Abstract
Background
Color vision deficiency (CVD) cause is the difficulty distinguishing colors, which can present vocational and avocational challenges. There is a lack of data on its overall prevalence of CVD. Therefore, this systematic review and meta-analysis aim to determine the prevalence of CVD in Africa.
Methods
The protocol was registered with the Prospective Register of Systematic Reviews (PROSPERO) database (protocol registration number: CRD42024510403). A comprehensive systematic literature search was conducted via PubMed/MEDLINE/EMBASE, Google, and Google Scholar from February 2024 to May 28, 2024. The Johanna Bridges Institute quality appraisal tool was used to assess the quality of eligible articles. The pooled prevalence of CVD among Africans was estimated using a random effect model and expressed as prevalence and odds ratios with 95% confidence intervals using Der Simonian-Laird weight. The I2 statistic test was used to measure heterogeneity, and subgroup analysis was performed based on country, source of population, and gender.
Result
A total of 502 initial studies were identified, and sixteen cross-sectional studies were included. The overall pooled prevalence of CVD in Africa was 2.71% (95% CI: 2.28,3.14, I2 = 72.6%, P<0.001). The prevalence among African males and females was 2.13% and 0.34%, respectively. The highest pooled prevalence was recorded in Ethiopia at 3.63% and the prevalence among primary and secondary school students was 2.96%. A funnel plot showed that all of the studies were symmetric, and the Egger test showed no publication bias.
Conclusion
The pooled prevalence of color vision deficiency in Africa was found to be 2.71%. The highest prevalence was reported in studies conducted among school-age children in Ethiopia. Establishing effective screening programs and raising public awareness are recommended as future steps.
Introduction
Color vision deficiency (CVD) is the inability or decreased ability to perceive color differences [1, 2]. The condition primarily affects males due to its X-linked inheritance pattern [3, 4], but it can also occur as a result of an ocular, neurologic, or systemic cause [5]. CVD is one of the most common eye disorders worldwide [3].
The prevalence and distribution of CVD vary significantly across the global population and geographic regions. Individual reports from North America have indicated a range of 1.4% [6] –29% [7] of CVD, while studies from Europe have reported CVD levels ranging from 0.05% [8]–7.33% [9]. Studies from Asia have indicated a range of 1.17% [10]- 6.8% [11]. However, data on CVD in Africa are limited and fragmented, with reported prevalence levels ranging from 1.2% [4] to 4.84% [12].
Individuals affected by CVD face restrictions in performing color-guided tasks, leading to difficulty in daily activities [13] and challenges in professions requiring precise color discrimination, such as driving, military service, piloting, air traffic control, and healthcare roles [14–16].
The socioeconomic impact of CVD in Africa is exacerbated by lack of awareness among the African population about CVD [17, 18], inadequate screening programs for early detection of the condition [19], limited coping options for those affected [17], and a lack of comprehensive data on its prevalence and distribution in Africa. Therefore, conducting a systematic review and meta-analysis of the pooled prevalence of CVD in Africa is important to understand its prevalence, identify populations at higher risk, guide the development of public health strategies, contribute to the global knowledge about CVD, support advocacy, and increase awareness about CVD.
Methods
Reporting
This systematic review and meta-analysis were conducted to compile evidence published on CVD in Africa, and the protocol was registered in the Prospective Register of Systematic Reviews (PROSPERO) database (protocol registration number: CRD42024510403). The review followed the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines [20] (S1 Checklist).
Study selection and search strategy
This review included all studies that reported on the prevalence of CVD in African countries. Participants in the studies included individuals of any race, gender, or age residing in Africa. A comprehensive systematic literature search was conducted in PubMed/MEDLINE/Embase, Google, and Google Scholar, regardless of publication timelines, from February 2024 to May 28, 2024, as no systematic review and meta-analysis on CVD in Africa had been conducted.
The key search terms included “magnitude, prevalence, level, incidence, color/color vision deficiency, color impairment, color blindness, color perception defect, and color/colour vision defect” combined with the Boolean operators "AND" and "OR". For the PubMed/MEDLINE advanced search strategy, the advanced search strategy used was (((((((magnitude[Title/Abstract]) OR (Prevalence[Title/Abstract])) OR (Level[Title/Abstract])) OR (Incidence[Title/Abstract]))) AND (((("color vision deficiency"[Title/Abstract]) OR ("color impairment"[Title/Abstract])) OR ("color blindness"[Title/Abstract])) OR ("color defect"[Title/Abstract])) OR ("color perception defect"[Title/Abstract]))) OR ("colour vision deficiency"[Title/Abstract])) OR ("colour vision defects"[Title/Abstract]). In addition to the electronic database search, literature published in PubMed unindexed journals and gray literature search was conducted using direct Google Search and Google Scholar.
Inclusion criteria
This systematic review and meta-analysis included articles that followed the Coco Pop mnemonic (Condition, Context, and Population) approach [21] and included studies of distinct levels of CVD published in English until May 28, 2024, in Africa.
Exclusion criteria
Studies that did not meet the minimum quality assessment, lacked full access, or were focused on unrelated topics were excluded from this systematic review and meta-analysis.
