Abstract
Purpose
To develop a risk stratification based on MRI features to predict hypervascular transformation for hepatobiliary-phase (HBP) hypointense nodules without arterial-phase hyperenhancement (APHE).
Materials and methods
This retrospective observational cohort study included 55 HBP hypointense nodules without APHE in 35 patients with chronic liver disease, cirrhosis, or current hepatocellular carcinoma (HCC) who underwent gadoxetic acid-enhanced MRI. The hypervascular transformation during the follow-up MRI(s) was the primary endpoint analyzed for the nodules. Univariable and multivariable Cox proportional hazard regression analyses were performed to identify risk features predicting transformation and assess their predictive value.
Results
Among the 55 nodules, 27 developed hypervascular transformation, while 28 did not. Diffusion-weighted imaging (DWI) hyperintensity (hazard ratio [HR], 4.98; 95% confidence interval [CI]: 1.60, 15.54; p = 0.006) and portal venous phase (PVP) hypointensity (HR, 4.08; 95% CI: 1.43, 11.64; p = 0.009) were associated with hypervascular transformation. DWI hyperintensity and PVP hypointensity had 44.4% (95% CI: 26.0%, 64.4%) and 81.9% (95% CI: 61.3%, 93.0%) sensitivity, while their specificity was 78.2% (95% CI: 64.6%, 87.8%) and 67.9 (95% CI: 47.6%, 83.4%), respectively. The specificity of the combination of two features was 100% (95% CI: 85.0%, 100%). The hypervascular transformation rates for nodules with both, either and neither of the risk MRI findings were 100% (10/10), 60.9% (14/23), and 13.6% (3/22), respectively; the median intervals for transformation were 312 (range: 73–838), 409 (range: 50–1643) and 555 (range: 423–968) days, respectively.
Conclusion
The combination of DWI hyperintensity and PVP hypointensity may be used as a high-risk indicator for the hypervascular transformation of HBP hypointense nodules without APHE; nodules without either feature may be treated as low-risk nodules and could adopt an extended interval follow-up schedule.
Keywords: hepatocellular carcinoma, magnetic resonance imaging, gadolinium ethoxybenzyl DTPA, cirrhosis, cancer risk
Introduction
Hepatocellular carcinoma (HCC) is the sixth most commonly occurring cancer and the third leading cause of cancer-related death worldwide, moreover, it represents the most common cause of death in patients with cirrhosis. 1 Early detection improved survival rate and recurrence-free survival. 2 Gadoxetic acid-enhanced magnetic resonance imaging (MRI) is included in the diagnostic algorithms of most international guidelines for HCC due to its liver-specific properties that allow for the assessment of both vascular behavior and hepatocellular function, which have led to an increase in the detection of borderline hepatic nodules in the gray area of multistep carcinogenesis.3,4 With the widespread use of this technique in the diagnostic and surveillance of high-risk HCC patients, more identified indeterminate nodules that appear non-hypervascular and hypointense in the hepatobiliary phase (HBP) have raised a new dilemma. The term HBP hypointense nodule without arterial-phase hyperenhancement (APHE) was proposed by Motosugi et al as a radiological definition for these nodules that are already visible on HBP images consisting mainly of HCC without APHE or a precursor to HCC.5,6 Many of these nodules transformed into hypervascular HCCs (ie, becoming HCCs with APHE) during follow-up,7,8 which draws current attention.
Elucidating the proportion and time of these nodules developing to hypervascular HCC, and clarifying the risk stratification for transformation might help balance the benefit of removing a tumor or precancerous lesion and the risk of surgical injury or impairment of liver function. Some researchers have attempted to answer the above questions and more advanced issues concerning the effect of such nodules on overall survival and disease-free survival in HCC patients when combining these nodules. 9 However, the results have been inconsistent. A radiologic-pathologic correlation study by Joo et al reported that the HBP hypointense nodules without APHE consisted of progressed HCCs, early HCCs (eHCCs), high-grade dysplastic nodules (HGDNs), and low-grade dysplastic nodules (LGDNs). 10 These diverse components explain why some researchers reported that combining these nodules was a significant predictive factor for recurrence after treatment of typical HCCs, and some did not.11–13 It is important to know that the pathologic results were variable nodule by nodule. A single treatment strategy cannot be determined for these nodules.
Liver biopsy is recommended by international guidelines in the case of atypical imaging features for HCC after the initial inconclusive imaging methods, also after the second inconclusive imaging technique used to analyze an indeterminate liver nodule of >1 cm in a patient under surveillance for HCC.14,15 However, its clinical use is limited by the invasive operation and the difficulty of obtaining specimens when the nodules are small, furthermore, the lesion may be focal, hence missing in needle biopsy specimens. Some liver segment locations are infeasible for biopsy, and there exist some possible biopsy-related risks such as bleeding, infection, or needle seeding. 16 Therefore, it is time to exact predictors from the many studies that have been conducted in recent years and establish evidence and imaging-based risk stratification that can be used in clinical scenarios regarding patients with these troubling nodules. To our knowledge, no data was published about creating a risk stratification for hypervascular transformation based on a combination of imaging predictors.
This study aimed to establish a risk stratification based on gadoxetic acid-enhanced MRI features to predict the hypervascular transformation for HBP hypointense nodules without APHE and assess the risk stratification's efficacy.
Materials and Methods
Patients
The institutional review board of Zhongshan Hospital, Fudan University (No. B2021-366) approved this retrospective observational cohort study. All patients signed informed consent forms agreeing that their images may be used for clinical research. The reporting of this study conforms to STROBE guidelines. 17 The patient details were de-identified. From January 2015 to January 2017, consecutive patients who underwent gadoxetic acid-enhanced MRI for surveillance of chronic liver disease, cirrhosis, or HCC which primarily based on the American Association for the Study of Liver Diseases (AASLD) practice guidelines 18 were initially included in this study.
The inclusion criteria for this study were as follows:
(i) chronic liver disease, including a. chronic hepatitis B virus (HBV); b. cirrhosis caused by HBV, HCV, alcoholic hepatitis, autoimmune hepatitis, nonalcoholic fatty disease, or other reasons except for congenital hepatic fibrosis or vascular disorders; and c. current or prior HCC;
(ii) an HBP hypointense nodule without APHE, defined as a nodule with iso- or hypointensity to the surrounding liver parenchyma in the arterial phase (AP) and hypointensity in the HBP (the maximum number of nodules was set to six to allow precise matched analysis; the size of the nodule was set at larger than 3 mm in consideration of the minimum slice thickness was 3 mm);
(iii) hypovascularity on at least one alternative examination within 1 month, as follows: contrast-enhanced ultrasonography (CEUS) using SonoVue® (Bracco Imaging SpA, Italy) or Sonazoid® (GE Healthcare, Norway), extracellular agent (ECA)–enhanced MRI, AP subtraction imaging (subtracting the unenhanced image from the AP image), or a contrast-enhanced CT scan;
(iv) follow-up contrast-enhanced MRI(s) (not confined to gadoxetic acid-enhanced MRI) performed at intervals of 3–6 months for at least 12 months or until transformation.
