Abstract
Background
Considering that the respective effects of obesity and hyperlipidemia on knee osteoarthritis (OA) have not been fully investigated, the purpose of this study was to determine the relationship of obesity or hyperlipidemia with the synovitis and structural abnormalities of knee OA, and the effect of obesity and hyperlipidemia on functional outcomes of total knee arthroplasty.
Methods
There were 99 OA patients without obesity and hyperlipidemia in Group 1, 100 OA patients only with obesity in Group 2, 98 OA patients only with hyperlipidemia in Group 3, and 97 OA patients with both obesity and hyperlipidemia in Group 4. Semi-quantitative synovial inflammatory markers were measured including effusion-synovitis, size and intensity of infrapatellar fat pad abnormality, and synovial proliferation score. The structural abnormalities of knee OA were evaluated using Whole-Organ Magnetic Resonance Imaging Score (WORMS). Functional outcomes were evaluated before surgery and at 2 years follow-up.
Results
There were significantly higher effusion-synovitis, size and intensity of infrapatellar fat pad abnormality, and synovial proliferation score, as well as higher cartilage, bone marrow edema, meniscus, and total WORMS scores in Group 2, Group 3, and Group 4 (P < 0.05), but with no significant difference between Group 2 and Group 3 (P > 0.05). Group 2, Group 3, Group 4 had significantly worse Western Ontario and McMaster Universities Osteoarthritis Index, Forgotten Joint Score, Oxford Knee Score, Knee Society Score at baseline and 2 years follow-up (P < 0.05), but with no significant difference between Group 2 and Group 3 (P > 0.05). There were significant associations of obesity or hyperlipidemia with all synovial inflammatory markers and cartilage, bone marrow edema, meniscus, and total WORMS scores as well as functional outcomes (P < 0.05).
Conclusions
Obesity and hyperlipidemia were associated with more severe synovitis and structural abnormalities of knee OA, as well as inferior preoperative and postoperative functional outcomes. The negative effects of obesity and hyperlipidemia on knee OA could be mutually enhanced. The findings emphasized the negative effects of obesity and hyperlipidemia on the symptoms and outcomes of knee OA, and highlighted the association of obesity and hyperlipidemia with synovitis.
Keywords: Osteoarthritis, Obesity, Hyperlipidemia, Synovitis, Structural abnormalities, Functional outcomes
Background
Knee osteoarthritis (OA), a common and debilitating disease, is classically defined as a degenerative condition of the whole joint involving progressive cartilage loss and periarticular bone remodeling, and is manifested by chronic pain, impaired physical function, and declining quality of life, which even leads to total knee arthroplasty (TKA) at advanced stage [1–3]. Nowadays, the deepening research and understanding of OA supported that OA is not just an age-related disorder, but rather a multifactorial condition with various risk factors associated with pro-inflammatory state, including sex, obesity, genetics, abnormal loading, injury, and so on [3–5].
Overweight and obesity are well-known major risk factors for knee OA [6, 7]. There was a dose-response relationship between higher body mass index (BMI) and inferior clinical manifestations of knee OA [8], while weight loss in overweight and obese individuals with mild to moderate or with risk factors for knee OA significantly decreased cartilage degeneration [9]. In addition, higher BMI in young men aged 20 to 29 years was associated with a higher risk of knee OA, which suggested that the effect of body weight on knee OA may not be less than that of physiological aging [10]. Recent literature has demonstrated positive clinical outcomes following unicompartmental knee arthroplasty or TKA in obese patients, including significantly improved functional outcomes and high implant survival rates, indicating that obese patients could enjoy the benefits of knee arthroplasty [11–14]. The traditional perspective attributed the association between obesity and knee OA to excessive joint loading, while obesity has also been reported to be related to OA of non-weight-bearing joints, such as hand and wrist, indicating that obesity-related OA may have more complicated systemic effects [15–18]. Emerging research has highlighted the significance of metabolic factors in the pathogenesis of OA, including obesity, hyperlipidemia, and insulin resistance [19].