Study selection
Three review authors (MMT, AGJ, and NHA) independently screened articles based on their titles and abstracts. The identified articles were then combined, exported, and managed using Endnote X9.2 (Thomson Reuters, Philadelphia, PA, USA) software [22]. After duplicate studies were excluded, full-text appraisal was done by review authors (AGJ, BAM, and FDS), and the disagreement between authors during abstract and full-text selection were solved based evidence-based discussion and the involvement of the remaining review authors (MMT).
Outcome measurement
The primary outcome was the prevalence of CVD in Africa, which shows the number of people who had CVD using different screening methods or tools (Ishihara, Color Vision Testing Made Easy (CVTME), and Richmond-HRR (Hardy-Rand-Rittler)).
Data extraction
Three review authors (MMT, AGJ, and FDS) extracted the data independently using a Microsoft Excel spreadsheet. The differences among the three review authors were resolved through discussion and agreement. Any discrepancies were resolved after the other authors’ review (NHA and BAM). Disagreements were resolved based on a Kappa statistic threshold of 0.8, which was used to ensure substantial agreement among reviewers. The first author’s name, the year of publication, the year of study, the study country, the study design, the sample size, the source population, the age difference among participants, the technique used to assess CVD, the type of CVD assessed, the prevalence of CVD, the prevalence CVD among gender, and the factors associated with CVD were extracted from each study. We derived estimates from each study, and when needed, variables were not directly reported. The full data extraction sheet in detail is available in S1 Table.
Quality assessment
The Johanna Bridges Institute (JBI) quality appraisal tool for cross-sectional studies, a methodological quality assessment tool with nine questions, was used to evaluate the quality of included articles and the risk of bias in each study [23]. Two authors (MMT and AGJ) independently evaluated the quality of the included articles. The assessment tool contains nine criteria: It was assessed using the JBI critical appraisal checklist options of "yes," "no," "unclear," and "not applicable." The weights of yes, no, and unclear were 1, 0, and 0, respectively. Bias risks were classified as low (5 to 9) or high (0 to 4). The study received a 50% or higher rating on all quality-assessed items, which were deemed low-risk and included in this review. Disagreements that occurred during the full-text quality assessment were resolved through evidence-based discussion with the other review authors (FDS, NHA, and BAM) The result of quality assessment/risk of bias are presented in Table 1 and S2 Table.
Table 1. JBI methodological guidance for systematic reviews of observational epidemiological studies included in this systematic review and meta-analysis,2024.
| Authors | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|
| Gudeta and Asrat et al., [12] | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Low |
| Darge et al., [24] | U/c | Yes | Yes | Yes | U/c | U/c | U/c | Yes | Yes | Low |
| Mashige et al., [25] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Dohvoma et al., [26] | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Wale et al., [27] | Yes | Yes | Yes | Yes | Yes | U/c | Yes | No | Yes | Low |
| Mitiku et al, [28] | Yes | U/c | U/c | Yes | Yes | Yes | U/c | Yes | Yes | Low |
| Ugalahi et al., [29] | Yes | Yes | Yes | Yes | Yes | Yes | U/c | No | Yes | Low |
| Oduntan et al.,[17] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Low |
| Tabansi et al., [30] | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes | Low |
| Woldeamanuel et al., [18] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Low |
| Mulusew et al., [31] | Yes | No | Yes | Yes | Yes | Yes | U/c | Yes | Yes | Low |
| Eze et al., [4] | Yes | Yes | U/c | Yes | U/c | Yes | Yes | Yes | U/c | Low |
| Mengesha et al., [32] | No | Yes | Yes | Yes | Yes | Yes | U/c | Yes | Yes | Low |
| Fakorede et al, [33] | Yes | U/c | Yes | Yes | Yes | U/c | Yes | U/c | Yes | Low |
| Ativie et al., [34] | Yes | Yes | Yes | Yes | Yes | Yes | U/c | No | Yes | Low |
| Nwobodo et al., [35] | Yes | Yes | No | Yes | Yes | Yes | U/c | No | Yes | Low |
Q1; was the sample frame appropriate to address the target population? Q2 = were study participants sampled in an appropriate way? Q3; was the sample size adequate? Q4; were the study subjects and the setting described in detail? Q5; was the data analysis conducted with sufficient coverage of the identified sample? Q6; were valid methods used for the identification of the condition? Q7; was the condition measured in a standard, reliable way for all participants? Q8; was there appropriate statistical analysis? Q9; was the response rate adequate, and if not, was the low response rate managed appropriately? U/c; unclear
Data analysis
The data was extracted using a Microsoft Excel spreadsheet and then exported to STATA version 11 for further analysis. Since the heterogenicity of this systematic review and meta-analysis is significant (I2 = 72.6%, P<0.001), the overall pooled prevalence of CVD among Africans was estimated using a random effect model and measured as prevalence and odds ratios with 95% confidence intervals using Der Simonian-Laird weight [36, 37]. The result was presented using tables and figures. Furthermore, the I2 statistic test was used to determine heterogeneity among the included studies, which describes the percentage of total variation caused by heterogeneity rather than chance. The forest plots were used to display the estimates and 95% confidence intervals from each individual study. In the meta-analysis, we used the inverse variance method to assign weights to each study. Sub-group analysis was performed based on the country in which the studies were conducted, gender, and the source of the population. We employed a funnel plot to detect publication bias, which allows us to assess whether smaller studies are more inclined to report extreme results. In addition, we conducted Egger’s test to statistically evaluate the symmetry observed in the funnel plot. To determine the impact of individual studies on the pooled estimate, a sensitivity analysis was performed. Missing data was handled by excluding the studies that missed the pertinent data and available case analysis was used. In addition, when needed, variables not reported directly, we derived estimates from each study.
