Abstract
There is limited access to molecular genetic testing in most low- and middle-income countries. The iHope program provides clinical genome sequencing (cGS) to underserved individuals with signs or symptoms of rare genetic diseases and limited or no access to molecular genetic testing. Here we describe the performance and impact of cGS in 247 patients from three clinics in Peru. Although most patients had at least one genetic test prior to cGS (70.9%), the most frequent was karyotyping (53.4%). The diagnostic yield of cGS was 54.3%, with candidate variants reported in an additional 22.3% of patients. Clinical GS results impacted clinician diagnostic evaluation in 85.0% and genetic counseling in 72.1% of cases. Changes in management were reported in 71.3%, inclusive of referrals (64.7%), therapeutics (26.3%), laboratory or physiological testing (25.5%), imaging (19%), and palliative care (17.4%), suggesting that increased availability of genomic testing in Peru would enable improved patient management.
Subject terms: Medical genomics, Genetic testing
Introduction
Peru is a low-middle-income country (LMIC), with the fifth-largest territory in South America. The Peruvian population has a complex genomic composition derived from a variable admixture of Amerindian, European, and African ancestries1. The main language spoken in Peru is Spanish, followed by Quechua and Aymara2. Peru has an emerging economy marked by pronounced and recent growth but significant challenges, such as social inclusion and access to healthcare, remain3–5. Persistent inequities in access to basic services and resources between regions are notable, and approximately 26% of the population lives in poverty6.
Health services in Peru are offered within a fragmented public healthcare system. The healthcare sector is comprised of five subsystems, each with its own separate facilities7. Health insurance coverage varies widely and is largely dependent on government programs. The Ministry of Health (MINSA) provides coverage for a majority of the population (63%), primarily those living in poverty, through its Integral Health insurance (SIS)7,8. Although SIS coverage has increased as part of Peru’s 2009 Universal Health Coverage Act, availability and coverage of complex testing are often limited9. Approximately 26% of Peruvians are covered through the social security program (EsSalud), administered by the Labor and Employment Minister to employees, which offers the most comprehensive insurance. Additionally, 10% of the population is covered by other insurance providers, such as private insurance or coverage associated with the military or police health systems2,7,8. It is not uncommon for most people, regardless of their type of health insurance, to pay out of pocket for healthcare services. In this context, a complex disease diagnosis can be catastrophic financially10,11. Healthcare providers often adjust the tests they order due to the financial constraints faced by many patients.
Genetic care services in Peru are growing, but access to genetic specialists remains limited. Only eight healthcare centers in the capital city of Lima have a genetic service (five specialized institutes, one national hospital, and two employee healthcare system hospitals), which are largely focused on cancer, pediatrics, and neurogenetic diseases7,12–14. Fewer than 40 accredited medical geneticists serve the Peruvian population of ~33 million people, with almost no formal genetic counseling training available in-country (https://www.cmp.org.pe/conoce-a-tu-medico/). Public laboratories have limited access to genetic testing technologies, which prevents patients and families from having timely access to services and an eventual diagnosis15,16. Although there have been notable advances in in-country genetic testing, such as molecular testing for the diagnosis of infectious diseases, cancer genomics, and neurogenetics, limited public investment has impeded the implementation of next-generation sequencing (NGS)-based genetic testing that is more readily available in high-income economy countries (HIC)7. Public health initiatives for genetic diseases have made some progress but remain limited in scope compared to those available in HIC. For example, in 2012, the Peruvian government passed legislation to implement universal newborn screening (NBS) for congenital hypothyroidism, phenylketonuria, congenital adrenal hyperplasia, cystic fibrosis, congenital cataracts, and hearing loss7. However, implementation has been limited to large hospitals in the urban centers, leaving a large portion of the population without access to NBS7,17.
Here, we report on the clinical deployment of cGS testing in a cohort of Peruvian patients through a partnership with the iHope program, a philanthropic clinical implementation program that provides access to clinical genome sequencing to underserved children with a suspected rare genetic disease18. Clinical genome sequencing allows for the simultaneous interrogation of single nucleotide variants (SNVs), small insertions and/or deletions (indels), copy number variants (CNVs), some structural chromosomal anomalies, short tandem repeat (STR) expansions and variation in the mitochondrial genome in a single assay. In an LMIC where many patients do not have access to testing routinely available to patients in HICs, including microarray and exome sequencing, utilization of cGS offers a potential advantage to reduce the burden on clinicians and families of serial molecular testing.
Partnerships were established with three clinical sites based in Lima, Peru: Hospital Nacional Edgardo Rebagliati Martins (HNERM), the Instituto Nacional de Ciencias Neurológicas (INCN), and the Instituto Nacional de Salud Niño-San Borja (INSNSB). The HNERM, affiliated with EsSalud, is the largest hospital in Peru, with more than 90 specialty departments serving patients of all ages across a broad spectrum of indications. At present, only cytogenetics testing through the hospital-affiliated laboratory is routinely available for patients with suspected genetic diseases. Through its genetics department, HNERM began a collaboration with the iHope program in 2019.
The INCN is a national referral center focused on neurological disorders that primarily serves patients with MINSA SIS health coverage, but also supports patients from other subsystems. The neurogenetics division at INCN, which initiated a partnership with the iHope program in 2019, provides outpatient consultations for adults and children nationally who are affected by neurogenetic disorders, mostly inherited movement disorders. The onsite molecular biology laboratory offers PCR-based testing for repeat expansion disorders (Huntington, inherited ataxias), primary dystonia DYT1 (TOR1A), MELAS, and some neuromuscular disorders14,19. INCN recently initiated a DNA bank facility within the public healthcare system, which supports diagnostic and research collaborative initiatives (https://gp2.org/news/first-of-its-kind-dna-biobank-opens-in-peru/).
In 2021, a third iHope site in Peru was established at the INSNSB, a MINSA-affiliated pediatric hospital that provides care to pediatric and adolescent patients referred nationally for complex surgical pathologies and for organ and hematopoietic cell transplants. The genetics department currently offers karyotyping in peripheral blood and bone marrow and PCR testing for frequent fusion genes in pediatric leukemias. Previously, gene panels for congenital heart disease and medullary failure syndromes and NGS-based somatic and germline leukemia panels were available through the hospital’s molecular genetics laboratory but were halted due to contracting issues between the hospital and the third-party equipment supplier.
