Abstract
Objectives
We aimed to determine the prevalence of female infertility in 204 countries and territories from 1990 to 2019.
Design
We evaluated the female infertility global burden between 1990 and 2019 in this systematic study.
Materials
The Global Health Data Exchange query tool (http://ghdx.healthdata.org/gbd-results-tool) and sociodemographic index (SDI) (http://ghdx.healthdata.org/data-type/estimate) provided data on the annual prevalence numbers, age-standardized prevalence rates (ASR), and disability-adjusted life-years (DALYs) of female infertility in 204 countries and territories from 1990 to 2019 for various age groups.
Setting
Female infertility has a devastating impact on the physical and mental health of individuals and national fertility. However, most of the previous studies on this subject were conducted on rather small sample sizes and have certain limitations.
Methods
We examined female infertility in terms of prevalence, ASR, and DALYs across different age groups in 204 countries and territories from 1990 to 2019 using data from the Global Health Data Exchange query tool.
Results
From 1990 to 2019, ASR and DALYs for female infertility increased globally. At the SDI quintile level, middle-SDI and high-middle-SDI countries exhibited a faster increase in the ASR of female infertility. In 2019, with the highest female infertility rate recorded among those between the ages of 30–34 years and the lowest among those between the ages of 45–49 years. In 2019, high-income North America recorded the highest proportion of primary infertility, while East Asia recorded the lowest proportion.
Limitations
First, the global burden of disease (GBD) database lacks data for some countries and regions. Second, data access and quality differ across locations. Third, the causes of infertility are not comprehensive, and data on Klinefelter in GBD 2019 in relation to primary infertility were absent.
Conclusion
Globally, the prevalence of DALYs and age-standardized female infertility rates increased from 1990 to 2019.
Keywords: Female infertility, Prevalence, Disability-adjusted life-years, Global burden of disease study, Sociodemographic index
Introduction
Female infertility is the inability of a woman of reproductive age to conceive without contraception with the same sexual partner for more than 1 year [1]. Some scholars believe that the prevalence of infertility is an “iceberg phenomenon” and that most couples with infertility remain undiagnosed [2]. Female infertility has a devastating impact on the physical and mental health of individuals and national fertility, resulting in a burden of medical costs [3]. The prevalence of infertility varies globally, affecting approximately 8–12% of couples of reproductive age [4].
Female infertility includes primary infertility, where the woman has not been pregnant without contraception with the same sexual partner for a minimum of 1 year, and secondary infertility, where the woman has not been pregnant without contraception with the same sexual partner for 12 months or more since the last pregnancy [5]. There are many causes of female infertility, including ovulation dysfunction, tubal blockage, endometriosis, uterine, and cervical causes [6]. The risk of infertility is heightened by other variables, including smoking, unsafe abortion, and sexually transmitted infections [7]. In addition, social factors such as exposure to toxic substances before/during pregnancy, delayed childbearing, poor socioeconomic status of women, intimate relationship violence, and sexually transmitted infections are important contributors to the reduction in female fertility [7].
Sun et al. [8] used the Global Health Data Exchange query tool to evaluate the regional, national, and global burden of infertility from 1990 to 2017. Additionally, from 1990 to 2019, Liu et al. [9] investigated the global disease burden associated with endometriosis-related primary and secondary infertility. Furthermore, many systematic studies related to female infertility have been published. However, most of these studies were conducted on rather small sample sizes and have certain limitations [10–12].
Therefore, we evaluated the female infertility global burden between 1990 and 2019 in this systematic study based on the following criteria: prevalence, disability-adjusted life-years (DALYs), age-standardized prevalence rates (ASR), average annual percent change (APC), and a sociodemographic index (SDI; combining indicators of per capita income, years of schooling, and fertility) and assessed their relationship with levels of development. This study also discusses the related causes of female infertility, classified the causes according to primary and secondary infertility, and analyzed the proportion of each cause in female infertility.
Materials and Methods
Overview
The design and standardization procedures of global burden of disease (GBD) investigations have been extensively reported in existing GBD literature [13–15]. GBD 2019 provides a systematic and comprehensive evaluation of 369 diseases and injuries across 204 countries and territories, covering age- and sex-specific incidence, prevalence, years of life lost, DALYs, mortality, and years of disability, between 1990 and 2019.
Data Sources
The Global Health Data Exchange query tool (http://ghdx.healthdata.org/gbd-results-tool) and SDI (http://ghdx.healthdata.org/data-type/estimate) provided data on the annual prevalence numbers, ASRs, and DALYs of female infertility in 204 countries and territories from 1990 to 2019 for various age groups.
Sociodemographic Index
The GBD 2019 research uses the SDI, a composite indicator, to provide a summary of sociodemographic progress in a specific area [13]. The average personal income per capita, the education level of residents above the age of 15, and the total population fertility rate are used to compute the SDI. The SDI, which ranges from 0 to 1, is a comprehensive marker of geographical development based on national per capita income, average educational attainment, and total fertility. 204 countries and territories were divided into five levels according to SDI: low (0–0.455), medium-low (0.456–0.608), medium (0.609–0.690), medium-high (0.691–0.805), and high (0.806–1) SDI regions [16].
Statistical Analysis
The burden of nonfatal diseases was modeled by GBD, employing the Bayesian meta-regression tool disMOd-MR 2.1. ASR, representing a weighted average of a specific age ratio, is calculated by aggregating measures of the ratio a population would have if it had a standard age structure. The standard population’s distribution is used to determine the weights using the following formula: . The specific age ratio, denoted as αi, highlights the number (or weight) of the chosen standard population per 100,000 individuals. The linear regression line is fitted to the ASR-related natural logarithm to determine the estimated annual percentage change (EAPC): EAPC = ln (ASR) = α+βx+ε, the x signifies the calendar year, and the ε denotes the error term. The 95% confidence interval for ε is computed as follows: 100 × (exp(β) − 1) [17]. As the EAPC value and its 95% confidence interval rise above zero, the corresponding ASR trends upward, and vice versa [18]. A smoothed spline model was employed in this study to evaluate the relationship between women’s burden of infertility and SDI. The Spearman order correlation technique was used for correlation analysis, considering a value of p < 0.05 as statistically significant. R 4.3.1 was utilized (R Foundation, Vienna, Austria, https://www.r-project.org/) for the analysis. Each step used to analyze the GBD database in this study complied with the cross-sectional study guidelines stipulated in the Guidelines for Accurate and Transparent Health Estimates Reporting [19].
