Abstract
We investigated the effectiveness and safety of nelarabine (NEL)‐combined chemotherapy for newly diagnosed adult T‐cell acute lymphoblastic leukemia (T‐ALL) patients. We conducted a phase II trial, T‐ALL213‐O, where adult T‐ALL patients aged 25 to 64 were treated by a regimen based on that used in our previous study, ALL202‐O. The main modifications from ALL202‐O to T‐ALL213‐O were as follows: (1) NEL‐combined chemotherapy, instead of consolidation (C)1, was used for non‐complete remission (CR) patients after induction therapy (IND)1 as IND2; (2) NEL treatments were inserted into C3 and C5 on day 29. Twenty‐four patients were analyzed. Ten patients did not receive NEL treatment due to therapy termination prior to C3. Three‐year event‐free survival (EFS) was 70%, with 52% as the lower limit of its 90% confidence interval, which exceeded the threshold of 25%; thus, the study treatment was considered effective. The CR rates by IND1, IND2, and both were 75%, 100%, and 88%, respectively. The 5‐year EFS and 5‐year overall survival rates were 66% and 70%, respectively, with median follow‐ups of 7.7 and 7.8 years. The addition of NEL improved the CR rate but not survival, compared with T‐ALL patients in ALL202‐O. Severe neuropathy after NEL administration was observed at a high frequency. Seven (50%) of 14 patients treated with NEL showed grade 3 peripheral neuropathy and/or gait disturbance. The neurotoxicity was considered stronger than that previously reported. Combination therapy of NEL at this dose and intensive multidrug chemotherapy is associated with a high risk of severe neurotoxicity (JALSG T‐ALL213‐O, UMIN000010642).
Keywords: acute lymphoblastic leukemia, clinical trial, nelarabine, neuropathy, T‐ALL
In T‐ALL213‐O, adult T‐ALL patients were treated by Nelarabine‐combined chemotherapy. Three‐year event‐free survival was 70%, and the study treatment was considered effective; however, severe neuropathy was observed in 50% of patients treated with Nelarabine.

1. INTRODUCTION
T‐cell acute lymphoblastic leukemia (T‐ALL) is an aggressive disease and an important cause of morbidity in children and adults. T‐ALL accounts for 15% and 25% of all childhood and adult ALL cases, respectively. 1 The outcome of pediatric patients with newly diagnosed T‐ALL is worse than that with B‐cell ALL (B‐ALL), 1 while the survival of adult ALL patients does not significantly differ between them 2 , 3 ; however, recent advances in immunotherapy for B‐cell‐specific antigen, such as blinatumomab, inotuzumab‐ozogamicin, and CD19‐targetting chimeric antigen receptor (CAR) T cells, are markedly improving the survival of B‐ALL patients. 4 Thus, the survival gap between B‐ and T‐ALL will also widen in adult patients. Development of monoclonal antibody‐ or CAR‐based immunotherapies for T‐ALL has been difficult because of secondary life‐threatening T‐cell immunodeficiency and fratricide of CAR‐T cells due to shared expression of targeted antigens among normal and malignant T cells and CAR‐T cells. 5 Development of other new therapeutic agents is eagerly awaited.
Nelarabine (NEL) is a DNA‐terminating nucleoside prodrug for araguanosine metabolized into arabinosylguanine nucleotide triphosphate, preferentially accumulating in T lymphoblasts secondary to slowed degradation kinetics. 6 Numerous studies have confirmed its antitumor activity in both adult and pediatric patients with relapsed/refractory T‐ALL/lymphoblastic lymphoma (LBL), resulting in its regulatory approval in many countries for this indication. 6 In several phase II trials of NEL monotherapy for relapsed/refractory T‐ALL/LBL, NEL induced complete or partial remission in 33%–46% of patients; however, it also caused frequent and severe central and peripheral neuropathies (PNs). The incidence of neurotoxicity greater than G2 was 7%–18%. 6 Although NEL has yet to be approved for primary T‐ALL, several phase II or III trials of NEL‐combined chemotherapies for newly diagnosed T‐ALL have been conducted. 7 , 8 , 9 , 10 , 11 Although many of them demonstrated good survival outcomes and acceptable safety, the efficacy and safety of NEL‐combined chemotherapy, especially for adult T‐ALL, have not been established (see Section 5).
In the present study, the Japan Adult Leukemia Study Group (JALSG) conducted a phase II trial of NEL‐combined chemotherapy for newly diagnosed adult T‐ALL patients. Although the treatment led to improved survival, its safety was unacceptably low due to severe neurotoxicity. Our results suggest that caution is necessary regarding the use of NEL for primary adult T‐ALL patients.
2. MATERIALS AND METHODS
2.1. Patients and eligibility criteria
The JALSG T‐ALL213‐O (T‐ALL213‐O) study was a prospective phase II trial conducted by JALSG and registered at UMIN‐CTR (ID: UMIN000010642). Patients with newly diagnosed and not previously treated T‐ALL between 25 and 64 years of age were eligible for this study. Detailed eligibility criteria are presented in Appendix S1.