Results
Study population
From July 2019 through May 2023, 247 probands with suspected rare genetic disease and their family members were enrolled in the iHope program at one of three sites in Lima, Peru: Hospital Edgardo Rebagliati Martins (HNERM) (n = 108), the Instituto Nacional de Ciencias Neurológicas (INCN) (n = 88), and the Instituto Nacional de Salud Niño-San Borja (INSNSB) (n = 51) (Table 1 and Supplementary Data 1).
Table 1.
iHope patient demographics
| Characteristic | Total Patients, No. (%) | HNERM | INCN | INSNSB |
|---|---|---|---|---|
| Total | 247 | 108 (43.7) | 88 (35.6) | 51 (20.6) |
| Age | ||||
| <1 | 13 (5.3) | 10 (9.3) | 0 (0.0) | 3 (5.9) |
| 1–5 | 79 (32.0) | 34 (31.5) | 17 (19.3) | 28 (54.9) |
| 6–18 | 107 (43.3) | 59 (54.6) | 28 (31.8) | 20 (39.2) |
| >18 | 48 (19.4) | 5 (4.6) | 43 (48.8) | 0 (0) |
| Median age at testing [interquartile range], y | 8.7 [3.1–15.8] | 8.4 [2.9–14.3] | 9.5 [4.1–16.2] | 6.5 [2.6–10.5] |
| Sex | ||||
| Male | 130 (52.6) | 49 (45.4) | 55 (62.5) | 26 (51.0) |
| Female | 117 (47.4) | 59 (54.6) | 33 (37.5) | 25 (49.0) |
| Family structure | ||||
| Proband only | 6 (2.4) | 1 (0.9) | 4 (4.5) | 1 (2.0) |
| Duo | 44 (17.8) | 16 (14.8) | 22 (25.0) | 6 (11.8) |
| Trio or Quad, including both unaffected parents | 180 (72.9) | 86 (79.6) | 55 (62.5) | 39 (76.4) |
| Other | 17 (6.9) | 5 (4.6) | 7 (7.9) | 5 (9.8) |
| Geography | ||||
| Lima | 156 (63.2) | 67 (62.0) | 66 (75.0) | 23 (45.1) |
| Outside of Lima | 91 (36.8) | 41 (37.9) | 22 (25%) | 29 (56.9) |
| Average distance traveled (km) | 194.5 | 191.1 | 185.9 | 195.2 |
Age, sex, family structure, and location distributions across the cohort and by site.
The median age at enrollment was 8.7 years (IQR, 3.1–15.8 years), and 130 (52.6%) were male. The cohort consisted mostly of children (199/247, 80.2%). Trio or higher-order family structures, including the affected child and both unaffected parents, were most common (180/247, 72.9%), but variable family structures were submitted depending on the expected inheritance mode and family members available for testing. Patients traveled an average of 195 km for genetics evaluation (range 0–1011 km), 36.8% (91/247) of whom resided outside of the capital city of Lima (Fig. 1). The median time from symptom onset to cGS testing was 5 years (IQR, 2.4–11.8 years). By site, INCN had the longest median time from symptom onset to cGS testing at 9 years (IQR, 3.8–18.1 years), followed by HNERM (5.4 years, IQR, 2.5–9.5 years) and INSNSB (2.2 years, IQR, 1.1–4.8 years).
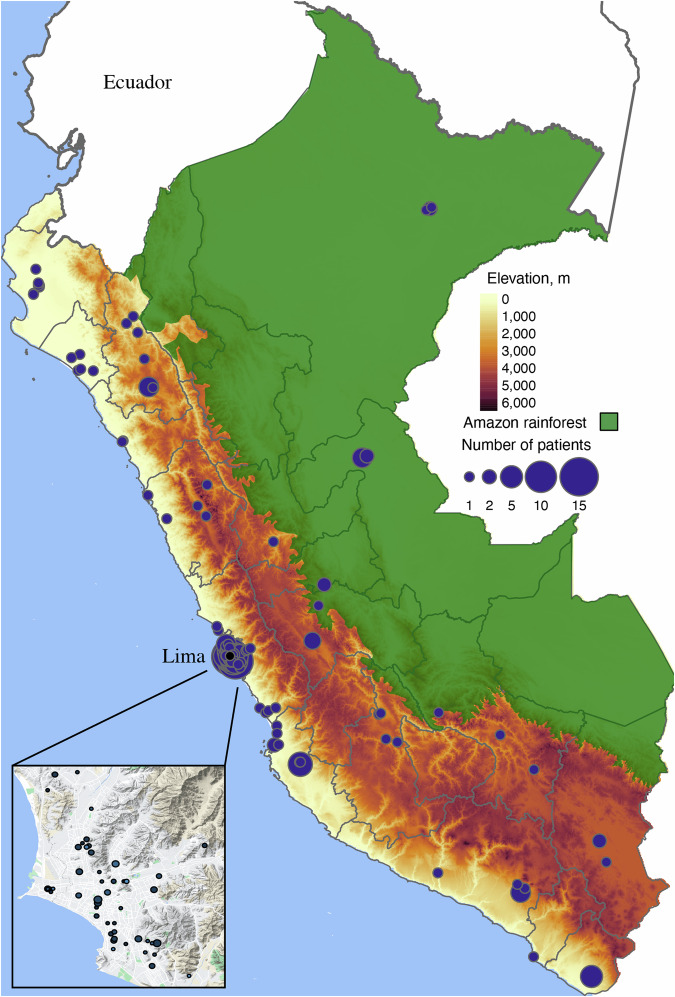
Fig. 1. Geographic distribution of iHope patients in Peru.
The distribution of patients participating in the iHope program is shown across Peru. Participating individuals are shown as purple circles, with the size of the circle denoting the number of patients from a given ZIP code. More than a third of patients (37%) were drawn from the capital city, Lima, which is shown in the inset in the lower left. Geographic barriers potentially impacting patient accessibility to clinical sites in Lima include both the Andes mountains (shown as the high-elevation band in dark brown) and the Amazon rainforest (shown in dark green). The cartographic and geographic data used to generate this figure were drawn from GPL3-licensed sources, as detailed in the “Statistical analysis” section.
All patients were evaluated by a clinical geneticist and had signs and symptoms suggestive of a rare, genetic disorder, consistent with current professional guidelines, and limited access to molecular testing (see “Methods”)20. Additional patient selection criteria considered by the ordering clinicians included a broad differential diagnosis (222/247; 89.9%), no other available next-generation sequencing test (198/247; 80.2%), non-diagnostic prior testing (180/247; 72.9%), a diagnostic odyssey lasting more than two years (173/247; 70.0%), a phenotypic presentation assessed by the clinician as severe (169/247; 68.4%), clinical suspicion of a disorder with an available treatment (91/274; 36.8%), parents of reproductive age (85/247; 34.4%), acute illness or ICU admission (29/247; 11.7%), and/or a first-degree relative with a similar clinical presentation (41/247; 16.6%) (Fig. 2a). Some differences in patient selection criteria were observed across sites (Supplementary Table 1).
