Abstract
Pediatric patients with recurrent metastatic neuroblastoma (NB) have a dismal 5-year survival. Novel therapeutic approaches are urgently needed. The melanoma cell adhesion molecule (MCAM/CD146/MUC18) is expressed in a variety of pediatric solid tumors, including NB, and constitutes a novel target for immunotherapy. Here, we developed a chimeric antigen receptor (CAR) expressing natural killer (NK) cell-targeting MCAM by non-viral electroporation of CAR mRNA into ex vivo expanded NK cells. Expression of anti-MCAM CAR significantly enhanced NK cell cytotoxic activity compared to mock NK cells against MCAMhigh but not MCAMlow/knockout NB cells in vitro. Anti-MCAM-CAR-NK cell treatment significantly decreased tumor growth and prolonged animal survival in an NB xenograft mouse model. NKTR-255, a polymer-conjugated recombinant human interleukin-15 agonist, significantly stimulated NK cell proliferation and expansion and further enhanced the in vitro cytotoxic activity and in vivo anti-tumor efficacy of anti-MCAM-CAR-NK cells against NB. Our preclinical studies demonstrate that ex vivo expanded and modified anti-MCAM-CAR-NK cells alone and/or in combination with NKTR-255 are promising novel alternative therapeutic approaches to targeting MCAMhigh malignant NB.
Keywords: MT: Regular Issue, MCAM, chimeric antigen receptor, natural killer cell, NKTR-255, neuroblastoma
Graphical abstract

Patients with recurrent neuroblastoma (NB) have a dismal prognosis, in large part secondary to NB TME chemoradiotherapy resistance. Here, Cairo and colleagues developed a CAR NK cell targeting MCAM and demonstrated its anti-tumor efficacy, alone and in combination with an IL-15 agonist (NKTR-255), to circumvent NB TME resistance.
Introduction
Neuroblastoma (NB) is the most common malignant solid tumor that occurs in infants.1 Patients with recurrent metastatic NB have a dismal survival.2 Current treatments, including surgery, radiation, high-dose chemotherapy, and autologous stem cell transplantation, have failed to improve patient outcome in advanced disease.3,4,5,6 Novel therapies are urgently needed.
Natural killer (NK) cells have long been recognized as important in treating pediatric solid tumors, including NB.7 Unlike T cells, NK cells target tumor cells without requiring prior sensitization or specific antigen recognition and have not been associated with significant cytokine-related side effects and neurotoxicity. To enhance the cytotoxicity of NK cells and facilitate specific targeting of tumor cells, we and others have engineered NK cells to express chimeric antigen receptors (CARs) against molecular targets.8,9 However, due to the scarcity of tumor-associated antigens in pediatric solid tumors,10 only few NK cell CARs have been developed to target NB.11
Melanoma cell adhesion molecule (MCAM) is a cell-surface protein overexpressed in a variety of pediatric cancers, including NB.10 Increased MCAM expression is associated with poor prognosis, increased metastasis, and recurrence rate in various cancers.12 MCAM is highly expressed in the embryo, but its expression in mature normal tissue is minimal.13 These features make MCAM a promising target for immunotherapy for patients with metastatic MCAM+ solid tumors. A fully humanized anti-MCAM antibody, ABX-MA1, was found to inhibit spontaneous pulmonary metastasis of osteosarcoma in an orthotopic mouse model.14 However, MCAM-targeted cellular immunotherapy against NB has not been investigated previously.
Two limitations of NK cell tumor immunity is the low number and poor persistence of NK cells in vivo. NKTR-255 is an investigational polymer-conjugated, recombinant human interleukin-15 (IL-15) receptor agonist that activates the IL-15 pathway and stimulates proliferation and survival of NK and CD8+ T cells15 and is currently in early-phase clinical trials with monoclonal antibodies or CAR T cell therapy (ClinicalTrials.gov: NCT04136756, NCT03233854, and NCT04616196), as reported by our group and others.16
In the current study, we engineered an ex vivo expanded CAR NK cell targeting MCAM and combined it with NKTR-255 in order to circumvent some of the limitations of NK cell therapy to facilitate increased anti-tumor efficacy against NB.