The exclusion criteria were as follows:
(i) a finding of hypervascularity or a nodule-in-nodule appearance in any alternative examination listed above;
(ii) a nodule that showed marked hyperintensity on T2-weighted imaging (T2WI) and could be diagnosed as a benign cyst or hemangioma;
(iii) resection of the nodule immediately or along with the surgical removal of de novo hypervascular HCC during follow-up;
(iv) transarterial chemoembolization (TACE) or radiofrequency ablation (RFA) of the lesion during follow-up;
(v) application of TACE or radiotherapy to de novo hypervascular HCC at another site in the liver during follow-up;
(vi) insufficient image quality for assessment.
MRI Protocols
All initial gadoxetic acid-enhanced MRI examinations were performed on a 1.5 T clinical scanner (MAGNETOM Aera, Siemens Healthcare, Erlangen, Germany). MRI protocol included axial fast spin-echo (FSE) T2WI with fat suppression; diffusion-weighted imaging (DWI) using 2 b-values of 0 and 500 s/mm2; in-phase and out-of-phase T1-weighted imaging (T1WI); a three-dimensional volumetric-interpolated breath-hold (3D-VIBE) pre-contrast T1WI, contrast-enhanced AP, portal venous phase (PVP), transitional phase (TP), and HBP. The AP was triggered automatically when the contrast medium reached the ascending aorta; the PVP began 14 s after the arterial phase; the TP began at 3 min, and the HBP at 20 min. The contrast agent and saline were injected with a power injector at a rate of 1 mL/s for hepatobiliary agent (Gd-EOB-DTPA, Primovist, Bayer Pharma) of 0.025 mmol/kg, followed by a 20-mL saline flush. The detailed parameters of the MRI sequences used are shown in Supplementary Table 1.
MRI Analysis
The MR imaging characteristics were reviewed by two abdominal radiologists with 7 and 16 years (NL and CY) of experience in liver MR imaging on Picture Archiving and Communication Systems (PACS), respectively. They had no prior knowledge of the endpoint of the nodules or the clinical information of the patients. When the two reviewers disagreed in their assessment of imaging characteristics, they reviewed the images jointly until a final consensus. The reviewers evaluated a set of selected MRI features including nodule size, Signal intensity (SI, on unenhanced T1WI, T2WI, and DWI and in the AP, PVP, TP, and HBP), intralesional fat, location (peripheral or central part), and margin (connected to vessels or not), most of which were identified as potential imaging characteristics for transformation by previous studies.19‐23
The nodule size was measured as the longest diameter on the HBP image. SI on T1WI was visually classified as hypointense, isointense, or hyperintense relative to the surrounding liver parenchyma. Intralesional fat was determined according to the signal drop on out-of-phase compared to in-phase T1WI. SI on T2WI was visually classified as non-hyperintense or intermediate hyperintense. SI on DWI was defined as non-hyperintense when the lesion was isointense (absence of diffusion restriction) or not observed; if it showed any hyperintensity, irrespective of the degree (from minimal hyperintensity to maximal hyperintensity similar to that of the spleen), it was classified as hyperintense. SI in AP was classified as hypointense or isointense compared with the enhancement of the surrounding liver parenchyma. SI in PVP and TP was classified as hypointense or isointense. The degree of HBP hypointensity was assigned to one of two categories: hypointense if the SI was equal to or lower than that of the intrahepatic portal vein, or slightly hypointense if the SI was lower than that of surrounding liver parenchyma but higher than that of the intrahepatic portal vein (Supplementary Figure 1). The location was categorized as “peripheral” or “central”: a nodule was considered “peripheral” if it was within one centimeter of the liver margin, while a nodule in any other location was considered “central”. The margin of the nodule was classified as connecting to the vessel(s) or not.
Endpoint
The follow-up contrast-enhanced MRI was conducted at intervals of 3 to 6 months. The primary endpoint was whether the nodule developed transformation (including diffuse hyperenhancement or nodule-in-nodule appearance in the AP) or not (ie, remained stable, size reduction, or spontaneously disappeared) during follow-up MRI(s).
Statistical Analysis
Categorical variables were analyzed using the chi-square test or Fisher's exact test (two-tailed). Continuous data were evaluated using a two-sample t-test or Mann-Whitney U test. Differences among more than two groups were analyzed by one-way ANOVA or the Kruskal–Wallis test, as appropriate. Receiver operator characteristic (ROC) curve analysis was used to determine the optimal size cutoff for transformation. The optimal cutoff was found to be 9.5 mm, and this value was used in the following analyses. Interobserver agreement for MRI features was evaluated by Cohen's kappa coefficient. The kappa values (levels of agreement) were classified as follows: 0.00–0.20, poor; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, good; and 0.81–1.0, excellent.
Before model selection, bivariate analysis was performed using Spearman rank correlation to test for collinearity among independent variables. The Spearman rank correlation coefficients for variables were generally below 0.5 except for T2WI and DWI, which had a significant coefficient of 0.62 (p = 0.000). Univariable and multivariable analyses using a Cox proportional hazard regression model were used to assess the MRI features for the prediction of hypervascular transformation. Variables with p < 0.05 in univariable Cox regression analysis were entered into the multivariable Cox regression model to identify independently significant MRI features. The hazard ratio (HR) and 95% confidence interval (CI) were calculated for each independent risk factor and then the combination of significant risk factors.
The sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) were calculated for each significant imaging feature and combination of features for predicting transformation. Hypervascular transformation rate curves were estimated by the Kaplan–Meier method, and differences in transformation intervals among subgroups were determined by log-rank analysis. Finally, the results were presented as a tree diagram, which makes it easier and more intuitive to understand the relevant interactions between the features and identify a suitable diagnostic algorithm.
A two-sided p-value of < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS for Windows version 26 (IBM, SPSS statistics) and Stata Version 15.0SE (StataCorp).
