Abstract
Introduction
The intestinal microbiota plays a crucial role in health and disease. This study aimed to assess the composition and functional diversity of the intestinal microbiota in donkeys and cows by examining samples collected from different segments of the digestive tract using two distinct techniques: direct swab sampling and faecal sampling.
Results
In this study, we investigated and compared the effects of multiple factors on the composition and function of the intestinal microbial community. Approximately 300 GB of metagenomic sequencing data from 91 samples obtained from various segments of the digestive tract were used, including swabs and faecal samples from monogastric animals (donkeys) and polygastric animals (cows). We assembled 4,004,115 contigs for cows and 2,938,653 contigs for donkeys, with a total of 9,060,744 genes. Our analysis revealed that, compared with faecal samples, swab samples presented a greater abundance of Bacteroidetes, whereas faecal samples presented a greater abundance of Firmicutes. Additionally, we observed significant variations in microbial composition among different digestive tract segments in both animals. Our study identified key bacterial species and pathways via different methods and provided evidence that multiple factors can influence the microbial composition. These findings provide new insights for the accurate characterization of the composition and function of the gut microbiota in microbiome research.
Conclusions
The results obtained by both sampling methods in the present study revealed that the composition and function of the intestinal microbiota in donkeys and cows exhibit species-specific and region-specific differences. These findings highlight the importance of using standardized sampling protocols to ensure accurate and consistent characterization of the intestinal microbiota in various animal species. The implications and underlying mechanisms of these associations provide multiple perspectives for future microbiome research.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12866-024-03696-5.
Keywords: Metagenomic analysis, Intestinal microbiota, Digestive tract segments, Microbiota composition
Background
The intestinal microbiota is a complex ecosystem consisting of diverse microbial communities that symbiotically coexist with their host organisms [1]. These microbial communities are known to influence various aspects of host physiology, including nutrient metabolism, immune system function, and overall health [2]. Therefore, it is important to study the composition, function and interactions of the intestinal microbiota, which can be influenced by factors such as diet, antibiotic use, lifestyle, genetics, and age [3–5]. However, while the variability in the results of experiments investigating the influences of these factors can be reduced by using a control group, sampling methods and different digestive tract segments and structures can still affect the results.
The collection of samples from specific segments of the digestive tract allows for a more detailed understanding of regional microbial communities [6]. Variations in microbial composition among different segments can be linked to unique physicochemical conditions and interactions between the host and microbes in each area. For example, the small intestine has fewer bacteria and higher levels of oxygen than the large intestine does [7], highlighting the importance of taking these regional distinctions into account when researching the gut microbiota.
A prerequisite for reliable microbiota analysis is an effective and consistent sampling method [8]. Many studies have explored how sampling methods affect results, and assessing different sampling techniques is crucial for accurately describing the gut microbiota and guiding clinical diagnosis and treatment [9, 10]. Rectal swabs and faecal samples present similar microbiome composition profiles, suggesting that faecal sampling is a viable approach for investigating the microbiome [8]. In contrast, another study indicated that such samples may not accurately represent the microbial communities present in different segments of the digestive tract [11]. Additionally, it is commonly believed that the distribution of intestinal bacteria in different intestinal segments varies and is related to the specific functions of each intestinal segment [12]. Previous studies have often used random sampling methods in gut microbiota research, which lack systematicity and do not consider the variations across different digestive tract segments in various animal types. This approach can result in incomplete representations of the complex microbial ecosystems within the gastrointestinal tract [13, 14].
To address these challenges, there is an urgent need to understand the effects of multiple factors on the composition and function of the intestinal microbiota. Donkeys and cows, as typical monogastric and polygastric animals, are essential domesticated animals that make valuable contributions to various agricultural industries and serve as food sources for humans. The different segments of the digestive tract in these animals are easily distinguishable, which can help achieve our goal of comparing different intestinal segments [10, 15, 16]. Furthermore, metagenomics is a specific approach used to study the genomes of entire microorganismal communities, allowing for the direct extraction of genetic information from environmental samples without the need for microbial isolation and cultivation. In gut microbiome research, the advantages of metagenomics include providing a comprehensive understanding of the microbial community, revealing the functional potential of microbes, and discovering new microbial species. The application of metagenomics also involves studying the connection between the gut microbiota and diseases such as obesity and diabetes [17–19].
In this study, we utilized two different sampling methods (faecal and swab sampling) to collect samples from various digestive tract segments of donkeys and cows (Fig. 1). Metagenomic sequencing and analysis were then used to determine the complete microbiome profile and functionally annotate each sample. We compared the microbial diversity in different digestive tract segments and identified potential key bacteria species and pathways using different sampling methods. Finally, further statistical analysis systematically revealed the shared and distinct features of both microbial composition and function associated with different sampling methods and digestive tract segments in monogastric and polygastric animals.
Fig. 1.
Graphical representation of samples collected from distinct sites along the digestive tract of cows and donkeys
Materials and methods
Animals and experimental design
Three healthy Huabei donkeys and three healthy Holstein cows were housed in tie stalls for the 8-month experiment. These animals were purchased and raised by us at the Northern Resource Center, which belongs to our institute. All the animals were raised according to standard livestock management methods. The offspring were fed the milk of their mothers, and the adults were fed mainly maize straw and alfalfa hay. They animals were provided sufficient drinking water. The animals were housed separately throughout the study to allow accurate daily monitoring of food and water intake and stool output. The animals used in this study had no diarrhoea or other digestive conditions and had not been administered antibiotics or other drugs for at least 2 months prior to collection. Prior to being exsanguinated, all the animals were fed a forage-based diet for 1 month (Table S1). This study was approved and authorized by the Ethics Committee of the Laboratory of Animal Sciences of Peking Union Medical College (no. XZG22001).
Sampling protocol
On the last day of the experiment, all the animals were stunned and exsanguinated, and their internal organs were immediately dissected. The gastrointestinal tract (GIT), which includes the stomach, rumen, reticulum, omasum, abomasum, small intestine, caecum, colon, and rectum, was separated for analysis. We collected samples simultaneously from different gut segments, including the stomach, rumen, reticulum, omasum, abomasum, small intestine, caecum, and colon, as well as fresh faeces from the rectum. Rectal swabs were obtained by inserting a sterile cotton swab (Puritan Medical, Guilford, ME, USA; Cat. Number-25-3306-U) 20–30 mm into the rectum and rotating the swab against the bowel wall. The swabs were withdrawn and placed in 5 ml Eppendorf tubes prefilled with 500 µl of phosphate-buffered saline (pH 7.0). Faecal material was collected in a sterile container (Sarstedt, Nümbrecht, Germany; Cat number 80.734.311) and kept on ice during transport to the laboratory, where it was stored at − 20 °C until further processing. Three different samples the each gut segment were collected via both the swab (S) and faecal (F) methods, pooled and homogenized in sterile potassium phosphate buffer (0.1 M, pH 7.2) containing 15% glycerol (v/v). The homogenized samples were then immediately dispensed into cryotubes. Next, a total of 91 samples were subjected to metagenome sequencing (Table S1). Each animal’s stomach, small intestine, caecum, colon and rectum samples were alphabetically designated A through E. The cow stomach samples were further designated A1, A2, A3, and A4 to represent the rumen, reticulum, omasum, and abomasum. The samples were placed in liquid nitrogen immediately after collection and then transferred to a -80 °C ultralow temperature freezer for storage. All the samples were subjected to DNA extraction within three days after collection. The sample groupings are displayed in Table S2.