2.2. Diagnostic procedure
Acute lymphoblastic leukemia was diagnosed when marrow lymphoblasts, esterase‐ and peroxidase‐negative blasts, were more than or equal to 25% and AML M0, M5a, and M7 were excluded based on cell surface marker examination. The criterion for T‐ALL was that lymphoblasts were positive for CD3 or CyCD3. When CD3 was negative and the positivity of CyCD3 could not be examined, the following cases were diagnosed as T‐ALL: (1) positive for CD2 or CD7 and positive for CD1, CD4, CD5, or CD8; (2) positive for CD5 and negative for CD19 and CD20. When lymphoblasts that matched the above criteria were also positive for CD19, they were diagnosed as B‐ALL if the lymphoblasts were positive for CyCD79a, CD20, or CD22, and if not, they were diagnosed as T‐ALL. Immunophenotyping and cytogenetic studies were performed as described previously. 12 The multiplex reverse transcription (RT)‐PCR test was described previously. 13
3. STUDY DESIGN
This was a single‐arm, multi‐institutional phase 2 study. The protocol was approved by the institutional review board of each hospital. Written informed consent was obtained from all patients before registration in accordance with the Declaration of Helsinki. This study was initiated in May 2013 and closed for patient inclusion in September 2015.
3.1. Study treatment
The treatment schedule for T‐ALL213‐O is shown in Table 1. The regimen was planned based on our previous regimen, the high‐dose‐methotrexate (HD‐MTX) arm of ALL202‐O. 14 The modifications from ALL202‐O to T‐ALL213‐O were as follows: (1) Induction therapy (IND)1 was preceded by a 7‐day prednisolone (PSL) prephase treatment. (2) Cumulative doses of daunorubicin (DNR) and L‐asparaginase (L‐asp) in IND1 were altered from 180 mg/m2 and 18,000 KU/m2 to 135 mg/m2 and 40,000 KU/m2, respectively. (3) Randomization of HD‐MTX and intermediate‐dose (ID)‐MTX in consolidation (C)2 and C5 were fixed to HD‐MTX. (4) NEL‐combined chemotherapy, IND2, was newly planned and used for non‐complete remission (CR) patients after IND1, instead of C1 that was also used for the second induction in ALL202‐O. (5) Seven‐day NEL treatments, 3 alternative‐day administrations of 1500 mg/m2, were inserted into C3 and C5 on day 29. (6) Additional intrathecal chemotherapies (ITs) were administered on days 8 and 15 in IND1. (7) All ITs were changed from MTX and dexamethasone (DEX) to MTX, DEX, and cytarabine (IT‐triple). A schema of these changes is shown as Figure S1.
TABLE 1.
JALSG‐T‐ALL213‐O schedule.
| Phases/drugs | Route | Doses | Days |
|---|---|---|---|
| Prephase | |||
| Prednisolone | PO | 60 mg/m2 | 1–7 |
| Induction (IND)1 | |||
| Vincristine | IV | 1.3 mg/m2 a | 8, 15, 22, 29 |
| Daunorubicin | IV | 45 mg/m2 (30 mg/m2 c ) | 8, 9, 10 |
| Cyclophosphamide | IV | 1200 mg/m2 (800 mg/m2 c ) | 8 |
| L‐asparaginase | IV | 5000 U/m2 | 15, 17, 19, 21, 23, 25, 27, 29 |
| Prednisolone | PO | 60 mg/m2 | 8–28 (8–21 b , 8–15 c ) |
| IT‐triple d | IT | 8, 15 | |
| IND2 e | |||
| Nelarabine | IV | 1500 mg/m2 | 1, 3, 5 |
| Cyclophosphamide | IV | 1000 mg/m2 (800 mg/m2 c ) | 8 |
| 6‐Mercaptopurine | PO | 60 mg/m2 | 8–21 |
| Cytarabine | IV | 75 mg/m2 | 8–12, 15–19 |
| IT‐triple d | IT | 8 | |
| Consolidation (C)‐1 | |||
| Cytarabine | IV (3 h) | 2 g/m2 (1 g/m2 b ) q12h | 1, 2, 3 |
| Etoposide | IV | 100 mg/m2 | 1, 2, 3 |
| Dexamethasone | IV | 40 mg | 1, 2, 3 |
| IT‐triple d | IT | 1 | |
| C‐2 | |||
| Methotrexate f | CI | 3 g/m2 (1.5 g/m2 g ) | 1, 15 |
| Vincristine | IV | 1.3 mg/m2 a | 1, 15 |
| 6‐Mercaptopurine | PO | 25 mg/m2 | 1–21 |
| IT‐triple d | IT | 1, 15 | |
| C‐3 | |||
| Vincristine | IV | 1.3 mg/m2 a | 1, 8, 15 |
| Adriamycin | IV | 30 mg/m2 | 1, 8, 15 |
| Dexamethasone | PO | 10 mg/m2 | 1–8, 15–22 |
| Nelarabine | IV | 1500 mg/m2 | 29, 31, 33 |
| Cyclophosphamide | IV | 1000 mg/m2 (800 mg/m2 c ) | 36 |
| 6‐Mercaptopurine | PO | 60 mg/m2 | 36–49 |
| Cytarabine | IV | 75 mg/m2 | 36–40, 43–47 |
| IT‐triple d | IT | 1, 36 | |
| C‐4 | |||
| Same as C‐1 | |||
| C‐5 | |||
| Methotrexate f | CI | 3 g/m2 (1.5 g/m2 g ) | 1, 15 |
| Vincristine | IV | 1.3 mg/m2 a | 1, 15 |
| 6‐Mercaptopurine | PO | 25 mg/m2 | 1–21 |
| Nelarabine | IV | 1500 mg/m2 | 29, 31, 33 |
| IT‐triple d | IT | 1, 15 | |
| Maintenance (every 4 weeks until 24 months after the start of induction) | |||
| Vincristine | IV | 1.3 mg/m2 a | 1 |
| Prednisolone | PO | 60 mg/m2 | 1–5 |
| Methotrexate | PO | 20 mg/m2 | 1, 8, 15, 22 |
| 6‐Mercaptopurine | PO | 60 mg/m2 | 1–28 |
Abbreviations: CI, continuous infusion; IT, intrathecally; IV, intravenously; PO, per os; q12h, every 12 h.
