Abstract
Background
Recurrent miscarriage (RM) is influenced by immune factors, particularly regulatory T cells, which can impact immune function and miscarriage risk. Vitamin D (VD) is known to regulate the immune system, potentially improving pregnancy outcomes in RM patients. This study aims to assess the effect of VD adjuvant therapy on regulatory T cells and pregnancy outcomes in RM patients.
Methods
Clinical data from 104 individuals with RM admitted to our hospital between March 2022 and February 2023 were allocated at random to either the VD group (VDG) or the control group (CG), with 52 patients in each group. Both groups received standard treatment; the CG was treated with aspirin, while the VDG received additional VD therapy. Outcomes measured included regulatory T cell proportion, metabolic factors, immune inflammatory markers, and pregnancy outcomes.
Results
After treatment, the proportion of regulatory T cells in VDG was considerably higher (p < 0.05). Additionally, triglyceride levels, leptin, fasting blood glucose, and fasting insulin were lower in the VDG, whereas adiponectin levels were higher (p < 0.05). Levels of progesterone, luteinizing hormone, and 25‐hydroxy VD were also higher in the VDG (p < 0.05). Furthermore, interleukin‐17, gamma interferon, tumor necrosis factor‐α, and C‐reactive protein were lower in the VDG (p < 0.05). The pregnancy success rate in the VDG was higher, and the preterm birth rate was lower (p < 0.05).
Conclusion
Adjuvant treatment with VD can increase the proportion of regulatory T cells in peripheral blood of individuals with recurrent abortion, regulate metabolic disorder, alleviate immune inflammation, and improve pregnancy outcome.
Keywords: adjuvant therapy, pregnancy outcome, recurrent miscarriage, regulatory T cells, vitamin D
INTRODUCTION
Recurrent miscarriage (RM) is a complex pathological condition that affects a considerable proportion of women of childbearing age globally. Reports indicate that the incidence of RM among women of childbearing age ranges from 1% to 5%, with the miscarriage rate for those with recurrent episodes reaching 70% to 80%. 1 , 2 , 3 The etiology of RM is multifaceted, involving factors such as genetics, immunity system dysfunction, endocrine abnormalities, infections, and anatomical structure, with immune factors playing an important role. Regulatory T cells re pivotal in the immune system, essential for maintaining maternal‐fetal immune tolerance and preventing autoimmune reactions. During a normal pregnancy, the proportion and function of regulatory T cells are well‐regulated to support fetal development. 4 In women with RM, however, disruptions in the proportion and function of these cells may be linked to the occurrence and progression of the condition. 5 Vitamin D (VD), a fat‐soluble steroid hormone, not only regulates calcium and phosphorus metabolism but also exhibits broad immunomodulatory effects. 6 Recent studies have highlighted the significant relationship between VD and immune function, particularly in the modulation of regulatory T cells. 7 , 8 This study hypothesizes that VD can promote the proliferation and functional activity of immune cells through its receptor, potentially improving pregnancy outcomes in patients with RM. This research seeks to investigate the impact of VD adjuvant treatment on the number of regulatory T cells in peripheral blood and pregnancy outcomes in individuals with RM, considering the possible function of VD in immunological regulation. The goal is to provide new insights and strategies for the clinical management of RM, thereby improving pregnancy success rates, reducing the risk of miscarriage, and enhancing reproductive health.
RESEARCH OBJECTS AND METHODS
Research objects
From March 2022 to February 2023, the clinical data of 104 individuals with RM at our hospital were split into two groups at random: a vitamin D group (VDG) and a control group (CG) with 52 cases in each group. There was no considerably difference in baseline data involving the two groupings (p > 0.05), as seen in Table 1. All individuals willingly joined in this research, gave written informed permission, and the research was approved by Shenzhen Longgang Central Hospital's Medical Ethics Committee (approval number SLC202203061), adhering to the ethical guidelines set forth in the Helsinki Declaration of the World Medical Association.
TABLE 1.
Comparison of baseline data involving two groupings (n = 52, ).
| Group | Age (years) | Number of miscarriages (times) | BMI (kg/m2) | Gestational weeks (weeks) |
|---|---|---|---|---|
| Vitamin D family | 31.03 ± 3.55 | 3.52 ± 0.40 | 22.78 ± 1.03 | 9.04 ± 1.03 |
| Control group | 30.99 ± 3.51 | 3.56 ± 0.43 | 22.75 ± 1.04 | 8.97 ± 1.01 |
| t | 0.057 | 0.491 | 0.147 | 0.349 |
| p | 0.954 | 0.624 | 0.882 | 0.727 |
Inclusion criteria:
Diagnosis of RM according to established criteria. 9
Singleton pregnancy.
Age between 22 and 35 years.
