Abstract
Aging is a complex process associated with multimorbidity. Hypertension, one of widespread states, is among main causes of age-related alterations in behavior, emotionality and sociability. We studied the effects of long-term isolated housing on anxiety, depressive-like and social behavior as well as changes in the adrenocortical and sympathetic systems in the aging normotensive Wistar Kyoto (WKY) and spontaneously hypertensive rats (SHR). Ten-month-old male rats of both strains were subjected to 90-day isolated or group housing. Surprisingly, social isolation induced only mild effect on anxiety without influencing other affective-related behaviors. No effects of isolated housing on sociability or social novelty preferences were revealed. Despite the adrenal gland hypertrophy in the SHRs, corticosterone levels remained stable within the period of isolation but the expression of nuclear glucocorticoid receptor (Nr3c1) mRNA in the adrenals was lower in the SHR as compared to WKY rats. Pre-existing hypertension, associated with SHR genotype, did not significantly contribute to the effects of social isolation. The data suggest that the aged WKY and SHR rats are relatively resilient to chronic social stress associated with isolated housing.
Keywords: Aging, Social isolation, Hypertension, Spontaneously hypertensive rats, Wistar-Kyoto rats, Anxiety, Depression, Social preference and novelty, Corticosterone
Subject terms: Neuroscience, Physiology
Introduction
Social isolation/loneliness in elderly people have become a problem in many developed and developing countries around the globe. About one half of people over 60 years old suffer from loneliness occasionally or frequently1,2. Social isolation has both objective and subjective dimensions. Objective social isolation means physical separation from or limited interaction of the subject with other individuals. However, a subject can interpret his/her own insufficiently close relationships with other family members or the limited involvement in social networks as a form of isolation or rather loneliness, thus shaping the subjective aspect of social isolation. The subjective feeling of social isolation, loneliness, is gaining much attention recently in the light of COVID-19 pandemic.
Isolation or separation of an individual from other members of society or population is an important determinant of health. An association was revealed between prolonged social isolation, loneliness and the development of affective disorders, cognitive impairments and dementia3. In older adults, the effects of isolation on mental health may depend significantly on the age of the person and be strongly associated with the individual’s perception of isolation3,4. However, the relationship between social isolation and mental health consequences in older people remain poorly studied, especially the underlying brain mechanisms5.
Aging is associated with multimorbidity, i.e. co-occurrence of two or more diseases, each lasting more than one year6. A relationship between aging and increased vulnerability to various cardiovascular diseases has been well established6,7. While cardiovascular diseases remain the leading cause of morbidity and mortality among senior people, depression is the second most important cause of illness, which often remains underestimated in the elderly8. Heart diseases and depression are often interrelated making vital studies on central mechanisms promoting this interaction necessary. Symptoms of depression, but not anxiety are dose-dependently related to an increased risk of heart failure9, and depression also increases the risk of hypertension10. In the elderly, loneliness not only aggravates various medical conditions, including heart diseases, cardiovascular diseases, hypertension, metabolic disorders, and other, but, becoming a chronic state, it is associated with psychological stress and depression11. However, the contribution of objective and subjective aspects of social isolation and loneliness in the mechanisms of this multifaceted medical condition among older people remains largely obscure.
To study these mechanisms of isolation consequences, models of social isolation in laboratory rodents are routinely used. Rats or mice are usually housed in small groups. After being introduced into a new cage, the rats exhibit some aggressive behaviors with fighting, but later they live together relatively peacefully, playing and sleeping together, crawling under or walking over each other, allogrooming, sniffing, and so on12. These different forms of “sociability” allow us to consider rats and mice as social animals, albeit with some caution13. Since sociability is less variable in rats than in mice12, rats seem to be a more suitable animal model for studying the effects of long-term social isolation.
In the present study, we used aging spontaneously hypertensive rats (SHRs) to investigate the effects of long-term social isolation on anxiety, depressive-like and social behavior. Wistar Kyoto (WKYs) rats were used as a normotensive control. In contrast to the effects of isolation or separation from parents during early ontogeny, the consequences of isolated housing in the adult and aging animals have been less studied. The SHR strain provides a unique opportunity to study the effects of isolated housing on the age-associated multimorbid conditions. SHRs are not only a model for genetically encoded hypertension, most widely distributed age-related human pathology, but also a model of attention deficit and hyperactivity disorders14,15, type 2 diabetes mellitus-like insulin resistance16, and several other pathologies. SHR and WKY rats exhibit practically opposite coping strategies in various stress-associated behavioral paradigms. For example, in the forced swim test (FST), SHRs were more active than WKYs17–21. WKY rats showed lower sucrose preference compared to SHRs or other rat strains indicating the presence of anhedonia22,23. The correlation between behavioral features in these two tests led some authors to consider the WKY strain as a model for depression24,25. Hyperactivity of SHRs in novelty conditions is a well-known feature, and this strain is often used as a model for attention deficit and hyperactivity disorder26,27. Rats of the SHR strain exhibited innate hyperlocomotion in the open field test (OFT)27–31, which was not significantly affected even by regular repeated testing during ontogeny32. Hyperactivity was also revealed during testing in smaller new boxes or actimeters33–35. Some authors have reported increased locomotion of SHRs in the center of the OFT arena32,36, demonstrating lower anxiety of these rats compared to normotensive strains. In some cases, this hyperactivity correlated with lower indices of anxiety in the elevated plus maze (EPM)36–38. However, SHRs exhibited higher anxiety in the novelty feeding test compared to other rat strains35. Seven-day isolation significantly affected the behavior of SHRs and normotensive Lewis rats in the OFT, reducing the number of squares crossed in both central and peripheral parts of the arena, whereas no significant alterations were observed in the EPM36. We hypothesized that isolated housing of animals for a longer period would enhance depressive-like or anxiety-related behaviors in aging rats with comorbid hypertension similar to observations in older people4,39. For this purpose, we subjected 10-month-old male SHR and WKY rats to group or isolated housing for 90 days and after the end of the isolation the behavior of the rats was studied using standard tests for locomotor activity, anxiety, depressiveness, and sociability. We also examined some stress-related biochemical indices in the blood and saliva of the rats during and after the end of the isolation period.
Results
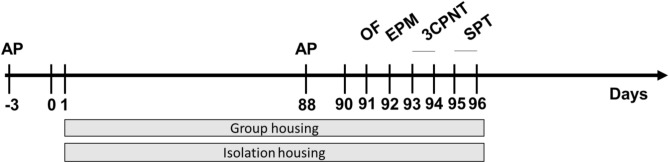
The experimental protocol is presented in Fig. 1 and is described in detail in the Materials and Methods section.
Fig. 1.
Schematic drawing of the experimental protocol.
Effects of chronic isolation on physiological indices
Mortality from unknown reasons was 2 of 12 rats in the SHRc group, 5 of 10 rats in the SHRi group, and 3 of 14 rats in each of the WKYc and WKYi groups. No mortality was observed in either group during first 4 weeks of the isolation period which started when the animals were 40–42 weeks old. Later, in the WKYc group one rat died in weeks 5, 7, and 10; WKYi – one rat in weeks 7, 9, and 14; SHRc—one rat in week 9 and 1 day before the end of the experiment; SHRi – one rat in week 12, three rats in week 13, and one rat in week 14. The main causes of mortality were vascular and renal deficits characteristic for SHRs. The differences in mortality between the groups were not significant (p = 0.16).
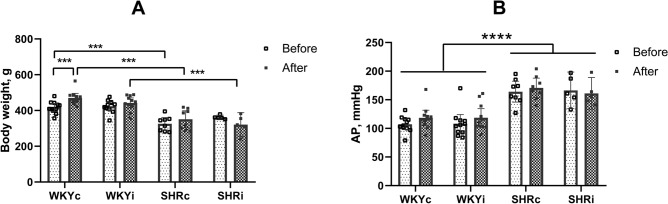
WKY and SHR rats significantly differed in their body weight at the start and during the whole course of the experiment. Data on the body weight of four groups of rats studied at the start and end of the experiment are presented in Fig. 2A. ANOVA-RM revealed the effect of “genotype” (F(1,31) = 56.74, p < 0.0001) with no effect of “isolation” (F(1,31) = 0.09, p = 0.80) or “genotype” × “isolation” interaction (F(1,31) = 0.28, p = 0.70). However, body weight gain during the experimental period changed significantly (factor “start–end” F(1,31) = 5.48, p < 0.05); significant interactions between the factors “start–end” × “genotype” (F(1,31) = 12.96, p < 0.01) and “start–end” × “isolation” (F(1,31) = 17.35, p < 0.001). These data indicate that the changes in the body weight were different in specific groups of animals. Post hoc multiple comparisons showed that body weight gain occurred in the WKYc but not WKYi group while in both SHR groups, the body weight did not change during the observation period.
Fig. 2.
Body weight and blood pressure in the WKY and SHR groups studied. (A) – body weight and (B) – averaged arterial pressure in the WKYc (n = 11), WKYi (n = 11), SHRc (n = 8), and SHRi (n = 5) groups at the start and the end of the experiment. Data are presented as mean and 95%CI. ***—p < 0.001 according to post hoc Tukey HST test. ****—p < 0.0001 – according to ANOVA (factor “genotype”).
Average arterial pressure (AP) was significantly higher in both SHR groups as compared to WKY rats (Fig. 2B; ANOVA “genotype” F(1,31) = 67.58, p < 0.0001). The differences between the control and isolated groups (F(1,31) = 0.053, p = 0.81) and between the initial and final data (F(1,31) = 2.083, p = 0.16) were not found. ANOVA did not reveal interactions between the factors studied.
Thus, we did not observe significant differences in mortality related to strain or chronic isolation. Social isolation for 90 days prevented body weight gain in WKY rats only, and did not affect the average AP level in either experimental group.