Hyperlipidemia, which is closely associated with obesity, typically included increased levels of total cholesterol (TC), triglycerides (TG), and low-density lipoprotein cholesterol (LDL-C) [20]. Patients with radiographic knee OA had increased TC, TG and LDL-C [21]. Higher TC and lower high-density lipoprotein cholesterol (HDL-C) were associated with more severe knee pain [22]. The meta-analysis of case-control and cross-sectional studies indicated a strong correlation between hyperlipidemia and OA, while it was not supported by the meta-analysis of cohort studies [23]. Another meta-analysis also suggested that there was no clear correlation between hyperlipidemia and OA in both radiological and symptomatic studies [24]. In addition, not all obese individuals are accompanied by hyperlipidemia, with a prevalence of 58.79% among those with compound obesity [25]. Besides, previous studies on the effect of obesity on knee OA usually did not consider the presence of hyperlipidemia, and may confuse the respective roles of obesity and hyperlipidemia. Therefore, it is necessary to further investigate the relationship of knee OA with obesity and hyperlipidemia, considering the important public health relevance of this topic.
There is inflammatory disorder in knee OA with 46% prevalence rate of synovitis in patients with symptomatic knee OA [26], which caused more severe pain, joint dysfunction, and cartilage damage, as well as increased morbidity and accelerated progression of OA [27, 28]. Previous studies reported that obese individuals had higher prevalence and severity of synovitis, and synovitis worsening mediated the association between BMI and knee OA progression [29, 30]. However, these studies did not consider whether there was hyperlipidemia, and thus the influence of obesity and hyperlipidemia on synovitis has not be completely revealed.
Therefore, considering that the respective effects of obesity and hyperlipidemia on synovitis and structural abnormalities of knee OA have not been fully studied, the purposes of this study were (1) to determine the relationship of obesity or hyperlipidemia with the synovitis and structural abnormalities of knee OA, and (2) to investigate the effect of obesity or hyperlipidemia on preoperative and postoperative functional outcomes of TKA. It was hypothesized that both obesity and hyperlipidemia could lead to more severe synovitis and structural abnormalities of knee OA as well as inferior functional outcomes.
Methods
Patient selection
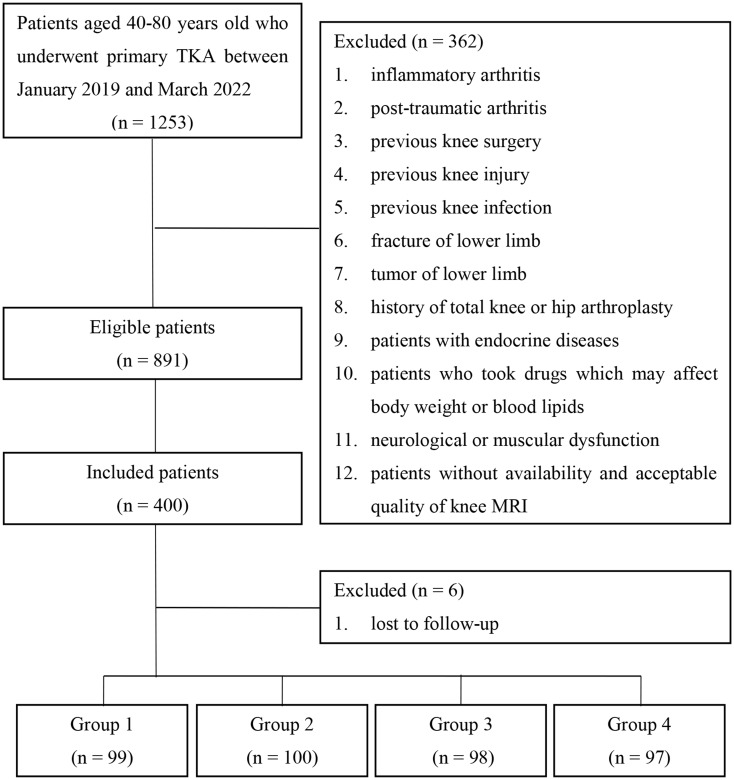
This retrospective study was reviewed and approved by Institutional Review Board of our hospital, and written informed consent was obtained from all included patients. All patients diagnosed with knee OA at our hospital between January 2019 and March 2022 were identified and screened. Inclusion criteria were as follows: (1) patients with primary knee OA, (2) patients with Kellgren-Lawrence grade of III or IV [31], (3) patients between 40 and 80 years of age, and (4) patients who received TKA. The exclusion criteria were: (1) inflammatory arthritis, (2) post-traumatic arthritis, (3) previous knee surgery, (4) previous knee injury, (5) previous