Fig. 2. Patient selection and prior genetic testing.
a The total proportion of patients, and the proportion from each site, that were associated with each of iHope program selection criteria. Patients could be associated with more than one selection criteria. Broad differential diagnosis was the most common rationale for program inclusion. b The proportion of iHope patients with genetic testing prior to cGS stratified by site and type of genetic test. WES: whole exome sequencing; HNERM Hospital Nacional Edgardo Rebagliati Martins, INCN Instituto Nacional de Ciencias Neurológicas, INSNSB Instituto Nacional de Salud Niño-San Borja.
Although the majority of patients had at least one genetic test prior to the iHope-related consult and cGS (175/247 [70.9%]), the most frequently ordered test was a karyotype (132/247 [53.4%]). Only 34.4% (85/247) had access to other genetic tests (Fig. 2b). The number of genetic investigations performed per patient ranged from 0 to 4, with most patients having pursued one (129/247 [52.2%]) prior test. Significant differences in the genetic tests ordered by site were observed. For example, 85.2% (92/108) of patients from HNERM pursued karyotype, compared to 3.4% (3/88) at INCN and 72.6% (37/51) at INSNSB (Fisher’s exact test (FET), p < 0.001). Microarray testing was more frequently pursued at INSNSB (11/51 [21.6%]) compared to HNERM (6/108 [5.6%]) and INCN (3/88 [3.4%]) (FET, p = 0.001), with the same trend noted for panel testing (INSNSB 15/51 [29.4%] vs HNERM 10/108 [9.3%] vs INCN 11/88 [12.5%], FET p = 0.003). Single gene testing was more frequently pursued at INCN (23/88 [26.1%]) compared to HNERM (3/108 [2.8%]) and INSNSB (1/51 [2.0%], FET p < 0.001) (Fig. 2b).
Patient phenotypes were diverse and complex, with abnormalities of the nervous system (184/247; 74.5%), skeletal system (143/247; 57.9%), and head or neck (134/257; 54.3%) the most frequently identified Human Phenotype Ontology root ancestor terms overall (Fig. 3). Differences in HPO root ancestor terms by site were observed (Supplementary Table 2).
Fig. 3. Patient phenotypes.
Summary distribution of top-level Human Phenotype Ontology terms nested beneath “Phenotypic abnormality” (HP:0000118) across the iHope cohort and grouped by clinical site. Patient phenotypes were diverse and complex, and with abnormalities of the nervous system, skeletal system, and head or neck, the most frequently identified Human Phenotype Ontology root ancestor terms overall and for each site. Differences in observed HPO root ancestor terms by site are detailed in Supplementary Table 2. HNERM Hospital Nacional Edgardo Rebagliati Martins, INCN Instituto Nacional de Ciencias Neurológicas, INSNSB Instituto Nacional de Salud Niño-San Borja.
Diagnostic yield and reported variants
Across the cohort, the diagnostic yield was 54.3% (134/247), with uncertain test results reported in an additional 22.3% (55/247), inclusive of variants of uncertain significance (Fig. 4a). Clinician review of uncertain results endorsed that 69.1% (38/55) were clinically suspected to be likely positive based on clinical correlation with the patient phenotype. Diagnostic yield varied across the sites, with observations of 43.1% at INSNSB (22/51), 53.4% at INCN (47/88), and 60.2% at HNERM (65/108) (Fig. 4a).
Fig. 4. Diagnostic yield and reported variants.
a Outcomes of cGS stratified by test result category and by clinical site. b Distribution of variant types reported across the iHope cohort and grouped by site. SNV single nucleotide variant; Indel: insertion or deletion, CNV copy number variant, STR short tandem repeat variant, MT SNV single nucleotide variant in the mitochondrial genome, SMA c.840 C allele in the SMN1 gene not detected, UPD uniparental disomy, HNERM Hospital Nacional Edgardo Rebagliati Martins, INCN Instituto Nacional de Ciencias Neurológicas, INSNSB Instituto Nacional de Salud Niño-San Borja.
Variants related to the indication for testing (248) included SNVs (162), small indels (46), CNVs ranging in size from 3 kb to 77 Mb (30), STRs (5), mitochondrial SNVs (3), regions of homozygosity suggestive of uniparental disomy (1) and spinal muscular atrophy detected by biallelic absence of the c.840 C allele (1) (Fig. 4b).
A total of 121 heterozygous variants in or encompassing single genes associated with disorders following an autosomal dominant mode of inheritance were reported, 87 variants in genes associated with autosomal recessive disorders, 28 variants in genes associated with X-linked disorders, and three variants in the mitochondrial genome (Supplementary Table 3). In 3 cases, chromosomal aneuploidy was detected, including one proband with Trisomy 18 and two probands with mosaic Trisomy 14, which were orthogonally confirmed via chromosomal microarray testing at an outside laboratory. In two probands, two de novo copy number variants were detected in each, with split-read evidence suggestive of a derivative chromosome present in the reportedly healthy parent. The most common recurrent primary diagnoses were Neurofibromatosis (4 probands), dopa-responsive dystonia (3 probands), Alagille syndrome (3 probands), and TTN-related disorders (3 probands).
Secondary and incidental findings
Positive secondary findings reports were issued for 24 individuals, including ten probands (10/247; 4.0.%) and 14 family members (14/456; 3.1%). In five probands, the reported secondary findings were also included on the primary clinical report due to overlap with the indication for testing, including one proband with a pathogenic FBN1 variant consistent with Marfan syndrome, one proband with bi-allelic BRCA2 variants consistent with Fanconi anemia, two probands with variants in LMNA consistent with dilated cardiomyopathy and one proband with a TTN variant consistent with dilated cardiomyopathy. Reported secondary findings also included variants in FLNC, KCNQ1, LDLR, MEN1, MYBPC3, PALB2, TNNI3, and TP53 (Supplementary Table 4).
