Abstract
Background
The use of khat, alcohol, and cigarettes during pregnancy is a serious public health problem associated with harmful outcomes for the fetus and the mother’s health. Studies that investigated khat, alcohol, and cigarettes usage during pregnancy yielded varied and contradictory results. This study used a systematic review and meta-analysis to estimate the pooled prevalence and associated factors of khat, alcohol, and cigarettes use among pregnant women in Africa.
Methods
A review of eligible studies was conducted using Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. This review has been registered in PROSPERO with protocol ID CRD42021289074. Literature published in English from January 1, 2002 to November 30, 2021 was retrieved from PubMed, Google Scholar, Cochrane, HINARI, African Journal Online, and Science Direct databases. The quality of included articles was assessed using the Joanna Briggs Institute’s (JBI) critical appraisal checklist. The I2 statistic and Cochran’s Q test were used to assess the presence of heterogeneity between studies. To assess publication bias, a funnel plot and Egger’s regression test were utilized. The random effect model was used to estimate the summary prevalence and the corresponding 95% confidence interval (CI) of risk factors for khat, alcohol, and cigarettes use.
Results
Out of the 1509 studies identified, 71 met the inclusion criteria. The pooled prevalence of khat chewing, alcohol drinking, active smoking, and secondhand smoke exposure during pregnancy was 18.93%, 22.20%, 11.85%, and 43.45%, respectively. The subgroup analysis by UN sub-region showed the highest pooled prevalence of alcohol use during pregnancy in Middle Africa (25.69%) and the lowest in Northern Africa (1.10%). Several factors were identified as risk factors for alcohol use, including low educational level, younger age women, pre-pregnancy alcohol use, unplanned pregnancy, history of abortion, poor social support, mental distress, poor knowledge on alcohol risks, and partner alcohol use.
Conclusion
This review indicated that the pooled magnitude of khat, alcohol and cigarette use during pregnancy was higher in Africa. Substance use screening and brief interventions (SBI) should be routinely delivered in antenatal care settings to reduce pregnant women’s substance use.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12884-024-06999-7.
Keywords: Khat, Alcohol, Cigarette, Pregnancy, Systematic review and meta-analysis, Africa
Background
The overall worldwide prevalence of alcohol intake during pregnancy is 9.8% [1]. According to a meta-analysis done in Sub-Saharan Africa, the pooled prevalence of alcohol use during pregnancy was 20.83% [2]. Consumption of psychoactive substances like alcohol, cigarette, and khat has long been identified as a major cause of human suffering and emerged as one of Africa’s significant socio-economic and health problems [3]. Alcohol, cigarette, and cannabis are prevalently used during pregnancy around the world [4]. Alcohol consumption during the antenatal period is a widely known risk factor for poor pregnancy outcomes; including stillbirth [5], spontaneous abortion [6], low birth weight [7], premature birth [8], and intrauterine growth retardation [9].
Smoking cigarettes during pregnancy causes significant health damage to the fetus [10–12]. The prevalence of smoking during pregnancy is around 3% in low- and middle-income countries [13]. Cigarette smoking during pregnancy has been found to vary across African countries, ranging from 1.1% in Tanzania [14] to 41.2% in Tunisia [15]. Secondhand smoke (SHS) exposure during pregnancy has nearly the same negative health consequences as active smoking [16, 17].
Khat is a plant with fresh green leaves that has been grown for centuries in the Horn of Africa [18]. People routinely chew the green leaves of khat for recreational purposes and for the euphoric effect it produces due to its capacity to enhance dopamine functions in the brain [19]. A national population survey of 7343 married women in Yemen found that 40.7% of respondents reported consuming khat while pregnant [20]. Khat chewing during pregnancy causes negative health outcomes such as low birth weight [21–23] and perinatal mortality [22].
There was no systematic review and meta-analysis on the pooled prevalence of khat use, active smoking, and SHS exposure during pregnancy in Africa. Therefore, this systematic review and meta-analysis aimed to determine the prevalence and risk factors of khat, alcohol, and cigarette use during pregnancy in Africa. The findings of this study would highlight the importance and urgency of scaling up substance use screening and its intervention throughout Africa.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines were used to conduct this review (Additional file 1: Check List 1) [24]. The protocol has been registered at PROSPERO with reference ID CRD42021289074 [25].
Search strategies and screening
A comprehensive literature search was performed using the following electronic databases to identify studies published between January 1, 2002 and November 30, 2021: PubMed, Cochrane Library, Google Scholar, HINARI, African Journals Online (AJOL), and Science Direct. In addition, potential articles were retrieved by checking the reference lists of the included studies. The following key terms and medical subject headings were used to identify relevant literature: “catha”, “khat”, “qat”, “qat plant”, “alcohol”, “alcohol drinking”, “binge drinking”, “tobacco”, “tobacco smoking”, “smoking”, “secondhand smoke”, “tobacco smoke pollution”, “substance abuse”, “substance use disorder”, “pregnancy”, “pregnant women”, “prenatal care”, “pregnant” and “ Africa” (all African countries were considered). The key terms were used in combination using Boolean operators like “AND” or “OR” (Additional file 2: Table S1).
Following the search, publications retrieved from electronic databases were exported to Endnote software (version X4). EndNote was used to identify, record, and remove duplicate records. Two authors (BW and TD) independently screened the titles and abstracts. Two reviewers (EW and MA) collected full texts independently and evaluated their suitability for final inclusion. Disagreements were resolved by discussion and in consultation with a third reviewer (KD).
Inclusion and exclusion criteria
The following criteria were used to select the articles: (i) cross-sectional, cohort, case-control studies of pregnant women residing in African countries reporting the prevalence of khat, alcohol, or smoking and/or associated factors; (ii) articles published in English language; and (iii) studies published between January 1, 2002 and November 30, 2021. Articles with the following criteria were excluded: (i) studies conducted among populations of African origin living outside of Africa; (ii) duplicates: for studies published in more than one paper, the most up-to-date was considered; (iii) qualitative studies, case reports, letters, reviews, commentaries, and editorials; (iv) studies conducted on animals; and (v) literatures that had not been formally published.
Outcome measurement
The main outcome of this review is to estimate the pooled prevalence of khat use, alcohol consumption, and cigarettes smoke exposure among pregnant women in Africa. Cigarettes smoke exposure includes active smoking and SHS exposure. In addition, determinants associated with psychoactive substance use during pregnancy were determined. Psychoactive substance use is defined as the percentage of pregnant women who are using any amount of khat, tobacco, or alcohol during pregnancy. All measurements of khat, cigarettes, and alcohol consumption during pregnancy were included. The measurements might be ASSIST (Alcohol, Smoking, and Substance Involvement Screening Test), Alcohol Use Disorder Identification Test-Consumption (AUDIT-C), Prenatal Questionnaire (PNQ), Tolerance-Annoyance, Cut Down, Eye Opener (T-ACE), and others. Odds ratio (OR), logarithms of OR, and standard error (SE) of logarithms of OR were computed for factors.
Quality assessment
The Joanna Briggs Institute (JBI) critical appraisal checklist was used for the quality assessment of the included studies, which includes a distinct appraisal checklist for each kind of study design. We used a checklist of 10 items, 8 items, and 11 items to evaluate case-control, cross-sectional, and cohort research, respectively [26]. The reviewers then classified the studies as having a high or low risk of bias. Articles with a critical appraisal score of 50% or greater were included in the final review.
Data extraction
Two independent reviewers (BW and TD) extracted data using a data extraction tool prepared on a Microsoft Excel spreadsheet (2010). The tool included information such as the primary author’s name, the year of publication, country, study setting, study design, sample size, the prevalence of substance use (khat, alcohol, and smoking), and the odds ratio of associated factors.
Data analysis
For meta-analysis, the extracted data were imported into STATA software (version 14.0). Tables and forest plots were used to present the findings. Cochran’s Q test (p-value < 0.05 indicating significant heterogeneity) and the I2 statistic were used to analyze heterogeneity [27]. As a result of the high heterogeneity among the included studies, the random-effects model was used to generate a pooled estimate. For factors, a random-effects model was also used to pool the odds ratios among the included studies. For each forest plot, the pooled estimate of heterogeneity test values (I2 and Chi2) and 95% confidence interval (CI) were reported. A visual inspection of funnel plots and Egger’s regression test were used to assess publication bias. A p-value below 0.05 was taken into account to indicate the presence of significant publication bias in Egger’s test [28]. A trim and fill analysis was conducted to adjust the publication bias.
Subgroup and sensitivity analyses were conducted in cases of substantial heterogeneity to find potential moderators of the heterogeneity. Subgroup analyses were conducted by various article characteristics like sub-regions of Africa (Northern, Southern, Eastern, Western, and Middle Africa), year of publication (2002–2015 and 2016–2021), study setting (community and health facility-based), study design (cohort, case control, and cross sectional), sample size (< 400 and ≥ 400), and income status (low, lower middle, and upper middle income). Moreover, we conducted a leave-one-out sensitivity meta-analysis to determine the key articles that have a significant effect on between-article heterogeneity.
