Summary
We proposed a personalized intervention that integrates computerized working memory (WM) training with real-time functional neuromonitoring and neurofeedback (NFB) to enhance frontoparietal activity and improve cognitive and clinical outcomes in children with attention-deficit/hyperactivity disorder (ADHD). The study involved 77 children with ADHD aged 7–11 years, who were assigned to either 12 sessions of NFB or treatment-as-usual (i.e., received standard clinical care) groups. Real-time neuromonitoring with functional near-infrared spectroscopy (fNIRS) and fMRI measured frontoparietal activity during n-back task at baseline and post-intervention. Thirty-six participants (21 NFB, 15 treatment-as-usual) completed the study. Significant improvements in NFB group were observed in frontoparietal brain activity and WM performance (primary outcomes). NFB group also showed improvements in Behavior Rating Inventory of Executive Function (BRIEF-2) WM t-scores and Conners 3 ADHD index scores (secondary outcomes) compared to treatment-as-usual group. These findings suggest that neuromonitoring-guided NFB effectively enhances cognitive and clinical outcomes in children with ADHD by targeting brain mechanisms underlying WM deficits.
Subject areas: Neuroscience, Clinical neuroscience, Cognitive neuroscience
Graphical abstract

Highlights
-
•
Integrating WM training and neuromonitoring for targeted enhancement of WM network
-
•
Targeting WM brain networks enhances neurocognitive outcomes in children with ADHD
-
•
Our intervention improved prefrontal activity, WM performance, and ADHD symptoms
Neuroscience; Clinical neuroscience; Cognitive neuroscience
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental condition that affects approximately 5%–7% of children globally.1 ADHD encompasses a broader spectrum of cognitive, behavioral, and emotional challenges. These include difficulties with working memory (WM), attention regulation, impulse control, emotional regulation, and executive functioning, which collectively impact learning, social interactions, and daily functioning.2,3 Particularly, WM deficits are a core underlying feature of ADHD, significantly relating to brain function.4 WM relies primarily on sustained active maintenance within the prefrontal cortex (PFC), which interacts with parietal regions in a task-dependent manner. Neuroimaging studies have shown reduced brain engagement, particularly in the PFC and parietal regions, during tasks involving WM in children with ADHD.5,6 WM deficits directly affect children’s abilities in learning, language, math, and social interactions and can have profound implications for behavioral, educational, social, and occupational performance1,5; highlighting the need for early interventions targeting WM to improve patient outcomes and enhance academic and occupational success.
The neural correlates of ADHD have been extensively studied using functional near-infrared spectroscopy (fNIRS) and fMRI.5,7,8 fNIRS offers child-friendly, non-invasive neuroimaging with high temporal resolution, ideal for studying children’s brain activity during cognitive tasks in ecologically valid settings.9 The unique characteristics of fNIRS, including its cost-effectiveness and ecological validity, position it as the preferred method for neuromonitoring and neurofeedback (NFB) interventions that often require measuring brain response across multiple sessions.10,11,12 fMRI is also a non-invasive neuroimaging method with high spatial resolution that is widely used to investigate functional brain activity and connectivity in a confined setting.13,14,15 Combining fNIRS and fMRI yields complementary insights, mitigates limitations inherent to each technique, and contributes to a more profound understanding of ADHD.
The dorsolateral prefrontal cortex (dlPFC) and the anterior cingulate cortex (ACC), along with the frontoparietal network (which includes prefrontal and parietal regions), are central for working memory (WM) function.7,16,17,18,19,20 Children with ADHD often display reduced activation in these brain regions during WM tasks.21,22,23 WM deficits are common in neurodevelopmental and psychiatric conditions, including ADHD, affecting learning, focus, and task completion.2,20,24 Early interventions targeting WM deficits appear vital for academic and occupational success.25,26 However, there is a notable gap in developing effective interventions that target the neural mechanisms associated with WM deficits.3 Here, we developed a personalized intervention that integrates computerized WM training with real-time fNIRS neuromonitoring and neurofeedback, focusing on enhancing the frontoparietal circuitry underlying WM deficits in children with ADHD. This intervention aims to enhance individualized neural systems by reinforcing strategies that maximize frontoparietal network engagement during WM performance; through use of real-time fNIRS imaging and computational techniques, this study examined how precise monitoring of frontoparietal network engagement impacts cognitive abilities in children with ADHD.
We hypothesize that targeting the brain mechanisms underlying WM deficits through the proposed neuromonitoring-guided WM training will lead to significant enhancement of frontoparietal function and further lead to improvement of cognitive and clinical outcomes. The proposed brain-focused intervention takes into account individual differences in brain networks and thus paves the way for developing methods for precision mental health and precision psychiatry. This study aims to establish a framework for creating customized, pathology-specific, and cross-diagnostic interventions that can contribute to a better understanding of the underlying pathophysiology of ADHD and ultimately improve patient outcomes.
Results
Demographics and clinical characteristics
Table 1 summarizes the descriptive statistics and neuropsychological outcomes at baseline. No differences on any demographic characteristics and key outcome measures were observed between the groups at baseline.
Table 1.
Baseline characteristics of the NFB and treatment-as-usual children with ADHD
| NFB (N = 21) | Treatment-as-usual (N = 15) | Statistics | |
|---|---|---|---|
| Age, mean (SD) | 9.92 (1.32) | 9.52 (1.46) | |
| Sex, female | 9 | 7 | |
| ADHD subtype, combined, inattentive, impulsive | 16,4,1 | 11,4,0 | |
| Medication, stimulant | 7 | 5 | |
| Race, Hispanic/Latino, | 3 | 2 | |
| BRIEF-2 WM scores (SD) | 75.28 (6.64) | 73.0 (6.26) | |
| Conners 3-ADHD index scores (SD) | 83.85 (10.51) | 84.93 (12.48) | |
| Conners 3 Inattention scores (SD) | 78.85 (10.96) | 78.46 (10.96) | |
| WRAML-2 | 96.87 (15.5) | 91.38 (8.66) | |
| NEPSY-II | 9.26 (2.54) | 7.65 (2.75) |
Neuromonitoring cognitive intervention outcomes
We observed an increase in target modulation in 10 participants (50%) in the left-vlPFC, 17 participants (85%) in the left-dlPFC, 17 participants (85%) in the right-dlPFC, and 15 participants (75%) in the right-dmPFC out of a total of 20 participants. Therefore, the majority of children with ADHD who participated in neurofeedback were able to control the protocol (Figure 1).
Figure 1.