DNA extraction and metagenomic sequencing
Total DNA was extracted from all GIT samples (approximately 200 mg per sample) via repeated bead beating using a mini-bead beater (Biospec Products, Bartlesville, USA) [20]. The integrity of the extracted DNA was measured via electrophoresis on 0.8% agarose gels, and the quality and quantity were determined using a Nanodrop ND-1000 (Thermo Scientific, Wilmington, USA). Following the manufacturer’s instructions for the TruSeq DNA PCR-Free Library Preparation Kit (Illumina, San Diego, CA, USA), high-quality DNA from each sample was used to construct a metagenomic library with an insert size of 350 bp, which was then sequenced on an Illumina NovaSeq platform. Metagenomic analysis was then performed to examine the microbial composition and functional potential of the intestinal microbiota.
Data quality control
Fastp [21] (version 0.23.2) software was used to filter out low-quality reads with ambiguous ‘N’ bases (more than 7 consecutive ‘N’ bases), and the quality value was set with the parameters ‘--q 20 --u 50 -n 7’. Next, sequence reads were mapped against reads mapping to the reference genome (hg37) using Burrows-Wheeler Aligner (BWA_software [22] with default parameters to remove the host genome. The remaining high-quality reads were further analysed.
Metagenomic assembly
Genome assembly: Using megahit [23] (version 1.2.9) and the parameters ‘--min-contig-len 500’, the high-quality sequences of each sample were spliced and assembled separately to construct the scaffolds. The K-mer was set for each sample between 21 and 141 to produce the optimal results. The clean data were compared to the assembled scaffold of each sample via Bowtie 2 [24] (version 2.2.6, parameter: -- unconc) to obtain the unused PE reads. The unmapped reads for mixed assembly were collected again to find low-abundance species in the sample via megaHit. Finally, scaffolds less than 500 bp in length were filtered out for further analysis.
Gene catalogue construction, taxonomic annotation, and abundance profiling
MetaGeneMark [25] (version 3.38) with default parameters was used to predict all open reading frames (ORFs) of the assembled scaffolds. Then, the ORFs whose lengths were less than 100 nt were filtered out. For the ORFs of each sample and mixed assembly, we used CD-HIT [26] software (version: 4.8.1; parameters: - c 0.95, - G 0, - aS 0.9, -g 1, -d 0) to remove redundancy and obtain a nonredundant initial gene catalogue using default settings with greater than 95% identity over 90% of the shorter ORF length clustered together via greedy pairwise comparison. To determine the quantity of genes and reads, these sequences were mapped to the gene catalogue (unigenes) via Bowtie 2 (version: 2.2.6) with the parameters ‘--end-to-end, --sensitive, -I 200, -X 400’. The genes that appeared in no more than 2 reads in each sample were filtered out, and the gene catalogue was ultimately used for subsequent analysis.
The unigenes were aligned to the integrated nonredundant (NR) database (version: 2018.01) via DIAMOND [27] (version: 0.9.22; parameter: BLASTP, value ≤ 1e-5). For the final aligned results of each gene, significant matches were defined according to a score ≥ maximum score * 0.9, and the taxonomic level was determined by using the lowest common ancestor (LCA) algorithm implemented in MEGAN [28]. A taxonomic group’s abundance in each sample was the sum of the abundance of genes annotated to a feature. To avoid multispecies classification and guarantee its biological significance simultaneously, the LCA algorithm was employed to take the last unambiguous classification level as the species annotation result.
Functional analysis
Unigenes were compared against functional databases, including the Kyoto Encyclopedia of Genes and Genomes (KEGG version: 2018.01), Nonsupervised Orthologous Groups (eggNOG version: 4.5), Carbohydrate-Active enZYmes (CAZy) database, Comprehensive Antibiotic Resistance Database (CARD version: 3.1.4) and Transporter Classification Database (TCDB). DIAMOND (with BLASTP, E value ≤ 1e-5) was used to analyse the KEGG, eggNOG and TCDB databases. To filter the results for each sequence, the DIAMOND parameter “--max-target-seqs 1” was used to retain unique comparison results. Carbohydrate-active enzymes (CAZymes) were predicted with the tool dbCAN 3.0 [29], using the HMMER: dbCAN (E value < 1e-15, coverage > 0.35), DIAMOND: CAZy (E value < 1e-102) and HMMER: dbCAN-sub (E value < 1e-15, coverage > 0.35) options. The comparison results therefore allowed the relative abundance of different functional levels to be calculated (i.e., where the relative abundance of each functional level is equal to the sum of the relative abundance of genes annotated at that functional level). The CARD was used in conjunction with the accompanying resistance gene identifier (RGI) software to search for antibiotic resistance ontology (ARO) associations among the unigenes (using RGI’s built-in BLASTP function, with the default E value of < 1e-30). The relative abundance of each ARO was calculated on the basis of the RGI and unigene abundance information [30].
Statistical analysis and graphing
Through the above computations, we were able to generate several datasets, including a gene catalogue and microbial compositions and functional annotations, for each sample. Using these data, we analysed the shared and unique paterrns across different samples, animals, sampling methods and digestive tract segments via multiple methods, such as principal coordinate analysis (PCoA) and linear discriminant analysis effect size (LEfSe). QIIME2 and R software were used to carry out these statistical analyses. Codifference networks were established on the basis of the significantly different functions and species between different sampling methods with Spearman’s test (|r| > 0.90, P < 0.05) and visualized via Cytoscape.
Results
Overview of the microbial composition and function
Here, 91 samples from monogastric (donkeys) and polygastric (cows) animals, representing five and eight digestive tract regions, respectively, were collected via two sampling methods and used for metagenome sequencing. We obtained approximately 1.66 TB of metagenomic sequencing data (4,605,907,824 raw reads) via high-throughput sequencing. After quality control, a total of 1.5 Tb of clean, high-quality data remained, with an effective data quality control rate of approximately 98.5%.
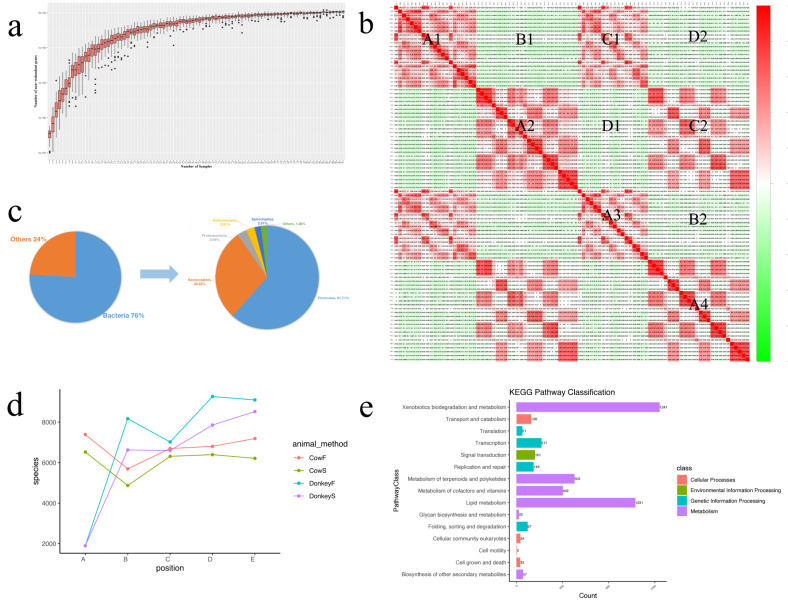
We assembled 4,004,115 contigs for cows and 2,938,653 for donkeys and obtained 9,060,744 genes with an average length of 553 bp (ranging from 102 bp ~ 10,912 bp),95% identity, and 90% coverage (Fig. S1a). The gene length was distributed primarily between 100 and 1,000 (Fig. S1b). To evaluate the rationality and adequacy of the identified genes, rarefaction analysis was performed with random sampling 50 times, and the core-pan gene rarefaction curve approached plateaus (Fig. 2a, Fig. S1c). A heatmap was used to calculate the correlations between the samples with the gene table (Fig. 2b). The results revealed a positive correlation between digestive tract segments within the same animal and a negative correlation otherwise. Additionally, the results of the digestive tract segments from different cows were highly consistent, especially the four stomachs and the large intestine (caecum, colon, rectum).
