Abstract
Both the incidence and prognosis of arterial atherothrombosis and venous thromboembolism are strongly correlated with increasing age. Over the past decade, clonal hematopoiesis of indeterminate potential (CHIP) has been identified as a novel biomarker for cardiovascular disease. Driven by somatic mutations in the hematopoietic system, the epidemiology of CHIP is highly age dependent: among individuals aged ≥70 years in the general population, estimated prevalence of CHIP exceeds 10%. Several additional risk factors for CHIP have emerged in recent years, including smoking, receipt of anticancer therapy, and germ line predispositions. CHIP carriers consistently have higher risk of incident arterial atherothrombosis, even after accounting for traditional cardiovascular risk factors. However, the magnitude of this association varies across studies. In addition, individuals with established cardiovascular disease and CHIP have higher risks of recurrence and all-cause mortality than their non-CHIP counterparts. An association between CHIP carriership and incident venous thromboembolism has recently been made, although additional studies are needed to confirm this finding. No approved therapy exists to modify the cardiovascular risk among CHIP carriers. However, canakinumab showed promise in a post-hoc analyses of patients with TET2-mutated CHIP, and other anti-inflammasome agents are actively under development or evaluation. In this review, we provide an overview of CHIP as a mediator of thromboembolic diseases and discuss emerging therapeutics aimed at intervening on this thrombo-inflammatory nexus.
Introduction
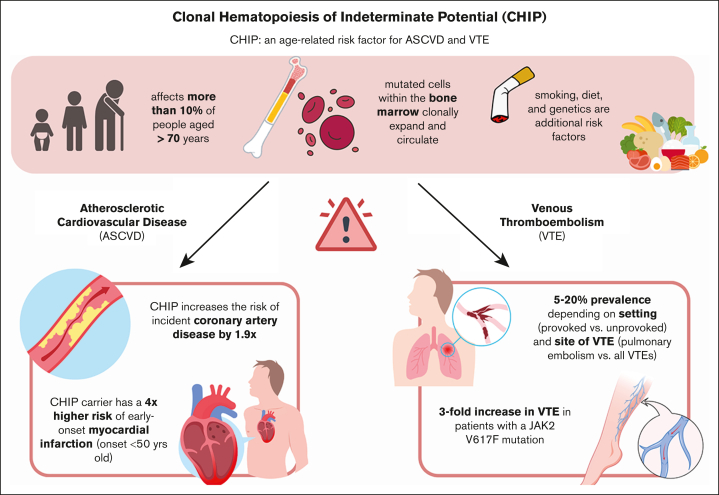
Approximately 1 in 4 deaths worldwide are attributable to thrombosis, manifested as atherothrombosis (eg, myocardial infarction, ischemic stroke, and peripheral artery disease) and venous thromboembolism (VTE; eg, pulmonary embolism [PE] and deep vein thrombosis).1 Risks of arterial and venous thrombosis are strongly correlated with age, and contemporary data have identified clonal hematopoiesis of indeterminate potential (CHIP), a set of age-dependent, inflammation-driven genetic changes in the hematopoietic system, as a novel cardiovascular biomarker. In this review, we aim to focus on the role of CHIP as a mediator of thromboembolic diseases, and emerging therapeutics aimed at intervening on this thrombo-inflammatory nexus (Figure 1).
Figure 1.
Overview of risk factors and thromboembolic sequelae of CHIP.
Nomenclature
Newly introduced in the revised 2022 World Health Organization definitions of myeloid neoplasm, CHIP is defined as the presence of a somatic (acquired) mutation of a myeloid malignancy–associated gene with a variant allele fraction (VAF) of ≥2%, in the absence of a diagnosed hematologic disorder.2 As a clonal bone marrow disorder, CHIP is considered a premalignant condition that predisposes to myeloid malignancies such as myelodysplastic syndrome and acute myeloid leukemia.2 A related condition termed clonal cytopenia of undetermined significance (CCUS) is distinguished from CHIP by the presence of cytopenia not meeting the formal criteria for a myeloid neoplasm and otherwise unexplained by a secondary hematologic or nonhematologic condition (eg, medications, hematinic deficiencies, and autoimmune conditions).2 Finally, micro-CHIP considers a group of conditions in which the VAF of a CHIP-qualifying gene mutation is <2%, a threshold that historically corresponded to the lower limit of detection for variant genetic alleles in early studies of clonal hematopoiesis.3
Gene mutations that qualify a diagnosis of CHIP has varied over time. Originally, there were 72 mutations associated with myeloid neoplasms identified in patients with myelodysplastic syndrome or acute myeloid leukemia. Through improvements in curation and delineation of candidate genes associated with thrombotic and myeloid complications, clonal hematopoietic mutations have now been trimmed to 58 genes. However, DNMT3A, TET2, and ASXL1 remain the most prevalent of CHIP mutations identified in the general population, accounting for >70% of all CHIP cases.4, 5, 6
Pathophysiologic mechanisms of CHIP acquisition and atherothrombosis
As an acquired condition associated with hematopoietic stem cells, CHIP-associated driver mutations arise stochastically through coding mutations. However, these mutations are advantaged because of selective pressure within the hematopoietic niches, allowing for clonal expansion that harbor such mutations. In fact, CHIP-associated mutations are often epigenetic regulators or chromatin modifiers. DNMT3A, the most prevalent locus mutated in CHIP carriers, is a methyltransferase involved in DNA methylation and gene expression.7 TET2 is another epigenetic regulator of DNA modification through 5-methylcytosine hydroxylation. Murine models with loss-of-function mutations of DNMT3A and TET2 are associated with loss of stem cell differentiation,8 increase in stem cell renewal,9 excess myeloproliferation, and extramedullary hematopoiesis. Similarly, ASXL1 is involved in assembly of chromatin modification complexes and transcription factors through homeobox genes (Hox genes).10
Although the rate of stochastical mutations acquired through DNA replication is considered to be constant, heightened activation of hematopoietic stem cells predisposes to the random acquisition of CHIP-associated driver mutations. Such conditions include anticancer therapy,11 smoking,12 and chronic inflammation.13 Importantly, CHIP captures a heterogenous group of gene loci and variants that exhibit differences in rates of acquisition and growth. For example, the rate of acquisition for JAK2V617F mutation is approximately twice that of DNMT3AR882C, the most frequently observed DNMT3A variant.14 Meanwhile, the growth rate of driver mutations can differ by 10-fold, from 5% to 50% per year between DNMT3A and SRSF2.15
In addition to growth dynamics, the mechanism of CHIP-driven atherothrombosis is variant dependent. Accelerated atherosclerosis in variants involving TET2 appears independent of systemic metabolism but is primarily mediated by the NLRP3 (NOD-like receptor family pyrin domain containing 3) inflammasome pathway.16 A multiprotein complex of the innate immune system, NLRP3 activation leads to conversion of pro–interleukin-1β (IL-1 β) to its active form. This in turn leads to increased expression of P-selectin and monocyte recruitment. In contrast, JAK2V617F variant carriers are predisposed to increased neutrophil extracellular trap formation and platelet reactivity in the setting of myeloproliferative neoplasm,17,18 which is abrogated through use of JAK1/2 inhibitors in preclinical models.18
Epidemiology and risk factors for CHIP
Age
A condition associated with aging and lifetime accumulation of somatic mutations, the prevalence of CHIP increases with each decade of life, with its prevalence rising from 0.7% among individuals aged <50 years in a Swedish population cohort,5 to >10% among individuals aged ≥70 years.3,19 The median age among CHIP carriers with VAF of ≥2% in the UK Biobank (UKB) and Geisinger MyCode Community Health Initiative (GHS) were 62 and 73 years, respectively, compared with 57 years among non-CHIP carriers in both cohorts.20 Because of differential growth dynamics, the composition of driver genes affected among CHIP carriers also differ by age categories. Although DNMT3A remains the most prevalent gene among CHIP carriers at younger age groups, TET2 mutations become more common among individuals aged ≥85 years.15