knee infection; (6) fracture of lower limb, (7) tumor of lower limb, (8) history of total knee or hip arthroplasty, (9) patients with endocrine diseases, such as diabetes, thyroid diseases, and adrenocortical diseases, (10) patients who took drugs which may affect body weight or blood lipids, such as lipid-lowering drugs, weight-loss drugs, and corticosteroids, and (11) neurological or muscular dysfunction. Patients without availability and acceptable quality of knee magnetic resonance imaging (MRI) were also excluded. Eligible patients were classified into four groups based on BMI and blood lipids: OA patients without obesity and hyperlipidemia in Group 1, OA patients only with obesity in Group 2, OA patients only with hyperlipidemia in Group 3, and OA patients with both obesity and hyperlipidemia in Group 4. One hundred patients in each group were consecutively selected from eligible patients, and followed-up for at least 2 years after TKA. Six patients were lost to follow-up. Finally, there were 99 patients in Group 1, 100 patients in Group 2, 98 patients in Group 3, and 97 patients in Group 4. The graphical flowchart for patient selection was shown in Fig. 1.
Fig. 1.
The graphical flowchart for patient selection. TKA: total knee arthroplasty; MRI: magnetic resonance imaging
Surgical technique
All surgeries were performed with the principle of mechanical alignment using the standard medial parapatellar approach under general anesthesia. The tibial resection was performed perpendicular to the tibial mechanical axis using extramedullary referencing. The distal femoral resection was performed using intramedullary referencing with a valgus angle of 5–7° depending on the deformity of each patient. Subsequent femoral resection was completed using a 4-in-1 distal femoral cutting block with external rotation of 3° relative to the posterior condylar axis due to the 3° physiological varus of the tibia to obtain a symmetrical rectangular gap. The Whiteside’s line, which was perpendicular to the femoral osteotomy line, was used to verify the osteotomy plane during the actual surgical operation to ensure the balance of flexion gap. Most of the infrapatellar fat pad was preserved. Most of the synovium was removed, especially the inflammatory synovium was completely removed. After achieving soft tissue balance and symmetrical gaps with the help of soft tissue release if necessary, the implants were fixed with bone cement.
Assessments of metabolic factors
Body weight was measured with patients without shoes using a calibrated balance beam scale in kilograms. Height was measured with patients without shoes using a stadiometer in millimeters. BMI was calculated as the weight divided by the square of the height. Body weight was classified into three groups according to BMI: normal weight (BMI < 24.9 kg/m2), overweight (BMI > 25 and ≤ 29.9 kg/m2), and obesity (BMI ≥ 30 kg/m2). Abdominal and hip circumferences were measured using a measuring tape around the abdomen or hip. TC, TG, LDL-C, and HDL-C were measured from venous blood collected after an overnight fast. Hyperlipidemia was defined as TC ≥ 6.22 mmol/L, TG ≥ 2.26 mmol/L, and LDL-C ≥ 4.14 mmol/L [20].