Results
Global Female Infertility Burden
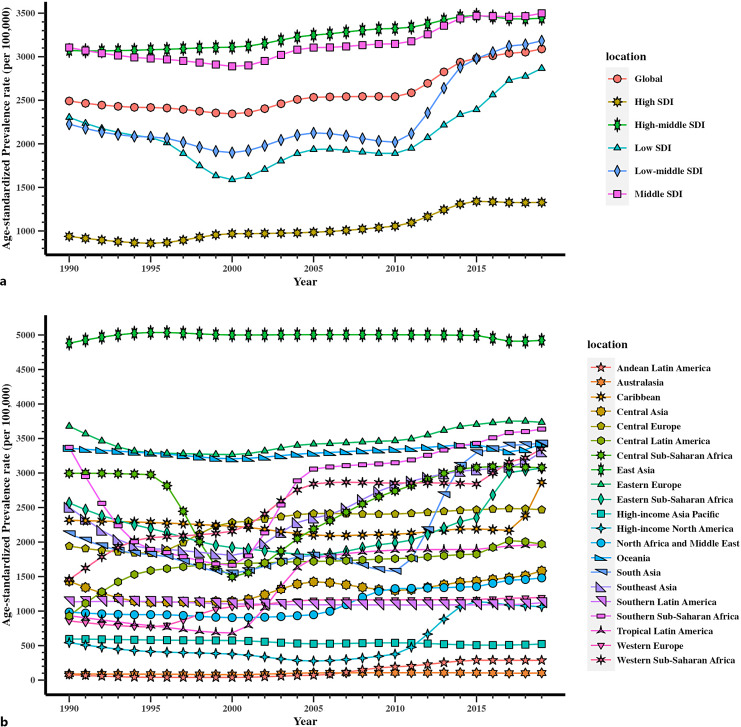
In 2019, 122.38 million (95% uncertainty interval [UI]: 58.38–209.12) cases of female infertility were reported globally among women at reproductive age (20–49 years), with an increase of 86.36% (95% UI: 74.01–97.98%) from 1990 to 2019 in all ages (online suppl. Table 1; for all online suppl. material, see https://doi.org/10.1159/000542408). The global ASR of infertility in reproductive-aged women was (3,089.18 [95% UI: 1,476.17–5,271.85] per 100,000 population) in 2019 (Fig. 1a), accounting for a rise of 23.97% (95% UI: 16.27–31.99%) compared with that in 1990 (online suppl. Table 1).
Fig. 1.
Trends in global disease burden of female infertility prevalence from 1990 to 2019. a Trends in global disease burden of female infertility prevalence by SDI from 1990 to 2019. b Trends in global disease burden of female infertility prevalence by region from 1990 to 2019.
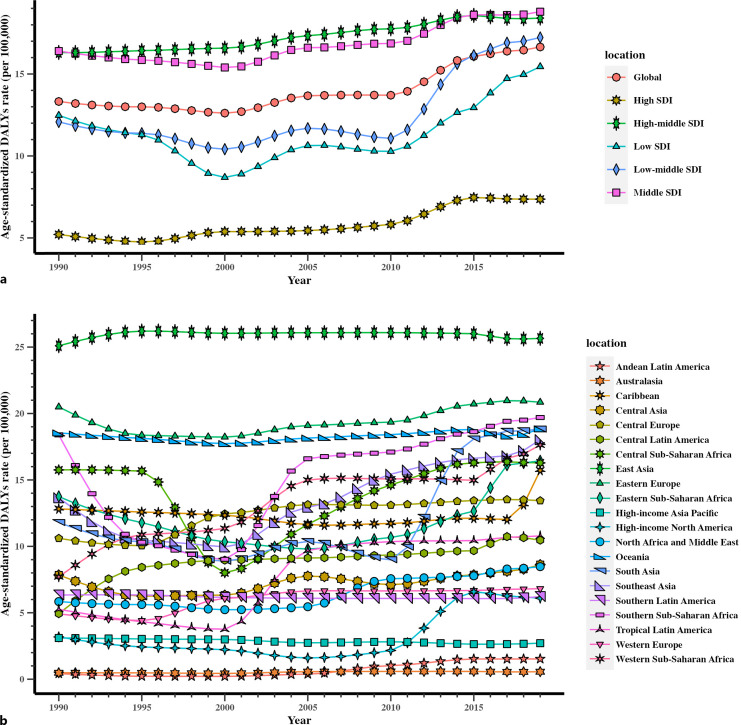
The total number of DALYs linked to female infertility was 0.66 (95% UI: 0.22–1.57) million globally in 2019, indicating an increase of 87.00% (95% UI: 74.93–99.39%) between 1990 and 2019 in all ages (online suppl. Table 1). In 2019, 16.65 (95% UI: 5.66–39.75) age-standardized DALYs per 100,000 women globally due to female infertility of reproductive years were reported (Fig. 2a), indicating an increase of 24.95% (95% UI: 17.07–32.82%) from 1990 to 2019 (online suppl. Table 1).
Fig. 2.
Trends in global disease burden of female infertility DALYs from 1990 to 2019. a Trends in global disease burden of female infertility DALYs by sociodemographic index from 1990 to 2019. b Trends in global disease burden of female infertility DALYs by region from 1990 to 2019.
Female Infertility Burden at the SDI Quintile Level
All SDI regions exhibited a growing trend when stratified by SDI quintiles. The ASR of middle-SDI and high-middle-SDI regions was consistently high from 1990 to 2019. GBD established seven super regions based on epidemiological similarity and geographic proximity, including “Southeast Asia, East Asia and Oceania,” “Southern Asia,” “North Africa and the Middle East,” “Sub-Saharan South Africa,” “Latin America and the Caribbean,” “Central and Eastern Europe” and “Central Asia.” However, among the seven super regions, regions with high-SDI recorded the lowest female infertility ASR. In 2019, the ASR of high-SDI region was (1,327.16 [95% UI: 470.52–2,609.23] per 100,000 population) (Fig. 1a), whereas the values for middle-SDI and high-middle-SDI regions were (3,498.50 [95% UI: 1,687.45–5,955.29])and (3,442.46 [95% UI: 1,628.77–5,789.99]), respectively (Fig. 1a).
The age-standardized DALYs rate per 100,000 women in high-SDI region was 7.37 (95% UI: 1.96–18.37) in 2019 (Fig. 2a). Middle SDI had the highest age-standardized DALYs rate (18.80 (95% UI: 6.41–45.04), per 100,000 population) in 2019 (Fig. 2a), followed by high-middle-SDI regions (18.41 (95% UI: 6.13–44.78) per 100,000 population) (Fig. 2a).
The percentage change in ASR of regions with high SDI (41.59% [95% UI: 24.82–56.32%]) increased significantly (online suppl. Table 1), ranking second among all SDI regions, with low-middle SDI exhibiting the highest increase (42.95% [95% UI: 24.53–68.54%]) (online suppl. Table 1). Conversely, the percentage change in ASR was lowest of regions with high-middle SDI (12.07% [95% UI: 5.52–18.34%]) (online suppl. Table 1).
Regional Burden of Female Infertility
In 2019, the highest age-standardized female infertility prevalence rates were observed in East Asia (4,922.57 [95% UI: 2,313.33–8,502.39] per 100,000 population), Eastern Europe (3,734.20 [95% UI: 1,844.37–6,311.99] per 100,000 population), and Southern Sub-Saharan Africa (3,636.60 [95% UI: 1,651.50–6,231.13] per 100,000 population) (Fig. 1b). Conversely, the lowest ASR was observed in Australasia (102.87 [95% UI: 31.40–429.00]) per 100,000 population), Andean Latin America (286.23 [95% UI: 51.66–793.09]) per 100,000 population), and high-income Asia Pacific (522.40 [95% UI: 53.18–1,622.97]) per 100,000 population). In addition, Andean Latin America and Australasia increased minimally from a very low base (Fig. 1b).