Results
Study selection
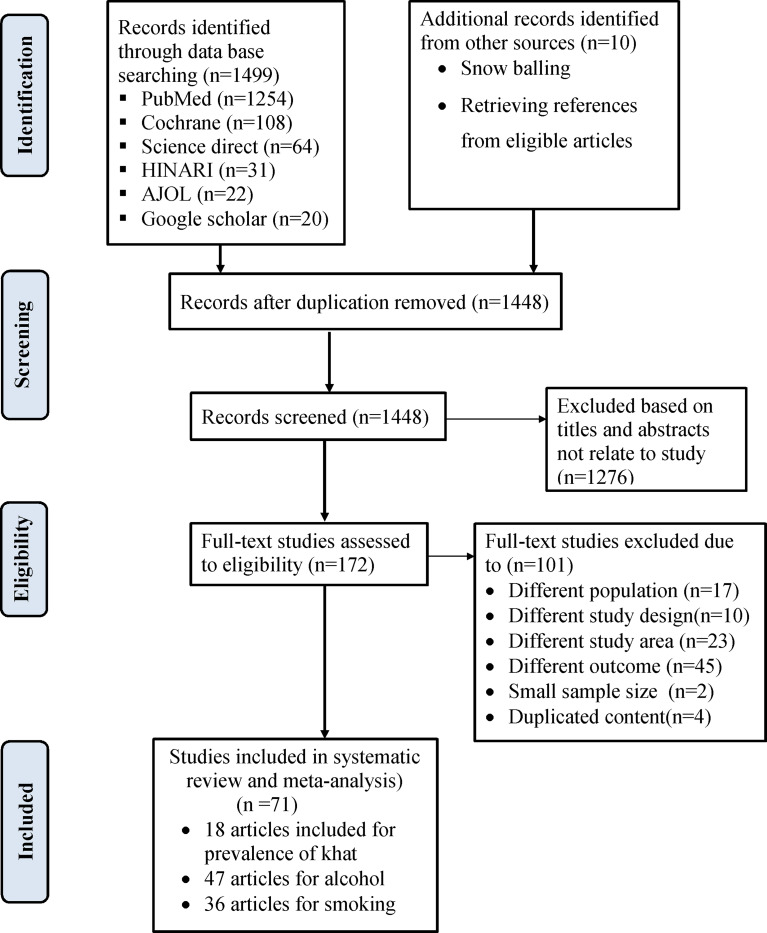
A total of 1499 studies were identified through the search of electronic databases. Ten studies were identified through reference tracing. From all identified articles, 61 studies were removed due to duplication, while 1448 articles were reserved for additional screening. Of these, 1276 were removed after reading the title and/or the abstract. Finally, 172 full-text studies were accessed and evaluated for eligibility using full-text screening, which resulted in further exclusion of 101 studies. Seventy-one articles met the inclusion criteria for the systematic review and meta-analysis (Fig. 1).
Fig. 1.
Flow diagram for study selection
Description of included studies
Overall, we selected a total of 71 articles in this systematic review and meta-analysis. We included 10 community-based and 61 health facility-based studies from 17 countries in Africa, across five geographical regions. Thirty-three studies were from the Eastern Africa region [14, 21, 29–59], twelve studies were from the Southern African region [60–70] and thirteen studies were from the Western African region [71–83].While eight studies were from the Northern Africa region [15, 84–90] and six studies were from the Middle Africa region [36, 91–95].
This review contains articles that were published between 2002 and 2021. Fifty-four studies used cross-sectional [14, 15, 29, 30, 32–37, 40–47, 49–59, 61, 62, 64, 66–68, 70–74, 76–78, 80–83, 85, 86, 89, 90, 92, 94, 95], eight studies used case-control [21, 31, 38, 39, 48, 75, 88, 93], and nine studies used cohort study design [60, 63, 65, 69, 79, 84, 87, 91, 96]. A total of 18 studies reported on khat use [21, 31–35, 37, 41, 42, 44, 45, 48, 49, 52, 59, 97–99], 47 studies reported on alcohol drinking [14, 29, 32, 33, 35, 40, 41, 46, 47, 49, 53, 54, 56, 57, 60–65, 70, 72, 73, 82, 87, 93, 95, 98–117], and 34 studies on smoking [14, 15, 33, 35, 36, 41, 42, 48, 53, 54, 56, 63–65, 69, 70, 76–78, 84–86, 88–90, 93, 98, 99, 105, 106, 110, 116–118] during pregnancy. In terms of response rate, the majority of included studies had a high response rate (> 85%) (Additional file 3: Table S2).
The pooled prevalence of khat use among pregnant women
A total of 18 studies were included in the meta-analysis to estimate the overall pooled prevalence of khat consumption during pregnancy. The included studies reported a sample size ranging from 279 participants [31] to 1688 [59]. The prevalence of khat use ranged from 3.50% [34] to 37.20% [59]. The pooled prevalence of khat use during pregnancy was 18.93% (95% CI 13.44–24.43%). The I2 test output showed high heterogeneity among the included articles (I2 = 98.5%; p-value < 0.001). Hence, a random effect analysis model was used (Fig. 2).
Fig. 2.
Forest plot of prevalence of khat use among pregnant women
The presence of publication bias was assessed using funnel plots and the Egger’s test. First, the effect sizes of the studies were plotted against their standard errors, and a visual evaluation of the funnel plot revealed publication bias because the graph appeared asymmetrical. Egger’s test was also conducted for khat use similar to the funnel plot; it showed evidence of publication bias (p-value < 0.001). Moreover, Begg’s test also indicated a similar finding (p-value = 0.002) (Additional file 4: Figure S1). Due to the presence of publication bias, a trim and fill analysis was conducted. After adding eight studies, the pooled prevalence of khat use was 9.38% (95%CI (2.99, 15.77%)) (Additional file 5: Table S3).
The random-effects model was used in a sensitivity analysis to examine the effect of specific studies on the aggregated prevalence of khat chewing among pregnant women. A single study had no effect on the pooled estimated prevalence of khat use during pregnancy, according to the sensitivity analysis. After removing a single study, the pooled estimated prevalence of khat use ranged between 17.81% (12.91, 22.71) [59] and 19.85% (14.21, 25.49) [34] (Additional file 6: Table S6).
Subgroup analysis of the prevalence of khat use during pregnancy
The meta-analysis of khat use revealed a significant degree of heterogeneity among the studies included. We computed subgroup analysis based on study design, study setting, publication period, and sample size when at least two studies are in the same category. Accordingly, the lowest heterogeneity of khat use during pregnancy was reported in a community based study; the heterogeneity between the groups was significant (p-value = 0.000). In reference to the sample size, the prevalence was lower in studies that included a sample of less than 600 pregnant women (18.83%, 95%CI 11.77–25.89). The prevalence of khat chewing did not differ significantly between cross-sectional and case-control studies (p-value = 0.505). According to the findings, the study design, study period, and sample size were not sources of heterogeneity (Additional file 7: Table S10).
Factors associated with khat chewing during pregnancy
Two articles [49, 52] were included to assess the association between partner khat use and khat use during pregnancy. Furthermore, a total of two articles [49, 52] were included to determine the association between alcohol use and khat use during pregnancy. One article showed a significant association between alcohol consumption and khat use during pregnancy [49]. The other article indicated a non-significant association between alcohol use and khat use during pregnancy [52].
The pooled prevalence of alcohol use during pregnancy in Africa
The meta-analysis included 47 studies to calculate the overall pooled prevalence of alcohol consumption during pregnancy in Africa. The prevalence of alcohol drinking during pregnancy for the included studies ranged from the highest observed in the Western Cape Province of South Africa (64.7%) [61] to the lowest in Egypt (1.1%) [87] (Additional file 3: Table S2). The estimated pooled prevalence of alcohol use during pregnancy, which was reported by 47 studies using the fixed effect model, indicated significant heterogeneity between the articles. Therefore, we did the analysis with a random effects model with 95% CI to account for the observed variability. Accordingly, the pooled prevalence of alcohol drinking during pregnancy was 22.20% (95% CI (17.72, 26.69%)) with significant heterogeneity between articles (I2 = 99.6, p-value < 0.001) (Fig. 3).
Fig. 3.
Forest plot of the prevalence of alcohol use among pregnant women in Africa
A visual assessment of the funnel plot revealed that the included studies were distributed asymmetrically. Additionally, the result of Egger’s test was statistically significant for the presence of publication bias (p-value < 0.001) (Additional file 4: Figure S2). Therefore, the trim and fill analysis was conducted, and with this analysis, the pooled prevalence of alcohol use among pregnant women in Africa was 7.72% (95%CI (2.52, 12.93%)) (Additional file 5: Table S4).
Sensitivity analysis of alcohol use
The sensitivity analysis results showed that there was no impactful article on the pooled estimated prevalence of alcohol consumption among pregnant women. The pooled estimated prevalence of alcohol consumption during pregnancy ranged from 18.20 (13.76, 22.65) [82] to 20.19 (15.40, 24.98) [112] after the deletion of a single study (Additional file 6: Table S7).
Subgroup analysis of the prevalence of alcohol use during pregnancy
Subgroup analysis was carried out by dividing all primary articles included in the analysis by the United Nations (UN) sub-region classification of Africa, country income level, study design, study setting, sample size, and publication year to make comparisons between them and as a method of investigating heterogeneity.
The subgroup analysis by UN sub-region showed the highest prevalence of alcohol use during pregnancy in Middle Africa, 25.69% (95%CI: 19.98, 31.40; I2 = 96.0, p < 0.001) and the lowest in Northern Africa, 1.10% (95%CI: 0.63, 1.57; I2 = 0.0). Whereas, the pooled prevalence of alcohol consumption during pregnancy in Eastern Africa, Southern Africa, and Western Africa was 20.55% (95%CI: 13.58, 27.52), 24.20% (95%CI: 17.41, 30.98), and 23.80% (95%CI: 15.54, 32.06), respectively. The overall heterogeneity between sub-regions was significant (p-value < 0.001).
