Abstract
Pasteurella aerogenes has been implicated in reproductive disorders in sows, yet its prevalence and characteristics in vaginal discharge are not well understood. This study aimed to detect P. aerogenes in sow vaginal discharge samples and investigate its antibiotic resistance profile, toxin genes, and toxicity. P. aerogenes was isolated from 40% (8/20) of samples. Antimicrobial susceptibility testing revealed universal resistance to amoxicillin-clavulanate (4:1), with 87.5% of isolates also resistant to oxytetracycline, amoxicillin, ceftriaxone, and enrofloxacin. The colistin resistance gene mcr-2 was detected in 75% of isolates, while class 1 integron (int1) was found in 12.5%. The pax toxin gene cluster was present in 75% of isolates. Toxicity assays using Panagrellus redivivus demonstrated dose-dependent effects of P. aerogenes supernatant containing pax toxins. This study represents the first report of P. aerogenes isolation from sow vaginal discharge in Thailand. The high prevalence of antibiotic resistance, presence of the mcr-2 gene, and toxicity of pax toxin-positive isolates suggest that P. aerogenes may be an underestimated factor in swine reproductive health. These findings highlight the need for further investigation into the role of P. aerogenes in sow reproductive disorders and its potential impact on swine production.
Keywords: Pasteurella aerogenes, Sow’s vaginal discharge, pax genes, mcr genes
Subject terms: Antimicrobials, Bacteria, Bacteriology
Introduction
Vaginal discharge is a prevalent clinical manifestation of sow endometritis, primarily caused by bacterial infections. Predominant pathogens include Escherichia coli (33.3%), Staphylococcus spp., and Streptococcus spp.1–3. The etiology of endometritis is multifactorial, with poor hygiene during artificial insemination and bacterial contamination of semen being significant contributors4. Although the morbidity incidence is relatively modest (7–5%), endometritis can substantially impact sow reproductive performance, leading to abortion, return to oestrus, and reduced litter size2,5. Acute endometritis typically responds to antibiotic treatment, restoring normal reproductive function. However, chronic cases may result in severe, irreversible damage to sow reproductive performance1. While Pasteurella aerogenes has been implicated in sow endometritis, its reported incidence is comparatively low. P. aerogenes is a Gram-negative, facultatively anaerobic coccobacillus belonging to the family Pasteurellaceae. While commonly found in the respiratory and digestive tracts of pigs as a commensal organism, it can become an opportunistic pathogen. Of particular concern is its potential presence in the reproductive tract of sows, where it may contribute to various health issues6. The pathogenicity of P. aerogenes is primarily attributed to RTX toxins encoded by the pax gene cluster7. P. aerogenes strains positive for pax genes have been associated with septicemia in newborn piglets and sow abortion. These strains typically exhibit hemolytic activity on sheep-blood agar and are CAMP-positive7. The RTX operon comprises four essential genes: paxC (activator), paxA (structural toxin), paxB, and paxD (secretion proteins). The PaxA toxin is the primary virulence factor of P. aerogenes7. Antibiotic therapy (e.g. penicillin G or oxytetracycline) serves a dual purpose in managing endometritis: treatment of active infections and prevention8. Preventive measures include the addition of antibiotics to semen extenders to mitigate bacterial contamination9–11.
The widespread use of antibiotics to maintain farm productivity has raised concerns regarding the emergence of antibiotic-resistant bacteria12. While antimicrobial resistance data for Pasteurella spp. infections in swine have primarily focused on P. multocida, with documented resistance to tetracycline and penicillin13. There is a notable lack of information regarding antibiotic resistance in P. aerogenes. Mobile colistin resistance (mcr) genes, plasmid-borne determinants conferring colistin resistance in bacteria, have been classified into ten variants (mcr-1 to mcr-10)14–19. The global dissemination of mcr genes across animal, human, and food samples has become a significant public health concern14. This is particularly alarming given that colistin is a last-resort antibiotic for infections caused by multidrug-resistant Gram-negative bacteria, including Enterobacteriaceae, underscoring the critical importance of monitoring mcr genes prevalence in these organisms14. Horizontal gene transfer of antibiotic resistance determinants is primarily facilitated by mobile genetic elements, including plasmids, transposons, and integrons20. Integrons play a crucial role in disseminating and transmitting resistance factors among bacterial populations owing to their ability to integrate several gene cassettes encoding resistance to various antibiotics20. Among the three classes of integrons, class 1 integrons, which contain the int1 gene, are the most prevalent21,22.
The present study aimed to characterize P. aerogenes isolates obtained from sow vaginal discharge in Thailand. This investigation encompassed multiple aspects: determining the antimicrobial susceptibility profiles of the isolates through standardized antimicrobial susceptibility testing (AST); detecting and identifying the presence of virulence-associated toxin genes (paxA, paxB, paxC, and paxD) and antimicrobial resistance genes (mcr-1 to mcr-10 and int1) using molecular techniques; and evaluating the toxicity of P. aerogenes isolates using an appropriate in vitro model. This comprehensive analysis seeks to elucidate the potential pathogenicity and antimicrobial resistance patterns of P. aerogenes in the context of sow reproductive health in Thailand, contributing to our understanding of this understudied pathogen in swine production.
