Abstract
Introduction
Neurofibrillary tangles (NFTs) are composed of hyperphosphorylated forms of microtubule-associated protein tau (Tau), which is responsible for neurodegeneration in Alzheimer’s disease (AD). The hippocampal region has been a major focus of AD research because the deposits of phosphorylated tau protein in these regions are correlated with early memory deficits. Despite extensive studies, therapeutic strategies to reduce tau hyperphosphorylation and NFTs deposition remain unclear. AL04, a recently developed recombinant fusion protein comprising Cystatin C, human serum albumin, and a novel blood brain barrier (BBB) penetrating peptide, is currently under investigation. Previous studies have demonstrated its effectiveness in reducing amyloid beta plaques in AD mouse model.
Methods
In this study, we investigated the effects of AL04 on lowering hyperphosphorylated tau and NFTs in JNPL3 mouse model harboring human tau-P301L mutation. 3-month-old female mice intraperitoneally received AL04 (5 mg/kg) or PBS treatment every other week for 24 weeks. We used confocal microscopy and western blot to visualize and analyze changes in hyperphosphorylated tau Ser202/Thr205 labeled with AT8 antibody in the brain.
Results
We found that the AL04 treatment decreases hyperphosphorylated tau at PP2A-sensitive epitope Ser202/Thr205 in the hippocampus of the brain. In the brain lysates of AL04 treated mice, we observed the reduction of I2PP2A, inhibitor of PP2A, and the induction of autophagy receptor proteins such as SQSTM-1/p62 and OPTN.
Conclusion
Our data suggests that AL04 can be used as an AD prophylactic/therapeutic agent as it lowers the hyperphosphorylated tau by downregulating I2PP2A. We also propose that AL04 can induce the degradation of hyperphosphorylated tau aggregates through the upregulation of the autophagy pathway.
Key words: Alzheimer’s disease, Anti-Tau Therapeutics, Hyperphosphorylated Tau, Phosphatase PP2A, Protein-degradation, Neurodegeneration
Highlights
-
•
AL04 lowers hyperphosphorylated tau in the hippocampus of JNPL3 mice.
-
•
AL04 attenuates the spread of tau pathology within the subfields of the CA3 and DG.
-
•
AL04 treatment may promote the degradation of hyperphosphorylated tau in the brain.
1. Introduction
NFTs pathology in AD is thought to be the result of the aggregation of an abnormal hyperphosphorylated tau due to the deregulation of kinase and/or phosphatase activities (Ballatore et al., 2007, Braak and Braak, 1995, Grundke-Iqbal et al., 1986). Hyperphosphorylation of tau causes the tau to be detached from the microtubules, thereby causing the destabilizing of microtubules, which will lead to the destabilization of microtubule while compromising axonal integrity (Bramblett et al., 1993, Ishihara et al., 1999; Rodríguez-Martín et al., 2013). Therefore, therapeutic strategies to inhibit tau hyperphosphorylation in AD are urgently needed. The phenotype of mice expressing tau P301L mutant mimics the features of human tauopathies and provides a model for the investigation of the pathogenesis of AD with NFTs (Goedert et al., 2000, Lewis et al., 2000).
The phosphorylation and dephosphorylation of tau are controlled by the equilibrium of protein kinase activities (GSK3β, Akt/PKB, PKA, ERK1/2), and phosphatases (PP1, PP2A and PP5) (Ballatore, 2007). Among them, PP2A is the major tau serine/threonine phosphatase in mammalian brain and accounts for 70 % of the total tau phosphatase activity in the human brain and dephosphorylates abnormally phosphorylated tau at Ser46, Ser199, Ser202, Thr 205, Ser396 and Ser404 (Gong et al., 1993, Taleski and Sontag, 2018). In the AD patient brain, the activity of PP2A was decreased and the expression of an endogenous PP2A inhibitor (Inhibitor 2 of protein phosphatase 2 A, I2PP2A) was increased (Chohan et al., 2006, Liu et al., 2005). Therefore, I2PP2A inhibits PP2A activity and leads to the increase of hyperphosphorylation tau in AD brain (Qian et al., 2010).
Because P301L mutation makes tau prone to phosphorylation and aggregation, the JNPL3 mice present a more challenging target for drug development than mouse models of human tau without mutations (Davidowitz et al., 2023, Shibuya et al., 2015). Therefore, reducing levels of P301L Tau may offer a promising therapeutic strategy to reduce Tau hyperphosphorylation and NFT deposition. However, progress against AD has been limited with only a few clinical trials over the past decades. This has prompted the need for the development of new and more effective treatments.
Autophagy is one of the main processes of degradation of pathological proteins such as phosphor-Tau (Xu et al., 2022). Autophagic malfunction has been suggested to be an element of pathogenesis of neurodegenerative disorders, including AD (Caballero et al., 2018; Rodríguez-Martín et al., 2013). Tizon et al. showed that Cystatin C induces autophagy and could be considered as a neuroprotective agent (Tizon et al., 2010). If the levels of mutant Tau (P301L Tau) and hyperphosphorylated tau are reduced in JNPL3 mice due to autophagy activated by AL04 (Cystatin C, action moiety), this may demonstrate a direct neuroprotective effect. The therapeutic strategies for the hyperphosphorylated tau would include inhibition of kinases, restoration of PP2A activity, inhibition of tau aggregation, induction of tau clearance and post-translational modifications (Khanna et al., 2016, Le Corre et al., 2006, Lee et al., 2001, Wang et al., 2007).
We have previously reported that the novel BBB-crossing human serum albumin-Cystatin C fusion protein, AL04, might be beneficial for the treatment of AD (Bang et al., 2022). In this study, we investigated the effect of AL04 for lowering the hyperphosphorylated tau in the hippocampus of JNPL3 mice. Thus, we explore the therapeutic possibility of AL04 to determine its ability to attenuate tau hyperphosphorylation aggregates and/or restore the protein-degradation system.
