Abstract
Breast cancer has become the most prevalent malignant tumor worldwide and remains one of the leading causes of cancer-related mortality among women globally. The prognosis for patients with metastatic breast cancer remains poor, necessitating the exploration of novel therapeutic strategies to improve survival rates. In the era of precision medicine, antibody-drug conjugates (ADCs) have gained significant attention as a targeted therapeutic strategy in breast cancer treatment. ADCs, a relatively new treatment for breast cancer, deliver cytotoxic drugs (payloads), directly into the tumor space, turning chemotherapy into a targeted agent, which enables patients to experience significant improvements with manageable drug toxicity. For the treatment of breast cancer, there are three ADCs approved for breast cancer treatment: Trastuzumab emtansine (T-DM1), Trastuzumab Deruxtecan (T-Dxd) targeting HER-2, and Sacituzumab Govitecan (SG) targeting Trop-2. Recent clinical studies have demonstrated that the benefits of ADC therapies extend beyond HER2-positive breast cancer toinclude hormone receptor (HR)-positive breast cancer, triple-negative breast cancer (TNBC), and HER2-low expressing breast cancer. Notably, the DESTINY-Breast series of studies, particularly focusing on T-Dxd, encompass neoadjuvant, adjuvant, and multiple lines of therapy for advanced breast cancer. This marks the advent of a comprehensive ADC era in breast cancer treatment. This review summarizes the efficacy and adverse effects of ADC therapies that have completed or are currently undergoing phase I-III clinical trials. Additionally, it analyzes potential combination strategies to overcome ADC resistance, aiming to provide clinicians with a comprehensive clinical guide to the use of ADCs in breast cancer treatment.
Keywords: Antibody-drug conjugate, Breast cancer, HER2-Low, HER2-Ultra-low, HER2, Trop2, Targeted therapy
Highlights
-
•
ADCs are transforming breast cancer (BC) treatment with unprecedented precision.
-
•
ADCs are expanding the therapeutic potential to HER2-low, ultra-low, and negative BC.
-
•
ADCs are poised to become a standard of care across various stages of BC.
-
•
Personalized ADC treatment strategies represent the future of BC therapy.
-
•
A Combination strategy and novel payload are essential to overcoming drug resistance.
1. Introduction
Breast cancer has emerged as the foremost prevalent malignancy among women globally [1]. Historically, the evolution of treatment modalities for breast cancer has been significantly influenced by advancements in molecular biology. Postoperative chemotherapy employing cytotoxic agents was the cornerstone of therapy aimed at minimizing tumor recurrence. Nevertheless, the limitations of chemotherapy, characterized by a narrow therapeutic window, pronounced systemic toxicity, and the propensity for drug resistance, necessitated alternative approaches [2]. Enhanced understanding of cellular carcinogenesis mechanisms, coupled with advancements in monoclonal antibody production technologies, has pivoted anti-tumor drug development towards targeted therapies targeting proteins that facilitate breast cancer cell growth, dissemination, and proliferation [3]. ADCs, colloquially termed "molecular missiles"—have undergone extensive research, particularly for Her-2-positive breast cancers [4]. ADCs ingeniously combine small-molecule cytotoxins with large-molecule monoclonal antibodies, yielding potent anti-tumor efficacy with minimal systemic toxicity. Recently, the exploration of cellular oncogenic signal transduction pathways and tumor markers has broadened the target repertoire of ADCs, exemplified by the advent of SG targeting Trop-2 in breast cancer, heralding the clinical emergence of novel targets [5]. This development underscores the potential of ADCs to undergo continual refinement and expansion, catering to new patient cohorts. This review aims to summarize the ADCs approved for breast cancer and describe the potential ADCs under investigation and new strategies of ADC in treating breast cancer.
2. Basic characteristics of ADC
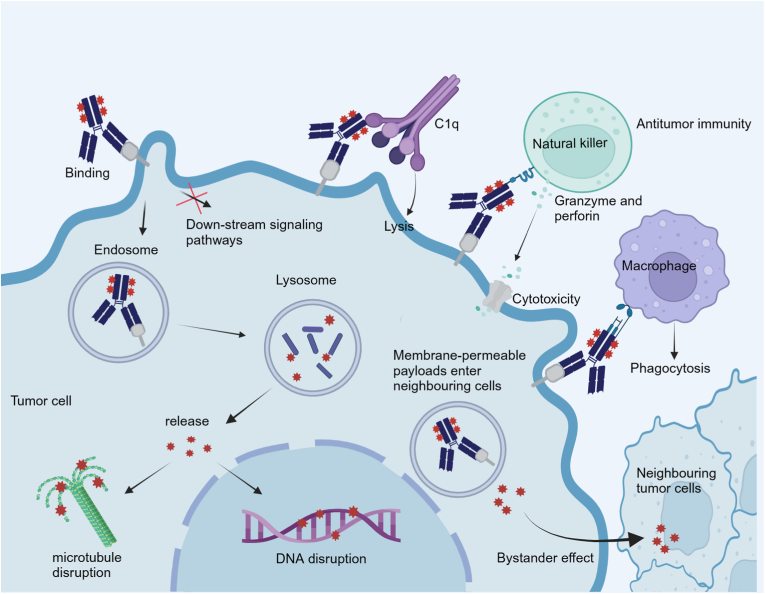
ADC represents a pivotal advancement in targeted cancer therapy, combining a monoclonal antibody specific to tumor antigens, a cytotoxic payload, and a linker. Upon administration, the antibody component of an ADC specifically binds to its antigen on the tumor cell surface, facilitating internalization via clathrin-mediated endocytosis [6]. This is followed by lysosomal degradation, releasing the payload within the cell to exert its cytotoxic effects, either by disrupting DNA or inhibiting tubulin function, thereby halting tumor cell division (Fig. 1). This dual mechanism—antigen-targeted blockade and payload-mediated cytotoxicity—enables ADCs to deliver targeted therapy with potentially superior clinical efficacy compared to conventional monoclonal antibodies or their fragments.
Fig. 1.
Schematic presentation of the mechanism of action for an ADC.
ADCs with different targets have different killing mechanisms which determine where the drug will be released and the adaptive populations. Her-2 overexpression occurs in 20%–30 % of breast tumors and predicts a poor clinical prognosis [7]. Therefore, Her-2 targeted ADCs, T-DM1 and T-Dxd, which have been approved by the U.S. Food and Drug Administration (FDA), the mechanism includes two parts: one is the anti-tumor effect mediated by trastuzumab: the Fab segment can block the extracellular domain of Her-2 on the cell surface and inhibit the proliferation of tumor cells by inhibiting the PI3K/AKT signaling pathway [8,9]; The Fc segment can induce antibody-dependent cell-mediated cytotoxicity (ADCC) to kill tumor cells [10]. The second is the anti-tumor effect mediated by payload. The payloads currently under development are mainly limited to tubulin inhibitors, DNA damage agents, and immunomodulators [11]. The payload of T-DM1 can block mitosis by destroying the microtubule network of target cells, resulting in mitotic disaster or apoptosis to inhibit tumor cell proliferation [12]. The second Her-2 targeted ADC approved for breast cancer, T-Dxd, the payload is a derivative of exatecan, has stronger membrane penetration, and can penetrate neighboring cells to eliminate neighboring antigen-negative tumor cells, a process known as the bystander effect, this process bears significance for tumor cells characterized by heterogeneous antigen expression [13].
Transmembrane calcium signal transducer Trop-2 is highly expressed in multiple tumor types, most notably in more than 90 % of breast cancer [14,15]. Trop-2 is related to a variety of transcription factors, resulting in the dysregulation of related pathways. It can increase the expression of CREB1, Jun, NF-κB, Rb, STAT1, and STAT3 by activating CyclinD1 and ERK/MEK pathways, thereby mediating tumor cell deterioration and metastasis [16]. Studies have shown that the use of small interfering RNA (siRNA) to silence the Trop-2 gene in breast cancer cell models can inhibit tumor cell deterioration, proliferation, invasion, and cell colony formation in vitro [17,18]. Therefore, Trop-2 as the target of SG can effectively block these signaling pathways, and the highly toxic payload SN-38 also has the bystander effect to kill the tumor cells with low expression of antigen [19,20].
3. For HER-2 positive breast cancer
3.1. Trastuzumab emtansine(T-DM1, Trastuzumab/DM1)
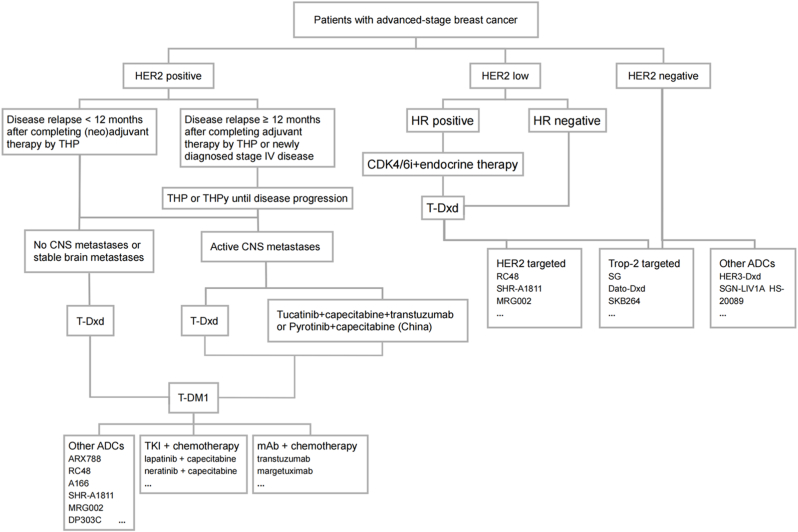
T-DM1, the first Her-2 targeted antibody-drug conjugate approved for BC, serves as a pivotal adjuvant therapy for Her-2+ BC. In the EMILIA trial, the findings revealed that the median progression-free survival (mPFS) in the T-DM1 monotherapy group exceeded that of the lapatinib plus capecitabine group by 3.2 months (9.6 months vs. 6.4 months), and median overall survival (OS) was 30.9 months compared to 25.1 months, respectively. This demonstrated a 32 % reduction in mortality risk with T-DM1 treatment [21]. Following this study, in 2013, FDA approved T-DM1 as the standard second-line treatment for patients with metastatic (Fig. 2), Her-2+ BC [22,23]. Subsequent research, the TH3RESA trial, evaluating T-DM1 against the treatment of physician's choice (TPC), supported these conclusions, showing a significant improvement in mPFS with T-DM1 (6.2 months vs. 3.3 months). The final mOS analysis indicated a considerable increase in survival for the T-DM1 group (22.7 months vs. 15.8 months) [24]. Importantly, the TH3RESA trial enrolled individuals who had received at least two prior anti-Her-2 therapies, suggesting the benefits of T-DM1 even after multiple lines of targeted Her-2 therapy. Further investigation has been conducted into the potential benefits of T-DM1 in earlier stages of treatment, specifically in the adjuvant setting. The KATHERINE trial found that for patients who did not achieve a pathological complete response (pCR), T-DM1 substantially lowered the recurrence risk by 50 % and increased the absolute invasive disease-free survival (iDFS) benefit by 11.3 % compared to trastuzumab, after a median follow-up of 41 months [25]. Consequently, the FDA has approved T-DM1 for monotherapy in the adjuvant treatment of Her-2+ early BC in patients with residual invasive disease post-neoadjuvant therapy involving taxanes and trastuzumab [26]. Moreover, the efficacy of T-DM1 in comparison to traditional chemotherapy combined with targeted therapy regimens in the neoadjuvant setting was assessed in the KRISTINE trial. It reported a higher probability of event-free survival (EFS) events in the T-DM1 group (13.9 %) compared to the control group (5.9 %), indicating that the traditional chemotherapy combined with dual anti-Her-2 blockade maintains its superiority [27]. Furthermore, the KAMILLA trial's exploratory subgroup analysis, representing the largest cohort of Her-2+ BC patients treated with T-DM1 in a prospective study, showed a median PFS and OS of 6.8 and 27.2 months, respectively [28].
