Abstract
Background
Chronic pain is a substantial problem in modern healthcare resulting in health care overutilization. The cumulative incidence of developing chronic pain after visiting the emergency department with acute pain has been determined for specific patient groups only. If the cumulative incidence of chronic pain in emergency department patients with acute pain is high, more proactive measures are justified to limit development of chronic pain. The primary objective was to study the cumulative incidence of chronic pain in patients visiting Dutch emergency departments with acute pain. In addition, we compared the Health-Related Quality of Life (HRQOL) and pain related interference with work.
Methods
In this prospective multicenter cohort study data was collected from adult patients visiting the emergency department with acute pain. Chronic pain was defined by means of a numeric rating scale (NRS) of ≥ 1 measured 90 days after the initial visit. HRQOL was measured with European Quality of Life (EQ-5D-5 L) and Short Form (SF-36) questionnaires.
Results
1906 patients were included of which 825 had complete data. Of these, 559 patients (67.8%; 95%CI: 64.5 − 70.9%) scored an NRS ≥ 1 after 90 days. Incidence with completed analyses (with imputed data) was similar. Patients with chronic pain reported a significantly lower HRQOL; EQ-5D-5 L index (median 0.82 vs. 1.00) and significantly more pain related hindrance (median 1.00 vs. 0.00).
Conclusions
67.8% of the responders scored NRS ≥ 1 90 days after ED-visit with acute pain. Regardless of the used definition, chronic pain is associated with a lower HRQOL and more pain related hindrance.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12871-024-02836-8.
Keywords: Acute pain, Emergency service, Chronic pain, Quality of life
Introduction
Most patients visit the emergency department in the Netherlands with acute pain [1–4]. This acute pain could evolve into chronic pain. Chronic pain is associated with mental health impairment, overutilization of healthcare, absenteeism, productivity loss and opioid dependency. Therefore, chronic pain is substantial and one of the costliest conditions in western society [5–10].
Prior to 2019, there was a lack of consensus on the definition of chronic pain. In 2019, a generally accepted definition was addopted stating that any pain persisting beyond the expected healing period (3 months according to International Classification of Diseases, 11th edition criteria) is pathological. The incidence of chronic pain varies from 11–62,7% depending on the definition of chronic pain and studied population [11–26]. The majority of these studies determined the incidence only for specific groups of patients and used different definitions of chronic pain. Furthermore, there are only a few initiatives towards the calculation of the cumulative incidence of chronic pain after acute pain [11, 16, 19, 21, 23, 26, 27]. A study on the incidence of chronic pain in patients in the emergency department with any form of acute pain is lacking.
The incidence of chronic pain in patients in the emergency department with any form of acute pain is of great importance in order to estimate the impact on patients, our society and the healthcare system. Multiple risk factors for chronic pain development include older age, female sex, pain catastrophizing, high-intensity acute pain, less than college education, low socio-economic status, anxiety, and depression [28]. Emergency department physicians could initiate measures in order to limit the development of chronic pain in patients with a high risk in developing chronic pain. Initiation of adequate pain medication, psychological interventions such as cognitive behavioral therapy, informing the general practitioner and other physicians of the high risk and more proactive follow-up could potentially reduce the incidence of chronic pain.
The primary objective was to determine the cumulative incidence of chronic pain in all patients visiting the emergency department with acute pain. Furthermore, we studied the impact of chronic pain on Health-Related Quality of Life (HRQOL) and pain-related hindrance.
Patients and methods
Study design
Data from the PRACTICE study was used for this descriptive analysis. The PRACTICE study is a prospective multicenter longitudinal study aiming to create a prediction model for the transitioning of acute into chronic pain.
Patients were recruited in 15 emergency departments throughout the Netherlands. These emergency departments varied from academic trauma care centers in major cities to emergency departments in rural areas. Characteristics of the different participating emergency departments and number of inclusions are summarized in supplemental Table 1.
Emergency departments in The Netherlands are part of hospitals. Patients visiting the emergency department are triaged by a trained emergency department nurse using a color code. Red: directly, Orange: within 10 min, Yellow: within 60 min, Green: within 120 min, Blue: within 240 min. Depending on the triage patients are seen directly by a physician or an emergency department nurse. Initial diagnostics, stabilization and treatment is initiated. Afterwards, patients can either be discharged or admitted to the hospital.
Participants
All consecutive patients visiting one of the 15 participating emergency departments with acute pain meeting the inclusion criteria were asked to participate in the study.
The following inclusion criteria were used:
Age ≥ 18 years, acute pain existing.
Duration of pain ≤ 48 h.
Pain as main complaint during emergency department visit.
Discharge after initial emergency department treatment.
Signed written informed consent.
Potential subjects meeting any of the following criteria were excluded from participation in this study:
Cognitive impairment.
Illiteracy.
Language barrier.
Current diagnosis of chronic pain located at or near the location of the acute pain with which the patient presents at the emergency department.
Hospital admission.
Acute pain within 7 days after surgery.
Patients with acute pain within 7 days after surgery were excluded because the etiology of their pain might differ from other patients presenting at the emergency department with pain. It was hypothesized that these patients had a high chance of procedure or complication related pain and thus a different chance of developing chronic pain.
The Medical Research Ethics Committee (MREC) concluded that the study did not fall under the scope of the Medical Research Involving Human Subjects Act. (Protocol 2018-39). Furthermore, the study was conducted in accordance to the principles of the Declaration of Helsinki and all participating hospitals approved the study locally.