HbO Beta value changes of targeted brain regions
(I) Left-vlPFC (p = 0.02); (II) left-dlPFC (p = 0.0001); (III) right-dlPFC (p = 0.0001); (IV) right-dmPFC (p = 0.003) between averages of the first two sessions and the last two sessions.
One participant (out of a total of 21) was excluded due to low data quality of intervention in targeted brain regions.
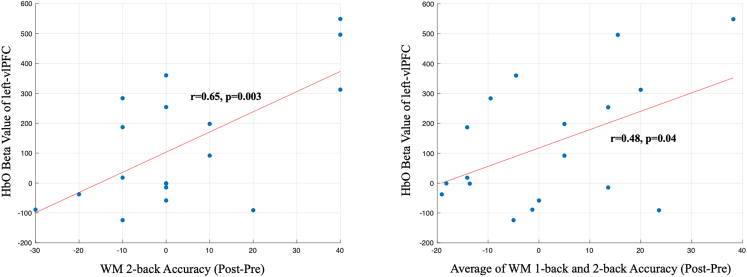
To assess the correlation between intervention performance and behavioral outcomes, we conducted Pearson correlation analyses. As shown in Figure 2, the results indicated a significant positive correlation between the degree of control over the neurofeedback protocol (NFB behavioral accuracy) and improvements in WM 2-back performance (r = 0.48, p < 0.03) and the average of WM 1-back and 2-back (r = 0.49, p < 0.03). In addition, the HbO findings in NFB interventions also revealed a significant positive correlation between the degree of control over the neurofeedback protocol (NFB fNIRS data) and improvements in WM 2-back performance (r = 0.65, p < 0.003) and the average of WM 1-back and 2-back (r = 0.48, p < 0.04) (See Figure 3). These findings suggest that participants who were better able to control the neurofeedback protocol (i.e., those who performed better along with higher HbO activation in ROI target during the intervention) also showed greater improvements in cognitive outcomes.
Figure 2.
Significant association between average behavioral accuracy across 12 intervention sessions and WM 2-back accuracy in post-session (left) and also the average of WM 1-back and 2-back accuracy (right)
Figure 3.
Significant association between HbO Beta value of left-vlPFC during NFB intervention and WM 2-back accuracy (left) and also the average of WM 1-back and 2-back accuracy between post-intervention and pre-intervention (right)
The effect of NFB intervention on fNIRS brain activation in children with ADHD
We examined changes in HbO during the 1-back+2-back versus 0-back conditions to evaluate the impact of NFB intervention on fNIRS hemodynamic activity in the NFB group compared to treatment-as-usual. LME model revealed significant group by session interaction effects primarily in four frontal cortical ROIs: right dorsolateral prefrontal cortex (right-dlPFC) (), right dorsomedial prefrontal cortex (right-dmPFC) (, left dorsolateral prefrontal cortex (left-dlPFC) (), and left ventrolateral prefrontal cortex (left-vlPFC) () (Figure 4). Overall, the NFB group demonstrated substantial improvements in cortical activity during the n-back working memory task, indicating the effectiveness of NFB cognitive training in enhancing frontal brain function in children with ADHD. More extended results are provided in the STAR∗METHODS section (also see Table S2; Figures S6 and S7).
Figure 4.
Channel map of brain regions that showed significant group by session interaction effect in cortical HbO activation
The graphs show corresponding changes in group-average HbO Beta values for each group and session. d indicates Cohen’s d effect size.
The effect of NFB intervention on fMRI brain activation in children with ADHD
Children with ADHD who underwent NFB cognitive training demonstrated significant improvements in brain activation during the n-back fMRI working memory task under the 2-back+1-back versus 0-back condition, particularly in the frontal regions depicted in Figure 5A. Analyzing the fMRI task data using LME analysis revealed a significant interaction effect between the group and session in the left-dlPFC (dorsal and lateral combined) (, −46, 24, 36) and in the left anterior prefrontal cortex ( , −30, 50, 14), when comparing the 2-back + 1-back condition to the 0-back condition at an uncorrected p value threshold of less than 0.001 (Figure 5B).
Figure 5.
Group by session interaction effect in (A) fMRI task activation and (B) fMRI signal changes in the left dlPFC (dorsal and lateral) and anterior PFC for each group and session during the working memory task (measured by 2-back + 1-back versus 0-back)
The violin plots represent the distribution of the fMRI signal and changes across the groups and sessions.
Working memory performance and neuropsychological outcomes
We aimed to explore the potential of NFB cognitive training in improving n-back working memory performance and neuropsychological functioning. Our results showed a significant group by session interaction effect for fNIRS n-back performance during the 2-back condition ( ) (Figure 6). The NFB group showed an average increase in accuracy by around 8%, whereas the treatment-as-usual group experienced a decrease in accuracy by 3.33%. However, the group by session interaction effect was not significant for n-back performance during the fMRI session.
Figure 6.
Changes in n-back WM performance accuracy during fNIRS and in BRIEF-2 WM T-scores and Conners 3 ADHD index scores baseline and post-intervention for each group
The results revealed highly promising findings on clinical and neuropsychological scores. First, we observed a significant group by session interaction effect in BRIEF-2 working memory T-scores ( < 0.01; 95% confidence interval [CI], 3.75 to 11.75; ). In the NFB group, a large proportion of participants (85.7%) showed a decrease (i.e., improvement) in BRIEF-2 T-scores (mean = −8.4 [±6.1]) versus only 40% of participants in the treatment-as-usual group (mean = −0.85 [±7.94]). Moreover, our study also revealed a significant group by session interaction effect in Conners 3 ADHD index scores ( = 0.04; 95% CI, −0.05 to 10.17, ). Within the NFB group, 52.4% of individuals exhibited a significant decrease (improvement) in their Conners 3 ADHD index scores, with an average reduction of −6.21 (±10.61), versus 13.3% of participants treatment-as-usual participants, whose mean reduction was −1 (±3.49) (Table S3). No significant group by session interaction effect was found for Conners 3 Inattention T-scores and the WRAML-2 and NEPSY-II composite scores.
Discussion
The goal of this randomized clinical trial study was to investigate the impact of a personalized neuromonitoring-guided cognitive intervention on enhancing brain activity underlying working memory performance and its impact on cognitive and clinical ADHD outcomes. The results confirmed our hypothesis, demonstrating a significant upregulation of brain activity in various prefrontal regions, with large effect size in the right dlPFC in the NFB group (Cohen’s d = 1.13). Notably, these areas were among the brain regions frequently exhibiting hypoactivity in ADHD, as reported in previous fNIRS and fMRI studies,7,8 reinforcing the efficacy of the personalized intervention in normalizing brain activity in these critical regions.
