Abstract
Background:
It is unclear whether extended lymphadenectomy is associated with improved disease-free and overall survival compared to standard lymphadenectomy in patients with localized muscle invasive bladder cancer undergoing radical cystectomy.
Methods:
Patients with localized muscle invasive bladder cancer cT2–4a N0–2 were randomized intra-operatively 1:1 to bilateral standard lymphadenectomy or extended lymphadenectomy involving removal of common iliac, pre-sciatic, and pre-sacral nodes. Patients were stratified by receipt and type of neoadjuvant chemotherapy, T2 vs. T3–4a and Performance status 0–1 vs. 2. The primary outcome was disease free survival.
Results:
Of 658 patients registered, 592 eligible patients were randomized to extended (n=292) or standard (n=300) lymphadenectomy, performed by 36 surgeons at 27 sites in the US and Canada. Neoadjuvant chemotherapy was given to 57%. At median follow-up 6.1 years, the number of patients who had recurrence or death was 130 (44.5%) with extended lymphadenectomy versus 127(42.3%) with standard lymphadenectomy. Estimated 5-year disease-free survival probability was 56% and 60%, respectively (HR 1.10; 95% CI 0.86, 1.40; p=0.45), and overall survival was 59% and 63%, respectively (HR 1.13; 95% CI 0.88; 1.45).. Grade 3–5 adverse events occurred in 157 (54%) of extended lymphadenectomy patients vs. 132 (44%) with standard lymphadenectomy; deaths within 90 days of surgery occurred in 19 (6.5%) vs. 7 (2.3%), respectively.
Conclusions:
Compared to standard lymphadenectomy, extended lymphadenectomy did not result in improved disease-free or overall survival for patients with curable muscle invasive bladder cancer undergoing radical cystectomy and was associated with increased peri-operative morbidity and mortality. (Funding: NIH/NCI and Canadian Cancer Society; NCT01224665)
Introduction
Bilateral pelvic lymphadenectomy is an essential component of radical cystectomy as it provides local control, accurately identifies pathologic nodal metastases, and is associated with long-term disease-free survival for some patients with proven nodal metastases (1). Despite no randomized trials confirming a benefit to more extensive lymphadenectomy, many academic centers have adopted this approach, with a cranial limit extending, outside of the pelvic cavity, up to the inferior mesenteric artery (IMA) (1–5). The average number of lymph nodes removed at these centers is 20–30 and it was proposed that a minimum number of lymph nodes (~25) as a surrogate for an extended template dissection should be considered a quality assurance measure for this operation (6). Nonetheless, in a large multi-institutional trial that demonstrated survival benefit of neoadjuvant chemotherapy before radical cystectomy, 9% of patients at major academic centers had no lymph node dissection and 37% underwent a limited dissection (obturator nodes only) (2). Moreover, population-based data from the SEER program cancer registry found that 40% of patients had a lymphadenectomy omitted during radical cystectomy (7). In a more recent analysis of SEER data (8), patients who underwent pelvic lymphadenectomy (performed in 81%, with the median yield 14 lymph nodes), had better cancer specific survival than those who did not undergo pelvic lymphadenectomy (HR 0.56; 95%CI 0.51–0.62). With recent increased attention to this important quality measure, two randomized trials of open vs. robotic radical cystectomy demonstrated a mean number of sampled nodes of 15–16 and 23–26, respectively (9,10).
Data from several retrospective series and one prospective observational study suggested that compared to a limited pelvic lymphadenectomy, an extended pelvic lymphadenectomy was associated with a survival benefit (11). In another report, as compared with extended lymphadenectomy, limited LND was associated with understaging of LN metastasis and lower 5-year recurrence survival (12). However, conclusions from these studies are limited by biases inherent in observational studies. Adoption of a more extensive lymphadenectomy became standard of care in many high-volume centers despite the low-level evidence supporting this approach (13). Of concern is the potential for increased post-operative morbidity associated with an extended node dissection that could outweigh any oncologic benefit. Randomized phase III trials in endometrial, gastric, and pancreatic cancers, did not show improved survival with extended lymphadenectomy contrary to what was expected, and with gastric cancer surgery-related complications were increased with the more extensive lymphadenectomy (14,15),
We conducted a randomized-controlled phase III trial of extended (ELND) versus standard (SLND) pelvic lymphadenectomy (LND) during RC for patients with MIBC with the primary objective to compare disease-free survival (DFS).
