Graphical abstract
Summary of the findings of the current study, defining high-volume centres for pulmonary endarterectomy in chronic thromboembolic pulmonary hypertension.
Abstract
Background
We conducted a volume–outcome meta-analysis of pulmonary endarterectomy procedures for chronic thromboembolic pulmonary hypertension to objectively determine the minimum required annual case load that can define a high-volume centre.
Methods
Three electronic databases were systematically queried up to 1 May 2024. Centres were divided in volume tertiles. The primary outcomes were early mortality and long-term survival. Restricted cubic splines were used to demonstrate the volume–outcome relationship and the elbow-method was applied to define high-volume centres. Long-term survival was assessed using Cox frailty models.
Results
We included 51 centres (52 consecutive cohorts) and divided them into tertiles (T1: <6 cases per year; T2: 6–15 cases per year, T3: >15 cases per year), comprising a total 11 345 patients (mean age 52.3 years). Overall early mortality was 6.0% (T1: 11.6%; T2: 7.2%; T3: 5.2%; p<0.001), for which a significant nonlinear volume–outcome relationship was observed (p=0.0437) with a statistically determined minimal required volume of 33 cases per year (95% CI 29–35 cases), and a modelled volume of 40 cases per year corresponding to a 5.0% mortality rate. Nevertheless, early mortality still progressively declined in higher volume centres (from 6.7% to 5.4% to 2.9% in centres performing 16–50, 51–100 and >100 procedures annually). In addition, a significant volume effect was observed for long-term survival (adjusted hazard ratio per tertile 0.75, 95% CI 0.63–0.89; p=0.001).
Conclusion
There is a significant association between procedural volume and early mortality in pulmonary endarterectomy. An annual procedural volume of >33–40 cases per year may be used to define a high‑volume centre, although higher volumes still lead to progressively lower mortality rates.
Shareable abstract
There is a significant nonlinear relationship between institutional volume and outcome in PEA for CTEPH, and a volume of 33–40 cases per year may define a high-volume centre https://bit.ly/4fFBwq4
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is diagnosed in 0.1–3.8% of pulmonary embolism survivors [1] and its incidence in the general population is 5.2–13.2 million per year [2]. CTEPH is characterised by a dismal prognosis, secondary to right-sided heart-failure. Although several therapies exist, pulmonary endarterectomy (PEA) is preferred in suitable patients because it is potentially curative [3, 4]. Still, PEA is a complex and technically demanding procedure, during which cardiopulmonary bypass is used in combination with deep hypothermic circulatory arrest. Consequently, the PEA procedure itself is also associated with significant morbidity and mortality rates, with reported mortality rates ranging between 0.8% and 24.4% [5], rendering patient selection crucial [6]. In addition, surgical expertise seems to play an important role. Given the relative infrequency of CTEPH as a disease, and PEA as a procedure, institutional volumes have historically been rather low, potentially resulting in suboptimal outcomes. However, high-volume centres have recently reported early mortality rates of <5% [5, 7–10] and the frequency of PEA as a procedure has steadily increased during the past decade [11–13]. Of note, in the absence of clear US recommendations [14, 15], the 2022 European Society for Cardiology (ESC)/European Respiratory Society (ERS) guidelines for the diagnosis and treatment of pulmonary hypertension state that PEA should be performed in centres performing >50 PEA cases annually [3], although a substantiated recommendation was not provided in that document.
Inherently, the volume–outcome (V-O) relationship is nonlinear and not absolute, because expertise is only part of the equation that determines the result [16]. We have recently introduced a new method to determine this nonlinear V-O relationship, and to estimate the minimum required caseload to define a high-volume centre for infrequently performed surgical procedures that require centralisation [17]. Given the call for centralisation of PEA procedures and its elective character, the current study aims to evaluate the V-O relationship in PEA procedures for CTEPH.
Materials and methods
Design and registration
The current study was designed as a systematic review and meta-analysis, and pre-registered in PROSPERO (CRD42024548185, 28 May 2024) [18]. We adhered to the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement [19].
Eligibility criteria
Studies published between conception of the three electronic databases and 1 May 2024 were eligible for inclusion. Inclusion criteria were studies reporting on 1) consecutive PEA patients per centre, 2) years of inclusion (to calculate the inclusion period and the subsequent annual case load) and 3) the primary outcome (early mortality). Both single- and multiple-arm studies were eligible for inclusion. The inclusion of duplicate cohorts of the same centre was prevented by including the study with the largest sample size and excluding other studies describing the same cohort. If a centre reported on separate cohorts in different timespans, these reports were both used and assessed separately. Finally, to mitigate for the risk of publication bias, we allowed conference abstracts to be eligible for inclusion, on the prerequisite of fulfilling the prespecified inclusion criteria.
Information sources
The search was performed in three electronic databases (PubMed, Embase and the Cochrane Library). Reference lists of included articles were screened for potentially missed articles (cross-referencing).
Search strategy
The first search was performed in September 2023, and was updated regularly until 1 May 2024 (supplementary table S1 presents the full search strategy). The search was performed by one experienced author (M.J. Kawczynski).
Selection process
Duplicate studies were removed by automated tools, and the remaining articles were screened by title and abstract using the semi-automated web-based application Rayyan (https://rayyan.qcri.org) [20], after which a full-text screening was conducted to determine the studies eligible for final inclusion. The selection was performed by two independent reviewers (S. Heuts and A. Leus), and potential disagreement was resolved by consultation with the senior author (T. Verbelen).
Data collection and items
Preoperative, perioperative and postoperative data were collected using a Microsoft Excel sheet (supplementary table S2) by two authors (S. Heuts and A. Leus).
