Abstract
Dorzolamide hydrochloride (DRZ) is a carbonic anhydrase inhibitor utilized in managing elevated intraocular pressure (IOP) associated with glaucoma. However, its clinical effectiveness is hindered by a short half-life, low residence time, and the need for frequent dosing, highlighting the necessity for innovative delivery systems. This work reviews recent advancements in DRZ delivery, particularly focusing on cyclodextrin complexation and nanotechnology applications. It explores the potential of cyclodextrin derivatives to enhance DRZ’s bioavailability. DRZ cyclodextrin complexes or nanoparticulate systems maintain high drug concentrations in the eye while minimizing irritation and viscosity-related issues. Nanotechnology introduces nanoparticle-based carriers such as polymeric nanoparticles, solid lipid nanoparticles, liposomes, niosomes, and nanoemulsions. These formulations enable sustained drug release, improved corneal permeation, and enhanced patient compliance. Clinical trials have shown that DRZ nanoparticle eye drops and nanoliposome formulations offer efficacy comparable to conventional therapies, with the potential for better tolerability. Overall, this review highlights significant progress in DRZ delivery systems, suggesting their potential to transform glaucoma treatment by addressing current limitations and improving therapeutic outcomes.
Keywords: Dorzolamide hydrochloride, Glaucoma, Drug delivery, Cyclodextrin complexation, Nanotechnology
Introduction
Glaucoma, a prevalent ocular disease, poses a significant risk of irreversible blindness if not promptly diagnosed and treated [1]. It stands as a leading cause of blindness in developed nations, emphasizing the urgent need for effective therapeutic interventions [2]. Glaucoma manifests in various forms, each presenting unique challenges, including primary, secondary, open-angle, and angle-closure glaucoma [3]. Despite the array of available treatments aimed at halting progressive optic nerve damage, each therapeutic option carries inherent risks, costs, and potential adverse effects [4]. Furthermore, glaucoma disproportionately affects African American and Latino populations, with a prevalence nearly six to eight times higher than that in Caucasians [5]. According to data from the Bright Focus Foundation, over 3 million Americans and nearly 80 million people globally continue to grapple with glaucoma. Alarming projections from the National Eye Institute suggest a staggering 58 percent increase in glaucoma incidence in the US by 2030. By 2020, an estimated 76 million individuals had primary open-angle glaucoma (POAG), the most prevalent form of the disease, with projections indicating a rise to 111.8 million cases by 2040 [6]. The glaucoma statistics from the past 20 years are presented in Fig. 1.
Fig. 1.
Glaucoma disease statistics involving number of review papers published, clinical trials conducted, meta analysis completed and genderwise patients distribution from year 2000 to 2024. The types of studies considered include randomized clinical trials, cohort studies, case–control studies, types of medical or procedural interventions, glaucoma progression, treatment efficacy, side effects, and patient demographics. (Created by using Pubmed database)
Several risk factors contribute to the development of glaucoma, including elevated intraocular pressure (IOP), a family history of the condition, advancing age, African ancestry, and certain medical comorbidities such as diabetes and hypertension [5]. Despite its widespread prevalence and severity, a significant portion of glaucoma cases remain undiagnosed, with up to 50 percent of affected individuals unaware of their condition [7].
Traditionally, the cornerstone of glaucoma management revolves around lowering IOP, a pivotal determinant in disease progression [8]. The management of increased intraocular pressure (IOP) and glaucoma has traditionally involved the oral administration of carbonic anhydrase inhibitors (CAIs), such as acetazolamide. However, the use of this approach is frequently restricted due to systemic side effects. Dorzolamide and brinzolamide are two novel topical CAIs that were released about 29 years ago [9]. Dorzolamide is sold under the brand name Trusopt® by Merck, Whitehouse Station, New Jersey, USA. It is an acidic, slightly viscous eye drop solution (pH 5.6) that contains 2% (w/v) dorzolamide hydrochloride. However, brinzolamide is available as an aqueous suspension (pH 7.5) with 1% (w/v) brinzolamide under the brand name Azopt® from Alcon Laboratories in Fort Worth, Texas, USA. Both medications are taken three times daily, and research indicates that while their effects on IOP are similar, they have slightly distinct adverse effects, primarily ocular pain for Trusopt® and impaired vision for Azopt® [1]. Improved topical bioavailability, clinical efficacy, and patient compliance could result from the drug’s reformulation. Clinical trials have underscored DRZ’s effectiveness in IOP reduction, especially when used adjunctively with other antiglaucoma medications [10].
However, conventional eye drop formulations encounter challenges such as short residence time, low corneal permeability, and rapid drainage, resulting in diminished bioavailability and patient adherence [11]. To overcome these obstacles, researchers have explored innovative drug delivery systems, including particulate delivery methods and mucoadhesive polymers [11–14].
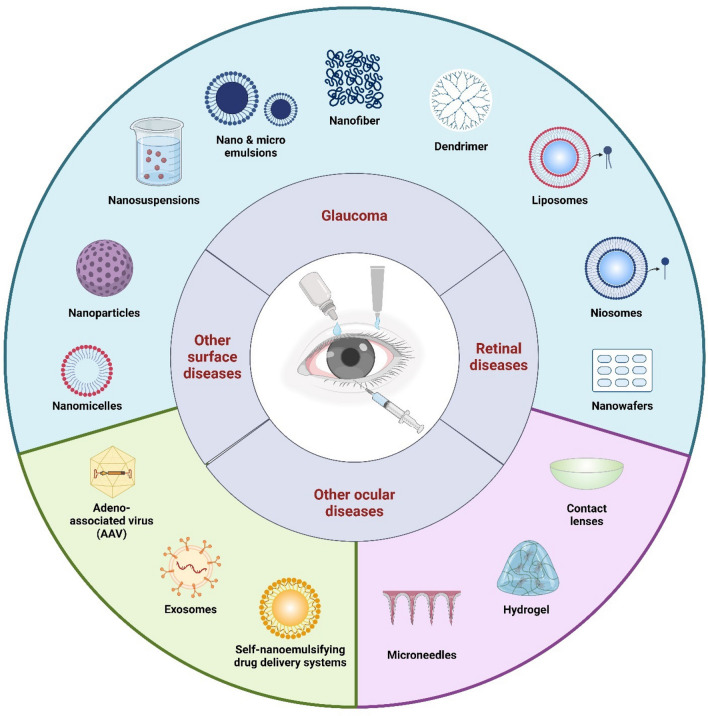
Particulate delivery systems, notably nanoparticles, offer prolonged drug release and heightened bioavailability, potentially bolstering patient adherence and preserving visual function [15]. The summary for ocular drug delivery systems is presented in Fig. 2. Among these, semi-interpenetrated polymer networks (IPNs) based on polymers like poly (sulfobetaine methacrylate) (pSBMA) and poly (vinyl alcohol) (PVA) hold promise in sustaining drug release and augmenting corneal drug concentration [16]. Additionally, mucoadhesive polymers like chitosan (CS) and its derivatives have demonstrated the capacity to prolong ocular residence time and enhance drug absorption [17]. By forming crosslinked nanoparticles, these polymers facilitate controlled drug release and improved bioavailability, mitigating the challenges inherent in traditional eye drop formulations [18].
Fig. 2.
A summary of ocular drug delivery systems, including ocular diseases, conventional treatments, and advanced approaches for enhanced therapeutic outcomes. (Created by using Biorender.com)
Moreover, many studies have attempted to develop drug formulations for topical delivery to the eye using hydrogels [19], or contact lenses [20, 21] with the aim to enhance the ocular bioavailability of DRZ and reduce dosing frequency. For example, nano-sized chitosan particles or in situ gelling hydrogel enhanced preocular retention, thereby increasing the bioavailability of DRZ. Similarly, contact lenses, when loaded with DRZ, could release the drug in a sustained manner and enhance ocular bioavailability. Despite these advancements, further research is warranted to optimize ocular drug delivery systems, ensuring compatibility, stability, and therapeutic efficacy. Continued exploration of innovative formulations, including those leveraging zwitterionic polymers [22] and advanced nanoparticle technologies, harbors the potential to revolutionize glaucoma management by offering sustained drug release, bolstered patient adherence, and enhanced therapeutic outcomes [23]. The development of effective drug delivery strategies for DRZ and other antiglaucoma medications represents a crucial stride in mitigating the debilitating impact of glaucoma. Through interdisciplinary collaboration and advancements in pharmaceutical technology, the preservation of vision and enhancement of the quality of life for glaucoma patients worldwide can be realized.
The present review critically examines and summarizes recent advancements in the delivery of dorzolamide hydrochloride (DRZ), with a particular focus on nanotechnology-based approaches. Given the limitations of conventional DRZ formulations—such as short residence time, low corneal permeability, and the need for frequent dosing—this review explores innovative strategies developed to enhance the bioavailability and therapeutic efficacy of DRZ. It investigates the potential of cyclodextrin complexation and various nanocarriers, including polymeric nanoparticles, liposomes, and nanoemulsions, to provide sustained drug release, improve corneal permeation, and enhance patient compliance. Additionally, it discusses the clinical outcomes of DRZ-loaded nanoparticle formulations, providing insights into their potential to revolutionize glaucoma treatment by addressing the limitations of existing therapies. This review serves as a comprehensive resource for understanding the current landscape and future potential of DRZ delivery systems in glaucoma management, emphasizing the critical role of nanotechnology in this evolution.
The octanol/water partition coefficient (P(octanol/water)) signifies the drug's lipophilicity, which is crucial for corneal permeation. DRZ's aqueous solubility and lipophilicity vary with pH, influencing its efficacy in different eye drop solutions. Research indicates that DRZ is more effective when administered in mildly acidic or basic eye drop solutions compared to neutral solutions. The ionized portion of the drug in the eye drop acts as an external reservoir, continuously providing unionized drug as the solution’s pH adjusts to tears. Moreover, a pH gradient across the corneal epithelium facilitates the transport of ionizable CAIs. DRZ's ampholyte nature, aqueous solubility, moderate lipophilicity, and potent activity against CA isoenzymes collectively contribute to its success as a topically active CAI [11].
Unveiling glaucoma: mechanisms and therapeutic insights
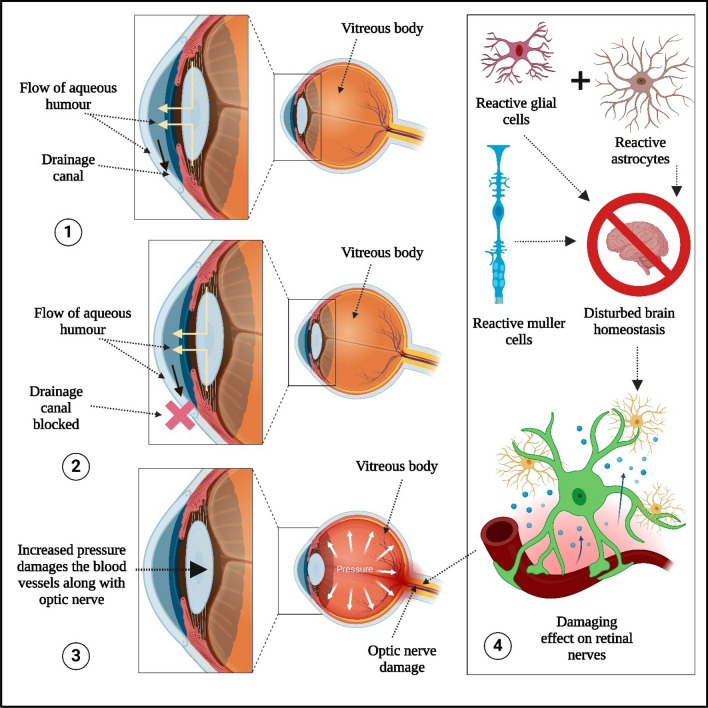
Glaucoma, a form of ocular neuropathy, progresses gradually and can lead to blindness, affecting over 76 million people worldwide. Understanding its mechanisms and therapeutic interventions is crucial for effective management [26]. This review aims to provide a comprehensive understanding of the fundamental mechanisms of glaucoma and its treatment options. Various aspects of glaucoma are illustrated in Fig. 3 [27], including:
The flow of aqueous humor and drainage canals. Aqueous humor is essential for eye health, supplying amino acids and glucose to nourish the cornea and lens. It is required to maintain intraocular pressure and provides inflation to the cornea for better protection against dust, wind, pollen, and pathogens.
