Abstract
Purpose: To determine the various factors affecting the duration of scleral buckle surgery, the percentage of profitable scleral buckle cases, and the operational break-even point. Methods: This single-center retrospective consecutive series comprised patients diagnosed with primary rhegmatogenous retinal detachment (RD) repaired with scleral buckling between 2019 and 2021. The primary outcome was operative time. Factors associated with longer surgery time were identified using regression analysis. A time-driven activity-based cost analysis was performed. Results: Following are the mean values: duration of 108 primary RD scleral buckle repairs, 106 ± 35 minutes (range, 52-231; median, 98); number of breaks, 2.15 ± 1.5 (range, 0-10); extent of the RD, 4.3 ± 2.0 clock hours (range, 0-9); duration of follow-up with a retina physician, 489 ± 355 days (range, 0-1316). Twenty eyes (19%) required subsequent RD repair. A regression analysis showed the following main risk factors for prolonged duration of RD repair via scleral buckling: number of breaks (β = 5.98; P = .005), use of radial elements (β = 52.09; P = .001), and gas injection (β = 31.27; P < .001). The median cost per case was $7674.64, which was $2713.64 (55%) more than the maximum Medicare reimbursement of $4961.00. The break-even time was 54.43 minutes. Conclusions: Independent risk factors for a prolonged duration of primary scleral buckle surgery include multiple breaks, use of radial elements, and gas injection. These additive steps could justify a separate complex Current Procedural Terminology code. The large majority of cases were not profitable, with losses proportional to operative time. This study demonstrates the clear need for greater reimbursements and economic incentives for scleral buckle surgery.
Keywords: operative time, cost analysis, scleral buckle, retinal detachment
Introduction
Rhegmatogenous retinal detachment (RD) is a sight-threatening condition in which the retina is physically separated from the choroid. 1 Risk factors for RD include older age and myopia.1,2 Although the incidence of RD is debated, a systematic review performed by Mitry et al 3 suggested that the approximate incidence lies between 6.3 and 17.9 per 100 000 population.
The current treatment for RD is primarily surgical, with 3 main options—pneumatic retinopexy, scleral buckling, and vitrectomy.4,5 Pars plana vitrectomy (PPV) is often the preferred intervention.6–9 Compared with scleral buckling, PPV offers better visualization of retinal tears, has better outcomes in aphakic and complex detachments,8,10,11 and reimburses physicians at a higher rate.8,12 Furthermore, PPV can require less operative time.11,13 A study by Brazitikos et al 11 found that the average PPV took 54.7 ± 8.3 minutes while scleral buckle procedures averaged 65.8 ± 9.3 minutes. Conversely, multiple recent studies found no difference in operative times between PPV and scleral buckling when repairing simple macula-on RDs,14–17 as Popovic et al 5 found in a meta-analysis of more than 2500 eyes. Sahanne et al 18 found that scleral buckle operative times were shorter than PPV times. Based on this lack of consensus, it is important to identify which specific factors contribute to prolonged operative times in a modern cohort of patients in an academic setting.
Although the answer remains unclear, physician trends continue to suggest that although less invasive, scleral buckling is often seen as a more complex procedure that requires prolonged operative time 13 and greater surgical skill. As a result, fewer scleral buckle procedures are being performed and fewer fellows are being trained in the technique. 8
Despite the perceived drawbacks, it has been suggested that scleral buckling produces better outcomes for phakic patients and for those with RDs of low or medium complexity.9,19–22 In addition, scleral buckling continues to be the first-line treatment for children and young adults with adherent vitreous. Therefore, scleral buckling retains its indications and ophthalmologists should continue to offer it to the appropriate patients. One aim of the current study was to understand the factors that prolong scleral buckle operative times to improve patient surgical selection, increase fellowship exposure, and guide reimbursement.
Two recent studies have used time-driven activity-based costing to evaluate vitreoretinal surgery.23,24 They found that for routine vitrectomy and RD repair, current Medicare reimbursements were not adequate to cover costs. These methods have not previously been applied to primary scleral buckle surgery. We aimed to determine the proportion of scleral buckle cases that were profitable and determine the operative break-even time.
Methods
The Research Patient Data Registry, a query tool available at Massachusetts Eye and Ear (MEE) through Massachusetts General Brigham, was used to identify eligible patients. Patients who had primary RD repair (operative code 67107) at MEE between January 1, 2019, and January 1, 2022, were included. Exclusion criteria were previous vitrectomy in the operative eye, concurrent bilateral procedures, or any other concomitant procedure (eg, vitrectomy, lensectomy, phacoemulsification). Cases from surgeons who performed fewer than 7 scleral buckle operations during the study were also excluded to establish a baseline for physician procedural and facility familiarity. The study conformed to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board, Mass General Brigham (protocol ID# 2022P001994). The requirement for informed consent was waived given the retrospective nature of the study.
