Abstract
Longevity is one of the most important characteristics in the life history of organisms. It is directly associated with growth, reproduction and age of sexual maturity. Despite this, little is known about longevity in South American anuran species, a region considered as a hotspot of world diversity. Thus, we carried out a literature review of publications on longevity of South American anurans that used the skeletochronology method to identify the main publication trends, as well as to point out the main information gaps and suggest future directions. We found clear biases when we analysed temporal, spatial and taxonomic patterns in publications on longevity: (i) studies are recent (mostly from 2015 onwards), (ii) bufonids and leptodactylids were the most studied groups, (iii) medium to large species are the most studied, (iv) species with wide geographic distribution, low risk of extinction, (v) the studies are concentrated in Brazil and Argentina, and (vi) most studies are in the Chaco biogeographical sub-region. We suggest that future work prioritizes little explored families and with high species diversity, small-bodied species, with restricted distribution, threatened with extinction, in order to expand the representation of different evolutionary lineages along the biogeographical units of South America.
Keywords: amphibia, demography, skeletochronology, review
1. Introduction
Life history studies aim at explaining development, growth pattern, reproductive investment and survival of species [1–6]. Longevity is one of the most important age aspects since it is directly associated with growth, reproduction and age of sexual maturity [5,7–9], while providing us with useful clues about species senescence [10].
In the last 50 years, skeletochronology, that is, the determination of age by counting the lines of bone growth of cross-sections of long bones such as phalanges [5,11,12], has been the most used method for determining age in different animal groups. Groups include fossil animals ([13,14]; fish [15], lizards [16,17], snakes [18], chelonians [19,20], birds [11,21], mammals [22,23] and amphibians [24,25]). This non-lethal method has proven highly effective and reliable for determining the age of species [12,24,26].
For amphibians, skeletochronology has been employed not only for age determination of temperate and subtropical regions, in which bone growth lines are formed during the hibernation period [12,24,25,27–30], but also for tropical species that slow down their growth during dry seasons [24,31–33]. Associating the age of organisms with environmental factors helps us understand population dynamics. Once we understand population dynamics, we can better explain population decline and design effective conservation strategies [27,34–36].
Amphibians are currently the most endangered vertebrate group in the world, with approximately 41% of species globally extinct or threatened with extinction [37,38]. South America is home to the greatest diversity of anuran species in the world, with more than 2600 species described and a high rate of endemism [37,39,40]. Despite the high species diversity in the Neotropical region, in which South America is inserted, little is known about age aspects of its species [5]. Therefore, the objective of this study was to carry out a literature review on the availability of longevity for South American anurans to identify the main trends in temporal, spatial and taxonomic publications, as well as to point out the main information gaps and provide suggestions for future directions.
2. Material and methods
We carried out a bibliographical survey of scientific articles published in national and international journals that included anuran species that occur in South America. We used the electronic databases Google Scholar (https://scholar.google.com.br/) and Scientific Electronic Library online (Scielo; www.scielo.org) with the following research queries: ‘longevity anura’, ‘age anura’, ‘skeletochronology anura’, demography anura’ and ‘age aspects anura’. We then filtered the articles to select only those with longevity data estimated by the skeletochronology technique for South American anurans. We excluded monographs, academic theses, dissertations and publications in annals. We also did not include the article by Brum et al. [41] since it aimed at describing a methodological protocol and used a very low sample number (three individuals).
We extracted the following information from the selected articles: (i) family and species used in the study, (ii) sampling country, (iii) sampling geographic coordinates, (iv) biome in which the species was collected, (v) habitat, distribution and threat category of the studied species, (vi) number of individuals analysed, (vii) average body size (SVL) of the species, (viii) maximum estimated longevity of females, (ix) maximum estimated longevity of males, (x) age of sexual maturity and reproductive potential of specie, and (xi) publication year.
We made a cumulative curve to see how the number of publications increased from 1990 to 2023, the overall period with publications. With this information, we plotted the geographic coordinates extracted from the articles to verify which countries and biogeographical regions (adapted from [42]) have more publications, as well as to detect geographic gaps and possible trends.
We defined the species’ habitat type according to Pincheira-Donoso et al. [43], which determines five categories: (a) aquatic: strict divers; (b) semi-aquatic: species that depend on intermittent contact with bodies of water; (c) terrestrial: species predominantly inhabiting the soil; (d) arboreal: species that land on bushes or trees; and (e) fossorial: species that live underground, except during reproductive seasons. We define species distribution as restricted, for species that occur in a single biome, and broad, for species that occur in two biomes or more. The threat category was defined following IUCN categories (https://www.iucnredlist.org/).
3. Results
We found 32 articles that provided information on South American species using the selected method out of the 160 articles on anuran longevity (electronic supplementary material, S1). Although publications on the longevity of South American anurans began in the 1990s (first publication by [44]), publications only became regular in 2009, with at least one article published per year and peaked from 2015 to 2019 (figure 1a). The cumulative curve of articles published from 1990 to 2023 showed an ascending trend, with gradual growth between 1990 and 2014 and a more pronounced increase from 2015 onwards (figure 1a). Studies on the longevity of anuran species from South America published in six countries such as Argentina (63.8%) and Brazil (27.7%) together concentrate more than 90% of scientific production in this area (figure 1b). Chile, Colombia, Ecuador and Peru hold 2.7% of publications each (figure 1b).
Figure 1.
(a) Number of articles on skeletochronology of South American anurans published by year. The brown line represents the cumulative number of articles. (b) Percentage of studies on the longevity of South American anurans published by country.
The 32 articles provided information on the longevity of 36 anuran species from eight families. Argentina provided information on 23 species distributed in six families. Brazil encompassed 10 species distributed in five families. Other countries (i.e. Chile, Colombia, Peru and Ecuador) had only one article, each with information on a single species (table 1, figure 2a). Most studies were carried out in the biogeographic region of Chaco/Pantanal. The regions of the Andes, Atlantic Forest and Pampa had three studies carried out in each, and only two and one study were carried out in the Patagonia and Cerrado regions, respectively (figure 2b).
Table 1.
Dataset used in this study. Lat = latitude; Long = longitude. Longevity is expressed in years. Displayed longevity = maximum longevity. All articles cited in the table are shown in the electronic supplementary material, S1.