Fig. 2.
Treatment algorithm for patients with advanced-stage breast cancer. T: taxane, H: transtuzumab, P: pertuzumab, Py: pyrotinib, CDK4/6i: CDK4/6 inhibitor.
Overall, the efficacy of T-DM1 in the adjuvant treatment of Her-2-positive early breast cancer and the multi-line treatment of advanced breast cancer is proved by robust evidence from trials such as EMILIA and TH3RESA. However, its comparative effectiveness against traditional chemotherapy, in conjunction with dual anti-Her-2 blockade as a neoadjuvant therapy, remains to be conclusively determined.
3.2. Trastuzumab deruxtecan(T-Dxd, trastuzumab/Dxd)
T-Dxd represents a pivotal shift in the therapeutic landscape for Her-2+ BC, emerging as a viable option for subsequent lines of therapy. In the DESTINY-Breast 01 study, patients with advanced Her-2 positive breast cancer treated with T-Dxd had a remarkable mPFS of 16.4 months [29]. Further validation came from the DESTINY-Breast 02 study, which supplemented the OS data for T-Dxd, the median OS of the T-Dxd group and the TPC group was 39.2 months and 26.5 months, respectively [30]. Moreover, data from the DESTINY-Breast 03 study unveiled that T-Dxd, when compared to T-DM1, significantly prolonged PFS in patients with advanced BC who had not responded to first-line therapy with trastuzumab, pertuzumab, and a taxane (THP), recording a PFS more than fourfold longer (28.8 months versus 6.8 months), at the 2024 ASCO Congress, the study published OS results, with 52.6 months of OS in the T-Dxd group, significantly longer than 42.7 months in the T-DM1 group [31,32]. In December 2019, FDA granted accelerated approval to T-Dxd for adult patients with unresectable or metastatic Her-2+ solid tumors who have received prior systemic treatment and have no satisfactory alternative treatment options. Historically, monoclonal antibody therapies have demonstrated limited efficacy in patients with brain metastases (BMs). However, T-Dxd has transformed the treatment landscape for these patients. The recent DESTINY-Breast12 single-arm clinical trial further substantiated this potential, yielding promising results. In the cohort with BMs, the 12-month PFS rate was 61.6 % (95 % confidence interval [CI]: 54.9–67.6), while the 12-month central nervous system (CNS) PFS was 58.9 % (95 % CI: 51.9–65.3). Notably, among patients with active brain metastases, the 12-month PFS rate was even more impressive, reaching 66.7 % (95 % CI: 53.4–76.9) [33]. In comparison, the best prior results were observed in the HER2CLIMB trial, where the combination of tucatinib, trastuzumab, and capecitabine achieved a 12-month PFS of only 35 % [34]. Currently, no head-to-head clinical trials have compared T-Dxd with tucatinib, leaving the relative efficacy of these agents inconclusive. Nonetheless, we can derive some clinical insights from the patient populations in both studies. The DESTINY-Breast12 trial included a higher proportion of untreated patients with active brain metastases (33.05 %), and these individuals achieved a 12-month PFS of 47 % with T-DXd, whereas the HER2CLIMB trial primarily enrolled previously treated patients. Additionally, according to the results of the PERMEATE study, the combination of pyrotinib and capecitabine has also shown promising efficacy in the Chinese population with brain metastases, with a median PFS of 11.3 months [35](Fig. 2).
3.3. Trastuzumab duocarmazine (SYD985, trastuzumab/duocarmycin)
SYD985 incorporates the duocarmycin derivative as its payload through an innovative technology platform. This platform engineers a seco-DUBA structure, enhancing water solubility and optimizing alkylation rates. The drug's linker, a peptide susceptible to cleavage by cathepsin B within cellular environments, safeguards the seco-DUBA structure, thus ensuring its high stability in the bloodstream and minimizing off-target toxicity [36]. Recent outcomes from the TULIP trial, part of the ongoing Phase 3 clinical evaluation of SYD-985 in patients with Her-2+ BC, were unveiled at the 2023 ESMO Congress. These findings demonstrate that SYD985 significantly extends PFS compared to the control group (7.9 vs 4.9 months, HR = 0.63) [37]. While the survival benefits confirm SYD-985's efficacy in treating Her-2-positive breast cancer, its performance does not surpass that of the already available drug, T-Dxd. This finding could help to explain why, despite its earlier development, the FDA has not yet approved SYD985. SYD985 is highly effective in treating heterogeneous tumors via the bystander effect. It has a 54 times greater killing effect on Her-2 negative cells compared to T-DM1 [38]. Further research is necessary to determine whether SYD-985 can produce positive outcomes in populations with low Her-2 expression.
3.4. FS-1502(Trastuzumab/MMAF)
FS-1502, leveraging Monomethyl auristatin F as its payload—a member of the tubulin inhibitors class—disrupts the cytoskeletal structure of tumor cells, impeding mitotic division. This cytotoxic agent exhibits a potency 100 to 1000 times greater than that of doxorubicin. In a Phase I dose-escalation trial (NCT03944499) targeting advanced Her-2-overexpressing solid tumors, FS-1502 demonstrated favorable tolerability and significant antitumor efficacy [39]. Specifically, among patients with Her-2+ BC who had not responded to multiple lines of therapy, the ORR reached 53.7 %, with a mPFS of 15.5 months. Notably, only mild ocular toxicity was reported, and no instances of interstitial lung disease were observed. Currently, a Phase III clinical trial (NCT05755048) is in progress to further assess the effectiveness and safety of FS-1502 in treating advanced Her-2+ BC.
3.5. A166(Trastuzumab/Duo-5)
A166, an innovative Her-2-targeted ADC, integrates trastuzumab with the microtubule inhibitor auristatin derivative, duostatin-5 (Duo-5), through a cleavable linker [40]. In this phase I study, at the recommended dose, the drug demonstrated a good safety profile with an ORR of 73.9 % and a median PFS of 12.3 months [41]. On May 11, 2023, the National Medical Products Administration (NMPA) accepted the marketing application for A166 for the treatment of Her-2-positive unresectable locally advanced, recurrent, or metastatic breast cancer following failure of second-line or higher anti-Her-2 therapies. To determine whether it can be used as an alternative to T-DM1 or overcome drug resistance in ADCs, we must await the results of the phase III clinical trial (CTR20231740).
3.6. SHR-A1811(Trastuzumab/SHR9265)
SHR-A1811, a domestically developed ADC in China, exhibits a structural similarity to T-Dxd. Characterized by its innovative payload, SHR169265, and an optimized DAR, SHR-A1811 has demonstrated an impressive pharmacokinetic profile along with a favorable preclinical safety profile [42]. Notably, phase I clinical trial results revealed that SHR-A1811 achieved an ORR of 81.5 % for the follow-up treatment of Her-2+BC, compared to the 62 % ORR reported for T-Dxd in the DESTINY-Breast01 trial. These findings position SHR-A1811 as a potential best-in-class anti-Her-2 ADC [43,44].
3.7. ARX788(Trastuzumab/MMAF)
ARX788 intricately conjugates a monoclonal antibody specific to Her-2 with Ambrastatin269, a potent cytotoxic agent targeting tubulin [45]. The initial findings from a Phase I clinical trial (CTR20171162) underscored ARX788's capacity to attain a satisfactory ORR in patients, while maintaining a safety profile devoid of dose-limiting toxicity or serious adverse effects [46]. Bolstering its potential further, data from a Phase II trial (NCT04829604) revealed an ORR of 51.7 % and a disease control rate (DCR) of 100 % in patients with Her-2+ metastatic BC, including those resistant or refractory to T-DM1. In the latest results of Phase II/III trial ACE-Breast-02, ARX788 significantly extended PFS compared to lapatinib plus capecitabine in patients with HER2+ advanced breast cancer previously treated with trastuzumab and taxane (11.33 months vs 8.25 months, HR = 0.64, P = 0.0006) [47,48]. Advancing on these promising results, a Phase III clinical trial (NCT05426486) is currently in progress. This trial is designed to assess the combined efficacy and safety of ARX788 and pyrotinib as a neoadjuvant therapy for Her-2+ BC, further cementing ARX788's potential as a foundational element in treating this formidable disease.
