Abstract
Introduction
Asthma is the most common chronic disease during pregnancy. Maternal asthma has been associated with a multitude of unwanted pregnancy outcomes, in some studies also with neurodevelopmental disorders. Here we investigated associations between maternal asthma and neurodevelopmental disorders.
Material and Methods
We studied a retrospective population‐based cohort of 1 271 439 mother–child pairs from singleton live births in Finland between the years 1996–2018. We used multiple high‐cover registers for data collection. Adjusted unconditional Cox regression models were used to investigate associations between maternal asthma, asthma medication used during pregnancy, and offspring's neurodevelopmental disorder diagnoses.
Results
We identified 106 163 mother–child pairs affected by maternal asthma. We found that maternal asthma was associated with offspring neurodevelopmental disorders, but the differences in absolute prevalence between the control and exposure groups were small. Attention‐deficit hyperactivity disorder (ADHD) was found in 4114 (3.9%) offspring with maternal asthma and in 32 122 (3.0%) controls (adjusted hazard ratio (HR): 1.49; 95% CI 1.44–1.54); autism in 1617 (1.5%) offspring vs 13 701 (1.3%) controls (HR: 1.33; 95% CI 1.26–1.40); motor‐developmental disorder in 1569 (1.5%) offspring vs 12 147 (1.1%) controls (HR: 1.37; 95% CI 1.30–1.45); language disorder in 3057 (2.9%) offspring vs 28 421 (2.7%) controls (HR: 1.13; 95% CI 1.08–1.17), learning disabilities in 849 (0.8%) offspring vs 6534 (0.6%) controls (HR: 1.51; 95% CI 1.41–1.62); mixed developmental disorder in 1633 (1.5%) offspring vs 14 434 (1.3%) controls (HR 1.20; 95% CI, 1.14–1.26); and intellectual disability in 908 (0.9%) vs 9155 (0.9%) controls (HR: 1.12; 95% CI 1.04–1.20). No substantial differences were found between allergic and non‐allergic asthma phenotypes, and neither allergic tendency nor respiratory infection was associated with a similar likelihood of neurodevelopmental disorders.
Conclusions
Maternal asthma and allergic and non‐allergic phenotypes showed weak associations with the offspring's neurodevelopmental disorders. The association is concerned especially with learning disabilities, ADHD, motor development, and autism.
Keywords: anti‐asthmatic agents, asthma, attention‐deficit disorder with hyperactivity, autism spectrum disorder, neurodevelopmental disorders, pregnancy
Maternal asthma was associated with the likelihood of a wide variety of offspring neurodevelopmental disorders in this large population‐based mother–child cohort study. The associations with maternal asthma are unlikely to be related to general maternal inflammation processes.
Abbreviations
- CI
confidence interval
- CRC
Care Register for Health Care
- DRR
Drug Reimbursement Register
- HR
hazard ratio
- MBR
Medical Birth Register
Key message.
Data from a large Finnish nationwide 1 271 439 mother–child pair cohort with 106 163 mothers with asthma suggest that maternal asthma is associated with offspring's neurodevelopmental disorders.
1. INTRODUCTION
Asthma is the most common chronic disease during pregnancy. 1 In Finland, the prevalence of physician‐diagnosed asthma among the adult population is about 10%, but asthma mortality and hospitalization rates are low, and asthma treatment results are considered excellent.2, 3
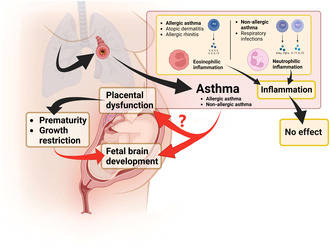
Asthma is related to an increase in airway hyperresponsiveness, with increased contractility of small airway bronchial muscles resulting from inflammation. 4 Allergic asthma is associated with eosinophilic inflammation, high blood immunoglobulin E (IgE) antibody levels, and high T‐helper cell type 2 (Th2HIGH) activity. In contrast, non‐allergic asthma is related to neutrophilic inflammation with no relation to high IgE levels or high Th2 activity (Th2LOW). Both eosinophilic and neutrophilic inflammation are present in mixed asthma. 5 Asthma is treated with specific medications and, according to international and national recommendations, begins in a stepwise manner.6, 7, 8, 9
Asthma is associated with increased risks for perinatal mortality, preterm birth, low birthweight, fetal growth restriction (SGA), and asphyxia, but why and by what mechanism remains unclear.10, 11, 12, 13, 14, 15, 16, 17, 18 Several mechanisms have been proposed relating to hypoxia, the effects of asthma medications, offsprings' DNA methylation changes, or similarities in hyperreactivity of uterine and bronchial muscle function.19, 20
Neurodevelopmental disorders are a group of lifelong conditions starting in early childhood (Figure S1). 21 Their etiology includes genetic, maternal, pregnancy‐related, and environmental factors.21, 22, 23 They are a continuum and sometimes do not cause disorder‐like functional problems and are only person characteristics. 21 In retrospective case–control and register‐based studies, maternal asthma and other inflammatory diseases have been associated with a likelihood of offspring ADHD and autism, but a recent meta‐analysis failed to confirm this association.24, 25, 26, 27
In this study, we investigated whether maternal asthma, asthma medication, asthma phenotypes, or related inflammation is associated with neurodevelopmental disorders in offspring. The study included 1 271 439 mother–child pairs.
2. MATERIAL AND METHODS
2.1. Cohort selection
We conducted a nationwide, multi‐register‐based study including all Finnish singleton mother–child pairs between the January 1, 1996 and December 31, 2018. We excluded stillbirths, offspring with triploids of chromosomes 13, 18, 21, and Fragile X from the study population (Figure 1). Maternal asthma has not been associated with offspring's chromosomal anomalies which are known to result in neurodevelopmental disorders. There was no age limit for the mothers. The follow‐up of the offspring ended on the 31st of December 2018.
FIGURE 1.