General activity
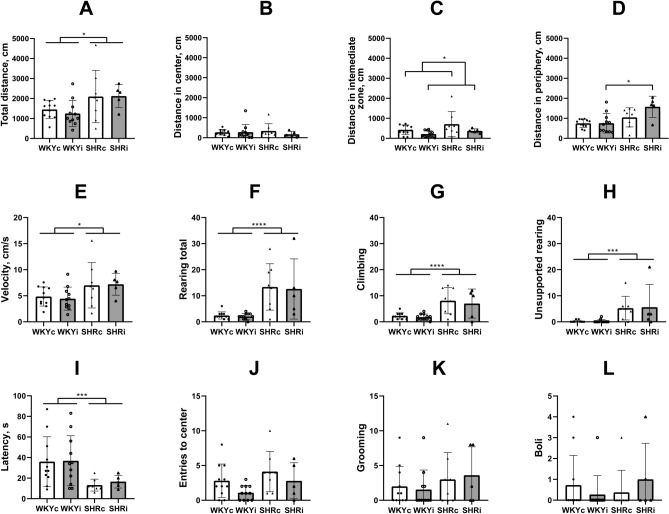
Locomotor activity was studied in the OFT. SHRs traveled longer distance in the arena as compared to WKY rats (Fig. 3A; factor “genotype” F(1,31) = 9.60, p < 0.01), but this effect did not depend on the isolation experience (F(1,31) = 0.016, p = 0.90). Moreover, animals of both SHRc and SHRi groups moved with higher velocity than rats of the WKYc and WKYi groups (Fig. 3E; factor “genotype” F(1,31) = 6.34, p < 0.05) and this phenomenon was not affected by chronic isolation (F(1,31) = 0.013, p = 0.95). However, some differences were revealed when the activity of animals was analyzed in the specific zones of the arena. The animals of all experimental groups did not differ in the length of the distance traveled in the central zone (Fig. 3B) whereas in the peripheral zone, SHRi animals traveled twice longer distance as compared to WKYi rats (Fig. 3D). ANOVA indicated the effect of “genotype” (F(1,31) = 13.81, p < 0.001) and trends to the effect of “isolation” (F(1,31) = 3.14, p = 0.09) and “genotype” × “isolation” interaction (F(1,31) = 3.04, p = 0.092). Isolated animals of both strains left the intermediate zone faster (Fig. 3C; ANOVA “isolation” F(1,31) = 5.02, p < 0.05; “genotype” F(1,31) = 3.26, p = 0.08).
Fig. 3.
Behavior of animals of the WKYc (n = 11), WKYi (n = 11), SHRc (n = 8), and SHRi (n = 5) groups in the open field test. (A) – total distance; (B) – distance in center; (C) – distance in the intermediate zone; (D) – distance in periphery; (E) – averaged velocity; (F) – total rearing; (G) – climbing or supported rearing; H – unsupported or free rearing; I – latency to leave the center; J – entries to center; K – grooming episodes; L – defecation boli. Data are presented as mean and 95%CI. *—p < 0.05, ***—p < 0.001, and ****—p < 0.0001 according to ANOVA (see details in the text).
In addition to higher locomotor activity SHRs showed increased rearing in comparison to WKYs (Fig. 3F; F(1,31) = 23.59, p < 0.0001) and long-term isolation housing did not affect this type of activity (F(1,31) = 0.073, p = 0.80). Same was true for climbing or supported rearing (Fig. 3G), contributing to general or exploratory activity (ANOVA effect of “genotype” (F(1,31) = 21.25, p < 0.0001; effect of “isolation” F(1,31) = 0.44, p = 0.55). The number of unsupported rearing (Fig. 3H) was reduced in WKY rats as compared to SHRs (F(1,31) = 13.75, p < 0.001) and long-term isolated housing did not affect this form of behavior in either strain studied (F(1,31) = 0.021, p = 0.90).
Thus, general activity in a new large arena was significantly higher in SHRs as compared to WKY rats. This was evident in both ambulation and rearing indices. Isolation did not significantly affect behavior in the OFT, although isolated SHRs traveled longer distance within the peripheral zone of the arena as compared to isolated WKY animals. This may be a result of increased thigmotaxis or tendency to avoid the central area because the rats of the WKYi and SHRi groups left the central and intermediate zones faster.
Emotionality and anxiety
Emotionality in the novel conditions was estimated as the number of defecation boli. However, since defecations in the OFT was observed in only small part of animals, we could not find differences in emotionality between strains or types of treatment (Fig. 3L). Additionally, we did not reveal any effect of strain or isolation stress on grooming, an index of emotional tension and/or anxiety in the OFT (Fig. 3K), and number of entries to the central zone of the arena (Fig. 3J). The differences were observed in the latency of leaving the central point of placement to the arena (Fig. 3I), probably because of the major difference in activity in WKY and SHR strains rather than in anxiety or fearfulness (ANOVA effect of “genotype” F(1,31) = 8.69, p < 0.01, effect of “isolation” F(1,31) = 0.084, p = 0.80).Total number of entries into the OA and CA of the EPM was significantly higher in both SHR groups as compared to the WKY groups (Fig. 4A; F(1,31) = 14.78, p < 0.001) directly supporting higher activity of SHRs in novel conditions. Isolated housing did not affect the activity of rats of either strain in the EPM (F(1,31) = 0.18, p = 0.70). The effect of strain was found on the number of OA entries (Fig. 4B; F(1,31) = 26.65, p < 0.0001) and to a lesser extent on the number of CA entries (Fig. 4C; F(1,31) = 5.21, p < 0.05). Long-term isolation did not affect either of these indices (F(1,31) = 0.23, p < 0.65 and F(1,31) = 0.091, p < 0.80 for OA and CA, respectively). In accordance with fewer number of entries into the OA of the EPM in WKY rats, rats of the WKYc and WKYi groups spent shorter time there in comparison with the SHRc and SHRi groups (Fig. 4D; F(1,31) = 24.38, p < 0.0001). However, the time spent by rats of each group in the CA did not differ (Fig. 4E; Fig. 2E; F(1,31) = 2.18, p = 0.16). Notably, only 4 of 11 rats in each of the WKY groups entered the OA of the EPM. On the contrary, all rats of the SHR groups entered the OA at least once (p < 0.01 and p < 0.05 according to Fisher exact test for WKYc vs. SHRc and WKYi vs. SHRi, respectively).
Fig. 4.
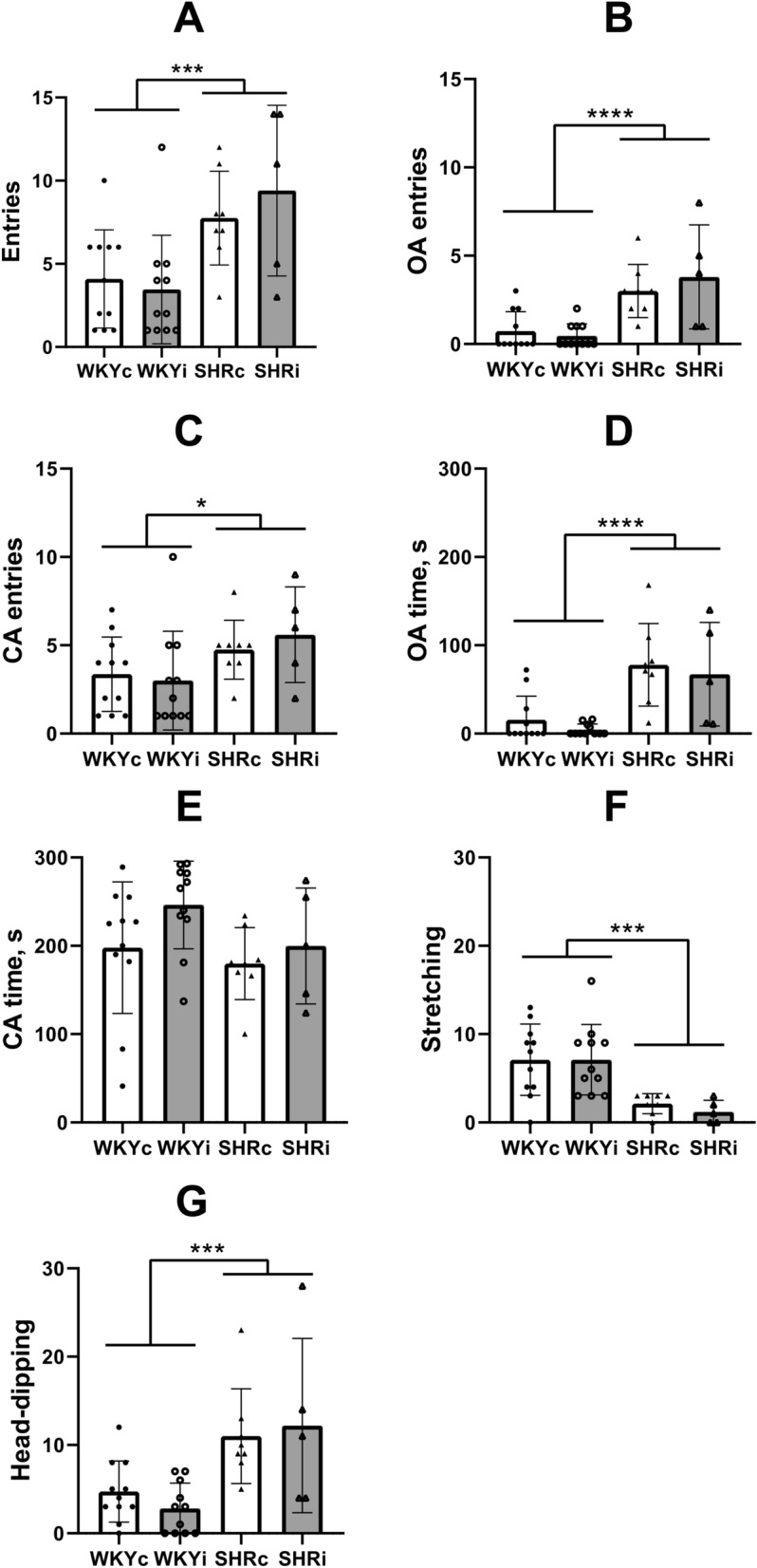
Behavior of animals of the WKYc (n = 11), WKYi (n = 11), SHRc (n = 8), and SHRi (n = 5) groups in the elevated plus maze. CA – closed arms, OA – open arms. (A) – total entries into the arms; (B) and (C) – number of entries into OA and CA, respectively; (D) and (E) – time spent in OA and CA, respectively; (F) – number of stretching postures; (G) – number of head-dipping. Data are presented as mean and 95%CI. *—p < 0.05, ***—p < 0.001, and ****—p < 0.0001 according to ANOVA (see details in the text).