Blocked drainage canals result in pressure buildup in the eye, which damages the optic nerve. The level of intraocular pressure is closely related to retinal ganglion cell death, and it is regulated by the balance between the trabecular meshwork and the uveoscleral outflow pathway, with the help of aqueous humor. Increased resistance to aqueous outflow through the trabecular meshwork is observed in open-angle glaucoma, while obstruction of the drainage pathways involving the iris is characteristic of angle-closure glaucoma.
Increased intraocular pressure damages blood vessels and the optic nerve.
-
Involvement of reactive glial cells, reactive Müller cells, and reactive astrocytes in optic nerve damage. Intraocular pressure-induced stress and strain may result in compression, deformation, and remodeling of the lamina cribrosa, leading to mechanical axonal damage and disruption of axonal transport. This interrupts the retrograde delivery of essential trophic factors from the brainstem to the retinal ganglion cells [2].
Reactive astrocytes create a toxic environment that damages and kills retinal ganglion cells. Reactive Müller cells release inflammatory cytokines, neurotransmitters, and gliotransmitters, inducing excitotoxicity [3]
Fig. 3.
Mechanisms of glaucoma involving 1. Flow of aqueous humor and drainage canal 2. Blocked drainage canal 3. Increased intraocular pressure induced damages into blood vessels along with optic nerve 4. Involvment of reactive glial cells, reactive muller cells and reactive astrocytes in optic nerve damages. (Created by using Biorender.com)
The primary alteration in glaucoma is the loss of retinal ganglion cells (RGCs), with increased intraocular pressure (IOP) being widely recognized as its main cause. Elevated IOP induces mechanical damage, resulting in RGC loss and axonal damage, accompanied by astrocytosis and increased nitric oxide production [29]. This cascade of events, including peroxynitrite formation and subsequent DNA and protein damage, ultimately leads to cell death. Moreover, elevated IOP affects retrograde axonal transport, impeding crucial elements needed for RGC homeostasis. Glaucoma is considered a chronic optic neuropathy characterized by progressive RGC loss, optic disc blood supply disorders, and glial cell activation [30].
Current treatment strategies focus on preventing optic nerve damage, preserving visual fields, and maintaining patients' quality of life by mitigating therapy-related adverse effects. Among the various risk factors, IOP stands out as the most significant and modifiable factor. Lowering IOP is pivotal in managing glaucoma, with treatment options ranging from topical anti-glaucoma eye drops to oral medications, laser procedures, and surgical interventions [31].
In glaucoma, although lowering intraocular pressure (IOP) is the primary and most direct method of managing the disease, recent research has also focused on neuroprotective strategies aimed at preserving retinal ganglion cells (RGCs) and the optic nerve, which are directly affected by the disease. Neuroprotection involves safeguarding these cells from degeneration and death, which is crucial for preventing vision loss, particularly in cases where IOP-lowering therapies are insufficient to halt disease progression. Many patients are refractory to IOP-lowering treatments, or they continue to progress toward blindness despite having normal or reduced IOP. Such cases highlight the importance of developing new therapies to protect ganglion cells. Nanomedicine offers potential solutions not only in reducing IOP but also in targeting the optic nerve and RGCs with neuroprotective agents. By focusing on the optic nerve, these advanced delivery systems aim to preserve visual function by addressing the neurodegenerative aspects of glaucoma [4].
Topical anti-glaucoma eye drops, although effective in lowering IOP, may be poorly tolerated, leading to compliance issues. To address this, fixed drug combinations and sustained-release injectable medications have been developed [32]. However, despite maximal medical therapy, glaucoma may progress, necessitating interventions such as trabeculoplasty or glaucoma surgery. Trabeculoplasty, utilizing either Argon laser or selective laser techniques, aims to enhance aqueous humor outflow [33].
Glaucoma surgeries, including trabeculectomy and glaucoma drainage implants, can effectively reduce intraocular pressure (IOP) but are associated with various complications. The advancement of pharmacotherapy has reduced the need for glaucoma surgeries [34]. Nonetheless, improving drug delivery remains a challenge, with a focus on developing safer, more effective means of delivering drugs to the optic nerve. Nanomedicine holds promise in this regard, offering alternative administration strategies to enhance patient compliance and therapeutic efficacy while treating glaucoma at the molecular level [35]. Therefore, overcoming the limitations of traditional anti-glaucoma treatments is imperative. Addressing challenges in drug delivery, particularly to the optic nerve, is key to enhancing glaucoma management and improving patient outcomes.
Exploring dorzolamide: understanding its mechanism, clinical applications, and ocular delivery challenges
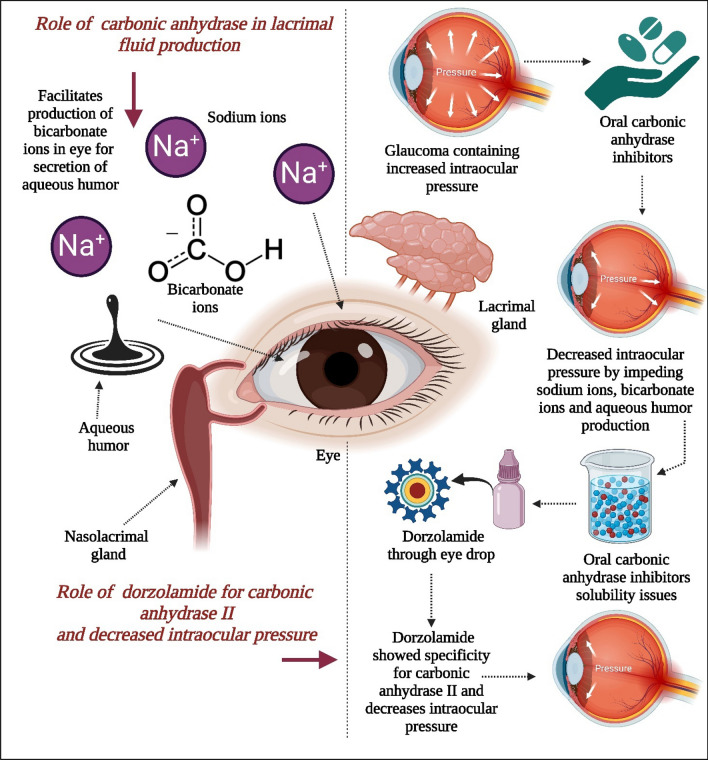
CA plays a pivotal role in the secretion of aqueous humor, a vital fluid in the eye. Initially identified in rabbit ciliary processes, subsequent research confirmed its presence in human ciliary processes as well [5]. CA facilitates the production of bicarbonate ions, which are then released into the posterior chamber of the eye from the ciliary process, with sodium acting as the counter ion [6]. Inhibition of CA in the ciliary processes leads to a reduction in aqueous humor secretion, primarily by slowing down the production of bicarbonate ions, thereby impeding sodium and fluid transfer as presented in Fig. 4 [7]. Maren's comprehensive review extensively covers the enzyme's function in aqueous humor secretion [8].
Fig. 4.
This figure illustrates the role of carbonic anhydrase in lacrimal fluid production and the role of dorzolamide in inhibiting carbonic anhydrase II, leading to decreased intraocular pressure. Carbonic anhydrase is essential for the production of lacrimal fluid. Parasympathetic agonists increase intracellular calcium ions, which in turn stimulate the sodium-hydrogen exchanger by activating the chloride-bicarbonate exchanger. The basolateral membrane is involved in this process, driving sodium and chloride ions into the duct cells. Intracellular carbonic anhydrase is required for the generation of hydrogen and bicarbonate ions, which facilitate the movement of sodium and chloride ions. The increase in intracellular potassium and chloride ions drives these ions out of the cell into the lumen through potassium and chloride channels, as well as potassium-chloride co-transporters. Oral carbonic anhydrase inhibitors are used in glaucoma cases with elevated IOP to reduce intraocular pressure by inhibiting sodium ion, bicarbonate ion, and aqueous humor production, although they present challenges related to targeted delivery and solubility. Dorzolamide has demonstrated better specificity for carbonic anhydrase II, effectively reducing intraocular pressure [11]. (Created using Biorender.com)
Patients with glaucoma experience a decrease in intraocular pressure when orally administered CAIs. However, the systemic administration of these drugs often results in adverse effects stemming from the extraocular inhibition of the enzyme [9]. Consequently, extensive research has been conducted to develop topically effective medications. Merck Sharp & Dohme Research Laboratories, engaged in synthesizing various chemicals since the 1980s, discovered several compounds that exhibited topical activity in humans. Unfortunately, many of these substances lacked adequate solubility. As a response, DRZ hydrochloride was developed as an effective and soluble CAI. Introduced for pharmacological testing in 1987, DRZ inhibits human CA isoenzymes, akin to other sulfonamide CAIs such as acetazolamide, ethoxzolamide, and methazolamide [10].
DRZ, marketed as a 2% ophthalmic solution, is licensed for the treatment of open-angle glaucoma and ocular hypertension to reduce increased intraocular pressure [12]. Reducing IOP is imperative in treating glaucoma, and CAIs are frequently employed for this purpose [7]. CA, an enzyme with diverse physiological functions, is widely distributed throughout the body. It regulates urine production in the kidneys by secreting hydrogen ions and reabsorbing Na+ at the glomerular filtration stage [13]. Additionally, it plays roles in maintaining blood pH, facilitating gas exchange in the lungs, and secreting hydrogen ions into gastric juice. Specifically, carbonic anhydrase (CA) isoforms I, II, and IV are the most prominent in ocular tissues. CA I and II are expressed in the corneal endothelium and eye lens, with CA II also present in the retina and ciliary processes. CA IV is found in the choriocapillaris and retinal pigment epithelium. [5]. Among these, CA II is particularly crucial in the formation of aqueous humor. While oral administration of CAIs has been a common treatment strategy, it often leads to systemic adverse effects [14]. Thus, DRZ was developed as an eye drop, offering good corneal permeability, high specificity against CA II, and an intraocular pressure-lowering action comparable to or better than that of oral CAIs [15].
The topical activities and potential adverse drug reactions of DRZ in the eyes necessitate an understanding of its intraocular pharmacokinetics, despite the belief that eye drop instillation has minimal side effects [16]. Current investigations focus on evaluating the effects of DRZ hydrochloride on CA activity in ocular tissues and its intraocular pharmacokinetics following eye drop instillation [17].
The study aims to assess the impact of topical 2% dorzolamide hydrochloride (DRZ) applied alone and in conjunction with topical 0.5% timolol on intraocular pressure (IOP) in healthy cats. Twenty-four domestic shorthair cats in good health were included. During the pretreatment phase (days 1–2), baseline IOP values were obtained at specific intervals. Subsequently, cats were assigned to different treatment groups: Group A received 2% DRZ HCl every 12 h, Group B received it every 8 h, Group C received 0.5% timolol maleate every 12 h, and Group D served as the control, receiving artificial tears every eight hours [18]. IOP measurements were recorded at the same intervals during the treatment phase as during the pretreatment phase. The average baseline IOP before therapy was 18.46 ± 2.99 mmHg for all cats. Modest reductions in mean IOP were observed in all treatment groups compared to pretreatment values (Group A: 16.40 ± 0.49 mmHg; Group B: 16.04 ± 0.49 mmHg; Group C: 17.76 ± 0.49 mmHg). In contrast, there was no statistically significant change in IOP between treatment groups A, B, and C; however, each treatment group showed a statistically significant difference in IOP compared to the control group (A–D; P = 0.0057; B–D, P = 0.0012; C–D, P = 0.0212). In summary, although the effect is modest, topical 2% DRZ significantly reduces IOP in healthy cats. The simultaneous administration of 0.5% timolol and 2% DRZ results in a slight reduction in IOP, but this change is not statistically significant.
DRZ hydrochloride is one of the topical carbonic anhydrase inhibitors (TCAIs) and is considered a potent antiglaucoma drug for humans. TCAIs selectively inhibit three isozymes of the zinc enzyme carbonic anhydrase, namely CA I, CA II, and membrane-bound CA IV. CA II, found in the nonpigmented ciliary body epithelium, is crucial for aqueous humor production [19]. Unlike systemic carbonic anhydrase inhibitors (CAIs), topical CAIs are considered safe, with no reports of systemic negative effects in humans, even with chronic dosing [20].
