Abstract
The loss of sesame capsule seed prior to harvest poses a significant economical challenge in mechanized production. The metabolites involved in capsule closure are still unclear. Using comparative metabolome and transcriptome analysis, this work investigated the molecular regulation and enrichment pathways in two sesame types of indehiscent capsule WanZhi28 (ND) and dehiscent capsule WanZhi2 (WZ2). The findings demonstrated that genes and metabolites were significantly enriched in lignin synthesis-related pathways. Furthermore, data suggests that lipid and sugar metabolism may have an impact on capsule closure. Apart from its function in cell signaling, the latter may contribute to the glycosylation of lignin monomers, while the former may provide ATP for cellular microtubule movement. This work concurrently focused on a large number of differentially expressed transcription factors linked to the sesame capsule's anti-cleft mechanism, providing new evidence for the discovery and use of functional markers and genes for capsule dehiscence. The identification of key pathways and regulatory mechanisms offers valuable information for developing strategies to mitigate seed loss during harvest, ultimately contributing to more efficient and profitable sesame production.
Keywords: Sesame, Capsule dehiscence, Metabolome, Transcriptome, Lignin synthesis, Lipid and sugar metabolism
Graphical abstract
Highlights
-
•
Lignin metabolism was associated with indehiscent capsule in sesame.
-
•
Lipid metabolism may supply ATP for microtubule movement during capsule closure.
-
•
Sugar metabolism may be involved in lignin monomers and cell signaling.
-
•
Multi-omics analysis provided reference for gene mining related to capsule closure.
1. Introduction
Research on sesame seeds has predominantly focused on physiological indices such as seed count, capsule quantity, plant growth, and the oil and organic acid content of sesame seeds (Dossou et al., 2021; Mi et al., 2022). However, there remains a significant gap in understanding sesame capsule dehiscence, which is a critical factor affecting yield and poses challenges for mechanized harvesting (Chellamuthu et al., 2022; Qureshi et al., 2022). To enhance the suitability of sesame varieties for automated harvesting, one of the key breeding objectives is to increase resistance to capsule breakage. Investigations into the genetic basis of dehiscence have identified several regulatory genes in Arabidopsis thaliana that influence the fruit valve margin governing the dehiscence zone. Notably, the AP2 gene has shown stronger expression in indehiscent fruits, suggesting its role in regulating this trait (Yong et al., 2023; Zhou et al., 2022). The different expression of lignification value and AP2 transcript has also been emphasized in studies examining cracking gene in almond fruit (Tamayo García et al., 2022). Previous research indicates that the indehiscent capsule trait in sesame is governed by two recessive genes, based on the progeny characteristics from crosses between dehiscent and indehiscent capsule varieties (Teboul et al., 2022). Furthermore, the gene SiCL1 has been highlighted for its dual role; it encodes the KAN1 protein, which acts as a transcription repressor, and also regulates leaf curling and capsule indehiscence in sesame (Zhang et al., 2018). While there has been significant research on various aspects of sesame seeds, understanding the mechanisms behind capsule dehiscence is essential for improving yield and facilitating mechanized harvesting. (Yong et al., 2023).
The maturation of sesame capsules is closely linked to the process of lignification, which is essential for the structural integrity of the plant (Zhang et al., 2024). Lignin, along with cellulose and hemicellulose, constitutes a significant portion of the plant cell wall and is a key component that forms the plant skeleton (Mazumder & Zhang, 2023; Singh et al., 2022). Research in other legumes, such as kidney beans, has indicated that genetic variations associated with lignin biosynthesis can influence pod dehiscence. Specifically, a base-pair substitution in a gene related to lignin production was linked to decreased pod dehiscence, suggesting that alterations in lignin content can directly affect the rigidity of the cell wall (Parker et al., 2020). The reduction of lignin content can reduce the rigidity of cell wall, which may have implications for seed retention (Zhao et al., 2022). Studies have hypothesized that the anatomical features of sesame capsules are related to seed retention, they stressed that during capsule senescence, endocarp cells shrank less than mesocarp cells, generating tension in the capsule wall during drying process, which forced the capsules to open along a zone of weakness between locules, thereby facilitating seed dispersal (Singla et al., 2023). Despite these observations, the underlying mechanisms of the metabolic, biosynthetic, and regulatory pathways involved in the sesame capsule indehiscence and dehiscence process remain largely unknown. This is the first ever attempt to investigate the process of capsule indehiscence and dehiscence using metabolomics and transcriptomics to target the pathways associated to lignification, cell mobility and arrangement of capsule tissue.
In our previous study (Zhang et al., 2021), we established a comprehensive framework for understanding the lipid and fatty acid biosynthesis mechanism during different growth periods of sesame seed formation. Based on this foundation, current investigation focuses on elucidating the regulatory and metabolic pathways associated with lignification in the indehiscent (ND) and dehiscent (WZ2) sesame capsule types. Through the analysis of transcriptomic and metabolomic data, we have clarified the complex processes involved in lignification. Our findings indicate that the closing of sesame capsules is intricately linked to several factors, including cell migration along microtubules, sugar modifications, and the involvement of multiple transcription factors that regulate this network. This comprehensive approach has allowed us to identify key genes and metabolic pathways that underpin the regulation of lignification in sesame capsules.
2. Materials and methods
2.1. Materials
The control group was from the dehiscent capsule variety of Wanzhi2 (WZ2, Fig. 1A), which was bred by the sesame research group which from Anhui Academy of Agricultural Sciences (Hefei, China). And the test group was derived from a new indehiscent capsule variety of Wanzhi 28 (ND, Fig. 1B), which was also bred by the sesame research group from Anhui Academy of Agricultural Sciences (Hefei, China), after natural maturity, the capsules were indehiscent. At the stage of sesame capsule dehiscence, 12 disease-free plants with the same growth vigor were selected from ND population and WZ2 population, respectively. Twelve samples in all, including three biological duplicates for the control and test groups of sesame capsules, were therefore collected. After the necessary fruit cheek was harvested, the seeds were taken out and combined into six repetitions per group so that they could be examined as metabolic groups (Zhang et al., 2024). Additionally, three replicates were created by combining six repetitions for transcriptome sequencing.
Fig. 1.
The differences in the capsule morphology, the capsule shell cross-sectional micromorphology and widely-targeted metabolomics in two sesame varieties. (A) and (B) Mature sesame capsules of dehiscent capsule (WZ2) and indehiscent capsule (ND); (C) and (D) Mature sesame capsules cross section of WZ2 and ND; (E) Total metabolite classification in the SuperClass about mature sesame capsules of ND and WZ2; (F) Classification map of phenylpropanoids and polyketides about mature sesame capsules of ND and WZ2.
Collected samples in the field, stored them in liquid nitrogen quickly and then transferred into the refrigerator of −80 °C until further use.
In addition, mature capsules of ND and WZ2 were collected separately after removing the seeds, cross-sectional samples of the capsule shell were used for microscopic observations. After staining by sarranine and fast green method, the differences in the structure between capsule cracking and closure were observed under the microscope (Olympus, BX53 + DP73 + cellSens, Tokyo, Japan).
