Abstract
Background
Climate change is driving increased extreme weather events that can impact ecology by moderating host–pathogen interactions. To date, few studies have explored how cold snaps affect disease prevalence and proliferation. Using the Daphnia magna–Ordospora colligata host-parasite system, a popular model system for environmentally transmitted diseases, the amplitude and duration of cold snaps were manipulated at four baseline temperatures, 10 days post-exposure, with O. colligata fitness recorded at the individual level.
Results
Cold snaps induced a fivefold increase or a threefold decrease in parasite burden relative to baseline temperature, with complex nuances and varied outcomes resulting from different treatment combinations. Both amplitude and duration can interact with the baseline temperature highlighting the complexity and baseline dependence of cold snaps. Furthermore, parasite fitness, i.e., infection prevalence and burden, were simultaneously altered in opposite directions in the same cold snap treatment.
Conclusions
We found that cold snaps can yield complicated outcomes that are unique from other types of temperature variation (for example, heatwaves). These results underpin the challenges and complexity in understanding and predicting how climate and extreme weather may alter disease under global change.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12915-024-02041-6.
Keywords: Climate change, Temperature variation, Cold snap, Cold spell, Host, Parasite, Disease, Pathogen, Daphnia magna, Ordospora colligata
Background
Global change has far-reaching impacts in natural, agricultural, and human systems. It has been implicated in the deterioration of ecosystem structure and function [1], food production [2], and human health [3]. For instance, biodiversity is particularly vulnerable to the influence of climate change (for review see Bellard et al. [4]). Moreover, climate change is also altering the incidence and distribution of infectious diseases [5], due to range shifts and changes in behavior of hosts, parasites, and vectors [6]. The important role temperature plays in disease dynamics and transmission has been well studied empirically [7–9] and theoretically [10–12]. For example, temperature can influence the host foraging rate [13] and food availability [14] which in turn can impact the encounter rate between the host and parasite. Furthermore, temperature can also lower host immunity when hosts need to expend more energy on maintaining homeostasis due to temperature fluctuations [15], potentially leading to an increased parasitic burden [16]. Conversely, other studies have reported no effect of temperature on host immunity [17] or even an increased parasite resistance with higher temperature [18]. Importantly, to successfully infect a host, the parasite needs to overcome a multitude of host defenses (avoidance behavior, physical barriers, innate and adaptive immunity) [19], each of which may be affected directly or indirectly by climate change [20]. Thus, shifts in disease prevalence and burden due to climate change can be complex [8, 21, 22]. While many studies have highlighted interactions between climate change (that is, rising mean temperatures) and disease [23–25], studies that have empirically investigated the role of temperature variation in the context of climate change and disease dynamics [26–31] have been less common.
Climate change is causing an increased occurrence and magnitude of anomalous weather events, including heatwaves, cold snaps, droughts, and flooding [32]. Trends in extreme temperature variation, frequency, and duration of heatwaves have accelerated since the 1950s [33], while cold snaps have increased in frequency since 1979 [34]. These climatic changes in variation may lead to shifts in host-parasite dynamics [26]. For example, natural disasters associated with extreme weather can lead to respiratory [35], waterborne [36], and vector borne diseases [37]. Moreover, shifts in temperature can influence host survival, which can indirectly alter the host-parasite relationship [22]. For example, a heatwave reaching 28 °C resulted in increased host immune activity in three-spined sticklebacks (Gasterosteus aculeatus) [38]. Not only are host traits (e.g., fecundity [39], survival [40], and behavior [41]) affected by extreme weather, but parasite traits can also be altered. For instance, recent work by Kunze et al. using water fleas (Daphnia magna) infected with a microsporidium parasite showed that a heatwave not only altered parasite burden, but that the direction of this effect depended on the mean temperature at which a heatwave occurred [42]. While recent studies have started exploring the impact of extreme heat events [26–31], the effect of cold snaps on disease outbreak and spread remains underexplored [27, 28, 43, 44] and not yet studied in the Daphnia system.
Surprisingly, few studies have focused on the effects of cold snaps on organisms [45], ecosystems [46, 47], and health [48], while even less is known about the impact of extreme cold events on disease dynamics [49]. Nonetheless, the effects of cold snaps may become increasingly relevant in spite of rising mean temperatures as, for example, the negative phases of the North Atlantic Oscillations lead to colder, drier winters in Northern Europe [50] and Southern Europe [51]. Like heatwaves, cold snaps can affect host-parasite relationships. For example, the establishment of the pathogenic trematode (Ribeiroia ondatrae) in its amphibian host is more successful at lower temperatures [43]. Similarly, a decrease in temperature has led to an increase in infections in frogs due to lowered resistance to chytrid fungus (Batrachochytrium dendrobatidis) [27]. Conversely, mice housed at cold temperatures had a lower metacestode intensity and growth than individuals housed at room temperature [44]. Indeed, how cold snaps alter disease outcomes remains an open question, to our knowledge.
Here, we examine the effect of different cold snap treatments across a broad range of baseline temperatures (the mean constant temperature throughout the experiment) using the Daphnia magna–Ordospora colligata host–pathogen system to test how cold snaps may alter disease prevalence and proliferation. This is a popular model system for environmentally transmitted diseases (horizontal transmission, infection by mass action), which are a common concern for humans, livestock, and agriculture [52]. As mentioned, in the study by Kunze et al. [42], an experimental heatwave event (characterized by a 6 °C increase in temperature occurring 20 days post-exposure and lasting for 3 days) led to an alteration in parasite burden. Building upon this observation, we hypothesized that cold snaps, like heatwaves, may alter parasite fitness in complex ways by increasing or decreasing infection prevalence and/or burden and may be dependent on the baseline temperature. We believed that at extreme temperatures, parasite fitness could decrease as a result of thermal mismatch [53], while parasite fitness could also be affected by differences in acclimation speed of host and parasite [29] and Jensen’s inequality [30]. To test how cold snaps affects Ordospora prevalence and burden, we manipulated the amplitude (− 3 °C and − 6 °C) and duration (3 days and 6 days) of a cold snap occurring 10 days post-exposure at four baseline temperatures (14, 17, 20, and 23 °C) (Fig. 1). In total, the impact of 16 different cold snaps on individual Daphnia (15 replicates per cold snap treatment) was tested. Infection prevalence and burden were observed upon natural death or at the end of the experiment, 27 days post-exposure. We demonstrated that cold snaps can alter parasite fitness in unexpected and nuanced ways depending on baseline temperature and cold snap properties.
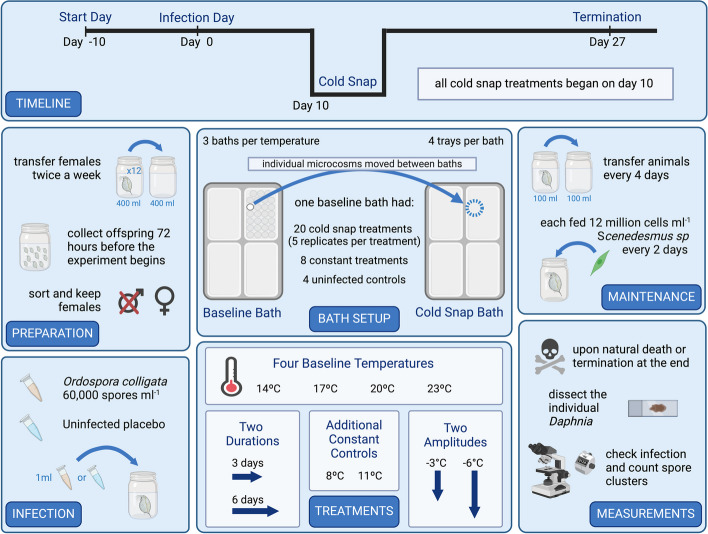
Fig. 1.