Methods
SWOG S1011 was reviewed and approved by the NCI Central Institutional Review Board (CIRB); patients provided written informed consent; it was conducted according to the Declaration of Helsinki guidelines. Eligible patients had predominant urothelial cancer clinical stage T2–4a, N0–2 (AJCC 7th edition, 2010) and elected radical cystectomy with curative intent. Exclusion criteria included prior partial cystectomy, prior pelvic surgery obviating a complete extended lymphadenectomy (e.g. aorto-femoral/iliac bypass) or surgeon-determined inability to perform a thorough pelvic node dissection. Prior pelvic radiation was not allowed. Patients were allowed neoadjuvant chemotherapy (NAC) completed within 70 days of registration. Surgery was scheduled within 28 days of registration. For patients who did not receive NAC, postoperative adjuvant chemotherapy for pT any, N+ or pT3–4, N0 was recommended but not required.
This trial was offered to institutions with experienced urologic oncology surgeons. Each performed ≥ 50 RCs over the previous 3 years and at least 30 per year by all surgeons at their respective hospital. Each surgeon underwent rigorous credentialing prior to registering their first patient and submitted operative and pathology reports and representative intra-operative photos from five recent RCs to verify compliance with the study protocol. Submissions were approved by the credentialing committee (ES chair, TK, RS, SL) prior to registering patients.
Surgical procedure
Intraoperative exploration was carried out prior to randomization to verify absence of T4b disease and no evidence of pelvic lymph node (LN) involvement (confirmed by frozen section if suspicious) at or above the bifurcation of the common iliac (CI) vessels. Patients were then randomized 1:1, and dynamically balanced by three stratification factors (clinical stage, performance status and receipt of neoadjuvant chemotherapy; see Fig 1), to either bilateral SLND or bilateral extended lymphadenectomy. SLND included external and internal iliac and obturator nodes. All potential lymph node bearing tissue was to be removed within the boundaries including the genitofemoral nerve laterally; distally Cooper’s ligament including the lymph node of Cloquet; proximally the CI bifurcation; medially the bladder to include the tissue medial to the hypogastric artery; and posteriorly the floor of the obturator fossa with circumferential mobilization of the external iliac artery and vein unless contraindicated due to extensive atherosclerotic vascular disease. ELND also included the node bearing tissue in the pre-sciatic region (fossa of Marcille) and pre-sacral nodes including the node-bearing tissue anterior to the left CI vein and medial to the CI arteries up to the bifurcation of the aorta. The node dissection could be extended proximally up to the inferior mesenteric artery (IMA) to include the distal paracaval, pre-caval, pre-aortic and para-aortic lymph nodes according to surgeon preference.
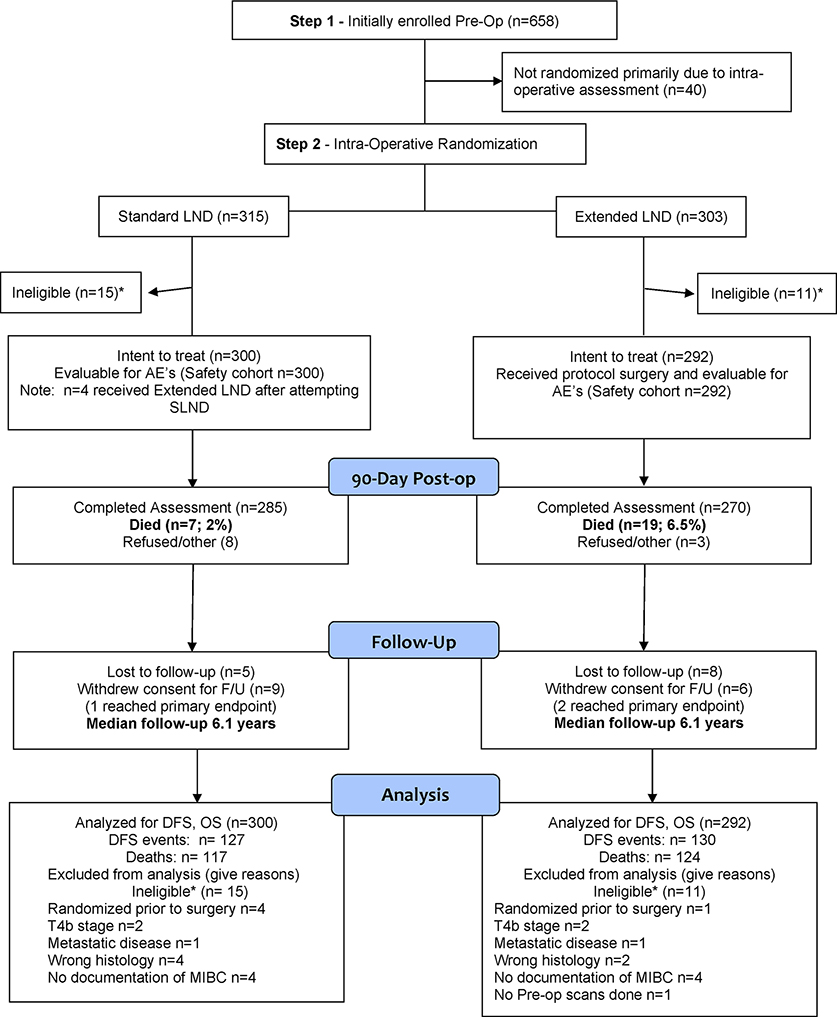
Figure 1.