Regular menstrual cycles.
Adherence to prescribed medication.
Exclusion criteria:
Allergy to any medication used in this study.
History of anticoagulation, fibrinolytic, or contraceptive use within the past 3 months.
Abnormal semen analysis of the partner, chromosome abnormalities in either partner or hereditary familial diseases.
Ectopic pregnancy.
Congenital or acquired reproductive system abnormalities.
Presence of endocrine, infectious, or hematologic disorders.
Tubal obstruction and ovulation disorder.
METHODS
Both groups received standard treatment. The CG was administered aspirin tablets (Shandong Kangwei Pharmaceutical Co., Ltd., National Medicine Zhunzi H37021425, 0.1 g/tablet) orally, at a dosage of 0.1 g per tablet, once daily. In addition to the CG's treatment, the VDG received VD drops (Star Shark Pharmaceutical [Xiamen] Co., Ltd., controlled by Sinopharm, containing 400 IU of VD per drop, National Medicine Zhunzi H35021450), at a dosage of 800 IU per dose, once daily. Both treatments continued until the 12th week of pregnancy. The administration of treatments started immediately after the baseline visit and was continued until the 12th week of pregnancy.
Observation index
The proportion of regulatory T cells in peripheral blood, glucose and lipid metabolism index, endocrine metabolism index, immune inflammatory factor, and pregnancy outcome were observed in the two groups.
Peripheral blood regulatory T‐cell ratio determination
Before and after treatment, 3 mL of peripheral blood was gathered from every individual and placed in an EDTA anticoagulant tube. For flow cytometry analysis, 100 μL of anticoagulated whole blood was transferred to a flow tube. To this, Pacific Blue‐CD3 antibody, FITC‐CD4 antibody, PE CyTM7‐CD25 antibody, and APC‐CD127 antibody were added sequentially (BD Company, clones UCHT1, OKT4, M‐A251, eBioRDR5). We measured 10 000 live lymphocyte gate events per sample to ensure adequate data for robust analysis. The negative control consisted of the corresponding isotype control antibodies. The mixture was incubated in the dark for 30 min.
Following incubation, 2 mL of red blood cell lysate (BD Company, Model 349 204) was added, and after a 20‐min dark reaction, the supernatant was thrown out. The specimen was thereafter rinsed with 2 mL of phosphate‐buffered saline (PBS), subjected to centrifugation for 5 min, and the liquid above the sediment was removed. Subsequently, 500 μL of phosphate‐buffered saline was added, and the sample was analyzed using the CytoFLEX flow cytometer (Beckman Coulter International Trading [Shanghai] Co., Ltd.). Regulatory T cells were identified as CD4+, CD25+, and CD127 dim/−.
Determination of glucose and lipid metabolism indexes
Fasting elbow venous blood (5 mL) was gathered from individuals before and after treatment. The blood was centrifuged to obtain serum, which was then analyzed for triglyceride levels using an automatic biochemical analyzer (Mindray, CA‐431B). The levels of adiponectin, fasting insulin, and leptin were assessed using DPC Company enzyme‐linked immunosorbent assay (ELISA) kits. A blood glucose meter (Luokangquan Company, GM505K) was used to measure the blood glucose levels during the fast.
Determination of endocrine and metabolic indexes
The sampling time, dosage, and method for collecting blood samples were consistent with those used for glucose and lipid metabolism indexes. After centrifugation, serum was collected and analyzed for progesterone, luteinizing hormone, and 25‐hydroxy VD levels using ELISA. The detection of progesterone and luteinizing hormone, particularly during ovulation, was conducted in strict accordance with the instructions supplied by the kit from Shanghai JiNing Biological Kit Company.
Determination of immune inflammatory factors
The sampling time, dosage, and method were identical to those used for glucose and lipid metabolism indices. Following centrifugation, serum was collected, and interleukin‐17 (IL‐17), interferon‐gamma (IFN‐γ), tumor necrosis factor‐alpha (TNF‐α), and C‐reactive protein (CRP) levels were calculated using a double antibody sandwich ELISA. All measurements were carried out in strict accordance with the instructions provided by the kit from Shanghai JiNing Biological Kit Company.
Blood samples were taken at the initial screening visit prior to beginning supplement intake (pre‐treatment) and again at the 12‐week follow‐up (post‐treatment). Blood samples were taken once every 4 weeks post‐treatment, aligning with menstrual cycles to capture peak hormone levels effectively.
Pregnancy outcome
Follow‐up was conducted for 12 months, during which occurrences of premature rupture of membranes, premature detachment of membranes, neonatal survival, successful pregnancy, and preterm delivery were observed and recorded for both groups. 10
Figure 1 depicts the course of the study.
FIGURE 1.
Research flow chart.