Results
Baseline Characteristics of the Patients and Nodules
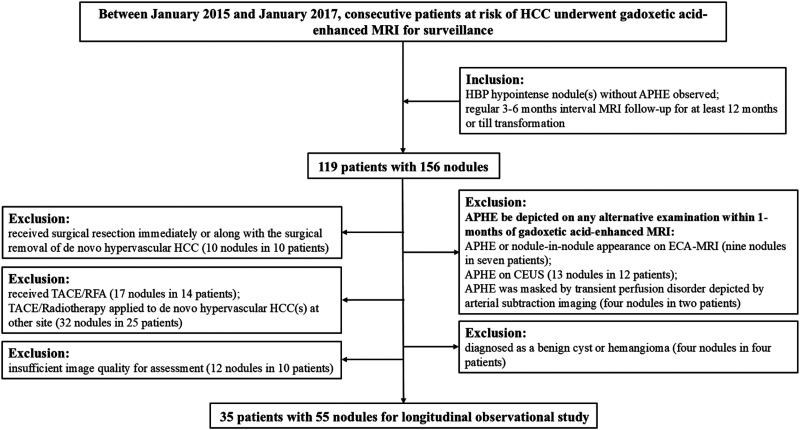
This study included 55 nodules in 35 patients (Figure 1). The clinical characteristics that were collected included age, sex, etiology of liver disease, Child-Pugh classification, serum α-fetoprotein (AFP) level within 1 month of the initial MRI, coexistence with hypervascular HCC, and previous history of HCC. Of these 35 patients (male: female, 27: 8), hepatitis B was the predominant cause of chronic liver disease (85.7%, 30/35), and all but one had cirrhosis (97.1%, 34/35). All were Child-Pugh class A. There are 22 patients with 1 nodule, and 13 patients with more than 1 nodule. Of whom, 22 patients combined with hypervascular HCC while 15 patients did not, 24 patients with previous HCC history while 11 patients did not. The median total follow-up time (or interval from initial MRI to hypervascular transformation) was 499 days (range: 50–2773 days), and the mean number of MRIs was 5.2 (range: 1–23). Among the 55 nodules, 27 developed hypervascular transformation, while 28 did not. All 27 nodules with hypervascular transformation fulfilled the diagnostic criteria of HCC (either by imaging or by pathology). Comparisons of baseline clinical characteristics between nodules that transformed and those that did not are summarized in Table 1. Nodules that later transformed were larger than those that did not (12.2 mm vs 9.6 mm, p = 0.043), and the cutoff size value for hypervascular transformation was 9.5 mm.
Figure 1.
Flow chart of the patient's inclusion. Abbreviations: HCC: hepatocellular carcinoma; HBP: hepatobiliary phase; APHE: arterial-phase hyperenhancement; TACE: transarterial chemoembolization; RFA: radiofrequency ablation; ECA: extracellular agent; CEUS: contrast-enhanced ultrasonography.
Table 1.
Characteristics of Patients and Comparison of Nodules with and Without Hypervascular Transformation During Follow-up.
| Characteristics | All patients n = 35 | Nodules developed hypervascular transformation | ||
|---|---|---|---|---|
| With, n = 27 | Without, n = 28 | p-value | ||
| Age, median, (range) [years] | 62.0 (43–75) | 62.0 (49–73) | 60.5 (43–75) | 0.825 |
| Sex, n (%) | 0.052 | |||
| Male | 27 (77.1) | 22 (81.5) | 16 (57.1) | |
| Female | 8 (22.9) | 5 (18.5) | 12 (42.9) | |
| Presence of cirrhosis, n (%) | 0.331 | |||
| Present | 34 (97.1) | 27 (100) | 27 (96.4) | |
| Absent | 1 (2.9) | 0 (0) | 1 (3.6) | |
| Etiology of liver disease, n (%) | 0.039* | |||
| HBV | 30 (85.7) | 21 (77.8) | 27 (96.4) | |
| Other reasons except for HBV | 5 (14.3) | 6 (22.2) | 1 (3.6) | |
| HCV | 1 | 1 (3.7) | 0 (0) | |
| HEV | 1 | 1 (3.7) | 0 (0) | |
| HBV + Alcoholic | 1 | 3 (11.1) | 0 (0) | |
| PBC | 1 | 1 (3.7) | 0 (0) | |
| Cryptogenic | 1 | 0 (0) | 1 (3.6) | |
| Previous history of HCC, n (%) | 0.844 | |||
| Present | 24 (68.6) | 8 (29.6) | 9 (32.1) | |
| Absent | 11 (31.4) | 19 (70.4) | 19 (67.9) | |
| Alpha-fetoprotein, mean (SD) [ng/mL] | 41.4 (95.0) | 32.6 (90.9) | 50.6 (100.3) | 0.520 |
| Child-Pugh (A/B/C) | 35/0/0 | 27/0/0 | 28/0/0 | 1.000 |
| Combined hypervascular HCC, n (%) | 0.233 | |||
| Present | 20 (57.1) | 14 (51.9) | 19 (67.9) | |
| Absent | 15 (42.9) | 13 (48.1) | 9 (32.1) | |
| Number of nodules per patient, n (%) | ||||
| 1 nodule | 22 (62.9) | |||
| 2 nodules | 9 (25.7) | |||
| 3 or more than 3 nodules | 4 (11.4) | |||
| Size, mean (SD), (range)[mm] | 10.9 (6.4) (3–32) | 12.2 (7.8) (3–32) | 9.6 (4.4) (4–23) | 0.043* |
| Proportion of size, n (%) | 0.124 | |||
| <10 mm | 32 (58.2) | 12 (44.4) | 20 (71.4) | |
| 10–19 mm | 17 (30.9) | 11 (40.7) | 6 (21.4) | |
| ≥20 mm | 6 (10.9) | 4 (14.8) | 2 (7.1) | |
| Interval, median, (range) [days] | 499 (50–2773) | 383 (50–1643) | 919 (394–2773) | < 0.001* |
| Test times, mean (range) | 5.2 (1–23) | 2.9 (1–8) | 7.3 (3–23) | 0.003* |
| Pre-contrast T1WI, n (%) | 0.085 | |||
| Isointensity | 10 (37.0) | 19 (67.9) | ||
| hypointensity | 12 (44.4) | 5 (17.9) | ||
| hyperintensity | 5 (18.5) | 4 (14.3) | ||
| Intralesional fat, n (%) | 0.422 | |||
| Present | 4 (14.8) | 2 (7.1) | ||
| Absent | 23 (85.2) | 26 (92.9) | ||
| T2WI, n (%) | 0.082 | |||
| intermediate hyperintensity | 8 (29.6) | 3 (10.7) | ||
| non-hyperintensity | 19 (70.4) | 25 (89.3) | ||
| DWI, n (%) | < 0.001* | |||
| hyperintensity | 12 (44.4) | 0 (0) | ||
| non-hyperintensity | 15 (55.6) | 28 (100) | ||
| Without APHE, n (%) | 0.701 | |||
| Hypointensity | 7 (25.9) | 6 (21.4) | ||
| Isointensity | 20 (74.1) | 22 (78.6) | ||
| Portal venous phase, n (%) | < 0.001* | |||
| Hypointensity | 22 (81.5) | 9 (32.1) | ||
| non-hypointensity | 5 (18.5) | 19 (67.9) | ||
| Transitional phase, n (%) | 0.744 | |||
| Hypointensity | 24 (88.9) | 23 (82.1) | ||
| non-hypointensity | 3 (11.1) | 5 (17.9) | ||
| Hepatobiliary phase, n (%) | 0.001* | |||
| Hypointensity | 27 (100) | 19 (67.9) | ||
| slightly hypointensity | 0 (0) | 9 (32.1) | ||
| Location, n (%) | 0.893 | |||
| peripheral | 14 (51.9) | 14 (50.0) | ||
| Central | 13 (48.1) | 14 (50.0) | ||
| Margin, n (%) | 0.010* | |||
| Connect to vessel(s) | 0 (0) | 6 (21.4) | ||
| Not connect to vessel(s) | 27 (100) | 22 (78.6) | ||
HBV, hepatitis B virus; HCV, hepatitis C virus; HEV, hepatitis E virus; PBC, primary biliary cirrhosis; HCC, hepatocellular carcinoma; SD, standard deviation; T1WI, T1-weighted imaging; T2WI, T2-weighted imaging; DWI, diffusion-weighted imaging; APHE, arterial-phase hyperenhancement.
