Abstract
Background
Device-related thrombosis (DRT) occurs in up to 4% of patients undergoing left atrial appendage occlusion (LAAO) and is associated with substantial morbidity and mortality. However, its pathophysiology, predictors, and optimal management remain unclear.
Objectives
This study aims to assess flow dynamic factors correlating to DRT.
Methods
A multicenter registry of patients who underwent LAAO and had pre- and post-computed tomography imaging was used. Patient-specific 3-dimensional digital models of the left atrium were created, and finite element simulations were performed to implant an LAAO device into each model in a position that matched the clinical deployment. Computational fluid dynamic simulations were performed to quantify the following flow dynamic parameters: time averaged wall shear stress, oscillatory shear index, and endothelial cell activation potential.
Results
A total of 38 patients (19 with DRT and 19 without DRT) were included. Left atrium volumes and mitral valve areas were larger in the DRT cohort compared with controls. Patients with DRT had a significantly lower time averaged wall shear stress (1.76 ± 1.24 Pa vs 2.90 ± 2.70 Pa), a higher oscillatory shear index (0.19 ± 0.11 vs 0.17 ± 0.11), and a higher endothelial cell activation potential (0.23 ± 0.58 Pa–1 vs 0.17 ± 0.30 Pa−1) than the controls (P < 0.001 for all). Thrombus locations identified from in-vivo images correlated well with the flow dynamic parameters tested.
Conclusions
Flow dynamic parameters may be able to predict the risk of DRT after LAAO. Further investigation with a larger patient cohort and long-term follow-up is needed to assess the role of computational fluid dynamics in the risk stratification of patients considered for LAAO.
Key words: device-related thrombosis, DRT, LAAO, left atrial appendage occlusion, thrombosis
Central Illustration
Atrial fibrillation is the most common arrhythmia affecting over 10 million individuals in the United States alone.1 Stroke prevention remains a major concern in the management of atrial fibrillation.2,3 While anticoagulants are effective in mitigating strokes, bleeding risk, noncompliance, and other side effects preclude their consistent use in >50% of eligible patients.3,4 To address this unmet need, left atrial appendage (LAA) occlusion (LAAO) emerged as an alternative approach to prevent potential strokes.5 The efficacy of LAAO has been documented in several randomized trials and a large body of observational studies, leading to a growing adoption in clinical practice. A previous computational study by our group evaluated the efficacy of LAAO.6 However, certain unresolved issues with the therapy remain, including device related thrombosis (DRT).7, 8, 9, 10 DRT occurs in 3% to 5% of patients undergoing LAAO and is associated with a 4-fold increase in cardioembolic events.7, 8, 9, 10, 11, 12 Furthermore, the treatment of DRT is challenging with a high frequency of persistence, bleeding risk, and recurrence rates.13 Therefore, considerable efforts have been made to identify risk factors for DRT.14
Given the close relationship between thrombosis and flow stasis, there has been a growing interest in investigating flow dynamics associated with DRT using computational fluid dynamics (CFD) techniques.15, 16, 17 However, studies assessing CFD correlates of DRT have been hindered by their small size and lack of a control group.18, 19, 20 To bridge this gap, we combined patient-specific CFD analyses with finite element simulations in a larger sample of patients with and without DRT from multiple institutions.
This study aims to characterize the fluid dynamic environment around LAAO devices in attempt to assess the flow dynamic factors correlating to DRT.
Methods
Patient data
This retrospective study used a database of 38 patients who underwent LAAO procedure from Mayo Clinic (Rochester, Minnesota, USA), Aarhus University Hospital (Aarhus, Denmark), and Copenhagen University Hospital (Copenhagen, Denmark) of which 19 developed DRT and 19 did not (control). All DRTs were verified by 2 separate expert cardiologists. Patient characteristics are shown in Table 1. The patient data sets included preprocedural and postprocedural computed tomography (CT) scans and echocardiographic waveforms. This study was performed under an approved Institutional Review Board protocol and data usage agreement between Michigan Technological University, Mayo Clinic, Aarhus, and Copenhagen University Hospitals.
Table 1.