Compared with fMRI, fNIRS outcomes revealed broader target engagement in the bilateral PFC. The observed improvement in bilateral prefrontal activity in the NFB group is supported by previous meta-analyses that demonstrated the involvement of bilateral PFC in n-back task performance in healthy controls27 and hypoactivation in bilateral PFC in children with ADHD across executive function tasks.7 Further, it underscores the importance of measuring brain activity in a more naturalistic environment, such as when participants are sitting on a chair rather than lying in a confined scanner. It also suggests that fNIRS can serve as a valuable and cost-effective neuroimaging tool for neuromonitoring and neurofeedback interventions in the field of psychiatry.
We also conducted a comprehensive assessment of cognitive and clinical outcomes, focusing on working memory and ADHD clinical outcomes. The premise of the proposed intervention was that engaging the brain networks underlying working memory performance would result in improved WM and ADHD clinical outcomes. Notably, the NFB group exhibited an improvement in working memory performance, whereas the treatment-as-usual group showed a decrease. Furthermore, the NFB group displayed significant improvements in real-world measures of WM (BRIEF-2 WM T-score) and in ADHD outcomes (Conners 3 ADHD index scores). These findings emphasize the potential benefits of the proposed intervention in enhancing cognitive performance and reducing ADHD symptoms. Further research is needed to fully explore the extent of these effects and their implications for individuals with neurodevelopmental disorders.
Previous studies have demonstrated improvements in ADHD symptoms and/or cognitive skills with passive fNIRS neurofeedback intervention in children.28,29 Our results corroborate these findings and extend them by showing improvements in both brain activity and cognitive/clinical outcomes. Particularly, our approach utilized a calibration period to localize the target WM network for each individual and thus accounting for individual variability in neuropathology. Further, we uniquely integrated cognitive training with real-time neuromonitoring and neurofeedback for real-time tracking of target network engagement and for reinforcing a strategy that maximizes the engagement of the target network. This personalized integrative approach represents a potentially more comprehensive and effective intervention than neurofeedback alone. We view these findings as a significant step forward in the development of comprehensive interventions for ADHD, encompassing not only symptom alleviation but also improvements in broader neural and cognitive domains. Previous literature also indicates that participants in the neurofeedback condition showed improvements in learning and reading abilities, whereas the transfer effect of stimulant medication in children was not as clearly established.30,31 It is noteworthy that the latest generation of NIRS systems is more portable, wearable, and affordable, with the potential for home-based and classroom-based use aligning with the future direction of personalized treatment approaches.32,33 These low-cost fNIRS platforms will significantly enhance the scalability of neuromonitoring and neurofeedback-based interventions.
Although previous research has demonstrated improvements in ADHD symptoms with neurofeedback interventions,34 these findings, alongside our current study, underscore the need to delve deeper into the factors influencing treatment outcomes. It is essential to explore whether NFB interventions can extend their efficacy to broader functional domains, especially given the mixed evidence from prior EEG neurofeedback studies in ADHD. Notably, a previous meta-analysis has raised questions about the effectiveness of EEG neurofeedback in addressing ADHD.35 The proposed intervention addresses the gap in previous passive neurofeedback intervention research by integrating cognitive training with fNIRS neuromonitoring and neurofeedback. This innovation has added benefits, as it specifically targets brain regions affected in ADHD, seeking to enhance the underlying mechanisms and alleviate ADHD symptoms.
The intervention findings suggest that participants who were better able to control the neurofeedback protocol also showed greater improvements in both cognitive outcomes (Figure 1). Moreover, the correlations highlight the potential efficacy of the neurofeedback intervention in enhancing cognitive and behavioral functions in children with ADHD (Figures 2 and 3). However, further research with larger sample sizes is needed to confirm these findings and establish the robustness of the correlations observed.
In conclusion, we found significant improvements in WM and ADHD outcomes among children who received personalized fNIRS NFB intervention. The targeted neural engagements mediated by the intervention, particularly in prefrontal regions, align with existing literature and support the efficacy of personalized NFB in modulating frontal neural activity. Most importantly, the proposed personalized integrative approach provides a foundation for developing effective interventions that focus on brain mechanisms responsible for cognitive deficits while taking into account individual differences in neuropathology. Overall, these findings support the efficacy of personalized neuromonitoring-guided cognitive intervention in improving brain activity and clinical outcomes in children with ADHD.
Limitations of the study
It is important to interpret these findings with caution due to the small sample size. Although the results are promising, the limited number of participants reduces the generalizability of the findings and increases the potential for type I and type II errors. Additionally, the small sample sizes within each ADHD sub-type—27 combined, 8 inattentive, and only 1 hyperactive-impulsive—further limit our ability to conduct meaningful statistical analyses across these groups. This prevents us from examining potential variations in how different sub-types may respond to the intervention, which could be crucial for tailoring treatment more effectively.
Future studies with larger, more balanced sample sizes are needed to validate these results and establish the efficacy and effectiveness of the intervention in broader populations, including a more detailed analysis by ADHD sub-type. This study provides a foundation for developing effective cognitive interventions that target system-level mechanisms responsible for symptoms. The results require replication in larger samples to validate efficacy and assess the potential for broader implementation in clinical practice, ensuring interventions are appropriately customized for diverse patient needs based on detailed sub-type analyses.
Further limitations are also noteworthy. An active or sham control group would improve the statistical power and strength of the conclusions regarding the efficacy of the proposed intervention. However, we have previously shown the efficacy of fNIRS neurofeedback intervention compared with sham in improving WM in a group of young adults,36 providing some contextual support for our findings. Future research should consider incorporating a healthy control group to establish a baseline for comparison. Further, the study procedures were halted due to COVID-19 pandemic, which resulted in significant loss of subjects available for follow-up. Additionally, both the NFB and treatment-as-usual groups underwent similar in-lab assessments throughout the study. However, the NFB group had additional in-lab visits (totaling 12 sessions) to receive the neurofeedback intervention. This increased frequency of visits for the NFB group represents a variance in participant experience that may influence study outcomes. We recognize this as a potential limitation in our study design, as it may have affected participant engagement and response to the intervention.
Therefore, collecting brain activity data in real-life settings and home environments could improve ecological validity by providing insights into daily challenges faced by individuals with ADHD. Our ongoing research explores wearable fNIRS headbands for at-home brain activity measurement, suggesting potential benefits for delivery of personalized fNIRS neurofeedback interventions at home. Finally, further investigation is required to assess the long-term effects and generalizability of the proposed intervention to larger and more diverse populations.
