Abstract
This viewpoint summarizes findings from analyses of large personal patient databases of myeloproliferative neoplasms (MPNs) to assess the impact of thrombosis on mortality, disease progression, and second cancers (SC). Despite advances, the current incidence of arterial and venous thrombosis remains a challenge. These events appear to signal a more aggressive disease course, as evidenced by their association with myelofibrosis progression and mortality using multistate models and time-dependent multivariable analysis. Inflammatory biomarkers, such as the neutrophil-to-lymphocyte ratio (NLR), are associated with the aggressiveness of polycythemia vera (PV) and essential thrombocythemia (ET), linking thrombosis to SC risk. This suggests a common inflammatory pathway likely influencing cardiovascular disease and cancer incidence. Notably, this is observed more frequently in younger patients, likely due to prolonged exposure to MPN and environmental inflammatory triggers. These data underscore the need for new studies to validate these associations, delineate the sequence of events, and identify therapeutic targets to mitigate thrombotic events and potentially improve overall patient outcomes in MPN.
Subject terms: Risk factors, Myeloproliferative disease
Introduction
The classic chronic myeloproliferative neoplasms (MPNs) include polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF) [1]. These disorders are characterized by a high incidence of arterial and venous thrombotic events [2, 3], potential progression to post-PV and post-ET myelofibrosis [4], and acute leukemia [5]. Understanding of the pathophysiology of MPNs has improved significantly with the identification of common genetic mutations, most notably the JAK2 V617F mutation, which is found in over 95% of PV and approximately 60–70% of ET and PMF cases [1, 6, 7]. Therapy is aimed at reducing the incidence of thrombosis, which is a major cause of death and severe disability in some patients [8, 9].
Recent reports suggest that the occurrence of thrombosis, particularly arterial events during the course of PV and ET, may pose additional risks such as progression to myelofibrosis [10, 11], increased mortality [10–13], and the development of secondary solid tumors [14, 15]. These risks arise from disease-related clonal hematopoiesis and subsequent chronic systemic inflammation, leading to thrombosis and genetic instability.
In our large databases of patients with MPN, we investigated the incidence and risk factors of thrombosis that may explain this association, culminating in an increased risk of mortality. This Viewpoint presents our findings with the goal of clarifying the current clinical evidence regarding key events that influence disease severity and may serve as potential therapeutic targets. This work is not intended to be a systematic review of the literature, but rather a perspective to stimulate interest and further research into these critical issues.
Rates of thrombosis in MPNs
Despite recommended treatments, thrombosis remains a significant challenge for patients diagnosed with MPNs today. It often heralds the diagnosis of MPN in approximately 20% of cases, with a persistent risk observed at subsequent follow-up. A recent study of 9429 MPN patients and 35,820 matched controls diagnosed between 1987 and 2009 and followed until 2010 documented significantly increased hazard ratios (HRs) for arterial and venous thrombosis compared to controls at various time intervals that at 3 months, 1 year, and 5 years were 3.0, 2.0, and 1.5, respectively; the corresponding HRs for venous thrombosis were even higher with values of 9.7, 4.7, and 3.2 [16]. While conventional therapy with hydroxyurea (HU) has shown overall efficacy in reducing arterial thrombosis [17–19], its effect is less pronounced in preventing venous thrombosis [20] and in older patient categories. In a systematic review and meta-analysis of patients with PV, thrombosis rates were 1.9%, 3.6%, and 6.8% person/year at a median age of 60, 70, and 80 years, respectively [21]. These figures are at least 4 times higher than in the normal population [22].
Polycythemia vera
In the largest epidemiologic study of PV (the European Collaboration on Low-dose Aspirin [ECLAP] study), which enrolled 1638 patients in the years before the discovery of JAK2V617F, cardiovascular mortality accounted for 45% of all recorded deaths. The rate of cardiovascular death was 1.7/100 person-years, with an overall incidence of 4.5% over a median follow-up of 2.8 years. The leading causes were coronary heart disease (15% of all deaths), congestive heart failure (8%), non-hemorrhagic stroke (8%), and pulmonary embolism (3.6%). During the same follow-up period, the cumulative rate of non-fatal thrombosis was 5.5% patients per year, with no discernible difference between arterial and venous thrombosis [23].
It is noteworthy that recent studies in contemporary PV patients have shown a lower incidence rate of major post-diagnosis thrombosis of 2.6/100 person-years [22, 24] (Table 1), a figure comparatively lower than that observed in the ECLAP cohort, but similar to the findings of the recent CYTO-PV randomized clinical trial, where the incidence rate was reported to be 2.7/100 person-years [25].
Table 1.
Frequency of thrombotic events in the different MPNs.
| PV [22, 24] N = 1545 | ET [29] N = 891 | pre-PMF [29] N = 180 | PMF [30] N = 707 | |
|---|---|---|---|---|
| Total thrombosis | ||||
| Follow-up (years), median (IQR) | 5.6 (2.8–9.4) | 5.6 (2.3–10.1) | 6.1 (2.5–10.8) | 2.92 (1.14–5.92) |
| N° events, n (%) | 290 (19%) | 109 (12%) | 27 (15%) | 47 (7%) |
| Incidence rate per 100 patients/year | 2.6 | 1.7 | 1.9 | 1.6 |
| Arterial thrombosis | ||||
| N° events, n (%) | 184 (12%) | 79 (9%) | 20 (11%) | 25 (4%) |
| Incidence rate per 100 patients-year | 1.6 | 1.2 | 1.4 | 0.9 |
| Venous thrombosis | ||||
| N° events, n (%) | 137 (9%) | 37 (4%) | 9 (5%) | 22 (3%) |
| Incidence rate per 100 patients/year | 1.1 | 0.6 | 0.6 | 0.8 |
Similarly low rates of thrombotic events were confirmed in a recent cohort of 1,545 PV patients recruited by the International Working Group for MPN Research and Treatment (IWG-MRT). After a median follow-up of 6.9 years, arterial thrombosis occurred in 184 patients (12%), and venous thrombosis in 137 patients (9%), with an overall annual incidence rate of 2.8% [22]. Regarding venous thrombosis, there are limited data on the prevalence of splanchnic vein thrombosis (SVT) and cerebral vein thrombosis in PV. The prevalence of SVT in PV has been reported to range between 5% and 10%, a rate similar to that observed in ET but notably higher than in PMF (0.6–1.0%) [26].