Physiochemical properties of dorzolamide
The physicochemical properties of DRZ, an ocular drug used for reducing intraocular pressure, are crucial for its effectiveness. It displays two pKa values, indicating its presence in cationic, unionized, and anionic forms at varying pH levels. While DRZ remains relatively stable in aqueous solutions, its sulfonamide moiety is susceptible to hydrolysis, with optimal stability observed at pH levels between 4 and 6 [24]. To prevent potential photochemical decomposition, DRZ eye drops should be stored in light-resistant containers at temperatures ranging from 15 to 30 °C. For effective permeation from tear fluid through the cornea into the aqueous humor, the drug molecule must possess adequate hydrophilicity to dissolve in tear fluid and sufficient lipophilicity to penetrate the cornea [25].
Chemistry and structure activity relationship (SAR) of dorzolamide
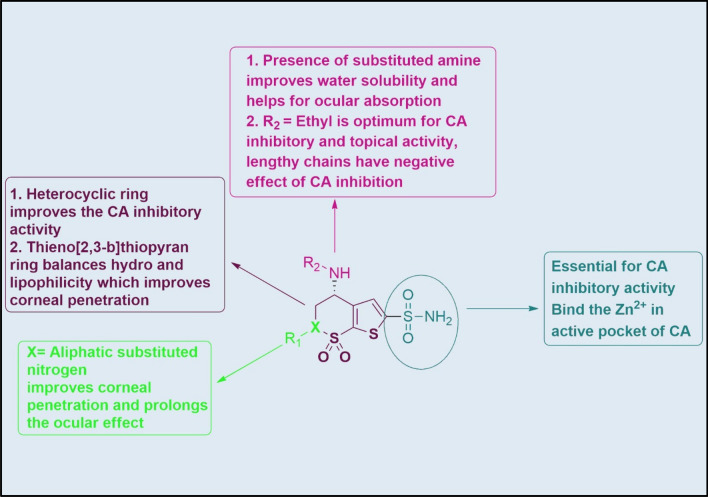
Dorzolamide belongs to the heterocyclic sulfonamides, a specific CAII. Apart from the heterocyclic sulfonamides, aromatic sulfonamides (dichlorophenamide) also possess non-selective inhibition of CA [21]. The enzyme contains zinc (Zn2+) with 260 amino acids with a molecular mass of 30 kDa. At the active site, Zn2+ has coordinated with the nitrogens three amino acids viz His 94, His 96, and His 119, along with oxygen of water molecule [22]. In an active pocket of the enzyme, the sulphonamide functional group interacted with the Zn2+ and His 119 through ionic and hydrogen bonding. Benzene ring in the aromatic sulphonamide interacted with Val 121, Leu 141, Val 143, Leu 198 and Val 207 via hydrophobic interactions. Substitution of the heterocyclic ring instead of benzene facilitates sulfonamide-CA complex formation. Thus, the substitution of 1,3,4-thiadiazole ring attached to the sulfonamido group (e.g. Acetazolamide) shows a 45-fold more significant inhibition constant than 4-acetamidobenzenesulfonamide (K = 7.1 × 10–6 versus 1.6 × 106 mol−1) [23]. The SAR of dorzolamide for CA inhibitory and ocular activity is presented in Fig. 5.
Fig. 5.
Chemistry and SAR of dorzolamide for CA inhibitory and ocular activity. (Created by using Biorender.com)
Although first-generation inhibitors were discovered decades ago, they are rarely used systemically, mainly as antiglaucoma drugs because of their side effects. These drugs have proved them topically (ocular) ineffective due to low aqueous solubility and no penetrability. Due to the compromised solubility, the drug could not arrive at the ciliary processes where CAs are present. In 1983, Maren et al. showed that the drug with balanced water and lipid solubility (able to penetrate cornea) with excellent CA inhibitory properties would be an effective IOP-lowering drug via the topical route. Immediately after this, water-soluble sulfonamide CAIs were developed, and in 1995, Merck launched its first pharmacological agent, dorzolamide, for clinical use as 2% eye drops. The addition of the polar secondary amine group and sulfonamide with non-polar thieno[2,3-b]thiopyran ring, respectively, made it sufficiently aqueous soluble and lipophilic to cross the cornea. To improve the ocular penetration, Alcon Laboratories substituted the thieno[2,3-b]thiopyran with thieno[3,2-e][1,2]thiazine ring along with the attachment of methoxypropyl at the nitrogen of thienothiazine ring. An increase or decrease of the aliphatic chain on the 4th amino group did not improve the CA inhibitory activity [24].
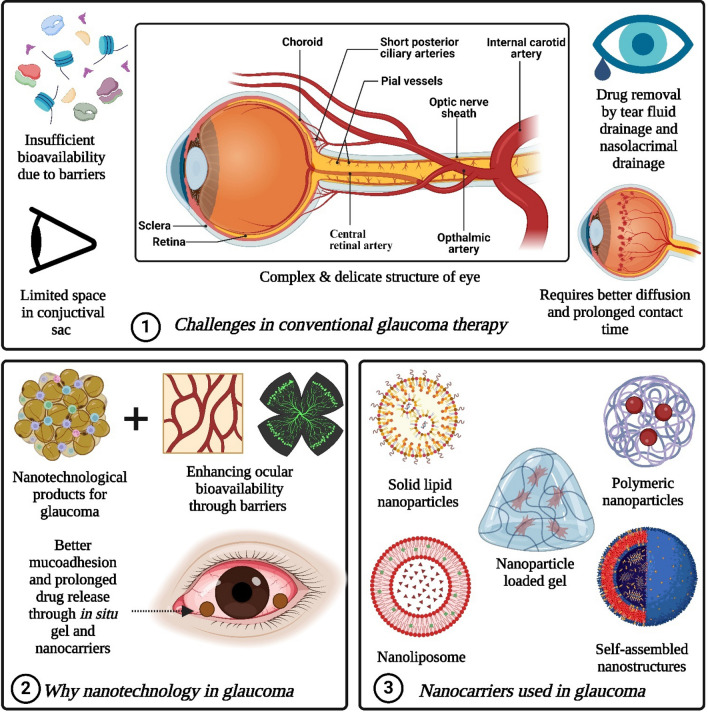
Challenges associated with conventional glaucoma therapy
Conventional ocular formulations represent the most basic delivery systems, suitable for patients across all age groups [25]. Among these, solution-based eye drops are widely employed for treating conditions localized in the front part of the eye [26]. Despite their prevalent usage, these delivery systems often encounter challenges in achieving sufficient bioavailability at the intended site due to various barriers as presented in Fig. 6 [27]. Nevertheless, topical administration remains the favored method for ocular drug delivery due to its non-invasive nature and ease of use. Unlike drugs administered systemically, those administered topically do not breach the blood-aqueous barrier (BAB) to access the anterior segment of the eye. This lowers the risk of systemic toxicity, making topical administration preferable even for drugs with potential systemic side effects, like β-blockers [28].
Fig. 6.
It represented 1. Challenges in conventional glaucoma therapy 2. Need of nanotechnology in glaucoma 3. Types of nanocarriers used in glaucoma. (Created by using Biorender.com)
Consequently, more than 90% of ocular preparations available in the market are in the form of topical eye drops [29]. However, it is crucial for formulators to ensure that an adequate quantity of the drug reaches the anterior segment with the appropriate concentration for effective treatment. Several factors, including the physicochemical properties of the drug, ocular metabolic mechanisms, and biological structure, can influence drug availability from topical ocular delivery systems [30]. Despite the benefits of the topical ocular route, poor bioavailability remains a significant hurdle. Approximately 90% of the administered drug is lost due to ocular clearance, the eye's primary defense mechanism [31]. Additionally, the conjunctival sac has a limited capacity, typically around 30 μl, while commercial droppers dispense approximately 40 μl, resulting in overflow [32].
Consequently, only about 10 μl of the administered volume remains in the eye after a single blink. Furthermore, tear fluid drainage and nasolacrimal drainage swiftly remove the drug, leaving a brief window of 5–7 min for absorption [33]. This underscores the need for developing topical ocular delivery systems capable of maximizing drug bioavailability within this short contact period. The ability of drugs to enter the eye from topically applied forms is influenced by various characteristics like molecular weight, solubility, particle size (for suspensions), and the composition of the vehicle (for solutions) [34].
However, it's becoming clear that just developing new medications isn't enough to ensure effective treatment. What's crucial is creating innovative delivery systems that can transport the drug to the target site in the right amounts and at the right time. Traditional forms of medication might not fulfill these needs for eye conditions like glaucoma. So, several unique ocular drug delivery systems have been created to specifically target tissues, enter cells, and release active substances in a controlled manner. Different methods, such as nano-emulsions [35–37], liposomes [38–40], and in situ forming gels [41–43], have been developed to overcome the challenge of limited bioavailability, especially for drugs that don't dissolve well [44].
Nanotechnology shows promise in enhancing ocular bioavailability and enabling drugs to penetrate the cornea better [45]. This is because nanoparticles are very small and can be designed with specific surface properties, allowing them to be quickly absorbed at the exact spot where they're needed, with a slow release over time. Moreover, incorporating supportive systems like mucoadhesives and in-situ gelling agents can help decrease elimination rates, extend the time the drug stays in the eye, and maintain a steady release of the medication [46]. Many research studies have focused on creating delivery systems that target specific sites for anti-glaucoma drugs [47]. Some of these have made it to the market, while others are still in the experimental phase, showing significant promise for future treatments.
Recent advances in dorzolamide hydrochloride delivery
Cyclodextrin complexation
Cyclodextrins, namely αCD, βCD, and γCD, are cyclic oligosaccharides composed of d-glucopyranose units. They possess a hydrophilic outer surface and a somewhat lipophilic central cavity. Although the natural forms of αCD, βCD, and γCD have limited aqueous solubility, various water-soluble derivatives such as hydroxypropyl derivatives of βCD and γCD, randomly methylated βCD, and sulfobutylether βCD have been synthesized and applied in ophthalmology. These derivatives form inclusion complexes with poorly soluble drugs in aqueous environments, enhancing their solubility and bioavailability. Initial efforts to incorporate cyclodextrin into aqueous DRZ eye drop formulations were unsuccessful due to inadequate formulation optimization and the drug's preference for mildly acidic or basic solutions over neutral ones. For instance, the addition of βCD to DRZ eye drops led to significantly lower drug tissue concentrations. Trusopt®, an aqueous DRZ eye drop solution, causes eye irritation due to its low pH and high viscosity, which can be mitigated by increasing the pH to physiological levels. Randomly methylated β-cyclodextrin (RMβCD), a water-soluble βCD derivative, did not improve DRZ availability compared to Trusopt® [1]. Although cyclodextrins have been effective in enhancing the delivery of certain drugs into the eye, they did not improve the topical delivery of DRZ due to its pH sensitivity and the viscosity of existing formulations [1].
While derivatives like HPβCD and HPγCD are highly water-soluble, their parent forms, βCD and γCD, have limited solubility. Exploiting the self-aggregation of drug-cyclodextrin complexes to form nanoparticles, aqueous dorzolamide eye drop formulations were developed using solid DRZ-CD complexes as a suspension. This formulation prolonged drug contact time with the eye surface, enhancing corneal penetration and leading to increased topical drug availability (Fig. 7) [1]. The developed DRZ eye drop suspension exhibited sustained high DRZ concentrations in the eye, particularly in the anterior and posterior segments, while minimizing systemic drug exposure. These promising results suggest the potential for developing this dorzolamide eye drop formulation into a once-a-day product.
Fig. 7.
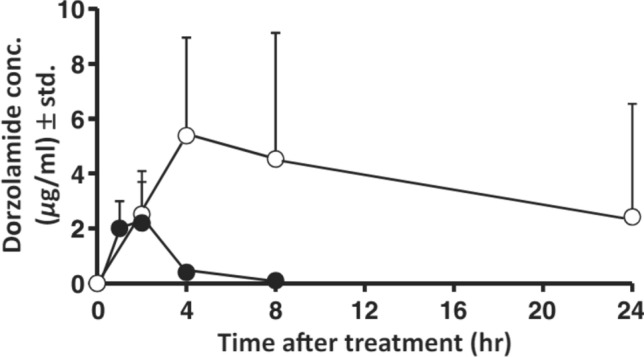
Dorzolamide concentration (mcg/mL) in aqueous humour after topical administration to rabbits (mean ± standard deviation; n = 6–8): formulation 3A (O) (3% dorzolamide ⁄ γCD eye drop microsuspension) and Trusopt® .