Fig. 2.
Overview of commonalities and differences among all samples. (a) Pangene rarefaction curve. The curve nearly plateaued when sufficient sequence data were inputted. (b) Heatmap showing the correlation between the samples and the gene table. We divided the heatmap into four parts, A, B, C, and D, whose primary variables were digestive tract segments, animals, sampling methods and animal and sampling methods, respectively. (c) Microbial composition of all samples at the kingdom level and the top 5 dominant phyla of bacteria. (d) The composition of microbial species depending on the animal (donkey, cow), sampling method (S: swab, F:faecal) and digestive tract segment (A-E: stomach, small intestine, caecum, colon, rectum). CowF denotes the cow faecal samples. (e) The enriched KEGG pathways
In our study, we focused on exploring the bacteria that made up 76% of the entire microbial community (Fig. 2c). There were 135 phyla (37 non-Candidatus phyla, 98 Candidatus phyla), 90 classes, 189 orders, 436 families, 2,133 genera and 12,213 species of microorganisms. The five most abundant phyla were Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria and Spirochaetes, and the microbial compositions of all the samples at different taxonomic levels are listed in additional file 2: Fig. S2. At the species level, the number of microbes was greater in donkey faecal samples and digestive tract segments aside from the stomach than that in cow samples (Fig. 2d). Additionally, we conducted a functional analysis of the microbiota using several databases to investigate functional differences (Fig. S3). We assessed the enrichment of KEGG metabolic pathways, and microbial functions were related to metabolism, particularly xenobiotic biodegradation and metabolism, lipid metabolism, and metabolism of terpenoids and polyketides (Fig. 2e). In other databases, ‘Replication, recombination and repair’ was the most enriched function in the Clusters of Orthologous Groups (COG) database, whereas the ‘ABC1, ABC2, ABC3 Superfamilies’ was enriched in 41% of the samples according to the TCDB, and ‘antibiotic efflux’ and ‘glycoside hydrolases’ were particularly enriched in the CARD and CAZy database (Fig. S3, S4).
The impact of faecal and rectal swab sampling on microbial community composition
To investigate the differences in the sampling methods used for studying the intestinal microbiota of monogastric and polygastric animals, we conducted correlation analyses on the donkey (Fig. 3) and cow (Fig. 4) samples.
Fig. 3.
Compared with the two donkey sampling methods. (a) Partial least squares discriminant analysis (PLS-DA) at the phylum level. (b) Analysis of similarities (ANOSIM) at the phylum level. (c) Top 30 phyla. (d) The species compositions of the samples collected using the two sampling methods. (e) The distribution of the unique species. (f) Species variable importance dot plot. The horizontal axis is the measure of importance, and the vertical axis is the phylum name in order of importance (the same applies below). (g) Linear discriminant analysis (LDA) effect size (LEfSe) analysis
Fig. 4.
The two sampling methods were compared. (a) PLS-DA results of bacteria at the phylum level. (b) ANOSIM results of bacteria at the phylum level. (b) Top 30 differentially abundant bacteria at the phylum level. (d) The microbial composition of the samples collected via the two methods. (e) The distribution of unique microbial species. (f) Species variable importance dot plot
Initially, we assessed the impact of the sampling method on the microbial composition in donkeys. At the phylum level, PLS-DA revealed that PC1 and PC2 explained 9.75% and 10.07% of the variation, respectively (Fig. 3a, Fig. S5). ANOSIM was subsequently conducted, which revealed significant differences between the donkey sampling methods (P < 0.05, R = 0.1454) (Fig. 3b, Fig. S6). The analysis of microbial composition at various taxonomic levels revealed Firmicutes (75.47%, 64.76%) and Bacteroidetes (17.23%, 24.69%) as the predominant bacteria in both the swab and faecal samples at the phylum level (Fig. 3c). This pattern was also consistent across other taxonomic levels (Fig. S7). At the species level, 389 and 109 unique species were identified by only one sampling method (Fig. 3d, Fig. S8). Following differential analysis via STAMP, bacteria unique to one sampling method, such as Pseudoscardovia, Raoultibacter, Enteroscipio and Senegalimassilia, were identified at the genus level. The results for other taxonomic levels can be found in the Supplementary files: Fig. S9 and Table S3. The differential functions identified through STAMP analysis are displayed in Table S4. Faecal samples presented a greater number of species recognized by only one sampling method compared to the digestive tract samples (Fig. 3e). We further utilized the random forest algorithm to classify microbial communities effectively and accurately, identifying Firmicutes, Bacilli, Eggerthellales, Eggerthellaceae, Libanicoccus and Hyointestinalis as the most abundant phyla (Fig. 3f, Fig. S10). To explore the differences in microorganisms among the donkey samples collected via different sampling methods, we conducted an LEfSe analysis (Fig. 3g). In the rectal swab samples, Proteobacteria was the most abundant phylum (LDA = 4.25), and Tannerellaceae in the phylum Bacteroidetes was significantly abundant at the family level. The genus Tannerella, also in the phylum Bacteroidetes, was the most abundant bacteria. Additionally, 18 other significantly abundant bacteria were identified at the species level, with Campylobacter jejuni being the most abundant (LDA = 4.18). In the faecal samples, Morganellaceae under Enterobacterales and Moraxellaceae under Pseudomonadales were the most abundant bacterial families, with increased levels of Xenorhabdus in the Morganellaceae family. All three of these families belong to the Proteobacteria phylum. Forty-five other significantly abundant microorganisms were identified at the species level, with Lactobacillus hayakitensis being the most abundant (LDA = 4.29).
Next, the cow samples were analysed using the same protocol. PLS-DA revealed that PC1 and PC2 explained 8.21% and 6.17% of the variation (Fig. 4a, Fig. S5), and ANOSIM yielded similar results (P < 0.05, R = 0.1454) (Fig. 4b, Fig. S6). The composition of microorganisms of the samples collected via different sampling methods displayed both similarities and differences at each taxonomic level (Fig. 4c, Fig. S7). At the species level, 630 and 96 species were recognized by only one method (Fig. 4d), and the results for the other levels are displayed in the Supplementary figures: Fig. S8. STAMP analysis revealed that Actinobacteria, Firmicutes, Fusobacteria, Chloroflexi, and Lentisphaerae were differentially abundant, and the results for the other taxonomic levels are displayed in the Supplementary files: Fig. S9 and Table S5. All differential functions identified by STAMP analysis are listed in Table S6. The number of unique species found in faecal samples across every digestive tract segment was significantly greater in cows than in donkeys (Fig. 4e). Actinobacteria was identified as an important phylum by the random forest algorithm for distinguishing between samples collected via the two sampling methods (Fig. 4f), with other key microbes at different taxonomic levels shown in Fig. S10. When comparing cows to donkeys, not only the number but also the diversity of differentially abundant bacteria identified by LEfSe analysis were greater in cows than donkeys (Fig. S11). In the swab samples, the phyla Bacteroidetes, Proteobacteria and Spirochaetes were more abundant. At the class, order, family, genus and species levels, there were 8, 10, 15, 16, and 70 bacteria, respectively. The most significantly abundant bacteria were Bacteroidetes, Bacteroidia, Bacteroidales, Prevotellaceae, Prevotella and Prevotella ruminicola. In the faecal samples, the differentially abundant bacteria were concentrated at the species level, with a total of 100 significantly abundant bacteria. The abundances of Fusobacteria, Fusobacteriia, Fusobacteriales, Fusobacteriaceae and Fusobacterium were significantly greater. Two differentially abundant genera, Alkalilimnicola in the family Ectothiorhodospiraceae and Ruminobacter in the family Succinivibrionaceae, both belong to the Proteobacteria phylum. The significantly abundant species in the faecal samples with LDA scores greater than 4 included Sharpea azabuensis, Sarcina sp. DSM 11,001, Kandleria vitulina and Bifidobacterium pseudolongum.