Statistical methods
The data analysis was conducted using the SPSS 26.0 program, and graphical representations were processed with Prism 9.4.1. Variables including age, number of miscarriages, body mass index (BMI), gestational age, regulatory T‐cell ratio, glucose and lipid metabolism indices, endocrine metabolism indices, and immune inflammatory factors were normally distributed and are presented as means ± standard deviations (). Independent sample and paired sample t‐tests were used for comparisons between groups and within groups, respectively. Pregnancy outcomes was presented as frequencies and percentages (n [%]), and comparisons were conducted using the χ 2 test. A p‐value below 0.05 was deemed statistically significant.
RESULTS
Comparison of two sets of baseline data
There was no considerable difference in age, number of abortions, BMI, and gestational age involving the two groupings (p > 0.05), as shown in Table 1. See Tables S1 and S2, Supporting Information, for full details.
Comparison of the proportion of regulatory T cells between two groups
There was no considerable difference in the proportion of regulatory T cells involving the two groupings before treatment (p > 0.05). After treatment, the proportion of regulatory T cells in the two groups increased, and the VDG was larger than that in the CG (p < 0.05), as seen in Table 2 and Figure 2.
TABLE 2.
Comparison of the proportion of regulatory T cells involving two groupings (n = 52, %, ).
| Group | Before treatment | After treatment |
|---|---|---|
| Vitamin D family | 3.59 ± 0.47 | 5.57 ± 0.72* |
| Control group | 3.61 ± 0.49 | 4.64 ± 0.60* |
| t | 0.212 | 7.155 |
| p | 0.832 | <0.001 |
Compared with the group before treatment, p < 0.05.
FIGURE 2.
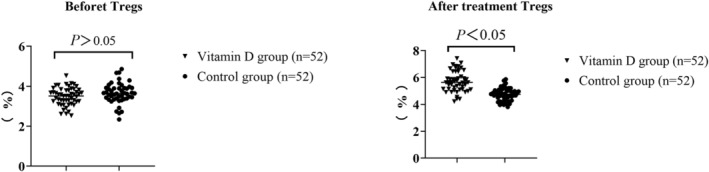
Comparison of the proportion of regulatory T cells between two groups.
Comparison of glucose and lipid metabolism indexes involving the two groupings after treatment
There was no considerable difference in triglyceride, leptin, fasting blood glucose, fasting insulin, and adiponectin involving the two groupings before treatment (p > 0.05). After treatment, the above indexes in both groups improved, and the triglyceride, leptin, fasting blood glucose, and fasting insulin in VDG were lower than those in CG, while adiponectin was higher than that in CG (p < 0.05), as shown in Table 3.
TABLE 3.
Comparison of glucose and lipid metabolism indexes involving the two groupings after treatment (n = 52, ).
| Groups | Triglyceride (mmol/L) | Leptin (mmol/L) | Fasting blood‐glucose (mmol/L) | Fasting insulin (pmol/mL) | Adiponectin (mg/L) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| BT | AT | BT | AT | BT | AT | BT | AT | BT | AT | |
| Vitamin D group | 3.83 ± 0.50 | 1.47 ± 0.19* | 34.23 ± 4.44 | 23.77 ± 3.09* | 6.33 ± 0.83 | 5.07 ± 0.65* | 12.85 ± 1.67 | 9.17 ± 1.19* | 6.27 ± 0.81 | 11.47 ± 1.49* |
| Control group | 3.81 ± 0.49 | 2.14 ± 0.27* | 34.21 ± 4.42 | 27.06 ± 3.50* | 6.35 ± 0.84 | 5.33 ± 0.68* | 12.87 ± 1.68 | 10.55 ± 1.37* | 6.30 ± 0.82 | 8.34 ± 1.08* |
| t | 0.206 | 14.634 | 0.023 | 5.081 | 0.122 | 1.993 | 0.060 | 5.483 | 0.187 | 12.265 |
| p | 0.837 | <0.001 | 0.981 | <0.001 | 0.903 | 0.048 | 0.951 | <0.001 | 0.851 | <0.001 |
Abbreviations: AT, after treatment; BT, before treatment.
Compared with the group before treatment, p < 0.05.
Comparison of endocrine and metabolic indexes between two groups
There was no significant difference in progesterone, luteinizing hormone, and 25‐hydroxy VD involving the two groupings before treatment (p > 0.05). After treatment, the levels of the above indexes in both groups increased, and the VDG was higher (p < 0.05, Figure 3), as seen in Table 4.
FIGURE 3.