2. Materials and methods
2.1. Purification of AL04 protein
This was conducted as described previously (Bang et al., 2022, Bang et al., 2024). In brief, the CHO-DG44 cells, which constitutively express the AL04 protein [CysC-HSA-CPP] (Bang et al., 2022) (Fig. 1) and were previously established as a stable cell line (Bang et al., 2022), were cultured in CD OptiCHO medium (Life Technologies) with 8 mM L-Glutamine for 10 days with rotation at 125 rpm at 37°C. The expression medium containing AL04 proteins were filtered (0.2 µm filter) and subsequently purified by Blue HP column (GE Healthcare, USA) chromatography followed by HiTrap QFF (GE Healthcare) anion-exchange column chromatography. The eluted AL04 protein was concentrated, and buffer exchanged with PBS using Tangential Flow Filtration (TFF) (Repligen). Purified proteins were analyzed by SDS-PAGE with purity greater than 95 %. Purified 1 mg/ml of AL04 proteins were filtered (0.2 µm filter) and stored at −80°C for further use.
Fig. 1.
Schematic illustration of AL04 (83 kDa) and roles of each fusion component. Cystatin C (CysC) as an action moiety protein, human serum albumin (HSA), modified TAT peptide as cell permeable peptide (CPP), GS linker (GGSAS), and cleavable linker (GFLG): The numbers on the boxes denote the amino acid numbers from the N-terminal of AL04.
2.2. Animals and treatment
Three-month-old female JNPL3 mice [Model # 2508-F, homozygote tg/tg] were purchased from Taconic Biosciences Inc. (Germantown, NY, USA). All animals [28.5–33.4 g; animal numbers: L09–101026, L09–101027, L09–101028, and L09–101032] were maintained and handled in accordance with a protocol approved by the Institutional Animal Care and Use Committee (IACUC) of Noble Life Science, Inc. [Approval no.NLS-511; Study No: NLS18-L09–101] (Sykesville, MD, USA). All animal experiments were planned by L&J Bio USA, Inc. and conducted by Noble Life Science, Inc. Starting at 3 months of age, JNPL3 mice were administered intraperitoneal injections of either PBS (100 µl) or AL04 (5 mg/kg) every other week for a period of 24 weeks. Animals were weighed prior to administration; the dose volume was adjusted according to their body weight. On the 25th week, the animals were anaesthetized with ketamine/xylazine for brain removal and euthanized after extraction. Brains were sectioned along the midline (sagittal), half of the brains were stored in formalin for 24 hours for immunohistochemical analysis, and the other half were snapped frozen using liquid nitrogen for protein analysis.
2.3. Histology and Immunohistochemistry analysis
Brains were post-fixed for another 24 hours and then embedded in paraffin using standard protocols. The coronal sections (5μm) were cut on a microtome and processed for immunohistochemistry using a mouse anti-pTau AT8 (phospho-tau Ser202/Thr205, Thermo), and a M.O.M. (Mouse on Mouse) ImmPRESS® HRP Polymer Kits (Vector Biolabs, Cat# MP-2400). The sections were incubated with a dilution of 1:3000 AT8 antibody in 2.5 % normal horse serum M.O.M. solution overnight at 4°C. The AT8 signal was revealed by the incubation with M.O.M ImmPRESS reagent and enhanced with Diaminobenzidine (DAB). The staining quality was checked by comparing AT8 positive staining using M.O.M. kit (AT8/M.O.M.) to negative control staining by using M.O.M. kit without primary antibody, as well as to a concept control staining, in which AT8 staining was done using regular anti-mouse IgG secondary antibody instead of using M.O.M. kit. After the AT8 signal process, they were counterstained with hematoxylin and immunohistochemistry images were taken on a Zeiss AxioScope Imager Z.1 using a 20X objective. Histology and immunohistochemistry imaging procedures/immunohistochemistry evaluation are planned by L&J Bio and performed by Histoserv Inc. (Germantown, MD, USA) and CVPath Institute Inc. [Study number: CP2966–1306] (Gaithersburg, MD, USA).
2.4. Western blot analysis
For western blot of the brain sample, half of the brain from JNPL3 was homogenized with ice-cold RIPA buffer (25 mM Tris [pH 7.6], 150 mM NaCl, 1 % NP-40, 1 % sodium deoxycholate, 0.1 % SDS) containing 1 mM PMSF, protease inhibitor cocktail and phosphatase inhibitor using tissue-tearor homogenizer. The homogenates were centrifuged at 14,000 rpm for 30 min at 4°C to separate supernatant. Following protein quantification by BCA (Pierce, USA), 4x Laemmli buffer containing a final concentration of 1 mM DTT was added, and the samples were boiled for 5 minutes. 100 ug protein lysates protein was separated by 4–12 % bis-tris polyacrylamide gels in MES-SDS buffer and transferred to polyvinylidene difluoride (Thermo). The membrane was then blocked in 5 % non-fat milk for 1 hour at room temperature. The membranes were incubated with one of the following antibodies: phospho-tau (AT8: Ser202/Thr205, Thermo), human Tau (HT-7, Thermo), I2PP2A (Santa Cruz Biotechnology), phospho-GSK3β (Ser9, Cell Signaling), GSK3β (Cell Signaling), phospho-AKT (Ser 473, Cell Signaling), AKT (Cell Signaling), phospho-ULK1 (Ser 757, Cell Signaling), ULK1 (Cell Signaling), Nrf2 (Abcam), p62/SQSTM-1 (Cell Signaling), OPTN (Cell Signaling), LC3 (Cell Signaling) and GAPDH (Cell Signaling) at 4°C overnight followed by incubation with goat anti-rabbit or anti-mouse IgG HRP-conjugated secondary antibodies. Quantification of the immunoblot was performed using the ImageJ software (NIH). The statistical significance was determined by Student's t-test.