Results
Isolation, identification, and antimicrobial susceptibility profiles of Pasteurella aerogenes
P. aerogenes was isolated from 40% (8/20) of sow vaginal discharge samples. Antimicrobial susceptibility testing was performed on all isolated P. aerogenes isolates (Table 1). All isolates (100%, 8/8) exhibited resistance to amoxicillin-clavulanate (4:1) (MIC ≥ 1 µg/mL). The majority of isolates (87.5%, 7/8) were susceptible to ceftazidime, with only one isolate showing resistance (MIC ≥ 16 µg/mL). The same proportion of isolates (87.5%, 7/8) demonstrated resistance to amoxicillin (MIC ≥ 32 µg/mL), ceftriaxone (MIC ≥ 4 µg/mL), enrofloxacin (MIC ≥ 1 µg/mL), and oxytetracycline (MIC ≥ 6 µg/mL) (Table 2). Resistance to trimethoprim: sulfamethoxazole (1:19) was observed in 25% (2/8) of the isolates (MIC ≥ 4 µg/mL). Gentamicin (MIC ≥ 16 µg/mL) resistance was detected in 62.5% (5/8) of the isolates, while colistin (MIC ≥ 4 µg/mL) resistance was observed in 62.5% (5/8) of the isolates. Notably, the mcr-2 gene was found in 75% (6/8) of the isolates, including two isolates that were phenotypically susceptible to colistin (MIC < 4 µg/mL). Ceftiofur resistance (MIC ≥ 8 µg/mL) was observed in 25% (2/8) of the isolates.
Table 1.
The minimum inhibitory concentrations (MICs) of various antimicrobial agents tested against Pasteurella aerogenes isolates (n = 20) obtained from sow vaginal samples.
| Sample ID | MIC (μg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CN | CAZ | CEF | CT | ENR | AMX | AMC | CRO | OTC | SXT | |
| V1-1 | 32* | 1 | 2 | 1 | 2* | >128* | 2* | 4* | >32* | 16* |
| V3-1 | 2 | 1 | 16* | 8* | 4* | >128* | 4* | 16* | >32* | 4 |
| V4-3 | 2 | 1 | 4 | 8* | 4* | >128* | 2* | 16* | >32* | 8* |
| V5-1 | 32* | 1 | 2 | 4* | 8* | >128* | 2* | 4* | >32* | >32* |
| V7-2 | 2 | 1 | 8* | 4* | 2* | >128* | 4* | 8* | >32* | 2* |
| V8-2 | 32* | 1 | 2 | 1 | 2* | >128* | 2* | 4* | >32* | 32* |
| V10-3 | 16* | 1 | 0.25 | 0.5 | 0.06 | 16* | 1* | 0.25 | >32* | 0.5 |
| V13-2 | 64* | >128* | 16* | 4* | 2* | 1 | 1* | >32* | 4 | >32* |
CN Gentamicin, CAZ Ceftazidime, CEF Ceftiofur, CT Colistin, ENR Enrofloxacin, AMX Amoxicillin, AMC amoxicillin-clavulanate (4:1), CRO Ceftriaxone, OTC Oxytetracycline, SXT Trimethoprim: sulfamethoxazole (1:19).
*Indicate the resistance zone.
Table 2.
The molecular screening for antibiotic resistance genes (mcr-1 to mcr-10 and int1) and the P. aerogenes virulence genes (pax) among the isolated strains.
| Sample ID | Resistance genes (mcr-1 to mcr-10 and int1) | Virulence (pax genes) | |||
|---|---|---|---|---|---|
| paxA | paxB | paxC | paxD | ||
| V1-1 | mcr-2 | + | + | + | + |
| V3-1 | mcr-2 | + | + | + | + |
| V4-3 | mcr-2 | + | + | + | + |
| V5-1 | mcr-2 | + | + | + | + |
| V7-2 | mcr-2 | + | + | + | + |
| V8-2 | mcr-2 | + | + | + | + |
| V10-3 | − | − | − | − | − |
| V13-2 | int-1 | − | − | − | − |
Detection of pax toxin gene cluster
The presence of the pax toxin gene cluster (paxA, paxB, paxC, and paxD) was assessed in all P. aerogenes isolates (Table 2). The complete pax gene cluster was detected in 75% (6/8) of the isolates. The remaining 25% (2/8) of isolates were negative for all pax genes.
Detection and phylogenetic analysis of mcr and int1 genes
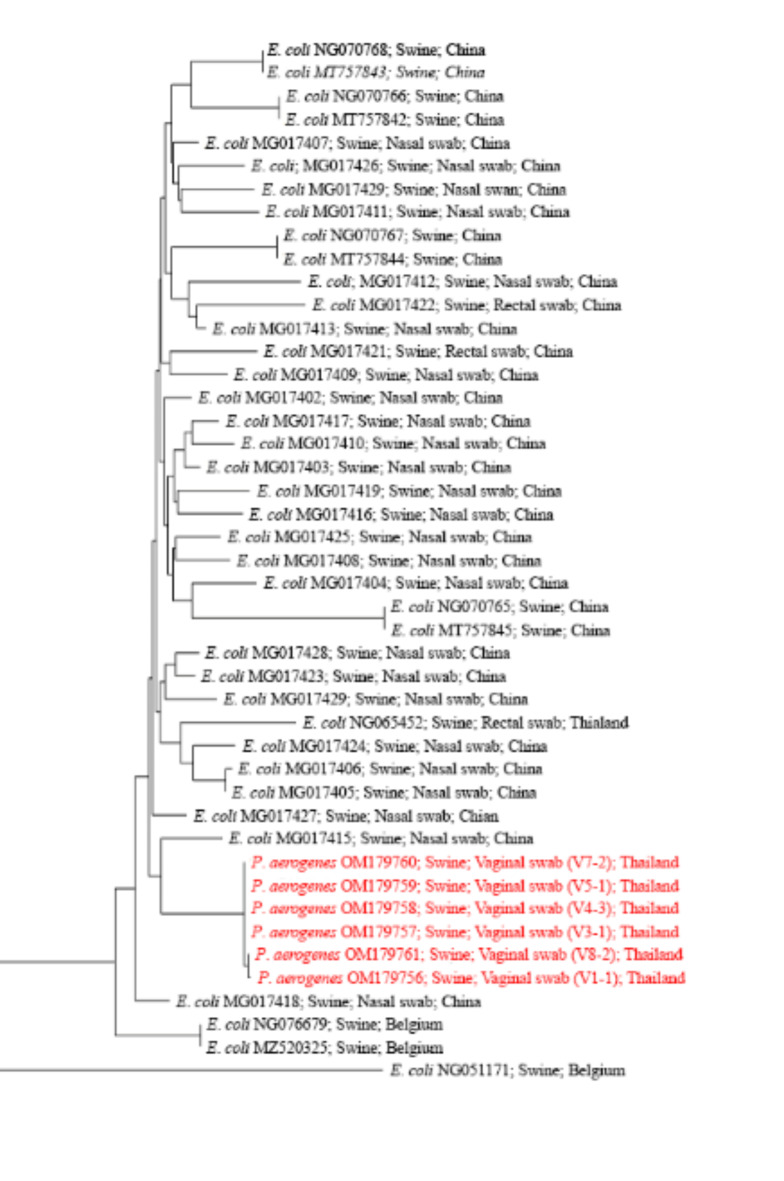