Experimental setup. TIMELINE; illustrates the timeline for the experiment starting at day − 10 and terminating at day 27, exposure occurred on day 0, and every cold snap began on day 10. PREPARATION; preparation started 3 weeks prior to exposure when 10–12 mothers were added to a 400 mL microcosm, 72 h before the experiment began offspring were collected and females were kept. INFECTION; a dose of ~ 60,000 Ordospora colligata spores was given to each exposed individual, while unexposed controls were given a placebo dose made of crushed up uninfected individuals. BATH SETUP; each temperature had three baths, and each bath held four trays with 27 microcosms in each, each bath contained 20 cold snap treated individuals (five replicates per cold snap treatment), eight constant temperature treatments, and four uninfected controls. To simulate a cold snap, microcosms were moved between baths and then returned to the baseline bath when the cold snap finished. TREATMENTS; 16 treatments were included in the experiment (four baseline temperatures · two amplitudes · two durations). Additionally, at the cold snap specific temperatures (8 °C and 11 °C), each bath was organized the same as the baseline temperatures; however, no cold snap treatments were included. MAINTAINENCE; maintenance and measurements were carried out between days − 4 and day 27. Figure created on Biorender.com
Results
Infection prevalence
The baseline temperature at which exposure occurred was important for predicting infection prevalence (quadratic effect, glmnet, effect = − 36.52, 95% CI: − 46.68 to − 27.89, linear effect, glmnet, effect = 21.66, 95% CI: 14.50 to 30.72 and feature importance of the combined polynomial = 58.51, Table 1). Infection prevalence followed a classic temperature response curve with the lowest infection prevalence at the extreme temperatures (~ 8 °C, ~ 23 °C) and highest infection prevalence at the optimal range (~ 17 °C) (Fig. 2). The strong and long cold snap (6 days of − 6 °C) in combination with the baseline temperature (3-way interaction between baseline temperature, duration 6 days, and amplitude − 6 °C) behaved differently from the other treatment combinations and was important for predicting infection prevalence (linear effect, glmnet, effect = − 34.08, 95% CI: − 61.39 to − 2.92 and feature importance of the combined polynomial = 62.63, Table 1). Here, this long and strong treatment behaved differently to other cold snap treatments by decreasing with increasing baseline temperature (Fig. 1), although no specific treatments in the same baseline temperature were statistically different (Additional file 1: Table S3). The finding of differences between cold snaps and a complex (3-way) interaction shows that baseline temperature, amplitude, and duration of extreme cold weather events modify and complicate the infection prevalence in the Daphnia-Ordospora system.
Table 1.
Coefficient estimates and feature importance of proportion infected
| A | |||
| Explanatory variable | Eff | Lower CI | Upper CI |
| Temperature (L): amplitude − 6: duration 6 | − 38.33 | − 71.71 | − 7.07 |
| Temperature (Q) | − 36.52 | − 46.68 | − 27.89 |
| Temperature (Q): amplitude − 6: duration 6 | 24.30 | 0 | 51.41 |
| Temperature (L): amplitude − 3: duration 6 | 21.99 | 0 | 48.87 |
| Temperature (L) | 21.66 | 14.50 | 30.72 |
| B | |||
| Explanatory variable | Importance | ||
| Temperature: amplitude − 6: duration 6 | 62.63 | ||
| Temperature | 58.51 | ||
A) Coefficient estimates and B) feature importance of proportion infected in Daphnia magna infected with Ordospora colligata. Coefficient effect (Eff) represents the estimated coefficient of the elastic net regression model, while lower (lower CI) and upper (upper CI) confidence intervals (95th) indicate the range. Non-significant values have CIs passing 0. Positive and negative effects indicate corresponding relationships. Temperature, modeled as a quadratic polynomial, is delineated by each degree (linear [L], quadratic [Q]). Feature importance is calculated by combining the absolute values of coefficients effect for each variable and polynomial degree. For clarity, only the most impactful variables are shown. Refer to Additional file 1: Table S1 for full coefficient estimate output and Table S4 for a full list of feature importance variables
Fig. 2.
The effect of cold snaps treatments on proportion infected at 10 days post-exposure, in Daphnia magna infected with Ordospora colligata. Error bars represent the standard error, and each dashed line is the quadratic fit of the elastic net regression (see Additional file 1: Table S1 for statistical results)
Burden
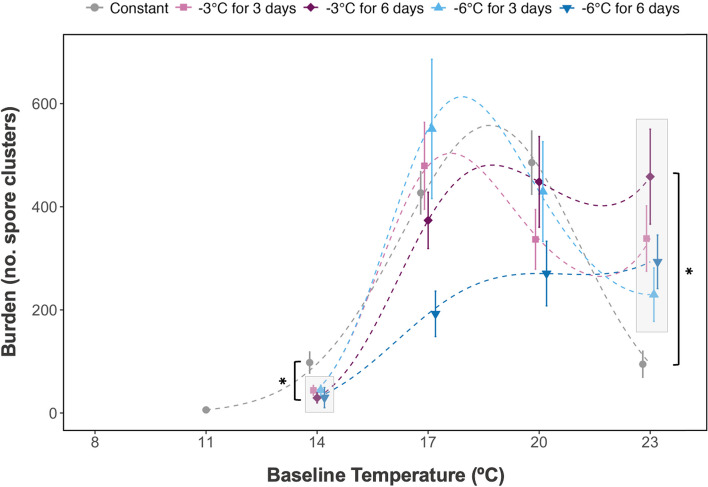
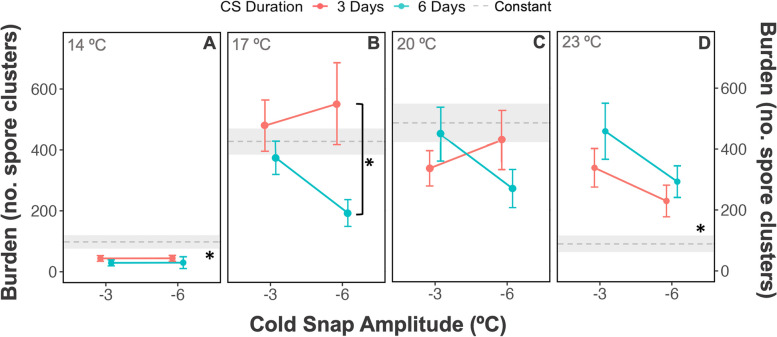
Of the infected individuals, cold snaps occurring 10 days post-exposure resulted in a 5.1-fold increase (485 vs 89 spores clusters, respectively) in disease proliferation or a 3.3-fold decrease (29 vs 98 spores clusters, respectively) depending on the treatment and the baseline temperature at which the cold snap occurred (Figs. 3 and 4). Generally, cold snaps led to similar or lower burden compared to the constant treatment at lower baseline temperatures (14–20 °C) and higher at hotter temperatures (23 °C) (Fig. 3). At 14 °C, all cold snap treatments resulted in a lower burden compared to the constant treatment, e.g., the mean spore cluster count of the treatment where the cold snap was reduced in temperature by − 3 °C for 6 days was 69 spore clusters less than that of the 14 °C constant treatment (Fig. 4A) (emmean, z(inf) = − 2.937, p = 0.002, see Additional file 1: Table S7 for significant p values of all cold snap custom contrasts, Fig. 3). Moreover, at intermediate temperatures (17 °C), the difference in burden between cold snaps and constant treatments was minimal, with only one lower burden for the 6 days at − 6 °C cold snap when compared to the constant treatment (emmean, z(inf) = − 2.508, p = 0.049, see Additional file 1: Table S7, Fig. 3). In contrast, all cold snap treatments at 23 °C had increased burden compared to constant temperature treatment; for example, the cold snap for 6 days at − 6 °C had a burden higher burden (204 spore clusters greater) than the constant temperature (emmean, z(inf) = 3.02, p = 0.018, see Additional file 1: Table S7, Fig. 3). While burden was differentially affected by baseline temperature, cold snap traits (amplitude and duration) also influenced the outcome of both spore burden and infection prevalence.