Using the study design, an additional subgroup analysis was performed. Accordingly, the highest pooled estimate of alcohol consumption during pregnancy was reported in the case-control study design, 25.85% (95% CI 4.95, 46.74). The lowest prevalence was reported in the cohort study design. The heterogeneity between the groups (case-control, cross-sectional, and cohort study design) was not significant (p-value = 0.794).
Concerning the sample size, the highest pooled prevalence was observed from 17 articles with a sample size less than 400, 27.69% (95% CI: 20.89, 34.49%). On the other hand, the lowest pooled prevalence of 19.13% (95% CI: 13.45, 24.80) was observed from studies with a sample size ≥ 400. Another subgroup analysis by study setting indicated that the prevalence of alcohol drinking was 31.80% (95% CI: 23.29, 40.32) and 20.22% (95% CI: 15.26, 25.17) among studies conducted at community level and health institutions, respectively.
Based on the World Bank income classification, the highest pooled prevalence was observed from 11 studies conducted in upper middle-income countries, 24.20% (95% CI: 17.41, 30.98%). On the other hand, the lowest pooled prevalence of 20.74% (95% CI: 11.45, 30.04%) was observed from studies conducted in lower middle income countries. The prevalence of alcohol drinking during pregnancy prior to the end of the Millennium Development Goals (MDGs) period (2002–2015 in the current review) was 22.88% (95%CI: 12.25, 33.50), which declined to 21.90% (95%CI: 18.00, 25.81) during the post-MDGs period (2016–2021). The heterogeneity between the groups (publication year) was not significant (p = 0.866) (Additional file 7: Table S11).
Factors associated with alcohol consumption during pregnancy
Socio-demographic characteristics
The socio-demographic factors included in this meta-analysis were age, place of residence, and educational status of pregnant women. A separate analysis was conducted for each independent variable. A total of six articles [29, 49, 82, 102, 113, 116] were included to assess the association between age and alcohol consumption during pregnancy. The pooled meta-analysis revealed that the pooled odds of alcohol drinking among pregnant women aged < 25 years was increased by 1.66 folds (OR = 1.66 (95%CI = 1.01, 2.76)). Similarly, a total of five articles [29, 35, 99, 101, 102] were also included to determine the association between the educational status of pregnant women and alcohol use. Five articles [29, 35, 99, 101, 102] found higher alcohol use among pregnant women who had no education. All researches showed significant association. Likewise, the final pooled meta-analysis using data from the five studies discovered that pregnant women who do not attend school are nearly two times (OR = 1.93; 95%CI = 1.47, 2.54) more likely to drink alcohol than those who do attend school (Fig. 4).
Fig. 4.
Forest plot which describes the association between socio-demographic characteristics and pregnancy-related factors with alcohol use during pregnancy in Africa
Moreover, five studies [49, 99, 101, 104, 113] were considered to investigate the relationship between place of residence and alcohol use during pregnancy. Three of the included articles [49, 104, 113] found significant association, while the rest two studies [99, 101] revealed non-significant association between alcohol drinking during pregnancy and residence. The pooled meta-analysis showed that there was no significant association between place of residence and alcohol consumption during pregnancy, OR = 1.60 (95%CI = 0.70, 3.69) (Additional file 8: Figure S5).
Gynecological and obstetric factors
The gynecological and obstetric factors included in this analysis were gravidity, parity, gestational age, pregnancy plan, and history of abortion. The results of the review indicated a significant association between pregnancy plan and alcohol use during pregnancy in the random model (OR = 2.14; 95%CI: 1.70, 2.70). The odds of drinking alcohol for women who had unplanned their pregnancy was two times that of women whose last pregnancy was planned. The heterogeneity test was too low, and the I2 value was 18.8%. Three studies [35, 37, 49] were also included to assess the relationship between abortion history and alcohol use during pregnancy. All of the articles included had a significant association. The final pooled meta-analysis found that women who had history of abortion were almost threefold more likely to consume alcohol during pregnancy as compared to women who had no abortion history, OR = 2.88 (95%CI = 2.09, 3.96) (Fig. 4). This review also demonstrated that gravidity and parity were not significant factors for alcohol use during pregnancy in Africa (Additional file 8: Figure S5).
Psychosocial and other factors
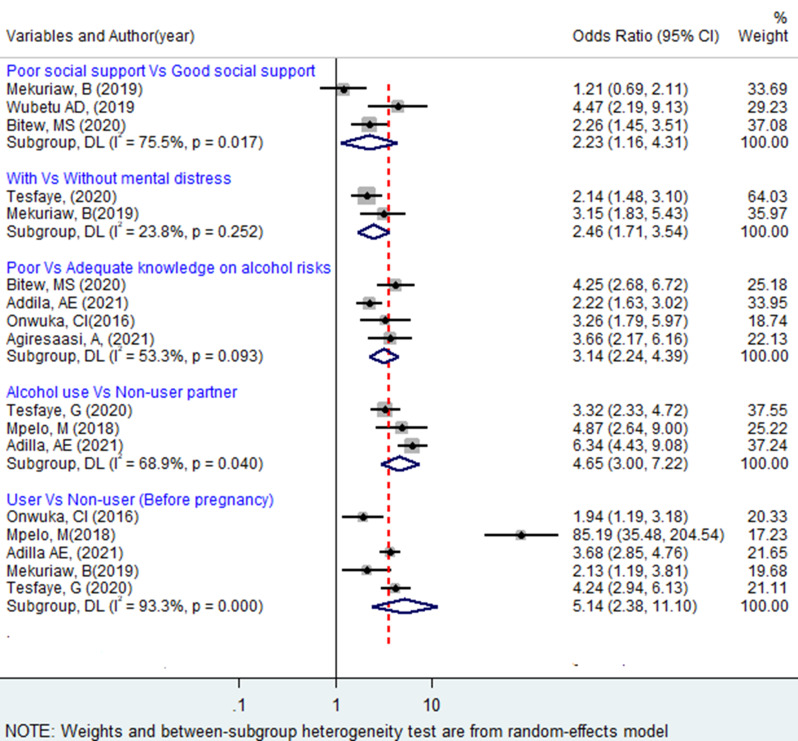
A total of three articles [35, 37, 49] were included to see the association between social support and alcohol consumption, of which two of them [35, 57] have shown a significant association with alcohol consumption. The pooled analysis showed that pregnant women with poor social support were two times more likely to use alcohol during pregnancy as compared to those with strong social support, OR = 2.23 (95%CI: 1.16,4.31). The association between mental distress and alcohol use during pregnancy was reported in two articles. The pooled estimate indicated that pregnant women who had mental distress were almost two and a half times more likely to use alcohol compared to those who did not (OR = 2.46; 95% CI: 1.71, 3.54). Articles considered for pooling the association between mental distress and alcohol use during pregnancy have no considerable heterogeneity (I2 = 23.8, p-value = 0.252).
The pooled effect of three articles [29, 99, 108] showed that partner alcohol use was positively associated with alcohol use during pregnancy. Women whose partners or husbands drink alcohol were 4.65 times more likely to drink alcohol during pregnancy compared with those whose partners or husbands do not drink alcohol (OR = 4.65; 95%CI: 3.00, 7.22). The heterogeneity test indicated I2 = 68.9. We examined the relation between pregnant women’s knowledge of the health risks of alcohol use and their intake of alcohol during pregnancy using 4 studies [29, 35, 101, 112] in this meta-analysis, and the results showed that alcohol use during pregnancy was positively associated with women’s knowledge of the health risks of alcohol use (OR = 3.14; 95% CI: 2.24, 4.39). Moderate heterogeneity (I2 = 53.3%, p-value = 0.093) was observed across the included articles.
A total of five studies [29, 49, 99, 108, 112] with pre-pregnancy alcohol consumption information were pooled. The review indicated that pre-pregnancy drinking behavior was found to have a significant effect on drinking during pregnancy. The estimate pooled value shows that women who drank alcohol before pregnancy were around 5 times more likely to continue to drink alcohol during pregnancy than women who did not report a previous history of drinking (OR = 5.14; 95% CI: 2.38, 11.10). The heterogeneity test (I2 = 93.3, p < 0.001) showed a significant variation across articles (Fig. 5).
Fig. 5.
Forest plot of odds ratio for the association of selected psychosocial and other factors with of alcohol use during pregnancy in Africa
The pooled prevalence of smoking exposure during pregnancy in Africa
The prevalence of active smoking ranged from 1.1% [14] to 36.8% [70] in Tanzania and South Africa, respectively. The pooled prevalence of smoking exposure during pregnancy in Africa from 36 studies was found to be 19.66% (95% CI: 15.13, 24.19%). There was a wide variation in smoking exposure prevalence. The heterogeneity was very high (I2 = 99.7%, p < 0.001). A greater disparity in the prevalence of active smoking was revealed in the studies. The prevalence ranges from 1.10% (95% CI: 0.28, 1.92) reported in Tanzania to 36.80% (95% CI: 31.55, 42.05) reported in South Africa. A total of 28 studies were included in the meta-analysis to estimate the overall pooled prevalence of active smoking during pregnancy. The pooled prevalence of SHS exposure and active cigarette smoking during pregnancy in Africa was 43.45% (95%CI (32.86, 54.04%) and 11.85% (95%CI (9.25, 14.45%)), respectively (Fig. 6).