The swab sampling method revealed a greater abundance of Bacteroidetes in both donkeys and cows than the faecal sampling method. Conversely, the faecal sampling method resulted in a relatively greater proportion of Firmicutes (Figs. 3c and 4c). To investigate the differences in microorganisms between the methods, we also used the Wilcoxon test at the genus and species levels for donkeys and cows. In cows, 401 of 2133 genera were significantly different between the two methods, with 21 of the 30 most abundant genera, such as Prevotella, Roseburia, Treponema, Bacteroides, Sharpea, Kandleria, Bifidobacterium, and Anaplasma, exhibiting varying distributions. Additionally, 2024 out of 9089 species were significantly differentially abundant, with 22 being among the 30 most abundant species. For donkeys, only 192 genera showed significant differences due to the sampling method, with 9 genera being among the 30 most abundant genera. At the species level, there were 995 and 10 significantly differentially abundant species for cows and donkeys, respectively. Compared with the swab sampling method, the faecal sampling method was able to identify more endemic species, particularly in cows. The microbial composition of the small intestine was found to be the most affected by the sampling method, with that of the stomach being less affected in donkeys. Whether it was differentially abundant, dominant, or endemic bacterial species, the sampling method had a significant effect on the microbial distribution, with cows being more sensitive to these changes.
The bacterial microbiomes of different digestive tract segments were compared
Next, we compared the bacterial compositions in various segment of the donkey digestive tract. The bacterial composition and abundance showed both similarities and differences at different levels, with the stomach being notably distinct from other intestinal segments (Fig. 5a and b, Fig. S12). All samples from donkeys contained 14 core phyla (Fig. 5c), with the majority of these phyla being present in all digestive tract segments (40/126) and intestinal segments (66/126) (Fig. 5d). At the species level, most microorganisms belonging to these phyla were found in intestinal segments as well as all digestive tract segments (Fig. 5e). The bacterial community in the stomach differed significantly from that in the other segments, and the rectum contained more endemic species than the other digestive tract segments did. When ranked by variable importance value, the top 30 microbial characteristics collectively represented 50.7% of the total microbial composition, with Armatimonadetes having the highest value (Fig. 5F).
Fig. 5.
Comparison of donkey digestive tract segments. (a-b) Bacterial composition of donkey digestive tract segments at the genus and species levels. (c) Venn diagram of bacteria in different donkey digestive tract segments at the species level. (d) Flower plot of bacteria in different donkey digestive tract segments at the phylum level. (e) UpSet plot of bacteria in different donkey digestive tract segments at the phylum level. The horizontal axis is the combination of different digestive tract segments, and the vertical axis is the intersection size of the segment combination (the same below). (f) Variable importance dot plot of bacteria at the phylum level
In terms of the bacterial composition of the cow, the stomach showed the most significant differences compared with the other intestinal segments, followed by the small intestine. The caecum, colon, and rectum all presented high similarity (Fig. 6a and b, Fig. S13). Most microbes were found in all segments of the digestive tract at both the phylum (138/176) and species levels (Fig. 6c and d). Compared with the stomach of donkeys, the stomach of cows contains the most endemic species relative to other the digestive tract segments. The number of unique species in cows’ stomachs was notably greater than that in other intestinal segments, and cows had more core species than donkeys did. The top 30 microbial characteristics collectively represented 44.9% of the total microbial composition. Elusimicrobia was identified as having the highest variable importance value, although it did not differ substantially from other phyla that were present in cows but not donkeys (Fig. 6e).
Fig. 6.
Comparison of digestive tract segments in cows. (a-b) Bacterial composition of cow digestive tract segments at the genus and species levels. (c) Venn diagram of bacteria in different cow digestive tract segments at the species level. (d) UpSet plot of bacteria in the different cow digestive tract segments at the phylum level. (e) Variable importance dot plot of bacteria at the phylum level. (f) The species composition of the cow rumen, reticulum, omasum, abomasum and donkey stomach
Firmicutes and Bacteroidetes were the most abundant phyla (> 80%) in the intestinal segment. There were some differences in the microbial compositions of the stomach between donkeys and cows, with Firmicutes being the most abundant bacteria in donkeys and Bacteroidetes being more prevalent in cows. Additionally, Proteobacteria and Actinobacteria were the other dominant bacterial phyla. The dominant genera in the stomachs of donkeys and cows were Lactobacillus (~ 80%) and Prevotella (40%~60%). The bacterial composition in the caecum, colon, and rectum was more uniform than that in the stomach. At the species level, Lactobacillus equigenerosi, Lactobacillus hayakitensi, Lactobacillus crispatus, and Lactobacillus equicursoris made up more than 60% of the dominant species in the donkey stomach (Figs. S12, S13). The majority of species in the donkey stomach (1,992/2,330) were also found in cows, with nearly 80% (5950/7503) of the microbes present in all of the cow stomachs (Fig. 6F). LEfSe analysis of the rumen, reticulum, omasum and abomasum revealed various differences at different taxonomic levels (Fig. S14). In the rumen samples, the genera Dongia and 15 species, such as Streptomyces sp. BK335 and Bacillus alkalitelluris, were significantly more abundant. The reticulum had 2 dominant genera (Psychrosphaera, Ochrobactrum) and 32 dominant species, including Clostridiales bacterium and Bacteroides_sp_43_108. Four genera (Trinickia, Sphaerochaeta, Fibrisoma, Mannheimia) and 10 species, such as Micromonospora globispora and Trinickia soli were abundant in the omasum. Moreover, the abomasum had a greater abundance of bacteria across different taxonomic levels, including the phylum Proteobacteria, class Alphaproteobacteria, 5 orders (Rickettsiales, Enterobacterales), 6 families (Anaplasmataceae, Enterobacteriaceae), 10 genera (Anaplasma, Klebsiella), and 42 species, such as Anaplasma phagocytophilum and Staphylococcus aureus.
The dominant and key species of the microbial community structure in cows and donkeys
To confirm the similarities between the animals, we analysed the bacterial composition of all the samples. A comparison of the bacterial composition and abundance at different levels revealed similarities and differences between donkeys and cows (Fig. 7a and b, Fig. S15). PLS-DA revealed that PC1 and PC2 explained 10.6% and 6.28% of the variation, respectively (Fig. 7c). Additionally, NMDS, OPLS-DA and PCA yielded results similar to those of PLS-DA (Fig. S16). A Venn diagram was used to determine the number of species shared by different animals (Fig. 7d). There are 6,860 common species, accounting for 68% and 78% of the total species in the donkey and cow, respectively. However, only six of the 30 most abundant bacterial species were common between cows and donkeys. Compared with cows, donkeys have more unique species. The donkey digestive tract, excluding the stomach, contains a greater number of distinct species (Fig. 7e).
Fig. 7.
