Abstract
Background
Inflammatory markers have been confirmed to be associated with the prognosis of cancer patients. In this study, we compared the impacts of intravenous anesthesia and inhalation anesthesia on the neutrophil-to-lymphocyte ratio (NLR), the platelet-to-lymphocyte ratio (PLR), and the systemic immune-inflammation index (SII) after esophageal cancer surgery.
Methods
We retrospectively reviewed the electronic medical records of patients who underwent esophagectomy from January 1, 2014 to December 31, 2016. Patients respectively received total intravenous anesthesia (TIVA) or inhalational anesthesia (INHA). Inverse probability of treatment weighting (IPTW) was employed to minimize differences. The Mann-Whitney U test or Kruskal Wallis test was utilized to compare the effect of the two groups on postoperative NLR, PLR and SII.
Results
A total of 519 patients who had undergone esophageal cancer resection were recruited in this study, among whom 339 patients were eligible (TIVA group, n = 201, INHA group, n = 138). After IPTW, there was no statistically significant difference in NLR, PLR, and SII on the first postoperative day(P = 0.1951), (P = 0.5611), (P = 0.9684) and on the third postoperative day(P = 0.5961), (P = 0.1804), (P = 0.9653) between the two groups.
Conclusions
In conclusion, there was no significant difference in NLR, PLR and SII between intravenous anesthesia or inhalational anesthesia. TIVA is not superior to INHA in reducing the perioperative inflammatory response of esophageal cancer.
Synopsis
Inflammatory markers play an important role in the recurrence, metastasis and survival of tumor patients after surgery. In this study, we will compare the effects of different anesthesia methods on inflammatory markers.
Keywords: TIVA, INHA, IPTW, Esophageal cancer, NLR, PLR, SII
Background
Esophageal cancer (EC) is one of the most common digestive system tumors, ranking seventh in incidence worldwide and sixth in terms of mortality [1]. China is one of the regions with high incidence of esophageal cancer in the world. Esophageal squamous cell carcinoma and adenocarcinoma are the main types of the disease [2]. Despite the constant changes in the treatment of esophageal cancer, surgery remains the optimal treatment option for esophageal cancer [3]. Stress and inflammatory responses during surgical procedures contribute to the recurrence and metastasis of tumors in patients [4]. Perioperative factors (such as the method of anesthesia, anesthetic drugs, and blood transfusions) may exacerbate or mitigate this effect [5, 6]. Among them, the influence of anesthesia methods and drugs on the progression of tumor patients, as well as postoperative recurrence and metastasis has been a hot topic [7]. Compared to inhalation anesthetics such as sevoflurane, propofol may lead to a better prognosis for oncology patients because of its anti-tumor and anti-inflammatory effects [8, 9].
Inflammation plays a crucial role in cancer patients, not only facilitating immune responses but also eliciting immune suppression. [10] Some inflammatory markers can monitor the systemic inflammatory response and the dynamic balance of immunity in tumor patients, such as the neutrophil-to-lymphocyte ratio (NLR), the platelet-to-lymphocyte ratio (PLR), and the systemic immunoinflammatory index (SII). Currently, NLR, PLR and SII are widely used to evaluate the prognosis of patients with tumors [11, 12]. Nevertheless, few studies have shown the impact of different anesthesia methods on perioperative inflammatory markers. In our study, we hypothesized that propofol-based total intravenous anesthesia has a beneficial effect on the inflammatory markers of esophageal cancer, and we compared the common inflammatory markers NLR, PLR, SII.
Methods
Inclusion and exclusion criteria for patients
Patients who underwent esophageal cancer resection at Harbin Medical University Cancer Hospital between January 1, 2014 and December 31, 2016 were collected for this study. The exclusion criteria were as follows: (1) Patients with esophageal tumors other than squamous and adenocarcinomas. (2) Emergency surgery. (3) Patients with preoperative metastases. (4) Patients with non-two anesthesia methods. (5) Patients with incomplete clinical data. We obtained the medical records of all included patients and data were extracted by researchers who were not involved in the study or data analysis.
Anesthesia technique and grouping method
According to the distinct anesthesia techniques, they were divided into total intravenous anesthesia group (TIVA) and inhalational anesthesia group (INHA). In both groups, patients underwent anesthesia induction with midazolam 0.05 ~ 0.15 mg/kg, 0.5 ug/kg fentanyl, 0.15 ~ 0.2 mg/kg cisatracurium and 1 ~ 2.5 mg/kg propofol. In the TIVA group, anesthesia was maintained with propofol and remifentanil [13]. In the INHA group, anesthesia was maintained with sevoflurane and remifentanil. The bispectral index (BIS) was maintained within the range of 40 to 60 for both anesthesia methods. During the anesthesia regimen, the mean systemic blood pressure was maintained within 20% of baseline or above 60 mmHg by using appropriate doses of vasopressors or inotropics. Patients received patient-controlled intravenous analgesia (PCIA) at dosages of 3 µg/mL of fentanyl or 0.5 µg/mL of sufentanil for 72 to 120 h after surgery.