Result
Study selection
A total of 502 initial records were extracted from search engines like PubMed/MEDLINE/Embase, Google, and Google Scholar. From the retrieved records, 398 studies were duplicated, and seventy-eight were excluded after the title and abstract were carefully reviewed based on the eligibility criteria. Full-text article assessment was performed for the rest of the 26 articles, and five, three, and two research articles were excluded from this systematic review and meta-analysis for not meeting the minimum quality assessment [38–42], lack of full-text article access [43–45] and similar studies published using a different topic [46, 47], respectively. Finally, this systematic review and meta-analysis included fifteen articles and one preprint study. The detailed result of all studies identified in the literature search, including those that were excluded from the analyses available in (Fig 1 and S3 Table).
Fig 1. Preferred reporting for systematic review and meta-analysis of CVD in Africa,2024.
Study characteristics
In this systematic review and meta-analysis, a total of sixteen cross-sectional studies were included [4, 12, 17, 18, 24–35], with a 21,167-study population. From all studies majority of the included studies were from Ethiopia and Nigeria, respectively. Only one study was included from South Africa and Cameron. All studies assessed congenital CVD. All studies used the Ishihara test to assess the prevalence of CVD, except those conducted in South Africa and Nigeria, which used CVTME and Richmond-HRR tests, respectively. The participants’ ages range from 5 to 60 years. About two-thirds of 11 (68.75%) of the studies, around quarter 4 (25%), and 1 (6.25%) of the studies were conducted in primary and secondary school, university students, and community, respectively (Table 2).
Table 2. Typical characteristics of cross-sectional studies included in systematic review and meta-analysis of color vision deficiency in Africa, 2024.
| Author (year) | Country | Sample size | Source population | Method | Diagnostic criteria | CVD (%) |
|---|---|---|---|---|---|---|
| Gudeta and Asrat et al., (2024) [12] | Ethiopia | 864 | School Children | Ishihara | > 5 typical red-green defective responses: plates 2 and 21 | 4.84 |
| Darge et al., (2017) [24] | Ethiopia | 378 | School Children | - | - | 4.2 |
| Mashige et al., (2019) [25] | South Africa | 1305 | School children | CVTME | > 3 errors: plates 1 and 14 | 2.2 |
| Dohvoma et al., (2018) [26] | Cameron | 303 | Higher education | Ishihara | > 3 three typical red-green defective responses: plates 2 and 21 | 1.7 |
| Wale et al., (2018) [27] | Ethiopia | 854 | School children | Ishihara | ≤ 9 read correctly | 4.24 |
| Mitiku et al., (2020) [28] | Ethiopia | 4004 | Higher education | Ishihara | ≤ 9 read correctly | 2.85 |
| Ugalahi et al., (2016) [29] | Nigeria | 1635 | Secondary school | Ishihara | Incorrect response in > 2 plates | 2.35 |
| Oduntan et al., (2019) [17] | Nigeria | 2326 | Primary and secondary school | Richmond-HRR | If any of plates 7–10 were not ticked | 2.5 |
| Tabansi et al., (2008) [30] | Nigeria | 1300 | Primary school | Ishihara | Incorrect response in >2 plates | 2.6 |
| Woldeamanuel and Geta, (2018) [18] | Ethiopia | 844 | School children | Ishihara | > 5 typical red-green defective responses: plates 2 and 21 | 4.1 |
| Mulusew et al., (2013) [31] | Ethiopia | 1040 | School children | Ishihara | > 5 typical red-green defective responses: plates 2 and 21 | 4.2 |
| Eze et al., 2020 [4] | Nigeria | 950 | Secondary school | Ishihara | Failed to read > 4 letters: plates 1–21 | 1.2 |
| Mengesha et al., (2021) [32] | Ethiopia | 2400 | Primary School | Ishihara | ≤ 9 read correctly | 2.29 |
| Fakorede et al., (2022) [33] | Nigeria | 1191 | Higher education | Ishihara | ≤ 9 read correctly | 2.85 |
| Ativie et al., (2017) [34] | Nigeria | 1500 | Community | Ishihara | ≤ 9 read correctly | 1.7 |
| Nwobodo et al., (2022) [35] | Nigeria | 291 | Higher education | Ishihara | Students that failed ≥ 8plates | 1.7 |
CVD; color/colour vision deficiency, CVTME; Color Vision Testing Made Easy, HRR; Hardy-Rand-Rittler
Pooled prevalence of color vision deficiency in Africa
From all sixteen studies, a total of 21,167 participants were included in this pooled estimate of the prevalence of CVD in Africa. The overall pooled prevalence of CVD in Africa was 2.71 percent (95% CI: 2.28,3.14, I2 = 72.6%, P<0.001). The lowest prevalence of CVD (1.20%)(95% CI:0.51,1.89) was recorded in Nigeria among secondary school students [4]. Whereas the higher prevalence of CVD of 4.84% (95% CI:3.39,6.29) was recorded in Ethiopia among primary school children [12] (Fig 2).