Outcomes and effect measures
The primary outcome was early mortality in relation to annual case volume per centre. Early mortality was defined as in-hospital mortality or 30-day mortality. Secondary outcomes were the need for postoperative extracorporeal membrane oxygenation (ECMO), duration of mechanical ventilation (days), intensive care unit (ICU) length of stay (days) and long-term survival. Long-term survival was expressed in absolute risk at 10 years, and absolute risk differences and numbers needed to treat were calculated.
The principal analysis focused on the statistical determination of the volume that could define a high-volume centre, using the elbow method (please see “Data synthesis” section). In addition, a 5.0% mortality rate has previously been proposed to be acceptable, and required, for this high-risk intervention [21]. Therefore, the annual case volume corresponding to this mortality rate threshold was studied, facilitating a combination of a statistically determined and clinically indicated high-volume threshold. Finally, we calculated the early mortality rates of centres performing less and more than the previously proposed required annual case load of 50 PEA procedures.
Risk of bias assessment
A risk of bias assessment was performed to evaluate the quality of the included articles, using the Newcastle–Ottawa scale, which can be modified for single-arm studies by excluding one of four questions in the “Selection” section (i.e. question 2), thereby assigning a maximal score of eight instead of nine to a study [17, 22]. This assessment was performed by two authors independently (S. Heuts and M.J. Kawczynski).
Data synthesis
All data are presented as mean±sd or n (%). Reported medians with interquartile range and/or minimum and maximum values were converted by Wan's method [23]. Annual procedural volume was calculated by dividing the number of patients per centre by their total inclusion time. Studies were categorised into tertiles (T1, T2, T3) according to their respective annual case volume. Early outcomes such as mortality and need for postoperative ECMO were pooled in a random-effects model (inverse variance weighting) and are presented for the overall cohort and per tertile, in n (%) and corresponding 95% confidence intervals. For early mortality, relative risk differences were calculated with both 10 cases per year and the threshold of a high-volume centre as a reference.
The nonlinear relationship between annual case volume and early mortality was examined using restricted cubic splines (RCS) (three knots) and presented graphically in a nonlinear mixed-effect model [17]. This outcome was studied both in unadjusted and adjusted analyses (adjusted for geographical location and median inclusion year, based on a complete case analysis, in a linear regression model, as proposed previously [17, 24]). To determine the volume that determined a high-volume centre, we applied the elbow method, which was first described by Antunes et al. [25] and Satopää et al. [26] for optimisation statistics and thereafter applied to the V-O relationship in surgical interventions by Kawczynski et al. [17] (graphically presented in supplementary figure S1).
For the secondary analysis of long-term survival, individual patient data derived from Kaplan–Meier curves were reconstructed as proposed by Liu et al. [27]. The presence of an association between annual case volume as a continuous parameter and as a tertile and long-term survival was evaluated using a univariable Cox frailty model [28], both in unadjusted and adjusted analyses. Of note, because only 12 out of 52 studies reported on long-term survival, new tertiles were constructed for this outcome, similarly based on the distribution of centre volume (four centres per tertile). For long-term survival, absolute risk differences were calculated with T1 as a reference, with subsequent derived numbers needed to treat.
Because the third tertile encompassed centres performing more than 16 annual procedures, sensitivity analyses were performed by dividing this tertile into smaller fractions (i.e. 16–50 procedures, 51–100 procedures and >100 procedures per year). Furthermore, sensitivity analyses were performed for studies published after 2014, and for studies only including patients after 2014 (which closely relates to the standardised introduction of balloon pulmonary angioplasty, which may have affected the patient selection for PEA from that era onwards).
All analyses were conducted using the R statistics software (“metafor”, “meta”, “rms”, “mgcv”, “survival”, “survminer”, “maps”, “ggplot2”, “pathviewr” and “discfrail” packages; www.r-project.org).
Publication bias assessment
The presence of publication bias was assessed for the primary outcome, visualised in a funnel plot and quantified by Egger's test.
Data availability
All data and coding for analyses will be openly shared through https://github.com/samuelheuts/V-O_in_PEA_for_CTEPH upon publication of this study.
Results
Study selection
The predefined systematic search produced 2148 results. After eliminating 738 duplicate studies, 1410 were left for title and abstract screening. This initial screening phase resulted in the exclusion of 1168 additional articles. One article could not be retrieved and was therefore excluded as well. The remaining 241 articles underwent full-text screening, ultimately resulting in the inclusion of 52 studies for final analysis (supplementary figure S2). These 52 studies originated from 51 centres, because one centre reported outcomes of two unique cohorts in two different time periods [29, 30].
Geography and tertiles
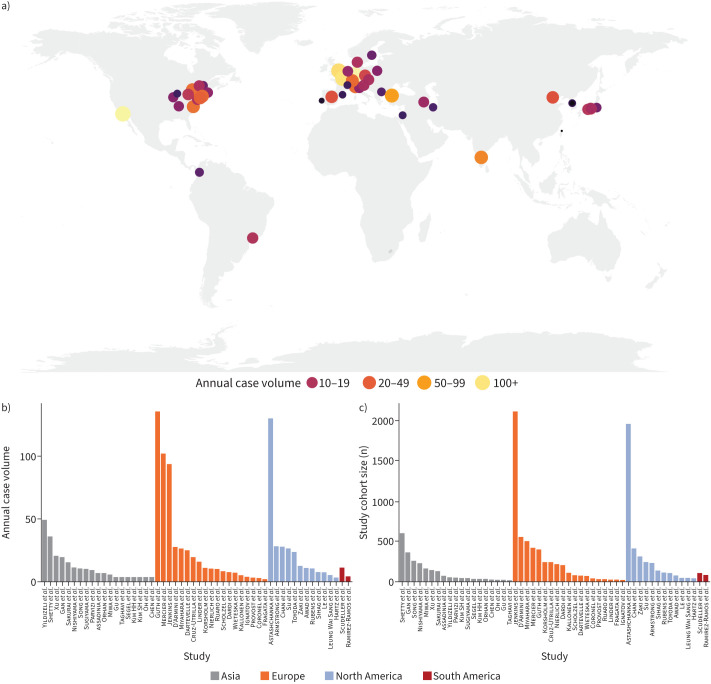
52 studies (51 unique centres, 52 unique consecutive cohorts) originating from 25 countries from four different continents were included. This resulted in a total number of 11 345 included patients. Figure 1 graphically presents the geographic distribution of centres (figure 1a) and their total sample sizes (figure 1b) and volumes (figure 1c).