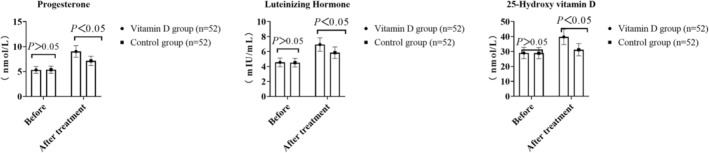
Comparison of endocrine and metabolic indexes involving the two groupings.
TABLE 4.
Comparison of endocrine and metabolic indexes involving the two groupings (n = 52, ).
| Group | Progesterone (nmol/L) | Luteinizing hormone (mIU/mL) | 25 hydroxyvitamin D (nmol/L) | |||
|---|---|---|---|---|---|---|
| BT | AT | BT | AT | BT | AT | |
| Vitamin D family | 5.33 ± 0.69 | 9.02 ± 1.17* | 4.55 ± 0.59 | 6.94 ± 0.90* | 28.97 ± 3.76 | 39.77 ± 5.17* |
| Control group | 5.37 ± 0.73 | 7.14 ± 0.93* | 4.51 ± 0.58 | 5.85 ± 0.76* | 28.88 ± 3.75 | 31.17 ± 4.05* |
| t | 0.287 | 9.070 | 0.348 | 6.672 | 0.122 | 9.442 |
| p | 0.774 | <0.001 | 0.728 | <0.001 | 0.902 | <0.001 |
Abbreviations: AT, after treatment; BT, before treatment.
Compared with the group before treatment, p < 0.05.
Comparison of immune inflammatory factors between two groups
There wasn't a noticeable variation in the levels of immune inflammatory factors involving the two groupings before treatment (p > 0.05). After treatment, the levels of immune inflammatory factors in both groups decreased, and those in VDG were lower than those in CG (p < 0.05), as seen in Table 5 and Figure 4.
TABLE 5.
Comparison of immune inflammatory factors between two groups (n = 52, ).
| Groups | Interleukin‐17 (pg/mL) | Interferon γ (ng/L) | Tumor necrosis factor‐α (ng/L) | C reactive protein (mg/L) | ||||
|---|---|---|---|---|---|---|---|---|
| BT | AT | BT | AT | BT | AT | BT | AT | |
| Vitamin D group | 344.71 ± 44.81 | 235.05 ± 30.43* | 192.74 ± 20.06 | 137.18 ± 17.83* | 37.55 ± 4.88 | 12.18 ± 1.58* | 24.35 ± 3.17 | 14.52 ± 1.89* |
| Control group | 342.04 ± 44.47 | 284.07 ± 36.93* | 187.94 ± 24.43 | 159.67 ± 20.76* | 36.44 ± 4.74 | 23.55 ± 2.45* | 23.72 ± 3.08 | 17.84 ± 2.31* |
| t | 0.304 | 7.387 | 1.094 | 5.926 | 1.176 | 28.124 | 1.027 | 8.021 |
| p | 0.761 | <0.001 | 0.276 | <0.001 | 0.242 | <0.001 | 0.306 | <0.001 |
Abbreviations: AT, after treatment; BT, before treatment.
Compared with the group before treatment, p < 0.05.
FIGURE 4.

Comparison of immune inflammatory factors involving two groupings.
Comparison of pregnancy outcomes between the two groups
There wasn't a noticeable variation in the rate of premature rupture of membranes, premature stripping of membranes, and neonatal survival rate involving the two groups (p > 0.05). However, the pregnancy success rate in VDG was higher than that in CG, and the premature delivery rate was lower than that in CG (p < 0.05), as seen in Table 6.
TABLE 6.
Comparison of pregnancy outcomes involving the two groupings (n = 52, n [%]).
| Group | Premature rupture of membranes | Premature abruption of fetal membranes | Successful pregnancy | Premature delivery | Neonatal survival |
|---|---|---|---|---|---|
| Vitamin D group | 1 (1.92) | 1 (1.92) | 42 (80.77) | 12 (23.08) | 44 (84.62) |
| Control group | 6 (11.54) | 4 (7.69) | 31 (59.61) | 27 (51.92) | 38 (73.08) |
| χ 2 | 3.829 | 1.891 | 5.561 | 9.231 | 2.075 |
| p | 0.050 | 0.169 | 0.018 | 0.002 | 0.150 |
DISCUSSION
Regulatory T cells are crucial components of the immune system, essential for maintaining immune tolerance and preventing autoimmune reactions, thereby playing a vital role in supporting a healthy pregnancy. 11 , 12 In patients with recurrent abortion, the function and proportion of regulatory T cells often deviate from the norm. These disturbances can lead to an impaired immune tolerance toward the embryo, increasing the risk of miscarriage. 13 Restoring the balance of regulatory T cells may become a new direction for the treatment of recurrent abortion. VD not only has an impact on calcium and phosphorus metabolism, but also plays a significant function in immune regulation. Numerous investigations have verified that VD has a positive effect on the function of regulatory T cells, which may inhibit excessive immune response by enhancing the activity of regulatory T cells. 14 , 15 As a potential immunomodulator, VD has attracted the attention of scholars in the treatment of recurrent abortion. While existing studies have explored the role of VD in recurrent abortion, much of the research has primarily focused on the correlation between serum VD levels and recurrent abortion. 16 , 17 There is still limited research on how VD adjuvant therapy specifically affects regulatory T cells in individuals with recurrent abortion and its subsequent impact on pregnancy outcomes.