Results
To evaluate the potential of NB cells to engage the NK cell activation or inhibitory receptors, we investigated the expression levels of the respective ligands, MHC class I chain-related protein A and B (MIC A/B), CD112, CD155, and human leukocyte antigen-ABC (HLA-ABC), in the NB cell lines SK-N-FI, Be2C, SK-N-DZ, and CHLA-255. We found that SK-N-FI and Be2C cells express intermediate levels of MIC A/B, high levels of CD112 and CD155, and low levels of HLA-ABC; CHLA-255 cells express high levels of CD155 and low levels of HLA-ABC (Figure S1). This suggests that these cells have the potential to induce more activating signals than inhibitory signals in NK cells, which renders them susceptible to NK cell cytotoxicity. SK-N-DZ cells, on the other hand, express a very low level of MIC A/B and an intermediate level of HLA-ABC, suggesting low sensitivity of these cells to NK cells. To evaluate MCAM as a target for CAR against NB cells, we analyzed MCAM expression levels in these cells and detected high levels of MCAM expression on SK-N-FI and CHLA-255 cells. In contrast, Be2C and SK-N-DZ cells express an intermediate and a low level of MCAM, respectively (Figure S1).
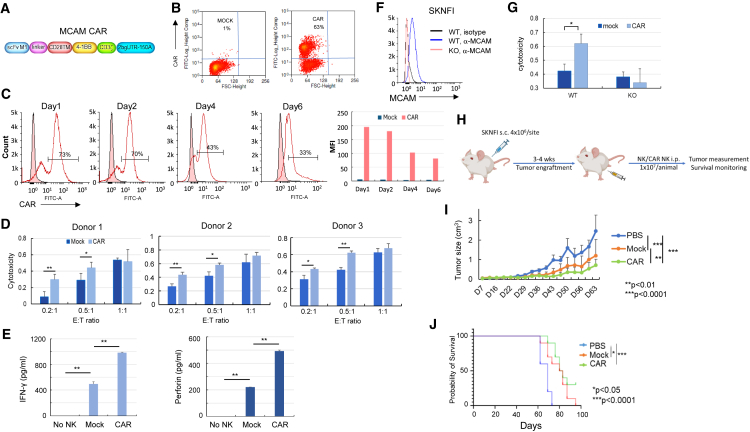
To assess cytotoxic activity of anti-MCAM-CAR-NK cells against NB cells, we engineered K562-membrane bound IL-21 (mbIL-21)-41BBL expanded NK (exNK) cells to express CARs against MCAM (Figures 1A and 1B). Electroporation of the CAR mRNA into exNK cells resulted in CAR expression in >60% of NK cells (Figure 1B), and the CAR expression lasted for at least 6 days (Figures 1C and S2). We performed in vitro cytotoxicity assays 1–2 days post CAR mRNA electroporation, when CAR expression on exNK cells was at the peak, and used SK-N-FI, CHLA-255, and Be2C cells, which express high and intermediate levels of MCAM and are potentially sensitive to NK cell cytotoxicity, as target cells. We found that, compared to the unmodified exNK cells (mock), expression of anti-MCAM CARs in exNK cells (CAR) significantly enhanced the NK cell cytotoxicity against the MCAMhigh SK-N-FI and CHLA-255 cells but not the Be2C cells (Figures 1D and S3) at effector-to-target (E:T) ratios of 0.2:1 and 0.5:1. We observed the significant differences between mock and CAR NK cells mostly at low E:T ratios (0.2:1 and 0.5:1), likely due to the high sensitivity of these tumor cells to NK cells at baseline. Next, we compared mock and CAR NK cell responses to SK-N-FI cells and found that CAR NK cells had significantly higher cytokine (interferon γ and perforin) secretion than mock NK cells when incubated with SK-N-FI cells (Figure 1E). To investigate whether the enhanced cytotoxic activity of CAR NK cells was due to specific targeting of MCAM, we knocked out MCAM in SK-N-FI cells (Figure 1F) and compared the cytotoxic activity of mock and CAR NK cells against wild-type (WT) and MCAM knockout (KO) cells. In the KO tumor cells, we did not observe a significant increase in cytotoxicity with CAR NK cells compared to mock NK cells as we did in the WT tumor cells (Figure 1G).
Figure 1.