2.2. Sample preparation and metabolite identification
The preparation of the sesame capsule samples, the analysis of capsule extracts and the quantification and identification of metabolites were performed at Novogene Co., LTD. (Shanghai, China). For metabolites analysis, each sample were repeated six times. The QC quality control samples were prepared to balance the state of chromatography-mass spectrometry system and monitoring and maintain the stability of the system during the experiment. Added the supernatant to the LC-MS/MS system (Waltham, MA, USA) (Zhang et al., 2024), then loaded the data which generated by LC-MS/MS into Compound Discoverer 3.1 (CD3.1, Thermo Fisher), and finally perform peak comparison, peak selection, and quantification for each metabolite, and the normalized data were used for a multivariate statistical analysis and were matched with the mzCloud (https://www.mzcloud.org/), mzVault (Waltham, MA, USA), and MassList databases (Waltham, MA, USA) to obtain the accurate result (Huang et al., 2021).
2.3. Metabolite screening
Utilizing KEGG database information (http://www.genome.jp/kegg/), HMDB database (http://www.hmdb.ca/), and Lipidmaps database (http://www.lipidmaps.org/) annotated the metabolites of sesame capsule samples. Performed the Principal Component Analysis (PCA) and Partial Least Squares Discriminant Analysis (PLS-DA) on Meta X. Metabolites with VIP > 1, P value<0.05, multiple change ≥2, or FC ≤ 0.5 were considered as differential metabolites between the samples of two groups. Volcano maps were used to filter metabolites of interest which based on Log2 (FC) and -log10 (P-value) of metabolites, the differential metabolites of the dehiscent capsule and indehiscent capsule were analyzed through clustering heat maps (Bai et al., 2021).
2.4. RNA extraction, quantification, and sequencing
To further investigate the potential regulatory molecular mechanisms between ND and WZ2, six cDNA libraries were constructed from the samples of three duplicate capsules of ND and WZ2 varieties respectively, and the six samples used for metabolite data analysis and subjected to high-throughput RNA-seq analysis. RNA extraction, quantification analysis, and transcriptome sequencing were carried out according to manufacturer's details using Trizol reagent (Invitrogen, Waltham, MA, USA). Total RNA was isolated from the collected seeds, including three biological duplicates, for transcriptomic and metabolomic analysis. The library preparations were sequenced on an Illumina Novaseq platform and 150 bp paired-end reads were generated.
In order to further investigate the potential regulatory molecular mechanisms between varieties of ND and WZ2, six cDNA libraries were constructed from the samples of three duplicate capsules of ND and WZ2 respectively. Also, the six samples were used for metabolite data analysis and high-throughput RNA seq analysis. RNA extraction (Invitrogen, Waltham, MA, USA), fluorescence quantitative detection used the PrimeScript RT Reagent Kit (TaKaRa, Dalian, China), and transcriptome sequencing were carried out according to the instructions by Novogene Co., LTD. (Shanghai, China). Sequenced the library formulation on the Illumina Novaseq platform and generated a paired-end reading of 150 bp (Liu et al., 2022; Zhang et al., 2020).
2.5. Identification of transcriptional data
Clean readings were retrieved after trimming the joining subsequence and removing low quality readings with unknown nucleotides. GC-content distribution was checked, and all downstream analysis was based on high-quality clean data (Bai et al., 2021). Map clean readings directly to the reference genome downloaded from the genome website using Hisat2 v2.0.5. The mapping readings for each sample were assembled by String Tie (v1.3.3b) according to the reference method. Quantify gene expression levels using feature counting v1.5.0-p3 based on the FPKM method. Performed differential expression analysis between two groups of samples using the DESeq2 R software package (1.16.1) and adjust the P-value (Zhang et al., 2020). The threshold for significant differential expression was set to 0.05 for the corrected P-value and 2 for the absolute fold change. Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses of differently expressed genes were further implemented employing the cluster Profiler R package (Liu et al., 2022).
2.6. Statistical analysis
For metabolites and Transcriptional analysis, each sample were repeated six times. SPSS software was used for significance analysis of differences between samples. The principal component analysis and radar plotting were performed using origin 2019b software. For qRT-PCR, each sample were repeated three times.
3. Results
3.1. Metabolome profiling
In this study, we utilized a widely-targeted metabolomics approach to analyze the indehiscent capsule (ND) and dehiscent capsule (WZ2) of sesame. A total of 895 metabolites were identified, with 368 of these annotated in the Human Metabolome Database (HMDB) and categorized into 11 super classes, containing 77 lipids and lipid-like molecules, 69 phenylpropanoids and polyketides, 68 organic acids and derivatives, 44 benzenoids, 43 organoheterocyclic compounds, 36 organic oxygen compounds, 19 nucleosides and analogues, 7 organic oxygen compounds, 2 alkaloids and derivatives, 2 organooxygen compounds, 1 homogeneous non-metal compounds (Fig.1E and 1F). Lignin was a compound polymerized from phenylpropane structural monomers and the classification was further refined and 69 metabolites of phenylpropanoids and polyketides were found closely related to lignin synthesis, including 30 flavonoids, 12 cinnamic acids and derivatives, 8 coumarins and derivatives, 8 isoflavanoids, 8 phynylpropanoic acids, and 1 cinnamaldehydes. These findings highlight the metabolic diversity present in the different capsule types and emphasize the significance of phenylpropanoid metabolism in lignin synthesis.
3.2. PCA and partial least squares discriminant analysis (PLS-DA)
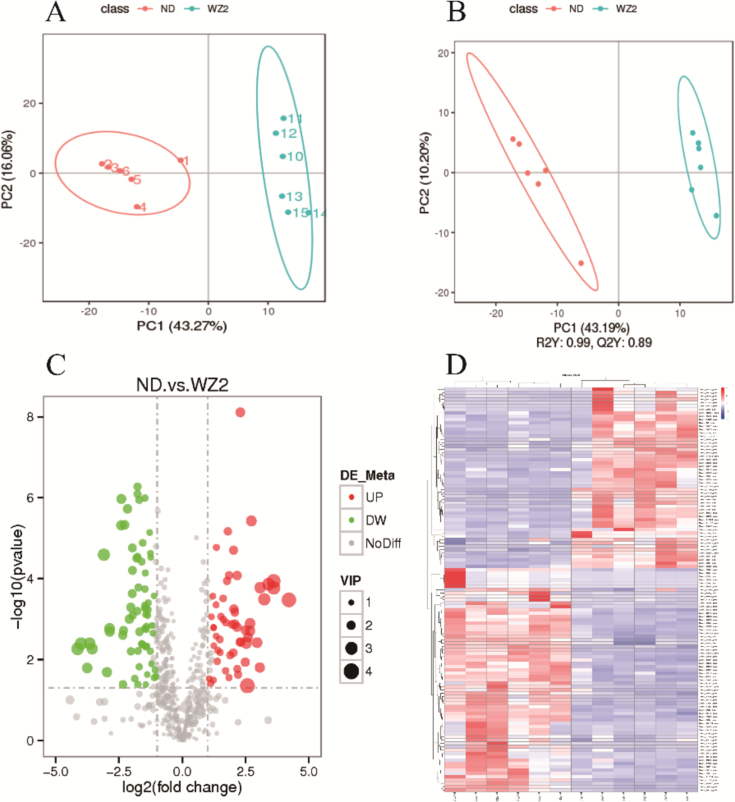
PCA combined with PLS-DA plots showed a clear and satisfactory separation between the ND and WZ2 (distinct metabolite profiles present in the two sesame varieties), the same treatment repeatability met the requirements, R2 of the PLS-DA model was greater than Q2 in both positive and negative mode. Q2 points of the regression line intersected the vertical axis below zero, which indicated that the PLS-DA models had no over fitting and good reliability for further analysis. As shown in the figure Fig. 2A and B, the test and the control group were obviously separated, suggesting that the metabolite profiles in these two samples were obviously distinct. The metabolite map in the positive mode was listed below, and the negative mode also showed similar distribution characteristics. These observations suggest that the metabolic differences between the two sesame varieties could be attributed to their distinct capsule traits, providing valuable insights for further research into the underlying biochemical pathways and their implications for capsule development and seed retention.