| family | species | longevity | country | coordinates | year | reference | ||
|---|---|---|---|---|---|---|---|---|
| male | female | lat | long | |||||
| Bufonidae | Atelopus lozanoi | 4 | — | Colombia | −4.5128 | −73.7382 | 2012 | [31] |
| Bufonidae | Atelopus peruensis | 6 | — | Peru | −6.9956 | −79.6809 | 2012 | [31] |
| Bufonidae | Melanophryniscus atroluteus | 7 | 9 | Argentina | −27.4902 | −55.6686 | 2023 | [45] |
| Bufonidae | Melanophryniscus atroluteus | 6 | 6 | Argentina | −29.0173 | −56.9324 | 2024 | [46] |
| Bufonidae | Melanophryniscus devincenzii | 7 | 7 | Argentina | −27.4902 | −55.6686 | 2023 | [45] |
| Bufonidae | Melanophryniscus krauczuki | 5 | 5 | Argentina | −27.4902 | −55.6686 | 2023 | [45] |
| Bufonidae | Melanophryniscus moreirae | 6 | 6 | Brazil | −22.3849 | −44.6782 | 2015 | [47] |
| Bufonidae | Rhinella achalensis | 9 | 11 | Argentina | −31.4397 | −64.875 | 2011 | [48] |
| Bufonidae | Rhinella arenarum | 6 | 8 | Argentina | −34.6083 | −58.3712 | 1990 | [44] |
| Bufonidae | Rhinella arenarum | 6 | 4 | Argentina | −30.1 | −64.4166 | 2015 | [49] |
| Bufonidae | Rhinella arenarum | 5 | 4 | Argentina | −33.1238 | −64.3490 | 2018 | [50] |
| Bufonidae | Rhinella arenarum | 5 | 5 | Argentina | −32.6198 | −64.9110 | 2018 | [51] |
| Bufonidae | Rhinella ornata | 4 | — | Brazil | −22.9222 | −43.7763 | 2019 | [52] |
| Bufonidae | Rhinella rubescens | 3 | 3 | Brazil | −15.5894 | −47.6963 | 2015 | [32] |
| Bufonidae | Rhinella diptycha | 5 | 4 | Brazil | −15.5894 | −47.6963 | 2015 | [32] |
| Ceratophryidae | Ceratophrys cranwelli | 2 | — | Argentina | −24.3456 | −61.1151 | 2009 | [53] |
| Ceratophryidae | Ceratophrys stolzmanni | 3 | 4 | Ecuador | −3.4886 | −80.1293 | 2018 | [54] |
| Ceratophryidae | Chacophrys pierottii | 5 | 5 | Argentina | −24.9411 | −61.4907 | 2018 | [55] |
| Cycloramphidae | Thoropa miliaris | 3 | — | Brazil | −22.9066 | −43.1727 | 2017 | [56] |
| Hylidae | Boana cordobae | 5 | 5 | Argentina | −32.6197 | −64.9111 | 2017 | [57] |
| Hylidae | Boana cordobae | 7 | 7 | Argentina | −32.5931 | −64.7108 | 2018 | [58] |
| Hylidae | Boana cordobae | 5 | 6 | Argentina | −32.6198 | −64.9110 | 2018 | [51] |
| Hylidae | Boana pulchella | 5 | — | Argentina | −33.1113 | −64.3046 | 2021 | [59] |
| Hylidae | Nyctimantis siemersi | 5 | 5 | Argentina | −27.4321 | −58.7466 | 2013 | [60] |
| Hylidae | Scinax fuscovarius | 5 | 6 | Argentina | −29.0173 | −56.9324 | 2023 | [46] |
| Hylodidae | Crossodactylus schmidti | 6 | 6 | Brazil | −27.2428 | −53.9538 | 2019 | [61] |
| Leptodactylidae | Leptodactylus bufonius | 4 | 5 | Argentina | −27.4314 | −58.7457 | 2019 | [62] |
| Leptodactylidae | Leptodactylus latinasus | 6 | — | Argentina | −31.7186 | −60.2555 | 2014 | [63] |
| Leptodactylidae | Leptodactylus latinasus | 3 | 2 | Argentina | −27.4314 | −58.7457 | 2019 | [62] |
| Leptodactylidae | Leptodactylus luctator | 5 | 5 | Argentina | −31.7047 | −60.6672 | 2017 | [64] |
| Leptodactylidae | Leptodactylus mystacinus | 7 | — | Argentina | −31.7186 | −60.2555 | 2014 | [63] |
| Leptodactylidae | Physalaemus biligonigerus | 5 | 4 | Argentina | −33.1116 | −64.3027 | 2018 | [65] |
| Leptodactylidae | Physalaemus cuvieri | 7 | 7 | Brazil | −29.7382 | −53.8431 | 2022 | [25] |
| Leptodactylidae | Physalaemus fernandezae | 6 | 6 | Argentina | −34.7981 | −58.0128 | 2012 | [66] |
| Leptodactylidae | Physalaemus riograndensis | 5 | 5 | Brazil | −29.7382 | −53.8431 | 2022 | [25] |
| Leptodactylidae | Pleurodema cordobae | 4 | 6 | Argentina | −32.3994 | −64.9263 | 2017 | [67] |
| Leptodactylidae | Pleurodema kriegi | 4 | 5 | Argentina | −31.6127 | −64.87472 | 2017 | [67] |
| Leptodactylidae | Pleurodema thaul | 5 | 5 | Chile | −30.6666 | −71.5166 | 2010 | [68] |
| Leptodactylidae | Pseudopaludicola falcipes | 4 | 5 | Brazil | −29.7382 | −53.8431 | 2022 | [25] |
| Microhylidae | Dermatonotus muelleri | 2 | — | Argentina | −24.3456 | −61.1151 | 2009 | [53] |
| Microhylidae | Dermatonotus muelleri | 5 | 5 | Argentina | −24.9411 | −61.4907 | 2016 | [69] |
| Odontophrynidae | Odontophrynus americanus | 3 | 4 | Argentina | −31.5166 | −67.85 | 2015 | [70] |
| Odontophrynidae | Odontophrynus asper | 10 | 7 | Brazil | −29.73828 | −53.8431 | 2020 | [29] |
| Odontophrynidae | Odontophrynus asper | 6 | — | Argentina | −32.7666 | −64.2666 | 2021 | [71] |
| Odontophrynidae | Odontophrynus asper | 5 | 7 | Argentina | −29.0173 | −56.9324 | 2023 | [46] |
| Odontophrynidae | Odontophrynus cordobae | 7 | — | Argentina | −32.7666 | −64.2666 | 2021 | [71] |
Figure 2.
Map of South America, highlighting countries (a) and biogeographical regions according to [42] (b) Yellow dots represent skeletochronology studies with anurans recovered in our survey.
Species that occupy arboreal habitats (11.1% of studies) are considerably less targeted for longevity studies, when compared with terrestrial, fossorial or semi-aquatic species (30.6%, 30.6% and 27.8% of studies, respectively) (table 2, figure 3a). Regarding geographical distribution pattern, more than 60% of the species studied have a wide geographic distribution, while species with a restricted distribution correspond to less than 39% of the studies (table 2, figure 3b). More than 80% of South American species used in skeletochronological studies are categorized by the IUCN as Last Concern (LC), followed by the categories Endangered (EN) and Near Threatened (NT), with 5.6% each, and Vulnerable (VU) and Critically Endangered (CR), with 2.8% each (table 2, figure 3c).
Table 2.
Dataset highlighting ecological data of the species. CR = critically endangered, EN = endangered, VU = vulnerable, NT = near threatened and LC = least concern.
| family | species | biome | habitat | distribution | IUCN category | reference |
|---|---|---|---|---|---|---|
| Bufonidae | Atelopus lozanoi | Andes | terrestrial | restricted | EN | [31] |
| Bufonidae | Atelopus peruensis | Andes | terrestrial | restricted | CR | [31] |
| Bufonidae | Melanophryniscus atroluteus | Chaco/Pantanal | fossorial | wide | LC | [45] |
| Bufonidae | Melanophryniscus atroluteus | Chaco/Pantanal | fossorial | wide | LC | [46] |
| Bufonidae | Melanophryniscus devincenzii | Chaco/Pantanal | fossorial | wide | LC | [45] |
| Bufonidae | Melanophryniscus krauczuki | Chaco/Pantanal | fossorial | restricted | LC | [45] |
| Bufonidae | Melanophryniscus moreirae | Atlantic Forest | fossorial | restricted | NT | [47] |
| Bufonidae | Rhinella achalensis | Chaco/Pantanal | terrestrial | restricted | EN | [48] |
| Bufonidae | Rhinella arenarum | Pampa | terrestrial | wide | LC | [44] |
| Bufonidae | Rhinella arenarum | Pampa | terrestrial | wide | LC | [49] |
| Bufonidae | Rhinella arenarum | Chaco/Pantanal | terrestrial | wide | LC | [49] |
| Bufonidae | Rhinella arenarum | Chaco/Pantanal | terrestrial | wide | LC | [51] |
| Bufonidae | Rhinella diptycha | Cerrado | terrestrial | wide | LC | [32] |
| Bufonidae | Rhinella ornata | Atlantic Forest | terrestrial | wide | LC | [52] |
| Bufonidae | Rhinella rubescens | Cerrado | terrestrial | wide | LC | [32] |
| Ceratophryidae | Ceratophrys cranwelli | Chaco/Pantanal | fossorial | wide | LC | [53] |
| Ceratophryidae | Ceratophrys stolzmanni | Andes | fossorial | restricted | VU | [54] |
| Ceratophryidae | Chacophrys pierottii | Chaco/Pantanal | fossorial | wide | LC | [55] |
| Cycloramphidae | Thoropa miliaris | Atlantic Forest | semi-aquatic | restricted | LC | [56] |
| Hylidae | Boana cordobae | Chaco/Pantanal | arboreal | restricted | LC | [57] |
| Hylidae | Boana cordobae | Chaco/Pantanal | arboreal | restricted | LC | [58] |
| Hylidae | Boana cordobae | Chaco/Pantanal | arboreal | restricted | LC | [51] |
| Hylidae | Boana pulchella | Chaco/Pantanal | arboreal | wide | LC | [59] |
| Hylidae | Nyctimantis siemersi | Chaco/Pantanal | arboreal | restricted | LC | [60] |
| Hylidae | Scinax fuscovarius | Chaco/Pantanal | arboreal | wide | LC | [46] |
| Hylodidae | Crossodactylus schmidti | Atlantic Forest | semi-aquatic | wide | LC | [61] |
| Leptodactylidae | Leptodactylus bufonius | Chaco/Pantanal | semi-aquatic | wide | LC | [62] |
| Leptodactylidae | Leptodactylus latinasus | Chaco/Pantanal | semi-aquatic | wide | LC | [63] |
| Leptodactylidae | Leptodactylus latinasus | Chaco/Pantanal | semi-aquatic | wide | LC | [62] |
| Leptodactylidae | Leptodactylus luctator | Chaco/Pantanal | semi-aquatic | wide | LC | [64] |
| Leptodactylidae | Leptodactylus mystacinus | Chaco/Pantanal | semi-aquatic | wide | LC | [63] |
| Leptodactylidae | Physalaemus biligonigerus | Chaco/Pantanal | semi-aquatic | wide | LC | [65] |
| Leptodactylidae | Physalaemus cuvieri | Pampa | semi-aquatic | wide | LC | [25] |
| Leptodactylidae | Physalaemus fernandezae | Pampa | semi-aquatic | restricted | LC | [66] |
| Leptodactylidae | Physalaemus riograndensis | Pampa | semi-aquatic | wide | LC | [25] |
| Leptodactylidae | Pleurodema cordobae | Patagonia | semi-aquatic | restricted | LC | [67] |
| Leptodactylidae | Pleurodema kriegi | Patagonia | semi-aquatic | restricted | NT | [67] |
| Leptodactylidae | Pleurodema thaul | Andes | semi-aquatic | restricted | LC | [68] |
| Leptodactylidae | Pseudopaludicola falcipes | Pampa | semi-aquatic | wide | LC | [25] |
| Microhylidae | Dermatonotus muelleri | Chaco/Pantanal | fossorial | wide | LC | [53] |
| Microhylidae | Dermatonotus muelleri | Chaco/Pantanal | fossorial | wide | LC | [69] |
| Odontophrynidae | Odontophrynus americanus | Patagonia | fossorial | wide | LC | [70] |
| Odontophrynidae | Odontophrynus asper | Pampa | fossorial | wide | LC | [29] |
| Odontophrynidae | Odontophrynus asper | Chaco/Pantanal | fossorial | wide | LC | [71] |
| Odontophrynidae | Odontophrynus asper | Chaco/Pantanal | fossorial | wide | LC | [46] |
| Odontophrynidae | Odontophrynus cordobae | Chaco/Pantanal | fossorial | restricted | LC | [71] |
Figure 3.