Study procedures
The PRACTICE study consisted of two study periods. During the first period in September 2018, data was collected by means of paper questionnaires. During the second period, ranging from October 2018 to April 2020, data was collected by means of electronically generated questionnaires using an application and/or website developed for the study. The questionnaires used in both study periods were internationally validated questionnaires. Further details are provided below in this section. It was assumed that paper and electronic modalities might differ in terms of response rate, but not in terms of primary outcome or prognostic ability [29]. Therefore, the data from both paper and electronic modalities were used for this study. All patients presenting with pain to the emergency department were screened for inclusion throughout the day by attending physicians or trained medical students. If eligible, patients were informed about the study and study procedures and asked for inclusion. After inclusion, the numeric rating scale (NRS) during emergency department-visit and at departure were collected. Furthermore, baseline characteristics were collected by means of the application. Baseline characteristics included: the type of referral, emergency department triage time, level of triage (MTS), age, (biological) sex, location of pain, fracture/trauma-related pain, comorbidities (yes/no), given medication at emergency department, medical advice at the emergency department, depression and or treatment for depression, pre-existing chronic pain (not located at or near the location of the acute pain), location of pre-existing chronic pain, alcohol consumption (yes/no), level of education, pain catastrophizing scale, marital status, current employment, sick leave because of pain and smoking status.
All patients were asked for NRS of pain, daily during the first week after emergency department visit and at day 90 and 180. Patients were specifically asked to score only the NRS remaining after, and related to, the initial emergency department visit. A continuous 90-day or 180-day NRS was not queried due to practical reasons. On a theoretical level the 90- or 180-day NRS combined with pain in the preceding 4 weeks (question 7 of the RAND 36-item Short Form Survey) (SF-36) (supplemental Table 2) gives information about experienced pain on day 62–90 or 152–180. This combination of the NRS and the preceding 4 weeks was named persisting pain. Besides question 7 of the SF-36, also question 8 (supplemental Table 3) of the SF-36 was queried after 90 and 180 days. (Ware & Sherbourne, 1992)
The Euroqol 5D-5 L (EQ-5D-5 L) Dutch tariff was collected on day 7, 90 and 180. The EQ-5D-5 L is a widely used tool to measure the HRQOL and comprises five dimensions: mobility, self-care, usual activities, pain/discomfort, anxiety/depression and interference with normal work due to pain [30–33]. In general, failures to respond resulted in application reminders and lastly reminder calls. Supplemental Table 4 shows the timetable of the questionnaires. Baseline characteristics were collected from the patient registry. Other variables were collected either on paper or with the specially developed application and website.
Primary and secondary outcomes
The primary objective was the cumulative incidence of chronic pain, defined as NRS ≥ 1, 90 days after an emergency department-visit for acute pain. The secondary objective was to estimate the cumulative incidence of chronic pain with other commonly used chronic pain definitions. These include an NRS ≥ 1 at 180 days, NRS ≥ 4 at 90 or 180 days and having continuous pain for 3 or 6 months after the acute pain. Finally, we also studied HRQOL and hindrance due to pain in relation to different definitions of chronic pain.
Statistical analysis
The cumulative incidence of chronic pain was calculated as the proportion of patients with an NRS ≥ 1, 90 days after emergency department visit. The baseline characteristics of the patients with and without chronic pain were compared using descriptive statistics. Means with standard deviation (SD) or medians with interquartile range (IQR) were used depending on the distribution. Ordinal/nominal values were presented as n (%). Patient scores on the Eq. 5D-5 L in the different groups were converted into utility values, using the algorithm of the Dutch general population (‘Dutch tariff’) [31]. A lower EQ-5D-5 L index (< 1,00) correlates with lower quality of life while an EQ-5D-5 L index of ‘1.00’ equals no loss. Differences in quality of life (measured by EQ-5D-5 L index and SF- 36 question 8) and pain-related hindrance between patients with or without chronic pain were tested with the Mann-Whitney U test. Additionally, in order to account for non-responders, we performed an analysis imputing missing data of NRS day 90 and 180 using multiple imputation by chained equations (MICE) with baseline variables and outcomes, generating m = 100 imputed datasets [34]. While there is no way to formally assess whether data is fully missing at random, we tried to make the assumption of missing at random plausible. Firstly, a large set of predictors was included in the multiple imputation model all measuring relevant aspects associated with health status (e.g. patient demographics, patient history). Secondly, we verified that several of these predictors indeed showed an association with responder status. Thirdly, for comparison, analyses were presented both for responders only group and the entire group with missing data completed by MICE. Detailed description of the multiple imputation model can be found in supplemental Table 5. A sensitivity analysis was performed to check whether differences existed between different hospitals. All analyses were performed using R version 3.6.3 [35]. For all analyses, a p-value < 0.05 was considered statistically significant.