3.8. MRG002(MAB802/MMAE)
MRG002, employing the Monomethyl auristatin E payload, has demonstrated initial anti-tumor efficacy in its first-in-human (FIH) study (MRG002-001) conducted in China, specifically in patients with Her-2+, late-stage BC. The ORR for 47 patients with evaluable HER2-positive breast cancer was 53 %, for 23 patients with liver metastasis it was 61 %, and for 5 patients with liver metastasis combined with brain metastasis it was 60 %, indicating that this drug has a high response rate in patients with metastasis, warranting further investigation [49]. A Phase III clinical trial is now in progress to assess the efficacy and safety of MRG002 for the treatment of Her-2+, unresectable locally advanced or metastatic BC.
3.9. RC-48(Hertuzumab/MMAE)
RC-48 is the first innovative ADC developed independently in China to enter clinical research. This innovative therapeutic has garnered regulatory approval for two distinct indications: the treatment of Her-2-overexpressing locally advanced or metastatic gastric cancer and gastroesophageal junction adenocarcinoma following at least two systemic chemotherapy regimens, as well as Her-2+ locally advanced or metastatic urothelial carcinoma in patients previously treated with systemic chemotherapy [50]. In the realm of breast cancer, the ASCO released pivotal data in 2021 concerning patients with either Her-2+ or Her-2-low expressing breast cancer. The findings highlighted a superior benefit-risk profile within the 2.0 mg/kg dosing cohort. Furthermore, the results from the C001 study demonstrated a notable ORR of 42.9 % in patients with metastatic Her-2+ BC, accompanied by a median PFS of 5.7 months [51].
4. For HER-2 low breast cancer
Breast cancer is classified as Her-2-positive if there is evidence of Her-2 overexpression, indicated by a score of 3+ in immunohistochemistry (IHC) assays, or gene amplification as detected by in situ hybridization (ISH) assays in at least one tumor sample. Conversely, the absence of these markers categorizes BC as Her-2-negative. Recently, a revised nomenclature has been suggested for cases exhibiting IHC scores of 1+ or 2+ coupled with negative ISH results, now termed Her-2-low BC [52]. Subsequent studies have revealed that this subtype may benefit from Her-2-targeted therapies, as demonstrated by the efficacy of novel anti-Her-2 agents like T-Dxd.
4.1. T-Dxd
Based on the membrane-permeable nature of the payload and the properties of the linker, T-Dxd can provide cytotoxic activity against off-targeted cancer cells, called the bystander effect. The pivotal Phase III DESTINY Breast04 (DB04) trial, involving participants with low Her-2 expression, showcased significant improvements in OS and PFS across various hormone receptor statuses, with T-Dxd outperforming the treatment of physician's choice [53]. Subsequently, the NMPA approved the indication for T-Dxd: monotherapy for adult breast cancer patients with unresectable or metastatic Her-2 low expression (IHC 1+ or IHC 2+/ISH-) who have previously received at least one systemic therapy in the metastatic stage of disease or who relapsed during or within 6 months after completion of adjuvant chemotherapy (Fig. 2).
4.2. SHR-A1811
The ORR of SHR-A1811 when used for posterior line treatment of breast cancer with low Her-2 expression can reach 55.8 %. In terms of safety, the incidence of interstitial pneumonia caused by T-Dxd has been condemned by the public, whereas the incidence of SHR-A1811 is just 3.2 % [43]. In addition, Phase III clinical trials (NCT05814354, NCT06126640) are underway in patients with different subtypes of breast cancer to evaluate whether SHR-A1811 has a dual benefit in survival time and quality of life compared to conventional therapies.
4.3. MRG-002
In preclinical studies, MRG002 demonstrated efficacy against BC characterized by low Her-2 expression. Subsequently, a Phase II trial was initiated to assess both the safety and antitumor activity of MRG002 in this patient subset. The trial, involving 49 evaluable patients with low Her-2 expression, reported an ORR of 34.7 % and a DCR of 75.5 %. Notably, the study included 8 patients with TNBC who had undergone at least two prior lines of therapy. The results from this subgroup were particularly promising, further substantiating the significant efficacy and safety of MRG002 for BC patients exhibiting low Her-2 expression [54].
4.4. RC-48
Due to its remarkable bystander effect, RC48 also demonstrated obvious efficacy in BC with low Her-2 expression. The Phase Ib/II trial C003 CANCER (NCT03052634) enrolled 48 patients with Her-2 low expression, to receive treatment with RC48. The ORR and mPFS for patients treated with a dose of 2.0 mg/kg were 39.6 % and 5.7 months, respectively. Overall, RC48 has shown promising potential in treating Her-2-negative BC, potentially bridging a significant gap in current treatment options [51].
5. For HER2-ultro-low/negative breast cancer (ADC drugs targeting Trop-2)
5.1. Sacituzumab govitecan (SG, IMMU-132, hRS7/SN-38)
SG is the first Trop-2-targeted ADC approved by the FDA, demonstrating significant efficacy in patients with Her-2-negative BC, which include HR+/HER-2 BC patients and TNBC patient [55]. TROPiCS-02 study indicated that the SG group achieved an extension of mPFS over the TPC group (5.5 months vs 4.0 months, P = 0.0003). And the OS data for SG and TPC was 14.5 months vs. 11.2 months (P = 0.01).The patients included in this study were all treated with CDK4/6 inhibitors and had received 2–4 lines of chemotherapy, suggesting that SG could be a new treatment option for HR+/Her-2-BC patients with poor response to endocrine therapy [56]. Additionally, the accelerated approval of SG in 2020 has introduced a targeted treatment alternative for TNBC. In the IMMU-132-01 study, 108 patients with metastatic TNBC treated with SG as a third-line or subsequent therapy showed an ORR of 33 %, with significant improvements in mPFS and OS—5.5 months and 13 months, respectively [57]. In the phase III ASCENT trial, SG significantly outperformed single-agent chemotherapy chosen by physicians, with the mPFS at the primary endpoint reaching 4.8 months versus 1.7 months, and the mOS at the secondary endpoint extending to 11.8 months versus 6.9 months [58]. These results underscore SG's potential as an effective option for treating advanced TNBC post-initial treatment.
5.2. Datopotamab deruxtecan (DS-1062, hTINA1/Dxd)
Datopotamab deruxtecan is an innovative ADC targeting Trop-2, currently under clinical trial. It is specifically designed to address Her-2-negative BC. The first Phase III data from the TROPION-Breast01 trial, involving HR+/Her-2- advanced BC, was unveiled at the 2023 ESMO Meeting. The results revealed that the median PFS in the Dato-DXd group was significantly superior than that in the investigator's choice of chemotherapy group (6.9 months vs 4.9 months) [59]. Additionally, the use of Dato-DXd extends to the treatment of TNBC which has a higher expression of Trop-2. Ongoing clinical trials include TROPION-Breast02, which compares Dato-DXd's efficacy and safety against chemotherapy in the first-line treatment of unresectable locally advanced or metastatic TNBC unsuitable for PD-1/PD-L1 inhibitor therapy [60]. Another trial, TROPION-Breast03, is evaluating Dato-DXd as postoperative adjuvant therapy in TNBC patients with residual lesions after neoadjuvant therapy.
5.3. SKB-264
The first phase II clinical data for SKB-264 showed that the monotherapy was effective in treating metastatic TNBC after multiple prior treatments [61]. As a result, SKB-264 has received two "breakthrough therapy" designations from the Center for Drug Evaluation of the NMPA. These designations are for the treatment of locally advanced or metastatic TNBC, as well as locally advanced or metastatic HR+/Her-2- BC that has previously undergone at least second-line chemotherapy. Ongoing clinical trials are examining whether SKB-264, alone (NCT05347134, NCT06081959) or in combination with other therapies, such as PD-L1 monoclonal antibody KL-A167 (NCT05445908), can offer improved survival benefits for patients with advanced TNBC and advanced HR+/Her-2- BC.
6. Other ADCs under pre/phase-II clinical development
In the evolving landscape of BC treatment, researchers are broadening their horizons beyond the well-known Her-2 and Trop-2 targets to explore the potential of other tumor-associated antigens as novel targets for ADCs. Notably, Nectin-4, a type I membrane protein, has been identified as a promising candidate due to its role in stimulating tumor cell proliferation and invasion through activation of the PI3K/AKT pathway [62]. Similarly, the interaction of the membrane protein associated with folate metabolism, FRα, and its interaction with LYN tyrosine kinase plays a pivotal role in tumor genesis by regulating PEAK1 phosphorylation and consequently activating ERK and STAT3 signaling pathways [63]. Additionally, the immunomodulatory molecule B7-H4 presents a significant interest in its ability to suppress anti-tumor T cell activity, leading to T cell exhaustion or dysfunction, and thus facilitating tumor immune evasion [64]. These emerging targets, supported by preclinical research, herald a new development frontier for the next generation of ADC therapeutics. We summarized the potential ADC medicines based on their respective targets (Table 1), which is an important step forward in the search for more effective breast cancer treatments.
Table 1.