(Reproduced from Loftsson et al. [1] with kind permision of copyright holder, Wiley)
Nanotechnology applications
Nanoparticles are extensively researched as drug delivery vehicles in medicine, with applications spanning cancer theranostics, tissue engineering, cell reprogramming, regenerative medicine, and treatments for eye-related conditions. Recent advancements in nanoparticle-based delivery systems offer significant advantages over conventional approaches, resulting in improved therapeutic outcomes. In the context of treating glaucoma, nanocarrier properties are particularly crucial due to biological barriers unique to ocular delivery. Parameters such as particle size, shape, structure, degradation behavior, dispersibility, bioavailability, and biocompatibility play pivotal roles in the effectiveness of nanoparticles for ocular drug delivery. Overcoming these barriers requires optimizing biological properties through strategies like chemical surface modifications, which enhance biocompatibility while reducing toxicity. This, in turn, prolongs drug retention in the eye and minimizes drug loss due to rapid tear turnover. The prime objective of formulation scientist is to enhance drug penetration into various layers of the cornea (epithelium, stroma, Descemet’s membrane, and endothelium) and the aqueous humor. Achieving this necessitates nanoparticles with favorable characteristics that facilitate efficient drug delivery while addressing the complexities of ocular physiology. This section will explore various types of Dorzolamide containing nanoparticles utilized in ocular drug delivery (Table 1), including particulate and vesicular based carriers, hydrogel systems, and other potential nanocarriers.
Table 1.
Dorzolamide micro/nanoformulations for the treatment of glaucoma
| Formulation | Therapeutics | Carriers used | Particle size | Outcomes | Ref |
|---|---|---|---|---|---|
| Microparticles based suspension | Dorzolamide HCl + Brinzolamide | γCD (18% w/v) and hydroxypropyl methyl cellulose (HPMC) (0.5% w/v) | 2.2 ± 0.1 µm | The drug permeation was significantly higher compared to the commercial product 8 h after topical application, maintaining sustained high dorzolamide concentrations in the aqueous humor for at least 24 h | [48] |
| Nanostructured Mucoadhesive Microparticles | Dorzolamide | Poly(lactic-co-glycolic acid) (PLGA), Polyethylene glycol (PEG) and Polyvinyl alcohol (PVA) | 1.61 ± 0.17 µm | The formulation showed significantly better results in lowering intraocular pressure compared to Trusopt®, with a 35% greater maximum decrease and longer duration | [49] |
| Microparticles | Dorzolamide | Poly(ethylene glycol)-co-poly(sebacic acid) (PEG3-PSA), sodium dodecyl sulfate (SDS) and sodium oleate (SO) | Subconjunctival microparticles reduced rabbit IOP significantly compared to untreated eyes; blank microparticles had no effect. Though inflammation was transient, fluorescently labeled microparticles persisted for at least 42 days, suggesting sustained efficacy and potential for long-term IOP reduction in glaucoma | [50] | |
| Nanoparticles loaded gel | Dorzolamide | Chitosan (CS) | 164 nm | A novel in situ gel nanoparticle formulation exhibited sustained drug release and superior corneal retention compared to a marketed formulation, as demonstrated through ex vivo release and gamma scintigraphic studies, respectively | [51] |
| Polymeric nanoparticles | Dorzolamide and Pramipexole | Chitosan | 300 ± 5 nm | The nanoparticles displayed reduced mucoadhesive properties with increasing drug content. Moreover, sustained in vitro drug release was achieved with DRZ-loaded chitosan nanoparticles in pH 7.4 PBS | [52] |
| Polymeric nanoparticles | Dorzolamide | 6-O-Carboxymethyl Chitosan (OCM) | 250.3 nm | In vivo studies showed that DRZ loaded OCM nanoparticles had a prolonged antiglaucoma effect without pulse entry, surpassing CSNPs. OCM-CSNPs featured enhanced drug entrapment, controlled release, and improved bioavailability, reducing pulse entry compared to CSNPs | [53] |
| Polymeric nanoparticles | Dorzolamide | Polycaprolactone (PCL) | 192.38 nm | DRZ release from CS-PCL-NPs showed biphasic behavior: initial burst release for 2 h followed by sustained release for 12 h. Corneal flux experiments demonstrated a significant enhancement in permeation across goat cornea | [54] |
| Polymeric nanoparticles | Dorzolamide | PLGA/vitamin E D-alpha-tocopheryl polyethylene glycol succinate (TPGS) | 129 nm | Transcorneal permeation study demonstrated a 1.8–2.5 fold increase compared to the solution. Efficacy studies indicated a significant 22.81% reduction in intraocular pressure with DRZ-PVA-NPs and DRZ-TPGS -NPs | [55] |
| Crosslinked polymeric nanoparticles | Dorzolamide | Chitosan-dextran sulphate NPs | 182.63 nm | Optimized formulations displayed excellent transcorneal permeation and strong mucoadhesive properties (93.37 ± 1.86%) and stability. In vivo testing revealed significantly higher ocular hypotensive activity with no signs of irritation | [56] |
| Solid lipid nanoparticles (SLNs) | Dorzolamide | Glyceryl monostearate and TPGS | 175.38 nm | The optimized DRZ-SLNs showed initial rapid release followed by sustained release in simulated tear fluids, with a 2.87-fold increase in transcorneal permeation compared to DRZ solution | [57] |
| Self-assembled nanostructures | Dorzolamide | L-α-Phosphatidylcholine (PC) | 100.1–604.1 nm | The optimized formulation demonstrated notably increased Cmax and area under curve (AUC) levels in the aqueous humor, compared to the marketed product Trusopt® | [58] |
| Cationic nanoemulsions | Dorzolamide | Isopropyl myristate, Tween 80 and Cetyl trimethyl ammonium bromide | 336.3 nm | The optimized formulation exhibited enhanced and extended reduction in intraocular pressure (IOP) in male albino New Zealand rabbits compared to both pure DRZ and commercially available DRZ eye drops | [36] |
| Nanoliposome | Dorzolamide | Phosphatidyl choline, cholesterol | 51 ± 3.24 nm | Twenty patients with primary open angle glaucoma (POAG) and ocular hypertension in both eyes were divided into two groups. Both groups (who received marketed dorzolamide solution or dorzolamide-loaded nanoliposome) experienced a significant reduction in IOP, with the lowest recorded IOP after DRZ-nanoliposome treatment being 10 mmHg | [59] |
| Ocular inserts | Dorzolamide | Chitosan/hydroxyethyl cellulose | – | Scintigraphic imaging revealed over 50% retention of 99mTc-dorzolamide in the eye 18 h post-insert administration, contrasting with about 30% after drop instillation | [60] |
| Ocular implant | Dorzolamide | Carboxymethyl cellulose, chitosan, and PEG 6000 | – | The release of DRZ from implants exhibited a biphasic pattern, characterized by an initial release enduring approximately 2 h, followed by a sustained release persisting for up to 6 h | [61] |
| Soluble ocular drug insert (SODI) | Dorzolamide | Polyvinyl Alcohol, Poloxamer 407, Propylene Glycol | – | The limitations of conventional dorzolamide eye drops possess poor bioavailability and to overcome these drawback several novel ophthalmic drug delivery systems have been developed like liposomes, nanoparticles, contact lenses etc. These systems showed limitation including ease of administration, lack of patient compliance, blurred vision and commercial applicability. To address these issues soluble ophthalmic drug inserts are presented to provide enhanced ocular residence, accurate dosing and better patient compliance | [62] |
| Contact lenses | Dorzolamide and Timolol | Vitamin E | – | Incorporating 20% vitamin E extended timolol and dorzolamide release by 35-fold and 14-fold, respectively. When released together, durations increased 1.7-fold and 1.2-fold, an effect seen with both control and vitamin E-loaded lenses | [21] |
Polymeric nanoparticles
Recent advancements in the delivery of DRZ, a medication used in treating glaucoma, have seen the emergence of various innovative approaches. Among these, polymeric nanoparticles stand out as promising carriers for sustained drug release [63]. These nanoparticles encompass a range of cutting-edge materials, including polymer microspheres, nanostructures, lipid nanoparticles, collagen shields, ocuserts, dendrimers, and iontophoresis techniques [64]. Of these, colloidal polymers have shown particular efficacy, offering prolonged drug retention on the ocular surface through mucoadhesive properties. By covalently bonding, adsorbing, encapsulating, or solubilizing the active moiety within polymeric nanoparticles, researchers aim to reduce the frequency of DRZ administration while enhancing its ocular bioavailability [65]. Glaucoma, characterized by progressive optic nerve degeneration and elevated intraocular pressure (IOP), presents a significant challenge in drug delivery due to biological barriers within the eye. To address this, researchers have developed in-situ gels of chitosan nanoparticles, demonstrating enhanced DRZ bioavailability and effectiveness [51]. These optimized nanoparticles exhibit prolonged drug release and superior corneal retention compared to commercial formulations, offering a less frequent dosing regimen and improved patient compliance.
Furthermore, innovative formulations of DRZ-loaded PLGA NPs, incorporating vitamin E TPGS as an emulsifier, have shown promising outcomes in enhancing drug encapsulation efficiency and permeability [55]. In vitro drug release data demonstrated an initial burst release of 34.89% and 28.15% for formulations containing polyvinyl alcohol (PVA) and vitamin E TPGS, respectively, in the first hour. After three days, cumulative releases reached 91.78% and 79.5%, respectively, due to the slow diffusion from the hydrophobic polymer matrix. The sustained release phase is governed by the hydrophobic polymer matrix, which controls the penetration of the release medium. These NPs have demonstrated significant improvements in trans-corneal permeation and the concentration of drug in the aqueous humor compared to conventional solutions. The research on trans-corneal permeation indicated a substantial enhancement in permeability, ranging from 1.8 to 2.5 fold, compared to traditional solutions. Moreover, an ex vivo biodistribution investigation showcased a notable increase, ranging from 1.5 to 2.3 fold, in the drug concentration in the aqueous humor. Histological and infrared camera experiments confirmed the formulations' non-irritating nature, contributing to their safety profile. Pharmacoscintigraphy investigations highlighted their efficacy by demonstrating a reduction in both naso-lachrymal drainage and corneal clearance compared to standard medication solutions. Furthermore, in efficacy trials following a single topical instillation into the eye, DRZ-loaded PLGA NPS, both emulsified with vitamin E TPGS (DRZ-T-NPs) and prepared with traditional emulsifiers (DRZ-P-NPs), exhibited remarkable reductions in intraocular pressure. Specifically, DRZ-P-NPs and DRZ-T-NPs led to reductions of 22.81% and 29.12%, respectively, underscoring their potential in effectively managing intraocular pressure in glaucoma patients. In another research, Pardeshi et al. [63] devised chitosan oligosaccharide (CSO)-dextran sulfate (DS) crosslinked nanoparticles (DRZ–CSO–DS-NPs) for delivering DRZ to the eyes. These nanoparticles were created through an ionic gelation method. The optimized formulation displayed a particle size of 142.8 nm and an entrapment efficiency of 92.12 ± 0.8%, ensuring sustained drug release over 12 h. Animal experiments showed a notable 41.56% decrease in intraocular pressure for 10 h. Additionally, the HET-CAM study affirmed the non-irritating and biocompatible nature, indicating their viability for ocular use. These results underscore the potential of DRZ–CSO–DS-NPs as mucoadhesive nanocarriers, enhancing the therapeutic effectiveness of DRZ for ocular delivery.
Solid lipid nanoparticles
Solid lipid nanoparticles (SLNs) are nano-sized carriers (10–1000 nm) formed by dispersing solid lipids in aqueous media with surfactants [66]. They are highly effective for ocular drug delivery due to their biocompatible and biodegradable nature, utilizing “generally recognized as safe” (GRAS) solid lipids from natural or synthetic sources, which exhibit non-toxic and non-irritant properties [33]. SLNs offer advantages such as sustained release, excellent colloidal stability, slow lipid degradation, and enhanced precorneal drug retention time, making them ideal for topical drug delivery [46].