Fig 2. Forest plot shows pooled estimate prevalence of color vision deficiency in Africa.
Publication bias
The funnel plot of this systematic review and meta-analysis showed that all the studies were symmetric (Fig 3), and the Egger test showed there was no significant publication bias (P-value = 0.067).
Fig 3. Funnel plot test of the sixteen studies included in the meta-analysis of color vision deficiency in Africa, 2024.
Pooled prevalence of color vision deficiency based on gender in Africa
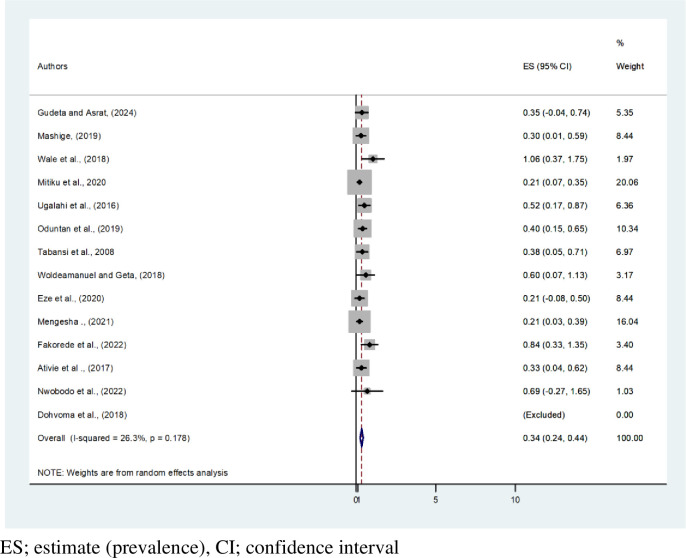
This systematic review and meta-analysis showed that the pooled estimated prevalence of CVD among African males and females is 2.13% (95% CI: 1.73, 2.52, I2 = 72%, P < 0.001) and 0.34% (95% CI: 0.24,0.44), respectively. The highest prevalence of CVD by gender, 4.49% [12], was recorded among males. Based on this systematic review and meta-analysis, CVD affects 1 in every 35 men and 1 in every 300 females in Africa (Figs 4 and 5). A tolerable heterogeneity level was observed, allowing for a valid comparison among females (I2 = 26.3%, P = 0.178).
Fig 4. Forest plot on pooled estimate prevalence of color vision deficiency among males in Africa.
Fig 5. Forest plot on pooled estimate prevalence of color vision deficiency among females in Africa.
Subgroup analysis based on country and source population
The subgroup analysis conducted based on the country showed that the highest pooled prevalence of CVD was recorded in Ethiopia (3.63%, 95% CI:2.88,4.38, I2; 73.5, P = 0.001), and the prevalence of CVD ranges from 4.84% (95% CI:3.39,6.29) [12] to 2.29 (95% CI:1.69,2.89) [32] in Ethiopia. Cameroon has the least pooled CVD: 1.70 (95% CI:0.24, 3.16) [26] (Fig 6).
Fig 6. Forest plot on subgroup estimate prevalence of color vision deficiency in Africa based on the country.
In addition, the sub group analysis was conducted based on the source of population, and the highest pooled prevalence of CVD was recorded among primary and secondary school students (2.96%, 95% CI: 2.36, 3.56, I2: 78.6%, P = 0.267) (Fig 7).
Fig 7. Forest plot on subgroup estimates the prevalence of color vision deficiency in Africa based on the source of population.
Sensitivity analysis
By excluding all 16 articles included step by step, a sensitivity analysis was conducted to test the effect of each study on the pooled prevalence (Table 3).
Table 3. Sensitivity analysis of systematic review and meta-analysis of color vision deficiency in Africa, 2024.