Potential ADC medicines based on their respective targets.
| Drug | Target | Linker | Payload | DAR | Phase | Clinical trial number | Disease status |
|---|---|---|---|---|---|---|---|
| ALT-P7 | HER-2 | Cleavable | Microtubule polymerization inhibitor, auristatin analogueMMAE | 2 | Ⅰ | NCT03281824 | R/R Advanced HER2+ BC |
| PF-06804103 | HER-2 | Cleavable | Microtubule polymerization inhibitor, auristatin analogueAur0101 | 4 | Ⅰ | NCT03284723 | R/R Advanced BC |
| ZW49 | HER-2 | Cleavable | Microtubule polymerization inhibitor, auristatin-basedpayload | NA | Ⅰ | NCT03821233 | R/R HER2+ cancer |
| XMT-1522 | HER-2 | Cleavable | Microtubule polymerization inhibitor, AF-HPA moiety | 12 | Ⅰ | NCT02952729 | R/R HER2+ cancer |
| BDC-1001 | HER-2 | Non-cleavable | TLR7/8 inhibitor | NA | Ⅱ | NCT04278144 | R/R Advanced HER2 expressing cancer |
| BB-1701 | HER2 | Cleavable | Eribulin | NA | Ⅱ | NCT06188559 | R/R Advanced HER2 ± BC |
| GQ1001 | HER2 | NA | Microtubule polymerization inhibitor, maytansinoidderivative DM1 | 2 | Ⅱ | NCT04450732 | R/R Advanced HER2+ cancer |
| NCT05575804 | R/R Advanced HER-2 BC | ||||||
| U3-1402 |
HER-3 |
Cleavable |
TOP1 inhibitor, camptothecin analogue DXd |
8 |
Ⅰ | NCT04610528 | Preoperative hormone receptor+/HER2-BC |
| Ⅱ | NCT04699630 | R/R mBC | |||||
| Ⅱ |
NCT02980341 |
R/R HER3+mBC |
|||||
| SGN-LIV1 | LIV-1 | Cleavable | Microtubule polymerization inhibitor, auristatin analogueMMAE | 4 | Ⅱ | NCT03310957 | First line mTNBC |
| Ⅰ | NCT01969643 | R/R mBC | |||||
| MORAb-202 | FRα | Cleavable | Microtubule inhibitor, eribulin | 4 | Ⅱ | NCT04300556 | R/R Advanced solid tumors |
| PRO1184 | FRα | Cleavable | Exatecan | 8 | Ⅱ | NCT05579366 | R/R Advanced solid tumors |
| BAT8006 | FRα | Cleavable | Topoisomerase I inhibitor (TOP1i) | 8 | Ⅰ | NCT05378737 | R/R Advanced solid tumors |
| CX-2009 | CCD166 | Cleavable | Microtubule polymerization inhibitor, maytansinoid derivative DM4 |
3.5 | Ⅱ | NCT04596150 | R/R Advanced HER-2 BC |
| Ⅱ | NCT03149549 | R/R Advanced solid tumors | |||||
| ASG-22ME | Nectin-4 | Cleavable | Microtubule polymerization inhibitor, auristatin analogueMMAE | 3–4 | Ⅱ | NCT04225117 | R/R Advanced solid tumors |
| NBE-002 | ROR1 | Non-cleavable | Highly potent anthracycline derivative PNU-19682 | NA | Ⅰ | NCT04410224 | R/R Advanced solid tumors |
| BA3021 | ROR2 | Cleavable | Microtubule polymerization inhibitor, auristatin analogueMMAE | NA | Ⅱ | NCT03504488 | R/R Advanced solid tumors |
| PF-0664720 | PTK7 | Cleavable | Microtubule polymerization inhibitor, auristatin-based payload Aur0101 |
4 | Ⅰ | NCT02222922 | R/R Advanced solid tumors |
| AZD8205 | B7-H4 | Cleavable | topoisomerase I inhibitor (TOP1i) | 8 | Ⅱ | NCT05123482 | R/R Advanced solid tumors |
| SGN-B7H4V | B7-H4 | Cleavable | Microtubule polymerization inhibitor, auristatin analogueMMAE | NA | Ⅰ | NCT05194072 | R/R Advanced solid tumors |
| PYX-201 | ED-B | Cleavable | Microtubule polymerization inhibitor, auristatin-based payload Aur0101 |
4 | Ⅰ | NCT05720117 | R/R Advanced solid tumors |
| MEN-1309 | CD205 | Cleavable | Microtubule polymerization inhibitor, maytansinoid derivative DM4 |
3.7 | Ⅰ | NCT04064359 | R/R Advanced solid tumors |
| IMGC936 | ADAM9 | Cleavable | maytansinoid linker-payload,DM21-C | 2 | Ⅱ | NCT04622774 | R/R Advanced solid tumors |
| ASN-004 | 5T4 | NA | Auristatin F hydroxypropylamide (AF-HPA) | 10–12 | Ⅰ | NCT04410224 | R/R Advanced solid tumors |
| TH1902 | SORT1 | Cleavable | Docetaxel | 2 | Ⅰ | NCT04706962 | R/R Advanced solid tumors |
Abbreviations: DAR, drug-antibody ratio; FRα, folate receptor alpha; HER, human epidermal growth factor receptor; MMAE, monomethyl auristatin E; MMAF, monomethyl auristatin F; NA, not acquired; PTK7, protein tyrosine kinase 7; ROR, receptor tyrosine kinase orphan receptor; TOP, topoisomerase; TROP2, trophoblast cell surface antigen 2; R/R, relapse/refractory.
7. ADC combination medication regimens
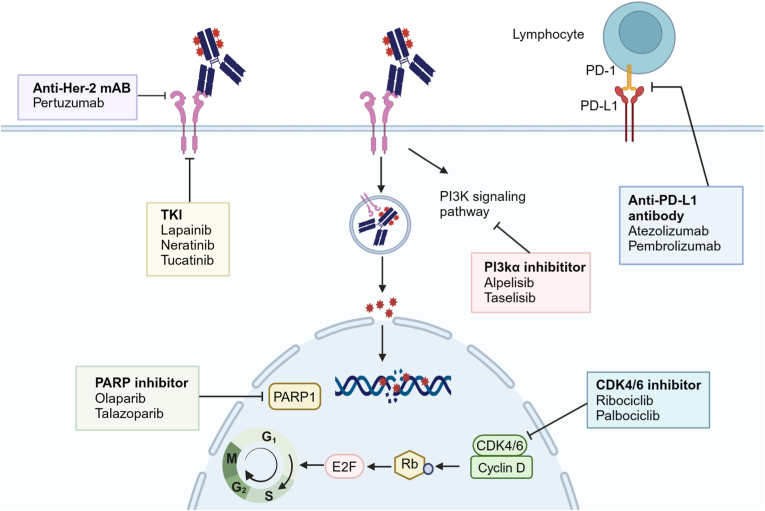
As new targets are explored, the range of indications for ADCs is expanding. However, in clinical settings, resistance to drugs like T-DM1 remains an issue for some patients. Drug resistance can manifest at various stages, including the downregulation or loss of antigen expression, defects in endocytic trafficking pathways, impaired lysosomal function, and limited toxicity of the payload [65]. We have investigated several combination drug regimens to counteract ADC resistance and enhance its efficacy (Fig. 2). The optimal strategy involves selecting combinations that produce additive or synergistic effects on tumor cells or the tumor microenvironment while avoiding overlapping toxicities.
7.1. Combine with chemotherapeutics
Many ADCs utilize microtubule inhibitors as payloads. To enhance the blockade of the G2/M phase of the cell cycle, DNA-damaging agents can be combined with microtubule inhibitors, with improved efficacy noted when microtubule inhibitors are used sequentially after DNA-damaging agents [66,67]. Additionally, chemotherapy may influence the expression of surface antigens targeted by ADCs. For instance, in pancreatic cancer cells, combining gemcitabine with T-DM1 has shown increased efficacy due to gemcitabine's ability to up-regulate Her-2 expression [68]. In a phase Ib/IIa study, the combination of T-DM1 and Paclitaxel for Her-2+ BC resulted in an ORR of 47.8 % and a mPFS of 7.4 months [69]. However, this combination treatment significantly increased toxicity. Another study involving T-DM1 and Pegylated doxorubicin reported an ORR of 40 % [70]. These findings indicate that while ADC and chemotherapy combinations may offer survival benefits, the associated increase in toxic side effects cannot be overlooked [71].
7.2. Combine with targeted therapies
ADCs, when combined with targeted therapies, can significantly improve the inhibition of oncogenic signaling pathways. This combination enhances the utilization of surface antigens and increases the sensitivity of tumors with low antigen expression, while also modifying the tumor microenvironment [72]. Research is ongoing to explore the potential synergies between ADCs and existing targeted treatments, including macromolecular monoclonal antibodies, small molecule TKIs, PI3K inhibitors (PI3Ki), CDK4/6i, and PARP inhibitors (PARPi).
The monoclonal antibody pertuzumab binds to domain II of the extracellular region of the Her-2 receptor, inhibiting its ability to form dimers with other HER receptors, particularly the Her-2-HER3 complex. This prevents the ligand regulator NRG-1β from diminishing the cytotoxic effects of T-DM1 in BC cell lines [73]. In a Phase Ib/IIa trial combining T-DM1, pertuzumab, and docetaxel in patients with Her-2+ BC, the treatment significantly improved the objective response rate to 80 % compared to 43.6 % [74]. However, a subsequent Phase III trial (NCT02131064) combining T-DM1 with pertuzumab did not yield the desired outcomes. The three-year EFS rate for the combination treatment was 85.3 %, lower than the 94.2 % observed in the T-DM1 monotherapy group. The specific mechanisms behind these results require further investigation [75].
Small molecule TKIs such as lapatinib, nilotinib, and tucatinib, bind to ATP binding sites and inhibit downstream signal transduction. These inhibitors are particularly effective in enhancing the inhibition of the PI3K-AKT and MAPK pathways when used in combination with T-DM1 [[76], [77], [78]]. At the 2023 SABCS, results from the Phase III Her-2CLIMB-02 study were presented. The findings indicate that combining tucatinib with T-DM1 significantly improves PFS in patients with Her-2+ advanced BC, notably in those with brain metastases. The study showed a mPFS of 9.5 months with the combination therapy compared to 7.4 months with the control group [79]. Importantly, tucatinib plus trastuzumab and capecitabine is approved for patients with metastatic HER 2+ breast cancer who have previously received one or more HER2-targeted therapies [80]. It is also suggested as the first option for patients who have disease progression after T-Dxd treatment, especially those with active brain metastases [81,82]. However, this strategy lacks prospective cohort research evidence and requires additional investigation. Besides, excessive activation of the PAM signaling pathway is also a key mechanism of drug resistance in BC. The combination of T-DM1 and PI3Kis has been found to have a synergistic effect in inhibiting the PI3K/AKT/mTOR pathway [83]. In a Phase I study of T-DM1 combined with the PI3Ki Alpelisib for treating Her-2+ BC, the ORR was 43 %, and the mPFS was 8.1 months [84].