Data collected from operative and clinic notes included the operative time, provider, day of the week, type of anesthesia, surgery completed before or after 5 pm, presence of a clinical fellow assisting in the surgery, macula status (on, off, splitting, or unavailable), presence of proliferative vitreoretinopathy (PVR), operative laterality, presence of lattice, use of radial elements, external drainage of subretinal fluid (SRF), gas injection, cryopexy, anterior chamber paracentesis, need for future same-eye RD repair, and length of follow-up. Operative timepoints were obtained from the intraoperative anesthesia log, which recorded procedure start and stop times. Start time was defined as starting after retrobulbar anesthetic was administered or after intubation. In both local anesthesia and general anesthesia cases, stop time was defined as the closing time. The primary outcome was procedure duration recorded in minutes.
Descriptive analysis of outcomes was performed and presented as the mean ± SD for continuous variables and as percentages for categorical variables. An analysis of variance (ANOVA) test and linear regression analysis were performed to establish the relationship between operative length and the above-listed variables. ANOVA was used to analyze the overall predictive value of the variables. Linear regression was used to parse out these relationships. Clinical significance was set at P < .05. Analysis and descriptive statistics were performed using SPSS software (version 28, SPSS Inc).
Time-driven activity-based costing was performed as previously described. 23 The published process flow map was used to determine the time spent in each setting and the resources used. The personnel costs per time were multiplied by the duration of involvement. The costs were adjusted for inflation. The Supplemental Material shows the specific dollars per resource. The break-even time was determined by developing a formula for cost using the components with a time variable. This was set equal to the Medicare reimbursement for the procedure, and the equation was solved for time.
Results
Of the total 148 scleral buckle procedures identified, 108 cases from 9 surgeons met the inclusion criteria. The 108 procedures were performed on 106 individual patients; 2 patients had the procedure performed in each eye on separate days. The racial breakdown of the cohort was predominantly White (Table 1). The mean patient age was 34.3 ± 13.9 years (range, 15-88), and 57.4% of patients were men.
Table 1.
Demographic Data of 108 Study Patients.
| Variable | Number (%) |
|---|---|
| Sex | |
| Male | 62 (57) |
| Female | 46 (43) |
| Race | |
| White | 69 (64) |
| Black | 8 (7) |
| Asian | 13 (12) |
| Other | 14 (13) |
| Declined/unknown/missing | 4 (4) |
| Ethnicity | |
| Non-Hispanic | 98 (91) |
| Hispanic | 3 (3) |
| Declined/unknown | 7 (6) |
Figure 1 shows a plot of case times and frequencies. The mean procedure duration was 106.4 ± 35.2 minutes (range, 52-231; median, 98). Table 2 shows the operative times for each surgeon. There was a significant difference in the mean procedure duration between surgeons (P < .001). One surgeon (#4) completed all cases with chandelier assistance (12.8% of all cases) with a duration close to the median time. The mean number of retinal breaks was 2.15 ± 1.5 (range, 0-10). Of the 104 cases for which the extent of the RD was described in clock hours, the mean size of the RD was 4.3 ± 2.0 clock hours (range, 0-9). The mean follow-up was 489 ± 356 days (range, 0-1316), with a redetachment rate of 19% (Table 3).
Figure 1.
Histogram showing the number of surgeries performed per surgical length in minutes.
Table 2.
Time per Surgeon.
| Surgeon | Cases (n) | Mean Min/Case | Median Case Time |
|---|---|---|---|
| 1 | 17 | 84.6 | 80.0 |
| 2 | 13 | 89.8 | 84.0 |
| 3 | 12 | 90.8 | 92.5 |
| 4 | 14 | 95.0 | 94.0 |
| 5 | 14 | 98.6 | 94.5 |
| 6 | 8 | 101.9 | 101.5 |
| 7 | 12 | 112.6 | 109.5 |
| 8 | 11 | 141.2 | 145.0 |
| 9 | 7 | 195.3 | 189.0 |
Table 3.
Procedural Findings and Follow-up Length.
| Variable | Minimum | Maximum | Mean ± SD |
|---|---|---|---|
| Age (y) | 15 | 88 | 34.3 ± 13.9 |
| Procedure duration (min) | 52 | 231 | 106.4 ± 35.2 |
| Breaks (n) | 0 | 10 | 2.2 ± 1.5 |
| Detachment clock hours | 0 | 9 | 4.3 ± 2 |
| Follow-up (d) | 0 | 1316 | 489.3 ± 355.8 |
Statistical Analysis
The regression model was significant (F(19,75) = 5.515; P < .001) with an R2 value of 0.583. Given these findings, a linear regression analysis was performed to further elaborate relationships between each variable and the operative time. These variables were age, provider, day of the week, type of anesthesia, surgery completed before or after 5 pm, presence of a clinical fellow assisting in the surgery, macula status, presence of PVR, operative laterality, presence of lattice, number of breaks, extent of the RD in clock hours, the use of radial elements, external drainage of SRF, gas injection, cryopexy, and anterior chamber paracentesis.