FIGURE 1.
Geographical distribution (a), annual case volumes (b) and total sample sizes (c) of the included centres. Additional reference details can be found in the supplementary material.
Based on the distribution of centres and centre volumes, studies/centres were evenly divided into tertiles. Tertile 1 (T1) comprised 17 studies (<6 cases per year, n=673 patients), T2 comprised 18 studies (6–15 cases per year, n=2316 patients) and T3 comprised 17 studies (>15 cases per year, n=8356 patients, table 1).
TABLE 1.
Centre, patient and procedural characteristics, for the overall cohort and divided in tertiles
| Annual case volume quartiles | p-value | ||||
|---|---|---|---|---|---|
| Variables | Overall | Tertile 1 (<6 cases per year) |
Tertile 2 (6–15 cases per year) |
Tertile 3 (>15 cases per year) |
|
| Patients | 11 345 | 673 | 2316 | 8356 | |
| Study characteristics | |||||
| Studies | 52 | 17 | 18 | 17 | NA |
| Patients, per continent | 11 345 (100) | 673 (100) | 2316 (100) | 8356 (100) | NA |
| Europe | 5316 (46.9) | 189 (28.0) | 820 (35.4) | 4307 (51.6) | |
| North America | 3640 (32.1) | 72 (10.7) | 655 (28.3) | 2913 (34.9) | |
| Asia | 2214 (19.5) | 339 (50.4) | 739 (31.9) | 1136 (13.5) | |
| South America | 175 (1.5) | 73 (10.9) | 102 (4.4) | NA | |
| Patient characteristics | |||||
| Age (years) | 52.3±5.5 | 51.9±6.9 | 54.2±3.6 | 53.9±6.3 | 0.670 |
| Sex | 8102 (100) | 649 (100) | 2010 (100) | 5463 (100) | <0.001 |
| Male | 4333 (53.5) | 302 (46.5) | 1013 (50.4) | 3018 (55.2) | |
| Female | 3789 (46.5) | 347 (53.5) | 997 (49.6) | 2445 (44.8) | |
| Preoperative mPAP (mmHg) | 48.1±7.8 | 48.5±4.0 | 48.4±3.9 | 52.0±13.8 | 0.653 |
| Preoperative cardiac index (L·min−1·m−2) | 2.3±0.2 | 2.3±0.2 | 2.4±0.3 | 2.1±0.1 | 0.480 |
| Preoperative PVR (WU) | 11.0±4.0 | 12.2±4.9 | 10.4±2.2 | 11.8±4.2 | 0.629 |
| Preoperative 6-min walking test (m) | 346.5±52.3 | 365.5±41.0 | 362.2±18.4 | 304.9±66.8 | 0.120 |
| NYHA classification (%) | 3763 (100) | 378 (100) | 1158 (100) | 2227 (200) | <0.001 |
| Class I | 19 (0.5) | 1 (0.3) | 4 (0.3) | 14 (0.6) | |
| Class II | 554 (14.7) | 58 (15.3) | 195 (16.8) | 301 (13.5) | |
| Class III | 2316 (61.5) | 239 (63.2) | 777 (67.2) | 1300 (58.4) | |
| Class IV | 874 (23.3) | 80 (21.2) | 182 (15.7) | 612 (27.5) | |
| Procedural characteristics | |||||
| CPB (min) | 230.4±56.9 | 231.5±41.9 | 238.5±41.4 | 200.3±52.1 | 0.395 |
| Aortic cross clamp time (min) | 103.7±34.7 | 109.4±24.7 | 141.1±12.9 | 95.6±26.5 | 0.140 |
| CA (min) | 52.3±49.3 | 42.8±16.1 | 83.4±85.1 | 29.7±7.5 | 0.223 |
Data are presented as n, n (%) or mean±sd, unless otherwise indicated. Bold text indicates statistical significance. mPAP: mean pulmonary artery pressure; PVR: pulmonary vascular resistance; NYHA: New York Heart Association dyspnoea class; CPB: cardiopulmonary bypass; CA: circulatory arrest.
Centre, patient and procedural characteristics
All available patient, haemodynamic, functional and procedural parameters per centre are presented in supplementary tables S3–7, including the early mortality rates. In the overall cohort the mean age was 52.3±5.5 years, without a statistically significant difference between volume tertiles (table 1). Higher volume tertiles operated more frequently on males (T3: 55.2% versus T2: 50.4% versus T1: 46.5%; p<0.001) and there was an important difference in symptomatology between tertiles based on the New York Heart Association classification. Nevertheless, there were no significant differences in relevant baseline haemodynamic and functional parameters, such as mean pulmonary arterial pressure (overall: 48.1±7.8 mmHg), cardiac index (overall: 2.3±0.2 L·min−1·m−2), pulmonary vascular resistance (overall: 11.0±4.0 WU) or 6-min walking distance (overall: 346±52.3 m) between tertiles (table 1). In addition, we did not observe a statistically significant difference in circulatory arrest time, aortic cross clamping time or cardiopulmonary bypass time, although these numbers may have been affected by the wide variability in reporting (table 1).