The study's findings indicate that the proportion of regulatory T cells in the VDG is considerably higher than that in the CG, suggesting that VD adjuvant therapy effectively enhances the proportion of regulatory T cells in the peripheral blood of individuals with recurrent abortion. Regulatory T cells play a vital part in preventing autoimmune reactions and maintaining immune tolerance, while Th17 cells are associated with inflammatory responses. In patients with recurrent abortion, VD appears to increase the proportion of regulatory T cells by influencing T cell proliferation, differentiation, and cytokine production. It has been reported that VD can promote the differentiation of T cells into regulatory T cells and enhance their inhibitory functions, potentially through molecular mechanisms involving the VD receptor signaling pathway, such as the regulation of the transcription factor Foxp3. 18 Furthermore, the level of VD is correlated with the percentage of regulatory T cells and Th17 cells in peripheral blood of individuals with recurrent abortion. 19 Therefore, VD supplementation can improve its level in patients with recurrent abortion, thus promoting regulatory T‐cell function, aiding to maintain immune balance and reducing the occurrence of abortion.
VD is recognized to play a pivotal role in the regulation of glucose and lipid metabolism. It functions primarily by modulating the expression and activity of various enzymes and hormones involved in these metabolic pathways. For glucose metabolism, VD enhances insulin secretion by the pancreatic beta cells and improves insulin sensitivity in peripheral tissues, which helps in reducing blood glucose levels. Regarding lipid metabolism, VD influences lipid profiles by decreasing the synthesis of triglycerides and increasing lipolysis, which assists in lowering serum triglyceride levels and improving overall lipid distribution. Thus, sufficient levels of VD are critical for maintaining proper metabolic functions and preventing disorders such as diabetes and dyslipidemia. Previous studies have shown that VD deficiency may also be related to endocrine abnormalities, insulin resistance, and metabolic problems, which are potential risk factors for patients with recurrent abortion. 20 The study's findings demonstrate that the levels of triglyceride, leptin, fasting blood glucose, and fasting insulin in VDG are lower, and the level of adiponectin is higher, indicating that VD adjuvant therapy can efficiently regulate the disorder of glucose and lipid metabolism in individuals with recurrent abortion. VD can affect the function of islet β cells, improving both insulin sensitivity and secretion, which helps regulate blood sugar levels. Additionally, adiponectin, a hormone secreted by adipocytes, plays a role in enhancing insulin sensitivity. VD promotes the synthesis and secretion of adiponectin, which in turn helps reduce triglyceride levels. 21 Inflammatory reactions are often associated with various metabolic disorders. VD can mitigate inflammation and reduce the production of inflammatory factors through its immunomodulatory effects, thereby improving leptin sensitivity and insulin resistance. Additionally, the antioxidant and anti‐inflammatory properties of VD help reduce oxidative stress and protect vascular endothelial function. These effects are crucial for maintaining healthy blood lipid and blood glucose levels. 22 Previous studies have reported that VD deficiency may lead to female reproductive endocrine disorders, affect embryo implantation and pregnancy maintenance, and thus increase the risk of abortion. 23 The findings of this investigation I indicate that progesterone, luteinizing hormone and 25‐hydroxy VD were higher in VDG compared to the CG. This indicates that VD adjuvant therapy effectively regulates endocrine and metabolic disorders in patients with recurrent abortion. Progesterone, an essential hormone for maintaining early pregnancy, is promoted by VD through its role in modulating pregnancy‐related immunity. Additionally, VD may influence luteinizing hormone levels indirectly by regulating placental hormone production and differentiation. Since 25‐hydroxyvitamin D is the primary circulating form of VD and reflects an individual's VD status, its supplementation helps normalize levels in patients with recurrent abortion, potentially improving pregnancy outcome.