The number of retinal breaks (β = 5.98; P = .005), the use of radial elements (β = 52.09; P = .001), and the use of gas tamponade (β = 31.27; P < .001) were significantly associated with a longer operative time (Table 4). The other factors, including surgeon, were not significantly associated with operative time.
Table 4.
Factors Associated with Greater Surgery Time Identified Using Linear Regression Analysis.
| Variable | Unstandardized β | P Value | 95% CI |
|---|---|---|---|
| Fellow present | 18.23 | .11 | −4.44, 40.9 |
| Macula status | −7.77 | .14 | −18.2, 2.7 |
| PVR | 1.84 | .85 | −17.5, 21.3 |
| Number of breaks | 5.98 | .01 | 1.8, 10.1 |
| Lattice present | 2.02 | .73 | −9.7, 13.7 |
| Total RD clock hours | 1.72 | .3 | −1.6, 5.0 |
| Radial elements used | 52.09 | .001 | 21.6, 82.6 |
| Gas injection | 31.27 | <.001 | 13.5, 49.1 |
| Anterior chamber tap | −6.45 | .25 | −17.4, 4.5 |
Abbreviations: PVR, proliferative vitreoretinopathy; RD, retinal detachment.
The linear regression analysis for the number of retinal breaks yielded an unstandardized β value of 6 and an error of 2.1. This suggests that on average, for each additional retinal break, the operative time increased by 6.0 ± 2.1 minutes. The standard coefficient β value of 0.25 suggests that for every SD increase in the number of retinal breaks (1.5 retinal breaks), the operative time increased by 0.25 SDs (8.8 minutes). Given the mean operative time of approximately 106 minutes, this finding suggests that each additional retinal break was associated with a 5.6% increase in operative time (P = .005).
The use of radial elements was analyzed as a binomial variable making the standardized coefficient of greater interest. The β value of 0.29 suggests that the use of radial elements was associated with an increase in operative time by 0.29 SDs (10.2 minutes). Given the mean operative time of approximately 106 minutes, this analysis suggests that the use of radial elements was associated with a 9.6% increase in operative time (P = .001).
Last, the use of gas tamponade was also analyzed as a binomial. Therefore, the statistical interpretation follows that of the radial elements. The standardized coefficient of 0.32 suggests that procedures in which gas injection was used were associated with an increase in operative time by 0.32 SDs (11.3 minutes). Given the mean operative time of approximately 106 minutes, this finding suggests that the use of gas injection is associated with a 10.7% increase in operative time (P < .001).
Cost Analysis
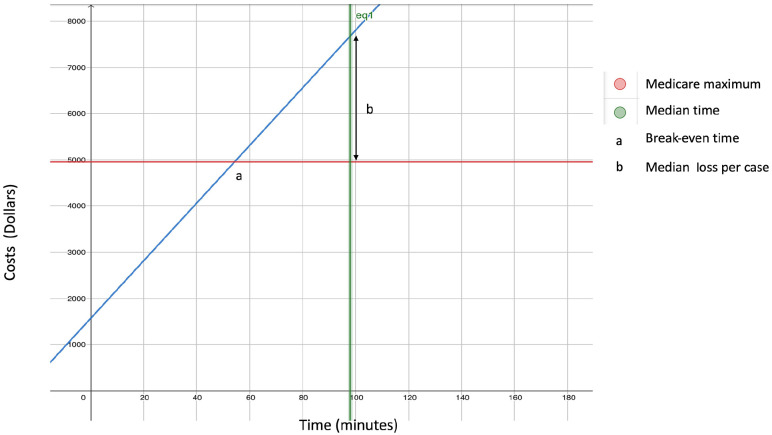
Figure 2 shows the plot of costs over time. The total preoperative, postoperative, and nonvariable operating room costs per case were $94.66, $547.56, $928.00, respectively. The total additional cost per operating room minute was $62.29. The following formula was created: y = (94.66 + 547.56 + 928.00) + (62.29x), where x is operative time in minutes and y is the total cost in dollars.
Figure 2.
Graph showing the cost of scleral buckle surgery vs surgical length.
The maximum allowable Medicare reimbursement in 2024 for CPT 67017 was $4961.00. The median estimated cost in the current series was $7674.64, which resulted in a $2713.64 loss per case. The cost was 55% more than the reimbursement. The break-even time was 54.43 minutes. In 1 case in this study, the operative time was less than the break-even time, showing that 99.1% of the included cases were unprofitable.
Conclusions
In this study, the key findings were as follows:
The number of retinal breaks, the use of radial elements, and the use of gas tamponade contributed to prolonged scleral buckle operative times.
The Medicare reimbursement was significantly less than the median procedural costs.
The break-even time was 54.43 minutes.