Comparison of monogastric and polygastric animals. (a-b) Bacterial composition of donkeys and cows at the genus and species levels. (c) PLS-DA results showing the differences in the bacterial species compositions of the animals. (d) Venn diagram of the number of species shared by different animals. (e) The number of unique species between the animals and their distributions. (f) Species variable importance dot plot
Spirochaetes was the phylum of bacteria that was more prevalent in cows, whereas Acidobacteria was substantially more abundant in donkeys (LDA > 2, P < 0.05; Fig. S17). The abundant classes in cows were Alphaproteobacteria, Spirochaetia and Gammaproteobacteria, whereas in donkeys, they were Flavobacteriia, Betaproteobacteria, Cytophagia, Sphingobacteriia, and Chitinophagia. Betaproteobacteria is a member of the class Proteobacteria, whereas the others belong to Bacteroidetes. At the order level, there were five significantly abundant bacteria in cows and eight in donkeys. In cows, these included Rickettsiales, Spirochaetales, Pseudomonadales, Aeromonadales, and Brachyspirales, whereas in donkeys, they included Flavobacteriales, Marinilabiliales, Burkholderiales, Pasteurellales, Cytophagales, Rhizobiales, Sphingobacteriales, and Chitinophagales. The number of significantly abundant families in cows and donkeys was nine and fourteen, respectively. The top three genera in cows were Prevotella in Bacteroidetes, Anaplasma in Proteobacteria, and Treponema in Spirochaetes. Prevotella was significantly greater in cows than in donkeys at all taxonomic levels (LDA = 4.70). The significantly abundant genera in donkeys included Bacteroides in Bacteroidaceae, Tannerella in Tannerellaceae, Xenorhabdus in Proteobacteria and ten more. In terms of species, there were 142 more abundant species in cows and 176 in donkeys. The more abundant species in cows included Prevotella ruminicola, Sharpea azabuensis and Kandleria vitulina, while the three most abundant species in donkeys were Lactobacillus equigeneros, Lactobacillus hayakitensis and Lactobacillus crispatus, which may be related to digestive function. Lactobacillus equigenerous was identified as a key species that can distinguish between groups (Fig. 7f, Fig. S18). All the differential microbes and functions identified through STAMP analysis are listed in Tables S7 and S8.
Functional comparison of the microbiomes obtained via different sampling methods
The functions of the bacteria were compared and grouped by animal, sampling method and digestive tract segment using the KEGG database, eggNOG, TCDB, CARD and the CAZy database. The differences were evaluated for statistical significance. To visualize the overall distribution of functions in each group, the top 30 most abundant results were selected and visualized in bar plots (Fig. S19, S20, S21S22, S23).
To further characterize the differential functions of the microbiome, we predicted the genes involved in the gut microbiome via Wilcoxon or Kruskal‒Wallis tests on the basis of the KEGG and CAZy databases (Fig. 8, Fig. S24). These genes were involved in various functions, such as the phospholipase D signalling pathway and betalain biosynthesis, and bacteria that performed these functions were significantly more abundant in the gut microbiome of cows than in that of donkeys. The majority of annotations related to toluene degradation and sesquiterpenoid and triterpenoid biosynthesis were significantly more prevalent in donkeys (Fig. S24a). Compared with the swab method, the faecal sampling method yielded results for 20 of the most abundant functions, with 11 in cows and 9 in donkeys (Fig. 8a and b). However, ‘biosynthesis of siderophore group nonribosomal peptides’ was an exception, as it was more abundant in the cow swab samples. Among the different digestive tract segments, the distributions of functions were more similar to each other in both cows (11/20) and donkeys (16/20) (Fig. 8c and d). In cows, ‘primary bile acid biosynthesis’, ‘atrazine degradation’, and ‘steroid degradation’ were more common in the caecum, colon and rectum, whereas ‘biosynthesis of siderophore group nonribosomal peptides’ was more common in the stomach and less common in the small intestine. In donkeys, retinol metabolism, primary bile acid biosynthesis, and toluene degradation were more common in the stomach, while the biosynthesis of the siderophore group of nonribosomal peptides was more common in the caecum, colon and rectum, and atrazine degradation was less common in the small intestine.
Fig. 8.
The results of microbiome functional comparisons and codifference networks. Comparison of the main KEGG functions of cows (a, c) and donkeys (b, d) grouped by sampling method and digestive tract segment. Comparison of the levels of GH, GT, CBM, CE, PL, and AA among different digestive tract segments of cows (e) and donkeys (f). (g) The distributions of function nodes (Nf), microbe nodes (Nm) and graph complexity (C) of the codifference network. Here, we used the formula (C = E-Nf-Nm + 1) to calculate the graph complexity (E represents the number of edges in the network). (h) The database sources of differential function nodes. (i) The major bacteria in different groups at the species level
In addition to the KEGG database, we also utilized the CAZy database to predict carbohydrate-active enzymes (CAZymes) in the gut microbiome to gain a deeper understanding of their activity. These CAZymes are categorized into six classes of enzymes, namely, glycoside hydrolase (GH), glycosyltransferase (GT), carbohydrate-binding module (CBM), carbohydrate esterase (CE), polysaccharide lyase (PL), and auxiliary activity (AA). Interestingly, genes encoding AAs were significantly more abundant in the gut microbiomes of donkey and faecal samples than in those of cow and swab samples (Fig. S24b, S24c, S24d). In cows, CE and PL were more abundant in swab samples. Additionally, the stomach presented a relatively high abundance of CBM, CE, GH, GT and PL, whereas the small intestine presented a relatively low abundance (Fig. 8e). Conversely, in donkeys, the stomach, small intestine and large intestine (caecum, colon, rectum) differed in the abundance of AA, CE, GT and PL (Fig. 8f). Cluster analysis was conducted on significantly different KEGG pathways among the groups, with most pathways being related to metabolism (Fig. S25).
On the basis of the distributions of significantly different functional classes and species, we created a codifference network to evaluate the relationships between species and functions. The networks between microbiomes and functions for various groups showed distinct differences, especially in the different digestive tract segments of donkeys (Fig. 8g). There were more connections in the groups representing different digestive tract segments in both species, with most nodes in the networks originating from the CAZy database and TCDB (Fig. 8h). After excluding less important nodes, we identified key functional nodes in the codifference network, which are listed in the Supplementary Note. Additionally, key subgraphs are listed in Figures S27-S31.
Discussion
In this study, a comprehensive analysis of the microbial communities in different segments of the digestive tract was conducted, and differences among species, digestive tract segments, and sampling techniques were evaluated. Previous studies have indicated that different sampling methods can influence the diversity of microbial communities in the samples [31], but the impact of sampling methods on the composition and functions of intestinal microbial communities has not been systematically investigated. This gap in research hinders our understanding of the complex relationship between the gut microbiome and human health. Taking the sampling method into account when determining the composition and diversity of the gut microbiome is crucial [32]. Research has shown that human microbiome composition can vary slightly depending on the type of sample collected, such as faecal samples versus rectal swab samples [11, 33]. However, studies have shown that rectal swabs and faecal samples obtained from pigs yield similar microbiome composition profiles [8]. The present study suggested that the microbial composition in samples collected from donkeys may be less affected by sampling methods; however, it was observed that faecal samples contained overall more bacteria, possibly due to the faecal sampling method, which enables the sampling of deeper surface crevices and the collection of more firmly attached microorganisms. LEfSe analysis revealed 69 bacterial species in donkeys that may be potentially associated with the sampling method, and most of these species belonged to the genus Lactobacillus and were distributed mainly in the stomach. On the other hand, the rectal swab samples contained relatively high levels of anaerobic species, such as Atopobium vaginae, Lannerella forsythia, and Clostridioides difficile. These findings revealed that the rectal swab samples contained relatively high levels of oxygen-tolerant mucosa-adherent microbiota [33, 34]. However, regardless of the dominant bacterial species, the sampling method had a greater impact on the microbial composition in cows than in donkeys. Humans, pigs, and donkeys are all monogastric animals, whereas cows are ruminants. We suspect that the difference in digestive tract structure between monogastric animals and ruminants may be a potential contributor to the differential impact of the sampling method on the microbial composition in cows. To explain this phenomenon, we further compared the codifference networks of cows and donkeys, which revealed that many species (such as Prevotella brevis and Prevotella sp. CAG:1124) belonging to Prevotella were involved in the biosynthesis of siderophore group nonribosomal peptides, implying that Prevotella plays an important role in metabolism. However, the cow codifference network lacked information and contained fewer connections between microbes and functions than the donkey codifference network, indicating that more exploration is needed in the future. Furthermore, in practical terms, rectal swab samples are more likely than faecal samples to come into contact with the external environment during the sampling and transfer processes, potentially resulting in the loss of some anaerobic microorganisms. In summary, in gut microbiota research, especially in ruminants, it would be preferable to perform both sampling methods simultaneously to complement each other and improve results, as the faecal sampling method tends to be used most often.
