Abstract
BACKGROUND:
In DanGer Shock (the Danish–German Cardiogenic Shock trial), use of a microaxial flow pump (mAFP) in patients with ST-segment–elevation myocardial infarction–related cardiogenic shock led to lower all-cause mortality but higher rates of renal replacement therapy (RRT). In this prespecified analysis, rates and predictors of acute kidney injury (AKI) and RRT were assessed.
METHODS:
In this international, randomized, open-label, multicenter trial, 355 adult patients with ST-segment–elevation myocardial infarction–related cardiogenic shock were randomized to mAFP (n=179) or standard care alone (n=176). AKI was defined according to RIFLE criteria (Risk, Injury, Failure, Loss, and End-stage kidney disease) and assessed using logistic regression models. Use of RRT was assessed accounting for the competing risk of death using Fine-Gray subdistribution hazard models.
RESULTS:
AKI (RIFLE ≥1) was recorded in 110 patients (61%) in the mAFP group and 79 patients (45%) in the control group (P<0.01); RRT was used in 75 (42%) and 47 (27%) patients, respectively (P<0.01). About two-thirds of the RRTs were initiated within the first 24 hours from admission (n=48 [64%] in the mAFP group and n=31 [66%] in the control group). Occurrence of AKI and RRT were associated with higher 180-day mortality in both study arms. At 180 days, all patients alive were free of RRT. mAFP use was associated with higher rates of RRT, even when accounting for competing risk of death (subdistribution hazard, 1.67 [1.18–2.35]). This association was largely consistent among prespecified subgroups. Allocation to mAFP was associated with lower 180-day mortality irrespective of AKI or RRT (Pinteraction=0.84). Relevant predictors of AKI in both groups comprised reduced left ventricular ejection fraction, baseline kidney function, shock severity, bleeding events, and positive fluid balance. Predictors of AKI specific to mAFP were suction events, higher pump speed, and longer duration of support.
CONCLUSIONS:
Shock severity, allocation to mAFP, and device-related complications were associated with an increased risk of AKI. AKI was generally associated with higher mortality, but the allocation to mAFP consistently led to lower mortality rates at 180 days irrespective of the occurrence of AKI with or without RRT initiation.
REGISTRATION:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT01633502.
Keywords: acute kidney injury; myocardial infarction; renal replacement therapy; shock, cardiogenic
Clinical Perspective.
What Is New?
Microaxial flow pump (mAFP) use increases rates of acute kidney injury (AKI) and short-term renal replacement therapy in patients with ST-segment–elevation myocardial infarction–related cardiogenic shock, even when accounting for the competing risk of death.
Irrespective of AKI or renal replacement therapy, mAFP consistently led to lower mortality rates at 180 days.
Relevant predictors of AKI in both groups were shock severity (including low left ventricular ejection fraction), high admission serum creatinine level, and bleeding events. Predictors specific to mAFP were suction events, higher pump speed, and longer duration of support.
What Are the Clinical Implications?
Risk of AKI and renal replacement therapy requirement is higher in patients with ST-segment–elevation myocardial infarction–related cardiogenic shock treated with mAFP, but this association provides little basis for clinicians to refrain from its use, as the mAFP-associated mortality benefit remains consistent independent of the occurrence of AKI or renal replacement therapy.
Addressing mechanisms of AKI, such as bleeding, suction events, and excessive mAFP unloading, may further improve the treatment effects of mAFP.
Editorial, see p 2004
Cardiogenic shock (CS) is a clinical syndrome defined as hypotension and hypoperfusion attributable to cardiac dysfunction. CS can occur as a complication of ST-segment–elevation myocardial infarction (STEMI)1 and is associated with a high in-hospital mortality rate of 40% to 60% depending on the definition of shock.2–4
Acute kidney injury (AKI) occurs frequently in CS, and is one of the strongest predictors of in-hospital death in CS,5,6 particularly in CS due to acute myocardial infarction.7,8 Mechanisms involve hypoperfusion, renal venous congestion, systemic inflammation, sepsis, neurohumoral activation, and volume extravasation.9 In addition, therapy with vasopressors and inotropes has been associated with AKI.9 Specific treatment options and recommendations beyond standard management of CS, careful fluid management, and timing of renal replacement therapy (RRT) are not available.10
DanGer Shock (the Danish-German Cardiogenic Shock trial) compared the safety and efficacy of use of a microaxial flow pump (mAFP) in addition to standard care with standard care alone in the treatment of patients with STEMI-related CS (STEMI-CS). The trial documented a lower risk of all-cause death at 180 days in the mAFP group.11 However, complication rates were higher in the mAFP group. Besides bleeding events and limb ischemia, the incidence of RRT was higher in the treatment group.
In this prespecified analysis, we sought to provide a detailed evaluation of incidence, outcomes, and possible underlying factors for AKI and the use of RRT in patients with STEMI-CS enrolled in DanGer Shock.
Methods
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design
DanGer Shock was an international, multicenter, randomized, open-label trial conducted in Denmark, Germany, and the United Kingdom. Detailed information about the trial design, inclusion and exclusion criteria, and statistical analysis plan are provided in the main study report and adjacent previous publications.11,12 The trial protocol was approved by the ethics committee at each participating site and the participants gave informed consent.
Patients ≥18 years of age with STEMI were included between 2013 and 2023 if they had CS, defined by hypotension (systolic blood pressure <100 mm Hg or need for vasopressor support), arterial lactate of ≥2.5 mmol/L, and left ventricular ejection fraction <45%. Key exclusion criteria were comatose out-of-hospital cardiac arrest, severe peripheral arterial obstructive disease, mechanical aortic valve prosthesis, septic shock, or right ventricular failure. Participants were randomized 1:1 to undergo either unloading with a mAFP (Impella CP; Abiomed, Johnson & Johnson Med Tech) plus standard of care or standard of care alone. Patients could be randomized before revascularization, while in the catheterization laboratory, or up to 12 hours after revascularization, depending on when shock criteria were met.
Primary Outcomes
For this prespecified, secondary analysis of DanGer Shock, the incidence of AKI according to RIFLE criteria (Risk, Injury, Failure, Loss, and End-stage kidney disease)13 and rates of RRT use throughout hospitalization were the main outcomes of interest. Both items were part of the study intake forms and adjudicated by clinical coordinators of the study at each respective site. AKI according to RIFLE criteria is defined as a ≥2-fold increase in serum creatinine or loss of glomerular filtration rate by ≥50% or a urine output of <0.5 mL·kg·h over 12 hours.13 As part of the electronic case report forms, duration of RRT was recorded as <30 days, 31 to 90 days, or >90 days according to the original RIFLE classification13 and the initiation of RRT was recorded with date and time after admission. For the purpose of this analysis, AKI status was assessed as no AKI, AKI without RRT, or AKI with RRT. AKI was recorded by the study sites and an independent contract research organization (www.KCRI.org) performed monitoring on all patients to ensure accuracy of recorded AKI class and use of RRT.
It was recommended in the study protocol (Supplemental Methods) to initiate RRT in case of oliguria (total diuresis of <50 mL in 6 hours) with volume overload (pulmonary edema), renal insufficiency with serum creatinine >300 to 400 mmol/L, treatment-resistant metabolic acidosis (arterial pH <7.30), or severe hyperkalemia (serum potassium >6.5 mmol/L). The final decision to initiate RRT was at the discretion of the treating shock team.
In addition, to characterize the relevance of AKI and RRT for the primary and secondary end points of the DanGer trial, we performed separate analyses treating 180-day mortality and escalation of treatment to additional mechanical circulatory support (short- or long-term), heart transplantation, or death of any cause as outcomes, and the occurrence of AKI and initiation of RRT as predictors, respectively.