Multiplex PCR was employed to detect colistin resistance genes (mcr-1 to mcr-10) and class 1 integrons (int1) in the P. aerogenes isolates (Table 2). The mcr-2 gene was detected in 75% (6/8) of the isolates, including two isolates that were phenotypically susceptible to colistin. The int1 gene was identified in 12.5% (1/8) of the isolates. The presence of antibiotic-resistance genes was correlated with the minimum inhibitory concentration (MIC) values for colistin (Table 3). When considering only P. aerogenes with mcr-2, colistin resistance was observed in 66.7% (4/6) of the mcr-2-positive isolates. Notably, one isolate exhibited colistin resistance despite being mcr-2-negative; this isolate was positive for the int1 gene. Phylogenetic analysis of the mcr-2 sequences was conducted using MEGA11 software. The resulting phylogenetic tree (Fig. 1) incorporated mcr-2 sequences from swine isolates available in GenBank and those obtained from P. aerogenes in this study. Three distinct lineages were identified among the six mcr-2-positive P. aerogenes isolates. The sequences OM179760, OM179759, OM1796758, and OM179757 formed a monophyletic group with MG017415, an mcr-2 sequence from Escherichia coli isolated from swine nasal swabs.
Table 3.
The distribution of minimum inhibitory concentrations (MICs) for colistin among the Pasteurella aerogenes isolates (n = 8) obtained in this study.
| MIC of colistin (μg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0.25 | 0.5 | 1 | 2 | 4# | 8 | 16 | 32 | |
| Pos. mcr-2 (n = 6) | 0 | 0 | 2 | 0 | 2 | 2 | 0 | 0 |
| Neg. mcr-2 (n = 2) | 0 | 1 | 0 | 0 | 1* | 0 | 0 | 0 |
# cut off of MIC value for colistin resistance (MIC ≥ 4 µg/mL).
*mcr-2 negative but positive for int1.
Fig. 1.
Phylogenetic analysis of mcr-2 sequences. The evolutionary relationships among 45 mcr-2 nucleotide sequences were inferred using the Neighbor-Joining method. Evolutionary distances, computed using the Maximum Composite Likelihood method, are expressed as the number of base substitutions per site. The mcr-2 sequences from P. aerogenes isolates in this study are highlighted in red. This phylogenetic tree demonstrates the evolutionary context of the mcr-2 gene identified in P. aerogenes relative to previously reported sequences.
Toxicity assessment of P. aerogenes using the Panagrellus redivivus model
The toxicity of P. aerogenes isolates was evaluated using Panagrellus redivivus as a model organism. Viability rates of P. redivivus after 24-h incubation with P. aerogenes supernatants are presented in Table 4. For pax toxin-positive P. aerogenes isolates, the viability of P. redivivus when incubated with 0.5 mL of supernatant was 54.65 ± 20.42%. A dose-dependent decrease in viability was observed with increasing supernatant volume (Table 4). In contrast, P. redivivus incubated with 0.5 mL of supernatant from pax toxin-negative P. aerogenes isolates showed higher viability (89.29 ± 3.05%), with a similar decreasing trend as supernatant volume increased (Table 4). The negative control group exhibited the highest viability across all tested volumes (0.5, 1, and 2 mL). Notably, at 2 mL supernatant volume, P. redivivus viability was significantly lower when exposed to pax toxin-positive isolates (11.03 ± 17.82%) compared to the negative control (p < 0.05, Table 4).
Table 4.
Cytotoxic effects of Pasteurella aerogenes supernatants on Panagrellus redivivus viability.
| Supernatant from P. aerogenes carrying toxin genes | Viability of Panagrellus redivivus (%) | ||
|---|---|---|---|
| P. aerogenes supernatants (mL) | |||
| 0.5 mL | 1 mL | 2 mL | |
| paxA, paxB, paxC, and paxD positive | 54.65 ± 20.47 | 42.09 ± 20.76 | 11.03 ± 17.82a |
| paxA, paxB, paxC, and paxD negative | 89.29 ± 3.05 | 68.05 ± 19.06 | 45.57 ± 30.10a, b |
| BHI media | 85.71 ± 1.41 | 85.00 ± 2.83 | 72.95 ±1.34b |
a, bSignificant difference between the represent of pax gene (p-value < 0.05).
Discussion
This study presents the first evidence of Pasteurella aerogenes isolation from sow vaginal discharge in Thailand, along with the novel detection of the mcr-2 gene in this species. Additionally, we demonstrate the toxicity of pax-positive P. aerogenes isolates using Panagrellus redivivus as a model organism.