Anti-MCAM-CAR-NK cells had superior cytotoxic activity against MCAMhigh NB cells in vitro and enhanced anti-tumor activity in NB xenograft mouse model compared to mock NK cells
(A) Schematic of the design of the anti-MCAM CAR construct. (B) CAR expression on ex vivo exNK cells mediated by electroporation of CAR mRNA. Ten micrograms of in vitro-transcribed CAR mRNA was introduced into 5 × 106 exNK cells using electroporation. Forty-eight hours after electroporation, CAR expression was detected by biotinylated MCAM protein, followed by fluorescein isothiocyanate (FITC)-streptavidin staining and flow cytometry. (C) Duration of expression of CAR on ex vivo exNK cells. CAR expression was detected 1, 2, 4, and 6 days post electroporation. Median fluorescence intensity for the CAR+ population was analyzed by FlowJo. (D) Compared to the unmodified exNK cells (mock), expression of anti-MCAM CAR in exNK cells (CAR) significantly enhanced the NK cytotoxic activity against MCAMhigh NB SK-N-FI cells. ∗p < 0.05, ∗∗p < 0.01 (two-tailed Student’s t test). Columns represent the mean values, and error bars indicate the standard deviation (SD) of triplicate samples in a representative experiment. The same trend was seen using three different donor-derived NK cells. (E) Compared to the mock NK cells, the anti-MCAM-CAR-NK cells (CAR) had significantly increased secretion of the cytokines interferon γ (IFN-γ; left) and perforin (right). ∗p < 0.05, ∗∗p < 0.01 (two-tailed Student’s t test). Columns represent the mean values, and error bars indicate the SD of triplicate samples in a representative experiment. The same trend was seen in three independent biological replicates. (F) Flow cytometry analysis showing MCAM KO by the CRISPR-Cas9 approach in SK-N-FI cells. (G) Specific targeting of MCAMhigh SK-N-FI cells by anti-MCAM-CAR-NK cells. Cytotoxic activities of mock and CAR NK cells against MCAM wild-type (WT) and knockout (KO) cells were compared. Columns indicate the mean, and error bars indicate the SD of triplicate samples in a representative experiment. ∗p < 0.05, ∗∗p < 0.01 (two-tailed Student’s t test). The same trend was seen in three independent biological replicates. (H) Schematic showing the in vivo study design and procedure in (I) and (J). NB SK-N-FI cells (4 × 106 cells/site) were implanted subcutaneously into 4- to 6-week-old female NSG mice. After tumor establishment, 1 × 107 of NK or CAR NK cells in PBS were injected intraperitoneally once a week for 5 weeks. Tumor growth was monitored by caliper measurement, and animals were followed until death or sacrificed upon reaching a tumor size of 2 cm in any dimension. (I) Anti-MCAM-CAR-NK treatment significantly decreased tumor growth in an NB xenograft mouse model. Mock NK or anti-MCAM-CAR-NK cells or PBS control were injected into NB xenograft tumor-bearing NSG mice intraperitoneally once a week for 5 weeks. Tumor growth was monitored by caliper measurement twice a week. Tumor size was estimated according to the following formula: tumor size (cm3) = length (cm) × width2 (cm) × 0.5. n = 10 per group. ∗∗p < 0.01, ∗∗∗p < 0.0001 (ANOVA). Growth rates between groups were analyzed using a mixed-effects model. (J) Anti-MCAM-CAR-NK cell treatment significantly prolonged animal survival in the NB xenograft mouse model. Mice were followed until death or sacrificed if any tumor size reached 2 cm in any dimension in (I). Probability of survival was determined by the Kaplan-Meier method using animal death/sacrifice as the terminal event using Prism v.8.0 (GraphPad). ∗p < 0.05, ∗∗∗p < 0.0001 (log rank test).
Next, we investigated the efficacy of anti-MCAM-CAR-NK cells in limiting NB xenograft tumor growth in vivo in NB (SK-N-FI)-xenografted NSG (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) mice. Consistent with the in vitro data, we found that treatment with CAR-modified exNK cells significantly decreased NB xenograft tumor growth compared to the vehicle and mock NK cells (Figure 1H) and prolonged animal survival compared to the vehicle (Figure 1I). Compared to the mock NK cell-treated mice, which all died at the end of the study (day 95), 30% of the mice treated with CAR NK cells were still alive (Figure 1I).
To improve CAR NK cell survival and persistence, we employed an IL-15 agonist, NKTR-255. We found that NKTR-255 treatment (40 ng/mL, 96 h) markedly increased the expression levels of NK cell-activating receptors including NKp30, natural killer group 2 member D (NKG2D), and NKp44, in the absence of IL-2 compared to the untreated exNK cells and only induced a mild increase in NKG2A and killer Ig-like receptors (KIR) expression levels (Figure 2A). Furthermore, NKTR-255 significantly improved NK cell survival and maintained NK cell expansion in the absence of IL-2 in vitro (Figure 2B). We therefore investigated the effect of NKTR-255 on the anti-tumor activity of the MCAM-CAR-NK cells. We first investigated whether NKTR-255 had any effect on CAR expression and observed no significant changes in CAR expression level or duration after 24, 48, 72, or 96 h of NKTR-255 treatment (Figure S4). We found that NKTR-255 significantly enhanced the cytotoxic activity of MCAM-CAR-NK cells targeting SK-N-FI cells at an E:T ratio of 0.5:1 (Figure 3A) and significantly enhanced secretion of interferon γ (Figure 3B) and perforin (Figure 3C) from MCAM-CAR-NK cells when incubated with SK-N-FI cells. In the NB (SK-N-FI)-xenografted mice, NKTR-255 alone did not have a significant effect on NB xenograft tumor growth or animal survival. However, combination with NKTR-255 further enhanced the anti-tumor effects of MCAM-CAR-NK cells (p < 0.001 compared to vehicle control, p < 0.05 compared to CAR NK cells) and further prolonged animal survival (100% vs. 30% survival on day 126 compared to CAR NK cells, p < 0.05) (Figures 3D–3F).