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Hadi Hosseini (hosseiny@stanford.edu).
Materials availability
This study did not generate new unique reagents or materials.
Data and code availability
Data
The data supporting the findings of this study, including neuroimaging (fNIRS and fMRI) and behavioral performance data, will be available upon request from the corresponding author, Hadi Hosseini (email: hosseiny@stanford.edu).
Code
All custom code used for data analysis, including the fNIRS and fMRI processing pipelines, is available upon request. The code will be provided by the lead contact and can be shared via GitHub or other repository upon request.
Additional information
Any additional materials or resources required for reanalysis of the data reported in this study are available upon request from the lead contact.
Acknowledgments
We thank the participants for their involvement in the study as well as the researchers involved in coordinating data collection for this project. We also thank Dr. David Boas for his feedback on fNIRS data processing. The study was funded by National Institute of Mental Health (R61MH119289). SMHH’s effort was supported in part by NIA (R01AG073362, R01AG072470, R21AG064263, R21AG073973) and National Institute of Mental Health (NIMH; R61MH119289, R21MH123873).
Author contributions
A.R., methodology, formal analysis, visualization, writing—original draft, and writing—review & editing. E.G., methodology, data curation, formal analysis, visualization, and writing—review & editing. L.D., methodology, data curation, and writing—review & editing. B.A.-P., methodology, formal analysis, visualization, and writing—review & editing. S.R., data curation and writing—review & editing. H.F., methodology, data curation, and writing—review & editing. D.H., writing—review & editing. G.E., writing—review & editing. A.H., writing—review & editing. S.H., writing—review & editing. S.M.H.H., conceptualization, methodology, resources, writing—review & editing, and funding acquisition.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Software and algorithms | ||
| MATLAB | MathWorks | R2022b |
| R Studio | lmer Package | R Version 4.3.0 |
| SPM 12 toolbox | https://www.fil.ion.ucl.ac.uk/spm/software/spm12/ | |
| FSL toolbox | https://fsl.fmrib.ox.ac.uk/fsl/docs/#/ | |
| AFNI toolbox | https://afni.nimh.nih.gov/ | |
| NIRS brain AnalyzIR toolbox | https://github.com/huppertt/nirs-toolbox | Santosa et al.32 |
| Other | ||
| NIH Clinical Trial (Clinical trial register number: NCT04002167) | https://clinicaltrials.gov/study/NCT04002167?tab=table | NCT04002167 |
Experimental model and study participant details
Participants were children with ADHD recruited from various sources, including local clinics, schools, and community providers. Seventy-seven children with ADHD (aged 7–11 years, mean ± SD = 9.81 ± 1.29) were consented to participate in the study. The cohort included 56% males and 44% females, with diverse racial backgrounds: 77% White, 13% Hispanic/Latino, 5% African American, and 5% Asian (see Table 1 for more detailed information). Forty-one participants were excluded or lost to follow-up primarily because of not meeting inclusion criteria (n = 20), non-compliance with MRI (n = 10) or a COVID-19-related halt (n = 11) in data collection. Ultimately, 36 participants (21 NFB and 15 treatment-as-usual) successfully completed the trial (Figure S1). Study was pre-registered on ClinicalTrials.gov under the following identifier: NCT04002167. The Stanford University Institutional Review Board approved this study, and written parental informed consent was obtained for every child. Every patient who began the intervention completed all the scheduled intervention visits, except for those whose involvement was interrupted due to COVID-19 pandemic halt in the study procedures.
Inclusion criteria included: (1) age between 7 and 11 years; (2) diagnosis of ADHD or presence of ADHD symptoms as determined by parent reports from the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS). The KSADS was administered to both parents and children. The version used was the KSADS-PL (Present and Lifetime Version), which is a comprehensive, semi-structured interview designed to assess current and past episodes of psychopathology in children and adolescents according to DSM-5 criteria. In addition to the KSADS, a comprehensive clinical evaluation was conducted prior to patient participation to ensure accurate diagnosis and eligibility. This evaluation included clinical interviews with the child and parents, review of medical and psychiatric history, and administration of standardized rating scales and questionnaires; (3) stability of permitted medications (Table 1) for at least three months; (4) an IQ ≥ 80 as determined by the Wechsler Abbreviated Scale of Intelligence (WASI); and (5) a Behavior Rating Inventory of Executive Function (BRIEF) Working Memory score >60. Comorbidities of Conduct Disorder (CD) and Oppositional Defiance Disorder (ODD) were allowed. Approximately 79% of children who meet the diagnostic criteria for ADHD would have a BRIEF WM score >60, thereby qualifying for study participation.
Exclusion criteria included IQ < 80 (n = 4), BRIEF Working Memory score <60 (n = 9), the presence of suicidality (n = 0), current regular use of psychiatric medications other than those permitted (e.g., opiates or thyroid medications) (n = 1), substance abuse (n = 1), severe neurological or psychiatric disorders (n = 3), history of significant head trauma (n = 0), history of alcohol abuse or dependence within the past two years (n = 0), any significant systemic or unstable medical condition (n = 0), or sensory deficits that could lead to difficulty complying with the study protocol (n = 2). Consequently, forty-one participants were deemed ineligible primarily due to not meeting the inclusion criteria (n = 20), non-compliance with MRI requirements (n = 10), and a COVID-19-related halt in data collection (n = 11). Of the participants who were excluded, 60% were male and 40% were female. The excluded psychiatric comorbidities included major depressive disorder (n = 1), generalized anxiety disorder (n = 1), and autism spectrum disorder (n = 1).
Clinical, neuropsychological, and neuroimaging outcomes were comprehensively assessed at both baseline and post-intervention. The clinical outcomes included the BRIEF-2, which measures executive functions such as working memory, and the Conners Rating Scales (3rd Edition), which assesses ADHD symptoms. The neuropsychological outcomes included the Wide Range Assessment of Memory and Learning 2 (WRAML-2) for working memory and general memory t-scores, as well as the Developmental NEuroPSYchological Assessment II (NEPSY-II) inhibition and word generation scales.
Neuroimaging outcomes were evaluated using fNIRS and fMRI to measure frontoparietal brain activity during an n-back working memory task. These neuroimaging techniques provided detailed data on the activation of prefrontal and parietal brain regions, which are critical for working memory function.
Primary outcomes included measurements of changes in the target frontoparietal activity and working memory performance using fMRI and fNIRS during an n-back working memory task. Secondary outcomes encompassed evaluations of real-world working memory behavior using the Behavior Rating Inventory of Executive Function (BRIEF-2)37 and the Wide Range Assessment of Memory and Learning 2 (WRAML-2, working memory t-score, general memory t-score).38 ADHD and inattention symptoms were assessed with the Conners 3rd Edition ADHD scale as well as the composite score from the Developmental NEuroPSYchological Assessment II (NEPSY-II) inhibition and word generation scales.