Fig. 2.
The results of differential metabolite analysis in two sesame varieties. (A) PCA between the ND and WZ2 of mature sesame capsules; (B) PLS-DA between the ND and WZ2 of mature sesame capsules; (C)Volcano plots of DEMs in positive mode about mature sesame capsules of ND and WZ2; (D) Heatmaps of differential metabolites in positive mode about mature sesame capsules of ND and WZ2.
3.3. Distribution and analysis of DEMs
The DEMs between ND and WZ2 were determined in projection (VIP) ≥ 1and fold change≥2 or fold change≤0.5 based on the variable importance. Volcano plots were constructed to confirm the number of significantly changed metabolites between the two groups, a total of 184 differently expressed metabolites were screened, of which 97 metabolites were down regulated and 87 metabolites were up regulated (Fig. 2C, Table S1A and S1B). The heat maps showed that the difference of metabolites among the replicates within the group was consistent (Fig. 2D), the test and control groups were obviously divided into two groups (Fig. 2C), all the biological replicates were grouped together suggesting that the generated metabolomic data with high reliability (Fig. 2D). The clear separation between the test and control groups further supports the reliability of the metabolomic data, indicating that all biological replicates clustered together. The direct synthesis of lignin in relation to phyloproponoid biosynthesis, in which the intermediate metabolites including phylopanaine, cinnamic acid, tyrosine, caffeic acid, ferulic acid, caffeoyl quinic acid, coniferyl alcohol, courine, sinapyl alcohol, syringin, etc. was detected. In particular, coumarin, sinapyl alcohol, and syringin showed differential expression. In addition, lignin was a micromolecular compound randomly polymerized from phenylpropane unit, and its synthesis was closely related to flavonoid biosynthesis, phynylalanine, tyrosine and tryptophan biosynthesis, and phynylalanine metropolis. According to previous studies, the synthesis of lignin in cell wall was related to glycosylated modification (Xie et al., 2022). The enrichment analysis of DEMs in both positive and negative modes in KEGG pathways confirmed that, similar to the identified pathways, galactose metabolism was also significantly enriched. Compared with the control group, the DEMs of the pathways was shown in Table S2.
3.4. Transcriptome profiling
The gene expression profiles of indehiscent and dehiscent capsule were further analyzed, and high-quality bioinformatics analysis was conducted on the six constructed RNA libraries. The 45.99 Gb clean data was obtained, the average value of Q30 in each library was 92.26 %, and the average value of GC content was 46.04 % (Table S3). After quality control, the clean reads were compared to the reference genome's unique sequence, and the map matching rate was higher than 94 % (Table S4). 517 additional genes were discovered after the transcript was put together, further enhancing the genome data for this particular plant type. The 517 genes have 55 GO items, 31 KEGG pathway entries, and 12 GO and KEGG enrichment entries (Table S5A and S5B). It was worthwhile to look into whether it impacts capsule closure further.
PCA analysis of gene expression values (FPKM) of all samples showed that PC1 and PC2 effectively separated the test group from the control group in two directions (Fig. 3A). Based on the screening criteria of |log2 (FoldChange)| > 0, padj <0.05, 7559 differential genes were screened, 3902 of which were down regulated and 3657 of which were up regulated (Fig. 3B, Table S6). The pronounced differences in expression levels were visually represented in a volcano plot, which illustrated the distribution of differentially expressed genes, emphasizing the greater variability in expression as the scatter increases upward and laterally. Overall, these findings provide valuable insights into the gene expression dynamics that contribute to the distinct characteristics of indehiscent and dehiscent sesame capsules.
Fig. 3.
The difference of gene expression values (FPKM) in two sesame varieties. (A) PCA distribution about mature sesame capsules of ND and WZ2; (B) Volcano plots of DEGs about mature sesame capsules of ND and WZ2.
3.5. The cluster and analysis of DEGs
We conducted a kinetic analysis using the FPKM data from ND and WZ2 to gain insight into the dynamic nature of gene expression changes across the sesame capsule closing phase. This analysis identified a total of 7559 DEGs, which were classified into four distinct kinetic clusters of co-expressed genes. Each cluster exhibited a significant transcriptional burst necessary for the transition from an indehiscent to a dehiscent capsule (Fig. 4). The DEGs from cluster 1 and cluster 3 showed a negative response in ND, while the DEGs from cluster 2 and cluster 4 showed a positive response. In particular, cluster 1 encompassed 110 genes significantly down regulated in the indehiscent capsule, which can be used as a potential marker gene to distinguish two strains, Meanwhile, cluster 1 also includes the genes encoding the transcription factors AP2 and UDPGT (Table S7). Similarly, 3792 genes in Cluster 3 exhibited a decline in gene expression in closed capsules (Table S8). In contrast, 3656 genes in Cluster 2 were positively related with the capsule-closed trait. Additionally, cluster 2 contains 74 % Myb and 90 % Kinesin transcription gene family genes in all discovered differential transcription factors (Table S9). In addition to the AP2 and the UDPGT, these molecules may be important in regulating the level of expression of the genes involved in capsule closure. It is important to note that cluster 4 contains the gene with the highest up-regulated amplitude during capsule closure. GO enrichment revealed that this gene was involved in the cofactor binding, pyridoxal phosphate binding, vitamin B6 binding, and vitamin binding pathways but not in the KEGG pathway enrichment. This raises the question of whether this gene is crucial for capsule closure, warranting further investigation. Overall, the kinetic analysis provides valuable insights into the regulatory networks governing capsule dehiscence in sesame.
Fig. 4.