The maximum dose was 2 mg.
Dose or schedule for patients 45–59 years old.
Dose or schedule for patients 60 years old or older.
IT‐triple consisted of methotrexate 15 mg, dexamethasone 4 mg, and cytarabine 40 mg.
Only for patients who could not obtain complete remission by IND1.
With leucovorin rescue (IV, 8 times every 6 h, and 50 mg for the first time and 15 mg after the second), beginning 36 h after the start of methotrexate infusion.
Dose for patients 50 years old or older. With leucovorin rescue (IV, 8 times every 6 h, and 15 mg), beginning 36 h after the start of methotrexate infusion.
Central nervous system (CNS) treatment was performed when CNS invasion was observed at the diagnosis or first examination of the cerebrospinal fluid (CSF). Details of CNS treatment are described in Appendix S1. The indication of allo‐stem cell transplantation (SCT) was decided based on institutional discretion. Each institution selected preparative and post‐transplant regimens for SCT according to its own discretion. Other detailed rules for treatments are described in Appendix S1.
3.2. Patient evaluation
Complete remission was defined as the presence of all of the following: <5% blasts in bone marrow (BM), no leukemic blasts in peripheral blood (PB), recovery of PB values to neutrophil counts of at least 1.0 × 109/L and platelet counts of at least 100 × 109/L, and no evidence of extramedullary leukemia. Relapse was defined as the presence of at least one of the following: recurrence of >5% leukemic cells in BM or any leukemic cells in PB or extramedullary sites. A PSL good response was defined as when the PB blast cell count was less than 1.0 × 109/L after the 7‐day PSL prephase. Toxicity was evaluated based on the National Cancer Institute Common Toxicity Criteria (NCI‐CTC) Version 4.0. Minimal residual disease (MRD) was not measured in this study.
3.3. Statistical analysis
The primary end point was event‐free survival (EFS) at 3 years after registration, which was defined as the time from registration to failure to achieve CR, relapse, death, or the last visit, whichever came first. The expected and threshold EFS rates at 3 years were estimated to be 50% and 25%, respectively. The threshold EFS rate of 25% was determined based on historical control data of JALSG ALL 90, 93, and 97. 12 , 15 , 16 With a statistical power of 80% and one‐sided, type I error of 5%, the minimum number of 26 eligible patients required for this study was calculated by means of binomial analysis. Allowing for a premature dropout rate of 8%, we aimed to include at least 28 patients. Primary end point analysis was performed with the Kaplan–Meier method to calculate the probability of EFS. The treatment was considered to be effective if the lower limit of the 90% confidence interval (CI) exceeded the threshold EFS (25%). Overall survival (OS) was defined as the time from registration to death or the last visit. Survival estimates and CIs were calculated with the Kaplan–Meier method and Greenwood's formula. The log‐rank test was used for group comparison.
The chi‐squared test was used to statistically analyze characteristic differences between patient groups. The Kaplan–Meier product limit method was performed to estimate EFS and OS. Univariate analysis of the effects of risk factors on survival was performed using the Log‐rank test. Stata SE 11.2 (Stata Co.) was used for all statistical analyses. All statistical tests were two sided.
3.4. Selection of T‐ALL202‐O patients
ALL202‐O was a study for both B‐ALL and T‐ALL patients aged from 25 to 64 years old, and patients were randomized into groups treated with HD‐MTX (HD‐MTX group) or ID‐MTX (ID‐MTX group). 14 For the comparison with T‐ALL213‐O, we selected T‐ALL patients and patients who were not assigned to the ID‐MTX group from ALL202‐O patients and designated those 36 patients as T‐ALL202‐O.
4. RESULTS
4.1. Patient entry and characteristics
Between May 2013 and September 2015, 28 patients from 24 hospitals participating in JALSG were enrolled in this study. A Consolidated Standards of Reporting Trials (CONSORT) diagram of this study is shown in Figure 1. Four patients were excluded: Three had been misdiagnosed and one had dropped out during the prephase treatment based on institutional discretion. Two of the misdiagnosed patients who had received at least one course of protocol therapy were included in the safety analysis set. Therefore, the full‐analysis and safety analysis sets contained 24 and 26 patients, respectively. Pretreatment characteristics are summarized in Supplemental Table S1. The median age was 42 years, and there were 15 men (62%) and 9 women. Cytogenetic evaluations were performed including all 24 patients. Results were classified according to the modified Medical Research Council (MRC) UKALLXII/ECOG E2993ALL cytogenetic subgroups 17 : The very‐high‐risk group (n = 4) included t(4;11), a complex karyotype, defined as >5 abnormalities without known translocations, or low hypodiploidy/near triploidy; the high‐risk group (n = 2) included other MLL translocations, monosomy 7 with <5 abnormalities, or t(1;19); the intermediate‐risk group (n = 15) included a normal karyotype or other miscellaneous abnormal karyotypes; the standard‐risk group (n = 0) included high hyperdiploidy. A multiplex RT‐PCR assay to detect the following eight distinct fusion gene transcripts and GAPDH as an internal control was performed for all patients: major and minor BCR::ABL1, ETV6::RUNX1, TCF3::PBX1, STIL::TAL1, KMT2A::AFF1, KMT2A::MLLT3, and KMT2A::MLLT1. All 24 patients were analyzed, and no chimera mRNA was detected except for the fact that one patient was positive for STIL::TAL1 (Table S1).