*p-value indicates statistical significance.
Primary Endpoint of Nodules
The endpoint outcomes of the nodules are shown in Supplementary Tables 2 and 3. The 27 nodules that were observed hypervascular transformation fulfilled the criteria of wash-in and wash-out proposed by the AASLD, 18 and the HCC diagnosis for eight nodules was also confirmed by histopathological results (six, one, and one by surgical resection, liver transplantation, and biopsy, respectively). Regarding size, 21 nodules showed enlargement, while the other six remained stable or diminished.
Table 2.
Univariable and Multivariable Analyses of Initial MRI Characteristics in Predicting Hypervascular Transformation.
| Univariable analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Nodule size ≥10 mm | 1.91 | 0.89, 4.09 | 0.096 | |||
| T1WI hypointensity (isointensity) | 0.60 | 0.21, 1.77 | 0.357 | |||
| T1WI hyperintensity (isointensity) | 2.19 | 0.76, 6.30 | 0.146 | |||
| Intralesional fat | 0.88 | 0.44, 1.76 | 0.722 | |||
| T2WI intermediate hyperintensity (non-hyperintensity) | 3.37 | 1.40, 8.11 | 0.007* | 0.74 | 0.22, 2.50 | 0.627 |
| DWI hyperintensity (non-hyperintensity) | 5.36 | 2.40, 11.95 | < 0.001* | 4.98 | 1.60, 15.54 | 0.006* |
| Without APHE- hypointensity (isointensity) | 1.22 | 0.51, 2.92 | 0.649 | |||
| PVP hypointensity (non-hypointensity) | 4.45 | 1.68, 11.78 | 0.003* | 4.08 | 1.43, 11.64 | 0.009* |
| TP hypointensity (non-hypointensity) | 1.48 | 0.44, 4.92 | 0.524 | |||
| HBP hypointensity (slightly hypointensity) | 40.78 | 0.84,1990.79 | 0.062 | |||
| Location- centrally (peripheral) | 0.68 | 0.31, 1.49 | 0.339 | |||
| Margin- connect to vessels (isolate) | 0.04 | 0.00, 8.25 | 0.238 | |||
Note: Characteristics in the parentheses are references.
T1WI, T1-weighted imaging; T2WI, T2-weighted imaging; DWI, diffusion-weighted imaging; APHE, arterial-phase hyperenhancement; PVP, portal venous phase; TP, transitional phase; HBP, hepatobiliary phase; HR, hazard ratio; CI, confidence interval
*p-value indicates statistical significance. Variables with p < 0.05 in univariable Cox regression analysis were applied to a multivariable Cox regression analysis.
Table 3.
Hazard Ratios for Hypervascular Transformation with 95% CI in Nodules with PVP Hypointensity Only, DWI Hyperintensity Only, or a Combination of Both.
| DWI hyperintensity | PVP hypointensity | Hazard ratio (95%CI) | p-value |
|---|---|---|---|
| No | No | 1 (reference) | |
| No | Yes | 4.26 (1.20, 15.18) | 0.025* |
| Yes | No | 5.79 (0.96, 35.06) | 0.056 |
| Yes | Yes | 19.57 (5.14, 74.51) | < 0.001* |
CI, confidence interval; DWI, diffusion-weighted imaging; PVP, portal venous phase.
*p-value indicates statistical significance.
MRI-Based Risk Factors for Hypervascular Transformation
The baseline MRI features of the nodules are summarized in Table 1. The results of univariable and multivariable analyses are summarized in Table 2. Cox analysis revealed that PVP hypointensity (p = 0.003), T2WI hyperintensity (p = 0.007), and DWI hyperintensity (p < 0.001) were associated with hypervascular transformation. The interobserver agreement for PVP hypointensity, T2WI hyperintensity, and DWI hyperintensity was 0.93, 0.82, and 0.77, respectively (Supplementary Table 4). According to the multivariable Cox analysis, PVP hypointensity (HR = 4.08; 95% CI: 1.43, 11.64; p = 0.009) and DWI hyperintensity (HR = 4.98; 95% CI: 1.60, 15.54; p = 0.006) were the only two significant features associated with subsequent hypervascular transformation (Figure 2, Supplementary Figure 2–4). The HRs and 95% CIs for PVP hypointensity only, DWI hyperintensity only, and the combination of both risk factors were 4.26 (1.20–15.18, p = 0.025), 5.79 (0.96–35.06, p = 0.056), and 19.57 (5.14–74.51, p < 0.001), respectively (Table 3).
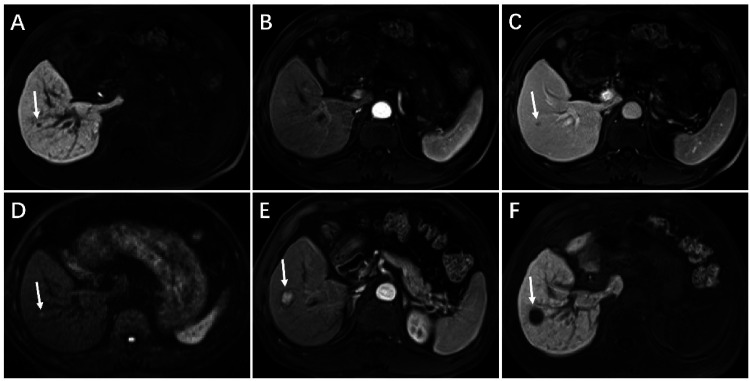
Figure 2.
Hypervascular transformation of a hepatobiliary phase hypointense nodule without arterial phase hyperenhancement manifested with the combination of DWI hyperintensity and PVP hypointensity in a 64-year-old man with hepatitis B–induced liver cirrhosis. On initial gadoxetic acid-enhance MRI (A-D), a 5-mm nodule shows hypointensity on 20-min hepatobiliary phase imaging (A, arrow), isointensity on arterial phase T1-weighted imaging (B), hypointensity on PVP imaging (C, arrow), and hyperintensity on DWI (b = 500 s/mm2) (D, arrow). Follow-up gadoxetic acid-enhanced MRI obtained 445 days later, the nodule shows marked enlargement as measuring 18-mm, unequivocally hyperenhancement than surrounding liver on arterial phase imaging (E, arrow), and hypointensity on 20-min hepatobiliary phase imaging (F, arrow). Nodule specimens after surgical resection confirmed the diagnosis of HCC. Abbreviations: DWI: diffusion-weighted imaging; PVP: portal venous phase; HCC: hepatocellular carcinoma.