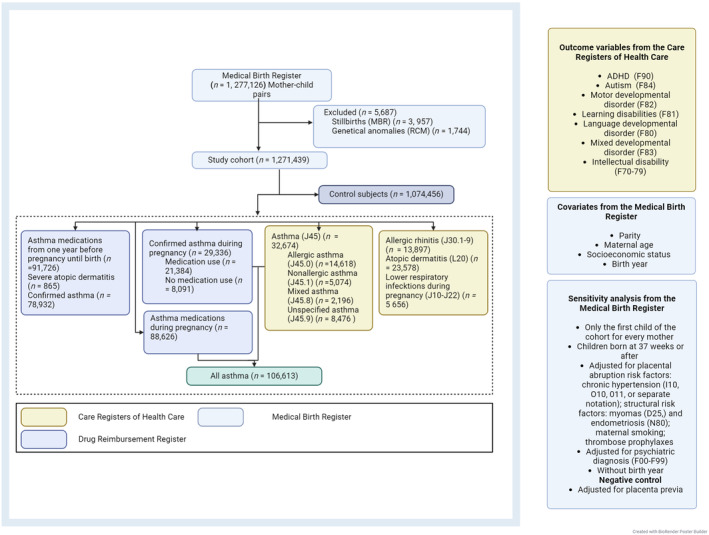
Participant flow of the population‐based sample. It is possible to set asthma diagnosis (J45) without subcategory (J45.0‐9). The numbers in all asthma groups do not balance out, because a mother with asthma can belong to multiple asthma groups. DRR, Drug Reimbursement Register; MBR, Medical Birth Register; CRC, Care Register for Health Care.
To access all needed information for the mothers and offspring, we combined information from four high‐cover national registers (Figure 1): the Medical Birth Register (MBR), the Drug Reimbursement Register (DRR), the Care Register for Health Care (CRC), and the Register of Congenital Malformations. In Finland, reliable register data linkage is possible through permanent personal identity coding.
The Medical Birth Register holds information on all live births and stillbirths (≥22 weeks of pregnancy or birthweight ≥500 g). The DRR contains individual data on medication purchases and data on entitlement for discounted medication for several diseases including asthma. The CRC includes inpatient care since 1969, hospital outpatient care since 1998, and primary care since 2011. International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD‐10) has been in use since 1996.
2.2. Exposure and outcomes
The CRC provided mothers' diagnoses, including asthma, asthma type, and related diseases (Figure 1). Diagnoses of asthma, atopy, and allergies covered the period from 5 years before the pregnancy until the end of the pregnancy while diagnoses of lower respiratory infections included only those during the pregnancy. Diagnoses end up in the CRC when set by a physician during clinical visits. In principle, a person with asthma is bound to visit a doctor at least every 2 years to renew asthma medication prescriptions. Allergic status evaluation is a recommended step in asthma diagnosis to determine the type of asthma. A confirmed diagnosis of asthma in the DRR requires that the diagnosis is based on documented reversibility of small airway obstruction in pulmonary function testing (PFT), together with a controlled 6‐month period of regular medication purchases (Figure 1, Tables S1–S3).6, 7, 16 In this study, purchased asthma medications were identifiable through the DRR. The use of asthma medications during pregnancy was defined as asthma medication purchases during the period of 3 months prior to pregnancy until the end of pregnancy. We used the 3‐month period prior to pregnancy as pharmacies in Finland are entitled to supply medications for up to 3 months at one time.
We included mothers to the maternal asthma group if they had an asthma diagnosis in the CRC or DRR registers, or if they had asthma medication purchases during pregnancy. We defined the control mother–child group as a mother with no diagnosis of asthma, no lower respiratory tract infections during pregnancy, no allergic rhinitis, no atopic dermatitis, or those not currently using any asthma medication (Figure 1, Tables S1–S3). We analyzed the asthma medications taken by therapeutic groups and by groups representing standard clinical asthma‐care entities (Figure 1, Tables S1–S3), that is, mothers using: only short‐acting β2 sympathomimetics; only inhaled corticosteroid; only short‐acting β2 sympathomimetics and inhaled corticosteroids; and monotherapy of the combination product of budesonide and formoterol.7, 9, 28
To evaluate allergic inflammation, the definition of allergic tendency was a combination of an ICD‐10 diagnosis of allergic spectrum diseases: allergic asthma (J45.0), allergic rhinitis (J30.1‐J30.9), and atopic dermatitis (L20). Lower respiratory tract infection diagnosis was acquired from the CRC (J10‐22). To study the effects of allergic or neutrophilic inflammation, we created two additional groups: mothers without asthma but with allergic tendency; and mothers without asthma but with respiratory infections during pregnancy.
To evaluate a possible role of placental dysfunction, we performed an analysis linking offsprings' neurodevelopmental disorder with maternal smoking and other maternal risk factors for placental abruption. Placental risk factors were divided into structural risk factors, including endometriosis, and leiomyomas (D25, N80) from the MBR; chronic hypertension (I10, O10, 011, or MBR code for hypertension before pregnancy), and the effect of smoking during pregnancy on the main outcomes. In this analysis, we further studied the effect of maternal thrombotic tendency, adjusting for the thrombose prophylaxis use, as registered since 2004 in the MBR. The use of thrombosis prophylaxis is based on updating international guidelines. 29
In the analysis of offspring's neurodevelopmental disorders, we included ADHD (ICD‐10 code F90), autism‐ (F84), learning disabilities (F81), motor‐developmental disorder (F82), language‐developmental disorder (F80), mixed developmental disorder (F83), and intellectual disability (F70‐79). These diagnostic entities constitute a standardized set of features of different neurodevelopment. 21
The CRC has high validity for neurodevelopmental screening and diagnosis,30, 31 where the diagnostic criteria meet international standards. The offspring are followed up during monthly visits in infanthood and later yearly visits in preventive primary care, in the high‐cover child welfare clinics, or in school health care by a nurse or a family doctor. If problems emerge, further evaluation by specialists and multidisciplinary teams is advised. The diagnoses were collected during this process. (Figure S1).32, 33 Neurodevelopmental features are considered to be present at birth, but functional disability becomes evident as the child grows and develops. 21 The diagnoses affecting speech and movement are typically made before or during preschool, and those affecting writing or nonverbal learning are usually during the school years.34, 35 Those with milder functional disability tend to be diagnosed later. For instance, ADHD or autism can be diagnosed as late as adulthood. The diagnoses are made only if the functional disability is at the stage of a disorder.32, 33, 34 The functional disability related to a diagnosis may entitle the patient to specific therapies, medication, or social support. However, the support from the community that commences prior to any diagnosis, may sometimes be sufficient and a child might thrive and require no diagnosis.