In SLN development, D-α-Tocopherol polyethylene glycol 1000 succinate (TPGS) serves as both a surfactant and stabilizer, addressing challenges in ocular drug delivery [67]. With its amphiphilic characteristics, TPGS facilitates the emulsification of hydrophilic drugs with solid lipids, resulting in SLNs with high encapsulation efficiency, colloidal stability, and sustained drug release [68]. Additionally, TPGS has been shown to inhibit corneal P-glycoprotein (P-gp), an efflux transporter, potentially enhancing total corneal permeation [69]. Furthermore, nanoparticles possess the ability to overcome dynamic barriers such as lymphatic clearance, excessive tear dilution, and systemic drainage. Considering this potential, Shahab et al. [57] conducted a study aimed at developing solid lipid nanoparticles (SLNs) loaded with dorzolamide (DRZ) for ocular delivery. The DRZ-SLNs were prepared using an ultrasonic emulsification method and statistically optimized using Box-Behnken design. The optimized DRZ-SLNs exhibited a particle size of 175.38 nm and an encapsulation efficiency (EE) of 80.47%. In vitro drug release data demonstrated an initial release of 47.36% within 2 h, followed by controlled release of up to 82.54% at 10 h (Fig. 8). The initial burst is attributed to the drug encapsulated in the outer matrix, with sustained release controlled by diffusion from the hydrophobic lipid core. The release mechanism follows the Korsmeyer-Peppas model, indicating Fickian diffusion.
Fig. 8.
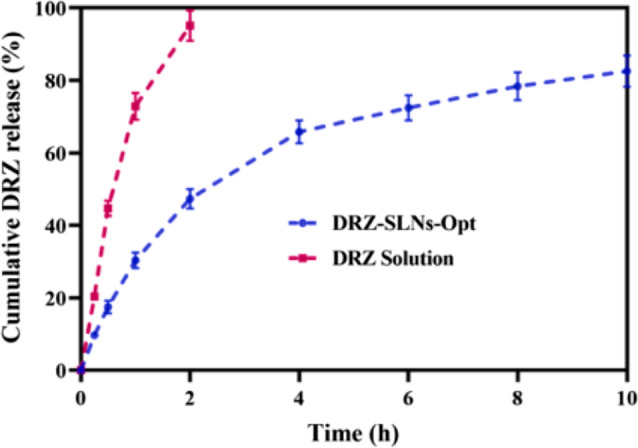
Graph illustrating the drug release study of the dorzolamide (DRZ) solution and optimized DRZ-SLNs.
Reproduced from Shahab et al. [57] with the kind permission of the copyright holder, Elsevier
DRZ-SLNs also showed a 2.87-fold increase in transcorneal permeation compared to DRZ solution, with non-irritant and safe properties confirmed by HET-CAM and histopathology studies. The study suggests that DRZ-SLNs hold promise as an effective nanoplatform for ocular administration.
Liposomes
Liposomes, characterized by bilayer phospholipid vesicles, offer a versatile solution for drug delivery [70]. They possess the ability to encapsulate both water-soluble and lipid-soluble drugs [71], thus presenting a flexible carrier system. One of the notable advantages of liposomes lies in their capacity to adhere to the cornea, attributed to their phospholipid membrane [72]. This property enables the gradual release of drugs onto the surface of the eye, ensuring sustained therapeutic effects with just a single application [73]. Additionally, liposomes are highly biocompatible and exhibit minimal toxicity, making them safe for medical use with low potential for inducing immune responses. Their nano-sized structure may also enhance penetration through physiological barriers such as the conjunctiva and sclera, facilitating efficient delivery to the target site, such as the ciliary body [74]. Recently, Kouchak et al. [75] developed a DRZ-loaded nanoliposome formulation with a lipid molar ratio of 7:4 (phosphatidylcholine: cholesterol) using the thin layer hydration method. In vitro release data demonstrated a rapid burst release of 70% within the first hour and almost complete release (95%) within 6 h. The fast release is attributed to the bilayer structure of the liposomes, which allows for the quick diffusion of dorzolamide (DRZ). In vivo evaluation in rabbit eyes showed that the nanoliposome formulation resulted in higher and more sustained therapeutic effects compared to DRZ solution and commercially available DRZ eye drops. The nanoliposomes effectively entrapped DRZ within their hydrophilic core and merged with the corneal epithelium, facilitating gradual drug release. Additionally, endocytosis of liposomes potentially facilitated the passage of loaded liposomes through the cornea, leading to drug release in the anterior eye space. In continuation with previous work, Kouchak and coworkers conducted a randomized study comparing DRZ-loaded nanoliposome eye drops with commercially available DRZ HCl eye drops in patients with primary open angle glaucoma or ocular hypertension [59]. Twenty participants were recruited, and intraocular pressure was measured on days 0, 14, and 28. The DRZ-loaded nanoliposome formulation, prepared using the thin layer hydration method, demonstrated significantly higher reductions in intraocular pressure compared to the control group (P < 0.05). This indicates the efficacy of the DRZ-loaded nanoliposome eye drops, possibly due to their similarity to biological membranes and small particle size.
In recent developments, a notable increase has been observed in the creation of self-assembled liposomal nanostructures utilizing biomaterials such as carbohydrates, nucleic acids, and peptides [76]. This trend is driven by the desire to enhance our understanding of self-assembly mechanisms and harness their potential for diverse biomedical applications like tissue regeneration, drug delivery, and biosensors. Through meticulous single-molecule design, these self-assembled drug nanostructures yield materials with customized morphologies and specialized functions. This capability allows for precise manipulation of bulk properties by modulating individual monomeric building blocks [76, 77]. Afify et al. [58] developed self-assembled nanostructures of DRZ and L-α-Phosphatidylcholine to improve pharmacokinetic parameters and extend drug action for glaucoma treatment. Utilizing a modified thin-film hydration technique, formulations were optimized based on response surface statistical design. Characterization included assessment of drug content, particle size, zeta potential, and release kinetics. The optimized formulations, prepared at pH 8.7 with varying ratios of L-α-Phosphatidylcholine to drug, exhibited enhanced drug levels in aqueous humor and prolonged control over intraocular pressure compared to Trusopt®. This study introduces promising self-assembled formulations for improved drug permeation and sustained pharmacological effects in glaucoma therapy.
Niosomes
Niosomes represent a highly promising avenue in ocular drug delivery, offering enhanced effectiveness and compliance. These nanometric vesicles, composed of non-ionic surfactants, are characterized by biodegradability, low toxicity, stability, and cost-effectiveness compared to liposomes [78]. Niosomes can manifest as Small Unilamellar Vesicles (SUVs), Multilamellar Vesicles (MLVs), or Large Unilamellar Vesicles (LUVs), all falling within the nanometric scale [79]. They not only facilitate prolonged and controlled drug release on the corneal surface but also regulate ocular delivery by inhibiting enzyme-mediated drug degradation at the tear/corneal epithelial interface [79].
Additionally, vesicles offer a promising solution for developing an ophthalmic drug delivery system that combines the convenience of eye drops with localized and sustained drug activity at the target site [33]. Recently, Hasan et al. [80] prepared niosomes using Cholesterol with sorbitan monoesters or sorbitan trioleate in a specific molar ratio, focusing on Span 40 formulations. They incorporated dicetylphosphate (DCP) and various polyoxyethylene fatty acid esters (Tween 20, 40, or 80) and characterized the batches using Zetasizer and transmission electron microscopy (TEM). Niosome sizes ranged from 25.9 to 165.5 nm, with a negative zeta potential charge. DRZ was successfully entrapped in all formulations with entrapment efficiencies between 34.81% and 97.66%. The variation in niosome size and entrapment efficiency is primarily attributed to the structure and properties of the surfactants and co-surfactants used. Span 40 and Span 60, which have higher phase transition temperatures (Tc), formed larger vesicles and exhibited higher entrapment efficiencies compared to Span 20 and Span 85, as presented in Fig. 9. The incorporation of DCP further increased entrapment efficiency and particle size due to electrostatic interactions. Additionally, the use of co-surfactants like Tween 20, 40, and 80 enhanced entrapment efficiency, with Tween 20 showing the highest due to its lower hydrophobicity and the resulting larger vesicle size. In vitro drug release data demonstrated a biphasic release pattern, with an initial rapid release lasting approximately 3 h, followed by sustained release over 36 h. There was a slower release with niosomes prepared using Span 40 and 60 compared to those prepared with Span 20 and 85. Niosomes with a more rigid bilayer (prepared with Tween 40) exhibited slower release due to increased membrane rigidity. The release mechanism is governed by Fickian diffusion. In summary, DRZ-loaded niosomal formulations exhibited a significant reduction in cumulative percent drug release compared to DRZ solution, indicating their potential as ophthalmic carriers for sustained intraocular pressure reduction.
Fig. 9.
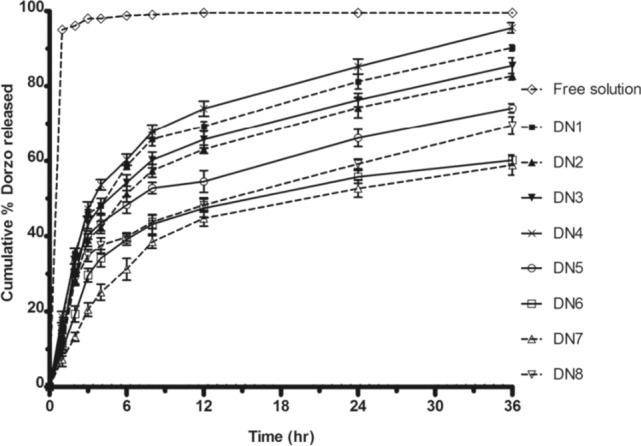
Effect of Lipid Composition on the Release Profile of Dorzolamide (Dorzo). Niosomes with Span 20 (DN1) exhibited a faster release, while those with Span 40 (DN2) and Span 60 (DN3) showed slower release due to higher phase transition temperatures (Tc). Span 85 (DN4) had a release rate similar to that of DN1. The addition of DCP (DN5) further reduced the release rate. Mixed niosomes containing Span 40 and Tween (DN6, DN7, DN8) delayed release, with the greatest reduction observed in DN7 (Tween 40) and the least in DN8 (Tween 80), likely due to differences in membrane rigidity and alkyl chain properties. The data represent the mean ± SD of six determinations.
Reproduced from Hasan et al. [80] with the kind permission of the copyright holder, Taylor & Francis
In another research, Dehaghi et al. [81] explored dorzolamide niosomes as a strategy to enhance drug corneal penetration and bioavailability. They compared passive and remote loading methods and assessed various formulation variables’ impact on encapsulation efficiency (EE). Results highlighted that higher cholesterol percentages and lipid concentrations improved dorzolamide encapsulation, with remote loading showing superior efficacy. Although TPGS incorporation decreased EE, it slowed drug release. Scanning electron microscopy confirmed spherical particles with smooth surfaces. Overall, the phosphate gradient method and 50% cholesterol in Span 60 niosomal formulation achieved the highest encapsulation efficiency.
Nanoemulsion
Nanoemulsions, transparent and kinetically stable formulations, typically contain inner-phase droplets ranging from 20 to 200 nm. In ophthalmic oil-in-water (o/w) nanoemulsions, the dispersed phase (oil) and continuous phase (water) are carefully combined with surfactants and cosurfactants to reduce surface tension at the interface of immiscible phases [82]. This composition facilitates prolonged precorneal retention and enhanced ocular bioavailability. The surfactants and cosurfactants act as penetration enhancers, aiding drug transport across the cornea by removing the mucus layer and disrupting tight junctional complexes [83]. Additionally, the submicron size of nanoemulsion particles promotes ocular drug absorption via endocytosis through corneal epithelial cells [84]. Recently, Kassem et al. [36] developed cationic nanoemulsions (CNEs) loaded with DRZ to improve ocular delivery. Using high-speed homogenization and ultrasonication, they optimized the formulation employing a Box-Behnken design. The optimized formulation (F-Opt) contained isopropyl myristate, Tween 80, and Cetyl trimethyl ammonium bromide, exhibiting desirable properties such as droplet size, zeta potential, and polydispersity index. F-Opt demonstrated sustained drug release and pronounced mucoadhesive properties, remaining stable under various conditions. In vitro and in vivo studies confirmed its enhanced and prolonged intraocular pressure-lowering effect compared to pure DRZ and marketed eye drops, with no ocular irritation observed. F-Opt shows promise for effective and safe ocular drug delivery for managing elevated intraocular pressure.