FIGURE 1.
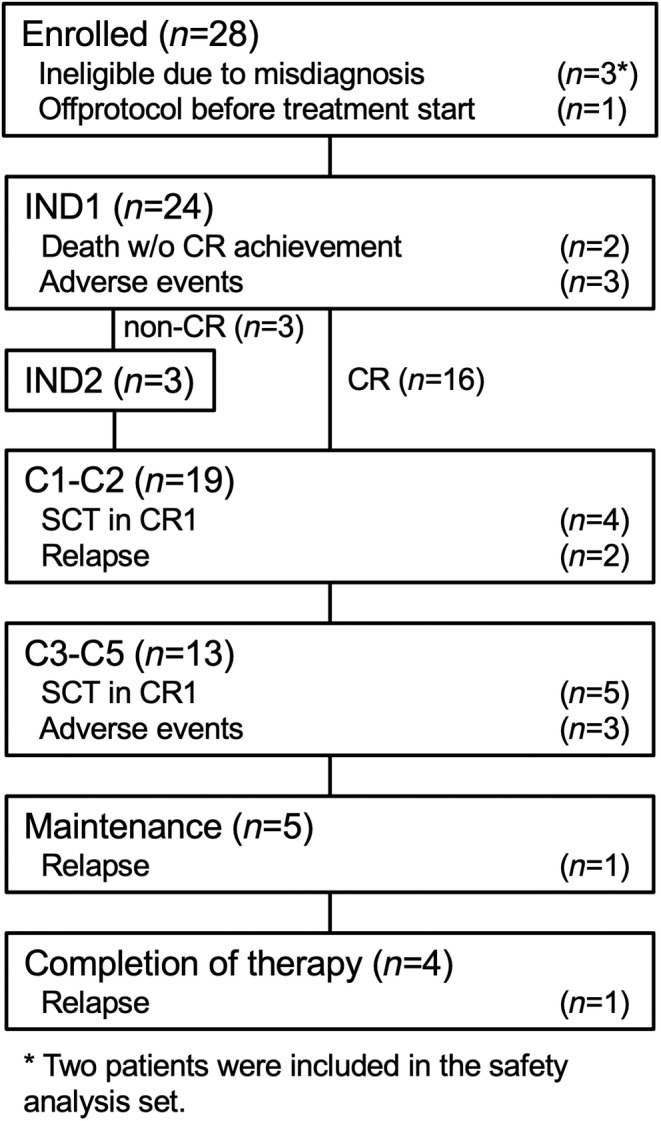
Consolidated Standards of Reporting Trials (CONSORT) diagram for the flow of patients through the study.
4.2. Response to induction therapy
Thirteen patients (54%) showed a good response to PSL in the prephase therapy. Two patients died of sepsis without CR achievement during IND1. Eighteen patients (75%) achieved CR after IND1. Three patients including a non‐CR patient dropped out of the study due to adverse events after IND1. Three non‐CR patients received IND2 and all of them achieved CR. A total of 21 (88%) out of 24 patients achieved CR.
4.3. Survival
Two patients died without CR achievement during IND1. One non‐CR patient dropped out of the study after IND1 due to adverse events and died. Of the 21 CR patients, three relapsed during the protocol therapy and died. Five patients dropped out of the study due to adverse events and were alive without relapse. Nine patients dropped out of the study due to SCT in CR1. All of them were alive, although one relapsed. Four patients completed the protocol study and one of them relapsed and died. Therefore, 7 patients died and 17 were alive.
In the primary endpoint analysis, the estimated 3‐year EFS rates were 70% (90% CI: 52%–83%, median observation period: 7.7 years) and 59% (90% CI: 34%–77%, median observation period: 7.8 years), when patients undergoing SCT in CR1 were uncensored and censored, respectively (Figure 2A and Figure S2A). In both cases, the lower limit of the 90% CI exceeded the threshold EFS at 25%; therefore, the treatment was considered to be effective.
FIGURE 2.
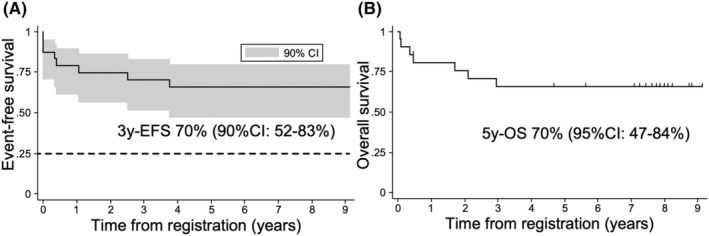
Survival analysis of T‐ALL213‐O. (A) Event‐free survival (EFS) curve of T‐ALL213‐O. Stem cell transplantation (SCT) cases were not treated as censored cases. The 90% CI is demonstrated as a gray area. The dashed line indicates threshold EFS. (B) OS curve of T‐ALL213‐O. SCT cases were not treated as censored cases.