Primary Exposures
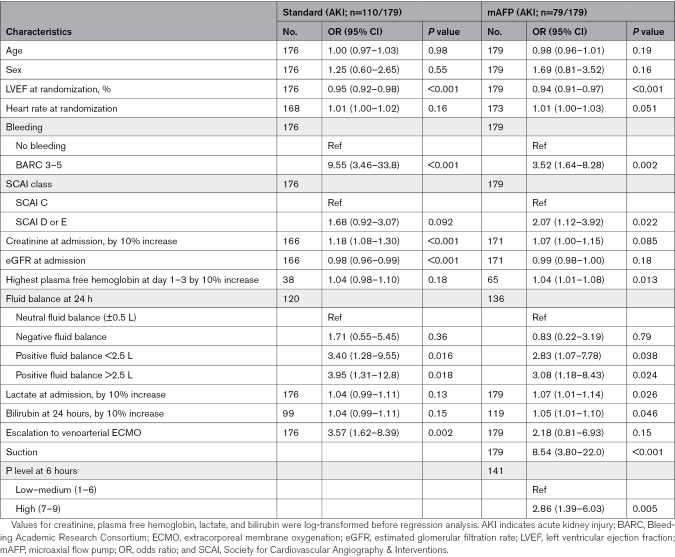
The primary exposure of interest was the study intervention (mAFP use), which was handled in the form of an intention-to-treat analysis. Other exposures assessed in multivariable models or by stratification were the key demographic, laboratory, and clinical baseline characteristics from the original report of the DanGer trial, as well as admission creatinine as an established predictor of AKI in critically ill patients and particularly in CS.7,14,15 Candidate postadmission predictors, based on previous literature and clinical experience suspected of being associated with AKI or RRT, included bleeding events (defined by Bleeding Academic Research Consortium criteria type 3–5), escalation to extracorporeal membrane oxygenation (ECMO), total bilirubin and fluid balance at 24 hours postadmission, and highest plasma free hemoglobin during the first 72 hours after admission. In addition, candidate predictors specifically in the mAFP group included suction events, P level at different available time points, and duration of support.
Statistical Analyses
All primary statistical analyses in this work were reported separately for both primary outcomes, AKI and RRT. AKI information was dichotomized as no AKI or any AKI during hospitalization, irrespective of the use of RRT and duration of RRT. Analyses relating to AKI as the outcome include between 2-group comparisons and corresponding logistic regression models. In addition, RRT information was assessed with timing information and treated as a time-to-event outcome.
In tables of patient characteristics with versus without AKI and with versus without RRT during their hospitalization, respectively, all continuous variables are reported as medians with interquartile ranges and compared between 2 groups with Wilcoxon rank sum tests. Categorical variables are expressed as counts and percentages and were compared with either χ2 tests or Fisher exact tests depending on the sample size. For figures and tables in which serial measurements are displayed, data are represented as estimated geometric means with 95% CIs calculated from linear mixed models.
To assess the association of AKI and RRT with the primary end point of the trial (180-day mortality) and secondary end point (escalation, left ventricular assist device, or death), we generated univariable logistic regression models with these end points as outcomes and AKI or RRT status as predictors, all stratified by treatment group.
The association of mAFP with 180-day mortality among patients with and without AKI or RRT was assessed using logistic regression models with mAFP and AKI or mAFP and RRT as predictors with an interaction term and 180-day mortality as the outcome. For the aforementioned models, logistic regression was chosen over Cox regression because follow-up for these outcomes was complete in all study participants, and from a clinical standpoint, we regarded the information whether these outcomes occurred as more important than when during the first 180 days.
In the figures depicting creatinine trajectories, creatinine values were log-transformed before analysis to approximate normal distribution. The results were then visualized as group-specific geometric means with CIs after back-transformation.
We then assessed whether the risk of AKI and RRT initiation in the mAFP group was increased, and whether this effect was consistent across prespecified subgroups. Here, we used univariable regression models with mAFP as the only predictor or bivariable regression models with mAFP and respective subgroups as predictors including interaction terms. For the outcome AKI, logistic regression models were used, whereas for the outcome RRT, Fine-Gray subdistribution hazard models were used. Fine-Gray models were chosen over Cox regression models because of the high early mortality rates in both study arms to account for the competing risk of death.
To assess the associations of candidate exposures and admission and postadmission predictors with these outcomes, univariable logistic regression and Fine-Gray models were used for AKI and RRT, respectively. Resulting associations are reported as odds ratios (ORs) or subdistribution hazard ratios with the corresponding 95% CIs, respectively. Skewed variables were log-transformed before regression to approximate normal distribution and resulting ORs or subdistribution hazard ratios are reported by 10% increase.
Estimated glomerular filtration rate (eGFR) was calculated according to the CKD-EPI formula (Chronic Kidney Disease Epidemiology Collaboration) in its most recent update from 2021.16 Logistic regression models were used to evaluate the relationship between eGFR at randomization and the risk of AKI, considering potential interactions with allocation. For illustration, we then plotted the risk of AKI across the range of eGFR, first stratified by treatment group and then including the treatment group as a predictor to show the treatment effect (mAFP versus standard) on the risk of AKI as a function of baseline eGFR with 95% CIs. Restricted cubic splines were initially used to assess potential nonlinear associations between eGFR and the risk of AKI in both study arms. However, a simpler model without splines adequately captured the relationship.
An exploratory analysis was performed only within the mAFP group to assess whether higher mAFP performance levels were associated with hemolysis and subsequent kidney injury. For this, linear regression models were generated with total bilirubin at 24 hours and serum creatinine at 48 hours as outcome variables and mAFP performance levels as predictors, stratified by higher levels (7 to 9) or lower levels of support (1 to 6). These associations were tested in univariate and multivariate regression models, the latter adjusted for the potential confounders age as well as left ventricular ejection fraction and admission lactate level as surrogates of CS severity.
Society for Cardiovascular Angiography & Interventions (SCAI) shock stage was assessed after completion of the study but before unblinding according to the Cardiogenic Shock Working Group approach.17 The vasoactive–inotropic score was calculated as previously described18 and square root transformed for regression modeling. A 2-sided α level of 0.05 was considered statistically significant. All statistical analyses and plots were conducted using R software, version 4.4.1 (R Foundation for Statistical Computing). Dr Møller had full access to all the data in the study and takes responsibility for its integrity and the data analysis.
Results
Baseline Characteristics of Patients With and Without Renal End Points
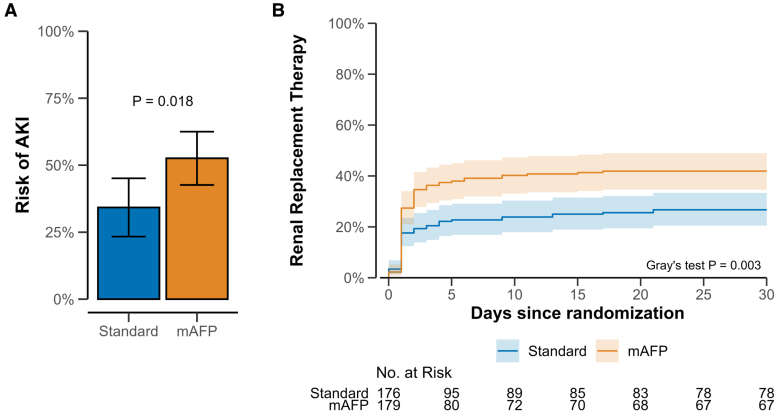
Of all study participants, 189 (53%) had AKI, including 110 (61%) in the mAFP group and 79 (45%) in the control group (P<0.01), and 122 (34%) required RRT (mAFP, 75 [42%] versus control, 47 [27%]; P<0.01; Figure 1).