Some ethological indices of anxiety were also assessed. Number of stretch-attend postures (SAP) was significantly lower in SHRs as compared to WKY rats (Fig. 4F; F(1,31) = 19.91, p < 0.001). Moreover, the number of SAPs negatively correlated with the OA entries (RS = -0.38, p < 0.01; 95%CI -0.59– -0.09) and time spent in the OA (RS = -0.41, p < 0.01; 95%CI -0.62– -0.13). The total number of head-dips was higher in SHRs preferring to move into the OA and explore the area around the EPM (Fig. 4G; F(1,31) = 17.68, p < 0.001). Positive correlations were revealed between the number of head-dips and the number of OA entries (RS = 0.75, p < 0.0001; 95%CI 0.58 – 0.85), number of CA entries (RS = 0.63, p < 0.01; 95%CI 0.41 – 0.77), and the time spent in the OA (RS = 0.76, p < 0.0001; 95%CI 0.60 – 0.86) whereas a negative correlation was found for the time spent in the CA (RS = -0.45, p < 0.01; 95%CI -0.65– -0.18). Isolation did not affect the number of SAPs and head-dips (data not shown). As well, neither strain, nor isolation significantly influenced the indices of emotionality such as defecation boli and grooming in the EPM (data not shown).
Social attachment and novelty
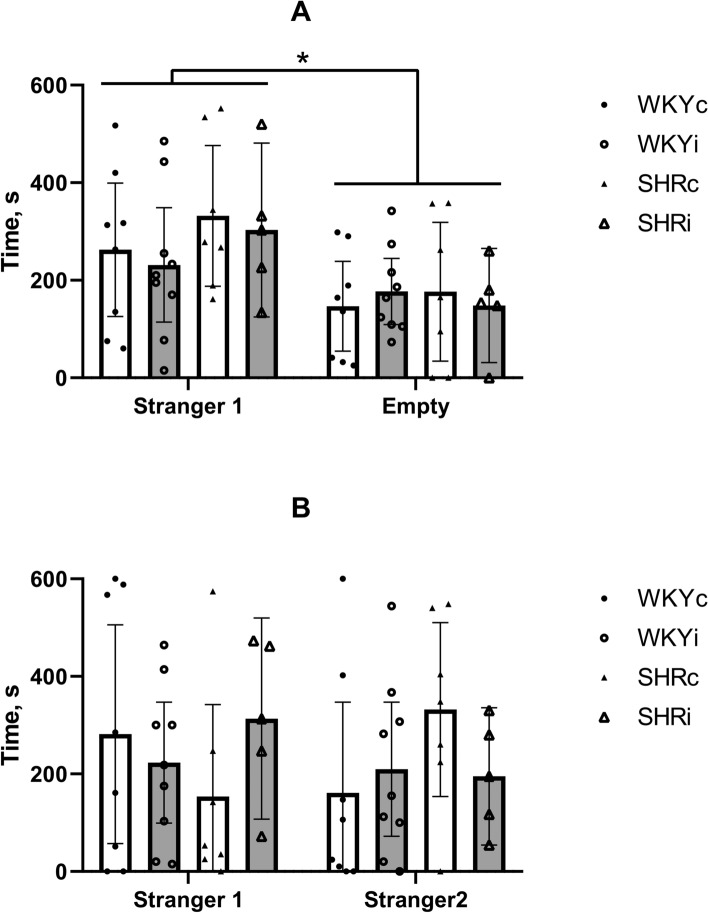
Social behavior was assessed using three-chamber social test (3CST). When the rats of all experimental groups had an opportunity to choose between staying with the previously unknown Stranger 1 rat of the same sex and empty part of the chamber, most of them preferred to be in the part of the chamber with Stranger 1 (Fig. 5A; RM-ANOVA time with Stranger 1 vs. empty part F(1,25) = 7.02, p < 0.05). However, 3 of 8 rats in the WKYc group, 2 of 9 in the WKYi group, 2 of 7 in the SHRc group, and 1 of 5 in the SHRi group spent more time in the chamber with empty basket (the differences not significant). This effect did not depend on the strain or long-term social isolation (F(1,25) = 2.02, p = 0.17 and F(1,25) = 0.34, p = 0.80, respectively).
Fig. 5.
Behavior of animals of the WKYc (n = 11), WKYi (n = 11), SHRc (n = 8), and SHRi (n = 5) groups in the three-chamber social test. (A) – social attachment test with the choice between the empty chamber and the chamber with Stranger 1; (B) – social novelty test with the choice between the chamber with the familiar Stranger 1 and new Stranger 2. Data are presented as mean and 95%CI. *—p < 0.05 according to ANOVA (see details in the text).
During the second part of the 3CST, when the rat was allowed to choose between the Stranger 1 and novel previously unknown Stranger 2, most of animals did not show significant social novelty preference. Time spent with Stranger 1 and Stranger 2 did not differ (Fig. 5B; RM-ANOVA F(1,25) = 0.074, p = 0.80) and it did not depend on genotype (F(1,25) = 0.84, p = 0.40) or isolation (F(1,25) = 0.01, p = 0.95). Interestingly, social novelty preference was observed in some rats in each experimental group: in 3 of 8 rats in the WKYc group, 3 of 9 in the WKYi group, 5 of 7 in the SHRc group, and 2 of 5 in the SHRi group (the differences not significant). Thus, the WKY and SHR rats demonstrated similar preference of social interactions in the conditions of choice between the stranger and empty partitions of the 3CST and did not prefer novel or familiar stranger. Behavior of rats in the 3CST was not significantly influenced by social isolation.
Sucrose preference test
To reveal whether long-term social isolation of rats induced the development of depression-like behavior, we used the sucrose preference test, which is believed to reflect the signs of anhedonia in rodents. In general, SHRs consumed significantly more liquid as compared to WKY rats (Fig. 6A; “genotype” F(1,31) = 9.60, p < 0.01). Furthermore, they also consumed more sucrose solution (Fig. 6B; “genotype” F(1,31) = 20.61, p < 0.0001). These indices were not influenced by isolated housing of rats (“isolation” F(1,31) = 0.016, p = 0.90 and F(1,31) = 0.18, p = 0.70, respectively). Though all animals exhibited very high level of sucrose preference, we did not reveal differences (Fig. 6C) between the rats of two genotypes (F(1,31) = 0.95, p = 0.35) as well as control and isolated animals within each strain (F(1,31) = 0.35, p = 0.56). Thus, long-term isolated housing of rats did not induce the development of depression-like state.
Fig. 6.
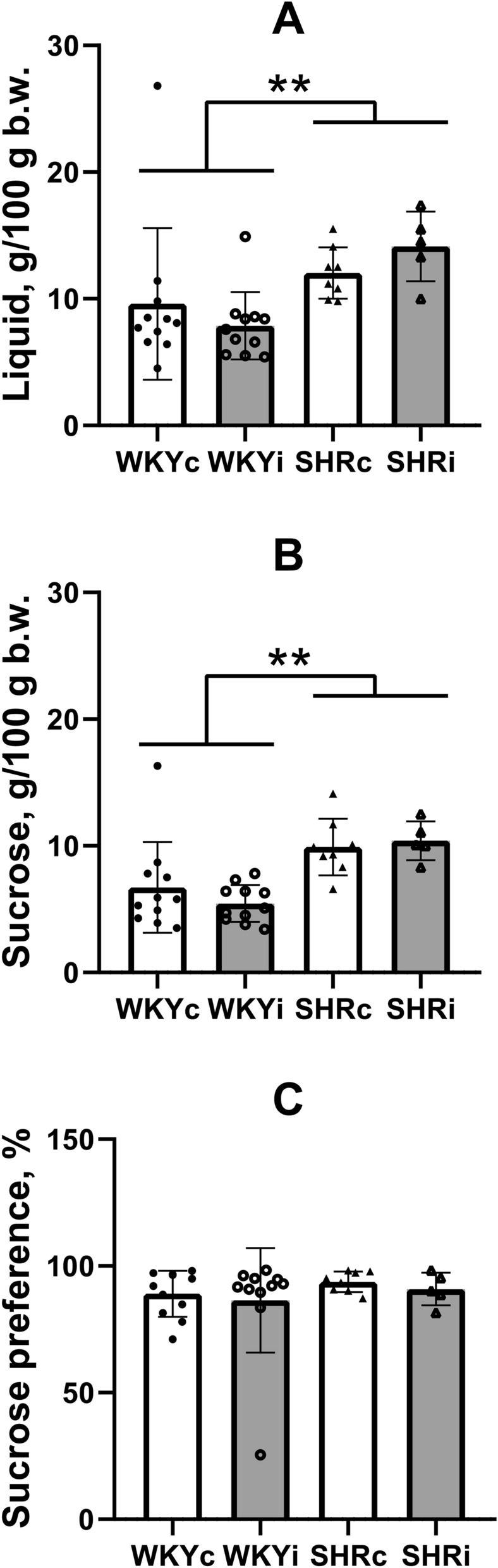
Behavior of animals of the WKYc (n = 11), WKYi (n = 11), SHRc (n = 8), and SHRi (n = 5) groups in the sucrose preference test. (A) and (B) – liquid and sucrose consumption, respectively; (C) – sucrose preference. Data are presented as mean and 95%CI. **—p < 0.01 according to ANOVA (see details in the text).