Study quality assessment
A detailed quality assessment per study is presented in supplementary table S8. Overall, risk of bias ranged from low (n=15) to intermediate (n=28) to high (n=9). Because all studies comprised consecutive patients and reported on the primary outcome of early mortality, all studies were included for this analysis.
Early mortality
The pooled early mortality rate for the entire cohort was 6.0% (95% CI 5.5–6.4%). Early mortality was significantly higher in the tertile containing the lowest volume centres (T1: 11.6% (9.4–14.2%) versus T2: 7.2% (6.2–8.3%) versus T3: 5.2% (4.7–5.7%); p<0.001) (table 2).
TABLE 2.
Postoperative outcomes
| Variables | Overall | Tertile 1 (<6 cases per year) |
Tertile 2 (6–15 cases per year) |
Tertile 3 (>15 cases per year) |
p-value |
|---|---|---|---|---|---|
| Patients | 11 345 | 673 | 2316 | 8356 | |
| Early mortality | |||||
| Reported patients# | 11 345 (100) | 673 (100) | 2316 (100) | 8356 (100) | |
| Pooled presence¶ | <0.001 | ||||
| Patients | 678 | 78 | 166 | 437 | |
| % (95% CI) | 6.0 (5.5–6.4) | 11.6 (9.4–14.2) | 7.2 (6.2–8.3) | 5.2 (4.7–5.7) | |
| Postoperative ECMO | |||||
| Reported patients# | 5065 (100) | 119 (100) | 1302 (100) | 3644 (100) | |
| Pooled presence¶ | <0.001 | ||||
| Patients | 306 | 20 | 112 | 174 | |
| % (95% CI) | 6.0 (5.4–6.7) | 16.8 (11.2–24.5) | 8.6 (7.2–10.3) | 4.8 (4.1–5.5) | |
| Postoperative ventilation (days) | 4.8±3.7 | 3.5±1.0 | 6.4±6.5 | 4.4±3.3 | 0.617 |
| Postoperative ICU duration (days) | 9.1±5.3 | 8.6±4.0 | 8.6±6.8 | 6.0±0.9 | 0.702 |
| Postoperative mPAP (mmHg) | 27.6±5.4 | 30.7±3.4 | 27.3±4.0 | 26.3±3.5 | 0.316 |
| Postoperative PVR (WU) | 3.6±1.1 | 3.7±0.7 | 4.1±1.1 | 4.1±1.3 | 0.856 |
Data are presented as n, n (%) or mean±sd, unless otherwise indicated. Bold text indicates statistical significance. ECMO: extracorporeal membrane oxygenation; ICU: intensive care unit; mPAP: mean pulmonary artery pressure; PVR: pulmonary vascular resistance. #: the total number of patients in which the presence and absence of the outcome was reported; ¶: actual pooled presence of the outcome.
The V-O association and the case load that defines a high-volume centre
The RCS analysis identified a significant unadjusted nonlinear V-O relationship between annual case volume and early mortality (p=0.0437) (figure 2a). After adjusting for the predefined variables (i.e. geographical location and median inclusion year) that were fully reported on, this significant nonlinear relationship persisted (p=0.0030) (figure 2b). Using the elbow method, we found that a procedural case load of 33 cases per year for both unadjusted and adjusted analyses may be used to define a high-volume centre (95% CI 29–35 cases per year for the unadjusted curves, 95% CI 31–34 cases per year for the adjusted curves) (figure 2c, d). This caseload corresponded to a modelled mean mortality rate of 5.3% (95% CI 2.4–8.1%) (supplementary table S9). With 10 cases per year as a reference, this would imply a 35% relative risk reduction for early mortality in high-volume centres (relative risk 0.65, 95% CI 0.29–0.99) (supplementary table S9). Of note, early mortality rates still progressively declined beyond the defined volume threshold (please see the sensitivity analyses).
FIGURE 2.
Unadjusted (a) and adjusted (b) volume–outcome (V-O) relationship for early mortality and estimation of the case load that can define a high-volume centre based on unadjusted (c) and adjusted (d) early mortality. a and b present the restricted cubic spline analysis to express the relationship between volume and (un)adjusted early mortality. The size of the dots corresponds to the variance of the data. In other words, if the variance is small (i.e. there is a high degree of certainty), dots are large and relatively more weight is assigned to these findings. c and d graphically present the application of the elbow method to these curves to determine the case load that can define a high-volume centre. The solid red line represents the mean and dashed red lines represent the 95% confidence intervals.
Previously, a 5.0% mortality threshold was advocated to define a high-volume centre [21]. Based on our analysis and the modelled mortality curve, this mortality rate would correspond to an annual case load of 40 cases per year (supplementary table S9, figure 2a). Finally, the previously proposed threshold of 50 annual PEA procedures was studied as well. In a pooled analysis of studies performing less and more than this threshold, the early mortality rate was 8.2% (95% CI 6.8–9.9%, <50 PEA procedures per year) and 3.4% (95% CI 2.0–6.9%, >50 PEA procedures per year).
Secondary outcomes
There was a significantly increased use of postoperative ECMO in lower volume centres as compared to higher volume centres (T1: 16.8% versus T2: 8.6% versus T3: 4.8%; p<0.001) (table 2). Postoperative mean ventilation duration and ICU length of stay did not differ significantly between volume tertiles (table 2).
