Abstract
Background:
We aimed to assess the likelihood of cause-specific death and other causes of death after gastrectomy for gastric cancer (GC). Additionally, a competing-risk nomogram was developed for patient counseling and decision-making.
Methods:
Eligible GC patients who had gastrectomy between 2007 and 2015 were included in the study from the Surveillance, Epidemiology, and End Results (SEER) database. Death from gastric cancer and death from other causes were considered as separate competing events. Cumulative incidence functions (CIF) were calculated for each event, and a competing-risk nomogram was developed.
Results:
Overall, 8,808 patients who underwent gastrectomy were analyzed. Among them, 4,659 (52.90%) died from gastric cancer and 1,284 (14.58%) died from other causes. The five-year cumulative incidence of cause-specific death for gastric cancer was 50.4%, and 10.2% for deaths from other causes. Several independent factors, such as age at diagnosis, tumor site, grade, size, lymph node examination results, pathological T status, pathological N status, metastatic status, Lauren classification, radiation, and chemotherapy, were found to be associated with gastric cancer-specific death. The nomogram, based on results from the competing risk regression model, demonstrated good performance.
Conclusion:
We have developed a nomogram aimed at predicting gastric cancer-specific mortality in patients following gastrectomy. The model has undergone internal validation, demonstrating good accuracy and reliability. It serves as useful tool that can assist physicians and patients in making more informed clinical decisions.
Keywords: Competing-risk nomogram, Gastric cancer, Survival
Introduction
Gastric cancer (GC) is a prevalent malignancy worldwide, with over one million new cases annually, constituting 5.6% of all cancer diagnoses (1). Despite advancements in treatment, the prognosis for GC remains generally unfavorable, with complete surgical eradication serving as the sole curative approach. Consequently, the accurate assessment of post-gastrectomy outcomes is imperative for both clinicians and patients. The American Joint Committee on Cancer (AJCC) has long provided a foundational framework for prognosis through its TNM staging system, which considers tumor depth (T), lymph node involvement (N), and distant metastases (M). However, the AJCC acknowledges the need for a more comprehensive approach, advocating for the integration of additional tumor-related and host-related factors to enhance prognostic accuracy (2).
Nomograms have been utilized extensively in medical research as a tool for survival analysis, providing a visual representation that simplifies the prediction of a clinical event by incorporating multiple prognostic factors. In the context of gastric cancer, nomograms have been developed to enhance the predictability of outcomes based on an array of postoperative pathological factors (3–6). Traditionally, these nomograms are built upon the Kaplan-Meier survival analysis or Cox proportional hazards regression—methods that might not fully account for the presence of competing risks, such as non-cancer-related mortality, which is particularly relevant given the older age of many GC patients (7–9).
To address the limitations inherent in traditional survival analysis and enhance the practical utility of nomograms for predicting gastric cancer outcomes, we employed Fine and Gray’s competing risk analysis using the Surveillance, Epidemiology, and End Results (SEER) database. This advanced approach allowed us to calculate CIF that accurately measure the unbiased probability of each competing event, such as death from causes other than gastric cancer (10–12). Based on these comprehensive analyses, we have developed a competing-risk nomogram. This tool refines previous models by integrating competing risks into its calculations, offering an advancement in the predictive accuracy of cancer-specific mortality for gastric cancer patients post-gastrectomy. This model not only builds upon the historical use of nomograms in survival analysis but also adapts them to more closely reflect the complexities of real-world clinical scenarios, thereby providing enhanced support for clinical decision-making and personalized patient care.
Materials and Methods
Data Extraction
We obtained US patient data for individuals over 20 yr of age from SEER Research Incidence database (Registration time: Mar 2023). To ensure a minimum of five years of follow-up, the year of diagnosis was restricted to the period between Jan 2007 and Dec 2015. We identified cases of malignant gastric tumors using the International Classification of Diseases for Oncology (ICD-O) codes: 8000/3, 8010/3, 8020/3, 8021/3, 8022/3, 8140/3, 8142/3-8145/3, 8210/3, 8211/3, 8255/3, 8260/3-8263/3, 8310/3, 8323/3, 8480/3, 8481/3, 8490/3. To maintain the integrity of our analysis, we excluded data with missing or incomplete details regarding demographics, clinical pathology, treatment, and follow-up results. Additional exclusion criteria were applied to ensure that patients had undergone gastrectomy and that gastric cancer was the primary tumor. The AJCC pathological TNM staging classification for stomach carcinoma (8th edition, 2017) was retrieved using the collaborative stage data collection system (Version 02.05). Although the SEER database does not include information on D1 gastrectomy (removal of the regional lymph nodes) and D2 gastrectomy (removal of the regional lymph nodes and the nodes along the celiac axis and the common hepatic artery), we analyzed the number of regional lymph nodes examined. The Institutional Review Board-approval was exempt as all patients were de-identified in the database.
