Abstract
Toscana virus (TOSV) is an emerging arthropod-borne virus (arbovirus) of medical importance that is increasing its range across much of the Mediterranean Basin, Europe and the Middle East. Transmitted by Phlebotomus spp. sand flies, it is the most clinically relevant sand fly-borne phlebovirus. Initially isolated in the Tuscany region of Central Italy, it has now been detected in multiple countries that surround this geographical area. Infection of the vertebrate host can cause fever and neurological disease, following the dissemination of the virus to the brain. The prevalence is high in some regions, with a notable percentage of individuals showing seroconversion. TOSV can be a leading cause of acute meningitis and encephalitis (AME) during the summer months. In this comprehensive review, we will focus on several key topics. We discuss how TOSV has spread to establish outbreaks of infection in both humans and animals around the Mediterranean and the wider region. Clinical aspects of TOSV infection in humans are described, along with the best standards in diagnosis. Finally, we focus our discussion on the role of the sand fly vector, describing their biology, vector competency, implications for putative vertebrate reservoirs, the effect of the climate emergency on sand fly distribution and the putative role that sand fly-derived salivary factors may have on modulating host susceptibility to TOSV infection.
Keywords: arbovirus, diagnostics, epidemiology, pathogenesis, vector-borne disease
Introduction
Toscana virus (TOSV) is a medically important virus belonging to the Phlebovirus of the Phenuiviridae family, in the recently classified Hareavirales order of the Bunyaviricetes class of negative-sense RNA viruses [1,2]. Transmitted by multiple species of sand flies, including several within the Phlebotomus genus, infection typically causes a febrile-like illness with occasional development of severe disease, following the dissemination of the virus to neural tissue. It was first isolated from sand flies in the Tuscany region of Central Italy in 1971 [3], and infection can cause fever followed by neurological disease as the virus disseminates to the brain [4,5], with TOSV being the leading cause of meningitis and encephalitis during the summer months in some regions [6]. Infection of multiple putative reservoir vertebrate species has been documented, including agriculturally important livestock, dogs, cats and bats. As such, it has a remarkable ability to persist within endemic areas, triggering sporadic outbreaks of infection in the warmer months, when its sand fly vector is more numerous. A combination of factors including the climate emergency and increasing globalization is expanding the range of both sand flies and TOSV to new geographic areas. Nonetheless, research on this agent has been neglected, with only a limited understanding of TOSV biology and the diseases that result from infection. The absence of licensed antivirals and vaccines indicates an urgent unmet need to better understand TOSV biology, pathogenesis and its ecology. In this review, we provide a comprehensive summary of the key findings relating to this increasingly important virus.
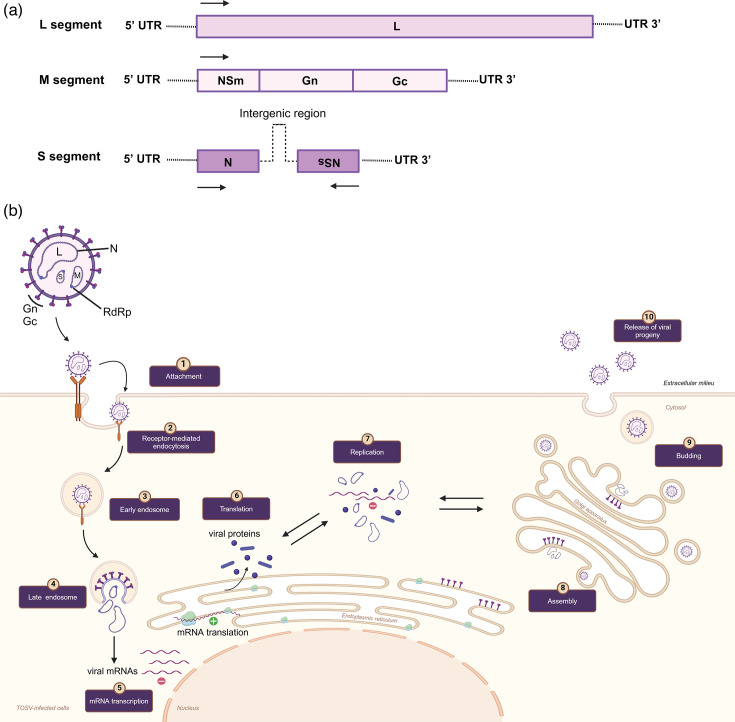
TOSV is a tri-segmented, negative-sense RNA with a diameter of ~100 nm [7,8]. Like all bunyaviruses, the genome consists of three segments called small (S), medium (M) and large (L), reflecting their nt length (summarized in Fig. 1a). The replication strategy of TOSV has mostly been inferred from the studies on other bunyaviruses (Fig. 1b) [8,13]. One recent study has defined TOSV’s entry mechanism into mammalian cells, demonstrating that it shares its acid-activated membrane fusion strategy with other Bunyavirales. Here, they found that TOSV cell entry interestingly relies on late endosomes, in the early stages of maturation, and thereby displayed greater resistance to endocytic degradation and flexibility in pH-dependent fusion [14]. However, vector receptors need to be defined for the virus entry mechanism. Similar to other Bunyaviricetes, the S segment encodes the nucleocapsid (N) protein responsible for encapsulating the viral RNA replication products to form the ribonucleoprotein complex (Fig. 1). The M segment encodes a polyprotein precursor, which then is cleaved into Gn and Gc components via host cell proteases in the endoplasmic reticulum. The Gn–Gc heterodimer is involved in virus assembly and attachment to new target cells. The L segment encodes the viral component of the RNA-dependent RNA polymerase (RdRp) [15]. The S and M segments also encode NSs and NSm, which are non-structural proteins, respectively [15,16]. Although NSs is not required for efficient Phlebovirus replication in cultured mammalian cells [e.g. Rift Valley fever virus (RVFV)] [15], NSs is required for efficient suppression of the IFN response [17,20] and therefore likely has an important role in suppressing anti-viral immunity during infection in vivo. The specific molecular aspects of TOSV replication and genetics are not well described compared to other Bunyaviricetes. In the absence of this, one can draw on findings from other genetically similar arboviruses, such as RVFV, an important human and animal pathogen [9,21], and the novel family of Phenuiviridae viruses (e.g. Uukuniemi uukuvirus). Together, these can serve as a guide for understanding TOSV structure and replication, which have been described elsewhere [14,22, 23].
Fig. 1. Genome organisation (a) and replication cycle (b) of TOSV. (a) TOSV contains a tripartite single-stranded RNA genome. L segment (negative-sense) encodes the viral component of the RNA-dependent RNA polymerase (RdRp). M segment (negative-sense) encodes Gn/Gc and a non-structural protein (NSm). S segment encodes (ambisense) the nucleocapsid (N) protein and a non-structural protein (NSs). (b) (1) Phleboviruses, including TOSV, use virus-encoded glycoproteins, Gn and Gc, to bind cell surface molecules DC-SIGN, L-SIGN and heparan sulphate (glycosaminoglycan). This is similar to the related phenuiviruses that also target DC-SIGN. Receptor use is likely cell specific, e.g., with DC-SIGN mediated entry occurring in dendritic cells (DCs). (2) Virus enters the cell via receptor-mediated endocytosis. (3) Once internalized, the viral particles move through early and late endosomes. (4) In late endosomes, acidification induces the membrane fusion activity of the Gc protein with the endosomal membrane. This fusion triggers the encapsidation of the viral genome, and virus RdRps are released into the cytoplasm, where primary transcription and replication occur. (5) N interactions with RdRp allow access to the ribonucleoprotein (RNP), which serves as a template for the transcription of new mRNA. Following the translation of the viral mRNAs and genome replication (6–7), viral Gn/Gc are cleaved by host cell proteases in the endoplasmic reticulum, allowing Gn–Gc glycoprotein heterodimers to reach the Golgi apparatus, (8) where viral assembly occurs. (9) Newly formed virions decorated with Gn and Gc in the Golgi bud via vesicles to the plasma membrane and (10) are released from the host cell by exocytosis. Replication within arthropod cells, although likely similar, is not well studied and requires definition. The figures were created on BioRender.com.
Phylogenetic analyses have revealed three distinct lineages of TOSV, denoted as A, B and C. Presently, there are no differences in virulence or clinical symptoms among these genetic lineages. In some countries, such as France (lineages A and B), Türkiye (lineages A and B) and Croatia (lineages B and C), at least two lineages are known to coexist [6]. Whether these distinct lineages have different animal reservoirs requires further research. Recently, the complete sequence of the TOSV strain 1500590, a lineage A virus, was made available, leading to the establishment of the first reverse genetic system capable of recovering infectious recombinant TOSV (rTOSV) from cDNA. This advancement allows for the creation of genetically modified versions of the virus. By generating an NSs-deficient rTOSV capable of expressing reporter genes, it enables the visualization and tracking of intracellular replication, essential for further research and vaccine development efforts [24]. A developed reverse genetic system is also available for a lineage B strain of TOSV [17]. In comparison, research with lineage C virus is challenging, as it only exists as a sequence, although it could be possible for a reverse genetics strategy to rescue lineage C.
To demonstrate the phylogeny of TOSV and related Bunyavirales, we have undertaken a de novo analysis of all Bunyaviricetes viruses by comparing RdRp sequences and plotting those viruses that are of medical importance to humans (Fig. 2). The main vector that transmits each virus is also annotated. This shows how TOSV and the other genetically related phleboviruses are all transmitted by sand fly vectors. The sequence similarity that these genetically related viruses have with other Bunyaviricetes viruses, which are transmitted by mosquito and tick vectors, is shown. This includes three other Phenuiviridae viruses that are transmitted by ticks. More distantly related are the Nairoviridae viruses, also transmitted by ticks, and the Peribunyaviridae viruses that are transmitted by either mosquito or midge vectors. This includes the Oropouche virus, which is an important emerging Bunyaviricetes virus responsible for ongoing outbreaks in South America.
Fig. 2. Phylogenetic tree of arboviruses of medical and veterinary importance. Maximum likelihood tree of the RdRp aligned using MAFFT and reconstructed using IQTREE2. Tips are annotated with the virus name, the species name in brackets and the arthropod vector. Values at the nodes represent the bootstrap support, only values above 70 are shown and 100 bootstrap support is represented with a *. The tree is mid-point rooted, the branch lengths are drawn to scale and the scale bar represents the number of amino acid changes per site.
Epidemiology of TOSV: a historical context
Due to a combination of factors including the climate emergency, globalization, urbanization and changes in countryside management, the burden of arboviral diseases (including those caused by TOSV) on human health is increasing. TOSV cases in humans have been reported across the wider Mediterranean region and, as such, pose a risk of infection to millions living in these regions. Importantly, the number of TOSV cases is on the rise [25]. After its first identification from sand flies in Italy, in 1971, early seroepidemiological studies frequently did not assess TOSV distribution, often referred to as ‘Phlebotomus fever’ or ‘Pappataci fever’ [26,27]. It was not until 10 years after its identification that TOSV was registered in the International Catalogue of Arthropod-Borne Viruses [28]. TOSV is not alone, as Phlebotomus spp. sand flies can also transmit the genetically related Phlebovirus napoliense and Phlebovirus siciliaense viruses. Infection with these two viruses is limited to febrile illness, with no neurological involvement. Importantly, TOSV is the only sand fly-borne phlebovirus that can cause neurological disease [6]. TOSV has since been detected across a wide geographic range, including much of Southern Europe, Africa and the Mediterranean region. In the Mediterranean region, the majority of TOSV infections occur in the warmer months between May and October, peaking in August. This time frame corresponds to the peak activity of the sand fly vector [5]. In some of these regions, antibody prevalence in humans for sand fly-borne viruses is greater than 50% [27], suggesting that TOSV and related viruses have long imposed a substantial, underappreciated burden on human health. The ability of TOSV to be neurovirulent was first shown following the investigation of cerebrospinal fluid (CSF) from a Swedish patient with encephalitis, who had recently visited Portugal [4]. TOSV infection has also been reported in Swedish United Nations soldiers following stays in Cyprus [29,30]. Perhaps not surprisingly, there is a high antibody prevalence rate of 20% to TOSV among Cyprus’s local population [27]. Travellers returning from TOSV-endemic regions also regularly present with evidence of TOSV infection [31,33]. Table 1 describes a complete list of epidemiological studies evaluating TOSV burden in these regions. However, it is important to note that cross-reactions might have influenced the estimation of the seroprevalence rate. Serological cross-reactions have been documented within the sand fly fever Naples virus complex, of which TOSV is a member [34]. Indeed, our new phylogenetic comparison shows that TOSV is highly related to a number of viruses including Naples phlebovirus, to which it is most related, and the Zerdali, Tehran, Massilia and Punique phleboviruses (Fig. 2).
Table 1. Epidemiological studies of TOSV.