Diagnostic Performance of Significant MRI Features
Overall, 22 of 55 nodules (40%) had no risk-associated MRI features, 23 of 55 (41.8%) had one of the two risk features, and 10 of 55 (18.2%) had both features. The sensitivity, specificity, accuracy, PPV, and NPV of the two significant imaging features and their combination are shown in Table 4. The accuracy, sensitivity, and specificity for predicting hypervascular transformation of PVP hypointensity were 74.5%, 81.9% (95%CI: 61.3% to 93.0%), and 67.9% (95%CI: 47.6% to 83.4%), respectively, and those of DWI hyperintensity was 72.7%, 44.4% (95%CI: 26.0% to 64.4%), and 78.2% (95%CI: 64.6% to 87.8%), respectively. When a combination of DWI hyperintensity and PVP hypointensity, the specificity increased to 100.0% (95%CI: 85.0% to 100.0%), and the sensitivity decreased to 37.0% (95%CI: 20.0% to 57.5%).
Table 4.
Diagnostic Performance of MRI Findings in Predicting Hypervascular Transformation.
| MRI findings | Sensitivity | Specificity | Accuracy | PPV | NPV |
|---|---|---|---|---|---|
| DWI hyperintensity | 44.4 (26.0, 64.4) | 78.2 (64.6, 87.8) | 72.7 (40/55) | 29.3 (20.0, 40.5) | 70.7 (59.5, 80.8) |
| PVP hypointensity | 81.9 (61.3, 93.0) | 67.9 (47.6, 83.4) | 74.5 (41/55) | 56.4 (42.4, 69.4) | 43.6 (30.6, 57.6) |
| Both findings | 37.0 (20.0, 57.5) | 100.0 (85.0, 100.0) | 69.1 (38/55) | 18.2 (9.5, 31.4) | 81.8 (68.6, 90.5) |
Data are presented as percentages. Data in parentheses are 95% confidence interval or the number of subjects used to calculate the percentage. MRI, magnetic resonance imaging; DWI, diffusion-weighted imaging; PVP, portal venous phase; PPV, positive predictive value; NPV, negative predictive value.
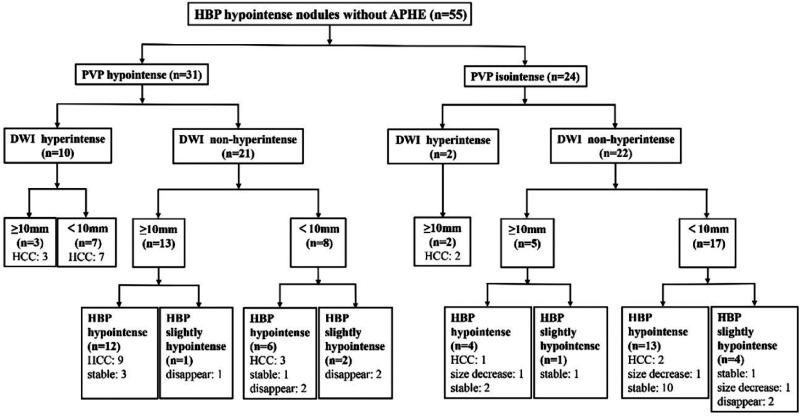
Tree Diagram
A tree diagram (Figure 3) was developed using four features (PVP hypointensity, DWI hyperintensity, nodule size, and SI in the HBP) capable of identifying nodules with a high risk of hypervascular transformation. In the first step, PVP hypointensity classified 22 of 27 nodules (81.5%) as suspicious of the transformation risk. In the second step, the SI on DWI in the PVP hypointense group was evaluated, and DWI hyperintensity was identified in 10 in 22 nodules (45.5%) that later transformed. In the third step, the sizes of the remaining PVP hypointense and DWI non-hyperintense nodules were evaluated, and a minimum size threshold of 10 mm detected nine nodules that transformed. In the last step, the SI in the HBP was evaluated; none of the nodules with slight HBP hypointensity developed transformation, while three nodules with marked HBP hypointensity did.
Figure 3.
Tree diagram for the nodules in the longitudinal observation. Abbreviations: HBP: hepatobiliary phase; APHE: arterial phase hyperenhancement; PVP: portal venous phase; DWI: diffusion-weighted imaging.
In the PVP isointensity group, at the second step of the tree diagram, all nodules with DWI hyperintensity (two nodules) developed hypervascular transformation. As the third step, one in five larger nodules (≥ 10 mm) developed hypervascularization. For the 17 nodules smaller than 10 mm, the SI in the HBP was evaluated; none of the nodules with slight HBP hypointensity transformed, while two of the 13 nodules with HBP hypointensity transformed.
Comparison of Hypervascular Transformation Rates and Intervals Based on MRI Risk Features
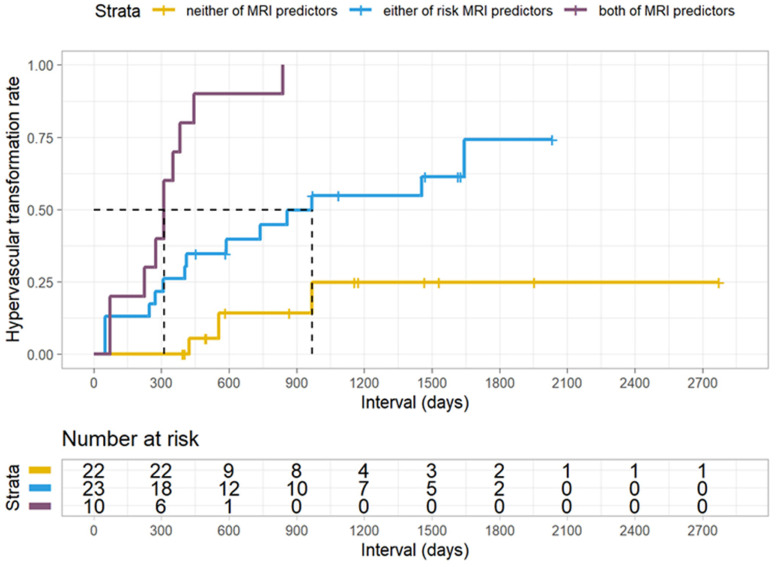
The hypervascular transformation rates for nodules with both, either and neither of the risk MRI findings were 100% (10/10), 60.9% (14/23), and 13.6% (3/22), respectively (p < 0.001) (Figure 4). The median intervals for transformation in nodules with both features was 312 days (range: 73–838 days), while with either was 409 days (range: 50–1643 days) and with neither was 555 days (range: 423–968 days), the difference was not significant (p = 0.226).
Figure 4.
Kaplan-Meier curves of hypervascular transformation rates according to the risk MRI findings. Hypervascular transformation rates of HBP hypointense nodules without APHE according to both (purple), either (blue), and neither (yellow) of the risk MRI features were evaluated using the Kaplan-Meier method. Nodules with both risk MRI features versus either feature versus neither of features, p < 0.001; Statistical significance was assessed with the log-rank test.