Figure 1.
Risk of acute kidney injury and renal replacement therapy in both study arms. A, Unadjusted cumulative risk of acute kidney injury (AKI) according to RIFLE criteria (Risk, Injury, Failure, Loss, and End-stage kidney disease). B, Unadjusted cumulative incidence of renal replacement therapy until 30 days after randomization (Gray test adjusting for competing risk of death). mAFP indicates microaxial flow pump.
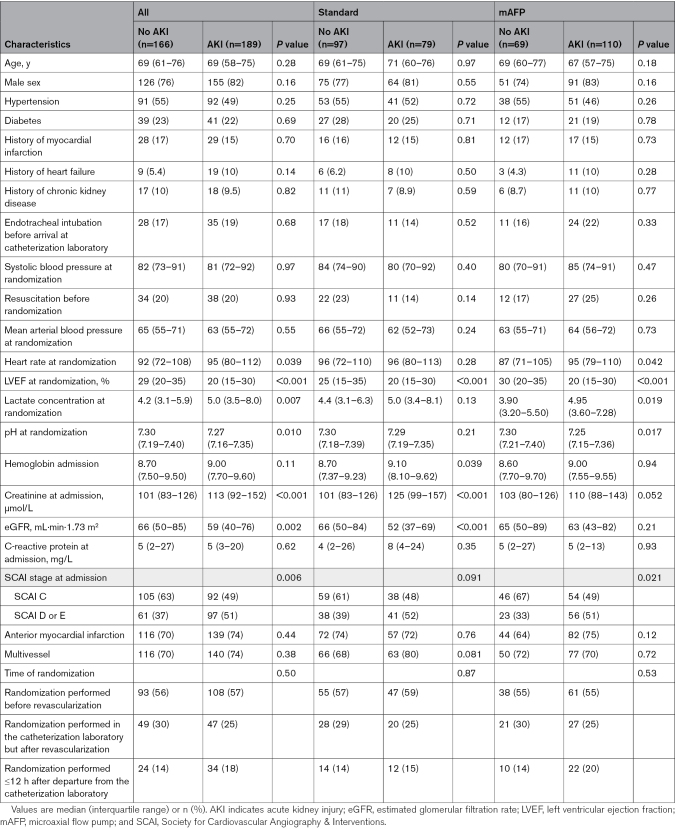
At admission, compared with patients with CS who did not develop AKI, those who later developed AKI presented with higher heart rate, higher creatinine levels, and more advanced SCAI shock stages (Table 1). Most of these differences were consistent among study participants in both study arms (Table 1).
Table 1.
Baseline Characteristics of Patients Developing AKI in Both Study Arms
The differences in baseline characteristics between patients who later received RRT and those who did not undergo RRT were similar, but study participants who later received RRT were also younger and more likely to be male (Table S1). Again, most of these differences were also consistent when the groups were stratified by study intervention (Table S1).
Association of AKI With Mortality
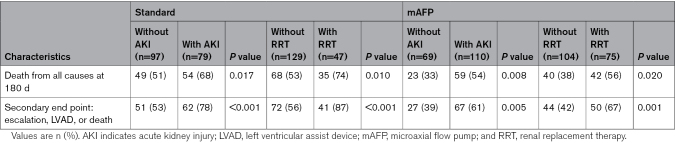
The occurrence of either AKI or AKI with RRT during the study period of 180 days was associated with higher 180-day mortality in both study arms (Table 2).
Table 2.
Primary Outcomes Based on Occurrence of AKI and RRT
There was no significant interaction between the effect of allocation to the mAFP on all-cause mortality and the occurrence of AKI or the requirement for RRT with respect to 180-day mortality. In patients without AKI, the OR for 180-day mortality when allocated to the mAFP compared with standard care was 0.49 (95% CI, 0.26–0.93); in patients with AKI, the OR was 0.55 (95% CI, 0.29–0.98; Pinteraction=0.84). In patients who did not receive RRT, the OR was 0.56 (95% CI, 0.33–0.95), compared with an OR of 0.44 (95% CI, 0.20–0.97) in those who received RRT (Pinteraction=0.61).
Long-Term Kidney Outcomes
Serum creatinine level before discharge among survivors was higher in the mAFP group (median, 127 µmol/L [1.33 mg/dL; interquartile range, 91–244] in the mAFP group versus 100 µmol/L [1.13 mg/dL; interquartile range, 76.5–148] in the standard care group; P<0.001). Time from symptom onset to randomization did not correlate with baseline creatinine levels (r=0.072, P=0.20; data not shown). At 180 days, none of the survivors (n=73 in the standard group, n=97 in the mAFP group) required RRT.
Incidence of AKI and RRT Based on Treatment Allocation
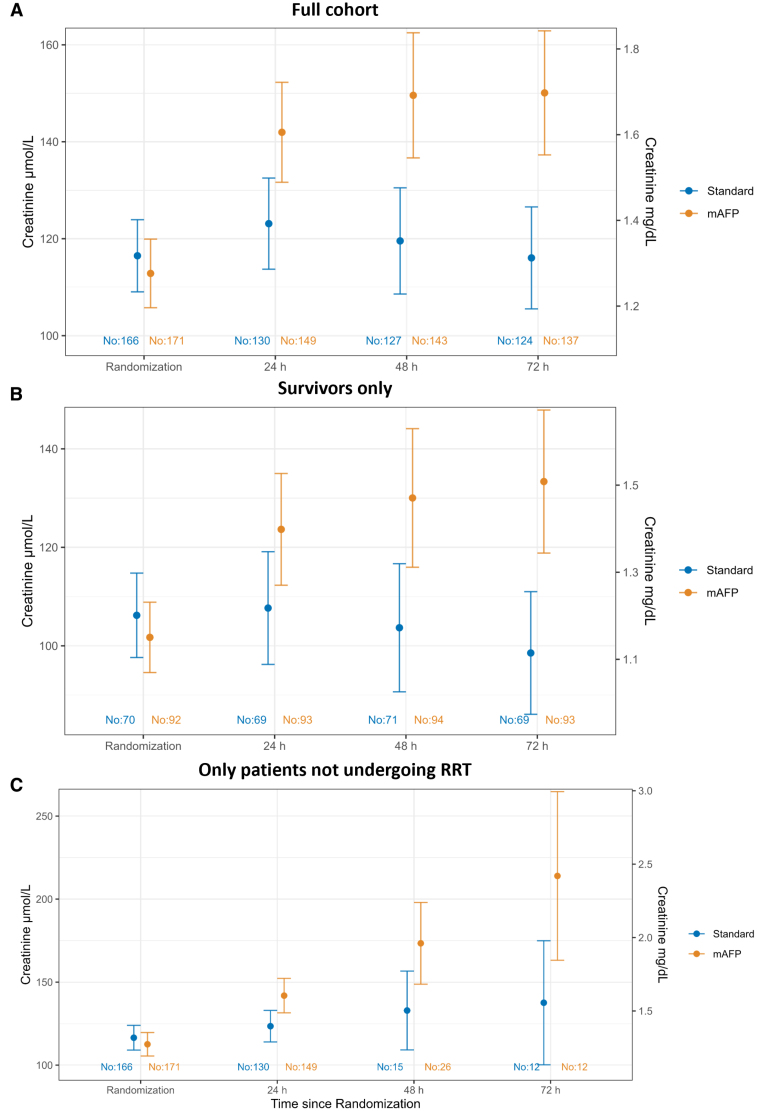
The majority of RRTs were initiated within the first 24 hours from admission (48 patients [64%] in the mAFP group, 31 patients [66%] in the control group). The probability of AKI and RRT was higher in the mAFP group (Figure 1). mAFP use was also associated with higher serum creatinine levels during the first 72 hours compared with controls in the full study population, in the subgroup of 180-day survivors only, and in the subgroup of those who did not receive RRT during the first 72 hours after admission (Figure 2). Use of diuretic stress tests and previous use of angiotensin-converting enzyme inhibitors are shown in Tables S3 and S4. The vasoactive–inotropic score was higher in the standard of care group during first hours in the intensive care unit (Table S2).