| Location | Year | Cases/findings | References |
| USA | 1985, 2009, 2015 | Three imported cases of TOSV meningitis or meningoencephalitis from Italy were reported | [31,52, 197] |
| UK | 2019 | One imported case was determined with TOSV encephalitis | [198] |
| Sweden | 1980s | An imported TOSV case was reported from Swedish tourists visiting Spain | [199] |
| Southern Europe | |||
| Southern Italy | 2000s | Meningitis and encephalitis cases were reported during the summer | [200,202] |
| Southern France | 2001, 2004 | Two TOSV cases manifested as aseptic meningitis and influenza-like illnesses, while one case was diagnosed as acute meningitis | [203,204] |
| Catalonia, Spain | Early 2000s | A 6% seroprevalence of anti-TOSV IgG was found, with two acute clinical cases with viral meningitis or meningoencephalitis | [205] |
| Spanish Mediterranean and Madrid | Early 2000s | The overall seroprevalence was 24.9%, with higher rates observed in rural populations compared to urban areas | [73,206,208] |
| Emilia-Romagna and Umbria, Italy | 2002, 2003 | The first reports of CNS infections caused by TOSV were documented | [209,210] |
| France | 2003, 2006 | Imported acute meningitis and meningoencephalitis cases due to TOSV infection were reported | [211,212] |
| Portugal | 2002–2005 | TOSV meningitis was confirmed in 6 (5.6%) cases out of 106 samples tested | [213] |
| Portugal | 2004–2008 | The prevalence was 4.2% in those with neurological symptoms and 1.3% in those without neurological symptoms | [50] |
| Kosovo | 2005 | The presence of TOSV in the population was suggested | [214] |
| Bosnia and Herzegovina | 2006–2008 | Anti-TOSV IgG and IgM were analysed in 68 human serum samples, revealing recent infection in 7 patients (10.29%) | [215] |
| Croatia | 2007–2009 | TOSV seropositivity was 37.5% among healthy residents | [216] |
| Ionian Islands, Greece | 2010 | TOSV IgG antibody prevalence was 51.7% in Corfu and 39% in Cephalonia | [217] |
| Northern Italy | 2010 | Acute meningitis cases due to TOSV infection were reported | [218] |
| Greece | 2010–2014 | TOSV was responsible for 10% of CNS infections | [219] |
| Greece | 2010–2014 | Three different TOSV cases were reported, two of which showed neurological symptoms | [51,62, 220] |
| Tuscany, Italy | 2012 | A total seropositivity of 10% was recorded for TOSV | [221] |
| Sicily, Italy | 2012 | TOSV-specific IgG prevalence was 25% in those with neurological symptoms and 10.8% in those without neurological symptoms | [222,223] |
| Emilia-Romagna, Italy | 2012 | Among 120 suspected neuroinvasive infection cases, TOSV was detected in 28.3%. Of these, 79.4% were in the acute phase of infection | [224] |
| Aegean Sea Islands, Greece | 2013 | TOSV seroprevalence was 21% | [225] |
| Northern Greece | 2013 | TOSV seroprevalence was 11.26% | [226] |
| Corsica, France | 2014 | TOSV RNA was detected in Phlebotomus species sand flies | [227] |
| Portugal | 2009–2018 | Six TOSV cases were identified from patients who had CNS infections | [228] |
| Madrid, Spain | 2007, 2018–2019 | The seroprevalence was 34.5% overall, with anti-TOSV IgG at 41.5% in 2007 and 21.3% in 2018–2019 | [229] |
| Elba Island, Italy | 2018 | Twelve cases of TOSV meningoencephalitis with symptoms were reported | [230] |
| Southern Tuscany, Italy | 2011–2019 | TOSV positivity was 4.6% in CSF samples, and TOSV-specific IgM was 27.1% in sera | [231] |
| Southwest Portugal | 2019 | Neutralizing antibodies to TOSV were found in 5.3% of healthy blood donors | [232] |
| Corsica, France | 2019 | TOSV antibodies were found in 22.5% of cases using virus microneutralization assay | [233] |
| Central Europe | |||
| Germany | 1993–1994 | Thirteen acute TOSV infections were reported in German citizens returning from Southern France, Greece, Italy and Portugal | [33,234, 235] |
| Germany | 1993–1995 | Out of 317 patients, 13 (4.1%) tested positive for TOSV antibodies; these cases were imported from Italy (11 cases, 84.6%), Portugal (1 case, 7.7%) and Turkey (1 case, 7.7%) | [236] |
| Netherlands, Germany | 2000s | Imported TOSV infectious-caused CNS diseases were reported | [237,238] |
| Switzerland | 2008, 2009, 2012 | Imported aseptic meningitis cases due to TOSV infection were reported from Swiss tourists who visited in Italy | [239,241] |
| Africa | |||
| Tunisia | 2003–2009 | IgM (10%) and IgG (7%) antibodies for TOSV were identified in patients with neurological diseases | [242] |
| Djibouti, Africa | 2010–2011 | The circulation of Toscana-related viruses was 3.7% | [243] |
| Tunisia | 2013 | Anti-TOSV IgG positivity was 9.5% in healthy individuals | [244] |
| Tunisia | 2013 | TOSV-neutralizing antibodies were present in 41% of human sera, with confirmed co-circulation with Punique virus | [91,245] |
| Northern Algeria | 2013 | The presence of TOSV was confirmed in sand flies and the population with almost 50% seropositivity | [246] |
| Tunisia | 2014 | Anti-TOSV IgM was 12.16% in serum samples, TOSV positivity was 12.86% in CSF samples and TOSV RNA was found in pooled sand fly samples | [247] |
| Algeria | 2016–2018 | TOSV infection was found in 3.8% of patients with neurological symptoms | [248] |
| Libya | 2013–2014 | TOSV seroprevalence was 25% in the population | [249] |
| Middle East | |||
| Türkiye | 2010 | Fourteen TOSV infection cases were reported in patients initially diagnosed with aseptic meningoencephalitis | [250] |
| Central, North and Southeast Anatolia, Türkiye | 2011–2012 | TOSV seroprevalence was 17.8% in asymptomatic blood donors and 15.7% in patients with CNS infections of unknown cause | [85,251] |
| Eastern Thrace, Türkiye | 2012 | TOSV seroprevalence in blood donors was 14.4%, and the first co-infections of WNV and TOSV were reported | [252] |
| Central Anatolia, Türkiye | 2012 | Among 94 patients investigated, TOSV seroreactivity was found in 37.2% (35 patients) | [77] |
| Mediterranean region, Türkiye | 2011–2012 | Neutralizing antibodies to TOSV were detected in 13.9% of healthy blood donors | [253] |
| Western border of Iran | 2013 | Among military personnel, 1% TOSV was revealed in serum samples | [254] |
| Türkiye | 2014 | A patient who was HIV positive was also found to have an acute TOSV infection | [255] |
| Western Saudi Arabia | 2012–2016, 2019 | The circulation of TOSV showed an overall seroprevalence of 0.8% in residents | [135] |
The time frame indicates the years during which the samples were collected or when TOSV diagnoses were made in patients. CNS, central nervous system; HIV, Human Immunodeficiency virus; WNV, West Nile virus.
The clinical importance of TOSV infections is underlined by studies assessing the viral aetiology of central nervous system (CNS) infection among children in Tuscany, Italy. Importantly, these showed that TOSV infection is responsible for at least 80% of summertime viral infections of the CNS in children [35,36]. This peak of CNS infection was coincident with a high frequency of adult insect vectors (Phlebotomus perniciosus and Phlebotomus perfiliewi), which typically peaks in August [37]. In addition, the seroprevalence studies suggest that an L proportion of infection occurs without obvious clinical CNS involvement. For example, in nine different regions of Spain, the seroprevalence of TOSV in a random cohort of individuals was 26% (n = 1268 individuals) that had not presented with meningitis or febrile illness [38]. Here, although antibodies to TOSV were found in younger age groups, they were detected at a higher frequency in older age groups [39]. This has also been shown in an Italian cohort, with an age-dependent seroprevalence of TOSV, with 19.8% in adults and 5.8% in children [40]. Interestingly, those who have exposure to sand fly-enriched environments, such as forestry workers, demonstrate seroprevalence rates as high as 77.2% [41]. A more recent assessment of sand fly-transmitted virus in Italy over a 10-year period described TOSV seropositivity between 22.95 and 26.75% [42].
Further evidence for increased geographical dissemination has come from a recent prevalence study in Bulgaria, which found seropositivity at 24.4% [43]. In Southwest Germany, 4% of individuals with probable viral meningoencephalitis in a retrospective cohort analysis exhibited neuroinvasive TOSV, despite having no prior history of visiting an endemic region [44]. To assess the exposure to sand fly-borne infections in general, one study from Spain assessed seropositivity to sand fly salivary proteins. Here, the seroprevalence to Phlebotomus perniciosus sand fly salivary gland homogenate and recombinant protein rSP03B were investigated to detect sand fly exposure in blood donors, with seroprevalences estimated at 69 and 88%, respectively. The same study showed 26% of TOSV seropositivity in blood samples [45].
In summary, TOSV infection is now widespread across multiple countries. It is likely that further spread to more temperate countries will occur as the climate warms and international travel continues apace [46,47].
Clinical manifestations of TOSV infection
Similar to many arboviruses, the majority of TOSV infections are either asymptomatic, mild or undiagnosed [48]; however, an increasing number of cases develop severe disease that can be life threatening or leave disabling sequelae, including those that involve deafness [49]. Typically, the infection manifests as a mild febrile illness, commonly marked by elevated body temperature, headaches, skin rashes (exanthema) with haemorrhagic features, feelings of sickness, muscle pain, joint pain, arthritis and occasionally nausea and vomiting [50,51]. Following a bite by an infected sand fly, the virus is assumed to replicate in the skin and then disseminates to the systemic circulation, at which point clinical signs can become apparent. This incubation period can range from 3 to 7 days, before the onset of more severe clinical signs. However, the incubation period of this infection can be considerable. In one case report, the incubation period extended to 17 days in a patient who developed extreme lethargy, malaise, anhedonia and decreased hearing [52], while another study assessing infection in a travel-acquired cohort estimated the incubation period at an average of 12 days [53]. During the early stage phase of infection that coincides with viraemia, TOSV RNA can also be detected in urine, a characteristic that is sometimes observed in virus infections that involve the nervous system [54,57].
Complications of TOSV infection
Signs of meningitis, sudden hearing loss and other neurological involvement can develop in some cases 2 weeks post-fever. These are diverse and can manifest differently and can encompass Kernig’s sign (resistance to knee extension with hip flexed), stiffness in the neck, light sensitivity, tremors, nystagmus (involuntary eye movements), muscle weakness, double vision, sleep disturbances, prolonged fatigue, altered mental alertness and changes in consciousness. Typically, these symptoms can endure for several weeks, while persistent alterations in personality linked to TOSV encephalitis have also been reported [58,60], which can also occur without concurrent meningitis [61,62]. TOSV has the potential to cause fatal encephalitis in humans, although this is rare. In one fatal case, the diagnosis was based on positive serological results and the patient’s travel history to Tuscany prior to the development of symptoms, which indicated progressive encephalitis linked to TOSV infection [63]. Moreover, in Romania, severe encephalitis and meningoencephalitis caused by TOSV were identified through real-time reverse transcription polymerase chain (RT-PCR) testing, following five deaths out of eight patients [55]. More frequent is the presentation of TOSV-linked hydrocephalus, a complication of viral meningoencephalitis that is otherwise highly uncommon. Patients with CNS TOSV infection have also reported testicular pain including epididymal-orchitis, epididymitis and genital vasculitis [64,68] although no evidence yet suggests direct infection of testes [65]. Interestingly, TOSV RNA was detected in seminal fluid samples from a patient with TOSV meningitis [69], suggesting possible transmission through sexual intercourse.
Persistent sequalae of infection have also been noted. In one case, a patient presented with severe neurological features after returning from Umbria, Italy. Here, serum and CSF were positive for antibodies against TOSV, and recovery was associated with persistent headaches [70]. There have also been reports of siblings who contracted severe life-threatening meningoencephalitis following TOSV infection and have since experienced long-lasting neurological complications, including hydrocephalus [64].
TOSV infection associated with peripheral neuropathy has been reported to mimic a Guillain–Barré-like syndrome. Although one case report was insufficient to demonstrate a definitive association [71], in a further case-control study, TOSV was recognized as a contributing factor to the development of Guillain–Barré syndrome [72]. Due to the scarcity of cases, it is not clear whether these TOSV neurological manifestations of the peripheral nervous system can be considered an established feature of this disease. TOSV infection also has the capacity to rarely cause persistent neurological infections accompanied by ischaemic complications [73], sensory polymyeloradiculopathy [66] and brachial plexus involvement [74].
Complications arising from TOSV infections do not always involve CNS disease [75]; lymphadenopathy [76], pancytopenia during acute infection [77], benign myositis and fasciitis [78] have been described. The connection between myositis and viral infections is well established [79]. The mechanisms behind these viral-induced pathologies remain unclear and warrant further research. In summary, TOSV infections are of growing clinical importance. In the absence of an animal model that recapitulates TOSV disease, the majority of our insights on TOSV pathogenesis have come from observations in the clinic. Understanding the molecular and cellular basis for these diverse pathologies, and the host response to infection, is important if we are to improve the care and treatment of these patients.
Diagnosis of TOSV infection
The diagnosis of TOSV infection typically involves clinical evaluation and laboratory testing, emphasizing the assessment of symptoms, medical history and travel to TOSV-endemic areas. Given the rise in imported cases, especially in countries where the virus is not endemic, it is increasingly important for medical professionals to consider TOSV as a differential diagnosis, particularly in cases of AME in individuals who have recently returned from the Mediterranean region [52,80].
For patients with CNS involvement, differential diagnosis can be aided through the use of virological tests to confirm TOSV infection and so differentiate from, e.g. West Nile virus (WNV), enterovirus and herpesvirus infections among others. These assays are typically PCR based and can detect low concentrations of viral RNA. PCR testing detects viral genetic material and is considered indirect virological testing. PCR does not confirm active viral infection, as residual RNA can persist in some tissues for many months post-infection. Sensitivity is an important consideration for cases with neurological involvement, as the prior acute viraemic phase is rapid and infectious virus can be cleared from the blood, most likely through binding neutralizing antibodies, before clinical presentation [81]. To date, several PCR assays have been established to detect TOSV, including nested RT-PCR of serum and CSF samples, which are additionally employed for those with the acute meningitis [82,83]. Typically, assay primers amplify the TOSV S fragment [36]. More recently, real-time PCR assays have been adopted due to their time-saving benefits and reduced risk of contamination [84]. Although CSF sampling is most informative, recent developments have optimized the detection of TOSV RNA using real-time RT-PCR in more accessible blood samples from patients with CNS disease [85,87]. Lately, a TOSV real-time RT-PCR assay has been established to target three specific genomic regions within the nucleoprotein gene. This assay offers a robust and sensitive method for detecting TOSV by targeting multiple genomic regions, enhancing the specificity and reducing the risk of false negatives [88]. Additionally, diluted urine samples have been shown to be suitable for TOSV RNA detection using this Trio TOSV real-time RT-PCR system [89]. Finally, there now exists a multiplex PCR where several sets of primers are employed in a single reaction to identify infections caused by either TOSV or enterovirus [81].
Serological assays, such as ELISA and immunofluorescence assay, can be used to detect current and previous infections. As such, they are less informative for diagnosis but may offer the only sign of TOSV infection if the acute viraemia has passed and TOSV RNA can no longer be detected. These assays detect specific antibodies (IgM and IgG) against TOSV in the patient’s blood serum. There is limited cross-reactivity among viruses belonging to the Phlebovirus genus, particularly between the TOSV and Phlebovirus napoliense, mainly because of the significant similarity in the N protein of these viruses [90]. As such, these tests are of most use when screening many specimens rapidly, e.g. for seroprevalence studies. Notably, the neutralization assay has a lower likelihood of cross-reactions compared to indirect immunofluorescence assay or ELISA [38,91]. Several commercial assays are available for the detection of TOSV antibodies, providing diagnostic options for healthcare professionals when assessing potential TOSV infections [92].
Alternatively, diagnosis can be based on isolating the virus. Here, clinical samples, especially CSF, are used to infect cells (e.g. Vero or BHK-21) and monitored for detection of cytopathic effects in cells. This approach is more powerful when combined with sequencing to define the aetiological agent. However, in practice, this is rarely undertaken due to its complexity and the availability of other diagnostic techniques. In addition, there are concerns that these cell culture-based approaches, which, e.g. monitor cytopathic effect, are not sufficiently sensitive compared to PCR [93]. For example, a recent study that incorporated metagenomic next-generation sequencing has been used as a differential diagnostic tool in undiagnosed meningitis cases and revealed 8 cases (8/23) caused by TOSV [94]. Much insight could be generated if sequencing of the full infectious genome was undertaken, especially alongside epidemiological studies. This could help define a number of key TOSV infection attributes such as mutation rates, strain selection and whether specific strains of TOSV are more likely to involve CNS tissue.