Results
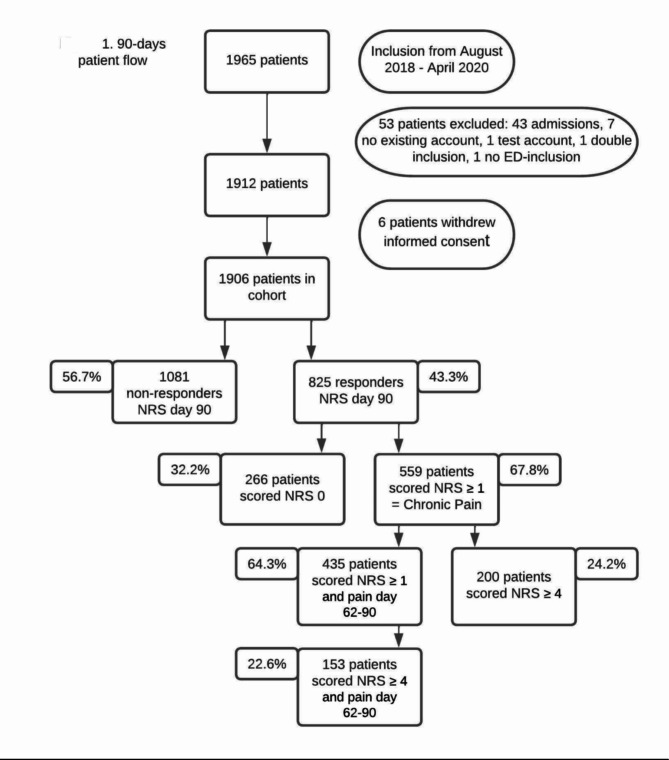
From October 2018 until April 2020, 1965 patients were included in the 15 participating hospitals. 53 patients (2.7%) were excluded due to various reasons and 6 patients (0.3%) withdrew their consent. The 90-day patient flow is shown in Fig. 1. 1906 patients were included of which 825 had complete data (responder group).
Fig. 1.
90-day patient flow
Baseline characteristics
Patient characteristics and emergency department characteristics for the non-responder and responder group are summarized in Table 1. In total 252 patients (13.2%) of the 1906 included patients already suffered from chronic pain in prior to their arrival at the emergency department. Patients were excluded if this pre-existing chronic pain was located at or near the location of the present acute pain.
Table 1.
Patient baseline characteristics NRS of pain day 90
| Total cohort (n = 1906) | Responders (n = 825) | ||||||
|---|---|---|---|---|---|---|---|
| Non-Responders n = 1081 |
Responders n = 825 |
P | NRS 0 n = 266 |
NRS ≥ 1 n = 559 |
P | ||
| Sociodemographic factors | |||||||
| Age (median [IQR]) | 45.00 [28.00–58.00] | 47.00 [31.00–59.00] | 0.011 | 43.00 [29.00–55.00] | 49.00 [33.00–60.00] | 0.001 | |
| Female sex (n (%)) | 491/1074 (45.7) | 434/824 (52.6) | 0.003 | 111/265 (41.9) | 323/559 (57.8) | < 0.001 | |
| Currently relationship (yes, %) | 119/425 (28.0) | 222/732 (30.3) | 0.402 | 73/220 (33.2) | 149/512 (29.1) | 0.271 | |
| Currently working (yes, %) | 271/365 (74.2) | 559/716(78.1) | 0.159 | 177/214 (82.7) | 382/502 (76.1) | 0.050 | |
| Acute pain characteristics | |||||||
| Location of pain (n (%)) | |||||||
|
Head Cervical spine Thoracic Thoracic and lumbar spine Abdomen Upper extremity/shoulder Lower extremity/hip Multiple Remaining Unknown Fracture related pain (n (%)) NRS arrival (median [IQR]) NRS departure (median [IQR]) |
56/1081 (5.2) 13/1081 (1.2) 47/1081 (4.4) 16/1081 (1.5) 64/1081 (5.9) 450/1081 (41.6) 314/1081 (29.1) 102/1081 (9.4) 13/1081 (1.2) 6/1081 (0.6) 515/1064 (48.4) 5.00 [3.00–7.00] 4.00 [3.00–6.00] |
36/825 (4.4) 5/825 (0.6) 37/825 (4.5) 13/825 (1.6) 43/825 (5.2) 356/825 (43.2) 257/825 (31.2) 71/825 (8.6) 5/825 (0.6) 2/825 (0.2) 471/813 (57.9) 5.00 [3.00–7.00] 4.00 [3.00–6.00] |
0.410 0.182 0.885 0.866 0.506 0.505 0.320 0.532 0.182 0.478 < 0.001 0.787 0.943 |
13/266 (4.9) 2/266(0.8) 20/266 (7.5) 2/266(0.8) 28/266 (10.5) 102/266 (38.4) 74/266 (27.8) 22/266(8.3) 2/266 (0.8) 1/266 (0.4) 121/261 (46.4) 4.50 [2.00–6.00] 4.00 [3.00–6.00] |
23/559 (4.1) 3/559 (0.5) 17/559 (3.0) 11/559 (2.0) 15/559(2.7) 254/559 (45.4) 183/559 (32.7) 49/559 (8.8) 3/559 (0.5) 1/559 (0.2) 350/552(63.4) 6.00 [3.00–7.00] 4.00 [3.00–6.00] |
0.612 0.660 0.004 0.242 < 0.001 0.055 0.154 0.813 0.660 0.841 < 0.001 < 0.001 0.255 |
|
| ED visit characteristics | |||||||
| Total minutes ED (median [IQR]) | 121 [83–174] | 118.50 [81–180] | 0.201 | 120 [79–187] | 118 [82–176] | 0.913 | |
| General Health | |||||||
| Comorbidity (Yes, %) | 478/864 (55.3) | 339/707 (47.9) | 0.004 | 100/228 (43.9) | 239 /479 (49.9) | 0.133 | |
| Pre-existent chronic pain (n (%)) | 92/393 (23.4) | 160/712 (22.5) | 0.722 | 38/213(17.8) | 122/499 (24.5) | 0.053 | |
| Psychosocial factors | |||||||
|
Smoking (yes, %) Alcohol (yes, %) Ever depressed (yes, %) |
70/288 (24.3) 181/396 (45.7) 87/424 (20.5) |
89/702 (12.7) 359/720 (49.9) 132/725 (18.2) |
< 0.001 0.184 0.336 |
26/214 (12.1) 122/216 (56.5) 31/216 (14.4) |
63/488 (12.9) 237/504 (47.0) 101/509 (19.8) |
0.780 0.020 0.080 |
|
Abbreviations:
IQR: Interquartile range
n: Number of patients
NRS: Rating Scale
*triage color and specified comorbidity is shown in supplemental Table 6
Primary outcome
Table 1 summarizes the characteristics from the 825 patients who replied to the NRS questionnaire of day 90. From these 825 responders, 559 (67.8%; 95%CI: 64.5 − 70.9%) scored NRS ≥ 1. These 559 patients were concluded to have chronic pain. 442 (79.1%) of these 559 patients scored NRS ≥ 1 for at least 5 days in the first week after the initial visit to the emergency department.
