Abstract
The efficacy of adjuvant denosumab in combination with hormonotherapy in breast cancer patients was investigated in two randomized trials, ABCSG-18 and D-Care, but the results were mixed with respect to the impact of this drug on disease-free survival. However, the ABCSG-18 study has achieved its primary goal: prevention of clinical fractures. Therefore, the protective role of Denosumab on bone fragility induced by estrogen deprivation, already demonstrated in post-menopausal women, has been validated in the breast cancer setting.
The D-Care study failed to confirm the denosumab efficacy in prolonging disease free survival, which was reported in the ABCSG-18 study. This may be attributed to the different patients enrolled (considering the tumor risk relapse and chemotherapy) but also bone-drug exposure of women in both studies: low in the ABCSG-18 study and high in the D-Care study. In this viewpoint, it is suggested that the strong inhibition of osteoclastic activity by high doses of denosumab and the resulting changes in the bone microenvironment could potentially compromise its effectiveness in reducing breast cancer relapse.
Denosumab, an anti-RANK ligand monoclonal antibody, is not recommended by international guidelines for breast cancer patients in the adjuvant setting due to mixed results in terms of disease outcomes reported by randomized studies [1,2]. Following these recommendations, oncologists today tend not to prescribe denosumab in association with adjuvant therapy in women with early breast cancer (EBC). On the other hand, bisphosphonates (BPs), which are another class of bone-resorption agents, are recommended as an adjuvant systemic therapy to decrease the risk of breast cancer recurrence, based on the results of the individual patient data (IPD) meta-analysis from the Early Breast Cancer Trialists' Collaborative Group (EBCTCG) [3].
However, is evidence from randomized studies so discordant that it is not advised to use denosumab in women with breast cancer who are receiving adjuvant therapy with drugs that have been known to promote bone fragility, such as aromatase inhibitors?
Two studies, the Austrian Breast and Colorectal Cancer Study Group (ABCSG)-18 [4,5] and the D-CARE trial [6] addressed adjuvant denosumab efficacy in EBC.
ABCSG-18 study enrolled 3420 patients with estrogen and/or progesterone receptor-positive EBC who completed loco-regional therapy. All patients were postmenopausal and were receiving aromatase inhibitors. D-CARE study included 4509 stage II or III breast cancer patients at high risk of recurrence (T3/T4 disease and/or lymph node positivity) who were either premenopausal or postmenopausal and received endocrine therapy alone or associated with chemotherapy and/or HER2-targeted therapy according to histology and risk state.
In the ABCSG-18, denosumab was prescribed at 60 mg sc every six months (average of administered doses 7, range: 4 to 10). Conversely, in the D-CARE study, denosumab was administered at the dose of 120 mg sc once monthly for six months, followed by maintenance with Denosumab 120 mg sc every three months (average of administered doses 25, range: 1 to 26).
Time to the first clinical fracture was the primary endpoint of the ABCSG-18 study. Clinical fractures were defined as fractures accompanied by noticeable symptoms. The secondary endpoints were categorized as bone-related or disease-related. The bone-related secondary endpoints were: percentage change in bone mineral density from baseline to 36 months after, incidence of new vertebral fracture, and new or worsening of pre-existing vertebral fractures. Disease outcome-related endpoints were disease-free survival, bone-metastasis-free survival, and overall survival.
The primary endpoint of the D-CARE trial was bone-metastasis-free survival (BMFS), i.e., the time between randomization and the first detection of bone metastasis. The secondary endpoints included disease-free survival and overall survival. Additionally, there were several exploratory endpoints, such as time to first bone metastasis, time to disease recurrence, time to first on-study fracture, and time to first on-study skeletal-related events following the development of bone metastasis.
The primary end point analysis of the ABCSG-18 trial [4] showed that denosumab significantly prolonged the time to the first clinical fracture compared to a placebo (hazard ratio [HR] 0.50 [95 % CI, 0.39–0.65], p < 0.0001). Five percent (95 % CI, 3.8–6.2 %) of patients in the denosumab group had experienced a fracture, compared with 9.6 % (95 % CI, 8.0–11.2 %) in the placebo group. These initial results were confirmed by a second analysis performed after 8-year follow-up [5]. In the ABCSG-18 trial, denosumab also improved bone metastases-free survival (HR, 0.81; 95 % CI, 0.65 to 1.00).