The percentage change in ASR declined between 1990 and 2019 in only two regions: high-income Asia Pacific (−12.24% [95% UI: −37.03–12.18%]), and Southern Latin America (−1.14% [95% UI: −21.32 to −16.95%]). Concurrently, the ASR in Andean Latin America (266.48% [95% UI: −12.51–908.62%]) and Western Sub-Saharan Africa (130.17% [95% UI: 60.80–196.57%]) had the steepest increases in the percentage change of age-standardized rate from 1990 to 2019, although the change in Andean Latin America was from a very low base. Similarly, Central Latin America, Tropical Latin America, high-income North America, South Asia, North Africa and Middle East also had obvious increase in percentage change from 1990 to 2019 (online suppl. Table 1).
The regions of East Asia (25.65 [95% UI: 8.29–62.94] per 100,000 population), Eastern Europe (20.85 [95% UI: 7.23–51.08] per 100,000 population), and Southern Sub-Saharan Africa (19.70 [95% UI: 6.69–47.71] per 100,000 population) had the highest age-standardized DALYs rates in 2019 (Fig. 2b), whereas Australasia (0.56 [95% UI: 0.12–2.53] per 100,000 population), Andean Latin America (1.53 [95% UI: 0.23–4.94]) per 100,000 population), and high-income Asia Pacific (2.71 [95% UI: 0.24–9.27]) per 100,000 population) had the lowest age-standardized DALYs rate (Fig. 2b).
Andean Latin America (262.81% [95% UI: −6.54–849.37%]), Western Sub-Saharan Africa (128.62% [95% UI: 62.28–193.14%]), and Central Latin America (112.08% [95% UI: 71.56–168.61%]) had the steepest increases in the percentage change of age-standardized DALYs from 1990 to 2019, whereas high-income Asia Pacific (−12.54% [95% UI: −37.34 to 11.90%]) and Southern Latin America (−1.85% [95% UI: −23.69 to 14.46%]) exhibited the downward trend during this period. Similar to the results of ASR, the age-standardized DALYs rate in Andean Latin America was still very low in 2019 (online suppl. Table 1).
SDI-Specific Female Infertility Burden in Various Age Groups
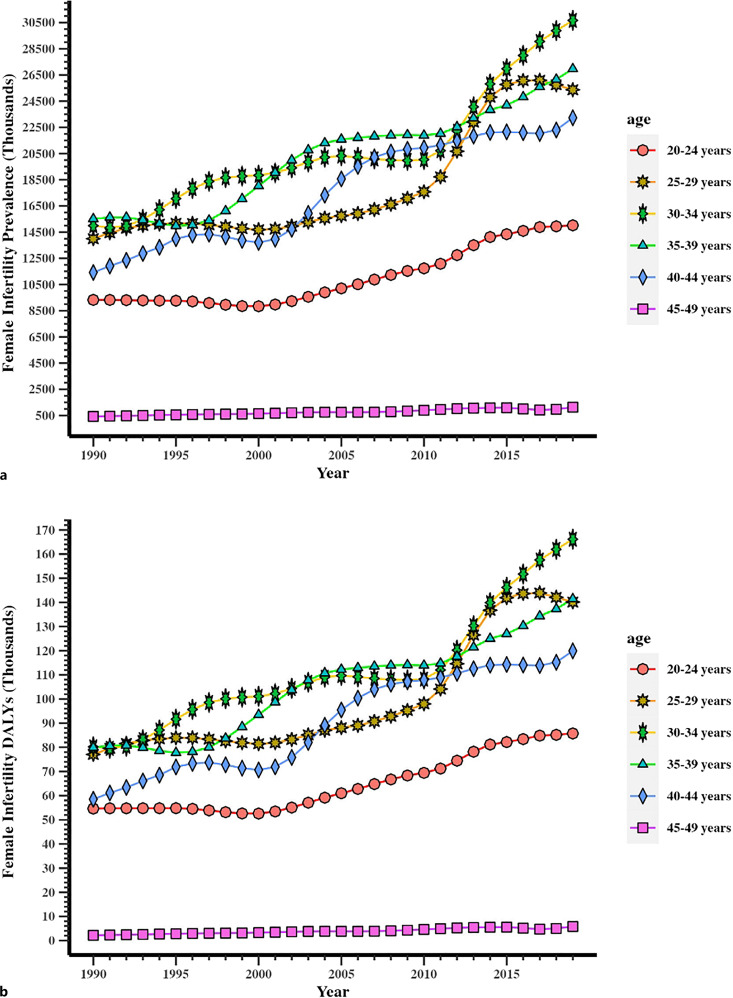
From 1990 to 2019, the prevalence of female infertility among women of reproductive age (20–49 years) increased globally. Regarding prevalence across the ages of 20–49 years in 2019, the 30–34 years age group recorded the highest value at 30.66 (95% UI: 9.39–68.77) million, while the 45–49 years age group recorded the lowest value at 1.13 (95% UI: 0.07–5.56) million in 2019 (online suppl. Table 2; Fig. 3a). In addition, the prevalence in the 45–49 years age group remained relatively low from 1990 to 2019. In addition, the other age groups all recorded a rapid growth trend. The age groups of female infertility in DALYs showed similar results (online suppl. Table 2; Fig. 3b).
Fig. 3.
Trends in global disease burden of female infertility prevalence and DALYs in age groups from 20 to 49 years old from 1990 to 2019. a Prevalence. b DALYs.
Countries with the Highest Female Infertility Burdens from 1990 to 2019
The following five countries ranked the highest in 2019 for the ASR of female infertility per 100,000 population: Central African Republic (5,847.42 [95% UI: 2,990.76–9,859.64]), Djibouti (5,558.83 [95% UI: 2,832.56–9,593.05]), the Philippines (5,308.07 [95% UI: 1,509.00–9,355.45]), China 4,968.91 (95% UI: 2,334.85–8,590.40), and Comoros 4,953.49 (95% UI: 2,674.50–8,229.69) (online suppl. Table 6). Conversely, the lowest ASR per 100,000 population in the same year was recorded in Australia (81.95 [95% UI: 28.92–360.62]), Peru (90.51 [95% UI: 42.87–215.45]), New Zealand (228.35 [95% UI: 45.00–934.96]), Denmark (282.08 [95% UI: 28.89–786.91]), and Singapore (326.08 [95% UI: 36.93–1,212.18]) (online suppl. Table 6).