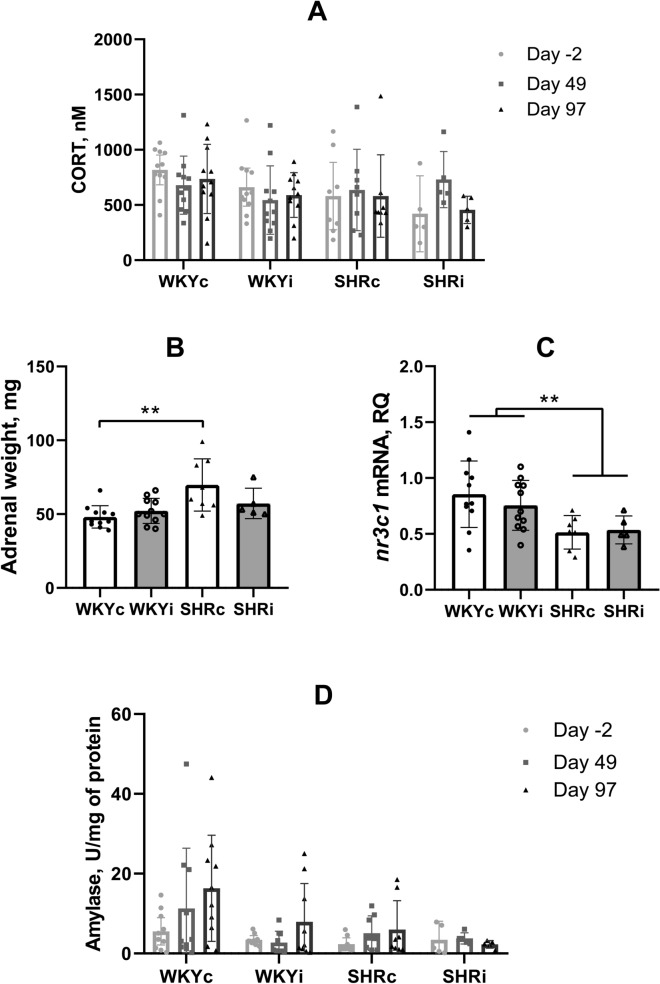
Effects of chronic isolation on blood corticosterone level, expression of corticosterone-associated genes in the adrenal glands and salivary alpha-amylase activity
The level of circulating corticosterone was measured in blood serum sampled from the tail vein before the start of isolation (Day -2), after 49 days of isolation, and after the end of behavioral experiments (Day 97). We did not reveal any effects of “genotype” or “isolation” on the levels of blood corticosterone (Fig. 7A; p = 0.30). Remarkably, the weight of the adrenal glands was significantly higher in SHRs as compared to WKY rats (Fig. 7B; ANOVA effect of “genotype” F(1,31) = 11.19, p < 0.01), the effect of “isolation” was not significant (F(1,31) = 1.12, p = 0.30), but there was a significant “genotype” × “isolation” interaction (F(1,31) = 4.35, p < 0.05). Multiple comparisons of means revealed a significant difference between the adrenal gland weight of the WKYc and SHRc groups, however, this difference was not evident in groups subjected to the isolated housing.
Fig. 7.
Effects of social isolation on the indices of the hypothalamus–pituitary–adrenal and sympathetic system in animals of the WKYc (n = 11), WKYi (n = 11), SHRc (n = 7–8), and SHRi (n = 5) groups. (A), level of corticosterone in blood serum, (B), weight of the adrenal glands, (C), nr3c1 mRNA content in the adrenal glands, and (D), salivary amylase activity. Data are presented as mean and 95%CI. **—p < 0.01 according to post hoc Tukey HST test.
Taking into account hypertrophy of the adrenal glands in the SHRs, we assessed the expression of several genes related to glucocorticoid synthesis and signaling in adrenal tissue. Decrease in the content of nr3c1 mRNA transcripts was revealed in SHRs compared to WKY rats (effect of genotype F(1,30) = 11.37, p < 0.01) and this difference was not influenced by isolated housing (effect of genotype F(1,30) = 11.37, p < 0.01) (Fig. 7C). Differences in the expression of hsd11b, cyp11b1, cyp11a1, mc2r, star, and fkbp5 mRNAs were not revealed (data not shown).
Salivary alpha-amylase activity seemed to be higher in WKY compared to SHR rats although RM-ANOVA revealed only a trend for the effect of “genotype” (Fig. 7D; F(1,31) = 3.57, p = 0.07). Similar trend was revealed for the effect of “isolation” (F(1,31) = 3.17, p = 0.09), indicating possible change in amylase activity during the period of observation and this was supported by the significant effect of “week” (F(2,62) = 3.64, p < 0.05). However, we did not reveal interactions between these factors. Since salivary alpha-amylase activity is believed to represent the level of sympathetic activation we can assume that it tended to be increased in WKYs as compared with SHRs and increased during the 14-week experiment in the WKYc, WKYi, and SHRc groups, but not in the SHRi group.
Discussion
Most previous studies have focused on the effects of isolation in early ontogeny whereas behavioral and biochemical consequences of isolation in adult or aged animals remained understudied. The aim of this study was to investigate the effect of prolonged social isolation of aging rats on locomotion, anxiety, depression-like symptoms, and sociability and whether the hypertensive phenotype of SHRs affected these features. In addition to the behavioral and emotional consequences we studied alterations in the hypothalamic-pituitary-adrenocortical and sympathoadrenal indices during or after isolation.
In addition to hypertension in SHRs, SHR and WKY rats (two strains established from the same parental Wistar stock) significantly differ phenotypically. SHRs exhibit higher locomotor activity, impulsivity, and inattention in various behavioral tests35,40–42. These differences are observed from 3 to 11 months of age43. In the present study, we confirmed increased locomotor and exploratory activity in SHRs as compared to WKY rats at the age of 13 months. Many authors have reported that WKY rats are more anxious than SHRs based on their behavior in the EPM test44–47. This conclusion is also supported by our data showing decreased number of OA visits and time, as well as increased stretching and lower risk-assessment behavior. However, we cannot suggest that the differences in animal behavior in the EPM are determined by strain-specific trait/state anxiety only, rather they are associated with the hyperactivity of SHRs. This is supported by a higher number of total entries into both the CA and OA of the EPM. We have previously shown that hyperactivity significantly affects animal performance in many behavioral tests including the EPM in olfactory bulbectomized mice48.
In the present study, WKY rats consumed less water or sucrose solution than SHRs, confirming the results previously reported23. This phenomenon may be related to the specific water metabolism and higher water excretion in the SHRs49. However, no effects of isolated housing on sucrose preference were found in either WKY or SHR rats, indicating that long-term social isolation did not result in the development of depressive-like anhedonia. Available data on the development of anhedonia in WKY rats are contradictory. Some authors reported that WKY rats consume less sweet solution but the data on sucrose preference remain inconsistent (see relevant references in two extensive reviews24,25). Together with data on reduced locomotion (35,40–42and several other studies, including the present one), increased learned helplessness20, and longer passive floating or immobility in the Porsolt’s test45,50–52, low consumption of sucrose solution may indicate a propensity for a depressive-like state in WKY rats. Yoshii et al53 have reported that solitary housing of young adult WKY rats for 3–10 days led to lower locomotor activity in the OFT, lower activity in the forced swim test and lower sucrose consumption compared to both paired or individually housed Wistar rats. This was associated with atrophy in some brain regions, including the ventral hippocampus, caudate putamen, lateral septum, and cerebellum; however, the authors did not use socially housed WKY rats as a control. Taking this into account, interpretation of the WKY behavior as a model of depression should be done with caution since such a “depressive-like state” may be a consequence of passive/defensive behavior prevalence in the WKY strain24.
Social avoidance was revealed in the adult 1.5-month-old male WKY rats compared to Wistar or Sprague–Dawley rats, but not in SHRs45. In another study, an increased duration of social interaction was revealed in 5-month-old male SHRs compared to Wistar rats54. Conversely, a social interaction deficit was observed in other studies using 1.5-month55 or 5-month old56 SHRs and Wistar rats. The SHRs did not differ from Wistar rats in the social recognition task57. These inconsistencies may be due to difference in experimental approaches in studies of social behavior. In the current study, we used a 3CST to study sociability and social novelty preference in 13-month-old male SHRs and WKY rats. However, this approach failed to reveal significant differences in the sociability or social novelty preference either between the strains or between individual and group housing.
SHRs and WKY rats differ in stress sensitivity. Ulcer formation was significantly higher in WKY rats after application of different stressors20,30,58 indicating that this strain is more susceptible to the harmful effects of acute stress. On the other hand, SHRs were more responsive to acute short-term handling as compared to WKYs judged by an increase in circulating corticosterone and adrenocorticotropic hormone59. Exposure of male rats of both strains to a 10-day chronic stress (restraining or unpredictable mild stress) resulted in a similar increase in the corticosterone level accompanied by adrenal gland hypertrophy independently on the type of stressors used60. Notably, SHRs had higher basal level of corticosterone. In 1.5-month-old WKY rats, chronic unpredictable stress for 42 days led to an increase in the AP value and sympathetic tone which returned to normal levels after the end of stress exposure61. However, psychosocial stress of confrontations applied to WKY, Sprague–Dawley or hybrid prehypertensive SHR-WKY F1 rats, housed in groups or individually for 7 weeks, did not produce the same effects62. These data show that at least some expected effects of chronic stress, such as hypertension, may significantly depend on the type of stressors and the effects of social isolation, even followed by the experience of social confrontations, may have a less severe impact than chronic restraining or unpredictable stress models.
In this study, chronic isolation was the only major stressor for aging male SHRs and WKY rats. We could not reveal significant effects of chronic isolation on the behavior of rats in either strain. The only significant effect of isolation was a decrease in the distance traveled by animals in the intermediate zone of the arena in the OFT. This effect was observed in both SHRs and WKY rats, probably, indicating a trend towards leaving a potentially dangerous zone that may reflect increased anxiety due to stress exposure. Moreover, the rats from the SHRi group seemed more anxious, as they traveled a longer distance in the periphery of the arena as compared to the WKYi rats, if this locomotion along the wall would be considered as thigmotaxis; however, in the absence of differences in the length of distance traveled in other parts of the arena, this may be a consequence of hyperactivity of SHRs. These data partially support previously reported results59, although we could not find effects of isolation on anxiety in the EPM. Notably, in 18-month-old Sprague–Dawley rats, 3-month isolation was followed by increased anxiety that correlated with an elevated level of corticosterone63. Considering contradictory data from different groups, we can assume that the lack of a strong effect from chronic isolation in the present experiment may be attributed to the use of younger animals or an insufficient level of restraint which was an additional stressor in the above paper.