In summary, prompt diagnosis allows for appropriate medical care and management of patients with TOSV infections [95]. To provide rapid diagnosis and minimize cross-reactivity, it is important that the clinician chooses the gold standard for TOSV detection, which can incorporate a combination of specific serology and PCR-based approaches. Although there are no specific treatments for TOSV infection, appropriate clinical management can improve outcomes, while a more widespread use of clinical TOSV detection would enable a more accurate definition of TOSV prevalence.
Phlebotomus spp. (Diptera: Psychodidae) sand fly: vectors of TOSV
Transmission features
Sand flies belong in the order Diptera, suborder Nematocera, family Psychodidae and subfamily Phlebotominae. Six primary sand fly genera are recognized, three of which are found in the Old World (Phlebotomus, 13 subgenera; Sergentomyia, 10 subgenera; and Chinius, 4 species) and three of which are found in the New World (Lutzomyia 26 subgenera and groups; Brumptomyia, 24 species; and Warileya, 6 species) [96]. Notably, the genera Lutzomyia and Phlebotomus and some Sergentomyia [97] are those that are anthropophilic and exhibit competence to transmit pathogens [98].
Geographically, sand flies are present between 50° N and 40° S latitudes, but are absent in New Zealand and the Pacific islands [98]. Phlebotomine sand flies principally exist in the warmer climates of Asia, Africa, Australia, Southern Europe and the Americas [99]. Importantly, it is predicted that this range will extend to new transmission zones because of climate change [100]. In addition to Phleboviruses, phlebotomine sand flies are also responsible for the spread of Leishmania (Leishmania) spp. (Kinetoplastida: Trypanosomatidae) and Bartonella bacilliformis [101,102]. Of the 900 sand fly species, less than 100 can transmit Leishmania parasites, while just nine species of sand flies transmit Phleboviruses, including TOSV [93,101, 102]. Phlebotomus perniciosus and Phlebotomus perfiliewi have been identified as vectors of TOSV [103,104]. Although not yet documented, it is likely that other related species such as Phlebotomus sergenti, Phlebotomus longicuspis, Phlebotomus neglectus, Phlebotomus tobbi and Sergentomyia minuta could also participate in TOSV transmission [105,107].
The contributing factors for TOSV maintenance in nature are not well known. Interestingly, both male and female sand flies have been identified with TOSV infection. As in other haematophagous insects, only females acquire blood meals; therefore, infection in male sand flies suggests vertical and/or transovarial transmission [104]. Indeed, experimentally infected sand fly species, including Phlebotomus perniciosus, can transmit TOSV transovarially [108,110]. In addition, transovarially infected female sand flies can transmit TOSV by biting a susceptible vertebrate [110]. Experimental evidence further suggests venereal infection in female sand flies, which might serve as an infection amplifier in the absence of other reservoirs [111]. However, the presence of vertebrate reservoirs is likely to be required for TOSV maintenance, as viral infection rates in sand fly colonies not exposed to viraemic vertebrates steadily drop with each succeeding generation of the colony [110,112, 113].
The capacity of TOSV to circulate horizontally among members of the same generation has also been suggested based on work on the related phlebovirus Massilia virus [114,115]. Interestingly, infection of sand flies was more efficient if included with a sugar meal. This could suggest that virus deposited by sand flies, as they seek nectar, may be an efficient method to infect other sand flies that feed from the same site [116].
Potential vertebrate reservoirs of TOSV
With the high prevalence of TOSV seroconversion in humans, the presence of a non-human vertebrate reservoir may not necessarily be a prerequisite. Nonetheless, numerous species of vertebrates have been proposed as TOSV reservoirs, although firm proof is still lacking. Temperature, humidity and airflow all have an impact on feeding activity. Though biting might occur indoors in darkened areas or among shaded vegetation during the day, most species feed around sunset and night when the temperature drops and humidity rises [99]. Adult sand flies can be found in caves and rock crevices, tree trunks or tree hollows, domestic animal enclosures, masonry crevices and other dark, humid locations such as basements and wells [117]. The vast range of vertebrate hosts that female sand flies feed on includes humans and various animals including canines, rodents, reptiles, amphibians and birds [101]. Phlebotomus perniciosus have a particularly varied feeding habit and typically are opportunistic feeders, biting whichever animal happens to be nearby [118,121].
Given the high frequency of either TOSV RNA detection or neutralizing antibodies found in canine blood samples taken during the sand fly season in Mediterranean Anatolia, Türkiye [121,122]; Portugal [123]; Corsica [124]; and Algeria [125,126], dogs have been proposed as a reservoir host. Indeed, the seroprevalence of TOSV was 6.8% in dogs and 3.7% in cats in the Portuguese study. Another seroprevalence study showed that guard dogs’ seroprevalence rate was 7.5% in two different regions of Tunisia [127], while there was 8.4% seropositivity in dogs from Greece [128]. Antibodies in cats to Phlebotomus perniciosus saliva (47.7%, 350/167) and neutralizing antibodies against TOSV (4.9%, 18/365) show that cats are bitten by sand flies and can be infected with TOSV [129]. However, it is not clear if either species supports the TOSV transmission cycle, due to the low level of viraemia that results from infection and inability to excrete virus [130]. However, experimental infection of dogs by Phlebotomus perniciosus feeding has been recently documented in a natural setting [131]. It has also been suggested that Leishmania infantum-infected canines demonstrate enhanced vectorial capacity for TOSV [132,133], compared to healthy dogs that do not have L. infantum. Livestock are also frequently bitten by sand flies, particularly Phlebotomus perniciosus [121]. These animals may also act as a reservoir, with 5–8% of serum samples exhibiting seropositivity (Kosovo, n = 1086 [134] and Saudi Arabia [135]). TOSV RNA is typically not detected in most cases, complicating host range definition [136], although infections of sheep and goat have also been suggested.
For several neurotropic arboviruses, such as WNV, birds have a role as viral amplification hosts, vector dispersion vehicles and sources of new strains by interspecies transmission. Birds passing through known migratory routes in the Hatay Province of Türkiye have been shown to be positive for TOSV RNA in samples taken from the brain and kidney [137]. In addition, the migratory common quail (Coturnix coturnix) exhibits a high seroprevalence rate of 42.45% in Spain [138]. Furthermore, a recent study conducted in the northern wetlands of Türkiye revealed the presence of both TOSV RNA and infectious virus in bird populations [139]. These findings indicate that birds may be a reservoir or act as an amplifying host for TOSV.
Bats have been recognized as important reservoirs of many zoonotic viruses worldwide [140]. However, there is little information about the role of bats in the ecology of Phleboviruses, including TOSV. While TOSV was once isolated from a bat’s brain (Pipistrellus kuhlii) [104], TOSV exposure rate can be considered low in bats as long-lived animals, with an antibody seroprevalence at 10%. Therefore, bat colonies are not likely to play a reservoir role for TOSV [141].
Effect of the climate emergency on sand fly distribution
Zika, dengue and chikungunya viruses are well-described examples of agents that are transmitted by mosquitoes whose distribution has spread to over 130 countries [142]. As climate change becomes more pronounced, it is anticipated that, in addition to mosquitoes, other haematophagous insects like sand flies will also undergo geographical expansion. Climate has multiple impacts on the dynamics and prevalence of arthropod-borne infections. Invertebrate vectors, including sand flies, are ectothermic, and changes in the environment due to climate change can impact life cycle, movement, feeding activity and survival [143,144]. Many sand fly species have already been established across the Mediterranean region. Models predicted that just a 1 °C increase could create optimal environmental conditions for certain sand fly species (Phlebotomus mascittii and Phlebotomus neglectus) [145]. Subsequently, Phlebotomus (Transphlebotomus) mascittii was newly documented in Austria and across the Western Europe [146], while Phlebotomus mascittii has now been confirmed in central Europe, north of the Alps, France, Switzerland, Belgium and Germany [147]. As such, the TOSV vectors Phlebotomus mascittii and Phlebotomus perniciosus are now not only confined to Southern Europe but also identified in Germany [148], where autochthonous cases of TOSV meningoencephalitis have been reported [44,149]. In addition, changes in Phlebotomus ariasi, Phlebotomus neglectus and Phlebotomus perfiliewi distribution are being observed, with these species spotted in northern regions and higher altitudes, a shift attributed to climate change. The occurrence of imported TOSV infections in these locations poses a risk for potential local outbreaks if the competent vector species become established, as observed with other diseases transmitted by vectors. In summary, TOSV could extend its activity to new temperate regions where suitable vector species exist [150,153].
TOSV and Sergentomyia sp.
Sand flies belonging to the genus Sergentomyia feed on a wide range of animals including reptiles, birds and a diverse array of mammals, occasionally including humans [154,155]. Some of these flies, including S. minuta, have been found positive for both human and Leishmania DNA [120] and can transmit Leishmania to reptiles [96] and possibly to humans [156,159]. Importantly, this species may also transmit TOSV to vertebrates as they have been found harbouring TOSV RNA [160]. Furthermore, the Phlebotomusvectored Chandipura virus (CHPV) has also been detected in Sergentomyia sand flies from India. CHPV is a member of Rhabdoviridae that can, like TOSV, cause encephalitis [161]. Together, this suggests that S. minuta may also be competent for transmitting Phleboviruses, including TOSV [162].
TOSV and Lutzomyia sp.
A pressing concern is the possibility that Lutzomyia sp. sand flies could transmit TOSV. This New World sand fly genus is found across South, Central and North America and consists of over 400 species, including Lutzomyia (Lu.) longipalpis (Diptera: Psychodidae: Phlebotominae). This species is an important vector of several medically important pathogens, including Leishmania and potentially also arboviruses [96]. Indeed, it is the main vector of L. infantum in the Americas that causes visceral leishmaniasis. Lu. longipalpis has broad-range feeding habits and has different habitats, including rural and urban areas feeding on humans, pets, livestock, rodents, bats and opossums [163,165]. However, studies investigating the potential capability of this key vector to become infected and transmit arbovirus are urgently required [166].
While laboratory-based infection of Lu. longipalpis with Phlebotomus-transmitted Phlebovirus siciliaense and Phlebovirus napoliense is inefficient [167], there is evidence to suggest competence as an arboviral vector. For example, Punta Toro virus (PTV, also a phlebovirus) is transmitted by Lutzomyia species in Panama [168], while the Candiru complex viruses (family Phenuiviridae) have been isolated from Lutzomyia species, some of which cause febrile illness in humans [169]. Viola phlebovirus, a putative new viral species and a novel Phlebotomus fever serogroup member, was identified in Lu. longipalpis species in Brazil. The ability of this new sand fly-derived virus to replicate within mammalian cell lines and express NSs and NSm proteins suggests that the virus may be a novel arbovirus [170].
A Lu. longipalpis cell line can be infected and replicate a wide range of arboviruses, although PTV infection was inefficient [171,172]. This is surprising as PTV has been isolated from both humans and sand flies and may suggest that the cell line may be derived from a cell type resistant to infection. Interestingly, the replication of Bunyavirales in Lu. longipalpis is nonetheless possible as RVFV can replicate following the intrathoracic inoculation (albeit not via blood feeding) and be transmitted to RVFV-susceptible mammalian hosts [173]. Despite RVFV’s ability to infect multiple species, this is intriguing considering the geographic separation of vector (Americas) with RVFV (Old World) and their lack of co-evolution to date [173]. Interestingly, Lu. longipalpis may mechanically transmit RVFV to other mammalian hosts after exposure to a virus donor blood meal [174]. The epidemiological significance of mechanical transmission of arboviruses needs to be clarified, as this transmission route could enable infection from vectors that are otherwise not considered competent vectors [174].
To date, with the geographic range of Lu. longipalpis and TOSV not overlapping, it is not surprising that there is no field evidence that this sand fly can be infected or transmit TOSV to vertebrate hosts. There may also be sequence adaptions required by TOSV for infection of Lutzomyia species for transmission to be sufficiently efficient. Nonetheless, in our globalized world of international travel, there is a risk of TOSV viraemic individuals becoming exposed to biting sand flies of the Americas [175].
Putative role of sand fly saliva in modulating TOSV infection
Saliva deposited by biting haematophagous arthropods is biologically active in vertebrates. Like all arbovirus life cycles, sand fly-vectored TOSV involves continual transfer between vertebrate hosts and vectors. Following a blood meal from an infected vertebrate host, the virus undergoes replication within the sand fly’s midgut. Subsequently, it migrates to the salivary glands, where it can be transmitted to a new host when the sand fly feeds on another blood meal. The transmission occurs when a female sand fly (males do not bite) inserts its mouthparts into the host’s skin, during which saliva and virus are deposited into the dermis [176].
Female sand fly saliva contains a blend of diverse pharmacologically active substances that have evolved to facilitate efficient feeding, including compounds with anti-haemostatic, vasoactive, immunomodulatory and anti-inflammatory properties [177]. These counteract vertebrate processes to enable efficient feeding but also have unintended consequences for mammalian susceptibility to pathogens including Leishmania infection. Leishmania major, the causative agent of cutaneous leishmaniasis, co-inoculated with salivary gland lysate from Lu. longipalpis, causes larger lesion size in the skin and higher parasite burden [178]. Indeed, compared to needle inoculation, Leishmania infection by sand fly bite increased the replication of this parasite in mice by many orders of magnitude and more severe disease. This is due to sand fly saliva/biting causing a rapid influx of neutrophils and monocytes, both which can become infected and support enhanced Leishmania infection [179,181].
Notably, mosquito saliva also plays an important role in determining the severity of arbovirus infections [182]. Factors in Aedes mosquito saliva enhance infection with a wide number of arboviruses including Bunyavirales RVFV, Cache Valley virus and Bunyamwera virus, resulting in increased mortality rates of mice [183,185]. In addition, the flavivirus dengue virus and alphaviruses (Semliki Forest virus and Chikungunya virus) infection are also enhanced by the presence of mosquito saliva in the inoculum. Similarly, WNV mixed with its vector (Culex mosquito) salivary factors caused higher viraemia, faster dissemination of the virus to tissues and earlier microinvasion compared to inoculation with WNV alone [186]. Besides mosquito-derived factors, tick saliva co-inoculated with Powassan virus (an encephalitic tick-borne flavivirus) also increases viral loads and alters the course of disease in mice, compared to mice infected with POWV alone [187].
It is not yet known whether sand fly saliva has a role in modulating bunyavirus infection, including TOSV infection, of the vertebrate host. Importantly, the lack of suitable immunocompetent mouse models of TOSV for investigating skin infection has hindered this, although a mouse model using a neuro-adapted strain of TOSV, with limited viral dissemination, has been defined [188]. The majority of salivary components of sand flies remain only partially characterized, and their specific roles are still unknown. Nonetheless, some interesting insights have been obtained, including salivary proteins such as antigen 5-related proteins, apyrases, odorant-binding proteins (including D7-related and PpSP15-like proteins), yellow-related proteins, silk-related proteins and lufaxin-like proteins [189]. The characterization of these salivary molecules and their biological activities have been discussed elsewhere [190,191].