In another research, Ammar et al. [83] explored dilutable nanoemulsions as effective drug carriers for treating glaucoma. Their study aimed to formulate dorzolamide hydrochloride into an ocular nanoemulsion with enhanced therapeutic efficacy and prolonged effect. Thirty-six formulations were developed and evaluated, resulting in seventeen nanoemulsions exhibiting acceptable physicochemical properties and slow drug release. Selected formulations were non-irritant based on Draize rabbit eye tests and histological examinations. Biological evaluation on normotensive albino rabbits demonstrated superior therapeutic efficacy, faster onset of action, and prolonged effect compared to standard drug solutions and marketed products. Overall, nanoemulsions offer a promising approach for more effective glaucoma treatment, reducing application frequency and improving patient compliance.
Among the various nanocarriers, polymeric nanoparticles (PNPs) demonstrate the most controlled and sustained release, making them ideal for applications that require prolonged therapeutic effects and reduced dosing frequency, such as glaucoma management. However, solid lipid nanoparticles (SLNs) also provide a well-balanced release profile with significant controlled release, positioning them as another strong candidate for the ocular instillation of dorzolamide. In contrast, liposomes and niosomes exhibit more rapid release, which may be advantageous in cases where faster drug action is necessary. Therefore, polymeric nanoparticles may be considered the best delivery system for dorzolamide ocular instillation due to their sustained release profile and ability to maintain therapeutic levels over an extended period.
Recent clinical trials and patents
Recent advancements in pharmaceutical research have focused on enhancing the efficacy and tolerability of existing ophthalmic medications, particularly those aimed at managing IOP in conditions like glaucoma [85]. Among these, DRZ, a CAI, has been widely used to reduce IOP. However, challenges related to dosing frequency and ocular discomfort have prompted the exploration of novel formulations, such as DRZ nanoparticle eye drops. These nanoformulations, designed to improve drug delivery and patient compliance, are under investigation in recent clinical trials. In a study conducted by Gudmundsdottir et al. [86] DRZ γ-cyclodextrin (γCD) nanoparticle eye drops were evaluated against Trusopt in managing IOP. Seventeen participants were enrolled in a prospective randomized trial, where the nanoparticle eye drops, administered once daily, demonstrated efficacy comparable to Trusopt given three times daily. Both treatments exhibited similar reductions in IOP levels at peak and trough periods. Moreover, participants reported less burning sensation with the nanoparticle eye drops compared to Trusopt®. These findings suggest that DRZ nanoparticle eye drops offer a promising alternative with comparable efficacy and potentially improved tolerability over conventional Trusopt therapy. The specific clinical trial details related to the dorzolamide nanoformulations are exemplified in Table 2 [87].
Table 2.
Recent clinical trials on dorzolamide containing micro/nanoformulations for the treatment of glaucoma
| Sponsor | Summary | Study phase | Study design | Clinical trial gov. ID | Study methods | |
|---|---|---|---|---|---|---|
| Comparative Study of Dorzol Eye Drops, 20 mg/ml Versus Trusopt® Eye Drops, 20 mg/ml | Jadran Galenski laboratorij d.d | This study compares the efficacy and safety of Dorzol Eye Drops (investigational drug) with TRUSOPT Eye Drops (reference drug) in patients with ocular hypertension and primary open-angle glaucoma. It involves 118 participants randomized into two groups, aiming to determine equality in efficacy and safety between the investigational and reference drugs | Phase 3 | Randomized open-label, controlled comparative study | NCT05973305 | The study evaluates changes in intraocular pressure (IOP) over 12 weeks, including reduction rates to target levels (≤ 18 mm Hg), and incidences of reductions exceeding 20% and 30%, with corresponding rates at visit 5 |
| Augmented Macular Pigment-containing Nutraceutical and Central Visual Function | University of the Incarnate Word | This study aims to prospectively examine alterations in macular pigment optical density and dermal carotenoid levels in patients receiving Lumega-Z medical food, combined with a topical carbonic anhydrase inhibitor, and their correlation with visual field function | Phase 4 | Randomized, parallel assignment | NCT04676126 | Both groups began with similar baseline levels of L, Z, and MZ. The LM group showed greater increases in these carotenoids compared to PV. Over 24 weeks, LM supplementation significantly raised MPOD levels, while contrast sensitivity improved in all groups but not significantly |
| A Study to Evaluate the Effectiveness of Cosopt® as First Line Therapy (MK-0507A-153) | Merck Sharp & Dohme LLC | Researchers evaluated the efficacy of dorzolamide-timolol (Cosopt®) as the initial therapy for lowering intraocular pressure (IOP) in individuals diagnosed with untreated Open Angle Glaucoma (OAG) or Ocular Hypertension (OH) | Phase 3 | Non-Randomized | NCT00546286 | Reduction in intraocular pressure (IOP) by 4 mmHg (or ≥ 20%) within 6 to 12 weeks |
| MK0507A Clinical Study in Patients With Glaucoma and Ocular Hypertension (0507A-149) | Merck Sharp & Dohme LLC |
The clinical trial compares the safety and efficacy of MK0507A (dorzolamide 1.0% / timolol 0.5%) with two other treatment regimens: 1. Timolol 0.5% alone 2. Concomitant therapy of dorzolamide 1.0% and timolol 0.5% This design includes three treatment groups, aiming to evaluate the reduction in intraocular pressure (IOP) and the outflow pressure reduction rate |
Phase 3 | Randomized, parallel assignment | NCT00449956 | The study measured the percentage change from baseline in IOP at 8 weeks, specifically evaluating IOP two hours post ocular instillation (at Hour 2). Additionally, it assessed the percentage change from baseline in outflow pressure reduction rate at 8 weeks, also evaluated two hours post ocular instillation (at Hour 2) |
| Dry Eye Study With Cosopt® Over 8 Weeks in Patients With Open-Angle Glaucoma or Ocular Hypertension (0507A-152) | Merck Sharp & Dohme LLC | The clinical trial assess the tolerability of preservative-free Cosopt in individuals diagnosed with Open Angle Glaucoma (OAG) or Ocular Hypertension (OH) who also experience dry eyes | Phase 4 | Non-Randomized, single group assignment | NCT00545064 | The study examined alterations in non-visual ocular symptoms, measured by the Glaucoma Symptom Scale (GSS) SYMP-6 scale, before and after 12 weeks of treatment with preservative-free Cosopt |
| Effectiveness and Safety of Timolol and Dorzolamide Loaded Contact Lenses | University of Florida | This study examines the safety and efficacy of drug-eluting contact lenses for glaucoma therapy. These lenses will contain timolol maleate and dorzolamide hydrochloride, common ophthalmic drugs, along with vitamin E for extended drug release. Effectiveness will be determined by a reduction in IOP after using the lenses | Phase 1 | Interventional, Single group assignment | NCT02852057 | No result posted |
| Dorzolamide + Timolol Multidose Preservative-free vs Dorzolamide + Timolol BAK Preserved Efficacy and Safety | Laboratorios Poen | This study aims to compare the tolerability of a new preservative-free Dorzolamide + Timolol formulation in OSD Aptar Pharma's multidose system with the Dorzolamide + Timolol formulation preserved with benzalkonium chloride (BAK) for ophthalmic use | Phase 4 | Randomized, parallel assignment | NCT05857267 | Over 48 weeks, confocal microscopy assessed changes in corneal and conjunctival parameters like epithelium density, corneal stroma reflectivity, nerve density, neuroma density, dendrite density, nerve and presence of inflammatory infiltrates compared to baseline |
| Safety and Efficacy of Triple Combination Therapy With Dorzolamide Hydrochloride / Brimonidine Tartrate / Timolol Ophthalmic Solution in Patients With Glaucoma or Ocular Hypertension | Allergan | This study will examine the safety and efficacy of Triple Combination Therapy with DRZ/brimonidine tartrate/timolol ophthalmic solution in patients with glaucoma or ocular hypertension who have elevated IOP while on dorzolamide hydrochloride/timolol maleate combination therapy | Phase 3 | Open label, Single group assignment | NCT01284166 | Change in intraocular pressure (IOP) from baseline will be evaluated at two time points: baseline and Week 12 |
Data obtained from https://clinicaltrials.gov/search?cond=Glaucoma
Recent advancements in nanotechnology have transformed drug delivery systems, offering improved effectiveness and fewer side effects. In glaucoma treatment, micro/nanoformulations of dorzolamide have emerged as promising options to traditional eye drop formulations, aiming to enhance patient adherence and therapeutic outcomes. Table 3 [88] below provides a comprehensive overview of recently published patents focusing on dorzolamide-based micro/nanoformulations for glaucoma treatment. These patents explore diverse innovative strategies and formulations aimed at optimizing drug delivery, increasing ocular bioavailability, and prolonging therapeutic efficacy.
Table 3.
Recent patents on dorzolamide-containing micro/nanoformulations for the treatment of glaucoma show that many inventors have used dorzolamide HCl as a model drug for effective comparison of newly invented ophthalmic formulations for glaucoma treatment, alongside other antiglaucoma drugs such as latanoprost, bimatoprost, brimonidine tartrate, and brinzolamide
| Title | Patent number | Current assignee | Status | Brief description |
|---|---|---|---|---|
| Use of directed droplet streams with controllable droplet charge for the preparation of a medicament | CN107970506B | Corinthian Ophthalmic Inc | Granted on 16-06-2020 | This disclosure discusses ejector mechanisms and devices for generating a directed stream of droplets, along with improved methods for delivering them to a target, especially for ocular applications. It emphasizes delivering low-dose pharmaceutical compositions effectively by controlling factors like charge and droplet size |
| Preservative removal from eye drops | US11051976B2 | University of Florida Research Foundation Inc | Granted on 06-07-2021 | The BAK removal device is a plug made of microparticles of a hydrophilic polymeric gel, featuring a hydraulic permeability over 0.01 Da. Primarily consisting of poly (2-hydroxyethyl methacrylate) (pHEMA), the microparticles measure 2–100 μm. The plug's surface area ranges from 30 mm2 to 2 mm2, with a length of 2–25 mm. These microparticles have pores sized between 3 and 60 μm |
| Ophthalmic drug delivery | US10073949B2 | Eyenovia Inc | Granted on 11-09-2018 | The invention involves delivering medication to the eye by generating droplets with specific size and velocity and ensuring a portion of the droplets' mass reaches the eye |
| Oil-in-water method for making polymeric implants containing a hypotensive lipid | US20210361670A1 | Allergan Inc | Published on 25-11-2021 | Biocompatible microparticles are developed to release a cyclic lipid component into both front and back parts of the eye for an extended period when placed in the subconjunctival space. These microparticles, formulated as oil-in-water emulsions, can treat symptoms of ocular conditions like glaucoma or age-related macular degeneration |
| Cyclodextrin nanotechnology for ophthalmic drug delivery | US7893040B2 | Oculis ehf | Granted on 22-02-2011 | The invention presents an ophthalmic composition—an aqueous suspension containing a drug, cyclodextrin, and water. With drug concentrations ranging from about 0.1% to 90% (w/v), solid drug/cyclodextrin particles dissolve in tear fluid within 24 h post-application. The composition, available as eye drops, gel, or mist, treats conditions of the eye's anterior and posterior segments. It also encompasses nasal compositions and powder forms for both nasal and ophthalmic use |
| Mucoadhesive nano particle delivery system | CN104507458B | University of Waterloo | Granted on 22-05-2018 | The present application discusses mucoadhesive nanoparticle delivery systems, formed by coating amphiphilic macromolecules with a mucous membrane targeting moiety. These nanoparticles allow precise targeting without compromising stability, with high payload-to-weight ratios and sustained release at mucosal sites. The disclosure also covers polymer preparation, composition, methods, packaging, kits, and applications |
Data obtained from https://patents.google.com/?q=(glaucoma)&oq=glaucoma
Conclusion
This comprehensive review underscores the transformative potential of nanotechnology in enhancing the delivery of Dorzolamide Hydrochloride (DRZ) for effective glaucoma management. Traditional DRZ delivery systems, while widely used, face significant challenges such as limited bioavailability, frequent dosing requirements, and suboptimal patient compliance. The integration of nanotechnology offers promising solutions to these challenges by utilizing advanced nanocarriers like liposomes, nanoparticles, micelles, and dendrimers.