Our study cohort included a diverse range of ADHD sub-types, enhancing the generalizability of our findings. Specifically, we had 27 participants with the combined sub-type, 8 with the inattentive sub-type, and 1 with the hyperactive-impulsive sub-type. The distribution within our intervention groups was as follows: In the NFB group, there were 16 combined, 4 inattentive, and 1 hyperactive-impulsive. The treatment-as-usual group included 11 combined and 4 inattentive participants (See Table 1).
Method details
Neuromonitoring cognitive intervention procedure
The NFB cognitive training involved a computerized working memory game with a neuromonitoring and neurofeedback program developed in-house.36 This program aimed to enhance working memory skills while monitoring brain activity using fNIRS technology. The cognitive training was based on a modified version of the Sternberg task, which is a delayed matching-to-sample task (Figure S5). In each trial, participants viewed a set of letters for 2 s during the encoding phase, followed by a variable delay (6–8 s) representing the retention phase. After the delay, a single inquiry letter was displayed for 2 s during the inquiry phase, and participants had to determine whether the inquiry letter was part of the original set. The probe phase was followed by a variable fixation period (6–8 s). Each training session consisted of 80 trials and lasted approximately 25 min. Participants underwent two training sessions per week, each lasting 20–25 min, for a total of six weeks (four weeks in some cases).
The training program commenced with a calibration period to identify the individualized target network associated with working memory load. During this phase, which included 12 trials and lasted about 4 min, regions within the dorsolateral prefrontal cortex (dlPFC) were identified using general linear modeling based on a t-value criterion (1 standard deviation above the mean) to pinpoint the target regions. These regions varied among participants due to individual differences in task performance but generally overlapped across subjects, ensuring consistency in targeting regions critical for working memory.
Once the target regions were identified, participants received feedback on both brain activity and task performance. The neurofeedback provided represented brain activity in the targeted channels during the encoding (2 s) and retention phases (7 s, accounting for hemodynamic delay). Hemodynamic signals (HbO) were band-pass filtered and averaged over a 9-s window, and the relative change from the calibration period was displayed to participants. Behavioral feedback, based on accuracy in the task, was also calculated relative to the calibration period.
Feedback was presented visually in the form of gold and cross coins, illustrating changes in neural activity and behavioral performance over the previous ten trials (Figure S5). A change in slope indicated corresponding changes in the average activity in the targeted channels. This feedback was delivered intermittently, updating 2 s after each inquiry phase during the inter-trial interval to minimize interference with task performance. Participants were encouraged to prioritize task performance during each trial and use the feedback during the inter-trial interval to adjust their cognitive strategies.
The program was designed to promote the development of personalized metacognitive strategies, where participants would reflect on and adjust their cognitive processes to better control neural activity without compromising task performance. These strategies involved self-monitoring, self-evaluation, and attention adjustment based on the feedback provided.
The fNIRS setup and probe layout used during the cognitive training sessions were consistent with the layout used during the n-back task. This ensured direct comparability of data collected across different cognitive tasks. Both tasks focused on measuring activity in the prefrontal cortex, particularly the frontoparietal regions crucial for working memory. This consistent layout across tasks allowed us to accurately monitor and compare brain activity changes attributable to the interventions rather than differences in the measurement setup.
Treatment-as-usual group
The treatment-as-usual group received standard clinical care, which included a medication management, or no therapy as deemed appropriate by their healthcare providers and their parents. Medication management included the use of stimulant and non-stimulant medications commonly prescribed for ADHD, such as methylphenidate or atomoxetine, with doses adjusted based on clinical response and side effects at least three months prior to participation in the study. To ensure the integrity of the intervention’s impact, participants in both the NFB and treatment-as-usual groups were required to maintain a stable regimen of ADHD medication, with no adjustments allowed three months prior to and during the study period. This protocol was enforced to ensure consistency in pharmacological influences across all participants.
The treatment-as-usual participants did not have scheduled study visits twice weekly as the neuromonitoring-guided NFB group did. Instead, they attended regular appointments as needed for their behavioral therapy and medication management. The treatment-as-usual group received mock MRI sessions and underwent the same baseline and post-intervention neuroimaging, clinical and neuropsychological assessments as the NFB group. The temporal relationship between the last treatment session and the outcome measures was carefully maintained. Outcome measures, including clinical, neuropsychological, and neuroimaging assessments, were conducted within one week after the final treatment session for both the NFB and treatment-as-usual groups. This ensured that the results reflected the immediate impact of the interventions.
Participants across both groups received standardized medical care according to best practices for ADHD management. No additional psychological, educational, or behavioral treatments were administered during the study period to ensure that any observed effects could be attributed primarily to the NFB intervention.
N-back working memory task
During the fMRI and fNIRS sessions, participants completed an n-back working memory task (Figure S2). The fMRI session was always conducted before the fNIRS session. The task involved the sequential presentation of numbers from 0 to 9. In the 0-Back conditions, participants identified and responded to the appearance of the number "0." For the 1-Back conditions, they determined if the current number matched the one presented in the previous trial. Similarly, in 2-Back, participants assessed if the current number matched the number from two trials earlier. Each n-back task block began with a 3-s initiation cue, followed by a number displayed at the center of the screen for 2 s, with 0.5-s intervals between each number. The order of number presentations was pseudorandomized for each participant and session while maintaining the same difficulty level. All participants completed runs lasting 476 s, approximately 8 min, consisting of four n-back blocks (totaling 12 sessions) interleaved with a rest block. Importantly, all participants had an identical number of trials across sessions, with ten trials for each n-back condition. Participants were briefed on task initiation and the importance of timely responses before starting, and response recording accuracy was verified.
Assessments
The psychological and cognitive measures included the following assessments.
Behavior Rating Inventory of Executive Function (BRIEF-2)
This parent-report questionnaire assesses executive functions in children, focusing on aspects such as working memory, inhibition, and planning/organization. The BRIEF-2 Working Memory scale was specifically used to determine inclusion criteria and to measure changes in working memory over the course of the intervention.
Conners Rating Scales (3rd Edition)
This is a comprehensive tool used to assess ADHD symptoms and behaviors in children. The Conners 3 ADHD Index score was used to evaluate changes in ADHD symptomatology pre- and post-intervention.