Long-term survival
A total of 12 centres, comprising 2198 patients, reported on long-term survival. The overall 1-year survival was 89.7% (95% CI 88.4–91.0%), with a 10-year survival of 75.9% (95% CI 73.4–78.5%) (supplementary table S10, figure 3a). Increased annual case volume (as a continuous variable) was associated with improved long-term survival in both unadjusted and Cox frailty-adjusted analyses (hazard ratio per case 0.98, 95% CI 0.97–0.99; unadjusted p=0.033, adjusted p=0.018) (supplementary table S11 presents the results of the overall Cox frailty model, without residual frailty).
FIGURE 3.
Long-term survival for the overall cohort (a) and divided into tertiles (b).
Because only 12 studies reported on long-term survival, new tertiles of four centres per tertile were constructed (T1: <5 cases per year; T2: 5–15 cases per year; T3: >15 cases per year). 1-, 3-, 5- and 10-year survival per tertile is presented in supplementary table S10 and figure 3b. The Cox frailty analysis demonstrated a significantly increased long-term survival in higher volume tertiles, with an unadjusted and adjusted hazard ratio per tertile of 0.77 (95% CI 0.66–0.90; p=0.001) and 0.75 (95% CI 0.63–0.89; p=0.001), respectively (supplementary table S11, without residual frailty).
With T1 as a reference (<5 cases per year, 10-year survival 66.0%, 95% CI 57.9–75.2%), 10-year survival was markedly increased in patients undergoing PEA in a T3 institution (>15 cases per year, 10-year survival 79.0%, 95% CI 75.7–82.5%), resulting in a 13.0% absolute risk difference, implying a number needed to treat to save a life at 10 years of 7.7 (supplementary table S12).
Sensitivity analyses
T3 was subdivided into smaller fractions to further assess the V-O relationship in this highest tertile. Interestingly, the pooled mortality rate progressively decreased from 6.7% to 5.4% to 2.9% in centres performing 16–50 (13 centres), 51–100 (one centre) and >100 (three centres) procedures annually (supplementary table S13). Of note, these mortality rates differed slightly from the modelled mortality rates, because the model was based on the totality of the data and these pooled rates only on the data from specific centres in this tertile.
When confined to studies published in the last decade (after 2014, supplementary figure S3a) or studies including patients in the last decade (after 2014, supplementary figure S3b), the statistical significance of the previously observed V-O relationship persisted (p=0.040 and p=0.001, respectively).
Publication bias
Publication bias assessment was performed for the primary outcome (early mortality). We did not observe funnel plot asymmetry, suggesting the absence of publication bias (supplementary figure S4). This was reflected statistically by the Egger's test, which showed no significant indication for publication bias (β 0.30, p=0.631).
Discussion
The current study comprised the largest accumulation of consecutive patients undergoing PEA for CTEPH in the literature and quantified the V-O association for this procedure, while objectively determining an annual case load that can define a high-volume PEA centre. Based on our findings, early mortality and long-term survival are significantly affected by procedural volume and subsequent expertise. Furthermore, operations in high-volume centres confer an important survival benefit, with a number needed to treat to save a life at 10 years of only 7.7 in a high- versus low-volume centre.
Contemporary US guidelines do not provide recommendations regarding PEA as a procedure, or a volume threshold to define PEA centres of expertise [14, 15]. The most recent ESC/ERS guidelines for the treatment of pulmonary hypertension suggest CTEPH centres ideally perform 50 PEA procedures per year [3], although a formal recommendation (i.e. class of recommendation with level of evidence) was lacking. This statement was supported by a reference to the 2011 study by Mayer et al. [7], who presented the results from an international multicentre registry. This commendable analysis comprised 386 surgical PEA patients from 27 centres, and primarily investigated short-term outcomes. In an exploratory analysis, Mayer et al. [7] observed in-hospital and 1-year mortality to be numerically lower in centres performing >50 cases per year, although this difference did not reach statistical significance (5.0% versus 8.2%, p=nonsignificant). The absence of a statistically significant relationship was also confirmed by Delcroix et al. [31], using longer term follow-up data from the same registry. Nevertheless, Jenkins et al. [21] built further on these findings and stated that, in an ideal world, one CTEPH centre would serve 50 million people, while performing 75–100 PEA procedures per year.
Although we firmly support the incentive to centralise CTEPH care, it is important to provide evidence-based recommendations for the required case load in high-volume centres. Based on our overview of published literature, only four centres in the world would meet the volume criterion as proposed by the ESC/ERS guidelines [10, 30, 32, 33], which appears stringent. However, in the studies by Mayer et al. [7] and Delcroix et al. [31], annual case volume was treated as a categorical parameter [7, 12, 31], which is known to reduce the power of a statistical analysis [34]. Moreover, the categorisation of volume disregards the nonlinear relationship between volume and outcome, a concept which is elegantly summarised by Vonlanthen et al. [16]. Also, these previous studies were based on the inclusion of 27 centres, potentially limiting the analysis’ power. Instead, the current pooled V-O analysis acknowledges the nonlinear relationship between volume and outcome by treating volume as a continuous parameter with the additional application of the RCS analysis. We were also able to include data from 51 centres (52 unique consecutive cohorts), further augmenting the statistical power of the analysis. As presented in figure 2, the modelled early mortality rate in low-volume centres (<10 cases per year) exceeds 10%, but sharply declines with the accrual of more cases, after which a deflection in the curve is observed between 20–30 cases per year. By virtue of the elbow method, a mathematical concept derived from optimisation statistics, this deflection point in the curve can be defined as the location at which the highest yield in performance is obtained, with respect to the annual volume. In this analysis, we found a “statistical threshold” of 33 cases per year, both in unadjusted and adjusted analyses, which may define a high-volume centre. Nevertheless, we also found that outcomes still improved further beyond this threshold, as presented in the sensitivity analyses. This progressive reduction in mortality was also demonstrated in studies by Madani et al. [9] and Jenkins et al. [10], with commendable early mortality rates of <2%.