| Author(year) | Pooled estimate | I 2 | P value |
|---|---|---|---|
| Gudeta and Asrat et al., (2024) [12] | 2.58% (2.18–2.99) | 68.5% | <0.001 |
| Darge et al., (2017) [24] | 2.66% (2.22–3.09) | 73.1% | <0.001 |
| Mashige et al., (2019) [25] | 2.75% (2.29–3.21) | 74.1% | <0.001 |
| Dohvoma et al., (2018) [26] | 2.76% (2.31–3.20) | 73.8% | <0.001 |
| Wale et al., (2018) [27] | 2.62% (2.19–3.04) | 71.0% | <0.001 |
| Mitiku et al., (2020) [28] | 2.71% (2.23–3.18) | 73.4% | <0.001 |
| Ugalahi et al., (2016) [29] | 2.75% (2.28–3.21) | 74.3% | <0.001 |
| Oduntan et al., (2019) [17] | 2.74% (2.26–3.21) | 74.4% | <0.001 |
| Woldeamanuel and Geta, (2018) [18] | 2.63% (2.20–3.05) | 71.5% | <0.001 |
| Tabansi et al., (2008) [30] | 2.72% (2.26–3.18) | 74.4% | <0.001 |
| Mulusew et al., (2013) [31] | 2.61% (2.19–3.03) | 70.3% | <0.001 |
| Eze et al., (2020) [4] | 2.81% (2.40–3.21) | 64.6% | <0.001 |
| Fakorede et al., (2022) [33] | 2.70% (2.25–3.16) | 74.2% | <0.001 |
| Ativie et al., (2017) [34] | 2.78% (2.33–3.23) | 72.5% | <0.001 |
| Nwobodo et al., (2022) [35] | 2.76% (2.31–3.12) | 73.8% | <0.001 |
| Mengesha et al., (2021) [32] | 2.56% (2.28–3.23) | 74.1% | <0.001 |
Discussion
Studies have shown that the prevalence of CVD in the African population ranges from 1.20% to 4.84% [4, 12]. Therefore, this systematic review and meta-analysis will be crucial for enhancing the overall understanding of CVD in Africa and providing valuable insights to advocate for better management of CVD. In this study, the pooled prevalence of CVD was found to be 2.71% (95% CI: 2.28, 3.14, I2 = 72.6%). Furthermore, the prevalence of CVD was 2.13% among African males and 0.34% among African females.
The findings of our current study are consistent with individual studies conducted in South America, which reported a prevalence of 2.36% [48], and a study conducted in Asia, which reported 2.72% [49]. This similarity could be attributed to comparable data collection methods, particularly the use of pseudoisochromatic plates [27, 28, 32].
The findings of the current study show a higher prevalence of color vision deficiency compared to individual reports from Asia: 1% in Bangladesh [50], 1.17% in Saudi Arabia [10], 2.18% in India [51], 2.1% in Nepal [52], and 3.8% in Iran [53]. In North America, an individual report showed a prevalence of 1.4% [54]. Previous single studies had limited sample sizes, particularly among children. In contrast, the current meta-analysis included studies conducted with both children and adults, which may have contributed to the higher prevalence of acquired color vision deficiency [5]. Additionally, ethnic differences among study participants could also accounts for discrepancy [6].
The pooled prevalence of CVD in Africa, as determined by this systematic review and meta-analysis, is lower than the prevalence reported in individual studies from North America, where a 29% prevalence was found among participants with high exposure to both hexane and non-hexane solvents, which was associated with a higher prevalence of acquired color vision defects [7]. In Europe, a single study reported a prevalence of 7.33% [9], and a study from Asia found a prevalence of 6.8% [11], which only included male participants. This might be a possible reason for the higher prevalence, as color vision deficiency is linked to the X linked inheritance pattern and is more prevalent in men [2].
In a systematic review and meta-analysis, the prevalence of CVD was found to be 2.13% among African males, and 0.34% among African females. In Iran, a systematic review and meta-analysis reported a prevalence of 4.7% among males and 0.7% among females [53]. The difference between the two populations could be attributed to ethnic variation, possibly related to variance in color vision among different ethnic groups [6].
The highest pooled prevalence of CVD was recorded in Ethiopia at 3.63%, followed by South Africa at 2.20%, and Nigeria at 2.16%, with the lowest prevalence in Cameroon at 1.70% [26]. A higher percentage of male participants were enrolled in Ethiopia studies compared to Cameroon, where there was a comparable number of male and female participants. The difference in prevalence may be attributed to the fact that CVD primarily affects male [53] and ethnic variation between the two population [6]. These factors should be considered in future studies.
The highest pooled prevalence of CVD was found among primary and secondary school students, followed by studies conducted among university students and community-based studies. More participants were involved in studies conducted among primary and secondary school students, with a higher percentage of male participants compared to studies conducted among university students and community-based studies. Since CVD follows an X-linked pattern [29], this could explain the higher prevalence among primary and secondary school students.
In a systematic review and meta-analysis, being male [4, 17, 18, 27, 29] and visually impaired [27, 47] were frequently reported as risk factors for CVD in Africa. Males had a higher prevalence of CVD than females due to genetic predisposition [29]. Another significant factor is visual impairment, as those who are visually impaired are more likely to acquired CVD [47].
Color vision deficiency poses a significant challenge in many occupational activities. A diagnosis of CVD can have a negative psychological impact on mental well-being [55] and can adversely affect job prospects [56], often leading individuals to invest significant time and resources, both financial and mental, toward an unattainable goal [14, 57]. Its high prevalence among school-age children and university students can negatively affect their academic performance [58]. Raising public awareness through mainstream media and social media is crucial. Parents should be informed about color vision deficiency in their children, enabling early diagnosis and management to support their kids [59].
Regular early screening and counseling programs at higher education centers are essential to minimize the impact of CVD on African students. It is critical to ensure that individuals and children receive proper care without it interfering with their future careers and education. Research should be conducted on the occupational impact of CVD in Africa, advocating for changes in recruitment process based on the type and severity of color vision loss. Additionally, critical color-related tasks in the work environment should be considered for future planning in Africa.