Sand fly saliva is also immunomodulatory, which may alter host susceptibility to TOSV. Both Lutzomyia and Phlebotomus species’ saliva has an inhibitory impact on the activation of T cells, while promoting the expression of Th2-type cytokines [192,193]. Whether T cell modulation by saliva in the skin occurs sufficiently quickly to alter infection with the rapidly replicating TOSV is not known. Sand fly saliva is also highly inflammatory, inducing chemokine CCL2 expression and the recruitment of macrophages [194] and the expression of pro-inflammatory cytokines TNF-α, IL-6, CXCL8 and IL-12 [195]. Interestingly, saliva also has an impact on dendritic cells, stimulating the expression of IL-10 and prostaglandin E2, while decreasing the expression of co-stimulatory molecules Cluster of Differentiation 86 and also Major Histocompatibility Complex Class II may suppress this cell’s antigen-presenting function [196]. Considering that saliva has multiple effects on host physiology and immunity, it will be crucial to define whether sand fly saliva might also only influence the vertebrate host’s susceptibility to TOSV infection.
In conclusion, TOSV is an important and yet poorly understood cause of infectious neurological disease, especially in children. The high levels of prevalence in some endemic regions suggest that it also constitutes a substantial burden to non-neurological health, e.g. febrile illness. For those infections that cause neurological disease, the more routine inclusion of TOSV as a differential diagnosis, combined with more accurate molecular and/or serological testing will improve our estimate of the true burden of health imposed by TOSV. For those infections that do spread to neural tissue, our understanding of TOSV pathogenesis has almost exclusively been informed by observations made in the clinic. The development of an animal model that recapitulates aspects of human disease is therefore urgently needed to aid both our understanding of TOSV disease and the development of novel therapeutics. This is key, as numbers of TOSV cases are predicted to increase in the coming years, including in more temperate regions, in which the majority of individuals are immunologically naïve to TOSV. As such there is a clear unmet need to undertake more TOSV research.
Acknowledgements
Y.K.T. acknowledges the financial support of the Study Abroad Postgraduate Education Scholarship (MEB1416) awarded by the Republic of Türkiye Ministry of National Education. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official, or as reflecting true views of the US Department of the Army, Navy or the Department of Defense. The material contained within this publication has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. This manuscript was prepared while K.E. held a National Research Council (NRC) Research Associateship Awards at the Walter Reed Biosystematics Unit, through the Walter Reed Army Institute of Research, Silver Spring, MD. J.H. is funded by the UK Medical Research Council (MC_UU_00034/5).
Abbreviations
- AME
acute meningitis and encephalitis
- CHPV
Chandipura virus
- CHPV
Chandipura virus
- CNS
central nervous system
- CSF
cerebrospinal fluid
- L
large
- M
medium
- N
nucleocapsid
- PTV
Punta Toro virus
- RdRp
RNA-dependent RNA polymerase
- rTOSV
recombinant TOSV
- RVFV
Rift Valley fever virus
- S
small
- TOSV
Toscana virus
- WNV
West Nile virus
Footnotes
Funding: The authors received no specific grant from any funding agency.
Contributor Information
Yonca Keskek Turk, Email: umykt@leeds.ac.uk.
Koray Ergunay, Email: korayergunay@gmail.com.
Alain Kohl, Email: Alain.Kohl@lstmed.ac.uk.
Joseph Hughes, Email: joseph.hughes@glasgow.ac.uk.
Clive S. McKimmie, Email: clive.mckimmie@york.ac.uk.
References
- 1.Kuhn JH, Abe J, Adkins S, Alkhovsky SV, Avšič-Županc T, et al. Annual (2023) taxonomic update of RNA-directed RNA polymerase-encoding negative-sense RNA viruses (realm Riboviria: kingdom Orthornavirae: phylum Negarnaviricota) J Gen Virol. 2023;104:1–55. doi: 10.1099/jgv.0.001864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King AMQ, Lefkowitz EJ, Mushegian AR, Adams MJ, Dutilh BE, et al. Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2018) Arch Virol . 2018;163:2601–2631. doi: 10.1007/s00705-018-3847-1. [DOI] [PubMed] [Google Scholar]
- 3.Verani P, Ciufolini MG, Nicoletti L, Balducci M, Sabatinelli G, et al. Ecological and epidemiological studies of Toscana virus, an arbovirus isolated from Phlebotomus. Ann Ist Super Sanita. 1982;18:397–399. [PubMed] [Google Scholar]
- 4.Ehrnst A, Peters CJ, Niklasson B, Svedmyr A, Holmgren B. Neurovirulent Toscana virus (a sandfly fever virus) in Swedish man after visit to Portugal. Lancet. 1985;1:1212–1213. doi: 10.1016/s0140-6736(85)92886-7. [DOI] [PubMed] [Google Scholar]
- 5.Nicoletti L, Verani P, Caciolli S, Ciufolini MG, Renzi A, et al. Central nervous system involvement during infection by Phlebovirus toscana of residents in natural foci in central Italy (1977-1988) Am J Trop Med Hyg. 1991;45:429–434. doi: 10.4269/ajtmh.1991.45.429. [DOI] [PubMed] [Google Scholar]
- 6.Ayhan N, Charrel RN. An update on Toscana virus distribution, genetics, medical and diagnostic aspects. Clin Microbiol Infect. 2020;26:1017–1023. doi: 10.1016/j.cmi.2019.12.015. [DOI] [PubMed] [Google Scholar]
- 7.Elliott RM. The Bunyaviridae. Springer Science & Business Media; 2013. [Google Scholar]
- 8.Koch J, Xin Q, Tischler ND, Lozach PY. Entry of Phenuiviruses into mammalian host cells. Viruses. 2021;13:11–14. doi: 10.3390/v13020299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lozach P-Y, Kühbacher A, Meier R, Mancini R, Bitto D, et al. DC-SIGN as a receptor for phleboviruses. Cell Host Microbe. 2011;10:75–88. doi: 10.1016/j.chom.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Spiegel M, Plegge T, Pöhlmann S. The role of Phlebovirus glycoproteins in viral entry, assembly and release. Viruses. 2016;8:202. doi: 10.3390/v8070202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lozach P-Y, Mancini R, Bitto D, Meier R, Oestereich L, et al. Entry of bunyaviruses into mammalian cells. Cell Host Microbe. 2010;7:488–499. doi: 10.1016/j.chom.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hornak KE, Lanchy J-M, Lodmell JS. RNA encapsidation and packaging in the phleboviruses. Viruses. 2016;8:194. doi: 10.3390/v8070194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wuerth JD, Weber F. Phleboviruses and the type I interferon response. Viruses. 2016;8:174. doi: 10.3390/v8060174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koch J, Xin Q, Obr M, Schäfer A, Rolfs N, et al. The phenuivirus Toscana virus makes an atypical use of vacuolar acidity to enter host cells. PLoS Pathog . 2023;19:e1011562. doi: 10.1371/journal.ppat.1011562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walter CT, Barr JN. Recent advances in the molecular and cellular biology of bunyaviruses. J Gen Virol. 2011;92:2467–2484. doi: 10.1099/vir.0.035105-0. [DOI] [PubMed] [Google Scholar]
- 16.Piper ME, Sorenson DR, Gerrard SR. Efficient cellular release of Rift Valley fever virus requires genomic RNA. PLoS One. 2011;6:e18070. doi: 10.1371/journal.pone.0018070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woelfl F, Léger P, Oreshkova N, Pahmeier F, Windhaber S, et al. Novel Toscana virus reverse genetics system establishes NSS as an antagonist of type I interferon responses. Viruses. 2020;12:400. doi: 10.3390/v12040400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gori-Savellini G, Valentini M, Cusi MG. Toscana virus NSs protein inhibits the induction of type I interferon by interacting with RIG-I. J Virol. 2013;87:6660–6667. doi: 10.1128/JVI.03129-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gori Savellini G, Anichini G, Gandolfo C, Prathyumnan S, Cusi MG. Toscana virus non-structural protein NSs acts as E3 ubiquitin ligase promoting RIG-I degradation. PLoS Pathog. 2019;15:e1008186. doi: 10.1371/journal.ppat.1008186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalveram B, Ikegami T. Toscana virus NSs protein promotes degradation of double-stranded RNA-dependent protein kinase. J Virol. 2013;87:3710–3718. doi: 10.1128/JVI.02506-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valassina M, Cusi MG, Valensin PE. A Mediterranean arbovirus: the Toscana virus. J Neurovirol. 2003;9:577–583. doi: 10.1080/13550280390247678. [DOI] [PubMed] [Google Scholar]
- 22.Elliott RM. Emerging viruses: the Bunyaviridae. Mol Med. 1997;3:572–577. [PMC free article] [PubMed] [Google Scholar]
- 23.Barker J, daSilva LLP, Crump CM. Mechanisms of bunyavirus morphogenesis and egress. J Gen Virol. 2023;104:1–11. doi: 10.1099/jgv.0.001845. [DOI] [PubMed] [Google Scholar]
- 24.Alexander AJT, Confort M-P, Desloire S, Dunlop JI, Kuchi S, et al. Development of A reverse genetics system for Toscana virus (Lineage A) Viruses. 2020;12:1–15. doi: 10.3390/v12040411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ayhan N, Baklouti A, Prudhomme J, Walder G, Amaro F, et al. Practical guidelines for studies on sandfly-borne Phleboviruses: Part I: important points to consider ante field work. Vector-Borne Zoo Dis. 2017;17:73–80. doi: 10.1089/vbz.2016.1957. [DOI] [PubMed] [Google Scholar]
- 26.Sabin AB, Philip CB, Paul JR. Phlebotomus (pappataci or sandfly) fever: a disease of military importance summary of existing knowledge and preliminary report of original investigations. J Am Med Assoc. 1944;125:693–699. [Google Scholar]
- 27.Eitrem R, Stylianou M, Niklasson B. High prevalence rates of antibody to three sandfly fever viruses (Sicilian, Naples, and Toscana) among Cypriots. Epidemiol Infect. 1991;107:685–691. doi: 10.1017/s0950268800049384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karabatsos N. Supplement to International Catalogue of Arboviruses including certain other viruses of vertebrates. Am J Trop Med Hyg. 1978;27:372–440. doi: 10.4269/ajtmh.1978.27.372. [DOI] [PubMed] [Google Scholar]
- 29.Eitrem R, Vene S, Niklasson B. Incidence of sand fly fever among Swedish United Nations soldiers on Cyprus during 1985. Am J Trop Med Hyg. 1990;43:207–211. doi: 10.4269/ajtmh.1990.43.207. [DOI] [PubMed] [Google Scholar]
- 30.Niklasson B, Eitrem R. Sandfly fever among Swedish UN troops in Cyprus. Lancet. 1985;325:1212. doi: 10.1016/S0140-6736(85)92885-5. [DOI] [PubMed] [Google Scholar]
- 31.Calisher C, Weinberg A, Muth D, Lazuick J. Toscana virus infection in United States citizen returning from Italy. Lancet. 1987;329:165–166. doi: 10.1016/S0140-6736(87)92005-8. [DOI] [PubMed] [Google Scholar]
- 32.Schwarz T, Gilch S, Jäger G. Travel-related Toscana virus infection. Lancet. 1993;342:803–804. doi: 10.1016/0140-6736(93)91568-7. [DOI] [PubMed] [Google Scholar]
- 33.Schwarz TF, Jäger G, Gilch S, Pauli C. Serosurvey and laboratory diagnosis of imported sandfly fever virus, serotype toscana, infection in Germany. Epidemiol Infect. 1995;114:501–510. doi: 10.1017/s0950268800052213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Depaquit J, Grandadam M, Fouque F, Andry PE, Peyrefitte C. Arthropod-borne viruses transmitted by Phlebotomine sandflies in Europe: a review. Euro Surveill. 2010;15:40–47. doi: 10.2807/ese.15.10.19507-en. [DOI] [PubMed] [Google Scholar]
- 35.Braito A, Corbisiero R, Corradini S, Fiorentini C, Ciufolini MG. Toscana virus infections of the central nervous system in children: a report of 14 cases. J Pediatr. 1998;132:144–148. doi: 10.1016/S0022-3476(98)70500-1. [DOI] [PubMed] [Google Scholar]
- 36.Valassina M, Meacci F, Valensin PE, Cusi MG. Detection of neurotropic viruses circulating in Tuscany: the incisive role of Toscana virus. J Med Virol. 2000;60:86–90. doi: 10.1002/(sici)1096-9071(200001)60:1<86::aid-jmv14>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 37.Valassina M, Cuppone AM, Bianchi S, Santini L, Cusi MG. Evidence of Toscana virus variants circulating in Tuscany, Italy, during the Summers of 1995 to 1997. J Clin Microbiol. 1998;36:2103–2104. doi: 10.1128/JCM.36.7.2103-2104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mendoza-Montero J, Gámez-Rueda MI, Navarro-Marí JM, de la Rosa-Fraile M, Oyonarte-Gómez S. Infections due to sandfly fever virus serotype Toscana in Spain. Clin Infect Dis. 1998;27:434–436. doi: 10.1086/514684. [DOI] [PubMed] [Google Scholar]