3. Results
3.1. AL04 attenuates the accumulation of hyperphosphorylated tau and NFTs in the subfields of hippocampus of JNPL3 mice brain
Phosphorylated forms of microtubule-associated protein tau accumulate in neurofibrillary tangles in AD (Rodríguez-Martín et al., 2013). We investigated whether AL04 treatment could reduce the levels of hyperphosphorylated tau in the hippocampus of JNPL3 mice using AT8 antibody. Mice received PBS or AL04 injections 12 times in 24 weeks, and their brain tissues were subsequently subjected to immunohistochemistry. The stained results showed that intense, confluent brown AT8-staining was observed in the cell bodies and the neurite of Cornu Ammonis3 (CA3) and dentate gyrus (DG) subfields of the hippocampus in PBS treated animal (Fig. 2A). On the other hand, AT8-staining was reduced in the hippocampus of AL04-treated mice (Fig. 2B). These results suggest that AL04 lowers hyperphosphorylated tau in the hippocampus of JNPL3 mice and may also attenuate the spread of tau pathology within the subfields of the CA3 and DG.
Fig. 2.
AL04 attenuates the accumulation of hyperphosphorylated tau in the hippocampal CA3 and DG of JNPL3 Mice. (A and B) Representative immunohistochemistry (IHC) images with antibody against AT8 (p-Tau, Ser202/Thr205) in hippocampal CA3 and DG in PBS-treated animal (A), AL04-treated animals (B). AT8-staining is brown, and nuclei counter staining are in light blue. The boxed areas in CA3 and DG are shown in the upper panels on the right side, respectively (10x magnification). Higher magnification (25x magnification) views of the corresponding dashed black squares are shown in the lower panel. Red arrowheads indicated AT8 in the DG. Scale bars = 100 μm (A and B).
3.2. AL04 decreases the level of hyperphosphorylated tau and mutant tau (P301L) in the brain parenchyma
Although we observed that AL04 reduced levels of hyperphosphorylated tau in the hippocampal region, we question whether the effects of AL04 could be attributed to the whole brain parenchyma. To investigate the effect of AL04 treatment on the levels of hyperphosphorylated tau in the brain, western blots were performed on brain samples from one PBS-treated mouse and three AL04-treated mice. Consistent with the data from immunohistochemistry staining, we observed remarkable reductions of hyperphosphorylated tau at PP2A-sensitive Ser202 and Thr205 (AT8) in the brains of AL04 treated animals compared to PBS-treated (Fig. 3A). We also observed reductions in total levels of P301L mutant tau (HT-7). Additionally, the reduced phospho-tau/total tau ratio in AL04-treated animals is statistically significant (p < 0.005 for AT8). Densitometric quantification revealed that AL04 treatment decreases the levels of hyperphosphorylated tau in the brain (Fig. 3B).
Fig. 3.
Treatment with AL04 lowers phospho-tau at PP2A-sensitive Ser202/Thr205 in the brain of JNPL3 mouse. (A) Representative blots of AT8 and total P301L tau (HT-7) upon the treatment of PBS or AL04 for 24 weeks. GAPDH was used as loading control. (B) Quantitative analysis of the ratio of phosphorylation level of tau at Ser202/Thr205 normalized against total tau in brain homogenates from PBS-treated (black, n=1) or AL04 (white, n=3) for 24 weeks. The results are expressed as mean ± SD of samples. *, P < 0.005, Student's t-test.
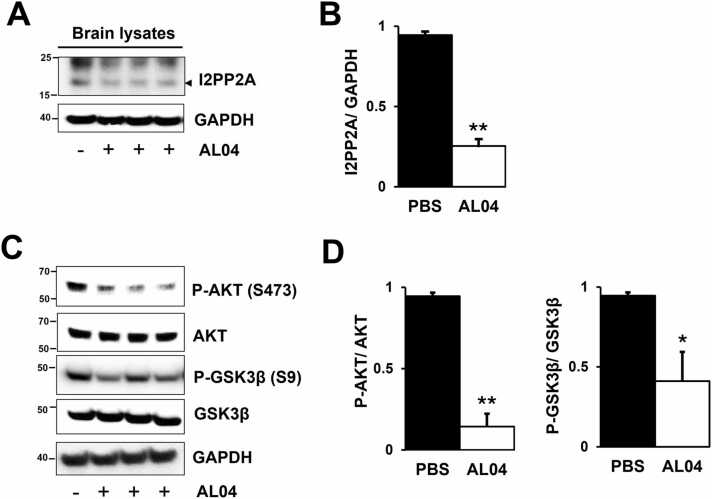
3.3. AL04 induces the downregulation of AEP-I2PP2A and the dephosphorylation of PP2A-sensitive kinases
It has been reported that the level of asparagine endopeptidase (AEP), which cleaves I2PP2A, has increased in the brains of AD patients (Basurto-Islas et al., 2013). The cleavage of I2PP2A is sufficient to induce AD-like pathology in rodent models (Bolognin et al., 2012). Since CysC is an inhibitor of AEP (Alvarez-Fernandez et al., 1999, van Kasteren et al., 2011), it is possible that AL04 containing CysC affects the regulation of PP2A activity associated with dephosphorylation of tau through inhibition of AEP and downregulation of I2PP2A, a PP2A inhibitor (Basurto-Islas et al., 2013, Li et al., 1996). Therefore, we investigated whether AL04 regulates PP2A by inhibiting I2PP2A cleavage in the brains of PBS-treated and AL04-treated mice, using western blot analysis. As shown in Fig. 4A and B, AL04 treatment significantly reduced 20 kDa active I2PP2A levels in the brains of AL04-treated (right three lanes) compared to PBS-treated (left lanes). These results indicated that the inhibitory effect of AL04 on I2PP2A cleavage contributes to PP2A recovery and reduces hyperphosphorylated tau at PP2A sensitive sites (S202 and T205).