At baseline the group of patients with chronic pain were significantly older (49 years [IQR 33.00–60.00] vs. 43 years [IQR 29.00–55.00], p = 0.001), predominately female (57.8% vs. 41.9%, p < 0.001), visited the ED more frequently due to a fracture (63.4% vs. 46.4%, p < 0.001) and had a significant higher NRS of pain at arrival (6 [IQR 3.00–7.00] vs. 4.5 [IQR 2.00–6.00], p < 0.001). A trend was observed for a higher incidence of pre-existing chronic pain (not located at or near the location of the acute pain) in the chronic pain group (24.5% vs. 17.8%, p = 0.053).
Furthermore, patients without chronic pain were more likely to consume alcohol (56.5% vs. 47.0%, p = 0.020) and were more often employed (82.7% vs. 76.1%, p = 0.050).
Secondary outcomes
Of the 825 previous mentioned responders, 200 patients (24.2%; 95%CI: 21.4 – 27.3%) reported an NRS ≥ 4 after 90 days. In total 435 patients (64.3%; 95%CI: 60.6 − 67.8%) scored an NRS ≥ 1 after 90 days and also experienced pain in the preceding 4 weeks (day 62–90). Finally, 153 patients (22.6%; 95%CI: 19.6 – 25.9%) scored NRS ≥ 4 after 90 days and also experienced pain from day 62–90. At 180 days NRS was available for 675 patients (35.4%) of which 393 patients (58.2%; 95%CI: 54.5-61.9%) reported an NRS ≥ 1. Of these, 164 (24.3%; 95%CI: 21.2-27.7%) patients scored an NRS ≥ 4. In total 568 patients reported a pain score at day 90 and 180. Of these, 308 (54.2%; 95%CI: 50.1 – 58.3%) reported an NRS ≥ 1 on day 90 as well as day 180 and experienced pain from day 62–90 and 152–180. These patients were concluded to have persisting pain beyond 180 days. Finally, 123 of 568 patients (21.7%; 95%CI: 18.5 − 25.2%) scored NRS ≥ 4 on day 90 and 180 and experienced pain from day 62–90 and 152–180. Table 2 summarizes the incidences of chronic pain for the different definitions, EQ-5D-5 L indices and hindrance due to pain for both responders and non-responders.
Table 2.
Results various definitions of chronic pain, responders only
| Definitions of chronic pain | Incidence n (%) * | EQ-5D-5 L index** Median (IQR) chronic pain vs. no chronic pain |
P (Wilcox. Test) | Hindrance due to pain Median (IQR) chronic pain vs. no chronic pain |
P (Wilcox. Test) |
|---|---|---|---|---|---|
| Day 90 NRS ≥ 1 (Primary outcome) | 559 (67.8%) |
0.82 (0.74–0.92) Vs. 1.00 (0.89–1.00) |
< 0.001 |
Day 62–90 1.00 (0.00–2.00) Vs. 0.00 (0.00–0.00) |
< 0.001 |
| Day 90 persisting pain NRS ≥ 1 | 435 (64.3%) |
0.81 (0.73–0.89) Vs. 1.00 (0.89 -1.00) |
< 0.001 |
Day 62–90 1.00 (1.00– 2.00) Vs. 0.00 (0.00–0.00) |
< 0.001 |
| Day 90 NRS ≥ 4 | 200 (24.2%) |
0.75 (0.65–0.85) Vs. 0.89 (0.81 -1.00) |
< 0.001 |
Day 62–90 2.00 (1.00–2.00) Vs. 1.00 (0.00–1.00) |
<0.001 |
| Day 90 persisting pain NRS ≥ 4 | 153 (22.6%) |
0.74 (0.64–0.81) Vs. 0.89 (0.81–1.00) |
< 0.001 |
Day 62–90 2.00 (1.00–2.00) Vs. 0.00 (0.00–1.00) |
< 0.001 |
| Day 180 NRS ≥ 1 | 393 (58.2%) |
0.85 (0.75–1.00) Vs. 1.00 (0.89–1.00) |
< 0.001 |
Day 152–180 1.00 (0.00–1.00) Vs. 0.00 (0.00–0.00) |
< 0.001 |
| Day 180 persisting pain NRS ≥ 1 | 308(54.2%) |
0.85 (0.74–0.89) Vs. 1.00 (0.89–1.00) |
< 0.001 |
Day 152–180. 1.00 (0.00–1.00) Vs. 0.00 (0.00–0.00) |
< 0.001 |
| Day 180 NRS ≥ 4 | 164 (24.3%) |
0.78 (0.67–0.88) Vs. 1.00 (0.85–1.00) |
< 0.001 |
Day 152–180 1.00 (1.00–2.00) Vs. 0.00 (0.00–1.00) |
< 0.001 |
| Day 180 persisting pain NRS ≥ 4 | 123 (21.7%) |
0.75 (0.64–0.85) Vs. 1.00 (0.85–1.00) |
< 0.001 |
Day 152–180 1.00 (1.00–2.00) Vs. 0.00 (0.00–1.00) |
< 0.001 |
* Number and percentage of patients suffering from chronic pain with previous given definitions
** A lower EQ-5D-5 L index correlates with lower quality of life
Patients with chronic pain on day 90 (n = 559) showed a lower mean of the EQ-5D-5 L index compared with patients without chronic pain (median 0.82 vs. 1.00, p < 0.001). Furthermore, patients with chronic pain on day 90 also reported significantly higher mean hindrance due to pain from day 62–90 (median 1.00 vs. 0.00, p < 0.001). Patients with chronic pain on day 180 (n = 393 showed a lower mean EQ-5D-5 L index (median 0.82 vs. 0.94, p < 0.001). Furthermore, patients with chronic pain on day 180 also reported significantly higher mean hindrance due to pain from day 152–180 (median 0.98 vs. 0.17, p < 0.001). Table 3 shows the results of the various definitions of chronic pain using mice multivariate imputation by chained equations (MICE). Results are similar to the analysis with responders only.