Figure 2.
NKTR-255 treatment increased the expression of NK-activating receptors and stimulated NK cell proliferation and expansion in vitro
(A) Expression of NKp30, NKG2D, NKp44, CD94, NKG2A, KIR, NKG2C, and NKp46, detected by flow cytometry on ex vivo exNK cells treated with or without NKTR-255 (40 ng/mL) for 96 h in the absence of IL-2. (B) Ex vivo exNK cells (2.5 × 106 cells per condition) were incubated with increasing concentrations of NKTR-255 (0, 1, 10, and 40 ng/mL) in the absence of IL-2 for various periods of time as indicated (0–144 h). The viable cells were counted every 24 h using the trypan blue staining method. ∗p < 0.05, ∗∗p < 0.01. Columns indicate mean values, and error bars indicate the SD of triplicate samples in a representative experiment. The same trend was seen in three independent biological repeats.
Figure 3.
Combination of NKTR-255 significantly increased the cytotoxic activity of anti-MCAM-CAR-NK cells against NB cells in vitro and further enhanced the anti-tumor activity of anti-MCAM-CAR-NK cells in vivo in the NB xenograft mouse model
(A–C) Mock NK or MCAM-CAR-NK cells were incubated with or without 40 ng/mL NKTR-255 for 72 h, followed by in vitro cytotoxicity assays against SK-N-FI cells at an E:T of 0.5:1. The number of donors tested is 3. NKTR-255 significantly enhanced the cytotoxic activity of MCAM CAR-engineered NK cells targeting NB SK-N-FI cells (A) and enhanced cytokine IFN-γ (B) and perforin (C) secretion from MCAM-CAR-NK cells. ∗p < 0.05, ∗∗p < 0.01. Columns represent mean values, and error bars indicate the SD of triplicate samples in a representative experiment. The same trend was seen in three independent biological replicates. (D) Schematic showing the in vivo study design and procedure in (E) and (F). NB SK-N-FI cells (4 × 106 cells/site) were implanted subcutaneously into 4- to 6-week-old female NSG mice. After tumor establishment, animals were divided into 4 groups and injected intraperitoneally with PBS or CAR NK cells in PBS (1 × 107/animal once a week for 5 weeks), NKTR-255 (0.3 mg/kg once every 2 weeks 3 times), or CAR NK cells combined with NKTR-255. Tumor growth was monitored by caliper measurement, and animals were followed until death or sacrificed upon reaching a tumor size of 2 cm in any dimension. (E) NKTR-255 further enhanced the efficacy of anti-MCAM-CAR-NK cells in significantly decreasing tumor growth in the NB xenograft mouse model. Tumor growth was monitored by caliper measurement twice a week. Tumor size was estimated according to the following formula: tumor size (cm3) = length (cm) × width2 (cm) × 0.5. n = 7 per group. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (ANOVA). Growth rates between groups were analyzed using a mixed-effects model. (F) Anti-MCAM-CAR-NK cell treatment combined with NKTR-255 further significantly prolonged animal survival compared to CAR NK cells alone in the NB xenograft mouse model. Mice were followed until death or sacrificed if any tumor size reached 2 cm in any dimension in (E). Probability of survival was determined by the Kaplan-Meier method using animal death/sacrifice as the terminal event using Prism v.8.0 (GraphPad). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (log rank test).