Pasteurella aerogenes is typically considered part of the normal porcine intestinal microbiota, with the potential to act as an opportunistic pathogen6. While bacterial and viral infections are the primary etiological agents in swine abortion, with Escherichia coli (19.8%, 64/323) and Porcine circovirus-2 (42.7%, 138/323) being the most prevalent bacterial and viral pathogens, respectively, Pasteurella spp. infections are less frequently reported (0.9%, 3/323)23. The association between P. aerogenes infection and sow reproductive efficiency remains underreported in current literature. However, several case studies have documented the presence of P. aerogenes in reproductive disorders. In 1991, P. aerogenes was isolated from aborted fetuses of a sow at 12 weeks gestation24. A subsequent study in 2011 described two cases implicating P. aerogenes in sow reproductive performance issues, with the pathogen isolated from the placenta and aborted swine fetuses using sequencing analysis25. Furthermore, P. aerogenes has been identified in the uterine and peritoneal cavity of an aborted rabbit, suggesting its potential role in reproductive disorders across species26.
Treatment of reproductive bacterial infections in sows typically involves antibiotic administration guided by antibiotic sensitivity testing. Commonly used antibiotics include penicillin, ampicillin, third or later-generation cephalosporins, and carbapenems5,27,28. However, our findings indicate a high prevalence of antibiotic resistance among P. aerogenes isolates, which is concerning given the widespread use of antibiotics in swine production. Subtherapeutic doses of antibiotics are frequently administered in pig farms to enhance growth and reproductive performance29. A survey of farming practices revealed that 75% of farmers acknowledged antibiotic use, although many were either unaware of or reluctant to disclose the antibiotic content in medicated feed. Notably, approximately 92% of sows were reported to receive antibiotic treatment30,31. This widespread use of low-dose antibiotics contributes significantly to the development of antibiotic resistance, a significant global public health challenge29,32. The high level of antibiotic resistance observed in P. aerogenes isolates in this study may be a consequence of these practices, highlighting the need for more judicious use of antimicrobials in swine production.
This study presents novel findings of mcr-2 and int1 genes in Pasteurella aerogenes isolates. The prevalence of mcr-2 is generally low, with a reported occurrence of 0.13% in 9,091 Escherichia coli isolates analyzed between 2010 and 202033. A systematic review of mcr gene prevalence in livestock revealed that mcr-1 was most frequently detected in pigs (40 isolates), followed by cattle (16 isolates) and poultry (31 isolates). In contrast, mcr-2 was identified in only five swine isolates, three of which were from bovine and poultry sources34. In Thailand, mcr-2 has been reported in healthy humans but not in livestock34,35. Interestingly, two P. aerogenes isolates in our study were mcr-2-positive yet colistin-susceptible. Our study revealed a significant discrepancy between genotype and phenotype in antimicrobial resistance. We identified two P. aerogenes isolates harboring the mcr-2 gene that remained susceptible to colistin (MIC < 4 µg/mL). This unexpected finding highlights the complex relationship between the presence of resistance genes and their phenotypic expression. The observed discordance could be attributed to various factors, including lack of gene expression, regulatory mechanisms, or environmental conditions unsuitable for gene activation. This phenomenon underscores the limitations of relying solely on genetic markers for predicting antimicrobial resistance and reinforces the importance of phenotypic testing in clinical settings36. The int1 gene has been investigated at various stages of pig production, with previous studies successfully identifying it in rectal swabs from sows and piglets and in boar semen samples37,38. However, to our knowledge, this is the first report of int1 detection in sow vaginal discharge. Moreover, this study represents the first documentation of both int1 and mcr-2 genes in P. aerogenes, expanding our understanding of antimicrobial resistance gene carriage in this species. Our results have important implications for antimicrobial resistance surveillance and clinical practice. They suggest the potential existence of ‘silent’ resistance genes that could activate under specific conditions, raising concerns about the future emergence of colistin resistance. Further research is needed to elucidate the mechanisms underlying this genotype-phenotype mismatch and to investigate the conditions that might trigger the expression of these currently silent mcr-2 genes.
In this study, six out of eight isolates of P. aerogenes examined were found to harbor the pax toxin genes. Kuhnert et al.7 previously associated P. aerogenes strains positive for the pax toxin gene with cases of swine abortion and septicemia in newborn piglets. Furthermore, they observed that paxA-positive P. aerogenes strains exhibited the CAMP phenomenon, while paxA-negative strains did not7.
Cytotoxicity assays using Panagrellus redivivus revealed that supernatants from pax toxin-positive P. aerogenes were lethal to the nematodes. This finding aligns with the work of Aryukarn et al.39 who studied the effects of supernatants from Klebsiella pneumoniae and Pseudomonas aeruginosa. The P. redivivus model for bacterial toxicity testing has been shown to yield results comparable to those obtained using Caenorhabditis elegans40. An advantage of P. redivivus is its ability to grow at 37 °C, in contrast to C. elegans, which requires temperatures between 15 and 25 °C40.
The high prevalence of pax toxin-positive P. aerogenes isolates observed in this study suggests that P. aerogenes infections may be an underappreciated factor contributing to productivity losses in swine farming. This is supported by previous reports of P. aerogenes detection in aborted fetuses and purulent vaginal discharge from sows41.
The high incidence of P. aerogenes observed in samples from a single pig farm may indicate a localized outbreak rather than a widespread phenomenon. This finding limits the generalizability of our results, and we caution against extrapolating these data to a national scale. Our study was designed to report on the findings from the specific sample set we investigated, and we acknowledge that a broader study involving multiple farms and regions is necessary to provide a more comprehensive picture of P. aerogenes prevalence in sow endometritis across the country.
Furthermore, the potential variation in the mobile genetic content of these strains, as observed in this study, underscores the need for further molecular investigations. Such studies will be essential to understand the mechanisms driving the spread and emergence of this pathogen, as well as the conditions that may favor localized outbreaks. Future research should focus on examining additional farms in different geographic locations and conducting in-depth genomic analyses to better understand the diversity and evolution of P. aerogenes strains in swine populations.