The estimated 5‐year OS rates were 70% (95% CI: 47%–84%, median observation period: 7.7 years) and 57% (95% CI: 26%–79%, median observation period: 7.8 years) when patients undergoing SCT in CR1 were uncensored and censored, respectively (Figure 2B and Figure S2B).
4.4. Comparison with T‐ALL202‐O
In order to investigate whether the addition of NEL conferred an advantage in the therapeutic effect, we compared the therapeutic effect of T‐ALL213‐O with T‐ALL202‐O, whose regimen was very similar to T‐ALL213‐O except for the lack of using NEL (Figure S1). Patients' characteristics were not significantly different between T‐ALL213‐O and T‐ALL202‐O (Table S1). The second induction therapy for non‐CR patients after IND1 was C1 in T‐ALL202‐O, but it was IND2, NEL‐combined chemotherapy, in T‐ALL213‐O (Figure S1). The CR rate of the second induction therapy in T‐ALL213‐O was significantly higher than in T‐ALL202‐O (100% vs. 29%, respectively, p = 0.038); however, probably due to the small number of patients who received IND2 therapy, the total CR rate was not significantly different between the two groups (88% vs. 75%, respectively, p = 0.236, Table 2). When SCT was not censored, both 5‐year EFS (66% vs. 45%, respectively, p = 0.0914) and 5‐year OS (70% vs. 58%, respectively, p = 0.3965) were higher in T‐ALL213‐O, but the difference was not significant (Figure 3A,B). These results were similar when SCT in CR1 was censored (Figures S3A and S3B).
TABLE 2.
Comparison of complete remission (CR) rate: T‐ALL213‐O versus T‐ALL202‐O.
| T‐ALL213‐O (n = 24) | T‐ALL202‐O (n = 36) | p‐Value | ||
|---|---|---|---|---|
| 1st induction | Total | 24 | 36 | |
| CR | 18 | 25 | 0.64 | |
| CR rate (%) | 75 | 69 | ||
| 2nd induction | Total | 3 | 7 | |
| CR | 3 | 2 | 0.038 | |
| CR rate (%) | 100 | 29 | ||
| Induction total | Total | 24 | 36 | |
| CR | 21 | 27 | 0.236 | |
| CR rate (%) | 88 | 75 |
FIGURE 3.
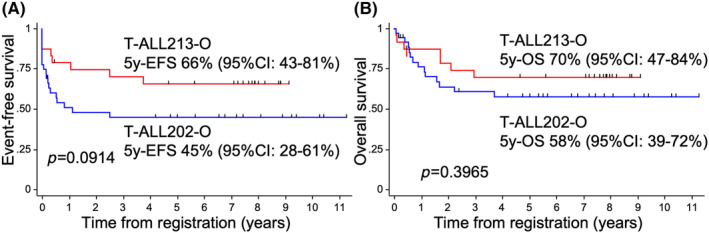
Comparison of survival between T‐ALL213‐O and T‐ALL202‐O. Event‐free survival (EFS) (A) and OS (B) were compared between T‐ALL213‐O (red line) and T‐ALL202‐O (blue line). Stem cell transplantation (SCT) cases were not treated as censored cases.
4.5. Safety
The frequency of severe adverse events (SAE) is summarized in Table 3. Of note, G3 PN developed in seven patients. All occurred after NEL treatment; therefore, it occurred in 50% of NEL‐treated patients. The clinical features of NEL‐associated neuropathies are summarized in Table 4. No association was observed with patients' age. PN occurred in 0 (0%), 6 (43%), and 1 (33%) of the patients who received IND2, C3, and C5, respectively. NEL was skipped thereafter when G3 or higher CNS disorders developed (also see Appendix S1). The median time from NEL administration to G3 PN development was 51 days. Six patients showed G3 gait disturbance (GD) and three of them had bladder bowel dysfunction (BBD). Treatments with steroid pulse, plasma exchange, and/or pregabalin were performed for five patients. PN improved to G2 or lower in six of seven cases.
TABLE 3.
Frequency of SAE.
| SAE | All patients (n = 26) | NEL‐treated patients (n = 14) | ||
|---|---|---|---|---|
| No. | (%) | No. | (%) | |
| AST/ALT increased | 13 | 50 | 11 | 79 |
| Pancreatitis | 4 | 15 | 1 | 7 |
| Thrombosis | 0 | 0 | 0 | 0 |
| Febrile neutropenia | 15 | 58 | 8 | 57 |
| Sepsis | 3 | 12 | 1 | 7 |
| Peripheral neuropathy | 7 | 27 | 7 | 50 |
| Ileus | 4 | 15 | 1 | 7 |
| Creatinine increased | 2 | 8 | 2 | 14 |
Abbreviations: NEL, nelarabine; SAE, severe adverse events.
TABLE 4.