- 39.Cusi MG, Savellini GG, Zanelli G. Toscana virus epidemiology: from Italy to beyond. Open Virol J. 2010;4:22–28. doi: 10.2174/1874357901004010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terrosi C, Olivieri R, Bianco C, Cellesi C, Cusi MG. Age-dependent seroprevalence of Toscana virus in central Italy and correlation with the clinical profile. Clin Vaccine Immunol. 2009;16:1251–1252. doi: 10.1128/CVI.00376-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valassina M, Valentini M, Pugliese A, Valensin PE, Cusi MG. Serological survey of Toscana virus infections in a high-risk population in Italy. Clin Vaccine Immunol. 2003;10:483–484. doi: 10.1128/CDLI.10.3.483-484.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marchi S, Trombetta CM, Kistner O, Montomoli E. Seroprevalence study of Toscana virus and viruses belonging to the sandfly fever naples antigenic complex in central and southern Italy. J Infect Public Health. 2017;10:866–869. doi: 10.1016/j.jiph.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Christova I, Panayotova E, Trifonova I, Taseva E, Gladnishka T, et al. Serologic evidence of widespread Toscana virus infection in Bulgaria. J Infect Public Health. 2020;13:164–166. doi: 10.1016/j.jiph.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 44.Dersch R, Sophocleous A, Cadar D, Emmerich P, Schmidt-Chanasit J, et al. Toscana virus encephalitis in Southwest Germany: a retrospective study. BMC Neurol. 2021;21:495. doi: 10.1186/s12883-021-02528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ortuño M, Muñoz C, Spitzová T, Sumova P, Iborra MA, et al. Exposure to Phlebotomus perniciosus sandfly vectors is positively associated with Toscana virus and Leishmania infantum infection in human blood donors in Murcia Region, southeast Spain. Transbound Emerg Dis. 2022;69:e1854–e1864. doi: 10.1111/tbed.14520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Erguler K, Pontiki I, Zittis G, Proestos Y, Christodoulou V, et al. A climate-driven and field data-assimilated population dynamics model of sand flies. Sci Rep. 2019;9:2469. doi: 10.1038/s41598-019-38994-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fischer D, Moeller P, Thomas SM, Naucke TJ, Beierkuhnlein C. Combining climatic projections and dispersal ability: a method for estimating the responses of sandfly vector species to climate change. PLoS Negl Trop Dis. 2011;5:e1407. doi: 10.1371/journal.pntd.0001407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weaver SC, Charlier C, Nikos V, Viral LM. Other emerging vector-borne. Annu Rev Med. 2018;69:395–408. doi: 10.1146/annurev-med-050715-105122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martínez-García FA, Moreno-Docón A, Segovia-Hernández M, Fernández-Barreiro A. Deafness as a sequela of Toscana virus meningitis. Med Clin. 2008;130:639. doi: 10.1157/13120347. [DOI] [PubMed] [Google Scholar]
- 50.Amaro F, Luz T, Parreira P, Marchi A, Ciufolini MG, et al. Serological evidence of Toscana virus infection in Portuguese patients. Epidemiol Infect. 2012;140:1147–1150. doi: 10.1017/S0950268811001403. [DOI] [PubMed] [Google Scholar]
- 51.Papa A, Kesisidou C, Kontana A, Arapidou Z, Petropoulou D. Phlebovirus infection in Greece: a case report. Hippokratia. 2015;19:189–191. [PMC free article] [PubMed] [Google Scholar]
- 52.Howell BA, Azar MM, Landry ML, Shaw AC. Toscana virus encephalitis in a traveler returning to the United States. J Clin Microbiol. 2015;53:1445–1447. doi: 10.1128/JCM.03498-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laroche L, Jourdain F, Ayhan N, Bañuls A-L, Charrel R, et al. Incubation period for neuroinvasive Toscana virus infections. Emerg Infect Dis . 2021;27:3147–3150. doi: 10.3201/eid2712.203172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ergunay K, Kaplan B, Okar S, Akkutay-Yoldar Z, Kurne A, et al. Urinary detection of Toscana virus nucleic acids in neuroinvasive infections. J Clin Virol. 2015;70:89–92. doi: 10.1016/j.jcv.2015.07.297. [DOI] [PubMed] [Google Scholar]
- 55.Popescu CP, Cotar AI, Dinu S, Zaharia M, Tardei G, et al. Emergence of Toscana virus, Romania, 2017-2018. Emerg Infect Dis . 2021;27:1482–1485. doi: 10.3201/eid2705.204598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mathur A, Khanna N, Kulshreshtha R, Maitra SC, Chaturvedi UC. Viruria during acute Japanese encephalitis virus infection. Int J Exp Pathol. 1995;76:103–109. [PMC free article] [PubMed] [Google Scholar]
- 57.Barzon L, Pacenti M, Franchin E, Pagni S, Martello T, et al. Excretion of West Nile virus in urine during acute infection. J Infect Dis. 2013;208:1086–1092. doi: 10.1093/infdis/jit290. [DOI] [PubMed] [Google Scholar]
- 58.Ludlow M, Kortekaas J, Herden C, Hoffmann B, Tappe D, et al. Neurotropic virus infections as the cause of immediate and delayed neuropathology. Acta Neuropathol. 2016;131:159–184. doi: 10.1007/s00401-015-1511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.García San Miguel L, Sierra MJ, Vazquez A, Fernandez-Martínez B, Molina R, et al. Phlebovirus-associated diseases transmitted by phlebotominae in Spain: are we at risk? Enfermedades Infecc y Microbiol Clin. 2021;39:345–351. doi: 10.1016/j.eimce.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 60.Serata D, Rapinesi C, Del Casale A, Simonetti A, Mazzarini L, et al. Personality changes after Toscana virus (TOSV) encephalitis in a 49-year-old man: a case report. Int J Neurosci. 2011;121:165–169. doi: 10.3109/00207454.2010.537412. [DOI] [PubMed] [Google Scholar]
- 61.Dionisio D, Valassina M, Ciufolini MG, Vivarelli A, Esperti F, et al. Encephalitis without meningitis due to sandfly fever virus serotype toscana. Clin Infect Dis. 2001;32:1241–1243. doi: 10.1086/319759. [DOI] [PubMed] [Google Scholar]
- 62.Papa A, Paraforou T, Papakonstantinou I, Pagdatoglou K, Kontana A, et al. Severe encephalitis caused by Toscana virus, Greece. Emerg Infect Dis . 2014;20:1417–1419. doi: 10.3201/eid2008.140248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bartels S, de Boni L, Kretzschmar HA, Heckmann JG. Lethal encephalitis caused by the Toscana virus in an elderly patient. J Neurol. 2012;259:175–177. doi: 10.1007/s00415-011-6121-y. [DOI] [PubMed] [Google Scholar]
- 64.Baldelli F, Ciufolini MG, Francisci D, Marchi A, Venturi G, et al. Unusual presentation of life-threatening Toscana virus meningoencephalitis. Clin Infect Dis. 2004;38:515–520. doi: 10.1086/381201. [DOI] [PubMed] [Google Scholar]
- 65.Zanelli G, Bianco C, Cusi MG. Testicular involvement during Toscana virus infection: an unusual manifestation? Infection. 2013;41:735–736. doi: 10.1007/s15010-012-0368-9. [DOI] [PubMed] [Google Scholar]
- 66.Gonen OM, Sacagiu T. Sensory polymyeloradiculopathy associated with Toscana virus infection. J Neurovirol. 2013;19:508–510. doi: 10.1007/s13365-013-0201-y. [DOI] [PubMed] [Google Scholar]
- 67.Tschumi F, Schmutz S, Kufner V, Heider M, Pigny F, et al. Meningitis and epididymitis caused by Toscana virus infection imported to Switzerland diagnosed by metagenomic sequencing: a case report. BMC Infect Dis. 2019;19:591. doi: 10.1186/s12879-019-4231-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mascitti H, Calin R, Dinh A, Makhloufi S, Davido B. Testicular pain associated with clear fluid meningitis: how many cases of Toscana virus are we missing? Int J Infect Dis. 2020;93:198–200. doi: 10.1016/j.ijid.2020.02.008. [DOI] [PubMed] [Google Scholar]
- 69.Matusali G, D’Abramo A, Terrosi C, Carletti F, Colavita F, et al. Infectious Toscana virus in seminal fluid of young man returning from Elba Island, Italy. Emerg Infect Dis. 2022;28:865–869. doi: 10.3201/eid2804.211920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oechtering J, Petzold GC. Acute hydrocephalus due to impaired CSF resorption in Toscana virus meningoencephalitis. Neurology. 2012;79:829–831. doi: 10.1212/WNL.0b013e3182661f1a. [DOI] [PubMed] [Google Scholar]
- 71.Rota E, Morelli N, Immovilli P, De Mitri P, Guidetti D. Guillain-Barré-like axonal polyneuropathy associated with Toscana virus infection. Medicine . 2017;96:e8081. doi: 10.1097/MD.0000000000008081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Okar SV, Bekircan-Kurt CE, Hacıoğlu S, Erdem-Özdamar S, Özkul A, et al. Toscana virus associated with Guillain-Barré syndrome: a case-control study. Acta Neurol Belg. 2021;121:661–668. doi: 10.1007/s13760-020-01279-5. [DOI] [PubMed] [Google Scholar]
- 73.Sanbonmatsu-Gámez S, Pérez-Ruiz M, Palop-Borrás B, Navarro-Marí JM. Unusual manifestation of toscana virus infection, Spain. Emerg Infect Dis . 2009;15:347–348. doi: 10.3201/eid1502.081001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vilibic-Cavlek T, Zidovec-Lepej S, Ledina D, Knezevic S, Savic V, et al. Clinical, virological, and immunological findings in patients with Toscana neuroinvasive disease in Croatia: report of three cases. Trop Med Infect Dis. 2020;5:144. doi: 10.3390/tropicalmed5030144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Braito A, Corbisiero R, Corradini S, Marchi B, Sancasciani N, et al. Evidence of Toscana virus infections without central nervous system involvement: a serological study. Eur J Epidemiol. 1997;13:761–764. doi: 10.1023/a:1007422103992. [DOI] [PubMed] [Google Scholar]
- 76.Ranaldi R, Goteri G, Biagetti S, Cusi MG, Rossini S. Histological description of the lymphadenopathy related to Toscana virus infection. Report of a case. Pathol - Res Pract. 2011;207:197–201. doi: 10.1016/j.prp.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 77.Ocal M, Orsten S, Inkaya AC, Yetim E, Acar NP, et al. Ongoing activity of Toscana virus genotype A and West Nile virus lineage 1 strains in Turkey: a clinical and field survey. Zoonos Public Health. 2014;61:480–491. doi: 10.1111/zph.12096. [DOI] [PubMed] [Google Scholar]
- 78.Mosnier E, Charrel R, Vidal B, Ninove L, Schleinitz N, et al. Toscana virus myositis and fasciitis. Med Mal Infect. 2013;43:208–210. doi: 10.1016/j.medmal.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 79.Crum-Cianflone NF. Bacterial, fungal, parasitic, and viral myositis. Clin Microbiol Rev. 2008;21:473–494. doi: 10.1128/CMR.00001-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Charrel RN, Bichaud L, de Lamballerie X. Emergence of Toscana virus in the Mediterranean area. World J Virol. 2012;1:135–141. doi: 10.5501/wjv.v1.i5.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Valassina M, Valentini M, Valensin PE, Cusi MG. Fast duplex one-step RT-PCR for rapid differential diagnosis of entero- or Toscana virus meningitis. Diagn Microbiol Infect Dis. 2002;43:201–205. doi: 10.1016/s0732-8893(02)00393-0. [DOI] [PubMed] [Google Scholar]
- 82.Valassina M, Cusi MG, Valensin PE. Rapid identification of Toscana virus by nested PCR during an outbreak in the Siena area of Italy. J Clin Microbiol. 1996;34:2500–2502. doi: 10.1128/jcm.34.10.2500-2502.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sánchez-Seco M-P, Echevarría J-M, Hernández L, Estévez D, Navarro-Marí J-M, et al. Detection and identification of Toscana and other phleboviruses by RT-nested-PCR assays with degenerated primers. J Med Virol. 2003;71:140–149. doi: 10.1002/jmv.10465. [DOI] [PubMed] [Google Scholar]
- 84.Pérez-Ruiz M, Collao X, Navarro-Marí JM, Tenorio A. Reverse transcription, real-time PCR assay for detection of Toscana virus. J Clin Virol. 2007;39:276–281. doi: 10.1016/j.jcv.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 85.Ergünay K, Saygan MB, Aydoğan S, Lo MM, Weidmann M, et al. Sandfly fever virus activity in central/northern Anatolia, Turkey: first report of Toscana virus infections. Clin Microbiol Infect. 2011;17:575–581. doi: 10.1111/j.1469-0691.2010.03346.x. [DOI] [PubMed] [Google Scholar]
- 86.Weidmann M, Sanchez-Seco MP, Sall AA, Ly PO, Thiongane Y, et al. Rapid detection of important human pathogenic Phleboviruses. J Clin Virol. 2008;41:138–142. doi: 10.1016/j.jcv.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 87.Brisbarre N, Plumet S, Cotteaux-Lautard C, Emonet SF, Pages F, et al. A rapid and specific real time RT-PCR assay for diagnosis of Toscana virus infection. J Clin Virol. 2015;66:107–111. doi: 10.1016/j.jcv.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 88.Thirion L, Pezzi L, Pedrosa-Corral I, Sanbonmatsu-Gamez S, de Lamballerie X, et al. Evaluation of a trio Toscana virus real-time RT-PCR assay targeting three genomic regions within nucleoprotein gene. Pathogens. 2021;10:1–11. doi: 10.3390/pathogens10030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mori A, Matucci A, Pomari E, Accordini S, Piubelli C, et al. Urine: a pitfall for molecular detection of Toscana virus? An analytical proof-of-concept study. Viruses. 2024;16:98. doi: 10.3390/v16010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Amodio E, Cusi MG, Valenti RM, Valentini M, Mammina C, et al. Immunoglobulin M seropositivity for Toscana virus in a random population sample in Sicily. Int J Infect Dis. 2012;16:e633–5. doi: 10.1016/j.ijid.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhioua E, Moureau G, Chelbi I, Ninove L, Bichaud L, et al. Punique virus, a novel phlebovirus, related to sandfly fever Naples virus, isolated from sandflies collected in Tunisia. J Gen Virol. 2010;91:1275–1283. doi: 10.1099/vir.0.019240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ergünay K, Litzba N, Lo MM, Aydoğan S, Saygan MB, et al. Performance of various commercial assays for the detection of Toscana virus antibodies. Vector Borne Zoonotic Dis. 2011;11:781–787. doi: 10.1089/vbz.2010.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Charrel RN, Gallian P, Navarro-Mari J-M, Nicoletti L, Papa A, et al. Emergence of Toscana virus in Europe. Emerg Infect Dis . 2005;11:1657–1663. doi: 10.3201/eid1111.050869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gámbaro F, Pérez AB, Prot M, Agüera E, Baidaliuk A, et al. Untargeted metagenomic sequencing identifies Toscana virus in patients with idiopathic meningitis, southern Spain, 2015 to 2019. Euro Surveill. 2023;28:1–12. doi: 10.2807/1560-7917.ES.2023.28.45.2200913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cusi MG, Savellini GG. Diagnostic tools for Toscana virus infection. Expert Rev Anti Infect Ther. 2011;9:799–805. doi: 10.1586/eri.11.54. [DOI] [PubMed] [Google Scholar]
- 96.Akhoundi M, Kuhls K, Cannet A, Votýpka J, Marty P, et al. A historical overview of the classification, evolution, and dispersion of leishmania parasites and sandflies. PLoS Negl Trop Dis. 2016;10:e0004349. doi: 10.1371/journal.pntd.0004349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Remadi L, Farjallah D, Chargui N, Belgacem S, Baba H, et al. Blood meal analysis and molecular detection of mammalian Leishmania DNA in wild-caught Sergentomyia spp. from Tunisia and Saudi Arabia. Parasitol Res . 2023;122:2181–2191. doi: 10.1007/s00436-023-07919-y. [DOI] [PubMed] [Google Scholar]