Additionally, CDK4/6 serves as a common downstream target for several growth-promoting signaling pathways, including RAS/MAPK, ER, and PI3K/mTOR [85]. CDK4/6i enhances cell cycle control and inhibits tumor cell proliferation by selectively targeting CDK4/6 [86]. In a phase I clinical trial, the combination of T-DM1 and the CDK4/6i Palbociclib for treating Her-2+ BC yielded an ORR of 33 % and a mPFS of 6 months [87]. When T-DM1 was combined with another CDK4/6i, Ribociclib, the ORR dropped to 16.7 % [88]. The efficacy of CDK4/6 inhibitors in combination with T-DM1 was not significant, this may be due to the CDK4/6i′s role in preventing tumor cells from entering the S/M phase, thereby diminishing the impact of T-DM1 [89].
Recently, a Phase 1b study indicates that the PARP inhibitor Rucaparib effectively disrupts DNA repair in cancer cells with BRCA gene mutations, which enhances cancer cell mortality and hampers tumor progression. The result showed that SG and Rucaparib achieved an ORR of 50 % and a CBR of 100 %, suggesting a synergistic interaction [[90], [91], [92]].
7.3. Combine with immunotherapy
Numerous studies suggest that ADCs could boost the efficacy of immunotherapy. The induction of immunogenic cell death by ADC leads to the maturation of dendritic cells, increases T-lymphocyte infiltration, and enhances immune memory. Additionally, these studies indicate increased expression of immunomodulatory proteins like PD-L1 and MHC [[93], [94], [95], [96]]. It is reported that T-DM1 could enhance tumor-specific immunity by increasing stromal tumor-infiltrating lymphocytes [97]. However, the Phase II KATE2 study, which investigated the combination of T-DM1 and Atezolizumab in treating Her-2- BC, did not yield significant improvements in PFS but instead showed a higher incidence of adverse effects [98,99]. Interestingly, a subset of patients with PD-L1 positive tumors did experience a PFS benefit, with an mPFS of 8.5 months for the T-DM1 plus Atezolizumab group compared to 4.1 months for the control group, though this finding was not statistically significant (HR = 0.60, 95 % CI: 0.32–1.11, P = 0.099) [99]. The limited sample size and variability at baseline temper these results, leaving the true benefit uncertain. Consequently, the ongoing Phase III KATE3 study continues to explore this combination [100]. Moreover, the preclinical study of Dato-Dxd has shown that combining its payload 'Dxd' with immunotherapy can enhance T-cell recognition of tumor cells, bolster the immune response, and increase anti-tumor activity, a finding supported by data from the BEGONIA study released at the 2023 ESMO [16,101]. When used with the immune checkpoint inhibitor Durvalumab in the first-line treatment of advanced TNBC, regardless of PD-L1 expression, Dato-Dxd demonstrated potential for additional benefits in ORR, warranting further investigation [102].
8. Discussion
HER2-targeted ADCs have established a pivotal role in treating both HER2-positive and HER2-low breast cancer patients, while TROP-2-targeted ADCs, such as SG, have demonstrated remarkable efficacy in TNBC. This review synthesizes clinical data to outline the therapeutic pathways for breast cancer patients treated with ADCs (Fig. 3). As depicted, ADCs exemplified by T-Dxd now span the full spectrum of breast cancer therapies. Although current clinical data for ADCs targeting HER2-low and TNBC patients are limited, the growing variety of ADCs in development holds promise for future breakthroughs. Target selection is pivotal in advancing effective ADCs [103]. While the development of HER2-targeted ADCs in breast cancer has progressed earlier and more comprehensively, a biomarker-agnostic strategy is currently applied to other ADCs targeting proteins like HER3 and Trop-2, which are highly expressed in breast cancer cells. In the TROPiCS-02 study, no significant differences in PFS and OS were observed between subgroups with high Trop-2 expression and low Trop-2 expression, potentially due to the bystander effect [104,105]. Notably, longitudinal evaluations revealed that anti-HER2 ADC agents, particularly T-DXd, displayed superior efficacy, specificity, and cytotoxicity compared to ADCs targeting non-driver oncogenes such as HER3-DXd and SG. The absolute difference in ORR among these drugs was substantial, ranging from 20% to 30 %, with mPFS and mOS differences nearly doubling. Despite similar designs between HER3-DXd and T-DXd, the primary difference lies in their antigen targets, while the linker, payload, and DAR remain consistent, highlighting the importance of target selection. Oncogenic targets significantly influence ADC behavior, potentially through enhanced internalization and ubiquitination of the target-ADC complex, reducing downregulation—a known ADC resistance mechanism that impairs drug uptake. Additionally, oncogenic drivers tend to be uniformly and highly expressed in tumor tissues due to evolutionary pressures [106]. ADCs targeting driver oncogenes also retain some intrinsic mAb functionality, impairing target protein function by blocking receptor ligands, disrupting dimerization, and inducing endocytosis and degradation. The Fc segment of ADC antibodies can engage with FcR on effector cells (e.g., NK cells, macrophages), promoting direct cytotoxic effects like ADCC, CDC, and ADCP, while inhibiting downstream antigen receptor signaling [8,107,108], reinforcing the hypothesis that ADCs targeting oncogenic or functional proteins may offer enhanced antitumor efficacy compared to those targeting non-functional proteins. Innovations in ADC drug development can be pursued in multiple dimensions: 1) Mechanism innovation: Combining novel ADC mechanisms, such as dual conjugation of cytotoxic agents or conjugating ADCs with PD-L1 inhibitors, especially for HER2-positive breast cancer. 2) Combination drug innovation: Concurrently conjugating cytotoxic agents with two large molecule monoclonal antibodies or combining ADCs with immune checkpoint inhibitors, monoclonal antibodies, or small molecule TKIs. 3) Combination therapy innovation: Integrating ADCs with immunotherapy, endocrine therapy, targeted therapy, or chemotherapy.
Fig. 3.
ADC combination regimens to address ADC resistance and enhance efficacy.
Resistance is a critical challenge in ADC therapy, driven by mechanisms such as antibody-mediated resistance, impaired drug transport, lysosomal dysfunction, and payload-specific resistance. Strategies to overcome resistance include developing drugs targeting novel mechanisms and exploring combination therapies addressing different resistance pathways. Designing effective ADC combination regimens should adhere to the following principles: minimizing additive toxicities to avoid exacerbating adverse effects, ensuring synergistic interactions to enhance therapeutic efficacy, and supporting the regimen with robust evidence-based feasibility. Current ADC combination regimens undergoing clinical trials encompass immunotherapy, endocrine therapy, targeted therapy, and chemotherapy. The advent of precision medicine has introduced a broader array of treatment options and reshaped the therapeutic landscape for breast cancer. From mechanistic innovations to clinical applications, ADCs continue to advance the frontier of breast cancer treatment, offering the potential for significant patient benefits.
CRediT authorship contribution statement
Lu Sun: Writing – review & editing, Writing – original draft, Formal analysis, Conceptualization. Xiaomeng Jia: Visualization, Methodology, Investigation, Data curation. Kainan Wang: Writing – review & editing, Methodology, Funding acquisition, Formal analysis, Conceptualization. Man Li: Writing – review & editing, Project administration, Methodology, Funding acquisition.
Ethical approval
Ethical approval was not required.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (82072934), the Dalian Medical University Interdisciplinary Research Cooperation Project Team Funding (JCHZ2023009) of M.L., and the Liaoning Province Science and Technology Plan Joint Fund Project - Doctoral Startup Fund (2023-BSBA-093), Dalian Medical Science Research Program (2312017) of K.W.We are grateful to BioRender for their exceptional image creation platform, which significantly facilitated the production of our high-quality figures.
Contributor Information
Lu Sun, Email: gddmusl@163.com.
Xiaomeng Jia, Email: jxm19970906@163.com.
Kainan Wang, Email: kainan_wang@dmu.edu.cn.
Man Li, Email: man_li@dmu.edu.cn.