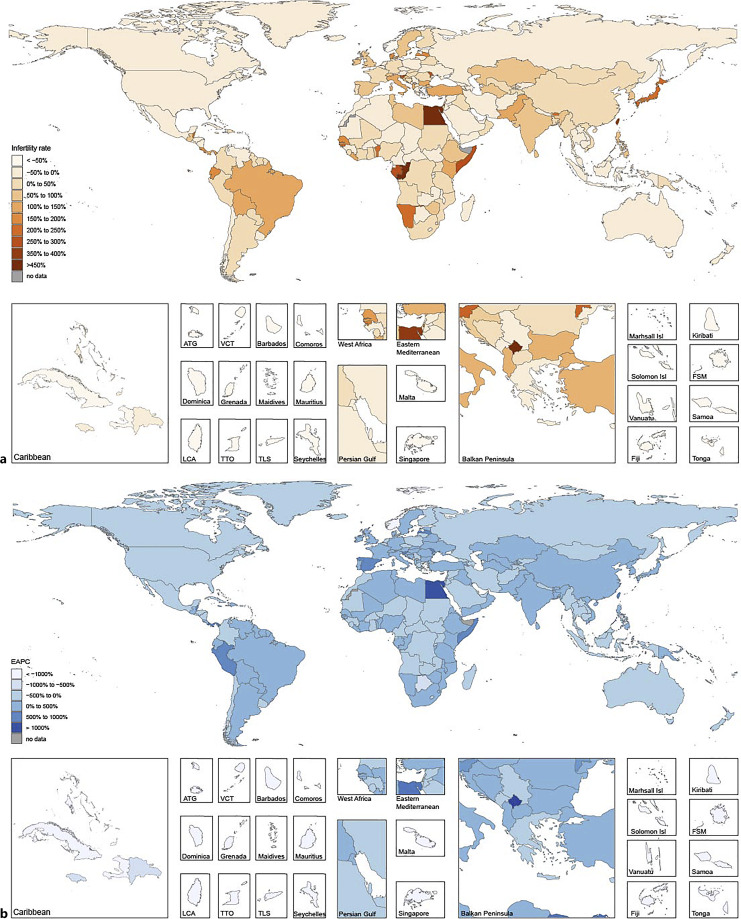
Significant differences in the percentage changes in female infertility ASR were observed between 1990 and 2019, with the steepest rises in Ecuador (490.73% [95% UI: −21.94 to 1,982.25%]), Colombia (480.94% [95% UI: 93.05–1,724.34%]), and Togo (353.47% [95% UI: 43.42–1,189.32%]). The steepest decreases were observed in Pakistan (−88.52% [95% UI: −95.35 to −82.87%]), Malawi (−24.89% [95% UI: −71.13 to 44.39%]), and Burundi (−19.60% [95% UI: −75.95 to 69.22%]) (online suppl. Table 3; Fig. 4a).
Fig. 4.
Global disease burden of female infertility prevalence in 204 countries and territories. a The percentage change in age-standardized prevalence of female infertility between 1990 and 2019. b The estimated annual percentage change (EAPC) of female infertility age-standardized prevalence from 1990 to 2019.
According to age-standardized DALYs related to female infertility per 100,000 population, the five top-ranking countries in 2019 were Central African Republic (31.76 [95% UI: 11.72–74.22]), Djibouti (29.58 [95% UI: 10.51–69.93]), the Philippines (29.41 [95% UI: 7.67–70.17]), Comoros (27.95 [95% UI: 10.44–62.25]), and Gabon (25.99 [95% UI: 9.04–62.95])(online suppl. Table 6). Conversely, the lowest age-standardized DALYs rates per 100,000 individuals were recorded in Australia (0.45 [95% UI: 0.10–2.00]), Peru (0.52 [95% UI: 0.14–1.57]), New Zealand (1.24 [95% UI: 0.17–5.26]), Denmark (1.66 [95% UI: 0.16–5.61]), and Singapore (1.68 [95% UI: 0.15–6.81]) (online suppl. Table 6).
Ecuador (475.25% [95% UI: −17.24 to 1,962.27%]), Colombia (459.48% [95% UI: 104.60–1,681.85%]), and Togo (326.55% [95% UI: 43.09–1,081.42%]) recorded the sharpest increases in the percentage changes of the age-standardized DALYs between 1990 and 2019, with steepest decline recorded in Pakistan (−88.02% [95% UI: −95.28 to −81.41%]), Malawi (−25.89% [95% UI: −69.53 to 40.23%]), and Vietnam (−19.71% [95% UI: −53.21 to −1.68%]) (online suppl. Table 3). Online supplementary Table 4 and Figure 4b present the EAPC of female infertility ASR from 1990 to 2019. Ecuador had the highest EAPC value (1,108.18% [95% UI: 1,381.96–840.99%]), and Pakistan had the lowest (−1,302.52% [95% UI: −1,025.11 to −1,571.36%]) (online suppl. Table 4; Fig. 4b).
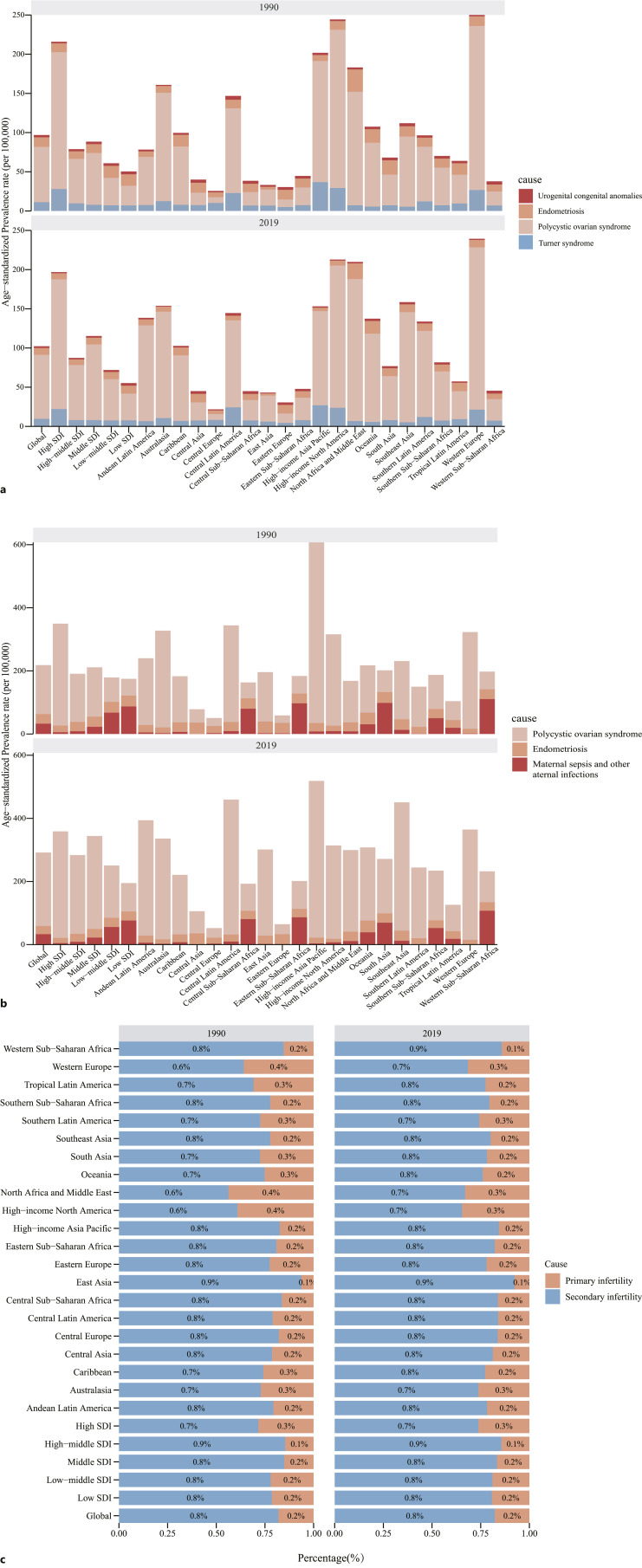
GBD Study on Causes Associated with Female Infertility
The data on the causes of infertility in the GBD 2019 database are not perfect, and we selected several of them for analysis and research. Infertility in women can be of two types: primary and secondary. Globally, between 1990 and 2019, secondary infertility accounted for a higher proportion of female infertility. In 2019, the highest proportion of secondary infertility in 21 regions was Western Sub-Saharan Africa, and East Asia, the highest proportion of secondary infertility in SDI regions was high-middle SDI. For primary infertility, we chose “polycystic ovarian syndrome, endometriosis, urogenital congenital anomalies, Turner syndrome, and Klinefelter syndrome,” of which the highest ASR in 2019 was polycystic ovarian syndrome (8,101.49% [3,953.49%–14550.96%]). For secondary infertility, we chose “polycystic ovarian syndrome, endometriosis, and maternal sepsis and other maternal infections,” of which the highest ASR in 2019 was polycystic ovarian syndrome (22,733.37% [13,503.66%–35737.41%]) (Tables 1, 2, Fig. 5a–c).