Sex
Using multivariate regression based on exome sequencing data from the UKB, Kessler et al reported an increased prevalence of CHIP among females compared with males (odds ratio [OR], 1.08; 95% confidence interval [CI], 1.05-1.11).20 However, this finding was not replicated in the GHS (OR, 1.01; 95% CI, 0.93-1.11), 20 The All of Us cohort (OR, 0.97; 95% CI, 0.89-1.06),21 or the Dutch Lifelines cohort (OR, 1.09; 95% CI, 0.90-1.30).22 Although evidence to date does not suggest sex as a consistent risk factor for CHIP, sex-specific differences exist in the type of mutation observed. In the Lifelines cohort, males had higher prevalence of ASXL1, SF3B1, and SRSF2, whereas DNMT3A mutations were more commonly observed in females.22
Lifestyle factors
Genotoxic and inflammatory effects of cigarette smoking are associated with CHIP, specifically those involving ASXL1 mutations.6,12 In contrast, JAK2 mutations were inversely associated with smoking history in the UKB, suggesting the heterogeneity of interactions between environmental factors and genetic subtypes within CHIP.6
Given lower inflammatory burden in diets high in fruits and vegetables,23, 24, 25 Bhattacharya et al interrogated data from the UKB to evaluate the association between self-reported dietary pattern and the presence of CHIP among adults aged 40 to 70 years.26 Using the 2015 Healthy Eating Index from the US Department of Health and Human Services, the investigators categorized dietary data into healthy (higher than median intake of fruits and vegetable intake, combined with lower than median intake of red meat, processed food, and added salt), unhealthy (converse of healthy diet), and intermediate (neither healthy nor unhealthy) dietary patterns. They found a step-wise increase in the prevalence of CHIP among individuals with healthy (5.1%), intermediate (5.7%), and unhealthy (7.1%) diets.26 Nonetheless, the impact of dietary factors on CHIP acquisition remains debated: using data from 8709 participants in the Women’s Health Initiative, Haring et al found individuals with higher adherence to dietary guidelines had a numerically greater prevalence of CHIP (10.7% among most adherent quintile vs 8.3% in least adherence quintile),27 and a multivariate model did not detect the association between dietary quality and prevalent CHIP (P = .14 for dose response).27
The Women’s Health Initiative study did not identify an association between physical activity and CHIP, but reported increased CHIP prevalence with higher body mass index (OR, 1.02; 95% CI, 1.01-1.04 for each point increase).27 It is therefore unclear whether the discrepant findings between the UKB and the Women’s Health Initiative analyses relate to proportion of females included (56% vs 100%), overall prevalence of CHIP (5.7% vs 8.6%), or body mass index as a factor along the causal pathway between diet, exercise, and CHIP acquisition.
Concurrent medical conditions
Consistent with the impact of systemic anticancer therapy on the development of secondary hematologic malignancies and arterial thromboembolism,28,29 CHIP is highly correlated with history of radiation or systemic chemotherapy receipt, in particular topoisomerase II inhibitors and platinum-based agents.11 Using prospective sequencing data from the Memorial Sloan Kettering Integrated Mutation Profiling of Actionable Cancer Targets database, Bolton et al observed a dose-dependent rise in CHIP burden among patients treated with systemic anticancer therapy, especially among individuals receiving platinum-based chemotherapy.11
Prevalence of CHIP is also increased among patients with antineutrophil cytoplasmic antibody–associated vasculitis compared with matched controls (30.4% vs 13.5%; P < .001).30 In addition, CHIP carriers were observed to have increased risk of giant cell arteritis, its vision-related complications (especially TET2-mutated CHIP), and history of relapsed disease.31,32 However, the association between CHIP carriership and other rheumatologic conditions are less consistent.33
Inherited (germ line) predisposition
In a single-variant association study using whole-genome sequences of 65 405 individuals enrolled in the US National Heart, Lung, and Blood Institute TOPMed project, Bick et al identified a 3.6% heritability of CHIP acquisition,34 which is considerably lower than contributions from acquired causes aforementioned. Three germ line genetic loci associated with increased risk of CHIP development were found: TERT, KPNA4-TRIM59, and TET2.34 Subsequent analysis using UKB and GHS genomic data identified 21 additional germ line loci harboring 57 variants that affect CHIP acquisition.20 One unique gene identified through analysis of TOPMed data is TCL1A, for which common inherited polymorphism in its promoter was associated with slower expansion rate of TET2- or ASXL1-mutated (but not DNMT3A) clones.35
Ethnic and racial groups
Data from population-based genomic cohorts suggest important differences in the prevalence of CHIP by race and ethnicity. Self-reported Hispanic ethnicity and East Asian ancestry are associated with a lower risk of CHIP than non-Hispanic White or Black individuals.21,34 Although CHIP is associated with markers of age-related inflammation, and lifestyle factors and dietary patterns, it is unknown whether these are mediators of ethnic and racial disparities in the CHIP epidemiology. Furthermore, emerging data point to the association between inflammation and socioeconomic factors such as social connectedness, everyday discrimination, and financial vulnerability,36, 37, 38 although whether this leads to CHIP has not been determined.37,38
CHIP and ASCVD
Over the past decade, CHIP has emerged as an important contributor to atherosclerotic cardiovascular disease (ASCVD; Table 1). Using whole-exome sequencing data from 2 prospective cohorts (1010 total participants), Jaiswal et al reported a 1.9-fold increased risk of incident coronary artery disease among individuals with CHIP at baseline compared with their non-CHIP counterparts (hazard ratio [HR], 1.9; 95% CI, 1.4-2.7).39 Presence of CHIP involving the JAK2 locus conferred the highest relative increase in incident coronary artery disease risk (HR, 12.0; 95% CI, 3.8-38.4), followed by ASXL1 (HR, 2.0; 95% CI, 1.0-3.9).39 In the same report, 7245 participants across 2 case-control studies were evaluated for the association between CHIP and early-onset myocardial infarction, the latter defined as events diagnosed before 50 years. This showed a more potent effect of CHIP on atherothrombosis, with a fourfold increased risk among CHIP carriers compared with matched controls (adjusted OR, 4.0; 95% CI, 2.4-6.7).39 In this group, early-onset myocardial infarction was strongly associated with mutations in TET2, ASXL1, and JAK2.39
Table 1.