Statistical Analysis
Our study introduces a competing risks analysis model to predict cause-specific mortality for gastric cancer patients. This model is based on the proportional subdistribution hazards regression, which allows us to determine the effect of various variables on the risk of cause-specific and all-other-cause mortality. To begin our analysis, we calculated the cumulative incidence functions (CIFs) for each event using the competing risks methodology, as described by Fine and Gray (12). We then compared the differences in CIFs among the groups using Gray’s test. The Cox proportional hazards regression model was also employed to analyze all-cause mortality. The validity of the proportional hazards’ assumption was rigorously assessed using the Schoenfeld residual test. This test is pivotal in confirming whether the underlying assumption of constant hazard ratios over time holds true for the variables included in the model.
Based on the results of the proportional subdistribution hazards regression analysis, we developed a competing-risk nomogram, which can predict cause-specific mortality for gastric cancer patients. We evaluated the accuracy of the nomogram using a calibration plot and assessed the discriminatory ability of the model using the concordance index (C-index) and Brier scores (13). Additionally, we performed a decision curve analysis (DCA) to determine the net benefit (14). The precision of the 1-, 3-, and 5-year mortality predictions was evaluated using the area under the receiver operating characteristic (ROC) curve (AUC). All analyses were conducted using the R software (https://www.r-project.org) and statistical significance was set at a 2-sided P-value of less than 0.05.
Results
Baseline characteristics and 5-years cumulative incidence of death
In the formal analysis, 8,808 gastric cancer patients who underwent gastrectomy were included. Among them, 4,659 (52.90%) died from gastric cancer, while 1,284 (14.58%) died from causes unrelated to gastric cancer during the follow-up period. The average age at diagnosis for individuals who died from other causes was 74.5, with an average survival time of 44.4 months. Conversely, those who died from gastric cancer had an average age at diagnosis of 67.3 yr and an average survival time of 20.6 months.
Table 1 provides an overview of the baseline characteristics and the 5-year cumulative incidence of mortality in the two groups. The majority of patients (80%) underwent non-total gastrectomy, and nearly half had fewer than 15 lymph nodes assessed. The 5-year cumulative incidence of death from causes other than gastric cancer was 10.2%, with this rate rising alongside increasing age (3.7% for those aged 18–59, 7.6% for 60–69, 11.9% for 70–79, and 20.4% for those over 79). In contrast, the 5-year cumulative incidence of death specifically from gastric cancer was 50.4%, and this risk escalated with larger tumor size, higher grade, advanced pathological T status, and elevated pathological N status. Among the 1,007 patients with metastasis, the likelihood of dying from gastric cancer within five years was nearly double that of patients without metastatic disease (88.6% versus 45.5%). Fig. 1 visually represents the cumulative incidence plots for all these variables.
Table 1:
baseline characteristics and 5-years cumulative incidence of death (%) among patients with surgically resected gastric cancer
| Variable | Total Patients (n=8,808) | Gastric cancer-specific death (n=4,659) | Other causes of death (n=1,284) | Total Patients (n=8,808) | Gastric cancer-specific death (n=4,659) | Other causes of death (n=1,284) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NO. | % | 5-Y | P | 5-Y | P | NO | % | 5-Y | P | 5-Y | P | ||