Nanocarrier-based DRZ delivery systems have demonstrated significant improvements in drug stability, bioavailability, and controlled release profiles. Preclinical and clinical studies reviewed in this manuscript reveal that these advanced formulations can enhance therapeutic efficacy, reduce dosing frequency, and minimize side effects. For instance, liposome-encapsulated DRZ has shown prolonged retention time in the ocular tissues, while nanoparticle formulations have exhibited superior penetration and sustained drug release.
Despite these promising advancements, several limitations and challenges remain. The scalability of nanocarrier production, potential toxicity issues, and regulatory hurdles need to be thoroughly addressed before widespread clinical adoption. Additionally, more extensive clinical trials are essential to establish the long-term safety and efficacy of these novel delivery systems.
Future research should focus on optimizing nanocarrier formulations, exploring combination therapies, and developing personalized medicine approaches to cater to individual patient needs. Collaborative efforts between researchers, clinicians, and regulatory bodies will be crucial in translating these innovative solutions from bench to bedside.
In conclusion, leveraging nanotechnology for DRZ delivery represents a significant leap forward in glaucoma treatment, offering the potential to improve patient outcomes and quality of life. Continued research and development in this field hold promise for revolutionizing ocular drug delivery and setting new standards in glaucoma therapy.
Author contribution
S.R.P., A.D. G., N.T. H., K.D. G., J.B. N., A.O. wrote the main manuscript text and S.R.P., A.D. G., N.T. H., K.D. G., J.B. N. prepared figures. All authors discussed the results and contributed to the final manuscript. A.O. supervised the project.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Loftsson T, Jansook P, Stefánsson E. Topical drug delivery to the eye: dorzolamide. Acta Ophthalmol. 2012;90:603–8. 10.1111/j.1755-3768.2011.02299.x. [DOI] [PubMed] [Google Scholar]
- 2.Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma. JAMA. 2014;311:1901. 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoo H-S, Shanmugalingam U, Smith PD. Harnessing astrocytes and müller glial cells in the retina for survival and regeneration of retinal ganglion cells. Cells. 2021;10:1339. 10.3390/cells10061339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tribble JR, Hui F, Quintero H, et al. Neuroprotection in glaucoma: mechanisms beyond intraocular pressure lowering. Mol Aspects Med. 2023;92:101193. 10.1016/j.mam.2023.101193. [DOI] [PubMed] [Google Scholar]
- 5.García-Llorca A, Carta F, Supuran CT, Eysteinsson T. Carbonic anhydrase, its inhibitors and vascular function. Front Mol Biosci. 2024. 10.3389/fmolb.2024.1338528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capasso C, Supuran CT. Carbonic anhydrase and bacterial metabolism: a chance for antibacterial drug discovery. Expert Opin Ther Pat. 2024. 10.1080/135437762332663. [DOI] [PubMed] [Google Scholar]
- 7.Patton GN, Lee HJ. Chemical insights into topical agents in intraocular pressure management: from glaucoma etiopathology to therapeutic approaches. Pharmaceutics. 2024;16:274. 10.3390/pharmaceutics16020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maren TH. Role of carbonic anhydrase in aqueous humour and cerebrospinal fluid formation. In: Barriers and fluids of the eye and brain. London: Macmillan Education UK; 1992. p. 37–48. [Google Scholar]
- 9.Schmidl D, Schmetterer L, Garhöfer G, Popa-Cherecheanu A. Pharmacotherapy of Glaucoma. J Ocul Pharmacol Ther. 2015;31:63–77. 10.1089/jop.2014.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Supuran CT. A simple yet multifaceted 90 years old, evergreen enzyme: Carbonic anhydrase, its inhibition and activation. Bioorg Med Chem Lett. 2023;93:129411. 10.1016/j.bmcl.2023.129411. [DOI] [PubMed] [Google Scholar]
- 11.Dartt DA. Neural regulation of lacrimal gland secretory processes: Relevance in dry eye diseases. Prog Retin Eye Res. 2009;28:155–77. 10.1016/j.preteyeres.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balfour JA, Wilde MI. Dorzolamide. Drugs Aging. 1997;10:384–403. 10.2165/00002512-199710050-00006. [DOI] [PubMed] [Google Scholar]
- 13.Purkerson JM, Schwartz GJ. The role of carbonic anhydrases in renal physiology. Kidney Int. 2007;71:103–15. 10.1038/sj.ki.5002020. [DOI] [PubMed] [Google Scholar]
- 14.Stoner A, Harris A, Oddone F, et al. Topical carbonic anhydrase inhibitors and glaucoma in 2021: where do we stand? Br J Ophthalmol. 2022;106:1332–7. 10.1136/bjophthalmol-2021-319530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jansook P, Hnin HM, Loftsson T, Stefánsson E. Cyclodextrin-based formulation of carbonic anhydrase inhibitors for ocular delivery – a review. Int J Pharm. 2021;606:120955. 10.1016/j.ijpharm.2021.120955. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal R, Gupta S, Agarwal P, et al. Current concepts in the pathophysiology of glaucoma. Indian J Ophthalmol. 2009;57:257. 10.4103/0301-4738.53049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inoue J, Oka M, Aoyama Y, et al. Effects of dorzolamide hydrochloride on ocular tissues. J Ocul Pharmacol Ther. 2004;20:1–13. 10.1089/108076804772745419. [DOI] [PubMed] [Google Scholar]
- 18.Dietrich UM, Chandler MJ, Cooper T, et al. Effects of topical 2% dorzolamide hydrochloride alone and in combination with 0.5% timolol maleate on intraocular pressure in normal feline eyes. Vet Ophthalmol. 2007;10:95–100. 10.1111/j.1463-5224.2007.00583.x. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi M, Naito K. Pharmacological profiles of the potent carbonic anhydrase inhibitor dorzolamide hydrochloride, a topical antiglaucoma agent. Folia Pharmacol Jpn. 2000;115:323–8. 10.1254/fpj.115.323. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y-C, Ling XC, Tsai W-H, et al. Risks of topical carbonic anhydrase inhibitors in glaucoma patients with chronic kidney disease: a nationwide population-based study. Am J Ophthalmol. 2023;253:49–55. 10.1016/j.ajo.2023.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Ghorai S, Pulya S, Ghosh K, et al. Structure-activity relationship of human carbonic anhydrase-II inhibitors: detailed insight for future development as anti-glaucoma agents. Bioorg Chem. 2020;95:103557. 10.1016/j.bioorg.2019.103557. [DOI] [PubMed] [Google Scholar]
- 22.Di FA, De SG, Menchise V, et al. Carbonic anhydrase inhibitors: X-ray crystal structure of a benzenesulfonamide strong CA II and CA IX inhibitor bearing a pentafluorophenylaminothioureido tail in complex with isozyme II. Bioorg Med Chem Lett. 2005;15:1937–42. 10.1016/j.bmcl.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 23.Vedani A, Meyer EF. Structure-activity relationships of sulfonamide drugs and human carbonic anhydrase C: modeling of inhibitor molecules into the receptor site of the enzyme with an interactive computer graphics display1. J Pharm Sci. 1984;73:352–8. 10.1002/jps.2600730316. [DOI] [PubMed] [Google Scholar]
- 24.Baldwin JJ, Ponticello GS, Anderson PS, et al. Thienothiopyran-2-sulfonamides: novel topically active carbonic anhydrase inhibitors for the treatment of glaucoma. J Med Chem. 1989;32:2510–3. 10.1021/jm00132a003. [DOI] [PubMed] [Google Scholar]
- 25.Paganini V, Chetoni P, Di Gangi M, et al. Nanomicellar eye drops: a review of recent advances. Expert Opin Drug Deliv. 2024. 10.1080/174252472323208. [DOI] [PubMed] [Google Scholar]
- 26.Jin K, Li Y, Wu H, et al. Integration of smartphone technology and artificial intelligence for advanced ophthalmic care: a systematic review. Adv Ophthalmol Pract Res. 2024. 10.1016/j.aopr.2024.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ezike TC, Okpala US, Onoja UL, et al. Advances in drug delivery systems, challenges and future directions. Heliyon. 2023;9:e17488. 10.1016/j.heliyon.2023.e17488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schopf LR, Popov AM, Enlow EM, et al. Topical ocular drug delivery to the back of the eye by mucus-penetrating particles. Transl Vis Sci Technol. 2015;4:11. 10.1167/tvst.4.3.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kagkelaris K, Panayiotakopoulos G, Georgakopoulos CD. Nanotechnology-based formulations to amplify intraocular bioavailability. Ther Adv Ophthalmol. 2022;14:251584142211123. 10.1177/25158414221112356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmed S, Amin MM, Sayed S. Ocular drug delivery: a comprehensive review. AAPS PharmSciTech. 2023;24:66. 10.1208/s12249-023-02516-9. [DOI] [PubMed] [Google Scholar]
- 31.del Amo EM, Rimpelä A-K, Heikkinen E, et al. Pharmacokinetic aspects of retinal drug delivery. Prog Retin Eye Res. 2017;57:134–85. 10.1016/j.preteyeres.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Agarwal P, Craig JP, Rupenthal ID. Formulation considerations for the management of dry eye disease. Pharmaceutics. 2021;13:207. 10.3390/pharmaceutics13020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pardeshi SR, More MP, Kulkarni AD, et al. Current perspectives in nanomedicine delivery for targeted ocular therapeutics. Bull Mater Sci. 2023;46:35. 10.1007/s12034-022-02869-0. [Google Scholar]
- 34.Subrizi A, del Amo EM, Korzhikov-Vlakh V, et al. Design principles of ocular drug delivery systems: importance of drug payload, release rate, and material properties. Drug Discov Today. 2019;24:1446–57. 10.1016/j.drudis.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Tau J, Passerini MS, del Papa M, et al. A novel ophthalmic latanoprost 0.005% nanoemulsion: a cytotoxicity study. Graefe’s Arch Clin Exp Ophthalmol. 2022;260:1941–6. 10.1007/s00417-021-05536-y. [DOI] [PubMed] [Google Scholar]
- 36.Kassem AA, Salama A, Mohsen AM. Formulation and optimization of cationic nanoemulsions for enhanced ocular delivery of dorzolamide hydrochloride using Box-Behnken design: in vitro and in vivo assessments. J Drug Deliv Sci Technol. 2022;68:103047. 10.1016/j.jddst.2021.103047. [Google Scholar]
- 37.Guo X, Zhang J, Liu X, et al. Antioxidant nanoemulsion loaded with latanoprost enables highly effective glaucoma treatment. J Control Release. 2023;361:534–46. 10.1016/j.jconrel.2023.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Londhe VY, Sharma S. Formulation, characterization, optimization and in-vivo evaluation of methazolamide liposomal in-situ gel for treating glaucoma. J Drug Deliv Sci Technol. 2022;67:102951. 10.1016/j.jddst.2021.102951. [Google Scholar]
- 39.Brugnera M, Vicario-de-la-Torre M, González-Cela Casamayor MA, et al. Enhancing the hypotensive effect of latanoprost by combining synthetic phosphatidylcholine liposomes with hyaluronic acid and osmoprotective agents. Drug Deliv Transl Res. 2024. 10.1007/s13346-024-01584-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vicario-de-la-Torre M, Benitez-del-Castillo JM, Vico E, et al. Design and characterization of an ocular topical liposomal preparation to replenish the lipids of the tear film. Invest Ophthalmol Vis Sci. 2014;55:7839–47. 10.1167/iovs.14-14700. [DOI] [PubMed] [Google Scholar]
