Abstract
Background
Nursing homes (NHs) are high-risk facilities with limited infection control resources and residents susceptible to infectious diseases. The evidence regarding World Health Organization (WHO) core components in NHs is lacking. This study evaluates the effectiveness of establishing an infection prevention and control (IPC) program with WHO’s core components in an NH.
Methods
The IPC program, encompassing evidence-based guidelines, education and training, surveillance, multimodal strategies, monitoring and feedback, workload and staffing considerations, and the built environment, was implemented in a 130-bed NH for one year. The effects were assessed based on the number of infections among residents, the level of knowledge, and the performance of infection control among staff. The risk of infection was analyzed across three phases: pre-implementation phase, implementation phase (6 and 12 months after intervention initiation), and sustainability phase (3, 6, and 12 months after intervention was finished). Staff data were analyzed before and after the intervention.
Results
Analysis of 18,124 resident-days revealed that during the sustainability phase, the risk of respiratory tract infection was significantly lower than before intervention implementation (odds ratio [OR] 0.51, 95% CI 0.30–0.86, p = 0.012). Moreover, a significant improvement was observed in staff knowledge (p = 0.002) and performance (p < 0.001) after the intervention compared to before.
Conclusions
WHO’s core components may have a potential effect on reducing healthcare-associated infections among residents and enhancing the infection control competency of staff in the NH with limited IPC resources.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13756-024-01492-4.
Keywords: Infection control, Nursing homes, Longitudinal studies, Resource-limited settings
Background
According to the United Nations [1], Korea is projected to experience one of the largest increases in the elderly population. Since the introduction of long-term care insurance in 2008 [2], the number of long-term care facilities (LTCFs), such as nursing homes (NHs), in South Korea has rapidly increased in response to the growing demand. An NH is a facility that provides care for older adults with chronic or gerontological diseases who have difficulty performing daily activities. Therefore, NHs house susceptible populations to infectious diseases due to aging, underlying medical conditions and chronic illnesses [3]. Facility and staff-related factors, such as the sharing of communal space, shortage of staff, and untrained staff, also hinder infection control in NHs [3]. These factors facilitate the transmission of infectious diseases and contribute to outbreaks of high-risk pathogens, such as severe acute respiratory syndrome coronavirus 2. In the United States, approximately 1–3 million severe infections occur annually in NHs and assisted-living facilities [4]. Furthermore, a cohort study showed that the mortality risk from the coronavirus disease of 2019 (COVID-19) among elderly residents in LTCFs is notably higher than among older adults living in the community [5]. The COVID-19 pandemic has highlighted several important lessons. First, the unclear boundaries of infectious diseases, which can extend beyond specific geographic areas or groups, allow the spread of infections within these facilities to be linked to community transmission. Second, effectively managing high-risk facilities that accommodate high-risk patients in communal living environments is essential, as infectious diseases do not impact all individuals equally. For these reasons, infection prevention and control (IPC) programs in NHs are crucial to ensuring the health and safety of residents and communities by preventing and reducing infectious disease transmission. Accordingly, the World Health Organization [6] proposed eight core components for the IPC in facilities: IPC programs, evidence-based guidelines, education and training, surveillance, multimodal strategies, monitoring and auditing of IPC practices and feedback, workload, staffing and bed occupancy, and built environment, materials and equipment [6].
Based on scientific evidence, expert consensus, and country experiences, the WHO core components for IPC are the foundation for strengthening effective national and facility-level programs. However, there is little data to support the efficacy of de novo implementation of IPC programs based on these WHO eight core components in NHs, particularly in South Korea. Aged care settings, such as NHs, are generally low-resourced, making it challenging to meet these requirements realistically. Low registered nurses (RN) staffing in NHs has been associated with an increased incidence of infectious diseases [7, 8], and RNs play a crucial role in infection control and prevention in NHs [9]. Nevertheless, over 70% of NHs in South Korea lack RNs [10]. While laws mandate staffing ratios per resident for RNs and certified nursing assistants, these ratios are often inadequate for effective infection control [11], and no regulations mandating designated personnel for infection control. Additionally, there is a lack of structures and resources for infection control, such as diagnostic and microbiological laboratory systems, infection control programs, and surveillance systems. The absence of an in-house diagnostic testing infrastructure delays the detection of infectious pathogens and disease. Consequently, due to these aforementioned challenges, NHs in Korea are generally considered under-resourced and understaffed [12].
The current study establishes an IPC program, including the WHO’s core components, in an NH in South Korea that did not previously have an IPC program. The primary objective was to evaluate the effectiveness of establishing an IPC program with WHO’s core components on the risk of healthcare-associated infection (HAI) (respiratory tract, urinary tract, skin and soft tissue, and gastrointestinal tract) among residents during a 2-year period and determine the role of the program in improving knowledge and compliance with infection control practices among nursing staff. Additionally, considering the lack of evidence on the long-term effects of IPC interventions in LTCFs [13], this study assesses the sustained effects over time. Hence, this study serves as a meaningful exemplar related to implementing an IPC program in an NH, offering valuable insights to complement the evidence regarding its effectiveness.