| Gender | 0.35 | 0.79 | Grade III | 5783 | 65.7 | 55.8 | 9.2 | ||||||
| Male | 5281 | 60.0 | 51.0 | 10.6 | Grade IV | 228 | 2.6 | 62.0 | 6.3 | ||||
| Female | 3527 | 40.0 | 49.5 | 9.7 | LNs examined | <0.001 | <0.001 | ||||||
| Age | <0.001 | <0.001 | <15 | 4372 | 49.6 | 50.2 | 11.9 | ||||||
| 18–59 | 2451 | 27.8 | 49.7 | 3.7 | 15–30 | 3352 | 38.1 | 50.4 | 9.2 | ||||
| 60–69 | 2133 | 24.2 | 48.7 | 7.6 | >30 | 979 | 11.1 | 49.1 | 6.6 | ||||
| 70–79 | 2532 | 28.7 | 50.2 | 11.9 | unknown | 105 | 1.2 | 69.2 | 6.9 | ||||
| >79 | 1692 | 19.2 | 53.8 | 20.4 | pT(8th) | <0.001 | <0.001 | ||||||
| Race | <0.001 | <0.001 | pT1a | 665 | 7.5 | 11.3 | 11.3 | ||||||
| Hispanic | 1759 | 20.0 | 49.3 | 7.7 | pT1b | 1164 | 13.2 | 17.6 | 13.8 | ||||
| NH AA | 64 | 0.7 | 54.6 | 15.6 | pT2 | 1153 | 13.1 | 28.7 | 14.4 | ||||
| NH AP | 1915 | 21.7 | 44.1 | 8.3 | pT3 | 3163 | 35.9 | 57.2 | 9.9 | ||||
| NH Black | 1272 | 14.4 | 53.0 | 11.7 | pT4a | 1914 | 21.7 | 74.2 | 7.2 | ||||
| NH White | 3784 | 43.0 | 53.2 | 11.8 | pT4b | 749 | 8.5 | 81.0 | 6.3 | ||||
| NH Unknown | 14 | 0.2 | 22.6 | 0 | pN(8th) | <0.001 | <0.001 | ||||||
| Marital status | 0.003 | <0.001 | pN0 | 3206 | 36.4 | 23.6 | 13.0 | ||||||
| Divorced | 762 | 8.7 | 52.3 | 13.0 | pN1 | 1718 | 19.5 | 51.2 | 10.9 | ||||
| Married | 5309 | 60.3 | 49.5 | 8.5 | pN2 | 1644 | 18.7 | 64.5 | 8.1 | ||||
| Single | 1066 | 12.1 | 53.3 | 9.7 | pN3a | 1565 | 17.8 | 74.8 | 7.8 | ||||
| Widowed | 1400 | 15.9 | 51.8 | 15.6 | pN3b | 675 | 7.7 | 85.8 | 6.2 | ||||
| Unknown | 271 | 3.1 | 43.6 | 9.9 | M(8th) | <0.001 | <0.001 | ||||||
| Primary site | <0.001 | 0.008 | M0 | 7801 | 88.6 | 45.5 | 11.0 | ||||||
| Cardia | 1521 | 17.3 | 52.0 | 9.4 | M1 | 1007 | 11.4 | 88.6 | 4.5 | ||||
| Fundus | 315 | 3.6 | 52.0 | 10.0 | Lauren | <0.001 | <0.001 | ||||||
| Body | 940 | 10.7 | 46.2 | 9.8 | Diffuse | 2380 | 27.0 | 57.6 | 7.1 | ||||
| Gastric antrum | 3010 | 34.2 | 47.6 | 11.3 | Intestinal | 6063 | 68.8 | 47.6 | 11.6 | ||||
| Pylorus | 489 | 5.6 | 57.2 | 8.4 | Mixed | 365 | 4.1 | 50.5 | 8.4 | ||||
| Lesser curvature | 1255 | 14.2 | 47.2 | 9.7 | Surgery | 0.004 | 0.293 | ||||||
| Greater curvature | 531 | 6.0 | 48.9 | 11.8 | Total G | 1764 | 20.0 | 53.6 | 9.9 | ||||
| Overlapping | 747 | 8.5 | 64.9 | 9.4 | Non-total G | 7044 | 80.0 | 49.6 | 10.3 | ||||
| Tumor size(mm) | <0.001 | <0.001 | Radiation | 0.004 | <0.001 | ||||||||
| <30 | 2308 | 26.2 | 27.7 | 11.5 | Yes | 2716 | 30.8 | 50.4 | 6.6 | ||||
| 30–49 | 2480 | 28.2 | 50.0 | 11.9 | No/unknown | 6092 | 69.2 | 50.4 | 11.9 | ||||
| 50–69 | 1980 | 22.5 | 59.7 | 9.3 | Chemotherapy | <0.001 | <0.001 | ||||||
| >69 | 2040 | 23.2 | 67.8 | 7.8 | Yes | 3724 | 42.3 | 56.5 | 6.0 | ||||
| Grade | <0.001 | <0.001 | No/unknown | 5084 | 57.7 | 45.9 | 13.4 | ||||||
| Grade I | 415 | 4.7 | 24.5 | 12.6 | |||||||||
| Grade II | 2382 | 27.0 | 40.7 | 12.8 | |||||||||
Abbreviations: NH: Non-Hispanic; AA: American Indian/Alaska Native; AP: Asian or Pacific Islander; LNs: Lymph nodes; G: gastrectomy.
Fig. 1:
Cumulative incidence curves of death based on characteristics (solid line represents cause-specific death; dotted line represents other cause of death)
A competing-risk nomogram
The results of the Fine and Gray competing-risk regression and Cox proportional hazards regression model are presented in Table 2. No major violations of the proportional hazards assumption were detected. Independent variables, including age at diagnosis, tumor site, grade, size, lymph nodes examined, pathological T status, pathological N status, metastatic status, Lauren classification, radiation and chemotherapy, were associated with gastric cancer-specific death.