Indicator and data
This study focused on comparing the influences of two anesthetic methods on the changes in NLR, PLR and SII on the first and third postoperative days. We gathered the following perioperative details: demographic data, concurrent disease, adjuvant therapy (radiotherapy/chemotherapy), preoperative hemoglobin (HB), American Society of Anesthesiologists (ASA) grade, duration of anesthesia, the length of the operation, surgical type, degree of differentiation, pathological classification, tumor location, cancer staging, preoperative and postoperative NLR, PLR, and SII. The criteria for cancer staging are based on the 8th edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual. Neutrophils, lymphocytes and platelets were collected within 3 days before surgery, the first day and the third day after surgery. NLR was defined as: NLR = neutrophil count/lymphocyte count. PLR was defined as: PLR = platelet count/lymphocyte count. SII was defined as: (platelet count × neutrophil count)/lymphocyte count.
Statistical analysis
The cases with unqualified data were excluded from the final analysis, and the cases that met the requirements of this study were statistically analyzed. Associations between categorical variables were assessed using the Fisher exact test or χ2 test. Continuous variables between patient groups were compared by T-tests or Manne Whitney U tests. Categorical data were represented by n (%), and analyzed by χ2 test, continuous data was expressed as the mean (standard deviation, SD) or median [interquartile range], and two independent samples were analyzed by T-tests. Inverse probability of treatment weighting (IPTW) was utilized to reduce the effect of confounding factors between the two groups [14]. The weighted model included the following variables: sex, age, BMI, smoke, drink, hypertension, diabetes, cardiovascular disease, cerebrovascular disease, ASA, adjuvant treatment, duration of anesthesia, duration of anesthesia, the length of the operation, blood transfusion, surgical type, pathological classification, degree of differentiation, tumor location, TNM, preoperative HB, preoperative NLR, preoperative PLR, preoperative SII. The balance of covariates between the TIVA and INHA groups was assessed by the standardized mean difference (SMD). An SMD < 0.1 indicated a good balance in the covariates between the two groups. All the analyses were performed by the R software (version 4.1.2). We utilized the package “survey” for inverse probability of treatment weighting. The package “ggprism”, “reshape2”, “tableone” were employed for graphing and p-value < 0.05 was regarded statistically significant.
Results
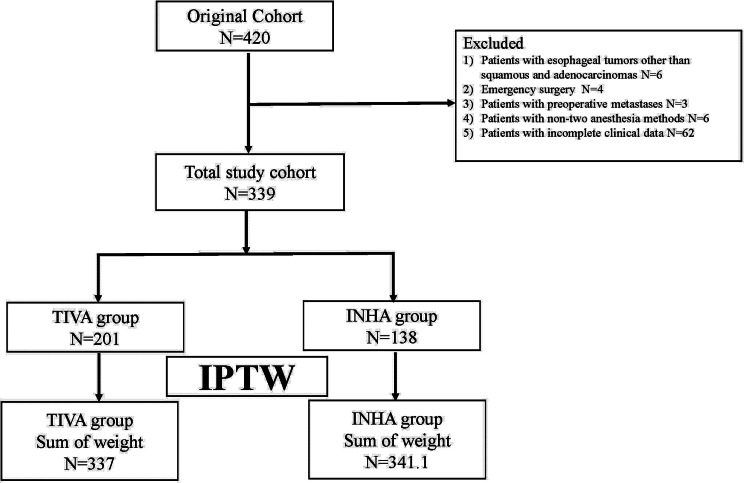
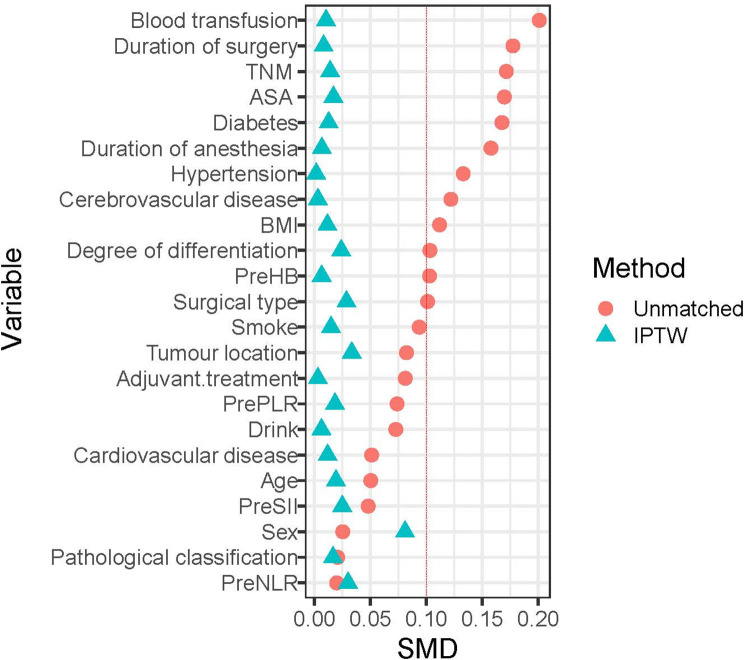
The clinical data of 519 patients with esophageal cancer who underwent radical resection were retrospectively examined. After the application of the inclusion criteria were applied, 201 patients assigned to the TIVA group and 138 patients to the INHA group. After IPTW, the sum of weights was 337 in the TIVA group, and 341.1 in the INHA group (Fig. 1). All standardized mean differences (SMD)for the study variables were less than 0.1 (Table 1) (Fig. 2).