The D-CARE trial [6] found no significant difference in terms of BMFS between the denosumab and placebo arms (HR 0.97, 95 % CI 0.82–1.14; p = 0.709). However, denosumab administration was associated with a significant risk reduction of bone fracture occurrence (HR 0.76, CI 0.63–0.92, p-value 0.0037).
Regarding DFS, the two studies yielded contrasting outcomes (HR 1.04, 95 % CI 0.91–1.19, p = 0.57 in the D-CARE trial; HR 0.83, 95 % CI 0.71–0.97 in ABCSG-18) and this is the reason why international guidelines report that there are mixed results [1,2].
Regarding OS outcome, 127 events were reported in the Long term (9 years) analysis by Gnant et al., and a 1 percentage point improvement was described [5].
The two above-mentioned studies were substantially different regarding study population, primary objectives, dose, and denosumab schedule. So, claiming that the results are mixed and contradictory is not correct in our opinion. If anything, a concordance emerges between the two studies regarding the reduction of the fracturing risk associated with denosumab, although this endpoint was exploratory in the D-CARE study. Denosumab, therefore should be considered among the bone resorption inhibitors used to reduce the risk of fracture in both postmenopausal women receiving aromatase inhibitors and premenopausal women whose menopause is induced by LHRH analogs associated with aromatase inhibitors, with level of evidence 1 [1,7].
The recommended dose should be 60 mg every six months, as used in the ABCSG-18 study, whose primary objective was reducing fracturing events. Women on adjuvant hormonotherapy are at risk of fractures due to worsening bone quality, which means that bone mineral density is not a reliable predictor, unlike women with postmenopausal osteoporosis [8,9]. Since bone quality impairment is irreversible, treatment with denosumab (or bisphosphonates) should be started early. Denosumab should not be administered in the adjuvant setting at 120 mg every 28 days, according to the D-CARE study results.
Moreover, it is worth asking if there is a plausible explanation for the difference in breast cancer outcomes between the two studies. Patient selection could have played a role, as previously mentioned. ABCSG-18 exclusively recruited postmenopausal patients with low-risk stage I/II ER+, while D-CARE recruited stage II/III patients that included both ER+ and ER-patients, and a significant number of premenopausal patients. According to the EBCTCG meta-analysis mentioned earlier, the advantages of adjuvant bisphosphonates are mostly limited to postmenopausal women [3]. In the D-CARE study, however, the lack of effect of denosumab treatment on bone metastasis-free survival was not influenced by menopausal status [6].
The post-hoc analyses of patients who were recruited in studies involving adjuvant bisphosphonates showed that the amplification of the transcription factor MAF (mesenchymal aponeurotic fibrosarcoma gene) is a predictor of poor efficacy of both zoledronic acid and clodronate administered in the adjuvant setting [10,11]. Premenopausal women with MAF-positive breast cancer even experienced adverse effects from adjuvant bisphosphonate administration in terms of invasive disease-free survival [10]. MAF is a prognostic factor, more frequently expressed in high-grade and highly proliferating tumors, and associated with increased metastasis, especially bone metastasis [12]. It is probable that patients enrolled in the D-CARE study had more MAF-positive tumors than those enrolled in the ABCSG-18 study. Unfortunately, this marker has not been evaluated in patients included in both studies, so it is not possible to determine if the predictive effect observed for bisphosphonates is also applicable to denosumab.
An important difference between the D-CARE and ABCSG-18 trials is the dose of denosumab administered to patients, which was much higher in the former than in the latter and the increased bone turnover inhibition could have limited the efficacy of denosumab in terms of reducing the metastatic potential. We previously conducted an explorative analysis on the relevance of BPs' potency on the efficacy of these bone-modifying agents in the adjuvant setting [13]. Firstly, we performed a literature-based meta-analysis of 12 randomized prospective trials comparing different BPs with either placebo or no antiresorptive therapy in women with early breast cancer. In four of these 12 trials (4981 patients), clodronate, a BP with low potency, was employed in the experimental arm. In the other eight trials (10,927 patients), a more potent nitrogen-containing BP was tested (zoledronic acid, pamidronate, ibandronate, or risedronate). Based on data extracted from the published reports, we calculated the HRs for overall survival in the two trial subgroups and found a point estimate of 0.86 and 0.96 for clodronate and nitrogen-containing BPs, respectively, although the interaction test was not significant.