Figure 2.
Creatinine trajectories throughout the first 72 hours after admission. Estimates of linear mixed models showing the geometric mean of serum creatinine levels at randomization and 24, 48, and 72 hours after randomization, along with the respective 95% CIs. A, Full cohort. B, Subgroups of patients who survived until the end of the study follow-up (180 days). C, Subgroups of patients who did not undergo renal replacement therapy (RRT) at the time of serum creatinine measurements. mAFP indicates microaxial flow pump.
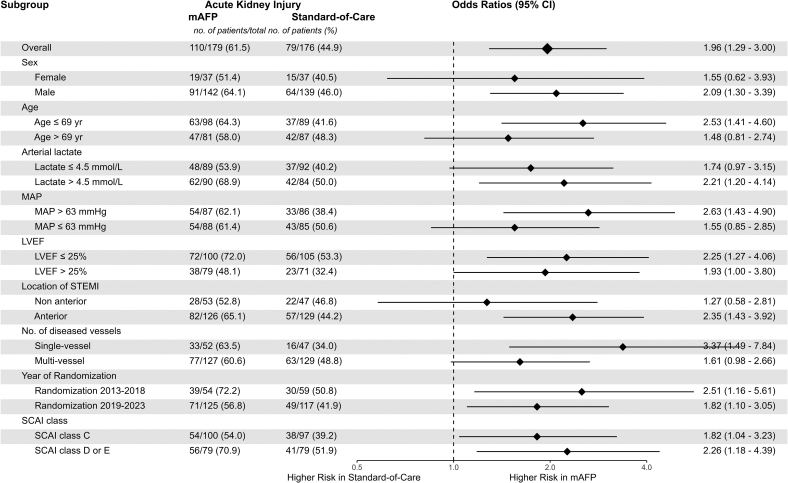
When accounting for the competing risk of death, mAFP use was associated with higher rates of RRT (Fine-Gray subdistribution hazard ratio, 1.67 [1.18–2.35]; Figure 3). mAFP use was consistently associated with a higher risk of AKI and RRT in all subgroups without signals of significant interaction (Figure 3; Figure S1).
Figure 3.
Risk of acute kidney injury in microaxial flow pump vs standard of care groups, stratified by prespecified subgroups. Odds ratios from logistic regression models with treatment group and respective subgroups as predictors with an interaction term and acute kidney injury as the outcome. LVEF indicates left ventricular ejection fraction; mAFP, microaxial flow pump; MAP, Mean arterial pressure; SCAI, Society for Cardiovascular Angiography & Interventions; and STEMI, ST-segment–elevation myocardial infarction.
Predictors of AKI in Patients of Both Study Arms
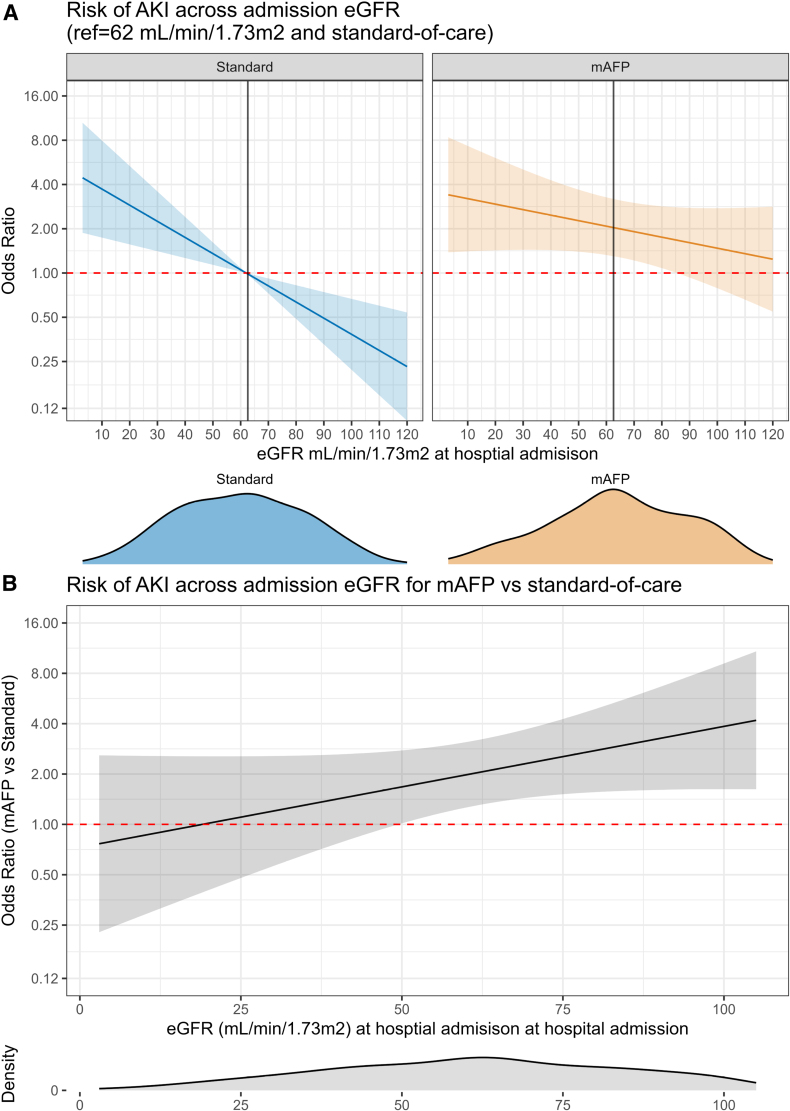
Low baseline eGFR was a predictor for AKI in the standard of care group (Table 3) and for RRT in the mAFP group (Table S5). mAFP-associated excess risk of AKI decreased with lower admission eGFR (Figure 4). Sensitivity analyses using restricted cubic splines suggested linear association between baseline eGFR and AKI risk in both treatment arms.
Table 3.
Univariable Predictors of AKI in Both Study Groups
Figure 4.
Acute kidney injury risk across baseline estimated glomerular filtration rate for microaxial flow pump vs standard group. A, Risk of acute kidney injury (AKI) according to admission estimated glomerular filtration rate (eGFR) in both treatment arms. The reference is median eGFR in standard of care group. B, Risk of AKI in microaxial flow pump (mAFP)–treated patients compared with standard across the range of admission eGFR estimated using a logistic regression model with mAFP and eGFR as predictors with an interaction term. The distribution of eGFR is depicted with density plots below each panel.
Baseline left ventricular ejection fraction was inversely associated with the risk of AKI (Table 3). Patients presenting with more severe SCAI stages (D/E versus C) and higher lactate levels also tended to have higher AKI risk (Table 3).