The findings of this investigation demonstrated that the levels of IL‐17, IFN‐γ, TNF‐α, and CRP were considerably lower in the VDG compared to the CG, indicating that VD adjuvant therapy can effectively mitigate the immune inflammatory state in patients with recurrent abortion. This effect may be attributed to VD's ability to modulate the functions of T and B cells, inhibit Th1 cell activity, and promote Th2 cell responses. As instance, VD increases the release of anti‐inflammatory cytokines while decreasing the synthesis of pro‐inflammatory cytokines like IL‐17 and IFN‐γ. Additionally, VD can directly inhibit the production of inflammatory mediators like TNF‐α and CRP, which are often overexpressed in recurrent abortion and contribute to embryo implantation failure and miscarriage. By reducing these inflammatory indicators, VD helps create a more stable environment for embryo development. 24 Previous research has shown that maintaining immune tolerance to the semi‐allogeneic nature of the embryo is crucial during pregnancy to prevent its rejection. 25 VD enhances this immune tolerance via promoting the proliferation and function of regulatory T cells, thereby protecting the embryos. Moreover, VD positively influences endometrial endometrium and function, promoting endometrial receptivity and improving embryo implantation conditions, which may reduce the incidence of abortion. 26
The findings of this investigation show that the pregnancy success rate in the VDG was higher, and the premature delivery rate was lower compared to the CG, suggesting that VD adjuvant therapy can considerably enhance pregnancy outcomes in patients with recurrent abortion. VD is crucial for maintaining pregnancy and supporting embryo implantation. Research has demonstrated that inadequate levels of VD are associated with pregnancy complications such as spontaneous abortion and premature delivery. 27 VD modulates the immune system by influencing the functions of T cells and natural killer cells, which helps establish and sustain maternal‐fetal immune tolerance, thereby facilitating a successful pregnancy. Additionally, VD plays a crucial part in placental development and function, including the regulation of calcium homeostasis, promotion of embryo implantation, and impact on placental angiogenesis. Consequently, VD supplementation supports optimal placental function, thereby reducing the risk of premature delivery. These findings align with prior studies linking VD deficiency to an increased risk of spontaneous abortion and suggest that VD supplementation may improve pregnancy outcomes. 28 In patients with recurrent abortion, VD enhances pregnancy success rates, supports normal placental development and function, and reduces premature delivery by interacting with its receptor to improve the immune environment at the maternal‐fetal interface, decrease autoantibody production, regulate Th1/Th2 cytokine balance, and modulate NK cell activity. 28 , 29 , 30
Additionally, this study has certain limitations in its design. Our inclusion criteria excluded endocrine disorders, genetic issues, and anatomical anomalies, however, antiphospholipid syndrome, a significant cause of RM, was not specifically excluded. The diagnosis of antiphospholipid syndrome depends on the detection of specific antibodies, such as lupus anticoagulant and anticardiolipin antibodies. The omission of these factors may affect the general applicability and interpretative power of our study results. In future research, we plan to include testing for these markers to more comprehensively assess the impact of VD supplementation therapy on patients with RMs due to diverse etiologies, thereby ensuring higher clinical relevance of the outcomes. Additionally, this underscores the need for a more thorough etiological assessment of patients with RM in clinical practice, to facilitate more targeted therapeutic strategies.
VD adjuvant therapy can enhance the proportion of regulatory T cells in the peripheral blood of individuals with recurrent abortion, regulate metabolic disorders, mitigate immune inflammation, and improve pregnancy outcomes. However, the single‐center design, small sample size, and lack of external validation severely restrict this study. Subsequent investigations will include multicenter studies with more extensive sample sizes to better authenticate and broaden these discoveries.
AUTHOR CONTRIBUTIONS
Shaoyun Ling was involved in the part of conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing – original draft, writing–review and editing.
CONFLICT OF INTEREST STATEMENT
The author declare that they have no competing interest.
ETHICS STATEMENT
All individuals willingly joined in this research, gave written informed permission, and the research was approved by Shenzhen Longgang Central Hospital's Medical Ethics Committee (approval number SLC202203061), adhering to the ethical guidelines set forth in the Helsinki Declaration of the World Medical Association.
Supporting information
Table S1. Comparison of biochemical indicators and clinical characteristics of patients in the VD group before and after treatment.
Table S2. Comparison of biochemical indicators and clinical characteristics of patients in the control group before and after treatment.
Ling S. Effect of vitamin D adjuvant therapy on the proportion of regulatory T cells in peripheral blood and pregnancy outcome of patients with recurrent miscarriage. J Obstet Gynaecol Res. 2025;51(1):e16151. 10.1111/jog.16151
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in Tables S1 and S2.