Ecological characteristics of the species, expressed as percentage. (a) habitat type, (b) species geographic distribution and (c) IUCN category.
Out of the 24 anuran families that occur in South America, only eight (33%) had information on species’ longevity. A total of eight publications comprised longevity data on 12 species of the Leptodactylidae family (which represents 6% of the described leptodactylids). For the Bufonidae family, information on longevity was found for 11 species (4% of described species), published in 11 scientific articles. For the Hylidae family, information on longevity was found for four species (0.76% of described species), published in six papers. The families Ceratophryidae and Odontophrynidae had information for only three species each (25% and 5.66% of the described species, respectively), published in three and four papers, respectively. Cycloramphidae, Hylodidae and Microhylidae were the families with the greatest deficits in knowledge on longevity, with information for only one species of each family (2.78%, 2.17% and 1.35% of the described species, respectively), within four articles (table 3, figure 4).
Table 3.
Table of South American species by family and number of species in these families with longevity data. Data presented in descending order of representativeness.
| family | species of South America | species with data about longevity | % |
|---|---|---|---|
| Ceratophryidae | 12 | 3 | 25 |
| Leptodactylidae | 200 | 12 | 6 |
| Odontophrynidae | 53 | 3 | 5.66 |
| Bufonidae | 274 | 11 | 4.01 |
| Cycloramphidae | 36 | 1 | 2.78 |
| Hylodidae | 46 | 1 | 2.17 |
| Microhylidae | 74 | 1 | 1.35 |
| Hylidae | 520 | 4 | 0.76 |
Figure 4.
Number of anuran species per family and the number of South American species for which longevity is known (inside the silhouettes). Hy = Hylidae; Bu = Bufonidae; Le = Leptodactylidae; Mi = Microhylidae; Od = Odontophrynidae; Hlo = Hylodidae; Cy = Cycloramphidae; Ce = Ceratophryidae; Of = other South American anuran families. Gray and black hatched bar = sum of species without longevity data. The silhouettes are not to scale. Anuran families according to Vasconcelos et al. [37].
In general, the sample size used in the studies differed between males and females, with males being more targeted for longevity studies than females (ratio >1) (electronic supplementary material, S2). When we analysed by family, we recovered those studies on species of Bufonidae, Hylidae and Leptodactylidae families typically used a larger sample number of males, while females were more used in studies on species from Ceratophryidae and Odontophrynidae families (figure 5a). It is worth mentioning that, of the 36 South American species on which there are longevity studies, for more than 20% of them (eight species) the longevity of females has not been investigated (electronic supplementary material, S1). The average snout-vent length (SVL) of the species for which longevity has been studied was 47.73 ± 23.93 mm for males, and 48.95 ± 24.82 mm for females, where only Bufonidae, Ceratophryidae, Hylidae and Microhylidae had an average SVL lower than 50 mm (figure 5b, electronic supplementary material, S1).
Figure 5.
(a) Ratio of the sample number of males and females per family used in the studies. (b) Average body size of anuran species used in skeletochronology studies per family, in South America. Average body size (SVL) data of the species were taken directly from the articles and the average of the SVL found for each family was calculated. Hy = Hylidae; Bu = Bufonidae; Le = Leptodactylidae; Mi = Microhylidae; Od = Odontophrynidae; Hlo = Hylodidae; Cy = Cycloramphidae; Ce = Ceratophryidae; Gray line = standard deviation; Gray and orange hatched bar = general average size of the species.
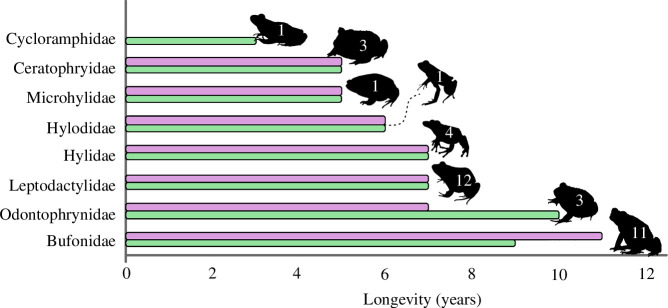
Regarding longevity, Bufonidae and Odontophrynidae are the families with the greatest longevity recorded for females (11 years) and males (10 years), respectively. (table 4). Species from the Leptodactylidae and Hylidae families had the same maximum longevity (7 years for males and females). The lowest longevity was recorded for species of the families Hylodidae (6 years for males and females), Ceratophryidae and Microhylidae (5 years for males and females). For the Cycloramphidae family, the only study provided longevity only for males (3 years) of one species (figure 6). The mean age found for the families analysed varied between 3 and 6 years (table 4; electronic supplementary material, S3), with Bufonidae presenting the highest average longevity (6 years for males and females), and Cycloramphidae the lowest (3 years for males). The average age of sexual maturity was the same for males and females in the families Hylidae, Leptodactylidae and Microhylidae (2 years), Ceratophryidae and Odontophrynidae (1 year), but it varied between males and females in the families Bufonidae and Hylodidae, where males reach sexual maturity, on average, at 2 years and females at 3 years (table 4; electronic supplementary material, S3). In general, the average reproductive potential of families varied between 2 and 5 years, with Odontophrynidae presenting the highest average reproductive potential (5 years for females and 4 for males) and males of Ceratophryidae, Leptodactylidae and Microhylidae had the lowest average reproductive potential (both 2 years) (table 4; electronic supplementary material, S3).
Table 4.
Table of species age data. Bu = Bufonidae; Ce = Ceratophryidae; Cy = Cycloramphidae; Hy = Hylidae; Hlo = Hylodidae; Le = Leptodactylidae; Mi = Microhylidae; Od = Odontophrynidae. n = total sample number used. SVL = snout vent length. ♂ = male. ♀ = female. SVL expressed in mm ± s.d.. Maximum and average longevity, sexual maturity and reproductive potential expressed in years.