Fig. 3.
The effect of cold snaps treatments on burden (average number of spore clusters) 10 days post-exposure, in Daphnia magna infected with Ordospora colligata. Each spore cluster contains 32–62 individual Ordospora. Error bars represent the standard error, and each dashed line is the cubic fit of the elastic net regression (see Additional file 1: Table S5 for statistical results). Absence of the data point at 8 °C, for the constant temperature, is due to the absence of infection at this temperature. The asterisk and bracket (*[) indicates significant contrasts between means of the cold snap treatments when compared to the constant treatment
Fig. 4.
Burden (average number of spore clusters) by baseline temperature, amplitude, and duration, for Daphnia magna infected with Ordospora colligata. Error bars represent the standard error; light gray area represents the standard error for the constant treatments. An asterisk (*) near the gray dashed line indicates that all cold snap treatments are different from the constant temperature treatment and an asterisk next to a solid black bracket (]*) indicates a difference between two single treatments
Each of the factors in our experiment (baseline temperature, cold snap amplitude, and duration) was statistically important for predicting burden of O. colligata. However, baseline temperature was the most important for predicting burden (quadratic main effect, glmnet,effect = − 12.12, 95% CI: − 14.58 to − 10.06 and feature importance of the combined polynomial = 14.19, Table 2). In general, burden also followed a classical temperature response curve with lower burdens at extreme temperatures (~ 11 °C, ~ 23 °C) and optimal performance at intermediate temperatures (~ 17–20 °C). Yet, the cold snap treatments can alter the outcome. Indeed, both the amplitude and duration of the cold snap influenced burden depending on the baseline temperature (i.e., both separately interacted with temperature). A weak cold snap (− 3 °C) had the largest influence on burden, where the burden increased with increasing baseline temperature (linear main effect, glmnet, effect = 6.28, 95% CI: 2.63 to 8.57 and feature importance of the combined polynomial = 9.27, Table 2). For example, at 23 °C a cold snap of − 3 °C resulted in a higher burden (SE = ± 124) than the constant at 23 °C (SE = ± 29) (Fig. 4D), and nearly equal to the performance of the constant three degrees lower (SE = ± 64) (Fig. 4C). A strong cold snap with an amplitude of − 6 °C showed similar results, that is, increasing burden with increasing temperature (linear effect, glmnet, effect = 4.49, 95% CI: 1.03 to 7.58, and feature importance of the combined polynomial = 6.84, Table 2). Similar to amplitude, cold snap duration increased burden with increasing baseline temperature for both the short (3-day) cold snap and long (6-day) cold snap (linear effect, glmnet, effect = 3.45, 95% CI: 0.41 to 5.92 and feature importance of the combined polynomial = 8.54, and linear effect, glmnet, effect = 7.15, 95% CI: 2.83 to 10.62 and feature importance of the combined polynomial = 8.76, respectively, Table 2). Higher order interactions between temperature, amplitude, and duration played a minimal role (low importance, see Additional file 1: Table S8, and confidence intervals overlapping zero, see Table S5). Thus, temperature and its interaction with both the amplitude and duration (independently) are most important in explaining the burden of O. colligata, and as a result, several combinations of amplitude and duration led to alternative outcomes.
Table 2.
Coefficient estimates and feature importance of burden
| A | |||
| Explanatory variable | Eff | Lower CI | Upper CI |
| Temperature (Q) | − 12.12 | − 14.58 | − 10.06 |
| Temperature (L): duration 6 | 7.15 | 2.83 | 10.62 |
| Temperature (L): amplitude − 3 | 6.28 | 2.63 | 8.57 |
| Temperature (L): amplitude − 6 | 4.49 | 1.03 | 7.58 |
| Temperature (C): duration 3 | 3.61 | 0 | 6.59 |
| Temperature (L): duration 3 | 3.45 | 0.41 | 5.92 |
| B | |||
| Explanatory variable | Importance | ||
| Temperature | 14.19 | ||
| Temperature: amplitude − 3 | 9.27 | ||
| Temperature: duration 6 | 8.76 | ||
| Temperature: duration 3 | 8.54 | ||
| Temperature: amplitude − 6 | 6.84 | ||
A) Coefficient estimates and B) feature importance of burden in Daphnia magna infected with Ordospora colligata. Coefficient effect (Eff) represents the estimated coefficient of the elastic net regression model, while lower (lower CI) and upper (upper CI) confidence intervals (95th) indicate the range. Non-significant values have CIs passing 0. Positive and negative effects indicate corresponding relationships. Temperature, modeled as a cubic polynomial, is delineated by each degree (linear [L], quadratic [Q], cubic [C]). Feature importance is calculated by combining the absolute values of coefficients effect for each variable and polynomial degree. For clarity, only the most impactful variables are shown. Refer to Additional file 1: Table S5 for full coefficient estimate output and Table S8 for a full list of feature importance variables
Certain cold snap treatment combinations led to differential outcomes, and as a result not all cold snaps resulted in the same burden. At 17 °C, a long and strong cold snap with a 6-day duration and an amplitude of − 6 °C had a lower burden compared to that of a short cold snap (3 days) with the same amplitude (Fig. 4B) whereby the difference in burden was 323 spore clusters (emmean, z(inf) = − 2.983, p = 0.018, see Additional file 1: Table S7). Overall, this long and strong cold snap had the lowest mean values at 17 °C and 20 °C and was also among the lowest values at 14 °C and 23 °C (Fig. 3). Finally, while this long and strong treatment tended to increase burden with increasing baseline temperature (Fig. 3), the opposite was true for infection prevalence which decreased with increasing baseline temperature (Fig. 2). Thus, not all cold snaps will lead to the same outcome in disease intensity, and parasite fitness can be simultaneously altered in opposite directions in the same cold snap treatment.
Discussion
This experiment demonstrated that cold snaps can alter the performance of Ordospora colligata; however, the direction and influence depends on the baseline temperature. At higher baseline temperatures (23 °C), a cold snap may increase the parasite’s performance as temperatures near the optimal growth temperature (17–20 °C), resulting in up to a fivefold increased burden. At lower temperatures (14 °C), burden instead decreased by a up to threefold in response to a cold snap, likely due to decreased parasite performance as temperatures reached or exceeded the thermal limits of the parasite [54]. As Ordospora has a smaller thermal range than its Daphnia host [8], the decrease in parasite performance at temperatures near the thermal maximum may be attributed to the thermal mismatch hypothesis [53]. This hypothesis suggests that the performance of either the host or the parasite may be most affected at temperatures where the difference in thermal performance between the two antagonists is the greatest. This is supported by the lower burden and infection prevalence of Ordospora at the edge of its thermal tolerance during cold snaps at lower temperatures, compared to the constant treatment. However, to formally test this hypothesis, separate measures of host and parasite thermal performance would be needed, which is not possible in this system, as Ordospora cannot survive outside the host. The fact that thermal variation near the maximum thresholds can lead to decreased or increased performance of diseases has been demonstrated in various study organisms. For example, daily temperature fluctuations are expected to increase malaria transmission at lower baseline temperatures while decreasing transmission at higher baseline temperatures [55]. Furthermore, the effect of a heatwave can also depend on the baseline temperature, as demonstrated in a related Daphnia-Ordospora experiment that resulted in a fourfold increase in burden at 16 °C but a threefold decrease at 22 °C [42]. Indeed, our results show that cold snaps, like heatwaves, may modify parasite proliferation, and the direction of this effect depends on the baseline temperature. While the impact of cold snaps on disease proliferation and outbreak have received less attention, these findings are in line with other studies that have shown that cold snaps can alter host morphology and physiology [56], immunity [57, 58], and behavior [59]. Thus, the baseline temperature at which the cold snap occurred was the leading factor in explaining the performance of O. colligata, yet both the duration and the amplitude of the cold snap also played an important role.