The co-expressed genes and their kinetic patterns clusters about mature sesame capsules of ND and WZ2.
3.6. Identification and enrichment of the DEGs
To further elucidate the biological functions of DEGs, GO and KEGG analysis were used to classify the functions of the DEGs between ND and WZ2. The enrichment of GO showed that DEGs were only significantly enriched on BP and MF, but not on CC, suggesting no significant association of capsule resistance with cellular components. There were many items in GO enrichment related to cell microtubule movement and phosphatase activity, in addition, items related to glucose metabolism were also significantly enriched (Fig. 5A).
Fig. 5.
The Identification and enrichment of DEGs in two sesame cultivars. (A) GO classification of DEGs about mature sesame capsules of ND and WZ2; (B) KEGG enrichment of DEGs about mature sesame capsules of ND and WZ2.
The KEGG analysis further identified that DEGs were significantly enriched in several critical metabolic pathways, including phenylpropanoid biosynthesis, flavonoid biosynthesis, tyrosine and tryptophan biosynthesis, phenylalanine metabolism, phenylalanine, galactose metabolism. This result was consistent with the enrichment of DEMs, implying that transcriptome studies confirm the changes in metabolic processes, the differential metabolic are closely controlled by the differential genes in their pathways. The identification of these DEGs provides valuable candidate genes that could be targeted for modifying lignin synthesis in plants or for enhancing molecular breeding efforts aimed at improving capsule traits. By focusing on genes involved in key biosynthetic pathways, researchers can explore strategies to optimize capsule characteristics, ultimately contributing to better seed retention and higher yields in sesame cultivation (Fig. 5B).
3.7. Molecular regulation mechanism
3.7.1. Enzyme activity of indehiscent capsule in lignin biosynthesis
This study focuses on the changes of lignin in indehiscent capsule, the results indicated that DEMs and DEGs were significantly enriched in the main pathway of lignin biosynthesis, especially in the pathway of phenylpropanoid biosynthesis (ko00940). In this study, a total of 34 differential genes were identified on this pathway, and 26 genes were up-regulated, indicating that the biological pathway of lignin synthesis has undergone tremendous changes at the transcriptional level during the process of sesame capsule indehiscent (Fig. 6A, Table S10). Compared with the dehiscent capsule, the activity of 11 enzymes in the indehiscent capsule variety was changed in the phenylpropanoid pathway of which 10 enzymes were enhanced, all or most of the enzyme regulation genes were enhanced. The phenylpropanoid pathway and the specific pathway, of which the phenylpropanoid pathway mostly consists of PAL, C4H, and 4CL, are the two components of lignin synthesis, as demonstrated by previous research (Cui et al., 2022). Their activities are closely related to the total amount of lignin synthesis. In this study, the activity about three enzymes of PAL, C4H and 4CL were all up-regulated. Furthermore, the 4CL was the key enzyme connecting the phenylpropanoid pathway and the lignin specific pathway, catalyzed by 4CL, the upstream pathway generates the corresponding acetyl-CoAs, which play as a substrate source for the specific pathway. Additionally, CCoAOMT, CAD and POD were involved in lignin specific pathways (Song et al., 2022), and the activities of CCoAOMT and POD were significantly enhanced, while the activities of CAD were slightly decreased (Fig. 6A, Table S10). Furthermore, we expanded our analysis to adjacent pathways related to lignin synthesis, noting significant changes in both genes and metabolites. In summary, the upregulation of key enzymes in the phenylpropanoid biosynthesis pathway highlights the dynamic nature of lignin synthesis in the indehiscent capsule.
Fig. 6.
The expression and qRT-PCR validation of Lignin Metabolic Pathway in two sesame varieties. (A) Expression of DEMs and DEGs in Lignin Metabolic Pathway about mature sesame capsules of ND and WZ2; (B) qRT-PCR validation of DEGs related to Lignin Metabolic in ND and WZ2 of mature sesame capsules (n = 3).
3.7.2. Combining DEMs to construct molecular regulation process
Pathways as for flavonoid biosynthesis, tyrosine and tryptophan biosynthesis, phenylalanine and galactose metabolism were overlapped with phenylpropanoid biosynthesis to varying degrees. In phenylalanine, tyrosine and tryptophan biosynthesis pathway, the substrate D-Erythrose 4-phosphate was promoted by KDPH to synthesize 3-Dehydroquinate (Fig. 6A). A series of reactions generate the phenylalanine and tyrosine, which then as substrates enters into the phenylpropanoid biosynthesis pathway (Fig. 6A). In Phenylalanine metabolism pathway, phenylalanine generated trans-Cinnamate to participate in the phenylpropanoid biosynthesis under the action of PAL (Fig. 6A). In flavonoid biosynthesis, cinnamoyl-CoA was up regulated by a series of enzymes (trans-cinnamate 4-monooxygenase, AT1 protein, 5-O-(4-coumaroyl)-D-quinate 3′-monooxygenase, caffeoyl-CoA O-methyltransferase) to generate Ferroyl-CoA, which was an important intermediate for lignin synthesis (Fig. 6A). Based on the above pathways, we combined the main differential metabolites and key regulatory genes to construct the lignin synthesis process.
It can be seen from the figure that the up regulation of genes LOC105158969 and LOC105176445 caused the accumulation of 3-Dehydroquinate, which generated phynylalanine and tyrosine through a series of enzymatic reactions and entered phynylpropanoid biosynthesis (Fig. 6A). Two up-regulated genes such as LOC105161378 and LOC105163854 controlled PAL and C4H at the early stage of the pathway, respectively. On the left side of the pathway, the substrate tran-Cinnamic acid, up regulated by genes LOC105160028, LOC105168898 and LOC105164008, eventually produced a large amount of Couraine, which has been described in previous studies to affect lignin formation (Hou et al., 2022). LOC105166823 and LOC105158263 controlled the activity of 4CL, which catalyzes the generation of corresponding acyl-CoA from p-coumaric acid, ferric acid, 5-hydroxyferric acid and sinapic acid, respectively. LOC105172712 and LOC105168408 controlled CCoAOMT, which promotes substrate caffeoyl-CoA, 5-hydroxyferuloyl-CoA to catalyze to feruloyl CoA and sinapoyl -CoA, respectively (Fig. 6A). CAD catalyzes p-coumaraldehyde, coniferyl aldehyde, 5-hydroxy coniferaldehyde and sinapaldehyde to generate corresponding alcohols, which are precursors of lignin synthesis. Under the control of six genes, the activity of CAD decreases, in which LOC105165982 and LOC105169503 were up-regulated, and LOC105156006, LOC105165970, LOC105179042 and LOC105175812 were down-regulated, leading to a decrease in the metabolites of S-type lignin synthesis precursors, but an increase in the amount of interchangeable synagin (Fig. 6A). Some carbohydrates contained in tissues can be converted into lignin by the action of POD, which increases the degree of tissue lignification. Moreover, POD can also serve as a physiological indicator of tissue aging (Yu et al., 2021). The activity of POD in the pathway was significantly increased, and the structural genes including LOC105174641, LOC105174327, LOC105163909, LOC105175601, LOC105176439, LOC105162056, LOC105175345, LOC105174640 were up regulated, and the genes including LOC105156219, LOC105169211, LOC105162172 were down regulated (Fig. 6A).