Methods
Study design and setting
This was a quasi-experimental before-and-after cohort study based on a cohort of all residents admitted to an NH in South Korea. The study site was purposively selected considering the feasibility and absence of any infection control intervention. The facility had a 130-bed capacity and approximately 70–80 workers. The NH did not have infection control guidelines, and no IPC program or professionals were dedicated to IPC activities before intervention. The institutional review board reviewed and approved this study (No. 1711/003–015, 1911/001–008). The intervention described below was implemented for 12 months, from January 1, 2018 to December 31, 2018 while data was collected between December 2017 and February 2020, after written informed consent was obtained from the workers and residents. Due to the health status of LTCF residents, individuals who had difficulty exercising autonomy required consent from proxies such as family members.
Intervention
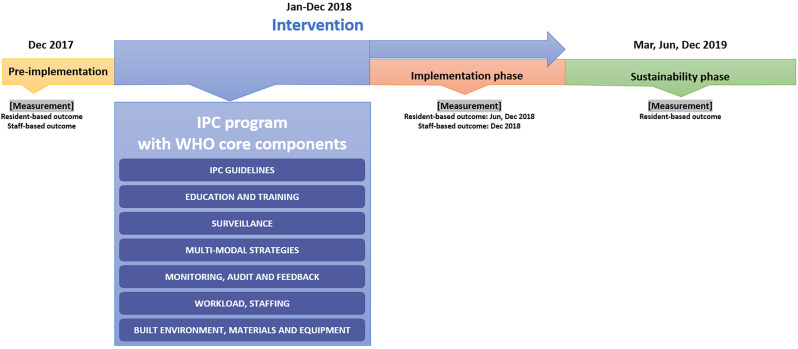
The multi-component IPC program was based on the IPC manual of the WHO [6] comprising evidence-based guidelines; education and training; surveillance; multi-modal strategies; monitoring and feedback; workload and staffing; and built environment, materials, and equipment (Fig. 1). The specific activities for each component are as follows:
Fig. 1.
The timeline of this study. IPC = infection prevention and control
IPC guidelines
Based on the guideline development process of the Scottish Intercollegiate Guidelines Network [14], the guidelines were developed [15]. During the development, the infection control guidelines for LTCF provided by the Society for Healthcare Epidemiology of America (SHEA)/ Association for Professionals in Infection Control and Epidemiology (APIC) guideline for infection control in LTCF [16] were used as a foundation, along with guidelines and literature on infection control [17–19]. Printed guidelines were provided to the NH. The IPC practice algorithm [15], structuring the infection control procedures, was posted in units, including nursing stations and corridors, to facilitate application in daily practice.
Education and training
Education specific to the IPC was implemented for all employees. Nursing staff, including nurses and certified caregivers, participated in 6 h of education delivered over 3 weeks, with one 2-hour session each week. The educational content included the characteristics of HAIs in LTCFs, standard precautions such as hand hygiene, transmission-based precautions, IPC guidelines, algorithms, nursing and assessment for infection prevention and control, occupational infection prevention and control (including immunization, medical evaluation, and management of exposure), visitor restrictions, environmental infection control, management of outbreaks, surveillance, and communication regarding infection-related information between healthcare facilities. An additional two-hour training session were provided after six months.
Surveillance
Facility-based surveillance was performed by an external infection control nurse once weekly to detect HAI occurrences and outbreaks. The nurse had approximately 5 years of experience working as an infection control nurse at a tertiary hospital and played a role as part of the intervention in this study, but was not involved in data collection, analysis, or evaluation of the effectiveness of this intervention. The infection control nurse monitored residents for symptoms and signs of HAIs.
Multi-modal strategies
Five elements of the WHO multi-modal strategy were applied, namely, system change, training and education, monitoring and feedback, reminders, and a culture of safety.
System change: Before intervention, alcohol-based hand sanitizers were only placed at central stations of each unit, limiting access to hand sanitizers at the point of care. To address this issue, alcohol-based hand sanitizers, including pocket-sized hand rubs and paper towels, were supplied during the intervention. An educational kit for HH, such as a UV lamp for effective hand-washing demonstrations, was placed in the units.
Training and education: In addition to the aforementioned education sessions for staff, the infection control nurse provided immediate on-site education and training to improve the staff’s infection control practices for 2 h per week.
Monitoring and feedback: The infection control nurse monitored HAI occurrence and infection control practice by conducting direct observations to assess hand hygiene, proper glove use, environmental management, and compliance with IPC guidelines. Timley feedback was provided to staff on-site based on the results of these observations and the results of HH compliance were analyzed monthly and posted in units.
Reminder and communication: Posters and checklists related to IPC activities were posted in each unit; the infection control nurse reminded the staff of proper IPC practices and infection control guidelines. The posters featured WHO’s hand hygiene indications, examples of nursing practices for each indication, proper techniques of hand hygiene, respiratory etiquette, visitor restrictions and the IPC practical algorithm.
Culture of safety: An HH campaign was held for all employees at the NH in February 2018 to increase awareness of the importance of HH. Heads of nurses and certified caregivers were encouraged to participate in the intervention and lead their members actively. Employees with excellent IPC practices were selected monthly as role models, the list was posted in units, and incentives were awarded.
Monitoring and feedback
The IPC practices of the NH were monitored weekly, and the infection control nurse provided timely feedback.
Workload and staffing
The NH did not have dedicated infection control personnel before the intervention. Thus, we assigned the infection control nurse, who was hired by the research project, to the facility as a facilitator for implementing the IPC program. The infection control nurse visited the NH once weekly.