Table 2:
Hazard models of probabilities of death for surgically resected gastric cancer
| Variable | Proportional subdistribution hazards model | Cox proportional hazards model | ||
|---|---|---|---|---|
| Characteristic | sdHR (95%CI) | P | HR (95%CI) | P |
| Female Gender | 1.01 (0.95–1.08) | 0.77 | 0.97 (0.92–1.02) | 0.3 |
| Age at diagnosis (yr) | ||||
| 60–69 | 1.08 (1.00–1.17) | 0.06 | 1.12 (1.04–1.21) | 0.003 |
| 70–79 | 1.24 (1.14–1.34) | <0.001 | 1.41 (1.31–1.51) | <0.001 |
| >79 | 1.29 (1.16–1.44) | <0.001 | 2.04 (1.89–2.20) | <0.001 |
| Race | ||||
| NH AA | 1.39 (0.96–2.03) | 0.08 | 1.50 (1.13–1.99) | 0.004 |
| NH AP | 0.93 (0.85–1.02) | 0.12 | 0.89 (0.82–0.97) | 0.006 |
| NH Black | 1.09 (0.98–1.21) | 0.10 | 1.22 (1.12–1.33) | <0.001 |
| NH White | 1.09 (1.00–1.18) | 0.05 | 1.24 (1.16–1.33) | <0.001 |
| NH Unknown | 0.62 (0.21–1.85) | 0.39 | 0.28 (0.09–0.87) | 0.03 |
| Marital status | ||||
| Married | 1.00 (0.90–1.12) | 0.94 | 0.85 (0.77–0.93) | <0.001 |
| Single | 1.06 (0.92–1.21) | 0.43 | 0.99 (0.88–1.11) | 0.84 |
| Widowed | 1.00 (0.88–1.15) | 0.96 | 1.17 (1.05–1.30) | 0.003 |
| Unknown | 0.95 (0.76–1.17) | 0.61 | 0.77 (0.64–0.92) | 0.003 |
| Primary site | ||||
| Fundus | 0.80 (0.66–0.96) | 0.01 | 1.04 (0.90–1.20) | 0.6 |
| Body | 0.72 (0.64–0.82) | <0.001 | 0.91 (0.82–1.00) | 0.06 |
| Gastric antrum | 0.73 (0.67–0.81) | <0.001 | 0.99 (0.92–1.07) | 0.77 |
| Pylorus | 0.84 (0.72–0.98) | 0.02 | 1.13 (1.00–1.28) | 0.04 |
| Lesser curvature | 0.71 (0.64–0.79) | <0.001 | 0.92 (0.84–1.01) | 0.07 |
| Greater curvature | 0.81 (0.70–0.94) | 0.01 | 1.00 (0.89–1.13) | 0.94 |
| Overlapping | 0.80 (0.70–0.91) | <0.001 | 1.47 (1.33–1.62) | <0.001 |
| Tumor size (mm) | ||||
| 30–49 | 1.07 (0.97–1.18) | 0.19 | 1.78 (1.65–1.92) | <0.001 |
| 50–69 | 1.11 (1.00–1.23) | 0.046 | 2.12 (1.96–2.29) | <0.001 |
| >69 | 1.12 (1.01–1.25) | 0.03 | 2.57 (2.39–2.78) | <0.001 |
| Grade | ||||
| Grade II | 1.02 (0.85–1.23) | 0.84 | 1.44 (1.25–1.67) | <0.001 |
| Grade III | 1.19 (0.99–1.43) | 0.06 | 1.90 (1.66–2.19) | <0.001 |
| Grade IV | 1.40 (1.09–1.80) | 0.008 | 2.13 (1.74–2.61) | <0.001 |
| LNs examined | ||||
| 15–30 | 0.73 (0.68–0.79) | <0.001 | 0.92 (0.87–0.97) | 0.002 |
| >30 | 0.61 (0.55–0.68) | <0.001 | 0.81 (0.75–0.89) | <0.001 |
| unknown | 1.46 (1.08–1.96) | 0.012 | 1.49 (1.19–1.85) | <0.001 |
| pT(8th) | ||||
| pT1b | 1.44 (1.13–1.83) | 0.003 | 1.33 (1.13–1.56) | <0.001 |
| pT2 | 2.12 (1.67–2.69) | <0.001 | 1.99 (1.70–2.32) | <0.001 |
| pT3 | 3.93 (3.13–4.94) | <0.001 | 3.48 (3.03–4.01) | <0.001 |
| pT4a | 5.09 (4.03–6.43) | <0.001 | 5.25 (4.55–6.06) | <0.001 |
| pT4b | 6.07 (4.73–7.78) | <0.001 | 6.86 (5.87–8.00) | <0.001 |
| pN(8th) | ||||
| pN1 | 1.83 (1.65–2.03) | <0.001 | 1.94 (1.80–2.10) | <0.001 |
| pN2 | 2.35 (2.12–2.60) | <0.001 | 2.48 (2.30–2.67) | <0.001 |
| pN3a | 3.01 (2.69–3.36) | <0.001 | 3.33 (3.09–3.59) | <0.001 |
| pN3b | 4.33 (3.78–4.97) | <0.001 | 4.58 (4.16–5.04) | <0.001 |
| M1(8th) | 1.74 (1.58–1.91) | <0.001 | 3.10 (2.89–3.33) | <0.001 |
| Lauren | ||||
| Intestinal | 0.90 (0.83–0.97) | 0.004 | 0.90 (0.85–0.96) | <0.001 |
| Mixed | 1.03 (0.88–1.21) | 0.7 | 0.94 (0.82–1.07) | 0.34 |
| Non-total gastrectomy | 1.03 (0.95–1.11) | 0.51 | 0.92 (0.86–0.98) | 0.007 |
| No/unknown Radiation | 1.32 (1.22–1.43) | <0.001 | 1.30 (1.23–1.37) | <0.001 |
| No/unknown Chemotherapy | 1.26 (1.17–1.37) | <0.001 | 1.05 (1.00–1.11) | 0.06 |
Abbreviations: sdHR: subdistribution hazard ratio; HR: hazard ratio; CI: confidence interval.