REFERENCES
- 1. Iordache O, Anastasiu‐Popov DM, Anastasiu DM, Craina M, Dahma G, Sacarin G, et al. A retrospective assessment of thrombophilia in pregnant women with first and second trimester pregnancy loss. Int J Environ Res Public Health. 2022;19(24):16500. doi: 10.3390/ijerph192416500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carbonnel M, Pirtea P, de Ziegler D, Ayoubi JM. Uterine factors in recurrent pregnancy losses. Fertil Steril. 2021;115(3):538–545. 10.1016/j.fertnstert.2020.12.003 [DOI] [PubMed] [Google Scholar]
- 3. Alecsandru D, Klimczak AM, Garcia Velasco JA, Pirtea P, Franasiak JM. Immunologic causes and thrombophilia in recurrent pregnancy loss. Fertil Steril. 2021;115(3):561–566. 10.1016/j.fertnstert.2021.01.017 [DOI] [PubMed] [Google Scholar]
- 4. Robertson SA, Moldenhauer LM, Green ES, Care AS, Hull ML. Immune determinants of endometrial receptivity: a biological perspective. Fertil Steril. 2022;117(6):1107–1120. 10.1016/j.fertnstert.2022.04.023 [DOI] [PubMed] [Google Scholar]
- 5. Piccinni MP, Raghupathy R, Saito S, Szekeres‐Bartho J. Cytokines, hormones and cellular regulatory mechanisms favoring successful reproduction. Front Immunol. 2021;12:717808. 10.3389/fimmu.2021.717808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tamblyn JA, Pilarski NSP, Markland AD, Marson EJ, Devall A, Hewison M, et al. Vitamin D and miscarriage: a systematic review and meta‐analysis. Fertil Steril. 2022;118(1):111–122. 10.1016/j.fertnstert.2022.04.017 [DOI] [PubMed] [Google Scholar]
- 7. Fernando M, Ellery SJ, Marquina C, Lim S, Naderpoor N, Mousa A. Vitamin D‐binding protein in pregnancy and reproductive health. Nutrients. 2020;12(5):1489. doi: 10.3390/nu12051489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abdollahi E, Rezaee SA, Saghafi N, Rastin M, Clifton V, Sahebkar A, et al. Evaluation of the effects of 1,25 vitamin D3 on regulatory T cells and T helper 17 cells in vitamin D‐deficient women with unexplained recurrent pregnancy loss. Curr Mol Pharmacol. 2020;13(4):306–317. 10.2174/1874467213666200303130153 [DOI] [PubMed] [Google Scholar]
- 9. Hong Li Y, Marren A. Recurrent pregnancy loss: a summary of international evidence‐based guidelines and practice. Aust J Gen Pract. 2018;47(7):432–436. 10.31128/AJGP-01-18-4459 [DOI] [PubMed] [Google Scholar]
- 10. Soper JT. Gestational trophoblastic disease: current evaluation and management. Obstet Gynecol. 2021;137(2):355–370. 10.1097/AOG.0000000000004240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Von Woon E, Greer O, Shah N, Nikolaou D, Johnson M, Male V. Number and function of uterine natural killer cells in recurrent miscarriage and implantation failure: a systematic review and meta‐analysis. Hum Reprod Update. 2022;28(4):548–582. 10.1093/humupd/dmac006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barbaro G, Inversetti A, Cristodoro M, Ticconi C, Scambia G, Di Simone N. HLA‐G and recurrent pregnancy loss. Int J Mol Sci. 2023;24(3):2557. doi: 10.3390/ijms24032557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Keller CC, Eikmans M, van der Hoorn MP, Lashley L. Recurrent miscarriages and the association with regulatory T cells; A systematic review. J Reprod Immunol. 2020;139:103105. 10.1016/j.jri.2020.103105 [DOI] [PubMed] [Google Scholar]
- 14. Harrison SR, Li D, Jeffery LE, Raza K, Hewison M. Vitamin D, autoimmune disease and rheumatoid arthritis. Calcif Tissue Int. 2020;106(1):58–75. 10.1007/s00223-019-00577-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Srichomchey P, Sukprasert S, Khulasittijinda N, Voravud N, Sahakitrungruang C, Lumjiaktase P. Vitamin D(3) supplementation promotes regulatory T‐cells to maintain immune homeostasis after surgery for early stages of colorectal cancer. In Vivo. 2023;37(1):286–293. 10.21873/invivo.13078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mousavi Salehi A, Ghafourian M, Amari A, Zargar M. Evaluation of CD3+ T cell percentage, function and its relationship with serum vitamin D levels in women with recurrent spontaneous abortion and recurrent implantation failure. Iran J Immunol. 2022;19(4):369–377. 10.22034/IJI.2022.91464.2083 [DOI] [PubMed] [Google Scholar]