Built environment, materials, and equipment
Products for HH, such as pocket-sized alcohol sanitizers and antimicrobial soaps containing chlorhexidine gluconate, were readily available at the point-of-care. Cleaning checklists were developed and used for environmental infection control on resident rooms, common areas, and bathrooms. These checklists offered guidelines for cleaning practices, detailing which environmental surfaces required cleaning, as well as specifying proper disinfectant use and verification of expiration dates.
Measurements
The effect of the intervention was evaluated using resident- and staff-based outcomes. Resident-based outcomes were evaluated by the incidence of respiratory tract, urinary tract, GI, and SST infections—the most common infections in LTCFs [15, 20, 21]—defined by McGeer’s revised criteria [22], which were used for surveillance purposes. The study periods for the resident outcome evaluation were divided into three phases: ‘pre-implementation phase’ preceding the intervention, ‘implementation phase’ as the first evaluation of the intervention’s effect (with surveillance conducted at 6 and 12 months after intervention began), and ‘sustainability phase’ as the evaluation of long-term effects (with surveillance conducted at 3, 6, and 12 months after intervention was finished). The incidence of HAIs was calculated based on the number of new cases occurring over a one-month at each surveillance point, and the infection risk was analysed accordingly. A case that satisfied the criteria was classified as a definite case, while those that did not meet the criteria but in which the resident presented with symptoms of a possible infection were classified as probable cases. Infections included (1) definite and probable cases, and those (1) with no evidence of infection at the time of admission, (2) attributed to the NH, and (3) in residents on more than two calendar days after admission. The authors evaluated resident-based outcomes (MHL, YMY, and EYN). Initially, two authors independently carried out the surveillance, and then the results were reviewed and discussed with the third author to determine whether each case met the criteria.
Staff-based outcomes included the self-reported level of knowledge and compliance with infection control practices measured before and after the intervention. Knowledge of infection control practices was measured using a tool modified by Baek [23] according to the revised guidelines of the Hospital Infection Control Practices Advisory Committee [24], based on the tool designed by Suh and Oh [25]. This tool consists of a total of 29 items assessing standard precaution, HH, personal protective equipment, respiratory etiquette, placement of patient, environmental management, and sharp injury prevention. Each item was measured on a dichotomous scale: 1 point for correct answer and 0 points for wrong answer. A higher score indicated greater infection control knowledge. The reliability of the tool in this study was assessed based on the Kuder–Richardson 20 (KR-20) = 0.71. The performance of infection control practices was measured using a tool of Park and Lim [26], based on the infection control guidelines for elderly care facilities developed by Kim and Chun [27]. This tool comprised seven categories: HH, personal hygiene, disinfection, medication management, urinary tract infection management, respiratory infection management, and environmental management, and included 35 items rated on a 4-point Likert scale (1-never to 4-always). A higher score indicated a higher level of infection control performance. The reliability of this tool was Cronbach’s alpha = 0.96 in the developer’s study [26] and 0.96. in this study.
Statistical analysis
Considering the characteristics of LTCFs, older adults who resided for ≥ 14 days were included in the final analysis. Data analysis was conducted using R 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org/). For comparisons between groups (time points) in Tables 1, 3, and 4, one-way analysis of variance (ANOVA), independent sample t-tests, and chi-squared tests were performed. A Poisson regression model was used to predict the risk of infection based on the number of infections across the three phases of the study periods. However, a substantial proportion of the data had zero counts, making applying a standard Poisson model inappropriate. Therefore, the number of infections was modeled using a zero-inflated Poisson model with the ‘pscl’ package for analysis in Table 2. When conducting the analysis using the zero-inflated Poisson model, we adjusted for sex and age as covariates. We treated the number of resident days for each phase of study periods as an offset, representing at-risk days. All tests were conducted with a significance threshold set at p < 0.05.
Table 1.
Resident characteristics across the three phases of outcome evaluation
| Characteristic | Total (n = 603) |
Pre-implementation phase (n = 121) |
Implementation phase (n = 249) |
Sustainability phase (n = 233) |
p-value |
|---|---|---|---|---|---|
| Mean age (years) | 84.1 ± 8.6 | 83.6 ± 8.8 | 84.0 ± 8.6 | 84.4 ± 8.5 | 0.667 |
| Female sex | 498 (82.6) | 101 (83.5) | 207 (83.1) | 190 (81.5) | 0.877 |
| Length of stay per month (days) | 30.1 ± 2.1 | 29.8 ± 1.6 | 30.1 ± 1.9 | 30.2 ± 2.6 | 0.213 |
| Long-term care insurance rating* | 0.473 | ||||
| 1 | 121 (20.1) | 28 (23.1) | 47 (18.9) | 46 (19.7) | |
| 2 | 230 (38.1) | 49 (40.5) | 102 (41.0) | 79 (33.9) | |
| 3 | 213 (35.3) | 37 (30.6) | 87 (34.9) | 89 (38.2) | |
| 4 | 39 (6.5) | 7 (5.8) | 13 (5.2) | 19 (8.2) |
*The long-term care insurance rating is determined by evaluating physical and mental functions to decide eligibility for long-term care insurance support. A lower rating indicates a higher dependency in performing activities of daily living
Table 3.