Characteristics and impact of CHIP among individuals without symptomatic cardiovascular disease
| Cohort name | Recruitment period | Geographical region | No. of participants | Age (mean or median), y | Ethnicity, race, or ancestry (%) | Female, % | Participants with CHIP (VAF of ≥2% unless otherwise specified) | Most common CHIP loci (% distribution) | Follow-up duration (y) from genome sequencing | Impact of CHIP (VAF of ≥2% unless otherwise specified) |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coronary artery disease∗ | Ischemic stroke† | Peripheral arterial disease | VTE‡ | ||||||||||
| UKB20,40, 41, 42, 47 | 2006-2010 | United Kingdom | 454 803 (arterial) 425 399 (venous) |
Eligibility: 40-69 Overall: 56 (mean) |
European: 95 African: 2 South Asian: 2 Other: 0.9 |
54 | 21 137 (4.6%) |
DNMT3A: 68.5 TET2: 19.5 ASXL1: 9.0 PPM1D: 3.0 TP53: 1.9 |
13.5 | HR, 1.0; 95% CI, 1.0-1.1; P = .12§ VAF ≥10%: HR, 1.1; 95% CI, 1.0-1.2; P = .004 TET2 CHIP: HR, 1.3; 95% CI, 1.1-1.5 |
HR, 1.1; 95% CI, 0.8-1.4|| | HR, 1.6; 95% CI, 1.1-2.3¶ VAF ≥10%: HR, 2.1; 95% CI, 1.4-3.3¶ |
VTE overall: HR 1.2; 95% CI, 1.0-1.3; P = .01 VAF ≥10%: HR 1.2; 95% CI, 1.1-1.4 PE: HR, 1.17; 95% CI, 1.1-1.3 VAF ≥10%: HR, 1.2; 95% CI, 1.1-1.4; P = .002 TET2 CHIP: HR, 1.4; 95% CI, 1.2-1.7 |
| Women’s Health Initiative40 | 1993-1998 | United States | 9 683 | Eligibility: 50-79 Overall: 68.9 |
White: 82.5 Black: 12.3 Other: 5.2 |
100 | 904 (9.3%) |
DNMT3A: 59.1 TET2: 23.5 ASXL1: 7.1 JAK2: 4.3 TP53: 1.8 |
10.8 | NR | Any ischemic stroke: HR, 1.2 (P = .03) Small vessel: HR, 1.6 (P = .001) Large artery: HR, 1.1 (P = .62) Cardioembolic: HR, 1.05 (P = .68) |
NR | NR |
| BioImage39 | 2008-2009 | United States | 370 | Eligibility: 55-80 Overall: 70 (median) |
European: 100 | 38.3 | 44 (11.9%) |
DNMT3A: 31.8 TET2: 15.9 ASXL1: 13.6 |
2.6 | HR, 1.8; 95% CI, 1.1-2.9 | NR | NR | NR |
| Malmo Diet and Cancer39 | 1991-1996 | Malmö, Sweden | 640 | Overall: 60 (median) | NR | 39.0 | 33 (5.1%) |
DNMT3A: 45.5 TET2: 18.2 ASXL1: 18.2 |
17.7 | HR, 2.0; 95% CI, 1.2-3.1 | NR | NR | NR |
| China-PAR44 | 1998-2008 | China | 6 181 | Overall: 53.8 | Chinese: 100 | 49.9 | 658 (10.6%) |
DNMT3A: 50.0 TET2: 28.0 |
12.2 | VAF 2%-10%: HR, 1.5; 95% CI, 1.1-1.9 VAF ≥10%: HR, 1.8; 95% CI, 1.1-3.1 |
NR | NR | NR |
| GESUS45 | January 2010-October 2013 | Naestved, Denmark | 19 958 | Eligibility: ≥30 and 25% of 20-30 | NR | Overall: NR JAK2 V617F CHIP carriers: 46 |
92 (0.5%) with JAK2 V617F ≥1% and no MPN |
JAK2 V617F: 100 Allele burden ≥1%: 12. Allele burden ≥10%: 5 |
NR | VAF ≥1%: OR, 0.7; 95% CI, 0.2-2.4 | VAF ≥1%: OR, 0.57; 95% CI, 0.14-2.4 | NR | VAF ≥1%: OR, 2.8; 95% CI, 1.1-7.0 |
MPN, myeloproliferative neoplasm; NR, not reported.
Adjusted for age, sex, lipid profile, smoking, body mass index, hypertension, and diabetes (or glycemic control) at minimum.
Adjusted for age, sex, smoking status, diabetes, hypertension, and principal components of genetic ancestry at minimum.
Adjusted for age, sex, ethnicity, or principal components of genetic ancestry, smoking, and body mass index at minimum.
Both 95% CIs and P values are reported in situations in which upper or lower bound of CI involves 1.0.
Earlier UKB release of whole-exome sequencing on 45 186 participants.
Earlier UKB release of whole-exome sequencing on 37 657 participants, adjusted for age, age2, sex, smoking status, Townsend deprivation index, principal components of genetic ancestry, body mass index, hypertension, hyperlipidemia, and type 2 diabetes.
Although CHIP has been established as a risk factor for ASCVD across several studies, the relative increase in ASCVD risk conferred by its presence is variable: for example, the UKB/GHS combined analysis only demonstrated a small increase in ASCVD risk among CHIP carriers.20 This may be, in part, because of the time-varying nature of CHIP carriership status: in a randomized controlled trial of aspirin vs placebo among healthy older adults aged ≥70 years, incidence rate of CHIP acquisition was 7% per year among those who did not harbor such genetic change at baseline.41 Studies that aim to evaluate the impact of CHIP on long-term incident ASCVD risk based on 1-time whole-genome sequencing are therefore at risk of being attenuated by such crossover bias.
Furthermore, CHIP may provide prognostic information among individuals with established ASCVD (Table 2). Gumuser et al evaluated 13 129 individuals with a diagnosis of coronary artery disease, ischemic stroke, or peripheral arterial disease before their enrollment in the UKB, and compared 665 (5.1%) individuals with CHIP at the time of study enrollment to their non-CHIP counterparts.42 In a multivariate analysis that adjusted for cardiovascular risk factors, race, and social deprivation index, a higher risk of recurrent ASCVD was observed among individuals with CHIP compared with those without CHIP (HR, 1.24; 95% CI, 1.08-1.43). The strength of association was higher among individuals with high CHIP burden (VAF ≥ 10%), which was not attenuated when adjusted for traditional markers of inflammation such as high sensitivity C-reactive protein.42
Table 2.