Conclusion
This study presents the first documented evidence of Pasteurella aerogenes in sow vaginal discharge in Thailand. The isolated P. aerogenes strains exhibited a high prevalence of antibiotic resistance, including the presence of the mcr-2 gene, which confers resistance to colistin. Furthermore, P. aerogenes isolates positive for the pax toxin demonstrated cytotoxicity against Panagrellus redivivus in vitro. These findings suggest that P. aerogenes infections may be an underappreciated factor contributing to production losses in swine farming. The combination of antibiotic resistance, including to last-resort antibiotics, and the presence of virulence factors such as the pax toxin, underscores the potential significance of P. aerogenes as a pathogen in porcine reproductive health. Further research is warranted to elucidate the prevalence, pathogenicity, and economic impact of P. aerogenes infections in swine production systems.
Materials and methods
Sample collection
A total of 20 samples from sow vaginal discharge were collected using cotton swabs from a commercial pig farm in Thailand. All specimens were preserved under the sterile repository at 4 °C and immediately shipped to the Laboratory of Bacteria, Veterinary Diagnostic Center, Faculty of Veterinary Science, Mahidol University. The research ethic was approved by the Faculty of Veterinary Science, Mahidol University-Institute Animal Care and Use Committee (FVS-MU-IACUC-Protocol No. MUVS-2021-10-41), Animal use license No. U1-01281-2558. All methods were performed in accordance with the relevant guidelines and regulations.
Isolation and identification of Pasteurella aerogenes
All collected samples were inoculated onto sheep blood agar and MacConkey agar (Oxoid, UK) and incubated at 37 °C for 18–24 h. Colonies displaying typical characteristics were subjected to standard biochemical tests followed by 16S rRNA sequencing for definitive identification. Genomic DNA was extracted using the G-spin™ genomic DNA extraction kit (iNtRON, Republic of Korea). PCR amplification of the 16S rRNA gene was performed using the BiometraTOne96G thermal cycler (AnalytikJena, Germany) with primers UFUL (5′-GCCTAACACATGCAAGTCGA-3′) and 800R (5′-TACCAGGGTATCTAATCC-3′). The PCR protocol included initial denaturation at 94 °C for 3 min, followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 45 s. A final extension step was conducted at 72 °C for 5 min. PCR products were purified using the MEGAquick-spin™ Plus Total Fragment DNA purification kit (iNtRON, Republic of Korea) and sequenced using an Applied Biosystems 3730XL DNA Analyzer (Bionics, Republic of Korea). Sequence identification was performed by comparing the obtained 16 S rRNA sequences against the NCBI nucleotide database (https://blast.ncbi.nlm.nih.gov).
Detection of pax toxin gene cluster
DNA extracted from P. aerogenes was used to detect the presence of the pax genes. The PCR amplification was carried out under the following conditions: initial denaturation at 95 °C for 3 min, followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 45 s. A final extension step was conducted at 72 °C for 5 min. The PCR products were visualized and confirmed by 1.5% agarose gel electrophoresis. Details of the primers used in the PCR procedure and product sizes are provided in Table 5.
Table 5.
Primer sequences for detection of virulence and antimicrobial resistance genes.
| Target | Primer name | Sequence | Product size (bp) | Source |
|---|---|---|---|---|
| Virulence genes | ||||
| paxA | PaxA-F | 5′-AAG TTC GCC TTG TAT CTC GT-3′ | 748 | This study |
| PaxA-R | 5′-TGG TTA CCT GAA GTA GAG CG-3′ | |||
| paxB | PaxB-F | 5′-GTC ATT GCA CCT GTT ATC CG-3′ | 397 | This study |
| PaxB-R | 5′-TAT CTT GTA GCA CAA CGC CT-3′ | |||
| paxC | PaxC-F | 5′-TTG CTT GGT TAT GGG CAA AT-3′ | 220 | This study |
| PaxC-R | 5′-TCG CCT GAA TTC CAA TCC TC-3′ | |||
| paxD | PaxD-F | 5′-ACA CAG GTT GCT TTA GGA CT-3′ | 584 | This study |
| PaxD-R | 5′-ACT GGT GCG CGA ATA ATA GA-3′ | |||
| Drug resistance genes | ||||
| mcr-1 | MCR1F | 5′-AGT CCG TTT GTT CTT GTG GC-3′ | 320 | 40 |
| MCR1R | 5′-AGA TCC TTG GTC TCG GCT TG-3′ | |||
| mcr-2 | MCR2F | 5′-CAA GTG TGT TGG TCG CAG TT-3′ | 715 | 40 |
| MCR2R | 5′-TCT AGC CCG ACA AGC ATA CC-3′ | |||
| mcr-3 | MCR3F | 5′-AAA TAA AAA TTG TTC CGC TTA TG-3′ | 929 | 40 |
| MCR3R | 5′-AAT GGA GAT CCC CGT TTT T-3′ | |||
| mcr-4 | MCR4F | 5′-TCA CTT TCA TCA CTG CGT TG-3′ | 1116 | 40 |
| MCR4R | 5′-TTG GTC CAT GAC TAC CAA TG-3′ | |||
| mcr-5 | MCR5F | 5′-ATG CGG TTG TCT GCA TTT ATC-3′ | 1644 | 40 |
| MCR5R | 5′-TAC TTG TGG TTG TCC TTT TCT G-3′ | |||
| mcr-6 | MCR6F | 5′-GTC CGG TCA ATC CCT ATC TGT-3′ | 556 | 40 |
| MCR6R | 5′-ATC CGG GAT TGA CAA GTA C-3′ | |||
| mcr-7 | MCR7F | 5′-TGC TAC AGC CCT TTT CGT-3′ | 892 | 40 |
| MCR7R | 5′-TTC ATC TGC GCC ACC TCG T-3′ | |||
| mcr-8 | MCR8F | 5′-AAC CGC CAG AGC ACA GAA TT-3′ | 667 | 40 |
| MCR8R | 5′-TTC CCC CAG CGA TTC TCC AT-3′ | |||
| mcr-9 | MCR9F | 5´-AGA ACA TGC ACG GAA CGG AT-3´ | 183 | 40 |
| MCR9R | 5´-CTC ACG AAA AAC CCA CGC TG-3´ | |||
| mcr-10 | MCR10F | 5′-AGC CGT CTT GAA CAT GTG AG-3′ | 744 | 40 |
| MCR10R | 5′-CAT ACA GGG CAC CGA GAC TG-3′ | |||
| int1 | INT1-F | 5′-CTC CCG CAC GAT GAT CGT-3′ | 450 | 40 |
| INT1-R | 5′-TTG CGT GAG CGC ATA CGC-3′ | |||
Detection and phylogenetic analysis of mcr and int1 genes
Plasmids from P. aerogenes were extracted using the QIAprep Spin Miniprep Kit (Qiagen, Germany). The mcr-1 to mcr-10 and int1 genes were detected using a multiplex PCR procedure based on Nguyet et al.42 Details of the primers utilized in the PCR procedure are provided in Table 5. The multiplex PCR conditions were as follows: initial denaturation at 94 °C for 3 min, followed by 25 cycles of denaturation at 94 °C for 30 s, annealing at 58 °C for 90 s, and extension at 72 °C for 60 s. A final extension step was performed at 72 °C for 5 min. PCR products were visualized and confirmed by 1.5% agarose gel electrophoresis.