Table 1.
Global ranking of female primary infertility by causes
| Rank | Cause | ASR in 1990 (95% UI) | ASR in 2019 (95% UI) |
|---|---|---|---|
| 1 | Polycystic ovarian syndrome | 6,697.89% (3,570.93%–11,158.79%) | 8,101.49% (3,953.49%–14,550.96%) |
| 2 | Endometriosis | 1,140.1% (551.8%–1,987.94%) | 844.66% (368.75%–1,545.37%) |
| 3 | Turner syndrome | 1,074.04% (793.18%–1,381.86%) | 968.04% (709.47%–1,247.8%) |
| 4 | Urogenital congenital anomalies | 265.32% (119.77%–491.99%) | 257.6% (116.02%–475.12%) |
| 5 | Klinefelter syndrome | 0 | 0 |
Table 2.
Global ranking of female secondary infertility by causes
| Rank | Cause | ASR in 1990 (95% UI) | ASR in 2019 (95% UI) |
|---|---|---|---|
| 1 | Polycystic ovarian syndrome | 15,652.07% (9,359.6%–24,669.5%) | 22,733.37% (13,503.66%–35,737.41%) |
| 2 | Maternal sepsis and other maternal infections | 3,533.44% (3,064.54%–4,047.04%) | 3,124.9% (2,799.1%–3,490.31%) |
| 3 | Endometriosis | 3,048.6% (1,832.43%–4,766.26%) | 2,535.37% (1,530.68%–3,990.87%) |
Fig. 5.
Female infertility age-standardized prevalence rate and its proportion by etiologies. a Primary infertility age-standardized prevalence rate and its proportion in different SDI regions and countries by etiologies in 1990 and 2019. b Secondary infertility age-standardized prevalence rate and its proportion in different countries and SDI regions by etiologies in 1990 and 2019. c The proportion of primary infertility and secondary infertility for female infertility in different SDI regions and geographical regions in 1990 and 2019.
Estimates of the Global Infertility Burden Related to SDI Levels
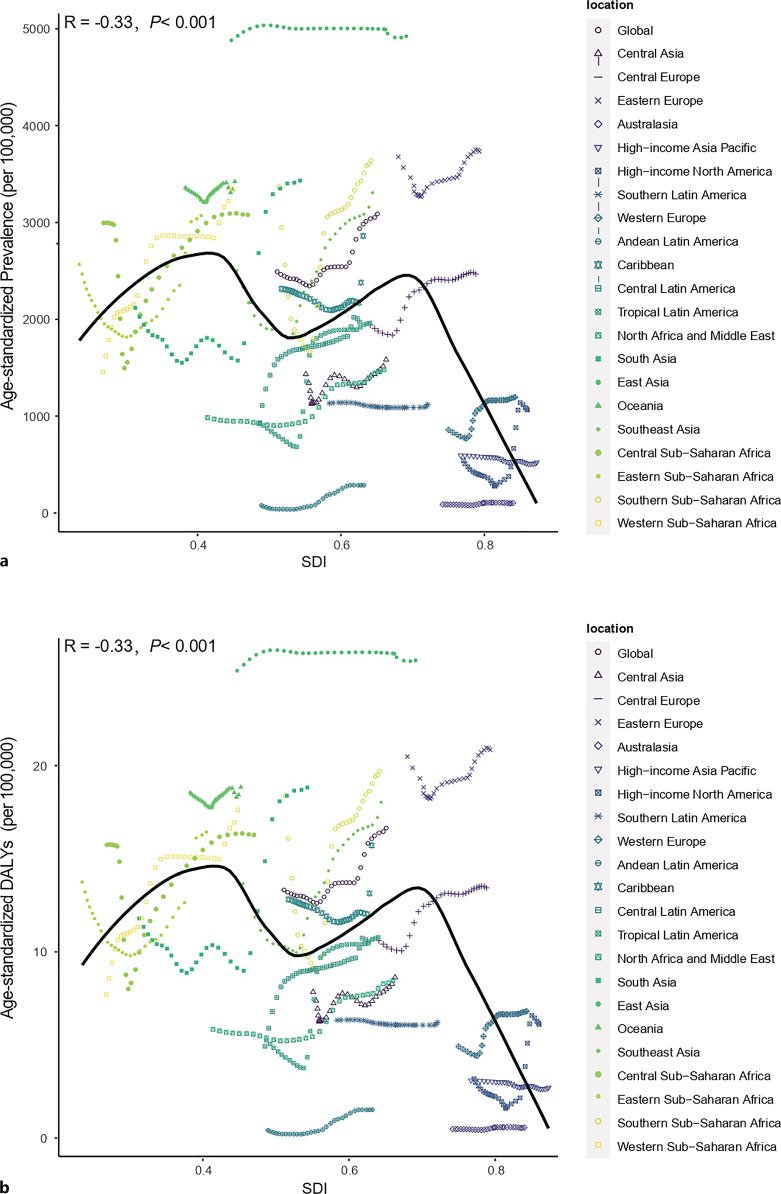
We presented the relationships between the SDI levels and the estimations of the global burden of female infertility for each of the 21 GBD regions and global for all individual years between 1990 and 2019. General negative correlations were observed between the SDI level and burden estimates. The expected pattern is essentially a nonlinear, bimodal pattern, with the first peak occurring at approximately SDI = 0.4, the second peak at approximately SDI = 0.7, and the turning point between the two peaks at approximately SDI = 0.55. When SDI <0.4, burden estimates increased; when SDI >0.7, the estimated burden exhibited a clear downward trend. In addition, fluctuations were observed when 0.4 < SDI < 0.7 (online suppl. Table 5; Fig. 6a, b).
Fig. 6.
Coevolution of age-standardized burden estimates with SDI and for GBD regions for female infertility from 1990 to 2019. a Prevalence. b DALYs. Colored lines show region values for age-standardized burden estimate rates. Each point in a line represents 1 year, starting in 1990 and ending in 2019. The black line represents the average expected relationship between SDI and burden estimate rates for female infertility based on values from each region in the 1990–2019 estimation period.