In addition to annual institutional volume, the definition of a high-volume centre also relies on excellent outcomes. As such, a 5.0% mortality threshold was previously proposed to define such expertise [10, 21]. Consequently, this mortality threshold was studied as well, which corresponded to a modelled case load of 40 cases per year. Therefore, we can hypothesise that the volume threshold to define a high-volume centre may range between the statistically determined and clinically indicated volumes of 33 and 40 cases per year, notwithstanding the further progressive decline in early mortality beyond these thresholds. However, we must note that these thresholds were based on statistical analyses and assumptions, which may not be in line with clinical judgment. Therefore, it remains to be determined whether these analyses justify lowering the previously proposed thresholds.
Although our findings for early mortality are also supported by the analyses of the secondary outcomes (among others, postoperative need for ECMO and long-term survival), less weight may be assigned to these findings because these parameters were incompletely reported. Still, the observed 10-year survival rate of 75.9% closely corresponds to a nationwide analysis from the UK by Cannon et al. [35] of 72.0%. Importantly, in our analysis, the progressively diverging nature of the survival curves between volume tertiles seems to confirm a robust and durable effect of the PEA procedure in higher volume centres. Moreover, the statistically significant and clinically relevant absolute risk difference between tertiles for 10-year survival further underline these findings. Based on these analyses, a life is saved at 10 years for every 7.7 patients that are treated in a T3 institution (>15 cases per year) as compared to a T1 institution (<5 cases per year).
Only six centres have reported annual procedural volumes exceeding the proposed threshold of 33 cases per year [8, 10, 30, 32, 36, 37]. In light of centralisation, PEA for CTEPH is an ideal procedure because it is performed infrequently, is associated with high morbidity and mortality rates, and has an elective character. Furthermore, CTEPH patients require a multidisciplinary approach, a thorough work-up, meticulous patient selection and dedicated peri-operative care [6]. Still, the balance between a high case load, number of operators per centre, sufficient training and exposure, and geographical distributions of centres can be difficult. Indeed, CTEPH is a disease that can be treated in an elective setting, and can therefore be diagnosed and treated without issue as an “orphan disease” in specialised centres. Still, if centralisation is pushed to an extreme, patients will have to travel excessive distances and the accessibility to CTEPH care may be compromised. This can in turn affect and aggravate socioeconomic disparities. In addition, a lesser number of individual surgeons will be exposed to PEA procedures in this context, and fewer surgeons will be trained in this technically demanding operation. In contrast, when criteria are too lenient, patients’ outcomes may be jeopardised. Consequently, the current study provides an objective and evidence-based formulation of the threshold that could define a high-volume centre. Based on our new findings derived from objective and reproducible statistical analyses, this threshold varies between 33 and 40 procedures per year, although outcomes still further improve beyond this threshold. Keeping in mind the reported incidence and surgery rate of CTEPH [38, 39], one high-volume specialised centre per 10–30 million inhabitants may eventually be justified. Such centralisation efforts would both lead to optimal outcomes while also guaranteeing CTEPH care accessibility in smaller countries.
Strengths and limitations
The current study provides a complete overview of the literature and included all reports of consecutive CTEPH patients treated by PEA. The need for centralisation of PEA for CTEPH is high, and substantiated recommendations to define high-volume centres are warranted. Based on a new statistical approach, the nonlinear relationship between volume and outcome was appreciated and an objective threshold for a high-volume centre was formulated.
Nevertheless, several limitations should be addressed. First, we only included single-centre studies because this allowed us to calculate the annual case load per centre. This prohibited the inclusion of multicentre experiences, such as the study by Mayer et al. [7]. Still, we believe this limitation is mitigated by the inclusion of almost all single-centre experiences from this multicentre analysis separately. In line with this, tertiles were divided based on the number of centres instead of the number of patients. Inherently, the latter is not possible in a pooled analysis. Second, volume can be measured at the institutional level or the individual operator level. Regrettably, individual operator volume was rarely reported in the included studies. Yet, CTEPH is such an exceptionally multidisciplinary disease that it may be overly simplistic to merely focus on operator volume, because institutional expertise can provide a more accurate reflection of the quality of integrated care pathways for CTEPH patients. In light of this, we should also acknowledge that the volume of PEA procedures is not the only determinant of a CTEPH centre of expertise, and other factors should be taken into account. These factors include the presence and experience of specialised cardiologists, pulmonary physicians, imagers and interventionalists with sufficient expertise in balloon pulmonary angioplasty and medical treatment (multimodality treatment [40]). Moreover, for PEA, expertise in cardiac anaesthesia and ICU care is equally important. Also, the training for this complex and time-sensitive surgery must be considered, because a sufficient number of cases is necessary for this aspect as well.
Of note, results may be less generalisable to Asian centres and/or populations, given the geographical distribution of centres publishing on PEA. The results may therefore particularly apply to US and European centres. Finally, all literature analyses are subject to publication bias, in which suboptimal outcomes of smaller centres are usually underreported. Although we did not find an indication of a statistically significant presence of publication bias, it is intuitively there. However, the reporting of results by smaller centres with worse outcomes would inherently only further strengthen the V-O association for this procedure.