Staff characteristics in pre- and post-test
| Characteristics | Pre-test (n = 77) |
Post-test (n = 66) |
t or χ2 | p |
|---|---|---|---|---|
| Age (year) | 52.22 ± 8.41 | 54.02 ± 8.90 | -1.24 | 0.218 |
| Sex | ||||
| Female | 74 (96.1) | 63 (95.5) | 0.037* | 0.999 |
| Male | 3 (3.9) | 3 (4.5) | ||
| Education level (year) | 13.18 ± 2.42 | 13.15 ± 2.26 | 0.08 | 0.939 |
| Job | 1.84* | 0.809 | ||
| Nurse | 8 (10.4) | 10 (15.2) | ||
| Licensed caregiver | 58 (75.3) | 48 (72.7) | ||
| Physical therapist | 2 (2.6) | 3 (4.5) | ||
| Social worker | 7 (9.1) | 4 (6.1) | ||
| Others | 2 (2.6) | 1 (1.5) | ||
| Working Career (month) | 76.43 ± 80.86† | 93.77 ± 105.30 | -1.11 | 0.270 |
| Career in current facility | ||||
| < 1 year | 17 (22.1) | 15 (22.7) | 4.97* | 0.294 |
| 1–2 years | 18 (23.4) | 8 (12.1) | ||
| 2–3 years | 6 (7.8) | 11 (16.7) | ||
| 3–5 years | 13 (16.9) | 13 (19.7) | ||
| ≥ 5 years | 23 (29.9) | 19 (28.8) |
*Fisher’s exact test, †n = 76
Table 4.
Intervention effectiveness on knowledge and performance of infection control practice among staff
| Variable | Pre-test (n = 77) |
Post-test (n = 66) |
t | p |
|---|---|---|---|---|
| Knowledge of infection control practice | 19.82 ± 3.25 | 21.26 ± 2.17 | -3.150 | 0.002* |
| Standard precautions | 2.34 ± 1.35 | 2.91 ± 1.00 | -2.892 | 0.004* |
| Hand hygiene | 3.12 ± 0.86 | 3.23 ± 0.74 | -0.817 | 0.415 |
| Personal protective equipment | 7.97 ± 1.46 | 8.27 ± 1.20 | -1.324 | 0.188 |
| Environmental control | 3.01 ± 0.84 | 3.11 ± 0.68 | -0.722 | 0.471 |
| Respiratory etiquette | 1.77 ± 0.46 | 1.91 ± 0.29 | -2.267 | 0.025* |
| Resident placement | 1.61 ± 0.57 | 1.83 ± 0.38 | -2.811 | 0.006* |
| Variable |
Pre-test (n = 72) |
Post-test (n = 66) |
t | P |
| Performance of infection control practice | 118.58 ± 23.54 | 133.59 ± 10.18 | -4.931 | < 0.001* |
| Hand hygiene | 39.76 ± 7.83 | 46.14 ± 3.29 | -6.327 | < 0.001* |
| Personal hygiene | 21.39 ± 2.97 | 22.73 ± 2.09 | -3.081 | 0.003* |
| Urinary care | 13.79 ± 4.16 | 15.44 ± 1.92 | -3.030 | 0.003* |
| Respiratory care | 19.58 ± 5.54 | 22.33 ± 2.75 | -3.737 | < 0.001* |
| Environmental control | 24.06 ± 5.69 | 26.95 ± 2.14 | -4.020 | < 0.001* |
*p < 0.005
Table 2.
Assessment of the intervention effectiveness on infection outcomes
| Infection | Incidence of HAI | Effectiveness* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-implementation phase | Implementation phase | Sustainability phase | Change after intervention (implementation phase vs. pre-implementation phase) |
Long-term effect (sustainability phase vs. pre-implementation phase) |
||||||
| Rate (per 100 residents) | Density (per 1,000 resident days) | Rate (per 100 residents) | Density (per 1,000 resident days) | Rate (per 100 residents) | Density (per 1,000 resident days) | OR(95%CI) | p | OR(95%CI) | p | |
| Respiratory tract | 19.0 | 6.39 | 16.9 | 5.61 | 9.9 | 3.27 | 0.88(0.53, 1.46) | 0.615 | 0.51(0.30, 0.86) | 0.012† |
| Urinary tract | 5.0 | 1.67 | 4.4 | 1.47 | 3.0 | 1.00 | 0.88(0.33, 2.38) | 0.803 | 0.60(0.20, 1.78) | 0.355 |
| Skin and soft tissue | 12.4 | 4.17 | 17.7 | 5.87 | 13.3 | 4.41 | 1.41(0.78, 2.53) | 0.251 | 2.23(0.75, 6.60) | 0.147 |
| Gastrointestinal tract | 1.7 | 0.56 | 3.6 | 1.20 | 3.4 | 1.14 | 2.16(0.45, 10.37) | 0.335 | 2.05(0.42, 9.99) | 0.376 |
| Total | 38.0 | 12.77 | 42.6 | 14.15 | 29.6 | 9.81 | 1.06(0.77, 1.48) | 0.709 | 1.19(0.70, 2.03) | 0.519 |
*adjusted for sex, age, and resident days, †p < 0.05, HAI: healthcare associated infection; OR: Odds ratio; CI: confidence interval
A power analysis was performed using the G*Power 3.1.9 program [28] based on Poisson regression, with a focus on the odds ratio (OR) of resident outcomes. The analysis indicated that all resident outcomes achieved a power of 0.9 or higher, suggesting an adequate sample size, except for short-term total infection (OR = 1.06).