Full-length amplification of mcr-2 positive samples was performed using a BiometraTOne96G thermal cycler (AnalytikJena, Germany). The following primers were employed: forward (5′-ATGACATCACAGCACTCTTGGTATCG-3′) and reverse (5′-TTACTGGATAAATGCCGTGCGGTCT-3′). The PCR protocol consisted of initial denaturation at 94 °C for 3 min, followed by 25 cycles of denaturation (94 °C, 30 s), annealing (60 °C, 30 s), and extension (72 °C, 90 s). A final extension step was conducted at 72 °C for 5 min. PCR products were purified using the MEGAquick-spin™ Plus Total Fragment DNA purification kit (iNtRON, Republic of Korea) and sequenced via BTSeq™ (Barcode-Tagged Sequencing; CELEMICS, South Korea). Sequence analysis and phylogenetic tree construction were performed using Molecular Evolutionary Genetics Analysis Version 11 software (MEGA11)43. The evolutionary history was inferred using the Neighbor-Joining method. Evolutionary distances, measured in base substitutions per site, were computed using the Maximum Composite Likelihood method.
Antimicrobial susceptibility testing
All P. aerogenes isolates were cultured on sheep blood agar and incubated at 37 °C for 18–24 h. Colonies were suspended in 0.85% NaCl, and turbidity was adjusted to 0.5 McFarland standard (approximately 108 CFU/mL). Minimum inhibitory concentrations (MICs) were determined using the broth dilution method in a 96-well plate, following Clinical and Laboratory Standards Institute (CLSI) guidelines44. The following ten antibiotics were tested using two-fold dilutions: amoxicillin (TCI, Japan) 1–128 µg/mL, amoxicillin-clavulanate (4:1; Sigma, Germany) 1–128 µg/mL, cefazidime (Sigma, Germany) 1–128 µg/mL, ceftriaxone (TCI, Japan) 0.25–32 µg/mL, ceftiofur (TCI, Japan) 0.25–32 µg/mL, colistin (Sigma, Germany) 0.25–32 µg/mL, enrofloxacin (Fluka Biochemika, Japan) 0.06–8 µg/mL, gentamicin (TCI, Japan) 0.5–64 µg/mL, oxytetracycline (AppliChem, USA) 0.25–32 µg/mL, and trimethoprim (1:19; TCI, Japan) 0.25–32 µg/mL. The 96-well plates were incubated at 37 °C for 16–20 h. The MIC was defined as the lowest concentration of each antibiotic at which visible growth of P. aerogenes was inhibited.
Toxicity assessment of P. aerogenes using the Panagrellus redivivus model
The research ethics was approved by the Faculty of Veterinary Science, Mahidol University-Institute Animal Care and Use Committee (FVS-MU-IACUC-Protocol No. MUVS-2021-10-38), Animal use license No. U1-01321-2558. All methods were performed in accordance with the relevant guidelines and regulations. The cytotoxicity assay was adapted from the protocol described by Aryukarn et al.39P. aerogenes isolates were cultured overnight in Brain Heart Infusion (BHI) broth (Oxoid, UK) at 37 °C with agitation at 200 rpm. Cultures were centrifuged at 5000 rpm for 2 min (Denville Micro 260D Microcentrifuge, Denville Scientific, Inc., Metuchen, USA). Supernatants were filtered through 0.22 μm sterile syringe filters (Guangzhou Jet Bio-Filtration Co., Ltd., Guangzhou, China) for toxicity testing. Panagrellus redivivus were cultured at 37 °C on oatmeal substrate. Prior to toxicity testing, approximately 30 worms were transferred to each well of a 6-well plate containing 5 mL of 2% yeast extract (Oxoid, UK) and incubated overnight at 37 °C. Filtered P. aerogenes supernatants were added to the worm-containing wells in volumes of 0.5, 1, and 2 mL. The plates were incubated at 37 °C, and worm viability was assessed microscopically after 24 h of exposure. BHI media served as a negative control39.
Statistical analysis
Descriptive statistics were employed to summarize the data. Statistical comparisons were performed using the Kruskal–Wallis test, a non-parametric method for comparing two or more independent samples. All analyses were conducted using PASW Statistics for Windows, version 18.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was defined as p < 0.05.