Fig. 4.
AL04-treated inhibition of Akt-GSK3β signaling in JNPL3 mice brain is dependent on PP2A. (A and C) Mice were treated with 5 mg/kg of AL04 every other week for 24 weeks. Brain lysates were subjected to SDS-PAGE and immunoblotting for 20 kDa active I2PP2A, phospho-GSK3β (P-GSK3β, Ser9) and its upstream kinase phospho-AKT (P-AKT, Ser473). GAPDH was used as a loading control. (B and D) Relative quantifications of active I2PP2A, P-GSK3β, and P-AKT levels are shown. Values are expressed as means ± SE of 3 determinations. *, P < 0.05; **, P < 0.005, Student's t test.
PP2A regulates phosphorylation of tau not only directly by dephosphorylation of tau but also by regulating the activities of several tau kinases (Taleski and Sontag, 2018). GSK3β-AKT and PP2A are the important enzymes controlling the tau hyperphosphorylation (Kohyanagi et al., 2024, Qian et al., 2010, Wang et al., 2015). I2PP2A also serves as a link between PP2A and GSK-3β (Liu et al., 2008). We investigated the effect of I2PP2A downregulation by AL04 on two kinases in the brain. As shown in Fig. 4C and D, inhibition of I2PP2A cleavage by AL04 treatment is associated with a 3-fold decrease in Ser9 phosphorylation of GSK3β and S473 phosphorylation of AKT (Fig. 4C, D). These results suggest that AL04 treatment rescues PP2A phosphatase activity and regulates PP2A-sensitive kinases (GSK3β and AKT) in JNPL3 mouse brain while reducing hyperphosphorylated tau levels. This is consistent with findings from other studies (Avila et al., 2012, Iqbal et al., 2010, Qian et al., 2010).
3.4. AL04 enhances autophagy initiation and upregulation of autophagy receptor proteins
Because the P301L Tau mutation causes tau to become aggregation-prone (Caballero et al., 2018), it is possible that the mutated tau influences autophagy mediated degradation due to the existence of increased number of protein aggregates. We examined the effect of AL04 on the levels of autophagy pathway-involved proteins in brain homogenates by western blot. As shown in Fig. 5A and B, the increase in p-ULK1 (S757) in brain homogenate from PBS-treated animal indicated that autophagy was inhibited. However, the decrease in the level of this protein upon AL04 treatment suggests the initiation of autophagy and is consistent with other reports showing that dephosphorylation at S757 of ULK1 activates ULK1 and initiates autophagy (Egan et al., 2015, Kim et al., 2011, Pyo et al., 2018). Meanwhile, AL04 treatment increased the levels of autophagy receptors (p62 and OPTN) (Kumar et al., 2022, Xu et al., 2022) and the ratio of LC3-II/LC3-I compared with PBS-treated (Fig. 5A, B). We further investigated whether the increase in p62 expression by AL04 was associated with the activation of Nrf2, an essential transcription factor that regulates autophagy-related proteins and can stimulate autophagy (Pajares et al., 2016). As shown in Fig. 5A and B, AL04 treated groups show a 3–5-fold increased protein expression level of Nrf2 as compared to PBS-treated. These results suggest that AL04 treatment may improve autophagy pathways that are involved in the degradation of hyperphosphorylated tau in the brain.
Fig. 5.
AL04 enhances the expressions of proteins involved in protein-degradation pathways. (A) Brain lysates obtained from PBS-treated or AL04-treated mice were immunoblotted with a P-ULK1 (Ser757), ULK1, LC3-II, p62, OPTN, Nrf2, and GAPDH antibody. GAPDH was used as a loading control. (B) The quantified ratio of P-ULK1 (Ser 757), p62, OPTN, Nrf2, or LC3-II are shown. Values are expressed as means ± SE of 3 determinations. *, P < 0.05; **, P < 0.005, Student's t test.
4. Discussion
It has been reported that the abnormally hyperphosphorylated tau proteins are responsible for the neurodegeneration in AD (Iqbal et al., 2010, Wang et al., 2007,). It was also reported that tau pathology correlates better with cognitive impairment than amyloid-β aggregates does. Therefore, the targeting of tau is likely to be a more effective way to improve cognitive decline in AD (Li et al., 2018).
The tau-targeting therapies for AD are focusing on the reduction of NFTs or aggregation, while also identifying numerous tau post-translational modifications associated with tau aggregation (Khanna et al., 2016, Le Corre et al., 2006, Lee et al., 2001, Li et al., 1996, Wang et al., 2007). PP2A activity negatively correlated to the level of tau phosphorylation at the most phosphorylation sites in human brains (Liu et al., 2005). Although some preclinical data from tauopathy AD animal models have looked promising, clinical trials have not been successful. This may be due to imperfect reproduction of human pathology in animal models. Animal models showed the deposition of hyperphosphorylated tau, but cognitive deficiency was not observed. On the other hand, JNPL3 mice with the P301L tau mutation showed the hyperphosphorylated tau and extensive NFTs with cognitive deficiency (Iqbal et al., 2010, Santacruz et al., 2005).