With advancing age at diagnosis, the risk of gastric cancer-specific death increased: 60–69 yr (sdHR 1.08, P=0.06), 70–79 yr (sdHR 1.24, P<0.001), and >79 yr (sdHR 1.29, P<0.001). The Cox proportional hazards model also showed a significant increase of all cause death in the hazard ratio (HR) for these age groups, being 1.12, 1.41, and 2.04 respectively, all with P<0.001.
Advanced pathological T status was linked to a higher probability of death, with significant sdHR and HR values for pT1b (sdHR 1.44, HR 1.33), pT2 (sdHR 2.12, HR 1.99), pT3 (sdHR 3.93, HR 3.48), pT4a (sdHR 5.09, HR 5.25), and pT4b (sdHR 6.07, HR 6.86), all with P<0.001 compared to pT1a.
Patients with advanced pN status were more likely to die of gastric cancer, with sdHR and HR values for pN1 (sdHR 1.83, HR 1.94), pN2 (sdHR 2.35, HR 2.48), pN3a (sdHR 3.01, HR 3.33), and pN3b (sdHR 4.33, HR 4.58), all with P<0.001 compared to pN0 stage.
Examining a higher number of lymph nodes was associated with a decreased probability of gastric cancer-specific death, with sdHR of 0.73 (95% CI 0.68–0.79) for 15–30 lymph nodes and 0.61 (95% CI 0.55–0.68) for >30 lymph nodes, both with P<0.001. Race was also considered in the final model to address differences in incidence and clinical prognosis across races (15–17).
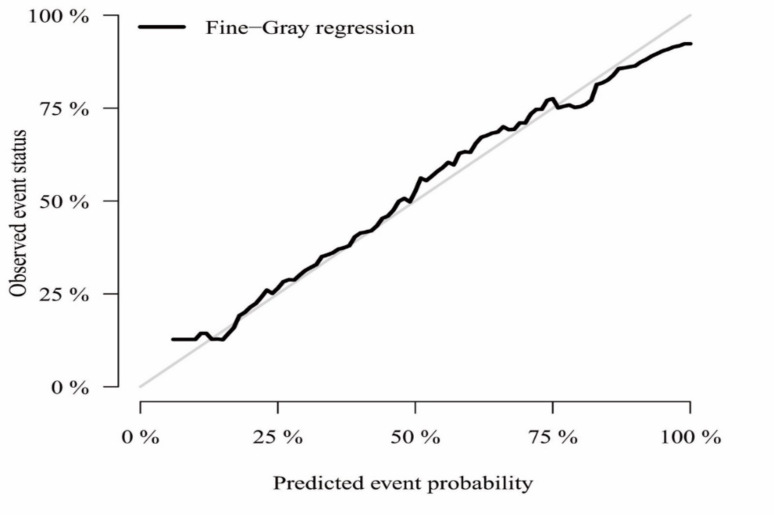
A nomogram was constructed from the competing-risk regression model’s results (Fig. 2). This device enables the estimation of 1-year, 3-year, and 5-year probabilities for gastric cancer-specific mortality. Calibration curves matched closely with the standard, indicating the nomogram’s accuracy (Fig. 3).
Fig. 2:
Competing-risk nomogram for predicting probabilities of cause-specific death of 1-, 3-, 5- year for postsurgical gastric cancer patients. CSD: cause-specific death
Fig. 3:
A calibration plot for assessing the accuracy of the competing-risk nomograms
The model’s precision was further substantiated by evaluations of the C-indexes, AUC, and Brier scores, achieving robust outcomes (with C-indexes of 0.76 across the board, AUCs of 0.79, 0.82, and 0.82, along with Brier scores of 0.14, 0.17, and 0.17 at 1, 3, and 5-year intervals, respectively) (Table 3). Decision curve analysis was utilized to gauge the clinical net benefit, with both the competing-risk model and the Cox model displaying utility (Fig. 4).
