol Chem. 2006;281:4931–4937. doi: 10.1074/jbc.M506911200. [DOI] [PubMed] [Google Scholar]
- 92.Guo F., Cheng X., Jing B., Wu H., Jin X. FGD3 binds with HSF4 to suppress p65 expression and inhibit pancreatic cancer progression. Oncogene. 2022;41:838–851. doi: 10.1038/s41388-021-02140-6. [DOI] [PubMed] [Google Scholar]
- 93.Yuan J., Liu H., Gao W., et al. MicroRNA-378 suppresses myocardial fibrosis through a paracrine mechanism at the early stage of cardiac hypertrophy following mechanical stress. Theranostics. 2018;8:2565–2582. doi: 10.7150/thno.22878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Raychaudhuri S., Loew C., Körner R., et al. Interplay of acetyltransferase EP300 and the proteasome system in regulating heat shock transcription factor 1. Cell. 2014;156:975–985. doi: 10.1016/j.cell.2014.01.055. [DOI] [PubMed] [Google Scholar]
- 95.Kourtis N., Moubarak R.S., Aranda-Orgilles B., et al. FBXW7 modulates cellular stress response and metastatic potential through HSF1 post-translational modification. Nat Cell Biol. 2015;17:322–332. doi: 10.1038/ncb3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Powell C.D., Paullin T.R., Aoisa C., Menzie C.J., Ubaldini A., Westerheide S.D. The heat shock transcription factor HSF1 induces ovarian cancer epithelial-mesenchymal transition in a 3D spheroid growth model. PLoS One. 2016;11(12) doi: 10.1371/journal.pone.0168389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Elsing A.N., Aspelin C., Bjork J.K., et al. Expression of HSF2 decreases in mitosis to enable stress-inducible transcription and cell survival. J Cell Biol. 2014;206:735–749. doi: 10.1083/jcb.201402002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rossi A., Riccio A., Coccia M., Trotta E., La Frazia S., Santoro M.G. The proteasome inhibitor bortezomib is a potent inducer of zinc finger AN1-type domain 2a gene expression: role of heat shock factor 1 (HSF1)-heat shock factor 2 (HSF2) heterocomplexes. J Biol Chem. 2014;289:12705–12715. doi: 10.1074/jbc.M113.513242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bjork J.K., Sandqvist A., Elsing A.N., Kotaja N., Sistonen L. miR-18, a member of Oncomir-1, targets heat shock transcription factor 2 in spermatogenesis. Development. 2010;137:3177–3184. doi: 10.1242/dev.050955. [DOI] [PubMed] [Google Scholar]
- 100.Meng X., Chen X., Lu P., et al. miR-202 promotes cell apoptosis in esophageal squamous cell carcinoma by targeting HSF2. Oncol Res. 2017;25:215–223. doi: 10.3727/096504016×14732772150541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gaglia G., Rashid R., Yapp C., et al. HSF1 phase transition mediates stress adaptation and cell fate decisions. Nat Cell Biol. 2020;22:151–158. doi: 10.1038/s41556-019-0458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang H., Shao S., Zeng Y., et al. Reversible phase separation of HSF1 is required for an acute transcriptional response during heat shock. Nat Cell Biol. 2022;24:340–352. doi: 10.1038/s41556-022-00846-7. [DOI] [PubMed] [Google Scholar]
- 103.Kribelbauer J.F., Rastogi C., Bussemaker H.J., Mann R.S. Low-affinity binding sites and the transcription factor specificity paradox in eukaryotes. Annu Rev Cell Dev Biol. 2019;35:357–379. doi: 10.1146/annurev-cellbio-100617-062719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ubersax J.A., Ferrell J.E., Jr Mechanisms of specificity in protein phosphorylation. Nat Rev Mol Cell Biol. 2007;8:530–541. doi: 10.1038/nrm2203. [DOI] [PubMed] [Google Scholar]
- 105.Yasuda K., Hirohashi Y., Mariya T., et al. Phosphorylation of HSF1 at serine 326 residue is related to the maintenance of gynecologic cancer stem cells through expression of HSP27. Oncotarget. 2017;8(19):31540–31553. doi: 10.18632/oncotarget.16361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Soncin F., Zhang X., Chu B., et al. Transcriptional activity and DNA binding of heat shock factor-1 involve phosphorylation on threonine 142 by CK2. Biochem Biophys Res Commun. 2003;303:700–706. doi: 10.1016/s0006-291x(03)00398-x. [DOI] [PubMed] [Google Scholar]