As most PV studies have included patients diagnosed over different time periods, caution must be exercised when reporting event rates, which should be evaluated taking into account the time of data collection, diagnostic criteria used, and treatments.
Essential thrombocythemia
In ET, the incidence of thrombosis is slightly lower than in PV (2.3/100 person-years), and arterial events are more common (70%) than venous thromboembolism (VTE), which includes conditions such as deep vein thrombosis (DVT) or pulmonary embolism, as well as thrombosis in unusual sites such as splanchnic or cerebral veins [27]. However, it should be emphasized that the epidemiology of thrombosis and bleeding in ET older studies needs to be re-evaluated according to the 2016/2022 World Health Organization (WHO) [28] and the International Consensus [1] diagnostic classifications, which highlighted the distinction between “prefibrotic” and “overtly fibrotic” PMF; the former entity with a presentation mimicking ET but a different natural history requiring specific monitoring and management. In an international study [29] of 891 patients diagnosed with ET and 180 patients diagnosed with prefibrotic PMF, the rates of total thrombosis after diagnosis were 1.70/100 person-years and 1.90/100 person-years, respectively (Table 1).
Myelofibrosis
In primary MF (PMF), the prevalence of major thrombosis was assessed in 707 patients in four European centers; the overall incidence rate of cardiovascular death and non-fatal thrombotic complications was 2.23 events/100 person-years, a figure comparable to that observed in ET; unlike ET, in which arterial events were more frequent (70%), no significant difference was observed between non-fatal venous and arterial thrombosis [30]. In the same study, the incidence rate of non-fatal thrombosis was 1.6/100 person-years [30] (Table 1). In another recent study of 642 PMF patients and 2568 matched controls, venous events were significantly more frequent than arterial events; interestingly, thrombosis was predominantly observed in atypical sites and more likely to occur around the time of PMF diagnosis [31]. In a large study of patients with myelofibrosis secondary to PV and ET (n = 1258), major thrombotic events were reported in 2.3% of patients per year, with venous events accounting for two-thirds of the total [32]. These figures are superimposable to the ones found in PMF.
Recurrence rates of thrombosis after the first event
While the above-mentioned thrombosis rates in ET and PV were calculated by evaluating the first episode occurring after diagnosis, there are few data available on recurrence rates. In a retrospective study, the recurrence of thrombosis after a single episode was evaluated in 235 patients with PV and 259 with ET. The primary thrombotic events of interest were ischemic stroke, transient ischemic attack, acute myocardial infarction, unstable angina, peripheral arterial thrombosis, retinal artery or vein occlusion, deep vein thrombosis (including cerebral and splanchnic vein thrombosis), and pulmonary embolism. Thrombosis recurred in 166 patients (33.6%), resulting in an incidence rate of 7.6% patient-years. It’s noteworthy that HU was more effective in reducing recurrent events after arterial thrombosis (hazard ratio HR 0.47, 95% CI 0.31–0.70), and less effective in preventing recurrent venous thromboses [33]. In another retrospective study of patients with MPN and stroke, HU confirmed efficacy in reducing recurrent stroke. On multivariable analysis, the HR was 0.24 (95% CI 0.08–0.76) [34].
In conclusion, despite currently recommended treatments, thrombosis remains a significant challenge in the management of MPNs. This highlights the need for continued efforts to improve prophylactic strategies and develop more effective therapies to reduce the risk of thrombotic events and likely improve other critical outcomes as reported in this paper. In particular, we highlight the role of lifestyle and all modifiable risk factors such as diabetes, obesity, smoking, hyperlipidemia and arterial hypertension [35]. A significant different lower thrombosis-free survival (p = 0.025) was documented in patients with hypertension (34%) in comparison with those without hypertension (66%); this difference was evident after 4 years of diagnosis and confirmed in an external cohort of comparable PV patients.
Arterial thrombosis affects survival and disease progression in ET and PV
The impact of thrombosis on mortality has predominantly been investigated through Cox regression analysis, often overlooking competing intermediate risks like progression to myelofibrosis and blast-phase. By the use of multistate models new light has been shed on the relationship between multiple events occurring after MPN diagnosis, particularly highlighting the influence of thrombosis not only on mortality but also on progression to myelofibrosis and blast phase [10, 11]. These models are designed to handle situations where there are multiple possible states or events of interest, and individuals can move between these states over time. In the context of MPNs, different states could represent various disease stages or events, such as thrombosis progression into myelofibrosis, acute leukemia or death.
For example, in pre-PMF, prior studies utilizing Kaplan-Meier curves, suggested that thrombotic events occurred at a frequency akin to that observed in ET. Therefore, the potential impact of a higher propensity for overt myelofibrosis progression than the occurrence of thrombosis over time in pre-PMF was not considered [29]. The likelihood of thrombosis within the first decade after diagnosis of pre-PMF was less than 5%, primarily due to the significant competing risk of myelofibrosis development, which affected approximately 11% of patients [10] (Fig. 1). This finding may have implications for the choice of primary thromboprophylaxis in ET and for the development of overt myelofibrosis in pre-PMF.
Fig. 1. Thrombosis affects survival and disease progression in prePMF, ET and PV.
A–C show the probability of progression to myelofibrosis -MF- (red lines) or to blast phase -BP- (blu lines) directly (solid lines) from the diagnosis of pre-PMF, ET and PV, respectively, or via thrombosis (dashed lines). Panels D, E and F show the probability of death directly (solid lines) from the diagnosis of pre-PMF, ET and PV, respectively, or via thrombosis (dashed lines).
Moreover, previous prediction of mortality in ET was based on Cox regression estimates that were incorporated into the IPSET-survival score [36]. In 791 patients with ET, mortality risk due to intermediate events preceding death (thrombosis, myelofibrosis, and blast-phase) was re-analyzed [10]. Beyond confirming IPSET-survival results in the prediction of direct mortality from ET diagnosis, the multistate model unveiled a 25% mortality increase when preceded by thrombosis (Fig. 1); this estimate was fourfold higher than in patients without thrombosis [10]. Furthermore, in a multivariable time-dependent analysis, arterial and not venous incident thrombosis was found an independent predictor of death together with baseline thrombocytosis ( > 1 million × 109/L) (HR = 4.43 and HR = 5.74, respectively).