Neuroendocrine abnormalities are a well-known specific feature of the SHR strain. The hypertensive young SHRs have impaired adrenal steroidogenesis compared to the age-matched WKY rats64–66. Three-week-old SHRs had higher corticosterone levels in blood plasma compared to WKY rats66. Adrenal hyperplasia develops in 8-week-old SHRs and it is associated with lower corticosterone secretion64; its level remains lower until 16 weeks, although, in general, steroidogenesis normalizes after the onset of hypertension65. Corticosterone levels may normalize with age, since in the present study, we found similar levels of this hormone in the blood serum of 13–14-month-old SHR and WKY rats, even though persisting adrenal hyperplasia was evident only in SHRs. Furthermore, in adrenal tissue the level of Nr3c1 mRNA, representing the gene encoding the glucocorticoid receptor, was lower in SHRs. This receptor can function as a transcription factor that binds to glucocorticoid response elements in DNA to activate the transcription of glucocorticoid dependent genes, as well as a regulator of other transcription factors67. In the adrenal glands, signaling through glucocorticoid receptors may have some genomic effects related to the negative feedback regulation of glucocorticoid synthesis and secretion68. Additionally, it has been shown that glucocorticoid receptor haploinsufficiency may result in adrenal hyperplasia69, which may be the case in the SHR strain. This may indicate the presence of an imbalance in glucocorticoid signaling despite the lack of differences in the levels of circulating corticosterone levels. We did not find a significant effect of individual housing on corticosterone contents of in the blood of animals of either group studied or on the expression of genes related to steroidogenesis and signaling in the adrenal glands. This is not very surprising as 8-week isolation of young adult Sprague–Dawley rats did not lead to an increase in plasma corticosterone content, although decreased the level of the brain-derived neurotrophic factor in the hippocampus70. A similar lack of effect of 20-day social isolation stress on corticosterone levels was observed in young adult mice71.
We used salivary alpha-amylase activity to assess changes in the sympathetic system during chronic isolation stress. It is believed that salivary alpha-amylase reflects the activity of the sympathetic nervous system in both humans and rodents, including stress-related situations72, such as psychosocial stress conditions in humans73 and exposure to constant light or chronic immobilization in animals74. In the present study no changes in the salivary alpha-amylase activity could be revealed in any of the studied groups. Together with data on corticosterone levels, these results may indicate that isolated housing in rats used as a major stress factor in our study is either not a severe enough stressor for the rat strains used, or the animals became well adapted to these conditions during the isolation period. Remarkably, Kvetnansky et al.75 have shown that SHRs had a more pronounced sympathoadrenal response to acute immobilization stress but decreased adrenal medullary secretion after repeated immobilization demonstrating better adaptive capabilities as compared to WKY rats.
Although there are numerous studies on the effects of chronic isolation on behavioral and neuroendocrine indices in rats, the analysis of available data shows that it is tricky, if possible, at all, to compare the reproducibility of the data between different groups. The experimental design in all studies differs in at least several of very important details, such as rat strain, sex, the age of isolation start, the extent of isolation period, experimental specific features of isolation process, and other. All these experimental differences prevent rational comparison of the data. Therefore, we have to state that both the hypertensive rat strain (SHR) and the strain widely used as the control to SHRs (WKY) were able to effectively adapt to a chronic 3-month-long isolation applied to 10-month-old animals. However, it does not necessarily mean that chronic isolation did not affect these animals. Most probably, the mechanisms affected by this impact may be revealed by challenging the animals using acute severe stressors.
Importantly, the type of isolation may be relevant for the possibility to form a firm long-term adaptation to this type of chronic stress. In our study, the main factor of isolation was the absence of tactile and visual contacts between the isolated animals housed in opaque cages. However, potentially, some olfactory contacts and ultrasound communications might be established, alleviating conditions of isolation and subjective isolation experience. Individual stress level may be reduced by effective adaptation and be insufficient to evoke a significant stress-response and subsequent development of depressive- and anxiety-like associated with significant changes in adrenocortical and sympathetic functions.
It should be noted that the present study has several limitations. First, the animal groups, which were equal at the start of the study, became relatively small and unequal in size at the end of the study due to mortality during the prolonged experiment. The SHRs have limited longevity with a 0–25% survival rate to the age of 24 months76,77. Most of them die after the age of 15 months. Therefore, we expected to finish our experiment when the rats were 14-month-old. Although mortality in our population was within the range reported by other groups, it led to a significant reduction in rat numbers in the experimental samples. Second, we performed our experiments only in male rats of both strains. On the one hand, this allowed us to exclude the effects of possible hormonal fluctuations on the results of behavioral experiments. However, some sex differences were reported in the SHR strain in mechanisms related to the maintenance of high blood pressure associated with sexual dimorphism in the sympathetic control, renin-angiotensin system, oxidative stress, nitric oxide bioavailability, and the immune system78. Moreover, this sexual dimorphism is age-dependent79. Including female rats in future experiments will enhance the translational potential of the study. Third, an important question is to what extent individual housing can be considered as strict isolation and whether this physical limitation to social interactions may be stressful enough to induce significant emotional disturbances leading to the development of depressive-like behavior in aging rodents. Probably individual housing is not equal to complete isolation. Alternative pathways of inter-subject communication, such as ultrasound and olfactory communication used by rats should be controlled more carefully. Though many people suffer from loneliness in different situations causing isolation, they still have the availability of phone and Internet communications. Fourth, aging is associated with a relatively good tolerance to stressful events and only long-term uncontrollable stress leading to significant changes in the hypothalamic–pituitary–adrenal response may result in depression. In our study isolation did not lead in an increase in the levels of circulating corticosterone. This may explain to some extent the absence of severe behavioral abnormalities.
Conclusion
In the present study, we were surprised to find that social isolation had a very mild effect on anxiety and did not influence other affective-related forms of behavior as well as sociability and social novelty preference in male 10–14-month-old SHR and WKY rats. The levels of corticosterone remained stable during the observation period in rats of both strains despite the hypertrophy of the adrenal gland in the SHRs. These data suggest that aged SHR and WKY rats are relatively resilient to chronic social stress associated with isolated housing, although definite mechanisms of adaptation may be strain-specific.
Materials and methods
All procedures performed in experiments involving animals were in accordance with the ethical standards of the Institution or practice at which the studies were conducted. Experiments were performed in accordance with the principles of Basel declaration and Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes, and the Order of the Ministry of Health Care of the Russian Federation no. 199n, April 1, 2016 “On approval of the rules of good laboratory practice”, and were supervised by the Ethical Commission of the Institute of Higher Nervous Activity and Neurophysiology, Russian Academy of Sciences, Moscow, Russia. The ARRIVE Guidelines (v. 2.0) were followed for data report.
Animals
Male SHR (n = 22) and WKY (n = 28) rats were supplied at the age of 4 months by The Animal Breeding Facility (the Unique Research Unit Bio-Model of the Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, Moscow Region, Russia). The animals were derived from stock supplied by Charles River Laboratories (USA). The rats were housed 4–5 per cage made of clear polycarbonate. The rats were housed in the conventional vivarium under 12:12 h light/dark cycle and food and water available ad libitum. The experiments were started in 10-month-old rats. Taking into account that averaged longevity in SHRs is about 73 weeks, i.e. approximately 18 months, with a 100% mortality at the age of 100 weeks77, we suggested that start of the isolation period at 10-month age with the start of behavioral testing at 13-month age would correspond to an aging period in this specific strain of rats. This period is unlikely related to the predominance of secondary pathology associated with the development of hypertensive target organ disease.
Social isolation
Isolated rats were housed individually in opaque cages (200 × 120 × 120 mm) for 90 days before the start of behavioral testing (groups WKYi and SHRi). After 90-day isolation period the animals, i.e. during the behavioral study, the rats of these two groups maintained in the same individual home cages. Thus, isolation continued until the end of the experiment. Control animals remained in their home cages (480 × 375 × 210 mm) in groups of 2–3 rats (groups WKYc and SHRc). Despite the small sizes of individual cages, the rats of 300–400 g body weight could move from the front to the back walls to get the feeder and drinking bottle although some restraint of movement also occurred. Isolated and control animals were housed in the same colony room, so that the isolated rats might have auditory and olfactory contacts with control rats. Animals were disturbed for cleaning purposes, which were accomplished daily because of increased urination in SHRs. Food and water were available ad libitum. Body weight was measured weekly in all animals.
Blood pressure measurement
Before the start of the isolation period and after isolation (at Day 88 of the experiment) blood pressure was measured using the tail cuff method. For this purpose, the animals were adopted to plastic holders, which were used for the procedure for 3 days. During the measurement, the rat was placed into a holder and a cuff was put onto the tail. The holder was placed onto a “Flogiston” heating plate (Neurobotics, Russia) to maintain a stable temperature of the body. The cuff was connected to a computer-assisted “Sistola” device (Neurobotics, Russia). Blood pressure was measured using original software supplied by a manufacturer. Three measurements were conducted with a 10-min interval between them. The averaged arterial pressure (AP) was calculated and used as an index of the AP in the animal.
Behavioral study
For behavioral tests we used devices supplied by “Open Science Ltd” (Krasnogorsk, Russia). Behavior of animals was recorded with DMK 23GV024 GigE camera connected to a personal computer, using IC-Capture Ver. 2.2.248.1000 software (The Imaging Source Europe GmbH, Germany). Behavioral tracing and analysis were performed using EthoVision 11XT software (Noldus, The Netherlands). Thirty min before each behavioral test, except of the sucrose preference test, the animals were transported into the behavioral room in their home cages and adopted to the experimental conditions. All behavioral tests were conducted between 10 a.m. and 1 p.m.