- 17. Radzinsky VE, Ramazanova FU, Khamoshina MB, Azova MM, Orazov MR, Orazmuradov AA. Vitamin D insufficiency as a risk factor for reproductive losses in miscarriage. Gynecol Endocrinol. 2021;37(Suppl 1):8–12. 10.1080/09513590.2021.2006451 [DOI] [PubMed] [Google Scholar]
- 18. Tafti FD, Zare F, Miresmaeili SM, Fesahat F. Evaluating vitamin D and foxp3 mRNA levels in women with recurrent spontaneous abortion. JBRA Assist Reprod. 2022;26(2):232–236. 10.5935/1518-0557.20210062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zolfaghari MA, Arefnezhad R, Parhizkar F, Hejazi MS, Motavalli Khiavi F, Mahmoodpoor A, et al. T lymphocytes and preeclampsia: the potential role of T‐cell subsets and related MicroRNAs in the pathogenesis of preeclampsia. Am J Reprod Immunol. 2021;86(5):e13475. 10.1111/aji.13475 [DOI] [PubMed] [Google Scholar]
- 20. Abdollahi E, Saghafi N, Rezaee SA, Rastin M, Jarahi L, Clifton V, et al. Evaluation of 1,25(OH)2D3 effects on FOXP3, ROR‐gammat, GITR, and CTLA‐4 gene expression in the PBMCs of vitamin D‐deficient women with unexplained recurrent pregnancy loss (URPL). Iran Biomed J. 2020;24(5):295–305. 10.29252/ibj.24.5.290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. D'Ippolito S, Capozzi A, Scambia G, Sorge R, Lello S, Simone ND. Glucose/insulin metabolism and vitamin D in women with recurrent pregnancy loss. Am J Reprod Immunol. 2022;87(1):e13505. 10.1111/aji.13505 [DOI] [PubMed] [Google Scholar]
- 22. Chen HY, Zhang HP, Yang J, Huang ZQ, Xu HX, Jin J, et al. The relationship between maternal vitamin D deficiency and glycolipid metabolism and adverse pregnancy outcome. Clin Endocrinol. 2020;93(6):713–720. 10.1111/cen.14298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saxtorph MH, Hallager T, Persson G, Petersen KB, Eriksen JO, Larsen LG, et al. Assessing endometrial receptivity after recurrent implantation failure: a prospective controlled cohort study. Reprod Biomed Online. 2020;41(6):998–1006. 10.1016/j.rbmo.2020.08.015 [DOI] [PubMed] [Google Scholar]
- 24. Barbosa O, Sim‐Sim M, Silvestre MP, Pedro C, Cruz D. Effects of vitamin D levels during pregnancy on prematurity: a systematic review protocol. BMJ Open. 2024;14(2):e076702. 10.1136/bmjopen-2023-076702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sinaci S, Ocal DF, Yucel Yetiskin DF, Uyan Hendem D, Buyuk GN, Goncu Ayhan S, et al. Impact of vitamin D on the course of COVID‐19 during pregnancy: a case control study. J Steroid Biochem Mol Biol. 2021;213:105964. 10.1016/j.jsbmb.2021.105964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ghanavatinejad A, Rashidi N, Mirahmadian M, Rezania S, Mosalaei M, Ghasemi J, et al. Vitamin D3 controls TLR4‐ and TLR2‐mediated inflammatory responses of endometrial cells. Gynecol Obstet Investig. 2021;86(1–2):139–148. 10.1159/000513590 [DOI] [PubMed] [Google Scholar]
- 27. Vahid F, Rahmani D, Davoodi SH, Hekmatdoost A. The association among maternal index of nutritional quality, dietary antioxidant index, and odds of miscarriage incidence: case‐control study. J Am Nutr Assoc. 2022;41(3):310–317. 10.1080/07315724.2021.1880987 [DOI] [PubMed] [Google Scholar]
- 28. Perez‐Lopez FR, Pilz S, Chedraui P. Vitamin D supplementation during pregnancy: an overview. Curr Opin Obstet Gynecol. 2020;32(5):316–321. 10.1097/GCO.0000000000000641 [DOI] [PubMed] [Google Scholar]
- 29. Gallo S, McDermid JM, Al‐Nimr RI, Hakeem R, Moreschi JM, Pari‐Keener M, et al. Vitamin D supplementation during pregnancy: an evidence analysis center systematic review and meta‐analysis. J Acad Nutr Diet. 2020;120(5):898–924.e4. 10.1016/j.jand.2019.07.002 [DOI] [PubMed] [Google Scholar]
- 30. Bialy L, Fenton T, Shulhan‐Kilroy J, Johnson DW, McNeil DA, Hartling L. Vitamin D supplementation to improve pregnancy and perinatal outcomes: an overview of 42 systematic reviews. BMJ Open. 2020;10(1):e032626. 10.1136/bmjopen-2019-032626 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of biochemical indicators and clinical characteristics of patients in the VD group before and after treatment.
Table S2. Comparison of biochemical indicators and clinical characteristics of patients in the control group before and after treatment.
Data Availability Statement
The data that supports the findings of this study are available in Tables S1 and S2.