References
- 1.Lei S., Zheng R., Zhang S., Wang S., Chen R., Sun K., et al. Global patterns of breast cancer incidence and mortality: a population-based cancer registry data analysis from 2000 to 2020. Cancer communications (London, England) 2021;41(11):1183–1194. doi: 10.1002/cac2.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kerr A.J., Dodwell D., McGale P., Holt F., Duane F., Mannu G., et al. Adjuvant and neoadjuvant breast cancer treatments: a systematic review of their effects on mortality. Cancer Treat Rev. 2022;105 doi: 10.1016/j.ctrv.2022.102375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finck A., Gill S.I., June C.H. Cancer immunotherapy comes of age and looks for maturity. Nat Commun. 2020;11(1):3325. doi: 10.1038/s41467-020-17140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ye F., Dewanjee S., Li Y., Jha N.K., Chen Z.S., Kumar A., et al. Advancements in clinical aspects of targeted therapy and immunotherapy in breast cancer. Mol Cancer. 2023;22(1):105. doi: 10.1186/s12943-023-01805-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chau C.H., Steeg P.S., Figg W.D. Antibody-drug conjugates for cancer. Lancet (London, England) 2019;394:793–804. doi: 10.1016/S0140-6736(19)31774-X. 10200. [DOI] [PubMed] [Google Scholar]
- 6.Staudacher A.H., Brown M.P. Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required? Br J Cancer. 2017;117(12):1736–1742. doi: 10.1038/bjc.2017.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooke T., Reeves J., Lanigan A., Stanton PJAoo. vol. 12. 2001. pp. S23–S28. (HER2 as a prognostic and predictive marker for breast cancer). [DOI] [PubMed] [Google Scholar]
- 8.Junttila T.T., Li G., Parsons K., Phillips G.L., Sliwkowski M.X., treatment Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive. Breast Cancer. 2011;128:347–356. doi: 10.1007/s10549-010-1090-x. [DOI] [PubMed] [Google Scholar]
- 9.Berns K., Horlings H.M., Hennessy B.T., Madiredjo M., Hijmans E.M., Beelen K., et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. 2007;12(4):395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 10.Barok M., Tanner M., Köninki K., Isola J. Trastuzumab-DM1 causes tumour growth inhibition by mitotic catastrophe in trastuzumab-resistant breast cancer cells in vivo. Breast Cancer Res. 2011;13(2) doi: 10.1186/bcr2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCombs J.R., Owen S.C. Antibody drug conjugates: design and selection of linker, payload and conjugation chemistry. AAPS J. 2015;17(2):339–351. doi: 10.1208/s12248-014-9710-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis Phillips G.D., Li G., Dugger D.L., Crocker L.M., Parsons K.L., Mai E., et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68(22):9280–9290. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 13.Bargh J.D., Isidro-Llobet A., Parker J.S., Spring D.R. Cleavable linkers in antibody-drug conjugates. Chem Soc Rev. 2019;48(16):4361–4374. doi: 10.1039/c8cs00676h. [DOI] [PubMed] [Google Scholar]
- 14.Rapani E., Sacchetti A., Corda D., Sjijoc Alberti. Human Trop‐2 is a tumor‐associated calcium signal transducer. 1998;76(5):671–676. doi: 10.1002/(sici)1097-0215(19980529)76:5<671::aid-ijc10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Zaman S., Jadid H., Denson A.C., Gray J.E.J.O., therapy . 2019. Targeting Trop-2 in solid tumors: future prospects; pp. 1781–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lombardi P., Filetti M., Falcone R., Altamura V., Paroni Sterbini F., Bria E., et al. Overview of trop-2 in cancer: from pre-clinical studies to future directions in clinical settings. Cancers. 2023;15(6) doi: 10.3390/cancers15061744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J., Zhang K., Grabowska D., Li A., Dong Y., Day R., et al. Loss of Trop2 promotes carcinogenesis and features of epithelial to mesenchymal transition in squamous cell carcinoma. Mol Cancer Res : MCR. 2011;9(12):1686–1695. doi: 10.1158/1541-7786.MCR-11-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trerotola M., Cantanelli P., Guerra E., Tripaldi R., Aloisi A.L., Bonasera V., et al. Upregulation of Trop-2 quantitatively stimulates human cancer growth. Oncogene. 2013;32(2):222–233. doi: 10.1038/onc.2012.36. [DOI] [PubMed] [Google Scholar]
- 19.Starodub A.N., Ocean A.J., Shah M.A., Guarino M.J., Picozzi V.J., Jr., Vahdat L.T., et al. First-in-Human trial of a novel anti-trop-2 antibody-SN-38 conjugate, sacituzumab govitecan, for the treatment of diverse metastatic solid tumors. Clin Cancer Res : an official journal of the American Association for Cancer Research. 2015;21(17):3870–3878. doi: 10.1158/1078-0432.CCR-14-3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldenberg D.M., Sharkey R.M. Sacituzumab govitecan, a novel, third-generation, antibody-drug conjugate (ADC) for cancer therapy. Expet Opin Biol Ther. 2020;20(8):871–885. doi: 10.1080/14712598.2020.1757067. [DOI] [PubMed] [Google Scholar]
- 21.Verma S., Miles D., Gianni L., Krop I.E., Welslau M., Baselga J., et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diéras V., Miles D., Verma S., Pegram M., Welslau M., Baselga J., et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(6):732–742. doi: 10.1016/S1470-2045(17)30312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krop I.E., Lin N.U., Blackwell K., Guardino E., Huober J., Lu M., et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol : official journal of the European Society for Medical Oncology. 2015;26(1):113–119. doi: 10.1093/annonc/mdu486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krop I.E., Kim S.B., González-Martín A., LoRusso P.M., Ferrero J.M., Smitt M., et al. Trastuzumab emtansine versus treatment of physician's choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(7):689–699. doi: 10.1016/S1470-2045(14)70178-0. [DOI] [PubMed] [Google Scholar]
- 25.Mamounas E.P., Untch M., Mano M.S., Huang C.S., Geyer C.E., Jr., von Minckwitz G., et al. Adjuvant T-DM1 versus trastuzumab in patients with residual invasive disease after neoadjuvant therapy for HER2-positive breast cancer: subgroup analyses from KATHERINE. Ann Oncol : official journal of the European Society for Medical Oncology. 2021;32(8):1005–1014. doi: 10.1016/j.annonc.2021.04.011. [DOI] [PubMed] [Google Scholar]
- 26.von Minckwitz G., Huang C.S., Mano M.S., Loibl S., Mamounas E.P., Untch M., et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380(7):617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 27.Hurvitz S.A., Martin M., Jung K.H., Huang C.S., Harbeck N., Valero V., et al. Neoadjuvant trastuzumab emtansine and pertuzumab in human epidermal growth factor receptor 2-positive breast cancer: three-year outcomes from the phase III KRISTINE study. J Clin Oncol : official journal of the American Society of Clinical Oncology. 2019;37(25):2206–2216. doi: 10.1200/JCO.19.00882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montemurro F., Ellis P., Delaloge S., Wuerstlein R., Anton A., Button P., et al. Abstract P1-12-10: safety and efficacy of trastuzumab emtansine (T-DM1) in 399 patients with central nervous system metastases: exploratory subgroup analysis from the KAMILLA study. 2017;77(4) [Google Scholar]
- 29.Modi S., Saura C., Yamashita T., Park Y.H., Kim S.B., Tamura K., et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382(7):610–621. doi: 10.1056/NEJMoa1914510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.André F., Hee Park Y., Kim S.B., Takano T., Im S.A., Borges G., et al. Trastuzumab deruxtecan versus treatment of physician's choice in patients with HER2-positive metastatic breast cancer (DESTINY-Breast02): a randomised, open-label, multicentre, phase 3 trial. Lancet (London, England) 2023;401:1773–1785. doi: 10.1016/S0140-6736(23)00725-0. 10390. [DOI] [PubMed] [Google Scholar]
- 31.Hurvitz S.A., Hegg R., Chung W.P., Im S.A., Jacot W., Ganju V., et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet (London, England) 2023;401:105–117. doi: 10.1016/S0140-6736(22)02420-5. 10371. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton E.P., Hurvitz S.A., Im S.-A., Iwata H., Curigliano G., Kim S.-B., et al. American Society of Clinical Oncology; 2024. Trastuzumab deruxtecan (T-DXd) vs trastuzumab emtansine (T-DM1) in patients (pts) with HER2+ metastatic breast cancer (mBC): updated survival results of DESTINY-Breast03. [Google Scholar]
- 33.Harbeck N., Ciruelos E., Jerusalem G., Müller V., Niikura N., Viale G., et al. Trastuzumab deruxtecan in HER2-positive advanced breast cancer with or without brain metastases: a phase 3b/4 trial. Nat Med. 2024:1–10. doi: 10.1038/s41591-024-03261-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin N.U., Borges V., Anders C., Murthy R.K., Paplomata E., Hamilton E., et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol : official journal of the American Society of Clinical Oncology. 2020;38(23):2610–2619. doi: 10.1200/JCO.20.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan M., Ouyang Q., Sun T., Niu L., Yang J., Li L., et al. Pyrotinib plus capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases (PERMEATE): a multicentre, single-arm, two-cohort, phase 2 trial. Lancet Oncol. 2022;23(3):353–361. doi: 10.1016/S1470-2045(21)00716-6. [DOI] [PubMed] [Google Scholar]
- 36.Xu Z., Guo D., Jiang Z., Tong R., Jiang P., Bai L., et al. Novel HER2-targeting antibody-drug conjugates of trastuzumab beyond T-DM1 in breast cancer: trastuzumab deruxtecan(DS-8201a) and (Vic-)Trastuzumab duocarmazine (SYD985) Eur J Med Chem. 2019;183 doi: 10.1016/j.ejmech.2019.111682. [DOI] [PubMed] [Google Scholar]
- 37.Aftimos P., Turner N., O'Shaughnessy J., van den Tweel E., Oesterholt M., Escrivá-de-Romaní S., et al. vol. 34. 2023. pp. S340–S341. (386MO Trastuzumab duocarmazine versus physician's choice therapy in pre-treated HER2-positive metastatic breast cancer: final results of the phase III TULIP trial). [Google Scholar]
- 38.Menderes G., Bonazzoli E., Bellone S., Black J., Predolini F., Pettinella F., et al. SYD985, a novel duocarmycin-based HER2-targeting antibody-drug conjugate, shows antitumor activity in uterine and ovarian carcinosarcoma with HER2/neu expression. Clin Cancer Res : an official journal of the American Association for Cancer Research. 2017;23(19):5836–5845. doi: 10.1158/1078-0432.CCR-16-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Q., Wang X., Cheng Y., Liu Y., Chang J., Wang Z., et al. Abstract P4-01-07: FS-1502, an anti-HER2 ADC, in patients with HER2-Expressing advanced solid tumors: a Phase 1a dose-escalation study. 2023;83 [Google Scholar]
- 40.NCI Drug Dictionary a166. Available online: [https://www.cancer.gov/publications/dictionaries/cancer-drug/def/795827 ].