The “Open field test” (OFT) was used to estimate locomotor and exploratory activity, anxiety, and emotionality in rats80,81. The OFT was performed in the gray circular arena with a diameter of 100 cm surrounded with the 30-cm high wall. The arena was situated in the center of a sound-protected room. The floor of the arena was evenly lit (450 lx) by reflected light with four diode lamps located on the walls of the room. The rat was placed into the center of the arena and its behavior was recorded for 5 min. The floor of the arena was divided into the central circle and intermediate and peripheral rings. The distance traveled in the arena and specific zones of interest, latency to leave the center, entries to center, rearing (total, free and climbing), grooming episodes, urinations and defecation boli were counted.
The “Elevated plus maze” (EPM) test was used to test anxiety in experimental animals82,83. The EPM consisted of four crossed arms with a size of 50 × 14 cm. Two closed arms (CA) had side walls of 30 cm high, and the open arms (OA) had a side boards of 1 cm high. The maze was elevated by 55 cm over the floor. The intensity of OA lighting was 300 lx. The rat was placed onto the central platform facing to an OA. During 5 min the latency to start movement, number of entries into and time spent in the OA and CA, number of head dips and stretching postures, grooming and defecation boli were recorded.
The “Three-chamber sociability and social novelty test” was applied to assay cognition on the form of sociability and interest in social novelty in rodents using a protocol adopted from previously published studies84,85. The chamber (120 × 80 × 40 cm) was divided onto three equal compartments by two clear partitions. In the center of each partition there was an opening (10 × 10 cm) closed by a guillotine door. The central compartment was empty whereas in both side compartments cylinder baskets (20 cm in diameter and 30 cm high) were located. The test was performed under dim light (15 lx) conditions. The 10-min habituation session started by placing the rat into the central compartment; the doors were opened immediately after the placement. After the end of the habituation the rat was returned into the central compartment. The never-been-met Stranger 1 rat was introduced into one of the baskets and the 10-min sociability session was initiated by opening the doors. In this session, the rat was allowed to explore both empty compartment and compartment with a new subject. After the session has elapsed, the rat was returned into the central compartment and the doors were closed. The never-been-met Stranger 2 rat was introduced into the other basket and the baskets were alternated between the side compartments. Then, the doors were opened and the rat was allowed to explore both side compartments with the Stranger 1 and the Stranger 2 during the next 10-min social novelty session. Behavior of rats was video recorded and then, the time spent sniffing each basket, the time spent in each compartment, and the number of entries into each compartment were recorded.
The “Sucrose preference test” (SPT) is often used to detect depressive-like features in animals86. For this purpose, the rats from the control groups were placed individually into clear Plexiglas cages to decrease the effect of individual housing. The isolated animals were tested in cages made of nontransparent plastic. The cages were located in the room separated from the colony room. The test was performed as previously described87,88. On day 94, after the end of the three-chamber social test, each rat was placed into an individual cage and exposed to two drinking bottles filled with fresh water. On day 95, water in one of the bottles was replaced with 2% sucrose solution. Then, drinking behavior was observed for 48 h. The positions of the bottles were changed every 12 h. Food pellets were available for the whole period of observation. Bottles with water or sucrose solution were weighted before the start and after the end of each 12-h interval. Sucrose consumption was calculated as total weight of consumed solution. Sucrose preference was calculated as sucrose weight referred to total weight of consumed liquid (sucrose solution + water) and presented as percentage.
Biochemical assays
In order to estimate circulating corticosterone, peripheral blood was collected by puncture of the tail vein. For this purpose, rats were anesthetized with 2% isoflurane, the tail was washed with water and disinfected with 70% alcohol. After puncture, 200 µl of blood was collected into a tube. After clot formation, the samples were centrifuged at 1500 × g for 15 min and the serum was collected and frozen in liquid nitrogen. The samples were stored at -85 °C until use. Corticosterone was measured using a solid phase enzyme-linked immunosorbent assay (ELISA), based on the principle of competitive binding with the Rat Corticosterone ELISA Kit (XEMA, Russia) according to the protocol of the manufacturer.
Salivary alpha-amylase activity was measured in the samples collected in anesthetized rats during blood sampling. For this purpose, a piece of filter paper (5 × 5 mm) was placed under the tongue for 10 min till the complete moistening. Then, the filter was placed into the tube containing 100 µl of 0.9% NaCl, frozen in liquid nitrogen, and stored at -85 °C until use. Activity of salivary alpha-amylase was measured by the kinetic method using the Amylase kit (Vector-Best, Russia) according to the protocol of the manufacturer. Protein content was measured by the Bradford method using the Pierce Bradford Protein Assay Kit (Thermo Scientific, USA). Alpha-amylase activity was calculated as Units/mg of protein.
RNA extraction and reverse transcription
Total RNA was isolated from the adrenal glands using Extract RNA reagent (#BC032, Evrogen, Russia) according to the manufacturer’s recommendations. Before cDNA synthesis, 2 μg of RNA was treated with DNase I (Thermo Fisher Scientific, USA) according to the manufacturer’s recommendations. Then, one half of DNase-treated RNA was used as a negative control without reverse transcription.
The second half of RNA was used to synthesize cDNA using the equimolar mix of random decaprimer (#SB002, Evrogen, Russia) and oligo(dT)-primer (#SB001, Evrogen, Russia) by means of the MMLV RT Kit (#SK021, Evrogen, Russia) and RiboCare RNase Inhibitor (#EK005M, Evrogen, Russia) in accordance with the manufacturer’s recommendations. The product of reverse transcription reaction was diluted 8 times.
Quantitative real-time polymerase chain reaction (qPCR)
The gene expression was analyzed using qPCRmix-HS SYBR + LowROX (#PK156L, Evrogen, Russia) in accordance with the manufacturer’s recommendations by means of a quantitative PCR system CFX384 (Bio-Rad, USA). The genes of interest were Hk1, Pik3c3, Mc2r, Star, Cyp11a1, Cyp11b1, Hsd11b1, Fkbp5. Nucleotide sequences of primers used are shown in Table 1. Primers were designed based on genes sequences from NCBI database using the Lasergene Primer Select Software Package.
Table 1.
Nucleotide sequences of primers used in the present study.
| Gene | Forward primer | Reverse primer |
|---|---|---|
|
Hk1 |
TTATTCGAAGGGCGCATCACTC | TCAACATCAGACGGCTCCACT |
|
Pik3c3 |
CGATGACGAGGATTTGCTGATGTA | CCGGAGGAAGAGGGTTGGTTAT |
|
Mc2r |
CTGATGTAGTTGTGCCAGAAGAGAT | TGGCCAAACTGCAGATGAAAAAG |
|
Star |
TGGCTGGAAGTCCCTCAAAGA | GTGGCTGGCGAACTCTATCTG |
|
Cyp11a1 |
GACGCATCAAGCAGCAAAACTCT | GGTCCACGATCTCCTCCAACAT |
|
Cyp11b1 |
TGCTCAGCACTAAAGCACAAATCT | AGTAGGCACAACCCAGTAATCTCA |
|
Hsd11b1 |
GCCTGGGAGGTTGTAGAAAGAG | AATAGTAGTAACCCAGGCAGAGCAC |
|
Fkbp5 |
GCCGGCAAGAAACACGAGAGT | GAGGAGGGCCGAGTTCATTAGGA |
The relative quantity (RQ) of transcripts was assessed using the 2-ΔΔCt method, taking into account the efficiency of the reaction with respect to the expression of the Hk1 and Pik3c3 genes and the data are presented as relative quantity.
Statistical analysis.
Statistical analysis was performed using Statistica 12 for Windows (StatSoft Corp., USA) or GraphPad Prism 8 (GraphPad Software, LLC, USA) software. All data were initially analyzed for correspondence to normal distribution using Shapiro–Wilk’s test. All samples passed the normality test were subjected to analysis of variances (ANOVA); the others were analyzed using non-parametrical methods. Mortality of rats and the proportions of animals in groups performed entries into the arms of the EPM were estimated using the two-tailed Fisher exact test. Physiological indices were evaluated using two-way ANOVA with repeated measures (ANOVA-RM) with “genotype” and “isolation” as between- and “start–end” as within-factor. Post hoc comparisons were performed using the Tukey HST for unequal N. Two-way factorial ANOVA was applied to most other group comparisons with “genotype” and “isolation” as between-factors and post hoc multiple comparisons were performed using the Tukey HST for unequal N. Spearman rank correlation analysis was used for calculation of interrelationships between specific behavioral measures in the EPM.
Acknowledgements
This study was supported by the Russian Science Foundation (project #22-15-00132).