Discussion
Hepatocarcinogenesis is a multifactorial process that includes changes in architecture, hepatocyte function, cellular density, and Kupffer cell numbers or function. 24 Accordingly, with recent technical advances in liver imaging and surveillance of patients at high risk of developing HCC, especially gadoxetic acid-enhanced MRI which can target three processes of hepatic carcinogenesis: hemodynamic changes, hepatocyte function, and tissue diffusivity been increasingly used in the diagnosis and surveillance of HCC, more and more borderline hepatic nodules in the gray area of multistep carcinogenesis were detected. In these nodules, hepatocyte function changes or tissue diffusivity may precede hemodynamic changes, thus manifested as HBP hypointensity but without APHE. In our study, PVP hypointensity and DWI hyperintensity on gadoxetic acid-enhanced MRI were independent and significant predictors of hypervascular transformation for nodules with HBP hypointense and without APHE. The hypervascular transformation rate was markedly increased in nodules with the combination of the two imaging predictors compared with either one or neither of the predictors. The combination of DWI intensity and PVP hypointensity yielded a specificity of 100% for the prediction of hypervascular transformation, with a median interval of 312 days (95% CI: 257–367 days).
DWI can target cellular density and architectural changes through differences in diffusivity as well as vascular changes and its hyperintensity is widely regarded as a radiological feature of eHCC, shortly preceding arterial hypervascularization. 25 In our study, DWI hyperintensity was also detected as an independent risk MRI finding to predict hypervascular transformation of HBP hypointense nodules without APHE (HR = 4.98, 95% CI: 1.60–15.54), this result was consistent with a recent meta-analysis which reported that DWI features reflected the risk of hypervascularization with pooled HRs of 5.83 (95% CI: 1.42–23.95). 8 In our study, all 12 nodules with DWI hyperintensity ultimately developed hypervascularization with a median interval of 312 days (95% CI: 225–399 days).
PVP was detected as another independent risk factor predicting hypervascular transformation. Twenty-two of 31 nodules with PVP hypointensity showed hypervascularization, with a median interval of 412 days (95% CI: 164-660 days). PVP images are necessary for assessing portal blood flow in focal liver lesions because the portal blood flow in HCC varies with the stage of carcinogenesis. 26 Kudo et al suggested that PVP hypointensity should be treated as the utmost important imaging feature in diagnosing eHCC of hypovascular nodules. For nodules with hypovascular arterial blood flow and reduced portal blood flow, biopsy is essential. 27 Renzulli et al stated that double hypointensity in the portal/venous and hepatobiliary phases could be considered a new MRI pattern which highly suggestive of hypovascular HCC. 28 We speculated that PVP hypointensity may indicate that nodules are in the process of multi-step carcinogenesis, and thus reduce the false-positive interpretations of DWI hyperintensity alone in eHCC diagnosis or hypervascularization prediction.
Combining these two features could be highly effective in predicting hypervascular transformation (both existence of them) and non-transformation (neither existence of them). When these two risk MRI predictors existed in HBP hypointense nodules with APHE, a close follow-up should be monitored, even treatment that seems radical might be applied when the nodules were located adjacent to major vessels or other structures that would easily induce vascular invasion or intrahepatic spread during the process that nodules developed malignant transformation. The high specificity may prevent unnecessary frequent follow-up procedures for relatively low-risk nodules and decrease physical and/or psychological harm to patients.
We also constructed a tree diagram to reflect the results in a simple and readily interpretable way, to be noted is that nodule size and SI of HBP provided additional information. In our study, 65.2% of nodules equal to or larger than 10 mm developed transformation while 37.5% of nodules less than 10 mm did. The size was also listed as a risk factor for transformation in previous studies, most of which suggested a cutoff value of 10 mm.29‐31 SI of HBP provided additional value in predicting non-transformation for non-DWI hyperintensity nodules. None of the nodules with slightly hypointense HBP signal SI transformed, while five with hypointense HBP signal developed transformation. Likewise, previous studies suggested that SI during the HBP is capable of identifying nodules at risk for carcinogenesis or HCC progression.32,33 SI during the HBP was assessed visually by the reviewers in this study; this method is subjective but easy to apply in routine clinical practice, and the interobserver agreement was excellent (k = 0.930).
We adopted a list of MRI parameters, mostly extracted from previous studies including size, signal intensity relative to the surrounding liver, location, margin, and some clinical characteristics including AFP, a combination of hypervascular HCC, and previous HCC history, to try to portray the nodules more comprehensively. It should be noted that the results were inconsistent across the previous studies; for example, T2WI hyperintensity was noted as a major predictor of transformation in several studies,7,20,31 but not in others,21,30,34 and also not identified in our study. Since dysplastic nodules (DNs), regenerative nodules (RNs), fibrosis, and scarring in the course of cirrhosis also occasionally appear as hyperintense on T2WI, it has been noted that T2WI does not provide added diagnostic value in the characterization of focal lesions in the cirrhotic liver. 35 There was a correlation between T2WI and DWI results in our study, and DWI was a more sensitive predictor than T2WI. T1WI hyperintensity and intralesional fat were reported as risk factors in previous studies,21,23 but not in ours. The explanation may be that intracellular lipids can appear in both early HCCs and DNs, and T1WI hyperintensity mainly owing to iron or copper accumulation is more commonly observed in DNs but not eHCCs. As was widely recognized, AFP was of limited value in eHCC detection, or more stringent circumstances, like in this kind of study, predicting the evolution of pre-malignant lesions. Previous HCC history and combination of hypervascular HCC were also listed as risk factors for hypervascular transformation in some studies,8,20,31 but most did not, possibly because of heterogeneous study populations and/or disease severity. Maybe some diagnostic biomarkers of tumor microenvironment may provide another perspective for assessing the circumstances of these nodules and promoting the prediction of carcinogenesis as well as early detection of cancers. 36 The nodule location and margin do not reflect an obvious impact on hypervascular transformation.
Our study had several limitations. First, its design as a single-center longitudinal observational study inevitably introduced a risk of selection bias. There was also a degree of selection bias because gadoxetic acid-enhanced MRI is more likely to be performed regularly in patients at high risk for HCC, such as those with advanced liver fibrosis or a history of prior HCC. On the other side, the inclusion criterion requiring at least 12 months of follow-up may favor patients with better liver function: although the patients were not deliberately selected by disease severity, all enrolled in this study had Child-Pugh class A disease. Second, approximately 86% of patients had chronic hepatitis B. Therefore, the results of this study should be interpreted carefully when applied to other populations. Third, SI was only visually evaluated, though it was easy to apply in routine clinical practice, in case of severe liver dysfunction, poor uptake of gadoxetic-acid by hepatocytes may reduce the contrast of lesions and hamper the SI assessment in the HBP. Fourth, the nodules were not all subjected to pathological evaluation. However, the diagnosis of HCC by imaging could provide enough accuracy. Finally, we used stringent inclusion criteria such as double-checking hypovascularization of nodules, simulating a real dilemma in routine clinical practice, some objects thus be excluded, and the sample size may be smaller than in some previous studies. A future study including a large number of these nodules, is required to validate these factors and this risk stratification.