Results
Effectiveness of the intervention on resident-based outcomes
In total, 18,124 resident-day records were analyzed: 121 residents at pre-implementation phase, 249 during implementation phase, and 233 during the sustainability phase (Supplementary Fig. 1). The mean age of residents was 84.1 years, and 82.6% were female (Table 1). There were no significant differences in the characteristics of residents across the three phases.
The effectiveness on infection outcome are shown in Table 2. There was no significant difference in the likelihood of infections during implementation compared to pre-implementation (p = 0.615, p = 0.803 for respiratory and urinary tract infections, respectively). There was no difference in SST or GI infection risk between the implementation and pre-implementation phases. The risk of respiratory tract infection was significantly lower during the sustainability phase than at pre-implementation phase (OR 0.51, 95%CI 0.30–0.86, p = 0.012). There was no significant difference in the risk of infection during the sustainability phase compared to the pre-implementation phase for urinary tract infections and other infections.
Effectiveness of the intervention on staff-based outcomes
A total of 77 staff members in pre-test and 66 in post-test were included in the final analysis (Table 3). The mean age was 52.22 years on pre-test and 54.02 years on post-test, showing no significant difference. Most staff were female (pre-test: n = 74, 96.1%, post-test: n = 63, 95.5%), and 10–15% were nurses (pre-test: n = 8, 10.4%, post-test: n = 10, 15.2%). There was also no statistically significant difference in the time employed in the facility between the pre- and post-groups.
A significant increase was observed in the knowledge of infection control in post-test compared to pre-test groups (p = 0.002; Table 4). Regarding the sub-categories, the level of knowledge regarding standard precautions (p = 0.004), respiratory etiquette (p = 0.025), and resident placement (p = 0.006) was significantly higher post-test than pre-test. Moreover, the level of performance for infection control practices significantly increased compared to pre-test (p < 0.001). The level of all infection control practices improved in post-test, including HH (p < 0.001), urinary care (p = 0.003), respiratory care (p < 0.001), personal hygiene (p = 0.003), and environmental control (p < 0.001).
Discussion
This study assessed the effects of establishing an IPC program, based on WHO’s core components, in an NH setting with limited infection control resources on residents and staff. Recently, infection control in high-risk facilities, where resources are limited and vulnerable populations reside, has become a critical public health focus. Within the current study, the overall decrease in the risk of respiratory tract infections suggests a possible effect of de novo implementation of the IPC program in NHs.
This IPC program may have a long-term effect on reducing the risk of respiratory tract infections, which aligns with a systematic review of 17 studies that found that IPC programs employing education, monitoring, feedback, and the four to five components of a multi-modal strategy, demonstrate effectiveness in LTCFs [13]. The introduction and maintenance of the IPC program with WHO’s core components in low-resourced settings can ultimately contribute to the prevention of HAIs and enhance the provision of safe care to residents. The IPC program in this study does not have a vertical approach targeting specific pathogens; rather, it mainly focuses on reinforcing standard precautions and hygiene management. A horizontal approach might be more effective than a vertical intervention, particularly in LTCFs with poor access to laboratory tests and monthly physician visits [29, 30].
The present study demonstrates a significant improvement in the level of knowledge and performance of infection control practices of staff after the intervention, indicating the successful promotion of behavioral change through the multi-component IPC program. In the case of HH, there was no significant difference in the level of knowledge, but the performance significantly improved. This suggests that because HH is a fundamental practice; the level of knowledge was already relatively high before the intervention. Therefore, it is interpreted that the intervention facilitated the application of knowledge to practice, leading to actual behavior changes. A previously reported study from our IPC program, a consistent improvement in staff HH compliance has also been observed as a process indicator [31], supporting this interpretation. This study offers insights into several key factors that can contribute to sustained adherence of staff for continuous improvement [32]. Firstly, the current study implemented evidence-based IPC guidelines and multi-modal interventions for 12 months to improve staff adherence. The year-long efforts of this study have contributed to establishing an organizational culture for infection control within the NH. These long-term efforts may be required to enhance staff performance in facilities with limited resources. Secondly, the multi-modal strategy appears to have been significant. Incentives and role models, as key elements of the culture of safety, help encourage institutional staff and administrators to actively participate in the IPC program and exhibit leadership. Indeed, previous studies have reported that effective leadership and administrative engagement improve staff performance [33, 34]. Furthermore, considering the general staff shortage and staff overload, previous studies have demonstrated that strategies including ongoing training and on-site education, such as those applied in our study, are beneficial to achieve behavioral changes in staff [35, 36]. Finally, the placement of the infection control nurse has been crucial in addressing the staff shortage and absence of dedicated infection control personnel. In low-resource settings, a substantial workload is a major obstacle to implementing effective infection control [37]. In the United States, ~ 10% of LTCF staff are RNs [38], generally lacking training and education regarding infection control [39]. Similarly, in South Korea, approximately 70% of NHs lack RNs, and no regulations mandating dedicated infection control personnel or structured IPC programs for NHs [10]. Therefore, we included the infection control nurse as an intervention element, drawing inspiration from the concept of an ‘infection control link nurse (ICLN)’ [40]. Since infection control nurses improved compliance on guidance and infection control practices, reducing infection risks, it would be worth reinforcing external IPC support in NHs with limited human resources by deploying ICLN nurses at the district level [41].