2.3. Statistical analyses
We analyzed the risk associated with asthma as the primary analysis. Adjusted hazard ratios (HR) and 95% confidence intervals (CIs) were calculated with unconditional Cox regression analysis. The use of HR was chosen based on statistician consultation as the follow‐up was incomplete within the given timeframe. The study setup also met the proportional hazard assumption.
We used parity, maternal age, year of child's birth, and socioeconomic status during pregnancy as confounding factors. We adjusted the risk by birth year as the prevalence of neurodevelopmental disorders increased during the study period, most likely due to the more sensitive and more extensive diagnostics.36, 37 Maternal age was selected due to the increase in pregnancy complications among older mothers. Parity was included since prior pregnancies and siblings may influence pregnancy outcome and offspring's development. 38 We did not adjust for risk factors possibly on a causal pathway from asthma to neurodevelopmental disorders. All statistical analyses used SAS version 9.4 (SAS Institute Inc).
The individuals lacking data on parity were excluded from the analysis. This included 0.1% of children (Table 1). European Union legislation (General Data Protection Regulation) does not allow the registration of race or ethnicity. This study was approved by the Helsinki University Hospital ethical committee (Reference: HUS/1828/2019) and Finnish Social and Health Data Permit Authority Findata. As the women were not contacted, patient consent was not required according to Finnish legislation.
TABLE 1.
Number and proportion of offspring diagnosed with neurodevelopmental disorders along with additional demographics by exposure groups.
| Entire cohort | Mothers with asthma | Control subjects | |
|---|---|---|---|
| Number of participants | 1 271 439 | 106 163 | 1 074 456 |
| Mothers | |||
| Age at delivery, mean (SD) | 29.6 (5.4) | 30.1 (5.2) | 29.6 (5.4) |
| Parity | |||
| 0 | 403 987 (31.8) | 32 402 (30.5) | 342 498 (31.9) |
| 1 | 380 109 (29.9) | 30 291 (28.5) | 323 353 (30.1) |
| 2 | 231 350 (18.2) | 19 647 (18.5) | 195 061 (18.2) |
| 3 or more | 254 931 (20.1) | 23 749 (22.4) | 212 619 (19.8) |
| Missing | 1062 (0.1) | 74 (0.1) | 925 (0.1) |
| Diabetes | 4705 (0.4) | 450 (0.4) | 3898 (0.4) |
| Epilepsy | 6682 (0.5) | 559 (0.5) | 5705 (0.5) |
| Hypertension | 39 125 (3.1) | 3901 (3.7) | 32 140 (3.0) |
| Smoking during pregnancy | |||
| Yes | 186 144 (14.6) | 18 380 (17.3) | 152 958 (14.2) |
| No | 1 049 543 (82.6) | 84 805 (79.9) | 891 183 (82.9) |
| Unknown | 35 752 (2.8) | 2978 (2.8) | 30 315 (2.8) |
| Socioeconomic status | |||
| Upper white‐collar | 196 627 (15.5) | 15 650 (14.7) | 168 420 (15.7) |
| Lower white‐collar | 432 305 (34.0) | 37 148 (35.0) | 362 686 (33.8) |
| Blue collar | 172 538 (13.6) | 12 592 (11.9) | 147 887 (13.8) |
| Other or unknown | 469 969 (37.0) | 40 773 (38.4) | 395 463 (36.8) |
| Structural placental abruption risk factors | 1150 (0.1) | 129 (0.1) | 939 (0.1) |
| Maternal psychiatric disease | 2959 (0.2) | 488 (0.5) | 2183 (0.2) |
|
Thrombo‐prophylaxis (2004 onwards) |
9743 (1.2) | 1489 (1.9) | 7398 (1.1) |
| Offspring | |||
| Pregnancy duration | |||
| Preterm (<37 weeks) | 55 511 (4.4) | 5570 (5.3) | 44 660 (4.2) |
| Full term (37 weeks or more) | 1 212 067 (95.3) | 100 337 (94.5) | 1 025 417 (95.4) |
| Missing | 3861 (0.3) | 256 (0.2) | 3379 (0.3) |
| Growth retardation 54 | 37 917 (3.0) | 3669 (3.5) | 31 529 (2.9) |
| Apgar score at 1 min | |||
| 0–3 | 16 578 (1.3) | 1584 (1.5) | 13 715 (1.3) |
| 4–6 | 47 298 (3.7) | 4471 (4.2) | 39 260 (3.7) |
| 7–10 | 1 204 067 (94.7) | 99 849 (94.1) | 1 018 479 (94.8) |
| Missing | 3496 (0.3) | 259 (0.2) | 3002 (0.3) |
| Neurodevelopmental disorders | |||
| ADHD | 39 400 (3.1) | 4114 (3.9) | 32 122 (3.0) |
| Autism | 16 595 (1.3) | 1617 (1.5) | 13 701 (1.3) |
| Motor development disorder | 14 870 (1.2) | 1569 (1.5) | 12 147 (1.1) |
| Learning disabilities | 8009 (0.6) | 849 (0.8) | 6534 (0.6) |
| Language‐developmental disorder | 33 945 (2.7) | 3057 (2.9) | 28 421 (2.7) |
| Mixed developmental disorder | 17 374 (1.4) | 1633 (1.5) | 14 434 (1.3) |
| Intellectual disability | 10 755 (0.9) | 908 (0.9) | 9155 (0.9) |
Note: Values are in numbers (percentage) unless otherwise stated.