MRI protocol
Knee MRI images were obtained when patients were in supine position, with their knees in extended position and their lower limbs in slightly external rotation using the cross-calibrated 3.0 Tesla scanners (Magnetom Trio, Siemens, Erlangen, Germany) with the identical protocol. Three sequences were obtained to semi-quantitatively analyze synovitis and structural abnormalities of knee OA: a coronal two-dimensional intermediate-weighted (IW) turbo spin-echo (TSE) sequence, a sagittal three-dimensional dual-echo steady-state (DESS) sequence with water excitation along with coronal and axial reformations, and a sagittal two-dimensional IW fat-suppressed TSE sequence.
Semi-quantitative assessment of synovitis
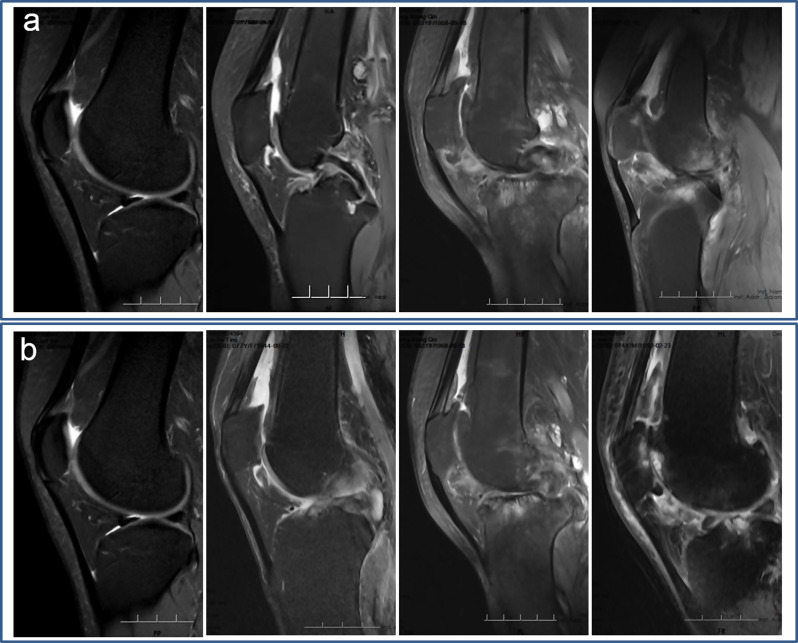
Knee MRI images were carefully evaluated in a random fashion by two independent researchers who had more than 10 years of experience in assessing musculoskeletal MRI images and were blinded to patient grouping and research purpose. In cases of equivocal images, a consensus reading was performed with a third independent radiologist with 20 years of experience. Because it is not always feasible to distinguish synovial thickening from intra-articular fluid on non-contrast enhanced MRI, the term effusion-synovitis was used as previously reported [29, 32, 33]. Six parameters of each knee were reviewed and scored semi-quantitatively in MRI images (Table 1), which were described in previous studies [29, 32–34]. First, according to the Anterior Cruciate Ligament OsteoArthritis Score (ACLOAS), effusion-synovitis was graded using the maximum anteroposterior diameter of the suprapatellar recess, which was assessed at the midline slice on sagittal fat-saturated IW or DESS images [33]. Specifically, effusion-synovitis was scored from 0 to 3 based on the degree of capsular distension, with an anteroposterior diameter of joint distension < 2 mm scoring as grade 0, ≥ 2 to < 5 mm scoring as grade 1, ≥ 5 to < 10 mm scoring as grade 2, and ≥ 10 mm scoring as grade 3 (Fig. 2a). Second, according to the MRI Osteoarthritis Knee Score (MOAKS), effusion-synovitis was scored from 0 to 4 on axial fat-saturated DESS images [32]. Specifically, a normal physiologic amount of fluid was graded as 0; a small amount of fluid continuously expanding into the retropatellar space was graded as 1; a medium amount of fluid with a mild convexity of the suprapatellar bursa was graded as 2; and a large amount of fluid with capsular distention was graded as 3 (Fig. 2b). Third, the size of the infrapatellar fat pad (IPFP) abnormality was evaluated on sagittal IW fat-suppressed images, with no signal abnormality being grade 0, ≤ 33% of the region with signal abnormality being grade 1, 34–66% of the region being grade 2, and ≥ 66% of the region being grade 3 [35] (Fig. 3a). Fourth, the highest signal intensity of the IPFP abnormality was assessed on sagittal IW fat-suppressed images with grade 0 = none, grade 1 = mild (lower than cartilage), grade 2 = moderate (equal to or higher than cartilage but lower than fluid), and grade 3 = severe (equal to fluid) [35] (Fig. 3b). Fifth, synovial proliferation score (SPS) in the knee was graded on fat-saturated IW and DESS images if joint effusion was present indicated by the effusion-synovitis score of ≥ 1 by either ACLOAS or MOAKS [29, 34, 36]. A smooth synovium without proliferation and synovial bands was graded as 1; a synovium with mild irregularity and some synovial bands or small bodies was graded as 2; and a synovium with extensive thickening and irregular villonodular proliferation was graded as 3 (Fig. 4a). Finally, SPS was graded in knees with a popliteal cyst using the same method (Fig. 4b).