Clinical features of NEL‐associated neuropathies.
| No. | Age | Responsible NEL administration | Days from NEL to G3 PN | Symptoms | Treatment | Improvement to ≦G2 |
|---|---|---|---|---|---|---|
| 1 | 33 | C5 | 51 | PSN | None | Y |
| 2 | 34 | C3 | 47 | PSN, PMN, GD, BBD | SP, PE | Y |
| 3 | 56 | C3 | 46 | PSN, PMN, GD, BBD | SP, PE | N |
| 4 | 54 | C3 | 65 | PSN, PMN, GD | None | Y |
| 5 | 45 | C3 | 36 | PSN, PMN, GD, BBD | SP | Y |
| 6 | 47 | C3 | 63 | PSN, PMN, GD | SP | Y |
| 7 | 25 | C3 | 64 | PSN, PMN, GD | Pregabalin | Y |
Abbreviations: BBD, bladder bowel dysfunction; GD, gait disturbance; N, no; NEL, nelarabine; PE, plasma exchange; PMN, peripheral motor neuropathy; PN, peripheral neuropathy; PSN, peripheral sensory neuropathy; SP, steroid pulse; Y, yes.
4.6. Prognostic factors
In order to investigate prognostic factors for patients treated with the T‐ALL213‐O regimen, univariate analysis of the effects of clinical and biological features on the EFS rate was performed. An age older than 44 years was the only factor significantly unfavorable for longer EFS (p = 0.018, Table S2).
5. DISCUSSION
In the present study, the incidence of severe PN was very high. All severe PN cases developed after NEL treatment, during C3–C5. The incidence of severe PN that newly developed during C3–C5 in our previous ALL202‐O study, whose regimen was very similar to T‐ALL213‐O except for the lack of using NEL (Figure S1), was 3% (6 out of 190 cases), being markedly less frequent than in T‐ALL213‐O. These findings indicate that the high incidence of PN was due to NEL. In the comparison between T‐ALL213‐O and T‐ALL202‐O, it is considered that the survival advantage of T‐ALL213‐O was small, if at all present, and could not be detected by such small‐scale comparison; therefore, T‐ALL213‐O, that is, the addition of NEL to ALL202‐O, did not have an advantage in therapeutic effects to overcome safety concerns.
There have been two randomized control trials (RCTs) investigating the effectiveness of NEL, and they showed opposite results. In the Children's Oncology Group (COG) trial AALL04343, a 2 × 2 RCT to receive escalating‐dose MTX or HD‐MTX and receive or not receive NEL (650 mg/m2 on 5 consecutive days) was conducted for 659 high‐risk T‐ALL patients aged 1–31 years old (median: 16 years old). NEL improved disease‐free survival (88% vs. 82%, respectively, p = 0.029). 8 On the other hand, in the UKALL14 trial, 175 T‐ALL patients aged 25–65 (median: 38 years old) were randomized to receive or not receive NEL (1500 mg/m2 on 3 alternative days). Addition of NEL did not significantly improve EFS (57% vs. 62%, respectively, p = 0.61). 10 These findings suggest that the effect of NEL in adult T‐ALL was limited, if at all present, and could be detected only by a large‐scale study like the AALL04343 trial.
In both trials, the incidence of neurotoxicity greater than G2 was not significantly different between NEL‐treated and untreated groups, being approximately 8% in the AALL04343 trial and 10% in the UKALL14 trial. 8 , 10 In the GMALL trial 08/2013, 281 T‐ALL and T‐lymphoblastic lymphoma patients aged 18–55 years old were treated with NEL (1500 mg/m2 on 3 alternative days)‐combined chemotherapy, and the incidence of neurotoxicity greater than G2 was less than 5%. 11 Furthermore, in a combined analysis of the two trials, JPLSG ALL‐T11 for patients aged 0–18 years old and JALSG T‐ALL211‐U for patients aged 15–24 years old, which used the same protocol including NEL (650 mg/m2 on 5 consecutive days), the incidence of PN greater than G2 in 142 NEL‐treated patients was 8%. 9 The incidence was not changed, at 7%, when only patients aged 15–24 years old were analyzed. 18 These findings indicate that the incidence of severe PN in T‐ALL213‐O was conspicuously frequent among studies of primary T‐ALL. The reason has yet to be clarified. The chemotherapies before and after NEL, their intervals until and from NEL, and the dose of NEL may be important. Delayed intensification (DI) therapy of the AALL04343 trial was similar to C3 therapy of T‐ALL213‐O, but the PN incidences in both therapies were quite different, 9% versus 43%, respectively. 8 The schema of the difference between the two regimens is demonstrated as Figure S4. One major difference possibly explaining the high PN incidence is the difference in the dose of NEL, 1500 mg/m2 × 3 versus 650 mg/m2 × 5, respectively, although this cannot be tested due to differences in the patients' race and age between the two trials. Taken together with the finding that NEL treatment (650 mg/m2 × 5) did not cause severe PN frequently in young Japanese adults in ALL‐T11/T‐ALL211‐U, 9 , 18 it may be better to use NEL at a dose of 650 mg/m2 × 5 for Japanese patients when combined with intensive chemotherapies.
Limitations of this study were the small number of cases and SCT indication decided by institutional discretion; the threshold EFS was determined based on the results of JALSG ALL 90, 93, and 97, 12 , 15 , 16 when the outcome of ALL202‐O was not yet known and the number of cases required was determined. However, it was subsequently found that the outcome of ALL202‐O was much better than in those trials, so the threshold EFS should have been set higher and the estimated number of cases required should also have been higher. The current case numbers are insufficient to assess the efficacy of adding NEL to the ALL202‐O regimen. In the absence of measuring MRD, the only established prognostic factor in T‐ALL, and with institutional determination of whether and when to perform SCT in CR1, many SCTs in CR1 were performed with unclear necessity. This makes it difficult to determine the efficacy of chemotherapy.