Fig. 1.
Patient identification and exclusion. Abbreviations: IPTW, Inverse probability of treatment weighting
Table 1.
Patient characteristics for before IPTW adjustment and after IPTW adjustment
| Variable | Before IPTW adjustment | After IPTW adjustment | |||||
|---|---|---|---|---|---|---|---|
| TIVA | INHA | P | TIVA | INHA | P | SMD | |
| Sum of weight | Sum of weight | ||||||
| n = 201 | n = 138 | n = 337 | n = 341.1 | ||||
| Sex (%) | |||||||
| Female | 5 (2.5) | 4 (2.9) | 1 | 7.0 (2.1) | 11.8 (3.4) | 0.451 | 0.083 |
| Male | 196 (97.5) | 134 (97.1) | 330.0 (97.9) | 329.3 (96.6) | |||
| Age | 59 | 58 | 0.525 | 58.93 | 58 | ||
| (median [IQR], year) | [53.00, 63.00] | [53.00, 62.00] | [53.00, 63.00] | [53.00, 63.00] | 0.992 | 0.02 | |
| BMI | 22.09 | 22.49 | 0.472 | 22.15 | 22.1 | ||
| (median [IQR], kg/m2) | [20.08, 24.82] | [20.29, 24.24] | [20.08, 25.09] | [20.28, 24.18] | 0.641 | 0.012 | |
| Smoke (%) | |||||||
| No | 79 (39.3) | 48 (34.8) | 0.465 | 127.0 (37.7) | 126.6 (37.1) | 0.92 | 0.012 |
| Yes | 122 (60.7) | 90 (65.2) | 210.0 (62.3) | 214.5 (62.9) | |||
| Drink (%) | |||||||
| No | 68 (33.8) | 42 (30.4) | 0.591 | 109.2 (32.4) | 112.1 (32.9) | 0.934 | 0.01 |
| Yes | 133 (66.2) | 96 (69.6) | 227.8 (67.6) | 229.0 (67.1) | |||
| Hypertension (%) | |||||||
| No | 173 (86.1) | 112 (81.2) | 0.288 | 283.5 (84.1) | 287.6 (84.3) | 0.959 | 0.006 |
| Yes | 28 (13.9) | 26 (18.8) | 53.5 (15.9) | 53.5 (15.7) | |||
| Diabetes (%) | |||||||
| No | 197 (98.0) | 131 (94.9) | 0.207 | 325.0 (96.4) | 329.5 (96.6) | 0.935 | 0.01 |
| Yes | 4 (2.0) | 7 (5.1) | 12.0 (3.6) | 11.6 (3.4) | |||
| Cardiovascular disease (%) | |||||||
| No | 195 (97.0) | 135 (97.8) | 0.91 | 327.8 (97.3) | 331.0 (97.0) | 0.915 | 0.013 |
| Yes | 6 (3.0) | 3 (2.2) | 9.2 (2.7) | 10.1 (3.0) | |||
| Cerebrovascular disease (%) | |||||||
| No | 191 (95.0) | 127 (92.0) | 0.371 | 316.6 (94.0) | 320.2 (93.9) | 0.979 | 0.003 |
| Yes | 10 (5.0) | 11 (8.0) | 20.4 (6.0) | 20.8 (6.1) | |||
| ASA (%) | |||||||
| I | 37 (18.4) | 19 (13.8) | 0.308 | 56.6 (16.8) | 55.6 (16.3) | 0.99 | 0.016 |
| II | 146 (72.6) | 101 (73.2) | 245.9 (73.0) | 249.5 (73.1) | |||
| III | 18 (9.0) | 18 (13.0) | 34.5 (10.2) | 36.0 (10.6) | |||
| Adjuvant treatment (%) | |||||||
| No | 113 (56.2) | 72 (52.2) | 0.533 | 184.3 (54.7) | 187.4 (55.0) | 0.963 | 0.005 |
| Yes | 88 (43.8) | 66 (47.8) | 152.7 (45.3) | 153.6 (45.0) | |||
| Duration of anesthesia | 6.08 | 6.29 | 6.17 | 6.17 | |||
| (median [IQR], h) | [5.00, 7.00] | [5.17, 7.40] | 0.265 | [5.00, 7.09] | [5.06, 7.25] | 0.876 | 0.006 |
| Duration of surgery | 5.17 | 5.25 | 5.17 | 5.13 | |||
| (median [IQR], h) | [4.00, 6.08] | [3.94, 6.40] | 0.214 | [4.08, 6.21] | [3.83, 6.13] | 0.816 | 0.007 |
| Blood transfusion (%) | |||||||