Fig. 6.
Forest plot of the pooled prevalence of second-hand smoke exposure and active smoking in Africa, 2002 − 2021
The presence of publication bias was investigated using visual inspection of the funnel plot and Egger’s test. We evaluated the funnel plot for asymmetry by visual inspection of the prevalence of smoking during pregnancy. The funnel plot figures (Additional file 4: Figure S3 and S4) show the presence of possible publication bias. The Egger’s regression asymmetry test also indicated significant publication bias (p-value less than 0.001). Based on trim-and-fill analysis, after adding ten studies, the pooled prevalence of active smoking in Africa was 2.64% (Additional file 5: Table S5).
Sensitivity analysis of smoking
To discover a significant single study effect on the combined estimate of active smoking and SHS exposure, the leave-one-out analysis was applied. Our result indicated that no study had a significant impact on the total pooled prevalence of active smoking and SHS exposure during pregnancy (Additional file 6: Table S8 and S9).
Subgroup analysis of prevalence of the active smoking during pregnancy
Because of the substantial variability across the studies, we conducted a subgroup analysis. We performed subgroup analysis using sub-region, study design, study setting, sample size, income classification, and publication year as the variables of interest. Subgroup analysis indicated that the highest pooled prevalence of active smoking during pregnancy was observed in Southern Africa 28.92% (95% CI: 26.94, 31.90), followed by 25.51% (95%CI: 6.61, 44.42) in Northern Africa, 6.97% (95%CI: 2.56, 11.38) in West Africa, 5.44% (95%CI: 0.26, 10.62) in Middle Africa, and the lowest was in Eastern Africa, 3.33% (95%CI: 2.24, 4.42). There was significant heterogeneity across the sub-regions (p-value < 0.0001).
Based on the study design, the pooled prevalence of active smoking during pregnancy for five studies, which applied a cohort study design, was the highest, at 22.97% (95% CI: 9.91, 36.03%). The subgroup analysis by country income level indicated that the highest prevalence of active smoking in upper middle income, 28.89% (95%CI: 26.37, 31.41) and the lowest in low income, 4.91% (95%CI: 3.38, 6.45). According to study setting, a higher pooled prevalence was observed from twenty five studies conducted in healthcare institutions, 12.06% (95% CI: 9.10, 15.03%).
In addition, subgroup analysis was also conducted using sample size, and those articles done with a sample size less than 400 had the highest pooled prevalence, 13.12% (95%CI: 7.32, 8.93), while studies done with a sample size greater than 400 had the lowest pooled prevalence, 11.27% (95%CI: 8.19,14.35). Lastly, we also performed subgroup analysis using the publication year (during MDG and after MDG) of the primary articles. The result of this analysis revealed that articles published from 2002 to 2015 had the highest pooled prevalence, 14.15% (95% CI: 9.16, 19.14), while articles published from 2016 to 2021 had the lowest prevalence, 10.66% (95% CI: 7.44, 13.89) (Additional file 7: Table S12).
Discussion
We conducted this review with the objective of determining the pooled prevalence of khat use, alcohol intake, and cigarette smoke exposure during pregnancy, and associated risk factors in Africa using studies published between January 1, 2002 and November 30, 2021.
According to the findings of 18 articles included in this meta-analysis, the overall pooled prevalence of khat consumption during pregnancy was 18.93% (95% CI: 13.44, 24.43). Generally, there was a great difference among individual studies. The pooled prevalence of khat use among pregnant women in our review is lower than the result of the primary studies conducted in Yemen, which indicated the prevalence of khat use was 27.97% in Sana’a [119] and 49.2% in Dhamar district [120]. One potential reason for this disparity is that the majority of respondents in the aforementioned articles were Muslims. As Muslim religion is one of the risk factors for using khat during pregnancy [121], this might increase the prevalence of khat use. Khat use is deeply imbedded in the culture and social life of the East Africa and Arabian Peninsula, particularly among Muslim-majority societies [122, 123]. For example, in Ethiopia, several Muslims chew khat while visiting pilgrimage sites and performing rituals such as singing, praying (du’a), blessing, and other activities [124].
The use of khat by one’s partner was one of the factors that contributed to khat chewing during pregnancy. This result was similar to the results of a Yemeni study [125]. This might be related to the fact that a partner plays a role as an important role model for a woman to decide to chew, and sometimes one can be invited to chew khat and becomes hard for the woman to resist.
The overall prevalence of alcohol drinking during pregnancy in this review is comparable to the result of a meta-analysis conducted in Sub-Saharan Africa (20.83%) [2]. This result could be explained by similarities in socio-demographics, methodologies, and the characteristics of individual studies included in both reviews. The pooled prevalence of alcohol drinking in this review is within the range of the prevalence reported from Latin America and Caribbean countries (4.8–23.3%) [126], and Sub-Saharan countries (3.0–32.4%) [127]. The overall pooled prevalence of alcohol use in this study was higher than what was reported from a meta-analysis of 328 population based studies from 50 countries across the world (9.8%) [1]. Likewise, the overall reported prevalence of alcohol use in this review is higher than the result of the meta-analysis conducted in four African countries (18.5%) [128]. Variations in prevalence may be explained by differences in socioeconomic status, study period, and access to and utilization of ANC services.
The highest alcohol use prevalence reported in this review was 64.7% in a study conducted in West Cape Province, South Africa [61]. This result is in line with a review conducted in Sub-Saharan Africa. In the subgroup analysis, the highest prevalence of alcohol drinking during pregnancy was reported in the Middle Africa region (25.69%) and followed by the Southern African region (24.20%). The lowest prevalence of alcohol drinking was seen in the North Africa region (1.1%). This variation could be attributed to differences in religion and cultural practices observed between geographic areas, countries, and societies, as well as their beliefs about alcohol intake. The majority of people in North Africa follow the Muslim religion. The Holy Quran and Al-Hadith, the two main sources of Islamic law, both state that drinking alcohol is generally forbidden [129].
We identified the following risk factors for alcohol use during pregnancy that may aid in the development of targeted interventions: women’s low educational status, younger age, unplanned pregnancy, mental distress, poor social support, a lack of knowledge about alcohol risks, pre-pregnancy alcohol use, and partner alcohol use. In line with a meta-analysis conducted in Sub-Saharan African countries [2], we also noticed that the odd of alcohol intake during pregnancy was found to be higher in pregnant women whose partners drink alcohol. It may be the case that having a husband or partner who uses alcohol places some pressure on pregnant women to drink with their husband. Enabling male partners to participate in ANC is an effective strategy for enhancing maternal health outcomes. Men are an essential stakeholder and should be considered as half of the equation in maternal and child health [130].
The odd of alcohol consumption during pregnancy among illiterate women was 1.93 times higher when compared with pregnant women having formal education. This result is in agreement with a study conducted in Ukraine, which reported that women with low education level had higher odds of alcohol use during pregnancy [131]. Pregnant women should be adequately empowered in education so as to improve awareness and knowledge of alcohol risks during pregnancy.
The review also revealed that the odds of having risky alcohol consumption behaviors were two times higher among women who had weak social support as compared with women who had strong social support. A similar result was found in a study conducted in Sweden [132]. In agreement with the result of a systematic review conducted in Sub-Saharan Africa [2], pregnant women with mental distress were 2.46 times more likely to use alcohol as compared to pregnant women without mental distress. This finding is supported by the fact that people experiencing mental distress are more likely to use alcohol to alleviate the difficulties associated with sleep initiation, social engagement, and a lack of happiness, all of which are major symptoms of mental distress.
Our review also revealed that pre-pregnancy alcohol use was significantly associated with alcohol consumption during pregnancy. This finding was consistent with the findings of studies conducted in Sweden [132] and Australia [133]. According to research in a range of areas, habits practiced in stable contexts are unlikely to be instantaneously reconsidered. Most women who use alcohol prior to becoming pregnant may experience withdrawal symptoms, making it tough for them to cease suddenly after becoming pregnant. One systematic review found that, preconception interventions were effective in lowering alcohol-exposed pregnancies risk during the preconception period by preventing unexpected pregnancy [134]. Therefore, a wide diversity of sectors and stakeholders must be involved to ensure full access to preconception care. According to the findings of this review, the pooled prevalence of active cigarette use during pregnancy was 11.85%. The pooled prevalence of this study was higher than the finding of the meta-analysis result conducted in 54 LMICs (2.6%) [13]. This figure is also higher than a previously published study conducted in sub-Saharan Africa, which reported a prevalence of 1.8% [135]. This variation might be because of differences in socio-demographic and economic characteristics, the definition of smoking, and the study period.
Limitations
Some limitations were observed during the present review. The large percentages of the primary articles included in the current review were cross-sectional researches, which might influence the outcome variable due to other confounding factors. This review did not include research articles written in languages other than English. The pooled prevalence of khat chewing was determined using 18 studies conducted in Ethiopia. As a result, it does not represent the whole African continent. The use of various information gathering tools in the included studies may have an impact on generalizing the findings. Self-reported measures were used to collect data on khat chewing, alcohol consumption, and smoking during pregnancy. Hence, it is vulnerable to recall and reporting biases. Hence, the pooled prevalence of khat, alcohol, and cigarette use during pregnancy might be underestimated in the current review. Furthermore, potentially unpublished articles were not considered, and hidden sources of heterogeneity may also have an impact on the overall finding of the result.