Wide Range Assessment of Memory and Learning 2 (WRAML-2)
This test measures various aspects of memory and learning, including working memory and general memory. The WRAML-2 working memory and general memory t-scores were used to assess cognitive changes resulting from the intervention.
Developmental NEuroPSYchological assessment II (NEPSY-II)
This assessment evaluates a range of neuropsychological functions in children, including executive function, language, memory, and attention. The inhibition and word generation scales were specifically used to assess executive function changes.
These measures were administered at baseline and post-intervention to both the NFB and treatment-as-usual groups to evaluate the effectiveness of the interventions on cognitive and psychological outcomes.
Neuroimaging
fNIRS data acquisition and analysis
For the n-back task, we used a multichannel continuous-wave fNIRS system (NIRScout, NIRx Medical Technologies, LLC) to monitor cortical hemodynamics. The system included a probe with 24 light source emitter positions emitting LED lights at 760 nm and 850 nm wavelengths, along with 15 APD light detectors. Data were recorded at 5.2083 Hz, with an average distance of 3 cm between the optodes for long channels and 0.8 cm for short channels. The source and detector layout, along with the channel configuration (55 long and 8 short channels), are shown in Figure S6. Table S1 provides the MNI coordinates corresponding to the channel numbers and source-detector pairs, which were determined using AtlasViewer.39
We began by assessing and controlling the quality of the fNIRS data. We used the QT-NIRS40 toolbox (https://github.com/lpollonini/qt-nirs) to identify and remove low-quality channels. Channels with a scalp coupling index (SCI) and peak spectral power (PSP) lower than the set thresholds (SCI = 0.6, Q threshold = 0.7, PSP threshold = 0.1) were classified as low quality (Figure S3). Additionally, channels with fewer than 50% data samples were excluded from further analysis, resulting in an average of 43 samples per selected channel (lowest = 33, highest = 58 out of 65 subjects). To improve signal quality, we applied principal component analysis (PCA)-based baseline corrections on the raw intensity signals to eliminate DC shifts and the global signal. Motion artifacts were further addressed through wavelet filtering using a sym8 basis function and an SD threshold of 5 to remove outliers and low-frequency characteristics. For computational efficiency, we adjusted the sampling rate of the fNIRS data to 2.4 Hz.
After this preprocessing step, we applied the AR-IRLS Regression41 Model using the BrainAnalyzIR toolbox42 to regress out the data from the short channels, effectively reducing physiological and motion-related artifacts and enhancing the reliability of the fNIRS measurements43 (Figure S4). Statistical analysis was performed using a mixed regression model to examine the effects of group (NFB and Treatment-as-usual), session (pre and post), age, sex, and medication use on brain activation. The dependent variable, Beta (β), represented the estimated coefficient of oxyhemoglobin (HbO) and deoxyhemoglobin (HbR) for the contrast between the WM task (1-Back and 2-Back conditions combined) and the control condition (0-Back). To account for multiple comparisons, we used the Benjamini-Hochberg False Discovery Rate (FDR) correction method, identifying statistically significant channels with .
fMRI data acquisition and analysis
The children in the NFB and treatment-as-usual groups, all diagnosed with ADHD, underwent baseline and follow-up MRI scans. MRI data were collected using a 3T GE system with a 32-channel head coil at the Richard M. Lucas Center for Imaging at Stanford University. Task functional MRI scans employed a multi-band acceleration factor of 6, with TR = 0.76 s, TE = 35 s, flip angle = 54°, 60 slices in ascending order, and a resolution of 1 mm isotropic.
Participants performed the n-back working memory (WM) task during 8-min runs, resulting in 630 functional brain volumes per run. The task began after the first seven volumes to avoid partial saturation effects, and a short acquisition with six functional volumes was conducted before the task to correct geometrical distortion due to field inhomogeneities.
Structural MRI data were acquired using an MPRAGE pulse sequence with a 45 ms inversion time (TI), flip angle = 12°, and 0.9 mm slice thickness. The fMRI data from all sessions were preprocessed using Statistical Parametric Mapping Software (SPM12; Welcome Department of Cognitive Neurology, University College London), FSL, and AFNI toolboxes in MATLAB R2022b (MathWorks Inc., Natick, MA). The preprocessing pipeline included removing the noisy functional brain volumes acquired before the task, correcting for susceptibility distortion, co-registration, realignment and unwarping, and identification of outlier frames (frame-wise displacement >0.5mm). Functional and structural images were then normalized to standard Montreal Neurological Institute (MNI) space and segmented into gray matter, white matter, and cerebrospinal fluid. The fMRI data were then smoothed using a spatial convolution with a 6 mm full width at half-maximum (FWHM) Gaussian filter.
Due to high motion, eight participants had to repeat either their baseline or post-session scans. Additionally, we excluded one or two N-back sessions from five participants for exceeding the motion threshold, but the number of sessions did not differ significantly between groups or sessions (p > 0.05). The preprocessed fMRI data were analyzed using a linear mixed-effect model with 3dLME (AFNI’s toolbox), incorporating age, sex, and medication use as covariates. Group and session were considered as main effects, and interaction effects were generated by comparing the 1-Back and 2-Back conditions to the 0-Back condition.
Quantification and statistical analysis
Statistical analysis plan
All of the outcomes of this study were a pre-planned measures documented under our study record on ClinicalTrials.gov. The primary outcomes included changes in frontoparietal brain activity as measured by fNIRS and fMRI and working memory performance. Secondary outcome measures included the BRIEF-2 Working Memory t-scores, Conners 3 ADHD Index scores, WRAML-2 general memory t-scores and the NEPSY-II inhibition and word generation scales. All these outcomes were pre-specified under the study record on ClinicalTrials.gov prior to start of the study.
-
1Primary Outcomes:
-
•Changes in frontoparietal brain activity were analyzed using fNIRS and fMRI data. A mixed regression model was used to examine the effects of group (NFB vs. treatment-as-usual) and session (baseline and post-intervention) on brain activation, with age, sex, and medication use as covariates. The group by session interaction effect was of primary interest.
-
•Working memory performance was assessed using accuracy scores from the n-back task. A similar mixed regression model was applied to these data.
-
•
-
2Secondary Outcomes:
-
•BRIEF-2 Working Memory t-scores, Conners 3 ADHD Index scores, WRAML-2 general memory t-scores and NEPSY-II inhibition and word generation scales were analyzed using paired t-tests and ANCOVA, controlling for baseline scores and other covariates. to examine changes from baseline to post-intervention.
-
•
-
3Multiple Comparisons Correction:
-
•To control for multiple comparisons, the Benjamini-Hochberg False Discovery Rate (FDR) correction method was applied to identify statistically significant outcomes ().