Incidental findings in genes not included on the ACMG Secondary Findings gene list but deemed actionable per the laboratory policy of the Illumina Clinical Services Laboratory were reported in a separate category on the primary clinical report in seven probands (7/247, 2.8%). Laboratory policy dictates that variants must be in the molecular state expected to cause disease (e.g., two variants in trans for an autosomal recessive condition, etc.), be classified as pathogenic or likely pathogenic by ACMG variant classification criteria (e.g., for a recessive condition with a compound heterozygous variant pair, each variant must be classified as P/LP), and have a ClinGen Actionability score of six or greater or have available National Comprehensive Cancer Network (NCCN) guidelines to guide the clinician in management recommendations based on the finding21–23. Incidental findings included variants in ATM, VWF, PALB2, FH, and G6PD. In two probands, incidental findings were the only reported variants (Supplementary Data 1).
Diagnostic evaluation and change of management
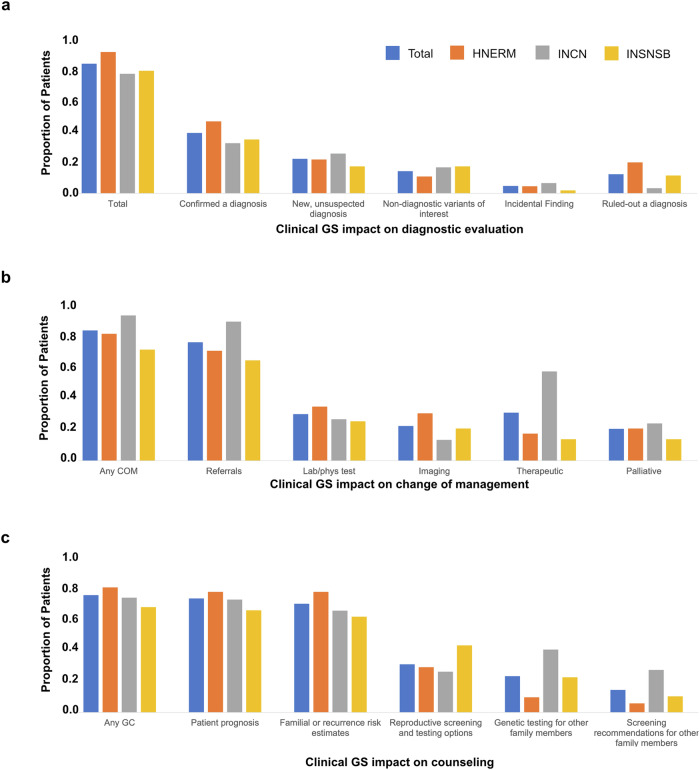
Clinical GS test results impacted clinician diagnostic evaluation in 85.0% (210/247) of cases, including confirmation of a clinical diagnosis or diagnosis in the differential (39.7%, 98/247), establishing a new diagnosis (22.7%, 56/247), identifying variants of potential interest in relation to the patient’s phenotype (14.6%, 36/247), ruling out a suspected diagnosis (12.6%, 31/247) and/or producing an incidental diagnosis (4.9%, 12/247) (Fig. 5a).
Fig. 5. Impact on the diagnostic evaluation (DE), change in management (COM), and genetic counseling (GC).
a The impact of cGS results on the diagnostic evaluation, b change of management, and c genetic counseling. For each, multiple response options could be endorsed for a single patient. Results are displayed for the cohort overall and by site. HNERM Hospital Nacional Edgardo Rebagliati Martins, INCN Instituto Nacional de Ciencias Neurológicas, INSNSB Instituto Nacional de Salud Niño-San Borja.
Changes in management were reported in 71.3% (176/247) of cases, inclusive of referrals (64.7%, 160/247), therapeutics (26.3%, 65/247), laboratory or physiological testing (25.5%, 63/247), imaging (19%, 47/247), and palliative care (17.4%. 43/247) (Fig. 5b). The need for additional tests or evaluations was eliminated in 33.6% (83/247) of cases.
Clinical GS test results impacted genetic counseling in 72.1% (178/247) of cases, including counseling regarding patient prognosis (70.0%, 173/247), familial recurrence risk estimates (66.8%, 165/247), reproductive screening and testing options (29.6%, 73/247), testing options for other family members (22.3%, 55/247) and clinical screening recommendations for other family member (13.8%, 34/247) (Fig. 5c).
Case examples
Notable case examples include a 3-month-old female in the intensive care unit on mechanical ventilation from one month of life with liver failure, jaundice, collateral circulation ascites, thrombocytopenia, coagulopathy, respiratory infections, and sepsis with a poor prognosis. At three months of age, cGS revealed a homozygous, pathogenic missense variant, c.443 G > A (p.Arg148Gln) in the GALT gene, consistent with a diagnosis of galactosemia. Her diet was changed to soy formula, resulting in normalized laboratory results (e.g., hemogram and biochemistry), regaining of consciousness, independent breathing, and resolved visceromegaly. As a result, she was discharged from the intensive care unit. Unfortunately, prolonged mechanical ventilation resulted in tracheomalacia, requiring a tracheostomy. Currently, she is two years old, able to stand, babbles, and continues to have a tracheostomy.
In another case, an 8-year-old male presented with walking difficulties and dysarthria since 1 year of age, together with recurrent respiratory infections, ocular telangiectasia, oculomotor apraxia, and head tremor. A compound heterozygous variant pair was identified in ATM, inherited from both parents, consistent with a diagnosis of ataxia–telangiectasia. This genetically confirmed diagnosis allowed the patient to receive IV immunoglobulin to prevent recurrent infections, as well as appropriate counseling for malignancies for the patient and his family members.
In a third case, a 15-year-old female presented with scoliosis, hyperpigmentation along Blaschko’s lines, hemihyperplasia of the right leg, and abnormal intramedullary signal on MRI of the iliac and long bones. She had a clinical diagnosis of Gaucher disease, for which she had been treated via enzyme replacement therapy (ERT) for 5 years. Clinical GS testing corrected the diagnosis to mosaic Trisomy 14, and ERT was discontinued.
Discussion
Globally, patients with suspected rare genetic diseases (RGD) remain underrecognized and underserved, particularly in low and middle-income countries (LMIC) where access to molecular genetic testing routinely available in high-income countries (HIC) is often limited or unavailable24,25. Here we have described a cohort of Peruvian patients with suspected RGD who were assessed with cGS and reported cumulative diagnostic yield, impact on diagnostic evaluation (DE), change of management (COM), and genetic counseling (GC).