The influence of incident thrombosis on the trajectory of death or disease progression was also investigated in PV by a parametric five-state Markov model in a cohort of 1545 patients [11]. During a median follow-up of 6.9 years, the cohort experienced 347 (23%) deaths, 50 (3%) blast phase (BP) events, and 138 (9%) fibrotic transformations (post-PV MF). Incident thrombosis was documented at a rate of 2.62% per patient-year, with arterial events occurring in 1.59% and venous events in 1.05%. Among the 280 (18%) patients who developed thrombosis during follow-up, the probability of death within the first 10 years was 40%, twice the rate observed in the absence of thrombosis (20%; p < 0.01), thus reproducing what observed in ET (see above) (Fig. 1). This adverse effect was particularly pronounced for arterial thrombosis (HR 1.74; p < 0.01) compared to venous events (HR = 1.32; p = 0.26) [11]. In the time-dependent multivariable analysis, this risk of arterial thrombosis remained independent of other fixed (e.g., age, previous venous thrombosis, leukocytosis) or other time-dependent (e.g., progression to BP or MF) variables [11]. The transition from PV diagnosis to MF was direct in 85% of cases. The remaining 15% progressed via thrombosis. Similarly, the transition to the blast phase occurred directly from diagnosis in 44% of cases, while 32% and 24% of cases progressed via myelofibrosis and thrombosis, respectively. Notably, thrombosis had a discernible impact on the acceleration of progression to MF and BP (Fig. 1).
Neutrophil to lymphocyte ratio (NLR) is an inflammatory biomarker that signals more aggressive ET and PV
The above presented findings underscore the aggressive nature of arterial thrombosis in PV and ET, impacting the progression and survival. This stems from a combination of gene mutations in both MPN driver and non-driver genes, leading to the activation of blood circulating cells and the establishment of a chronic subclinical inflammatory state [37]. The link between elevated white blood cell count and thrombosis has been extensively explored in numerous studies within MPNs [38–40] and a robust connection between leukocytosis and thrombosis, particularly in ET and PV was also confirmed in a meta-analysis [41]. Very recently, in the comprehensive REVEAL study, which prospectively followed 2510 patients with PV, researchers discovered a notable link between elevated leukocyte count and the onset of first thrombosis, regardless of the patients’ risk state. Intriguingly, when the hematocrit level was maintained at or below 45%, a white blood cell count exceeding 12 × 10^9/L emerged as a significant predictor for cardiovascular events, with a hazard ratio of 1.95, yielding a p-value of 0.030 [42]. Conversely, other investigators have not confirmed these findings, while suggesting that persistent leukocytosis in PV may prognosticate hematologic evolution to myelofibrosis rather than thrombosis [43]. The question of whether leukocytosis is merely a marker and not a causative factor of vascular disease remains unexplored and can be addressed only by prospective randomized trials. In this regard, the application of epidemiologic causality criteria has demonstrated that leukocytosis may indeed play a causal role in the occurrence of vascular events [44].
Further evidence for the role of leukocytes in thrombogenesis comes from experimental studies showing that neutrophils and platelets play an important role in thrombus formation and vascular occlusion. Platelets not only adhere to the endothelium during plaque formation but also facilitate leukocyte recruitment, while neutrophils contribute to thrombus propagation through NETosis, linking thrombosis and inflammation [45]. Non-myeloid inflammatory cells such as T lymphocytes also play a role in this context. Experimental studies consistently showed T-reg lymphocytes’ involvement in regulating the prothrombotic activity of activated neutrophils and contributing to mitigate the systemic chronic inflammation [46].
Interestingly, inflammation-induced arterial thrombosis itself may exacerbate clonal hematopoiesis, which in turn may directly amplify inflammation through the release of IL-1beta cytokines from monocytes [47]. Monocytes infiltrate lesions and, together with macrophages, elicit inflammation and deliver proteolytic enzymes that digest extracellular matrix and render atherosclerotic plaques unstable [48].
After myocardial infarction, leukocytosis predicts the risk of re-infarction and death [49, 50], indicating that the superimposed inflammation on a pre-existing lesion may increase the plaque size and display high protease activity making it more vulnerable for thrombosis [51].
This triggers a vicious cycle of innate immunity, cross-activation of platelets and neutrophils, culminating in clot formation [52]. The phenomenon of “immunothrombosis” is more evident in arteries after rupture or erosion of an atherosclerotic plaque with strong platelet involvement, which favors leukocyte recruitment and NET formation. In contrast, the thrombo-inflammatory profile in venous thrombosis induced by stasis of blood flow is different, with less intense platelet activation compared to arteries, given the integrity of the endothelial surface [53, 54].
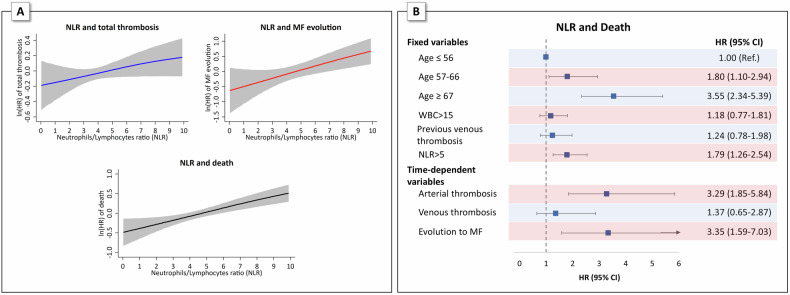
Several biochemical biomarkers are associated with the inflammatory state in MPNs [55–61] and are prognostic risk for myelofibrosis evolution and mortality [57–61]. Pro-inflammatory cytokines such as IL-6, IL-1β and TNF-α, are shown to trigger the C-reactive protein (CRP) formation that is strikingly correlated with JAK2V617F mutation allele burden [55, 56, 59]. Elevated CRP levels were associated with mortality in MF [57, 58] and major thrombotic events in patients with ET and PV [55, 56]. More recently, new hematologic biomarkers routinely measured in common blood tests have been the subject of several investigations, including absolute neutrophil and lymphocyte counts [62, 63], and the neutrophil-to-lymphocyte ratio (NLR) [64–66]. Elevated NLR values are considered as expression of hyper-proliferative state of the innate myeloid immune system and dysregulation of the adaptive immune system [67, 68]. Importantly, the increasing NLR values were associated with thrombosis [64], evolution of myelofibrosis [69], and high mortality rate in ET, PV and MF [70]. These notions have been deeply investigated in PV (Fig. 2). In a prospective cohort of 1508 PV patients enrolled in the ECLAP study, the relationship between the continuous variables of NLR, absolute lymphocyte and neutrophil counts were correlated with survival. There was a linear upward trend in the risk of death with increasing absolute neutrophil count and decreasing lymphocyte count, with log HRs greater than 0 (i.e., HR > 1) for neutrophil and lymphocyte counts greater than 8 × 109/L and less than 2 × 109/L, respectively. The NLR showed a similar trend of linear increase in risk as the neutrophil count, but with more precise 95% confidence intervals, especially for extreme values, reaching HR > 1 when NLR > 5 (unpublished data).