- 107.Chu B., Zhong R., Soncin F., Stevenson M.A., Calderwood S.K. Transcriptional activity of heat shock factor 1 at 37 degrees C is repressed through phosphorylation on two distinct serine residues by glycogen synthase kinase 3 and protein kinases Calpha and Czeta. J Biol Chem. 1998;273:18640–18646. doi: 10.1074/jbc.273.29.18640. [DOI] [PubMed] [Google Scholar]
- 108.Wang X., Khaleque M.A., Zhao M.J., Zhong R., Gaestel M., Calderwood S.K. Phosphorylation of HSF1 by MAPK-activated protein kinase 2 on serine 121, inhibits transcriptional activity and promotes HSP90 binding. J Biol Chem. 2006;281:782–791. doi: 10.1074/jbc.M505822200. [DOI] [PubMed] [Google Scholar]
- 109.Budzyński M.A., Puustinen M.C., Joutsen J., Sistonen L. Uncoupling stress-inducible phosphorylation of heat shock factor 1 from its activation. Mol Cell Biol. 2015;35(14):2530–2540. doi: 10.1128/MCB.00816-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zheng X., Krakowiak J., Patel N., et al. Dynamic control of Hsf1 during heat shock by a chaperone switch and phosphorylation. eLife. 2016;5 doi: 10.7554/eLife.18638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chou S.D., Prince T., Gong J., Calderwood S.K. mTOR is essential for the proteotoxic stress response, HSF1 activation and heat shock protein synthesis. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Holmberg C.I., Hietakangas V., Mikhailov A., et al. Phosphorylation of serine 230 promotes inducible transcriptional activity of heat shock factor 1. EMBO J. 2001;20:3800–3810. doi: 10.1093/emboj/20.14.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang Y., Murshid A., Prince T., Calderwood S.K. Protein kinase A regulates molecular chaperone transcription and protein aggregation. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dai R., Frejtag W., He B., Zhang Y., Mivechi N.F. c-Jun NH2-terminal kinase targeting and phosphorylation of heat shock factor-1 suppress its transcriptional activity. J Biol Chem. 2000;275(24):18210–18218. doi: 10.1074/jbc.M000958200. [DOI] [PubMed] [Google Scholar]
- 115.Chu B., Soncin F., Price B.D., Stevenson M.A., Calderwood S.K. Sequential phosphorylation by mitogen-activated protein kinase and glycogen synthase kinase 3 represses transcriptional activation by heat shock factor-1. J Biol Chem. 1996;271(48):30847–30857. doi: 10.1074/jbc.271.48.30847. [DOI] [PubMed] [Google Scholar]
- 116.Wang X., Grammatikakis N., Siganou A., Calderwood S.K. Regulation of molecular chaperone gene transcription involves the serine phosphorylation, 14-3-3 epsilon binding, and cytoplasmic sequestration of heat shock factor 1. Mol Cell Biol. 2003;23:6013–6026. doi: 10.1128/MCB.23.17.6013-6026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schulz R., Streller F., Scheel A.H., et al. HER2/ErbB2 activates HSF1 and thereby controls HSP90 clients including MIF in HER2-overexpressing breast cancer. Cell Death Dis. 2014;5(1):e980. doi: 10.1038/cddis.2013.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lu W.C., Omari R., Ray H., et al. AKT1 mediates multiple phosphorylation events that functionally promote HSF1 activation. FEBS J. 2022;289:3876–3893. doi: 10.1111/febs.16375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dai C., Santagata S., Tang Z., et al. Loss of tumor suppressor NF1 activates HSF1 to promote carcinogenesis. J Clin Invest. 2012;122(10):3742–3754. doi: 10.1172/JCI62727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dai S., Tang Z., Cao J., et al. Suppression of the HSF1-mediated proteotoxic stress response by the metabolic stress sensor AMPK. EMBO J. 2015;34(3):275–293. doi: 10.15252/embj.201489062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Narita T., Weinert B.T., Choudhary C. Functions and mechanisms of non-histone protein acetylation. Nat Rev Mol Cell Biol. 2019;20:156–174. doi: 10.1038/s41580-018-0081-3. [DOI] [PubMed] [Google Scholar]