Discussion
While CAR T cells and checkpoint inhibitors have been successful in other pediatric and adult malignancies, these approaches in NB are severely limited,17 in part due to low expression of major histocompatibility complex (MHC) molecules, low mutational burden in NB, and severe side effects, including cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome.18 An alternative approach is to utilize NK cells that demonstrate anti-tumor cytolytic activity in an MHC-independent manner. In vitro data from us and others have indicated that NB cell lines are highly susceptible to NK cell-mediated cell lysis due to the low expression of MHC and high expression of NK cell-activating receptor ligands (Figure S1).19 Consistent with this, increased NK cell frequency in NB patient tumors has been associated with a favorable prognosis.20
However, NK cell number and function are low in patients with NB tumors, largely due to the low number of active NK cells; poor NK cell function, activation, and persistence; and lack of specific targeting, among others.21 To address low NK cell number and function, our group has developed a genetically engineered antigen-presenting cell (K562) expressing mbIL-21 and 4-1BBL to expand peripheral blood mononuclear cells into NK cells.22 This approach results in over 35,000-fold expansion in NK cells and significant NK cell functional activation.22
To enhance NK cell targeting specificity, we developed an mRNA electroporation technology to efficiently engineer exNK cells to express CARs.8 We and others have demonstrated that CAR NK or T cells generated by the mRNA electroporation approach are effective and safe without neurologic or systemic toxicity in lymphoma, medulloblastoma, and high-grade glioma.8,23,24 Here, we demonstrated significantly enhanced in vitro cytotoxic activity and in vivo anti-tumor efficacy of MCAM-targeting CAR NK cells against NB compared to mock NK cells (Figure 1). NB is an extremely heterogeneous tumor.25 We observed heterogeneity in MCAM and NK cell receptor ligands expression in our study (Figure S1). SK-N-FI and CHLA-255 cells, which express high levels of MCAM and NK cell-activating receptor ligands, were sensitive to the MCAM-CAR-NK cells, while Be2C and SK-N-DZ cells were not, due to their relatively low expression of MCAM or high expression of NK cell-inhibitory receptor ligands. This is consistent with a previous report that low surface density of the target (CD22), rather than total loss of its expression on tumor cells, is sufficient to allow escape from CAR therapy.26 This underscores the importance of monitoring the target expression level on patient tumors before and during the CAR therapy. In addition, these results, together with the cytotoxicity data for MCAM KO cells (Figure 1G), demonstrated the targeting specificity of the MCAM-CAR-NK cells.
IL-15 plays important roles in stimulating the proliferation and cytolytic activity of NK cells and CD8+ T cells with limited effect on regulatory T cells.21,27 Due to obstacles in production, a poor pharmacokinetic profile, weak potency, and its toxicity profile, the clinical application of recombinant human IL-15 (rhIL-15) has been limited.21 IL-15 agonists have been developed to address these limitations. We have reported previously that N-803, consisting of an IL-15 agonist mutein (IL-15N72D) and a dimeric IL-15 receptor alpha (IL-15Rα)/Fc fusion protein, significantly increased the viability, proliferation, and antibody-dependent cellular cytotoxicity (ADCC) of exNK cells.28 The combination of dinutuximab and N-803 significantly enhanced the in vitro cytotoxicity and in vivo anti-tumor effect of exNK cells against osteosarcoma, NB, and glioblastoma, as we have reported previously.28 Different from N-803, NKTR-255 is a polyethylene glycol conjugate of rhIL-15 that exhibits a longer half-life, reduced clearance, and prolonged receptor affinity in comparison to rhIL-15.15 Functionally, NKTR-255 induces the proliferation and activation of NK and CD8+ T cells, increases the CD8+:regulatory T cell ratio, increases the accumulation and persistence of anti-CD19 CAR T cells in the bone marrow, and synergizes with monoclonal antibodies to enhance ADCC in cancer models.15,16 Consistent with our previous report, we found that NKTR-255 stimulated NK cell activation and maintained NK cell ex vivo expansion in the absence of IL-2 (Figure 2). By combining the specific and effective targeting of MCAMhigh NB cells via anti-MCAM-CAR and the enhancement of NK in vivo persistence by NKTR-255, we were able to improve the therapeutic efficacy of NK cells against NB (Figure 3). Interestingly, the combinatorial effect of NKTR-255 and CAR NK cells was not due to increased CAR expression or duration because we did not observe significant effects of NKTR-255 on either (Figure S4).
Due to heterogeneous expression of MCAM on NB cell lines, we only evaluated the efficacy of MCAM-CAR-NK cells by utilizing a xenograft mouse model based on one NB cell line that expresses a high level of MCAM. In addition, the NSG mouse lacks mature murine T, B, and functional NK cells and is deficient in cytokine signaling. These characteristics are supportive of stable engraftment of human tumor cells and the evaluation of human NK cell anti-tumor efficacy. However, a validation of the study result in a syngeneic or humanized mouse model is warranted.
Nevertheless, our findings demonstrate that ex vivo expanded MCAM-CAR-NK cells are effective alone and with NKTR-255 in NB. MCAM-CAR-NK cells in combination with NKTR-255 are a promising novel alternative therapeutic approach for NB and potentially other MCAMhigh-expressing malignancies.