In summary, NEL‐combined chemotherapy, T‐ALL213‐O, led to a good survival outcome but also showed frequent severe neurotoxicity. NEL should be used carefully for Japanese adults when combined with intensive chemotherapies.
AUTHOR CONTRIBUTIONS
Fumihiko Hayakawa: Formal analysis; investigation; visualization; writing – original draft; writing – review and editing. Naoki Mori: Investigation; project administration; writing – review and editing. Kiyotoshi Imai: Conceptualization; investigation; project administration; writing – review and editing. Yasuhisa Yokoyama: Investigation; resources; writing – review and editing. Yuna Katsuoka: Investigation; resources; writing – review and editing. Takeshi Saito: Investigation; resources; writing – review and editing. Tohru Murayama: Conceptualization; investigation; writing – review and editing. Etsuko Yamazaki: Conceptualization; investigation; writing – review and editing. Shinya Sato: Data curation; writing – review and editing. Yoshiko Atsuta: Conceptualization; formal analysis; writing – review and editing. Yuichi Ishikawa: Investigation; supervision; writing – review and editing. Emiko Sakaida: Funding acquisition; writing – review and editing. Yoshihiro Hatta: Conceptualization; investigation; project administration; writing – review and editing. Itaru Matsumura: Supervision; writing – review and editing. Yasushi Miyazaki: Supervision; writing – review and editing. Hitoshi Kiyoi: Supervision; writing – review and editing.
FUNDING INFORMATION
This work was supported by Grants for Practical Research for Innovative Cancer Control Grant nos. JP16ck0106129, JP19ck0106331, JP22ck0106607 (to F.H.), and JP24ck0106851 (to E.S.) from the Japan Agency for Medical Research and Development (AMED). We sincerely thank all these support providers.
CONFLICT OF INTEREST STATEMENT
Lecture fees: S.E. from Pfizer Inc., Novartis AG, and Janssen Pharmaceutical K.K.; M.I. from Pfizer Inc., Novartis AG, and Janssen Pharmaceutical K.K; M.Y. from Novartis AG and Nippon Shinyaku Co., Ltd. Research funds: M.I. from Novartis AG; K.H. from Kyowa Kirin Co., Ltd. Scholarship (incentive) endowments: M.I. from Kyowa Kirin Co., Ltd. and Nippon Shinyaku Co., Ltd.; K.H. from Kyowa Kirin Co., Ltd. K.H. is an editorial board member of Cancer Science. Other authors do not have any conflicts of interest.
ETHICS STATEMENT
Approval of the research protocol by an Institutional Review Board: YES.
Informed Consent: YES.
Registry and the Registration No. of the study/trial: UMIN000010642.
Animal Studies: N/A.
Supporting information
Figure S1. Schema of difference between ALL202‐O HD‐MTX arm and T‐ALL213‐O.
Figure S2. Survival analysis of T‐ALL213‐O, CR1 stem cell transplantation (SCT) censored.
Figure S3. Comparison of survival between T‐ALL213‐O and T‐ALL202‐O, CR1 stem cell transplantation (SCT) censored.
Figure S4. Schema of the difference between T‐ALL213‐O C3 and AALL0434 delayed intensification.
Table S1. Patient characteristics.
Table S2. Univariate analysis of prognostic factors.
Appendix S1. Doc S1 Detailed information about the T‐ALL213‐O protocol.
ACKNOWLEDGMENTS
We thank the former members of the JALSG ALL213 committee: Itsuro Jinnai, M.D., Ph.D., Isamu Sugiura, M.D., Ph.D., Takeshi Yasunami, M.D., Ph.D., Toru Sakura, M.D., Ph.D., Yuichi Yahagi, M.D., Ph.D., Toshiya Yokozawa, M.D., Ph.D., Jin Takeuchi, M.D., Ph.D., and all physicians and staff at the participating centers and JALSG. In addition to the authors, the investigators listed in Appendix S1 are acknowledged for contributing to this trial.
Hayakawa F, Mori N, Imai K, et al. Nelarabine‐combined chemotherapy improves outcome of T‐cell acute lymphoblastic leukemia but shows more severe neurotoxicity: JALSG T‐ALL213‐O. Cancer Sci. 2025;116:453‐461. doi: 10.1111/cas.16405
Contributor Information
Fumihiko Hayakawa, Email: bun-hy@med.nagoya-u.ac.jp.