In this study, we attempted to investigate the efficacy of AL04 for lowering hyperphosphorylated tau in JNPL3 mice to explore the possibility of AL04 as the therapeutic agent for AD. We evaluated the potential of AL04 treatment to reduce hyperphosphorylated tau levels in the hippocampus of JNPL3 mice at therapeutic doses, utilizing an empirical approach to optimize dosing regimens. We reported that a dramatic reduction in Aβ in the hippocampal subfields and cortex of 11- to 12-month-old Tg2576 mice following AL04 treatment (10 mg/kg, weekly for 8 weeks via intraperitoneal injection) (Bang et al., 2022). In this current study, we tested a lower, extended dosing regimen (5 mg/kg of AL04 every other week for 24 weeks) and observed a reduction in hyperphosphorylated tau levels in the hippocampus of JNPL3 mice. Future investigations will include logBB (brain-to-blood drug concentration ratio) predictions and brain distribution analyses to further clarify the brain disposition of AL04 as a blood-brain barrier–permeable drug aimed at reducing hyperphosphorylated tau in the brain.
We focused on the hippocampus because of its involvement in memory formation. The subfields of the hippocampus were affected by tau pathology at a relatively early age of AD patients (Braak and Braak, 1991, Braak and Braak, 1995). In the present study, we encountered challenges due to variations in the location and area of specific cortical regions (e.g., the entorhinal cortex) relative to the hippocampus across coronal sections at varying distances from bregma (data not shown). A significant increase in AT8 staining was observed in both the cortex (data not shown) and hippocampus of PBS-treated brain sample (n = 1). However, only the hippocampus could be analyzed in AL04-treated brain slices because part of the cortex was accidentally lost during dissection (Fig. 2). AT8 immunohistochemical analysis revealed abnormal hyperphosphorylation at Ser202/Thr205 in the CA3 and DG field of the hippocampus in JNPL3 mice. The presence of AT8-immunoreactive tau suggests that axonal tau may be abnormally phosphorylated, raising the possibility that axonal dysfunction may occur in its projections to the hippocampus (Braak and Braak, 1995, Lace et al., 2009). In both CA3 and DG regions, a significant reduction of AT8 was observed in the AL04 treated mice in comparison to the PBS-treated mouse.
It has been reported that the level of asparagine endopeptidase (AEP), which cleaves I2PP2A, has increased in the brains of AD patients (Basurto-Islas et al., 2013). The cleavage of I2PP2A is sufficient to induce AD-like pathology in rodent models (Bolognin et al., 2012). Cystatin C, an AEP inhibitor (Alvarez-Fernandez et al., 1999), is the action moiety of AL04 and may be involved in lowering hyperphosphorylated tau. Previously, we reported that the dose-dependent lowering effects of AL04 on the levels of phospho-Tau at Ser202/Thr205, Thr231 and active I2PP2A in the differentiated PC12 cells under NGF deprivation (Bang et al., 2024). In this current study, we showed that attenuation of hyperphosphorylated tau by AL04 treatment likely occurs through inhibition of I2PP2A cleavage, and recovery of PP2A activity in JNPL3 mice. Downregulation of PP2A has been reported to inhibit autophagy and induce intraneuronal accumulation of protein inclusions, a neuropathological hallmark of AD (Magnaudeix et al., 2013). However, PP2A cannot dephosphorylate S757 of ULK1 (Kim et al., 2011). Therefore, we suggested the possibility that another phosphatase (e.g. PP1 (Pyo et al., 2018)) may regulate autophagy induction through dephosphorylation of ULK1 at S757, as seen in AL04-treated mice.
Tau protein is degraded by both the proteasome and autophagy systems, and these systems have been reported to be defective in AD (Dikic, 2017, Khanna et al., 2016). The induction of tau protein-degradation by AL04 treatment may be exploited as a strategy to reduce the burden of hyperphosphorylated tau aggregates. Increasing evidence indicates that p62 has an important role in the degradation of tau protein (Ramesh Babu et al., 2008). The lack of p62 protein expression provokes the tau pathology in mice. A recent study showed that overexpression of OPTN protein significantly reduced pathological tau in mice expressing P301L-tau (Xu et al., 2022). A decline in the levels of p62 protein can disturb the signaling pathways of Nrf2 and increase the oxidative stress and impair neuronal survival (Babu et al., 2005). The level of Nrf2 decreases with age (Zhang et al., 2015) and is reduced in AD patients (Ramsey et al., 2007). Nrf2 activation may facilitate tau degradation through autophagy (Pajares et al., 2016).
The AL04-induced reduction of hyperphosphorylated tau levels is likely the result of increased autophagic clearance.
We investigated strategies to improve impaired autophagy, focusing on how identifying autophagy regulators in AL04-treated mice could offer benefits. Interestingly, AL04 treatment causes increased expressions of autophagy receptor (p62 and OPTN) and Nrf2 protein. This data is relevant to the role of p62-OPTN-Nrf2 proteins in the modulation of autophagy and the clearance of hyperphosphorylated tau aggregates in neurodegenerative diseases. Further studies will be needed to elucidate the changes in autophagy pathways that can be mediated by AL04 treatment.
It has been reported that the P301L tau mutation, located within the repeat domain of tau, is susceptible to conformational changes and phosphorylation (Shibuya et al., 2015). It interferes with tau’s autophagic degradation and is prone to aggregation (Caballero et al., 2018). Based on Roberson's paper on the protective effect of tau reduction (Roberson et al., 2007), we propose that P301L mutant tau reduction by AL04 treatment in JNPL3 brains is a protective effect (Fig. 3A). Therefore, inhibition or elimination of P301L tau may provide a new strategy for AD drug development. After treatment with AL04 for over six months, we found that reductions in hyperphosphorylated tau and P301L tau did not have negative health effects in terms of body weight change (data not shown).