Postadmission predictors of AKI/RRT in both groups were Bleeding Academic Research Consortium 3 through 5 bleeding, a positive fluid balance at 24 hours after admission to the intensive care unit, and escalation to ECMO (Table 3; Table S5). In the mAFP group, we also identified device-related suction events, higher mAFP performance levels, and longer duration of mAFP support. In addition, among patients in whom bilirubin or plasma free hemoglobin levels were recorded (218 of 355 for bilirubin, 101 of 355 for plasma free hemoglobin), bilirubin levels at day 1 and highest plasma free hemoglobin at day 1 to 3 were also associated with AKI or RRT (Table 3; Table S5).
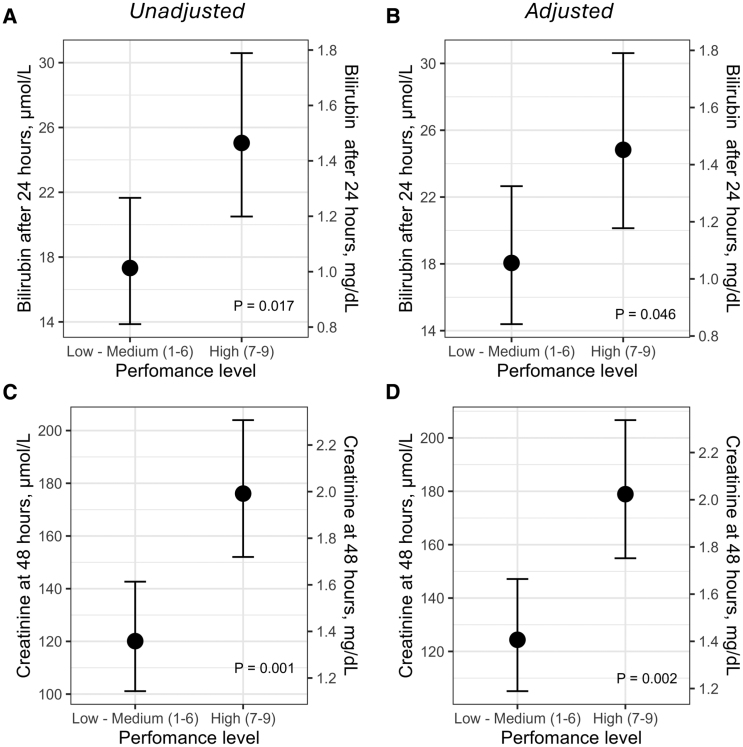
Higher mAFP performance levels in the mAFP arm were associated with both higher levels of bilirubin at 24 hours and creatinine levels at 24 hours in crude and adjusted linear regression analyses (Figure 5).
Figure 5.
Association between microaxial flow pump performance levels, markers of hemolysis, and renal function. Estimated marginal means from linear regression models with total bilirubin (A and B) and serum creatinine (C and D) as outcome variables. P values correspond to the respective coefficients of microaxial flow pump performance level as a dichotomous predictor. A and C, Unadjusted models. B, Adjusted for age, left ventricular ejection fraction, lactate, and alanine aminotransferase at arrival. D, Adjusted for age, left ventricular ejection fraction, lactate, and estimated glomerular filtration rate at arrival.
Discussion
This analysis provides several insights into the specific risk of AKI and RRT associated with the use of mAFP in patients with STEMI-CS. Shock severity, bleeding, baseline eGFR, and allocation to mAFP were associated with an increased risk of AKI. in addition, specifically in the mAFP group, suction events, high mAFP performance levels, and longer duration of support were also associated with an increased risk of AKI. Whereas AKI occurrence was generally associated with higher mortality, allocation to mAFP was associated with comparable relative survival benefit regardless of whether patients experienced AKI or RRT.
AKI is a frequent complication in CS, often leading to the requirement of RRT, and is a well-established predictor of mortality in patients with STEMI-CS.5,7,8 The prevalence of AKI in the current study, even in the control arm, was higher than that reported in other contemporary acute myocardial infarction–related CS trials, which may be due to differences in study design, among other factors.19–21 Most CS trials did not record or report the incidence of AKI, but the IABP-SHOCK-II (Intraaortic Balloon Pump in Cardiogenic Shock II), CULPRIT-SHOCK (Culprit Lesion Only PCI versus Multivessel PCI in Cardiogenic Shock), and ECLS-SHOCK (Extracorporeal Life Support in Cardiogenic Shock) shock trials have reported the rates of RRT.19–22 The rates of RRT were significantly lower in IABP-SHOCK-II (20.6% in the intra-aortic balloon pump arm, 15.7% in the control arm; risk ratio [RR], 1.31), CULPRIT-SHOCK (overall 14.0%), and ECLS-SHOCK (8.1% in the extracorporeal life support [ECLS] arm, 13.9% in the control arm; RR, 0.58) compared with both study arms in DanGer Shock (41.9% in the mAFP arm, 26.7% in the control arm; RR, 1.57).11,19–21 The differences in RRT initiation rates in the control arms of these trials reflect the differences of the underlying trial populations, as reviewed previously.23 Nevertheless, the magnitude of the RRs within each respective trial hints toward intrinsic pathophysiologic mechanisms specific for the mAFP, which could lead to the reported higher rates of RRT in DanGer Shock. This risk difference associated with mAFP use in CS is in line with previous observational studies. For example, Schrage et al24 report an RR of 1.50 for RRT use in patients treated with ECLS and mAFP compared with those treated with ECLS alone.
AKI- and RRT-Related Survival in Patients With STEMI-CS in DanGer Shock
In line with other observational and nonobservational trials, AKI and RRT were associated with higher mortality at 180 days in the DanGer Shock population.5,7,8 The current analysis shows that mAFP use increased the risk of AKI and RRT in selected patients with STEMI-CS, even when accounting for competing risks. However, despite this increased risk, mAFP use increased survival in patients with and without AKI or RRT as indicated by the subgroup analysis. The results of the mediation analysis should be interpreted with caution as this analysis was not prespecified and may be subject to potential survivor bias. In addition, none of the survivors in the study required dialysis at the end of follow-up at 180 days. These data suggest that rather than avoiding the use of mAFP for fear of AKI, a better approach for clinicians and researchers may be to focus on strategies to minimize the risks and complications associated with mAFP use. The current analysis of predictors of AKI, particularly in the mAFP group, may help identify such strategies.
Predictors of AKI and RRT
Even though the trial design of DanGer Shock does not allow for causal inference regarding the pathophysiologic mechanisms leading to higher rates of RRT and AKI in patients with CS treated with mAFP, we identified several factors at admission as well as throughout hospitalization that were tightly associated with these renal end points.
Previous studies hint toward low admission eGFR as a predictor of AKI risk in CS.9,15 This was also the case in our study, but to a lesser extent in the mAFP group as compared with the standard group. The AKI risk attributable to mAFP seemed to decrease with lower admission eGFR, even though patients with low eGFR were at higher a priori risk of AKI and RRT. The underlying reasons remain uncertain.
Sex differences represent a mostly unmet challenge in CS research, and DanGer Shock is no exception, given the high proportion of male participants and the uncertainty of benefit from the primary study intervention in women.11,25 With respect to renal outcomes in this trial, sex was no clear predictor of AKI risk, in line with previous observational studies reporting similar rates of RRT use in male and female patients with CS.25
Beyond baseline factors, we identified several postadmission predictors of AKI and RRT in patients with and without mAFP. Of these, in both arms, bleeding events, low left ventricular ejection fraction, escalation to venoarterial ECMO, and positive fluid balance at 24 hours were highly relevant predictors of AKI. In the mAFP arm, hemolysis, suction events, higher pump speed, and longer duration of support were also associated with higher AKI risk.