Conclusion
The combination of DWI hyperintensity and PVP hypointensity may be used as a high-risk indicator for the hypervascular transformation of HBP hypointense nodules without APHE; nodules without either feature may be treated as low-risk nodules and could adopt an extended interval follow-up schedule.
Supplemental Material
Supplemental material, sj-docx-1-tct-10.1177_15330338241299003 for Hepatobiliary-Phase Hypointense Nodules Without Arterial-Phase Hyperenhancement: Developing a Risk Stratification for Hypervascular Transformation Based on a Real-World Observational Cohort Study by Na Li, Yi Wang, Li Yang, Chun Yang and Mengsu Zeng in Technology in Cancer Research & Treatment
Acknowledgments
Not applicable.
Abbreviations
- HCC
Hepatocellular carcinoma
- MRI
Magnetic resonance imaging
- HBP
Hepatobiliary phase
- APHE
Arterial phase hyperenhancement
- eHCC
Early HCC
- HGDN
High-grade dysplastic nodule
- LGDN
Low-grade dysplastic nodule
- AASLD
Association for the Study of Liver Diseases
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- AP
Arterial phase
- CEUS
Contrast-enhanced ultrasonography
- ECA
Extracellular agent
- CT
Computed tomography
- T2WI
T2-weighted imaging
- TACE
Transarterial Chemoembolization
- RFA
Radiofrequency ablation
- FSE
Fast spine echo
- DWI
Diffusion-weighted imaging
- T1WI
T1-weighted imaging
- 3D-VIBE
Three-dimensional volumetric-interpolated breath-hold
- PVP
Portal venous phase
- TP
Transitional phase
- SI
Signal intensity
- ROC
Receiver operating characteristic curve
- HR
Hazard ratio
- CI
Confidence interval
- PPV
Positive predictive value
- NPV
Negative predictive value
- AFP
Serum α-fetoprotein
- DN
Dysplastic nodule
- RN
Regenerative nodule
Authors contributions: All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Data Availability Statement: The authors confirm that the data supporting the findings of this study are available within the article and its Supplemental materials, the original research data are available if requested.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The institutional review board of Zhongshan Hospital, Fudan University (No. B2021-366) approved this retrospective observational cohort study.
Funding: This work was supported by the Clinical Research Plan of SHDC [grant number SHDC2020CR1029B]; National Natural Science Foundation of China [grant number 82171897]; Shanghai Municipal Key Clinical Specialty [grant number shslczdzk03202]; and Clinical Research Project of Zhongshan Hospital, Fudan University [grant number 2020ZSLC61].
Informed Consent: All patients signed informed consent forms agreeing that their images may be used for clinical research.
ORCID iD: Mengsu Zeng https://orcid.org/0009-0000-9899-1778
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Takayama T, Makuuchi M, Hirohashi S, et al. Early hepatocellular carcinoma as an entity with a high rate of surgical cure. Hepatology. 1998;28(5):1241‐1246. doi: 10.1002/hep.510280511 [DOI] [PubMed] [Google Scholar]
- 3.Ichikawa S, Goshima S. Gadoxetic acid-enhanced liver MRI: Everything you need to know. Invest Radiol. 2024;59(1):53‐68. doi: 10.1097/rli.0000000000000990 [DOI] [PubMed] [Google Scholar]
- 4.Brandi N, Renzulli M. Liver lesions at risk of transformation into hepatocellular carcinoma in cirrhotic patients: Hepatobiliary phase hypointense nodules without arterial phase hyperenhancement. J Clin Transl Hepatol. 2024;12(1):100‐112. doi: 10.14218/jcth.2023.00130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motosugi U, Murakami T, Lee JM, Fowler KJ, Heiken JP, Sirlin CB. Recommendation for terminology: Nodules without arterial phase hyperenhancement and with hepatobiliary phase hypointensity in chronic liver disease. J Magn Reson Imaging. 2018;48(5):1169‐1171. doi: 10.1002/jmri.26515 [DOI] [PubMed] [Google Scholar]
- 6.Motosugi U. Treat or wait? Hepatobiliary phase hypointense nodule without arterial phase hyperenhancement. Radiology. 2020;296(2):346‐347. doi: 10.1148/radiol.2020201726. [DOI] [PubMed] [Google Scholar]
- 7.Inoue T, Hyodo T, Murakami T, et al. Hypovascular hepatic nodules showing hypointense on the hepatobiliary-phase image of Gd-EOB-DTPA-enhanced MRI to develop a hypervascular hepatocellular carcinoma: A nationwide retrospective study on their natural course and risk factors. Dig Dis. 2013;31(5–6):472‐479. doi: 10.1159/000355248 [DOI] [PubMed] [Google Scholar]
- 8.Kim TH, Woo S, Han S, Suh CH, Do RKG, Lee JM. Risk factors for hypervascularization in hepatobiliary phase hypointense nodules without arterial phase hyperenhancement: A systematic review and meta-analysis. Acad Radiol. 2022;29(2):198‐210. doi: 10.1016/j.acra.2020.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim TH, Woo S, Han S, Suh CH, Lee DH, Lee JM. Hepatobiliary phase hypointense nodule without arterial phase hyperenhancement: Are they at risk of HCC recurrence after ablation or surgery? A systematic review and meta-analysis. Eur Radiol. 2020;30(3):1624‐1633. doi: 10.1007/s00330-019-06499-9 [DOI] [PubMed] [Google Scholar]
- 10.Joo I, Kim SY, Kang TW, et al. Radiologic-pathologic correlation of hepatobiliary phase hypointense nodules without arterial phase hyperenhancement at gadoxetic acid-enhanced MRI: A multicenter study. Radiology. 2020;296(2):335‐345. doi: 10.1148/radiol.2020192275. [DOI] [PubMed] [Google Scholar]
- 11.Lee DH, Lee JM, Lee JY, et al. Non-hypervascular hepatobiliary phase hypointense nodules on gadoxetic acid-enhanced MRI: Risk of HCC recurrence after radiofrequency ablation. J Hepatol. 2015;62(5):1122‐1130. doi: 10.1016/j.jhep.2014.12.015 [DOI] [PubMed] [Google Scholar]
- 12.Toyoda H, Kumada T, Tada T, et al. Non-hypervascular hypointense nodules detected by Gd-EOB-DTPA-enhanced MRI are a risk factor for recurrence of HCC after hepatectomy. J Hepatol. 2013;58(6):1174‐1180. doi: 10.1016/j.jhep.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 13.Takeishi K, Yoshizumi T, Itoh S, et al. Surgical indications for hepatocellular carcinoma with non-hypervascular hypointense nodules detected by Gd-EOB-DTPA-enhanced MRI. Ann Surg Oncol. 2020;27(9):3344‐3353. doi: 10.1245/s10434-020-08419-4 [DOI] [PubMed] [Google Scholar]