CONSORT Flow Diagram
LN specimens were submitted in anatomically specified packets to maximize LN yield (12) and optimize sensitivity for detecting LN metastases (7). A minimum of two separate packets (left and right pelvic) were required. The ELND nodes were submitted in a minimum of 3 packets labeled left and right CI (including the pre-sciatic) and pre-sacral. If the nodes were dissected from the bifurcation of the aorta proximally then these were submitted as a separate packet(s). Intraoperative photographs were required for every randomized patient and submitted for central review for protocol-specified compliance and surgeon feedback.
We designed a peer-reviewed protocol-specified quality control assessment on 100 randomly selected patients including at least one patient per surgeon and 50 patients in each arm (75% of patients had been accrued at the time of this analysis). We recruited 2 experienced urologic oncology surgeons with extensive experience with radical cystectomy who were not associated with the trial to perform this review (John Taylor, Karim Chamie). We recorded several metrics of surgical quality and describe each stratified by arm.
Outcomes
The primary outcome was disease free survival (DFS), determined from randomization to first documentation of relapse/recurrence or death due to any cause, with censoring at last contact. Criteria for recurrence included measurable disease on cross-sectional imaging or plain radiography targeting lung, liver, and bone. If local pelvic recurrence was identified by digital rectal exam, biopsy was required for confirmation. Second primary tumors of the upper urinary tract or retained urethra were not considered recurrence.
Secondary outcomes included overall survival (OS); operative time; utilization of erectile nerve preservation; intra-operative, peri-operative, and 90-day morbidity and mortality; length of hospital stay; histology (pure urothelial versus mixed); LN counts; LN density, adjuvant chemotherapy received; and local and retroperitoneal soft tissue recurrence.
We employed a detailed reporting and assessment of toxicity within the first 90 days following surgery to determine whether ELND was associated with higher peri-operative morbidity and mortality.
Statistical Analyses
We estimated the targeted hazard ratio using estimated 3-year DFS rates of 55% for patients undergoing SLND and 65% for those undergoing ELND (16–23). This 10% improvement translates into a hazard ratio of 0.72, which was considered clinically meaningful. Assuming exponential DFS, 5 years of patient accrual, 3 additional years of follow-up and a sample size of 564 eligible, randomized patients, the study had 85% power to detect a 28% reduction in the hazard rate of recurrence or death with ELND vs. SLND. Using an ITT analysis, a stratified logrank test with a one-sided alpha= 0.025 was specified. The final analysis was to be conducted when 184 events occurred on the SLND arm with a significance level of 0.022 to account for interim testing. Patients were to be followed for a maximum of six years. Kaplan-Meier methods were used to estimate DFS and OS curves. Cox regression was used to estimate treatment hazard ratios, adjusting for stratification factors. CT takes responsibility for the accuracy and completeness of analyses and the fidelity of reporting to the study protocol (available at nejm.org).
Results
We registered 658 patients between August 2011 and February 2017 (Figure 1). 36 credentialed surgeons at 27 US and Canadian sites registered at least one patient and 618 patients were randomized (Table S1). There were 26 ineligible patients post randomization due to wrong histology (6), T4b or metastasis (6), no MIBC (8), no pre-study scans (1) and randomization prior to intra-op assessment (5) resulting in 300 SLND and 292 ELND patients respectively, in the intent to treat population. Four patients randomized to SLND who underwent ELND are included in the AE assessment based on intent to treat.