Over the past 20 years, there has been a gradual shift away from scleral buckling toward PPV for the treatment of RD.6–9 This shift in practice has resulted in reduced exposure to scleral buckling for vitreoretinal surgery fellows throughout their training.8–10 Despite these trends, scleral buckling has been shown to achieve high single-operation success and is the operation of choice in RD in young patients and phakic patients,9,19–22,25–29 showing superiority over PPV in preventing cataract progression 19 and achieving better best-corrected visual acuity. 27
Operative time is a critical component to surgical planning, efficiency, patient satisfaction, and net cost recovery. Most RD repairs are performed under local anesthesia. Unlike surgical procedures performed under general anesthesia, patients with RD more actively experience their procedures. The patient experience is therefore affected by operative time, with shorter procedure durations being preferred. Furthermore, prolonged exposure to operative light has been shown to damage the retina.28–31
The reported results on comparative operative times between scleral buckling and PPV are mixed (Table 5).7,10,11,15–18,21,30–36 Recent data from 2022 suggest that when repairing simple phakic macula-on RDs, there is no difference in operative time between PPV and scleral buckling.14–17 Furthermore, reported operative times have wide ranges. A review of the literature found a range in mean scleral buckle operative times from 65.8 minutes 11 to 105.1 minutes. 30 The mean scleral buckle operative time in our study was 106.4 ± 35.2 minutes (range, 52-231; median, 98.5 minutes). The skew toward longer operative times in our study may be a consequence of the higher complexity and involvement of trainees in an academic surgical environment. 37 A study by Baldwin et al 10 collected scleral buckle operative times from a single surgeon at our academic center. They also found longer operative times (median, 107 minutes) than those reported in the literature. Meanwhile, mean PPV operative times ranged from 54.69 ± 8.3 minutes 11 to 95.9 minutes. 21
Table 5.
Literature on the Operative Time of Scleral Buckle Surgery and PPV.
| Mean Operative Time (Min) ± SD | |||
|---|---|---|---|
| Author a | Article Title | Scleral Buckling | PPV |
| Brazitikos 11 | Primary Pars Plana Vitrectomy Versus Scleral Buckle Surgery for the Treatment of Pseudophakic Retinal Detachment: A Randomized Clinical Trial | 65.8 ± 9.34 | 54.69 ± 8.3 |
| Ho 30 | Selection of Scleral Buckling for Primary Retinal Detachment | 76.4 ± 22.3 (radial segmental) | — |
| Ho 30 | Selection of Scleral Buckling for Primary Retinal Detachment | 78.1 ± 24.0 (circumferential segmental) | — |
| Ho 30 | Selection of Scleral Buckling for Primary Retinal Detachment | 105.1 ± 21.8 (encircling) | — |
| Ho 30 | Selection of Scleral Buckling for Primary Retinal Detachment | 77.0 ± 22.8 (segmental) | — |
| Singh 31 | Pneumoretinopexy Versus Scleral Buckling in Retinal Detachments with Superior Breaks: A Comparative Analysis of Outcome and Cost | 97 ± 62.8 | — |
| Angerman 16 | Efficiency Benchmarks in the Surgical Management of Primary Rhegmatogenous Retinal Detachment: A Monocentric Register Cohort Study of Operating Room Time Metrics and Influential Factors | 62.1 ± 24.6 (retrobulbar block) | 74.0 ± 32.6 (retrobulbar anesthesia) |
| Angerman 16 | Efficiency Benchmarks in the Surgical Management of Primary Rhegmatogenous Retinal Detachment: A Monocentric Register Cohort Study of Operating Room Time Metrics and Influential Factors | 76.0 ± 22.5 (general anesthesia) | 112.0 ± 52.0 (general anesthesia) |
| Dhoot 15 | Pars Plana Vitrectomy Versus Scleral Buckle: A Comprehensive Meta-analysis of 15,947 Eyes | 66.4 ± 28.0 | 75.8 |
| Baldwin 10 | A Comparative Study of Traditional Scleral Buckling to a New Technique: Guarded Light Pipe with Heads-Up Three-Dimensional Visualization | Median, 107 Range, 94, 123 |
— |
| Baldwin 10 | A Comparative Study of Traditional Scleral Buckling to a New Technique: Guarded Light Pipe with Heads-Up Three-Dimensional Visualization | Median, 113 Range, 100, 135 (with drainage of SRF) |
— |
| Ciulla 7 | Evolution of Primary Scleral Buckling Surgery: A Modified Lean Six Sigma Technique to Improve Surgical Efficiency | 86.7 | — |
| Governatori 32 | Chandelier-Assisted Scleral Buckling: A Literature Review | 102.48 ± 43.76 | — |
| Kawano 14 | Scleral Buckling Versus Pars Plana Vitrectomy in Simple Phakic Macula-on Retinal Detachment: A Propensity Score-Matched, Registry-Based Study | 72 | 71 |
| Mafi 33 | Modified Encircling Scleral Buckle Technique Without Subretinal Fluid Drainage or Retinopexy | 72.2 ± 13.2 | — |
| Narayanan 34 | Scleral Buckling with Wide-Angled Endoillumination As a Surgical Educational Tool | 95.71 ± 26.59 | — |
| Jo 35 | Scleral Buckling Using a Non-contact Wide-Angle Viewing System with a 25-Gauge Chandelier Endoilluminator | 76.8 ± 16.1 | — |
| Park 36 | Comparison of Scleral Buckling and Vitrectomy Using Wide Angle Viewing System for Rhegmatogenous Retinal Detachment in Patients Older Than 35 Years | — | 60.8 |
| Ahmadieh 21 | Anatomic and Visual Outcomes of Scleral Buckling versus Primary Vitrectomy in Pseudophakic and Aphakic Retinal Detachment: Six-Month Follow-up Results of a Single Operation—Report No. 1 | — | 95.9 |
| Sahanne 18 | A Retrospective Study Comparing Outcomes of Primary Rhegmatogenous Retinal Detachment Repair by Scleral Buckling and Pars Plana Vitrectomy in Finland | — | 78 |
| Hong 17 | Comparison of Scleral Buckling and Vitrectomy Using Wide Angle Viewing System for Rhegmatogenous Retinal Detachment | — | 78.16 ± 27.6 |
Abbreviations: PPV, pars plana vitrectomy; SRF, subretinal fluid.