Discussion
This study is based on GBD estimates and trends in the burden of female infertility in 204 countries and regions from 1990 to 2019. The findings indicate a global increase in ASRs of female infertility over the observation period, with the burden of female infertility increasing in all countries at the SDI quintile level and in all regions at the regional level. The increased prevalence of female infertility may be related to the delayed childbearing age, as well as the increased prevalence of mental disorders such as depression and anxiety in modern women [20, 21]. Ovarian reserve function declines in older women, female reproductive potential steadily declines with age, and fertility rates drop sharply in women over the age of 35 [22, 23]. Women over 35 experience a rapid reduction in fertility rates, with their reproductive capacity and their ovarian reserve function continuously declining with age [24]. Infertility in women is also linked to several physical and psychological conditions, including anxiety, depression, obsessive-compulsive disorders, and sleep disturbances. Mental health issues and female infertility are related, although the exact mechanism of the two is uncertain. Women with infertility have higher levels of psychological stress and are more prone to developing mental health problems. Additionally, mental disorders will increase the difficulty of pregnancy in women with infertility [21, 25].
Our study revealed that from 1990 to 2019, the ASR of female infertility was consistently high in middle-SDI and high-middle-SDI regions, while high-SDI countries exhibited the lowest ASR of female infertility among all seven SDI countries, consistent with the findings of Sun et al. [8]. This may be related to lower infertility detection rates in countries with low SDI. The rate of female infertility has risen in low-SDI countries with economic progress. Differences in female infertility may be caused by differences in the distribution of factors, such as education, culture, race, economic conditions, women’s status, medical level, and social security system. Higher income and educational attainment are socioeconomic benefits linked to infertility and delayed childbirth in developed countries [26]. The increase in late marriages and late childbearing in developed countries may also be related to the significant increase in the percentage change in ASR observed in high-SDI countries in this study, although policy related to infertility in developed countries are superior to those in low- and upper-middle-income countries [27]. While the average age of conception in developing countries is low, infertility rates among women in developing countries are also exacerbated by the increase in environmental pollution unwanted pregnancies, unsafe abortions, and the limitations of medical standards [28]. Higher rates of reproductive tract tuberculosis and female smoking in developing countries can also increase female infertility prevalence [29, 30]. Furthermore, in countries with high fertility rates and insufficient health resources, infertility is often considered a secondary issue by the government [31]. These factors may be partly responsible for the largest age-standardized prevalence increases observed in low-middle-SDI countries in this study.
The highest ASR in 2019 was observed in East Asia and Eastern Europe, whereas the lowest ASR was observed in Australasia and Andean Latin America. Between 1990 and 2019, ASR decreased in only two regions: high-income Asia Pacific and Southern Latin America. Additionally, between 1990 and 2019, the ASR displayed the relatively obvious increasing trends in Andean Latin America and Western Sub-Saharan Africa, but the ASR in Andean Latin America was still at a very low level in 2019. Secondary infertility is relatively prevalent in Central/Eastern Europe and Central Asia, and this may be attributed to greater rates of abortions. In these regions, abortion rates declined between 1995 and 2003 but remained higher than the global average [32]. In countries with limited resources, secondary infertility is more prevalent [33]. As East Asia’s largest country, China has a 25% infertility prevalence among couples of childbearing age, and nearly half of the couples with infertility choose not to seek medical assistance [34]. The high rate of infertility in Central and Southern Africa has led to the region being referred to as an “infertility belt” [35]. Pelvic infections and Fallopian tube injury after abortion are the leading cause of infertility in women of childbearing age in Central and Southern Africa. Therefore, the elimination of unsafe abortion and the early detection and treatment of postabortion complications are extremely important [36]. Female infertility is a major risk factor for intimate partner violence in low- and middle-income countries [37]. The prevalence of ASR in high-income North America has been at a low level in the past 30 years, but it has increased significantly in the last 10 years, which may be related to the degree of economic development and people’s fertility intention.
Social and economic support policies for female infertility also have a notable role in promoting fertility recovery [27]. Developed countries such as the USA and Europe have relatively effective healthcare policies, but the specific policies vary greatly [38, 39]. Conversely, comparatively few medical protection policies for female infertility exist in several low- and middle-income countries [40].
Among the women, aged 20–49 years, with female infertility worldwide between 1990 and 2019, the 45–49 years age group had the fewest cases, maintaining a relatively stable situation. In addition, the growth of the 20–24 years age group was relatively stable, while other age groups exhibited a rapid growth trend. The 30–34 years age range represented the largest proportion of female infertility cases in 2019, closely followed by the 35–39 years age group. Women in the 45–49 years age group are at the end of their reproductive age, with significantly lower reproductive demand and fertility compared with other reproductive age groups. Therefore, the number of women in this age group was the lowest among all age groups, with no significant fluctuation. Since the 1970s, more women have been delaying childbearing due to increasing access to higher education, full-time employment, and improved contraceptive techniques. Assisted reproductive technologies have also prolonged reproductive life. Furthermore, some women overvalue their fertility, being unaware of the effects of advanced age on fertility or acknowledging the risks of old age but not perceiving them as imminent. Delayed childbearing is now a global phenomenon [41, 42]. Pregnant women aged between 30 and 39 years constitute the primary childbearing population after delayed childbearing. Since 1978, the number of first births in women aged 35–39 years in the USA has increased by an average of 2% per year [43]. Between 1971 and 2013, the average age of new mothers in Australia increased from 25.4 to 29.3 years [44]. According to a review of natural childbearing populations in developed countries, age-related losses in fertility rose from 4.5% at age 25 to 20% at the age of 38 [41]. Global data reveal that fertility rates have declined over time, with the steepest declines among young women and regions with higher income and education levels [45]. Research illustrates that first-time mothers aged 35 years and older have shorter intervals between their second pregnancies than mothers aged 20–29 years [46]. This is because some women are aware of the negative effects of old age, such as decreased fertility [47]. Conversely, excessively lengthy or short gestation intervals are linked to a higher risk of unfavorable pregnancy outcomes, such as a fetus small for gestational age, low birth weight, and premature birth [48]. Additionally, some types of cancer, metabolic diseases, congenital abnormalities, and chromosomal diseases are more common in older mothers, who also have a higher annual morbidity of their children [49].
In addition to the etiology of infertility included in this study, many factors affect the prevalence of infertility, such as thyroid disease, pituitary disease, adrenal hyperplasia, or adrenal tumors caused by androgen elevation [50]. Several review articles offer reliable proof that abnormal sleep patterns are linked to several detrimental effects on women’s reproductive health; however, further research is required [51, 52]. A North American cohort study revealed that difficulty sleeping at night was linked to moderately reduced fertility, although the negative association between shorter sleep duration and fertility was weak, and shift work or a night job was not associated with fertility [53]. Obesity is also considered to be a cause of infertility. Obesity can have an adverse effect on embryo implantation [54]. Additionally, infertility raises the risk of ovarian, endometrial, and breast cancers; however, whether fertility treatment itself elevates these risks is uncertain [55].