| No | 191 (95.0) | 136 (98.6) | 0.154 | 324.9 (96.4) | 329.5 (96.6) | 0.943 | 0.01 |
| Yes | 10 (5.0) | 2 (1.4) | 12.1 (3.6) | 11.6 (3.4) | |||
| Surgical type (%) | |||||||
| lvor Lewis | 79 (39.3) | 53 (38.4) | 0.823 | 129.3 (38.4) | 127.2 (37.3) | 0.994 | 0.03 |
| MMcKeown | 49 (24.4) | 35 (25.4) | 83.5 (24.8) | 85.3 (25.0) | |||
| Sweet | 72 (35.8) | 48 (34.8) | 122.1 (36.2) | 125.8 (36.9) | |||
| Thoracoa | 1 (0.5) | 2 (1.4) | 2.0 (0.6) | 2.7 (0.8) | |||
| Pathological classification (%) | |||||||
| adenocarcinoma | 5 (2.5) | 3 (2.2) | 1 | 7.1 (2.1) | 6.3 (1.9) | 0.859 | 0.019 |
| Squamous carcinoma | 196 (97.5) | 135 (97.8) | 329.8 (97.9) | 334.7 (98.1) | |||
| Degree of differentiation (%) | |||||||
| G1 | 41 (20.4) | 25 (18.1) | 0.651 | 64.4 (19.1) | 61.1 (17.9) | 0.963 | 0.031 |
| G2 | 93 (46.3) | 62 (44.9) | 156.4 (46.4) | 161.9 (47.5) | |||
| G3 | 67 (33.3) | 51 (37.0) | 116.2 (34.5) | 118.0 (34.6) | |||
| Tumour location (%) | |||||||
| Bottom | 90 (44.8) | 64 (46.4) | 0.756 | 151.2 (44.9) | 147.4 (43.2) | 0.957 | 0.034 |
| Middle | 97 (48.3) | 62 (44.9) | 158.5 (47.0) | 166.0 (48.7) | |||
| Top | 14 (7.0) | 12 (8.7) | 27.3 (8.1) | 27.6 (8.1) | |||
| TNM (%) | |||||||
| I | 44 (21.9) | 22 (15.9) | 0.308 | 65.3 (19.4) | 64.7 (19.0) | 0.99 | 0.016 |
| II | 69 (34.3) | 46 (33.3) | 114.2 (33.9) | 118.2 (34.6) | |||
| III | 88 (43.8) | 70 (50.7) | 157.4 (46.7) | 158.1 (46.4) | |||
| Pre.HB | 145 | 145.45 | 0.439 | 146.03 | 145.04 | ||
| (median [IQR]) | [135.20, 153.70] | [137.12, 154.95] | [136.24, 154.00] | [137.00, 154.65] | 0.896 | 0.004 | |
| Pre.PLR | 121.83 | 121.92 | 0.82 | 121.93 | 117.11 | ||
| (median [IQR]) | [99.48, 148.84] | [90.51, 162.02] | [99.54, 148.82] | [86.59, 152.20] | 0.312 | 0.02 | |
| Pre.NLR | 1.97 | 1.98 | 0.528 | 1.96 | 1.97 | ||
| (median [IQR]) | [1.55, 2.81] | [1.56, 2.62] | [1.53, 2.76] | [1.56, 2.60] | 0.544 | 0.032 | |
| Pre.SII | 469.62 | 455.86 | 0.467 | 463.51 | 439.45 | ||
| (median [IQR]) | [346.50, 704.19] | [324.30, 635.68] | [347.59, 700.55] | [313.07, 615.04] | 0.179 | 0.026 | |
Abbreviations: IQR, inter-quartile range; Cancer stages: stage I: T1, N0, M0/T2, N0, M0/T1, N1, M0; stage II: T3, N0, M0/T4a, N1, M0/T3, N1, M0/T2, N2, M0/T1, N3, M0; stage III: T2, N3, M0/T3, N2, M0/T3, N3, M0/T4a, N2, M0/T4a, N3, M0/any T4b, any N, M0; stage IV: any T, any N, M1.ASA, American Society of Anesthesiologists; BMI, body mass index; INHA, inhalational anesthesia; TIVA, total intravenous anesthesia, IPTW, inverse probability of treatment weighting; SMD, Standardized Mean Difference; Pre, preoperative; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; SII, systemic immune-inflammatory index
Because the weighted values were presented, the number of patients were not an integer
Fig. 2.