Bleeding Events
In the context of the aforementioned other large randomized controlled trials, bleeding events should in general be expected as more common in ECLS-treated patients compared with mAFP-treated patients. Still, in ECLS-SHOCK, rates of AKI were lower in the ECLS arm.20 Thus, despite being associated with higher AKI risk, bleeding events alone are unlikely to solely explain the mAFP-specific excess risk of AKI and RRT observed in DanGer Shock. This, and the strong association between device-related measures and AKI risk, indicate other, more mAFP-specific risk factors.
Low Output and Severe CS
In this secondary analysis, we identified that in both groups, several measures indicative of low cardiac output were associated with higher risk of AKI and RRT. As such, admission left ventricular ejection fraction was inversely related to AKI risk. We also found that higher admission heart rate tended to be associated with AKI risk. Even though higher heart rate in general could indicate volume demand, specifically in CS it is more likely to represent a signal of reduced stroke volume and compensatory tachycardia to maintain cardiac output, and thus again a measure of disease severity. Increased heart rate may also be a surrogate of minor bleeding events or a systemic inflammatory response. The fact that we identified positive fluid balance 24 hours after admission as being associated with a higher AKI risk again may also point against heart rate as a surrogate for volume demand. Yet positive fluid balance may be evident in different clinical scenarios, such as no diuretic treatment or a lack of response to a diuretic stress test, which makes this measure difficult to interpret without further information. In addition, escalation to ECMO could simply be interpreted as another surrogate for more severe CS cases with low output, and was associated with a higher risk of RRT in the entire study population.
Hemolysis
Hemolysis can cause direct renal damage through the release of free hemoglobin and development of pigment nephropathy.26,27 The DanGer Shock protocol mandated 48 hours of mAFP support at the highest possible P level while avoiding suction. This may have led to overly aggressive unloading in some individuals and concurrently may have increased the risk of hemolysis. This assumption is supported by the current association between high performance levels and higher levels of bilirubin at 24 hours. Hemolysis may also be the major driver of the strong association between suction events and AKI risk that was evident in the current study. This high risk of AKI in patients with suction events calls for strict surveillance for early indicators of imminent suction events, such as changes in motor current signal, and should prompt immediate imaging study to evaluate device placement, right ventricular function, volume status, and (until resolved) lowering of the P level on the device.
In addition, redesigning an mAFP to minimize suction forces and improve hemocompatibility could potentially reduce hemolysis. Direct reduction of plasma free hemoglobin (eg, by haptoglobin infusion) may be another promising, albeit purely experimental, approach.28 Implementation of these strategies could potentially improve renal outcomes and overall prognosis in patients with STEMI-CS requiring mAFP support.
If prolonged support is required, an axillary approach has been proposed for more stable device placement. Yet, recent data from observational studies of higher-volume mAFPs, such as the Impella 5.0 or 5.5, suggest that the RRT rate may be similar to that of the Impella CP at 41.3% in acute myocardial infarction–related CS.29 Therefore, it remains unclear whether an axillary approach could lower incidence of hemolysis and AKI, and this question cannot be answered by the current trial, the results of which should be interpreted only for the device tested (Impella CP).
Limitations
DanGer Shock was a pragmatic trial, and the data captured were based on data collected for the main trial; therefore, the granularity of information on renal characteristics could have been higher, and the data collected were based on knowledge at the time of study initiation >10 years ago. For example, hemolysis likely played an important role in the development of AKI in mAFP-supported patients, but some markers of hemolysis, such as fibrinogen, were not assessed, and the measurements of plasma free hemoglobin, haptoglobin, or bilirubin were not mandated by the study protocol and were recorded only if measured. Thus, the association between these markers and AKI could be influenced by selection bias.
Furthermore, only the history of previous chronic kidney disease was available, not whether these patients required dialysis before the study. However, the overall number of patients with a history of chronic kidney disease was low (10%), and no survivors required dialysis at 6 months, suggesting that the proportion of patients requiring dialysis at baseline was low and would be expected to be evenly distributed between treatment groups given the randomized design.
Serum creatinine was not assessed at 180 days, but only the latest available serum creatinine before discharge, which limits possible inference on the long-term renal outcomes. Contrast volume used during the initial procedure in the catheterization laboratory was not assessed in the study, but there were no differences in the extent of revascularization between groups. Hemodynamic data obtained from pulmonary artery catheters were not available for this study.
The initiation of RRT remained subject to the bedside clinician’s decision, who was not blinded to treatment allocation as blinding was not possible in this trial. Bearing this in mind, RRT use in this study should reflect real-life clinical practice. Another advantage of this analysis is that AKI (independent of RRT) was also assessed as part of the electronic case report forms, and this outcome is less likely to be affected by treatment bias than RRT.
Whereas the randomized controlled trial setting may allow for causal inference with respect to mAFP-associated AKI and risk of RRT, the analyses of predictors of AKI and RRT may be subject to residual confounding and require further testing in clinical trials with the appropriate study design.
Conclusions
In patients with STEMI-CS, shock severity, allocation to mAFP, and device-related complications were associated with an increased risk of AKI. AKI was generally associated with higher mortality rates, but the allocation to mAFP consistently led to lower mortality rates at 180 days irrespective of the occurrence of AKI with or without RRT initiation. Addressing mechanisms of AKI, such as bleeding, suction events, and excessive mAFP unloading, may further improve the treatment effects of mAFP.
Article Information
Sources of Funding
The work of Dr Zweck is supported by grants from the German Research Foundation (DFG; grants 527448911 and 493659010) and the German Heart Foundation (grant F/22/18). DanGer Shock was funded by the Danish Heart Foundation and Abiomed.
Disclosures
Dr Zweck reports a travel grant from Abiomed Inc. Dr Hassager reports institutional research grants from Novo Nordisk Foundation, Lundbeck Foundation, and Danish Heart Foundation outside the submitted work. Dr Beske has nothing to disclose. Dr Jensen reports unrestricted institutional research grants from Biosensors International Group and Biotronik. Dr Eiskjær reports institutional research grants from Novo Nordisk Foundation and Independent Research Fund Denmark and speaker fees from Novartis outside the submitted work. Dr Mangner received a research and an educational grant from Abiomed to his institution, outside the submitted work; an educational grant from Boston Scientific to his institution, outside the submitted work; and personal fees from Edwards Lifesciences, Medtronic, Biotronik, Novartis, Sanofi Genzyme, AstraZeneca, Pfizer, Daiichi Sankyo, Abbott, Abiomed, B. Braun, and Boston Scientific, outside the submitted work. Dr Polzin reports institutional research grants and personal speaker honoraria from Abiomed Inc. Dr Schulze reports grants from Boehringer Ingelheim, Abiomed Inc, Edwards Inc, Cytosorb Inc, and Boston Sci; consulting fees or honoraria from Bayer, Astra Zeneca, Daiichi Sankyo, Novartis, Actelion, Roche, Sanofi Aventis, Pharmacosmos, Medtronic, Thoratec, Boehringer Ingelheim, Heartware, Coronus, Abbott, Boston Scientific, St Jude Medical, Abiomed, and DGK; and trial committee work for Abbott and Abiomed. Dr Skurk reports speaker honoraria from Abiomed. Dr Nordbeck reports speaker honoraria from Abiomed, Bayer Healthcare, Boehringer Ingelheim, Daiichi Sankyo Europe GmbH, and Pfizer. Dr Clemmensen reports consultant fees from Acarix AB, is an investigator for Acarix AB and Bayer Healthcare, and is on End Point Review Committees for Boehringer Ingelheim and WGC, outside the submitted work. Dr Panoulas reports consultant fees, honoraria, and an educational grant from Abiomed J&J. Dr Zimmer reports speaking engagements and serves on the scientific advisory board for Abiomed. Dr Schäfer received honoraria from Abiomed, ZOLL Circulation, AstraZeneca, Amgen, BMS, Pfizer, Daiichi-Sankyo, Eli Lilly, and Boehringer Ingelheim, as well as an institutional grant from Abiomed and Daiichi-Sankyo. Dr Kelm reports grant support from Abbott Vascular, Abiomed, Amgen, B. Braun, Boston Scientific, Daiichi Sankyo, Mars, Edwards LifeSciences, Medtronic, Microvision Medical Holding B.V., Philips, and the German Research Foundation, and is on the Scientific Advisory Board for Mars. Dr Engstrøm reports speakers fees from Boston Scientific, Abbott, and Novo Nordisk, and advisory board fees from Novo Nordisk and Abbott; and has received educational grants from Novo Nordisk and The Danish Heart Foundation. Dr Holmvang received a travel grant from Boston Scientific. Drs Junker and Schmidt have nothing to disclose. Dr Terkelsen reports research grants from Novo Nordisk and Danish Heart Foundation not related to this topic; unrestricted research grants from Meril and Terumo not related to this topic; and lecture and proctor fees from Meril and Terumo not related to this topic. Dr Linke reports consultant and speaker honoraria from Abbott Laboratories, Abiomed, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific Corporation, Corvia, Daiichi Sankyo Europe GmbH, Edwards Lifesciences, Medtronic USA, Novartis, and Pfizer, and stock options for Picardia, Transverse Medical Inc. Dr Westenfeld is employed by Abiomed Inc. Dr Møller reports institutional research grants from Novo Nordik Foundation and Abiomed and serves as an advisor for Boston Scientific.