To validate the data, 12 differentially expressed genes was selected for qRT-PCR to test the high-throughput sequencing results (Fig. 6B). The primers and gene names of qPCR were listed in Table S2. The results of qRT-PCR showed that LOC105174640, LOC105172712, and LOC105158263, was higher relative expression in WZ2 than ND, which control the synthesis of lignin metabolites. Similarly, LOC105175658, LOC105174410, LOC105171765, LOC105166061, and LOC105158017 was higher relative expression in WZ2 than ND as well, which code the protein of ABC transporter and kinesin. Interestingly, LOC105175081, LOC105171122, LOC105157416, and LOC105155381 was lower relative expression in WZ2 than ND, and the function of these genes are ethylene-responsive transcription factor. The expression pattern of qPCR was consistent with that of RNA-Seq, and the consistency between qPCR and RNA-Seq expression suggesting that the RNA-Seq expression profile of this study were dependable (Fig. 6B).
4. Discussion
4.1. Lipid metabolism provides ATP for microtubule movement during capsule indehiscence
Research into sesame capsule development is critical for the improvement of this crop variety. Previous studies have indicated that during capsule senescence, mesocarp cells shrank more than endocarp cells creating tension in the drying capsule wall (Sorokin et al., 2024; Yanasan et al., 2024). The tension forced capsules open along a zone of weakness between locules, the capsule dehiscence was influenced by the parenchyma cell layers in mesocarp (Ferreira et al., 2023). GO enrichment showed that DEGs were largely enriched in microtubule movement related items and phosphatase activity related items including GTPase activity. Furthermore, the analysis of metabolites revealed that lipids and lipid-like molecules were the most abundant components. DEMs and DEGs were significantly enriched in fatty acid precipitation, glycerophospholipid metabolism, phosphatidylinositol signaling system, etc. Moreover, previous studies have found that the transmembrane transport of coniferin in the xylem meristem was very dependent on ATP, the transport of lignin precursors across plasmalemma and their sequestration into vacuoles are ATP-dependent primary-transport processes, involving ATP-binding cassette-like transporters (Shimada et al., 2021; Kern-Cardoso et al., 2022). Based on the above results, it is reasonable to speculate that lipid metabolites may provide energy for transmembrane movement through the energy cycle of tricarboxylic acid or participate in the form of the second messenger cAMP, cGMP, IP, DAG in cell signal transduction to control cell movement and arrangement, thus significantly affecting capsule opening/closing (Wang et al., 2023). Moreover, GTPases are known to regulate the reorganization of myofibrils and the dynamics of microtubules, serving as molecular switches in cell signaling transduction (Basu et al., 2022). This regulation could be crucial for coordinating the structural changes necessary for capsule dehiscence.
4.2. Sugar metabolism and glycosylation modification associated with capsule indehiscence
In this study, the enrichment of sugar related items and pathways also led us to speculate that carbohydrate metabolites may play a crucial role in the physiological processes underlying capsule cracking by regulating cell osmotic pressure to cause cell wall contraction or affect signal transduction (Oliveira et al., 2024; Shi et al., 2022). So far, studies on the sesame indehiscence capsule about internal connection between hormone signaling and lignin biosynthesis are scarce. Our focus on phenylpropanoid secondary metabolites emphasizes their impact on capsule dehiscence and indehiscence. Their synthesis, localization and biochemical activity were not only regulated by the transcriptional level, but also the glycosylation of compound structure was an important regulatory mechanism (Xie et al., 2022). Glycosyltransferases (UGTS) use UDP glucose as sugar donor to modify lignin monomer metabolites (Pei et al., 2022). GO showed that DEGs were largely enriched on the sugar-associated terms, the processes of polysaccharide metabolic, glucan metabolic, glucan metabolic and cellular polysaccharide metabolic were enriched in BP category. And in MF category, cellulose synthase activity, cellulose synthase (UDP-forming) activity, cellulose synthase (UDP-forming) activity, glucosyltransferase activity, glucosyltransferase activity was enriched. Further, several studies found that most of the DEGs enriched on the above terms were down-regulated in the indehiscent capsules. KEGG showed that DEMs and DEGs were enriched in the galactose metabolism pathway, on which several enzymes including aldose 1-epimerase were changed and the metabolite galactitol, as a synthetic precursor of the raffinose series oligosaccharide RFO (soluble sugar raffinose, salicylose, and Trichinella sugar) decreased in indehiscent capsules. Previous studies have suggested that galactitol serves as a major translocated sugar in the vascular bundles of cucumber, facilitating transport and accumulation in the collateral and stalk vascular bundles (Hao et al., 2016; Kalaivani et al., 2021; Zhang et al., 2023).
4.3. Analysis of the transcriptional factors associated with capsule indehiscence
In this study, we identified several genes related to lignin synthesis within the enriched pathways, highlighting the significant role of TFs in the resistance to dehiscence in sesame capsules. Analysis implied that some regulatory genes, including, LOC105174640, LOC105172712, and LOC105158263, exhibited differential expression between WZ2 and ND, as validated by qRT-PCR. The TFs affect the opening or closure of the capsule by regulating the gene expression involved in lignin biosynthesis and related pathways (Yuan et al., 2024). DEGs were introduced into Pfam database using InterProScan software to make out TFs, the result turned out that the key enzymes of lignin synthesis-glu, 4CL, CCR and CAD were regulated by Glyco_hydro_1/3, amp-binding enzyme, isomerase, ADH_N/FAD_bing_4 transcription factor family, respectively. The 7559 DEGs contained a large number of transcription factors distributed in more than 400 families. We focused on the analysis of families associated with lignin synthesis, including HLH, UDPGT, AP2, Kinesin, ABC_membrane/ABC2_membrane/ABC_tran, MYB DNA-binding et al. UDPGT affects the synthesis of lignin monomer by glycosyating phenylpropane metabolites through the regulation of glucose metabolism (Almeida et al., 2021). The MYB transcription factor family is also well-documented for its involvement in phenylpropanoid synthesis, with multiple MYB factors linked to aberrant lignification processes (Wu et al., 2023; Zhao et al., 2022).
It has been reported that the AP2/ERF transcription factors family is involved in the control of primary and secondary metabolism, conservatively widespread in the plant kingdom, and it has been reported that the more intense expression of AP2 in indehiscent capsules is a potential gene worth further investigation (Tamayo García et al., 2022). The kinetic analysis's findings demonstrated that the AP2 coding genes displayed a negative response. Meanwhile, LOC105175081, LOC105171122, LOC105157416, and LOC105155381, which coding for ERF, displayed different relative expressions between WZ2 and ND, suggesting that ethylene may be involved in the regulation of capsule indehiscent.