- 122.Westerheide S.D., Anckar J., Stevens S.M., Sistonen L., Morimoto R.I. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT. Science. 2009;323(5917):1063–1066. doi: 10.1126/science.1165946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zelin E., Zhang Y., Toogun O.A., Zhong S., Freeman B.C. The p23 molecular chaperone and GCN5 acetylase jointly modulate protein-DNA dynamics and open chromatin status. Mol Cell. 2012;48:459–470. doi: 10.1016/j.molcel.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zelin E., Freeman B.C. Lysine deacetylases regulate the heat shock response including the age-associated impairment of HSF1. J Mol Biol. 2015;427:1644–1654. doi: 10.1016/j.jmb.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Attar N., Kurdistani S.K. Exploitation of EP300 and CREBBP lysine acetyltransferases by cancer. Cold Spring Harb Perspect Med. 2017;7(3) doi: 10.1101/cshperspect.a026534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.de Thonel A., Ahlskog J.K., Daupin K., et al. CBP-HSF2 structural and functional interplay in Rubinstein-Taybi neurodevelopmental disorder. Nat Commun. 2022;13:7002. doi: 10.1038/s41467-022-34476-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wilkinson K.A., Henley J.M. Mechanisms, regulation and consequences of protein SUMOylation. Biochem J. 2010;428:133–145. doi: 10.1042/BJ20100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hietakangas V., Ahlskog J.K., Jakobsson A.M., et al. Phosphorylation of serine 303 is a prerequisite for the stress-inducible SUMO modification of heat shock factor 1. Mol Cell Biol. 2003;23:2953–2968. doi: 10.1128/MCB.23.8.2953-2968.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Anckar J., Hietakangas V., Denessiouk K., Thiele D.J., Johnson M.S., Sistonen L. Inhibition of DNA binding by differential sumoylation of heat shock factors. Mol Cell Biol. 2006;26:955–964. doi: 10.1128/MCB.26.3.955-964.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Goodson M.L., Hong Y., Rogers R., Matunis M.J., Park-Sarge O.K., Sarge K.D. Sumo-1 modification regulates the DNA binding activity of heat shock transcription factor 2, a promyelocytic leukemia nuclear body associated transcription factor. J Biol Chem. 2001;276:18513–18518. doi: 10.1074/jbc.M008066200. [DOI] [PubMed] [Google Scholar]
- 131.Boellmann F., Guettouche T., Guo Y., Fenna M., Mnayer L., Voellmy R. DAXX interacts with heat shock factor 1 during stress activation and enhances its transcriptional activity. Proc Natl Acad Sci USA. 2004;101:4100–4105. doi: 10.1073/pnas.0304768101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xu M., Lin L., Ram B.M., et al. Heat shock factor 1 (HSF1) specifically potentiates c-MYC-mediated transcription independently of the canonical heat shock response. Cell Rep. 2023;42 doi: 10.1016/j.celrep.2023.112557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fujimoto M., Takii R., Matsumoto M., et al. HSF1 phosphorylation establishes an active chromatin state via the TRRAP-TIP60 complex and promotes tumorigenesis. Nat Commun. 2022;13:4355. doi: 10.1038/s41467-022-32034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nitika, Porter C.M., Truman A.W., Truttmann M.C. Post-translational modifications of Hsp70 family proteins: expanding the chaperone code. J Biol Chem. 2020;295:10689–10708. doi: 10.1074/jbc.REV120.011666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nitika, Zheng B., Ruan L., et al. Comprehensive characterization of the Hsp70 interactome reveals novel client proteins and interactions mediated by posttranslational modifications. PLoS Biol. 2022;20 doi: 10.1371/journal.pbio.3001839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Omkar S., Rysbayeva A., Truman A.W. Understanding chaperone specificity: evidence for a 'client code’. Trends Biochem Sci. 2023;48:662–664. doi: 10.1016/j.tibs.2023.