| family | species | N | SVL | maximun longevity | mean Age | sexual maturity | reproductive potential | reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ||||
| Bu | Atelopus lozanoi | 5 | 28.65 | 42.45 | 4 | 4 | — | — | — | — | — | — | [31] |
| Bu | Atelopus peruensis | 14 | 39.55 | 43.4 | 6 | — | — | — | — | — | — | — | [31] |
| Bu | Melanophryniscus atroluteus | 38 | 23.63 ± 1.18 | 25.76 ± 1.41 | 7 | 9 | 4 | 5 | 3 | 3 | 4 | 6 | [45] |
| Bu | Melanophryniscus atroluteus | 53 | 21.41 ± 1.89 | 22.49 ± 1.33 | 6 | 6 | 3 | 4 | 2 | 2 | 4 | 4 | [46] |
| Bu | Melanophryniscus devincenzii | 30 | 23.49 ± 1.16 | 27.45 ± 1.54 | 7 | 7 | 5 | 5 | 3 | 4 | 4 | 3 | [45] |
| Bu | Melanophryniscus krauczuki | 35 | 20.88 ± 1.21 | 23.52 ± 1.44 | 5 | 5 | 3 | 3 | 2 | 2 | 3 | 3 | [45] |
| Bu | Melanophryniscus moreirae | 55 | 23.2 ± 0.2 | 26.2 ± 0.2 | 6 | 6 | 4 | 5 | 2 | 3 | 4 | 3 | [47] |
| Bu | Rhinella achalensis | 205 | 57.96 ± 6.75 | 54.59 ± 6.8 | 9 | 11 | 5 | 4 | — | — | — | — | [48] |
| Bu | Rhinella arenarum | 88 | 82.5 ± 11.45 | 69.17 ± 8.13 | 6 | 8 | — | — | — | — | — | — | [44] |
| Bu | Rhinella arenarum | 138 | 100.45 ± 7.95 | 108.6 ± 9.6 | 6 y | 4 | 3 | 2 | 1 | 2 | 5 | 2 | [49] |
| Bu | Rhinella arnarum | 114 | 96.34 ± 9.02 | 106.18 ± 5.09 | 5 | 4 | 2 | 3 | 1 | 2 | 3 | [49] | |
| Bu | Rhinella arenarum | 76 | 90.16 ± 9.4 | 96.53 ± 7.73 | 5 | 5 | 3 | 3 | 2 | 2 | 3 | 3 | [51] |
| Bu | Rhinella diptycha | 29 | 118.4 ± 25.44 | 102.6 ± 41.55 | 5 | 4 | — | — | — | — | — | — | [32] |
| Bu | Rhinella ornata | 116 | — | — | 4 | — | 1 | — | — | — | — | — | [52] |
| Bu | Rhinella rubescens | 52 | 51.4 ± 21.35 | 45.2 ± 19.57 | 3 | 3 | — | — | — | — | — | — | [32] |
| Ce | Ceratophrys cranwelli | 6 | 88.9 ± 3.46 | — | 2 | — | — | — | — | — | — | — | [53] |
| Ce | Ceratophrys stolzmanni | 152 | 59.79 ± 3.67 | 64.87 ± 4.67 | 3 | 4 | 2 | 2 | 1 | 1 | 2 | 3 | [54] |
| Ce | Chacophrys pierottii | 26 | 51.44 ± 2.33 | 59.14 ± 4.16 | 5 | 5 | 3 | 4 | 1 | 1 | 4 | 4 | [55] |
| Cy | Thoropa miliaris | 92 | 43.8 ± 15.2 | — | 3 | — | 1 | — | — | — | — | — | [56] |
| Hy | Boana cordobae | 60 | 48.01 ± 4.99 | 51.27 ± 5.06 | 5 | 5 | 3 | 3 | 2 | 2 | 3 | 3 | [57] |
| Hy | Boana cordobae | 129 | 48.85 ± 3.32 | 53.61 ± 5.26 | 7 | 7 | 3 | 3 | 2 | 2 | 5 | 5 | [58] |
| Hy | Boana cordobae | 102 | 49.16 ± 3.83 | 52.5 ± 3.8 | 5 | 6 | 3 | 3 | 3 | 3 | 2 | 3 | [51] |
| Hy | Boana pulchella | 63 | 46.34 ± 2.97 | — | 5 | — | 3 | — | 2 | — | 3 | — | [59] |
| Hy | Nyctimantis siemersi | 56 | 69.17 ± 3.56 | 74.19 ± 4.14 | 5 | 5 | 3 | 4 | 2 | 3 | 3 | 2 | [60] |
| Hy | Scinax fuscovarius | 43 | 38.96 ± 4.85 | 37.78 ± 4.31 | 5 | 6 | 3 | 2 | 2 | 1 | 3 | 5 | [46] |
| Hlo | Crossodactylus schmidti | 103 | 25.03 ± 1.33 | 27.68 ± 2.26 | 6 | 6 | 4 | 4 | 2 | 3 | 4 | 3 | [61] |
| Le | Leptodactylus bufonius | 31 | 55.3 ± 1.8 | 56.33 ± 2 | 4 | 5 | 2 | 1 | 1 | 1 | 3 | 4 | [62] |
| Le | Leptodactylus latinasus | 17 | 33.05 ± 0.75 | — | 6 | — | 4 | — | 3 | — | 3 | — | 63] |
| Le | Leptodactylus lati-sus | 24 | 32.38 ± 2.94 | 33.02 ± 3.15 | 3 | 2 | 2 | 1 | 1 | 1 | 2 | 1 | [62] |
| Le | Leptodactylus luctator | 183 | 65.41 ± 28.84 | 63.59 ± 26.72 | 5 | 5 | 2 | 2 | 1 | 1 | 4 | 4 | [64] |
| Le | Leptodactylus mystacinus | 18 | 47.65 ± 2.5 | — | 7 y | — | 4 y | — | 3 y | — | 3 y | — | [63] |
| Le | Physalaemus biligonigerus | 29 | 34.69 ± 2.44 | 35.27 ± 2.54 | 5 | 4 | 3 | 3 | 2 | 3 | 3 | 1 | [72] |
| Le | Physalaemus cuvieri | 35 | 26.15 ± 3.1 | 28.14 ± 2.7 | 7 | 7 | 4 | 3 | 2 | 2 | 5 | 5 | [25] |
| Le | Physalaemus fernandezae | 64 | 20.49 ± 0.77 | 22.29 ± 1.15 | 6 | 6 | 4 | 4 | 2 | 3 | 4 | 3 | [66] |
| Le | Physalaemus riograndensis | 22 | 18.01 ± 0.89 | 18.55 ± 2.2 | 5 | 5 | 3 | 3 | 1 | 2 | 4 | 3 | [25] |
| Le | Pleurodema cordobae | 50 | 35.69 ± 1.74 | 40.43 ± 3.63 | 4 | 6 | 3 | 5 | 3 | 5 | 2 | 2 | [67] |
| Le | Pleurodema kriegi | 41 | 34.41 ± 2.6 | 37.76 ± 1.81 | 4 | 5 | 3 | 4 | 3 | 3 | 2 | 3 | [67] |
| Le | Pleurodema thaul | 83 | 32 ± 1.04 | 34.3 ± 1.02 | 5 | 5 | — | — | 2 | 2 | — | — | [68] |
| Le | Pseudopaludicola falcipes | 35 | 14.22 ± 1.26 | 15.04 ± 0.91 | 4 | 5 | 3 | 3 | 1 | 1 | 3 | 3 | [25] |
| Mi | Dermatonotus muelleri | 8 | 52.84 ± 3.08 | — | 2 | — | — | — | — | — | — | — | [53] |
| Mi | Dermatonotus muelleri | 43 | 70.2 ± 2.92 | 75.86 ± 3.78 | 5 | 5 | 3 | 3 | 2 | 2 | 3 | 3 | [69] |
| Od | Odontophrynus americanus | 38 | 51.46 ± 4.64 | 52.42 ± 4.13 | 3 | 4 | 2 | 2 | 1 | 1 | 1 | 3 | [70] |
| Od | Odontophrynus asper | 48 | 41.68 ± 5.8 | 43.13 ± 4.8 | 10 | 7 | 4 | 4 | 1 | 1 | 9 | 6 | [29] |
| Od | Odontophrynus asper | 34 | 46.36 ± 2.58 | — | 6 | — | 4 | — | 2 | — | 4 | — | [71] |
| Od | Odontophrynus asper | 25 | 40.58 ± 4.31 | 39.68 ± 3.04 | 5 | 7 | 4 | 3 | 1 | 1 | 4 | 4 | [46] |
| Od | Odontophrynus cordobae | 34 | 47.2 ± 2.97 | — | 7 | 7 | 4 | — | 2 | — | 5 | — | [71] |
Figure 6.
Estimated longevity (in years) for each South American anuran family using skeletochronology. Green bars = male longevity; purple bars = female longevity; the values within the silhouettes represent the number of species in each family for which longevity work has been published. The silhouettes are out of scale.
4. Discussion
Although determining the age of amphibians using the skeletochronology method has been used for more than 50 years, in South America, there are few studies using this approach. The lack of studies in the Neotropics is likely due to regional differences in research effort, as well as the considerable difficulty in accessing amphibian habitats in tropical forests, as they are characterized by dense vegetation and hot and humid climates [5]. In addition, longevity information through skeletochronology requires specific laboratory infrastructure, which includes equipment (rotating microtome and electronic microscope with coupled camera), and reagents (decalcifier, historesin and dyes) for correct histological processing of the samples. Laboratory cost is often not accessible in countries with little investment in basic research. Moreover, South America has the greatest diversity of anuran species in the world [37,40], with regions considered hotspots of biodiversity [73,74]. When exploring such diverse regions, researchers tend to focus on identifying and describing species rather than identifying parameters such as longevity.
In the last 33 years, 32 studies on the longevity of South American frogs were published, mostly between 2015 and 2019. Most of these are concentrated in the Chaco/Pantanal biogeographical region, in Argentina (22 studies) and in Brazil (seven studies), followed by Chile, Colombia, Peru and Ecuador, each with only one study published. This discrepancy in the number of publications per country can be explained because Brazil and Argentina have greater access to research funds and a higher proportion of herpetologists [75]. Furthermore, the divergence in the number of publications among biogeographic regions seems to be related to the geographic location of the research laboratories working in this line, since scientific investigations tend to concentrate near locations that offer convenient access, infrastructure and logistics [76,77].