- 98.Cecílio P, Cordeiro-da-Silva A, Oliveira F. Sand flies: basic information on the vectors of leishmaniasis and their interactions with Leishmania parasites. Commun Biol. 2022;5:305. doi: 10.1038/s42003-022-03240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Killick-Kendrick R. The biology and control of phlebotomine sand flies. Clin Dermatol. 1999;17:279–289. doi: 10.1016/s0738-081x(99)00046-2. [DOI] [PubMed] [Google Scholar]
- 100.Chalghaf B, Chemkhi J, Mayala B, Harrabi M, Benie GB, et al. Ecological niche modeling predicting the potential distribution of Leishmania vectors in the Mediterranean basin: impact of climate change. Parasit Vectors. 2018;11:461. doi: 10.1186/s13071-018-3019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ready PD. Biology of phlebotomine sand flies as vectors of disease agents. Annu Rev Entomol. 2013;58:227–250. doi: 10.1146/annurev-ento-120811-153557. [DOI] [PubMed] [Google Scholar]
- 102.Ayhan N, Charrel RN. Of phlebotomines (sandflies) and viruses: a comprehensive perspective on a complex situation. Curr Opin Insect Sci. 2017;22:117–124. doi: 10.1016/j.cois.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 103.Nicoletti L, Ciufolini MG, Verani P. Imported Virus Infections. Vienna, Austria: Springer Vienna; Sandfly fever viruses in Italy; pp. 41–47. [DOI] [Google Scholar]
- 104.Verani P, Ciufolini MG, Caciolli S, Renzi A, Nicoletti L, et al. Ecology of viruses isolated from sand flies in Italy and characterized of a new Phlebovirus (Arabia virus) Am J Trop Med Hyg. 1988;38:433–439. doi: 10.4269/ajtmh.1988.38.433. [DOI] [PubMed] [Google Scholar]
- 105.Ayhan N, Charrel RN. Emergent sand fly–borne Phleboviruses in the Balkan region. Emerg Infect Dis. 2018;24:2324–2330. doi: 10.3201/eid2412.171626. [DOI] [Google Scholar]
- 106.Es-sette N, Ajaoud M, Anga L, Mellouki F, Lemrani M. Toscana virus isolated from sandflies, Morocco. Parasit Vectors. 2015;8:205. doi: 10.1186/s13071-015-0826-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ergunay K, Kasap OE, Orsten S, Oter K, Gunay F, et al. Phlebovirus and Leishmania detection in sandflies from eastern Thrace and northern Cyprus. Parasit Vectors. 2014;7:575. doi: 10.1186/s13071-014-0575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ciufolini MG, Maroli M, Verani P. Growth of two phleboviruses after experimental infection of their suspected sand fly vector, Phlebotomus perniciosus (Diptera: Psychodidae) Am J Trop Med Hyg. 1985;34:174–179. doi: 10.4269/ajtmh.1985.34.174. [DOI] [PubMed] [Google Scholar]
- 109.Tesh RB, Modi GB. Studies on the biology of Phleboviruses in sand flies (Diptera: Psychodidae). I. Experimental infection of the vector. Am J Trop Med Hyg. 1984;33:1007–1016. doi: 10.4269/ajtmh.1984.33.1007. [DOI] [PubMed] [Google Scholar]
- 110.Ciufolini MG, Maroli M, Guandalini E, Marchi A, Verani P. Experimental studies on the maintenance of Toscana and Arbia viruses (Bunyaviridae: Phlebovirus) Am J Trop Med Hyg. 1989;40:669–675. doi: 10.4269/ajtmh.1989.40.669. [DOI] [PubMed] [Google Scholar]
- 111.Tesh RB, Lubroth J, Guzman H. Simulation of arbovirus overwintering: survival of Toscana virus (Bunyaviridae:Phlebovirus) in its natural sand fly vector Phlebotomus perniciosus. Am J Trop Med Hyg. 1992;47:574–581. doi: 10.4269/ajtmh.1992.47.574. [DOI] [PubMed] [Google Scholar]
- 112.Maroli M, Ciufolini MG, Verani P. Vertical transmission of Toscana virus in the sandfly, Phlebotomus perniciosus, via the second gonotrophic cycle. Med Vet Entomol. 1993;7:283–286. doi: 10.1111/j.1365-2915.1993.tb00689.x. [DOI] [PubMed] [Google Scholar]
- 113.Bergren NA, Kading RC. The ecological significance and implications of transovarial transmission among the vector-borne Bunyaviruses: a review. Insects. 2018;9:173. doi: 10.3390/insects9040173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jancarova M, Bichaud L, Hlavacova J, Priet S, Ayhan N, et al. Experimental infection of sand flies by Massilia virus and viral transmission by co-feeding on sugar meal. Viruses. 2019;11:332. doi: 10.3390/v11040332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Charrel RN, Moureau G, Temmam S, Izri A, Marty P, et al. Massilia virus, a novel Phlebovirus (Bunyaviridae) isolated from sandflies in the Mediterranean. Vector Borne Zoonotic Dis. 2009;9:519–530. doi: 10.1089/vbz.2008.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Laroche L, Ayhan N, Charrel R, Bañuls A-L, Prudhomme J. Persistence of Toscana virus in sugar and blood meals of phlebotomine sand flies: epidemiological and experimental consequences. Sci Rep . 2023;13:1–7. doi: 10.1038/s41598-023-32431-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Durden LA, Mullen GR. Medical and Veterinary Entomology. Elsevier; 2018. [DOI] [Google Scholar]
- 118.De Colmenares M, Portús M, Botet J, Dobaño C, Gállego M, et al. Identification of blood meals of Phlebotomus perniciosus (Diptera: Psychodidae) in Spain by a competitive enzyme-linked immunosorbent assay biotin/avidin method. J Med Entomol. 1995;32:229–233. doi: 10.1093/jmedent/32.3.229. [DOI] [PubMed] [Google Scholar]
- 119.Svobodová M, Sádlová J, Chang KP, Volf P. Short report: distribution and feeding preference of the sand flies Phlebotomus sergenti and P. papatasi in a cutaneous leishmaniasis focus in Sanliurfa, Turkey. Am J Trop Med Hyg. 2003;68:6–9. [PubMed] [Google Scholar]
- 120.Maia C, Parreira R, Cristóvão JM, Freitas FB, Afonso MO, et al. Molecular detection of Leishmania DNA and identification of blood meals in wild caught phlebotomine sand flies (Diptera: Psychodidae) from southern Portugal. Parasites Vectors. 2015;8:1–10. doi: 10.1186/s13071-015-0787-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Messahel NE, Benallal KE, Halada P, Lafri I, Manseur H, et al. Identification of blood source preferences and Leishmania infection in sand flies (Diptera: Psychodidae) in north-eastern Algeria. Vet Parasitol Reg Stud Reports . 2022;31:100729. doi: 10.1016/j.vprsr.2022.100729. [DOI] [PubMed] [Google Scholar]
- 122.Dincer E, Karapinar Z, Oktem M, Ozbaba M, Ozkul A, et al. Canine infections and partial S segment sequence analysis of Toscana virus in Turkey. Vector Borne Zoonotic Dis. 2016;16:611–618. doi: 10.1089/vbz.2016.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Alho AM, Pita J, Amaro A, Amaro F, Schnyder M, et al. Seroprevalence of vector-borne pathogens and molecular detection of Borrelia afzelii in military dogs from Portugal. Parasites Vectors. 2016;9:1–6. doi: 10.1186/s13071-016-1509-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dahmani M, Alwassouf S, Grech-Angelini S, Marié J-L, Davoust B, et al. Seroprevalence of Toscana virus in dogs from Corsica, France. Parasit Vectors. 2016;9:381. doi: 10.1186/s13071-016-1665-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tahir D, Alwassouf S, Loudahi A, Davoust B, Charrel RN. Seroprevalence of Toscana virus in dogs from Kabylia (Algeria) Clin Microbiol Infect. 2016;22:e16–7. doi: 10.1016/j.cmi.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 126.Sellali S, Lafri I, Hachid A, Ayhan N, Benbetka C, et al. Presence of the sandfly-borne phlebovirus (Toscana virus) in different bio-geographical regions of Algeria demonstrated by a microneutralisation-based seroprevalence study in owned dogs. Comp Immunol Microbiol Infect Dis. 2022;88:101861. doi: 10.1016/j.cimid.2022.101861. [DOI] [PubMed] [Google Scholar]
- 127.Sakhria S, Alwassouf S, Fares W, Bichaud L, Dachraoui K, et al. Presence of sandfly-borne phleboviruses of two antigenic complexes (Sandfly fever Naples virus and Sandfly fever Sicilian virus) in two different bio-geographical regions of Tunisia demonstrated by a microneutralisation-based seroprevalence study in dogs. Parasit Vectors. 2014;7:476. doi: 10.1186/s13071-014-0476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Alwassouf S, Christodoulou V, Bichaud L, Ntais P, Mazeris A, et al. Seroprevalence of sandfly-borne Phleboviruses belonging to three serocomplexes (sandfly fever naples, sandfly fever sicilian and salehabad) in dogs from Greece and Cyprus using neutralization test. PLoS Negl Trop Dis. 2016;10:e0005063. doi: 10.1371/journal.pntd.0005063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pereira A, Ayhan N, Cristóvão JM, Vilhena H, Martins Â, et al. Antibody response to Toscana virus and sandfly fever sicilian virus in cats naturally exposed to phlebotomine sand fly bites in Portugal. Microorganisms. 2019;7:339. doi: 10.3390/microorganisms7090339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Muñoz C, Ayhan N, Ortuño M, Ortiz J, Gould EA, et al. Experimental infection of dogs with Toscana virus and sandfly fever Sicilian virus to determine their potential as possible vertebrate hosts. Microorganisms. 2020;8:596. doi: 10.3390/microorganisms8040596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dachraoui K, Chelbi I, Labidi I, Ben Osman R, Sayadi A, et al. The role of the Leishmania infantum infected dogs as a potential reservoir host for Toscana virus in a zoonotic visceral leishmaniasis focus of northern Tunisia. Viruses. 2023;15:1–16. doi: 10.3390/v15041012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dincer E, Gargari S, Ozkul A, Ergunay K. Potential animal reservoirs of Toscana virus and coinfections with Leishmania infantum in Turkey. Am J Trop Med Hyg. 2015;92:690–697. doi: 10.4269/ajtmh.14-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Maia C, Alwassouf S, Cristóvão JM, Ayhan N, Pereira A, et al. Serological association between Leishmania infantum and sand fly fever Sicilian (but not Toscana) virus in sheltered dogs from southern Portugal. Parasit Vectors. 2017;10:92. doi: 10.1186/s13071-017-2023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ayhan N, Sherifi K, Taraku A, Bërxholi K, Charrel RN. High rates of neutralizing antibodies to Toscana and sandfly fever Sicilian viruses in Livestock, Kosovo. Emerg Infect Dis . 2017;23:989–992. doi: 10.3201/eid2306.161929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Al-numaani SA, Al-Nemari AT, El-Kafrawy SA, Hassan AM, Tolah AM, et al. Seroprevalence of Toscana and sandfly fever Sicilian viruses in humans and livestock animals from western Saudi Arabia. One Health . 2023;17:100601. doi: 10.1016/j.onehlt.2023.100601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Navarro-Marí JM, Palop-Borrás B, Pérez-Ruiz M, Sanbonmatsu-Gámez S. Serosurvey study of Toscana virus in domestic animals, Granada, Spain. Vector Borne Zoonotic Dis. 2011;11:583–587. doi: 10.1089/vbz.2010.0065. [DOI] [PubMed] [Google Scholar]
- 137.Hacioglu S, Dincer E, Isler CT, Karapinar Z, Ataseven VS, et al. A snapshot avian surveillance reveals West Nile virus and evidence of wild birds participating in Toscana virus circulation. Vector Borne Zoonotic Dis. 2017;17:698–708. doi: 10.1089/vbz.2017.2138. [DOI] [PubMed] [Google Scholar]
- 138.Ayhan N, Rodríguez-Teijeiro JD, López-Roig M, Vinyoles D, Ferreres JA, et al. High rates of antibodies against Toscana and Sicilian phleboviruses in common quail Coturnix coturnix birds. Front Microbiol. 2022;13:1091908. doi: 10.3389/fmicb.2022.1091908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hacioglu S, Ozkul A. Do birds play a role in the transmission of Toscana virus? Initial isolation results from birds in northernmost Türkiye. Zoonoses and Public Health. 2024;71:225–235. doi: 10.1111/zph.13100. [DOI] [PubMed] [Google Scholar]
- 140.Moratelli R, Calisher CH. Bats and zoonotic viruses: can we confidently link bats with emerging deadly viruses? Mem Inst Oswaldo Cruz. 2015;110:1–22. doi: 10.1590/0074-02760150048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ayhan N, López-Roig M, Monastiri A, Charrel RN, Serra-Cobo J. Seroprevalence of Toscana virus and sandfly fever Sicilian virus in European bat colonies measured using a neutralization test. Viruses. 2021;13:88. doi: 10.3390/v13010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kraemer MUG, Reiner RC, Jr, Brady OJ, Messina JP, Gilbert M, et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat Microbiol. 2019;4:854–863. doi: 10.1038/s41564-019-0376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Caminade C, McIntyre KM, Jones AE. Impact of recent and future climate change on vector-borne diseases. Ann N Y Acad Sci. 2019;1436:157–173. doi: 10.1111/nyas.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Volf P, Volfova V. Establishment and maintenance of sand fly colonies. J Vector Ecol. 2011;36:S1–S9. doi: 10.1111/j.1948-7134.2011.00106.x. [DOI] [PubMed] [Google Scholar]
- 145.Aspöck H, Gerersdorfer T, Formayer H, Walochnik J. Sandflies and sandfly-borne infections of humans in central Europe in the light of climate change. Wien Klin Wochenschr. 2008;120:24–29. doi: 10.1007/s00508-008-1072-8. [DOI] [PubMed] [Google Scholar]
- 146.Naucke TJ, Lorentz S, Rauchenwald F, Aspöck H. Phlebotomus (Transphlebotomus) mascittii Grassi, 1908, in Carinthia: first record of the occurrence of sandflies in Austria (Diptera: Psychodidae: Phlebotominae) Parasitol Res. 2011;109:1161–1164. doi: 10.1007/s00436-011-2361-0. [DOI] [PubMed] [Google Scholar]
- 147.Poeppl W, Obwaller AG, Weiler M, Burgmann H, Mooseder G, et al. Emergence of sandflies (Phlebotominae) in Austria, a Central European country. Parasitol Res. 2013;112:4231–4237. doi: 10.1007/s00436-013-3615-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Oerther S. Phlebotomus mascittii as potential vector of human pathogens in southwest Germany. Entomol Ornithol Herpetol. 2020;9:1–3. [Google Scholar]
- 149.Naucke TJ, Menn B, Massberg D, Lorentz S. Sandflies and leishmaniasis in Germany. Parasitol Res. 2008;103:65–68. doi: 10.1007/s00436-008-1052-y. [DOI] [PubMed] [Google Scholar]