2.4. Sensitivity analyses
As a sensitivity analysis, we analyzed asthma in the ways we gathered the information, asthma with or without medication, asthma phenotypes, and asthma medication groups. We further repeated the analysis for asthma groups: first, selecting only the first liveborn child in the cohort for every mother; second, including only term births at 37 gestational weeks or later; third, we performed an analysis adjusting for maternal psychiatric diagnosis (ICD‐10 code F00‐F99); fourth was an analysis without adjustment for birth year; and fifth we also performed these analyses for boys and girls separately. We studied the effects of placenta previa as a negative control that would unlikely be associated with neurodevelopment.
3. RESULTS
3.1. Main findings
The study cohort included 1 271 439 singleton live births (Figure 1, Table 1). Of these, 106 163 mothers had asthma, and 88 626 mothers were using asthma medication (Figure 1, Table 1).
Maternal asthma was associated with an increased likelihood of offspring ADHD (HR: 1.49; 95% CI 1.44–1.54), autism (HR: 1.33; 95% CI 1.26–1.40), motor‐developmental disorder (HR: 1.37; 95% CI 1.30–1.45), and learning disability (HR: 1.51; 95% CI 1.41–1.62). Somewhat increased likelihoods were also evident for language‐development disorder (HR: 1.13; 95% CI 1.08–1.17), mixed developmental disorder (HR: 1.20; 95% CI 1.14–1.26), and intellectual disability (HR: 1.12; 95% CI 1.04–1.20) (Figures 2 and 3, Tables S4–S6). Similar risk profiles were noted in relation to both maternal allergic and non‐allergic asthma (Figure 2).
FIGURE 2.
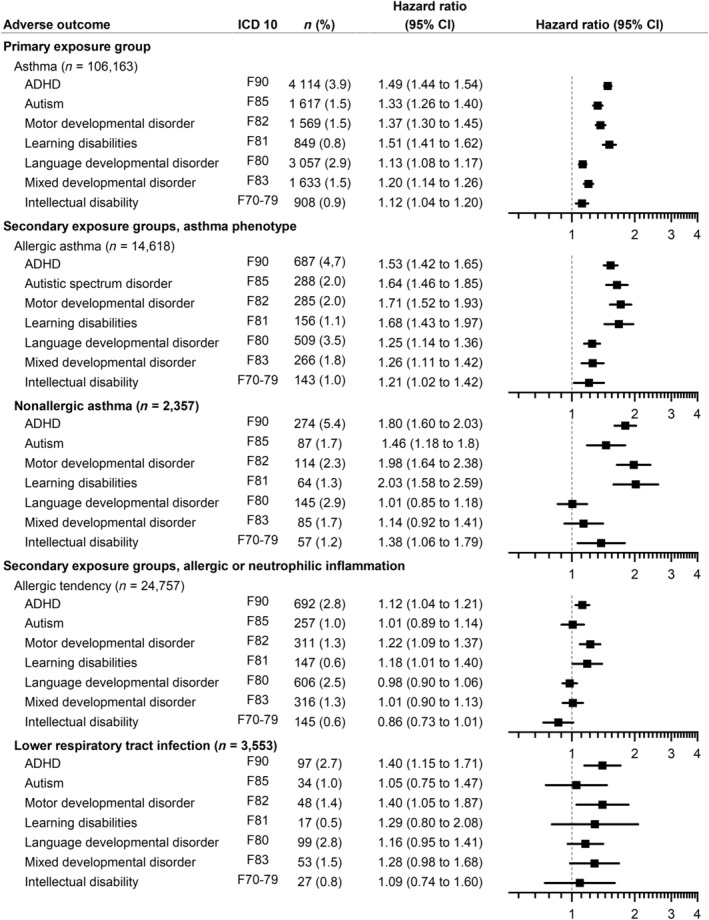
Associations between maternal asthma, atopy, lower respiratory tract infections during pregnancy, and neurodevelopmental disorders in offsrping compared to control subjects adjusted for maternal age, parity, year of birth, and socioeconomic status. Number (n) in the group (percentage). In the allergic tendency and lower respiratory infection group, we excluded mothers with asthma. HR, hazard ratio; CI, confidence interval.
FIGURE 3.
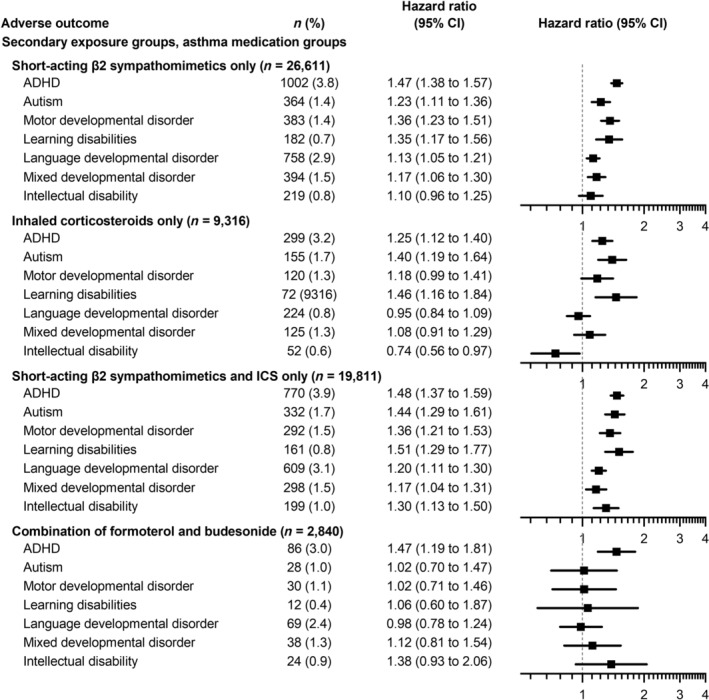
Associations between maternal asthma medication and neurodevelopmental disorders in offspring compared to control subjects adjusted for maternal age, parity, year of birth, and socioeconomic status. Number (n) in the group (percentage). Mothers in the formoterol‐budesonide group used no other inhaled corticosteroids, β2 inhaled sympathomimetics, or their other related combination products. HR, hazard ratio CI, confidence interval.