Pre-randomization clinical variables are described in Table 1. The majority of patients were clinical stage T2, one quarter had hydronephrosis, and 13% had mixed histology. Neoadjuvant chemotherapy was used in 57% of patients in both groups, and the majority (86% and 89% in SLND and ELND, respectively) received cisplatin-based standard of care. Pathologic tumor staging and the presence and extent of pathologic LN metastasis was similar between groups (Table 2). The median number of lymph nodes identified by the pathologist was 24 in the standard lymphadenectomy arm, versus 39 in the ELND arm (Table 2). The median number of positive nodes was 1 and 2, respectively.
Table 1.
Patient and clinical disease characteristics at randomization
| SLND (n=300) | ELND (n=292) | |
|---|---|---|
| Male n (%) | 234 (78%) | 236 (81%) |
| Age median (range) | 68 (38, 90) | 69 (37, 92) |
| Race | ||
| White n (%) | 271 (90%) | 265 (91%) |
| Black n (%) | 12 (4%) | 10 (3%) |
| Asian n (%) | 7 (2%) | 5 (2%) |
| Other, Multi-racial, Unknown n (%) | 10 (3%) | 12 (4%) |
| Hispanic ethnicity | 6 (2%) | 21 (7%) |
| BMI median (range) | 27.3 (15.2, 45.2) | 27.3 (15.6, 42.8) |
| Performance Status 0–1 n (%) | 295 (98%) | 288 (99%) |
| Clinical Stage | ||
| T2 | 213 (71%) | 208 (71%) |
| T3-T4a | 87 (29%) | 84 (29%) |
| Hydronephrosis | 74 (25%) | 78 (27%) |
| Variant histologic subtypes | 36 (12%) | 40 (14%) |
| Neoadjuvant Chemotherapy | 170 (57%) | 166 (57%) |
| Cisplatin-based | 146 (49%) | 147 (50%) |
| Carboplatin-based | 10 (3%) | 12 (4%) |
| Other | 14 (5%) | 7 (2%) |
| None | 130 (43%) | 126 (43%) |
Table 2:
Pathologic Tumor and node stage findings from surgery (AJCC 7th edition, 2010), lymph nodes removed and positive nodes.
| SLND (n=300**) | ELND (n=292) | |
|---|---|---|
| Pathologic stage | ||
| pT0 N0 | 61 (20%) | 59 (20%) |
| pTis/Ta/Tl N0 | 55 (18%) | 49 (17%) |
| pT2(T2, T2a, T2b)N0 | 52 (17%) | 54 (18%) |
| pT3/T4 N0 | 61 (20%) | 55 (19%) |
| pTany N+ | 71 (24%) | 75 (26%) |
| pTany N1 | 36 (12%) | 34 (12%) |
| pTany N2 | 31 (10%) | 20 (7%) |
| pTany N3 | 4** (1.3%) | 21 (7.2%) |
| Total Number Nodes Removed Median (range) | 24 (6, 61) | 39 (15, 94) |
| Number of Positive Nodes if N+ Median (range) |
1 (1, 16) | 2 (1, 35) |
| 1 | 37 (12%) | 34 (12%) |
| 2–5 | 23 (8%) | 26 (9%) |
| 6–10 | 9 (3%) | 9 (3%) |
| > 10 | 2 (1%) | 6 (2%) |
4 patients randomized to the SLND arm received an ELND
Compliance with the protocol-specified LND was high with the exception of documentation of the pre-sciatic (fossa of Marcille) dissection in the extended template (Table S2). Surgery time, blood loss, and length of stay favored the standard lymphadenectomy group, though blood transfusion rates were similar. Nerve-sparing surgery was performed in 32% of patients in both groups and was bilateral in the majority of patients. Type of urinary diversion was also similar between groups and neobladder was performed in 48% of SLND patients vs. 47% of ELND patients (Table S3). Eleven percent of patients in each group had either received adjuvant therapy or reported plans to start adjuvant treatment.
Estimated 5-year disease free survival was 56% for ELND and 60% for SLND (HR 1.10; 95% CI 0.86,1.40; p=0.45) (Figure 2A). Overall 5-year survival was 59% and 63%, respectively (HR 1.13; 95% CI 0.88, 1.45) (Figure 2). Table S4 shows results for both DFS and OS according to pre-specified stratification factors, pathologic tumor, or node stage.
Figure 2.


A-Disease-free survival; B-Overall survival
The first site of recurrence was local in 23% of standard lymphadenectomy patients versus 35% of extended lymphadenectomy patients; distant in 62% versus 51%, and both local and distant in 12% vs. 11%, respectively. The overall local recurrence (LR) rate was 9% with SLND and 12.7% with ELND.