A large number of TFs derived from the Kinesin and ABC transporter family were significantly differentially expressed, the GO enrichment showed the DEGs enriched on the terms related to cell microtubule movement or ATP were almost all derived from the Kinesin transcription factor family and ABC transporter family, respectively. Similarly, LOC105175658, coding for the ABC transporter B family member 9-like, and LOC105166061, LOC105174410, LOC105171765, and LOC105158017, coding for the Kinesin-like protein, had higher relative expression in WZ2 than ND as well. And almost all of these DEGs were all up regulated in indehiscent capsules, indicating that these TFs mainly responded to the dehiscence-resistance through positive feedback regulation (Chen et al., 2024). The enrichment of these transcription factors supports a conventional cell biological mechanism: motor proteins interact with cell microtubules to convert the chemical energy of ATP into kinetic energy, facilitating microtubule movement. Concurrently, lignin precursor molecules are transported by ABC proteins across the inner and outer cell membranes, utilizing ATP for energy (Beckett & Voth, 2023; Kankaanpää et al., 2024).
5. Conclusion
In this study, we conducted a joint analysis of the metabolome and transcriptome of mature sesame pods with contrasting dehiscence traits: the dehiscent pod variety WZ2 and the indehiscent pod variety ND to explore the metabolites and molecular regulation mechanism of capsule -closed traits. The correlation analysis between DEMs and DEGs revealed that both groups were jointly enriched in phenylpropane biosynthesis, flavonoid biosynthesis, phenylalanine, tyrosine and tryptophan biosynthesis, phenylalanine metabolism, and galactose metabolism in KEGG. Notably, the phenylpropane biosynthesis pathway, which is essential for lignin synthesis, ranked relatively high in the KEGG enrichment analysis of both DEMs and DEGs. Combined with the related pathway to reconstruct the lignin synthesis pathway, a total of 4 DEMs were detected in this pathway, namely 3-dehydroquinate, coumarine, syringin and sinapyl alcohol. The first two metabolites were found to be elevated in closed capsules, whereas sinapyl alcohol, a precursor for S-lignin synthesis, showed decreased levels in the indehiscent capsules, with an increase in syringin content noted instead. Further gene mining focused on enzymes with obvious changes in pathway activity led to the identification of 28 differentially expressed structural and regulatory genes. At the same time, GO enrichment analysis showed that DEGs were enriched in multiple pathways related to cellular microtubule movement, as well as lipid and glucose metabolism. Multiple TFs families were mined in the enrichment pathway, including HLH, UDPGT, AP2, Kinesin, ABC_membrane/ABC2_membrane/ABC_tran, MYB DNA-binding, which may affect or participate in the regulation of structural genes or regulatory genes in lignification, glycosylation, cell microtubule movement, energy supply and other biosynthesis. While the regulatory mechanisms identified warrant further investigation and validation, these findings provide valuable insights for gene mining related to lignin synthesis and the mechanisms of capsule closure in sesame.
CRediT authorship contribution statement
Yinping Zhang: Writing – original draft, Software, Resources, Methodology, Investigation, Funding acquisition. Ruirui Chen: Resources, Methodology, Investigation. Yujun Liu: Validation, Software, Resources. Shuwen Xu: Validation, Software, Resources. Shuguang Gao: Writing – review & editing, Validation. Haiyang Zhang: Supervision, Software, Resources. Hongmei Miao: Supervision, Resources. Lingling Qin: Writing – review & editing, Validation. Xiangyu Zhou: Resources, Methodology. Kiran Thakur: Writing – review & editing, Validation. Cheng Li: Writing – review & editing, Validation. Juan Li: Visualization, Supervision, Project administration. Pengcheng Wei: Validation, Supervision, Project administration. Zhao-Jun Wei: Writing – review & editing, Visualization, Validation, Supervision, Project administration, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by the Sesame Mechanization Positions in the National Characteristic Oil Industry Technology System (CARS-14-1-26); The Special Fund for Anhui Agriculture Research System (2024); Oil Industry Technology System of Anhui Province (2023); The Special Capacity Building Project of Anhui Province (202106b03020004, 202206b03020003); The Anhui Province Traditional Chinese Medicine Resource Protection and Sustainable Utilization Engineering Laboratory (TCMRPSU-2022-09); Postdoctoral Foundation of Henan Academy of Agricultural Sciences (2024).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochms.2024.100231.
Contributor Information
Haiyang Zhang, Email: zhy@hnagri.org.cn.
Hongmei Miao, Email: miaohongmeichina@yahoo.com.
Kiran Thakur, Email: kumarikiran@hfut.edu.cn.
Zhao-Jun Wei, Email: zjwei@hfut.edu.cn.
Appendix A. Supplementary data
Supplementary material: Table S1A DEMs in negative mode about mature sesame capsules of ND and WZ2. Table S2B DEMs in positive mode about mature sesame capsules between ND and WZ2. Table S2 Differential metabolites of pathways related to lignin synthesis. Table S3 The results of high-quality bioinformatics analysis. Table S4 The map matching rate of reference genome's unique sequence. Table S5A The GO enrichment entries. Table S5B The KEGG enrichment entries. Table S6 Some transcription factor families listed in this paper. Table S7 DEGs in cluster 1. Table S8 DEGs in cluster 3. Table S9 DEGs in cluster 2. Table S10 Expression of DEGs in lignin metabolic pathway. Table S11 the main phytochemical strucrures of sesame and their biological activities.
Data availability
No data was used for the research described in the article.