Atopic and allergic tendencies without maternal asthma showed barely any association with neurodevelopmental disorders. Maternal lower respiratory tract infections without asthma showed some increase in the likelihood of offspring's ADHD and motor‐developmental disorder (Figure 2). There were no differences by sex in the analysis (Table S6).
In the subgroup of 2840 mothers using combined formoterol and budesonide as a monotherapy, the offspring presented with only an increased likelihood of ADHD but no other neurodevelopmental disorders (Figure 3).
When searching for possible linking mechanisms between maternal asthma and neurodevelopmental disorders, placental abruption risk factors and smoking were associated with an increased likelihood of ADHD, motor‐developmental, language‐developmental disorder, and intellectual disability (Figure S2, Table S8). These neurodevelopmental disorders were also noted in association with thrombotic tendencies. Maternal psychiatric diseases showed an increased likelihood of all neurodevelopmental disorders, but adjustment had no effect on asthma‐related associations.
The analyses gave similar results for the main exposure groups when taking only the first‐born child of the mother into account (Table S7) and the first‐born children born at 37 weeks of gestation or later. The negative control, placenta previa, showed no significant influence on any of the parameters (Table S9).
4. DISCUSSION
This large, population‐based, retrospective cohort study shows that maternal asthma holds clear but weak associations with a variety of offspring's neurodevelopmental disorders, including ADHD, autism, motor‐developmental disorder, and learning disabilities. Some increase was also evident for the likelihood of language‐developmental disorder, mixed developmental disorder, and intellectual disability. These increased likelihoods were related to both allergic and non‐allergic asthma. An atopic and allergic tendency without asthma was not associated with similar likelihoods; neither were lower respiratory tract infections without asthma. The associations with asthma are thus unlikely to be related to allergic inflammation or any other types of inflammation cascade. Of the maternal asthma medications, only formoterol and budesonide as combination monotherapy seemed to have no association with the likelihood of offspring's neurodevelopmental disorders seen with asthma. Only the likelihood of ADHD remained.
An attractive first‐line theory would include relationships between asthma, allergic inflammation, and their resultant effects on pregnancy outcome, however, our study results do not support this assumption. We observed no significant difference between allergic and non‐allergic asthma. Furthermore, an atopic and allergic tendency without asthma (ICD‐10 codes J30.1‐J30.9, L20, but no asthma by any definition) showed no link to an increased likelihood of offspring's neurodevelopmental disorders. Similarly, we observed no increased likelihood of neurodevelopmental disorders associated with maternal lower respiratory tract infections. Thus, neither eosinophilic nor short‐term neutrophilic inflammatory exposure of a mother seems to alter the offspring's neurodevelopment.
Asthma medication purchases by mothers, implying a likely use of asthma medication, showed no different association with the offspring's neurodevelopmental disorders than maternal asthma by itself. However, combined treatment with formoterol and budesonide as a monotherapy was the exception. The offspring of mothers taking this combination lacked an increased likelihood of neurodevelopmental disorders, except for ADHD. This smaller likelihood was not observable for all the combination medications, including mothers using budesonide and formoterol with an additional short‐lasting bronchodilator. The reason for the difference is not obvious. It is possible that the mothers using formoterol and budesonide combination as monotherapy (the Smart treatment protocol) had higher compliance with asthma therapy than with other types of asthma management.39, 40 Because the inhaled corticosteroid by itself did not change the likelihood associated with asthma, and given the tocolytic effect of formoterol, it is also possible that formoterol was the effective remedy (Figures S3 and S4).41, 42, 43 Formoterol is rarely used by itself without a combined inhaled steroid for asthma.6, 7, 8, 9 The regular use of this combination product in a monotherapy makes the formoterol exposure both long‐lasting and stable. We present a theory that this effect is mediated by the direct action of formoterol on the uterus (Figures S3 and S4). 19
Maternal asthma is associated with risk for a wide variety of pregnancy complications: pre‐eclampsia, gestational diabetes, placental abruption, placenta previa, preterm birth, breech presentation, hemorrhage, pulmonary embolism, and maternal intensive care unit admission. 44 Conversely, asthma shows an inverse correlation with spontaneous labor and vaginal delivery. 45 Placental insufficiency due to an ischemic placenta has an inherent risk profile similar to that of maternal asthma and causes an increased risk for pre‐eclampsia, placental abruption, perinatal mortality, preterm birth, and neurodevelopmental disorders.46, 47, 48, 49 Similar findings also exist for thrombophilia and maternal hypertension.29, 50, 51 Interestingly in the current study, placental abruption risk factors, thrombosis risk, and asthma each had a similar risk profile related to the offspring's neurodevelopmental outcomes (Figure S2).
Our results are in accordance with earlier research related to maternal asthma and pregnancy outcomes which reported associations with offspring neurodevelopmental disorders.24, 25, 26 In the future, an intervention study or study at birth would possibly provide more information as to whether the medications or mother's asthma treatment balance is beneficial for the neurodevelopmental health of offspring.
Because maternal asthma has been associated with several adverse perinatal outcomes, we made no adjustment for perinatal factors.,12, 16, 52 which we considered to be potentially on causal pathway for impaired neurodevelopment. Maternal psychiatric diseases failed to impact the results.