The amplitude and the duration of a cold snap can affect parasite fitness, and the effect of both depends on the baseline temperature (amplitude ⋅ °C and duration ⋅ °C interaction). Both amplitude and duration independently decreased burden at low temperatures while increasing proliferation at higher temperatures. Infection prevalence, however, showed a more context-specific response as illustrated by the long and strong cold snap which resulted in a decrease in parasite fitness with increasing temperatures. Additionally, some combinations of amplitude and duration led to alternate patterns, indicating an interaction between amplitude, duration, and baseline temperature. Similar to burden, if the temperature exceeds the parasite’s thermal range, then infection prevalence (i.e., ability to establish an infection in the host) may decrease [53], for example, as seen at 14 °C (Fig. 2). Moreover, it has previously been noted that short fluctuations can reduce infection prevalence in the same system [42]. Indeed, previous studies have highlighted the importance of the strength of temperature fluctuations, for example, in Aedes albopictus [60] where duration influenced the egg hatching response at the parasite’s thermal limits, and in herbivore-parasitoid [61] and plant-endoparasite [62] systems the fitness of the parasite depended on the amplitude of a heatwave. Additionally, both factors (amplitude and duration) can influence human systems, for instance, population dynamics in Anopheles infected with Plasmodium were affected by the amplitude and type of fluctuation around a baseline temperature [63], heatwave amplitude and duration can influence Salmonella serotypes and phage types resulting in increased risk of infection with increasing amplitude and/or duration [64], and heatwave amplitude and duration increased infection risk for children infected with shiga toxin-producing Escherichia coli [65]. While for heatwaves we have some insight into the effect of duration and amplitude on disease proliferation [42], for cold snaps such insight is lacking. We show here that in addition to the baseline temperature, both the amplitude and duration of a cold snap can alter parasite fitness, with variable outcomes.
Different combinations of the duration and amplitude of a cold snap can lead to divergent disease outcomes. The findings for burden at 17 °C demonstrate that cold snaps can result in differential outcomes at the same baseline temperature, with the highest burden found in response to a short, strong cold snap, while a long, strong cold snap led to the lowest burden. Another such example is the decreasing infection prevalence with increasing baseline temperature in the long, strong cold snap which exhibits a different response compared to the other cold snap treatments. These context-specific differences may be explained by trade-offs associated with the temperature variability hypothesis [27] and thermal mismatch hypothesis [53]. High parasite fitness as observed in the short weak cold snap is expected under fluctuating temperatures according to the temperature variability hypothesis [26]. This theory posits that since parasites are almost always smaller than their hosts and therefore have higher metabolic rates (according to metabolic theory of ecology [66]), they should acclimatize faster to shifts in temperature, giving them an advantage over their hosts as the host’s thermal acclimation is slower following temperature shifts [29]. In line with this hypothesis, parasite fitness is increased in the Daphnia-Ordospora system when temperature fluctuates (both in individuals [42] and whole populations [30]) which is also found in other systems (caribou-nematode [67], Cuban tree frog-chytrid fungus [27], and protist-bacteria [68]). However, thermal mismatch [53] or thermal stress [54] can also play an important role alongside variable temperatures and may decrease or reverse the advantage of the parasite, especially if the parasite has a narrower thermal performance than its host, as is the case for Ordospora colligata [69]. Moreover, the reduced burden of Ordospora when exposed to the long and strong cold snap over much of the temperature range suggests that the parasite may be experiencing higher levels of stress and a decrease in performance due to temperatures outside the optimal range. Yet, the increased burden at high temperatures suggests that even a small temporary relief from stressful temperatures can help Ordospora to establish higher levels of burden [7]. Furthermore, cold snaps with a short duration and weak amplitude showed the same increase in burden, highlighting that even weak cold snaps may alter disease patterns. Indeed, previous studies have shown the advantage of weak and short cold snaps. For example, short-term fluctuations ± 6 °C in the same Daphnia-Ordospora system have also been found to increase endemic infection prevalence [30], and short-term cold snaps increased burden in Alytes obstetricans tadpoles infected with Batrachochytrium [70]. Thus, even weak and short cold snaps may have substantial effect on disease, and this effect can be different depending on the duration and amplitude of the cold snap and the parasite trait.
Cold snaps are different from other types of temperature variation and can affect parasite traits in opposing directions. Comparisons to previous studies using the same Daphnia-Ordospora model system reveal that cold snaps alter within-host proliferation in a different direction than heatwaves or daily fluctuating temperatures. Both Kunze et al. [42] who studied heatwaves and daily fluctuation in the same host-parasite system and our study (cold snaps) found large differences in burden for different types of temperature variation. However, there are clear differences between heatwaves, daily fluctuations, and cold snaps at higher temperatures, with cold snaps increasing disease burden at 23 °C while daily fluctuations and heatwaves lead to a decrease. Moreover, in line with previous findings, which showed that different host and parasite traits respond in a unique way to changes in baseline temperature [7, 8, 71], here, we demonstrated that two parasite traits (burden and infection prevalence) can be affected in opposite directions for some but not all combinations of duration and amplitude. This is illustrated by the decrease in infection prevalence with increasing temperature for the long and strong cold snap, while burden increased with increasing temperature for the same treatment. Our findings address an outstanding question in the field [26] and show that the direction of the temperature shift, the type of variation and the life history trait studied, matters.
Conclusions
In conclusion, we show that in the Daphnia-Ordospora system, cold snaps are distinct from other forms of temperature variation, and these can lead to increased or decreased disease proliferation depending on the prevailing baseline temperature. Moreover, different combinations of amplitude and duration lead to differential outcomes, and that parasite’s fitness traits can be simultaneously affected in opposite directions. Overall, the impact of cold snaps on Ordospora fitness is therefore complex. Thus, the effect of cold snaps and temperature variation on disease may result in unexpected outcomes in the face of climate change. Given Daphnia’s key ecological role [52], this may result in far-reaching ecological impacts as Daphnia diseases are known to alter inter- and intra-specific competition [72], cause trophic cascades [73], influence host evolution [74], and host stoichiometry [75]. For example, the infection of Daphnia dentifera led to a reduction in host density, and consequently, alterations in the food web which could lead to potential losses in biodiversity [73]. Similarly in other systems, for example, sea star wasting disease in Pisaster ochraceus, infection led to a trophic cascade, and the collapse of a keystone predator with unexpected repercussions [76]. Adding to the complexity of host-parasite dynamics, there is often a disconnect between thermal performance and parasite burden in many systems where no correlation is found between heat tolerance and burden in a parasite (see Hector et al. [77] for a review). Indeed, understanding temperature variation has been a challenge for years [78], and although some theoretical studies are highlighting that the addition of temperature shifts to predictive models is key to accurate predictions [79] we still lack the needed empirical data and mechanisms. Thus, there is an urgent need to understand the underlying mechanisms responsible for the impact of temperature variation on diseases, both with this host-parasite genotype combination and with others to facilitate in generalization of the mechanisms and conclusions. Future research is needed on disease interactions in response to different types of extreme weather (such as heatwaves and cold snaps) to better understand how disease outbreaks will respond to intensifying global change.
Methods
Study system
Daphnia magna (genotype FI-OER-3–3), previously isolated from a rockpool at Tvärminne archipelago, Finland, was infected with its microsporidian parasite Ordospora colligata (isolate 3), sampled from the same location. Daphnia magna is a popular model organism for the study of host–pathogen interactions, given their fast generation time of a new clutch every 3–4 days, clonal reproduction via parthenogenesis which allows for experiments to be conducted on genetically identical individuals [80], their well-known ecology, and their key role in ecosystems [52, 81]. Ordospora colligata is a microparasite (that is, an infectious disease) which invades the upper gut epithelium of its host upon ingestion of spores via filter feeding and is then horizontally transmitted (from host to host) via feces into the water [82]. This parasite has been well studied with respect to climate change (and especially the specific host genotype and parasite isolate used here [7, 30, 42, 71, 74]), with multiple papers focused on the effects of baseline temperature and temperature variation [7, 42]. Daphnia have a temperature tolerance ranging from 6 to 33.3 °C with an optimum temperature of 20 °C, while Ordospora has a similar, but somewhat narrower thermal tolerance of 11.8–29.7 °C [8].