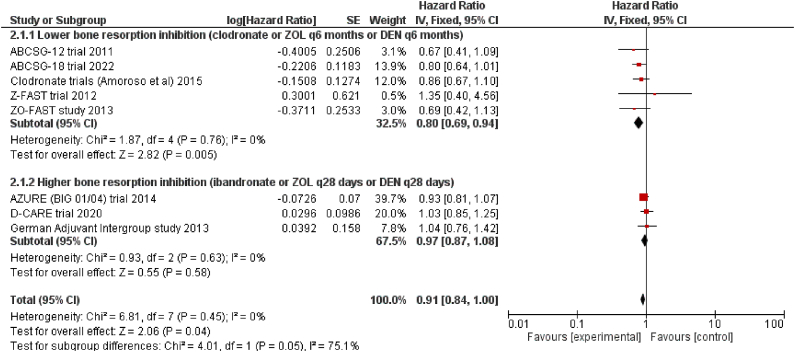
The evidence gathered from randomized trials that tested bone resorption inhibitors in the adjuvant setting in EBC patients suggests that the efficacy of intensive intravenous BPs or subcutaneous denosumab on a close schedule, which significantly inhibits bone turnover, is lower than delayed schedules of BPs and denosumab, or oral clodronate, which have a less significant impact on bone metabolism. Therefore, it would appear that the favorable effect of bone resorption inhibitors on outcomes in women with EBC may be obscured by a strong inhibition of bone resorption. To explore this further, we conducted a meta-analysis incorporating overall survival outcomes from the two studies discussed in this paper, along with pivotal randomized trials assessing the efficacy of BPs in EBC [5,6,[13], [14], [15], [16], [17], [18]]. The forest plot (Fig. 1a) indicates that bone resorption inhibitors have a significant impact on the overall survival of patients with EBC (HR 0.91, 95 % CI 0.84–1.00), similar to the effects on breast cancer mortality reported by the EBCTCG meta-analysis [3]. This positive effect appears to be more pronounced with zoledronic acid and denosumab administered at longer intervals or with the less potent oral BP clodronate, compared to zoledronic acid or denosumab administered at shorter intervals, or the more potent amino-BP ibandronate (point estimate of the HR for overall survival in the two trial subgroups: 0.80 vs 0.97, respectively). However, a key limitation of our analysis is the inability to account for differences in patient selection and concomitant therapies across the included studies. It is worth noting that the immunosuppressive effects of chemotherapy, administered to over 90 % of patients in the AZURE and D-CARE studies, may have negatively impacted the patient's outcome.
Fig. 1a.
Explorative meta-analysis assessing the pooled effect on the overall survival of bone resorption inhibitors vs control in early breast cancer patients. Included studies were divided into two subgroups according to the intensity of the antiresorptive therapy in the experimental arm, Abbreviations: ZOL, zoledronic acid; DEN, denosumab; ABCSG, Austrian Breast and Colorectal Cancer Study Group.
The possibility that over-suppression of bone turnover can result in a hostile environment is indirectly suggested by the results of the D-CARE study. Despite not meeting its primary endpoint (BMFS), which also involved relapses beyond bone metastases and patient death, the trial had a positive impact on prolonging the time to first bone metastasis. These data imply a tendency for an excess of recurrences in visceral sites that may have contributed to the overall nil effect on BMFS and disease-free survival.