Limitations
High heterogenicity was observed in the estimate of the pooled prevalence of CVD and in sub group estimate based on country and source population. This variation may be attributed to the difference in the percentage of sex and ethnicity across the studies included in the analysis, which could influence the prevalence. Different studies used different pass/fail criteria on the Ishihara test and this could impact the measured prevalence of CVD. Additionally, the inability to access three full text articles was a limitation of this systematic review and meta-analysis.
Conclusion
The prevalence of color vision deficiency in Africa was found to be 2.71%, with 2.13% among African males and 0.34% among African females. It is critical to increase public awareness and establish proper screening programs for school children and university students.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Fareed M, Anwar MA, Afzal M. Prevalence and gene frequency of color vision impairments among children of six populations from North Indian region. Genes & diseases. 2015;2(2):211–8. doi: 10.1016/j.gendis.2015.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simunovic MP. Colour vision deficiency. Eye (Lond). 2010;24(5):747–55. doi: 10.1038/eye.2009.251 [DOI] [PubMed] [Google Scholar]
- 3.Birch J. Worldwide prevalence of red-green color deficiency. J Opt Soc Am A Opt Image Sci Vis. 2012;29(3):313–20. doi: 10.1364/JOSAA.29.000313 [DOI] [PubMed] [Google Scholar]
- 4.Eze GC, Kizor-Akaraiwe N, Chime AA, Anajekwu CC, Asimadu IN, Edoga CE, et al. Colour vision defect among secondary school students in enugu, Nigeria: prevalence, pattern and impact. Advance in Ophthalmology & Visual System. 2020;10(5):113–9. [Google Scholar]
- 5.Simunovic MP. Acquired color vision deficiency. Survey of ophthalmology. 2016;61(2):132–55. doi: 10.1016/j.survophthal.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 6.Xie JZ, Tarczy-Hornoch K, Lin J, Cotter SA, Torres M, Varma R. Color vision deficiency in preschool children: the multi-ethnic pediatric eye disease study. Ophthalmology. 2014;121(7):1469–74. doi: 10.1016/j.ophtha.2014.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beckman S, Eisen EA, Bates MN, Liu S, Haegerstrom-Portnoy G, Hammond SK. Acquired Color Vision Defects and Hexane Exposure: A Study of San Francisco Bay Area Automotive Mechanics. American journal of epidemiology. 2016;183(11):969–76. doi: 10.1093/aje/kwv328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrington S, Davison PA, O’Dwyer V. Prevalence of colour vision deficiency in the Republic of Ireland schoolchildren and associated socio-demographic factors. Clinical & experimental optometry. 2021;104(1):48–55. doi: 10.1111/cxo.13072 [DOI] [PubMed] [Google Scholar]
- 9.Citirik M, Acaroglu G, Batman C, Zilelioglu O. Congenital color blindness in young Turkish men. Ophthalmic epidemiology. 2005;12(2):133–7. doi: 10.1080/09286580590932743 [DOI] [PubMed] [Google Scholar]
- 10.Khairoalsindi OA, Almasoudi BM, Bamahfouz AY, Alghamdi AA, Siddiqui MI. Prevalence and Determinants of Color Vision Defects among Preparatory University Students at Makkah, Saudi Arabia. Middle East African journal of ophthalmology. 2019;26(3):133–7. doi: 10.4103/meajo.MEAJO_29_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahadi M, Ebrahimi A, Rahmani S, Baghban AA. Prevalence of refractive errors and color vision deficiency in a population of industry-workers in Abhar, Iran. Medicine. 2021;100(46):e27758. doi: 10.1097/MD.0000000000027758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gudeta TB, Asrat TJBp. Prevalence and genotypic frequency of color vision defects among primary schoolchildren in Adama Town, Eastern Ethiopia. BMC pediatrics. 2024;24(1):72. doi: 10.1186/s12887-024-04529-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Male SR, Shamanna BR, Bhardwaj R, Gandhi R, Bhagvati C, Theagarayan B. Impact of color vision deficiency on the quality of life in a sample of Indian population: Application of the CVD-QoL tool. Indian journal of ophthalmology. 2023;71(5):2204–11. doi: 10.4103/ijo.IJO_1975_22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cumberland P, Rahi JS, Peckham CS. Impact of congenital colour vision defects on occupation. Archives of disease in childhood. 2005;90(9):906–8. doi: 10.1136/adc.2004.062067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hathibelagal AR. Implications of inherited color vision deficiency on occupations: A neglected entity! Indian journal of ophthalmology. 2022;70(1):256–60. doi: 10.4103/ijo.IJO_1100_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah A, Hussain R, Fareed M, Afzal M. Prevalence of Red-Green Color Vision Defects among Muslim Males and Females of Manipur, India. Iranian journal of public health. 2013;42(1):16–24. [PMC free article] [PubMed] [Google Scholar]