The distribution of standardized mean difference for variables included before and after matching. Abbreviations: Cancer stages: stage I: T1, N0, M0/T2, N0, M0/T1, N1, M0; stage II: T3, N0, M0/T4a, N1, M0/T3, N1, M0/T2, N2, M0/T1, N3, M0; stage III: T2, N3, M0/T3, N2, M0/T3, N3, M0/T4a, N2, M0/T4a, N3, M0/any T4b, any N, M0; stage IV: any T, any N, M1.ASA, American Society of Anesthesiologists; BMI, body mass index; Pre, preoperative; NLR, neutrophil to lymphocyte ratio; PLR, platelet to lymphocyte ratio; SII, systemic immune-inflammatory index
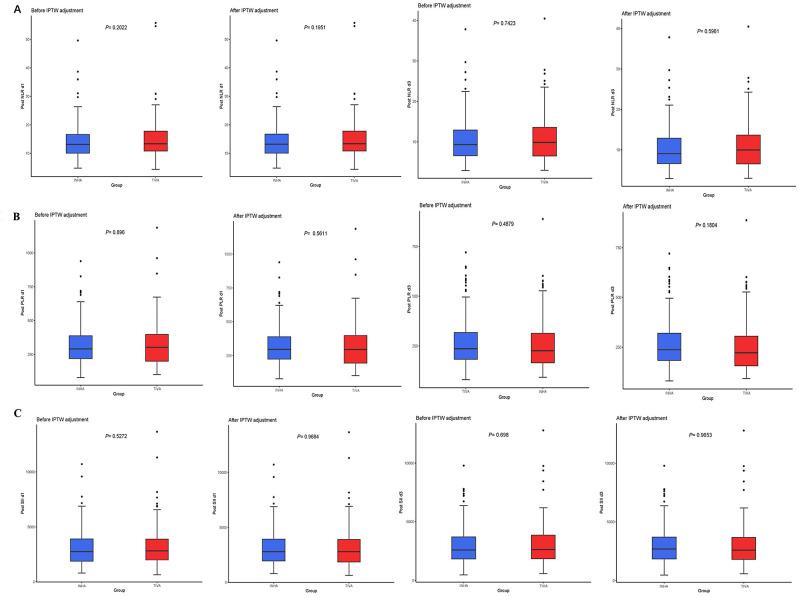
In this study, NLR, PLR, and SII were collected at 1 and 3 days after surgery for comparison between the two groups. The median NLR in the TIVA and INHA groups on the first postoperative day was 13.32(interquartile range,10.78 to 15.07) vs. 13.12(interquartile range,10.5 to 14.01), there was no statistically significant difference between the two groups (P = 0.2022). The median NLR at TIVA and INHA on the third postoperative day was 9.89(interquartile range,6.52 to 13.60) vs. 9.35(interquartile range,6.59 to 12.94), there was no statistically significant difference between the two groups (P = 0.7423). After IPTW, there remained no significant difference in NLR between the two groups on the first (P = 0.1951) and third postoperative days (P = 0.5961) (Fig. 3A).
Fig. 3.
(A) Postoperative NLR at 1 and 3 days before and after IPTW adjustment. 3(B). Postoperative PLR at 1 and 3 days before and after IPTW adjustment. 3 (C). Postoperative SII at 1 and 3 days before and after IPTW adjustment. Abbreviations: NLR, neutrophil to lymphocyte ratio. PLR, platelet to lymphocyte ratio. SII, systemic immune-inflammatory index
The median PLR in the TIVA and INHA groups on the first postoperative day was 302.7(interquartile range,199.5 to 398.1) vs. 291.07(interquartile range,219.38 to 387.8), there was no statistically significant difference between the two groups (P = 0.896). The median PLR in the TIVA and INHA groups on the third postoperative day was 9.89(interquartile range,6.52 to 13.60) vs. 9.35(interquartile range,6.59 to 12.94), there was no statistically significant difference between the two groups (P = 0.4879). After IPTW, there still remained no significant difference in PLR between the two groups on the first (P = 0.5611) and third postoperative days (P = 0.1804) (Fig. 3B).