Experimental setup
This experiment had a fully factorial design, whereby the amplitude and duration of cold snaps were manipulated and assessed at four baseline temperatures (14, 17, 20, and 23 °C). Here, we define a cold snap as an event which reduced the baseline temperature at a set amplitude for a specific duration. The experiment began 10 days pre-exposure giving the juvenile animals time to mature, while the cold snap itself was initiated 10 days post-exposure. During the cold snap, the temperature was lowered by either − 3 °C or − 6 °C (two amplitudes), and the duration was varied by 3 days or 6 days (two durations) (Fig. 1). A change of 6 °C is within the limits of naturally encountered temperature fluctuations in the rockpools these animals inhabit [83]. A constant temperature control (amplitude 0 °C) was included for each of the four baseline temperatures with additional temperature constant controls at 8 °C and 11 °C. Additionally, we included uninfected controls for parasite exposure for each of the constant treatments. In total, there were 16 different cold snap treatments (four baseline temperatures · two amplitudes · two durations), each with 15 replicates per treatment that were organized into trays. Trays were submerged into 18 water baths (four trays per bath, three baths per temperature) that regulated the temperature, and trays were repositioned daily to avoid positioning effects. Each baseline temperature bath held one hundred and eight 100 mL microcosms (filled with 80 mL of Artificial Daphnia Medium (ADaM [84])) of which four microcosms were uninfected controls, eight were constant-temperature replicates, and 20 were parasite exposed cold snap treated microcosms (four treatments · five replicates, per bath) (Fig. 1), remaining microcosms only contained water and were present for stability when moving the trays. In total, the experiment contained 456 individual Daphnia, each placed in a separate microcosm (i.e., 240 cold snap treatments, and 144 constant treatments and 72 uninfected controls).
The 18 temperature-controlled water baths ranged from 8 to 23 °C (three baths per temperature). Each bath was fitted with an aquarium chiller (Hailea HC-150A, DC300, or DC750), an aquarium heater (EHEIM JÄGER 300W), and pumps (Micro-Jet Oxy and Oase Optimax 500) to create a constant flow and ensure even water and temperature distribution. The temperature for each bath was regulated using a programmable controller (Inkbird ITC-308 or ITC-310 T) and monitored daily using HOBO temperature loggers (HOBO UA-001–08). Baths were kept at a constant water level and any evaporation was offset by adding tap water. The baths were kept under natural lighting conditions of 16:8, light:dark. To simulate the cold snaps, individual microcosms were moved between assigned baths, while each bath was maintained at a constant temperature throughout the experiment. The microcosms were added directly to the target temperature bath, upon which, the microcosm would immediately start to equilibrate, and the target cold snap temperature was reached within an hour. Once the cold snap treatment was concluded, the microcosm was then returned to its original position in the baseline temperature water bath. For example, five replicates of a cold snap treatment which decreased by 6 °C for 6 days would stay in the first 14 °C water bath until day 10. These five treatments would then be transferred to the first 8 °C water bath for 6 days, then after the duration of the cold snap they were then returned to the original 14 °C water bath.
In preparation for the experiment, Daphnia were grown asexually under ideal laboratory conditions for 3 weeks. Each small population of 10–12 females was held in a 400 mL microcosm containing ~ 300 mL of ADaM, under continuous light at 20 °C. The populations were transferred twice a week to fresh ADaM and fed ad libitum with batch culture algae (Scenedesmus species) [85]. Seventy-two hours prior to the beginning of the experiment the animals were transferred to fresh medium with no juveniles present. Any juveniles born over the next 72 h were collected and sexed (using a dissecting microscope at 8 × to 12 × magnification). Any males were removed, as Daphnia have environmental sex determination and are therefore not truly clonal [80]. To start the experiment, one female juvenile was added to each of the 456, 100 mL microcosms. Microcosms were filled with 50 mL of ADaM, and a pinch of cetyl alcohol was also added to break the surface tension (which can entangle young animals). On day − 10, the experiment began; Daphnia were unexposed until day 0 when the exposed individuals were given 1 mL of ADaM containing ~ 60,000 O. colligata spores, to ensure near 100% infection prevalence. The spore dose was created by crushing (using a mortar and pestle) 1942 infected D. magna with a known average burden (quantified by using phase-contrast microscopy at 400 × magnification) and the resulting slurry was diluted to 500 mL. Unexposed controls were exposed to a placebo dose made by crushing uninfected individuals. Individuals were transferred to fresh ADaM every 4 days (to remove any offspring produced) and fed every 2 days (from 5 million algae/mL at the start of the experiment to 12 million algae/mL by day 5, which was maintained until the end of the experiment) (Fig. 1).
Measurement of parasite fitness
Upon natural death or at the end of the experiment (day 27), the infection prevalence (i.e., presence or absence of spores) and burden (i.e., number of spore clusters inside the host) were observed for each individual. At the end of the experiment, animals were terminated within 5 days of day 27. If infected, spore clusters (each cluster holds up to 64 individual spores) were counted using phase-contrast microscopy with 400 × magnification (Fig. 1). Deaths occurring before day 12 were omitted (n = 6) from the analysis as infections could not be accurately diagnosed prior to this period. Any misidentified male Daphnia (n = 4) and inconclusive infections (n = 12) were also omitted.
Data analysis
Analysis was performed using R version 4.0.3 [86]. Infection prevalence (i.e., presence or absence of infection) and burden (i.e., the number of clusters of Ordospora spores) were the response variables, while the explanatory variables were baseline temperature, amplitude, and duration. Infection prevalence data included all exposed individuals, while burden data included confirmed infections only. Baseline temperature was a continuous centered variable to help reduce issues present with collinearity. A polynomial (quadratic for infection prevalence and cubic for burden) was added to account for non-linearity in the response to temperature. Amplitude and duration were ordered factors with two levels each (“ − 3” and “ − 6” for amplitude and “3” and “6” for duration), both also included the constant treatments with the reference level “0,” this allowed for statistical testing on the two variables both independently and combined. Elastic net regression using the “glmnet” package [87] was used for variable selection and regression to model infection prevalence and burden; this regression fits a generalized linear model (GLM) via penalized maximum likelihood reducing the issues associated with collinearity [88] which were present in a regular “GLM” with a binomial (infection prevalence) or a negative binomial (burden) distribution. Thus, a “glmnet” can effectively handle multicollinearity by shrinking the coefficients, preventing overfitting, and providing more stable estimates. While a random effect for “bath” could have accounted for batch-specific variability, “glmnet” does not support random effects, as it focuses on regularization of fixed-effects models. Mixed-effects models could address this, but “glmnet” was chosen for its ability to handle multicollinearity and high-dimensional data. For infection prevalence data, a “cv.glmnet” function was used for cross-validation and to find the optimal lambda (lambda = 0.00533), the regression fit (alpha = 0.5) was chosen based on the lowest cross-validation error value, and a binomial family was used. For burden data, a “glmnet” function was used for modeling, and the regression fit (alpha = 0.5) was chosen based on the lowest AIC score (Akaike information criterion score) to find a good balance between model complexity and data fit. The strength of regularization in the model (lambda = 0.0711) was selected using the lowest AIC score and post-model assessments, and the family used was negative binomial (theta = 1.2208) to account for overdispersion. Bootstrapping with 25,000 iterations was used for both infection prevalence and burden to assess the model stability and significance, resulting in the estimated coefficients (effect), and their 95% confidence intervals. Finally, feature importance was calculated for each factor by finding the absolute value of each coefficient to indicate which features are the most important for predicting the outcome (response variable) based on the elastic net regression model. This was carried out individually per polynomial degree and then added together as a whole per variable. Additionally, custom contrasts were created using the “emmeans” package [89] to compare the average infection prevalence/burden of treatments. For this, amplitude and duration were combined into one factor and modeled with temperature to avoid issues with collinearity and ensure clarity in interpretation. The contrast p values were then adjusted for multiple comparisons using the “Benjamini-Hochberg” method [90]. Analysis code and supporting data sets are available in an online repository [91].