Family socioeconomic status served as an adjusted cofactor. It may correlate with both neurodevelopmental disorders and smoking. Because smoking correlates with socioeconomic status, it was not a primary choice as a covariate. The adjustment for smoking had no significant effect on the results (Figure S2). Smoking, like socioeconomic status, seems to serve as a risk factor for adverse neurodevelopment by itself (Figure S2, Table S4).
Since the number and proportion of children diagnosed with neurodevelopmental disorders has grown globally, we made an adjustment for birth year.36, 37 Our analysis without birth year showed the likelihood of neurodevelopmental disorders to be greater than without adjustment. This disparity may be explained by the parallel increase in the prevalence of asthma and neurodevelopmental disorder diagnoses. Our analyses of the first‐born child of any mother of our cohort, and children born at 37 weeks or later did not alter these results (Table S7). In conclusion, our sensitivity analyses support our main results.
The strengths of our study include, first, that it was based on a population of over a million mother–child pairs, enabling sensitivity analysis and subgrouping. Second, the cohort from the Finnish national registers comprised all live‐born children with complete follow‐up over the study period, ensuring minimal recall and selection biases. Third, the validity of the diagnosis was high, recorded by physicians in the Care Register for Health Care and the Medical Birth Register, and especially in the drug reimbursement documents requiring objective test results for drug reimbursement.
This study might have several limitations, including, first, the fact that we were unable to adjust for any heritable family diseases increasing the likelihood of neurodevelopmental disorders. Second, the follow‐up of these children was incomplete, because some neurodevelopmental disorders may be diagnosed as late as in adulthood. Third, we had no documentation of the severity of maternal asthma symptoms and the treatment balance by PFTs or asthma severity questionnaires. Because Finland's level of asthma control is considered high,16, 53 it is unlikely that the associations with neurodevelopmental disorders and maternal asthma would be secondary to dyspnea‐ or ventilation‐related factors. Fourth, although we assume that each asthma diagnosis was confirmed highly reliably, some inaccuracy may be evident for asthma phenotyping via ICD‐10 codes (J45.0, J45.1, J45.8, and J45.9). Findings involving phenotyping should therefore be interpreted with caution. Our findings are, however, supported by their comparison to allergic tendency and respiratory infection, each analyzed alone without asthma. Fifth, we do not know why 16% of the mothers with asthma diagnoses failed to use asthma medication during pregnancy.
We performed multiple comparisons, thus some results may be coincidental, even though we had complete data on all children born during the study period in Finland. The study was hypothesis‐driven. The results are similar in different sub‐ and sensitivity groups, for example, when defining atopic inflammation or asthma in different ways. The main analysis results stayed significant after Bonferroni correction and the p‐values in the main analysis were <0.001.
5. CONCLUSION
Maternal asthma was associated with the likelihood of a wide variety of offspring neurodevelopmental disorders in this large population‐based mother–child cohort study. The specific type of inflammation associated with the mothers' asthma seemed, however, to make no difference in the associations with the offspring's neurodevelopment. Maternal atopic or allergic tendency or any respiratory tract infection showed no similar associations. The associations with maternal asthma are therefore unlikely to be related to general maternal inflammation processes.
AUTHOR CONTRIBUTIONS
The conception and planning were performed by all the authors. The work was carried out and analyzed mostly by Mari Kemppainen, with guidance from Mika Gissler and medical knowledge from Turkka Kirjavainen. Writing was performed by all the authors.
FUNDING INFORMATION
This study was supported by grants from the Foundation for Pediatric Research in Finland as a group grant to TK (#160268, #190140, #200184). MK received funds from the State research fund and Tampere Tuberkuloosisäätiö foundation. The State research fund is granted by the Finnish state based on both scientific evaluation and evaluation of health benefits. The Foundation for Pediatric Research and Tampere Tuberkuloosisäätiö foundation are independent charity organizations committed to pediatric and pulmonary health improvement, respectively. MG was funded by FLUX Consortium ‘Family Formation in Flux—Causes, Consequences and Possible Futures, by the Strategic Research Council, Research Council of Finland (DEMOGRAPHY 345130).
CONFLICT OF INTEREST STATEMENT
The authors have no competing interests to declare.
ETHICS STATEMENT
This study was approved by the Helsinki University Hospital ethical committee (Reference: HUS/1828/2019) on September 13, 2019 and Finnish Social and Health Data Permit Authority Findata.
Supporting information
Data S1. Supporting Information.
ACKNOWLEDGMENTS
We thank Dr. Paula Kauppi for her assistance in data interpretation.
Kemppainen M, Gissler M, Kirjavainen T. Maternal asthma during pregnancy and the likelihood of neurodevelopmental disorders in offspring. Acta Obstet Gynecol Scand. 2025;104:235‐244. doi: 10.1111/aogs.15008
DATA AVAILABILITY STATEMENT
The register data used in this study have been given for this specific study, and the data cannot be shared. Similar data can be acquired from the Finnish Social and Health Data Permit Authority Findata (https://findata.fi/en/), as regulated by Finnish legislation.
REFERENCES
- 1. Kwon HL, Triche EW, Belanger K, Bracken MB. The epidemiology of asthma during pregnancy: prevalence, diagnosis, and symptoms. Immunol Allergy Clin N Am. 2006;26:29‐62. [DOI] [PubMed] [Google Scholar]
- 2. Kauppi P, Linna M, Martikainen J, Makela MJ, Haahtela T. Follow‐up of the Finnish asthma Programme 2000‐2010: reduction of hospital burden needs risk group rethinking. Thorax. 2013;68:292‐293. [DOI] [PubMed] [Google Scholar]
- 3. Kainu A, Pallasaho P, Piirila P, Lindqvist A, Sovijarvi A, Pietinalho A. Increase in prevalence of physician‐diagnosed asthma in Helsinki during the Finnish asthma Programme: improved recognition of asthma in primary care? A cross‐sectional cohort study. Prim Care Respir J. 2013;22:64‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Holgate ST, Wenzel S, Postma DS, Weiss ST, Renz H, Sly PD. Asthma. Asthma Nat Rev Dis Primers. 2015;1:15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lourenço LO, Ribeiro AM, Lopes F, Tibério I, Tavares‐de‐Lima W, Prado CM. Different phenotypes in asthma: clinical findings and experimental animal models. Clin Rev Allergy Immunol. 2022;62:240‐263. [DOI] [PubMed] [Google Scholar]
- 6. Duodecim WgsubtFMSDatFCSHTFMS . Asthma: Current Care Guidelines 2022 (Previous Version 2012). 2022.