- 17.Oduntan OA, Mashige KP, Kio FE. Colour vision deficiency among students in Lagos State, Nigeria. African health sciences. 2019;19(2):2230–6. doi: 10.4314/ahs.v19i2.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woldeamanuel GG, Geta TG. Prevalence of color vision deficiency among school children in Wolkite, Southern Ethiopia. BMC research notes. 2018;11(1):838. doi: 10.1186/s13104-018-3943-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burnett AM, Yashadhana A, Lee L, Serova N, Brain D, Naidoo K. Interventions to improve school-based eye-care services in low- and middle-income countries: a systematic review. Bulletin of the World Health Organization. 2018;96(10):682–94d. doi: 10.2471/BLT.18.212332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open medicine: a peer-reviewed, independent, open-access journal. 2009;3(3):e123–30. doi: 10.1136/bmj.327.7423.1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munn Z, Stern C, Aromataris E, Lockwood C, Jordan Z. What kind of systematic review should I conduct? A proposed typology and guidance for systematic reviewers in the medical and health sciences. BMC medical research methodology. 2018;18(1):5. doi: 10.1186/s12874-017-0468-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan ZY, Yang Y, Zhang F. Association between health literacy and mortality: a systematic review and meta-analysis. Archives of public health = Archives belges de sante publique. 2021;79(1):119. doi: 10.1186/s13690-021-00648-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. International journal of evidence-based healthcare. 2015;13(3):147–53. doi: 10.1097/XEB.0000000000000054 [DOI] [PubMed] [Google Scholar]
- 24.Darge HF, Shibru G, Mulugeta A, Dagnachew YM. The Prevalence of Visual Acuity Impairment among School Children at Arada Subcity Primary Schools in Addis Ababa, Ethiopia. Journal of ophthalmology. 2017;2017:9326108. doi: 10.1155/2017/9326108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mashige KP. Impact of congenital color vision defect on color-related tasks among schoolchildren in Durban, South Africa. Clinical optometry. 2019;11:97–102. doi: 10.2147/OPTO.S204332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dohvoma VA, Ebana Mvogo SR, Kagmeni G, Emini NR, Epee E, Mvogo CE. Color vision deficiency among biomedical students: a cross-sectional study. Clinical ophthalmology (Auckland, NZ). 2018;12:1121–4. doi: 10.2147/OPTH.S160110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wale MZ, Abebe Y, Adamu Y, Zelalem A. Prevalence of color blindness among school children in three primary schools of Gish -Abay town district, Amhara regional state, north-west Ethiopia. BMC ophthalmology. 2018;18(1):306. doi: 10.1186/s12886-018-0970-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitiku RG, Tolera BS, Tolesa ZG. Prevalence and allele frequency of Congenital Colour Vision Deficiency (CCVD) among students at Hawassa University, Ethiopia. The Journal of the Egyptian Public Health Association. 2020;95(1):10. doi: 10.1186/s42506-020-00037-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ugalahi MO, Fasina O, Ogun OA, Ajayi BG. Prevalence of congenital colour vision deficiency among secondary school students in Ibadan, South-West Nigeria. The Nigerian postgraduate medical journal. 2016;23(2):93–6. doi: 10.4103/1117-1936.186301 [DOI] [PubMed] [Google Scholar]
- 30.Tabansi PN, Anochie IC, Nkanginieme KE, Pedro-Egbe CN. Screening for congenital color vision deficiency in primary children in Port Harcourt City; teachers’ knowledge and performance. Nigerian journal of medicine: journal of the National Association of Resident Doctors of Nigeria. 2008;17(4):428–32. doi: 10.4314/njm.v17i4.37427 [DOI] [PubMed] [Google Scholar]
- 31.Asferaw M, A Y. Prevalence of congenital color vision defects among school children in five schools of Abeshge District, Central Ethiopia. Journal of Ophthalmology of Eastern, central and southern Africa. 2013;17:10–4. [Google Scholar]
- 32.Mengesha WA, Mengistu AG, Tolesa ZG. Prevalence and Allele Frequency of Red-Green Color Vision Defects among School Children in Repi Primary School in Addis Ababa, Ethiopia. 2021. [Google Scholar]
- 33.Fakorede S, Akpan L, Adekoya K, Oboh B. Prevalence and population genetic data of colour vision deficiency among students from selected tertiary institutions in Lagos State, Nigeria. Egyptian Journal of Medical Human Genetics. 2022;23. [Google Scholar]
- 34.Ativie R, Ubom R, Aigbiremolen A, Mukoro O, Odigie O, Igweh J. Prevalence of Congenital Colour Vision Deficiency in Nigerians Living in Ugep, Cross River State. Ophthalmology Research: An International Journal. 2017;7:1–6. [Google Scholar]
- 35.Nwobodo E, Nneoma D, Ikwuka D. Prevalence of Colour Blindness in Nigeria: Nnamdi Azikiwe University Medical. Cyprus Journal of Medical Sciences. 2021. [Google Scholar]
- 36.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 37.Fletcher J. What is heterogeneity and is it important? BMJ (Clinical research ed). 2007;334(7584):94–6. doi: 10.1136/bmj.39057.406644.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muthuswamy M, Oljira T, Geletu T. Identification of colorblindness among selected primary school children in Hararghe Region, Eastern Ethiopia. Alexandria Journal of Medicine. 2018;54. [Google Scholar]