Japan Adult Leukemia Study Group (JALSG):
Itsuro Jinnai, Isamu Sugiura, Takeshi Yasunami, Toru Sakura, Yuichi Yahagi, Toshiya Yokozawa, and Jin Takeuchi
REFERENCES
- 1. Vadillo E, Dorantes‐Acosta E, Pelayo R, Schnoor M. T cell acute lymphoblastic leukemia (T‐ALL): new insights into the cellular origins and infiltration mechanisms common and unique among hematologic malignancies. Blood Rev. 2018;32:36‐51. [DOI] [PubMed] [Google Scholar]
- 2. Marks DI, Paietta EM, Moorman AV, et al. T‐cell acute lymphoblastic leukemia in adults: clinical features, immunophenotype, cytogenetics, and outcome from the large randomized prospective trial (UKALL XII/ECOG 2993). Blood. 2009;114:5136‐5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huguet F, Chevret S, Leguay T, et al. Intensified therapy of acute lymphoblastic leukemia in adults: report of the randomized GRAALL‐2005 clinical trial. J Clin Oncol. 2018;36:2514‐2523. [DOI] [PubMed] [Google Scholar]
- 4. Kantarjian H, Thomas D, Wayne AS, O'Brien S. Monoclonal antibody‐based therapies: a new dawn in the treatment of acute lymphoblastic leukemia. J Clin Oncol. 2012;30:3876‐3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Testa U, Chiusolo P, Pelosi E, Castelli G, Leone G. CAR‐T cell therapy for T‐cell malignancies. Mediterr J Hematol Infect Dis. 2024;16:e2024031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kadia TM, Gandhi V. Nelarabine in the treatment of pediatric and adult patients with T‐cell acute lymphoblastic leukemia and lymphoma. Expert Rev Hematol. 2017;10:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abaza Y, M Kantarjian H, Faderl S, et al. Hyper‐CVAD plus nelarabine in newly diagnosed adult T‐cell acute lymphoblastic leukemia and T‐lymphoblastic lymphoma. Am J Hematol. 2018;93:91‐99. [DOI] [PubMed] [Google Scholar]
- 8. Dunsmore KP, Winter SS, Devidas M, et al. Children's oncology group AALL0434: a phase III randomized clinical trial testing Nelarabine in newly diagnosed T‐cell acute lymphoblastic leukemia. J Clin Oncol. 2020;38:3282‐3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sato A, Hatta Y, Imai C, et al. Nelarabine, intensive L‐asparaginase, and protracted intrathecal therapy for newly diagnosed T‐cell acute lymphoblastic leukaemia in children and young adults (ALL‐T11): a nationwide, multicenter, phase 2 trial including randomisation in the very high‐risk group. Lancet Haematol. 2023;10:e419‐e432. [DOI] [PubMed] [Google Scholar]
- 10. Rowntree CJ, Kirkwood AA, Hadley LC, et al. First analysis of the UKALL14 randomized trial to determine whether the addition of nelarabine to standard chemotherapy improves event free survival in adults with T‐cell acute lymphoblastic leukaemia (CRUK/09/006). 63rd ASH Annual Meeting Abstracts . 2021.
- 11. Goekbuget N, Fiedler W, Alakel N, et al. Results of the risk‐adapted, MRD‐Stratifed GMALL Trial 08/2013 in 281 T‐ALL/T‐Lbl patients: excellent outcome of standard risk thymic T‐ALL. 64th ASH Annual Meeting Abstracts . 2022.
- 12. Jinnai I, Sakura T, Tsuzuki M, et al. Intensified consolidation therapy with dose‐escalated doxorubicin did not improve the prognosis of adults with acute lymphoblastic leukemia: the JALSG‐ALL97 study. Int J Hematol. 2010;92:490‐502. [DOI] [PubMed] [Google Scholar]
- 13. Towatari M, Yanada M, Usui N, et al. Combination of intensive chemotherapy and imatinib can rapidly induce high‐quality complete remission for a majority of patients with newly diagnosed BCR‐ABL‐positive acute lymphoblastic leukemia. Blood. 2004;104:3507‐3512. [DOI] [PubMed] [Google Scholar]
- 14. Sakura T, Hayakawa F, Sugiura I, et al. Effectiveness of high‐dose MTX therapy for adult Ph‐negative ALL by randomized trial: JALSG ALL202‐O. ASH Annual Meeting Abstracts. 2015;126:79. [Google Scholar]
- 15. Ueda T, Miyawaki S, Asou N, et al. Response‐oriented individualized induction therapy with six drugs followed by four courses of intensive consolidation, 1 year maintenance and intensification therapy: the ALL90 study of the Japan adult leukemia study group. Int J Hematol. 1998;68:279‐289. [DOI] [PubMed] [Google Scholar]
- 16. Takeuchi J, Kyo T, Naito K, et al. Induction therapy by frequent administration of doxorubicin with four other drugs, followed by intensive consolidation and maintenance therapy for adult acute lymphoblastic leukemia: the JALSG‐ALL93 study. Leukemia. 2002;16:1259‐1266. [DOI] [PubMed] [Google Scholar]
- 17. Pullarkat V, Slovak ML, Kopecky KJ, Forman SJ, Appelbaum FR. Impact of cytogenetics on the outcome of adult acute lymphoblastic leukemia: results of southwest oncology group 9400 study. Blood. 2008;111:2563‐2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hatta Y, Sato A, Kada A, et al. Risk stratifed therapy with nelarabine and intensifed administration of L‐asparaginase for newly diagnosed T‐cell acute lymphoblastic leukemia in adolescents and young adults (JPLSG T‐11/JALSG T‐ALL‐211‐U): An intergroup phase 2 study. 64th ASH Annual Meeting Abstracts . 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Schema of difference between ALL202‐O HD‐MTX arm and T‐ALL213‐O.
Figure S2. Survival analysis of T‐ALL213‐O, CR1 stem cell transplantation (SCT) censored.
Figure S3. Comparison of survival between T‐ALL213‐O and T‐ALL202‐O, CR1 stem cell transplantation (SCT) censored.
Figure S4. Schema of the difference between T‐ALL213‐O C3 and AALL0434 delayed intensification.
Table S1. Patient characteristics.
Table S2. Univariate analysis of prognostic factors.
Appendix S1. Doc S1 Detailed information about the T‐ALL213‐O protocol.