- 41.El-Feky YA, Fares AR, Zayed G, et al. Repurposing of nifedipine loaded in situ ophthalmic gel as a novel approach for glaucoma treatment. Biomed Pharmacother. 2021;142:112008. 10.1016/j.biopha.2021.112008. [DOI] [PubMed] [Google Scholar]
- 42.Nair AB, Shah J, Jacob S, et al. Experimental design, formulation and in vivo evaluation of a novel topical in situ gel system to treat ocular infections. PLoS ONE. 2021;16:e0248857. 10.1371/journal.pone.0248857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Makwana SB, Patel VA, Parmar SJ. Development and characterization of in-situ gel for ophthalmic formulation containing ciprofloxacin hydrochloride. Results Pharma Sci. 2016;6:1–6. 10.1016/j.rinphs.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agarwal P, Rupenthal ID. Non-aqueous formulations in topical ocular drug delivery – A paradigm shift? Adv Drug Deliv Rev. 2023;198:114867. 10.1016/j.addr.2023.114867. [DOI] [PubMed] [Google Scholar]
- 45.Wang C, Pang Y. Nano-based eye drop: topical and noninvasive therapy for ocular diseases. Adv Drug Deliv Rev. 2023;194:114721. 10.1016/j.addr.2023.114721. [DOI] [PubMed] [Google Scholar]
- 46.Naik JB, Pardeshi SR, Patil RP, et al. Mucoadhesive micro-/nano carriers in ophthalmic drug delivery: an overview. Bionanoscience. 2020;10:564–82. 10.1007/s12668-020-00752-y. [Google Scholar]
- 47.Patel KD, Barrios Silva L, Park Y, et al. Recent advances in drug delivery systems for glaucoma treatment. Mater Today Nano. 2022;18:100178. 10.1016/j.mtnano.2022.100178. [Google Scholar]
- 48.Jansook P, Stefánsson E, Thorsteinsdóttir M, et al. Cyclodextrin solubilization of carbonic anhydrase inhibitor drugs: formulation of dorzolamide eye drop microparticle suspension. Eur J Pharm Biopharm. 2010;76:208–14. 10.1016/j.ejpb.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 49.Park CG, Kim YK, Kim S-N, et al. Enhanced ocular efficacy of topically-delivered dorzolamide with nanostructured mucoadhesive microparticles. Int J Pharm. 2017;522:66–73. 10.1016/j.ijpharm.2017.02.035. [DOI] [PubMed] [Google Scholar]
- 50.Fu J, Sun F, Liu W, et al. Subconjunctival delivery of dorzolamide-loaded poly(ether-anhydride) microparticles produces sustained lowering of intraocular pressure in rabbits. Mol Pharm. 2016;13:2987–95. 10.1021/acs.molpharmaceut.6b00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katiyar S, Pandit J, Mondal RS, et al. In situ gelling dorzolamide loaded chitosan nanoparticles for the treatment of glaucoma. Carbohydr Polym. 2014;102:117–24. 10.1016/j.carbpol.2013.10.079. [DOI] [PubMed] [Google Scholar]
- 52.Papadimitriou S, Bikiaris D, Avgoustakis K, et al. Chitosan nanoparticles loaded with dorzolamide and pramipexole. Carbohydr Polym. 2008;73:44–54. 10.1016/j.carbpol.2007.11.007. [Google Scholar]
- 53.Shinde U, Ahmed MH, Singh K. Development of dorzolamide loaded 6-O -carboxymethyl chitosan nanoparticles for open angle glaucoma. J Drug Deliv. 2013;2013:1–15. 10.1155/2013/562727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shahab MS, Rizwanullah M, Alshehri S, Imam SS. Optimization to development of chitosan decorated polycaprolactone nanoparticles for improved ocular delivery of dorzolamide: in vitro, ex vivo and toxicity assessments. Int J Biol Macromol. 2020;163:2392–404. 10.1016/j.ijbiomac.2020.09.185. [DOI] [PubMed] [Google Scholar]
- 55.Warsi MH, Anwar M, Garg V, et al. Dorzolamide-loaded PLGA/vitamin E TPGS nanoparticles for glaucoma therapy: Pharmacoscintigraphy study and evaluation of extended ocular hypotensive effect in rabbits. Colloids Surf B Biointerfaces. 2014;122:423–31. 10.1016/j.colsurfb.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 56.Manchanda S, Sahoo PK. Fabrication and characterization of mucoadhesive topical nanoformulations of dorzolamide HCl for ocular hypertension. J Pharm Investig. 2018;48:323–32. 10.1007/s40005-017-0324-x. [Google Scholar]
- 57.Shahab MS, Rizwanullah M, Sarim Imam S. Formulation, optimization and evaluation of vitamin E TPGS emulsified dorzolamide solid lipid nanoparticles. J Drug Deliv Sci Technol. 2022;68:103062. 10.1016/j.jddst.2021.103062. [Google Scholar]
- 58.Afify EAMR, Elsayed I, Gad MK, et al. Enhancement of pharmacokinetic and pharmacological behavior of ocular dorzolamide after factorial optimization of self-assembled nanostructures. PLoS ONE. 2018;13:e0191415. 10.1371/journal.pone.0191415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kouchak M, Malekahmadi M, Bavarsad N, et al. Dorzolamide nanoliposome as a long action ophthalmic delivery system in open angle glaucoma and ocular hypertension patients. Drug Dev Ind Pharm. 2018;44:1239–42. 10.1080/03639045.2017.1386196. [DOI] [PubMed] [Google Scholar]
- 60.Franca JR, Foureaux G, Fuscaldi LL, et al. Chitosan/hydroxyethyl cellulose inserts for sustained-release of dorzolamide for glaucoma treatment: In vitro and in vivo evaluation. Int J Pharm. 2019;570:118662. 10.1016/j.ijpharm.2019.118662. [DOI] [PubMed] [Google Scholar]
- 61.Özdemir S, Çakirli E, Sürücü B, et al. Preparation and characterization studies of dorzolamide-loaded ophthalmic implants for treating glaucoma. Turkish J Pharm Sci. 2023;20:149–56. 10.4274/tjps.galenos.2022.95752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patil SS, Bade A, Tagalpallewar A. Design, optimization and pharmacodynamic comparison of dorzolamide hydrochloride soluble ocular drug insert prepared by using 3 2 factorial design. J Drug Deliv Sci Technol. 2018;46:138–47. 10.1016/j.jddst.2018.05.010. [Google Scholar]
- 63.Pardeshi SR, More MP, Pardeshi CV, et al. Novel crosslinked nanoparticles of chitosan oligosaccharide and dextran sulfate for ocular administration of dorzolamide against glaucoma. J Drug Deliv Sci Technol. 2023;86:104719. 10.1016/j.jddst.2023.104719. [Google Scholar]
- 64.Sahoo S, Dilnawaz F, Krishnakumar S. Nanotechnology in ocular drug delivery. Drug Discov Today. 2008;13:144–51. 10.1016/j.drudis.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 65.Paolicelli P, de la Fuente M, Sánchez A, et al. Chitosan nanoparticles for drug delivery to the eye. Expert Opin Drug Deliv. 2009;6:239–53. 10.1517/17425240902762818. [DOI] [PubMed] [Google Scholar]
- 66.Arabestani MR, Bigham A, Kamarehei F, et al. Solid lipid nanoparticles and their application in the treatment of bacterial infectious diseases. Biomed Pharmacother. 2024;174:116433. 10.1016/j.biopha.2024.116433. [DOI] [PubMed] [Google Scholar]
- 67.Pham CV, Cho C-W. Application of d-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) in transdermal and topical drug delivery systems (TDDS). J Pharm Investig. 2017;47:111–21. 10.1007/s40005-016-0300-x. [Google Scholar]
- 68.Mohammed HA, Khan RA, Singh V, et al. Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development. Nanotechnol Rev. 2023. 10.1515/ntrev-2022-0517. [Google Scholar]
- 69.Alkholief M, Albasit H, Alhowyan A, et al. Employing a PLGA-TPGS based nanoparticle to improve the ocular delivery of acyclovir. Saudi Pharm J. 2019;27:293–302. 10.1016/j.jsps.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Manca ML, Aroffu M, Fulgheri F, et al. Conventional methods for preparing liposomes of various types (MLVs, LUVs, SUVs). In: Liposomes in Drug Delivery. Amsterdam: Elsevier; 2024. p. 461–88. [Google Scholar]
- 71.Nii T, Ishii F. Encapsulation efficiency of water-soluble and insoluble drugs in liposomes prepared by the microencapsulation vesicle method. Int J Pharm. 2005;298:198–205. 10.1016/j.ijpharm.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 72.Paramshetti S, Angolkar M, Talath S, et al. Unravelling the in vivo dynamics of liposomes: Insights into biodistribution and cellular membrane interactions. Life Sci. 2024;346:122616. 10.1016/j.lfs.2024.122616. [DOI] [PubMed] [Google Scholar]
- 73.Biswas A, Kumar S, Choudhury AD, et al. Polymers and their engineered analogues for ocular drug delivery: enhancing therapeutic precision. Biopolymers. 2024. 10.1002/bip.23578. [DOI] [PubMed] [Google Scholar]
- 74.Tasharrofi N, Nourozi M, Marzban A. How liposomes pave the way for ocular drug delivery after topical administration. J Drug Deliv Sci Technol. 2022;67:103045. 10.1016/j.jddst.2021.103045. [Google Scholar]
- 75.Kouchak M, Bahmandar R, Bavarsad N, Farrahi F. Ocular dorzolamide nanoliposomes for prolonged IOP reduction: in-vitroand in-vivo evaluation in rabbits. Iran J Pharm Res IJPR. 2016;15:205–12. [PMC free article] [PubMed] [Google Scholar]
- 76.Sharma A, Singh M, Sharma V, et al. Current paradigms in employing self-assembled structures: drug delivery implications with improved therapeutic potential. Colloids Surf B Biointerfaces. 2024;234:113745. 10.1016/j.colsurfb.2024.113745. [DOI] [PubMed] [Google Scholar]
- 77.Liu Y, Castro Bravo KM, Liu J. Targeted liposomal drug delivery: a nanoscience and biophysical perspective. Nanoscale Horizons. 2021;6:78–94. 10.1039/D0NH00605J. [DOI] [PubMed] [Google Scholar]
- 78.Alyami H, Abdelaziz K, Dahmash EZ, Iyire A. Nonionic surfactant vesicles (niosomes) for ocular drug delivery: development, evaluation and toxicological profiling. J Drug Deliv Sci Technol. 2020;60:102069. 10.1016/j.jddst.2020.102069. [Google Scholar]
- 79.Moammeri A, Chegeni MM, Sahrayi H, et al. Current advances in niosomes applications for drug delivery and cancer treatment. Mater Today Bio. 2023;23:100837. 10.1016/j.mtbio.2023.100837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hasan AA. Design and in vitro characterization of small unilamellar niosomes as ophthalmic carrier of dorzolamide hydrochloride. Pharm Dev Technol. 2014;19:748–54. 10.3109/10837450.2013.829095. [DOI] [PubMed] [Google Scholar]
- 81.Hashemi Dehaghi M, Haeri A, Keshvari H, et al. Dorzolamide loaded niosomal vesicles: comparison of passive and remote loading methods. Iran J Pharm Res IJPR. 2017;16:413–22. [PMC free article] [PubMed] [Google Scholar]
- 82.Gawin-Mikołajewicz A, Nartowski KP, Dyba AJ, et al. Ophthalmic nanoemulsions: from composition to technological processes and quality control. Mol Pharm. 2021;18:3719–40. 10.1021/acs.molpharmaceut.1c00650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ammar HO, Salama HA, Ghorab M, Mahmoud AA. Nanoemulsion as a potential ophthalmic delivery system for dorzolamide hydrochloride. AAPS PharmSciTech. 2009;10:808. 10.1208/s12249-009-9268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gorantla S, Rapalli VK, Waghule T, et al. Nanocarriers for ocular drug delivery: current status and translational opportunity. RSC Adv. 2020;10:27835–55. 10.1039/D0RA04971A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Asendrych-Wicik K, Zarczuk J, Walaszek K, et al. Trends in development and quality assessment of pharmaceutical formulations - F2α analogues in the glaucoma treatment. Eur J Pharm Sci. 2023;180:106315. 10.1016/j.ejps.2022.106315. [DOI] [PubMed] [Google Scholar]
- 86.Gudmundsdottir BS, Petursdottir D, Asgrimsdottir GM, et al. γ-Cyclodextrin nanoparticle eye drops with dorzolamide: effect on intraocular pressure in man. J Ocul Pharmacol Ther. 2014;30:35–41. 10.1089/jop.2013.0060. [DOI] [PubMed] [Google Scholar]
- 87.ClinicalTrials.gov (2023) ClinicalTrials.gov. In: ClinicalTrials.gov
- 88.Google Patents (2024)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.