Furthermore, there have been numerous studies on the microbial differences among digestive tract segments [35], and our results confirmed these findings. Donkeys and cows shared similar characteristics, such as noticeable differences in the number of bacteria between the stomach and other segments, with the colon containing the highest number of bacteria in both animals owing to longer bowel transit times [36]. The top 5 dominant phyla in both donkeys and cows were Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria and Spirochaetes, which is consistent with findings in other monogastric [37] and polygastric [38] animals. Ruminococcus, a genus of bacteria associated with saccharide degradation [39], was the dominant genus in both animals. The more abundant species in both donkeys and cows belonged to the Lachnospiraceae family, and bacteria in this family are closely related to the production of butyrate [40]. Compared with those in other digestive tract segments, the microbial community in the donkey stomach thrives in a lower pH environment. Lactobacillus, a representative genus of Firmicutes, is abundant in the digestive tract of donkeys, particularly in the stomach (80%), and consists of various species important for digestion and health [41]. Despite the smaller size of the donkey digestive tract compared with that of cows, it harbours a richer microbial community, possibly due to the need for more microbes involved in digestion or differences in diet between monogastric and polygastric animals.
Moreover, the small intestine is another segment that is characterized by a series of adverse factors, such as low pH, faster bowel transit time, and ethe presence of bile acids and antimicrobial peptides [42]. Studies have shown that Firmicutes and Proteobacteria, which are the dominant bacteria in the small intestine, are more tolerant of these factors [43]. This explains why the proportion of Proteobacteria in the small intestine was greater than that in the other segments. However, the abnormal microbial community of the small intestine is reflected mainly in faecal samples. Prevotella, a representative genus of Bacteroidetes, has been identified as a key microbe that can improve disease prognosis [44]. An increased abundance of Prevotella has been linked to the activation of various amino acid and carbohydrate metabolism pathways, including branched chain amino acid metabolism [45] and proteolysis of cereal grains [46]. Kamke J et al. revealed a Sharpea-enriched microbiome characterized by lactic acid formation and utilization [47]. Sharpea was found to be a dominant microbe in cows, potentially compensating for the lack of Lactobacillus bacteria. Unlike in donkeys, where Lactobacillus was dominant through the digestive tract, cows presented different dominant genera in different segments of the digestive tract, such as Prevotella in the stomach; Kandleria in the small intestine; and Clostridium in the caecum, colon, and rectum. This variation highlights the complexity and diversity of microbial communities among different digestive tract segments, emphasizing the importance of considering multiple factors in future research.
Conclusion
Through metagenomic comparative analysis, we discovered that the composition of microbial communities varied between digestive tract segments in both cows and donkeys. We also found that different dominant species were present in the bacterial microbiome on the basis of whether faecal or swab sampling methods were used. Moreover, the results between monogastric and ruminant animals were different, shedding light on the methods used in microbiome studies and raising further questions. Integrating multiple omics approaches, such as metagenomics, metabolomics, and transcriptomics, could help us better understand the functional capabilities of the gut microbiome. Importantly, our study had a small sample size, and larger sequencing efforts will be necessary in the future to fully grasp the importance of sampling methods and digestive tract segments in comparative microbiome research. These associations provide various insights for future microbiome research, contributing to a deeper understanding of human gut health.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1: Fig. S1 Results of assembly and gene prediction. Fig. S2 Top 30 microbial species of all the samples at different taxonomic levels. Fig. S3 Functional annotation. Fig. S4 Functional annotation of different samples and at different levels using different databases. Fig. S5 PLS-DA results for different sampling methods. Fig. S6 ANOSIM for different sampling methods. Fig. S7 Bacterial composition of the samples analysed using different sampling methods. Fig. S8 Venn diagram for different sampling methods. Fig. S9 STAMP analysis for different sampling methods. Fig. S10 Microbe variable importance dot plot for different sampling methods. Fig. S11 LEfSe analysis of different sampling methods in cows. Fig. S12 Bacterial compositions of the digestive tract segments of donkeys at different taxonomic levels. Fig. S13 Bacterial compositions of the digestive tract segments of cows at different taxonomic levels. Fig. S14 LEfSe analysis of cow stomach samples. Fig. S15 Bacterial compositions of samples from cows and donkeys at different taxonomic levels. Fig. S16 NMDS, OPLS-DA and PCA. Fig. S17 Supplementary LEfSe analysis of different animals. Fig. S18 Microbe variable importance dot plot at different taxonomic levels for donkeys and cows. Fig. S19 Functional annotation of samples from different animals using different databases. Fig. yS20 Functional annotation of cow samples analysed via different methods using different databases. Fig. S21 Functional annotation of different digestive tract segments of cows using different databases. Fig. S22 Functional annotation of donkey samples analysed via different methods used different databases. Fig. S23 Functional annotation of different digestive tract segments of donkeys using different databases. Fig. S24 Comparison of the main functional annotations. Fig. S25 Bubble plot of differentially expressed genes identified from the cluster analysis. Fig. S26 Information on the codifference networks. Fig. S27 Network of functions and microbes at the species level grouped by animal. Fig. S28 Network of functions and microbes at the species level grouped by cow sampling method. Fig. S29 Network of functions and microbes at the species level grouped by donkey sampling method. Fig. S30 Network of functions and microbes at the species level grouped by the cow digestive tract segment. Fig. S31 Network of functions and microbes at the species level grouped by the donkey digestive tract segment.
Supplementary S3: Supplementary S1: Sample information.
Supplementary Material 4: Supplementary S2: Microbial composition.
Supplementary Material 5: Node and edge information of the codifference network.
Supplementary Material 6: Table S1 Product composition analysis of the cow and donkey diets. Table S2 Information on samples collected from different intestinal regions using different methods. Table S3 Differentially abundant microbes between donkey sampling methods. Table S4 Functions of differentially abundant microbes between donkey sampling methods. Table S5 Differentially abundant microbes between cow sampling methods. Table S6 Differentially abundant microbes between cow sampling methods. Table S7 Differentially abundant microbes between animals. Table S8 Functions of the differentially abundant microbes between animals.
Acknowledgements
We thank the many contributors to the Sequence Read Achieve for making their data publicly available. This research was funded by the National Key Research and Development Program of China (2021YFF0702900) and the CAMS initiative for Innovative Medicine of China (2021-I2M-1-039, 2021-I2M-1-034), Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2023-PT180-01).
Author contributions
Jindan Guo, Weixiong Shi and Lei Su: Investigation, Data curation, Data analysis, Formal analysis, Writing - original draft. Wei Tong: Conceptualization, Data curation and Methodology. Jindan Guo, Xue Li and Wei Tong: Data curation, Data Analysis, Validation. Lei Su: Writing - review & editing. Zhiguang Xiang and Bochao Yang: Resource collection, Validation. Lei Su, Zhiguang Xiang and Chuan Qin: Conceptualization, Supervision, Funding acquisition. All authors read, edited, and approved the final manuscript.
Data availability
Data are available in a public, open access repository. All metagenomic sequencing data generated and analysed in the present study have been deposited to the NCBI Sequence Read Archive under BioProject accession number PRJNA1119043.
Declarations
Ethics approval and consent to participate
Our animal experiments were approved and authorized by the Ethics Committee of the Laboratory of Animal Sciences of Peking Union Medical College (no. XZG22001).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lei Su, Jindan Guo, Weixiong Shi and Wei Tong contributed equally to this work.