Table 1.
Semi-quantitative assessment of synovitis in MRI
| MRI-inflammatory markers | Criteria |
|---|---|
| Effusion-synovitis according to ACLOAS | |
| 0 | < 2 mm anteroposterior diameter of joint distension |
| 1 | ≥ 2 to < 5 mm anteroposterior diameter of joint distension |
| 2 | ≥ 5 to < 10 mm anteroposterior diameter of joint distension |
| 3 | ≥ 10 mm anteroposterior diameter of joint distension |
| Effusion-synovitis according to MOAKS | |
| 0 | Normal physiologic amount of fluid |
| 1 | Small amount of fluid continuously expanding into the retropatellar space |
| 2 | Medium amount of fluid with a mild convexity of the suprapatellar bursa |
| 3 | Large amount of fluid with capsular distention |
| Size of IPFP abnormality | |
| 0 | No signal abnormality |
| 1 | ≤ 33% of the region with signal abnormality |
| 2 | 34–66% of the region with signal abnormality |
| 3 | ≥ 66% of the region with signal abnormality |
| Highest signal intensity of IPFP abnormality | |
| 0 | None |
| 1 | Mild (lower than cartilage) |
| 2 | Moderate (equal to or higher than cartilage but lower than fluid) |
| 3 | Severe (equal to fluid) |
| SPS in knee1 | |
| 1 | Smooth synovium without proliferation and synovial bands |
| 2 | Synovium with mild irregularity and some synovial bands or small bodies |
| 3 | Synovium with extensive thickening and irregular villonodular proliferation |
| SPS in popliteal cyst2 | |
| 1 | Smooth synovium without proliferation and synovial bands |
| 2 | Synovium with mild irregularity and some synovial bands or small bodies |
| 3 | Synovium with extensive thickening and irregular villonodular proliferation |
1SPS in knee was graded if joint effusion was present indicated by the effusion-synovitis score of ≥ 1 by either ACLOAS or MOAKS
2Used only in knees with a popliteal cyst
MRI: magnetic resonance imaging; ACLOAS: Anterior Cruciate Ligament OsteoArthritis Score; MOAKS: MRI Osteoarthritis Knee Score; IPFP: infrapatellar fat pad; SPS: synovial proliferation score
Fig. 2.
Effusion-synovitis according to (a) the Anterior Cruciate Ligament OsteoArthritis Score and (b) the MRI Osteoarthritis Knee Score. MRI: magnetic resonance imaging
Fig. 3.
(a) The size of the infrapatellar fat pad abnormality. (b) The highest signal intensity of the infrapatellar fat pad abnormality
Fig. 4.