First author.
Table 4 shows a full list of scleral buckle and PPV operative times. Factors contributing to this wide range in reported values likely included patient selection, case complexity, surgeon familiarity, anesthesia choice, and type of scleral buckle used. Overall, it appears that scleral buckle operative times in our study are greater than in most reports of PPV operative times.
Longer operating times associated with the scleral buckle procedure may have contributed to the trend away from the procedure. 13 This makes it critical to understand what factors prolong scleral buckle operative times. The study by Baldwin et al 10 found that drainage of SRF was associated with longer operative times (113 minutes vs 93 minutes; P = .035). A study by Ho et al 30 found that the use of an encircling band as opposed to a radial, circumferential, or segmental band increased the operative time from 77 minutes to 108 minutes.
To our knowledge, ours is the first study to report that factors prolonging scleral buckle procedures also include the number of breaks in the retina (β = 5.98; P = .005), the use of radial elements (β = 52.09; P = .001), and the use of gas tamponade to flatten the retina (β = 31.27; P < .001). In contrast to results reported by Baldwin et al, 10 our study did not find a statistically significant difference in operating time caused by the drainage of SRF. On average, each additional retinal break, the use of radial elements, and gas injection increased the operative time by 6.0 minutes, 10.2 minutes, and 11.3 minutes, respectively. These findings contribute to our understanding of the wide range of reported scleral buckle operative times. It could be argued that an RD repaired by scleral buckling that requires cryotherapy to multiple areas, gas injection, and/or an element should be reimbursed at a higher rate or be associated with a different Current Procedural Terminology (CPT) code that reflects the level of complexity.
Some variables, such as the status of the macula, the presence of PVR, external drainage of SRF, and performing an anterior chamber tap, were not significantly associated with longer operative times. It is possible that although each of these steps adds to the operative time, the amount of time added was negligible or the study was not sufficiently powered to assess their contributions.
In terms of finances, this study is the first to our knowledge to use time-driven activity-based costing to assess profitability and determine the break-even time of scleral buckle surgery. Time-driven activity-based costing is a helpful tool to calculate costs because it may represent true costs more accurately by accounting for both personnel and operative resources. It is concerning that more than 99% of cases in this study were longer than the break-even time, making them unprofitable for their level of Medicare reimbursement. The break-even time may be achievable with the right patient and in the right setting; however, it is far less than the average time and median time required in an academic setting. This evaluation builds on the previous time-driven activity-based costing analyses for vitreoretinal surgery. Berkowitz et al 23 found an average loss of $2053.85 per case for vitrectomy, while Pan et al 24 found a net negative margin of $976.93 for RD repaired with PPV, increasing to $3271.10 in complex cases.
In our study, a median loss per scleral buckle case of $2713.64 echoes previous sentiments that surgery is being under-reimbursed. Specifically, scleral buckle repairs in this series were under-reimbursed by 55%. Our institution has seen a rise in referrals by vitreoretinal specialists for routine surgical cases. Economic pressures from opportunity costs 38 and the availability of resources may be playing a role in this phenomena. The reimbursement was based on a hospital outpatient department setting rather than an ambulatory surgical center, where reimbursement is lower (facility fee for CPT code 67107 is $2045 in ambulatory surgical centers vs $3873 in outpatient departments). Although the costs may be marginally less in an ambulatory surgical setting, it is possible that the decrease in costs is not proportional to the decrease in reimbursements and that the net loss could be even greater than what was reported in this cohort.