Patient Characteristics
| DRT (n = 19) | Control (n = 19) | P Value | |
|---|---|---|---|
| Age >75 (y) | 8 (42.1%) | 9 (47.4%) | 0.6550 |
| Male | 14 (73.7%) | 10 (52.6%) | 0.1902 |
| BMI | 29.2 ± 6.3 | 26 ± 5.2 | 0.0932 |
| CHA2DS2-VASc | 4.1 ± 1.2 | 4.4 ± 1.7 | 0.5902 |
| Has bled score | 3 ± 0.9 | 2.7 ± 1.3 | 0.3547 |
| AF type | 0.3253 | ||
| Paroxysmal | 8 (42.1%) | 12 (63.2%) | |
| Permanent | 10 (52.6%) | 5 (26.3%) | |
| Unspecified | 1 (5.3%) | 2 (10.5%) | |
| History | |||
| Heart failure | 5 (26.3%) | 4 (21.1%) | 0.7213 |
| Diabetes | 6 (31.6%) | 3 (15.8%) | 0.2671 |
| Ischemic stroke or TIA | 6 (31.6%) | 5 (26.3%) | 0.7381 |
| Other vascular disease | 7 (36.8%) | 4 (21.1%) | 0.2982 |
| Hypertension | 15 (78.9%) | 13 (68.4%) | 0.4790 |
| Abnormal liver function | 0 (0%) | 0 (0%) | 1.0000 |
| Abnormal renal function | 5 (26.3%) | 8 (42.1%) | 0.3200 |
| Ischemic heart disease | 9 (47.4%) | 8 (42.1%) | 0.7604 |
| PCI | 5 (26.3%) | 4 (21.1%) | 0.7213 |
| CABG | 4 (21.1%) | 1 (5.3%) | 0.1628 |
| LVEF (%) | 55.7 ± 5.1 | 54 ± 10.3 | 0.6774 |
| Valvular heart disease | 3 (15.8%) | 5 (26.3%) | 0.4445 |
| CRT/pacemaker/ICD | 2 (10.5%) | 3 (15.8%) | 0.6537 |
| Indications | |||
| Intracranial hemorrhage | 6 (31.6%) | 3 (15.8%) | 0.2671 |
| GI bleed | 5 (26.3%) | 5 (26.3%) | 1.0000 |
| Urinary tract bleeding | 0 (0%) | 0 (0%) | 1.0000 |
| Other spontaneous bleeding | 1 (5.3%) | 2 (10.5%) | 0.5739 |
| Stroke despite OAC | 5 (26.3%) | 5 (26.3%) | 1.0000 |
| Cerebral amyloid angiopathy | 0 (0%) | 2 (10.5%) | 0.1627 |
| Cognitive impairment | 3 (15.8%) | 0 (0%) | 0.0802 |
| Patient preference/lack of compliance/side effects | 7 (36.8%) | 5 (26.3%) | 0.5025 |
| Device size | 0.6530 | ||
| 20 mm | 1 (5.3%) | 0 (0%) | |
| 22 mm | 0 (0%) | 1 (5.3%) | |
| 24 mm | 0 (0%) | 2 (10.5%) | |
| 25 mm | 2 (10.5%) | 0 (0%) | |
| 27 mm | 10 (52.6%) | 7 (36.8%) | |
| 30 mm | 0 (0%) | 1 (5.3%) | |
| 31 mm | 4 (21.1%) | 7 (36.8%) | |
| 34 mm | 1 (5.3%) | 0 (0%) | |
| 35 mm | 1 (5.3%) | 1 (5.3%) | |
| Device type | |||
| WATCHMAN | 1 (5.3%) | 3 (15.8%) | |
| WATCHMAN FLX | 14 (73.7%) | 13 (68.4%) | |
| Amplatzer Amulet | 4 (21.1%) | 3 (15.8%) | |
| Discharge antithrombotics | |||
| Acetylsalicylic acid (ASA) therapy (aspirin) | 16 (84.2%) | 14 (73.7%) | 0.4445 |
| P2Y12-inhibitor (clopidogrel, ticagrelor, etc.) | 8 (42.1%) | 9 (47.4%) | 0.7604 |
| Vitamin K antagonist (VKA) (warfarin) | 2 (10.5%) | 3 (15.8%) | 0.6537 |
| DOAC (apixaban, rivaroxaban, edoxaban, dabigatran) | 4 (21.1%) | 4 (21.1%) | 1.0000 |
| Low-molecular-weight heparin (LMWH) | 0 (0%) | 0 (0%) | 1.0000 |
| No antithrombotic therapy | 0 (0%) | 0 (0%) | 1.0000 |
| Time from follow-up (LAAO to post-CT) | 112.5 ± 118 | 105.8 ± 108.4 | 0.8100 |
Values are n (%) or mean ± SD.
AF = atrial fibrillation; CABG = coronary artery bypass graft surgery; CRT = cardiac resynchronization therapy; CT = computed tomography; DOAC = direct oral anti-coagulation; DRT = device-related thrombosis; GI = gastrointestinal; ICD = implantable cardioverter-defibrillator; LAAO = left atrial appendage occlusion; LVEF = left ventricular ejection fraction; OAC = oral anti-coagulation; P2Y12 = purinergic receptor P2Y, G-protein coupled, 12 protein; PCI = percutaneous coronary intervention; TIA = transient ischemic attack.