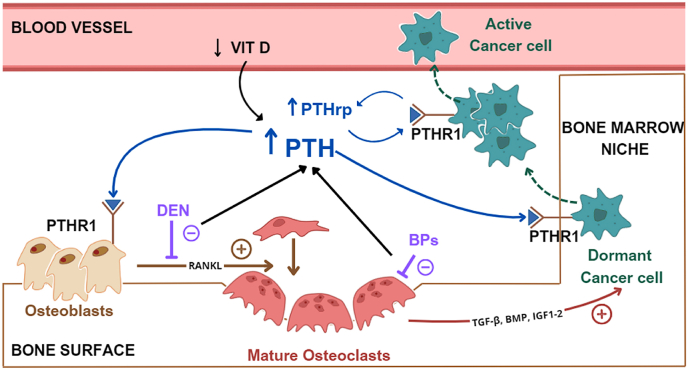
The explanation for these findings is not clear. Bone resorption inhibitors are used in adjuvant settings due to their ability to modulate the bone marrow microenvironment, favoring the dormant state of the tumor cell in the pre-metastatic niche [19]. However, the relationship between cancer cells and the bone microenvironment is complex and largely unexplored, and the interference of bone resorption inhibitors on this connection is poorly understood [20,21]. A recent preclinical study has shown that denosumab can increase skeletal stem cells as a compensatory mechanism for the lack of RANK ligand [22]. Of course, it is uncertain whether these data have any impact on bone marrow niches organization by neoplastic cells. Higher osteoclast inhibition can induce calcium entrapment in bone and secondary hyperparathyroidism in response to the increased calcium demand [13,23], which is also favored by a hypovitaminosis D status that is highly prevalent in breast cancer patients who receive adjuvant therapies [24,25]. PTH levels were not measured in patients enrolled in the two denosumab studies; however, the proportion of hypocalcemia was 0.1 % in women enrolled in the ABCSG-18 study and 11 % in those enrolled in the D-CARE study, suggesting higher PTH values in the second study than the first. High PTH levels in the bone marrow can favor metastatic progression through several mechanisms as detailed in a published paper by our group (Fig. 1b) [13]. Of note, a post hoc analysis of a randomized placebo-controlled trial evaluating zoledronic acid for metastatic prostate cancer reported that elevated serum PTH levels could predict worse outcomes in patients treated with zoledronic acid [26]. This is certainly a hypothesis and an interesting field of future research. The synergistic role of denosumab in reducing the recurrence risk in EBC patients treated with adjuvant aromatase inhibitors at doses used to preserve bone health in patients with postmenopausal osteoporosis (i.e., 60 mg every six months) deserves to be further evaluated in prospective clinical studies.
Fig. 1b.
High doses of denosumab and high-potency bisphosphonates favor calcium entrapment in bone and a marked increase in PTH levels, which in turn favor reactivation of dormant tumor cells in the bone marrow niche. PTH promotes tumor cell reactivation by various mechanisms, including binding to its receptor PTHR1 expressed by osteoblasts and triggering the release of molecular mediators (e.g. RANKL) of osteoclast maturation. PTH also increase the availability of growth factors such as TGF-β, BMP, and IGF1-2 in the bone microenvironment, which stimulate tumour cells to proliferate, further accelerating the cycle. Moreover tumour cells themselves secrete PTHrp, which binds to the same PTH receptor (PTHR1), contributing to the cycle. PTH = parathyroid hormone, PTHrp = parathyroid hormone related protein, PTHR1 = parathyroid hormone receptor 1, RANKL = receptor activator of nuclear factor kappa-B lingand, TGF-β = transforming growth factor beta, BMP = bone morphogenetic protein, IGF1-2 = insulin-like growth factor 1 and 2, DEN = denosumab, BPs = bisphosphonates.
1. Conclusion
Bone resorption inhibitors should be prescribed in women with EBC during adjuvant therapy, primarily to preserve bone health. In this view, denosumab, at the dose used to prevent osteoporotic fractures (i.e. 60 mg every 6 months) in postmenopausal women, should be considered in women receiving adjuvant treatment with aromatase inhibitors. Preventing breast cancer recurrence may also be possible with denosumab at these same doses, as suggested by the results of the ABCSG study. Due to being a secondary objective of the study, this result cannot be generalized. Adjuvant treatment of EBC with denosumab at a dose of 120 mg every month is not recommended, as evidenced by the D-CARE study.
CRediT authorship contribution statement
Laura Moretti: Writing – review & editing, Writing – original draft, Validation, Data curation. Laura Richelmi: Writing – review & editing, Writing – original draft, Validation, Data curation. Deborah Cosentini: Supervision, Formal analysis. Rebecca Pedersini: Validation, Supervision, Methodology, Conceptualization. Salvatore Grisanti: Supervision, Formal analysis, Conceptualization. Vito Amoroso: Supervision, Software, Methodology, Conceptualization. Alfredo Berruti: Validation, Supervision, Funding acquisition, Conceptualization. Marta Laganà: Supervision, Data curation, Conceptualization.