Contributor Information
Lei Su, Email: sulei@cnilas.org.
Zhiguang Xiang, Email: xiangzhiguang@cnilas.org.
References
- 1.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–48. 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 2.Kartjito MS, Yosia M, Wasito E, Soloan G, Agussalim AF, Basrowi RW. Defining the relationship of gut microbiota, immunity, and cognition in early life-a narrative review. Nutrients. 2023;15:2642. 10.3390/nu15122642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu D, Meng X, de Vos WM, Wu H, Fang X, Maiti AK. Implications of gut microbiota in complex human diseases. Int J Mol Sci. 2021;22:12661. 10.3390/ijms222312661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tee MZ, Er YX, Easton AV, Yap NJ, Lee IL, Devlin J, Chen Z, Ng KS, Subramanian P, Angelova A, Oyesola O, Sargsian S, Ngui R, Beiting DP, Boey CCM, Chua KH, Cadwell K, Lim YAL, Loke P, Lee SC. Gut microbiome of helminth-infected indigenous Malaysians is context dependent. Microbiome. 2022;10:214. 10.1186/s40168-022-01385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo J, Shi W, Li X, Yang B, Qin C, Su L. Comparative analysis of gut microbiomes in laboratory chinchillas, ferrets, and marmots: Implications for pathogen infection research. Microorganisms. 2024;12:646. 10.3390/microorganisms12040646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright RD, Bartelli TF, Baydogan S, White JR, Kim MP, Bhutani MS, McAllister F. Bacterial and fungal characterization of pancreatic adenocarcinoma from Endoscopic Ultrasound-guided biopsies. Front Immunol. 2023;14:1268376. 10.3389/fimmu.2023.1268376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ladjimi MH, Barka ZB, Lahbib K, Miled HB, Rhouma KB, Sakly M, Tebourbi O. Antidiarrheal and antioxidant activities of Ajuga iva (L.) leave extract. Heliyon. 2023;9:e21139. 10.1016/j.heliyon.2023.e21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choudhury R, Middelkoop A, Bolhuis JE, Kleerebezem M. Legitimate and reliable determination of the age-related intestinal microbiome in young piglets; rectal rwabs and fecal samples provide comparable insights. Front Microbiol. 2019;10:1886. 10.3389/fmicb.2019.01886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahnic A, Breznik V, Bombek Ihan M, Rupnik M. Comparison between cultivation and sequencing based approaches for microbiota analysis in swabs and biopsies of chronic wounds. Front Med (Lausanne). 2021;8:607255. 10.1101/2020.09.08.288779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang Q, Jin G, Wang G, et al. Current Sampling Methods for Gut Microbiota: A Call for More Precise Devices. Front Cell Infect Microbiol. 2020;10:151. 10.3389/fcimb.2020.00151. Published 2020 Apr 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bassis CM, Moore NM, Lolans K, Seekatz AM, Weinstein RA, Young VB, Hayden MK, CDC Prevention Epicenters Program. Comparison of stool versus rectal swab samples and storage conditions on bacterial community profiles. BMC Microbiol. 2017;17(1):78. 10.1186/s12866-017-0983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lagler H, Bangert C, Quint T, Österreicher Z, Nussbaumer-Pröll A, Eberl S, Weber M, Karer M, Sommer MOA, Zeitlinger M. Comparison of non-invasive Staphylococcus aureus sampling methods on lesional skin in patients with atopic dermatitis. Eur J Clin Microbiol Infect Dis. 2022;41:245–52. 10.1007/s10096-021-04365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gysens L, Martens A, Haspeslagh M. Cross-sectional comparison of superficial swab and fine-needle aspiration: Improving the diagnostic workup of horses with sarcoids. Vet J. 2022;289:105916. 10.1016/j.tvjl.2022.105916. [DOI] [PubMed] [Google Scholar]
- 14.Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65–80. 10.1053/j.gastro.2008.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagpal S, Srivastava SK. Colon or semicolon: gut sampling microdevices for omics insights. NPJ Biofilms Microbiomes. 2024;10(1):97. 10.1038/s41522-024-00536-2. Published 2024 Oct 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milani C, Alessandri G, Mancabelli L, et al. Multi-omics Approaches To Decipher the Impact of Diet and Host Physiology on the Mammalian Gut Microbiome. Appl Environ Microbiol. 2020;86(23):e01864–20. 10.1128/AEM.01864-20. Published 2020 Nov 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao B, Chi L, Zhu Y, Shi X, Tu P, Li B, Yin J, Gao N, Shen W, Schnabl B. An introduction to next generation sequencing bioinformatic analysis in gut microbiome studies. Biomolecules. 2021;11:530. 10.3390/biom11040530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yen S, Johnson JS. Metagenomics: a path to understanding the gut microbiome. Mamm Genome. 2021;32(4):282–96. 10.1007/s00335-021-09889-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan Y, Li H, Fayyaz A, Gai Y. Metagenomic and network analysis revealed wide distribution of antibiotic resistance genes in monkey gut microbiota. Microbiol Res. 2022;254:126895. 10.1016/j.micres.2021.126895. [DOI] [PubMed] [Google Scholar]
- 20.Yu Z, Morrison M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques. 2004;36:808–12. 10.2144/04365ST04. [DOI] [PubMed] [Google Scholar]
- 21.Chen S, Zhou Y, Chen Y, Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–90. 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–60. 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li D, Liu CM, Luo R, Sadakane K, Lam TW. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics. 2015;31:1674–6. 10.1093/bioinformatics/btv033. [DOI] [PubMed] [Google Scholar]
- 24.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu W, Lomsadze A, Borodovsky M. Ab initio gene identification in metagenomic sequences. Nucleic Acids Res. 2010;38:e132. 10.1093/nar/gkq275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li W, Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006;22:1658–9. 10.1093/bioinformatics/btl158. [DOI] [PubMed] [Google Scholar]
- 27.Buchfink B, Xie C, Huson DH. Fast and sensitive protein alignment using DIAMOND. Nat Methods. 2015;12:59–60. 10.1038/nmeth.3176. [DOI] [PubMed] [Google Scholar]
- 28.Huson DH, Auch AF, Qi J, Schuster SC. MEGAN analysis of metagenomic data. Genome Res. 2007;17:377–86. 10.1101/gr.5969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng J, Ge Q, Yan Y, Zhang X, Huang L, Yin Y. dbCAN3: automated carbohydrate-active enzyme and substrate annotation. Nucleic Acids Res. 2023;51:W115–21. 10.1093/nar/gkad328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma K, Bai T, Hu P, Zhao M, Xiu Z, Dalintai S, Zhang Q, Wan Q. Sanwei sandalwood decoction improves function of the gut microbiota in heart failure. Front Microbiol. 2023;14:1236749. 10.3389/fmicb.2023.1236749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sisk-Hackworth L, Brown J, Sau L, Levine AA, Tam LYI, Ramesh A, Shah RS, Kelley-Thackray ET, Wang S, Nguyen A, Kelley ST, Thackray VG. Genetic hypogonadal mouse model reveals niche-specific influence of reproductive axis and sex on intestinal microbial communities. Biol Sex Differ. 2023;14:79. 10.1186/s13293-023-00564-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Little S, Braff J, Duncan K, Elsemore D, Hanna R, Hanscom J, Lee A, Martin KA, Sobotyk C, Starkey L, Sundstrom K, Tyrrell P, Verocai GG, Wu T, Beall M. Diagnosis of canine intestinal parasites: Improved detection of Dipylidium caninum infection through coproantigen testing. Vet Parasitol. 2023;324:110073. 10.1016/j.vetpar.2023.110073. [DOI] [PubMed] [Google Scholar]