- 150.Ayhan N, Prudhomme J, Laroche L, Bañuls A-L, Charrel RN. Broader geographical distribution of Toscana virus in the mediterranean region suggests the existence of larger varieties of sand fly vectors. Microorganisms. 2020;8:114. doi: 10.3390/microorganisms8010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Knechtli R, Jenni L. Distribution and relative density of three sandfly (Diptera: Phlebotominae) species in southern Switzerland. Ann Parasitol Hum Comp. 1989;64:53–63. doi: 10.1051/parasite/198964153. [DOI] [Google Scholar]
- 152.Liang G, Gao X, Gould EA. Factors responsible for the emergence of arboviruses; strategies, challenges and limitations for their control. Emerg Microb Infect. 2015;4:1–5. doi: 10.1038/emi.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Maroli M, Feliciangeli MD, Bichaud L, Charrel RN, Gradoni L. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Med Vet Entomol. 2013;27:123–147. doi: 10.1111/j.1365-2915.2012.01034.x. [DOI] [PubMed] [Google Scholar]
- 154.Remadi L, Chargui N, Jiménez M, Molina R, Haouas N, et al. Molecular detection and identification of Leishmania DNA and blood meal analysis in Phlebotomus (Larroussius) species. PLoS Negl Trop Dis. 2020;14:e0008077. doi: 10.1371/journal.pntd.0008077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pombi M, Giacomi A, Barlozzari G, Mendoza-Roldan J, Macrì G, et al. Molecular detection of Leishmania (Sauroleishmania) tarentolae in human blood and Leishmania (Leishmania) infantum in Sergentomyia minuta: unexpected host-parasite contacts. Med Vet Entomol. 2020;34:470–475. doi: 10.1111/mve.12464. [DOI] [PubMed] [Google Scholar]
- 156.Campino L, Cortes S, Dionísio L, Neto L, Afonso MO, et al. The first detection of Leishmania major in naturally infected Sergentomyia minuta in Portugal. Mem Inst Oswaldo Cruz. 2013;108:516–518. doi: 10.1590/S0074-02762013000400020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Berdjane-Brouk Z, Koné AK, Djimdé AA, Charrel RN, Ravel C, et al. First detection of Leishmania major DNA in Sergentomyia (Spelaeomyia) darlingi from cutaneous leishmaniasis foci in Mali. PLoS One. 2012;7:e28266. doi: 10.1371/journal.pone.0028266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Maia C, Depaquit J. Can Sergentomyia (Diptera, Psychodidae) play a role in the transmission of mammal-infecting Leishmania? Parasite. 2016;23:55. doi: 10.1051/parasite/2016062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Latrofa MS, Iatta R, Dantas-Torres F, Annoscia G, Gabrielli S, et al. Detection of Leishmania infantum DNA in phlebotomine sand flies from an area where canine leishmaniosis is endemic in southern Italy. Vet Parasitol. 2018;253:39–42. doi: 10.1016/j.vetpar.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 160.Charrel RN, Izri A, Temmam S, de Lamballerie X, Parola P. Toscana virus RNA in Sergentomyia minuta flies. Emerg Infect Dis. 2006;12:1299–1300. doi: 10.3201/eid1208.060345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Geevarghese G, Arankalle VA, Jadi R, Kanojia PC, Joshi MV, et al. Detection of Chandipura virus from sand flies in the genus Sergentomyia (Diptera: Phlebotomidae) at Karimnagar district, Andhra Pradesh, India. J Med Entomol. 2005;42:495–496. doi: 10.1093/jmedent/42.3.495. [DOI] [PubMed] [Google Scholar]
- 162.Ticha L, Volfova V, Mendoza-Roldan JA, Bezerra-Santos MA, Maia C, et al. Experimental feeding of Sergentomyia minuta on reptiles and mammals: comparison with Phlebotomus papatasi. Parasites Vectors. 2023;16:1–9. doi: 10.1186/s13071-023-05758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Afonso MMDS, Duarte R, Miranda JC, Caranha L, Rangel EF. Studies on the feeding habits of Lutzomyia (Lutzomyia) longipalpis (Lutz & Neiva, 1912) (Diptera: Psychodidae: Phlebotominae) populations from endemic areas of American visceral Leishmaniasis in Northeastern Brazil. J Trop Med. 2012;2012:858657. doi: 10.1155/2012/858657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Soares VYR, Silva JC da, Silva KR da, Cruz M do SP e, Santos MPD, et al. Identification of blood meal sources of Lutzomyia longipalpis using polymerase chain reaction-restriction fragment length polymorphism analysis of the cytochrome B gene. Mem Inst Oswaldo Cruz. 2014;109:379–383. doi: 10.1590/0074-0276130405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dias F de OP, Lorosa ES, Rebêlo JMM. Blood feeding sources and peridomiciliation of Lutzomyia longipalpis (Lutz & Neiva, 1912) (Psychodidae, Phlebotominae) Cad saúde Pública / Ministério da Saúde, Fundação Oswaldo Cruz, Esc Nac Saúde Pública. 2003;19:1373–1380. doi: 10.1590/S0102-311X2003000500015. [DOI] [PubMed] [Google Scholar]
- 166.Salomon OD. Lutzomyia longipalpis, gone with the wind and other variables. Neotrop Entomol. 2021;50:161–171. doi: 10.1007/s13744-020-00811-9. [DOI] [PubMed] [Google Scholar]
- 167.Jennings M, Boorman J. The susceptibility of Lutzomyia longipalpis (Lutz and Neiva), Diptera, Psychodidae, to artificial infection with three viruses of the Phlebotomus fever group. Ann Trop Med Parasitol. 1980;74:455–462. doi: 10.1080/00034983.1980.11687367. [DOI] [PubMed] [Google Scholar]
- 168.Tesh RB, Chaniotis BN, Peralta PH, Johnson KM. Ecology of viruses isolated from Panamanian. Am J Trop Med Hyg. 1974;23:258–269. doi: 10.4269/ajtmh.1974.23.258. [DOI] [PubMed] [Google Scholar]
- 169.Palacios G, Tesh R, Travassos da Rosa A, Savji N, Sze W, et al. Characterization of the Candiru antigenic complex (Bunyaviridae: Phlebovirus), a highly diverse and reassorting group of viruses affecting humans in tropical America. J Virol. 2011;85:3811–3820. doi: 10.1128/JVI.02275-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.De Carvalho MS, De Lara Pinto AZ, Pinheiro A, Rodrigues JSV, Melo FL, et al. Viola phlebovirus is a novel phlebotomus fever serogroup member identified in lutzomyia (lutzomyia) longipalpis from brazilian pantanal. Parasit Vectors. 2018;11:1–10. doi: 10.1186/s13071-018-2985-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tesh RB, Modi GB. Development of a continuous cell line from the sand fly Lutzomyia longipalpis (Diptera: Psychodidae), and its susceptibility to infection with arboviruses. J Med Entomol. 1983;20:199–202. doi: 10.1093/jmedent/20.2.199. [DOI] [PubMed] [Google Scholar]
- 172.Ferreira FV, Aguiar ERGR, Olmo RP, de Oliveira KPV, Silva EG, et al. The small non-coding RNA response to virus infection in the Leishmania vector Lutzomyia longipalpis. PLoS Negl Trop Dis. 2018;12:e0006569. doi: 10.1371/journal.pntd.0006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hoch AL, Turell MJ, Bailey CL. Replication of Rift valley fever virus in the sand fly Lutzomyia longipalpis. Am J Trop Med Hyg. 1984;33:295–299. doi: 10.4269/ajtmh.1984.33.295. [DOI] [PubMed] [Google Scholar]
- 174.Hoch AL, Gargan TP, 2nd, Bailey CL. Mechanical transmission of Rift Valley fever virus by hematophagous Diptera. Am J Trop Med Hyg. 1985;34:188–193. doi: 10.4269/ajtmh.1985.34.188. [DOI] [PubMed] [Google Scholar]
- 175.Peterson AT, Campbell LP, Moo-Llanes DA, Travi B, González C, et al. Influences of climate change on the potential distribution of Lutzomyia longipalpis sensu lato (Psychodidae: Phlebotominae) Int J Parasitol. 2017;47:667–674. doi: 10.1016/j.ijpara.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 176.Schneider CA, Calvo E, Peterson KE. Arboviruses: how saliva impacts the journey from vector to host. Int J Mol Sci. 2021;22:9173. doi: 10.3390/ijms22179173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Volf P, Tesarová P, Nohýnkova EN. Salivary proteins and glycoproteins in phlebotomine sandflies of various species, sex and age. Med Vet Entomol. 2000;14:251–256. doi: 10.1046/j.1365-2915.2000.00240.x. [DOI] [PubMed] [Google Scholar]
- 178.Titus RG, Ribeiro JMC. Salivary gland lysates from the sand fly Lutzomyia longipalpis enhance Leishmania infectivity. Science. 1988;239:1306–1308. doi: 10.1126/science.3344436. [DOI] [PubMed] [Google Scholar]
- 179.Samuelson J, Lerner E, Tesh R, Titus R. A mouse model of Leishmania braziliensis Braziliensis infection produced by coinjection with sand fly saliva. J Exp Med. 1991;173:49–54. doi: 10.1084/jem.173.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rohousová I, Volf P. Sand fly saliva: effects on host immune response and Leishmania transmission. Folia Parasitol. 2006;53:161–171. [PubMed] [Google Scholar]
- 181.Peters NC, Egen JG, Secundino N, Debrabant A, Kimblin N, et al. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science . 2008;321:970–974. doi: 10.1126/science.1159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pingen M, Schmid MA, Harris E, McKimmie CS. Mosquito biting modulates skin response to virus infection. Trends Parasitol. 2017;33:645–657. doi: 10.1016/j.pt.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 183.Le Coupanec A, Babin D, Fiette L, Jouvion G, Ave P, et al. Aedes mosquito saliva modulates Rift Valley fever virus pathogenicity. PLoS Negl Trop Dis. 2013;7:e2237. doi: 10.1371/journal.pntd.0002237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Pingen M, Bryden SR, Pondeville E, Schnettler E, Kohl A, et al. Host inflammatory response to mosquito bites enhances the severity of arbovirus infection. Immunity. 2016;44:1455–1469. doi: 10.1016/j.immuni.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Edwards JF, Higgs S, Beaty BJ. Mosquito feeding-induced enhancement of Cache valley virus (Bunyaviridae) infection in mice. J Med Entomol. 1998;35:261–265. doi: 10.1093/jmedent/35.3.261. [DOI] [PubMed] [Google Scholar]
- 186.Styer LM, Lim P-Y, Louie KL, Albright RG, Kramer LD, et al. Mosquito saliva causes enhancement of West Nile virus infection in mice. J Virol. 2011;85:1517–1527. doi: 10.1128/JVI.01112-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hermance ME, Thangamani S. Tick saliva enhances powassan virus transmission to the host, influencing its dissemination and the course of disease. J Virol. 2015;89:7852–7860. doi: 10.1128/JVI.01056-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Grazia Cusi M, Gori Savellini G, Terrosi C, Di Genova G, Valassina M, et al. Development of a mouse model for the study of Toscana virus pathogenesis. Virology. 2005;333:66–73. doi: 10.1016/j.virol.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 189.Abdeladhim M, V. Coutinho-Abreu I, Townsend S, Pasos-Pinto S, Sanchez L, et al. Molecular diversity between salivary proteins from new world and old world sand flies with emphasis on Bichromomyia olmeca, the sand fly vector of Leishmania mexicana in Mesoamerica. PLoS Negl Trop Dis. 2016;10:e0004771. doi: 10.1371/journal.pntd.0004771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lestinova T, Rohousova I, Sima M, de Oliveira CI, Volf P. Insights into the sand fly saliva: blood-feeding and immune interactions between sand flies, hosts, and Leishmania. PLoS Negl Trop Dis. 2017;11:e0005600. doi: 10.1371/journal.pntd.0005600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Abdeladhim M, Kamhawi S, Valenzuela JG. What’s behind a sand fly bite? The profound effect of sand fly saliva on host hemostasis, inflammation and immunity. Infect Genet Evol. 2014;28:691–703. doi: 10.1016/j.meegid.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Soares MBP, Titus RG, Shoemaker CB, David JR, Bozza M. The vasoactive peptide maxadilan from sand fly saliva inhibits TNF-α and induces IL-6 by mouse macrophages through interaction with the Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) Receptor. J Immunol. 1998;160:1811–1816. doi: 10.4049/jimmunol.160.4.1811. [DOI] [PubMed] [Google Scholar]
- 193.Mbow ML, Bleyenberg JA, Hall LR, Titus RG. Phlebotomus papatasi sand fly salivary gland lysate down-regulates a Th1, but up-regulates a Th2, response in mice infected with Leishmania major. J Immunol. 1998;161:5571–5577. [PubMed] [Google Scholar]
- 194.Teixeira CR, Teixeira MJ, Gomes RBB, Santos CS, Andrade BB, et al. Saliva from Lutzomyia longipalpis induces CC chemokine ligand 2/monocyte chemoattractant protein-1 expression and macrophage recruitment. J Immunol. 2005;175:8346–8353. doi: 10.4049/jimmunol.175.12.8346. [DOI] [PubMed] [Google Scholar]
- 195.Costa DJ, Favali C, Clarêncio J, Afonso L, Conceição V, et al. Lutzomyia longipalpis salivary gland homogenate impairs cytokine production and costimulatory molecule expression on human monocytes and dendritic cells. Infect Immun. 2004;72:1298–1305. doi: 10.1128/IAI.72.3.1298-1305.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Carregaro V, Valenzuela JG, Cunha TM, Verri WA, Jr, Grespan R, et al. Phlebotomine salivas inhibit immune inflammation-induced neutrophil migration via an autocrine DC-derived PGE2/IL-10 sequential pathway. J Leukoc Biol. 2008;84:104–114. doi: 10.1189/jlb.1107797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kay MK, Gibney KB, Riedo FX, Kosoy OL, Lanciotti RS, et al. Toscana virus infection in American traveler returning from Sicily, 2009. Emerg Infect Dis . 2010;16:1498–1500. doi: 10.3201/eid1609.100505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Farrugia J, Maxwell-Scott H, Brooks T, Tang JW, Pareek M. Toscana virus as a cause of short-term fever and encephalitis in returning travellers from Mediterranean Europe. Clinical Infection in Practice . 2020;6:100018. doi: 10.1016/j.clinpr.2020.100018. [DOI] [Google Scholar]
- 199.Eitrem R, Niklasson B, Weiland O. Sandfly fever among Swedish tourists. Scand J Infect Dis. 1991;23:451–457. doi: 10.3109/00365549109075093. [DOI] [PubMed] [Google Scholar]