References
- 1.Loibl S., André F., Bachelot T., Barrios C.H., Bergh J., Burstein H.J., et al. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2024;35:159–182. doi: 10.1016/j.annonc.2023.11.016. [DOI] [PubMed] [Google Scholar]
- 2.Gradishar W.J., Moran M.S., Abraham J., Abramson V., Aft R., Agnese D., et al. Breast cancer, version 3.2024, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2024;22:331–357. doi: 10.6004/jnccn.2024.0035. [DOI] [PubMed] [Google Scholar]
- 3.Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet. 2015;386:1353–1361. doi: 10.1016/S0140-6736(15)60908-4. [DOI] [PubMed] [Google Scholar]
- 4.Gnant M., Pfeiler G., Dubsky P.C., Hubalek M., Greil R., Jakesz R., et al. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Lond Engl. 2015;386:433–443. doi: 10.1016/S0140-6736(15)60995-3. [DOI] [PubMed] [Google Scholar]
- 5.Gnant M., Frantal S., Pfeiler G., Steger G.G., Egle D., Greil R., et al. Long-term outcomes of adjuvant denosumab in breast cancer. NEJM Evid. 2022;1 doi: 10.1056/EVIDoa2200162. [DOI] [PubMed] [Google Scholar]
- 6.Coleman R., Finkelstein D.M., Barrios C., Martin M., Iwata H., Hegg R., et al. Adjuvant denosumab in early breast cancer (D-CARE): an international, multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21:60–72. doi: 10.1016/S1470-2045(19)30687-4. [DOI] [PubMed] [Google Scholar]
- 7.Kanis J.A., Cooper C., Rizzoli R., Reginster J.-Y. On behalf of the scientific advisory board of the European society for clinical and economic aspects of osteoporosis (ESCEO) and the committees of scientific advisors and national societies of the international osteoporosis foundation (IOF). Correction to: European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2020;31 doi: 10.1007/s00198-019-05184-3. 209–209. [DOI] [Google Scholar]
- 8.Dalla Volta A., Mazziotti G., Maffezzoni F., Grisanti S., Palumbo C., Pedersini R., et al. Bone mineral density and FRAX score may not predict fracture risk in patients with cancer undergoing hormone deprivation therapies. J Clin Oncol Off J Am Soc Clin Oncol. 2020;38:3363–3366. doi: 10.1200/JCO.20.00434. [DOI] [PubMed] [Google Scholar]
- 9.Mazziotti G., Vena W., Pedersini R., Piccini S., Morenghi E., Cosentini D., et al. Prediction of vertebral fractures in cancer patients undergoing hormone deprivation therapies: reliability of who fracture risk assessment tool (frax) and bone mineral density in real-life clinical practice. J Bone Oncol. 2022;33 doi: 10.1016/j.jbo.2022.100421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coleman R., Hall A., Albanell J., Hanby A., Bell R., Cameron D., et al. Effect of MAF amplification on treatment outcomes with adjuvant zoledronic acid in early breast cancer: a secondary analysis of the international, open-label, randomised, controlled, phase 3 AZURE (BIG 01/04) trial. Lancet Oncol. 2017;18:1543–1552. doi: 10.1016/S1470-2045(17)30603-4. [DOI] [PubMed] [Google Scholar]
- 11.Paterson A.H.G., Lucas P.C., Anderson S.J., Mamounas E.P., Brufsky A., Baez-Diaz L., et al. MAF amplification and adjuvant clodronate outcomes in early-stage breast cancer in NSABP B-34 and potential impact on clinical practice. JNCI Cancer Spectr. 2021;5:pkab054. doi: 10.1093/jncics/pkab054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pavlovic M., Arnal-Estapé A., Rojo F., Bellmunt A., Tarragona M., Guiu M., et al. vol. 107. JNCI J Natl Cancer Inst; 2015. p. djv256. (Enhanced MAF oncogene expression and breast cancer bone metastasis). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amoroso V., Petrelli F., Pedersini R., Simoncini E.L., Clézardin P., Barni S., et al. Adjuvant bisphosphonates in patients with breast cancer: does the potency matter? Future Oncol. 2015;11:2853–2856. doi: 10.2217/fon.15.210. [DOI] [PubMed] [Google Scholar]