Supplementary Information
Additional file 1: Tables S1–S8. Table S1. Coefficient estimates and feature importance of infection prevalence. Table S2. Estimated marginal means (emmean) of infection prevalence. Table S3. Custom contrasts of infection prevalence “emmeans” between treatments at different baseline temperatures. Table S4. Combined feature importance for infection prevalence with importance. Table S5. Coefficient estimates and feature importance of burden. Table S6. Estimated marginal means (emmean) of burden. Table S7. Custom contrasts of burden “emmeans” between treatments at different baseline temperatures. Table S8. Combined feature importance for burden with importance.
Acknowledgements
The authors thank Dieter Ebert and Jürgen Hottinger for provision of the biological materials; Alison Boyce for technical assistance in creating the water baths and maintenance of electrical equipment; Floriane O’Keeffe, Peter Mc Cartan, and Sean Hoare for helping with empirical work; and Whitney Parker for helping with empirical work and revisions.
Abbreviations
- ADaM
Artificial Daphnia Medium
- AIC
Akaike information criterion
- C
Cubic
- CI
Confidence interval
- D. magna
Daphnia magna
- Eff
Coefficient effect
- emmean
Estimated marginal means
- GLM
Generalized Linear Model
- L
Linear
- O. colligata
Ordospora colligata
- Q
Quadratic
Authors’ contributions
NMcC and PL designed the experiment. NMcC conducted the experiment with assistance of PL and SDiC. NMcC conducted the analysis with the aid of PL and JP. NMcC generated figures. NMcC and PL wrote the first draft of the manuscript and all authors contributed to revisions. All authors read and approved the final manuscript.
Authors’ Twitter handles
Twitter handles: @niamhasaurus (Niamh McCartan); @jaypiggott (Jeremy Piggott); @SickWaterFlea (Pepijn Luijckx).
Funding
Pepijn Luijckx and Niamh McCartan were funded by a Science Foundation Ireland Frontiers for the Future grant 19/FFP/6839. Jeremy Piggott was funded by Irish Research Council Laureate Award IRCLA/2017/112.
Data availability
The datasets generated and code used to analyze during the current study are available in the GitHub repository: https://github.com/niamhmccartan/cold_snap_ten (copy archived at https://zenodo.org/records/13254101). McCartan N., Piggott J., DiCarlo S., and Luijckx P. Cold snaps lead to a fivefold increase or a threefold decrease in disease proliferation depending on the baseline temperature. Zenodo repository https://doi.org/10.5281/zenodo.13254101 (2024).
Declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to declare.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Doney SC, Ruckelshaus M, Emmett Duffy J, Barry JP, Chan F, English CA, et al. Climate change impacts on marine ecosystems. Ann Rev Mar Sci. 2012;4:11–37. [DOI] [PubMed] [Google Scholar]
- 2.Gomez-Zavaglia A, Mejuto JC, Simal-Gandara J. Mitigation of emerging implications of climate change on food production systems. Food Res Int. 2020;134:109256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.IPCC. Summary for policymakers. In: Climate change 2022: impacts, adaptation, and vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK and New York, NY, USA: Cambridge University Press; 2022. [Google Scholar]
- 4.Bellard C, Bertelsmeier C, Leadley P, Thuiller W, Courchamp F. Impacts of climate change on the future of biodiversity. Ecol Lett. 2012;15(4):365–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altizer S, Ostfeld RS, Johnson PT, Kutz S, Harvell CD. Climate change and infectious diseases: from evidence to a predictive framework. Science. 2013;341(6145):514–9. [DOI] [PubMed] [Google Scholar]
- 6.Bouchard C, Dibernardo A, Koffi J, Wood H, Leighton PA, Lindsay LR. N Increased risk of tick-borne diseases with climate and environmental changes. Can Commun Dis Rep. 2019;45(4):83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirk D, Luijckx P, Jones N, Krichel L, Pencer C, Molnár P, et al. Experimental evidence of warming-induced disease emergence and its prediction by a trait-based mechanistic model. Proc Biol Sci. 2020;287(1936):20201526-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirk D, Jones N, Peacock S, Phillips J, Molnár PK, Krkošek M, et al. Empirical evidence that metabolic theory describes the temperature dependency of within-host parasite dynamics. PLoS Biol. 2018;16(2): e2004608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hector TE, Sgrò CM, Hall MD. Temperature and pathogen exposure act independently to drive host phenotypic trajectories. Biol Lett. 2021;17(6):20210072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ewing DA, Cobbold CA, Purse BV, Nunn MA, White SM. Modelling the effect of temperature on the seasonal population dynamics of temperate mosquitoes. J Theor Biol. 2016;400:65–79. [DOI] [PubMed] [Google Scholar]
- 11.Lusekelo E, Helikumi M, Kuznetsov D, Mushayabasa S. Modeling the effects of temperature and heterogeneous biting exposure on chikungunya virus disease dynamics. Informatics Med Unlocked. 2022;32: 101007. [Google Scholar]
- 12.Shi P, Dong Y, Yan H, Zhao C, Li X, Liu W, et al. Impact of temperature on the dynamics of the COVID-19 outbreak in China. Sci Total Environ. 2020;728: 138890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shocket MS, Strauss AT, Hite JL, Šljivar M, Civitello DJ, Duffy MA, et al. Temperature drives epidemics in a zooplankton-fungus disease system: a trait-driven approach points to transmission via host foraging. Am Nat. 2018;191(4):435–51. [DOI] [PubMed] [Google Scholar]
- 14.McKee D, Ebert D. The interactive effects of temperature, food level and maternal phenotype on offspring size in Daphnia magna. Oecologia. 1996;107(2):189–96. [DOI] [PubMed] [Google Scholar]
- 15.Seppälä O, Jokela J. Immune defence under extreme ambient temperature. Biol Lett. 2011;7(1):119–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mignatti A, Boag B, Cattadori IM. Host immunity shapes the impact of climate changes on the dynamics of parasite infections. Proc Natl Acad Sci. 2016;113(11):2970–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manzi F, Agha R, Lu Y, Ben-Ami F, Wolinska J. Temperature and host diet jointly influence the outcome of infection in a Daphnia-fungal parasite system. Freshwater Biol. 2020;65(4):757–67. [Google Scholar]
- 18.Blanford S, Thomas MB, Pugh C, Pell JK. Temperature checks the Red Queen? Resistance and virulence in a fluctuating environment. Ecol Lett. 2003;6(1):2–5. [Google Scholar]
- 19.Ebert D, Duneau D, Hall MD, Luijckx P, Andras JP, Du Pasquier L, et al. Chapter five - a population biology perspective on the stepwise infection process of the bacterial pathogen Pasteuria ramosa in Daphnia. In: Rollinson D, Stothard JR, editors. Adv Parasitol. 2016;91:265–310 Academic Press. [DOI] [PubMed] [Google Scholar]
- 20.Lõhmus M, Björklund M. Climate change: what will it do to fish–parasite interactions? Biol J Linn Soc. 2015;116(2):397–411. [Google Scholar]
- 21.Marcogliese D. The impact of climate change on the parasites and infectious diseases of aquatic animals. Rev Sci Tech. 2008;27(2):467–84. [PubMed] [Google Scholar]
- 22.Claar DC, Wood CL. Pulse heat stress and parasitism in a warming world. Trends Ecol Evol. 2020;35(8):704–15. [DOI] [PubMed] [Google Scholar]
- 23.El-Sayed A, Kamel M. Climatic changes and their role in emergence and re-emergence of diseases. Environ Sci Pollut Res. 2020;27:22336–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fouque F, Reeder JC. Impact of past and on-going changes on climate and weather on vector-borne diseases transmission: a look at the evidence. Infect Dis Poverty. 2019;8(03):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurane I. The effect of global warming on infectious diseases. Osong Public Health Res Perspect. 2010;1(1):4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rohr JR, Raffel TR, Blaustein AR, Johnson PT, Paull SH, Young S. Using physiology to understand climate-driven changes in disease and their implications for conservation. Conserv Physiol. 2013;1(1):cot022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raffel TR, Romansic JM, Halstead NT, McMahon TA, Venesky MD, Rohr JR. Disease and thermal acclimation in a more variable and unpredictable climate. Nat Clim Chang. 2013;3(2):146–51. [Google Scholar]
- 28.Rohr JR, Cohen JM. Understanding how temperature shifts could impact infectious disease. PLoS Biol. 2020;18(11): e3000938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rohr JR, Raffel TR. Linking global climate and temperature variability to widespread amphibian declines putatively caused by disease. Proc Natl Acad Sci. 2010;107(18):8269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krichel L, Kirk D, Pencer C, Hönig M, Wadhawan K, Krkošek M. Short-term temperature fluctuations increase disease in a Daphnia-parasite infectious disease system. PLoS Biol. 2023;21(9):e3002260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raffel TR, Halstead NT, McMahon TA, Davis AK, Rohr JR. Temperature variability and moisture synergistically interact to exacerbate an epizootic disease. Proc Biol Sci. 2015;282(1801):20142039. [DOI] [PMC free article] [PubMed]
- 32.Leahy P, Gonzalez LH, Hickey K, Kiely G, Allen M, Nowbakht P, et al. ClimAtt: tools for climate change attribution of extreme weather events. (Report No. 384). Dublin: EPA; 2021. p. 43. Available from: https://www.epa.ie/publications/research/climate-change/Research_Report_384.pdf.