Digital 3-Dimensional model development
The workflow adopted in this study is shown in Figure 1. CT images of patients with and without DRT after LAAO were imported into Mimics Research 23.0 (Materialise) for processing. A mask (using a threshold that isolates blood from soft tissue) was applied to the CT images and segmentation was performed to create a patient-specific 3-dimensional digital model of the left atrium (LA) and LAA. The mitral valve (MV) was segmented as a saddle shaped opening and the pulmonary veins were cut at their ostia. Finite-element computational simulations were performed by FEops NV to deploy an LAAO device into each model in a position that matched the postprocedural CT positioning for each patient (Supplemental Figure 1).21 The output of the finite element analysis simulations, that is, the patient-specific anatomy deformed after the virtual deployment of the LAA device and the device itself, were retained and used as input for the CFD simulations. The novelty of this technique is twofold: 1) an accurate model of the device as seen in postprocedural imaging is virtually deployed in the atrium models as opposed to merely creating a sealing surface at the LAA ostium,22 which accounts for the potential presence of peri-device leak paths, and 2) the walls at the region of the deployment are deformed as the device is expanded into position, accurately depicting real-world device deployment. Thrombi identified in in-vivo imaging were not included in the digital models, thus simulating the device-related flow environment immediately after deployment. Geometric measurements were recorded for LA volumes, MV areas, and thrombus volumes.
Figure 1.
Workflow Adopted for This Study, Utilizing a Sequence of Finite Element Analysis and Computational Fluid Dynamics
CT = computed tomography; ECAP = endothelial cell activation potential; OSI = oscillatory shear index; TAWSS = time averaged wall shear stress; TTE = transthoracic echocardiogram.
Computational fluid dynamics
The digital models (after virtual device deployment) were imported into Ansys Workbench 2020 R1 for the CFD simulations. Prior to that, extensions equivalent to ten times the atrial inlets and outlet diameters were created to overcome entrance effects and to have an appropriate velocity profile.23 An element size of 0.8 mm was selected (∼14,000,000 elements) based on a grid independence study; in addition, the initial tetrahedral mesh was converted to a polyhedral mesh before performing CFD simulations.24 Boundary conditions (BCs) necessary to run the computational simulations were extracted from echocardiographic data. Briefly, 0-gauge pressure inlet BCs were applied at the pulmonary veins and a time-dependent velocity waveform from the MV (Vout), which matched the rhythm of each patient, was applied at the outlet (Figure 1). These BCs were in accordance with our previous work and similar published studies in literature.24,25 CFD simulations were performed using Ansys Fluent and based on the finite volume approach to discretize the Navier-Stokes equations (Supplemental Appendix). Blood was simulated as an incompressible Newtonian fluid with a density ρ = 1,060 kg/m3 and a dynamic viscosity μ = 0.0035 Pa s. Because the Reynolds number, estimated from peak MV at the E-wave and the MV diameter, was <2000 in all cases, a laminar model was chosen. This was also in accordance with other studies in literature.24,25 Three cycles of a transient simulation were carried out to ensure stability and to overcome transitional effects.23,25 For data analysis, the final cycle was used for stability.
Flow dynamic parameters
Nonphysiological levels of wall shear stress (WSS) have been associated with platelet and endothelial cell activation resulting in thrombogenesis.26 In this study, we computed several flow dynamic parameters with WSS as a function. This includes time averaged wall shear stress (TAWSS), oscillatory shear index (OSI), and endothelial cell activation potential (ECAP). The derivations for each parameter are listed in the Supplemental Appendix.
TAWSS is the average WSS over the cardiac cycle. Averaging the change in WSS across the cardiac cycle may highlight regions of nonphysiological WSS levels, which might in turn, be associated with thrombogenesis.27 OSI describes the deflection (change in direction) of the WSS vectors from the main flow direction throughout a cardiac cycle.28 OSI has an interval of 0 to 0.5, where 0 and 0.5 indicate no deflection and a deflection of 180°, respectively. Regions of high OSI have been shown to induce an inflammatory response,27 promoting thrombogenesis as described within the triad of Virchow.29 ECAP consists of the ratio between OSI and TAWSS. It has been proposed as a useful flow dynamic parameter as it may be utilized for identifying vasculature with a higher possibility of thrombogenesis.30 A high ECAP indicates low TAWSS and high OSI, which have been shown to be associated with thrombogenesis.27
Statistics
Statistical analysis was performed using JMP Pro, version16.0.0 (SAS Institute Inc). All data are presented as mean ± SD. A t-test test was used to compare the means (if the distribution was normal) and the Wilcoxon test was used for non-normal data distributions. P < 0.05 was considered statistically significant. Statistical analysis was conducted on the value taken from the nodal elements of each patient’s device, resulting in tens of thousands of data points for both groups while verifying the normality assumption.