Peng et al. [5] highlighted a global trend of amphibian species with terrestrial habitats are one of the most targeted for longevity studies, and this pattern also was recovered for South American anurans. In addition, our results also highlight the arboreal anuran group as the least targeted in longevity studies in South America. This can be explained because animals that use vegetation can sometimes be difficult to collect [78,79], compared with those associated with the soil, resulting in less representation of the first group in scientific collections.
Regarding threat categories, in South America there is a greater prevalence of studies on the longevity of Least Concern (LC) species (>80% of species) than those allocated to other categories, contradicting the argument that scientific research efforts are driven by global risk of extinction of a species [80,81]. In fact, Silva et al. [82] argue that the low probability of threatened species being associated with no or few studies show that, often, the need for conservation is overcome by more practical factors (e.g. local conservation priority, abundant and easily accessible species) when researchers need to decide which species are most appropriate for a given scientific study. Also, in general, LC species are those with a wide geographic distribution, present in high abundance across various types of habitats (including modified environments), and therefore with more individuals deposited in collections than species falling into any threat category. Unfortunately, comparison with patterns recorded in other studies is unfeasible at this moment, since we failed to find previous analysis on this subject.
Although the age composition of a population is a key demographic trait, with implications for the population dynamics of the species [27,34], South American anurans are still little investigated regarding this parameter. South America has a high diversity of anuran amphibians, which are distributed in 24 families [37]. However, only eight of them have any information about longevity. Hylidae, for example, is the second richest family in number of species [83] but presented the least known longevity of species (0.76%), following the same pattern reported by Peng et al. [5] in the global review. On the other hand, longevity data are available for 25% of Ceratophryidae species. This number is even more discrepant when we consider the total number of anuran species in South America. In fact, out of the more than 2623 anuran species known for South America [37], only 36 of them have available longevity data, which represents 1.29% of the species in this region.
Following the global pattern evidenced by Peng et al. [5], Bufonidae and Leptodactylidae families are the most studied regarding species longevity: 10 studies published with bufonids (11 species) and eight with leptodactylids (12 species). This is probably due to the ease of working with these anurans that generally have medium to large body size (e.g. [29,32,52,57,64,67]). Furthermore, the predominance of studies on Bufonids and Leptodactylids can be related to species in this group that are commonly found in peri-urban environments, facilitating the specimen collection [84]. On the other hand, there are few studies with small-bodied or hard-to-find species (e.g. [25,45,47,66], and therefore we recommend future research effort directed at species in these categories.
Regarding sampling biases, and contrary to the global standard in studies with anurans [5], our results suggest a male-biased tendency in South America. This sex-biased pattern makes it difficult to evaluate sex-specific life history strategies, such as reproductive rate and survival, in addition to making it impossible to carry out a more in-depth analysis of how sexual differences can affect ecological and evolutionary processes [43,85–87]. Analysing the male/female ratio used within families, we observed that there is a greater use of males in studies on Leptodactylidae and Hylidae, probably associated with the facility to detect calling males than females [88]. For Bufonidae and Ceratophryidae, we found a female-biased tendency, which may be related to the explosive reproductive dynamics of some species in these families, which allow for a greater collection of females during reproductive peaks [89,90]. Also, in bufonids, we find species that live associated with human dwellings, facilitating the encounter of males and females in the peri-anthropic landscape [84].
Longevity evolves in response to local abiotic and biotic factors [10]. The maximum longevity of South American anurans did not exceed 10 years for males and 11 years for females, with the families Odontophrynidae and Bufonidae having the species with the longest longevity. The average longevity varied between families from 3 to 6 years. Although the estimated maximum longevity for Odontophrynidae and Bufonidae is high when compared with other families, the average longevity of the families studied did not exceed that expected for anurans living in tropical and subtropical regions, where it normally does not exceed 9 years [91,92]. Overall, longevity within families did not vary between sexes, contrary to what is expected, since males tend to live for a shorter time than females due to the high predation pressure that they experience during the calling season [93,94]. Thus, extracting overall patterns remains challenging as only a small fraction of anurans has been studied regarding male and female longevity.
Although [5] provided a global review of studies involving amphibian longevity, information such as average age, maximum longevity, age of sexual maturity and reproductive potential of the species has not been investigated in depth. Thus, our work provides the first general information on family/species patterns for these parameters. As expected for anurans living in temperate and subtropical regions, the average age of sexual maturity was 2 years for males and females from the families Hylidae, Leptodactylidae and Microhylidae, as well as for males from the families Bufonidae and Hylodidae. This pattern occurs because, generally, anurans that live in these regions experience a well-marked climatic seasonality, determined by the variation in photoperiod and air temperature [25,29,30,61,66,67,95]. In Ceratophryidae and Odontophrynidae, males and females follow the pattern proposed for anurans that live in tropical regions, but which has already been demonstrated for anurans from subtropical regions (refer to [25,29,64–66], reaching sexual maturity at just 1 year old [33]). The anticipation of sexual maturity in these species may be the result of factors such as predation pressure, female competition or growth rate [96–98], as well as the reproductive strategies adopted by them (i.e. explosive reproduction) [89,99].
The average reproductive potential of the families varied between 2 and 5 years, with males of Ceratophryidae, Leptodactylidae and Microhylidae showing the lowest average reproductive potential (only 2 years). This low average reproductive potential in ceratoprhyids, leptodactylids and microhylids is unexpected, as it implies that males from these families have a low reproductive life expectancy [65]. Furthermore, we emphasize that the low reproductive potential is even more worrying when it comes to the Ceratophryidae family, which has members with ephemeral demography, with explosive reproduction [89] and threatened with extinction (refer to [54].
Our results suggest that efforts for future studies of longevity of South American anurans should focus mainly on families few or not yet studied (e.g. Craugastoridae, Hylidae, Centrolenidae and Dendrobatidae), with high species diversity, including small-bodied ones. Also, we suggest that future work should also be aimed at species with terrestrial habitats, with more restricted geographical distribution, as well as for species at risk of extinction. Furthermore, we suggest that sampling should be carried out in other countries than Argentina and Brazil, in order to expand the representation of different evolutionary lineages along the biogeographical units of South America. Moreover, considering the basic laboratory infrastructure demands for longevity studies and the low scientific investment in South American countries, we suggest that researchers who are interested in studying the longevity of South American anurans seek collaboration with more experienced South American researchers in the area (e.g. Argentinian and/or Brazilian researchers), or even with researchers from Europe, North America and China [5].
Acknowledgements
We thank Cinthia A. Brasileiro, Cynthia P. A. Prado, Elaine M. L. Gonsales, and Mariana Baraquet for all suggestions that improved the manuscript, and colleagues from the Herpetology Lab (UFSM), for help and discussions during the entire work. Also, we would like to thank the reviewers and associate editor Laura Porro for their excellent suggestions. Amanda J. C. Brum is grateful to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for a doctoral fellowship. Sonia Z. Cechin thanks the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), for a research fellowship (307135/2020-9).
Contributor Information
Amanda J. C. Brum, Email: amanda.c.brum94@gmail.com.
Tiago G. dos Santos, Email: frogomes@gmail.com.
Sonia Z. Cechin, Email: soniacechin@gmail.com.
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
This article has no additional data.
Supplementary material is available online [100].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors’ contributions
A.J.C.B.: conceptualization, formal analysis, investigation, methodology, project administration, validation, visualization, writing—original draft, writing—review and editing; T.GdS.: conceptualization, supervision, visualization, writing—review and editing; S.Z.C.: conceptualization, supervision, visualization, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This work was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior.