- 41.Zhang J., Liu R., Gao S., Li W., Chen Y., Meng Y., et al. Phase I study of A166, an antibody‒drug conjugate in advanced HER2-expressing solid tumours. 2023;9(1):28. doi: 10.1038/s41523-023-00522-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang T., Xu J., Yin J., Gao Y., Zheng H., Fu B., et al. 2023. SHR-A1811, a novel anti-HER2 antibody–drug conjugate with optimal drug-to-antibody ratio, superior bystander killing effect and favorable safety profiles. [Google Scholar]
- 43.Yao H., Yan M., Tong Z., Wu X., Ryu M.-H., Kim J.H., et al. Abstract CT175: safety, tolerability, pharmacokinetics, and antitumor activity of SHR-A1811 in HER2-expressing/mutated advanced solid tumors: a global phase 1, multi-center, first-in-human study. 2023;83(8_Supplement):CT175. [Google Scholar]
- 44.Manich C.S., Modi S., Krop I., Park Y., Kim S., Tamura K., et al. vol. 32. 2021. pp. S485–S486. (279P Trastuzumab deruxtecan (T-DXd) in patients with HER2-positive metastatic breast cancer (MBC): updated survival results from a phase II trial (DESTINY-Breast01)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Humphreys R.C., Kirtely J., Hewit A., Biroc S., Knudsen N., Skidmore L., et al. Site specific conjugation of ARX-788, an antibody drug conjugate (ADC) targeting HER2, generates a potent and stable targeted therapeutic for multiple cancers. 2015;75(15_Supplement):639. [Google Scholar]
- 46.Zhang Y., Qiu M.Z., Wang J.F., Zhang Y.Q., Shen A., Yuan X.L., et al. Phase 1 multicenter, dose-expansion study of ARX788 as monotherapy in HER2-positive advanced gastric and gastroesophageal junction adenocarcinoma. Cell reports Medicine. 2022;3(11) doi: 10.1016/j.xcrm.2022.100814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hurvitz S., Kalinsky K., Tripathy D., Sledge G., Gradishar W., O'Shaughnessy J., et al. 273TiP ACE-Breast-03: a phase II study patients with HER2-positive metastatic breast cancer whose disease is resistant or refractory to T-DM1, and/or T-DXd, and/or tucatinib-containing regimens treated with ARX788. 2022;33:S662–S663. [Google Scholar]
- 48.Hu X., Wang L., Zhang J., Zhang Q., Ouyang Q., Wang X., et al. American Society of Clinical Oncology; 2024. ACE-Breast-02: a pivotal phase II/III trial of ARX788, a novel anti-HER2 antibody-drug conjugate (ADC), versus lapatinib plus capecitabine for HER2+ advanced breast cancer (ABC) [Google Scholar]
- 49.Li H., Zhang X., Xu Z., Li L., Liu W., Dai Z., et al. Preclinical evaluation of MRG002, a novel HER2-targeting antibody-drug conjugate with potent antitumor activity against HER2-positive solid tumors. Antibody therapeutics. 2021;4(3):175–184. doi: 10.1093/abt/tbab017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deeks E.D., Vedotin Disitamab. First approval. Drugs. 2021;81(16):1929–1935. doi: 10.1007/s40265-021-01614-x. [DOI] [PubMed] [Google Scholar]
- 51.Wang J., Liu Y., Zhang Q., Feng J., Fang J., Chen X., et al. Wolters Kluwer Health; 2021. RC48-ADC, a HER2-targeting antibody-drug conjugate, in patients with HER2-positive and HER2-low expressing advanced or metastatic breast cancer: a pooled analysis of two studies. [Google Scholar]
- 52.Mahtani R., Holmes F.A., Badve S., Caldera H., Coleman R., Mamounas E., et al. A roundtable discussion of the breast cancer therapy expert group (BCTEG): clinical developments and practice guidance on human epidermal growth factor receptor 2 (HER2)-positive breast cancer. Clin Breast Cancer. 2020;20(3):e251–e260. doi: 10.1016/j.clbc.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 53.Modi S., Ohtani S., Lee C., Wang Y., Saxena K., Cameron D.A.J.C.R. Abstract OT1-07-02: a phase 3, multicenter, randomized, open-label trial of [fam-] trastuzumab deruxtecan (T-DXd; DS-8201a) vs investigator's choice in HER2-low breast cancer. DESTINY-Breast04) 2020;80 4_Supplement):OT1-07-02-OT01-07-02. [Google Scholar]
- 54.Jiang Z., Sun T., Wang X., Liu Q., Yan M., Tong Z., et al. American Society of Clinical Oncology; 2022. A multiple center, open-label, single-arm, phase II clinical trial of MRG002, an HER2-targeted antibody-drug conjugate, in patients with HER2-low expressing advanced or metastatic breast cancer. [Google Scholar]
- 55.Syed Y.Y. Sacituzumab govitecan: first approval. Drugs. 2020;80(10):1019–1025. doi: 10.1007/s40265-020-01337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rugo H.S., Bardia A., Marmé F., Cortés J., Schmid P., Loirat D., et al. Overall survival with sacituzumab govitecan in hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer (TROPiCS-02): a randomised, open-label, multicentre, phase 3 trial. Lancet (London, England) 2023;402:1423–1433. doi: 10.1016/S0140-6736(23)01245-X. 10411. [DOI] [PubMed] [Google Scholar]
- 57.Bardia A., Mayer I.A., Vahdat L.T., Tolaney S.M., Isakoff S.J., Diamond J.R., et al. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med. 2019;380(8):741–751. doi: 10.1056/NEJMoa1814213. [DOI] [PubMed] [Google Scholar]
- 58.Bardia A., Rugo H.S., Tolaney S.M., Loirat D., Punie K., Oliveira M., et al. Final results from the randomized phase III ASCENT clinical trial in metastatic triple-negative breast cancer and association of outcomes by human epidermal growth factor receptor 2 and trophoblast cell surface antigen 2 expression. J Clin Oncol : official journal of the American Society of Clinical Oncology. 2024 doi: 10.1200/JCO.23.01409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bardia A., Jhaveri K., Kalinsky K., Pernas S., Tsurutani J., Xu B., et al. TROPION-Breast01: Datopotamab deruxtecan vs chemotherapy in pre-treated inoperable or metastatic HR+/HER2–breast cancer. 2023;(0) doi: 10.2217/fon-2023-0188. [DOI] [PubMed] [Google Scholar]
- 60.Dent R.A., Cescon D.W., Bachelot T., Jung K.H., Shao Z.-M., Saji S., et al. TROPION-Breast02: datopotamab deruxtecan for locally recurrent inoperable or metastatic triple-negative. Breast Cancer. 2023;19(35):2349–2359. doi: 10.2217/fon-2023-0228. [DOI] [PubMed] [Google Scholar]
- 61.Yin Y., Wu X., Ouyang Q., Yan M., Song L., Liu Y., et al. Abstract OT1-03-02: efficacy and safety of SKB264 for previously treated metastatic triple negative breast cancer in Phase 2 study. 2023;83 [Google Scholar]
- 62.Takai Y., Miyoshi J., Ikeda W., Ogita H. Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat Rev Mol Cell Biol. 2008;9(8):603–615. doi: 10.1038/nrm2457. [DOI] [PubMed] [Google Scholar]
- 63.Scaranti M., Cojocaru E., Banerjee S., Banerji U. Exploiting the folate receptor α in oncology. Nat Rev Clin Oncol. 2020;17(6):349–359. doi: 10.1038/s41571-020-0339-5. [DOI] [PubMed] [Google Scholar]
- 64.John P., Wei Y., Liu W., Du M., Guan F., Zang X. The B7x immune checkpoint pathway: from discovery to clinical trial. Trends in pharmacological sciences. 2019;40(11):883–896. doi: 10.1016/j.tips.2019.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen Y.F., Xu Y.Y., Shao Z.M., Yu K.D. Resistance to antibody-drug conjugates in breast cancer: mechanisms and solutions. Cancer communications (London, England) 2023;43(3):297–337. doi: 10.1002/cac2.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Quanz M., Hagemann U.B., Zitzmann-Kolbe S., Stelte-Ludwig B., Golfier S., Elbi C., et al. Anetumab ravtansine inhibits tumor growth and shows additive effect in combination with targeted agents and chemotherapy in mesothelin-expressing human ovarian cancer models. Oncotarget. 2018;9(75):34103–34121. doi: 10.18632/oncotarget.26135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moore K.N., O'Malley D.M., Vergote I., Martin L.P., Gonzalez-Martin A., Malek K., et al. Safety and activity findings from a phase 1b escalation study of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with carboplatin in patients with platinum-sensitive ovarian cancer. 2018;151(1):46–52. doi: 10.1016/j.ygyno.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 68.Kan S., Koido S., Okamoto M., Hayashi K., Ito M., Kamata Y., et al. Up-regulation of HER2 by gemcitabine enhances the antitumor effect of combined gemcitabine and trastuzumab emtansine treatment on pancreatic ductal adenocarcinoma cells. 2015;15:1–9. doi: 10.1186/s12885-015-1772-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krop I.E., Modi S., LoRusso P.M., Pegram M., Guardino E., Althaus B., et al. Phase 1b/2a study of trastuzumab emtansine (T-DM1), paclitaxel, and pertuzumab in HER2-positive metastatic breast cancer. Breast Cancer Res. 2016;18(1):34. doi: 10.1186/s13058-016-0691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.López-Miranda E., Pérez-García J.M., Di Cosimo S., Brain E., Ravnik M., Escrivá-de-Romaní S., et al. Trastuzumab emtansine plus non-pegylated liposomal doxorubicin in HER2-positive metastatic breast cancer (thelma): a single-arm, multicenter, phase Ib trial. Cancers. 2020;12(12) doi: 10.3390/cancers12123509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fuentes-Antrás J., Genta S., Vijenthira A., Siu L.L. Antibody-drug conjugates: in search of partners of choice. Trends in cancer. 2023;9(4):339–354. doi: 10.1016/j.trecan.2023.01.003. [DOI] [PubMed] [Google Scholar]
- 72.Ocaña A., Amir E., Pandiella A.J.B.C.R. vol. 22. 2020. pp. 1–3. (HER2 heterogeneity and resistance to anti-HER2 antibody-drug conjugates). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Patel T.A., Dave B., Rodriguez A.A., Chang J.C., Perez E.A., Colon-Otero G. Dual HER2 blockade: preclinical and clinical data. Breast Cancer Res. 2014;16(4):419. doi: 10.1186/s13058-014-0419-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miller K.D., Diéras V., Harbeck N., Andre F., Mahtani R.L., Gianni L., et al. Phase IIa trial of trastuzumab emtansine with pertuzumab for patients with human epidermal growth factor receptor 2–positive, locally advanced, or metastatic. Breast Cancer. 2014;32(14):1437–1444. doi: 10.1200/JCO.2013.52.6590. [DOI] [PubMed] [Google Scholar]