- 7. Mauer Y, Taliercio RM. Managing adult asthma: the 2019 GINA guidelines. Cleve Clin J Med. 2020;87:569‐575. [DOI] [PubMed] [Google Scholar]
- 8. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343‐373. [DOI] [PubMed] [Google Scholar]
- 9. Reddel HK, Bacharier LB, Bateman ED, et al. Global initiative for asthma strategy 2021: executive summary and rationale for key changes. Arch Bronconeumol. 2022;58:35‐51. [DOI] [PubMed] [Google Scholar]
- 10. Kallen B, Otterblad OP. Use of anti‐asthmatic drugs during pregnancy. 2. Infant characteristics excluding congenital malformations. Eur J Clin Pharmacol. 2007;63:375‐381. [DOI] [PubMed] [Google Scholar]
- 11. Kallen B, Otterblad OP. Use of anti‐asthmatic drugs during pregnancy. 1. Maternal characteristics, pregnancy and delivery complications. Eur J Clin Pharmacol. 2007;63:363‐373. [DOI] [PubMed] [Google Scholar]
- 12. Rejno G, Lundholm C, Gong T, Larsson K, Saltvedt S, Almqvist C. Asthma during pregnancy in a population‐based study—pregnancy complications and adverse perinatal outcomes. PLoS One. 2014;9:e104755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rejno G, Lundholm C, Larsson K, et al. Adverse pregnancy outcomes in asthmatic women: a population‐based family design study. J Allergy Clin Immunol Pract. 2018;6:916‐922. e6. [DOI] [PubMed] [Google Scholar]
- 14. Murphy VE, Namazy JA, Powell H, et al. A meta‐analysis of adverse perinatal outcomes in women with asthma. BJOG. 2011;118:1314‐1323. [DOI] [PubMed] [Google Scholar]
- 15. Wang G, Murphy VE, Namazy J, et al. The risk of maternal and placental complications in pregnant women with asthma: a systematic review and meta‐analysis. J Matern Fetal Neonatal Med. 2014;27:934‐942. [DOI] [PubMed] [Google Scholar]
- 16. Kemppainen M, Lahesmaa‐Korpinen AM, Kauppi P, et al. Maternal asthma is associated with increased risk of perinatal mortality. PLoS One. 2018;13:e0197593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stenius‐Aarniala B, Piirila P, Teramo K. Asthma and pregnancy: a prospective study of 198 pregnancies. Thorax. 1988;43:12‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Powell H, Murphy VE, Taylor DR, et al. Management of asthma in pregnancy guided by measurement of fraction of exhaled nitric oxide: a double‐blind, randomised controlled trial. Lancet. 2011;378:983‐990. [DOI] [PubMed] [Google Scholar]
- 19. Murphy VE, Gibson PG, Smith R, Clifton VL. Asthma during pregnancy: mechanisms and treatment implications. Eur Respir J. 2005;25:731‐750. [DOI] [PubMed] [Google Scholar]
- 20. Gunawardhana LP, Baines KJ, Mattes J, Murphy VE, Simpson JL, Gibson PG. Differential DNA methylation profiles of infants exposed to maternal asthma during pregnancy. Pediatr Pulmonol. 2014;49:852‐862. [DOI] [PubMed] [Google Scholar]
- 21. Thapar A, Cooper M, Rutter M. Neurodevelopmental disorders. Lancet. Psychiatry. 2017;4:339‐346. [DOI] [PubMed] [Google Scholar]
- 22. Ismail FY, Shapiro BK. What are neurodevelopmental disorders? Curr Opin Neurol. 2019;32:611‐616. [DOI] [PubMed] [Google Scholar]
- 23. Maher GM, O'Keeffe GW, Kearney PM, et al. Association of Hypertensive Disorders of pregnancy with risk of neurodevelopmental disorders in offspring: a systematic review and meta‐analysis. JAMA Psychiatry. 2018;75:809‐819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gong T, Lundholm C, Rejno G, et al. Parental asthma and risk of autism spectrum disorder in offspring: a population and family‐based case‐control study. Clin Exp Allergy. 2019;49:883‐891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Han VX, Patel S, Jones HF, et al. Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: a systematic review. Transl Psychiatry. 2021;11:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Langridge AT, Glasson EJ, Nassar N, et al. Maternal conditions and perinatal characteristics associated with autism spectrum disorder and intellectual disability. PLoS One. 2013;8:e50963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Whalen OM, Karayanidis F, Murphy VE, Lane AE, Mallise CA, Campbell LE. The effects of maternal asthma during pregnancy on child cognitive and behavioral development: a systematic review. J Asthma. 2019;56:130‐141. [DOI] [PubMed] [Google Scholar]
- 28. Edwards SJ, Gruffydd‐Jones K, Ryan DP. Systematic review and meta‐analysis of budesonide/formoterol in a single inhaler. Curr Med Res Opin. 2007;23:1809‐1820. [DOI] [PubMed] [Google Scholar]
- 29. Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO. VTE, thrombophilia, antithrombotic therapy, and pregnancy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141:e691S‐e736S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sund R. Quality of the Finnish hospital discharge register: a systematic review. Scand J Public Health. 2012;40:505‐515. [DOI] [PubMed] [Google Scholar]
- 31. Lampi KM, Sourander A, Gissler M, et al. Brief report: validity of Finnish registry‐based diagnoses of autism with the ADI‐R. Acta Paediatr. 2010;99:1425‐1428. [DOI] [PubMed] [Google Scholar]
- 32. Working group set up by the Finnish Medical Society Duodecim and the Finnish Foniatric Society FPNSHTFMSD , 2019. (referred Janury 24, 2019). Available online at: www.kaypahoito.fi. Language developmental disorder (children and adolescent). Current Care Guidelines. 2019.