- 200.Di Nicuolo G, Pagliano P, Battisti S, Starace M, Mininni V, et al. Toscana virus central nervous system infections in southern Italy. J Clin Microbiol. 2005;43:6186–6188. doi: 10.1128/JCM.43.12.6186-6188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Venturi G, Madeddu G, Rezza G, Ciccozzi M, Pettinato ML, et al. Detection of Toscana virus central nervous system infections in Sardinia Island, Italy. J Clin Virol. 2007;40:90–91. doi: 10.1016/j.jcv.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 202.Venturi G, El-Sawaf G, Arpino C, Madeddu G, Fiorentini C, et al. Arboviral infections in Egyptian and Sardinian children and adults with aseptic meningitis and meningo-encephalitis. Scand J Infect Dis. 2009;41:898–899. doi: 10.3109/00365540903225179. [DOI] [PubMed] [Google Scholar]
- 203.Hemmersbach-Miller M, Parola P, Charrel RN, Paul Durand J, Brouqui P. Sandfly fever due to Toscana virus: an emerging infection in southern France. Eur J Intern Med. 2004;15:316–317. doi: 10.1016/j.ejim.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 204.Peyrefitte CN, Devetakov I, Pastorino B, Villeneuve L, Bessaud M, et al. Toscana virus and acute meningitis, France. Emerg Infect Dis. 2005;11:778–780. doi: 10.3201/eid1105.041122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Cardeñosa N, Kaptoul D, Fernández-Viladrich P, Aranda C, Ory F, et al. Toscana virus infection in catalonia (Spain) Vector-Borne Zoonotic Dis. 2013;13:273–275. doi: 10.1089/vbz.2011.0903. [DOI] [PubMed] [Google Scholar]
- 206.Echevarría J-M, de Ory F, Guisasola M-E, Sánchez-Seco M-P, Tenorio A, et al. Acute meningitis due to Toscana virus infection among patients from both the Spanish Mediterranean region and the region of Madrid. J Clin Virol. 2003;26:79–84. doi: 10.1016/S1386-6532(02)00041-0. [DOI] [PubMed] [Google Scholar]
- 207.Navarro JM, Fernández-Roldán C, Pérez-Ruiz M, Sanbonmatsu S, Rosa M, et al. Meningitis by Toscana virus in Spain: description of 17 cases. Med Clin. 2004;122:420–422. doi: 10.1157/13059543. [DOI] [PubMed] [Google Scholar]
- 208.de Ory-Manchón F, Carlos Sanz-Moreno J, Aranguez-Ruiz E, Ramírez-Fernández R. Seroprevalencia edad dependiente frente al virus Toscana en la Comunidad de Madrid: años 1993-1994 y 1999-2000. Enfermedades Infecciosas y Microbiología Clínica. 2007;25:187–189. doi: 10.1157/13099371. [DOI] [PubMed] [Google Scholar]
- 209.Portolani M, Sabbatini AM, Beretti F, Gennari W, Tamassia MG, et al. Symptomatic infections by Toscana virus in the Modena province in the Triennium 1999-2001. New Microbiol. 2002;25:485–488. [PubMed] [Google Scholar]
- 210.Francisci D, Papili R, Camanni G, Morosi S, Ferracchiato N, et al. Evidence of Toscana virus circulation in Umbria: First report. Eur J Epidemiol. 2002;18:457–459. doi: 10.1023/A:1024295710118. [DOI] [PubMed] [Google Scholar]
- 211.Defuentes G, Rapp C, Imbert P, Debord T. Acute meningitis owing to phlebotomus fever Toscana virus imported to France. J Travel Med. 2006;12:295–296. doi: 10.2310/7060.2005.12512. [DOI] [PubMed] [Google Scholar]
- 212.Epelboin L, Hausfater P, Schuffenecker I, Riou B, Zeller H, et al. Meningoencephalitis due to Toscana virus in a French traveler returning from central Italy. J Travel Med. 2008;15:361–363. doi: 10.1111/j.1708-8305.2008.00221.x. [DOI] [PubMed] [Google Scholar]
- 213.Santos L, Simões J, Costa R, Martins S, Lecour H. Toscana virus meningitis in Portugal, 2002-2005. Euro Surveill. 2007;12:3–4. doi: 10.2807/esm.12.06.00715-en. [DOI] [PubMed] [Google Scholar]
- 214.Venturi G, Marchi A, Fiorentini C, Ramadani N, Quaglio G, et al. Prevalence of antibodies to phleboviruses and flaviviruses in Peja, Kosovo. Clin Microbiol Infect. 2011;17:1180–1182. doi: 10.1111/j.1469-0691.2010.03445.x. [DOI] [PubMed] [Google Scholar]
- 215.Hukić M, Salimović-Bešić I. Sandfly – Pappataci fever in Bosnia and Herzegovina: the new-old disease. Bosn J of Basic Med Sci. 2009;9:39–43. doi: 10.17305/bjbms.2009.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Punda-Polić V, Jerončić A, Mohar B, Kraljevićc KŠ. Prevalence of Toscana virus antibodies in residents of Croatia. Clin Microbiol Infect. 2012;18:E200–E203. doi: 10.1111/j.1469-0691.2012.03840.x. [DOI] [PubMed] [Google Scholar]
- 217.Papa A, Andriotis V, Tzilianos M. Prevalence of Toscana virus antibodies in residents of two Ionian islands, Greece. Travel Med Infect Dis. 2010;8:302–304. doi: 10.1016/j.tmaid.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 218.Vocale C, Bartoletti M, Rossini G, Macini P, Pascucci MG, et al. Toscana virus infections in northern Italy: laboratory and clinical evaluation. Vector-Borne Zoonot Dis. 2012;12:526–529. doi: 10.1089/vbz.2011.0781. [DOI] [PubMed] [Google Scholar]
- 219.Papa A, Kontana A, Tsergouli K. Phlebovirus infections in Greece. J Med Virol. 2015;87:1072–1076. doi: 10.1002/jmv.24163. [DOI] [PubMed] [Google Scholar]
- 220.Papa A, Mallias J, Tsergouli K, Markou F, Poulou A, et al. Neuroinvasive Phlebovirus infection in Greece: a case report. Intervirology. 2014;57:393–395. doi: 10.1159/000363505. [DOI] [PubMed] [Google Scholar]
- 221.Remoli ME, Fiorentini C, Marchi A, Renzi S, Vonesch N, et al. Seroprevalence survey of arboviruses in workers from Tuscany, Italy. 2018;109:125–131. doi: 10.23749/mdl.v109i2.5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Colomba C, Saporito L, Ciufolini MG, Marchi A, Rotolo V, et al. Prevalence of Toscana sandfly fever virus antibodies in neurological patients and control subjects in Sicily. New Microbiol. 2012;35:161–165. [PubMed] [Google Scholar]
- 223.Calamusa G, Valenti RM, Vitale F, Mammina C, Romano N, et al. Seroprevalence of and risk factors for Toscana and Sicilian virus infection in a sample population of Sicily (Italy) J Infect. 2012;64:212–217. doi: 10.1016/j.jinf.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Pierro A, Landini MP, Gaibani P, Rossini G, Vocale C, et al. A model of laboratory surveillance for neuro-arbovirosis applied during 2012 in the Emilia-Romagna region, Italy. Clin Microbiol Infect. 2014;20:672–677. doi: 10.1111/1469-0691.12436. [DOI] [PubMed] [Google Scholar]
- 225.Anagnostou V, Papa A. Seroprevalence of Toscana virus among residents of Aegean Sea islands, Greece. Travel Med Infect Dis. 2013;11:98–102. doi: 10.1016/j.tmaid.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 226.Anagnostou V, Papa A. Prevalence of antibodies to phleboviruses within the sand fly fever Naples virus species in humans, northern Greece. Clin Microbiol Infect. 2013;19:566–570. doi: 10.1111/j.1469-0691.2012.03957.x. [DOI] [PubMed] [Google Scholar]
- 227.Bichaud L, Izri A, de Lamballerie X, Moureau G, Charrel RN. First detection of Toscana virus in Corsica, France. Clin Microbiol Infect. 2014;20:O101–O104. doi: 10.1111/1469-0691.12347. [DOI] [PubMed] [Google Scholar]
- 228.Amaro F, Zé-Zé L, Luz MT, Alves MJ. Toscana virus: ten years of diagnostics in Portugal. Acta Med Port. 2021;34:677–681. doi: 10.20344/amp.13308. [DOI] [PubMed] [Google Scholar]
- 229.Martinez JG, García SG, Walter S, Gil-Prieto R, Lacomba DL, et al. Seroprevalence against Toscana virus in Spain: the case of the autonomous community of Madrid. J Vector Borne Dis. 2022;59:172–177. doi: 10.4103/0972-9062.335771. [DOI] [PubMed] [Google Scholar]
- 230.Quattrone F, Mazzetti P, Aquino F, Sani S, Carneglia L, et al. Two clusters of Toscana virus meningo-encephalitis in Livorno Province and Elba Island, July-September 2018. Ann di Ig Med Prev e di Comunita. 2020;32:674–681. doi: 10.7416/ai.2020.2387. [DOI] [PubMed] [Google Scholar]
- 231.Gori Savellini G, Gandolfo C, Cusi MG. Epidemiology of Toscana virus in South Tuscany over the years 2011-2019. J Clin Virol. 2020;128:104452. doi: 10.1016/j.jcv.2020.104452. [DOI] [PubMed] [Google Scholar]
- 232.Maia C, Ayhan N, Cristóvão JM, Pereira A, Charrel R. Human seroprevalence of Toscana virus and Sicilian phlebovirus in the southwest of Portugal. Eur J Clin Microbiol Infect Dis. 2022;41:137–141. doi: 10.1007/s10096-021-04332-0. [DOI] [PubMed] [Google Scholar]
- 233.Masse S, Ayhan N, Capai L, Bosseur F, de Lamballerie X, et al. Circulation of Toscana Virus in a Sample Population of Corsica, France. Viruses. 2019;11:817. doi: 10.3390/v11090817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Dobler G, Treib J, Haass A, Frösner G, Woesner R, et al. Toscana virus infection in German travellers returning from the Mediterranean. Infection. 1997;25:325. doi: 10.1007/BF01720413. [DOI] [PubMed] [Google Scholar]
- 235.Schwarz TF, Gilch S, Jäger G. Travel-related Toscana virus infection. Lancet. 1993;342:803–804. doi: 10.1016/0140-6736(93)91568-7. [DOI] [PubMed] [Google Scholar]
- 236.Schwarz TF, Jäger G, Gilch S, Pauli C, Eisenhut M, et al. Imported Virus Infections. Vienna: Springer Vienna; Travel-related vector-borne virus infections in Germany; pp. 57–65. [DOI] [PubMed] [Google Scholar]
- 237.Beersma MF, Grimbergen YA, Kroon FP, Veldkamp PJ. Meningitis caused by Toscana virus during a summer stay in Italy. Ned Tijdschr Geneeskd. 2004;148:286–288. [PubMed] [Google Scholar]
- 238.Imirzalioglu C, Schaller M, Bretzel RG. Sandfly fever Naples virus (serotype Toscana) infection with meningeal involvement after a vacation in Italy. Dtsch Med Wochenschr. 2006;131:2838–2840. doi: 10.1055/s-2006-957210. [DOI] [PubMed] [Google Scholar]
- 239.Sonderegger B, Hächler H, Dobler G, Frei M. Imported aseptic meningitis due to Toscana virus acquired on the Island of Elba, Italy, August 2008. Eurosurveillance . 2009;14:9–10. doi: 10.2807/ese.14.01.19079-en. [DOI] [PubMed] [Google Scholar]
- 240.Gabriel M, Resch C, Günther S, Schmidt-Chanasit J. Toscana virus infection imported from Elba into Switzerland. Emerg Infect Dis. 2010;16:1034–1036. doi: 10.3201/eid1606.091763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Cordey S, Bel M, Petty TJ, Docquier M, Sacco L, et al. Toscana virus meningitis case in Switzerland: an example of the ezVIR bioinformatics pipeline utility for the identification of emerging viruses. Clin Microbiol Infect. 2015;21:387. doi: 10.1016/j.cmi.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 242.Bahri O, Fazaa O, Ben Alaya-Bouafif N, Bouloy M, Triki H, et al. Rôle du virus Toscana dans les infections neuroméningées en Tunisie. Pathologie Biologie. 2010;59:2010–2012. doi: 10.1016/j.patbio.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 243.Andayi F, Charrel RN, Kieffer A, Richet H, Pastorino B, et al. A sero-epidemiological study of arboviral fevers in Djibouti, Horn of Africa. PLoS Negl Trop Dis. 2014;8:e3299. doi: 10.1371/journal.pntd.0003299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Fezaa O, Bahri O, Alaya Bouafif NB, Triki H, Bouattour A. Seroprevalence of Toscana virus infection in Tunisia. Int J Infect Dis. 2013;17:e1172-5. doi: 10.1016/j.ijid.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 245.Sakhria S, Bichaud L, Mensi M, Salez N, Dachraoui K, et al. Co-circulation of Toscana virus and punique virus in northern Tunisia: a microneutralisation-based seroprevalence study. PLoS Negl Trop Dis. 2013;7:e2429. doi: 10.1371/journal.pntd.0002429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Alkan C, Allal-Ikhlef AB, Alwassouf S, Baklouti A, Piorkowski G, et al. Virus isolation, genetic characterization and seroprevalence of Toscana virus in Algeria. Clin Microbiol Infect. 2015;21:1040. doi: 10.1016/j.cmi.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 247.Fezaa O, M’ghirbi Y, Savellini GG, Ammari L, Hogga N, et al. Serological and molecular detection of Toscana and other Phleboviruses in patients and sandflies in Tunisia. BMC Infect Dis. 2014;14:598. doi: 10.1186/s12879-014-0598-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Benbetka C, Hachid A, Benallal KE, Khardine FA, Ayhan N, et al. Epidemiology, isolation, and genetic characterization of Toscana virus in Algerian patients displaying neurological infection, 2016-2018. IJID Reg . 2023;7:193–198. doi: 10.1016/j.ijregi.2023.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Saadawi WK, Abozaid FD, Almukhtar M, Annajar BB, Shaibi T. Seroprevalence study of Toscana virus in Yafran area, Libya. J Vector Borne Dis. 2022;59:186–189. doi: 10.4103/0972-9062.335728. [DOI] [PubMed] [Google Scholar]
- 250.Ergunay K, Sayiner AA, Litzba N, Lederer S, Charrel R, et al. Multicentre evaluation of central nervous system infections due to Flavi and Phleboviruses in Turkey. J Infect. 2012;65:343–349. doi: 10.1016/j.jinf.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 251.Ergunay K, Aydogan S, Ilhami Ozcebe O, Cilek EE, Hacioglu S, et al. Toscana virus (TOSV) exposure is confirmed in blood donors from Central, North and South/Southeast Anatolia, Turkey. Zoonoses Public Health. 2012;59:148–154. doi: 10.1111/j.1863-2378.2011.01436.x. [DOI] [PubMed] [Google Scholar]
- 252.Erdem H, Ergunay K, Yilmaz A, Naz H, Akata F, et al. Emergence and co-infections of West Nile virus and Toscana virus in Eastern Thrace, Turkey. Clin Microbiol Infect. 2014;20:319–325. doi: 10.1111/1469-0691.12310. [DOI] [PubMed] [Google Scholar]
- 253.Tezcan S, Di̇nçer E, Ülger M, Özgür D, Erdoğan S, et al. Serological investigation of phlebovirus exposure in blood donors from the Mediterranean Province of Mersin, Turkey. Mikrobiyol Bul. 2015;49:403–413. doi: 10.5578/mb.9765. [DOI] [PubMed] [Google Scholar]
- 254.Shiraly R, Khosravi A, Farahangiz S. Seroprevalence of sandfly fever virus infection in military personnel on the western border of Iran. J Infect Public Health. 2017;10:59–63. doi: 10.1016/j.jiph.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 255.Kuşçu F, Menemenlioğlu D, Öztürk DB, Korukluoğlu G, Uyar Y. Anti-HIV Pozitif Bir Hastada Saptanan Akut Toskana Virus Enfeksiyonu. Mikrobiyol Bul. 2014;48:168–173. doi: 10.5578/mb.5401. [DOI] [PubMed] [Google Scholar]