Table 3:
The discriminatory ability of three models
| Variable | Competing risk model | Cox model | AJCC staging system (8th) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1-year | 3-year | 5-year | 1-year | 3-year | 5-year | 1-year | 3-year | 5-year | |
| C-index | 0.76 | 0.76 | 0.75 | 0.76 | 0.75 | 0.75 | 0.69 | 0.70 | 0.69 |
| AUC | 0.79 | 0.82 | 0.82 | 0.80 | 0.82 | 0.82 | 0.72 | 0.78 | 0.78 |
| Brier score | 0.14 | 0.17 | 0.17 | 0.15 | 0.16 | 0.16 | 0.17 | 0.18 | 0.18 |
Abbreviations: AUC: Area Under The Curve.
Fig. 4:
Decision curve analysis for the risk model for cause-specific mortality and the Cox model for any causes of death.
Discussion
While the incidence of gastric cancer is on a decline, it remains a significant health concern, ranking as the fifth most common cancer globally (18). Surgery is acknowledged as the primary treatment choice, and accurately assessing a patient’s prognosis post-surgery is of paramount importance. The nomogram model, which integrates multiple prognostic variables, offers physicians a straightforward and readily available method to make precise prognoses and anticipate survival rates. Due to their precision, practicality, and sophisticated capacity to differentiate between outcomes, nomogram models have become increasingly favored in the field of oncology. One of the classic models is the 2003 Memorial Sloan Kettering Cancer Center (MSKCC) nomogram for projecting gastric cancer outcomes (3). Although previous studies have presented various useful nomograms for this disease, they have commonly neglected the significance of non-gastric cancer-related death (2, 3, 19). In our research, we have developed a competing-risk nomogram that is capable of predicting the risk of death specifically from gastric cancer in individual patients following gastrectomy. This model employs independent prognostic factors that have been identified using proportional subdistribution hazards regression analysis, with the aim of enhancing patient prognosis and treatment outcomes.
Our model marks advancement in predictive tools by distinguishing between deaths caused by gastric cancer and those resulting from other causes, treating these as two separate competing risks. During the follow-up period, 4,659 patients, accounting for 52.90% of the cohort, died from gastric cancer, while more than 1,200, representing 14.6%, died from other causes. Recognizing the substantial number of deaths unrelated to gastric cancer is critical for accurate prognosis as well as for informing patient counseling and decision-making processes. To accommodate this, we estimated the cumulative incidence for each category of competing risks. Using the findings from the proportional subdistribution hazards regression, we performed a competing-risk nomogram that delivers precise estimations of the likelihood of cause-specific mortality for patients who have undergone gastrectomy. Our nomogram is particularly beneficial for patients with fewer than 16 examined lymph nodes, who are not adequately categorized by the current AJCC TNM staging system. Additionally, it proves valuable for patients undergoing palliative surgery, which is gaining importance as a viable treatment option in light of advancements in adjuvant therapies (20, 21).
The existing literature offers a multifaceted view on the impact of age on the prognosis of gastric cancer patients’ post-surgical resection. On one hand, older age did not increase the risk of severe postoperative complications and did not significantly impact surgical outcomes (22). However, Dutch researchers studied a large cohort of 2,315 surgically treated patients with gastric cancer and found that the elderly had a higher thirty-day postoperative mortality rate than younger adults (7.9% vs. 3.2%, with a P-value of less than 0.001) (23). Advanced age correlates with an increased risk of death from gastric cancer, adding another dimension to the ongoing debate by suggesting that older patients may possess inherent vulnerabilities that affect cancer-related outcomes post-resection. The divergent findings in the literature, coupled with our research, highlight the intricate relationship between age and prognosis in gastric cancer surgery. This relationship may be influenced by a multitude of factors, including the patient’s overall health, the stage of cancer at diagnosis, the type of surgical intervention, and the quality of postoperative care. This complexity underscores the importance of adopting personalized medical approaches that take into account age alongside other patient-specific factors to optimize outcomes for gastric cancer patients undergoing surgery.
Elderly patients frequently exhibit a favorable histological phenotype, specifically the Lauren’s intestinal type, and are more likely to have well-differentiated cancers (24, 25). These characteristics are associated with improved outcomes following gastrectomy surgery. Our research corroborates this trend, demonstrating that elderly individuals generally experience longer survival times compared to their younger counterparts (mean survival time: 44.4 months vs. 20.6 months), aligning with previous findings (24, 26). This survival advantage may be partly attributed to the underdeveloped screening programs for early detection of gastric cancer in younger populations in the United States, which results in fewer young patients being diagnosed early and more instances of delayed diagnoses (7). Additionally, young gastric cancer patients often present with fewer or less pronounced symptoms, which can further contribute to the delay in diagnosis (27, 28).
Previous research has yielded inconsistent findings regarding the influence of gender on overall survival in gastric cancer patients. Men experience poorer survival outcomes (29). whereas others have suggested that women face worse prognoses due to factors like younger age, poorly differentiated cancer, and the presence of signet ring cell carcinoma (30). In contrast, our findings did not reveal a significant difference in gastric cancer-specific death rates between men and women. This discrepancy underscores the necessity for further investigation to elucidate the gender-related disparities in gastric cancer outcomes from alternative perspectives. Additional research is essential to provide a clearer understanding of this complex issue.