- 33. Working Group Set up by the Finnish Medical Society Duodecim and the Finnish Child Psychiatric Society FAPs, and Finnish Pediatric Neurologic Society. The Finnish Medical Society Duodecim; 2019. (referred April 4th, 2019). Available online at: www.kaypahoito. Hyperkinetic disorder. Current Care Guidelines. 2019 [Google Scholar]
- 34. Working group set up by Finnish Child Psychiatric Society YPSaK . Autism Current Guidelines. 2024.
- 35. Falkmer T, Anderson K, Falkmer M, Horlin C. Diagnostic procedures in autism spectrum disorders: a systematic literature review. Eur Child Adolesc Psychiatry. 2013;22:329‐340. [DOI] [PubMed] [Google Scholar]
- 36. Graf WD, Miller G, Epstein LG, Rapin I. The autism "epidemic": ethical, legal, and social issues in a developmental spectrum disorder. Neurology. 2017;88:1371‐1380. [DOI] [PubMed] [Google Scholar]
- 37. Sayal K, Prasad V, Daley D, Ford T, Coghill D. ADHD in children and young people: prevalence, care pathways, and service provision. Lancet Psychiatry. 2018;5:175‐186. [DOI] [PubMed] [Google Scholar]
- 38. Stormshak EA, Bullock BM, Falkenstein CA. Harnessing the power of sibling relationships as a tool for optimizing social‐emotional development. New Dir Child Adolesc Dev. 2009;2009:61‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Crossingham I, Turner S, Ramakrishnan S, et al. Combination fixed‐dose beta agonist and steroid inhaler as required for adults or children with mild asthma. Cochrane Database Syst Rev. 2021;5:CD013518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lalloo UG, Malolepszy J, Kozma D, et al. Budesonide and formoterol in a single inhaler improves asthma control compared with increasing the dose of corticosteroid in adults with mild‐to‐moderate asthma. Chest. 2003;123:1480‐1487. [DOI] [PubMed] [Google Scholar]
- 41. Shinkai N, Takayama S. Tocolytic effects of a long‐acting beta2‐adrenoceptor agonist, formoterol, in rats. J Pharm Pharmacol. 2000;52:1417‐1423. [DOI] [PubMed] [Google Scholar]
- 42. Shinkai N, Takasuna K, Takayama S. Tocolytic activity of formoterol against premature delivery in mice. J Pharm Pharmacol. 2002;54:1637‐1643. [DOI] [PubMed] [Google Scholar]
- 43. Yurtcu N, Cetin A, Karadas B, et al. Comparison of effects of formoterol and BRL 37344 on isolated term‐pregnant rat myometrial strips in vitro. Eur J Pharmacol. 2006;530:263‐269. [DOI] [PubMed] [Google Scholar]
- 44. Mendola P, Mannisto TI, Leishear K, Reddy UM, Chen Z, Laughon SK. Neonatal health of infants born to mothers with asthma. J Allergy Clin Immunol. 2014;133(85–90):e1‐e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mendola P, Laughon SK, Mannisto TI, et al. Obstetric complications among US women with asthma. Am J Obstet Gynecol. 2013;208(127):e1‐e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ananth CV, Vintzileos AM. Ischemic placental disease: epidemiology and risk factors. Eur J Obstet Gynecol Reprod Biol. 2011;159:77‐82. [DOI] [PubMed] [Google Scholar]
- 47. Ananth CV, Friedman AM. Ischemic placental disease and risks of perinatal mortality and morbidity and neurodevelopmental outcomes. Semin Perinatol. 2014;38:151‐158. [DOI] [PubMed] [Google Scholar]
- 48. Nijman TA, van Vliet EO, Benders MJ, et al. Placental histology in spontaneous and indicated preterm birth: a case control study. Placenta. 2016;48:56‐62. [DOI] [PubMed] [Google Scholar]
- 49. Wright E, Audette MC, Ye XY, et al. Maternal vascular Malperfusion and adverse perinatal outcomes in low‐risk nulliparous women. Obstet Gynecol. 2017;130:1112‐1120. [DOI] [PubMed] [Google Scholar]
- 50. Sinkey RG, Battarbee AN, Bello NA, Ives CW, Oparil S, Tita ATN. Prevention, diagnosis, and Management of Hypertensive Disorders of pregnancy: a comparison of international guidelines. Curr Hypertens Rep. 2020;22:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jenabi E, Salimi Z, Ayubi E, Bashirian S, Salehi AM. The environmental risk factors prior to conception associated with placental abruption: an umbrella review. Syst Rev. 2022;11:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jensen ME, Barrett HL, Peek MJ, Gibson PG, Murphy VE. Maternal asthma and gestational diabetes mellitus: exploration of potential associations. Obstet Med. 2021;14:12‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kauppi P, Peura S, Salimaki J, Jarvenpaa S, Linna M, Haahtela T. Reduced severity and improved control of self‐reported asthma in Finland during 2001‐2010. Asia Pac Allergy. 2015;5:32‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sankilampi U, Hannila ML, Saari A, Gissler M, Dunkel L. New population‐based references for birth weight, length, and head circumference in singletons and twins from 23 to 43 gestation weeks. Ann Med. 2013;45:446‐454. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.
Data Availability Statement
The register data used in this study have been given for this specific study, and the data cannot be shared. Similar data can be acquired from the Finnish Social and Health Data Permit Authority Findata (https://findata.fi/en/), as regulated by Finnish legislation.