The median SII in the TIVA and INHA groups on the first postoperative day was 2822.1 (interquartile range, 1993.9 to 3897.1) vs. 2747.8 (interquartile range, 1885.0 to 3900.9), there was no statistically significant difference between the two groups (P = 0.5272). The median SII in the TIVA and INHA groups on the third postoperative day was 2626.0 (interquartile range, 1843.3 to 3860.7) vs. 2593.7 (interquartile range, 1825.0 to 3700.2), there was no statistically significant difference between the two groups (P = 0.698). After IPTW, there was no significant difference in SII between the two groups on the first (P = 0.9684) and third postoperative days (P = 0.9653) (Fig. 3C).
Discussion
This study showed that there was no difference in the effects of propofol-based total intravenous anesthesia and sevoflurane-based inhalation anesthesia on NLR, PLR, and SII at 1 and 3 days after surgery in EC surgery. The choice of anesthesia method may have no impact on the inflammatory markers in EC patients.
It is well known that systemic inflammation and immunity play an important role in tumor progression [10]. NLR, PLR, and SII are inflammatory markers based on common peripheral blood cells, and they reflect the relative changes between systemic inflammation and immunity. The higher the perioperative NLR, the worse the prognosis of cancer patients [15, 16]. This may be attributed to a higher tendency for intratumoral neutrophils in patients with high NLR [17]. Intratumoral neutrophils promote tumor cell growth and metastasis through paracrine [18]. PLR has also been demonstrated to be a potent marker of systemic inflammation, and high PLR is associated with distant metastasis in patients with tumors [19, 20]. The main cause for this is that platelets protect tumor cells within the circulatory system from immune elimination and promote tumor cell metastasis [21]. However, these two inflammatory markers integrate only two cell types. SII is a new inflammatory marker proposed by Hu et al., which includes neutrophils, platelets and lymphocytes [22]. The prognosis of patients with SII tumors is predicted by the function of the three cell types and their close relationship with circulating tumor cells (CTCs), which play a significant role in the initiation of postoperative recurrence and metastasis [23]. Compared to NLR, PLR, SII can reflect the link between inflammation, immunity and tumor more effectively and comprehensively [11, 12, 24].
In our study, we discovered that propofol-based total intravenous anesthesia, when compared with sevoflurane-based inhalation anesthesia, failed to reduce NLR in EC patients on the first and third postoperative days. This is similar to the results of a recent prospective study on colorectal cancer. In this study, propofol anesthesia compared to sevoflurane did not reduce postoperative NLR levels in colorectal cancer patients at 1 and 24 h [25]. It is possible that clinical doses of anesthetic drugs have a restricted effect on reducing immunosuppression and inflammation. Another retrospective study on postoperative NLR in colorectal cancer found that NLR levels were decreased in the TIVA group at 1, 2, and 5 days after surgery [26]. The results of the above study are contrary to ours, which may be attributed to differences in tumor and type of surgery. In addition, retrospective studies often use propensity score matching to reduce confounding, which may result in distinct outcomes with some significant missing data. In our research, we utilized the method of IPTW [14] to minimize confounding factors without reducing the amount of data, and the statistical results were more convincing. Similarly, we investigated PLR and SII in EC patients on the first and third postoperative days. The outcomes demonstrated that clinical concentrations of propofol anesthesia did not provide an advantage over sevoflurane-based anesthesia in short-term postoperative PLR and SII.
Propofol is a commonly utilized anesthetic, and its immune and inflammatory effects on cancer patients have been a heated topic [27, 28]. In the domain of immunity, one study revealed that propofol-based total intravenous anesthesia significantly increased postoperative NKCC (Natural killer cell cytotoxicity) in breast cancer patients [29]. In another study comparing the immune effects of propofol and sevoflurane in cervical cancer, the postoperative sevoflurane group had significantly lower CD3 + cells, CD4 + cells, NK cells, and CD4+/CD8 + ratios than the TIVA group [30]. Conversely, some studies have indicated that the function of immune cells including natural killer cells and T lymphocytes is not influenced by sevoflurane and propofol anesthesia [31, 32]. Gu et al. also claimed in the review that propofol may be more beneficial than sevoflurane and isoflurane in terms of tumor postoperative immune regulation, but there is still some disagreement and more randomized clinical trials are needed [33]. In terms of inflammation, in vitro experiments disclosed that propofol, compared with sevoflurane, reduced inflammatory responses through the NF-κB signaling pathway and induced apoptosis in human neuroglioma cells [34]. In clinical practice, O’Bryan et al. noted in a meta-analysis that the choice of anesthesia method has little effect on the inflammatory response during the perioperative period [35]. The available data demonstrating the effect of anesthesia on inflammation in vitro and in vivo studies are still controversial. The above might explain the fact that postoperative inflammatory markers in EC patients did not show significant differences between the two anesthesia methods.