Table 3.
Results various definitions of chronic pain, responders and non-responders using MICE
| Definitions of chronic pain | Incidence (%) * | EQ-5D-5 L index** Median (IQR) chronic pain Vs. no chronic pain |
P
(Wilcox. Test) |
Hindrance due to pain Median (IQR) Chronic pain Vs. no chronic pain |
P
(Wilcox. Test) |
|
|---|---|---|---|---|---|---|
| Day 90 NRS ≥ 1 (Primary outcome) | 65.3% |
0.82 (0.74–0.92) Vs. 1.00 (0.89–1.00) |
< 0.001 | Day 62–90 |
1.00 (0.00–2.00) Vs. 0.00 (0.00–0.00) |
< 0.001 |
| Day 90 persisting pain NRS ≥ 1 | 56.5% |
0.81 (0.72–0.89) Vs. 1.00 (0.89–1.00) |
< 0.001 | Day 62–90 |
1.00 (1.00–2.00) Vs. 0.00(0.00–0.00) |
< 0.001 |
| Day 90 NRS ≥ 4 | 25.2% |
0.75 (0.64–0.85) Vs. 0.89 (0.81–1.00) |
< 0.001 | Day 62–90 |
2.00 (1.00–2.00) Vs. 1.00 (0.00–1.00) |
< 0.001 |
| Day 90 persisting pain NRS ≥ 4 | 23.8% |
0.74(0.64–0.81) Vs. 0.89 (0.81–1.00) |
< 0.001 | Day 62–90 |
2.00 (1.00–2.00) Vs. 0.00 (0.00–1.00) |
< 0.001 |
| Day 180 NRS ≥ 1 | 56.3% |
0.85 (0.75–1.00) Vs. 1.00 (0.88–1.00) |
< 0.001 | Day 152–180 |
1.00 (0.00–1.00) Vs. 0.00 (0.00–0.00) |
< 0.001 |
| Day 180 persisting pain NRS ≥ 1 | 46.0% |
0.85 (0.74–0.89) Vs. 1.00 (0.89–1.00) |
< 0.001 | Day 152–180 |
1.00 (0.00–1.00) Vs. 0.00 (0.00–0.00) |
< 0.001 |
| Day 180 NRS ≥ 4 | 26.6% |
0.78 (0.67–0.89) Vs. 1.00 (0.85–1.00) |
< 0.001 | Day 152–180 |
1.00 (1.00–2.00) Vs. 0.00 (0.00–0.00) |
< 0.001 |
| Day 180 persisting pain NRS ≥ 4 | 23.3% |
0.75 (0.64–0.85) Vs. 1.00 (0.85–1.00) |
< 0.001 | Day 152–180 |
1.00 (1.00–2.00) Vs. 0.00 (0.00–1.00) |
< 0.001 |
* Percentage of patients suffering from chronic pain with previous given definitions
** A lower EQ-5D-5 L index correlates with lower quality of life
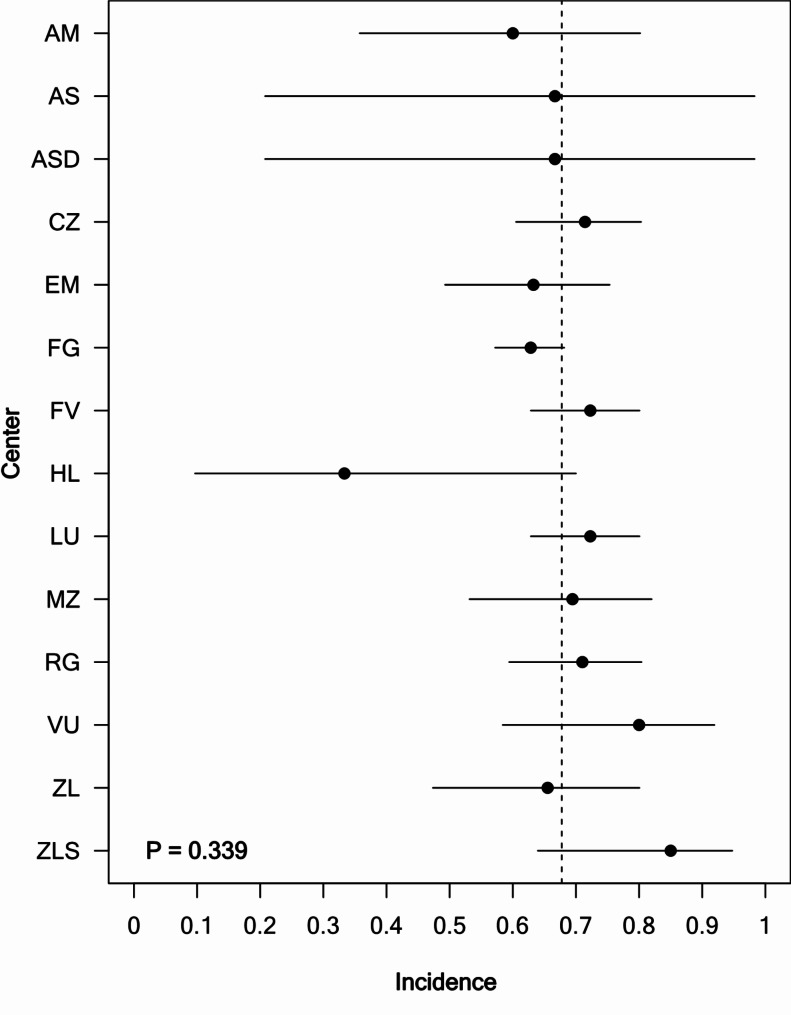
As a sensitivity analysis, we analyzed the incidence of chronic pain among centers. The incidence rate did not vary between different centers. (P = 0.339). Figure 2 shows a forest plot with the incidence of chronic pain for each center (NRS ≥ 1) at day 90 n = 825 patients.