Results
Time averaged wall shear stress
The TAWSS contour plots are shown in Supplemental Figure 2; only the portion of the device exposed to the atrial flow is shown. To quantify the differences between patient populations, Table 2 shows a global account of the TAWSS values and Figure 2A shows a sample of contours. The mean TAWSS was found to be 1.76 ± 1.24 Pa for the DRT cohort and 2.90 ± 2.70 Pa for the control cohort with P < 0.0001.
Table 2.
Flow Dynamics Metrics and Geometric Measurements of Device-Related Thrombosis and Control Patients
| Group | Mean | Median | SD | IQR | P Value | |
|---|---|---|---|---|---|---|
| Flow dynamics metrics | ||||||
| TAWSS (Pa) | DRT | 1.76 | 1.43 | 1.24 | 1.42 | <0.0001 |
| CTL | 2.90 | 2.14 | 2.70 | 2.86 | ||
| OSI | DRT | 0.19 | 0.17 | 0.11 | 0.17 | <0.0001 |
| CTL | 0.17 | 0.15 | 0.11 | 0.17 | ||
| ECAP (Pa-1) | DRT | 0.23 | 0.12 | 0.58 | 0.20 | <0.0001 |
| CTL | 0.17 | 0.07 | 0.30 | 0.15 | ||
| Geometric measurements | ||||||
| Left atrium volume (mL) | DRT | 171.49 | 143.11 | 69.16 | 88.91 | <0.05 |
| CTL | 127.90 | 127.36 | 33.82 | 61.30 | ||
| Mitral valve area (mm2) | DRT | 1,340.64 | 1,309.88 | 401.37 | 573.25 | <0.05 |
| CTL | 1,061.89 | 963.94 | 380.07 | 491.83 | ||
| Thrombus volume (mL) | DRT | 1.44 | 0.53 | 2.06 | 1.72 |
CTL = control; DRT = device-related thrombosis; ECAP = endothelial cell activation potential; OSI = oscillatory shear index; TAWSS = time averaged wall shear stress.
Low TAWSS, high OSI, and high ECAP are associated with thrombosis.
Figure 2.
Sample of TAWSS, OSI, and ECAP Contours for the Top Surface of Each Device for the DRT and CTL Populations
Sample of time averaged wall shear stress (A), oscillatory shear index (B), and endothelial cell activation potential (C) contours for the top surface of each device for the device-related thrombosis, and control populations. Thrombus locations from in-vivo imaging are shown by the black line for each drt patient displayed. The line represents the border of the thrombus. DRT = device-related thrombosis; CTL = control; other abbreviations as in Figure 1.
Oscillatory shear index
The OSI contour plots are shown in Supplemental Figure 3; only the portion of the device exposed to the atrial flow is shown. To quantify the differences between patient populations, Table 2 shows a global account of the OSI values and Figure 2B shows a sample of contours. The mean OSI was found to be 0.19 ± 0.11 for the DRT cohort and 0.17 ± 0.11 for the control cohort with P < 0.0001.
Endothelial cell activation potential
The ECAP contour plots are shown in Supplemental Figure 4; only the portion of the device exposed to the atrial flow is shown. To quantify the differences between patient populations, Table 2 shows a global account of the ECAP values and Figure 2C shows a sample of contours. The mean ECAP was found to be 0.23 ± 0.58 Pa-1 for the DRT cohort and 0.17 ± 0.30 Pa-1 for the control cohort with P < 0.0001.
Thrombus location and volume from in-vivo imaging
The boundaries of the thrombi found in each DRT patient’s post-CT scan are overlayed for each flow dynamic parameter (Supplemental Figures 2 to 4). The thrombi were mainly observed in regions of low WSS, high OSI, and high ECAP. The total mean volume of the thrombi was 1.44 ± 2.06 mL (Table 2).
Geometric measurements
Measurements of the LA volume and MV area are shown in Table 2. Mean LA volume was larger among the DRT cohort compared with the controls (171.49 ± 69.16 mL and 127.90 ± 33.82 mL, respectively; P < 0.05). Also, the mean MV area was larger for the DRT cohort (1,340.64 ± 401.37 mm2 and 1,061.89 ± 380.07 mm2, respectively; P < 0.05).