Characteristics and impact of CHIP among individuals with established cardiovascular disease
| Cohort name | Recruitment period | Geographical region | No. of participants | Age, y | Female, % | Prevalence of CHIP | Most common CHIP loci (% distribution) | Follow-up duration | Impact of CHIP (VAF of ≥2%, unless otherwise specified)∗ |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Recurrent events | All-cause mortality | Other | |||||||||
| ASCVD | |||||||||||
| UK Biobank42 | 2006-2010 | United Kingdom | 13 129 | 63 (median) | 24.4 | 665 (5.1%) |
DNMT3A: 50.6 ASXL1: 21.2 TET2: 13.8 PPM1D: 6.1 SF3B1: 4.5 |
10.8 y | Recurrent ASCVD: HR, 1.3; 95% CI, 1.2-1.5 VAF ≥10%: HR, 1.5; 95% CI, 1.3-1.7 |
HR, 1.5; 95% CI, 1.3-1.8 VAF ≥10%: HR, 1.7; 95% CI, 1.4-2.0 |
NR |
| Acute coronary syndrome | |||||||||||
| CULPRIT-SHOCK46 | April 2013-April 2017 | Global | 446 | 69 (median) | 24.4 | 129 (28.9%) |
DNMT3A: 47 TET2: 36 ASXL1: 13 JAK2: 2% |
1 y | Recurrent myocardial infarction: 1.6% vs 1.3%; P > .99 (unadjusted) Repeat revascularization: 10.9% vs 14.8%; P = .27 (unadjusted) |
OR at 30 d: 1.67; 95% CI, 1.0-2.9; P = .07† OR at 1 y: 1.52; 95% CI, 0.87-2.63 |
NR |
| Wang et al46 | January 2017-September 2019 | China | 485 | 61.8 y (mean) | 27.4 | 60 (12.4%) |
DNMT3A: 42.1 TET2: 27.5 ASXL1: 12.7 SF3B1: 4.9 PPM1D: 2.9 |
3.0 y | Recurrent nonfatal MI or stroke: HR, 2.6; 95% CI, 1.3-5.1 | HR, 2.0; 95% CI, 1.1-3.5 | NR |
| AIS | |||||||||||
| PROSCIS-B and BeLOVE44 | March 2010-May 2013 | Berlin, Germany | 581 | 68 (median) | 55.1 | 236 (40.6%) with VAF ≥ 1% |
DNMT3A: 50.4 TET2: 30.9 ASXL1: 10.6 PPM1D: 4.7 |
36.2 mo | No difference in time to recurrent vascular event between VAF of ≥10% vs <1% (P = .55) | Shorter survival time among VAF of ≥10% vs <1% (P = .0021) | NR |
| Lee et al47 | May 2016-January 2021 | South Korea | 380 | 67.2 (mean) | 41.3 | 110 (29.0%) with VAF ≥ 1.5% |
DNMT3A: 57.1 TET2: 19.4 PPM1D: 3.1 |
90 d | NR | NR | Mean NIHSS at presentation: 8.1 vs 5.6 (adjusted β coefficient 1.67; P = .022) Hemorrhagic transformation: aOR, 5.6; 95% CI, 3.2-9.8 Modified Rankin score at 90 d: 2.5 vs 1.4; P < .001 |
| VTE | |||||||||||
| EDITH48 | May 2000-December 2004 | France | 394 first-episode unprovoked proximal DVT and PE | 67.4 (mean) | 58.5 | 4 (1.0%) with JAK2 V617F ≥1% and no MPN |
JAK2 V617F: 100. Allele burden ≥10%: 50% |
Up to 40 mo | No recurrent events | 2 (50%) | 1 (25%) episode of fatal anticoagulant-associated intracranial hemorrhage |
| Haque et al49 | 2017-2020 | Ohio, United States | 167 patients (81% unprovoked; 14% cancer associated) | 60.3 (median) | 55.5 | 22 (13.2%) |
DNMT3A: 46 TET2: 13 PPM1D: 10 |
400 d | No difference (rates NR) | NR | Clinically relevant bleeds: 23% vs 10%; P = .14 |
DVT, deep vein thrombosis; MI, myocardial infarction; MPN, myeloproliferative neoplasm; NIHSS, National Institutes of Health stroke scale; NR, not reported.
Unless specified, comparative statistics reported are adjusted for baseline covariates.
Both 95% CIs and P values are reported in situations in which upper or lower bound of CI involves 1.0.
Similar findings have been observed in acute ischemic strokes (AIS; Table 2). In a case-control study of 380 patients with AIS and 446 age-matched individuals, the presence of CHIP was associated with incident AIS, higher stroke severity, and risk of hemorrhagic transformation.47 Furthermore, a prospective cohort of 581 patients with AIS showed CHIP carriership (specifically those involving TET2 and PPM1D) to be associated with increased risk of recurrent vascular events and death (Table 2).44
Crucially, lifestyle factors can mitigate the cardiovascular effects of CHIP: in the UKB study that evaluated dietary patterns and CHIP prevalence, individuals with CHIP who had a healthy diet had a similar risk of incident ASCVD compared with those without CHIP who had an intermediate diet (HR, 0.99; 95% CI, 0.62-1.58).26 This is further supported by preclinical data, in which the deleterious effect of TET2-deficient hematopoietic stem cells on aortic atherosclerosis was prevented with a normal diet compared with a high-fat, high-cholesterol diet.16
Although CHIP has not formally been incorporated into ASCVD prediction tools to date, its presence appears to confer enhanced prediction of atherothrombotic events compared with traditional predictors (eg, age, sex, smoking status, blood pressure, and lipid profile). For example, Lee et al showed a synergistic impact of CHIP and high low-density lipoprotein levels compared with high low-density lipoprotein levels alone.50 Nonetheless, literature on cost-effectiveness of incorporating CHIP testing in routine ASCVD risk stratification remains scant, because of paucity of data on validated ASCVD prevention strategies among CHIP carriers. As discussed hereafter, although CHIP is a powerful prognostic biomarker, it has not yet been shown to be a predictive tool to inform effective interventions.51
CHIP and VTE
Age is a common risk factor in the development of both VTE and CHIP. Although VTE is rare among children aged <15 years at a rate of <5 per 100 000 person-years, its incidence sharply rises by >100-fold to >700 per 100 000 person-years (or ∼0.5% per year) among individuals aged 80 and above.52 Overall, the cumulative probability of a VTE diagnosis is estimated to be 11% among individuals aged 50 to 80 years in a longitudinal cohort from Malmö, Sweden.53
However, evidence on the impact of CHIP on VTE remains limited (Table 1). In a retrospective study, Soudet et al performed next-generation sequencing for targeted CHIP variants at 5 loci (DNMT3A, ASXL1, SF3B1, TET2, and TP53) among 61 patients with a first symptomatic unprovoked PE who did not harbor an inherited thrombophilia. In this group of patients (median age, 54 years) without unexplained cytopenia or established diagnosis of myeloproliferative neoplasm, these investigators detected somatic mutations consistent with CHIP in 12 patients (20% prevalence).54 Similar findings were observed by Haque et al (Table 2), who performed next-generation sequencing using a custom panel to detect CHIP among 167 patients with VTE, of whom 81% were unprovoked and 14% were cancer associated. In this study, the prevalence of CHIP was 13.2%, with no difference between the etiology of index VTE.49 More recently, Zon et al reported a 1.2-fold higher risk of incident VTE among individuals with CHIP in then UKB than their non-CHIP counterparts over a median follow-up of 11.8 years.43 However, in the same study, it was determined that CHIP was not associated with an increased risk of prevalent VTE (OR, 1.0; 95% CI, 0.8-1.2; P = .81).43 Among individuals with CHIP involving JAK2 mutations, risk of incident VTE was 4.2-fold higher (HR, 4.2; 95% CI, 2.2-8.1).43 This latter group of JAK2-mutated CHIP carriers is of special interest, with the Danish General Suburban Population Study reporting a 2.6% prevalence of JAK2V617F mutations in the general population,45 who do not meet the diagnostic criteria of a myeloproliferative neoplasm. Using a population-wide screening approach with pooled multiplex droplet digital polymerase chain reaction (sensitivity of 0.009% for JAK2V617F mutations), Cordua et al reported a threefold increase in risk of VTE among those with subclinical JAK2V617F mutations compared with those without such mutation (OR, 3.0; 95% CI, 1.2-7.8), after adjustment for vascular risk factors and complete blood count parameters.45
Although JAK2V617F mutation is known to be present in 20% and 5% of individuals with normal blood counts who are diagnosed with unprovoked splanchnic and cerebral vein thrombosis, respectively,55,56 studies using systematic screening of patients diagnosed with lower-limb deep vein thrombosis and PE reported low frequency of JAK2V617F mutation.48,57 It is worth noting that the JAK2-mutated CHIP was detected in <0.2% of prevalent VTE cases in the UKB,58 and its clinical impact on primary VTE prevention therefore requires further study.