In conclusion, AL04 reduces hyperphosphorylated tau at PP2A-sensitive Ser202/Thr205 (AT8 antibody recognized) in the hippocampus. AL04 inhibits the I2PP2A cleavage, an inhibitor of phosphatase PP2A. This may lead to the recovery of PP2A, which dephosphorylates tau kinases and hyperphosphorylated tau. Additionally, AL04 restores the impaired protein-degradation system of autophagy pathway in the brains of JNPL3 mice. We believe that AL04 treatment can be a potential therapy for AD because it inhibits NFTs through the reduction of tau hyperphosphorylation. The present study suggests that AL04 shows beneficial effects in treating AD.
Compliance with ethical standard
This study will be conducted in compliance with the current version of the following 1) Animal Welfare Act Regulations (9 CFR); 2) U.S. Public Health Service Office of Laboratory Animal Welfare (OLAW) Policy on Humane Care and Use of Laboratory Animals; 3) Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, 1996); and 4) AAALAC accreditation. The protocol for animal studies was approved by the Institutional Animal Care and Use Committee of Noble Life Science, Inc., Sykesville, MD, USA. Approval No: NLS-511, Study No: NLS18-L09–101.
CRediT authorship contribution statement
Kwan Hee Lee: Writing – review & editing, Supervision, Funding acquisition, Conceptualization. SOOKHEE BANG: Writing – original draft, Visualization, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Jeong Kuen Song: Writing – review & editing, Methodology, Investigation, Formal analysis, Conceptualization.
Declaration of Competing Interest
S.B., J.K.S. are current employees and K.H. Lee serves as CTO of L&J Bio.
L&J Bio has filed certain patent applications pertaining to AL04 naming S.B., J.K.S., and K.H.L. as inventors.
Acknowledgements and Funding
These studies were funded by L&J Bio.
Conflict of Interest and Compliance with ethical standards
S.B., J.K.S. are current employees and K.H. Lee serves as CTO of L&J Bio.
L&J Bio has filed certain patent applications pertaining to AL04 naming S.B., J.K.S., and K.H.L. as inventors.
This study will be conducted in compliance with the current version of the following 1) Animal Welfare Act Regulations (9 CFR); 2) U.S. Public Health Service Office of Laboratory Animal Welfare (OLAW) Policy on Humane Care and Use of Laboratory Animals; 3) Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, 1996); and 4) AAALAC accreditation. The protocol for animal studies was approved by the Institutional Animal Care and Use Committee of Noble Life Science, Inc., Sykesville, MD, USA. Approval No: NLS-511, Study No: NLS18-L09–101.
Consent for publication
The authors of this manuscript have all approved the manuscript and provided consent for its publication.
Contributor Information
Sookhee Bang, Email: sookhee.bang@lnjbio.com.
Kwan Hee Lee, Email: ortholee@lnjbio.com.
References
- Alvarez-Fernandez M., Barrett A.J., Gerhartz B., Dando P.M., Ni J., Abrahamson M. Inhibition of mammalian legumain by some cystatins is due to a novel second reactive site. J. Biol. Chem. 1999;274(27):19195–19203. doi: 10.1074/jbc.274.27.19195. [DOI] [PubMed] [Google Scholar]
- Avila J., Leon-Espinosa G., Garcia E., Garcia-Escudero V., Hernandez F., Defelipe J. Tau Phosphorylation by GSK3 in Different Conditions. Int. J. Alzheimers Dis. 2012;2012 doi: 10.1155/2012/578373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu J.R., Geetha T., Wooten M.W. Sequestosome 1/p62 shuttles polyubiquitinated tau for proteasomal degradation. J. Neurochem. 2005;94(1):192–203. doi: 10.1111/j.1471-4159.2005.03181.x. [DOI] [PubMed] [Google Scholar]
- Ballatore C., Lee V.M., Trojanowski J.Q. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat. Rev. Neurosci. 2007;8(9):663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- Bang S., Song J.K., Shin S.W., Lee K.H. Human serum albumin fusion protein as therapeutics for targeting amyloid beta in Alzheimer's diseases. Neurosci. Lett. 2022;767 doi: 10.1016/j.neulet.2021.136298. [DOI] [PubMed] [Google Scholar]
- Bang, S., Song, J.K., Shin, S.-W., Lee, K.H., Lee, H.J., inventors; L & J Bio Co., Ltd., assignee. Methods of decreasing Amyloid Beta (Aβ) plaque deposition and Hyperphosphorylated Tau plaque deposition in Alzheimer’s disease using a Cystatin C fusion protein. United States Patent US 12,115,212 B2. 2024 Oct. 15.