Implications of the findings
Our systematic review provides a comprehensive overview of the prevalence and associated factors of khat, alcohol, and cigarette use among pregnant women across different African regions. The findings of our review may help policymakers, researchers, and other stakeholders identify the regions in Africa with a high prevalence of khat, alcohol, or cigarette use during pregnancy. Identifying regions in Africa with a high prevalence of khat, alcohol, or cigarette use during pregnancy allows policymakers to allocate resources more effectively, ensuring that interventions reach those who need them the most. Our reviews identify the most significant risk factors for khat and alcohol use during pregnancy, allowing policymakers to design targeted interventions to prevent the risk factors. Moreover, identifying and addressing these risk factors can lead to more effective public health campaigns and educational programs aimed at reducing khat and alcohol use among pregnant women. Our review also provides a benchmark for monitoring and evaluating the effectiveness of implemented policies and interventions over time. With a clearer understanding of risk factors, health professionals can implement targeted interventions to prevent khat, alcohol, and cigarette use during pregnancy.
Our systematic review and meta-analysis can highlight gaps in the existing literature, guiding future research priorities and funding towards areas that need further investigation. First, there were some countries that were not covered by the studies included in this review and meta-analysis. Second, the influence of community-level factors such as the density of khat, alcohol, or cigarette outlets per area on khat, alcohol, or cigarette use was not explored. Thirdly, there is a scarcity of prospective cohort studies that assess patterns of khat, alcohol, or cigarette use throughout pregnancy. Therefore, our systematic review contributes to advancing the research agenda on khat, alcohol, and cigarette use during pregnancy in Africa by highlighting priority areas for future research, and methodological improvements.
Conclusion
This systematic review and meta-analysis indicated that the pooled magnitude of khat, alcohol, and cigarette use during pregnancy was higher in Africa. Wide differences in the magnitude of alcohol and cigarette use were observed across the different sub-regions of Africa, with the highest levels of alcohol use and smoking being in the Southern Africa region. Husband or partner khat use was a risk factor for khat use during pregnancy. The associated factors for alcohol intake during pregnancy include women with low educational levels; younger women; unplanned pregnancy; poor social support; mental distress; poor knowledge of alcohol risks; pre-pregnancy alcohol drinking; and partner alcohol use.
As a result, efforts should be increased to address the reported risk factors in order to decrease the burden of khat, alcohol, and cigarette use during pregnancy. Substance use screening and brief interventions (SBI) should be routinely delivered in antenatal care settings to reduce pregnant women’s substance use. Community-based health education programs and point-of-sale warnings on khat, alcohol, and cigarettes should also be strengthened. Additionally, governments should monitor the price of khat, alcohol, and cigarettes in relation to real income to make sure that these substances do not become more affordable.
But there are still many data shortages in terms of research coverage, and community-level factors contribute to the prevalence of alcohol, khat, and cigarette use during pregnancy and distribution across the continent, which urges future studies. Future studies should examine in greater detail the effects of khat on fetal and postnatal physical and mental development.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank our colleagues who gave useful advice on the completion of this review.
Abbreviations
- AJOL
African Journals Online
- ANC
Antenatal Care
- ASSIST
Alcohol, Smoking and Substance Involvement Screening Test
- AUDIT-C
Alcohol Use Disorder Identification Test-Consumption
- CI
Confidence Interval
- JBI
Joanna Briggs Institute
- MDGs
Millennium Development Goals
- MeSH
Medical Subject Heading
- OR
Odds ratio
- PNQ
Prenatal Questionnaire
- PRISMA
The preferred reporting items for systematic reviews and meta-analysis
- SE
Standard Error
- SHS
Secondhand Smoke
- SNNP
South Nations, Nationalities and People
- T-ACE
Tolerance-Annoyance, Cut Down, Eye Opener
- UN
United Nation
Author contributions
BW, TD, MA, EW and KD contributed to the conception of the study. BW, TD, EW, MA and KD designed the study. BW and TD searched and screened the literatures. EW and MA critically appraised. BW, TD, MA, EW and KD extracted and analyzed data. BW, TD, MA, EW and KD reviewed literatures and rewrote the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Popova S, Lange S, Probst C, Gmel G, Rehm J. Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(3):e290–9. [DOI] [PubMed] [Google Scholar]
- 2.Addila AE, Bisetegn TA, Gete YK, Mengistu MY, Beyene GM. Alcohol consumption and its associated factors among pregnant women in Sub-saharan Africa: a systematic review and meta-analysis’ as given in the submission system. Subst Abuse Treat Prev Policy. 2020;15(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onaolapo OJ, Olofinnade AT, Ojo FO, Adeleye O, Falade J, Onaolapo AY. Substance use and substance use disorders in Africa: an epidemiological approach to the review of existing literature. World J Psychiatry. 2022;12(10):1268–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang G. Maternal substance use: consequences, identification, and interventions. Alcohol Res. 2020;40(2):06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broccia M, Hansen BM, Winckler JM, Larsen T, Strandberg-Larsen K, Torp-Pedersen C, et al. Heavy prenatal alcohol exposure and obstetric and birth outcomes: a Danish nationwide cohort study from 1996 to 2018. Lancet Public Health. 2023;8(1):e28–35. [DOI] [PubMed] [Google Scholar]
- 6.Sundermann AC, Velez Edwards DR, Slaughter JC, Wu P, Jones SH, Torstenson ES, et al. Week-by-week alcohol consumption in early pregnancy and spontaneous abortion risk: a prospective cohort study. Am J Obstet Gynecol. 2021;224(1):97. e1-.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pereira P, Mata F, Figueiredo A, Silva RB, Pereira MG. Maternal exposure to Alcohol and low birthweight: a systematic review and Meta-analysis. Rev Bras Ginecol Obstet. 2019;41(5):333–47. [DOI] [PubMed] [Google Scholar]
- 8.Ikehara S, Kimura T, Kakigano A, Sato T, Iso H, Group tJECsS. Association between maternal alcohol consumption during pregnancy and risk of preterm delivery: the Japan Environment and Children’s study. BJOG: Int J Obstet Gynecol. 2019;126(12):1448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oh SS, Jee Y, Park EC, Kim YJ. Alcohol Use disorders and increased risk of adverse birth complications and outcomes: an 11-Year Nationwide Cohort Study. Int J Environ Res Public Health. 2020;17(22). [DOI] [PMC free article] [PubMed]
- 10.Diabelková J, Rimárová K, Urdzík P, Dorko E, Houžvičková A, Andraščíková Š, et al. Influence of maternal smoking during pregnancy on birth outcomes. Cent Eur J Public Health. 2022;30(Supplement):S32–6. [DOI] [PubMed] [Google Scholar]
- 11.Hamadneh S, Hamadneh J. Active and Passive maternal smoking during pregnancy and birth outcomes: a study from a developing country. Ann Glob Health. 2021;87(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarasi B, Cornuz J, Clair C, Baud D. Cigarette smoking during pregnancy and adverse perinatal outcomes: a cross-sectional study over 10 years. BMC Public Health. 2022;22(1):2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caleyachetty R, Tait CA, Kengne AP, Corvalan C, Uauy R, Echouffo-Tcheugui JB. Tobacco use in pregnant women: analysis of data from demographic and health surveys from 54 low-income and middle-income countries. Lancet Glob Health. 2014;2(9):e513–20. [DOI] [PubMed] [Google Scholar]
- 14.Nombo AP, Mwanri AW, Brouwer-Brolsma EM, Ramaiya KL, Feskens EJM. Gestational diabetes mellitus risk score: a practical tool to predict gestational diabetes mellitus risk in Tanzania. Diabetes Res Clin Pract. 2018;145:130–7. [DOI] [PubMed] [Google Scholar]
- 15.Fakhfakh R, Jellouli M, Klouz A, Ben Hamida M, Lakhal M, Belkahia C, et al. Smoking during pregnancy and postpartum among Tunisian women. J Matern Fetal Neonatal Med. 2011;24(6):859–62. [DOI] [PubMed] [Google Scholar]
- 16.Sobh E, Mohammed AM, Adawy Z, Nassef AH, Hasheesh A. The impact of secondhand smoke exposure on the pregnancy outcome: a prospective cohort study among Egyptian community. Egypt J Bronchol. 2021 2021/11/06;15(1):50.
- 17.Rang NN, Hien TQ, Chanh TQ, Thuyen TK. Preterm birth and secondhand smoking during pregnancy: a case-control study from Vietnam. PLoS ONE. 2020;15(10):e0240289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gebissa E. Khat in the Horn of Africa: historical perspectives and current trends. J Ethnopharmacol. 2010;132(3):607–14. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Shabat S, Goloubinoff P, Dudai N, Lewinsohn E. Farming amphetamines: Khat (Catha edulis Forsk.) A traditional plant with mild stimulating psychoactive and medicinal properties. Medicinal and aromatic plants of the Middle-East. Springer; 2014. pp. 181–97.
- 20.Khawaja M, Al-Nsour M, Saad G. Khat (Catha edulis) chewing during pregnancy in Yemen: findings from a national population survey. Matern Child Health J. 2008;12(3):308–12. [DOI] [PubMed] [Google Scholar]
- 21.Tesfay K, Abera M, Wondafrash M, Tesfaye M. Effect of Khat Use during pregnancy on the Birth Weight of Newborn in Jimma, Ethiopia. Int J Mental Health Addict. 2019 2019/12/01;17(6):1432–41.