Materials and methods
Additional methods are detailed in the supplemental information.8,29,30,31
Animal studies
All animal studies were performed in accordance with protocols approved by the New York Medical College Institutional Animal Care and Use Committee. SK-N-FI cells (4 × 106 cells/site) with growth factor-reduced Matrigel matrix (Corning) were implanted subcutaneously into 4- to 6-week-old female NSG mice (The Jackson Laboratory). After tumor establishment, 1 × 107 of NK/CAR NK cells in PBS were injected intraperitoneally once a week for 5 weeks. We and others have shown that intraperitoneally injected NK cells are effective in solid tumor mouse models.32 NKTR-255 (0.3 mg/kg) was injected intraperitoneally once every 2 weeks. To compare the anti-tumor efficacy of mock NK and CAR NK cells, NB xenograft tumor-bearing NSG mice were treated with mock NK cells, anti-MCAM-CAR-NK cells, or PBS control. To investigate the effect of CAR NK cells combined with NKTR-255 on NB xenograft tumor growth, tumor-bearing animals were treated with PBS control, CAR NK cells, NKTR-255, or CAR NK cells + NKTR-255. Before conducting mouse experiments, sample sizes achieving 80% power to detect an effect size >2 were determined at a significance level of 0.05. No randomization or blinding was used. Tumor growth was monitored by caliper measurement twice a week, as we have described previously.28 Mice were followed until death or sacrificed upon reaching a tumor size of 2 cm in any dimension.
Data and code availability
Data and material are available upon reasonable request from mitchell_cairo@nymc.edu.
Acknowledgments
The authors would like to thank Bin Liu, PhD (University of California, San Francisco, USA) for providing the scFv sequence; Carl June, MD, and Yangbing Zhao, MD, PhD (University of Pennsylvania, USA), for providing the CAR optimization construct; Nektar Therapeutics for providing NKTR-255; Nina Slivinsky, LVT, RLATG, for her technical assistance; and Ginny Davenport, RN, and Erin Morris, BSN, for their assistance with the preparation of this manuscript. This work was supported primarily by a grant from the National Cancer Institute Cancer Moonshot U54 CA232561 (to M.S.C., D.A.L., and T.P.C.). Additional support was from grants from the Pediatric Cancer Research Foundation and Children’s Cancer Fund (to M.S.C.).
Author contributions
W.L., D.A.L., and M.S.C. conceived and designed the study. W.L., A.G., H.H., and X.M. developed the methodology, performed the analyses and interpreted the data. W.L., X.M., Y.C., T.P.C., and M.S.C. wrote, reviewed, and revised the manuscript. Y.C., M.T., Y.L., J.A., J.M.R., A.M.M., W.W.O., T.P.C., D.A.L., and M.S.C. provided administrative, technical and material support. All authors approved the final manuscript for submission.
Declaration of interests
M.S.C. has served as a consultant for Jazz Pharmaceuticals, Omeros Pharmaceuticals, Servier Pharmaceuticals, Abbvie, and Novartis Pharmaceuticals; with the Speakers Bureau for Jazz Pharmaceuticals, Servier Pharmaceuticals, Amgen, Inc., Sanofi, and Sobi; and on the Advisory Board for Astra Zeneca and receives research funding from Celularity, Merck, Miltenyi Biotec, Servier, Omeros, Jazz, and Janssen. D.A.L. reports personal fees and others from Kiadis Pharma, CytoSen Therapeutics, Courier Therapeutics, and Caribou Biosciences outside of the submitted work. In addition, D.A.L. has a patent broadly related to NK cell therapy of cancer with royalties paid to Kiadis Pharma. T.P.C. recently served as a one-time consultant to Blueprint, Incyte, and Oncopeptides and as a DSMB chair for SpringWorks and is a cofounder of Vironexis Biotherapeutics, Inc.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omton.2024.200894.
Contributor Information
Dean A. Lee, Email: dean.lee@nationwidechildrens.org.
Mitchell S. Cairo, Email: mitchell_cairo@nymc.edu.