Author contributions
D.M., O.N., A.M., V.O., P.K., N.L., Yu.M., L.T., A.K., M.O., V.A., M.N., M.S. were involved in conception and design, material preparation, data collection and analysis. M.S. and N.G. wrote the main manuscript text and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to the requirements of the Institute of Higher Nervous Activity and Neurophysiology, but are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gerst-Emerson, K. & Jayawardhana, J. Loneliness as a public health issue: the impact of loneliness on health care utilization among older adults. Am. J. Public Health105, 1013–1019 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Voronin, G. L., Zakharov, V. & Kozyreva, P. Lonely old aged: surviving or living an active life?. Sociol. J24, 32–55 (2018). [Google Scholar]
- 3.Guarnera, J., Yuen, E. & Macpherson, H. The impact of loneliness and social isolation on cognitive aging: a narrative review. J. Alzheimers Dis. Rep.7, 699–714 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finley, A. & Schaefer, S. M. Affective neuroscience of loneliness: potential mechanisms underlying the association between perceived social isolation, health, and well-being. J. Psychiatr. Brain Sci.7, e220011 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ong, A. D., Uchino, B. N. & Wethington, E. Loneliness and health in older adults: a mini-review and synthesis. Gerontology62, 443–449 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forman, D. E. et al. The present and future JACC state-of-the-art review. multimorbidity older adults with Cardiovasc Dis.71, 2149–2161 (2018). [Google Scholar]
- 7.Moturi, S., Ghosh-Choudhary, S. K. & Finkel, T. Cardiovascular disease and the biology of aging. J. Mol. Cell Cardiol167, 109–117 (2022). [DOI] [PubMed] [Google Scholar]
- 8.Novak Sarotar, B. & Lainscak, M. Psychocardiology in the elderly. Wien Klin Wochenschr128, 474–479 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Gustad, L. T., Laugsand, L. E., Janszky, I., Dalen, H. & Bjerkeset, O. Symptoms of anxiety and depression and risk of heart failure: the HUNT Study. Eur. J. Heart Fail16, 861–870 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergantin, L. B. Depression rises the risk of hypertension incidence: discussing the link through the Ca2+/cAMP signalling. Curr. Hypertens Rev.16, 73–78 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Yanguas, J., Pinazo-Henandis, S. & Tarazona-Santabalbina, F. J. The complexity of loneliness. Acta Biomed.89, 302–314 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kondrakiewicz, K., Kostecki, M., Szadzińska, W. & Knapska, E. Ecological validity of social interaction tests in rats and mice. Genes Brain Behav.18, e12525 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Mcmahon, E. K., Farhan, S. & Cavigelli, S. A. How do we characterize temperament? Broad testing of temperament across time and contexts in low-variable conditions. Animal Behav.195, 29–42 (2023). [Google Scholar]
- 14.Sagvolden, T., Russell, V. A., Aase, H., Johansen, E. B. & Farshbaf, M. Rodent models of attention-deficit/hyperactivity disorder. Biol. Psychiat.57, 1239–1247 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Russell, V. Overview of animal models of attention deficit hyperactivity disorder (ADHD). Curr. Protoc. Neurosci.10.1002/0471142301.ns0935s54 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Shimamoto, K. & Ura, N. Mechanisms of insulin resistance in hypertensive rats. Clin. Exp. Hypertens28, 543–552 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Armario, A., Gavaldà, A. & Martí, J. Comparison of the behavioural and endocrine response to forced swimming stress in five inbred strains of rats. Psychoneuroendocrinology20, 879–890 (1995). [DOI] [PubMed] [Google Scholar]
- 18.Paré, W. P. ‘Behavioral despair’ test predicts stress ulcer in WKY rats. Physiol. Behav.46, 483–487 (1989). [DOI] [PubMed] [Google Scholar]
- 19.Marti, J. & Armario, A. Forced swimming behavior is not related to the corticosterone levels achieved in the test: a study with four inbred rat strains. Physiol. Behav.59, 369–373 (1996). [DOI] [PubMed] [Google Scholar]
- 20.Paré, W. P. Stress ulcer susceptibility and depression in Wistar Kyoto (WKY) rats. Physiol. Behav.46, 993–998 (1989). [DOI] [PubMed] [Google Scholar]
- 21.Nam, H., Clinton, S. M., Jackson, N. L. & Kerman, I. A. Learned helplessness and social avoidance in the Wistar-Kyoto rat. Front. Behav. Neurosci.8, 109 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, Q. et al. Brain structure and synaptic protein expression alterations after antidepressant treatment in a Wistar-Kyoto rat model of depression. J. Affect. Disord.314, 293–302 (2022). [DOI] [PubMed] [Google Scholar]
- 23.Dommett, E. J. & Rostron, C. L. Appetitive and consummative responding for liquid sucrose in the spontaneously hypertensive rat model of attention deficit hyperactivity disorder. Behav. Brain Res.238, 232–242 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Redei, E. E., Udell, M. E., Solberg Woods, L. C. & Chen, H. The Wistar Kyoto rat: a model of depression traits. Curr. Neuropharmacol.21, 1884–1905 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aleksandrova, L. R., Wang, Y. T. & Phillips, A. G. Evaluation of the Wistar-Kyoto rat model of depression and the role of synaptic plasticity in depression and antidepressant response. Neurosci. Biobehav. Rev.105, 1–23 (2019). [DOI] [PubMed] [Google Scholar]
- 26.Kantak, K. M. Rodent models of attention-deficit hyperactivity disorder: An updated framework for model validation and therapeutic drug discovery. Pharmacol. Biochem. Behav.216, 173378 (2022). [DOI] [PubMed] [Google Scholar]
- 27.Regan, S. L., Williams, M. T. & Vorhees, C. V. Review of rodent models of attention deficit hyperactivity disorder. Neurosci. Biobehav. Rev.132, 621–637 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gungor Aydin, A. & Adiguzel, E. The mesocortical dopaminergic system cannot explain hyperactivity in an animal model of attention deficit hyperactivity disorder (ADHD)- Spontaneously hypertensive rats (SHR). Lab. Anim Res.39, 20 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai, M. L., Kozłowska, A., Li, Y. S., Shen, W. L. & Huang, A. C. W. Social factors affect motor and anxiety behaviors in the animal model of attention-deficit hyperactivity disorders: A housing-style factor. Psychiatry Res.254, 290–300 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Paré, W. P. Stress ulcer and open-field behavior of spontaneously hypertensive, normotensive, and Wistar rats. Pavlov. J. Biol. Sci.24, 54–57 (1989). [DOI] [PubMed] [Google Scholar]
- 31.Mc Fie, S., Sterley, T.-L., Howells, F. M. & Russell, V. A. Clozapine decreases exploratory activity and increases anxiety-like behaviour in the Wistar-Kyoto rat but not the spontaneously hypertensive rat model of attention-deficit/hyperactivity disorder. Brain Res.1467, 91–103 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Granzotto, N. et al. Sustained hyperactivity and lower blood pressure are influenced by the ANXRR16 chromosomic region in rats. J. Clin. Rep. Med. Images Health Sci.3, 1–9 (2023). [Google Scholar]
- 33.Schaefer, C. F., Brackett, D. J., Gunn, C. G. & Wilson, M. F. Behavioral hyperreactivity in the spontaneously hypertensive rat compared to its normotensive progenitor. Pavlov. J. Biol. Sci.13, 211–216 (1978). [DOI] [PubMed] [Google Scholar]
- 34.Langen, B. & Dost, R. Comparison of SHR, WKY and Wistar rats in different behavioural animal models: effect of dopamine D1 and alpha2 agonists. Atten Defic. Hyperact. Disord3, 1–12 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Tchekalarova, J., Krushovlieva, D., Ivanova, P. & Kortenska, L. Spontaneously hypertensive rats vs Wistar Kyoto and Wistar rats: An assessment of anxiety, motor activity, memory performance, and seizure susceptibility. Physiol. Behav.269, 114268 (2023). [DOI] [PubMed] [Google Scholar]
- 36.Mazur, F. G. et al. Effects of physical exercise and social isolation on anxiety-related behaviors in two inbred rat strains. Behav. Proc.142, 70–78 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Hinojosa, F. R. et al. Evaluation of two genetic animal models in behavioral tests of anxiety and depression. Behav. Brain Res.168, 127–136 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Kulikov, A. et al. Central serotonergic systems in the spontaneously hypertensive and Lewis rat strains that differ in the elevated plus-maze test of anxiety. J. Pharmacol. Exp. Ther.281, 775–784 (1997). [PubMed] [Google Scholar]
- 39.Santini, Z. I. et al. Social disconnectedness, perceived isolation, and symptoms of depression and anxiety among older Americans (NSHAP): a longitudinal mediation analysis. Lancet Public Health5, e62–e70 (2020). [DOI] [PubMed] [Google Scholar]
- 40.Knardahl, S. & Sagvolden, T. Open-field behavior of spontaneously hypertensive rats. Behav. Neural. Biol.27, 187–200 (1979). [DOI] [PubMed] [Google Scholar]
- 41.Sagvolden, T. Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD). Neurosci. Biobehav. Rev.24, 31–39 (2000). [DOI] [PubMed] [Google Scholar]
- 42.Wultz, B., Sagvolden, T., Moser, E. I. & Moser, M. B. The spontaneously hypertensive rat as an animal model of attention-deficit hyperactivity disorder: Effects of methylphenidate on exploratory behavior. Behav. Neural Biol.53, 88–102 (1990). [DOI] [PubMed] [Google Scholar]
- 43.Ferguson, S. A. & Gray, E. P. Aging effects on elevated plus maze behavior in spontaneously hypertensive, Wistar-Kyoto and Sprague-Dawley male and female rats. Physiol. Behav.85, 621–628 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Berton, O., Ramos, A., Chaouloff, F. & Mormede, P. Behavioral reactivity to social and nonsocial stimulations: a multivariate analysis of six inbred rat strains. Behav. Genet.27, 155–166 (1997). [DOI] [PubMed] [Google Scholar]