Physicians can use these data to better predict operative time and guide surgical and anesthesia selection. This retrospective study was conducted using data collected from surgeons operating at a single center; therefore, the population in this study skews toward the population treated at this tertiary hospital. Further studies should aim to collect a more diverse patient population to improve the external validity of the study.
In an attempt to increase the power of the study, surgeons who performed at least 7 scleral buckle procedures between 2019 and 2021 were included. It is possible that setting this threshold higher may have reduced operative times because the cohort of surgeons could have greater facility with scleral buckle surgery.
Last, it is possible that the lack of associations found between macula status, PVR, external drainage of SRF, and anterior chamber taps may be the result of a type II error. Specific to the cost analysis, there are inherent limitations and organizational variations in estimating the cost of components, wages, and overhead that could potentially be improved by advancements in perioperative process efficiency. Moreover, variations are anticipated given the differences in population demographics and payer mix. Taking these factors into consideration is imperative for effective resource allocation and policy formulation across the entire spectrum of surgical care. 39
In summary, the number of retinal breaks, the use of radial elements, and the addition of gas tamponade were found to be independent factors that prolong the scleral buckle procedure. These extra steps could justify a separate complex CPT code. The results show that the large majority of cases were not profitable, with losses proportional to the operative time. There is a need for higher reimbursements and economic incentives to reverse the national trend away from scleral buckle surgery.
Supplemental Material
Supplemental material, sj-docx-1-vrd-10.1177_24741264241293904 for Operative Times in Scleral Buckle Surgery: Influencing Factors and Cost Analysis by Jonah Blumenthal, Ryan S. Meshkin, Sandra Hoyek, Yilin Feng and Nimesh A. Patel in Journal of VitreoRetinal Diseases
Footnotes
Ethical Approval: This study was conducted in accordance with the Declaration of Helsinki. The collection and evaluation of all protected patient health information were performed in a US Health Insurance Portability and Accountability Act–compliant manner. This study was approved by the Institutional Review Board, Massachusetts General Brigham (protocol ID# 2022P001994)
Statement of Informed Consent: The requirement for informed consent was waived given the retrospective nature of the study.
Dr. Patel is a consultant to Regeneron, Dutch Ophthalmic, Genentech, EyePoint Pharmaceuticals, and Alcon Vision. None of the other authors declared potential conflicts of interest with respect to the research, authorship, and/or publication of the article.
Funding: Dr. Hoyek is supported by the VitreoRetinal Surgery Foundation. Dr. Patel is supported by the Retina Innovation Fund, Massachusetts Eye and Ear, Boston, MA, USA. The funding organizations had no role in design or conduct of this research.
ORCID iD: Nimesh A. Patel  https://orcid.org/0000-0002-6681-6104
https://orcid.org/0000-0002-6681-6104
Supplemental Material: Supplemental material is available online with this article.
References
- 1. Ghazi NG, Green WR. Pathology and pathogenesis of retinal detachment. Eye (Lond). 2002;16(4):411-421. doi: 10.1038/sj.eye.6700197 [DOI] [PubMed] [Google Scholar]
- 2. Polkinghorne PJ, Craig JP. Northern New Zealand Rhegmatogenous Retinal Detachment Study: epidemiology and risk factors. Clin Exp Ophthalmol. 2004;32(2):159-163. doi: 10.1111/j.1442-9071.2004.00003.x [DOI] [PubMed] [Google Scholar]
- 3. Mitry D, Charteris DG, Fleck BW, Campbell H, Singh J. The epidemiology of rhegmatogenous retinal detachment: geographical variation and clinical associations. Br J Ophthalmol. 2010;94(6):678-684. doi: 10.1136/bjo.2009.157727 [DOI] [PubMed] [Google Scholar]
- 4. Soni C, Hainsworth DP, Almony A. Surgical management of rhegmatogenous retinal detachment: a meta-analysis of randomized controlled trials. Ophthalmology. 2013;120(7):1440-1447. doi: 10.1016/j.ophtha.2012.12.033 [DOI] [PubMed] [Google Scholar]
- 5. Popovic MM, Muni RH, Nichani P, Kertes PJ. Pars plana vitrectomy, scleral buckle, and pneumatic retinopexy for the management of rhegmatogenous retinal detachment: a meta-analysis. Surv Ophthalmol. 2022;67(1):184-196. doi: 10.1016/j.survophthal.2021.05.008 [DOI] [PubMed] [Google Scholar]