Fig. 2.
Forest plot showing the incidence of chronic pain (pain (NRS ≥ 1) at day 90 in n = 825 patients for each center. The Haaglanden Medical Center location Bronovo and Westeinde are combined in this forest plot and named “HL” (n = 17 inclusions). Error bars indicate 95% Wilson score confidence intervals. Overall incidence is indicated by the vertical dashed line. The null-hypothesis that centers were different with respect to incidence was not rejected (Likelihood ratio test comparing a logistic regression model with centers included as covariates to an intercept only model, P = 0.339). Abbreviations: AM: Amsterdam University Medical Center location AMC, Amsterdam, AS: Albert Schweitzer Hospital, Zwijndrecht, ASD: Albert Schweitzer Hospital location Dordwijk, Dordrecht, CZ: Catharina hospital, Eindhoven, EM: Erasmus Medical Center, Rotterdam, FG: Franciscus Gasthuis, Rotterdam, FV: Franciscus Vlietland, Schiedam, HL: Haaglanden Medical Center location Bronovo and Westeinde, Den Haag, LU: Leiden University Medical Center, Leiden, MZ: Maasstad Hospital, Rotterdam, RG: Reinier de Graaf Hospital, Delft, VU: Amsterdam University Medical Center location VU, Amsterdam, ZL: Zuyderland Medical Center, Heerlen, ZLS: Zuyderland Medical Center, Sittard-Geleen
Discussion
In this prospective study 67.8% (95%CI: 64.5 − 70.9%) of all responders suffered from chronic pain (NRS ≥ 1) at day 90 after visiting the emergency department with acute pain. The other commonly used definitions showed incidences varying between 24.3 and 58.2%. In the past, studies reported incidences of chronic pain varying from 11 to 62,7 [11, 16, 19, 21, 23, 26, 27]. Our concluded high cumulative incidence of chronic pain is explained by differences in the used chronic pain definitions. Using an NRS ≥ 1 at 90 days as cutoff for chronic pain, our incidence rate is quite comparable to studies reporting any degree of pain (NRS > 1). Using a higher cutoff of NRS ≥ 4, our incidence of chronic pain (24.2%) is comparable to other studies using similar cutoff values. Until 2019, the definition of chronic pain was a point of discussion. In 2019, the International Association for the Study of Pain reached a consensus defining chronic pain as any pain persisting after the expected healing period of three months. Although defining chronic pain as an NRS ≥ 1 after three months might lead to an overestimation of the incidence of chronic pain, we did see a significant decrease in the HRQOL and EQ-5D-5 L using this definition. One might argue that any pain (NRS ≥ 1) after 90 days can be considered chronic pain. Setting a stricter definition might lead to an underestimation of chronic pain.
With a more liberal threshold of chronic pain, more patients will be diagnosed with chronic pain and could potentially benefit from specialized chronic pain treatment. This could include extra surveillance by the general practitioner as well as extra outpatient appointments with for example the surgeon or the neurologist.
This study has several limitations. Firstly, consistent with literature, the percentage of patients with incomplete follow-up was considerable. Although this could have led to a false estimate of the incidence of chronic pain, the similar incidence of chronic pain in the complete and completed (with imputed data) analyses and the similar patient characteristics of the responders and non-responders, indicates that our estimate is valid. Using MICE with missing data above 40–50% shows increasing variability of effect estimates and should only be considered as hypothesis generating [36]. Nevertheless, MICE was used in addition to our results as a means of a sensitivity analysis and because of the large number and nearly 100% availability of patient characteristics in the non-responder group. Another limitation is of the difference in baseline characteristics of responders and non-responders. These differences could influence the incidence rate, the EQ-5D and HRQOL scores and perceived pain-related hindrance. As mentioned above, we took the baseline characteristics into account in the imputation process. Since calculating the incidence rate in non-imputated and imputated data yielded similar results, the differences in baseline characteristics probably did not influence our findings.