Incorporating CHIP into clinical decision-making among patients with an established diagnosis of VTE also warrants careful consideration. Anticoagulant treatment duration after a first episode of VTE requires consideration of net clinical benefit by estimating the cumulative incidence and case fatality rates of recurrent VTE and major bleeding with indefinite or time-limited treatment (Figure 2). Older adults, in whom CHIP carriership is prevalent, are at higher risks of both major bleeding events with indefinite anticoagulation and recurrent VTE with time-limited treatment. Although traditional risk stratification for recurrent VTE considers the presence of monogenic risk factors (eg, factor V Leiden, prothrombin gene mutation, and inherited deficiencies of natural anticoagulants), major society guidelines continue to recommend long-term anticoagulation in most patients with a first unprovoked VTE. In fact, inherited thrombophilia testing in this context is discouraged because of limitations of current methods to identify patients at sufficiently low risk of VTE recurrence.59 Finally, although patients with VTE are at higher subsequent risk of ASCVD, this end point is often not considered in the framework to VTE treatment.60 Therefore, whether CHIP may be a shared risk factor in the causal pathway that results in higher ASCVD risk among patients with VTE remains an evidentiary gap with practice-changing implications.
Figure 2.
Traditional parameters to inform treatment duration in unprovoked or mildly provoked VTE. ∗Patients with mildly provoked VTE as index event. †Patients with unprovoked VTE as index event. N/A, not available.
Predicting and managing thrombotic risk
Predicting thrombotic events
Because the risk of hematologic malignancy transformation among individuals with CHIP is 1% per year, accurate risk stratification to identify those at highest risk is essential to ensure appropriate monitoring. Using UKB data that included 438 890 persons with median 11.7 years of follow-up, Weeks et al derived a prognostic score (Clonal Hematopoiesis Risk Score [CHRS]) that incorporates age, mutation type, mutation number, VAF, presence of cytopenia, and 2 complete blood count parameters (red cell distribution width and mean corpuscular volume) to classify individuals as low, intermediate, or high risk of transformation to hematologic malignancy.61 The score showed excellent discrimination in the derivation cohort (c-statistic = 0.81), and was subsequently validated in the Dana Farber Cancer Institute/Brigham and Women’s Hospital’s CHIP/CCUS cohort (c-statistic = 0.80).
Although CHRS was derived to predict the risk of malignant transformation, it also provided risk stratification for thrombotic end points. For example, individuals classified as high risk by CHRS had an 8.1-fold higher risk of arterial thrombotic events than individuals at low risk (HR, 8.1; 95% CI, 2.0-32.5).61 The model was subsequently evaluated in 3871 community-dwelling older adults (aged 67-90 years) participating in the Atherosclerosis Risk in Communities study who had whole-exome sequencing in 2014. Using a Fine and Gray model to account for the competing risks of noncardiovascular death, Saadatagah et al demonstrated a 2.9-fold increased risk of cardiovascular mortality among individuals with high-risk CHIP (defined by CHRS) compared with their non-CHIP counterparts.62 In addition, cardiovascular mortality was the most common cause of death among individuals with CHIP in the Atherosclerosis Risk in Communities cohort, even among those with high risk of malignant transformation as predicted by CHRS.62 This further supports the utility of CHRS to risk stratify individuals with CHIP for ASCVD in addition to its original intended use as a prognostic tool for hematologic malignancy.
Primary or secondary prevention of thrombotic complications
Although aspirin is at the cornerstone of secondary ASCVD prevention, it has not been shown to be an effective modality of reducing ASCVD risk in the primary prevention setting, including individuals with CHIP. In a secondary analysis of the ASPREE trial that randomized 19 144 healthy older adults in the United States and Australia (aged ≥65 years among African Americans, and aged ≥70 years among other racial and ethnic groups) to aspirin 100 mg daily or placebo, McQuilten et al evaluated whether aspirin conferred a protective effect on major adverse cardiovascular events among CHIP carriers. Presented as an abstract, CHIP carriership with VAF of ≥10% was associated with increased all-cause mortality. However, aspirin use was not associated with improved cardiovascular outcomes among CHIP carriers identified at baseline visit or at year-3 follow-up.41 Furthermore, aspirin led to a reduction in the disease-free survival of individuals with detectable JAK2V617F at baseline who did not meet criteria for a myeloproliferative neoplasm.41 Although results of this analysis await full publication, the role of antithrombotic agents for primary ASCVD prevention among patients with CHIP remains unclear. As discussed previously, the predictive impact of CHIP to inform antithrombotic intensity or duration among CHIP carriers with established ASCVD or VTE remains a crucial evidence gap.