Supplemental information
References
- 1.Yan P., Qi F., Bian L., Xu Y., Zhou J., Hu J., Ren L., Li M., Tang W. Comparison of Incidence and Outcomes of Neuroblastoma in Children, Adolescents, and Adults in the United States: A Surveillance, Epidemiology, and End Results (SEER) Program Population Study. Med. Sci. Monit. 2020;26 doi: 10.12659/MSM.927218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whittle S.B., Smith V., Doherty E., Zhao S., McCarty S., Zage P.E. Overview and recent advances in the treatment of neuroblastoma. Expert Rev. Anticancer Ther. 2017;17:369–386. doi: 10.1080/14737140.2017.1285230. [DOI] [PubMed] [Google Scholar]
- 3.Maris J.M. Recent advances in neuroblastoma. N. Engl. J. Med. 2010;362:2202–2211. doi: 10.1056/NEJMra0804577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozkaynak M.F., Gilman A.L., London W.B., Naranjo A., Diccianni M.B., Tenney S.C., Smith M., Messer K.S., Seeger R., Reynolds C.P., et al. A Comprehensive Safety Trial of Chimeric Antibody 14.18 With GM-CSF, IL-2, and Isotretinoin in High-Risk Neuroblastoma Patients Following Myeloablative Therapy: Children's Oncology Group Study ANBL0931. Front. Immunol. 2018;9:1355. doi: 10.3389/fimmu.2018.01355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park J.R., Kreissman S.G., London W.B., Naranjo A., Cohn S.L., Hogarty M.D., Tenney S.C., Haas-Kogan D., Shaw P.J., Kraveka J.M., et al. Effect of Tandem Autologous Stem Cell Transplant vs Single Transplant on Event-Free Survival in Patients With High-Risk Neuroblastoma: A Randomized Clinical Trial. JAMA. 2019;322:746–755. doi: 10.1001/jama.2019.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu A.L., Gilman A.L., Ozkaynak M.F., London W.B., Kreissman S.G., Chen H.X., Smith M., Anderson B., Villablanca J.G., Matthay K.K., et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho D., Shook D.R., Shimasaki N., Chang Y.H., Fujisaki H., Campana D. Cytotoxicity of activated natural killer cells against pediatric solid tumors. Clin. Cancer Res. 2010;16:3901–3909. doi: 10.1158/1078-0432.CCR-10-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu Y., Hochberg J., Yahr A., Ayello J., van de Ven C., Barth M., Czuczman M., Cairo M.S. Targeting CD20+ Aggressive B-cell Non-Hodgkin Lymphoma by Anti-CD20 CAR mRNA-Modified Expanded Natural Killer Cells In Vitro and in NSG Mice. Cancer Immunol. Res. 2015;3:333–344. doi: 10.1158/2326-6066.CIR-14-0114. [DOI] [PubMed] [Google Scholar]
- 9.Liu E., Marin D., Banerjee P., Macapinlac H.A., Thompson P., Basar R., Nassif Kerbauy L., Overman B., Thall P., Kaplan M., et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020;382:545–553. doi: 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orentas R.J., Yang J.J., Wen X., Wei J.S., Mackall C.L., Khan J. Identification of cell surface proteins as potential immunotherapy targets in 12 pediatric cancers. Front. Oncol. 2012;2:194. doi: 10.3389/fonc.2012.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esser R., Müller T., Stefes D., Kloess S., Seidel D., Gillies S.D., Aperlo-Iffland C., Huston J.S., Uherek C., Schönfeld K., et al. NK cells engineered to express a GD2 -specific antigen receptor display built-in ADCC-like activity against tumour cells of neuroectodermal origin. J. Cell Mol. Med. 2012;16:569–581. doi: 10.1111/j.1582-4934.2011.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sechler M., Parrish J.K., Birks D.K., Jedlicka P. The histone demethylase KDM3A, and its downstream target MCAM, promote Ewing Sarcoma cell migration and metastasis. Oncogene. 2017;36:4150–4160. doi: 10.1038/onc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J., Tang X., Weng W., Qiao Y., Lin J., Liu W., Liu R., Ma L., Yu W., Yu Y., et al. The membrane protein melanoma cell adhesion molecule (MCAM) is a novel tumor marker that stimulates tumorigenesis in hepatocellular carcinoma. Oncogene. 2015;34:5781–5795. doi: 10.1038/onc.2015.36. [DOI] [PubMed] [Google Scholar]
- 14.McGary E.C., Heimberger A., Mills L., Weber K., Thomas G.W., Shtivelband M., Lev D.C., Bar-Eli M. A fully human antimelanoma cellular adhesion molecule/MUC18 antibody inhibits spontaneous pulmonary metastasis of osteosarcoma cells in vivo. Clin. Cancer Res. 2003;9:6560–6566. [PubMed] [Google Scholar]
- 15.Miyazaki T., Maiti M., Hennessy M., Chang T., Kuo P., Addepalli M., Obalapur P., Sheibani S., Wilczek J., Pena R., et al. NKTR-255, a novel polymer-conjugated rhIL-15 with potent antitumor efficacy. J. Immunother. Cancer. 2021;9 doi: 10.1136/jitc-2020-002024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah N., Perales M.A., Turtle C.J., Cairo M.S., Cowan A.J., Saeed H., Budde L.E., Tan A., Lee Z., Kai K., et al. Phase I study protocol: NKTR-255 as monotherapy or combined with daratumumab or rituximab in hematologic malignancies. Future Oncol. 2021;17:3549–3560. doi: 10.2217/fon-2021-0576. [DOI] [PubMed] [Google Scholar]