- 22.Yitayih Y, Vanderplasschen W, Vandewalle S, Rita VD, Gilbert L. The effects of khat use during pregnancy on perinatal and maternal outcomes: a meta-analysis. Arch Womens Ment Health. 2023;26(1):11–27. [DOI] [PubMed] [Google Scholar]
- 23.Wondemagegn AT, Bekana M, Bekuretsion Y, Afework M. The effect of possible mediators on the association between chewing khat during pregnancy and fetal growth and newborn size at birth in Eastern Ethiopia. BMC Pregnancy Childbirth. 2024;24(1):63. 2024/01/13;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG, The PG. Preferred reporting items for systematic reviews and Meta-analyses: the PRISMA Statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wogayehu B, Demissie T, Daka K, Alemayehu M. Magnitude and risk factors of psychoactive substance use among pregnant women in Africa: a systematic review and meta-analysis. Int Prospect Regist Syst Rev [Internet]. 2021; (CRD42021289074):1–6.
- 26.Aromataris E, Munn Z, editors. JBI Manual for evidence synthesis. JBI; 2020.
- 27.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Aert RCM, Wicherts JM, van Assen MALM. Publication bias examined in meta-analyses from psychology and medicine: a meta-meta-analysis. PLoS ONE. 2019;14(4):e0215052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Addila AE, Azale T, Gete YK, Yitayal M. Individual and community-level predictors of maternal alcohol consumption during pregnancy in Gondar town, Northwest Ethiopia: a multilevel logistic regression analysis. BMC Pregnancy Childbirth. 2021;21(1):419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agiresaasi A, Nassanga G, Maina GW, Kiguli J, Nabiwemba E, Tumwesigye NM. Various forms of alcohol use and their predictors among pregnant women in post conflict northern Uganda: a cross sectional study. Subst Abuse Treat Prev Policy. 2021;16(1):020–00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmed S, Hassen K, Wakayo T. A health facility based case-control study on determinants of low birth weight in Dassie town, Northeast Ethiopia: the role of nutritional factors. Nutr J. 2018;17(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmed SM, Sundby J, Aragaw YA, Abebe F. Self-Medication and Safety Profile of Medicines used among pregnant women in a Tertiary Teaching Hospital in Jimma, Ethiopia: a cross-sectional study. Int J Environ Res Public Health. 2020;17(11). [DOI] [PMC free article] [PubMed]
- 33.Alamneh AA, Endris BS, Gebreyesus SH. Caffeine, alcohol, khat, and tobacco use during pregnancy in Butajira, South Central Ethiopia. PLoS ONE. 2020;15(5):e0232712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belayhun Y, Kassa Y, Mekonnen N, Binu W, Tenga M, Duko B. Determinants of pregnancy-Induced hypertension among mothers attending public hospitals in Wolaita Zone, South Ethiopia: findings from unmatched case-control study. Int J Hypertens. 2021;2021:6947499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bitew MS, Zewde MF, Wubetu M, Alemu AA. Consumption of alcohol and binge drinking among pregnant women in Addis Ababa, Ethiopia: prevalence and determinant factors. PLoS ONE. 2020;15(12):e0243784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chomba E, Tshefu A, Onyamboko M, Kaseba-Sata C, Moore J, McClure EM, et al. Tobacco use and secondhand smoke exposure during pregnancy in two African countries: Zambia and the Democratic Republic of the Congo. Acta Obstet Gynecol Scand. 2010;89(4):531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Debella A, Dheresa M, Geda B, Tiruye G, Fage SG. A third of pregnant women are affected by Anemia in Eastern Ethiopia: a facility-based study. J Blood Med. 2021;12:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Demelash H, Motbainor A, Nigatu D, Gashaw K, Melese A. Risk factors for low birth weight in Bale Zone hospitals, South-East Ethiopia: a case-control study. BMC Pregnancy Childbirth. 2015;15:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dendir E, Deyessa N. Substance use and birth weight among mothers attending public hospitals: a case control study. Ethiop J Health Dev. 2017;31.
- 40.English LL, Mugyenyi G, Nightingale I, Kiwanuka G, Ngonzi J, Grunau BE, et al. Prevalence of ethanol use among pregnant women in Southwestern Uganda. Matern Child Health J. 2016;20(10):2209–15. [DOI] [PubMed] [Google Scholar]
- 41.Fetene MT, Teji K, Assefa N, Bayih WA, Tsehaye G, Hailemeskel HS. Magnitude and associated factors of substance use among pregnant women attending antenatal care in public hospitals of eastern Ethiopia. BMC Psychiatry. 2021;21(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Getachew B, Etefa T, Asefa A, Terefe B, Dereje D. Determinants of low fifth minute apgar score among Newborn delivered in Jimma University Medical Center, Southwest Ethiopia. Int J Pediatr. 2020;2020:9896127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Isaksen AB, Østbye T, Mmbaga BT, Daltveit AK. Alcohol consumption among pregnant women in Northern Tanzania 2000–2010: a registry-based study. BMC Pregnancy Childbirth. 2015;15(205):015–0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jember DA, Menji ZA, Yitayew YA. Low Birth Weight and Associated Factors among Newborn Babies in Health Institutions in Dessie, Amhara, Ethiopia. J Multidiscip Healthc. 2020;13:1839–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kedir H, Berhane Y, Worku A. Khat chewing and restrictive dietary behaviors are associated with anemia among pregnant women in high prevalence rural communities in eastern Ethiopia. PLoS ONE. 2013;8(11):e78601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kimbui E, Kuria M, Yator O, Kumar M. A cross-sectional study of depression with comorbid substance use dependency in pregnant adolescents from an informal settlement of Nairobi: drawing implications for treatment and prevention work. Ann Gen Psychiatry. 2018;17:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mdoe MB, Kibusi SM, Munyogwa MJ, Ernest AI. Prevalence and predictors of gestational diabetes mellitus among pregnant women attending antenatal clinic in Dodoma region, Tanzania: an analytical cross-sectional study. BMJ Nutr Prev Health. 2021;4(1):69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mekonnen AG, Hordofa AG, Kitila TT, Sav A. Modifiable risk factors of congenital malformations in bale zone hospitals, Southeast Ethiopia: an unmatched case-control study. BMC Pregnancy Childbirth. 2020;20(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mekuriaw B, Belayneh Z, Yitayih Y. Magnitude of Khat use and associated factors among women attending antenatal care in Gedeo Zone health centers, southern Ethiopia: a facility based cross sectional study. BMC Public Health. 2020;20(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moise IK. Alcohol use, pregnancy and associated risk factors: a pilot cross-sectional study of pregnant women attending prenatal care in an urban city. BMC Pregnancy Childbirth. 2019;19(1):019–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mpelo M, Kibusi SM, Moshi F, Nyundo A, Ntwenya JE, Mpondo BCT. Prevalence and factors influencing alcohol use in pregnancy among women attending Antenatal Care in Dodoma Region, Tanzania: a cross-sectional study. J Pregnancy. 2018;18:8580318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakajima M, Jebena MG, Taha M, Tesfaye M, Gudina E, Lemieux A, et al. Correlates of khat use during pregnancy: a cross-sectional study. Addict Behav. 2017;73:178–84. [DOI] [PubMed] [Google Scholar]
- 53.Namagembe I, Jackson LW, Zullo MD, Frank SH, Byamugisha JK, Sethi AK. Consumption of alcoholic beverages among pregnant urban Ugandan women. Matern Child Health J. 2010;14(4):492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sema A, Tesfaye F, Belay Y, Amsalu B, Bekele D, Desalew A. Associated factors with low birth weight in dire Dawa City, Eastern Ethiopia: a cross-sectional study. Biomed Res Int. 2019;2019:2965094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tesfaye G, Demlew D, Habte MGT, Molla F, Kifle G. The prevalence and associated factors of alcohol use among pregnant women attending antenatal care at public hospitals Addis Ababa, Ethiopia, 2019. BMC Psychiatry. 2020;20(1):337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wagura P, Wasunna A, Laving A, Wamalwa D. Ng’Ang’a P. Prevalence and factors associated with preterm birth at kenyatta national hospital. BMC Pregnancy Childbirth. 2018;18(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wubetu AD, Habte S, Dagne K. Prevalence of risky alcohol use behavior and associated factors in pregnant antenatal care attendees in Debre Berhan, Ethiopia, 2018. BMC Psychiatry. 2019;19(1):250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wynn A, Nabukalu D, Lutalo T, Wawer M, Chang LW, Kiene SM et al. Alcohol use during pregnancy in Rakai, Uganda. PLoS ONE. 2021;16(8). [DOI] [PMC free article] [PubMed]