References
- Almeida G.H.G.D., Siqueira-Soares R.D.C., Mota T.R., Oliveira D.M.D., Abrahão J., Foletto-Felipe M.D.P.…Marchiosi R. Aluminum oxide nanoparticles affect the cell wall structure and lignin composition slightly altering the soybean growth. Plant Physiology and Biochemistry. 2021;159:335–346. doi: 10.1016/j.plaphy.2020.12.028. [DOI] [PubMed] [Google Scholar]
- Bai Y., Liu H., Pan J., Zhang S., Guo Y., Xian Y.…Zhang Z. Transcriptomics and metabolomics changes triggered by inflorescence removal in Panax notoginseng (Burk.) Frontiers in Plant Science. 2021;12 doi: 10.3389/fpls.2021.761821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu D., Majumdar S., Mandal N., Dastidar S.G. Mechanisms of influence of the microtubule over-stabilizing ligands on the structure and intrinsic dynamics of α,β-Tubulin. Computational Biology and Chemistry. 2022;96 doi: 10.1016/j.compbiolchem.2021.107617. [DOI] [PubMed] [Google Scholar]
- Beckett D., Voth G.A. Unveiling the catalytic mechanism of GTP hydrolysis in microtubules. Proceedings of the National Academy of Sciences. 2023;120(27) doi: 10.1073/pnas.2305899120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellamuthu M., Subramanian S., Eswaran K. Enhancement of sesame omega-3 fatty acid content using fusarium moniliforme bifunctional desaturase gene. South African Journal of Botany. 2022;150:285–295. doi: 10.1016/j.sajb.2022.07.031. [DOI] [Google Scholar]
- Chen X., Tang S., Gao X., Niu F., Yang X., Song X., Zhang L. Characterization and validation of TaAGL66, a gene related to fertility conversion of wheat in the presence of Aegilops kotschyi cytoplasm. Planta. 2024;260(1):6. doi: 10.1007/s00425-024-04416-z. [DOI] [PubMed] [Google Scholar]
- Cui W., Zhuang Z., Jiang P., Pan J., Zhao G., Xu S., Shen W. Characterization, expression profiling, and biochemical analyses of the Cinnamoyl-CoA reductase gene family for lignin synthesis in alfalfa plants. International Journal of Molecular Sciences. 2022;23(14):7762. doi: 10.3390/ijms23147762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossou S.S.K., Xu F., Cui X., Sheng C., Zhou R., You J.…Wang L. Comparative metabolomics analysis of different sesame (Sesamum indicum L.) tissues reveals a tissue-specific accumulation of metabolites. BMC Plant Biology. 2021;21(1):352. doi: 10.1186/s12870-021-03132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira P.S., Freitas S.P., Almeida E.L. Scientific and technological advancements in the utilisation of by-products from babassu oil extraction: A bibliometric review. International Journal of Food Science & Technology. 2023;58(10):4980–4991. doi: 10.1111/ijfs.16650. [DOI] [Google Scholar]
- Hao J., Gu F., Zhu J., Lu S., Liu Y., Li Y.…Xian C.J. Low night temperature affects the phloem ultrastructure of lateral branches and Raffinose family oligosaccharide (RFO) accumulation in RFO-transporting plant melon (Cucumismelo L.) during fruit expansion. PLoS One. 2016;11(8) doi: 10.1371/journal.pone.0160909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou D., Lu H., Zhao Z., Pei J., Yang H., Wu A.…Lin X. Integrative transcriptomic and metabolomic data provide insights into gene networks associated with lignification in postharvest lei bamboo shoots under low temperature. Food Chemistry. 2022;368 doi: 10.1016/j.foodchem.2021.130822. [DOI] [PubMed] [Google Scholar]
- Huang D., Shang X., Wang Y., Xiao L., Ming R., Zeng W.…Yan H. Identification of nutritional ingredients and medicinal components of Pueraria lobata and its varieties using uplc-ms/ms-based metabolomics. Molecules. 2021;26(21) doi: 10.3390/molecules26216587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaivani V., Nikarika R., Shoma N., Arunraj R. Delayed hydrolysis of Raffinose family oligosaccharides (RFO) affects critical germination of chickpeas. 3 Biotech. 2021;11(6):298. doi: 10.1007/s13205-021-02764-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankaanpää S., Väisänen E., Goeminne G., Soliymani R., Desmet S., Samoylenko A., Vainio S., Wingsle G., Boerjan W., Vanholme R., Kärkönen A. Extracellular vesicles of Norway spruce contain precursors and enzymes for lignin formation and salicylic acid. Plant Physiology. 2024;196(2):788–809. doi: 10.1093/plphys/kiae287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern-Cardoso K.A., Mito M.S., Sánchez-Moreiras A.M., Reigosa M.J., Ishii-Iwamoto E.L. Cellular and ultrastructural alterations of Arabidopsis thaliana roots in response to exogenous trans-aconitic acid. Acta Physiologiae Plantarum. 2022;44(12):129. doi: 10.1007/s11738-022-03464-w. [DOI] [Google Scholar]
- Liu L., Zhang K., Bai J., Lu J., Lu X., Hu J., Pan C., He S., Yuan J., Zhang Y., Zhang M., Guo Y., Wang X., Huang Z., Du Y., Cheng F., Li J. All-flesh fruit in tomato is controlled by reduced expression dosage of AFF through a structural variant mutation in the promoter. Journal of Experimental Botany. 2022;73(1):123–138. doi: 10.1093/jxb/erab401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder S., Zhang N. Cellulose–hemicellulose–lignin interaction in the secondary Cell Wall of coconut endocarp. Biomimetics. 2023;8(2):188. doi: 10.3390/biomimetics8020188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S., Wang Y., Zhang X., Sang Y., Wang X. Discrimination of black and white sesame seeds based on targeted and non-targeted platforms with Chemometrics: From profiling towards identification of chemical markers. Foods. 2022;11(14):2042. doi: 10.3390/foods11142042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira M.R.B., Douradinho R.S., Sica P., Mota L.A., Pinto A.U., Faria T.M., Baptista A.S. Silica wort supplementation as an alternative for yeast stress relief on corn ethanol production with cell recycling. Stresses. 2024;4(3):421–435. doi: 10.3390/stresses4030028. [DOI] [Google Scholar]
- Parker T.A., Berny Mier Y., Teran J.C., Palkovic A., Jernstedt J., Gepts P. Pod indehiscence is a domestication and aridity resilience trait in common bean. New Phytologist. 2020;225(1):558–570. doi: 10.1111/nph.16164. [DOI] [PubMed] [Google Scholar]
- Pei T., Yan M., Li T., Li X., Yin Y., Cui M.…Zhao Q. Characterization of UDP-glycosyltransferase family members reveals how major flavonoid glycoside accumulates in the roots of Scutellaria baicalensis. BMC Genomics. 2022;23(1):169. doi: 10.1186/s12864-022-08391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi M., Langham D.R., Lucas S.J., Uzun B., Yol E. Breeding history for shattering trait in sesame: Classic to genomic approach. Molecular Biology Reports. 2022;49(7):7185–7194. doi: 10.1007/s11033-022-07636-2. [DOI] [PubMed] [Google Scholar]
- Shi L., Liu Q., Qiao Q., Zhu Y., Huang W., Wang X., Ren Z. Exploring the effects of pectate and pectate lyase on the fruit softening and transcription profiling of Solanum lycopersicum. Food Control. 2022;133 doi: 10.1016/j.foodcont.2021.108636. [DOI] [Google Scholar]