Also, there is a discrepancy between the number of patients reporting an NRS ≥ 1 at day 90 (559 patients) and the number of patients reporting pain at the four weeks prior to day 90 (64% of those 559 patients). Given the burden for patients, we did not ask patients for a daily pain score from day 0 (ED visit) till day 90. Asking an NRS score every day might have decreased the response rate even further. There are several possible explanations for this discrepancy. Firstly, there might be a recall bias by patients when asked for pain the last 4 weeks. Secondly, patients could have misunderstood the question. For example, the interpretation could have been that we wanted to know whether they had continuous pain the previous 4 weeks. Regardless of the reason for this discrepancy, we have shown that patients with an NRS ≥ 1 at day 90 have a decreased quality of life and increased hindrance due to pain at day 62–90. This suggest that, although not all patients report pain in the previous four weeks, there is a significantly increased burden of disease compared to patients who did not report pain at day 90.
Furthermore, not all eligible patients were included by our students and researchers because of the crowded and busy emergency departments. This was countered with inclusions 24/7 and with extra dedicated students and researchers for inclusion during the busiest hours. Despite these measures, probably eligible patients were missed. Unfortunately, due to privacy restrictions, baseline characteristics of included and non-included patients could not be compared. Not including eligible patients might have led to a selection bias.
Patients were included in 15 different hospitals, with different ED populations and attendance. The variability between these hospitals ensured the generalizability of our study population to the general Dutch population. However a large number of patients were included in just a few of the participating hospitals. This could influence the generalizability of the results. There were no differences among hospitals regarding the incidence rate of chronic pain. This makes a bias less likely.
We could not study whether the aspect of pain, namely neuropathic or nociceptive pain, influences the risk of chronification. Due to our study design, with patients self-reporting their pain, we lacked data on this topic.
Finally, inclusions were stopped in the end of April 2020 due to COVID-19. The early termination of the study due to covid-19 resulted in a lower number of included patients. This lower number of included patients, combined with a higher percentage of loss to follow-up, may have had consequences for the calculated cumulative incidence of chronic pain.
Despite the above mentioned limitations we think our study offers a valuable contribution on the incidence of chronic pain in patients visiting the emergency department with any form of acute pain. This study can be used to direct further research in the prevention of chronic pain after emergency department visit. We showed that the incidence of chronic pain after visiting the emergency department with any form of acute pain is high. Furthermore, regardless of the used definition, chronic pain is associated with a lower HRQOL and more hindrance due to pain. Future research could study whether the risk for chronification is different for nociceptive or neuropathic pain. Ultimately, the goal is to implement interventions to prevent the development of chronic pain.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The author thanks all the researchers and physicians of all the 15 participating inclusion sites* for the collection of data for this study. The PRACTICE study group thanks Euroqol Research Foundation for the use of the Euroqol-5D-5 L Dutch tariff, the RAND Corporation for the use of the RAND Short Form 36 and MD Anderson Centre for the use Brief Pain Inventory (Hays & Shapiro, 1992; Steward, 1992; Ware & Sherbourne, 1992; Versteegh et al., 2016). This survey was reprinted with permission from the RAND Corporation. Copyright © the RAND Corporation. RAND’s permission to reproduce the survey is not an endorsement of the products, services, or other uses in which the survey appears or is applied.The application used for the PRACTICE study is developed by Brightfish B.V.
Author contributions
S. MOL: Collected data, performed the analyses and wrote the manuscript. Coordinating investigator/project leader.A. V. BROWN: Conceived and designed the study, principal investigator of the PRACTICE study.T. M. KUIJPER: Statistician and edited the manuscript.M. G. BOUWHUIS: Collected data and edited the manuscript.B. de GROOT: Collected data and edited the manuscript.A. J. OUT: collected data and edited the manuscript.M. G.IBELINGS: collected data and edited the manuscript.J. S. H.A. KOOPMAN: Coordinating investigator Maasstad Hospital and edited the manuscript.
Funding
The authors received unrestricted grants from the Dutch Emergency Medicine Research fund (SGO-fonds) and the Stichting Coolsingel research fund for conducting this study.
Data availability
Data is available upon reasonable request to the corresponding author (koopmanj@maasstadziekenhuis.nl).
Declarations
Ethics approval and consent to participate
The Medical research ethics committee (METC, Protocol 2018-39) approved the study. Local approval was obtained by all participating centres and was conducted in accordance to the principles of the Declaration of Helsinki. Patients provided written informed consent according to the procedure approved by the METC.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Berben SA, et al. Pain prevalence and pain relief in trauma patients in the Accident & Emergency department. Injury. 2008;39(5):578–85. [DOI] [PubMed] [Google Scholar]
- 2.Cordell WH, et al. The high prevalence of pain in emergency medical care. Am J Emerg Med. 2002;20(3):165–9. [DOI] [PubMed] [Google Scholar]
- 3.Gaakeer MI, et al. [Acute pain at the emergency department: better treatment required]. Ned Tijdschr Geneeskd. 2011;155:A2241. [PubMed] [Google Scholar]
- 4.Tcherny-Lessenot S, et al. Management and relief of pain in an emergency department from the adult patients’ perspective. J Pain Symptom Manage. 2003;25(6):539–46. [DOI] [PubMed] [Google Scholar]
- 5.Gupta A, et al. Evidence-based review of the pharmacoeconomics related to the management of chronic nonmalignant pain. J Pain Palliat Care Pharmacother. 2010;24(2):152–6. [DOI] [PubMed] [Google Scholar]
- 6.Gustavsson A, et al. Socio-economic burden of patients with a diagnosis related to chronic pain–register data of 840,000 Swedish patients. Eur J Pain. 2012;16(2):289–99. [DOI] [PubMed] [Google Scholar]
- 7.Hruschak V, Cochran G. Psychosocial predictors in the transition from acute to chronic pain: a systematic review. Psychol Health Med. 2018;23(10):1151–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meerding WJ, et al. Demographic and epidemiological determinants of healthcare costs in Netherlands: cost of illness study. BMJ. 1998;317(7151):111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raftery MN, et al. The economic cost of chronic noncancer pain in Ireland: results from the PRIME study, part 2. J Pain. 2012;13(2):139–45. [DOI] [PubMed] [Google Scholar]
- 10.Sleed M, et al. The economic impact of chronic pain in adolescence: methodological considerations and a preliminary costs-of-illness study. Pain. 2005;119(1–3):183–90. [DOI] [PubMed] [Google Scholar]
- 11.Althaus A, et al. Development of a risk index for the prediction of chronic post-surgical pain. Eur J Pain. 2012;16(6):901–10. [DOI] [PubMed] [Google Scholar]
- 12.Bouhassira D, et al. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136(3):380–7. [DOI] [PubMed] [Google Scholar]
- 13.Breivik H, et al. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287–333. [DOI] [PubMed] [Google Scholar]
- 14.Elliott AM, et al. The epidemiology of chronic pain in the community. Lancet. 1999;354(9186):1248–52. [DOI] [PubMed] [Google Scholar]
- 15.Long DM. Bonica’s management of pain.. 3rd edition. ed, ed. J.D.C. Loeser, S.R. Vol. Volume 21. 2001: Lippincott Williams & Wilkins,. Issue 6, 527–528.