44% (132/300) of patients in the SLND arm, versus 54% (157/292) in the ELND arm had Grade 3–5 adverse events (AEs) reported (Table 3; Table S5). The most common AEs were anemia requiring blood transfusion, urinary tract infections, sepsis, wound complications, ileus and VTE events.
Table 3.
Select Grade 3–5 adverse events* occurring within 90 days of surgery
| SLND (n=300#) | ELND (n=292) | |||||||
|---|---|---|---|---|---|---|---|---|
| Grade | 3 | 4 | 5 | 3–5 | 3 | 4 | 5 | 3–5 |
|
| ||||||||
| Anemia | 53 | 1 | 0 | 54 (18%) | 45 | 0 | 0 | 45 (15%) |
| UTI | 28 | 0 | 0 | 28 (9.3%) | 24 | 1 | 0 | 25 (8.6%) |
| Sepsis | 0 | 12 | 2 | 14 (4.7%) | 0 | 18 | 1 | 19 (6.5%) |
| Wound complications | 12 | 0 | 0 | 12 (4.0) | 14 | 1 | 0 | 15 (5.1%) |
| Leukocytosis | 7 | 0 | 0 | 7 (2.3%) | 11 | 1 | 0 | 12 (4.1%) |
| Thromboembolic event | 5 | 1 | 2 | 8 (2.7%) | 7 | 4 | 1 | 12 (4.1%) |
| Ileus | 7 | 0 | 0 | 7 (2.3%) | 10 | 1 | 0 | 11 (3.8%) |
| Hyponatremia | 8 | 0 | 0 | 8 (2.7%) | 6 | 2 | 0 | 8 (2.7%) |
| Hypertension | 8 | 0 | 0 | 7 (2.7%) | 9 | 0 | 0 | 9 (3.1%) |
| Surg/med procedures | 9 | 0 | 0 | 9 (3.0%) | 12 | 5 | 0 | 17 (5.8%) |
| Acidosis | 7 | 0 | 0 | 7 (2.3%) | 6 | 0 | 0 | 6 (2.1%) |
| Hyperkalemia | 4 | 1 | 0 | 5 (1.7%) | 4 | 1 | 0 | 5 (1.7%) |
| Dehydration | 6 | 0 | 0 | 6 (2.0%) | 3 | 0 | 0 | 3 (1.0%) |
| Hypotension | 3 | 1 | 0 | 4 (1.3%) | 3 | 1 | 1 | 5 (1.7%) |
| Respiratory failure | 0 | 1 | 0 | 1 (0.3%) | 0 | 6 | 1 | 7 (2.4%) |
| Myocardial infarction | 2 | 1 | 0 | 3 (1.0%) | 0 | 0 | 3 | 3 (1.0%) |
| Death NOS | 0 | 0 | 0 | 0 (0%) | 0 | 0 | 4 | 4 (1.4%) |
| Cardiac other | 1 | 0 | 0 | 1 (0.3%) | 0 | 0 | 1 | 1 (0.3%) |
| Stroke | 1 | 0 | 0 | 1 (0.3%) | 0 | 0 | 1 | 1 (0.3%) |
| Aspiration | 0 | 0 | 0 | 0 (0%) | 1 | 0 | 1 | 2 (0.7%) |
| Multi-organ failure | 0 | 0 | 0 | 0 (0%) | 0 | 1 | 1 | 2 (0.7%) |
| DIC | 0 | 0 | 1 | 1 (0.3%) | 0 | 0 | 0 | 0 (0%) |
| Ventricular fibrillation | 0 | 0 | 0 | 0 (0%) | 0 | 0 | 1 | 1 (0.3%) |
| Maximum grade any AE | 104 | 21 | 4 | 129 (43.6%) | 116 | 29 | 12 | 157 (53.8%) |
1 death occurred at 102 days due to multiple surgical complications and is included.
CTCAE version 4.0 Grade 3 is serious, Grade 4 is life-threatening and Grade 5 resulted in death
four patients randomized to the SLND arm received an ELND after initially attempting a SLND. They are included in the SLND arm.
Table S5 includes all AEs and grades
Deaths within 30 days of surgery occurred in 1 (0.8%) participant in the SLND arm vs. 8 (2.7%) participants in the ELND arm. Deaths within 90 days of surgery occurred in 7 (2.4%) vs. 19 (6.5%), respectively. The majority of deaths within 90 days were due to post-operative complications, while 7 (27%; 2 SLND and 5 ELND) were due to disease progression. The proportion of patients in the SLND with a G3–5 AE or death within 90 days of surgery was 44% (132/300) and 55% (161/292) for ELND (includes 4 additional patients who died without reporting a G3–5 AE) (p=0.007).