-
•
-
4Software:
-
•All statistical analyses were conducted using MATLAB R2022b and RStudio (R version 4.3.0) with the lmer package for linear mixed-effect models.
-
•
Interventions
-
•
NFB Group: The NFB cognitive training involved a computerized working memory game with neuromonitoring and neurofeedback program developed in-house. The training program consisted of two clinic sessions per week, each lasting 20 min, for a total of six weeks.
-
•
Treatment-as-usual Group: This group received standard clinical care, including medication management or no therapy as deemed appropriate by their healthcare providers and parents. Treatment-as-usual participants attended regular appointments as needed for their medication management.
Additional resources
This study is part of a clinical trial (Clinical trial register number: NCT04002167): https://clinicaltrials.gov/study/NCT04002167?tab=table.
Published: September 30, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.111087.
Supplemental information
References
- 1.Polanczyk G.V., Willcutt E.G., Salum G.A., Kieling C., Rohde L.A. ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int. J. Epidemiol. 2014;43:434–442. doi: 10.1093/ije/dyt261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kowalczyk O.S., Cubillo A.I., Criaud M., Giampietro V., O'Daly O.G., Mehta M.A., Rubia K. Single-dose effects of methylphenidate and atomoxetine on functional connectivity during an n-back task in boys with ADHD. Psychopharmacology. 2023;240:2045–2060. doi: 10.1007/s00213-023-06422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roording-Ragetlie S., Spaltman M., de Groot E., Klip H., Buitelaar J., Slaats-Willemse D. Working memory training in children with borderline intellectual functioning and neuropsychiatric disorders: a triple-blind randomised controlled trial. J. Intellect. Disabil. Res. 2022;66:178–194. doi: 10.1111/jir.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinussen R., Hayden J., Hogg-Johnson S., Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2005;44:377–384. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- 5.Biederman J., Monuteaux M.C., Doyle A.E., Seidman L.J., Wilens T.E., Ferrero F., Morgan C.L., Faraone S.V. Impact of executive function deficits and attention-deficit/hyperactivity disorder (ADHD) on academic outcomes in children. J. Consult. Clin. Psychol. 2004;72:757–766. doi: 10.1037/0022-006X.72.5.757. [DOI] [PubMed] [Google Scholar]
- 6.Silk T.J., Vance A., Rinehart N., Bradshaw J.L., Cunnington R. Dysfunction in the fronto-parietal network in attention deficit hyperactivity disorder (ADHD): an fMRI study. Brain Imaging and Behavior. 2008;2:123–131. [Google Scholar]
- 7.Cortese S., Kelly C., Chabernaud C., Proal E., Di Martino A., Milham M.P., Castellanos F.X. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am. J. Psychiatr. 2012;169:1038–1055. doi: 10.1176/appi.ajp.2012.11101521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gossé L.K., Bell S.W., Hosseini S.H. Functional near-infrared spectroscopy in developmental psychiatry: a review of attention deficit hyperactivity disorder. Eur. Arch. Psychiatr. Clin. Neurosci. 2021;272:273–290. doi: 10.1007/s00406-021-01288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quaresima V., Ferrari M. MDPI; 2019. A Mini-Review on Functional Near-Infrared Spectroscopy (fNIRS): Where Do We Stand, and where Should We Go? Photonics; p. 87. [Google Scholar]
- 10.Green A.L., Rabiner D.L. What do we really know about ADHD in college students? Neurotherapeutics. 2012;9:559–568. doi: 10.1007/s13311-012-0127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinti P., Aichelburg C., Gilbert S., Hamilton A., Hirsch J., Burgess P., Tachtsidis I. A review on the use of wearable functional near-infrared spectroscopy in naturalistic environments. Jpn. Psychol. Res. 2018;60:347–373. doi: 10.1111/jpr.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartholdy S., Musiat P., Campbell I.C., Schmidt U. The potential of neurofeedback in the treatment of eating disorders: A review of the literature. Eur. Eat Disord. Rev. 2013;21:456–463. doi: 10.1002/erv.2250. [DOI] [PubMed] [Google Scholar]
- 13.Tong Y., Frederick B. Concurrent fNIRS and fMRI processing allows independent visualization of the propagation of pressure waves and bulk blood flow in the cerebral vasculature. Neuroimage. 2012;61:1419–1427. doi: 10.1016/j.neuroimage.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vloet T.D., Gilsbach S., Neufang S., Fink G.R., Herpertz-Dahlmann B., Konrad K. Neural mechanisms of interference control and time discrimination in attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49:356–367. [PubMed] [Google Scholar]
- 15.Güven A., Altınkaynak M., Dolu N., İzzetoğlu M., Pektaş F., Özmen S., Demirci E., Batbat T. Combining functional near-infrared spectroscopy and EEG measurements for the diagnosis of attention-deficit hyperactivity disorder. Neural Comput. Appl. 2020;32:8367–8380. [Google Scholar]
- 16.Fuster J.M. Principles of frontal lobe function. 2013. Cognitive functions of the prefrontal cortex; pp. 11–22. [Google Scholar]
- 17.Owen A.M., McMillan K.M., Laird A.R., Bullmore E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vogel E.K., Machizawa M.G. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428:748–751. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- 19.Baddeley A. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- 20.Shanmugan S., Wolf D.H., Calkins M.E., Moore T.M., Ruparel K., Hopson R.D., Vandekar S.N., Roalf D.R., Elliott M.A., Jackson C., et al. Common and dissociable mechanisms of executive system dysfunction across psychiatric disorders in youth. Am. J. Psychiatr. 2016;173:517–526. doi: 10.1176/appi.ajp.2015.15060725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bush G. Cingulate, frontal, and parietal cortical dysfunction in attention-deficit/hyperactivity disorder. Biol. Psychiatr. 2011;69:1160–1167. doi: 10.1016/j.biopsych.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vance A., Silk T.J., Casey M., Rinehart N.J., Bradshaw J.L., Bellgrove M.A., Cunnington R. Right parietal dysfunction in children with attention deficit hyperactivity disorder, combined type: a functional MRI study. Mol. Psychiatr. 2007;12 doi: 10.1038/sj.mp.4001999. 826-32, 793. [DOI] [PubMed] [Google Scholar]