- 33.Perkins-Kirkpatrick SE, Lewis SC. Increasing trends in regional heatwaves. Nat Commun. 2020;11(1):3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kug J-S, Jeong J-H, Jang Y-S, Kim B-M, Folland CK, Min S-K, et al. Two distinct influences of Arctic warming on cold winters over North America and East Asia. Nat Geosci. 2015;8(10):759–62. [Google Scholar]
- 35.Mirsaeidi M, Motahari H, Khamesi MT, Sharifi A, Campos M, Schraufnagel DE. Climate change and respiratory infection. Ann Am Thorac Soc. 2016;13(8):1223–30. [DOI] [PubMed] [Google Scholar]
- 36.Cann KF, Thomas DR, Salmon RL, Wyn-Jones AP, Kay D. Extreme water-related weather events and waterborne disease. Epidemiol Infect. 2013;141(4):671–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Filho WL, Scheday S, Boenecke J, Gogoi A, Maharaj A, Korovou S. Climate change, health and mosquito-borne diseases: trends and implications to the Pacific Region. Int J Environ Res Public Health. 2019;16(24): 5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dittmar J, Janssen H, Kuske A, Kurtz J, Scharsack JP. Heat and immunity: an experimental heat wave alters immune functions in three-spined sticklebacks ( Gasterosteus aculeatus). J Anim Ecol. 2014;83(4):744–57. [DOI] [PubMed] [Google Scholar]
- 39.Moreno J, Møller AP. Extreme climatic events in relation to global change and their impact on life histories. Curr Zool. 2011;57(3):375–89. [Google Scholar]
- 40.Frederiksen M, Daunt F, Harris MP, Wanless S. The demographic impact of extreme events: stochastic weather drives survival and population dynamics in a long-lived seabird. J Anim Ecol. 2008;77(5):1020–9. [DOI] [PubMed] [Google Scholar]
- 41.van de Pol M, Jenouvrier S, Cornelissen JHC, Visser ME. Behavioural, ecological and evolutionary responses to extreme climatic events: challenges and directions. Philos Trans R Soc Lond B Biol Sci. 2017;372(1723):20160134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kunze C, Luijckx P, Jackson AL, Donohue I. Alternate patterns of temperature variation bring about very different disease outcomes at different mean temperatures. eLife. 2022;11:e72861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paull SH, LaFonte BE, Johnson PTJ. Temperature-driven shifts in a host-parasite interaction drive nonlinear changes in disease risk. Glob Change Biol. 2012;18(12):3558–67. [Google Scholar]
- 44.Novak M. Environmental temperature and the growth of Taenia crassiceps cysticerci in mice. Experientia. 1978;34(9):1149. [DOI] [PubMed] [Google Scholar]
- 45.Williams CM, Henry HAL, Sinclair BJ. Cold truths: how winter drives responses of terrestrial organisms to climate change. Biol Rev. 2015;90(1):214–35. [DOI] [PubMed] [Google Scholar]
- 46.Boucek RE, Gaiser EE, Liu H, Rehage JS. A review of subtropical community resistance and resilience to extreme cold spells. Ecosphere. 2016;7(10): e01455. [Google Scholar]
- 47.Schlegel RW, Darmaraki S, Benthuysen JA, Filbee-Dexter K, Oliver EC. Marine cold-spells. Prog Oceanogr. 2021;198:102684. [Google Scholar]
- 48.Sun Q, Sun Z, Chen C, Yan M, Zhong Y, Huang Z, et al. Health risks and economic losses from cold spells in China. Sci Total Environ. 2022;821: 153478. [DOI] [PubMed] [Google Scholar]
- 49.Morley NJ, Lewis JW. Temperature stress and parasitism of endothermic hosts under climate change. Trends Parasitol. 2014;30(5):221–7. [DOI] [PubMed] [Google Scholar]
- 50.Wade S, Sanderson M, Golding N, Lowe J, Betts R, Reynard N, et al. Developing H++ climate change scenarios for heat waves, droughts, floods, windstorms and cold snaps. UK: Met Office, University of Reading, CEH 2015; 2015. [Google Scholar]
- 51.D’Errico M, Pons F, Yiou P, Thao S, Nardini C, Lunkeit F, et al. Present and future synoptic circulation patterns associated with cold and snowy spells over Italy. Earth Syst Dynam. 2022;13(2):961–92. [Google Scholar]
- 52.Ebert D. Daphnia as a versatile model system in ecology and evolution. EvoDevo. 2022;13(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen JM, Venesky MD, Sauer EL, Civitello DJ, McMahon TA, Roznik EA, et al. The thermal mismatch hypothesis explains host susceptibility to an emerging infectious disease. Ecol Lett. 2017;20(2):184–93. [DOI] [PubMed] [Google Scholar]
- 54.Paull SH, Raffel TR, LaFonte BE, Johnson PTJ. How temperature shifts affect parasite production: testing the roles of thermal stress and acclimation. Funct Ecol. 2015;29(7):941–50. [Google Scholar]
- 55.Paaijmans KP, Blanford S, Bell AS, Blanford JI, Read AF, Thomas MB. Influence of climate on malaria transmission depends on daily temperature variation. Proc Natl Acad Sci. 2010;107(34):15135–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kristan DM, Hammond KA. Combined effects of cold exposure and sub-lethal intestinal parasites on host morphology and physiology. J Exp Biol. 2000;203(22):3495–504. [DOI] [PubMed] [Google Scholar]
- 57.van der Lans AA, Boon MR, Haks MC, Quinten E, Schaart G, Ottenhoff TH, et al. Cold acclimation affects immune composition in skeletal muscle of healthy lean subjects. Physiol Rep. 2015;3(7). [DOI] [PMC free article] [PubMed]
- 58.Huang D, Taha MS, Nocera AL, Workman AD, Amiji MM, Bleier BS. Cold exposure impairs extracellular vesicle swarm–mediated nasal antiviral immunity. J Allergy Clin Immunol. 2023;151(2):509–25.e8. [DOI] [PubMed] [Google Scholar]
- 59.Hance T, van Baaren J, Vernon P, Boivin G. Impact of extreme temperatures on parasitoids in a climate change perspective. Annu Rev Entomol. 2007;52:107–26. [DOI] [PubMed] [Google Scholar]
- 60.Thomas SM, Obermayr U, Fischer D, Kreyling J, Beierkuhnlein C. Low-temperature threshold for egg survival of a post-diapause and non-diapause European aedine strain, Aedes albopictus (Diptera: Culicidae). Parasit Vectors. 2012;5(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Y-B, Yang A-P, Zhang G-F, Liu W-X, Wan F-H. Effects of simulated heat waves on life history traits of a host feeding parasitoid. Insects. 2019;10(12):419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schreven SJJ, Frago E, Stens A, de Jong PW, van Loon JJA. Contrasting effects of heat pulses on different trophic levels, an experiment with a herbivore-parasitoid model system. PLoS ONE. 2017;12(4):e0176704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beck-Johnson LM, Nelson WA, Paaijmans KP, Read AF, Thomas MB, Bjørnstad ON. The importance of temperature fluctuations in understanding mosquito population dynamics and malaria risk. R Soc Open Sci. 2017;4(3):160969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Milazzo A, Giles LC, Zhang Y, Koehler AP, Hiller JE, Bi P. Heatwaves differentially affect risk of Salmonella serotypes. J Infect. 2016;73(3):231–40. [DOI] [PubMed] [Google Scholar]