Fig. 2. Neutrophil-to-lymphocyte ratio (NLR) and the risk of total thrombosis, MF evolution and death.
A Generalized additive proportional hazard models (GAM) for the prediction of total thrombosis, MF evolution and death according to Neutrophil-to-lymphocyte ratio (NLR): the effect of NLR is analyzed on a continuous scale by GAM smooth function with cubic splines. Hazard-ratio estimates (solid line) along with their 95% confidence intervals (gray areas) are plotted in logarithmic scale. B Multivariable Cox proportional hazards regression model with fixed and time-dependent variables showing the significant independent effect of NLR on the risk of death.
In addition, as shown in Fig. 2, time-dependent analysis showed that mortality was also significantly affected by events occurring after a median follow-up of 2.8 years from PV diagnosis. This was expected from the occurrence of myelofibrosis (HR 3.34, p = 0.001), but also occurred from arterial thrombosis, which conferred a similar risk of death as the development of myelofibrosis (HR 3.29, p < 0.001). These findings suggest implications for appropriate monitoring of patients with NLR > 5 who develop arterial thrombosis after PV diagnosis.
Aberrant inflammatory responses can also occur in clonal hematopoiesis of indeterminate potential (CHIP), which occurs in individuals without the MPN phenotype owing to acquired genetic mutations typically in DNMT3A, TET2, ASXL1, and JAK2 [71, 72]. Although less than 0.5% of CHIP cases annually progress to overt hematologic and non-hematologic cancers, CHIP is associated with an approximately 40% increased risk of mortality, primarily due to non-hematologic conditions such as cardiovascular arterial events [73].
Thus, clonal hematopoiesis, whether in the form of CHIP or in fully manifested phenotypes such as PV and ET, has been associated with arterial and venous thrombosis and other critical events such as myelofibrosis development as a consequence of pathological inflammatory responses mediated by the myeloid clonal disease and also favored by generic cardiovascular risk factors such as smoking, obesity, hypertension [74–76].
Incident arterial thrombosis as a significant risk factor for secondary cancer
Emerging evidence suggests a strong association between cardiovascular disease and an increased risk of developing cancer in the general population, particularly among current and former smokers [77–79]. The link between these events may be due to the fact that they may share common risk factors and biological pathways. In a recent large epidemiologic study of the general population, the incidence of cancer in patients with heart failure was significantly higher than in controls, and cancer mortality was also increased, particularly in those under the age of 70 [80].
It was hypothesized that clonal hematopoiesis of CHIP variants and the consequent systemic chronic inflammation could underlie these results, as also observed in animal models [81].
Myeloproliferative neoplasms exemplify the sequence of these events. PV, ET and MF are considered human models of persistent systemic inflammation that predispose to increased risk of cardiovascular disease and subsequent cancers [82, 83]. In MPN patients, events such as stroke, myocardial infarction, or peripheral arterial thrombosis resulting from chronic exposure to disease-related inflammatory factors and other common risks (hypertension, diabetes, smoking, obesity) represent an additional source of inflammation. This triggers a vicious cycle, amplifying inflammation and contributing to a cytokine storm that drives recurrent thrombotic events, progression to myelofibrosis, blast phase, increased mortality, and the occurrence of secondary cancers.
Supporting this notion are the results of a nested case-control study involving 647 cases of MPN with second cancer (SC) and 1234 cancer-free MPN patients and matched controls [14, 82]. This study found that the first occurrence of thrombosis after MPN diagnosis was independently associated with an increased risk of SC, with carcinoma being the most common type (65.8%) [14]. Cases were matched to controls for age, sex, year of MPN diagnosis, and disease duration. Over a median follow-up of 4.5 years for cases and 3.7 years for controls, a higher incidence of thrombosis was observed in cases compared to controls (75 of 647 [11.6%] vs. 100 of 1234 [8.1%]; p = 0.013). The excess of thrombosis in cases was mainly due to arterial events such as myocardial infarction and stroke (40 of 647 [6.2%] vs. 46 of 1234 [3.7%]; p = 0.015), whereas no significant difference was observed for venous thrombosis (35 of 647 [5.4%] vs. 53 of 1234 [4.3%]) [14]. Interestingly, in this case-control study, cytoreductive drugs such as hydroxyurea (HU), ruxolitinib (Ruxo) and interferon-alfa were not associated with an increased risk of carcinoma. Instead, HU and Ruxo were independently associated with the risk of non-melanoma skin cancer. The method used to match cases and controls might have hidden or masked the effect of age differences. To address this, both cases and controls were split into two age groups: those younger than 60 years, and those 60 years or older. The new analysis revealed that, in patients younger than 60 years, arterial thrombosis was found to be an independent predictor of SC (HR = 2.53; p = 0.011) and that venous thrombosis exhibited a non-significant trend (HR = 1.78, p = 0.15) (Fig. 3). This suggests there may be a link between venous thrombosis and cancer, especially in patients under 60, even if the data isn’t strong enough to prove it definitively.
Fig. 3. Arterial thrombosis is a risk factor of second cancer in MPNs.
Odds Ratios (ORs) of second cancer obtained from a multivariable conditional regression model stratified for age at MPN diagnosis: red triangles represent OR of second cancer for MPN patients with age < 60 years; blue circles represent the OR of second cancer for MPN patients with age ≥ 60 years. Solid lines represent the corresponding 95% Confidence Intervals (CIs).
The findings might be explained by the fact that younger patients with myeloproliferative neoplasms (MPNs, a type of blood cancer) have had the disease for a longer time. Since these patients have lived longer with the disease, they have been exposed to inflammatory triggers for a longer period, which could increase their risk for complications like thrombosis and secondary cancer.