- 45.Nam, H., Clinton, S. M., Jackson, N. L. & Kerman, I. A. Learned helplessness and social avoidance in the Wistar-Kyoto rat. Front. Behav. Neurosci8, 109 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pardon, M. C. et al. Stress reactivity of the brain noradrenergic system in three rat strains differing in their neuroendocrine and behavioral responses to stress: implications for susceptibility to stress-related neuropsychiatric disorders. Neuroscience115, 229–242 (2002). [DOI] [PubMed] [Google Scholar]
- 47.Zubcevic, J. et al. MEMRI reveals altered activity in brain regions associated with anxiety, locomotion, and cardiovascular reactivity on the elevated plus maze in the WKY vs SHR rats. Brain Imaging Behav.12, 1318–1331 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Nedogreeva, O. A., Stepanichev, M. Y. & Gulyaeva, N. V. Removal of the olfactory bulbs in mice leads to changes in affective behavior. Neurosci. Behav. Physiol.50, 892–899 (2020). [Google Scholar]
- 49.Matsunaga, M. et al. Plasma renin and vascular complications in substrains of the spontaneously hypertensive rat, with a reference to water and electrolyte balance. Jpn. Circ. J.40, 889–894 (1976). [DOI] [PubMed] [Google Scholar]
- 50.Wang, Z. et al. Wistar-Kyoto rats and chronically stressed Wistar rats present similar depression- and anxiety-like behaviors but different corticosterone and endocannabinoid system modulation. Prog. Neuropsychopharmacol. Biol .Psychiatry127, 110825 (2023). [DOI] [PubMed] [Google Scholar]
- 51.López-Rubalcava, C. & Lucki, I. Strain differences in the behavioral effects of antidepressant drugs in the rat forced swimming test. Neuropsychopharmacology.222(22), 191–199 (2000). [DOI] [PubMed] [Google Scholar]
- 52.Lahmame, A., Grigoriadis, D. E., De Souza, E. B. & Armario, A. Brain corticotropin-releasing factor immunoreactivity and receptors in five inbred rat strains: relationship to forced swimming behaviour. Brain Res.750, 285–292 (1997). [DOI] [PubMed] [Google Scholar]
- 53.Yoshii, T. et al. Validation of Wistar-Kyoto rats kept in solitary housing as an animal model for depression using voxel-based morphometry. Sci. Rep.14, 3601 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Almeida, V. et al. Cannabidiol exhibits anxiolytic but not antipsychotic property evaluated in the social interaction test. Prog. Neuropsychopharmacol. Biol. Psychiatry41, 30–35 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Niigaki, S. T. et al. Young spontaneously hypertensive rats (SHRs) display prodromal schizophrenia-like behavioral abnormalities. Prog. Neuropsychopharmacol. Biol. Psychiatry90, 169–176 (2019). [DOI] [PubMed] [Google Scholar]
- 56.Calzavara, M. B. et al. Effects of antipsychotics and amphetamine on social behaviors in spontaneously hypertensive rats. Behav. Brain Re.225, 15–22 (2011). [DOI] [PubMed] [Google Scholar]
- 57.Pamplona, F. A., Pandolfo, P., Savoldi, R., Prediger, R. D. S. & Takahashi, R. N. Environmental enrichment improves cognitive deficits in Spontaneously Hypertensive Rats (SHR): relevance for attention deficit/hyperactivity disorder (ADHD). Prog. Neuropsychopharmacol Biol. Psychiatry33, 1153–1160 (2009). [DOI] [PubMed] [Google Scholar]
- 58.Paré, W. P. & Schimmel, G. T. Stress ulcer in normotensive and spontaneously hypertensive rats. Physiol. Behav.36, 699–705 (1986). [DOI] [PubMed] [Google Scholar]
- 59.Roman, O., Seres, J., Pometlova, M. & Jurcovicova, J. Neuroendocrine or behavioral effects of acute or chronic emotional stress in Wistar Kyoto (WKY) and spontaneously hypertensive (SHR) rats. Endocrin. Regul.38, 151–166 (2004). [PubMed] [Google Scholar]
- 60.Vieira, J. O., Duarte, J. O., Costa-Ferreira, W. & Crestani, C. C. Influence of pre-existing hypertension on neuroendocrine and cardiovascular changes evoked by chronic stress in female rats. Psychoneuroendocrinology97, 111–119 (2018). [DOI] [PubMed] [Google Scholar]
- 61.Zhou, J. J., Shao, J. Y., Chen, S. R., Li, D. P. & Pan, H. L. α2δ-1-Dependent NMDA receptor activity in the hypothalamus is an effector of genetic-environment interactions that drive persistent hypertension. J. Neurosci.41, 6551–6563 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harrap, S. B., Louis, W. J. & Doyle, A. E. Failure of psychosocial stress to induce chronic hypertension in the rat. J. Hypertens.2, 653–662 (1984). [DOI] [PubMed] [Google Scholar]
- 63.Dong, H. et al. Corticotrophin releasing factor receptor 1antagonists prevent chronic stress-induced behavioral changes and synapse loss in aged rats. Psychoneuroendocrinology90, 92 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moll, D., Dale, S. L. & Melby, J. C. Adrenal steroidogenesis in the spontaneously hypertensive rat (SHR). Endocrinology96, 416–420 (1975). [DOI] [PubMed] [Google Scholar]
- 65.Komanicky, P., Reiss, D. L., Dale, S. L. & Melby, J. C. Role of adrenal steroidogenesis in etiology of hypertension in the spontaneously hypertensive rat. Endocrinology111, 219–224 (1982). [DOI] [PubMed] [Google Scholar]
- 66.Kenyon, C. J. et al. The role of glucocorticoid activity in the inheritance of hypertension: studies in the rat. J. Steroid Biochem. Mol. Biol.45, 7–11 (1993). [DOI] [PubMed] [Google Scholar]
- 67.Nicolaides, N. C. & Chrousos, G. P. The human glucocorticoid receptor. Vitam. Horm.123, 417–438 (2023). [DOI] [PubMed] [Google Scholar]
- 68.Briassoulis, G., Damjanovic, S., Xekouki, P., Lefebvre, H. & Stratakis, C. A. The glucocorticoid receptor and its expression in the anterior pituitary and the adrenal cortex: a source of variation in hypothalamic-pituitary-adrenal axis function; implications for pituitary and adrenal tumors. Endocr. Pract.17, 941–948 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bouligand, J. et al. Familial glucocorticoid receptor haploinsufficiency by non-sense mediated mRNA decay, adrenal hyperplasia and apparent mineralocorticoid excess. PLoS One5, e13563 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scaccianoce, S. et al. Social isolation selectively reduces hippocampal brain-derived neurotrophic factor without altering plasma corticosterone. Behav. Brain Res.168, 323–325 (2006). [DOI] [PubMed] [Google Scholar]
- 71.Hilakivi, L. A., Ota, M. & Lister, R. Effect of isolation on brain monoamines and the behavior of mice in tests of exploration, locomotion, anxiety and behavioral ‘despair’. Pharmacol. Biochem. Behav.33, 371–374 (1989). [DOI] [PubMed] [Google Scholar]
- 72.Nater, U. M. & Rohleder, N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: current state of research. Psychoneuroendocrinology34, 486–496 (2009). [DOI] [PubMed] [Google Scholar]
- 73.Rohleder, N., Nater, U. M., Wolf, J. M., Ehlert, U. & Kirschbaum, C. Psychosocial stress-induced activation of salivary alpha-amylase: an indicator of sympathetic activity?. Ann. N. Y. Acad. Sci.1032, 258–263 (2004). [DOI] [PubMed] [Google Scholar]
- 74.Bellavia, S. & Gallará, R. Effect of photic stimuli on rat salivary glands. Role of sympathetic nervous system. Acta. Odontol. Latinoam13, 3–19 (2000). [PubMed] [Google Scholar]
- 75.Kvetnansky, R., McCarthy, R. & Thoa, N. B. Sympatho-adrenal responses of spontaneously hypertensive rats to immobilization stress. Am. J. Physiol.10.1152/ajpheart.1979.236.3.H457 (1979). [DOI] [PubMed] [Google Scholar]
- 76.Di Nicolantonio, R., Silvapulle, M. J., Spargo, S. & Morgan, T. O. High salt diet decreases longevity in the spontaneously hypertensive rat. Clin. Exp. Pharmacol. Physiol.15, 357–359 (1988). [DOI] [PubMed] [Google Scholar]
- 77.Freis, E. D. & Ragan, D. Effect of treatment on longevity in spontaneously hypertensive rats. Proc. Soc. Exp. Biol. Med.150, 422–424 (1975). [DOI] [PubMed] [Google Scholar]
- 78.Elmarakby, A. A. & Sullivan, J. C. Sex differences in hypertension: lessons from spontaneously hypertensive rats (SHR). Clin. Sci. (Lond)135, 1791–1804 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fortepiani, L. A., Zhang, H., Racusen, L., Roberts, L. J. & Reckelhoff, J. F. Characterization of an animal model of postmenopausal hypertension in spontaneously hypertensive rats. Hypertension41, 640–645 (2003). [DOI] [PubMed] [Google Scholar]
- 80.Sturman, O., Germain, P. L. & Bohacek, J. Exploratory rearing: a context- and stress-sensitive behavior recorded in the open-field test. Stress21, 443–452 (2018). [DOI] [PubMed] [Google Scholar]
- 81.Kraeuter, A. K., Guest, P. C. & Sarnyai, Z. The open field test for measuring locomotor activity and anxiety-like behavior. Methods Mol. Biol.1916, 99–103 (2019). [DOI] [PubMed] [Google Scholar]
- 82.Kraeuter, A. K., Guest, P. C. & Sarnyai, Z. The elevated plus maze test for measuring anxiety-like behavior in rodents. Methods Mol. Biol.1916, 69–74 (2019). [DOI] [PubMed] [Google Scholar]
- 83.Carobrez, A. P. & Bertoglio, L. J. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci. Biobehav. Rev.29, 1193–1205 (2005). [DOI] [PubMed] [Google Scholar]
- 84.Varlinskaya, E. I., Spear, L. P. & Diaz, M. R. Stress alters social behavior and sensitivity to pharmacological activation of kappa opioid receptors in an age-specific manner in sprague dawley rats. Neurobiol. Stress.9, 124 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tzanoulinou, S., Riccio, O., De Boer, M. W. & Sandi, C. Peripubertal stress-induced behavioral changes are associated with altered expression of genes involved in excitation and inhibition in the amygdale. Transl. Psychiatry10.1038/tp.2014.54 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Scheggi, S., De Montis, M. G. & Gambarana, C. Making sense of rodent models of anhedonia. Int. J. Neuropsychopharmacol21, 1049–1065 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stepanichev, M. Y. et al. Anhedonia but not passive floating is an indicator of depressive-like behavior in two chronic stress paradigms. Acta. Neurobiol. Exp. (Wars)76, 324–333 (2016). [DOI] [PubMed] [Google Scholar]
- 88.Stepanichev, M. et al. Specific Activity Features in the forced swim test: brain neurotrophins and development of stress-induced depressive-like behavior in rats. Neuroscience375, 49–61 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to the requirements of the Institute of Higher Nervous Activity and Neurophysiology, but are available from the corresponding author on reasonable request.