- 33.Jones RB, Zhu X, Moan E, Murff HJ, Ness RM, Seidner DL, Sun S, Yu C, Dai Q, Fodor AA, Azcarate-Peril MA, Shrubsole MJ. Inter-niche and inter-individual variation in gut microbial community assessment using stool, rectal swab, and mucosal samples. Sci Rep. 2018;8(1):4139. 10.1038/s41598-018-22408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Budding AE, Grasman ME, Eck A, Bogaards JA, Vandenbroucke-Grauls CMJE, van Bodegraven AA, Savelkoul PHM. Rectal swabs for analysis of the intestinal microbiota. PLoS ONE. 2014;9(7):e101344. 10.1371/journal.pone.0101344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tong F, Wang T, Gao NL, Liu Z, Cui K, Duan Y, Wu S, Luo Y, Li Z, Yang C, Xu Y, Lin B, Yang L, Pauciullo A, Shi D, Hua G, Chen WH, Liu Q. The microbiome of the buffalo digestive tract. Nat Commun. 2022;13:823. 10.1038/s41467-022-28402-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Vos WM, Tilg H, Hul MV, Cani PD. Gut microbiome and health: mechanistic insights. Gut. 2022;71(5):1020–32. 10.1136/gutjnl-2021-326789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’ Donnell MM, Harris HMB, Jeffery IB, Claesson MJ, Younge B, O’ Toole PW, Ross RP. The core faecal bacterial microbiome of Irish Thoroughbred racehorses. Lett Appl Microbiol. 2013;57:492–501. 10.1111/lam.12137. [DOI] [PubMed] [Google Scholar]
- 38.Khatoon M, et al. Rumen and fecal microbial profiles in cattle fed high lignin diets using metagenome analysis. Anaerobe. 2022;73:102508. 10.1016/j.anaerobe.2021.102508. [DOI] [PubMed] [Google Scholar]
- 39.Xue Y, Lin L, Hu F, Zhu W, Mao S. Disruption of ruminal homeostasis by malnutrition involved in systemic ruminal microbiota-host interactions in a pregnant sheep model. Microbiome. 2020;8:138. 10.1186/s40168-020-00916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haas KN, Blanchard JL. Kineothrix alysoides, gen. nov., sp. nov., a saccharolytic butyrate-producer within the family Lachnospiraceae. Int J Syst Evol Microbiol. 2017;67:402–10. 10.1099/ijsem.0.001643. [DOI] [PubMed] [Google Scholar]
- 41.Morita H, Nakano A, Shimazu M, Toh H, Nakajima F, Nagayama M, Hisamatsu S, Kato Y, Takagi M, Takami H, Akita H, Matsumoto M, Masaoka M, Murakami T. Lactobacillus hayakitensis, L. equigenerosi and L. equi, predominant lactobacilli in the intestinal flora of healthy thoroughbreds. Anim Sci J. 2009;80:339–46. 10.1111/j.1740-0929.2009.00633.x. [DOI] [PubMed] [Google Scholar]
- 42.Ma Y, Deng X, Yang X, Wang J, Li T, Hua G, Han D, Da L, Li R, Rong W, Deng X. Characteristics of bacterial microbiota in different intestinal segments of aohan fine-wool sheep. Front Microbiol. 2022;13:874536. 10.3389/fmicb.2022.874536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Angelakis E, Armougom F, Carrière F, Bachar D, Laugier R, Lagier JC, Robert C, Michelle C, Henrissat B, Raoult D. A metagenomic investigation of the duodenal microbiota reveals links with obesity. PLoS ONE. 2015;10:e0137784. 10.1371/journal.pone.0137784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huh JW, Kim MJ, Kim J, Lee HG, Ryoo SB, Ku JL, Jeong SY, Park KJ, Kim D, Kim JF, Park JW. Enterotypical Prevotella and three novel bacterial biomarkers in preoperative stool predict the clinical outcome of colorectal cancer. Microbiome. 2022;10:203. 10.1186/s40168-022-01388-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petersen LM, Bautista EJ, Nguyen H, Hanson BM, Chen L, Lek SH, Sodergren E, Weinstock GM. Community characteristics of the gut microbiomes of competitive cyclists. Microbiome. 2017;5:98. 10.1186/s40168-017-0320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xia Y, Kong Y, Huang H, Yang HE, Forster R, McAllister TA. In situ identification and quantification of protein-hydrolyzing ruminal bacteria associated with the digestion of barley and corn grain. Can J Microbiol. 2016;62:1063–7. 10.1139/cjm-2016-0293. [DOI] [PubMed] [Google Scholar]
- 47.Kamke J, Patel SH, Pandit RJ, Jakhesara SJ, Rank DN, Joshi CG, Kunjadiya AP. Rumen metagenome and metatranscriptome analyses of low methane yield sheep reveals a Sharpea-enriched microbiome characterised by lactic acid formation and utilisation. Microbiome. 2016;4:56. 10.1186/s40168-016-0201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Fig. S1 Results of assembly and gene prediction. Fig. S2 Top 30 microbial species of all the samples at different taxonomic levels. Fig. S3 Functional annotation. Fig. S4 Functional annotation of different samples and at different levels using different databases. Fig. S5 PLS-DA results for different sampling methods. Fig. S6 ANOSIM for different sampling methods. Fig. S7 Bacterial composition of the samples analysed using different sampling methods. Fig. S8 Venn diagram for different sampling methods. Fig. S9 STAMP analysis for different sampling methods. Fig. S10 Microbe variable importance dot plot for different sampling methods. Fig. S11 LEfSe analysis of different sampling methods in cows. Fig. S12 Bacterial compositions of the digestive tract segments of donkeys at different taxonomic levels. Fig. S13 Bacterial compositions of the digestive tract segments of cows at different taxonomic levels. Fig. S14 LEfSe analysis of cow stomach samples. Fig. S15 Bacterial compositions of samples from cows and donkeys at different taxonomic levels. Fig. S16 NMDS, OPLS-DA and PCA. Fig. S17 Supplementary LEfSe analysis of different animals. Fig. S18 Microbe variable importance dot plot at different taxonomic levels for donkeys and cows. Fig. S19 Functional annotation of samples from different animals using different databases. Fig. yS20 Functional annotation of cow samples analysed via different methods using different databases. Fig. S21 Functional annotation of different digestive tract segments of cows using different databases. Fig. S22 Functional annotation of donkey samples analysed via different methods used different databases. Fig. S23 Functional annotation of different digestive tract segments of donkeys using different databases. Fig. S24 Comparison of the main functional annotations. Fig. S25 Bubble plot of differentially expressed genes identified from the cluster analysis. Fig. S26 Information on the codifference networks. Fig. S27 Network of functions and microbes at the species level grouped by animal. Fig. S28 Network of functions and microbes at the species level grouped by cow sampling method. Fig. S29 Network of functions and microbes at the species level grouped by donkey sampling method. Fig. S30 Network of functions and microbes at the species level grouped by the cow digestive tract segment. Fig. S31 Network of functions and microbes at the species level grouped by the donkey digestive tract segment.
Supplementary S3: Supplementary S1: Sample information.
Supplementary Material 4: Supplementary S2: Microbial composition.
Supplementary Material 5: Node and edge information of the codifference network.
Supplementary Material 6: Table S1 Product composition analysis of the cow and donkey diets. Table S2 Information on samples collected from different intestinal regions using different methods. Table S3 Differentially abundant microbes between donkey sampling methods. Table S4 Functions of differentially abundant microbes between donkey sampling methods. Table S5 Differentially abundant microbes between cow sampling methods. Table S6 Differentially abundant microbes between cow sampling methods. Table S7 Differentially abundant microbes between animals. Table S8 Functions of the differentially abundant microbes between animals.
Data Availability Statement
Data are available in a public, open access repository. All metagenomic sequencing data generated and analysed in the present study have been deposited to the NCBI Sequence Read Archive under BioProject accession number PRJNA1119043.