These findings align with the results of two recent large European Leukemia Net surveys on MPN patients with thrombosis [34, 83, 84]. In the first study, which included 597 MPN patients with cerebrovascular arterial ischemic events, the incidence of secondary cancers following arterial thrombosis was 8.5% [34]. In the second study, involving 387 MPN patients with venous thromboembolism (VTE) [83, 84], the incidence of secondary cancer after VTE was lower, at 4.9% (p = 0.036). This suggests that, in MPN patients, arterial thrombosis may be more predictive of secondary cancer development than venous thrombosis.
Interestingly, in a multivariable analysis, low-dose aspirin administered during 4.4 years of follow-up resulted statistically associated with a reduction of risk of female genital tract tumors (odds ratio 0.47, 95% CI 0.25–0.89) including ovarian and endometrial cancers and breast cancer, independently of MPN subtype [85]. This finding is consistent with other findings in various cancers, where long-term aspirin use in randomized trials for the prevention of vascular events showed a clear protection against cancer development [86]. However, the efficacy of aspirin in the general population, which has been supported in some studies but refuted in others [87], remains uncertain.
Over the last twenty years, extensive basic research and clinical trials have demonstrated promising advantages of targeting inflammation in atherosclerosis. Notably, canakinumab, a human monoclonal antibody that effectively neutralizes IL-1β, emerged as a significant contender in this regard. A randomized clinical trial underscored its efficacy, revealing a marked reduction in cardiovascular events. Intriguingly, it also demonstrated a decrease in the occurrence of lung cancer [88]. However, despite these encouraging findings, subsequent clinical trials aimed specifically at cancer primary or secondary prevention, did not yield evidence of its protective effect [89].
Conclusion
The limitations of this perspective article must be acknowledged. Most of the studies reviewed here were retrospective, which introduces potential biases and limits the strength of the evidence. The role of cytoreductive therapy and associated comorbidities were not thoroughly investigated, which may have influenced the results.
Therefore, we emphasize that overall, this paper serves as a hypothesis-generating exploration of the complex relationship between thrombosis and MPN outcomes. It highlights the need for future prospective studies to fully elucidate the sequence of critical events leading to death in MPN patients.
We believe that arterial, and possibly venous thrombosis occurring during follow-up should be considered in the context of long-term occurring outcomes, including an increased incidence of solid tumors. Future therapies should focus on targeting the complex mechanisms involved in both atherogenesis and thrombogenesis, including new cytoreductive drugs targeting the somatic mutations, such as interferon and Jak2 inhibitors, and anti-inflammatory drugs for primary and secondary prevention of thrombosis.
Acknowledgements
This work is part of the RICO project (Ricerca Istituzionale Collaborativa-Ospedale Papa Giovanni XXIII di Bergamo), BCC, Milano, Italy; it is sponsored by FROM-Fondazione per la Ricerca Ospedale di Bergamo-ETS and endorsed by the AIRC - Gruppo Italiano Malattie Mieloproliferative (AGIMM) with the program number 1005 and MYNERVA project, program number 21267, website at https://progettomynerva.it.
Author contributions
TB, AG, AC, VD, AR, AT, AMV contributed to the conceptualization of the Viewpoint. TB, AG, AC contributed to the literature review, data collection, tables and figures. TB wrote the original draft. TB, AG, AC, VD, AR, AT, AMV reviewed the manuscript and approved the final draft.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka H-M, et al. International consensus classification of myeloid neoplasms and acute leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140:1200–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rungjirajittranon T, Owattanapanich W, Ungprasert P, Siritanaratkul N, Ruchutrakool T. A systematic review and meta-analysis of the prevalence of thrombosis and bleeding at diagnosis of Philadelphia-negative myeloproliferative neoplasms. BMC Cancer. 2019;19:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbui T, Carobbio A, De Stefano V. Thrombosis in myeloproliferative neoplasms during cytoreductive and antithrombotic drug treatment. Res Pr Thromb Haemost. 2022;6:e12657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barosi G, Mesa RA, Thiele J, Cervantes F, Campbell PJ, Verstovsek S, et al. Proposed criteria for the diagnosis of post-polycythemia vera and post-essential thrombocythemia myelofibrosis: a consensus statement from the International Working Group for Myelofibrosis Research and Treatment. Leukemia. 2008;22:437–8. [DOI] [PubMed] [Google Scholar]
- 5.Tefferi A, Alkhateeb H, Gangat N. Blast phase myeloproliferative neoplasm: contemporary review and 2024 treatment algorithm. Blood Cancer J. 2023;13:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loscocco GG, Gesullo F, Capecchi G, Atanasio A, Maccari C, Mannelli F, et al. One thousand patients with essential thrombocythemia: the Florence-CRIMM experience. Blood Cancer J. 2024;14:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guglielmelli P, Loscocco GG, Mannarelli C, Rossi E, Mannelli F, Ramundo F, et al. JAK2V617F variant allele frequency >50% identifies patients with polycythemia vera at high risk for venous thrombosis. Blood Cancer J. 2021;11:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbui T, Tefferi A, Vannucchi AM, Passamonti F, Silver RT, Hoffman R, et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia. 2018;32:1057–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerds AT, Gotlib J, Ali H, Bose P, Dunbar A, Elshoury A, et al. In: Vachhani P, Wadleigh M, Wall S, Ward DC, Bergman MA, Hochstetler C (eds). Myeloproliferative Neoplasms, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20:1033–62. [DOI] [PubMed]