- Basurto-Islas G., Grundke-Iqbal I., Tung Y.C., Liu F., Iqbal K. Activation of asparaginyl endopeptidase leads to Tau hyperphosphorylation in Alzheimer disease. J. Biol. Chem. 2013;288(24):17495–17507. doi: 10.1074/jbc.M112.446070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognin S., Blanchard J., Wang X., Basurto-Islas G., Tung Y.C., Kohlbrenner E., Grundke-Iqbal I., Iqbal K. An experimental rat model of sporadic Alzheimer's disease and rescue of cognitive impairment with a neurotrophic peptide. Acta Neuropathol. 2012;123(1):133–151. doi: 10.1007/s00401-011-0908-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H., Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol. Aging. 1995;16(3):271–278. doi: 10.1016/0197-4580(95)00021-6. discussion 8-84. [DOI] [PubMed] [Google Scholar]
- Bramblett G.T., Goedert M., Jakes R., Merrick S.E., Trojanowski J.Q., Lee V.M. Abnormal tau phosphorylation at Ser396 in Alzheimer's disease recapitulates development and contributes to reduced microtubule binding. Neuron. 1993;10(6):1089–1099. doi: 10.1016/0896-6273(93)90057-x. [DOI] [PubMed] [Google Scholar]
- Caballero B., Wang Y., Diaz A., Tasset I., Juste Y.R., Stiller B., Mandelkow E.-M., Cuervo A.M. Interplay of pathogenic forms of human tau with different autophagic pathways. Aging Cell. 2018;17(1) doi: 10.1111/acel.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chohan M.O., Khatoon S., Iqbal I.G., Iqbal K. Involvement of I2PP2A in the abnormal hyperphosphorylation of tau and its reversal by Memantine. FEBS Lett. 2006;580(16):3973–3979. doi: 10.1016/j.febslet.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Davidowitz E.J., Lopez P., Jimenez H., Adrien L., Davies P., Moe J.G. Small molecule inhibitor of tau self-association in a mouse model of tauopathy: A preventive study in P301L tau JNPL3 mice. PLoS One. 2023;18(8) doi: 10.1371/journal.pone.0286523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikic I. Proteasomal and Autophagic Degradation Systems. Annu. Rev. Biochem. 2017;86:193–224. doi: 10.1146/annurev-biochem-061516-044908. [DOI] [PubMed] [Google Scholar]
- Egan D.F., Chun M.G., Vamos M., Zou H., Rong J., Miller C.J., Lou H.J., Raveendra-Panickar D., Yang C.C., Sheffler D.J. Small Molecule Inhibition of the Autophagy Kinase ULK1 and Identification of ULK1 Substrates. Mol. Cell. 2015;59(2):285–297. doi: 10.1016/j.molcel.2015.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Satumtira S., Jakes R., Smith M.J., Kamibayashi C., White C.L., 3rd., Sontag E. Reduced binding of protein phosphatase 2A to tau protein with frontotemporal dementia and parkinsonism linked to chromosome 17 mutations. J. Neurochem. 2000;75(5):2155–2162. doi: 10.1046/j.1471-4159.2000.0752155.x. [DOI] [PubMed] [Google Scholar]
- Gong C.X., Singh T.J., Grundke-Iqbal I., Iqbal K. Phosphoprotein phosphatase activities in Alzheimer disease. J. Neurochem. 1993;61:921–927. doi: 10.1111/j.1471-4159.1993.tb03603.x. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Tung Y.C., Quinlan M., Wisniewski H.M., Binder L.I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. USA. 1986;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal K., Wang X., Blanchard J., Liu F., Gong C.X., Grundke-Iqbal I. Alzheimer's disease neurofibrillary degeneration: pivotal and multifactorial. Biochem. Soc. Trans. 2010;38(4):962–966. doi: 10.1042/BST0380962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara T., Hong M., Zhang B., Nakagawa Y., Lee M.K., Trojanowski J.Q., Lee V.M. Age-dependent emergence and progression of a tauopathy in transgenic mice overexpressing the shortest human tau isoform. Neuron. 1999;24(3):751–762. doi: 10.1016/s0896-6273(00)81127-7. [DOI] [PubMed] [Google Scholar]
- Khanna M.R., Kovalevich J., Lee V.M., Trojanowski J.Q., Brunden K.R. Therapeutic strategies for the treatment of tauopathies: Hopes and challenges. Alzheimers Dement. 2016;12(10):1051–1065. doi: 10.1016/j.jalz.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Kundu M., Viollet B., Guan K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell. Biol. 2011;13(2):132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohyanagi N., Kitamura N., Ikeda S., Shibutani S., Sato K., Ohama T. PP2A inhibitor SET promotes mTORC1 and Bmi1 signaling through Akt activation and maintains the colony-formation ability of cancer cells. J. Biol. Chem. 2024;300(1) doi: 10.1016/j.jbc.2023.105584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A.V., Mills J., Lapierre L.R. Selective Autophagy Receptor p62/SQSTM1, a Pivotal Player in Stress and Aging. Front Cell Dev. Biol. 2022;10 doi: 10.3389/fcell.2022.793328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lace G., Savva G.M., Forster G., de Silva R., Brayne C., Matthews F.E., Barclay J.J., Dakin L., Ince P.G., Wharton S.B., MRC-CFAS Hippocampal tau pathology is related to neuroanatomical connections: an ageing population-based study. Brain. 2009;132(Pt 5):1324–1334. doi: 10.1093/brain/awp059. [DOI] [PubMed] [Google Scholar]
- Le Corre S., Klafki H.W., Plesnila N., Hubinger G., Obermeier A., Sahagun H., Monse B., Seneci P., Lewis J., Eriksen J., Zehr C., Yue M., McGowan E., Dickson D.W., Hutton M., Order H.M. An inhibitor of tau hyperphosphorylation prevents severe motor impairments in tau transgenic mice. Proc. Natl. Acad. Sci. USA. 2006;103(25):9673–9678. doi: 10.1073/pnas.0602913103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee V.M., Goedert M., Trojanowski J.Q. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- Lewis J., McGowan E., Rockwood J., Melrose H., Nacharaju P., Van Slegtenhorst M. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat. Genet. 2000;25(4):402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- Li H., Liu C.C., Zheng H., Huang T.Y. Amyloid, tau, pathogen infection and antimicrobial protection in Alzheimer's disease -conformist, nonconformist, and realistic prospects for AD pathogenesis. Transl. Neurodegener. 2018;7:34. doi: 10.1186/s40035-018-0139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Makkinje A., Damuni Z. The myeloid leukemia-associated protein SET is a potent inhibitor of protein phosphatase 2A. J. Biol. Chem. 1996;271(19):11059–11062. doi: 10.1074/jbc.271.19.11059. [DOI] [PubMed] [Google Scholar]