From the above research results, it is not difficult to see that the global infertility burden is still very severe and the overall trend is rising. This suggests that we must pay attention to this disease. We should call on patients to actively seek treatment, and the clinicians should improve the diagnosis and treatment system, strive to achieve early detection and early treatment. From the perspective of policymakers, they should improve the medical and health security system and reduce the economic concerns of patients seeking medical treatment. We believe that with the efforts of all sectors of society, the disease of female infertility will be better controlled, and the life treatment of patients will also be improved to a certain extent.
This study has a few limitations. First, the GBD database lacks data for some countries and regions. Second, data access and quality differ across locations. In some developing countries, women with infertility have low consultation rates, which lead to scarce or missing data, leading to bias in study results. Third, the causes of infertility are not comprehensive, and data on Klinefelter syndrome in GBD 2019 in relation to primary infertility were absent. Nevertheless, this study is the most recent in-depth analysis of the epidemiological state and trends of the global burden of female infertility, lending it considerable unique advantages.
In conclusion, from 1990 to 2019, the ASR of female infertility and DALYs increased globally. These increases may be related to various factors such as population aging, delayed childbearing, socioeconomic development, access to healthcare, and health awareness. This report presents comprehensive findings on the global burden of female infertility and can inform policymakers on healthcare priorities related to infertility, strengthening education, raising public awareness of female infertility, regulating intimate relationship violence, safeguarding the status and safety of women, and implementing necessary prevention and management interventions, to reduce the prevalence of female infertility.
Acknowledgments
We highly appreciate the works of the Global Burden of Disease Study 2019 collaborators. We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Statement of Ethics
The data in this study are from the GBD open database, the informed consent waiver for the GBD 2019 data was reviewed and approved by the Institutional Review Board of the University of Washington.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Sources
This study was supported by the National Nature Science Foundation of China (No. 82274571), the Shanghai Medical Innovation, and Development Foundation (WL-HBMS-2021004K).
Author Contributions
D.Y.S. wrote the original manuscript and conceived the study; S.Y. performed the data analysis; D.Y.S. and S.Y. revised the final manuscript; C.Q. and H.Y. supervised the study process.
Funding Statement
This study was supported by the National Nature Science Foundation of China (No. 82274571), the Shanghai Medical Innovation, and Development Foundation (WL-HBMS-2021004K).
Data Availability Statement
The data underlying this article are available in the Global Health Data Exchange at http://ghdx.healthdata.org/gbd-results-tool.
Supplementary Material.
Supplementary Material.
Supplementary Material.
Supplementary Material.
Supplementary Material.
Supplementary Material.
References
- 1. Krueger RB, Reed GM, First MB, Marais A, Kismodi E, Briken P. Proposals for paraphilic disorders in the international classification of diseases and related health problems, eleventh revision (ICD-11). Arch Sex Behav. 2017;46(5):1529–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patra SA-O, Unisa S. Addressing reproductive health knowledge, infertility and coping strategies among rural women in India. J Biosoc Sci. 2021;53(4):557–65. [DOI] [PubMed] [Google Scholar]
- 3. Ethics Committee of the American Society for Reproductive Medicine Electronic address asrm@asrmorg . Disparities in access to effective treatment for infertility in the United States: an Ethics Committee opinion. Fertil Steril. 2021;116(1):54–63. [DOI] [PubMed] [Google Scholar]
- 4. Vander Borght M, Wyns C. Fertility and infertility: definition and epidemiology. Clin Biochem. 2018;62:2–10. [DOI] [PubMed] [Google Scholar]
- 5. Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The international glossary on infertility and fertility care, 2017. Fertil Steril. 2017;108(3):393–406. [DOI] [PubMed] [Google Scholar]
- 6. Zhao YX, Chen SR, Su PP, Huang FH, Shi YC, Shi QY, et al. Using mesenchymal stem cells to treat female infertility: an update on female reproductive diseases. Stem Cells Int. 2019;2019:9071720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med. 2012;9(12):e1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun H, Gong TT, Jiang YT, Zhang S, Zhao YH, Wu QJ. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990-2017: results from a global burden of disease study, 2017. Aging. 2019;11(23):10952–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu J, Han W, Wang H, Wang Z, Li B, Hong LA-O. Spatiotemporal trends and age-period-cohort analysis for the burden of endometriosis-related infertility: an analysis of the global burden of disease study 2019. J Pers Med. 2023;13(9):1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zauner G, Girardi G. Potential causes of male and female infertility in Qatar. J Reprod Immunol. 2020;141:103173. [DOI] [PubMed] [Google Scholar]
- 11. Zarif Golbar Yazdi H, Aghamohammadian Sharbaf H, Kareshki H, Amirian M. Infertility and psychological and social health of Iranian infertile women: a systematic review. Iran J Psychiatry. 2020;15(1):67–79. [PMC free article] [PubMed] [Google Scholar]
- 12. Liang SJ, Chen YH, Wang Q, Chen HH, Cui CC, Xu XH, et al. Prevalence and associated factors of infertility among 20-49 year old women in Henan Province, China. Reprod Health. 2021;18(1):254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. GBD 2019 Diseases and Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. GBD 2019 Risk Factors Collaborators . Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1223–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. GBD 2019 Demographics Collaborators . Global age-sex-specific fertility, mortality, Healthy Life Expectancy (HALE), and population estimates in 204 countries and territories, 1950-2019: a comprehensive demographic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1160–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mi Y, Huai L, Yin Y, Yuan J, Liu Y, Huang J, et al. Burden of stroke in China and the different SDI regions over the world. J Glob Health. 2023;13:04169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang H, Zhao S, Wang S, Zheng Y, Wang S, Chen H, et al. Global magnitude of encephalitis burden and its evolving pattern over the past 30 years. J Infect. 2022;84(6):777–87. [DOI] [PubMed] [Google Scholar]
- 18. Cao G, Liu J, Liu M. Global, regional, and national incidence and mortality of neonatal preterm birth, 1990-2019. JAMA Pediatr. 2022;176(8):787–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stevens GA, Alkema L, Black RE, Boerma JT, Collins GS, Ezzati M, et al. Guidelines for accurate and transparent health estimates reporting: the GATHER statement. PLoS Med. 2016;13(6):e1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW, et al. Comprehensive meta-analysis of excess mortality in depression in the general community versus patients with specific illnesses. Am J Psychiatry. 2014;171(4):453–62. [DOI] [PubMed] [Google Scholar]