Marital status did not seem to affect gastric cancer-specific mortality, which contradicts a previous study using the SEER cohort (31). The discrepancy between the two studies may stem from the differing criteria used to define endpoints. Deaths unrelated to gastric cancer were treated as censored events, whereas in our analysis, they were considered competing events. This methodological difference could explain the divergent results regarding the impact of marital status on gastric cancer outcomes.
Following the development of a nomogram designed to forecast outcomes for patients who have undergone gastrectomy for gastrointestinal cancer, we assessed its predictive accuracy through various evaluation methods. The calibration curves indicated a robust concordance between the predicted and observed outcomes, confirming the model’s reliability. Subsequent analyses, including the calculation of the C-index, AUC (Area Under the Curve), and Brier score, demonstrated the model’s exceptional predictive capabilities at 1, 3, and 5 yr post-gastrectomy. Additionally, the decision curve analysis affirmed that the model offers a clinically significant net benefit, underscoring its practical utility in clinical decision-making.
While the study presents valuable insights, it is important to acknowledge its limitations. Firstly, despite leveraging data from a publicly accessible database rich with gastric cancer cases, the nomograms developed require further external validation to ascertain their predictive precision and effectiveness. Secondly, the constraints of the variables available within the SEER database meant we could not examine the relationship between specific clinical variables like genetics, biomarkers, family history, and complications. Thirdly, the patient data was exclusively sourced from the U.S., the applicability of the nomogram’s conclusions may be limited in different international contexts. Additionally, the retrospective nature of database-based studies introduces the potential for selection bias. Prospective studies conducted across multiple centers with extensive sample sizes are essential for the nomogram’s validation. Lastly, our nomogram may not encompass all prognostic variables related to gastric cancer; however, it incorporates and validates key variables, which minimizes the likelihood of significant inaccuracy.
Conclusion
Our comprehensive analysis has identified key factors such as age at diagnosis, tumor characteristics, and treatment modalities as significant predictors of gastric cancer-specific death post-surgery. By integrating these factors into a competing-risk nomogram, we have developed a tool that enhances the accuracy of prognostic assessments for individual patients. This model is not only a resource for clinicians in tailoring treatment strategies but also serves as a guide for patient counseling, ensuring that patients are well-informed about their prognosis and potential outcomes.
Journalism Ethics considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Footnotes
Data Availability
All data for this study was provided to us and abstracted from Surveillance, Epidemiology, and End Results (SEER) Program, which is publicly available upon request.
Fund
None.
Conflict of Interest
The authors declare that they have no conflict of interest
References
- 1.Sung H, Ferlay J, Siegel RL, et al. (2021). Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin, 71 (3):209–249. [DOI] [PubMed] [Google Scholar]
- 2.Woo Y, Son T, Song K, et al. (2016). A Novel Prediction Model of Prognosis After Gastrectomy for Gastric Carcinoma: Development and Validation Using Asian Databases. Ann Surg, 264 (1):114–20. [DOI] [PubMed] [Google Scholar]
- 3.Kattan MW, Karpeh MS, Mazumdar M, Brennan MF. (2003). Postoperative nomogram for disease-specific survival after an R0 resection for gastric carcinoma. J Clin Oncol, 21 (19):3647–50. [DOI] [PubMed] [Google Scholar]
- 4.Han DS, Suh YS, Kong SH, et al. (2012). Nomogram predicting long-term survival after d2 gastrectomy for gastric cancer. J Clin Oncol, 30 (31):3834–40. [DOI] [PubMed] [Google Scholar]
- 5.Song KY, Park YG, Jeon HM, Park CH. (2014). A nomogram for predicting individual survival of patients with gastric cancer who underwent radical surgery with extended lymph node dissection. Gastric Cancer, 17 (2):287–93. [DOI] [PubMed] [Google Scholar]
- 6.Hirabayashi S, Kosugi S, Isobe Y, et al. (2014). Development and external validation of a nomogram for overall survival after curative resection in serosa-negative, locally advanced gastric cancer. Ann Oncol, 25 (6):1179–84. [DOI] [PubMed] [Google Scholar]