Discussion
This study represents the most comprehensive assessment to identify flow dynamics patterns that correlate with DRT to date. Using a novel approach that combined patient-specific modeling with CFD and finite element simulations to obtain a more realistic patient-specific and device deformation, we documented a strong association between certain flow parameters and DRT. Accordingly, patients with clinically adjudicated DRT displayed lower TAWSS, higher OSI, and higher ECAP than controls (P < 0.001 for all), supporting existing pathophysiological concepts. This computational approach approximates a realistic model for assessing flow dynamics in relation to DRT and represents a step forward in the growing trends to incorporate computational simulations in the planning of LAAO procedures.
Endothelial cell dysfunction is a known predictor of thrombus formation.26,31,32 Numerous studies have explored WSS as a possible factor in endothelial dysfunction,26,31,33 particularly noting the association of low WSS levels with thrombogenesis.27,34 In line with these previous findings, our study shows that DRT patients had a lower TAWSS compared to the controls. A low TAWSS indicates a region of blood stasis, which can induce thrombosis due to the accumulation of procoagulant components.35 Furthermore, our study documented that DRT was mainly located within or close by to regions with low levels of TAWSS. These lower TAWSS values may be related to device deployment. Zhong et al18 have shown that a deep device deployment depth resulted in lower TAWSS whereas a device deployed at the LAA ostium was higher. Clinical studies have also shown a higher incidence of DRT with deeper device deployments.36,37 When the device is deployed deep into the LAA, a shallow “valley” is created above the device that serves as a nidus for blood stagnation and thrombus formation. The larger atrial volumes and MV areas observed in the DRT patients might lead to lower velocities, ultimately yielding an increased risk of flow stagnation and thrombus formation.
Another important flow dynamic parameter investigated was OSI. Oscillatory flow can promote an inflammatory state which can result in endothelial cell dysfunction, a potential nidus for thrombus formation.27,32 The presence of an LAAO device may influence the flow patterns within the atrium, potentially resulting in increased oscillatory shear. In the case of a deeper device deployment, a recirculation region develops on top of the device increasing the oscillatory shear around the device.38,39 In this study, the DRT cohort was observed to have higher OSI than the controls. In addition, there was a correlation between the location of the thrombus identified from in-vivo imaging and regions of high OSI. Although not seen in this study, residual (micro) peri-device leaks may also influence OSI.
ECAP has been shown in various studies to confer areas of susceptibility at the vessel walls.18,30,40,41 ECAP is defined as the ratio of OSI and TAWSS, where a high value indicates a high OSI and a low TAWSS, both of which have been shown to be related to thrombogenesis. Endothelial cells naturally provide an antithrombotic surface,32,42 but when activated, this property is negated, which can result in thrombus formation.32,35 In this study, we observed an elevated ECAP for the DRT cohort compared to the controls further confirming the potential role of CFD parameters in predicting DRT.
Our findings have important practical implications. Mitigating the occurrence of DRT remains a key challenge in LAAO. The first step to address this problem is to understand the possible causes and to identify potential predictors of DRT. Clinical studies attempted to identify predictors of DRT but studies identifying risk factors did not account for flow dynamics, despite DRT being pathophysiologically intimately related to flow patterns. Therefore, our study provides a comprehensive understanding and characterization of the flow environment around the LAAO device to see what the differences are between patients with DRT and without DRT. Our study provides evidence that certain flow parameters (lower TAWSS, higher OSI, and higher ECAP) are associated with DRT. This information may, in time: 1 help estimate the likelihood of DRT prior to LAAO, guiding shared decision-making and improving appropriate patient selection; and 2) assist the design of future research to assess whether a tailored device implant or positioning might further mitigate the risk of DRT in patient-specific anatomies.
The uniqueness of this work stems from combining the finite element with the CFD approach, while implementing the deformation of the atrium and the device after deployment. In addition, this work involves a unique data set of LAAO patients who developed DRT with full imaging from three different medical institutions.
Study Limitations
The cohort used included only 19 individuals in each group. A larger patient population may help to better identify the threshold for each parameter studied associated with DRT. In addition, the study did not employ fluid structure interaction analysis, which might improve reproducibility of the LA flow dynamics. Due to CT quality and limitations in the FE process, not all patients are matched by age and sex. Therefore, we have opted to show a global comparison between the two groups as opposed to a direct comparison of each patient.
Conclusions
In this comprehensive study of flow dynamics in patients undergoing LAAO, we documented a characteristic flow profile in patients with DRT (low TAWSS, high OSI, and high ECAP) as compared with controls. Our novel approach combining CFD assessment with finite element simulations may improve risk stratification among patients referred for LAAO (Central Illustration). Further research involving a larger patient cohort and long-term follow-up is necessary to understand the impact of the routine evaluation of flow dynamic parameters on risk stratification of LAAO candidates.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: In this analysis, we demonstrated through a combined finite element analysis and computational fluid dynamic approach—where the device and LAA deformation were replicated similar to in-vivo—that patients with DRT exhibit larger LA volumes and MV areas in addition to more elevated oscillatory shear and ECAP combined with lower shear stress. The location of the thrombi verified the hemodynamic findings.