There are certain inevitable limitations to our study. Firstly, the study only included a few inflammatory markers (NLR, PLR, SII), which may not comprehensively reflect the entire spectrum of perioperative inflammatory and immune responses. Secondly, the study acknowledges missing data regarding the amount of opioid used, which could significantly influence postoperative inflammatory responses and affect the study outcomes. Thirdly, since the data were collected from a single hospital, the study may lack generalizability. The findings may not apply to different populations or hospitals with varying practices.
In conclusion, TIVA and INHA had no impact on NLR, PLR and SII in patients with EC. This suggests that numerous perioperative factors may influence the inflammatory response in patients with esophageal cancer, and our study demonstrates that the type of anesthesia has a negligible effect on it.
Acknowledgements
Not applicable.
Abbreviations
- EC
Esophageal cancer
- IPTW
Inverse probability of treatment weighting
- TIVA
Total intravenous anesthesia
- INHA
Inhalational anesthesia
- NLR
Neutrophil to lymphocyte ratio
- PLR
Platelet to lymphocyte ratio
- SII
Systemic immune-inflammatory index
- ASA
American Society of Anesthesiologists
- AJCC
American Joint Committee on Cancer
- CTC
Circulating tumor cell
Author contributions
Z.L. had contributions to study conception, design. J.R. and Y.M. drafting the article and acquisition of data.M.W. had contributions to interpretation of data. Z.L. had responsibility for the revision of important intellectual content and final approval of the version to be published.All authors reviewed the manuscript.
Funding
This study was supported by the Fundamental Research Funds for the Provincial Universities (Grant#2021-KYYWF-0258, Z.L.)
Data availability
All data generated or analyzed during this study are included in this published.
article.
Declarations
Ethics approval and consent to participate
This study was performed in accordance with the ethical principles of the Declaration of Helsinki. The study procedures were approved by the Ethics Committee of The Harbin Medical University Cancer Hospital. This is a retrospective study and individual informed consent to participate for this retrospective analysis with routine clinical data was waived by the Ethics Committee of The Harbin Medical University Cancer Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jie Ren and Yue Ma contributed equally to this work.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Ferlay J, van Berge Henegouwen MI, Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. 2020;69(9):1564–71. [DOI] [PubMed] [Google Scholar]
- 3.Mariette C, Piessen G, Triboulet JP. Therapeutic strategies in oesophageal carcinoma: role of surgery and other modalities. Lancet Oncol. 2007;8(6):545–53. [DOI] [PubMed] [Google Scholar]
- 4.Hiller JG, Perry NJ, Poulogiannis G, Riedel B, Sloan EK. Perioperative events influence cancer recurrence risk after surgery. Nat Rev Clin Oncol. 2018;15(4):205–18. [DOI] [PubMed] [Google Scholar]
- 5.Wall T, Sherwin A, Ma D, Buggy DJ. Influence of perioperative anaesthetic and analgesic interventions on oncological outcomes: a narrative review. Br J Anaesth. 2019;123(2):135–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hee HZ, Chang KY, Huang CY, Chang WK, Tsou MY, Lin SP. Perioperative Blood transfusion is dose-Dependently Associated with Cancer recurrence and mortality after Head and Neck Cancer surgery. Cancers (Basel). 2022. 15(1). [DOI] [PMC free article] [PubMed]
- 7.Costa B, Mourão J, Vale N. Personalized medicine for classical anesthesia drugs and Cancer Progression. J Pers Med. 2022. 12(11). [DOI] [PMC free article] [PubMed]
- 8.Zhou X, Shao Y, Li S, et al. An intravenous anesthetic drug-propofol, influences the biological characteristics of malignant tumors and reshapes the tumor microenvironment: a narrative literature review. Front Pharmacol. 2022;13:1057571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xue F, Xu Y, Song Y, Zhang W, Li R, Zhu X. The effects of Sevoflurane on the Progression and Cisplatinum Sensitivity of Cervical Cancer cells. Drug Des Devel Ther. 2019;13:3919–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greten FR, Grivennikov SI. Inflammation and Cancer: triggers, mechanisms, and consequences. Immunity. 2019;51(1):27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q, Pu Y, Gong Z, et al. Preoperative systemic immune-inflammation index for predicting the prognosis of thymoma with radical resection. Thorac Cancer. 2023;14(13):1192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J, Li S, Zhang S, et al. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. J Clin Lab Anal. 2019;33(8):e22964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma Y, Ren J, Chen Z, et al. Outcomes of intravenous and inhalation anesthesia on patients undergoing esophageal cancer surgery: a retrospective observational study. BMC Anesthesiol. 2023;23(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahmud Z, Rahman A, Mishu ID, Kabir Y. Mechanistic insights into the interplays between neutrophils and other immune cells in cancer development and progression. Cancer Metastasis Rev. 2022;41(2):405–32. [DOI] [PubMed] [Google Scholar]