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Alasady M.J., Koeva M., Takagishi S.R., et al. An HSF1-JMJD6-HSP feedback circuit promotes cell adaptation to proteotoxic stress. Proc Natl Acad Sci USA. 2024;121 doi: 10.1073/pnas.2313370121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ham H., Woolery A.R., Tracy C., Stenesen D., Kramer H., Orth K. Unfolded protein response-regulated Drosophila Fic (dFic) protein reversibly AMPylates BiP chaperone during endoplasmic reticulum homeostasis. J Biol Chem. 2014;289:36059–36069. doi: 10.1074/jbc.M114.612515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Preissler S., Rohland L., Yan Y., Chen R., Read R.J., Ron D. AMPylation targets the rate-limiting step of BiP’s ATPase cycle for its functional inactivation. Elife. 2017;6 doi: 10.7554/eLife.29428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wilkerson D.C., Skaggs H.S., Sarge K.D. HSF2 binds to the Hsp90, Hsp27, and c-Fos promoters constitutively and modulates their expression. Cell Stress Chaperones. 2007;12:283–290. doi: 10.1379/csc-250.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang J., Goodson M.L., Hong Y., Sarge K.D. MEL-18 interacts with HSF2 and the SUMO E2 UBC9 to inhibit HSF2 sumoylation. J Biol Chem. 2008;283:7464–7469. doi: 10.1074/jbc.M707122200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Murphy L.A., Wilkerson D.C., Hong Y., Sarge K.D. PRC1 associates with the hsp70i promoter and interacts with HSF2 during mitosis. Exp Cell Res. 2008;314:2224–2230. doi: 10.1016/j.yexcr.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ahlskog J.K., Bjork J.K., Elsing A.N., et al. Anaphase-promoting complex/cyclosome participates in the acute response to protein-damaging stress. Mol Cell Biol. 2010;30:5608–5620. doi: 10.1128/MCB.01506-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang J., Hu Y.Z., Xueli L., et al. The inhibition of CMV promoter by heat shock factor 4b is regulated by Daxx. Int J Biochem Cell Biol. 2010;42:1698–1707. doi: 10.1016/j.biocel.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 145.Hu Y., Mivechi N.F. Association and regulation of heat shock transcription factor 4b with both extracellular signal-regulated kinase mitogen-activated protein kinase and dual-specificity tyrosine phosphatase DUSP26. Mol Cell Biol. 2006;26:3282–3294. doi: 10.1128/MCB.26.8.3282-3294.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tu N., Hu Y., Mivechi N.F. Heat shock transcription factor (Hsf)-4b recruits Brg1 during the G1 phase of the cell cycle and regulates the expression of heat shock proteins. J Cell Biochem. 2006;98:1528–1542. doi: 10.1002/jcb.20865. [DOI] [PubMed] [Google Scholar]
- 147.Amici D.R., Jackson J.M., Truica M.I., Smith R.S., Abdulkadir S.A., Mendillo M.L. FIREWORKS: a bottom-up approach to integrative coessentiality network analysis. Life Sci Alliance. 2020;4(2) doi: 10.26508/lsa.202000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Brockway S., Wang G., Jackson J.M., et al. Quantitative and multiplexed chemical-genetic phenotyping in mammalian cells with QMAP-Seq. Nat Commun. 2020;11:5722. doi: 10.1038/s41467-020-19553-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pasqua A.E., Sharp S.Y., Chessum N.E.A., et al. HSF1 pathway inhibitor clinical candidate (CCT361814/NXP800) developed from a phenotypic screen as a potential treatment for refractory ovarian cancer and other malignancies. J Med Chem. 2023;66:5907–5936. doi: 10.1021/acs.jmedchem.3c00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Westerheide S.D., Bosman J.D., Mbadugha B.N., et al. Celastrols as inducers of the heat shock response and cytoprotection. J Biol Chem. 2004;279:56053–56060. doi: 10.1074/jbc.M409267200. [DOI] [PubMed] [Google Scholar]
- 151.Yoon Y.J., Kim J.A., Shin K.D., et al. KRIBB11 inhibits HSP70 synthesis through inhibition of heat shock factor 1 function by impairing the recruitment of positive transcription elongation factor b to the hsp70 promoter. J Biol Chem. 2011;286:1737–1747. doi: 10.1074/jbc.M110.179440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dong Q., Xiu Y., Wang Y., et al. HSF1 is a driver of leukemia stem cell self-renewal in acute myeloid leukemia. Nat Commun. 2022;13:6107. doi: 10.1038/s41467-022-33861-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were generated for the research described in the article.