References
- 1. Tejeda MT, Arredondo J, Liedo P, Pérez-Staples D, Ramos-Morales P, Díaz-Fleischer F. 2016. Reasons for success: rapid evolution for desiccation resistance and life-history changes in the polyphagous fly Anastrepha ludens. Evolution. 70, 2583–2594. ( 10.1111/evo.13070) [DOI] [PubMed] [Google Scholar]
- 2. Qin F, Liu G, Huang G, Dong T, Liao Y, Xu X. 2018. Zinc application alleviates the adverse effects of lead stress more in female Morus alba than in males. Environ. Exp. Bot. 146, 68–76. ( 10.1016/j.envexpbot.2017.10.003) [DOI] [Google Scholar]
- 3. Wu Q, Tang Y, Dong T, Liao Y, Li D, He X, Xu X. 2018. Additional AM fungi inoculation increase Populus cathayana intersexual competition. Front. Plant Sci. 9, 607. ( 10.3389/fpls.2018.00607) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liang T, Meiri S, Shi L. 2022. Sexual size dimorphism in lizards: Rensch’s rule, reproductive mode, clutch size, and line fitting method effects. Integr. Zool. 17, 787–803. ( 10.1111/1749-4877.12569) [DOI] [PubMed] [Google Scholar]
- 5. Peng Z, Zhang L, Lu X. 2022. Global gaps in age data based on skeletochronology for amphibians. Integr. Zool. 17, 752–763. ( 10.1111/1749-4877.12584) [DOI] [PubMed] [Google Scholar]
- 6. Krasnov BR, Surkova EN, Shenbrot GI, Khokhlova IS. 2023. Latitudinal gradients in body size and sexual size dimorphism in fleas: males drive Bergmann’s pattern. Integr. Zool. 18, 414–426. ( 10.1111/1749-4877.12686) [DOI] [PubMed] [Google Scholar]
- 7. Duellman WE, Trueb L. 1994. Biology of amphibians. Baltimore: The Johns Hopkins University Press. [Google Scholar]
- 8. Stearns SC. 1992. The evolution of life histories. Oxford: Oxford University Press. [Google Scholar]
- 9. Misawa Y, Matsui M. 1999. Age determination by skeletochronology of the Japanese Salamander Hynobius kimurae (Amphibia, Urodela). Zool. Sci. 16, 845–851. ( 10.2108/zsj.16.845) [DOI] [Google Scholar]
- 10. Ricklefs RE. 2008. The evolutionary ecology of senescence: the evolution of senescence from a comparative perspective. Funct. Ecol. 22, 379–392. ( 10.1111/j.1365-2435.2008.01420.x) [DOI] [Google Scholar]
- 11. Castanet J, Vieillot HF, Ricqles A, Zylberberg L. 2003. The skeletal histology of the amphibia. Amph. Biol. (eds Heatwole H, Davies M), 5, 1587–1683. [Google Scholar]
- 12. Sinsch U. 2015. Review: skeletochronological assessment of demographic life-history traits in amphibians. Herpetol. J. 25, 5–13. [Google Scholar]
- 13. Ezcurra MD. 2017. A new early coelophysoid neotheropod from the late triassic of Northwestern Argentina. Ameghiniana 54, 506. ( 10.5710/AMGH.04.08.2017.3100) [DOI] [Google Scholar]
- 14. Tütken THU, Pfretzschner TW, Vennemann GS, Wang YD. 2004. Paleobiology and skeletochronology of jurassic dinosaurs: implications from the histology and oxygen isotope compositions of bones. Palaeogeogr. Palaeoclimatol. Palaeoecol. 206, 217–238. [Google Scholar]
- 15. Meunier FJ. 2012. Skeletochronological studies of cyclical growth of freshwater fishes in french guiana: a review. Cybium 36, 55–62. [Google Scholar]
- 16. Smirina E, Ananjeva N. 2017. On the longevity, growth and reproductive characteristics of Lichtenstein’s toadhead agama, Phrynocephalus Interscapularis Lichtenstein, 1856 (Agamidae, Sauria). Amphib-reptil. 38, 31–39. ( 10.1163/15685381-00003080) [DOI] [Google Scholar]
- 17. Altunisik A, Yildiz MZ, Akman B, Igci N, Karis M, Sömer M. 2022. Variations in age structure and growth in congeners Lacerta viridis and Lacerta media. The Anat. Rec. Adv. Integr. Anat. Evol. Biol. 306, 527–536. ( 10.1002/ar.25099) [DOI] [PubMed] [Google Scholar]
- 18. Loebens L, Theis TF, Almeida-Santos SM, Cechin SZ. 2022. Reproductive biology, sperm storage, and sexual maturity of Thamnodynastes Strigatus (Serpentes: Dipsadidae). An. Acad. Bras. Cienc. 94, e20211087. ( 10.1590/0001-3765202220211087) [DOI] [PubMed] [Google Scholar]
- 19. Avens L, Goshe L, Pajuelo M, Bjorndal K, MacDonald B, Lemons G, Bolten A, Seminoff J. 2013. Complementary skeletochronology and stable isotope analyses offer new insight into juvenile loggerhead sea turtle oceanic stage duration and growth dynamics. Mar. Ecol. Prog. Ser. 491, 235–251. ( 10.3354/meps10454) [DOI] [Google Scholar]
- 20. Turner Tomaszewicz CN, et al. 2022. Age-specific growth and maturity estimates for the flatback sea turtle (Natator depressus) by skeletochronology. PLoS One 17, e0271048. ( 10.1371/journal.pone.0271048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bourdon E, Castanet J, de Ricqlès A, Scofield P, Tennyson A, Lamrous H, Cubo J. 2009. Bone growth marks reveal protracted growth in New Zealand kiwi (Aves, Apterygidae). Biol. Lett. 5, 639–642. ( 10.1098/rsbl.2009.0310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Castanet J, Croci S, Aujard F, Perret M, Cubo J, de Margerie E. 2004. Lines of arrested growth in bone and age estimation in a small primate: Microcebus murinus. J. Zool. 263, 31–39. ( 10.1017/S0952836904004844) [DOI] [Google Scholar]
- 23. Marín-Moratalla N, Jordana X, Köhler M. 2013. Bone histology as an approach to providing data on certain key life history traits in mammals: implications for conservation biology. Mamm. Biol. 78, 422–429. ( 10.1016/j.mambio.2013.07.079) [DOI] [Google Scholar]
- 24. Zhong M, Yu X, Liao WB. 2018. A review for life-history traits variation in frogs especially for anurans in China. Asian Herpetol. Res. 9, 165–174. ( 10.16373/j.cnki.ahr.180052) [DOI] [Google Scholar]
- 25. Brum AJC, dos Santos TG, Cechin SZ. 2022. Reproductive phenology of neotropical leptodactylid frogs (genera Physalaemus and Pseudopaludicola): integrating gametogenic cycle, sexual maturity and age. Zool. Anz. 301, 11–22. ( 10.1016/j.jcz.2022.08.004) [DOI] [Google Scholar]
- 26. Rozenblut B, Ogielska M. 2005. Development and growth of long bones in european water frogs (amphibia: anura: ranidae), with remarks on age determination. J. Morphol. 265, 304–317. ( 10.1002/jmor.10344) [DOI] [PubMed] [Google Scholar]
- 27. Middleton J, Green DM. 2015. Adult age-structure variability in an amphibian in relation to population decline. Herpetologica 71, 190–195. ( 10.1655/HERPETOLOGICA-D-14-00074) [DOI] [Google Scholar]
- 28. Pascual-Pons M, Oromi N, Pujol-Buxó E, Fibla M, Sanuy D. Mantori A. 2017. Life history traits of spadefood toad (Pelobates cultripes) population from a semiarid zone in the north east of the Iberian Peninsula. Herpetol. J. 27, 57–61. [Google Scholar]
- 29. Brum AJC, Loebens L, Prado CPA, Cechin SZ. 2022. Reproductive cycle, sexual maturity and longevity of Odontophrynus americanus (Anura: Odontophrynidae) in South Brazil. Acta Zool. 103, 99–111. ( 10.1111/azo.12359) [DOI] [Google Scholar]
- 30. Zhang K, Qiu D, Zhao L, Yan C, Jin L, Liao W. 2023. Geographical variation in body size in the Asian common toad (Duttaphrynus melanostictus). Life 13, 2219. ( 10.3390/life13112219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lindquist E, Redmer M, Brantner E. 2012. Annular bone growth in phalanges of five neotropical harlequin frogs (Anura: Bufonidae: Atelopus). Phyllomedusa 11, 117–124. ( 10.11606/issn.2316-9079.v11i2p117-124) [DOI] [Google Scholar]
- 32. Arantes IC, Vasconcellos MM, Boas TCV, Veludo LBA, Colli GR. 2015. Sexual dimorphism, growth, and longevity of two toad species (Anura, Bufonidae) in a neotropical savanna. Copeia 103, 329–342. ( 10.1643/CH-14-092) [DOI] [Google Scholar]
- 33. Sinsch U, Dehling JM. 2017. Tropical anurans mature early and die young: evidence from eight Afromontane Hyperolius species and a meta-analysis. PLoS One 12, e0171666. ( 10.1371/journal.pone.0171666) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Biek R, Funk WC, Maxell BA, Mills LS. 2002. What is missing in amphibian decline research: insights from ecological sensitivity analysis. Conserv. Biol. 16, 728–734. ( 10.1046/j.1523-1739.2002.00433.x) [DOI] [Google Scholar]
- 35. Green DM. 2003. The ecology of extinction: population fluctuation and decline in amphibians. Biol. Conserv. 111, 331–343. ( 10.1016/S0006-3207(02)00302-6) [DOI] [Google Scholar]
- 36. Blaustein AR, Bancroft BA. 2007. Amphibian population declines: evolutionary considerations. Bioscience 57, 437–444. ( 10.1641/B570517) [DOI] [Google Scholar]
- 37. Vasconcelos TS, Silva FR, Santos TG, Prado VHM, Provete DB. 2019. Biogeographic patterns of south american anurans. ed, p. 149. Switzerland: Springer Cham. ( 10.1007/978-3-030-26296-9) [DOI] [Google Scholar]
- 38. IUCN . 2024. The IUCN Red List of Threatened Species. Version 2024-1. See https://www.iucnredlist.org.