Acknowledgements
We appreciated to Semen Laboratory, Veterinary Diagnostic Center, Faculty of Veterinary Science, Mahidol University and other supportive staff for providing us materials and kind assistance.
Abbreviations
- BHI
Brain heart infusion
- CAMP
Christie–Atkins–Munch-Peterson
- CLSI
Clinical and laboratory standards institute
- int1
Class 1 integron
- mcr
Mobile colistin resistance
- MIC
Minimum inhibitory concentration
- PCR
Polymerase chain reaction
- RTX
Repeats-in-toxin
Author contributions
Kr.K, K.K., P.O., D.L.W., and N.N. conducted a study design and conceptualization. Kr.K. and N.N. conducted experiments. Kr.K. performed statistical analysis and data visualization. Kr.K. conducted the first draft of the manuscript. Kr.K., K.K., P.O., D.L.W., T.C. and N.N. performed writing, critical reviewing, editing the manuscript. K.K. conducted as a coordinator with the swine farm, provided sample collection and financial support. N.N. conducted as a laboratory supervisor, microbiological materials provider, and corresponding author. All authors contributed to this research article and approved the final version of the manuscript.
Funding
This project is financially supported by National Research Council of Thailand (NRCT) and Mahidol University (NRCT5-RSA63015-05).
Data availability
The datasets generated and analysed during the current study are available in the NCBI GenBank database under the accession numbers OM179756, OM179757, OM179758, OM179759, OM179760, and OM179761.
Declarations
Competing interests
The authors declare no competing interests.
Ethics declarations
The study was conducted in compliance with the ARRIVE guidelines. The research ethics was approved by the Faculty of Veterinary Science, Mahidol University-Institute Animal Care and Use Committee FVS-MU-IACUC-Protocol No. MUVS-2021-10-41, Animal use license No. U1-01281-2558 and FVS-MU-IACUC-Protocol No. MUVS-2021-10-38, Animal use license No. U1-01321-2558.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Farnum, D. & Riese, R. L. Urogenital infections in sows and gilts; differential diagnosis, diagnostic techniques and control. Iowa State Univ. Vet.51, 1–15 (1989). [Google Scholar]
- 2.de Winter, P., Verdoncka, M., de Kruif, A., Devriese, L. & Haesebrouck, F. Bacterial endometritis and vaginal discharge in the sow: Prevalence of different bacterial species and experimental reproduction of the syndrome. Anim. Reprod. Sci.37, 325–335 (1995). [Google Scholar]
- 3.Tummaruk, P., Kesdangsakonwut, S., Prapasarakul, N. & Kaeoket, K. Endometritis in gilts: Reproductive data, bacterial culture, histopathology, and infiltration of immune cells in the endometrium. Comp. Clin. Pathol.19, 575–584 (2010). [Google Scholar]
- 4.de Winter, P., Verdonck, M., de Kruif, A., Devriese, L. A. & Haesebrouck, F. Endometritis and vaginal discharge in the sow. Anim. Reprod Sci.28, 51–58 (1992). [Google Scholar]
- 5.Dee, S. A. Porcine urogenital disease. Vet. Clin. North Am. Food Anim. Pract.8, 641–660 (1992). [DOI] [PubMed] [Google Scholar]
- 6.Alifragki, A., Kontogianni, A., Protopapa, I., Baliou, S. & Ioannou, P. Infective endocarditis by Pasteurella Species: A systematic review. J. Clin. Med.11, 5037 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuhnert, P., Heyberger-Meyer, B., Nicolet, J. & Frey, J. Characterization of PaxA and its operon: A cohemolytic RTX toxin determinant from pathogenic Pasteurellaaerogenes. Infect. Immun.68, 6–12 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosman, A. L. et al. Antimicrobial use in lactating sows, piglets, nursery, and grower-finisher pigs on swine farms in Ontario, Canada during 2017 and 2018. Porc. Health. Manag.8, 17 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulze, M., Dathe, M., Waberski, D. & Muller, K. Liquid storage of boar semen: Current and future perspectives on the use of cationic antimicrobial peptides to replace antibiotics in semen extenders. Theriogenology85, 39–46 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Schulze, M. et al. Dose rates of antimicrobial substances in boar semen preservation-time to establish new protocols. Reprod. Domest. Anim.52, 397–402 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Vickram, A. S. et al. Antimicrobial peptides in semen extenders: A valuable replacement option for antibiotics in cryopreservation—A prospective review. J. Exp. Biol. Agric. Sci.5, 578–588 (2017). [Google Scholar]
- 12.Morrell, J. M. Antimicrobials in boar semen extenders—A risk/benefit analysis. J Antimicro.2, 1–2 (2016). [Google Scholar]
- 13.Michael, G. B., Bossé, J. T. & Schwarz, S. Antimicrobial resistance in Pasteurellaceae of veterinary origin. Microbiol. Spectr.10.1128/microbiolspec.ARBA-0022-2017 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gharaibeh, M. H. & Shatnawi, S. Q. An overview of colistin resistance, mobilized colistin resistance genes dissemination, global responses, and the alternatives to colistin: A review. Vet. World.12, 1735–1746 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wannigama, D. L. et al. A rapid and simple method for routine determination of antibiotic sensitivity to biofilm populations of Pseudomonas aeruginosa. Ann. Clin. Microbiol. Antimicrob.19, 8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luk-in, S. et al. Occurrence of mcr-mediated colistin resistance in Salmonella clinical isolates in Thailand. Sci. Rep.11, 14170 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shein, A. M. S. et al. Will there ever be cure for chronic, life-changing colistin-resistant Klebsiella pneumoniae in urinary tract infection?. Front. Med.8, 806849 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shein, A. M. S. et al. High prevalence of mgrB-mediated colistin resistance among carbapenem-resistant Klebsiella pneumoniae is associated with biofilm formation, and can be overcome by colistin-EDTA combination