Conclusion
There is a significant nonlinear relationship between annual institutional PEA procedural volume and early mortality in the treatment of CTEPH by PEA. Long-term survival is also markedly influenced by procedural volume. When combining our findings with previous definitions of high-volume centres in the literature, the minimum annual required case volume that can define a high-volume centre ranges between 33 and 40 cases per year. Whether these analyses justify lowering the previously proposed thresholds remains to be determined based on clinical judgment. The application of such thresholds could optimise outcomes while guaranteeing accessibility of care.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01865-2024.Supplement (1.3MB, pdf)
Shareable PDF
Footnotes
This article has an editorial commentary: https://doi.org/10.1183/13993003.02399-2024
This study was pre-registered in PROSPERO (as CRD42024548185).
Conflict of interest: The authors have no potential conflicts of interest to disclose.
Data availability
All data and coding for analyses will be openly shared through https://github.com/samuelheuts/V-O_in_PEA_for_CTEPH upon publication of this study.
References
- 1.Luijten D, Talerico R, Barco S, et al. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: an updated systematic review and meta-analysis. Eur Respir J 2023; 62: 2300449. doi: 10.1183/13993003.00449-2023 [DOI] [PubMed] [Google Scholar]
- 2.Durrington C, Hurdman JA, Elliot CA, et al. Systematic pulmonary embolism follow-up increases diagnostic rates of chronic thromboembolic pulmonary hypertension and identifies less severe disease: results from the ASPIRE Registry. Eur Respir J 2024; 63: 2300846. doi: 10.1183/13993003.00846-2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2023; 61: 2200879. doi: 10.1183/13993003.00879-2022 [DOI] [PubMed] [Google Scholar]
- 4.Delcroix M, Pepke-Zaba J, D'Armini AM, et al. Worldwide CTEPH registry: long-term outcomes with pulmonary endarterectomy, balloon pulmonary angioplasty, and medical therapy. Circulation 2024; 150: 1354–1365. doi: 10.1161/CIRCULATIONAHA.124.068610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brookes JDL, Li C, Chung STW, et al. Pulmonary thromboendarterectomy for chronic thromboembolic pulmonary hypertension: a systematic review. Ann Cardiothorac Surg 2022; 11: 68–81. doi: 10.21037/acs-2021-pte-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verbelen T, Godinas L, Maleux G, et al. Chronic thromboembolic pulmonary hypertension: diagnosis, operability assessment and patient selection for pulmonary endarterectomy. Ann Cardiothorac Surg 2022; 11: 82–97. doi: 10.21037/acs-2021-pte-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayer E, Jenkins D, Lindner J, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg 2011; 141: 702–710. doi: 10.1016/j.jtcvs.2010.11.024 [DOI] [PubMed] [Google Scholar]
- 8.Guth S, D'Armini AM, Delcroix M, et al. Current strategies for managing chronic thromboembolic pulmonary hypertension: results of the worldwide prospective CTEPH Registry. ERJ Open Res 2021; 7: 00850-2020. doi: 10.1183/23120541.00850-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madani MM, Auger WR, Pretorius V, et al. Pulmonary endarterectomy: recent changes in a single institution's experience of more than 2,700 patients. Ann Thorac Surg 2012; 94: 97–103. doi: 10.1016/j.athoracsur.2012.04.004 [DOI] [PubMed] [Google Scholar]
- 10.Jenkins DP, Tsui SS, Taghavi J, et al. Pulmonary thromboendarterectomy – the Royal Papworth experience. Ann Cardiothorac Surg 2022; 11: 128–132. doi: 10.21037/acs-2021-pte-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vohra AS, Olonoff DA, Ip A, et al. Nationwide trends of balloon pulmonary angioplasty and pulmonary thromboendarterectomy for chronic thromboembolic pulmonary hypertension (2012–2019). Pulm Circ 2024; 14: e12374. doi: 10.1002/pul2.12374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergquist CS, Wu X, McLaughlin VV, et al. Pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension: an STS database analysis. Ann Thorac Surg 2022; 114: 2157–2162. doi: 10.1016/j.athoracsur.2021.11.005 [DOI] [PubMed] [Google Scholar]
- 13.Hobohm L, Keller K, Munzel T, et al. Time trends of pulmonary endarterectomy in patients with chronic thromboembolic pulmonary hypertension. Pulm Circ 2021; 11: 20458940211008069. doi: 10.1177/20458940211008069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klinger JR, Elliott CG, Levine DJ, et al. Therapy for pulmonary arterial hypertension in adults: update of the CHEST guideline and expert panel report. Chest 2019; 155: 565–586. doi: 10.1016/j.chest.2018.11.030 [DOI] [PubMed] [Google Scholar]
- 15.Rajagopal S, Ruetzler K, Ghadimi K, et al. Evaluation and management of pulmonary hypertension in noncardiac surgery: a scientific statement from the American Heart Association. Circulation 2023; 147: 1317–1343. doi: 10.1161/CIR.0000000000001136 [DOI] [PubMed] [Google Scholar]
- 16.Vonlanthen R, Lodge P, Barkun JS, et al. Toward a consensus on centralization in surgery. Ann Surg 2018; 268: 712–724. doi: 10.1097/SLA.0000000000002965 [DOI] [PubMed] [Google Scholar]