- 16.Moore CM, Leonardi-Bee J. The prevalence of pain and disability one year post fracture of the distal radius in a UK population: a cross sectional survey. BMC Musculoskelet Disord. 2008;9:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nickel R, Raspe HH. [Chronic pain: epidemiology and health care utilization]. Nervenarzt. 2001;72(12):897–906. [DOI] [PubMed] [Google Scholar]
- 18.Ohayon MM, Stingl JC. Prevalence and comorbidity of chronic pain in the German general population. J Psychiatr Res. 2012;46(4):444–50. [DOI] [PubMed] [Google Scholar]
- 19.Peters ML, et al. Predictors of physical and emotional recovery 6 and 12 months after surgery. Br J Surg. 2010;97(10):1518–27. [DOI] [PubMed] [Google Scholar]
- 20.Picavet HS, Schouten JS. Musculoskeletal pain in the Netherlands: prevalences, consequences and risk groups, the DMC(3)-study. Pain. 2003;102(1–2):167–78. [DOI] [PubMed] [Google Scholar]
- 21.Pierik JG, et al. Incidence and prognostic factors of chronic pain after isolated musculoskeletal extremity injury. Eur J Pain. 2016;20(5):711–22. [DOI] [PubMed] [Google Scholar]
- 22.Smith BH, et al. Is chronic pain a distinct diagnosis in primary care? Evidence arising from the Royal College of General Practitioners’ oral Contraception study. Fam Pract. 2004;21(1):66–74. [DOI] [PubMed] [Google Scholar]
- 23.Traeger AC, et al. Estimating the risk of Chronic Pain: Development and Validation of a Prognostic Model (PICKUP) for patients with Acute Low Back Pain. PLoS Med. 2016;13(5):e1002019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Treede RD, et al. A classification of chronic pain for ICD-11. Pain. 2015;156(6):1003–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Tulder MW, et al. Chronic low back pain in primary care: a prospective study on the management and course. Fam Pract. 1998;15(2):126–32. [DOI] [PubMed] [Google Scholar]
- 26.Williamson OD, et al. Predictors of moderate or severe pain 6 months after orthopaedic injury: a prospective cohort study. J Orthop Trauma. 2009;23(2):139–44. [DOI] [PubMed] [Google Scholar]
- 27.Rivara FP et al. Prevalence of pain in patients 1 year after major trauma. Arch Surg, 2008. 143(3): pp. 282-7; discussion 288. [DOI] [PubMed]
- 28.Berube M, et al. Acute to chronic pain transition in extremity trauma: a narrative review for future preventive interventions (part 2). Int J Orthop Trauma Nurs. 2017;24:59–67. [DOI] [PubMed] [Google Scholar]
- 29.Shervin N, et al. Comparison of paper and computer-based questionnaire modes for measuring health outcomes in patients undergoing total hip arthroplasty. J Bone Joint Surg Am. 2011;93(3):285–93. [DOI] [PubMed] [Google Scholar]
- 30.Hays RD, Shapiro MF. An overview of generic health-related quality of life measures for HIV research. Qual Life Res. 1992;1(2):91–7. [DOI] [PubMed] [Google Scholar]
- 31.Versteegh M, et al. Dutch tariff for the five-level version of EQ-5D. Value Health. 2016;19(4):343–52. [DOI] [PubMed] [Google Scholar]
- 32.Steward ALS, Hayes C. R.D., Summary and discussion of MOS measures, in measuring functioning and Well-Being: the Medical Outcome Study Approach. Duke University Press; 1992. pp. 345–71.
- 33.Ware JE Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 34.van Buuren S. G.-O.K., Mice: multivariate imputation by chained equations in R. Journal of Statistical Software. J Stat Softw, 2011. 45(3).
- 35.R Development Core Team. R: A Language and Environment for Statistical Computing,. 2017.
- 36.Madley-Dowd P, et al. The proportion of missing data should not be used to guide decisions on multiple imputation. J Clin Epidemiol. 2019;110:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available upon reasonable request to the corresponding author (koopmanj@maasstadziekenhuis.nl).