- 75.Hurvitz S.A., Martin M., Jung K.H., Huang C.-S., Harbeck N., Valero V., et al. Neoadjuvant trastuzumab emtansine and pertuzumab in human epidermal growth factor receptor 2–positive breast cancer: three-year outcomes from the phase III KRISTINE study. 2019;37(25):2206. doi: 10.1200/JCO.19.00882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Patel T.A., Ensor J., Rodriguez A.A., Belcheva A., Darcourt J.G., Niravath P.A., et al. American Society of Clinical Oncology; 2018. Phase ib study of trastuzumab emtansine (TDM1) in combination with lapatinib and nab-paclitaxel in metastatic HER2-neu overexpressed breast cancer patients: stela results. [Google Scholar]
- 77.Borges V.F., Ferrario C., Aucoin N., Falkson C., Khan Q., Krop I., et al. Tucatinib combined with ado-trastuzumab emtansine in advanced ERBB2/HER2-positive metastatic breast cancer: a phase 1b clinical trial. 2018;4(9):1214–1220. doi: 10.1001/jamaoncol.2018.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abraham J., Montero A.J., Jankowitz R.C., Salkeni M.A., Beumer J.H., Kiesel B.F., et al. Safety and efficacy of T-DM1 plus neratinib in patients with metastatic HER2-positive breast cancer: NSABP foundation trial FB-10. 2019;37(29):2601. doi: 10.1200/JCO.19.00858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hurvitz S., Vahdat L., Harbeck N., Wolff A.C., Tolaney S.M., Loi S., et al. Abstract OT-28-01: HER2CLIMB-02: a randomized, double-blind, phase 3 study of tucatinib or placebo with T-DM1 for unresectable locally-advanced or metastatic HER2+ breast cancer. 2021;81(4_Supplement) [Google Scholar]
- 80.Curigliano G., Mueller V., Borges V., Hamilton E., Hurvitz S., Loi S., et al. Tucatinib versus placebo added to trastuzumab and capecitabine for patients with pretreated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB): final overall survival analysis. 2022;33(3):321–329. doi: 10.1016/j.annonc.2021.12.005. [DOI] [PubMed] [Google Scholar]
- 81.Gennari A., André F., Barrios C., Cortes J., de Azambuja E., DeMichele A., et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic. breast cancer☆. 2021;32(12):1475–1495. doi: 10.1016/j.annonc.2021.09.019. [DOI] [PubMed] [Google Scholar]
- 82.Lin N.U., Murthy R.K., Abramson V., Anders C., Bachelot T., Bedard P.L., et al. Tucatinib vs placebo, both in combination with trastuzumab and capecitabine, for previously treated ERBB2 (HER2)-positive metastatic breast cancer in patients with brain metastases: updated exploratory analysis of the HER2CLIMB randomized clinical trial. 2023;9(2):197–205. doi: 10.1001/jamaoncol.2022.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zouein J., Noujaim C. Kourie HRJBiM: targeting PIK3CA in HER2-positive breast cancer: what are the opportunities and the challenges? Future Medicine. 2021;15:609–613. doi: 10.2217/bmm-2021-0236. [DOI] [PubMed] [Google Scholar]
- 84.Jain S., Shah A.N., Santa-Maria C.A., Siziopikou K., Rademaker A., Helenowski I., et al. vol. 171. 2018. pp. 371–381. (Phase I study of alpelisib (BYL-719) and trastuzumab emtansine (T-DM1) in HER2-positive metastatic breast cancer (MBC) after trastuzumab and taxane therapy). [DOI] [PubMed] [Google Scholar]
- 85.Goel S., Wang Q., Watt A.C., Tolaney S.M., Dillon D.A., Li W., et al. Overcoming therapeutic resistance in HER2-positive breast cancers with CDK4/6 inhibitors. 2016;29(3):255–269. doi: 10.1016/j.ccell.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Witkiewicz A.K., Cox D., Knudsen E.S.J.G., cancer CDK4/6 inhibition provides a potent adjunct to Her2-targeted therapies in preclinical breast cancer models. 2014;5(7–8):261. doi: 10.18632/genesandcancer.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Haley B., Batra K., Sahoo S., Froehlich T., Klemow D., Unni N., et al. A phase I/Ib trial of PD 0332991 (palbociclib) and T-DM1 in HER2-positive advanced breast cancer after trastuzumab and taxane therapy. 2021;21(5):417–424. doi: 10.1016/j.clbc.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 88.Spring L.M., Clark S.L., Li T., Goel S., Tayob N., Viscosi E., et al. Phase 1b clinical trial of ado-trastuzumab emtansine and ribociclib for HER2-positive metastatic breast cancer. 2021;7(1):103. doi: 10.1038/s41523-021-00311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saatci Ö., Borgoni S., Akbulut Ö., Durmuş S., Raza U., Eyüpoğlu E., et al. Targeting PLK1 overcomes T-DM1 resistance via CDK1-dependent phosphorylation and inactivation of Bcl-2/xL in HER2-positive breast cancer. 2018;37(17):2251–2269. doi: 10.1038/s41388-017-0108-9. [DOI] [PubMed] [Google Scholar]
- 90.Singh D.D., Parveen A., Yadav D.K.J.B. Role of PARP in TNBC: mechanism of inhibition, clinical applications, and resistance. 2021;9(11):1512. doi: 10.3390/biomedicines9111512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yap T.A., Hamilton E., Bauer T., Dumbrava E.E., Jeselsohn R., Enke A., et al. vol. 6. 2022. (Phase Ib SEASTAR study: combining rucaparib and sacituzumab govitecan in patients with cancer with or without mutations in homologous recombination repair genes). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cardillo T.M., Sharkey R.M., Rossi D.L., Arrojo R., Mostafa A.A., Goldenberg D.M. Synthetic lethality exploitation by an anti–trop-2-SN-38 antibody–drug conjugate, IMMU-132, plus PARP inhibitors in BRCA1/2–wild-type triple-negative breast cancer. 2017;23(13):3405–3415. doi: 10.1158/1078-0432.CCR-16-2401. [DOI] [PubMed] [Google Scholar]
- 93.Nicolò E., Giugliano F., Ascione L., Tarantino P., Corti C., Tolaney S.M., et al. vol. 106. 2022. (Combining antibody-drug conjugates with immunotherapy in solid tumors: current landscape and future perspectives). [DOI] [PubMed] [Google Scholar]
- 94.D'Amico L., Menzel U., Prummer M., Müller P., Buchi M., Kashyap A., et al. A novel anti-HER2 anthracycline-based antibody-drug conjugate induces adaptive anti-tumor immunity and potentiates PD-1 blockade in breast cancer. 2019;7:1–15. doi: 10.1186/s40425-018-0464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Müller P., Martin K., Theurich S., Schreiner J., Savic S., Terszowski G., et al. Microtubule-depolymerizing agents used in antibody–drug conjugates induce antitumor immunity by stimulation of dendritic cells. 2014;2(8):741–755. doi: 10.1158/2326-6066.CIR-13-0198. [DOI] [PubMed] [Google Scholar]
- 96.Boshuizen J., Pencheva N., Krijgsman O., Altimari D.D.E., Castro P.G., de Bruijn B., et al. Cooperative targeting of immunotherapy-resistant melanoma and lung cancer by an AXL-targeting antibody–drug conjugate and immune checkpoint blockade. 2021;81(7):1775–1787. doi: 10.1158/0008-5472.CAN-20-0434. [DOI] [PubMed] [Google Scholar]
- 97.Müller P., Kreuzaler M., Khan T., Thommen D.S., Martin K., Glatz K., et al. Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci Transl Med. 2015;7(315) doi: 10.1126/scitranslmed.aac4925. [DOI] [PubMed] [Google Scholar]
- 98.Emens L.A., Esteva F.J., Beresford M., Saura C., De Laurentiis M., Kim S.-B., et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): a phase 2, multicentre, randomised, double-blind trial. 2020;21(10):1283–1295. doi: 10.1016/S1470-2045(20)30465-4. [DOI] [PubMed] [Google Scholar]
- 99.Waks A.G., Keenan T., Li T., Tayob N., Wulf G.M., Richardson E.T., et al. American Society of Clinical Oncology; 2020. A phase Ib study of pembrolizumab (pembro) plus trastuzumab emtansine (T-DM1) for metastatic HER2+ breast cancer (MBC) [Google Scholar]
- 100.Loi S., Schneeweiss A., Song E., Harries M., De Laurentiis M., Li Y., et al. 329TiP KATE3: a phase III study of trastuzumab emtansine (T-DM1) in combination with atezolizumab or placebo in patients with previously treated HER2-positive and PD-L1–positive locally advanced or metastatic breast cancer. 2021;32 [Google Scholar]
- 101.Bardia A., Jhaveri K., Im S., Simon S.P., De Laurentiis M., Wang S., et al. LBA11 Datopotamab deruxtecan (Dato-DXd) vs chemotherapy in previously-treated inoperable or metastatic hormone receptor-positive. HER2-negative (HR+/HER2–) breast cancer (BC): Primary results from the randomised phase III TROPION-Breast01 trial. 2023;34:S1264–S1265. [Google Scholar]
- 102.Krop I., Juric D., Shimizu T., Tolcher A., Spira A., Mukohara T., et al. Abstract GS1-05: datopotamab deruxtecan in advanced/metastatic HER2-breast cancer: results from the phase 1 TROPION-PanTumor01 study. 2022;82(4_Supplement) doi: 10.1200/JCO.23.01909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Qu F., Lu R., Liu Q., Wu X., Huang X., Yin Y., et al. Antibody-drug conjugates transform the outcome of individuals with low-HER2-expression advanced breast cancer. Cancer. 2024;130(S8):1392–1402. doi: 10.1002/cncr.35205. [DOI] [PubMed] [Google Scholar]
- 104.Zhou Y., Li J., JjeoTA-tT Ying. Anti-PD-1/L1 antibody plus anti-VEGF antibody vs. plus VEGFR-targeted TKI as first-line therapy for unresectable hepatocellular carcinoma: a network meta-analysis. 2024;5:568–580. doi: 10.37349/etat.2024.00236. Open Exploration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bardia A., Tolaney S., Punie K., Loirat D., Oliveira M., Kalinsky K., et al. Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative. Breast Cancer. 2021;32(9):1148–1156. doi: 10.1016/j.annonc.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 106.Damelin M., Zhong W., Myers J., Sapra PJPr. Evolving strategies for target selection for antibody-drug conjugates. 2015;32:3494–3507. doi: 10.1007/s11095-015-1624-3. [DOI] [PubMed] [Google Scholar]
- 107.Radocha J., van de Donk N.W., Weisel K.J.C. Monoclonal antibodies and antibody drug conjugates in multiple myeloma. 2021;13(7):1571. doi: 10.3390/cancers13071571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ogitani Y., Aida T., Hagihara K., Yamaguchi J., Ishii C., Harada N., et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. 2016;22(20):5097–5108. doi: 10.1158/1078-0432.CCR-15-2822. [DOI] [PubMed] [Google Scholar]