Given the role of inflammation at the center of CHIP pathophysiology, anti-inflammatory agents are another group that have been proposed as a potential candidate to halt the cardiovascular complications associated with CHIP. In the CANTOS trial, 10 061 patients with a previous myocardial infarction and high sensitivity C-reactive protein of ≥2 mg/L despite optimal secondary prevention strategy were randomized to receive canakinumab, an anti–IL-1β monoclonal antibody, at 50 mg, 150 mg, 300 mg, or placebo administered every 3 months. Over a median of 3.7 years, canakinumab 150 mg (HR, 0.85; 95% CI, 0.74-0.98) and 300 mg (HR, 0.86; 95% CI, 0.75-0.99) reduced the incidence of nonfatal myocardial infarction, stroke, or cardiovascular death compared with placebo (3.9 vs 3.9 vs 4.5 per 100 person-years).63 In a secondary analysis of the CANTOS trial, participants who harbored a CHIP mutation in the TET2 locus benefited particularly from the use of canakinumab compared with placebo (HR, 0.38; 95% CI, 0.15-0.96), an effect that was less pronounced in non-TET2 CHIP carriers enrolled in the trial (HR, 1.08; 95% CI, 0.58-2.00).64 Although interaction for effect modification by TET2 CHIP status was not statistically significant (Pinteraction = 0.14) and the results were from a post-hoc analysis, these findings support further evaluation of anti-inflammatory agents in ASCVD prevention among individuals with CHIP.
Colchicine is an anti-NLRP3 inflammasome agent that has demonstrated efficacy in ASCVD. Low-dose colchicine has shown a 30% relative risk reduction in recurrent cardiovascular events in randomized, placebo-controlled trials involving acute myocardial infarction and chronic coronary disease.65,66 Expanding on preclinical data demonstrating prevention of accelerated atherosclerosis with colchicine in TET2-mutated murine models, Zuriaga et al analyzed data from the Mass General Brigham Biobank and the UKB to demonstrate effect modification of TET2 CHIP status on the protective effect of colchicine in human ASCVD.67 Compared with individuals without CHIP, TET2-CHIP carriers prescribed colchicine in the UKB had a 70% lower risk of incident myocardial infarction (HR, 0.3; 95% CI, 0.08-1.22), a protective effect that was not observed among colchicine nonusers (HR, 1.08; 95% CI, 0.95-1.24; Pinteraction = .05). However, prospective clinical trials that incorporate CHIP into patient selection are needed, given imbalances in baseline characteristics and potential for unmeasured confounding in this retrospective analysis.
Emerging therapies
Although there is an emerging focus on altering the trajectory of CHIP and CCUS progression through agents that primarily affect the NLRP3/IL-1β pathway, few clinical studies list ASCVD or VTE as part of its outcomes to be captured (Table 3). This underscores the urgent need for clinical trials with adequately powered cardiovascular end points to compare management strategies among CHIP carriers.
Table 3.
Novel therapeutic targets in CHIP and CCUS
| Agent | Trial registration | Study populations | Phase | Cardiovascular outcome? | Mechanism of action |
|---|---|---|---|---|---|
| Selnoflast | ISRCTN 10520571 | CHIP, CAD, and CRP of ≥2 mg/L | 1 | No | NLRP3 inflammasome inhibitor |
| Canakinumab | NCT 05641831 | CCUS with VAF of ≥10% | 2 | Yes, as secondary end point | IL-1β blockade |
| Enasidenib | NCT 05102370 | IDH2-mutated CCUS | 1 | No | IDH2 inhibitor |
| Ivosidenib | NCT05030441 | IDH1-mutant CCUS | 2 | No | IDH1 inhibitor |
| Curcumin | NCT 06063486 | CCUS, low-risk MDS and MPN | 2 | No | Pleiotropic |
| IV ascorbic acid | NCT 03418038 | TET2-mutated CCUS | 2 | No | TET2 cofactor |
| Oral ascorbic acid | NCT 03682029 | CCUS, low-risk MDS and MPN | 2 | No | TET2 cofactor |
| Statins | NCT 05483010 | CCUS, low-risk MDS | 2 | No | Pleiotropic |
CAD, coronary artery disease; CRP, C-reactive protein; IDH1/2, isocitrate dehydrogenase 1/2; ISRCTN, International Standard Randomised Controlled Trial Number; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; NCT, National Clinical Trial; TET1/2, ten-eleven translocation 1/2.
Conclusion
As a condition fueled by low-grade inflammation associated with aging, CHIP is a potent risk factor for ASCVD. Nonetheless, no therapeutics have been prospectively evaluated as a disease-modifying agent to address the cardiovascular impact of CHIP. Furthermore, the association between CHIP and VTE remains inconclusive, and whether CHIP can be used as a prognostic and predictive tool in anticoagulant management of VTE remains to be seen. Intervention-focused research is therefore direly needed to address a crucial and ongoing treatment gap in mitigating the cardiovascular outcomes of individuals living with CHIP.
Conflict-of-interest disclosure: T.-F.W. reports advisory board honoraria from Valeo and research funding to the institution from Leo Pharma. M.C. reports research grants from Pfizer, Bristol Myers Squibb, and Leo Pharma, and consultancy honoraria from Pfizer, Bayer, Sanofi, Servier, and Leo Pharma. The remaining authors declare no competing financial interests.
Acknowledgments
Y.X., T.-F.W., and M.C. are members of the Canadian Venous Thromboembolism Research (CanVECTOR) Network.
The CanVECTOR Network receives funding from the Canadian Institutes of Health Research (grant CDT-142654). M.C. is supported by the Clinical Research Chair program from the Department of Medicine, University of Ottawa. Y.X. is supported by a Tier 2 Canada Research Chair in Thrombosis.
Authorship
Contribution: Y.X., T.-F.W., and M.C. conceived of the review and its structure; A.T. and Y.X. drafted the initial manuscript, to which all authors provided key revisions; and all authors read and approved the final version of the manuscript.