TRANSLATIONAL OUTLOOK: Further research involving a larger patient cohort and long-term follow-up is necessary to understand the impact of the routine evaluation of flow dynamic parameters on risk stratification of LAAO candidates.
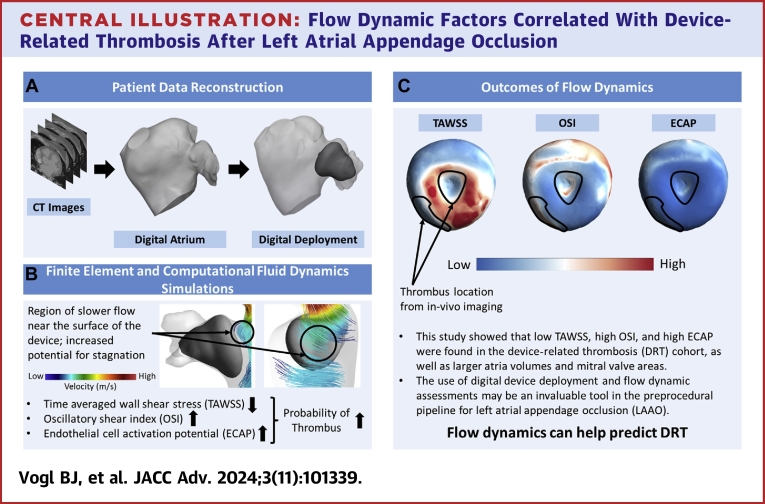
Central Illustration.
Flow Dynamic Factors Correlated With Device-Related Thrombosis After Left Atrial Appendage Occlusion
Patient-specific modeling (A) of the left atrium and left atrial appendage occlusion devices for use in a combined finite element and computational fluid dynamics simulations (B) to identify fluid dynamics predictors of device related thrombosis (DRT). (C) Patients with DRT had lower time averaged wall shear stress, high oscillatory shear index, and high endothelial cell activation potential, indicating an increased likelihood of thrombosis. ECAP = endothelial cell activation potential; OSI = oscillatory shear index; TAWSS = time averaged wall shear stress; abbreviations as in Figures 1 and 2.
Funding support and author disclosures
This research was partly supported by the American Heart Association (AHA) under award number 24CDA1269661. Dr De Backer has received consulting fees and institutional research grants from Abbott and Boston Scientific. Dr Nielsen-Kudsk has received institutional research grants from Abbott, Boston Scientific, and the Novo Nordic Foundation. Dr Bavo is an employee of FEops. Dr De Beule is a shareholder of FEops. Dr Vogl was partially supported by the Blue Cross Blue Shield of Michigan Foundation, the DeVlieg Foundation, and the Health Research Institute fellowship at Michigan Tech. Dr Jia has received a scholarship from China Scholarship Council under the State Scholarship Fund of China. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental information and figures, please see the online version of this paper.
Contributor Information
Mohamad Alkhouli, Email: Alkhouli.Mohamad@mayo.edu.
Hoda Hatoum, Email: hhatoum@mtu.edu.
Supplementary Data
References
- 1.Kornej J., Börschel C.S., Benjamin E.J., Schnabel R.B. Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ Res. 2020;127:4–20. doi: 10.1161/CIRCRESAHA.120.316340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reddy V.Y., Doshi S.K., Kar S., et al. 5-year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J Am Coll Cardiol. 2017;70:2964–2975. doi: 10.1016/j.jacc.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 3.Alkhouli M., Ellis C.R., Daniels M., Coylewright M., Nielsen-Kudsk J.E., Holmes D.R. Left atrial appendage occlusion: current advances and remaining challenges. JACC Adv. 2022 doi: 10.1016/j.jacadv.2022.100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmes D.R., Jr., Alkhouli M., Reddy V. Left atrial appendage occlusion for the unmet clinical needs of stroke prevention in nonvalvular atrial fibrillation. Mayo Clin Proc. 2019;94:864–874. doi: 10.1016/j.mayocp.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 5.Alkhouli M., Alqahtani F., Aljohani S., Alvi M., Holmes D.R. Burden of atrial fibrillation–associated ischemic stroke in the United States. JACC Clin Electrophysiol. 2018;4:618–625. doi: 10.1016/j.jacep.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 6.Bshennaty A., Vogl B.J., Bavo A.M., et al. Understanding the role of the left atrial appendage on the flow in the atrium. Catheter Cardiovasc Interv. 2024 doi: 10.1002/ccd.31153. [DOI] [PubMed] [Google Scholar]
- 7.Alkhouli M., Ellis C.R., Daniels M., Coylewright M., Nielsen-Kudsk J.E., Holmes D.R. Left atrial appendage occlusion. JACC Adv. 2022;1 doi: 10.1016/j.jacadv.2022.100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alkhouli M., Busu T., Shah K., Osman M., Alqahtani F., Raybuck B. Incidence and clinical impact of device-related thrombus following percutaneous left atrial appendage occlusion: a meta-analysis. JACC Clin Electrophysiol. 2018;4:1629–1637. doi: 10.1016/j.jacep.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Simard T.J., Hibbert B., Alkhouli M.A., Abraham N.S., Holmes D.R., Jr. Device-related thrombus following left atrial appendage occlusion. Eurointervention. 2022;18(3):224–232. doi: 10.4244/EIJ-D-21-01010. [DOI] [PubMed] [Google Scholar]