therapy. Sci. Rep.12, 12939 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srisakul, S. et al. Overcoming addition of phosphoethanolamine to lipid A mediated colistin resistance in Acinetobacter baumannii clinical isolates with colistin–sulbactam combination therapy. Sci. Rep.12, 11390 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akrami, F., Rajabnia, M. & Pournajaf, A. Resistance integrons; A Mini review. Caspian. J. Intern. Med.10, 370–376 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baltazar, M. et al. Activation of class 1 integron integrase is promoted in the intestinal environment. PLoS genetics.18, e1010177 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boucher, Y., Labbate, M., Koenig, J. E. & Stokes, H. W. Integrons: Mobilizable platforms that promote genetic diversity in bacteria. Trends in microbiology.15, 301–309 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Salogni, C. et al. Infectious agents identified in aborted swine fetuses in a high-density breeding area: A three-year study. J. Vet. Diagn. Invest.28, 550–554 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Fodor, L., Hajtós, I. & Glávits, R. Abortion of a sow caused by Pasteurella aerogenes. Acta Vet. Hung.39, 13–19 (1991). [PubMed] [Google Scholar]
- 25.Levent, S. et al. Two cases of P. aerogenes induced swine abortion: Pathological, immunohistochemical, bacteriological and molecular biological examinations. Magyar Allatorvosok Lapja.133, 214–219 (2011). [Google Scholar]
- 26.Thigpen, J. E., Clements, M. E. & Gupta, B. N. Isolation of P. aerogenes from the uterus of a rabbit following abortion. Lab. Anim. Sci.28, 444–447 (1978). [PubMed] [Google Scholar]
- 27.Citron, D. M. et al. Broth microdilution and disk diffusion tests for susceptibility testing of Pasteurella species isolated from human clinical specimens. J. Clin. Microbiol.43, 2485–2488 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldstein, E. J. C., Citron, D. M., Merriam, C. V. & Tyrrell, K. L. Ceftaroline versus isolates from animal bite wounds: Comparative in vitro activities against 243 isolates, including 156 Pasteurella species isolates. Antimicrob Agents Chemother.56, 6319–6323 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cromwell, G. L. Why and how antibiotics are used in swine production. Anim. Biotechnol.13, 7–27 (2002). [DOI] [PubMed] [Google Scholar]
- 30.Lekagul, A., Tangcharoensathien, V., Mills, A., Rushton, J. & Yeung, S. How antibiotics are used in pig farming: A mixed-methods study of pig farmers, feed mills and veterinarians in Thailand. BMJ Glob. Health.5, e001918 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bosman, A. L. et al. Antimicrobial use in lactating sows, piglets, nursery, and grower-finisher pigs on swine farms in Ontario, Canada during 2017 and 2018. Porc. Health Manag.8, 1–20 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wannigama, D. L. et al. Ca-EDTA restores the activity of ceftazidime-avibactam or aztreonam against carbapenemase-producing Klebsiella pneumoniae infections. iScience26, 107215 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ewers, C. et al. Occurrence of mcr-1 and mcr-2 colistin resistance genes in porcine Escherichia coli isolates (2010–2020) and genomic characterization of mcr-2-positive E. coli. Front. Microbiol.13, 1–17 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valiakos, G. & Kapna, I. Colistin resistant mcr genes prevalence in livestock animals (swine, bovine, poultry) from a multinational perspective. A systematic review. Vet. Sci.8, 1–32 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phuadraksa, T., Wichit, S., Arikit, S., Songtawee, N. & Yainoy, S. Co-occurrence of mcr-2 and mcr-3 genes on chromosome of multidrug-resistant Escherichia coli isolated from healthy individuals in Thailand. Int. J. Antimicrob. Agents.60, 106662 (2022). [DOI] [PubMed] [Google Scholar]
- 36.García, V. et al. Co-occurrence of mcr-1, mcr-4 and mcr-5 genes in multidrug-resistant ST10 Enterotoxigenic and Shiga toxin-producing Escherichia coli in Spain (2006–2017). Int. J. Antimicrob. Agents.52, 104–108 (2018). [DOI] [PubMed] [Google Scholar]
- 37.de Latorre, E. et al. Detection of integrase gene in E. coli isolated from pigs at different stages of production system. Int. J. Microbiol.2014, 1–7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keeratikunakorn, K., Kaewchomphunuch, T., Kaeoket, K. & Ngamwongsatit, N. Antimicrobial activity of cell free supernatants from probiotics inhibits against pathogenic bacteria isolated from fresh boar semen. Sci. Rep.13, 5995 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aryukarn, A., Wannigama, D. L., Ounjai, P. & Chatsuwan, T. Panagrellus redivivus as a model for the study of gram-negative bacteria pathogenesis and antibiotics efficacy. Int. J. Antimicrob. Agents.58, 68 (2021). [Google Scholar]
- 40.Laws, R. T. et al. The nematode Panagrellus redivivus is susceptible to killing by human pathogens at 37°C. FEMS Microbiol. Lett.205, 77–83 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Hommez, J. & Devriese, L. A. P. Aerogenes isolations from swine. Zentralbl Veterinarmed B.23, 265–268 (1976). [DOI] [PubMed] [Google Scholar]
- 42.Nguyet, L. T. Y., Keeratikunakorn, K., Kaeoket, K. & Ngamwongsatit, N. Antibiotic resistant Escherichia coli from diarrheic piglets from pig farms in Thailand that harbor colistin-resistant mcr genes. Sci. Rep.12, 9083 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tauma, K., Stecher, G. & Kuma, S. MEGA11: Molecular evolutionary genetics analysisversion11. Mol. Biol. Evol.38, 3022–3027 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. 31 ed. (2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed during the current study are available in the NCBI GenBank database under the accession numbers OM179756, OM179757, OM179758, OM179759, OM179760, and OM179761.