- 21. Teklemicheal AG, Kassa EM, Weldetensaye EK. Prevalence and correlates of infertility related psychological stress in women with infertility: a cross-sectional hospital based survey. BMC Psychol. 2022;10(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu XM, Liu YB, Xu J, Cao X, Zhang DD, Liu M, et al. Mitochondrial dysfunction in cumulus cells is related to decreased reproductive capacity in advanced-age women. Fertil Steril. 2022;118(2):393–404. [DOI] [PubMed] [Google Scholar]
- 23. Cedars MI. Evaluation of female fertility-AMH and ovarian reserve testing. J Clin Endocrinol Metab. 2022;107(6):1510–9. [DOI] [PubMed] [Google Scholar]
- 24. Chua SJ, Danhof NA, Mochtar MH, van Wely M, McLernon DJ, Custers I, et al. Age-related natural fertility outcomes in women over 35 years: a systematic review and individual participant data meta-analysis. Hum Reprod. 2020;35(8):1808–20. [DOI] [PubMed] [Google Scholar]
- 25. Madero S, Gameiro S, Garcia D, Cirera D, Vassena R, Rodriguez A. Quality of life, anxiety and depression of German, Italian and French couples undergoing cross-border oocyte donation in Spain. Hum Reprod. 2017;32(9):1862–70. [DOI] [PubMed] [Google Scholar]
- 26. Skakkebaek NE, Rajpert-De Meyts E, Buck Louis GM, Toppari J, Andersson AM, Eisenberg ML, et al. Male reproductive disorders and fertility trends: influences of environment and genetic susceptibility. Physiol Rev. 2016;96(1):55–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang X, Guan Q, Yu Q, Xiao W, Chen Z, Dong C, et al. Estimating the effects of policies on infertility prevalence worldwide. BMC Public Health. 2022;22(1):1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kulczycki A, Potts M, Rosenfield A. Abortion and fertility regulation. Lancet. 1996;347(9016):1663–8. [DOI] [PubMed] [Google Scholar]
- 29. Ghosh K, Ghosh K, Chowdhury JR. Tuberculosis and female reproductive health. J Postgrad Med. 2011;57(4):307–13. [DOI] [PubMed] [Google Scholar]
- 30. Bhanji S, Andrades M, Taj F, Khuwaja AK. Factors related to knowledge and perception of women about smoking: a cross sectional study from a developing country. Bmc Womens Health. 2011;11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2015;21(4):411–26. [DOI] [PubMed] [Google Scholar]
- 32. Sedgh G, Singh S, Shah IH, Ahman E, Henshaw SK, Bankole A. Induced abortion: incidence and trends worldwide from 1995 to 2008. Lancet. 2012;379(9816):625–32. [DOI] [PubMed] [Google Scholar]
- 33. Dhont N, Luchters S, Muvunyi C, Vyankandondera J, De Naeyer L, Temmerman M, et al. The risk factor profile of women with secondary infertility: an unmatched case-control study in Kigali, Rwanda. BMC Womens Health. 2011;11:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou Z, Zheng D, Wu H, Li R, Xu S, Kang Y, et al. Epidemiology of infertility in China: a population-based study. BJOG. 2018;125(4):432–41. [DOI] [PubMed] [Google Scholar]
- 35. Okonofua FE. The case against new reproductive technologies in developing countries. Br J Obstet Gynaecol. 1996;103(10):957–62. [DOI] [PubMed] [Google Scholar]
- 36. Sharma S, Mittal S, Aggarwal P. Management of infertility in low resource countries. BJOG. 2009;116(Suppl 1):77–83. [DOI] [PubMed] [Google Scholar]
- 37. Stellar C, Garcia-Moreno C, Temmerman M, van der Poel S. A systematic review and narrative report of the relationship between infertility, subfertility, and intimate partner violence. Int J Gynaecol Obstet. 2016;133(1):3–8. [DOI] [PubMed] [Google Scholar]
- 38. Chambers GM, Sullivan EA, Ishihara O, Chapman MG, Adamson GD. The economic impact of assisted reproductive technology: a review of selected developed countries. Fertil Steril. 2009;91(6):2281–94. [DOI] [PubMed] [Google Scholar]
- 39. Salonia A, Matloob R, Gallina A, Abdollah F, Saccà A, Briganti A, et al. Are infertile men less healthy than fertile men? Results of a prospective case-control survey. Eur Urol. 2009;56(6):1025–31. [DOI] [PubMed] [Google Scholar]
- 40. Morshed-Behbahani B, Lamyian M, Joulaei H, Rashidi BH, Montazeri A. Infertility policy analysis: a comparative study of selected lower middle- middle- and high-income countries. Global Health. 2020;16(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Eijkemans MJ, van Poppel F, Habbema DF, Smith KR, Leridon H, te Velde ER, et al. Too old to have children? Lessons from natural fertility populations. Hum Reprod. 2014;29(6):1304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cooke A, Mills TA, Lavender T. Informed and uninformed decision making’-Women’s reasoning, experiences and perceptions with regard to advanced maternal age and delayed childbearing: a meta-synthesis. Int J Nurs Stud. 2010;47(10):1317–29. [DOI] [PubMed] [Google Scholar]
- 43. Matthews TJ, Hamilton BE. Delayed childbearing: more women are having their first child later in life. NCHS Data Brief. 2009;21:1–8. [PubMed] [Google Scholar]
- 44. Kearney AL, White KM. Examining the psychosocial determinants of women's decisions to delay childbearing. Hum Reprod. 2016;31(8):1776–87. [DOI] [PubMed] [Google Scholar]
- 45. GBD 2017 Population and Fertility Collaborators . Population and fertility by age and sex for 195 countries and territories, 1950-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1995–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nabukera SK, Wingate MS, Salihu HM, Owen J, Swaminathan S, Alexander GR, et al. Pregnancy spacing among women delaying initiation of childbearing. Arch Gynecol Obstet. 2009;279(5):677–84. [DOI] [PubMed] [Google Scholar]
- 47. Carolan M. The graying of the obstetric population: implications for the older mother. J Obstet Gynecol Neonatal Nurs. 2003;32(1):19–27. [DOI] [PubMed] [Google Scholar]
- 48. Conde-Agudelo A, Rosas-Bermudez A, Kafury-Goeta AC. Birth spacing and risk of adverse perinatal outcomes: a meta-analysis. JAMA. 2006;295(15):1809–23. [DOI] [PubMed] [Google Scholar]
- 49. Joseph KS, Allen AC, Dodds L, Turner LA, Scott H, Liston R. The perinatal effects of delayed childbearing. Obstet Gynecol. 2005;105(6):1410–8. [DOI] [PubMed] [Google Scholar]
- 50. Practice Committee of the American Society for Reproductive Medicine . Obesity and reproduction: a committee opinion. Fertil Steril. 2015;104(5):1116–26. [DOI] [PubMed] [Google Scholar]
- 51. Beroukhim G, Esencan E, Seifer DB. Impact of sleep patterns upon female neuroendocrinology and reproductive outcomes: a comprehensive review. Reprod Biol Endocrinol. 2022;20(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sciarra F, Franceschini E, Campolo F, Gianfrilli D, Pallotti F, Paoli D, et al. Disruption of circadian rhythms: a crucial factor in the etiology of infertility. Int J Mol Sci. 2020;21(11):3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Willis SK, Hatch EE, Wesselink AK, Rothman KJ, Mikkelsen EM, Wise LA. Female sleep patterns, shift work, and fecundability in a North American preconception cohort study. Fertil Steril. 2019;111(6):1201–10.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Silvestris E, de Pergola G, Rosania R, Loverro G. Obesity as disruptor of the female fertility. Reprod Biol Endocrinol. 2018;16(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rizzuto I, Behrens RF, Smith LA. Risk of ovarian cancer in women treated with ovarian stimulating drugs for infertility. Cochrane Database Syst Rev. 2019;6:CD008215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the Global Health Data Exchange at http://ghdx.healthdata.org/gbd-results-tool.