- 14.Kim TH, Kim SY, Tang A, Lee JM. Comparison of international guidelines for noninvasive diagnosis of hepatocellular carcinoma: 2018 update. Clin Mol Hepatol. 2019;25(3):245‐263. doi: 10.3350/cmh.2018.0090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol Int. 2017;11(4):317‐370. doi: 10.1007/s12072-017-9799-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Renzulli M, Pecorelli A, Brandi N, et al. The feasibility of liver biopsy for undefined nodules in patients under surveillance for hepatocellular carcinoma: Is biopsy really a useful tool? J Clin Med. 2022;11(15):4399. doi: 10.3390/jcm11154399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573‐577. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 18.Bruix J, Sherman M. Management of hepatocellular carcinoma: An update. Hepatology. 2011;53(3):1020‐1022. doi: 10.1002/hep.24199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang J, Kim YK, Jeong WK, Choi D, Rhim H, Lee WJ. Nonhypervascular hypointense nodules at gadoxetic acid-enhanced MR imaging in chronic liver disease: Diffusion-weighted imaging for characterization. Radiology. 2015;277(1):309. doi: 10.1148/radiol.2015154031 [DOI] [PubMed] [Google Scholar]
- 20.Hyodo T, Murakami T, Imai Y, et al. Hypovascular nodules in patients with chronic liver disease: Risk factors for development of hypervascular hepatocellular carcinoma. Radiology. 2013;266(2):480‐490. doi: 10.1148/radiol.12112677 [DOI] [PubMed] [Google Scholar]
- 21.Motosugi U, Ichikawa T, Sano K, et al. Outcome of hypovascular hepatic nodules revealing no gadoxetic acid uptake in patients with chronic liver disease. J Magn Reson Imaging. 2011;34(1):88‐94. doi: 10.1002/jmri.22630 [DOI] [PubMed] [Google Scholar]
- 22.Suh CH, Kim KW, Pyo J, Lee J, Kim SY, Park SH. Hypervascular transformation of hypovascular hypointense nodules in the hepatobiliary phase of gadoxetic acid-enhanced MRI: A systematic review and meta-analysis. AJR Am J Roentgenol. 2017;209(4):781‐789. doi: 10.2214/ajr.16.17711 [DOI] [PubMed] [Google Scholar]
- 23.Kim YS, Song JS, Lee HK, Han YM. Hypovascular hypointense nodules on hepatobiliary phase without T2 hyperintensity on gadoxetic acid-enhanced MR images in patients with chronic liver disease: Long-term outcomes and risk factors for hypervascular transformation. Eur Radiol. 2016;26(10):3728‐3736. doi: 10.1007/s00330-015-4146-9 [DOI] [PubMed] [Google Scholar]
- 24.Kojiro M. Pathology of hepatocellular carcinoma. Blackwell; 2006. [PubMed] [Google Scholar]
- 25.Renzulli M, Biselli M, Brocchi S, et al. New hallmark of hepatocellular carcinoma, early hepatocellular carcinoma and high-grade dysplastic nodules on Gd-EOB-DTPA MRI in patients with cirrhosis: A new diagnostic algorithm. Gut. 2018;67(9):1674‐1682. doi: 10.1136/gutjnl-2017-315384 [DOI] [PubMed] [Google Scholar]
- 26.Midorikawa Y, Takayama T, Shimada K, et al. Marginal survival benefit in the treatment of early hepatocellular carcinoma. J Hepatol. 2013;58(2):306‐311. doi: 10.1016/j.jhep.2012.09.026 [DOI] [PubMed] [Google Scholar]
- 27.Kudo M. Multistep human hepatocarcinogenesis: Correlation of imaging with pathology. J Gastroenterol. 2009;44(Suppl 19):112‐118. doi: 10.1007/s00535-008-2274-6 [DOI] [PubMed] [Google Scholar]
- 28.Renzulli M, Golfieri R. Proposal of a new diagnostic algorithm for hepatocellular carcinoma based on the Japanese guidelines but adapted to the western world for patients under surveillance for chronic liver disease. J Gastroenterol Hepatol. 2016;31(1):69‐80. doi: 10.1111/jgh.13150 [DOI] [PubMed] [Google Scholar]
- 29.Akai H, Matsuda I, Kiryu S, et al. Fate of hypointense lesions on Gd-EOB-DTPA-enhanced magnetic resonance imaging. Eur J Radiol. 2012;81(11):2973‐2977. doi: 10.1016/j.ejrad.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Kumada T, Toyoda H, Tada T, et al. Evolution of hypointense hepatocellular nodules observed only in the hepatobiliary phase of gadoxetate disodium-enhanced MRI. AJR Am J Roentgenol. 2011;197(1):58‐63. doi: 10.2214/ajr.10.5390 [DOI] [PubMed] [Google Scholar]
- 31.Cho YK, Kim JW, Kim MY, Cho HJ. Non-hypervascular hypointense nodules on hepatocyte phase gadoxetic acid-enhanced MR images: Transformation of mr hepatobiliary hypointense nodules into hypervascular hepatocellular carcinomas. Gut Liver. 2018;12(1):79‐85. doi: 10.5009/gnl17046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mulé S, Chalaye J, Legou F, et al. Hepatobiliary MR contrast agent uptake as a predictive biomarker of aggressive features on pathology and reduced recurrence-free survival in resectable hepatocellular carcinoma: Comparison with dual-tracer 18F-FDG and 18F-FCH PET/CT. Eur Radiol. 2020;30(10):5348‐5357. doi: 10.1007/s00330-020-06923-5 [DOI] [PubMed] [Google Scholar]
- 33.Wang F, Numata K, Chuma M, et al. The value of hepatobiliary phase in EOB-MRI in predicting hypervascularization outcome of non-hypervascular hypointense lesions in high-risk patients for hepatocellular carcinoma. Abdom Radiol (NY). 2021;46(6):2527‐2539. doi: 10.1007/s00261-020-02881-0 [DOI] [PubMed] [Google Scholar]
- 34.Briani C, Di Pietropaolo M, Marignani M, et al. Non-hypervascular hypointense nodules at gadoxetic acid MRI: Hepatocellular carcinoma risk assessment with emphasis on the role of diffusion-weighted imaging. J Gastrointest Cancer. 2018;49(3):302‐310. doi: 10.1007/s12029-017-9952-7 [DOI] [PubMed] [Google Scholar]
- 35.Kim T, Baron RL, Nalesnik MA. Infarcted regenerative nodules in cirrhosis: CT and MR imaging findings with pathologic correlation. AJR Am J Roentgenol. 2000;175(4):1121‐1125. doi: 10.2214/ajr.175.4.1751121 [DOI] [PubMed] [Google Scholar]
- 36.Siminzar P, Tohidkia MR, Eppard E, et al. Recent trends in diagnostic biomarkers of tumor microenvironment. Mol Imaging Biol. 2023;25(3):464‐482. doi: 10.1007/s11307-022-01795-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tct-10.1177_15330338241299003 for Hepatobiliary-Phase Hypointense Nodules Without Arterial-Phase Hyperenhancement: Developing a Risk Stratification for Hypervascular Transformation Based on a Real-World Observational Cohort Study by Na Li, Yi Wang, Li Yang, Chun Yang and Mengsu Zeng in Technology in Cancer Research & Treatment