- 39.Osman S, Khalaf S, Mohammed H, El-Sebaity D, Osman D. Prevalence and predictors of colour vision defects among Egyptian university students. Eastern Mediterranean health journal = La revue de sante de la Mediterranee orientale = al-Majallah al-sihhiyah li-sharq al-mutawassit. 2021;27(4):399–406. doi: 10.26719/emhj.20.128 [DOI] [PubMed] [Google Scholar]
- 40.Pickford RW, Pickford R. Frequency of colour vision defects among Zulus in Natal. Journal of biosocial science. 1981;13(2):241–8. doi: 10.1017/s0021932000013407 [DOI] [PubMed] [Google Scholar]
- 41.Rahman SA, Singh PN, Nanda PK. Comparison of the incidence of colour blindness between sections of Libyan and Indian populations. Indian journal of physiology and pharmacology. 1998;42(2):271–5. [PubMed] [Google Scholar]
- 42.Rosa PJM. The Distribution of Red and Green Colourblindness in Kenya. Environmental Science 1981:286–99. [Google Scholar]
- 43.Borges da Silva G, Gabaye Borges da Silva G. [The prevalence of color blindness in Senegal in a population of 4500 workers]. Bulletin de la Societe de pathologie exotique et de ses filiales. 1983;76(5 Pt 2):841–5. [PubMed] [Google Scholar]
- 44.Odeigah PG, Okon EE. Colour vision defects and gene flow in Nigerians. East African medical journal. 1986;63(10):666–71. [PubMed] [Google Scholar]
- 45.Ohta Y, Kogure S, Izutsu Y, Miyamoto T, Nagai I. Clinical analysis of colour vision deficiencies with The City University test. Modern problems in ophthalmology. 1978;19:126–30. [PubMed] [Google Scholar]
- 46.Mashige KP, van Staden DB. Prevalence of congenital colour vision deficiency among Black school children in Durban, South Africa. BMC research notes. 2019;12(1):324. doi: 10.1186/s13104-019-4374-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zelalem M, Abebe Y, Adamu Y, Getinet T. Prevalence of visual impairment among school children in three primary schools of Sekela Woreda, Amhara regional state, north-west Ethiopia. SAGE open medicine. 2019;7:2050312119849769. doi: 10.1177/2050312119849769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mueller WH, Weiss KM. Colour-blindness in Colombia. Annals of human biology. 1979;6(2):137–45. doi: 10.1080/03014467900003471 [DOI] [PubMed] [Google Scholar]
- 49.Chia A, Gazzard G, Tong L, Zhang X, Sim EL, Fong A, et al. Red-green colour blindness in Singaporean children. Clinical & experimental ophthalmology. 2008;36(5):464–7. [PubMed] [Google Scholar]
- 50.Yasmin A, N J, Akhter R. Assessment of Color Blindness and Erythrocyte G6PD Enzyme Status among the School Children of Dhaka City. Journal of Bangladesh Society of Physiologist. 2010;4. [Google Scholar]
- 51.B S, K VP. The Prevalence of Congenital Color Vision Abnormality Among Patients Attending a Tertiary Eye Care Center in Southern India. Cureus. 2023;15(8):e43837. doi: 10.7759/cureus.43837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shrestha RK, Joshi MR, Shakya S, Ghising R. Color vision defects in school going children. JNMA; journal of the Nepal Medical Association. 2010;50(180):264–6. [PubMed] [Google Scholar]
- 53.Rezaei L, Hawasi E, Salari N, Mohammadi M. Prevalence of Color Blindness in Iranian Students: A Meta-analysis. Journal of ophthalmic & vision research. 2022;17(3):413–23. doi: 10.18502/jovr.v17i3.11580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choi TB, Lee DA, Oelrich FO, Amponash D, Bateman JB, Christensen RE. A retrospective study of eye disease among first grade children in Los Angeles. Journal of the American Optometric Association. 1995;66(8):484–8. [PubMed] [Google Scholar]
- 55.Barry JA, Mollan S, Burdon MA, Jenkins M, Denniston AK. Development and validation of a questionnaire assessing the quality of life impact of Colour Blindness (CBQoL). BMC ophthalmology. 2017;17(1):179. doi: 10.1186/s12886-017-0579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steward JM, Cole BL. What do color vision defectives say about everyday tasks? Optometry and vision science: official publication of the American Academy of Optometry. 1989;66(5):288–95. doi: 10.1097/00006324-198905000-00006 [DOI] [PubMed] [Google Scholar]
- 57.Stoianov M, de Oliveira MS, Dos Santos Ribeiro Silva MCL, Ferreira MH, de Oliveira Marques I, Gualtieri M. The impacts of abnormal color vision on people’s life: an integrative review. Quality of life research: an international journal of quality of life aspects of treatment, care and rehabilitation. 2019;28(4):855–62. doi: 10.1007/s11136-018-2030-1 [DOI] [PubMed] [Google Scholar]
- 58.Cole BL. Assessment of inherited colour vision defects in clinical practice. Clinical & experimental optometry. 2007;90(3):157–75. doi: 10.1111/j.1444-0938.2007.00135.x [DOI] [PubMed] [Google Scholar]
- 59.Rigaudière F, Leid J, Viénot F, Le Gargasson JF. [Neurophysiological basis and clinical tests for assessment of X-linked color vision deficiencies in school children]. Journal francais d’ophtalmologie. 2006;29(1):87–102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.