(a) Synovial proliferation score in the knee. (b) Synovial proliferation score in knees with a popliteal cyst
Whole-organ MRI score system
The structural abnormalities of knee OA were evaluated using the semi-quantitative Whole-Organ MRI Score (WORMS) system [37–40]. The following parameters were evaluated separately, including meniscus, cartilage, bone marrow edema, and subchondral cyst. Meniscal lesions were graded in each of three subregions (anterior horn, body segment, and posterior horn) in the medial and lateral meniscus from 0 (intact) to 4 (complete maceration/destruction or resection) [37]. Cartilage lesions were graded from 0 (normal thickness and signal) to 6 (full-thickness cartilage loss in ≥ 75% of the region), bone marrow edema from 0 (none) to 3 (≥ 50% of the region), and subchondral cyst from 0 (none) to 3 (≥ 50% of the region) in each of six regions (medial femur, lateral femur, trochlea, patella, medial tibia, and lateral tibia). The total WORMS score was calculated as the sum of meniscus, cartilage, bone marrow edema, and subchondral cyst.
Clinical evaluation
Functional outcomes were evaluated using patient-reported outcome measures before TKA and at 2-year follow-up after TKA to objectively evaluate knee function, including Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) [41], Forgotten Joint Score (FJS) [42], Oxford Knee Score (OKS) [43], and Knee Society Score (KSS), which consists of Knee Society Knee Score (KSKS) and Knee Society Function Score (KSFS) [44]. Patient satisfaction was evaluated using a five-point Likert scale, including very satisfied, satisfied, neutral, unsatisfied, and very unsatisfied, which were divided into satisfied (very satisfied or satisfied) and not satisfied (neutral, unsatisfied or very unsatisfied). Major complications and reoperations were recorded during the follow-up.
Intra- and interobserver reproducibility of measurements
To assess interobserver reproducibility, all MRI images were reviewed and graded by two independent researchers. To assess intraobserver reproducibility, twenty patients were randomly selected and scored twice by one researcher with 4 weeks apart. Cohen’s Kappa value was used to assess both intra- and interobserver reproducibility. A Kappa value of 0.61–0.80 indicates substantial agreement, and a value of 0.81-1.00 indicates almost perfect agreement [45].
Statistical analysis
Descriptive statistics included means and standard deviations for continuous variables, and frequency counts for categorical variables. The normality was assessed using Shapiro-Wilk tests, and homogeneity was assessed using Levene tests. Differences in synovial inflammatory markers, WORMS scores, and preoperative functional outcomes among Group 1, Group 2, Group 3, and Group 4 were assessed with analysis of variance and LSD tests or Kruskal-Wallis tests and Bonferroni tests depending on normality. Differences in postoperative functional outcomes were assessed with analysis of covariance to control confounding bias because there were significant differences in baseline functional outcomes. In addition, the standardized mean difference (SMD), which was expressed as the absolute value, was also used to indicate the difference in preoperative and postoperative functional outcomes among the four groups. SMDs of 0.2, 0.5 and 0.8 represent small, medium and large differences respectively. Categorical variables were evaluated with Chi-square tests. Comparisons between preoperative and postoperative functional outcomes were performed using paired t tests. The association between obesity or hyperlipidemia and synovitis or structural abnormalities of knee OA was evaluated using Spearman’s rank correlation coefficient. A P value < 0.05 was set as statistical significance. All statistical analyses were performed using IBM SPSS software version 22 (SPSS Inc., Chicago, IL, USA). An a priori power analysis using G-Power software version 3.1.9.4 (Heinrich-Heine-Universitat Dusseldorf, Dusseldorf, Germany) showed that 60 patients in each group could detect significant difference at 80% power.
Results
Demographic information and metabolic factors were shown in Table 2. There was no significant difference in age, sex, length of stay, and Kellgren-Lawrence grade among the four groups. Group 2 and Group 4 had significantly larger BMI, abdominal circumference, and hip circumference than those of Group 1 and Group 3. Group 3 and Group 4 had significantly larger TC, TG, and LDL-C than those of Group 1 and Group 2. There was no significant decrease in HDL-C in Group 3 and Group 4.
Table 2.