Supplemental Material
DanGer Shock Investigators
Methods
Figure S1
Tables S1–S5
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AKI
- acute kidney injury
- CKD-EPI
- Chronic Kidney Disease Epidemiology Collaboration
- CS
- cardiogenic shock
- DanGer Shock
- Danish–German Cardiogenic Shock trial
- ECLS
- extracorporeal life support
- ECMO
- extracorporeal membrane oxygenation
- eGFR
- estimated glomerular filtration rate
- mAFP
- microaxial flow pump
- OR
- odds ratio
- RIFLE
- Risk, Injury, Failure, Loss, and End-stage kidney disease
- RR
- risk ratio
- RRT
- renal replacement therapy
- SCAI
- Society for Cardiovascular Angiography & Interventions
- STEMI
- ST-segment–elevation myocardial infarction
A list of DanGer Shock trial investigators is provided in the Supplemental Material.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.124.072370.
This work was presented as an abstract at the Transcatheter Cardiovascular Therapeutics conference, Washington, DC, October 27–30, 2024.
For Sources of Funding and Disclosures, see page 2002.
Circulation is available at www.ahajournals.org/journal/circ
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
Contributor Information
Elric Zweck, Email: Elric.Zweck@med.uni-duesseldorf.de.
Christian Hassager, Email: Christian.Hassager@regionh.dk.
Lisette O. Jensen, Email: okkels@dadlnet.dk.
Hans Eiskjær, Email: heis@dadlnet.dk.
Norman Mangner, Email: norman.mangner@tu-dresden.de.
Amin Polzin, Email: amin.polzin@med.uni-duesseldorf.de.
Carsten Skurk, Email: carsten.skurk@dhzc-charite.de.
Peter Nordbeck, Email: p.clemmensen@uke.de.
Peter Clemmensen, Email: p.clemmensen@uke.de.
Vasileios Panoulas, Email: v.panoulas@rbht.nhs.uk.
Sebastian Zimmer, Email: sebastian.zimmer@ukbonn.de.
Andreas Schäfer, Email: schaefer.andreas@mh-hannover.de.
Malte Kelm, Email: malte.kelm@med.uni-duesseldorf.de.
Thomas Engstrøm, Email: thomas.engstroem@regionh.dk.
Lene Holmvang, Email: Lene.Holmvang@regionh.dk.
Anders Junker, Email: anders.junker@rsyd.dk.
Henrik Schmidt, Email: henrik.schmidt@rsyd.dk.
Christian J. Terkelsen, Email: Chriterk@rm.dk.
Axel Linke, Email: axel.linke@tu-dresden.de.
Ralf Westenfeld, Email: ralf.westenfeld@med.uni-duesseldorf.de.
Collaborators: Jacob Eifer Møller, Lisette Okkels Jensen, Anders Junker, Karsten Tange Veien, Nanna Louise Junker Udesen, Hanne Berg Ravn, Jens Flensted Lassen, Kristian Wachtell, Henrik Schmidt, Christian Hassager, Thomas Engstrøm, Lene Holmvang, Jesper Kjaergaard, Rikke Sørensen, Jacob Lønborg, Martin Fryldand, Rasmus Paulin Beske, Søren Boesgaard, Hans Eiskjær, Steffen Christensen, Evald Høj Christiansen, Steffen Christensen, Christian Juhl Terkelsen, Andreas Schäfer, Axel Linke, Felix J. Woitek, Jennifer Hommel, Norman Mangner, Amin Polzin, Ralf Westenfeld, Christian Schulze, Sven Moebius-Winkler, Carsten Skurk, Peter Nordbeck, Peter Clemmensen, Dirk Westermann, Vasileios Panoulas, Sebastian Zimmer, Nikos Werner, and Inge De Haas
References
- 1.Kapur NK, Thayer KL, Zweck E. Cardiogenic shock in the setting of acute myocardial infarction. Methodist Debakey Cardiovasc J. 2020;16:16–21. doi: 10.14797/mdcj-16-1-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolte D, Khera S, Aronow WS, Mujib M, Palaniswamy C, Sule S, Jain D, Gotsis W, Ahmed A, Frishman WH, et al. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J Am Heart Assoc. 2014;3:e000590. doi: 10.1161/JAHA.113.000590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sterling LH, Fernando SM, Talarico R, Qureshi D, van Diepen S, Herridge MS, Price S, Brodie D, Fan E, Di Santo P, et al. Long-term outcomes of cardiogenic shock complicating myocardial infarction. J Am Coll Cardiol. 2023;82:985–995. doi: 10.1016/j.jacc.2023.06.026 [DOI] [PubMed] [Google Scholar]
- 4.Helgestad OKL, Josiassen J, Hassager C, Jensen LO, Holmvang L, Sorensen A, Frydland M, Lassen AT, Udesen NLJ, Schmidt H, et al. Temporal trends in incidence and patient characteristics in cardiogenic shock following acute myocardial infarction from 2010 to 2017: a Danish cohort study. Eur J Heart Fail. 2019;21:1370–1378. doi: 10.1002/ejhf.1566 [DOI] [PubMed] [Google Scholar]
- 5.Tarvasmaki T, Haapio M, Mebazaa A, Sionis A, Silva-Cardoso J, Tolppanen H, Lindholm MG, Pulkki K, Parissis J, Harjola VP, et al. ; CardShock Study Investigators. Acute kidney injury in cardiogenic shock: definitions, incidence, haemodynamic alterations, and mortality. Eur J Heart Fail. 2018;20:572–581. doi: 10.1002/ejhf.958 [DOI] [PubMed] [Google Scholar]
- 6.Zweck E, Thayer KL, Helgestad OKL, Kanwar M, Ayouty M, Garan AR, Hernandez-Montfort J, Mahr C, Wencker D, Sinha SS, et al. Phenotyping cardiogenic shock. J Am Heart Assoc. 2021;10:e020085. doi: 10.1161/JAHA.120.020085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marenzi G, Assanelli E, Campodonico J, De Metrio M, Lauri G, Marana I, Moltrasio M, Rubino M, Veglia F, Montorsi P, et al. Acute kidney injury in ST-segment elevation acute myocardial infarction complicated by cardiogenic shock at admission. Crit Care Med. 2010;38:438–444. doi: 10.1097/CCM.0b013e3181b9eb3b [DOI] [PubMed] [Google Scholar]
- 8.Vallabhajosyula S, Dunlay SM, Barsness GW, Vallabhajosyula S, Vallabhajosyula S, Sundaragiri PR, Gersh BJ, Jaffe AS, Kashani K. Temporal trends, predictors, and outcomes of acute kidney injury and hemodialysis use in acute myocardial infarction-related cardiogenic shock. PLoS One. 2019;14:e0222894. doi: 10.1371/journal.pone.0222894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh S, Kanwar A, Sundaragiri PR, Cheungpasitporn W, Truesdell AG, Rab ST, Singh M, Vallabhajosyula S. Acute kidney injury in cardiogenic shock: an updated narrative review. J Cardiovasc Dev Dis. 2021;8:88. doi: 10.3390/jcdd8080088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adler C, Reuter H, Seck C, Hellmich M, Zobel C. Fluid therapy and acute kidney injury in cardiogenic shock after cardiac arrest. Resuscitation. 2013;84:194–199. doi: 10.1016/j.resuscitation.2012.06.013 [DOI] [PubMed] [Google Scholar]