References
- 1.Wendelboe AM, Raskob GE. Global burden of thrombosis: epidemiologic aspects. Circ Res. 2016;118(9):1340–1347. doi: 10.1161/CIRCRESAHA.115.306841. [DOI] [PubMed] [Google Scholar]
- 2.Khoury JD, Solary E, Abla O, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36(7):1703–1719. doi: 10.1038/s41375-022-01613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weeks LD, Ebert BL. Causes and consequences of clonal hematopoiesis. Blood. 2023;142(26):2235–2246. doi: 10.1182/blood.2023022222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steensma DP. Clinical consequences of clonal hematopoiesis of indeterminate potential. Blood Adv. 2018;2(22):3404–3410. doi: 10.1182/bloodadvances.2018020222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genovese G, Kähler AK, Handsaker RE, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371(26):2477–2487. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kar SP, Quiros PM, Gu M, et al. Genome-wide analyses of 200,453 individuals yield new insights into the causes and consequences of clonal hematopoiesis. Nat Genet. 2022;54(8):1155–1166. doi: 10.1038/s41588-022-01121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang L, Rau R, Goodell MA. DNMT3A in haematological malignancies. Nat Rev Cancer. 2015;15(3):152–165. doi: 10.1038/nrc3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Challen GA, Sun D, Jeong M, et al. Dnmt3a is essential for hematopoietic stem cell differentiation. Nat Genet. 2012;44(1):23–31. doi: 10.1038/ng.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moran-Crusio K, Reavie L, Shih A, et al. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell. 2011;20(1):11–24. doi: 10.1016/j.ccr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medina EA, Delma CR, Yang FC. ASXL1/2 mutations and myeloid malignancies. J Hematol Oncol. 2022;15(1):127. doi: 10.1186/s13045-022-01336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolton KL, Ptashkin RN, Gao T, et al. Cancer therapy shapes the fitness landscape of clonal hematopoiesis. Nat Genet. 2020;52(11):1219–1226. doi: 10.1038/s41588-020-00710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawoud AAZ, Tapper WJ, Cross NCP. Clonal myelopoiesis in the UK Biobank cohort: ASXL1 mutations are strongly associated with smoking. Leukemia. 2020;34(10):2660–2672. doi: 10.1038/s41375-020-0896-8. [DOI] [PubMed] [Google Scholar]
- 13.Avagyan S, Henninger JE, Mannherz WP, et al. Resistance to inflammation underlies enhanced fitness in clonal hematopoiesis. Science. 2021;374(6568):768–772. doi: 10.1126/science.aba9304. [DOI] [PubMed] [Google Scholar]
- 14.Watson CJ, Papula AL, Poon GYP, et al. The evolutionary dynamics and fitness landscape of clonal hematopoiesis. Science. 2020;367(6485):1449–1454. doi: 10.1126/science.aay9333. [DOI] [PubMed] [Google Scholar]
- 15.Fabre MA, de Almeida JG, Fiorillo E, et al. The longitudinal dynamics and natural history of clonal haematopoiesis. Nature. 2022;606(7913):335–342. doi: 10.1038/s41586-022-04785-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuster JJ, MacLauchlan S, Zuriaga MA, et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355(6327):842–847. doi: 10.1126/science.aag1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu W, Pircher J, Schuermans A, et al. Jak2 V617F clonal hematopoiesis promotes arterial thrombosis via platelet activation and cross talk. Blood. 2024;143(15):1539–1550. doi: 10.1182/blood.2023022260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolach O, Sellar RS, Martinod K, et al. Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Sci Transl Med. 2018;10(436) doi: 10.1126/scitranslmed.aan8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaiswal S, Ebert BL. Clonal hematopoiesis in human aging and disease. Science. 2019;366(6465) doi: 10.1126/science.aan4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kessler MD, Damask A, O’Keeffe S, et al. Common and rare variant associations with clonal haematopoiesis phenotypes. Regeneron Genetics Center GRDC, ed. Nature. 2022;612(7939):301–309. doi: 10.1038/s41586-022-05448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vlasschaert C, Mack T, Heimlich JB, et al. A practical approach to curate clonal hematopoiesis of indeterminate potential in human genetic datasets. Blood. 2023;141(18):2214–2223. doi: 10.1182/blood.2022018825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamphuis P, Van Zeventer IA, De Graaf AO, et al. Sex differences in the spectrum of clonal hematopoiesis. HemaSphere. 2023;7(2) doi: 10.1097/HS9.0000000000000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calder PC, Ahluwalia N, Brouns F, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr. 2011;106(suppl 3):S5–S78. doi: 10.1017/S0007114511005460. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Lee DH, Hu J, et al. Dietary inflammatory potential and risk of cardiovascular disease among men and women in the U.S. J Am Coll Cardiol. 2020;76(19):2181–2193. doi: 10.1016/j.jacc.2020.09.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation. J Am Coll Cardiol. 2006;48(4):677–685. doi: 10.1016/j.jacc.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 26.Bhattacharya R, Zekavat SM, Uddin MM, et al. Association of diet quality with prevalence of clonal hematopoiesis and adverse cardiovascular events. JAMA Cardiol. 2021;6(9):1069–1077. doi: 10.1001/jamacardio.2021.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haring B, Reiner AP, Liu J, et al. Healthy lifestyle and clonal hematopoiesis of indeterminate potential: results from the women’s health initiative. J Am Heart Assoc. 2021;10(5) doi: 10.1161/JAHA.120.018789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morton LM, Dores GM, Tucker MA, et al. Evolving risk of therapy-related acute myeloid leukemia following cancer chemotherapy among adults in the United States, 1975-2008. Blood. 2013;121(15):2996–3004. doi: 10.1182/blood-2012-08-448068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Y, Mallity C, Collins E, Siegal DM, Wang TF, Carrier M. Anticoagulation for the prevention of arterial thromboembolism in cancer patients by primary tumor site: a systematic review and meta-analysis of randomized trials. Eur Heart J Cardiovasc Pharmacother. Published online 13 September 2024 doi: 10.1093/ehjcvp/pvae068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arends CM, Weiss M, Christen F, et al. Clonal hematopoiesis in patients with anti-neutrophil cytoplasmic antibody-associated vasculitis. Haematologica. 2020;105(6):e264–e267. doi: 10.3324/haematol.2019.223305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinette ML, Weeks LD, Kramer RJ, et al. Association of somatic TET2 mutations with giant cell arteritis. Arthritis Rheumatol. 2024;76(3):438–443. doi: 10.1002/art.42738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gutierrez-Rodrigues F, Wells KV, Jones AI, et al. Clonal haematopoiesis across the age spectrum of vasculitis patients with Takayasu’s arteritis, ANCA-associated vasculitis and giant cell arteritis. Ann Rheum Dis. 2024;83(4):508–517. doi: 10.1136/ard-2023-224933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Savola P, Lundgren S, Keränen MAI, et al. Clonal hematopoiesis in patients with rheumatoid arthritis. Blood Cancer J. 2018;8(8):69. doi: 10.1038/s41408-018-0107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bick AG, Weinstock JS, Nandakumar SK, et al. Inherited causes of clonal haematopoiesis in 97,691 whole genomes. Nature. 2020;586(7831):763–768. doi: 10.1038/s41586-020-2819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinstock JS, Gopakumar J, Burugula BB, et al. Aberrant activation of TCL1A promotes stem cell expansion in clonal haematopoiesis. Nature. 