This study did not find a significant reduction in the risk of HAIs other than respiratory tract infections. This may be due to various challenges. First, due to the nature of NH, private rooms are limited; thus, the shared living spaces may have influenced nosocomial transmission. In particular, all residents share bathrooms, which could act as pathogen reservoirs related to GI and SST infections [42]. It is possible that the absence of risk reductions in GI and SST infections could be attributed to these factors. Moreover, considering that GI infections are the most prevalent type of infection in LTCFs, also in the context of outbreaks [43], the absence of GI infection outbreaks during the intervention might indicate a positive effect of the IPC program. Second, barriers related to human resources have impeded effective infection control. In fact, staff turnover in LTCFs is reportedly ~ 50% [44]. Similarly, in this study, the frequent nursing staff turnover acted as a barrier to the IPC program. Finally, diagnosing infections may be challenging in the NH population [45]. The case definition applied in this study was used for surveillance purposes and is meaningful only for identifying the possibility of an event, not for diagnosing an actual infection. In particular, the older residents in NHs may exhibit atypical presentations that may not meet the surveillance definition, which does not indicate the absence of infection. Additionally, the absence of microbiological laboratory capacity in the NH might have influenced the surveillance process.
To the best of our knowledge, this is the first study to confirm the positive effects of a de novo implementation of an IPC program applying all core components of the WHO on both infection risk of residents as well on knowledge and performance on infection control among staff, and to evaluate its long-term effects. Despite these strengths, this study has several limitations. First, the absence of a control group leads to the impact of exogenous variables, such as maturation, necessitating caution in interpreting the intervention effects. Furthermore, given the challenges of diagnostic testing, the number of HAIs identified through surveillance may have been underestimated. This low number of cases could have affected the statistical power to detect the effectiveness of the intervention. The analysis of short-term effect on total infection showed a power of approximately 0.7, suggesting that this specific analysis may be underpowered. Second, blinding was not performed when measuring the effects on infectious diseases; however, this did not likely impact the results given the increased number of certain infectious diseases. Third, the effects on staff were measured through self-reporting, making it difficult to exclude the Hawthorne effect. Fourth, during the sustainability phase coinciding with the COVID-19 pandemic, visitor restrictions policy led to the inability to obtain consent from proxies, resulting in the loss of follow-up for residents. The dropout rate during this phase may have influenced the results. Finally, seasonal effects may have confounded the effect of the intervention. Future studies should consider these seasonal factors.
Conclusions
Establishing the IPC program that addresses the WHO’s core components in an NH may have contributed to a reduction in the risk of respiratory infections among residents and improvements in infection control performance among staff. Applying the WHO’s core components in a low-resource setting, an LTCF, has demonstrated a positive impact. The findings of this study contribute to the design and development of effective IPC programs for NHs. Further studies that include control groups are necessary to validate the effects of the intervention.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Flowchart of participants in this study.
Acknowledgements
we would like to thank all participants and participating nursing home for their involvement in this study.
Abbreviations
- NHs
Nursing Homes
- LTCFs
Long-Term Care Facilities
- COVID-19
Coronavirus Disease 2019
- IPC
Infection Prevention and Control
- WHO
World Health Organization
- RNs
Registered Nurses
- HAI
Healthcare-Associated Infection
- GI
Gastrointestinal
- SST
Skin and Soft Tissue
- HH
Hand Hygiene
- SHEA
Society for Healthcare Epidemiology of America
- APIC
Association for Professionals in Infection Control and Epidemiology
- ANOVA
Analysis of Variance
- OR
Odds Ratio
- CI
Confidence Interval
- ICLN
Infection Control Link Nurse
Author contributions
MHL: conceptualization, investigation, conductig study interventions, data analysis, writing original draft, review and editing, YMY: investigation, conductig study interventions, writing review and editing, EYN: investigation, conductig study interventions, writing review and editing, project administration, YHP: conceptualization, supervision, data analysis, writing original draft, review.
Funding
This work was supported by the National Research Foundation of Korea grant funded by the Korean Government (Ministry of Science and ICT) [grant numbers NRF-2016R1A2B4008890, NRF-2019R1A6A3A01095343].
Data availability
The data collected and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study obtained approval from institutional review board (IRB) of Seoul National University of South Korea (No. 1711/003–015, 1911/001–008). Written informed consent was obtained from all participants.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.United Nations. World Population Prospects 2019: Highlights (ST/ESA/SER.A/423). https://population.un.org/wpp/publications/files/wpp2019_highlights.pdf; 2019. Accessed 5 March 2023.
- 2.Ga H. New system for Korean nursing home contracted physicians began in 2017. Ann Geriatr Med Res. 2017;21:35–6. 10.4235/agmr.2017.21.1.35. [Google Scholar]
- 3.Giri S, Chenn LM, Romero-Ortuno R. Nursing homes during the COVID-19 pandemic: a scoping review of challenges and responses. Eur Geriatr Med. 2021;12(6):1127–36. 10.1007/s41999-021-00531-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC). Nursing Homes and Assisted Living (Long-term Care Facilities [LTCFs]). https://www.cdc.gov/longtermcare/index.html; 2023. Accessed 7 Aug 2023.