- 16.Russo A, Russano M, Franchina T, et al. Neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and outcomes with Nivolumab in Pretreated Non-small Cell Lung Cancer (NSCLC): a large Retrospective Multicenter Study. Adv Ther. 2020;37(3):1145–55. [DOI] [PubMed] [Google Scholar]
- 17.Takakura K, Ito Z, Suka M, et al. Comprehensive assessment of the prognosis of pancreatic cancer: peripheral blood neutrophil-lymphocyte ratio and immunohistochemical analyses of the tumour site. Scand J Gastroenterol. 2016;51(5):610–7. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Chen J, Yang L, et al. Tumor-contacted neutrophils promote metastasis by a CD90-TIMP-1 Juxtacrine-Paracrine Loop. Clin Cancer Res. 2019;25(6):1957–69. [DOI] [PubMed] [Google Scholar]
- 19.Abu-Shawer O, Abu-Shawer M, Haimour A, et al. Hematologic markers of distant metastases in gastric cancer. J Gastrointest Oncol. 2019;10(3):529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato T, Oshikiri T, Goto H, et al. Impact of the platelet-to-lymphocyte ratio as a biomarker for esophageal squamous cell carcinoma. Anticancer Res. 2022;42(5):2775–82. [DOI] [PubMed] [Google Scholar]
- 21.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11(2):123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–22. [DOI] [PubMed] [Google Scholar]
- 23.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–64. [DOI] [PubMed] [Google Scholar]
- 24.He K, Si L, Pan X, et al. Preoperative systemic Immune-inflammation index (SII) as a Superior Predictor of Long-Term Survival Outcome in patients with stage I-II gastric Cancer after radical surgery. Front Oncol. 2022;12:829689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh CS, Park HJ, Piao L, et al. Expression profiles of Immune cells after Propofol or Sevoflurane Anesthesia for Colorectal Cancer surgery: a prospective double-blind Randomized Trial. Anesthesiology. 2022;136(3):448–58. [DOI] [PubMed] [Google Scholar]
- 26.Lee S, Pyo DH, Sim WS, Lee WY, Park M. Early and Long-Term outcomes after Propofol-and sevoflurane-based anesthesia in colorectal Cancer surgery: a retrospective study. J Clin Med. 2022;11(9):2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saha P, Das A, Chatterjee N, Chakrabarti D, Sinha D. Impact of anesthetics on oncogenic signaling network: a review on propofol and isoflurane. Fundam Clin Pharmacol. 2022;36(1):49–71. [DOI] [PubMed] [Google Scholar]
- 28.Gao X, Mi Y, Guo N, et al. The mechanism of propofol in cancer development: an updated review. Asia Pac J Clin Oncol. 2020;16(2):e3–11. [DOI] [PubMed] [Google Scholar]
- 29.Cho JS, Lee MH, Kim SI, et al. The effects of Perioperative Anesthesia and Analgesia on Immune function in patients undergoing breast Cancer resection: a prospective Randomized Study. Int J Med Sci. 2017;14(10):970–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu S, Gu X, Zhu L, et al. Effects of propofol and sevoflurane on perioperative immune response in patients undergoing laparoscopic radical hysterectomy for cervical cancer. Med (Baltim). 2016;95(49):e5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oh CS, Lee J, Yoon TG, et al. Effect of Equipotent doses of Propofol versus Sevoflurane Anesthesia on Regulatory T Cells after breast Cancer surgery. Anesthesiology. 2018;129(5):921–31. [DOI] [PubMed] [Google Scholar]
- 32.Lim JA, Oh CS, Yoon TG, et al. The effect of propofol and sevoflurane on cancer cell, natural killer cell, and cytotoxic T lymphocyte function in patients undergoing breast cancer surgery: an in vitro analysis. BMC Cancer. 2018;18(1):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gu L, Pan X, Wang C, Wang L. The benefits of propofol on cancer treatment: decipher its modulation code to immunocytes. Front Pharmacol. 2022;13:919636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian Y, Guo S, Guo Y, Jian L. Anesthetic Propofol attenuates apoptosis, Aβ Accumulation, and inflammation Induced by Sevoflurane through NF-κB pathway in human neuroglioma cells. Cell Mol Neurobiol. 2015;35(6):891–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Bryan LJ, Atkins KJ, Lipszyc A, Scott DA, Silbert BS, Evered LA. Inflammatory biomarker levels after propofol or sevoflurane anesthesia: a Meta-analysis. Anesth Analg. 2022;134(1):69–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published.
article.