- 59.Yadeta TA, Egata G, Seyoum B, Marami D. Khat chewing in pregnant women associated with prelabor rupture of membranes, evidence from eastern Ethiopia. Pan Afr Med J. 2020;36:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cluver CA, Charles W, van der Merwe C, Bezuidenhout H, Nel D, Groenewald C, et al. The association of prenatal alcohol exposure on the cognitive abilities and behaviour profiles of 4-year-old children: a prospective cohort study. BJOG. 2019;126(13):1588–97. [DOI] [PubMed] [Google Scholar]
- 61.Fletcher OV, May PA, Seedat S, Sikkema KJ, Watt MH. Attitudes toward alcohol use during pregnancy among women recruited from alcohol-serving venues in Cape Town, South Africa: a mixed-methods study. Soc Sci Med. 2018;215:98–106. [DOI] [PubMed] [Google Scholar]
- 62.Macleod CI, Young C, Molokoe K. Alcohol use during pregnancy: prevalence and patterns in selected Buffalo City areas, South Africa. Afr J Reprod Health. 2021;25(1):114–21. [DOI] [PubMed] [Google Scholar]
- 63.Maré KT, Pellowski JA, Koopowitz SM, Hoffman N, van der Westhuizen C, Workman L, et al. Perinatal suicidality: prevalence and correlates in a South African birth cohort. Arch Womens Ment Health. 2021;24(5):737–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morojele NK, London L, Olorunju SA, Matjila MJ, Davids AS, Rendall-Mkosi KM. Predictors of risk of alcohol-exposed pregnancies among women in an urban and a rural area of South Africa. Soc Sci Med. 2010;70(4):534–42. [DOI] [PubMed] [Google Scholar]
- 65.Myers B, Koen N, Donald KA, Nhapi RT, Workman L, Barnett W, et al. Effect of Hazardous Alcohol Use during pregnancy on growth outcomes at birth: findings from a South African cohort study. Alcohol Clin Exp Res. 2018;42(2):369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Onah MN, Field S, van Heyningen T, Honikman S. Predictors of alcohol and other drug use among pregnant women in a peri-urban South African setting. Int J Ment Health Syst. 2016;10(38):016–0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peltzer K, Pengpid S. Maternal alcohol use during pregnancy in a general national population in South Africa. S Afr J Psychiatr. 2019;25(0). [DOI] [PMC free article] [PubMed]
- 68.Petersen Williams P, Jordaan E, Mathews C, Lombard C, Parry CD. Alcohol and other Drug Use during pregnancy among women attending midwife obstetric units in the Cape Metropole, South Africa. Adv Prev Med. 2014;871427(10):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vanker A, Barnett W, Brittain K, Gie RP, Koen N, Myers B, et al. Antenatal and early life tobacco smoke exposure in an African birth cohort study. Int J Tuberc Lung Dis. 2016;20(6):729–37. [DOI] [PubMed] [Google Scholar]
- 70.Vythilingum B, Roos A, Faure SC, Geerts L, Stein DJ. Risk factors for substance use in pregnant women in South Africa. S Afr Med J. 2012;102(11 Pt 1):851–4. [DOI] [PubMed] [Google Scholar]
- 71.Abasiubong F, Bassey EA, Udobang JA, Akinbami OS, Udoh SB, Idung AU. Self-Medication: potential risks and hazards among pregnant women in Uyo, Nigeria. Pan Afr Med J. 2012;13(15):19. [PMC free article] [PubMed] [Google Scholar]
- 72.Adebowale OO, James BO. Psychoactive substance use and psychiatric morbidity among pregnant women attending an ante-natal clinic in Benin City, Nigeria. Niger Postgrad Med J. 2018;25(1):8–12. [DOI] [PubMed] [Google Scholar]
- 73.Adusi-Poku Y, Edusei AK, Bonney AA, Tagbor H, Nakua E, Otupiri E. Pregnant women and alcohol use in the Bosomtwe district of the Ashanti region-Ghana. Afr J Reprod Health. 2012;16(1):55–60. [PubMed] [Google Scholar]
- 74.Da Pilma Lekettey J, Dako-Gyeke P, Agyemang SA, Aikins M. Alcohol consumption among pregnant women in James Town Community, Accra, Ghana. Reprod Health. 2017;14(1):017–0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kargbo DK, Nyarko K, Sackey S, Addo-Lartey A, Kenu E, Anto F. Determinants of low birth weight deliveries at five referral hospitals in Western Area Urban district, Sierra Leone. Ital J Pediatr. 2021;47(1):021–01160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mutihir J, Musa J, Daru P, Nyango D, Audu M. Substance abuse among antenatal patients at Jos university teaching hospital, north central Nigeria. J West Afr Coll Surg. 2012;2(2):50–62. [PMC free article] [PubMed] [Google Scholar]
- 77.Obiora CC, Dim CC, Uzochukwu BS, Ezugwu FO. Cigarette smoking and perception of its advertisement among antenatal clinic attendees in referral health facilities in Enugu, Nigeria. Niger J Clin Pract. 2015;18(1):80–5. [DOI] [PubMed] [Google Scholar]
- 78.Odukoya O, Oyekan G, Igwilo U. Awareness, attitudes and factors associated with second hand smoke exposure among non-smoking pregnant women attending the ante-natal clinic of the Lagos University Teaching Hospital. Nigerian Q J Hosp Med. 2015;25(4):292–6. [Google Scholar]
- 79.Ome-Kaius M, Unger HW, Singirok D, Wangnapi RA, Hanieh S, Umbers AJ, et al. Determining effects of areca (betel) nut chewing in a prospective cohort of pregnant women in Madang Province, Papua New Guinea. BMC Pregnancy Childbirth. 2015;15(177):015–0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Onunkwor OF, Dubai SARA, Shuaibu HO. Second hand smoke exposure among pregnant women in Nigeria. J Adv Med Med Res. 2020;32(13):29–36. [Google Scholar]
- 81.Onwuka CI, Ugwu EO, Dim CC, Menuba IE, Iloghalu EI. Prevalence and predictors of Alcohol Consumption during pregnancy in South-Eastern Nigeria. J Clin Diagn Res. 2016;10(9):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ordinioha B, Brisibe S. Alcohol consumption among pregnant women attending the ante-natal clinic of a tertiary hospital in South-South Nigeria. Niger J Clin Pract. 2015;18(1):13–7. [DOI] [PubMed] [Google Scholar]
- 83.Sanou AS, Diallo AH, Holding P, Nankabirwa V, Engebretsen IMS, Ndeezi G et al. Maternal alcohol consumption during pregnancy and child’s cognitive performance at 6–8 years of age in rural Burkina Faso: an observational study. PeerJ. 2017;30(5). [DOI] [PMC free article] [PubMed]
- 84.Ben Salah A, Lemieux A, Mlouki I, Amor I, Bouanene I, Ben Salem K, et al. Impact of social violence and childhood adversities on pregnancy outcomes: a longitudinal study in Tunisia. J Glob Health. 2019;9(2):020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.El-Shahawy O, Labib K, Stevens E, Kahn LG, Anwar W, Oncken C et al. Exclusive and dual cigarette and Hookah Smoking is Associated with adverse perinatal outcomes among pregnant women in Cairo, Egypt. Int J Environ Res Public Health. 2021;18(24). [DOI] [PMC free article] [PubMed]
- 86.Hassan NE, Shalaan AH, El-Masry SA. Relationship between maternal characteristics and neonatal birth size in Egypt. East Mediterr Health J. 2011;17(4):281–9. [PubMed] [Google Scholar]
- 87.Ibrahim ZM, Sayed Ahmed WA, El-Hamid SA, Hagras AM. Intimate partner violence among Egyptian pregnant women: incidence, risk factors, and adverse maternal and fetal outcomes. Clin Exp Obstet Gynecol. 2015;42(2):212–9. [PubMed] [Google Scholar]
- 88.Maged A, Elsherbini M, Ramadan W, Elkomy R, Helal O, Hatem D, et al. Periconceptional risk factors of spina bifida among Egyptian population: a case-control study. J Matern Fetal Neonatal Med. 2016;29(14):2264–7. [DOI] [PubMed] [Google Scholar]
- 89.Saeed OA, Ahmed HA, Ibrahim AM, Mahmood EA, Abdu-Allah TO. Risk factors of low birth weight at three hospitals in Khartoum State, Sudan. Sudan J Paediatr. 2014;14(2):22–8. [PMC free article] [PubMed] [Google Scholar]
- 90.Yaya S, Bishwajit G. Exposure to second-hand smoking as a predictor of fetal loss: Egypt Demographic and Health Survey 2014. Int Health. 2019;11(6):561–7. [DOI] [PubMed] [Google Scholar]
- 91.Greenmyer JR, Klug MG, Nkodia G, Popova S, Hart B, Burd L. High prevalence of prenatal alcohol exposure detected by breathalyzer in the Republic of the Congo, Africa. Neurotoxicol Teratol. 2020;80(106892):15. [DOI] [PubMed] [Google Scholar]
- 92.Ishoso DK, Tshefu AK, Delvaux T, Coppieters Y. Extent of induced abortions and occurrence of complications in Kinshasa, Democratic Republic of the Congo. Reprod Health. 2019;16(1):019–0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mbuyi-Musanzayi S, Kayembe TJ, Kashal MK, Lukusa PT, Kalenga PM, Tshilombo FK, et al. Non-syndromic cleft lip and/or cleft palate: epidemiology and risk factors in Lubumbashi (DR Congo), a case-control study. J Craniomaxillofac Surg. 2018;46(7):1051–8. [DOI] [PubMed] [Google Scholar]
- 94.Nimi T, Fraga S, Costa D, Campos P, Barros H. Prevalence, determinants, and effects of violence during pregnancy: a maternity-based cross-sectional study in Luanda, Angola. J Public Health Afr. 2020;10(2):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Williams AD, Nkombo Y, Nkodia G, Leonardson G, Burd L. Prenatal alcohol exposure in the Republic of the Congo: prevalence and screening strategies. Birth Defects Res Clin Mol Teratol. 2013;97(7):489–96. [DOI] [PubMed] [Google Scholar]
- 96.Zar HJ, Pellowski JA, Cohen S, Barnett W, Vanker A, Koen N et al. Maternal health and birth outcomes in a South African birth cohort study. PLoS ONE. 2019;14(11). [DOI] [PMC free article] [PubMed]