- 23.Passarotti A.M., Sweeney J.A., Pavuluri M.N. Emotion processing influences working memory circuits in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49:1064–1080. doi: 10.1016/j.jaac.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klorman R., Hazel-Fernandez L.A., Shaywitz S.E., Fletcher J.M., Marchione K.E., Holahan J.M., Stuebing K.K., Shaywitz B.A. Executive functioning deficits in attention-deficit/hyperactivity disorder are independent of oppositional defiant or reading disorder. J. Am. Acad. Child Adolesc. Psychiatry. 1999;38:1148–1155. doi: 10.1097/00004583-199909000-00020. [DOI] [PubMed] [Google Scholar]
- 25.Biederman J., Petty C.R., Fried R., Doyle A.E., Spencer T., Seidman L.J., Gross L., Poetzl K., Faraone S.V. Stability of executive function deficits into young adult years: a prospective longitudinal follow-up study of grown up males with ADHD. Acta Psychiatr. Scand. 2007;116:129–136. doi: 10.1111/j.1600-0447.2007.01008.x. [DOI] [PubMed] [Google Scholar]
- 26.Gathercole S.E., Lamont E., Alloway T.P. Working Memory and Education. Elsevier; 2006. Working memory in the classroom; pp. 219–240. [Google Scholar]
- 27.Yaple Z.A., Stevens W.D., Arsalidou M. Meta-analyses of the n-back working memory task: fMRI evidence of age-related changes in prefrontal cortex involvement across the adult lifespan. Neuroimage. 2019;196:16–31. doi: 10.1016/j.neuroimage.2019.03.074. [DOI] [PubMed] [Google Scholar]
- 28.Marx A.-M., Ehlis A.-C., Furdea A., Holtmann M., Banaschewski T., Brandeis D., Rothenberger A., Gevensleben H., Freitag C.M., Fuchsenberger Y., et al. Near-infrared spectroscopy (NIRS) neurofeedback as a treatment for children with attention deficit hyperactivity disorder (ADHD)—a pilot study. Front. Hum. Neurosci. 2014;8:1038. doi: 10.3389/fnhum.2014.01038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu W.-J., Cui L.-B., Cai M., Peng Z.W., Zhang W.C., Lv S., Xu J.Y., Hu Y., Li G., von Deneen K.M., et al. A parallel-group study of near-infrared spectroscopy-neurofeedback in children with attention deficit hyperactivity disorder. Psychiatr. Res. 2022;309 doi: 10.1016/j.psychres.2021.114364. [DOI] [PubMed] [Google Scholar]
- 30.Steiner N.J., Frenette E.C., Rene K.M., Brennan R.T., Perrin E.C. Neurofeedback and cognitive attention training for children with attention-deficit hyperactivity disorder in schools. J. Dev. Behav. Pediatr. 2014;35:18–27. doi: 10.1097/DBP.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 31.Blume F., Quixal M., Hudak J., Dresler T., Gawrilow C., Ehlis A.C. Lernen und Lernstörungen; 2020. Development of reading abilities in children with ADHD following fNIRS-neurofeedback or EMG-biofeedback. [Google Scholar]
- 32.Chen W.-L., Wagner J., Heugel N., Sugar J., Lee Y.W., Conant L., Malloy M., Heffernan J., Quirk B., Zinos A., et al. Functional near-infrared spectroscopy and its clinical application in the field of neuroscience: advances and future directions. Front. Neurosci. 2020;14:724. doi: 10.3389/fnins.2020.00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsow F., Kumar A., Hosseini S.H., Bowden A. A low-cost, wearable, do-it-yourself functional near-infrared spectroscopy (DIY-fNIRS) headband. HardwareX. 2021;10 doi: 10.1016/j.ohx.2021.e00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fullen T., Jones S.L., Emerson L.M., Adamou M. Psychological treatments in adult ADHD: a systematic review. J. Psychopathol. Behav. Assess. 2020;42:500–518. [Google Scholar]
- 35.Begemann M.J., Florisse E.J., van Lutterveld R., Kooyman M., Sommer I.E. Efficacy of EEG neurofeedback in psychiatry: A comprehensive overview and meta-analysis. Translational Brain Rhythmicity. 2016;1:19–29. [Google Scholar]
- 36.Hosseini S.M.H., Pritchard-Berman M., Sosa N., Ceja A., Kesler S.R. Task-based neurofeedback training: a novel approach toward training executive functions. Neuroimage. 2016;134:153–159. doi: 10.1016/j.neuroimage.2016.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gioia G.A., Isquith P.K., Guy S.C., Kenworthy L. BRIEF: Psychological Assessment Resources Odessa, FL. 2000. Behavior rating inventory of executive function. [Google Scholar]
- 38.Adams W.P., Ahrens R.C., Chen M.L., Christopher D., Chowdhury B.A., Conner D.P., Dalby R., Fitzgerald K., Hendeles L., Hickey A.J., et al. Wide-Range Assessment of Memory and Learning. J. Aerosol Med. Pulm. Drug Deliv. 2010;23:1–29. doi: 10.1089/jamp.2009.0803. [DOI] [PubMed] [Google Scholar]
- 39.Aasted C.M., Yücel M.A., Cooper R.J. Anatomical guidance for functional near-infrared spectroscopy: AtlasViewer tutorial. Neurophotonics. 2015;2 doi: 10.1117/1.NPh.2.2.020801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernandez S.M., Pollonini L. Optica Publishing Group; 2020. NIRSplot: A Tool for Quality Assessment of fNIRS Scans. Optics and the Brain. [Google Scholar]
- 41.Santosa H., Zhai X., Fishburn F., Sparto P.J., Huppert T.J. Quantitative comparison of correction techniques for removing systemic physiological signal in functional near-infrared spectroscopy studies. Neurophotonics. 2020;7 doi: 10.1117/1.NPh.7.3.035009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santosa H., Zhai X., Fishburn F., Huppert T. The NIRS brain AnalyzIR toolbox. Algorithms. 2018;11:73. doi: 10.3390/a11050073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gagnon L., Yücel M.A., Dehaes M., Cooper R.J., Perdue K.L., Selb J., Huppert T.J., Hoge R.D., Boas D.A. Quantification of the cortical contribution to the NIRS signal over the motor cortex using concurrent NIRS-fMRI measurements. Neuroimage. 2012;59:3933–3940. doi: 10.1016/j.neuroimage.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data
The data supporting the findings of this study, including neuroimaging (fNIRS and fMRI) and behavioral performance data, will be available upon request from the corresponding author, Hadi Hosseini (email: hosseiny@stanford.edu).
Code
All custom code used for data analysis, including the fNIRS and fMRI processing pipelines, is available upon request. The code will be provided by the lead contact and can be shared via GitHub or other repository upon request.
Additional information
Any additional materials or resources required for reanalysis of the data reported in this study are available upon request from the lead contact.