- 17.Kawczynski MJ, van Kuijk SMJ, Olsthoorn JR, et al. Type A aortic dissection: optimal annual case volume for surgery. Eur Heart J 2023; 44: 4357–4372. doi: 10.1093/eurheartj/ehad551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Booth A, Clarke M, Dooley G, et al. The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Syst Rev 2012; 1: 2. doi: 10.1186/2046-4053-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan – a web and mobile app for systematic reviews. Syst Rev 2016; 5: 210. doi: 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenkins D, Madani M, Fadel E, et al. Pulmonary endarterectomy in the management of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26: 160111. doi: 10.1183/16000617.0111-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heuts S, Denessen EJS, Daemen JHT, et al. Meta-analysis evaluating high-sensitivity cardiac troponin T kinetics after coronary artery bypass grafting in relation to the current definitions of myocardial infarction. Am J Cardiol 2022; 163: 25–31. doi: 10.1016/j.amjcard.2021.09.049 [DOI] [PubMed] [Google Scholar]
- 23.Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14: 135. doi: 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buis ML. Predict and adjust with logistic regression. Stata Jo 2007; 7: 221–226. doi: 10.1177/1536867X0700700206 [DOI] [Google Scholar]
- 25.Antunes M, Gomes D, Aguiar RL, et al. A. knee/elbow estimation based on first derivative threshold. In: IEEE International Conference on Big Data Computing Service and Applications (BigDataService). Bamberg, Germany, 2018, pp. 237–240. doi: 10.1109/BigDataService.2018.00042 [DOI] [Google Scholar]
- 26.Satopää V, Albrecht J, Irwin D, et al. Finding a kneedle in a haystack: detecting knee points in system behavior. In: IEEE 31st International Distributed Computing Systems Workshops. Bamberg, Germany, 2011; pp. 166–171. [Google Scholar]
- 27.Liu N, Zhou Y, Lee JJ. IPDfromKM: reconstruct individual patient data from published Kaplan–Meier survival curves. BMC Med Res Methodol 2021; 21: 111. doi: 10.1186/s12874-021-01308-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gasperoni F, Ieva F, Paganoni AM, et al. Non-parametric frailty Cox models for hierarchical time-to-event data. Biostatistics 2020; 21: 531–544. doi: 10.1093/biostatistics/kxy071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dartevelle P, Fadel E, Chapelier A, et al. Angioscopic video-assisted pulmonary endarterectomy for post-embolic pulmonary hypertension. Eur J Cardiothorac Surg 1999; 16: 38–43. doi: 10.1016/S1010-7940(99)00116-5 [DOI] [PubMed] [Google Scholar]
- 30.Mercier O, Dubost C, Delaporte A, et al. Pulmonary thromboendarterectomy: the Marie Lannelongue hospital experience. Ann Cardiothorac Surg 2022; 11: 143–150. doi: 10.21037/acs-2021-pte-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delcroix M, Lang I, Pepke-Zaba J, et al. Long-term outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. Circulation 2016; 133: 859–871. doi: 10.1161/CIRCULATIONAHA.115.016522 [DOI] [PubMed] [Google Scholar]
- 32.Astashchanka A, Kerr KM, Yang JZ, et al. Repeat pulmonary thromboendarterectomy outcomes: a 15-year single-center retrospective review. J Thorac Cardiovasc Surg 2023; 166: 1512–1519. doi: 10.1016/j.jtcvs.2023.02.028 [DOI] [PubMed] [Google Scholar]
- 33.Guth S, Wiedenroth CB, Wollenschlager M, et al. Short-term venoarterial extracorporeal membrane oxygenation for massive endobronchial hemorrhage after pulmonary endarterectomy. J Thorac Cardiovasc Surg 2018; 155: 643–649. doi: 10.1016/j.jtcvs.2017.09.045 [DOI] [PubMed] [Google Scholar]
- 34.Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ 2006; 332: 1080. doi: 10.1136/bmj.332.7549.1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cannon JE, Su L, Kiely DG, et al. Dynamic risk stratification of patient long-term outcome after pulmonary endarterectomy: results from the United Kingdom national cohort. Circulation 2016; 133: 1761–1771. doi: 10.1161/CIRCULATIONAHA.115.019470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shetty V, Punnen J, Natarajan P, et al. Experience with pulmonary endarterectomy: lessons learned across 17 years. Asian Cardiovasc Thorac Ann 2022; 30: 532–539. doi: 10.1177/02184923211044035 [DOI] [PubMed] [Google Scholar]
- 37.Yildizeli B, Tas S, Yanartas M, et al. Pulmonary endarterectomy for chronic thrombo-embolic pulmonary hypertension: an institutional experience. Eur J Cardiothorac Surg 2013; 44: e219–e227. doi: 10.1093/ejcts/ezt293 [DOI] [PubMed] [Google Scholar]
- 38.Ende-Verhaar YM, Cannegieter SC, Vonk Noordegraaf A, et al. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: a contemporary view of the published literature. Eur Respir J 2017; 49: 1601792. doi: 10.1183/13993003.01792-2016 [DOI] [PubMed] [Google Scholar]
- 39.Nossent EJ, Meijboom LJ, Bogaard HJ, et al. Chronic thromboembolic pulmonary hypertension anno 2021. Curr Opin Cardiol 2021; 36: 711–719. doi: 10.1097/HCO.0000000000000907 [DOI] [PubMed] [Google Scholar]
- 40.Delcroix M, de Perrot M, Jais X, et al. Chronic thromboembolic pulmonary hypertension: realising the potential of multimodal management. Lancet Respir Med 2023; 11: 836–850. doi: 10.1016/S2213-2600(23)00292-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-01865-2024.Supplement (1.3MB, pdf)
This PDF extract can be shared freely online.
Shareable PDF ERJ-01865-2024.Shareable (749KB, pdf)
Data Availability Statement
All data and coding for analyses will be openly shared through https://github.com/samuelheuts/V-O_in_PEA_for_CTEPH upon publication of this study.
All data and coding for analyses will be openly shared through https://github.com/samuelheuts/V-O_in_PEA_for_CTEPH upon publication of this study.