- 7.Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. (2014). Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev, 23 (5):700–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergmann MM, Rehm J, Klipstein-Grobusch K, et al. (2013). The association of pattern of lifetime alcohol use and cause of death in the European prospective investigation into cancer and nutrition (EPIC) study. Int J Epidemiol, 42 (6):1772–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lacny S, Wilson T, Clement F, et al. (2018). Kaplan-Meier survival analysis overestimates cumulative incidence of health-related events in competing risk settings: a meta-analysis. J Clin Epidemiol, 93:25–35. [DOI] [PubMed] [Google Scholar]
- 10.Scrucca L, Santucci A, Aversa F. (2010). Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transplant, 45 (9):1388–95. [DOI] [PubMed] [Google Scholar]
- 11.Han SS, Rivera GA, Tammemagi MC, et al. (2017). Risk Stratification for Second Primary Lung Cancer. J Clin Oncol, 35 (25):2893–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fine JP. (2001). Regression modeling of competing crude failure probabilities. Biostatistics, 2 (1):85–97. [DOI] [PubMed] [Google Scholar]
- 13.Wolbers M, Koller MT, Witteman JC, Steyerberg EW. (2009). Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology, 20 (4):555–61. [DOI] [PubMed] [Google Scholar]
- 14.Vickers AJ, Elkin EB. (2006). Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making, 26 (6):565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theuer CP, Kurosaki T, Ziogas A, Butler J, Anton-Culver H. (2000). Asian patients with gastric carcinoma in the United States exhibit unique clinical features and superior overall and cancer specific survival rates. Cancer, 89 (9):1883–92. [DOI] [PubMed] [Google Scholar]
- 16.Strong VE, Song KY, Park CH, et al. (2010). Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg, 251 (4):640–6. [DOI] [PubMed] [Google Scholar]
- 17.Howard JH, Hiles JM, Leung AM, Stern SL, Bilchik AJ. (2015). Race influences stage-specific survival in gastric cancer. Am Surg, 81 (3):259–67. [PubMed] [Google Scholar]
- 18.Bray F, Laversanne M, Sung H, et al. (2024). Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 74 (3):229–263. [DOI] [PubMed] [Google Scholar]
- 19.Chen QY, Zhong Q, Wang W, et al. (2019). Development and external validation of a nomogram for predicting the conditional probability of survival after D2 lymphadenectomy for gastric cancer: A multicentre study. Eur J Surg Oncol, 45(10):1934–1942. [DOI] [PubMed] [Google Scholar]
- 20.Karpeh MS., Jr (2013). Palliative treatment and the role of surgical resection in gastric cancer. Dig Surg, 30 (2):174–80. [DOI] [PubMed] [Google Scholar]
- 21.Li C, Yan M, Chen J, et al. (2010). Survival benefit of non-curative gastrectomy for gastric cancer patients with synchronous distant metastasis. J Gastrointest Surg, 14 (2):282–8. [DOI] [PubMed] [Google Scholar]
- 22.Kim MG, Kim HS, Kim BS, Kwon SJ. (2013). The impact of old age on surgical outcomes of totally laparoscopic gastrectomy for gastric cancer. Surg Endosc, 27 (11):3990–7. [DOI] [PubMed] [Google Scholar]
- 23.Nelen SD, Bosscha K, Lemmens V, et al. (2018). Morbidity and mortality according to age following gastrectomy for gastric cancer. Br J Surg, 105 (9):1163–1170. [DOI] [PubMed] [Google Scholar]
- 24.Tavares A, Gandra A, Viveiros F, Cidade C, Maciel J. (2013). Analysis of clinicopathologic characteristics and prognosis of gastric cancer in young and older patients. Pathol Oncol Res, 19 (1):111–7. [DOI] [PubMed] [Google Scholar]
- 25.Hsieh FJ, Wang YC, Hsu JT, Liu KH, Yeh CN. (2012). Clinicopathological features and prognostic factors of gastric cancer patients aged 40 years or younger. J Surg Oncol, 105 (3):304–9. [DOI] [PubMed] [Google Scholar]
- 26.Saito H, Takaya S, Fukumoto Y, Osaki T, Tatebe S, Ikeguchi M. (2012). Clinicopathologic characteristics and prognosis of gastric cancer in young patients. Yonago Acta Med, 55 (3):57–61. [PMC free article] [PubMed] [Google Scholar]
- 27.Maconi G, Kurihara H, Panizzo V, et al. (2003). Gastric cancer in young patients with no alarm symptoms: focus on delay in diagnosis, stage of neoplasm and survival. Scand J Gastroenterol, 38 (12):1249–55. [DOI] [PubMed] [Google Scholar]
- 28.Boldys H, Marek TA, Wanczura P, Matusik P, Nowak A. (2003). Even young patients with no alarm symptoms should undergo endoscopy for earlier diagnosis of gastric cancer. Endoscopy, 35 (1):61–7. [DOI] [PubMed] [Google Scholar]
- 29.Camargo MC, Goto Y, Zabaleta J, et al. (2012). Sex hormones, hormonal interventions, and gastric cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev, 21 (1):20–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim HW, Kim JH, Lim BJ, et al. (2016). Sex Disparity in Gastric Cancer: Female Sex is a Poor Prognostic Factor for Advanced Gastric Cancer. Ann Surg Oncol, 23 (13):4344–4351. [DOI] [PubMed] [Google Scholar]
- 31.Jin JJ, Wang W, Dai FX, et al. (2016). Marital status and survival in patients with gastric cancer. Cancer Med, 5 (8):1821–9. [DOI] [PMC free article] [PubMed] [Google Scholar]