Patient selection was consistent with genetic testing guidelines for patients with suspected RGD. However, a review of patient selection criteria supports that the prior probability of a genetic diagnosis in the cohort was high as a majority had a phenotypic presentation that the geneticist assessed as severe (68.4%) and who otherwise lacked access to a next-generation sequencing assay (80.2%). The diagnostic yield of cGS across the cohort, 54.3%, was higher than other large studies in genetically and phenotypically diverse cohorts, but comparable to smaller cohorts comprised of patients in LMIC with reduced access to genetic testing26–29.
Although the three clinics that participated in this investigation serve patient populations with heterogenous phenotypes suggestive of RGD, they also have unique specializations and differences in routinely available genetic tests, which likely contributed to differences in study outcomes by the site. For example, INCN specializes in neurological indications, including patients with phenotypes suggestive of short tandem repeat expansion disorders, and is skewed toward the older patient population (Table 1), a higher proportion of patients who pursued a single gene genetic test prior to cGS (Fig. 2b), a lower proportion of patients who pursued karyotype (which is not available at INCN and generally not a test pursued in patients with a later-onset neurologic presentation, Fig. 2b) and a greater proportion of patients with STRs detected by cGS compared to HNERM and INSNSB (Fig. 4b). Additionally, INCN reported a higher rate of therapeutic COM compared to other sites (Fig. 5b, INCN 49% vs INSNSB 12% vs HNERM 15%), which may be explained by its focus on neurologic disorders which generally have more therapeutic options available (e.g., dopa–responsive dystonia) compared to rare genetic disorders which require specialized treatments. The vast majority of patients at HNERM pursued karyotype prior to cGS (85%), which is available through the onsite laboratory, but only 6% had chromosomal microarray testing (Fig. 2b). Consistent with this testing profile, HNERM had the highest proportion of reported CNVs by cGS (16%) compared to INCN (9%) and INSNSB (10%) (Fig. 4b). These comparisons highlight the nuanced differences in referral populations and access to genetic testing even within institutions residing in the same city, likely reflecting broader access disparities across Peru.
Analysis of COM revealed that 70% of patients had a change in clinical care, with more than a quarter of patients receiving therapeutic COM. This potentially challenges the presumption that limited resources, in combination with a need for highly specialized therapeutics for rare diseases, are unlikely to yield meaningful COM for patients in LMIC. While a detailed analysis of therapeutic access in Peru is beyond the scope of this study, and differences in therapeutic access certainly exist between HIC and LMIC, our findings suggest that a precise diagnosis may result in a therapeutic benefit to almost a quarter of patients. As detailed above, cGS detected a homozygous, pathogenic variant in GALT consistent with galactosemia in a 3-month-old female in the ICU. A diagnosis of galactosemia does not require comprehensive genomic testing, but because it is not included in the limited newborn screening offered through SIS insurance, this patient remained undiagnosed prior to cGS. A simple and accessible diet change to soy formula produced a marked improvement in her presentation. Additionally, in two unrelated probands and one parent, molecular diagnoses of dopa-responsive dystonia facilitated treatment with low doses of levodopa, resulting in near cessation of symptoms. Taken together, our findings suggest that precision diagnoses can enable accessible treatment options for some RGD patients in Peru.
Limitations of this study include a country-specific investigation that may not be generalizable to other LMIC, collaboration amongst three institutions that may not be representative of the Peruvian healthcare system writ large, and potential unknown barriers to genetics referral within Peru impacting access to the iHope program. Medical records were reviewed by the clinicians to assess COM, however follow-up may have occurred at outside institutions, and recommendations may not have been followed. The survey captured any COM related to cGS results, and therefore, some response endorsements may pertain to secondary or incidental findings, however, we expect the potential impact to be low as only five patients (2.0%) had a reported secondary finding that did not overlap their indication for testing and only two patients (0.8%) had an incidental finding reported in isolation. Additionally, testing was performed at a centralized laboratory in the United States, which added transit time to the testing timeline and did not address sustainable testing access solutions that can be integrated into the Peruvian healthcare system. To begin addressing this, the iHope program recently expanded to offer cGS through a network of laboratories both within and outside the US. Over time, iHope also aims to grow to expand the number of clinical sites and to use the data generated in the program to support efforts to garner local and federal support to expand access to genetic testing.
In conclusion, this study provides an exploration of the impact of cGS within a Peruvian patient cohort with RGD across three national-level referral centers. Our findings highlight the largely unmet needs of this patient population and underscore the potential benefits of integrating cGS, or other broad genomic testing assays such as microarray and exome sequencing, into routine care for individuals suspected of having RGD. This is aligned with the global objective of advancing healthcare access for patients grappling with rare diseases in LMICs30,31.
Methods
Observational, retrospective analysis of test outcomes and clinical utility survey responses, captured as part of the routine implementation of this clinical program, was conducted in accordance with requirements of approval by the Western Copernicus Group Institutional Review Board, which granted an Institutional Review Board Exemption with a HIPAA Full Waiver of Authorization (WCG IRB Work Order #1-1493034-1) as defined in US Department of Health and Human Services 45CFR46.104(d)(4), which requires an adequate plan to protect identifiers from improper use and disclosure, to destroy the identifiers at the earliest opportunity, and written assurances that protected health information will not be reused or redisclosed to any other person or entity, except as required by law. Additional ethics review board approvals were obtained at the Peruvian institutions, including the Ethical Research Committee at Instituto Nacional de Ciencias Neurológicas (N° 043-2023-CIEI-INCN), the Institutional Research Ethics Committee National Institute of Child Health—San Borja (N° 072-2023-CIEI-INSN-SAN BORJA), and the Ethics Committee of the Edgardo Rebagliati Martins National Hospital (N°119-CE-GHNERM-GRPR-ESSALUD-2024). Informed consent for cGS testing was facilitated in Spanish and obtained in accordance with local rules and regulations of the clinical institution and per standard operating procedures of Illumina Laboratory Services, San Diego. This study complied with all relevant ethical regulations, including the Declaration of Helsinki. iHope clinicians from each clinical site were directly involved in this study, including design, data analysis, and authorship; and the clinical leads at each iHope Peru site are co-first authors. Roles and responsibilities were agreed upon ahead of data analysis. The research does not result in stigmatization, incrimination, discrimination, or other personal risk to participants; it aims to identify a molecular etiology for existing signs and symptoms. Technical datafiles (e.g., gVCF or BAM files) for all individuals sequenced through the iHope program are available on request from the patient or ordering clinician. Relevant local research is included in the citations. The long term goals of the iHope program, including potential support of in-country capacity-building, have been discussed with clinical leads.