- 65.Acquaotta F, Ardissino G, Fratianni S, Perrone M. Role of climate in the spread of shiga toxin-producing Escherichia coli infection among children. Int J Biometeorol. 2017;61(9):1647–55. [DOI] [PubMed] [Google Scholar]
- 66.Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. Toward a metabolic theory of ecology. Ecology. 2004;85(7):1771–89. [Google Scholar]
- 67.Kutz SJ, Hoberg EP, Molnár PK, Dobson A, Verocai GG. A walk on the tundra: host–parasite interactions in an extreme environment. Int J Parasitol Parasites Wildl. 2014;3(2):198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Duncan AB, Gonzalez A, Kaltz O. Stochastic environmental fluctuations drive epidemiology in experimental host-parasite metapopulations. Proc R Soc B Biol Sci. 2013;280(1769):20131747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wharton DA. Parasites and low temperatures. Parasitol. 1999;119(S1):S7–17. [DOI] [PubMed] [Google Scholar]
- 70.Fernández-Beaskoetxea S, Carrascal LM, Fernández-Loras A, Fisher MC, Bosch J. Short term minimum water temperatures determine levels of infection by the amphibian chytrid fungus in Alytes obstetricans tadpoles. PLoS ONE. 2015;10(3):e0120237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kirk D, Luijckx P, Stanic A, Krkošek M. Predicting the thermal and allometric dependencies of disease transmission via the metabolic theory of ecology. Am Nat. 2019;193(5):661–76. [DOI] [PubMed] [Google Scholar]
- 72.Decaestecker E, Verreydt D, De Meester L, Declerck SAJ. Parasite and nutrient enrichment effects on Daphnia interspecific competition. Ecology. 2015;96(5):1421–30. [DOI] [PubMed] [Google Scholar]
- 73.Duffy MA. Selective predation, parasitism, and trophic cascades in a bluegill–Daphnia–parasite system. Oecologia. 2007;153(2):453–60. [DOI] [PubMed] [Google Scholar]
- 74.Capaul M, Ebert D. Parasite-mediated selection in experimental Daphnia magna populations. Evolution. 2003;57(2):249–60. [DOI] [PubMed] [Google Scholar]
- 75.Miner BE, De Meester L, Pfrender ME, Lampert W, Hairston NG. Linking genes to communities and ecosystems: Daphnia as an ecogenomic model. Proc R Soc B Biol Sci. 2012;279(1735):1873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Menge BA, Cerny-Chipman EB, Johnson A, Sullivan J, Gravem S, Chan F. Sea star wasting disease in the keystone predator Pisaster ochraceus in Oregon: insights into differential population impacts, recovery, predation rate, and temperature effects from long-term research. PLoS ONE. 2016;11(5):e0153994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hector TE, Gehman ALM, King KC. Infection burdens and virulence under heat stress: ecological and evolutionary considerations. Philos Trans R Soc B Biol Sci. 1873;2023(378):20220018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rodó X, Pascual M, Doblas-Reyes FJ, Gershunov A, Stone DA, Giorgi F, et al. Climate change and infectious diseases: can we meet the needs for better prediction? Clim Change. 2013;118(3):625–40. [Google Scholar]
- 79.Costas AV, Yuri AM. Future temperature extremes will be more harmful: a new critical factor for improved forecasts. Int J Environ Res Public Health. 2019;16(20):4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ebert D. Ecology, epidemiology, and evolution of parasitism in Daphnia. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information: National Library of Medicine; 2005. ISBN 1932811060.
- 81.Sarnelle O. Daphnia as keystone predators: effects on phytoplankton diversity and grazing resistance. J Plankton Res. 2005;27(12):1229–38. [Google Scholar]
- 82.Pombert JF, Haag KL, Beidas S, Ebert D, Keeling PJ, Boothroyd JC. The Ordospora colligata genome: evolution of extreme reduction in microsporidia and host-to-parasite horizontal gene transfer. mBio. 2015;6(1):e02400–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jocque M, Vanschoenwinkel B, Brendonck L. Freshwater rock pools: a review of habitat characteristics, faunal diversity and conservation value. Freshwater Biol. 2010;55(8):1587–602. [Google Scholar]
- 84.Klüttgen B, Dülmer U, Engels M, Ratte HT. ADaM, an artificial freshwater for the culture of zooplankton. Water Res. 1994;28(3):743–6. [Google Scholar]
- 85.Kilham SS, Kreeger DA, Lynn SG, Goulden CE, Herrera L. COMBO: a defined freshwater culture medium for algae and zooplankton. Hydrobiologia. 1998;377(1):147–59. [Google Scholar]
- 86.RStudio Team. RStudio: integrated development environment for R. Boston, MA: RStudio, PBC; 2022. [Google Scholar]
- 87.Friedman JH, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- 88.Zou H, Hastie T. Regularization and variable selection via the elastic net. J R Stat Soc Series B Stat Methodol. 2005;67(2):301–20. [Google Scholar]
- 89.Russell V. Lenth. emmeans: estimated marginal means, aka least-squares means 2022. Available from: https://CRAN.R-project.org/package=emmeans.
- 90.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Methodol. 1995;57(1):289–300. [Google Scholar]
- 91.McCartan N, Piggott J, DiCarlo S, Luijckx P. Cold snaps lead to a 5-fold increase or a 3-fold decrease in disease proliferation depending on the baseline temperature. Zenodo. 2024. 10.5281/zenodo.13254101. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Tables S1–S8. Table S1. Coefficient estimates and feature importance of infection prevalence. Table S2. Estimated marginal means (emmean) of infection prevalence. Table S3. Custom contrasts of infection prevalence “emmeans” between treatments at different baseline temperatures. Table S4. Combined feature importance for infection prevalence with importance. Table S5. Coefficient estimates and feature importance of burden. Table S6. Estimated marginal means (emmean) of burden. Table S7. Custom contrasts of burden “emmeans” between treatments at different baseline temperatures. Table S8. Combined feature importance for burden with importance.
Data Availability Statement
The datasets generated and code used to analyze during the current study are available in the GitHub repository: https://github.com/niamhmccartan/cold_snap_ten (copy archived at https://zenodo.org/records/13254101). McCartan N., Piggott J., DiCarlo S., and Luijckx P. Cold snaps lead to a fivefold increase or a threefold decrease in disease proliferation depending on the baseline temperature. Zenodo repository https://doi.org/10.5281/zenodo.13254101 (2024).