- Liu F., Grundke-Iqbal I., Iqbal K., Gong C.X. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur. J. Neurosci. 2005;22(8):1942–1950. doi: 10.1111/j.1460-9568.2005.04391.x. [DOI] [PubMed] [Google Scholar]
- Liu G.P., Zhang Y., Yao X.Q., Zhang C.E., Fang J., Wang Q., Wang J.Z. Activation of glycogen synthase kinase-3 inhibits protein phosphatase-2A and the underlying mechanisms. Neurobiol. Aging. 2008;29(9):1348–1358. doi: 10.1016/j.neurobiolaging.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Magnaudeix A., Wilson C.M., Page G., Bauvy C., Codogno P., Leveque P., Labrousse F., Corre-Delage M., Yardin C., Terro F. PP2A blockade inhibits autophagy and causes intraneuronal accumulation of ubiquitinated proteins. Neurobiol. Aging. 2013;34(3):770–790. doi: 10.1016/j.neurobiolaging.2012.06.026. [DOI] [PubMed] [Google Scholar]
- Pajares M., Jimenez-Moreno N., Garcia-Yague A.J., Escoll M., de Ceballos M.L., Van Leuven F., Rábano A., Yamamoto M., Rojo A.I., Cuadrado A. Transcription factor NFE2L2/NRF2 is a regulator of macroautophagy genes. Autophagy. 2016;12(10):1902–1916. doi: 10.1080/15548627.2016.1208889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyo K.E., Kim C.R., Lee M., Kim J.S., Kim K.I., Baek S.H. ULK1 O-GlcNAcylation is crucial for activating VPS34 via ATG14L during autophagy initiation. Cell Rep. 2018;25(10):2878–2890. doi: 10.1016/j.celrep.2018.11.042. e4. [DOI] [PubMed] [Google Scholar]
- Qian W., Shi J., Yin X., Iqbal K., Grundke-Iqbal I., Gong C.X., Liu F. PP2A regulates tau phosphorylation directly and also indirectly via activating GSK-3beta. J. Alzheimers Dis. 2010;19(4):1221–1229. doi: 10.3233/JAD-2010-1317. [DOI] [PubMed] [Google Scholar]
- Ramesh Babu J., Seibenhener L.M., Peng J., Strom A.L., Kemppainen R., Cox N., Zhu H.N., Wooten M.C., Diaz-Meco M.T., Moscat J. Genetic inactivation of p62 leads to accumulation of hyperphosphorylated tau and neurodegeneration. J. Neurochem. 2008;106(1):107–120. doi: 10.1111/j.1471-4159.2008.05340.x. [DOI] [PubMed] [Google Scholar]
- Ramsey C.P., Glass C.A., Montgomery M.B., Lindl K.A., Ritson G.P., Chia L.A. Expression of Nrf2 in neurodegenerative diseases. J. Neuropathol. Exp. Neurol. 2007;66(1):75–85. doi: 10.1097/nen.0b013e31802d6da9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson E.D., Scearce-Levie K., Palop J.J., Yan F., Cheng I.H., Wu T., Gerstein H., Yu G.Q., Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316(5825):750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Martin T., Cuchillo-Ibanez I., Noble W., Nyenya F., Anderton B.H., Hanger D.P. Tau phosphorylation affects its axonal transport and degradation. Neurobiol. Aging. 2013;34(9):2146–2157. doi: 10.1016/j.neurobiolaging.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santacruz K., Lewis J., Spires T., Paulson J., Kotilinek L., Ingelsson M., Guimaraes A., DeTure M., Ramsden M., McGowan E., Forster C., Yue M., Orne J., Janus C., Kuskowski M., Hyman B., Hutton M., Ashe K.H. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309(5733):476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya Y., Niu Z., Bryleva E.Y., Harris B.T., Murphy S.R., Kheirollah A., Bowen Z.D., Chang C.C.Y., Chang T.Y. Acyl-coenzyme A: cholesterol acyltransferase 1 blockage enhances autophagy in the neurons of triple transgenic Alzheimer's disease mouse and reduces human P301L-tau content at the presymptomatic stage. Neurobiol. Aging. 2015;36(7):2248–2259. doi: 10.1016/j.neurobiolaging.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taleski G., Sontag E. Protein phosphatase 2A and tau: an orchestrated 'Pas de Deux. FEBS Lett. 2018;592(7):1079–1095. doi: 10.1002/1873-3468.12907. [DOI] [PubMed] [Google Scholar]
- Tizon B., S. S, Yu H., Gauthier S., Kumar A.R., Mohan P., Figliola M., Pawlik M., Grubb A., Uchiyama Y., Bandyopadhyay U., Cuervo A.M., Nixon R.A., Levy E. Induction of autophagy by cystatin C: a mechanism that protects murine primary cortical neurons and neuronal cell lines. PLoS One. 2010;5(3) doi: 10.1371/journal.pone.0009819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kasteren S.I., Berlin I., Colbert J.D., Keane D., Ovaa H., Watts C. A multifunctional protease inhibitor to regulate endolysosomal function. ACS Chem. Biol. 2011;6(11):1198–1204. doi: 10.1021/cb200292c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.Z., Grundke-Iqbal I., Iqbal K. Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur. J. Neurosci. 2007;25(1):59–68. doi: 10.1111/j.1460-9568.2006.05226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Yang R., Gu J., Yin X., Jin N., Xie S. Cross talk between PI3K-AKT-GSK-3beta and PP2A pathways determines tau hyperphosphorylation. Neurobiol. Aging. 2015;36(1):188–200. doi: 10.1016/j.neurobiolaging.2014.07.035. [DOI] [PubMed] [Google Scholar]
- Xu Y., Liu Y., Chen X., Xu Q., Liu L., Liu H., Guo R., Qin Y. OPTN attenuates the neurotoxicity of abnormal Tau protein by restoring autophagy. Transl. Psychiatry. 2022;12(1):230. doi: 10.1038/s41398-022-02004-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Davies K.J.A., Forman H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015;88(Pt B)):314–336. doi: 10.1016/j.freeradbiomed.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]