- 10.Carobbio A, Vannucchi AM, Rumi E, De Stefano V, Rambaldi A, Carli G, et al. Survival expectation after thrombosis and overt-myelofibrosis in essential thrombocythemia and prefibrotic myelofibrosis: a multistate model approach. Blood Cancer J. 2023;13:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbui T, Carobbio A, Thiele J, Gangat N, Rumi E, Rambaldi A, et al. The impact of thrombosis on probabilities of death and disease progression in polycythemia vera: a multistate transition analysis of 1545 patients. Blood Cancer J. 2023;13:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pemmaraju N, Gerds AT, Yu J, Parasuraman S, Shah A, Xi A, et al. Thrombotic events and mortality risk in patients with newly diagnosed polycythemia vera or essential thrombocythemia. Leuk Res. 2022;115:e106809. [DOI] [PubMed] [Google Scholar]
- 13.Barbui T, Carobbio A. Prediction models for essential thrombocythemia from two longitudinal studies involving 2000 patients. Blood Cancer J. 2024;14:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Stefano V, Ghirardi A, Masciulli A, Carobbio A, Palandri F, Vianelli N, et al. Arterial thrombosis in philadelphia-negative myeloproliferative neoplasms predicts second cancer: a case-control study. Blood. 2020;135:381–6. [DOI] [PubMed] [Google Scholar]
- 15.Ghirardi A, Carobbio A, Guglielmelli P, Rambaldi A, Stefano V, Vannucchi AM, et al. Age-stratified analysis reveals arterial thrombosis as a predictor for gender-related second cancers in myeloproliferative neoplasms: a case-control study. Blood Cancer J. 2024;14:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hultcrantz M, Björkholm M, Dickman PW, Landgren O, Derolf ÅR, Kristinsson SY, et al. Risk for arterial and venous thrombosis in patients with myeloproliferative neoplasms: a population-based cohort study. Ann Intern Med. 2018;168:317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbui T, Vannucchi AM, Finazzi G, Finazzi MC, Masciulli A, Carobbio A, et al. A reappraisal of the benefit-risk profile of hydroxyurea in polycythemia vera: a propensity-matched study. Am J Hematol. 2017;92:1131–6. [DOI] [PubMed] [Google Scholar]
- 18.Cortelazzo S, Finazzi G, Ruggeri M, Vestri O, Galli M, Rodeghiero F, et al. Hydroxyurea for patients with essential thrombocythemia and a high risk of thrombosis. N. Engl J Med. 1995;332:1132–6. [DOI] [PubMed] [Google Scholar]
- 19.Harrison CN, Campbell PJ, Buck G, Wheatley K, East CL, Bareford D, et al. Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia. N. Engl J Med. 2005;353:33–45. [DOI] [PubMed] [Google Scholar]
- 20.Barbui T, Stefano V, Ghirardi A, Masciulli A, Finazzi G, Vannucchi AM. Different effect of hydroxyurea and phlebotomy on prevention of arterial and venous thrombosis in Polycythemia Vera. Blood Cancer J. 2018;8:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrari A, Carobbio A, Masciulli A, Ghirardi A, Finazzi G, De Stefano V, et al. Clinical outcomes under hydroxyurea treatment in polycythemia vera: a systematic review and meta-analysis. Haematologica. 2019;104:2391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barbui T, Carobbio A, Rumi E, Finazzi G, Gisslinger H, Rodeghiero F, et al. In contemporary patients with polycythemia vera, rates of thrombosis and risk factors delineate a new clinical epidemiology. Blood. 2014;124:3021–3. [DOI] [PubMed] [Google Scholar]
- 23.Marchioli R, Finazzi G, Landolfi R, Kutti J, Gisslinger H, Patrono C, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005;23:2224–32. [DOI] [PubMed] [Google Scholar]
- 24.Tefferi A, Rumi E, Finazzi G, Gisslinger H, Vannucchi AM, Rodeghiero F, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27:1874–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchioli R, Finazzi G, Specchia G, Cacciola R, Cavazzina R, Cilloni D, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N. Engl J Med. 2013;368:22–33. [DOI] [PubMed] [Google Scholar]
- 26.Liu A, Naymagon L, Tremblay D. Splanchnic vein thrombosis in myeloproliferative neoplasms: treatment considerations and unmet needs. Cancers. 2022;15:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405. [DOI] [PubMed] [Google Scholar]
- 28.Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the World Health Organization Classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36:1703–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbui T, Thiele J, Passamonti F, Rumi E, Boveri E, Ruggeri M, et al. Survival and disease progression in essential thrombocythemia are significantly influenced by accurate morphologic diagnosis: an international study. J Clin Oncol. 2011;29:3179–84. [DOI] [PubMed] [Google Scholar]
- 30.Barbui T, Carobbio A, Cervantes F, Vannucchi AM, Guglielmelli P, Antonioli E, et al. Thrombosis in primary myelofibrosis: incidence and risk factors. Blood. 2010;115:778–82. [DOI] [PubMed] [Google Scholar]
- 31.Saliba W, Mishchenko E, Cohen S, Rennert G, Preis M. Association between myelofibrosis and thromboembolism: a population-based retrospective cohort study. J Thromb Haemost. 2020;18:916–25. [DOI] [PubMed] [Google Scholar]
- 32.Mora B, Guglielmelli P, Kuykendall A, Rumi E, Maffioli M, Palandri F, et al. Prediction of thrombosis in post-polycythemia vera and post-essential thrombocythemia myelofibrosis: a study on 1258 patients. Leukemia. 2022;36:2453–60. [DOI] [PubMed] [Google Scholar]
- 33.De Stefano V, Za T, Rossi E, Vannucchi AM, Ruggeri M, Elli E, et al. Recurrent thrombosis in patients with polycythemia vera and essential thrombocythemia: incidence, risk factors, and effect of treatments. Haematologica. 2008;93:372–80. [DOI] [PubMed] [Google Scholar]
- 34.De Stefano V, Carobbio A, Di Lazzaro V, Guglielmelli P, Iurlo A, Finazzi MC, et al. Benefit-risk profile of cytoreductive drugs along with antiplatelet and antithrombotic therapy after transient ischemic attack or ischemic stroke in myeloproliferative neoplasms. Blood Cancer J. 2018;8:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barbui T, Vannucchi AM, Carobbio A, Rumi E, Finazzi G, Gisslinger H, et al. The effect of arterial hypertension on thrombosis in low-risk polycythemia vera. Am J Hematol. 2017;92:E5–E6. [DOI] [PubMed] [Google Scholar]
- 36.Passamonti F, Thiele J, Girodon F, Rumi E, Carobbio A, Gisslinger H, et al. A prognostic model to predict survival in 867 World Health Organization-defined essential thrombocythemia at diagnosis: a study by the International Working Group on Myelofibrosis Research and Treatment. Blood. 2012;20:1197–201. [DOI] [PubMed] [Google Scholar]