2023;616(7958):755–763. doi: 10.1038/s41586-023-05806-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berger E, Castagné R, Chadeau-Hyam M, et al. Multi-cohort study identifies social determinants of systemic inflammation over the life course. Nat Commun. 2019;10(1):773. doi: 10.1038/s41467-019-08732-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis TT, Cogburn CD, Williams DR. Self-reported experiences of discrimination and health: scientific advances, ongoing controversies, and emerging issues. Annu Rev Clin Psychol. 2015;11(1):407–440. doi: 10.1146/annurev-clinpsy-032814-112728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samuel L, Szanton SL, Fedarko NS, Simonsick EM. Leveraging naturally occurring variation in financial stress to examine associations with inflammatory burden among older adults. J Epidemiol Community Health. 2020;74(11):892–897. doi: 10.1136/jech-2020-213807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaiswal S, Natarajan P, Silver AJ, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377(2):111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhattacharya R, Zekavat SM, Haessler J, et al. Clonal hematopoiesis is associated with higher risk of stroke [published correction appears in Stroke.2022;53(9):e440] Stroke. 2022;53(3):788–797. doi: 10.1161/STROKEAHA.121.037388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McQuilten Z, Wong NC, Leichter A, et al. The effect of clonal hematopoiesis of indeterminate potential (CHIP) and aspirin on clinical outcomes in the healthy elderly: a sub-study of the aspirin in reducing events in the elderly (ASPREE) randomized controlled trial. Blood. 2022;140(suppl 1):2238–2239. [Google Scholar]
- 42.Gumuser ED, Schuermans A, Cho SMJ, et al. Clonal hematopoiesis of indeterminate potential predicts adverse outcomes in patients with atherosclerotic cardiovascular disease. J Am Coll Cardiol. 2023;81(20):1996–2009. doi: 10.1016/j.jacc.2023.03.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zon B, Sekar A, Clapham K, et al. Clonal hematopoiesis and venous thromboembolism in the UK Biobank. Blood. 2023;142(suppl 1):568. [Google Scholar]
- 44.Arends CM, Liman TG, Strzelecka PM, et al. Associations of clonal hematopoiesis with recurrent vascular events and death in patients with incident ischemic stroke. Blood. 2023;141(7):787–799. doi: 10.1182/blood.2022017661. [DOI] [PubMed] [Google Scholar]
- 45.Cordua S, Kjaer L, Skov V, Pallisgaard N, Hasselbalch HC, Ellervik C. Prevalence and phenotypes of JAK2 V617F and calreticulin mutations in a Danish general population. Blood. 2019;134(5):469–479. doi: 10.1182/blood.2019001113. [DOI] [PubMed] [Google Scholar]
- 46.Bohme M, Desch S, Rosolowski M, et al. Impact of clonal hematopoiesis in patients with cardiogenic shock complicating acute myocardial infarction. J Am Coll Cardiol. 2022;80(16):1545–1556. doi: 10.1016/j.jacc.2022.08.740. [DOI] [PubMed] [Google Scholar]
- 47.Lee E, An HY, Lim J, et al. Clonal hematopoiesis and acute ischemic stroke outcomes. Ann Neurol. 2023;94(5):836–847. doi: 10.1002/ana.26754. [DOI] [PubMed] [Google Scholar]
- 48.Ugo V, Le Gal G, Lecucq L, Mottier D, Oger E, EDITH Collaborative Study Group Prevalence of the JAK2 V617F mutation is low among unselected patients with a first episode of unprovoked venous thromboembolism. J Thromb Haemost. 2008;6(1):203–205. doi: 10.1111/j.1538-7836.2007.02811.x. [DOI] [PubMed] [Google Scholar]
- 49.Haque T, Lozanski A, Doong TJ, et al. Rate of clonal hematopoiesis in patients with venous thromboembolism. Blood. 2021;138(suppl 1):4297. [Google Scholar]
- 50.Lee H, Song H, Choi S-Y, et al. Impact of clonal haematopoiesis on atherosclerotic cardiovascular disease according to low-density lipoprotein cholesterol levels in general population. Eur J Prev Cardiol. 2024;31(9):1162–1171. doi: 10.1093/eurjpc/zwae055. [DOI] [PubMed] [Google Scholar]
- 51.Ballman KV. Biomarker: predictive or prognostic? J Clin Oncol. 2015;33(33):3968–3971. doi: 10.1200/JCO.2015.63.3651. [DOI] [PubMed] [Google Scholar]
- 52.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158(6):585–593. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 53.Nordström M, Lindblad B, Bergqvist D, Kjellström T. A prospective study of the incidence of deep-vein thrombosis within a defined urban population. J Intern Med. 1992;232(2):155–160. doi: 10.1111/j.1365-2796.1992.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 54.Soudet S, Jedraszak G, Evrard O, Marolleau JP, Garcon L, Pietri MAS. Is hematopoietic clonality of indetermined potential a risk factor for pulmonary embolism? TH Open. 2021;5(3):e338–e342. doi: 10.1055/s-0041-1733856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Stefano V, Fiorini A, Rossi E, et al. Incidence of the JAK2 V617F mutation among patients with splanchnic or cerebral venous thrombosis and without overt chronic myeloproliferative disorders. J Thromb Haemost. 2007;5(4):708–714. doi: 10.1111/j.1538-7836.2007.02424.x. [DOI] [PubMed] [Google Scholar]
- 56.Passamonti SM, Biguzzi E, Cazzola M, et al. The JAK2 V617F mutation in patients with cerebral venous thrombosis. J Thromb Haemost. 2012;10(6):998–1003. doi: 10.1111/j.1538-7836.2012.04719.x. [DOI] [PubMed] [Google Scholar]
- 57.Rodger MA, Kekre N, Le Gal G, et al. Low prevalence of JAK2 V617F mutation in patients with first unprovoked venous thromboembolism. Br J Haematol. 2011;155(4):511–513. doi: 10.1111/j.1365-2141.2011.08701.x. [DOI] [PubMed] [Google Scholar]
- 58.Zon RL, Sekar A, Clapham K, et al. JAK2-mutant clonal hematopoiesis is associated with venous thromboembolism. Blood. 2024;144(20):2149–2154. doi: 10.1182/blood.2024024187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ortel TL, Neumann I, Ageno W, et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020;4(19):4693–4738. doi: 10.1182/bloodadvances.2020001830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sørensen HT, Horvath-Puho E, Pedersen L, Baron JA, Prandoni P. Venous thromboembolism and subsequent hospitalisation due to acute arterial cardiovascular events: a 20-year cohort study. Lancet Lond Engl. 2007;370(9601):1773–1779. doi: 10.1016/S0140-6736(07)61745-0. [DOI] [PubMed] [Google Scholar]
- 61.Weeks LD, Niroula A, Neuberg D, et al. Prediction of risk for myeloid malignancy in clonal hematopoiesis. NEJM Evid. 2023;2(5) doi: 10.1056/evidoa2200310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saadatagah S, Uddin MM, Weeks LD, et al. Clonal hematopoiesis risk score and all-cause and cardiovascular mortality in older adults. JAMA Netw Open. 2024;7(1) doi: 10.1001/jamanetworkopen.2023.51927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 64.Svensson EC, Madar A, Campbell CD, et al. TET2 -driven clonal hematopoiesis and response to canakinumab: an exploratory analysis of the CANTOS randomized clinical trial. JAMA Cardiol. 2022;7(5):521–528. doi: 10.1001/jamacardio.2022.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nidorf SM, Fiolet ATL, Mosterd A, et al. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383(19):1838–1847. doi: 10.1056/NEJMoa2021372. [DOI] [PubMed] [Google Scholar]
- 66.Tardif JC, Kouz S, Waters DD, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381(26):2497–2505. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 67.Zuriaga MA, Yu Z, Matesanz N, et al. Colchicine prevents accelerated atherosclerosis in TET2-mutant clonal haematopoiesis. Eur Heart J. 2024;45(43):4601–4615. doi: 10.1093/eurheartj/ehae546. [DOI] [PMC free article] [PubMed] [Google Scholar]