- 11.Moller JE, Engstrom T, Jensen LO, Eiskjaer H, Mangner N, Polzin A, Schulze PC, Skurk C, Nordbeck P, Clemmensen P, et al. Microaxial flow pump or standard care in infarct-related cardiogenic shock. N Engl J Med. 2024;390:1382–1393. doi: 10.1056/NEJMoa2312572 [DOI] [PubMed] [Google Scholar]
- 12.Udesen NJ, Moller JE, Lindholm MG, Eiskjaer H, Schafer A, Werner N, Holmvang L, Terkelsen CJ, Jensen LO, Junker A, et al. Rationale and design of DanGer Shock: Danish-German cardiogenic shock trial. Am Heart J. 2019;214:60–68. doi: 10.1016/j.ahj.2019.04.019 [DOI] [PubMed] [Google Scholar]
- 13.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure: definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380:756–766. doi: 10.1016/S0140-6736(11)61454-2 [DOI] [PubMed] [Google Scholar]
- 15.Patsalis N, Kreutz J, Chatzis G, Syntila S, Choukeir M, Schieffer B, Markus B. Early risk predictors of acute kidney injury and short-term survival during Impella support in cardiogenic shock. Sci Rep. 2024;14:17484. doi: 10.1038/s41598-024-68376-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, Crews DC, Doria A, Estrella MM, Froissart M, et al. ; Chronic Kidney Disease Epidemiology Collaboration. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385:1737–1749. doi: 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapur NK, Kanwar M, Sinha SS, Thayer KL, Garan AR, Hernandez-Montfort J, Zhang Y, Li B, Baca P, Dieng F, et al. Criteria for defining stages of cardiogenic shock severity. J Am Coll Cardiol. 2022;80:185–198. doi: 10.1016/j.jacc.2022.04.049 [DOI] [PubMed] [Google Scholar]
- 18.Vallabhajosyula S, Faugno AJ, Li B, John K, Kong Q, Sinha SS, Hernandez-Montfort J, Kanwar MK, Abraham J, Blumer V, et al. Prognostic implications of quantifying vasoactive medications in cardiogenic shock. J Card Fail. 2024; doi: 10.1016/j.cardfail.2024.06.010 [DOI] [PubMed] [Google Scholar]
- 19.Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, et al. ; IABP-SHOCK II trial investigators. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287–1296. doi: 10.1056/NEJMoa1208410 [DOI] [PubMed] [Google Scholar]
- 20.Thiele H, Zeymer U, Akin I, Behnes M, Rassaf T, Mahabadi AA, Lehmann R, Eitel I, Graf T, Seidler T, et al. ; ECLS-SHOCK Investigators. Extracorporeal life support in infarct-related cardiogenic shock. N Engl J Med. 2023;389:1286–1297. doi: 10.1056/NEJMoa2307227 [DOI] [PubMed] [Google Scholar]
- 21.Thiele H, Akin I, Sandri M, Fuernau G, de Waha S, Meyer-Saraei R, Nordbeck P, Geisler T, Landmesser U, Skurk C, et al. ; CULPRIT-SHOCK Investigators. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017;377:2419–2432. doi: 10.1056/NEJMoa1710261 [DOI] [PubMed] [Google Scholar]
- 22.Ostadal P, Rokyta R, Karasek J, Kruger A, Vondrakova D, Janotka M, Naar J, Smalcova J, Hubatova M, Hromadka M, et al. ; ECMO-CS Investigators. Extracorporeal membrane oxygenation in the therapy of cardiogenic shock: results of the ECMO-CS randomized clinical trial. Circulation. 2023;147:454–464. doi: 10.1161/CIRCULATIONAHA.122.062949 [DOI] [PubMed] [Google Scholar]
- 23.Lusebrink E, Binzenhofer L, Thiele H. The DanGer Shock trial: a new dawn but much to uncover. Eur Heart J. 2024;45:4181–4183. doi: 10.1093/eurheartj/ehae516 [DOI] [PubMed] [Google Scholar]
- 24.Schrage B, Becher PM, Bernhardt A, Bezerra H, Blankenberg S, Brunner S, Colson P, Cudemus Deseda G, Dabboura S, Eckner D, et al. Left ventricular unloading is associated with lower mortality in patients with cardiogenic shock treated with venoarterial extracorporeal membrane oxygenation: results from an international, multicenter cohort study. Circulation. 2020;142:2095–2106. doi: 10.1161/CIRCULATIONAHA.120.048792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ton VK, Kanwar MK, Li B, Blumer V, Li S, Zweck E, Sinha SS, Farr M, Hall S, Kataria R, et al. Impact of female sex on cardiogenic shock outcomes: a cardiogenic shock working group report. JACC Heart Fail. 2023;11:1742–1753. doi: 10.1016/j.jchf.2023.09.025 [DOI] [PubMed] [Google Scholar]
- 26.Giuliani KTK, Kassianos AJ, Healy H, Gois PHF. Pigment nephropathy: novel insights into inflammasome-mediated pathogenesis. Int J Mol Sci. 2019;20:1997. doi: 10.3390/ijms20081997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Avondt K, Nur E, Zeerleder S. Mechanisms of haemolysis-induced kidney injury. Nat Rev Nephrol. 2019;15:671–692. doi: 10.1038/s41581-019-0181-0 [DOI] [PubMed] [Google Scholar]
- 28.Baldetti L, Labanca R, Belletti A, Dias-Frias A, Peveri B, Kotani Y, Fresilli S, Calvo F, Fominskiy E, Pieri M, et al. Haptoglobin administration for intravascular hemolysis: a systematic review. Blood Purif. 2024;1–9. doi: 10.1159/000539363 [DOI] [PubMed] [Google Scholar]
- 29.Fried J, Farr M, Kanwar M, Uriel N, Hernandez-Montfort J, Blumer V, Li S, Sinha SS, Garan AR, Li B, et al. Clinical outcomes among cardiogenic shock patients supported with high-capacity Impella axial flow pumps: a report from the Cardiogenic Shock Working Group. J Heart Lung Transplant. 2024;43:1478–1488. doi: 10.1016/j.healun.2024.05.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.