Discussion
In this multicenter randomized trial involving patients with localized muscle invasive bladder cancer undergoing radical cystectomy, the performance of extended (versus standard) lymphadenectomy did not result in improvement in disease free survival or overall survival. Moreover, ELND was associated with greater morbidity and higher peri-operative 90-day mortality.
A unique study feature was a surgeon credentialing process to optimize adherence to protocol-specified lymphadenectomy and radical cystectomy. Rigorous review of all operative and pathology reports and intra-operative photos may have contributed to the completeness of the SLND and ELND. We allowed neoadjuvant chemotherapy, and 57% of patients in both groups received it; in 87% of these patients, neoadjuvant chemotherapy was standard of care cisplatin-based chemotherapy. This rate is higher than rates of neoadjuvant therapy in other multi-center studies. (9,10)
The median number of nodes removed - 24 in the SLND arm and 39 in the ELND arm - are consistent with those reported in other contemporary cohorts, as are the percentages of patients found to have nodal metastases - 71 of 300 (24%) and 75 out of 292 (26%), respectively (24,25). The data confirm that the SLND in both arms provided a >95% probability of identifying nodal metastasis. The frequency of local recurrence (alone or with distant metastasis) was relatively low and similar between groups, suggesting that a thorough bilateral standard lymphadenectomy with removal of all potential lymph node bearing tissue and occult lymph node metastasis is associated with optimum local control.
Another multicenter randomized trial in Germany (AB 25–02; LEA) that compared extended with standard bilateral pelvic lymphadenectomy in patients undergoing radical cystectomy similarly found no differences between groups in recurrence-free survival (primary outcome) or cancer-specific or overall survival (26). Their study differed from ours in including patients with lower stage disease (i.e., clinical T1) and they did not allow neoadjuvant chemotherapy. Also, lymphadenectomy in the control group did not include deep obturator nodes and thus did not remove all of the potential lymph node bearing tissue in the obturator fossa, which is within the primary lymph node drainage of the bladder.
The 5-year observed DFS and OS estimates for SLND were 60% and 63%, respectively; both values are higher than what we had estimated for the control arm (DFS: 55% at 3 years). Our estimate was derived from retrospective cohort studies that pre-date the common use of neoadjuvant chemotherapy; its frequent use in our trial may have led to improved outcomes. It is possible that ELND has a benefit without NAC, but we did not observe heterogeneity in results in a post hoc analysis according to receipt of NAC. In addition, the LEA trial, which did not allow NAC, also did not show a benefit for ELND. Plausible differences in DFS between the extended and standard lymphadenectomy groups, based on the 95% CI around the point estimate, ranged from a 14% reduction in risk to an increase of 40%.
Serious AEs and 90-day mortality were higher in the ELND arm, further supporting standard over extended lymphadenectomy. Our study was conducted during the time that enhanced recovery after surgery (ERAS) protocols were being adopted. We did not collect data on use of ERAS other than pre-and post-op anticoagulation, which were widely considered standard of care prior to trial inception. Broader adoption of ERAS protocols might reduce risk of G3–5 adverse events associated with radical cystectomy and pelvic lymphadenectomy.
Muscle invasive bladder cancer is a heterogeneous disease, and the results of this trial only apply to patients with predominant urothelial histology, clinical stage T2–4aN0–2 disease, and an intra-operative exploration that ruled out disease in the extended template. Our patient population was predominantly Non-Hispanic White (90%), and median age was slightly younger than the most recent SEER data for localized invasive and in situ cancer (Table S6). We did not allow robotic surgery; however, the anatomic templates are independent of the surgical approach whether performed open or robotic assisted.
In summary, as compared with standard lymphadenectomy, extended lymphadenectomy did not improve disease free survival or overall survival in patients with localized muscle invasive bladder cancer undergoing radical cystectomy and was associated with higher rates of serious adverse events.
Supplementary Material
Acknowledgments
We wish to thank Dr. Eila Skinner for chairing the surgeon credentialing committee and members of the committee Drs. Theresa Koppie and Robert Svatek. We wish to thank Drs. Karim Chamie and John Taylor for conducting the protocol specified quality control assessment for compliance with the protocol specified lymph node dissection templates. We also thank Jean Barce, Laura Wells and Gabby Lopez for their data coordination. Bart Kiemeney assisted with the SEER population data (Table S6). We are grateful to all the patients, family members and caregivers who volunteered for this innovative surgical trial.