- 37.Hasselbalch HC, Elvers M, Schafer AI. The pathobiology of thrombosis, microvascular disease, and hemorrhage in the myeloproliferative neoplasms. Blood. 2021;137:2152–60. [DOI] [PubMed] [Google Scholar]
- 38.Carobbio A, Finazzi G, Antonioli E, Guglielmelli P, Vannucchi AM, Delaini F, et al. Thrombocytosis and leukocytosis interaction in vascular complications of essential thrombocythemia. Blood. 2008;112:3135–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Landolfi R, Gennaro L, Barbui T, Stefano V, Finazzi G, Marfisi R, et al. Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood. 2007;109:2446–52. [DOI] [PubMed] [Google Scholar]
- 40.Carobbio A, Finazzi G, Antonioli E, Vannucchi AM, Barosi G, Ruggeri M, et al. Hydroxyurea in essential thrombocythemia: rate and clinical relevance of responses by European LeukemiaNet criteria. Blood. 2010;116:1051–5. [DOI] [PubMed] [Google Scholar]
- 41.Carobbio A, Ferrari A, Masciulli A, Ghirardi A, Barosi G, Barbui T. Leukocytosis and thrombosis in essential thrombocythemia and polycythemia vera: a systematic review and meta-analysis. Blood Adv. 2019;3:1729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerds AT, Mesa R, Burke JM, Grunwald MR, Stein BL, Squier P, et al. Association between elevated white blood cell counts and thrombotic events in polycythemia vera: analysis from REVEAL. Blood. 2024;143:1646–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ronner L, Mascarenhas J, Moshier EL. Response to meta-analysis of leukocytosis and thrombosis in essential thrombocythemia and polycythemia vera. Blood Adv. 2019;3:3010–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barbui T, Carobbio A, Rambaldi A, Finazzi G. Perspectives on thrombosis in essential thrombocythemia and polycythemia vera: is leukocytosis a causative factor? Blood. 2009;114:759–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolach O, Sellar RS, Martinod K, Cherpokova D, McConkey M, Chappell RJ, et al. Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Sci Transl Med. 2018;10:eaan8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schuhmann MK, Kraft P, Stoll G, Lorenz K, Meuth SG, Wiendl H, et al. CD28 superagonist-mediated boost of regulatory T cells increases thrombo-inflammation and ischemic neurodegeneration during the acute phase of experimental stroke. J Cereb Blood Flow Metab J Int Soc Cereb Blood Flow Metab. 2015;35:6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steensma DP. Clinical consequences of clonal hematopoiesis of indeterminate potential. Blood Adv. 2018;2:3404–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–25. [DOI] [PubMed] [Google Scholar]
- 49.Hammerschmidt DE, Bagge U, Matrai A, Dormandy JA, Ernst E. Leukocytes and the risk of ischemic diseases. JAMA. 1987;257:3553628 [PubMed] [Google Scholar]
- 50.Sabatine MS, Morrow DA, Cannon CP, Murphy SA, Demopoulos LA, DiBattiste PM, et al. Relationship between baseline white blood cell count and degree of coronary artery disease and mortality in patients with acute coronary syndromes: a TACTICS-TIMI 18 (treat angina with aggrastat and determine cost of therapy with an invasive or conservative strategy- thrombolysis in myocardial infarction 18 trial) substudy. J Am Coll Cardiol. 2002;40:1761–8. [DOI] [PubMed] [Google Scholar]
- 51.Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487:325–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mereweather LJ, Constantinescu-Bercu A, Crawley JTB, Salles-Crawley I. Platelet-neutrophil crosstalk in thrombosis. Int J Mol Sci. 2023;24:1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Döring Y, Soehnlein O, Weber C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ Res. 2017;120:736–43. [DOI] [PubMed] [Google Scholar]
- 54.Stark K, Massberg S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat Rev Cardiol. 2021;18:666–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barbui T, Carobbio A, Finazzi G, Vannucchi AM, Barosi G, Antonioli E, et al. Inflammation and thrombosis in essential thrombocythemia and polycythemia vera: different role of C-reactive protein and pentraxin 3. Haematologica. 2011;96:315–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lussana F, Carobbio A, Salmoiraghi S, Guglielmelli P, Vannucchi AM, Bottazzi B, et al. Driver mutations (JAK2V617F, MPLW515L/K or CALR), pentraxin-3 and C-reactive protein in essential thrombocythemia and polycythemia vera. J Hematol Oncol J Hematol Oncol. 2017;10:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tefferi A, Vaidya R, Caramazza D, Finke C, Lasho T, Pardanani A. Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: a comprehensive cytokine profiling study. J Clin Oncol. 2011;29:1356–63. [DOI] [PubMed] [Google Scholar]
- 58.Barbui T, Carobbio A, Finazzi G, Guglielmelli P, Salmoiraghi S, Rosti V, et al. Elevated C-reactive protein is associated with shortened leukemia-free survival in patients with myelofibrosis. Leukemia. 2013;27:2084–6. [DOI] [PubMed] [Google Scholar]
- 59.Barosi G, Massa M, Campanelli R, Fois G, Catarsi P, Viarengo G, et al. Primary myelofibrosis: older age and high JAK2V617F allele burden are associated with elevated plasma high-sensitivity C-reactive protein levels and a phenotype of progressive disease. Leuk Res. 2017;60:18–23. [DOI] [PubMed] [Google Scholar]
- 60.Barosi G, Campanelli R, Catarsi P, Amici M, Abbà C, Viarengo G, et al. Plasma sIL-2Rα levels are associated with disease progression in myelofibrosis with JAK2V617F but not CALR mutation. Leuk Res. 2020;90:106319. [DOI] [PubMed] [Google Scholar]
- 61.Campanelli R, Massa M, Rosti V, Barosi G. New markers of disease progression in myelofibrosis. Cancers. 2021;13:5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tefferi A, Loscocco GG, Farrukh F, Szuber N, Mannelli F, Pardanani A, et al. A globally applicable ‘triple A’ risk model for essential thrombocythemia based on age, absolute neutrophil count, and absolute lymphocyte count. Am J Hematol. 2023;98:1829–37. [DOI] [PubMed] [Google Scholar]