- 39. Villalobos F, Dobrovolski R, Provete DB, Gouveia SF. 2013. Is rich and rare the common share? Describing biodiversity patterns to inform conservation practices for South American anurans. PLoS One 8, e56073. ( 10.1371/journal.pone.0056073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Antonelli A, et al. 2018. Conceptual and empirical advances in neotropical biodiversity research. PeerJ 6, e5644. ( 10.7717/peerj.5644) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brum AJC, Loebens L, Santos MBD, Cechin SZ. 2019. First record of growth rings for 11 native subtropical anuran species of South America. An. Acad. Bras. Cienc. 91, e20190154. ( 10.1590/0001-3765201920190154) [DOI] [PubMed] [Google Scholar]
- 42. Olson DM, Dinerstein E, Wikramanayake ED. 2001. Terrestrial ecoregions of the world: a new map of life on earth. Bioscience 51, 933–938. ( 10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2) [DOI] [Google Scholar]
- 43. Pincheira-Donoso D, Harvey LP, Grattarola F, Jara M, Cotter SC, Tregenza T, Hodgson DJ. 2020. The multiple origins of sexual size dimorphism in global amphibians. Glob. Ecol. Biogeogr. 00, 1–16. ( 10.1111/geb.13230) [DOI] [Google Scholar]
- 44. Echeverria DD, Filipello AM. 1990. Edad y crescimiento en Bufo arenarum (Anura, Bufonidae). Cuad. Herpetol. 3, 25–31. [Google Scholar]
- 45. Marangoni F, Baldo D. 2023. Life-history traits of three syntopic species of the South American redbelly toads (Anura: Bufonidae: Melanophryniscus) from the alantic forest of Argentina. Herpetol. Conserv. Biol. 18, 2013–2228. [Google Scholar]
- 46. Piñero JM, Cajade R, Marangoni F. 2023. Body size, age and growth pattern of the most represented anurans in inselbergs of northeastern argentina. Cuad. Herpetol. 37, 171–188. [Google Scholar]
- 47. Jeckel AM, Saporito RA, Grant T. 2015. The relationship between poison frog chemical defenses and age, body size, and sex. Front. Zool. 12, 27. ( 10.1186/s12983-015-0120-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sinsch U, Di-Tada IE, Martino AL. 2001. Longevity, demography and sex-specific growth of the pampa de achala toad, bufo achalensis CEI, 1972. Stud. on Neotrop. Fauna Environ. 3692, 95–104. [Google Scholar]
- 49. Bionda CL, Kost S, Salas NE, Lajmanovich RC, Sinsch U, Martino AL. 2015. Age structure, growth and longevity in the common toad, rhinella arenarum, from argentina. Acta Herpetol. 10, 55–62. [Google Scholar]
- 50. Bionda CL, Babini S, Martino AL, Salas NE, Lajmanovich RC. 2018. Impact assessment of agriculture and livestock over age, longevity and growth of populations of common toad rhinella arenarum (anura: bufonidae), central area of argentina. Glob. Ecol. Conserv. 14, e00398. [Google Scholar]
- 51. Otero MA, Pollo FE, Grenat PR, Salas NE, Martino AL. 2018. Differential effects on life history traits and body size of two anuran species inhabiting an environment related to fluorite mine. Ecol. Indic. 93, 36–44. [Google Scholar]
- 52. Rebouças R, da Silva HR, Sanuy D, Solé M. 2019. Sexual maturity and growth of male toads (Rhinella ornata): acomparison between insular and mainland populations. Zool. Anz. 283, 12–19. ( 10.1016/j.jcz.2019.07.002) [DOI] [Google Scholar]
- 53. Marangoni F, Schaefer E, Cajade R, Tejedo M. 2009. Growth-mark formation and chronology of two neotropical anuran species. J. Herpetol. 43, 546–550. [Google Scholar]
- 54. Székely D, Székely P, Stanescu F, Cogalniceanu D, Sinsch U. 2018. Breed fast, die young: demography of a poorly known fossorial frog from the xeric neotropics. Salamandra 51, 37–44. [Google Scholar]
- 55. Marangoni F, Stanescu F, Courtis A, Piñero JM, Ingaramo MR, Cajade R, Cogalniceanu D. 2018. Coping with aridity: life history of chacophrys pierottii, a fossorial anuran of gran chaco. S. Am. J. Herpetol. 13, 230–237. [Google Scholar]
- 56. Rebouças R, Silva HR, Sanuy D. 2017. Froghood: postmetamorphic devolepment in the rock river frog thoropa miliaris (spix, 1824)(anura, cycloramphidae). Acta Zool. 1–7. [Google Scholar]
- 57. Otero MA, Valetti JA, Bionda CL, Salas NE, Martino AL. 2017. Are ploidy and age size-related? A comparative study on tetraploid Pleurodema kriegi and octoploid P. cordobae (Anura: Leptodactylidae) from Central Argentina. Zool. Anz. 268, 136–142. ( 10.1016/j.jcz.2016.07.005) [DOI] [Google Scholar]
- 58. Baraquet M, Otero MA, Valetti JA, Grenat PR, Martino AL. 2018. Age, body size and growth of boana cordobae (anura: hylidae) along an elevational gradient in argentina. Herpetol. Conserv. Biol. 13, 391–398. [Google Scholar]
- 59. Baraquet M, Pollo FE, Otero MA, Grenat PR, Salas NE, Martino AL. 2021. Body size, age and growth in males populations of boana pulchella (anura, hylidae). An. Acad. Bras. Cienc. 93, e20200991. ( 10.1590/0001-3765202120200991) [DOI] [PubMed] [Google Scholar]
- 60. Cajade R, Marangoni F, Gangenova E. 2013. Age, body size and growth pattern of argenteohyla siemersi pederseni (anura: hylidae) in northeaster argentina. J. Nat. Hist. 47, 237–251. [Google Scholar]
- 61. Caldart VM, Loebens L, Brum AJC, Bataioli L, Cechin SZ. Reproductive cycle, size and age at sexual maturity, and sexual dimorphism in the stream-breeding frog Crossodactylus schmidti (Hylodidae). S. Am. J. Herpetol. 14, 1. ( 10.2994/SAJH-D-17-00060.1) [DOI] [Google Scholar]
- 62. Marangoni F, Courtis A, Piñeiro JM, Ingaramo MDR, Cajade R, Stănescu F. 2019. Contrasting life-histories in two syntopic amphibians of the leptodactylus fuscus group (HEYER 1978). An. Acad. Bras. Cienc. 91, e20180507. ( 10.1590/0001-3675201920180507) [DOI] [PubMed] [Google Scholar]
- 63. Attademo AM, Bionda CL, Peltzer PM, Lajmanovich RC, Seib SN, Basso A, Junges CM. 2014. Edad, tamaño corporal en la madurez sexual, longevidad y potencial reproductivo de leptodactylus latinasus y leptodactylus mystacinus em um cultivo de soja y um bosque nativo del centro este de argentina. Rev. Mex. Biodivers. 85, 315–317. [Google Scholar]
- 64. López JA, Antoniazzi CE, Llanes RE, Ghirardi R. 2017. Age structure, growth pattern, sexual maturity, and longevity Ofleptodactylus latrans (Anura: Leptodactylidae) in temperate wetlands. Amphib-reptil. 38, 371–379. ( 10.1163/15685381-00003117) [DOI] [Google Scholar]
- 65. Nicolino AM, Bionda CL, Salas NE, Martino AL. 2018. Historias de vida y demografa de Physalaemus biligonigerus (Anura: Leptodactylidae) en una charca periurbanadel centro de Argentina. R.B.T. 66, 765. ( 10.15517/rbt.v66i2.33407) [DOI] [Google Scholar]
- 66. Marangoni F, Barrasso DA, Cajade R, Agostin G. 2012. Body size, age and growth pattern of Physalaemus fernandezae (Anura: leiuperidae) from Argentina. N.W. J. Zool. 8, 36–71. [Google Scholar]
- 67. Otero MA, Baraquet M, Pollo F, Grenat P, Salas N, Martino A. 2017. Sexual size dimorphism in relation to age and growth in Hypsiboas cordobae (Anura: Hylidae) from Cordoba, Argentina. Herpetol. Conserv. Biol. 12, 141–148. [Google Scholar]
- 68. Iturra-Cid M, Ortiz JC, Ibargüengoytía NR. 2010. Age, size, and growth of the chilean frog pleurodema thaul (anura: leiuperidae): latitudinal and altitudinal effects. Copeia 4, 609–617. ( 10.1643/CG-09-193) [DOI] [Google Scholar]