- 17.Richards R.M., Sotillo E., Majzner R.G. CAR T Cell Therapy for Neuroblastoma. Front. Immunol. 2018;9:2380. doi: 10.3389/fimmu.2018.02380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richman S.A., Nunez-Cruz S., Moghimi B., Li L.Z., Gershenson Z.T., Mourelatos Z., Barrett D.M., Grupp S.A., Milone M.C. High-Affinity GD2-Specific CAR T Cells Induce Fatal Encephalitis in a Preclinical Neuroblastoma Model. Cancer Immunol. Res. 2018;6:36–46. doi: 10.1158/2326-6066.CIR-17-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sivori S., Parolini S., Marcenaro E., Castriconi R., Pende D., Millo R., Moretta A. Involvement of natural cytotoxicity receptors in human natural killer cell-mediated lysis of neuroblastoma and glioblastoma cell lines. J. Neuroimmunol. 2000;107:220–225. doi: 10.1016/s0165-5728(00)00221-6. [DOI] [PubMed] [Google Scholar]
- 20.Melaiu O., Chierici M., Lucarini V., Jurman G., Conti L.A., De Vito R., Boldrini R., Cifaldi L., Castellano A., Furlanello C., et al. Cellular and gene signatures of tumor-infiltrating dendritic cells and natural-killer cells predict prognosis of neuroblastoma. Nat. Commun. 2020;11:5992. doi: 10.1038/s41467-020-19781-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nayyar G., Chu Y., Cairo M.S. Overcoming Resistance to Natural Killer Cell Based Immunotherapies for Solid Tumors. Front. Oncol. 2019;9:51. doi: 10.3389/fonc.2019.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denman C.J., Senyukov V.V., Somanchi S.S., Phatarpekar P.V., Kopp L.M., Johnson J.L., Singh H., Hurton L., Maiti S.N., Huls M.H., et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS One. 2012;7 doi: 10.1371/journal.pone.0030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chu Y., Yahr A., Huang B., Ayello J., Barth M., S Cairo M. Romidepsin alone or in combination with anti-CD20 chimeric antigen receptor expanded natural killer cells targeting Burkitt lymphoma in vitro and in immunodeficient mice. OncoImmunology. 2017;6 doi: 10.1080/2162402X.2017.1341031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foster J.B., Griffin C., Rokita J.L., Stern A., Brimley C., Rathi K., Lane M.V., Buongervino S.N., Smith T., Madsen P.J., et al. Development of GPC2-directed chimeric antigen receptors using mRNA for pediatric brain tumors. J. Immunother. Cancer. 2022;10 doi: 10.1136/jitc-2021-004450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He W.G., Yan Y., Tang W., Cai R., Ren G. Clinical and biological features of neuroblastic tumors: A comparison of neuroblastoma and ganglioneuroblastoma. Oncotarget. 2017;8:37730–37739. doi: 10.18632/oncotarget.17146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fry T.J., Shah N.N., Orentas R.J., Stetler-Stevenson M., Yuan C.M., Ramakrishna S., Wolters P., Martin S., Delbrook C., Yates B., et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 2018;24:20–28. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang F., Zhao L.T., Jiang Y., Ba D.N., Cui L.X., He W. Activity of recombinant human interleukin-15 against tumor recurrence and metastasis in mice. Cell. Mol. Immunol. 2008;5:189–196. doi: 10.1038/cmi.2008.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chu Y., Nayyar G., Jiang S., Rosenblum J.M., Soon-Shiong P., Safrit J.T., Lee D.A., Cairo M.S. Combinatorial immunotherapy of N-803 (IL-15 superagonist) and dinutuximab with ex vivo expanded natural killer cells significantly enhances in vitro cytotoxicity against GD2(+) pediatric solid tumors and in vivo survival of xenografted immunodeficient NSG mice. J. Immunother. Cancer. 2021;9 doi: 10.1136/jitc-2020-002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bidlingmaier S., He J., Wang Y., An F., Feng J., Barbone D., Gao D., Franc B., Broaddus V.C., Liu B. Identification of MCAM/CD146 as the target antigen of a human monoclonal antibody that recognizes both epithelioid and sarcomatoid types of mesothelioma. Cancer Res. 2009;69:1570–1577. doi: 10.1158/0008-5472.CAN-08-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao Y., Moon E., Carpenito C., Paulos C.M., Liu X., Brennan A.L., Chew A., Carroll R.G., Scholler J., Levine B.L., et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 2010;70:9053–9061. doi: 10.1158/0008-5472.CAN-10-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanjana N.E., Shalem O., Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat. Methods. 2014;11:783–784. doi: 10.1038/nmeth.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geller M.A., Knorr D.A., Hermanson D.A., Pribyl L., Bendzick L., McCullar V., Miller J.S., Kaufman D.S. Intraperitoneal delivery of human natural killer cells for treatment of ovarian cancer in a mouse xenograft model. Cytotherapy. 2013;15:1297–1306. doi: 10.1016/j.jcyt.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and material are available upon reasonable request from mitchell_cairo@nymc.edu.