- 63.Warny M, Helby J, Nordestgaard BG, Birgens H, Bojesen SE. Incidental lymphopenia and mortality: a prospective cohort study. CMAJ. 2020;192:E25–E33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Larsen MK, Skov V, Kjær L, Eickhardt-Dalbøge CS, Knudsen TA, Kristiansen MH, et al. Neutrophil-to-lymphocyte ratio and all-cause mortality with and without myeloproliferative neoplasms-a Danish longitudinal study. Blood Cancer J. 2024;14:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Adamstein NH, MacFadyen JG, Rose LM, Glynn RJ, Dey AK, Libby P, et al. The neutrophil-lymphocyte ratio and incident atherosclerotic events: analyses from five contemporary randomized trials. Eur Heart J. 2021;42:896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Song M, Graubard BI, Rabkin CS, Engels EA. Neutrophil-to-lymphocyte ratio and mortality in the United States general population. Sci Rep. 2021;11:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carobbio A, Vannucchi AM, De Stefano V, Masciulli A, Guglielmelli P, Loscocco GG, et al. Neutrophil-to-lymphocyte ratio is a novel predictor of venous thrombosis in polycythemia vera. Blood Cancer J. 2022;12:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Laganà A, Passucci M, Pepe S, Scalzulli E, Carmosino I, Costa A, et al. Neutrophil to lymphocyte ratio in myelofibrosis patients treated with ruxolitinib may predict prognosis and rate of discontinuation. Eur J Haematol. 2024;112:938–43. [DOI] [PubMed] [Google Scholar]
- 69.Nathan DI, Dougherty M, Bhatta M, Mascarenhas J, Marcellino BK. Clonal hematopoiesis and inflammation: a review of mechanisms and clinical implications. Crit Rev Oncol Hematol. 2023;192:104187. [DOI] [PubMed] [Google Scholar]
- 70.Weeks LD, Ebert BL. Causes and consequences of clonal hematopoiesis. Blood. 2023;142:2235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Calvillo-Argüelles O, Jaiswal S, Shlush LI, Moslehi JJ, Schimmer A, Barac A, et al. Connections between clonal hematopoiesis, cardiovascular disease, and cancer: a review. JAMA Cardiol. 2019;4:380–7. [DOI] [PubMed] [Google Scholar]
- 72.Libby P, Sidlow R, Lin AE, Gupta D, Jones LW, Moslehi J, et al. Clonal hematopoiesis: crossroads of aging, cardiovascular disease, and cancer. JACC rev top week. J Am Coll Cardiol. 2019;74:567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hasselbalch HC. The role of cytokines in the initiation and progression of myelofibrosis. Cytokine Growth Factor Rev. 2013;24:133–45. [DOI] [PubMed] [Google Scholar]
- 74.Hasselbalch HC, Kristiansen MH, Kjær L, Skov V, Larsen MK, Ellervik C, et al. CHIP-JAK2V617F, chronic inflammation, abnormal megakaryocyte morphology, organ failure, and multimorbidties. Blood Adv. 2024;8:681–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Stefano V. Arterial thrombosis and cancer: the neglected side of the coin of Trousseau syndrome. Haematologica. 2018;103:1419–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilcox NS, Amit U, Reibel JB, Berlin E, Howell K, Ky B. Cardiovascular disease and cancer: shared risk factors and mechanisms. Nat Rev Cardiol. 2024. 10.1038/s41569-024-01017-x. [DOI] [PMC free article] [PubMed]
- 77.Reed SC, Croessmann S, Park BH. CHIP happens: clonal hematopoiesis of indeterminate potential and its relationship to solid tumors. Clin Cancer Res. 2023;29:1403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hasselbalch HC. Perspectives on chronic inflammation in essential thrombocythemia, polycythemia vera, and myelofibrosis: is chronic inflammation a trigger and driver of clonal evolution and development of accelerated atherosclerosis and second cancer? Blood. 2012;119:3219–25. [DOI] [PubMed] [Google Scholar]
- 79.Coombs CC, Gillis NK, Tan X, Berg JS, Ball M, Balasis ME, et al. Identification of clonal hematopoiesis mutations in solid tumor patients undergoing unpaired next-generation sequencing assays. Clin Cancer Res J Am Assoc Cancer Res. 2018;24:5918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bertero E, Robusto F, Rulli E, D’Ettorre A, Bisceglia L, Staszewsky L, et al. Cancer incidence and mortality according to pre-existing heart failure in a community-based cohort. JACC Cardio Oncol. 2022;4:98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brahmbhatt DH, Scolari FL, Billia F. Could clonal hematopoiesis explain the link between increased cancer mortality incidence in heart failure? JACC Cardio Oncol. 2022;4:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barbui T, Ghirardi A, Masciulli A, Carobbio A, Palandri F, Vianelli N, et al. Second cancer in Philadelphia negative myeloproliferative neoplasms (MPN-K). A nested case-control study. Leukemia. 2019;33:1996–2005. [DOI] [PubMed] [Google Scholar]
- 83.De Stefano V, Ruggeri M, Cervantes F, Alvarez-Larrán A, Iurlo A, Randi ML, et al. High rate of recurrent venous thromboembolism in patients with myeloproliferative neoplasms and effect of prophylaxis with vitamin K antagonists. Leukemia. 2016;30:2032–8. [DOI] [PubMed] [Google Scholar]
- 84.De Stefano V, Vannucchi AM, Ruggeri M, Cervantes F, Alvarez-Larrán A, Iurlo A, et al. Splanchnic vein thrombosis in myeloproliferative neoplasms: risk factors for recurrences in a cohort of 181 patients. Blood Cancer J. 2016;6:e493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barbui T, Ghirardi A, Vannucchi AM, Marchetti M, De Stefano V. MPN-K authors. Reply to: Second primary malignancies in myeloproliferative neoplasms and the role of aspirin. Leukemia. 2020;34:1208–9. [DOI] [PubMed] [Google Scholar]
- 86.Elwood P, Morgan G, Watkins J, Protty M, Mason M, Adams R, et al. Aspirin and cancer treatment: systematic reviews and meta-analyses of evidence: for and against. Br J Cancer. 2024;130:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen WY, Ballman KV, Partridge AH, Hahn OM, Briccetti FM, Irvin WJ, et al. Aspirin vs placebo as adjuvant therapy for breast cancer: The Alliance A011502 Randomized Trial. JAMA. 2024;331:1714–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ, et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:1833–42. [DOI] [PubMed] [Google Scholar]
- 89.Lythgoe MP, Prasad V. Repositioning canakinumab for non-small cell lung cancer-important lessons for drug repurposing in oncology. Br J Cancer. 2022;127:785–7. [DOI] [PMC free article] [PubMed] [Google Scholar]