- 6. Reeves MG, Pershing S, Afshar AR. Choice of primary rhegmatogenous retinal detachment repair method in US commercially insured and medicare advantage patients, 2003-2016. Am J Ophthalmol. 2018;196:82-90. doi: 10.1016/j.ajo.2018.08.024 [DOI] [PubMed] [Google Scholar]
- 7. Ciulla TA, Hariprasad SM, Hussain RM, Townsend JH. Evolution of primary scleral buckling surgery: a modified lean six sigma technique to improve surgical efficiency. Ophthalmic Surg Lasers Imaging Retina. 2020;51(5):256-261. doi: 10.3928/23258160-20200501-02 [DOI] [PubMed] [Google Scholar]
- 8. Cruz-Pimentel M, Huang CY, Wu L. Scleral buckling: a look at the past, present and future in view of recent findings on the importance of photoreceptor re-alignment following retinal re-attachment. Clin Ophthalmol Auckl NZ. 2022;16:1971-1984. doi: 10.2147/OPTH.S359309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fallico M, Alosi P, Reibaldi M, et al. Scleral buckling: a review of clinical aspects and current concepts. J Clin Med. 2022;11(2):314. doi: 10.3390/jcm11020314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baldwin G, Sokol JT, Ludwig CA, Miller JB. A comparative study of traditional scleral buckling to a new technique: guarded light pipe with heads-up three-dimensional visualization. Clin Ophthalmol Auckl NZ. 2022;16:3079-3088. doi: 10.2147/OPTH.S378179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brazitikos PD, Androudi S, Christen WG, Stangos NT. Primary pars plana vitrectomy versus scleral buckle surgery for the treatment of pseudophakic retinal detachment: a randomized clinical trial. Retina. 2005;25(8):957-964. [DOI] [PubMed] [Google Scholar]
- 12. Sodhi A, Leung L-S, Do DV, Gower EW, Schein OD, Handa JT. Recent trends in the management of rhegmatogenous retinal detachment. Surv Ophthalmol. 2008;53(1):50-67. doi:10.1016/j.survophthal.2007.10.007 [DOI] [PubMed] [Google Scholar]
- 13. Young BK, Zacks DN. The role of scleral buckling in 2021. Retina Today. March 2021. Accessed July 11, 2023. https://retinatoday.com/articles/2021-mar/the-role-of-scleral-buckling-in-2021
- 14. Kawano S, Imai T, Sakamoto T. Scleral buckling versus pars plana vitrectomy in simple phakic macula-on retinal detachment: a propensity score-matched, registry-based study. Br J Ophthalmol. 2022;106(6):857-862. doi: 10.1136/bjophthalmol-2020-318451 [DOI] [PubMed] [Google Scholar]
- 15. Dhoot AS, Popovic MM, Nichani PAH, et al. Pars plana vitrectomy versus scleral buckle: a comprehensive meta-analysis of 15,947 eyes. Surv Ophthalmol. 2022;67(4):932-949. doi: 10.1016/j.survophthal.2021.12.005 [DOI] [PubMed] [Google Scholar]
- 16. Angermann R, Huber AL, Hofer M, et al. Efficiency benchmarks in the surgical management of primary rhegmatogenous retinal detachment: a monocentric register cohort study of operating room time metrics and influential factors. BMJ Open. 2021;11(12):e052513. doi: 10.1136/bmjopen-2021-052513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hong IH, Jeon GS, Han JR. Comparison of scleral buckling and vitrectomy using wide angle viewing system for rhegmatogenous retinal detachment. Semin Ophthalmol. 2020;35(5-6):307-312. doi: 10.1080/08820538.2020.1842468 [DOI] [PubMed] [Google Scholar]
- 18. Sahanne S, Tuuminen R, Haukka J, Loukovaara S. A retrospective study comparing outcomes of primary rhegmatogenous retinal detachment repair by scleral buckling and pars plana vitrectomy in Finland. Clin Ophthalmol. 2017;11:503-509. doi: 10.2147/OPTH.S128746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heimann H, Bartz-Schmidt KU, Bornfeld N, et al. Scleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment: a prospective randomized multicenter clinical study. Ophthalmology. 2007;114(12):2142-2154. doi: 10.1016/j.ophtha.2007.09.013 [DOI] [PubMed] [Google Scholar]
- 20. Adelman RA, Parnes AJ, Ducournau D; European Vitreo-Retinal Society (EVRS) Retinal Detachment Study Group. Strategy for the management of uncomplicated retinal detachments: the European vitreo-retinal society retinal detachment study report 1. Ophthalmology. 2013;120(9):1804-1808. doi: 10.1016/j.ophtha.2013.01.070 [DOI] [PubMed] [Google Scholar]
- 21. Ahmadieh H, Moradian S, Faghihi H, et al. Anatomic and visual outcomes of scleral buckling versus primary vitrectomy in pseudophakic and aphakic retinal detachment: six-month follow-up results of a single operation—report no. 1. Ophthalmology. 2005;112(8):1421-1429. doi: 10.1016/j.ophtha.2005.02.018 [DOI] [PubMed] [Google Scholar]