- 5.Lai C-C, Wang J-H, Ko W-C, Yen M-Y, Lu M-C, Lee C-M, et al. COVID-19 in long-term care facilities: an upcoming threat that cannot be ignored. J Microbiol Immunol Infect. 2020;53:444–6. 10.1016/j.jmii.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Improving infection prevention and control at the health facility: interim practical manual supporting implementation of the WHO guidelines on core components of infection prevention and control programs. &isAllowed=y; 2018. https://apps.who.int/iris/bitstream/handle/10665/279788/WHO-HIS-SDS-2018.10-eng.pdf?sequence=1.
- 7.Carter MW, Porell FW. Vulnerable populations at risk of potentially avoidable hospitalizations: the case of nursing home residents with Alzheimer’s Disease. Am J Alzheimer’s Dis Other Demen. 2005;20(6):349–58. 10.1177/153331750502000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutt E, Radcliff TA, Liebrecht D, Fish R, McNulty M, Kramer AM. Associations among nurse and certified nursing assistant hours per resident per day and adherence to guidelines for treating nursing home-acquired pneumonia. J Gerontol Biol Sci Med Sci. 2008;63(10):1105–11. 10.1093/gerona/63.10.1105. [DOI] [PubMed] [Google Scholar]
- 9.Harrington C, Ross L, Chapman S, Halifax E, Spurlock B, Bakerjian D. Nurse staffing and coronavirus infections in California nursing homes. PPNP. 2020;21(3):174–86. 10.1177/1527154420938707. [DOI] [PubMed] [Google Scholar]
- 10.Bakerjian D, Boltz M, Bowers B, Gray-Miceli D, Harrington C, Kolanowski A, et al. Expert nurse response to workforce recommendations made by the coronavi Rus commission for safety and quality in nursing homes. Nurs Outlook. 2021;69(5):735–43. 10.1016/j.outlook.2021.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahn SR. Current status and challenges in response to infectious diseases in social welfare living facilities. Health Welf Issue Focus. 2020;381:1–11. http://repository.kihasa.re.kr/handle/201002/34980. [Google Scholar]
- 12.Park YH, Bang HL, Kim GH, Oh S, Jung YI, Kim H. Current status and barriers to health care services for nursing home residents: perspectives of staffs in Korean nursing homes. KJAN. 2015;27(4):418–27. 10.7475/kjan.2015.27.4.41. [Google Scholar]
- 13.Lee M, Lee G, Lee S, Park Y. Effectiveness and core components of infection prevention and control programs in long-term care facilities: a systematic review. J Hosp Infect. 2019;102:377–93. 10.1016/j.jhin.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Scottish Intercollegiate Guidelines Network. SIGN 50: a guideline developers’ handbook. Edinburgh: Scottish Intercollegiate Guidelines Network; 2015. [Google Scholar]
- 15.Park Y-H, Lee SH, Yi YM, Lee CY, Lee MH. Development of evidence-based guidelines for nursing home’s infection control in Korea. J Muscle Joint Health. 2018;25(2):135–47. 10.5953/JMJH.2018.25.2.135. [Google Scholar]
- 16.Smith PW, Bennett G, Bradley S, Drinka P, Lautenbach E, Marx J, et al. SHEA/APIC Guideline: infection prevention and control in the long-term care facility. Am J Infect Control. 2008;36:504–35. 10.1016/j.ajic.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Australian Government Department of Health. Infection prevention and control in residential and community aged care. Canberra: National Health and Medical Research Council; 2013. [Google Scholar]
- 18.Flanagan E, Cassone M, Montoya A, Mody L. Infection control in alternative health care settings: an update. Infect Dis Clin North Am. 2016;30(3):785–804. 10.1016/j.idc.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montoya A, Cassone M, Mody L. Infections in nursing homes: epidemiology and prevention programs. Clin Geriatr Med. 2016;32(3):585–607. 10.1016/j.cger.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Dwyer LL, Harris-Kojetin LD, Valverde RH, Frazier JM, Simon AE, Stone ND, et al. Infections in long‐term care populations in the United States. J Am Geriatr Soc. 2013;61:341–9. 10.1111/jgs.12153. [DOI] [PubMed] [Google Scholar]
- 21.Suetens C, Latour K, Kärki T, Ricchizzi E, Kinross P, Moro ML, et al. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: results from two European point prevalence surveys, 2016 to 2017. Euro Surveill. 2018;23(46):pii–. 10.2807/1560-7917.ES.2018.23.46.1800516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stone ND, Ashraf MS, Calder J, Crnich CJ, Crossley K, Drinka PJ, et al. Surveillance definitions of infections in long-term care facilities: revisiting the McGeer criteria. Infect Control Hosp Epidemiol. 2012;33:965–77. 10.1086/667743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baek KS. Effects of nurses’ knowledge, administrative support and environment for infection control on compliance of standard precautions in geriatric hospital. Seoul: Yonsei University; 2016. [Google Scholar]
- 24.Siegel J, Rhinehart E, Jackson M, Chiarello L, the Healthcare Infection Control Practices Advisory Committee. Guideline for isolation precautions: preventing transmission of Infectious agents in Healthcare Settings. Am J Infect Control. 2007;35:S65–164. 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suh YH, Oh HY. Knowledge, perception, safety climate, and compliance with hospital infection standard precautions among hospital nurses. J Korean Clin Nurs Res. 2010;16:61–70. [Google Scholar]