Funding Statement:
NIH/NCI grant awards U10CA180888 U10CA180819, U10CA180820, U10CA180821, and U10CA180863 and Canadian Cancer Society grant# 707213.
Abbreviations:
- CI
common iliac
- DFS
disease free survival
- ELND
extended lymph node dissection
- HR
hazard ratio
- IMA
Inferior mesenteric artery
- MIBC
muscle invasive bladder cancer
- NAC
neoadjuvant chemotherapy
- OS
overall survival
- RC
radical cystectomy
- SLND
standard lymph node dissection
Footnotes
Disclosures provided by the authors are available with the full text of this article at NEJM.org.
Publisher's Disclaimer: This is an Author Accepted Manuscript, which is the version after external peer review and before publication in the Journal. The publisher’s version of record, which includes all New England Journal of Medicine editing and enhancements, is available at https://www.nejm.org/doi/full/10.1056/NEJMoa2401497.
References
- 1.Lerner SP, Skinner DG, Lieskovsky G, Boyd SD, Groshen SL, Ziogas A, et al. The rationale for en bloc pelvic lymph node dissection for bladder cancer patients with nodal metastases: long-term results. J Urol. 1993. Apr;149(4):758–64; discussion 764–765. [DOI] [PubMed] [Google Scholar]
- 2.Herr HW, Faulkner JR, Grossman HB, Natale RB, deVere White R, Sarosdy MF, et al. Surgical factors influence bladder cancer outcomes: a cooperative group report. J Clin Oncol Off J Am Soc Clin Oncol. 2004. Jul 15;22(14):2781–9. [DOI] [PubMed] [Google Scholar]
- 3.Skinner DG. Management of invasive bladder cancer: a meticulous pelvic node dissection can make a difference. J Urol. 1982. Jul;128(1):34–6. [DOI] [PubMed] [Google Scholar]
- 4.Stein JP, Skinner DG. The role of lymphadenectomy in high-grade invasive bladder cancer. Urol Clin North Am. 2005. May;32(2):187–97. [DOI] [PubMed] [Google Scholar]
- 5.Poulsen AL, Horn T, Steven K. Radical cystectomy: extending the limits of pelvic lymph node dissection improves survival for patients with bladder cancer confined to the bladder wall. J Urol. 1998. Dec;160(6 Pt 1):2015–9; discussion 2020. [PubMed] [Google Scholar]
- 6.Capitanio U, Suardi N, Shariat SF, Lotan Y, Palapattu GS, Bastian PJ, et al. Assessing the minimum number of lymph nodes needed at radical cystectomy in patients with bladder cancer. BJU Int. 2009. May;103(10):1359–62. [DOI] [PubMed] [Google Scholar]
- 7.Konety BR, Joslyn SA. Factors influencing aggressive therapy for bladder cancer: an analysis of data from the SEER program. J Urol. 2003. Nov;170(5):1765–71. [DOI] [PubMed] [Google Scholar]
- 8.Sodagum L, Passarelli R, Pfail J, Patel HV, Chua K, Doppalapudi SK, et al. Pelvic lymphadenectomy: Evaluating nodal stage migration and will rogers effect in bladder cancer. Urol Oncol. 2024. Jan;42(1):21.e9–21.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catto JWF, Khetrapal P, Ricciardi F, Ambler G, Williams NR, Al-Hammouri T, et al. Effect of Robot-Assisted Radical Cystectomy With Intracorporeal Urinary Diversion vs Open Radical Cystectomy on 90-Day Morbidity and Mortality Among Patients With Bladder Cancer: A Randomized Clinical Trial. JAMA. 2022. Jun 7;327(21):2092–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parekh DJ, Reis IM, Castle EP, Gonzalgo ML, Woods ME, Svatek RS, et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): an open-label, randomised, phase 3, non-inferiority trial. Lancet Lond Engl. 2018. Jun 23;391(10139):2525–36. [DOI] [PubMed] [Google Scholar]
- 11.Steven K, Poulsen AL. Radical cystectomy and extended pelvic lymphadenectomy: survival of patients with lymph node metastasis above the bifurcation of the common iliac vessels treated with surgery only. J Urol. 2007. Oct;178(4 Pt 1):1218–23; discussion 1223–1224. [DOI] [PubMed] [Google Scholar]