- 10.Holmes D.R., Jr., Korsholm K., Rodes-Cabau J., Saw J., Berti S., Alkhouli M.A. Left atrial appendage occlusion. EuroIntervention. 2023;18:e1038–e1065. doi: 10.4244/EIJ-D-22-00627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kramer A.D., Korsholm K., Jensen J.M., et al. Cardiac computed tomography following Watchman FLX implantation: device-related thrombus or device healing? Eur Heart J Cardiovasc Imaging. 2023;24:250–259. doi: 10.1093/ehjci/jeac222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sedaghat A., Nickenig G., Schrickel J.W., et al. Incidence, predictors and outcomes of device-related thrombus after left atrial appendage closure with the WATCHMAN device—insights from the EWOLUTION Real World Registry. Cathet Cardiovasc Interv. 2021;97:E1019–E1024. doi: 10.1002/ccd.29458. [DOI] [PubMed] [Google Scholar]
- 13.Mesnier J., Simard T., Jung R.G., et al. Persistent and recurrent device-related thrombus after left atrial appendage closure: incidence, predictors, and outcomes. JACC Cardiovasc Interv. 2023;16:2722–2732. doi: 10.1016/j.jcin.2023.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Alkhouli M., Alarouri H., Kramer A., et al. Device-related thrombus after left atrial appendage occlusion: clinical impact, predictors, classification, and management. JACC Cardiovasc Interv. 2023;16:2695–2707. doi: 10.1016/j.jcin.2023.10.046. [DOI] [PubMed] [Google Scholar]
- 15.Vogl B., El Shaer A., Ponce A.C., et al. TCT-380 predicting device-related thrombosis using computational fluid dynamics. J Am Coll Cardiol. 2022;80:B153. [Google Scholar]
- 16.Lowe G.D. Virchow’s triad revisited: abnormal flow. Pathophysiol Haemost Thromb. 2003;33:455–457. doi: 10.1159/000083845. [DOI] [PubMed] [Google Scholar]
- 17.Alkhouli M., Hatoum H., Piazza N. Computational modeling to guide structural Heart interventions: measure twice (or thrice) but cut once. JACC Cardiovasc Interv. 2023;16:667–669. doi: 10.1016/j.jcin.2023.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Zhong Z., Gao Y., Kovács S., et al. Impact of left atrial appendage occlusion device position on potential determinants of device-related thrombus: a patient-specific in silico study. Clin Res Cardiol. 2023;113(10):1405–1418. doi: 10.1007/s00392-023-02228-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aguado A.M., Olivares A.L., Yagüe C., et al. In silico optimization of left atrial appendage occluder implantation using interactive and modeling tools. Front Physiol. 2019;10 doi: 10.3389/fphys.2019.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mill J., Agudelo V., Olivares A.L., et al. Sensitivity analysis of in silico fluid simulations to predict thrombus formation after left atrial appendage occlusion. Mathematics. 2021;9:2304. [Google Scholar]
- 21.Bavo A.M., Wilkins B.T., Garot P., et al. Validation of a computational model aiming to optimize preprocedural planning in percutaneous left atrial appendage closure. J Cardiovasc Comput Tomogr. 2020;14:149–154. doi: 10.1016/j.jcct.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 22.D’Alessandro N., Falanga M., Masci A., Severi S., Corsi C. Preliminary findings on left atrial appendage occlusion simulations applying different endocardial devices. Front Cardiovasc Med. 2023;10 doi: 10.3389/fcvm.2023.1067964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trusty P.M., Wei Z.A., Fogel M.A., Maher K., Deshpande S.R., Yoganathan A.P. Computational Modeling of a right-sided Fontan assist device: effectiveness across patient anatomies and cannulations. J Biomech. 2020;109 doi: 10.1016/j.jbiomech.2020.109917. [DOI] [PubMed] [Google Scholar]
- 24.Vogl B.J., Shaer A.E., Van Zyl M., Killu A.M., Alkhouli M., Hatoum H. Effect of catheter ablation on the hemodynamics of the left atrium. J Intervent Card Electrophysiol. 2022;65:83–96. doi: 10.1007/s10840-022-01191-3. [DOI] [PubMed] [Google Scholar]