- 14.Gnant M., Mlineritsch B., Stoeger H., Luschin-Ebengreuth G., Heck D., Menzel C., et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol. 2011;12:631–641. doi: 10.1016/S1470-2045(11)70122-X. [DOI] [PubMed] [Google Scholar]
- 15.Brufsky A.M., Harker W.G., Beck J.T., Bosserman L., Vogel C., Seidler C., et al. Final 5-year results of Z-FAST trial: adjuvant zoledronic acid maintains bone mass in postmenopausal breast cancer patients receiving letrozole. Cancer. 2012;118:1192–1201. doi: 10.1002/cncr.26313. [DOI] [PubMed] [Google Scholar]
- 16.Coleman R., de Boer R., Eidtmann H., Llombart A., Davidson N., Neven P., et al. Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): final 60-month results. Ann Oncol Off J Eur Soc Med Oncol. 2013;24:398–405. doi: 10.1093/annonc/mds277. [DOI] [PubMed] [Google Scholar]
- 17.Coleman R., Cameron D., Dodwell D., Bell R., Wilson C., Rathbone E., et al. Adjuvant zoledronic acid in patients with early breast cancer: final efficacy analysis of the AZURE (BIG 01/04) randomised open-label phase 3 trial. Lancet Oncol. 2014;15:997–1006. doi: 10.1016/S1470-2045(14)70302-X. [DOI] [PubMed] [Google Scholar]
- 18.von Minckwitz G., Möbus V., Schneeweiss A., Huober J., Thomssen C., Untch M., et al. German adjuvant intergroup node-positive study: a phase III trial to compare oral ibandronate versus observation in patients with high-risk early breast cancer. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31:3531–3539. doi: 10.1200/JCO.2012.47.2167. [DOI] [PubMed] [Google Scholar]
- 19.Coleman R. Metastasis prevention with bone-targeted agents: a complex interaction between the microenvironment and tumour biology. J Bone Miner Metabol. 2023;41:290–300. doi: 10.1007/s00774-023-01434-x. [DOI] [PubMed] [Google Scholar]
- 20.Coleman R., Gnant M., Morgan G., Clezardin P. Effects of bone-targeted agents on cancer progression and mortality. J Natl Cancer Inst. 2012;104:1059–1067. doi: 10.1093/jnci/djs263. [DOI] [PubMed] [Google Scholar]
- 21.Gnant M., Van Poznak C., Schnipper L. Therapeutic bone-modifying agents in the nonmetastatic breast cancer setting: the controversy and a value assessment. Am Soc Clin Oncol Educ Book Am Soc Clin Oncol Annu Meet. 2017;37:116–122. doi: 10.1200/EDBK_177357. [DOI] [PubMed] [Google Scholar]
- 22.Schiavone M.L., Crisafulli L., Camisaschi C., De Simone G., Liberati F.R., Palagano E., Rucci N., Ficara F., Sobacchi C. Rankl genetic deficiency and functional blockade undermine skeletal stem and progenitor cell differentiation. Stem Cell Res Ther. 2024 Jul 6;15(1):203. doi: 10.1186/s13287-024-03803-3. PMID: 38971808; PMCID: PMC11227705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soki F.N., Park S.I., McCauley L.K. The multifaceted actions of PTHrP in skeletal metastasis. Future Oncol. 2012;8:803–817. doi: 10.2217/fon.12.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman C.F., DeMichele A., Su H.I., Feng R., Kapoor S., Desai K., et al. Vitamin d deficiency in postmenopausal breast cancer survivors. J Womens Health. 2002 2012;21:456–462. doi: 10.1089/jwh.2011.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crew K.D., Shane E., Cremers S., McMahon D.J., Irani D., Hershman D.L. High prevalence of vitamin D deficiency despite supplementation in premenopausal women with breast cancer undergoing adjuvant chemotherapy. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27:2151–2156. doi: 10.1200/JCO.2008.19.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berruti A., Cook R., Saad F., Buttigliero C., Lipton A., Tampellini M., et al. Prognostic role of serum parathyroid hormone levels in advanced prostate cancer patients undergoing zoledronic acid administration. Oncol. 2012;17:645–652. doi: 10.1634/theoncologist.2011-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]