- 12.Dhar NB, Klein EA, Reuther AM, Thalmann GN, Madersbacher S, Studer UE. Outcome after radical cystectomy with limited or extended pelvic lymph node dissection. J Urol. 2008. Mar;179(3):873–8; discussion 878. [DOI] [PubMed] [Google Scholar]
- 13.Ghodoussipour S, Daneshmand S. Current controversies on the role of lymphadenectomy for bladder cancer. Urol Oncol. 2019. Mar;37(3):193–200. [DOI] [PubMed] [Google Scholar]
- 14.Farnell MB, Pearson RK, Sarr MG, DiMagno EP, Burgart LJ, Dahl TR, et al. A prospective randomized trial comparing standard pancreatoduodenectomy with pancreatoduodenectomy with extended lymphadenectomy in resectable pancreatic head adenocarcinoma. Surgery. 2005. Oct;138(4):618–28; discussion 628–630. [DOI] [PubMed] [Google Scholar]
- 15.Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008. Jul 31;359(5):453–62. [DOI] [PubMed] [Google Scholar]
- 16.Dalbagni G, Genega E, Hashibe M, Zhang ZF, Russo P, Herr H, et al. Cystectomy for bladder cancer: a contemporary series. J Urol. 2001. Apr;165(4):1111–6. [PubMed] [Google Scholar]
- 17.Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol Off J Am Soc Clin Oncol. 2001. Feb 1;19(3):666–75. [DOI] [PubMed] [Google Scholar]
- 18.Madersbacher S, Hochreiter W, Burkhard F, Thalmann GN, Danuser H, Markwalder R, et al. Radical cystectomy for bladder cancer today--a homogeneous series without neoadjuvant therapy. J Clin Oncol Off J Am Soc Clin Oncol. 2003. Feb 15;21(4):690–6. [DOI] [PubMed] [Google Scholar]
- 19.Hautmann RE, Gschwend JE, de Petriconi RC, Kron M, Volkmer BG. Cystectomy for transitional cell carcinoma of the bladder: results of a surgery only series in the neobladder era. J Urol. 2006. Aug;176(2):486–92; discussion 491–492. [DOI] [PubMed] [Google Scholar]
- 20.Shariat SF, Karakiewicz PI, Palapattu GS, Lotan Y, Rogers CG, Amiel GE, et al. Outcomes of radical cystectomy for transitional cell carcinoma of the bladder: a contemporary series from the Bladder Cancer Research Consortium. J Urol. 2006. Dec;176(6 Pt 1):2414–22; discussion 2422. [DOI] [PubMed] [Google Scholar]
- 21.Ghoneim MA, Abdel-Latif M, el-Mekresh M, Abol-Enein H, Mosbah A, Ashamallah A, et al. Radical cystectomy for carcinoma of the bladder: 2,720 consecutive cases 5 years later. J Urol. 2008. Jul;180(1):121–7. [DOI] [PubMed] [Google Scholar]
- 22.Manoharan M, Ayyathurai R, Soloway MS. Radical cystectomy for urothelial carcinoma of the bladder: an analysis of perioperative and survival outcome. BJU Int. 2009. Nov;104(9):1227–32. [DOI] [PubMed] [Google Scholar]
- 23.Volkmer BG, Kuefer R, Bartsch GC, Gust K, Hautmann RE. Oncological followup after radical cystectomy for bladder cancer-is there any benefit? J Urol. 2009. Apr;181(4):1587–93; discussion 1593. [DOI] [PubMed] [Google Scholar]
- 24.Leissner J, Ghoneim MA, Abol-Enein H, Thüroff JW, Franzaring L, Fisch M, et al. Extended radical lymphadenectomy in patients with urothelial bladder cancer: results of a prospective multicenter study. J Urol. 2004. Jan;171(1):139–44. [DOI] [PubMed] [Google Scholar]
- 25.Vazina A, Dugi D, Shariat SF, Evans J, Link R, Lerner SP. Stage specific lymph node metastasis mapping in radical cystectomy specimens. J Urol. 2004. May;171(5):1830–4. [DOI] [PubMed] [Google Scholar]
- 26.Gschwend JE, Heck MM, Lehmann J, Rübben H, Albers P, Wolff JM, et al. Extended Versus Limited Lymph Node Dissection in Bladder Cancer Patients Undergoing Radical Cystectomy: Survival Results from a Prospective, Randomized Trial. Eur Urol. 2019. Apr;75(4):604–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.