Patient selection
Patient selection was consistent with genetic testing guidelines and aligned with broad consensus in the application of genome sequencing for patients with suspected rare genetic disorders described in the literature20,26,28. All patients were evaluated by a clinical geneticist and assessed to have signs or symptoms suggestive of a rare genetic disease. Patients lacked an identified molecular etiology for their symptoms and access to genetic testing to obtain a molecular diagnosis. The additional factors considered for patient selection are detailed in Fig. 2a and were developed by clinicians who ordered cGS testing. The categories are intended to capture the complexities of test ordering for patients with suspected rare genetic diseases. To offer one example, if the differential diagnosis was broad enough to consider multiple potential diagnoses, but suspicion of a metabolic disorder was within the differential, both “broad differential” and “suspicion of a disorder with a medical treatment” might be endorsed. As another example, the clinical diagnosis before cGS testing may be epilepsy, and medication could be initiated without a genetic diagnosis, and often is, but a precision diagnosis may guide a more effective medication selection.
Genome sequencing
Whole-blood samples were submitted for cGS testing at the Illumina Clinical Services Laboratory in San Diego, California, a CLIA-approved and CAP-accredited laboratory. cGS was performed on DNA extracted from whole blood. A PCR-free library preparation protocol was utilized, and samples were sequenced with paired-end 150 base pair reads. The data were aligned according to build 37 of the Human Reference Genome (http://www.ncbi.nlm.nih.gov/projects/genome/assembly/grc/human) and sequenced to an average of ≥30- or ≥40-fold coverage depending on the sequencing instrument used for cGS testing. The analysis included the interrogation of single nucleotide variants (SNVs) and small insertions and deletions (indels) that fall within 15 bp of a RefSeq exon boundary. Copy number variants (CNVs) greater than 10 kb were assessed, however sensitivity has only been determined for events greater than 20 kb and was approximately 85%. CNV interpretation was limited to events that either overlapped an exon or had a boundary that was within 1 kb upstream or downstream of an exon. SNVs detected in the mitochondrial genome at an allele fraction greater than or equal to 3% were interpreted. Cases were assessed for the absence of the ‘C’ allele at GRCh37 Chr5:70247773 (NM_000344.3:c.840 C > T) in the SMN1 gene. An absence of the c.840 C allele is consistent with an absence of exon 7 of SMN1 and is reported as a positive result for Spinal Muscular Atrophy (SMA). Samples analyzed from November 2019 onward utilized Expansion Hunter (EH) to detect short tandem repeat (STR) expansions in the following genes: DMPK, CNBP, FXN, FMR1, HTT, JPH3, AR, ATXN1, ATXN2, ATXN3, CACNA1A, ATXN7, ATXN8OS, ATXN10, PPP2R2B, TBP, NOP56, ATN1, CSTB, TCF4, C9orf72. Only ‘expanded’ alleles were included on the clinical report, carrier status was not reported, and the specific repeat number was not reported. Orthogonal characterization of all clinically significant expanded STRs was facilitated through an external laboratory, and results were communicated in an addended report. cGS pipelines used over time for variant calling are detailed in Supplementary Table 5.
Variants of interest were identified using an in-house developed software system that considers population allele frequency, variant consequence, evolutionary conservation, occurrence in a gene with an established gene-disease relationship, occurrence in a gene whose disease association overlaps with the patient’s reported phenotype, and inheritance mode, as appropriate. Variant interpretation for SNVs and CNVs was performed according to the ACMG guidelines for variant classification.
A clinical report was issued for the proband and affected siblings to include genomic findings of potential clinical significance related to the indication for testing. Test result categories include “positive”, in which a likely pathogenic or pathogenic variant(s) is reported in a disease-associated gene(s) consistent with the clinical presentation and disease inheritance pattern; “see below”, which is referred to in the manuscript text as “uncertain” for readability, in which variants of potential clinical significance are reported, inclusive of variants of unknown significance (VUS); and “negative”, in which no variants are reported for the individual. Ancillary reports for a pharmacogenomics screen were provided for all individuals tested in a family. Optional reports for secondary findings were provided for all individuals tested in a family unless an individual opted out. Results from the pharmacogenomics screen were not considered here. Results from the secondary findings report were considered in DE and COM assessments due to the potential impact on clinical management.
Phenotype distribution assessment
PhenoTagger was utilized to systematically extract Human Phenotype Ontology (HPO) terms from clinician-provided patient phenotypes32. The top-level HPO terms were taken to be all immediate descendants of the term Phenotypic Abnormality (HP:0000118)33. To provide a simple summary of the phenotype of each case, the unique top-level terms were identified where at least one descendant of each term was present in the low-level phenotypic assignments using custom code.
cGS test results impact the clinical evaluation and patient management
A clinical utility survey was completed by a clinical provider for all probands (Supplementary Note, Clinical Utility Survey). Survey development was informed by the Medical Genome Initiative and included multiple choice questions and free-text responses to assess the impact of cGS results on the clinician’s diagnostic evaluation of the patient and change of management. Clinicians were instructed to endorse responses based on cGS test results, not due to other factors.
Impact on diagnostic evaluation (DE) was defined as endorsement of any of the following response options to the clinical utility survey (Supplementary Note, Clinical Utility Survey): confirmed a clinical diagnosis; confirmed a diagnosis within the differential diagnosis prior to testing; established/produced a new diagnosis that was not previously suspected; did not confirm or establish a diagnosis but did identify variants of potential interest in relation to the patient’s phenotype; produced an incidental diagnosis unrelated to the indication for testing; and/or ruled out a suspected diagnosis/diagnoses. The response options of confirmed a clinical diagnosis and confirmed a diagnosis within the differential diagnosis prior to testing were collapsed into a single response, confirmed a diagnosis, in Fig. 5a of the primary text.
Change of management (COM) was defined as endorsement of any of the following response options to the cGS impact survey (Supplementary Note, Clinical Utility Survey): referral for specialty consultation; laboratory testing; imaging; physiological testing; a medication, treatment or therapy specific to the diagnosis was recommended; nutritional or metabolic treatment; protein replacement therapy; disease-specific medication; organ transplant; stem cell transplant; and/or gene therapy; or an endorsement of ‘yes’ to the question ‘did the WGS result(s) contribute to decisions related to palliative or end of life care?’. The following response options were collapsed into a single category, therapeutic, in Fig. 5b of the primary text: a medication, treatment or therapy specific to the diagnosis was recommended; nutritional or metabolic treatment; protein replacement therapy; disease-specific medication; organ transplant; stem cell transplant; and/or gene therapy.