- 22. Ung T, Comer MB, Ang AJS, et al. Clinical features and surgical management of retinal detachment secondary to round retinal holes. Eye (Lond). 2005;19(6):665-669. doi: 10.1038/sj.eye.6701618 [DOI] [PubMed] [Google Scholar]
- 23. Berkowitz ST, Sternberg P, Patel S. Cost analysis of routine vitrectomy surgery. Ophthalmol Retina. 2021;5(6):496-502. doi: 10.1016/j.oret.2021.02.003 [DOI] [PubMed] [Google Scholar]
- 24. Pan WW, Portney DS, Mian SI, Rao RC. The cost of standard and complex pars plana vitrectomy for retinal detachment repair exceeds its reimbursement. Ophthalmol Retina. 2023;7(11):948-953. doi: 10.1016/j.oret.2023.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lincoff H, Kreissig I. Extraocular repeat surgery of retinal detachment. A minimal approach. Ophthalmology. 1996;103(10):1586-1592. doi: 10.1016/s0161-6420(96)30459-4 [DOI] [PubMed] [Google Scholar]
- 26. Ryan EH, Jr, Mittra RA. Scleral buckling vs vitrectomy: the continued role for scleral buckling in the vitrectomy era. Arch Ophthalmol. 2010;128(9):1202-1205. doi: 10.1001/archophthalmol.2010.192 [DOI] [PubMed] [Google Scholar]
- 27. Solaiman KAM, Dabour SA. Supplemental scleral buckling for inferior retinal detachment in silicone oil-filled eyes. Retina. 2014;34(6):1076-1082. doi: 10.1097/IAE.0000000000000037 [DOI] [PubMed] [Google Scholar]
- 28. Tillery WV, Lucier AC. Round atrophic holes in lattice degeneration–an important cause of phakic retinal detachment. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1976;81(3 Pt 1):509-518. [PubMed] [Google Scholar]
- 29. Velez-Montoya R, Jacobo-Oceguera P, Flores-Preciado J, et al. Primary repair of moderate severity rhegmatogenous retinal detachment: a critical decision-making algorithm. Med Hypothesis Discov Innov Ophthalmol. 2016;5(1):18-31. [PMC free article] [PubMed] [Google Scholar]
- 30. Ho CL, Chen KJ, See LC. Selection of scleral buckling for primary retinal detachment. Ophthalmologica. 2002;216(1):33-39. doi: 10.1159/000048294 [DOI] [PubMed] [Google Scholar]
- 31. Singh A, Behera U. Pneumoretinopexy versus scleral buckling in retinal detachments with superior breaks: a comparative analysis of outcome and cost. Indian J Ophthalmol. 2021;69(2):314. doi: 10.4103/ijo.IJO_1574_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Governatori L, Scampoli A, Culiersi C, et al. Chandelier-assisted scleral buckling: a literature review. Vision (Basel). 2023;7(3):47. doi: 10.3390/vision7030047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mafi M, Mirghorbani M, Ghahvehchian H, et al. Modified encircling scleral buckle technique without subretinal fluid drainage or retinopexy. Ophthalmol Ther. 2020;9(3):641-651. doi: 10.1007/s40123-020-00279-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Narayanan R, Tyagi M, Hussein A, Chhablani J, Apte RS. Scleral buckling with wide-angled endoillumination as a surgical educational tool. Retina. 2016;36(4):830-833. doi: 10.1097/IAE.0000000000000792 [DOI] [PubMed] [Google Scholar]
- 35. Jo J, Moon BG, Lee JY. Scleral buckling using a non-contact wide-angle viewing system with a 25-gauge chandelier endoilluminator. Korean J Ophthalmol. 2017;31(6):533-537. Accessed July 11, 2023. https://pubmed.ncbi.nlm.nih.gov/29230977/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Park SW, Kwon HJ, Kim HY, Byon IS, Lee JE, Oum BS. Comparison of scleral buckling and vitrectomy using wide angle viewing system for rhegmatogenous retinal detachment in patients older than 35 years. BMC Ophthalmology. 2015;15:121. Accessed August 17, 2023. https://www.proquest.com/docview/1780048303/abstract/C8DD435A306242B3PQ/1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sagong M, Chang W. Learning curve of the scleral buckling operation: lessons from the first 97 cases. Ophthalmologica. 2010;224(1):22-29. doi: 10.1159/000233232 [DOI] [PubMed] [Google Scholar]
- 38. Leung EH, Patel S, Reddy R, et al. Opportunity cost of vitreoretinal surgeries. J Vitreoretin Dis. 2023;7(4):275-280. doi: 10.1177/24741264231178590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Anaya JA, Regillo CD, Haller JA. Re: Berkowitz et al: Cost analysis of routine vitrectomy surgery (Ophthalmology Retina. 2021;5:496-502). Ophthalmol Retina. 2021;5(7):e8-e9. doi: 10.1016/j.oret.2021.04.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-vrd-10.1177_24741264241293904 for Operative Times in Scleral Buckle Surgery: Influencing Factors and Cost Analysis by Jonah Blumenthal, Ryan S. Meshkin, Sandra Hoyek, Yilin Feng and Nimesh A. Patel in Journal of VitreoRetinal Diseases