Demographic information and metabolic factors
| Variables | Group 1 | Group 2 | Group 3 | Group 4 | P Value |
|---|---|---|---|---|---|
| Patients (n) | 99 | 100 | 98 | 97 | - |
| Age (years) | 64.1 ± 7.3 | 65.4 ± 8.0 | 64.0 ± 8.2 | 64.5 ± 7.9 | 0.598 |
| Sex (man/women, n) | 32/67 | 38/62 | 44/54 | 29/68 | 0.123 |
| Length of stay (day) | 7.7 ± 1.4 | 8.0 ± 1.3 | 7.8 ± 1.5 | 8.1 ± 1.4 | 0.133 |
| Kellgren-Lawrence grade (III/IV, n) | 19/80 | 14/86 | 23/75 | 25/72 | 0.182 |
| BMI (Kg/m2) | 22.3 ± 1.4 | 34.8 ± 2.6 | 22.7 ± 1.3 | 34.6 ± 2.9 | < 0.001 |
| Abdominal circumference (cm) | 100.2 ± 5.9 | 107.3 ± 6.1 | 101.1 ± 6.1 | 107.2 ± 8.5 | < 0.001 |
| Hip circumference (cm) | 104.5 ± 6.5 | 111.9 ± 8.1 | 104.7 ± 6.6 | 111.2 ± 8.5 | < 0.001 |
| TC (mmol/L) | 4.18 ± 0.64 | 4.36 ± 0.68 | 5.16 ± 0.92 | 4.94 ± 0.47 | < 0.001 |
| TG (mmol/L) | 1.07 ± 0.43 | 1.13 ± 0.35 | 1.88 ± 1.25 | 1.72 ± 0.95 | < 0.001 |
| LDL-C (mmol/L) | 2.64 ± 0.39 | 2.15 ± 0.56 | 3.47 ± 0.99 | 3.28 ± 1.07 | < 0.001 |
| HDL-C (mmol/L) | 1.33 ± 0.16 | 1.37 ± 0.12 | 1.57 ± 0.65 | 1.74 ± 0.85 | < 0.001 |
BMI: body mass index; TC: total cholesterol; TG: triglycerides; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol. The values were presented as mean ± standard deviation otherwise indicated
Synovitis
The synovial inflammatory markers were shown in Table 3; Fig. 5. There were significantly higher synovial inflammatory markers in Group 2, Group 3, and Group 4 compared with Group 1, including effusion-synovitis, IPFP abnormality, and SPS. Group 4 had significantly higher synovial inflammatory markers than Group 2 and Group 3. There was no significant difference in all synovial inflammatory markers between Group 2 and Group 3.
Table 3.
MRI-synovial inflammatory markers
| Inflammatory markers | Group 1 | Group 2 | Group 3 | Group 4 | P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | 1 vs. 2 | 1 vs. 3 | 1 vs. 4 | 2 vs. 3 | 2 vs. 4 | 3 vs. 4 | |||||
| Effusion-synovitis | |||||||||||
| ACLOAS method | 0.97 ± 0.80 | 1.52 ± 0.80 | 1.55 ± 0.78 | 1.79 ± 0.77 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.788 | 0.016 | 0.032 |
| MOAKS method | 1.03 ± 0.76 | 1.43 ± 0.56 | 1.46 ± 0.58 | 1.77 ± 0.69 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.745 | < 0.001 | 0.001 |
| IPFP abnormality | |||||||||||
| Size | 1.01 ± 0.72 | 1.33 ± 0.47 | 1.36 ± 0.48 | 1.60 ± 0.49 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.700 | 0.001 | 0.002 |
| Highest signal intensity | 1.05 ± 0.69 | 1.36 ± 0.58 | 1.38 ± 0.58 | 1.66 ± 0.57 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.816 | 0.001 | 0.001 |
| Synovial proliferation score | |||||||||||
| Knee | 1.14 ± 0.70 | 1.34 ± 0.48 | 1.37 ± 0.49 | 1.71 ± 0.69 | < 0.001 | 0.018 | 0.007 | < 0.001 | 0.722 | < 0.001 | < 0.001 |
| Popliteal cyst | 0.31 ± 0.46 | 0.50 ± 0.64 | 0.53 ± 0.67 | 0.73 ± 0.81 | < 0.001 | 0.043 | 0.019 | < 0.001 | 0.748 | 0.014 | 0.033 |
ACLOAS: Anterior Cruciate Ligament OsteoArthritis Score; MOAKS: MRI Osteoarthritis Knee Score; IPFP: Infrapatellar fat pad; SPS: synovial proliferation score. The values were presented as mean ± standard deviation
Fig. 5.