- 25.Bosi G.M., Cook A., Rai R., et al. Computational fluid dynamic analysis of the left atrial appendage to predict thrombosis risk. Front Cardiovasc Med. 2018;5:34. doi: 10.3389/fcvm.2018.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiu J.-J., Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91:327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hathcock J.J. Flow effects on coagulation and thrombosis. Arterioscler Thromb Vasc Biol. 2006;26:1729–1737. doi: 10.1161/01.ATV.0000229658.76797.30. [DOI] [PubMed] [Google Scholar]
- 28.Soulis J.V., Lampri O.P., Fytanidis D.K., Giannoglou G.D. 2011 10th International Workshop on Biomedical Engineering; 2011. Relative Residence Time and Oscillatory Shear Index of Non-Newtonian Flow Models in Aorta. [Google Scholar]
- 29.Esmon C.T. Inflammation and thrombosis. J Thromb Haemostasis. 2003;1:1343–1348. doi: 10.1046/j.1538-7836.2003.00261.x. [DOI] [PubMed] [Google Scholar]
- 30.Di Achille P., Tellides G., Figueroa C.A., Humphrey J.D. A haemodynamic predictor of intraluminal thrombus formation in abdominal aortic aneurysms. Proc R Soc A. 2014;470 [Google Scholar]
- 31.Chien S. Effects of disturbed flow on endothelial cells. Ann Biomed Eng. 2008;36:554–562. doi: 10.1007/s10439-007-9426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yau J.W., Teoh H., Verma S. Endothelial cell control of thrombosis. BMC Cardiovasc Disord. 2015;15:130. doi: 10.1186/s12872-015-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hyun S., Kleinstreuer C., Archie J.P., Jr. Hemodynamics analyses of arterial expansions with implications to thrombosis and restenosis. Med Eng Phys. 2000;22:13–27. doi: 10.1016/s1350-4533(00)00006-0. [DOI] [PubMed] [Google Scholar]
- 34.Buck A.K.W., Groszek J.J., Colvin D.C., et al. Combined in silico and in vitro approach predicts low wall shear stress regions in a hemofilter that correlate with thrombus formation in vivo. ASAIO J. 2018;64:211–217. doi: 10.1097/MAT.0000000000000649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mackman N. New insights into the mechanisms of venous thrombosis. J Clin Invest. 2012;122:2331–2336. doi: 10.1172/JCI60229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cepas-Guillén P., Flores-Umanzor E., Leduc N., et al. Impact of device implant depth after left atrial appendage occlusion. JACC Cardiovasc Interv. 2023;16:2139–2149. doi: 10.1016/j.jcin.2023.05.045. [DOI] [PubMed] [Google Scholar]
- 37.Sedaghat A., Vij V., Al-Kassou B., et al. Device-related thrombus after left atrial appendage closure: data on thrombus characteristics, treatment strategies, and clinical outcomes from the EUROC-DRT-registry. Circ Cardiovasc Interv. 2021;14 doi: 10.1161/CIRCINTERVENTIONS.120.010195. [DOI] [PubMed] [Google Scholar]
- 38.DiCarlo A.L., Holdsworth D.W., Poepping T.L. Study of the effect of stenosis severity and non-Newtonian viscosity on multidirectional wall shear stress and flow disturbances in the carotid artery using particle image velocimetry. Med Eng Phys. 2019;65:8–23. doi: 10.1016/j.medengphy.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 39.Compagne K.C.J., Dilba K., Postema E.J., et al. Flow patterns in carotid webs: a patient-based computational fluid dynamics study. AJNR Am J Neuroradiol. 2019;40:703–708. doi: 10.3174/ajnr.A6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.García-Isla G., Olivares A.L., Silva E., et al. Sensitivity analysis of geometrical parameters to study haemodynamics and thrombus formation in the left atrial appendage. Int J Numer Method Biomed Eng. 2018;34 doi: 10.1002/cnm.3100. [DOI] [PubMed] [Google Scholar]
- 41.Paliwal N., Ali R.L., Salvador M., et al. Presence of left atrial fibrosis may contribute to aberrant hemodynamics and increased risk of stroke in atrial fibrillation patients. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.657452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neubauer K., Zieger B. Endothelial cells and coagulation. Cell Tissue Res. 2022;387:391–398. doi: 10.1007/s00441-021-03471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.