- 97.Demelash H, Motbainor A, Nigatu D, Gashaw K, Melese A. Risk factors for low birth weight in Bale Zone hospitals, South-East Ethiopia: a case–control study. BMC Pregnancy Childbirth. 2015;15(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dendir E, Deyessa N. Substance use and birth weight among mothers attending public hospitals: a case control study. Ethiop J Health Dev. 2017;31(1):27–35. [Google Scholar]
- 99.Tesfaye G, Demlew D, G/tsadik M, Habte F, Molla G, Kifle Y et al. Correction to: the prevalence and associated factors of alcohol use among pregnant women attending antenatal care at public hospitals Addis Ababa, Ethiopia, 2019. BMC Psychiatry. 2020 2020/10/06;20(1):493. [DOI] [PMC free article] [PubMed]
- 100.Abasiubong F, Bassey EA, Udobang JA, Akinbami OS, Udoh SB, Idung AU. Self-Medication: potential risks and hazards among pregnant women in Uyo, Nigeria. Pan Afr Med J. 2012;13:15. [PMC free article] [PubMed] [Google Scholar]
- 101.Agiresaasi A, Nassanga G, Maina GW, Kiguli J, Nabiwemba E, Tumwesigye NM. Various forms of alcohol use and their predictors among pregnant women in post conflict northern Uganda: a cross sectional study. Subst Abuse Treat Prev Policy. 2021;16(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Da Pilma Lekettey J, Dako-Gyeke P, Agyemang SA, Aikins M. Alcohol consumption among pregnant women in James Town Community, Accra, Ghana. Reprod Health. 2017;14(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Greenmyer JR, Klug MG, Nkodia G, Popova S, Hart B, Burd L. High prevalence of prenatal alcohol exposure detected by breathalyzer in the Republic of the Congo, Africa. Neurotoxicol Teratol. 2020 Jul-Aug;80:106892. [DOI] [PubMed]
- 104.Isaksen AB, Østbye T, Mmbaga BT, Daltveit AK. Alcohol consumption among pregnant women in Northern Tanzania 2000–2010: a registry-based study. BMC Pregnancy Childbirth. 2015;15:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ishoso DK, Tshefu AK, Delvaux T, Coppieters Y. Extent of induced abortions and occurrence of complications in Kinshasa, Democratic Republic of the Congo. Reprod Health. 2019;16(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kargbo DK, Nyarko K, Sackey S, Addo-Lartey A, Kenu E, Anto F. Determinants of low birth weight deliveries at five referral hospitals in Western Area Urban district, Sierra Leone. Ital J Pediatr. 2021;47(1):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moise IK. Alcohol use, pregnancy and associated risk factors: a pilot cross-sectional study of pregnant women attending prenatal care in an urban city. BMC Pregnancy Childbirth. 2019;19(1):472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mpelo M, Kibusi SM, Moshi F, Nyundo A, Ntwenya JE, Mpondo BCT. Prevalence and factors influencing alcohol use in pregnancy among women attending Antenatal Care in Dodoma Region, Tanzania: a cross-sectional study. J Pregnancy. 2018;2018:8580318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nimi T, Fraga S, Costa D, Campos P, Barros H. Prevalence, determinants, and effects of violence during pregnancy: a maternity-based cross-sectional study in Luanda, Angola. J Public Health Afr. 2019;10(2):1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ome-Kaius M, Unger HW, Singirok D, Wangnapi RA, Hanieh S, Umbers AJ, et al. Determining effects of areca (betel) nut chewing in a prospective cohort of pregnant women in Madang Province, Papua New Guinea. BMC Pregnancy Childbirth. 2015;15:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Onah MN, Field S, van Heyningen T, Honikman S. Predictors of alcohol and other drug use among pregnant women in a peri-urban South African setting. Int J Ment Health Syst. 2016;10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Onwuka CI, Ugwu EO, Dim CC, Menuba IE, Iloghalu EI. Prevalence and predictors of Alcohol Consumption during pregnancy in South-Eastern Nigeria. J Clin Diagn Res. 2016;10(9):QC10–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Peltzer K, Pengpid S. Maternal alcohol use during pregnancy in a general national population in South Africa. S Afr J Psychiatr. 2019;25(0):1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Petersen Williams P, Jordaan E, Mathews C, Lombard C, Parry CD. Alcohol and other Drug Use during pregnancy among women attending midwife obstetric units in the Cape Metropole, South Africa. Adv Prev Med. 2014;2014:871427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sanou AS, Diallo AH, Holding P, Nankabirwa V, Engebretsen IMS, Ndeezi G, et al. Maternal alcohol consumption during pregnancy and child’s cognitive performance at 6–8 years of age in rural Burkina Faso: an observational study. PeerJ. 2017;5:e3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wynn A, Nabukalu D, Lutalo T, Wawer M, Chang LW, Kiene SM, et al. Alcohol use during pregnancy in Rakai, Uganda. PLoS ONE. 2021;16(8):e0256434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zar HJ, Pellowski JA, Cohen S, Barnett W, Vanker A, Koen N, et al. Maternal health and birth outcomes in a South African birth cohort study. PLoS ONE. 2019;14(11):e0222399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Onunkwor OF, Al-Dubai S, Shuaibu H. Second hand smoke exposure among pregnant women in Nigeria. J Adv Med Med Res. 2020;32(13):29–36. [Google Scholar]
- 119.Shuaib A, Frass K. Occurrence and risk factors of low birth weight in Sana’a. Yemen J High Inst Public Health. 2017;47(1):8–12. [Google Scholar]
- 120.Al-Adhroey AH, Mehrass AA-KO, Al-Shammakh AA, Ali AD, Akabat MY, Al-Mekhlafi HM. Prevalence and predictors of Toxoplasma gondii infection in pregnant women from Dhamar, Yemen. BMC Infect Dis. 2019;19(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Alemu WG, Zeleke TA, Takele WW, Mekonnen SS. Prevalence and risk factors for khat use among youth students in Ethiopia: systematic review and meta-analysis, 2018. Ann Gen Psychiatry. 2020;19:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gebissa E. Scourge of life or an economic lifeline? Public discourses on khat (Catha edulis) in Ethiopia. Subst Use Misuse. 2008;43(6):784–802. [DOI] [PubMed] [Google Scholar]
- 123.Patel SL, Murray R, Britain G. Khat use among somalis in four English cities. Citeseer; 2005.
- 124.Geda GJ. Pilgrimages and syncretism: religious transformation among the Arsi Oromo of Ethiopia 2015.
- 125.Al-Abed AA, Sutan R, Al-Dubai SA, Aljunid SM. Family context and khat chewing among adult Yemeni women: a cross-sectional study. Biomed Res Int. 2014;2014:505474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lange S, Probst C, Heer N, Roerecke M, Rehm J, Monteiro MG, et al. Actual and predicted prevalence of alcohol consumption during pregnancy in Latin America and the Caribbean: systematic literature review and meta-analysis. Rev Panam Salud Publica. 2017;41:e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mulat B, Alemnew W, Shitu K. Alcohol use during pregnancy and associated factors among pregnant women in Sub-saharan Africa: further analysis of the recent demographic and health survey data. BMC Pregnancy Childbirth. 2022;22(1):361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Popova S, Lange S, Probst C, Shield K, Kraicer-Melamed H, Ferreira-Borges C, et al. Actual and predicted prevalence of alcohol consumption during pregnancy in the WHO African Region. Trop Med Int Health. 2016;21(10):1209–39. [DOI] [PubMed] [Google Scholar]
- 129.Tarighat-Esfanjani A, Namazi N. Nutritional concepts and frequency of Foodstuffs mentioned in the Holy Quran. J Relig Health. 2016;55(3):812–9. [DOI] [PubMed] [Google Scholar]
- 130.WHO. PMTsCT strategic vision 2010–2015: preventing mother-to-child transmission of HIV to reach the UNGASS and Millennium development goals: moving towards the elimination of pediatric HIV. Geneva: WHO; 2010. [Google Scholar]
- 131.Chambers CD, Yevtushok L, Zymak-Zakutnya N, Korzhynskyy Y, Ostapchuk L, Akhmedzhanova D, et al. Prevalence and predictors of maternal alcohol consumption in 2 regions of Ukraine. Alcohol Clin Exp Res. 2014;38(4):1012–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Skagerström J, Alehagen S, Häggström-Nordin E, Årestedt K, Nilsen P. Prevalence of alcohol use before and during pregnancy and predictors of drinking during pregnancy: a cross sectional study in Sweden. BMC Public Health. 2013;13:780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tsang TW, Kingsland M, Doherty E, Anderson AE, Tully B, Crooks K, et al. Predictors of alcohol use during pregnancy in Australian women. Drug Alcohol Rev. 2022;41(1):171–81. [DOI] [PubMed] [Google Scholar]
- 134.Reid N, Schölin L, Erng MN, Montag A, Hanson J, Smith L. Preconception interventions to reduce the risk of alcohol-exposed pregnancies: a systematic review. Alcohol Clin Exp Res. 2021;45(12):2414–29. [DOI] [PubMed] [Google Scholar]
- 135.Aychiluhm SB, Mare KU, Dagnew B, Seid AA, Melaku MS, Sabo KG, et al. Determinants of tobacco use among pregnant women in sub-saharan Africa. A multilevel mixed-effect logistic regression model. PLoS ONE. 2024;19(5):e0297021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and its supplementary information files].