Endorsement of any of the following cGS impact survey response options was considered genetic counseling (Supplementary Note, Clinical Utility Survey): counseling about patient prognosis, familial/recurrence risk estimates, reproductive screening and testing options, genetic screening or testing options for other family members, or clinical screening recommendations for other family members.
Statistical analysis
Categorical variables were reported as frequencies and percentages. Continuous variables with a skewed distribution were reported as median with interquartile ranges (IQR). Fisher exact or Pearson χ2 test was performed for comparative analysis. A 2-sided P value of less than 0.05 was considered statistically significant. Statistical analysis was performed with Stata version 17 (StataCorp LLC) and 95% CI.
In Fig. 1, patient zip codes were transformed to latitude and longitude using zipcodeR 0.3.5, and mapped using raster 3.6-26 database and the sf 1.0-14 package. Amazon rainforest borders were obtained from Google Earth satellite images, and altitude data were downloaded from raster and maps 3.4.1.1 databases. The administrative map of Peru was sourced from the GADM database (https://gadm.org/). All data was available under a GPL3 license.
Supplementary information
Supplementary Information_Bazalar-Montoya, Cornejo-Olivas, X Duenas-Roque_iHope Peru
Acknowledgements
We thank the patients and parents for participating in the iHope program in Peru. We acknowledge the Illumina Laboratory Services wet lab and shipping and logistics teams, and Julia Ortega for past management of the iHope program. We thank John Belmont for sharing his custom code for HPO phenotype mapping. The iHope program was philanthropically funded by the Illumina Foundation. We are grateful to Andrea Rivera-Valdivia for her collaboration on the clinical assessment of iHope patients at INCN. We thank the DNA-Neurogenetics Bank of the Instituto Nacional de Ciencias Neurológicas (BADN-INCN) for logistic support for the iHope program. Jeny Bazalar-Montoya and Richard S. Rodriguez are master’s students in Epidemiological Research at Universidad Peruana Cayetano Heredia, a program supported by Emerge, the Emerging Diseases Epidemiology Research Training D43 TW007393 training grant awarded by the Fogarty International Center of the US National Institutes of Health.
Author contributions
Conceptualization—E. T., M.C-O., M.D-R., J.B-M., and R.T. Data collection—M.C.-O., M.D.-R., J.B.-M., N.P.-R., R.S.R., K.M.-N., C.D.-H., E.S.-C. C.I.G., G.M.-M., G.C.-P., L.C.-G. J.L., E.C., J.O., and E.T. Data generation—Illumina Laboratory Services Bioinformatics, Software, Interpretation and Reporting and Customer Support Teams. Data and statistical analysis E.T., J.B.-M., R.S.R., C.I.G., and E.C. Writing original draft—E.T., M.C.-O., M.D.-R., J.B.-M., C.I.G., and R.T. Review and final approval—all authors. J.B-M., M.C-O., and M.D.-R. are co-first authors. E.T. and R.T. are co-senior authors.
Data availability
The de-identified outcomes data used and analyzed during the current study are available in Supplementary Data 1. Per Illumina Laboratory Services policy, all reported variants are donated to ClinVar. The sequencing data have not been deposited in a public repository to ensure anonymity and privacy.
Code availability
Custom R code was utilized to translate proband zip codes to latitude and longitude coordinates to generate Fig. 1. Custom R code was utilized to assign Phenotype terms extracted by Phenotagger to one of 25 immediate descendants of the term Phenotypic Abnormality (HP:0000118). Custom code is available on reasonable request of the corresponding author.
Competing interests
Ryan J. Taft, Erin Thorpe, Subramanian S. Ajay, James Avecilla, Krista Bluske, Carolyn M. Brown, Amanda Buchanan, Brendan Burns, Nicole Burns, Anjana Chandrasekhar, Amanda Clause, Katie Golden-Grant, R. Tanner Hagelstrom, Rueben Hejja, Basil Juan, Alka Malhotra, Philip Medrano, Becky Milewski, Felipe Mullen, Viswateja Nelakuditi, Vani Rajan, Revathi Rajkumar, Samin Sajan, Zinayida Schlachetzki, Sarah Schmidt, Julie Taylor, and Brittany Thomas, Evgenii Chekalin, Max Arseneault, Maren Bennett, Aditi Chawla, Alison J. Coffey, Akanchha Kesari, Denise L. Perry, Ajay Ramakrishnan Sylwia Urbaniak, Andrew Warren were employees of and stockholders in Illumina, Inc. during this study.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jeny Bazalar-Montoya, Mario Cornejo-Olivas, Milagros M. Duenas-Roque.
These authors jointly supervised this work: Erin Thorpe, Ryan J. Taft.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Ryan J. Taft, Email: rtaft@geneticalliance.org
Illumina Laboratory Services Bioinformatics, Software, Interpretation and Customer Support:
Max Arseneault, Subramanian S. Ajay, James Avecilla, Maren Bennett, Krista Bluske, Carolyn M. Brown, Amanda Buchanan, Brendan Burns, Nicole Burns, Anjana Chandrasekhar, Aditi Chawla, Amanda Clause, Alison J. Coffey, Katie Golden-Grant, R. Tanner Hagelstrom, Rueben Hejja, Basil Juan, Akanchha Kesari, Alka Malhotra, Philip Medrano, Becky Milewski, Felipe Mullen, Viswateja Nelakuditi, Denise L. Perry, Vani Rajan, Revathi Rajkumar, Ajay Ramakrishnan, Samin Sajan, Zinayida Schlachetzki, Sarah Schmidt, Julie Taylor, Brittany Thomas, Sylwia Urbaniak, and Andrew Warren
Supplementary information
The online version contains supplementary material available at 10.1038/s41525-024-00434-8.
References
- 1.Harris, D. N. et al. Evolutionary genomic dynamics of Peruvians before, during, and after the Inca Empire. Proc. Natl. Acad. Sci. USA115, E6526–E6535 (2018). [DOI] [PMC free article] [PubMed]
- 2.Instituto Nacional de Estadistica E. Informatica. Censos Nacionales 2017: XII de Poblacion, VII Vivienda y III de Comunidades Indigenas. https://www.inei.gob.pe/media/MenuRecursivo/publicaciones_digitales/Est/Lib1437/libro.pdf (2017).
- 3.Azam, M. Governance and economic growth: evidence from 14 Latin America and Caribbean Countries. J. Knowl. Econ.13, 1470–1495 (2022). [Google Scholar]
- 4.Varona-Castillo, L. & Gonzales-Castillo, J. R. Crecimiento económico y distribución del ingreso en Perú. Probl. Desarro. Rev. Latinoam. Econ. 52, 79–107 (2021).