- Shimada N., Munekata N., Tsuyama T., Matsushita Y., Fukushima K., Kijidani Y.…Kamei I. Active transport of lignin precursors into membrane vesicles from lignifying tissues of bamboo. Plants. 2021;10(11):2237. doi: 10.3390/plants10112237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S.K., Saulnier B.K., Hodge D.B. Lignin properties and cell wall response to deconstruction by alkaline pretreatment and enzymatic hydrolysis in brown midrib sorghums. Industrial Crops and Products. 2022;178 doi: 10.1016/j.indcrop.2022.114566. [DOI] [Google Scholar]
- Singla D., Sangha M.K., Singh M., Pathak M., Bala M. Variation of mineral composition in different fruit parts of bitter gourd (Momordica charantia L.) Biological Trace Element Research. 2023;201(10):4961–4971. doi: 10.1007/s12011-022-03546-3. [DOI] [PubMed] [Google Scholar]
- Song J.L., Wang Z.Y., Wang Y.H., Du J., Wang C.Y., Zhang X.Q.…Zhong T.X. Overexpression of Pennisetum purpureum CCoAOMT contributes to lignin deposition and drought tolerance by promoting the accumulation of flavonoids in transgenic tobacco. Frontiers in Plant Science. 2022;13 doi: 10.3389/fpls.2022.884456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin A.N., Yatsenko O.V., Bobrov A.V.F.C., Romanov M.S., Zdravchev N.S., Iovlev P.S.…Kuptsov K.V. The pericarp structure and histogenesis in Enkianthus: On the ancestral fruit type in Ericaceae family. Botanical Journal of the Linnean Society. 2024;204(1):76–85. doi: 10.1093/botlinnean/boad041. [DOI] [Google Scholar]
- Tamayo García R., Narváez Zapata J.A., Ku González A., Aguilar Espinosa M., Gutiérrez Pacheco L.C., Rivera-Madrid R. Gene expression analysis during the fruit development in dehiscent and indehiscent Bixa orellana L. accessions. Physiology and Molecular Biology of Plants. 2022;28(4):709–718. doi: 10.1007/s12298-022-01180-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teboul N., Magder A., Zilberberg M., Peleg Z. Elucidating the pleiotropic effects of sesame KANADI1 locus on leaf and capsule development. The Plant Journal. 2022;110(1):88–102. doi: 10.1111/tpj.15655. [DOI] [PubMed] [Google Scholar]
- Wang L., Wang G., Cui J., Wang X., Li M., Qi X.…Ma L. Transcriptomics, metabolomics, antioxidant enzymes activities and respiration rate analysis reveal the molecular responses of rice to Cd stress and/or elevated CO2 concentration. Plant and Soil. 2023;485(1–2):259–280. doi: 10.1007/s11104-022-05827-1. [DOI] [Google Scholar]
- Wu Z., Zeng W., Li C., Wang J., Shang X., Xiao L.…Yan H. Genome-wide identification and expression pattern analysis of R2R3-MYB transcription factor gene family involved in puerarin biosynthesis and response to hormone in Pueraria lobata var. thomsonii. BMC Plant Biology. 2023;23(1):107. doi: 10.1186/s12870-023-04115-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie D., Yang X., He R., Huo H., Ye Z., Ren X.…Su J. Comprehensive analysis of the UDP-glycosyltransferase gene family in flax [Linum usitatissimum L.] and functional verification of the role of LuUGT175 in the regulation of lignin biosynthesis. Industrial Crops and Products. 2022;188 doi: 10.1016/j.indcrop.2022.115720. [DOI] [Google Scholar]
- Yanasan N., Wangkananon W., Natakankitkul S., Kiattisin K. Nanoemulsions containing Passiflora quadrangularis L. fruit extracts for cosmetic application and skin efficacy study. Cosmetics. 2024;11(2):57. doi: 10.3390/cosmetics11020057. [DOI] [Google Scholar]
- Yong B., Zhu W., Wei S., Li B., Wang Y., Xu N., Lu J., Chen Q., He C. Parallel selection of loss-of-function alleles of Pdh1 orthologous genes in warm-season legumes for pod indehiscence and plasticity is related to precipitation. New Phytologist. 2023;240(2):863–879. doi: 10.1111/nph.19150. [DOI] [PubMed] [Google Scholar]
- Yu L., Pei J., Zhao Y., Wang S. Physiological changes of bamboo (Fargesia yunnanensis) shoots during storage and the related cold storage mechanisms. Frontiers in Plant Science. 2021;12 doi: 10.3389/fpls.2021.731977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L., Dang J., Zhang J., Wang L., Zheng H., Li G., Li J., Zhou F., Khan A., Zhang Z., Hu X. A glutathione S-transferase regulates lignin biosynthesis and enhances salt tolerance in tomato. Plant Physiology. 2024;kiae504 doi: 10.1093/plphys/kiae504. [DOI] [PubMed] [Google Scholar]
- Zhang C., Yue N., Li X., Shao H., Wang J., An L., Jin F. Potential translocation process and effects of polystyrene microplastics on strawberry seedlings. Journal of Hazardous Materials. 2023;449 doi: 10.1016/j.jhazmat.2023.131019. [DOI] [PubMed] [Google Scholar]
- Zhang H., Miao H., Wei L., Li C., Duan Y., Xu F.…Chang S. Identification of a SiCL1 gene controlling leaf curling and capsule indehiscence in sesame via cross-population association mapping and genomic variants screening. BMC Plant Biology. 2018;18(1):296. doi: 10.1186/s12870-018-1503-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Wang L., Liu Z., Zhao Z., Zhao J., Wang Z.…Liu M. Transcriptome and metabolome profiling unveil the mechanisms of Ziziphus jujuba mill. Peel coloration. Food Chemistry. 2020;312 doi: 10.1016/j.foodchem.2019.125903. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhang Y., Zhou X., Yang N., Zhou D., Thakur K.…Wei Z. Unraveling the amino acid synthesis in maturity sesame seeds based on the integrative analysis of transcriptome and metabolome. Food Frontiers. 2024 doi: 10.1002/fft2.509. [DOI] [Google Scholar]
- Zhang Y.P., Zhang Y.Y., Thakur K., Zhang F., Hu F., Zhang J.G.…Wei Z.-J. Integration of miRNAs, Degradome, and transcriptome omics uncovers a complex regulatory network and provides insights into lipid and fatty acid synthesis during sesame seed development. Frontiers in Plant Science. 2021;12 doi: 10.3389/fpls.2021.709197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Niu Y., Bai X., Mao T. Transcriptomic and metabolic profiling reveals a lignin metabolism network involved in Mesocotyl elongation during maize seed germination. Plants. 2022;11(8):1034. doi: 10.3390/plants11081034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Song S., Segla Koffi Dossou S., Zhou R., Wei X., Wang Z.…Wang L. Genome-wide association analysis and transcriptome reveal novel loci and a candidate regulatory gene of fatty acid biosynthesis in sesame (Sesamum indicum L.) Plant Physiology and Biochemistry. 2022;186:220–231. doi: 10.1016/j.plaphy.2022.07.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material: Table S1A DEMs in negative mode about mature sesame capsules of ND and WZ2. Table S2B DEMs in positive mode about mature sesame capsules between ND and WZ2. Table S2 Differential metabolites of pathways related to lignin synthesis. Table S3 The results of high-quality bioinformatics analysis. Table S4 The map matching rate of reference genome's unique sequence. Table S5A The GO enrichment entries. Table S5B The KEGG enrichment entries. Table S6 Some transcription factor families listed in this paper. Table S7 DEGs in cluster 1. Table S8 DEGs in cluster 3. Table S9 DEGs in cluster 2. Table S10 Expression of DEGs in lignin metabolic pathway. Table S11 the main phytochemical strucrures of sesame and their biological activities.
Data Availability Statement
No data was used for the research described in the article.