- 69. Stanescu F, Marangoni F, Reinko I, Cogalniceanu D. 2016. Life history of neotropical microhylid (dermantonotus muelleri, boettger 1885) from the arid chaco, argentina. Herpetol. J. 26, 41–48. [Google Scholar]
- 70. Quiroga LB, Sanabria EA, Marangoni F. 2015. Sexual size dimorphism and age in Odontophrynus cf. barrioi (Anura: Odontoprhynidae) from the Monte Desert, Argentina. J. Herpetol. 49, 267–632. ( 10.1670/13-216) [DOI] [Google Scholar]
- 71. Otero MA, Grenat PR, Bionda CL, Baraquet M, Pollo FE, Salas NE, Martino AL. 2021. Age and growth in as anuran hybrid zone: fitness-related traits of the diploid/polyploid ground frog complex (genus odontophrynus) from central argentina. Zool. Anz. 293, 257–262. [Google Scholar]
- 72. Nicolino AM, Bionda CL, Salas NE, Martino AL. 2018. Historia de vida y demografa de physalaemus biligonigerus (anura: leptodactylidae) em uma charca periurbana del centro de argentina. Rev. Biol. Trop. 66, 76–775. 10.15517/rbt.v66i2.33407 [DOI] [Google Scholar]
- 73. Myers N. 1990. The biodiversity challenge: expanded hot-spots analysis. Environmentalist 10, 243–256. ( 10.1007/BF02239720) [DOI] [PubMed] [Google Scholar]
- 74. Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GA, Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature 403, 853–858. ( 10.1038/35002501) [DOI] [PubMed] [Google Scholar]
- 75. Van Noorden R. 2014. The impact gap: South America by the numbers. Nature 510, 202–203. ( 10.1038/510202a) [DOI] [PubMed] [Google Scholar]
- 76. Hortal J, Jiménez‐Valverde A, Gómez JF, Lobo JM, Baselga A. 2008. Historical bias in biodiversity inventories affects the observed environmental niche of the species. Oikos 117, 847–858. ( 10.1111/j.0030-1299.2008.16434.x) [DOI] [Google Scholar]
- 77. Hortal J, de Bello F, Diniz-Filho JAF, Lewinsohn TM, Lobo JM, Ladle RJ. 2015. Seven shortfalls that beset large-scale knowledge of biodiversity. Annu. Rev. Ecol. Evol. Syst. 46, 523–549. ( 10.1146/annurev-ecolsys-112414-054400) [DOI] [Google Scholar]
- 78. Catling PC, Burt RJ, Kooyman R. 1997. A comparison of techniques used in a survey of the ground-dwelling and arboreal mammals in forests in North-Eastern New South Wales. Wildl. Res. 24, 417. ( 10.1071/WR96073) [DOI] [Google Scholar]
- 79. Dodd CK. 2016. Reptile ecology and conservation: a handbook of techniques. United Kingdom: Oxford University Press. [Google Scholar]
- 80. Jarić I, Correia RA, Roberts DL, Gessner J, Meinard Y, Courchamp F. 2019. On the overlap between scientific and societal taxonomic attentions - insights for conservation. Sci. Total Environ. 648, 772–778. ( 10.1016/j.scitotenv.2018.08.198) [DOI] [PubMed] [Google Scholar]
- 81. Zhang H, Hu Y, Zhang Y, Li W. 2015. Evidence of the matthew effect in scientific research on mammals in the chinese first-class national protected animals list. Biodivers. Conserv. 24, 2883–2886. ( 10.1007/s10531-015-0983-8) [DOI] [Google Scholar]
- 82. Silva AF da, Malhado ACM, Correia RA, Ladle RJ, Vital MVC, Mott T. 2020. Taxonomic bias in amphibian research: are researchers responding to conservation need? J. Nat. Conserv. 56, 125829. ( 10.1016/j.jnc.2020.125829) [DOI] [Google Scholar]
- 83. Frost DR. 2024. Amphibian Species of the World: an Online Reference. Version 6.2. New York, USA: American Museum of Natural History. ( 10.5531/db.vz.0001). See https://amphibiansoftheworld.amnh.org/index.php. [DOI] [Google Scholar]
- 84. Lourenço-de-Moraes R, Malagoli LR, Guerra V, Ferreira RB, Affonso I de P, Haddad CFB, Sawaya RJ, Bastos RP. 2018. Nesting patterns among neotropical species assemblages: can reserves in urban areas be failing to protect anurans? Urban Ecosyst. 21, 933–942. ( 10.1007/s11252-018-0767-5) [DOI] [Google Scholar]
- 85. Zhang LX, Lu X. 2013. Ontogenetic mechanisms underlying sexual size dimorphism in urodele amphibians: an across-species approach. Curr. Zool. 59, 142–150. ( 10.1093/czoolo/59.1.142) [DOI] [Google Scholar]
- 86. Zhang LX, Lu X. 2013. Sexual size dimorphism in anurans: ontogenetic determination revealed by an across-species comparison. Evol. Biol. 40, 84–91. ( 10.1007/s11692-012-9187-2) [DOI] [Google Scholar]
- 87. Lemaître JF, et al. 2020. Sex differences in adult lifespan and aging rates of mortality across wild mammals. Proc. Natl Acad. Sci. USA 117, 8546–8553. ( 10.1073/pnas.1911999117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Solla SR, Shirose LJ, Fernie KJ, Barrett GC, Brousseau CS, Bishop CA. 2005. Effect of sampling effort and species detectability on volunteer based anuran monitoring programs. Biol. Conserv. 121, 585–594. ( 10.1016/j.biocon.2004.06.018) [DOI] [Google Scholar]
- 89. Wild ER. 1997. The ontogeny and phylogeny of Ceratophryinae frogs (Anura: Leptodactylidae). Unpublished PhD Thesis, [USA: ]: University of Kansas. [Google Scholar]
- 90. Maneyro R, Carreira S. 2012. Guía de anfíbios del Uruguay, p. 207. Montevideo: Ediciones de la Fuga. [Google Scholar]
- 91. Morrison C, Hero JM, Browning J. 2004. Altitudinal variation in the age at maturity, longevity, and reproductive lifespan of anurans in subtropical Queensland. Herpetologica 60, 34–44. ( 10.1655/02-68) [DOI] [Google Scholar]
- 92. Liao WB, Lu X. 2010. Age and growth of a subtropical high-elevation torrent frog, Amolops mantzorum, in Western China. J. Herpetol. 44, 172–176. ( 10.1670/08-104.1) [DOI] [Google Scholar]
- 93. Woolbright LL. 1989. Sexual dimorphism in Eleutherodactylus coqui: selection pressures and growth rates. Herpetologica 45, 68–74. [Google Scholar]
- 94. Wells KD, Schwartz JJ. 2007. The behavioral ecology of anuran communication. In Hearing and sound communication in amphibians (eds Narins P, Feng AS, Fay RR), pp. 44–86. New York: Springer. ( 10.1007/978-0-387-47796-1_3) [DOI] [Google Scholar]
- 95. Both C, Kaefer ÍL, Santos TG, Cechin STZ. 2008. An austral anuran assemblage in the neotropics: seasonal occurrence correlated with photoperiod. J. Nat. Hist. 42, 205–222. ( 10.1080/00222930701847923) [DOI] [Google Scholar]
- 96. Haddad CFB, Bastos RP. Predation on the toad bufo crucifer during reproduction (Anura: Bufonidae). Amphib-reptil. 18, 295–298. ( 10.1163/156853897X00170) [DOI] [Google Scholar]
- 97. Hinshaw SH, Sullivan BK. 1990. Predation on Hyla versicolor and Pseudacris crucifer during reproduction. J. Herpetol. 24, 196. ( 10.2307/1564228) [DOI] [Google Scholar]
- 98. Leivas PT, Moura MO, Fávaro LF. 2012. The reproductive biology of the invasive Lithobates catesbeianus (Amphibia: Anura). J. Herpetol. 46, 153–161. ( 10.1670/11-045) [DOI] [Google Scholar]
- 99. Wells KD. 1977. The social behaviour of anuran amphibians. Anim. Behav. 25, 666–693. ( 10.1016/0003-3472(77)90118-X) [DOI] [Google Scholar]
- 100. Brum A, dos Santos TG, Cechin SZ. 2024. Data from: Research on longevity and associated age data of South American anurans: trends, gaps and recommendations. Figshare. ( 10.6084/m9.figshare.c.7431941) [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.
Supplementary material is available online [100].