- 26.Park E-J, Lim Y-J, Cho B-H, Sin I-J, Kim S-O. A Survey on performance of infection control by workers in nursing homes for the elderly. J Korean Gerontol Nurs. 2011;13:79–90. [Google Scholar]
- 27.Kim YS, Chun CY, Kim CJ, Park JW. A study on the awareness level and the performance level of the guidelines for the prevention of nosocomial infection. Infect Chemother. 1990;22:131–46. [Google Scholar]
- 28.Faul F, Erdfelder E, Lang AG, Buchner A. G* power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91. [DOI] [PubMed] [Google Scholar]
- 29.Stevens MP. Horizontal versus vertical infection prevention strategies. Int J Infect Dis. 2014;21:37. 10.1016/j.ijid.2014.03.492. [DOI] [PubMed] [Google Scholar]
- 30.Jump RL, Crnich CJ, Mody L, Bradley SF, Nicolle LE, Yoshikawa TT. Infectious diseases in older adults of long-term care facilities: update on approach to diagnosis and management. J Am Geriatr Soc. 2018;66:789–803. 10.1111/jgs.15248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noh E-Y, Lee MH, Yi YM, Park Y-H. Implementation of a multimodal infection control strategy in the nursing home. Geriatr Nurs. 2021;42:767–71. 10.1016/j.gerinurse.2021.03.020. [DOI] [PubMed] [Google Scholar]
- 32.Wong VWY, Huang Y, Wei WI, Wong SYS, Kwok KO. Approaches to multidrug-resistant organism prevention and control in long-term care facilities for older people: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2022;11:7. 10.1186/s13756-021-01044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinkowitz-Cochran RL, Burkitt KH, Cuerdon T, Harrison C, Gao S, Obrosky DS, et al. The associations between organizational culture and knowledge, attitudes, and practices in a multicenter Veterans affairs quality improvement initiative to prevent methicillin-resistant Staphylococcus aureus. Am J Infect Control. 2012;40:138–43. 10.1016/j.ajic.2011.04.332. [DOI] [PubMed] [Google Scholar]
- 34.Atwood MA, Mora JW, Kaplan AW. Learning to lead: evaluating leadership and organizational learning. Leadersh Organ Dev J. 2010;31:576–95. 10.1108/01437731011079637. [Google Scholar]
- 35.Sassi HP, Sifuentes LY, Koenig DW, Nichols E, Clark-Greuel J, Wong LF, et al. Control of the spread of viruses in a long-term care facility using hygiene protocols. Am J Infect Control. 2015;43:702–6. 10.1016/j.ajic.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 36.Koo E, McNamara S, Lansing B, Olmsted RN, Rye RA, Fitzgerald T, et al. Making infection prevention education interactive can enhance knowledge and improve outcomes: results from the targeted infection Prevention (TIP) study. Am J Infect Control. 2016;44:1241–6. 10.1016/j.ajic.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barker AK, Brown K, Siraj D, Ahsan M, Sengupta S, Safdar N. Barriers and facilitators to infection control at a hospital in northern India: a qualitative study. Antimicrob Resist Infect Control. 2017;6:35. 10.1186/s13756-017-0189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eliopoulos C. The clinical and business case for improving nurse staffing in long-term care. Ann Longterm Care. 2015;23.
- 39.Roup BJ, Scaletta JM. How Maryland increased infection prevention and control activity in long-term care facilities, 2003–2008. Am J Infect Control. 2011;39:292–5. 10.1016/j.ajic.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Ward D. Role of the infection prevention and control link nurse. Prim Health Care. 2016;26(5).
- 41.European Centre for Disease Prevention and Control. Point prevalence survey of healthcare associated infections and antimicrobial use in European long-term care facilities: 2016–2017. Stockholm: ECDC; 2023. [Google Scholar]
- 42.Nakamura I, Yamaguchi T, Miura Y, Watanabe H. Transmission of extended-spectrum β-lactamase-producing Klebsiella pneumoniae associated with sinks in a surgical hospital ward, confirmed by single-nucleotide polymorphism analysis. J Hosp Infect. 2021;118:1–6. 10.1016/j.jhin.2021.08.013. [DOI] [PubMed] [Google Scholar]
- 43.Lee MH, Lee GA, Lee SH, Park Y-H. A systematic review on the causes of the transmission and control measures of outbreaks in long-term care facilities: back to basics of infection control. PLoS ONE. 2020;15:e0229911. 10.1371/journal.pone.0229911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sutherland S, Meyer R. Long-term care facility National Healthcare Safety Network enrollment challenges, 2016. Am J Infect Control. 2018;46:726–8. 10.1016/j.ajic.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 45.Yoshikawa TT, Norman DC. Geriatric infectious diseases: current concepts on diagnosis and management. J Am Geriatr Soc. 2017;65:631–41. 10.1111/jgs.14731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Flowchart of participants in this study.
Data Availability Statement
The data collected and analysed during the current study are available from the corresponding author on reasonable request.