- 5.Cambra-Fierro, J. J., Fuentes-Blasco, M., Huerta-Álvarez, R. & Olavarría-Jaraba, A. Destination recovery during COVID-19 in an emerging economy: Insights from Perú. Eur. Res. Manag. Bus. Econ.28, 100188 (2022). [Google Scholar]
- 6.World Bank. Rising Strong: Peru Poverty and Equity Assessment. (The World Bank, Washington, D.C., 2023). https://documents1.worldbank.org/curated/en/099042523145533834/pdf/P17673806236d70120a8920886c1651ceea.pdf.
- 7.Guio, H. et al. Genetics and genomics in Peru: clinical and research perspective. Mol. Genet. Genom. Med.6, 873–886 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.SUSALUD Boletin Estadistico. https://cdn.www.gob.pe/uploads/document/file/5076147/Bolet%C3%ADn%20Estad%C3%ADstico%202023%201er%20trimestre.pdf.pdf. (2023)
- 9.De Habich, M. Leadership politics and the evolution of the universal health insurance reform in Peru. Health Syst. Reform5, 244–249 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Bernal, N., Carpio, M. A. & Klein, T. J. The effects of access to health insurance: evidence from a regression discontinuity design in Peru. J. Public Econ.154, 122–136 (2017). [Google Scholar]
- 11.Silva-Paredes, G., Urbanos-Garrido, R. M., Inca-Martinez, M., Rabinowitz, D. & Cornejo-Olivas, M. R. Economic burden of Huntington’s disease in Peru. BMC Health Serv. Res.19, 1017 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poterico, J., Purizaca-Rossillo, N. & Taype-Rondan Alvaro, A. Genética y genómica médica en el Perú. Acta Méd.34, 152–153 (2017). [Google Scholar]
- 13.Manrique, J., ullcahuamán-Allende, Y. & Limache-García, A. Genetic counseling about cancer in Peru. Rev. Peru Med. Exp. Salud Publica30, 118–123 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Mazzetti, P. et al. Neurogenetics in Peru, example of translational research. Rev. Peru Med. Exp. Salud Publica32, 787–793 (2015). [PubMed] [Google Scholar]
- 15.Penchaszadeh, V. B. Genetic testing and services in Argentina. J. Commun. Genet.4, 343–354 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Encina, G. et al. Rare diseases in Chile: challenges and recommendations in universal health coverage context. Orphanet J. Rare Dis.14, 289 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galán-Rodas, E., Dueñas, M., Obando, S. & Saborio, M. Newborn screening in Peru: where are we going?. Rev. Peru Med. Exp. Salud Publica30, 724–725 (2013). [PubMed] [Google Scholar]
- 18.Thorpe, E. et al. The impact of clinical genome sequencing in a global population with suspected rare genetic disease. Am. J. Hum. Genet.10.1016/j.ajhg.2024.05.006 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cornejo-Olivas, M. et al. Neurogenetics in Peru: clinical, scientific and ethical perspectives. J. Commun. Genet.6, 251–257 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manickam, K. et al. Exome and genome sequencing for pediatric patients with congenital anomalies or intellectual disability: an evidence-based clinical guideline of the American College of Medical Genetics and Genomics (ACMG). Genet. Med.23, 2029–2037 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Brown, C. M. et al. A framework for the evaluation and reporting of incidental findings in clinical genomic testing. Eur. J. Hum. Genet. 10.1038/s41431-024-01575-1 (2024). [DOI] [PMC free article] [PubMed]
- 22.Hunter, J. E. et al. A standardized, evidence-based protocol to assess clinical actionability of genetic disorders associated with genomic variation. Genet. Med.18, 1258–1268 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webber, E. M. et al. Evidence‐based assessments of clinical actionability in the context of secondary findings: updates from ClinGen’s Actionability Working Group. Hum. Mutat.39, 1677–1685 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Núñez-Samudio, V., Arcos-Burgos, M. & Landires, I. Rare diseases: democratising genetic testing in LMICs. Lancet401, 1339–1340 (2023). [DOI] [PubMed] [Google Scholar]
- 25.Wainstock, D. & Katz, A. Advancing rare disease policy in Latin America: a call to action. Lancet Reg. Health18, 100434 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark, M. M. et al. Meta-analysis of the diagnostic and clinical utility of genome and exome sequencing and chromosomal microarray in children with suspected genetic diseases. Npj Genom. Med.3, 16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertoli-Avella, A. M. et al. Successful application of genome sequencing in a diagnostic setting: 1007 index cases from a clinically heterogeneous cohort. Eur. J. Hum. Genet.29, 141–153 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung, C. C. Y. et al. Meta-analysis of the diagnostic and clinical utility of exome and genome sequencing in pediatric and adult patients with rare diseases across diverse populations. Genet. Med.25, 100896 (2023). [DOI] [PubMed] [Google Scholar]
- 29.Scocchia, A. et al. Clinical whole genome sequencing as a first-tier test at a resource-limited dysmorphology clinic in Mexico. Npj Genom. Med.4, 5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization Science Council. Accelerating Access to Genomics for Global Health: Promotion, Implementation, Collaboration, and Ethical, Legal, and Social Issues: A Report of the WHO Science Council. https://www.who.int/publications/i/item/9789240052857 (2022).
- 31.Rare Diseases International. Rare Diseases: Leaving No One Behind in Universal Health Coverage. https://d254mlohi4u805.cloudfront.net/rdi/2019/RDI%20UHC%20Paper%20Final%20October%202019.pdf.
- 32.Luo, L. et al. PhenoTagger: a hybrid method for phenotype concept recognition using human phenotype ontology. Bioinformatics37, 1884–1890 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Köhler, S. et al. The Human Phenotype Ontology in 2021. Nucleic Acids Res.49, D1207–D1217 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information_Bazalar-Montoya, Cornejo-Olivas, X Duenas-Roque_iHope Peru
Data Availability Statement
The de-identified outcomes data used and analyzed during the current study are available in Supplementary Data 1. Per Illumina Laboratory Services policy, all reported variants are donated to ClinVar. The sequencing data have not been deposited in a public repository to ensure anonymity and privacy.
Custom R code was utilized to translate proband zip codes to latitude and longitude coordinates to generate Fig. 1. Custom R code was utilized to assign Phenotype terms extracted by Phenotagger to one of 25 immediate descendants of the term Phenotypic Abnormality (HP:0000118). Custom code is available on reasonable request of the corresponding author.