The synovial inflammatory markers. ACLOAS: Anterior Cruciate Ligament OsteoArthritis Score; MOAKS: MRI Osteoarthritis Knee Score; IPFP: Infrapatellar fat pad; SPS: synovial proliferation score
Structural abnormalities of knee OA
The structural abnormalities of knee OA indicated by WORMS scores were shown in Table 4; Fig. 6. There were significantly higher cartilage, bone marrow edema, meniscus, and total WORMS scores in Group 2, Group 3, and Group 4 compared with Group 1. Group 4 had significantly higher cartilage, bone marrow edema, meniscus, and total WORMS scores than those of Group 2 and Group 3. There was no significant difference in all WORMS scores between Group 2 and Group 3. No significant difference in subchondral cyst score was found among four groups.
Table 4.
The structural abnormalities of knee OA indicated by WORMS scores
| WORMS | Group 1 | Group 2 | Group 3 | Group 4 | P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | 1 vs. 2 | 1 vs. 3 | 1 vs. 4 | 2 vs. 3 | 2 vs. 4 | 3 vs. 4 | |||||
| Cartilage | 3.6 ± 1.2 | 4.1 ± 1.0 | 4.3 ± 1.1 | 4.8 ± 1.2 | < 0.001 | 0.002 | < 0.001 | < 0.001 | 0.207 | < 0.001 | 0.005 |
| Bone marrow edema | 1.1 ± 1.0 | 1.4 ± 1.1 | 1.5 ± 1.1 | 2.0 ± 1.0 | < 0.001 | 0.044 | 0.027 | < 0.001 | 0.840 | < 0.001 | < 0.001 |
| Meniscus | 1.9 ± 1.4 | 2.6 ± 1.2 | 2.7 ± 1.1 | 3.2 ± 1.0 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.593 | 0.002 | 0.009 |
| Subchondral cyst | 1.2 ± 0.8 | 1.3 ± 0.7 | 1.3 ± 0.7 | 1.4 ± 0.8 | 0.327 | 0.235 | 0.361 | 0.068 | 0784 | 0.522 | 0.361 |
| Total | 7.9 ± 23 | 9.5 ± 2.2 | 9.8 ± 2.1 | 11.4 ± 2.2 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.353 | < 0.001 | < 0.001 |
OA: osteoarthritis; WORMS: Whole-Organ Magnetic Resonance Imaging Score. The values were presented as mean ± standard deviation
Fig. 6.
The structural abnormalities of knee osteoarthritis indicated by WORMS scores. WORMS: Whole-Organ Magnetic Resonance Imaging Score
Relationship between obesity or hyperlipidemia and synovitis or structural abnormalities of knee OA
Relationship between obesity or hyperlipidemia and synovitis or structural abnormalities of knee OA was shown in Table 5. There were significant positive associations of obesity with effusion-synovitis using ACLOAS method (P < 0.001), effusion-synovitis using MOAKS method (P < 0.001), size of IPFP abnormality (P < 0.001), highest signal intensity of IPFP abnormality (P < 0.001), SPS in knee (P < 0.001), and SPS in popliteal cyst (P = 0.013), as well as cartilage (P < 0.001), bone marrow edema (P < 0.001), meniscus (P < 0.001), and total WORMS scores (P < 0.001). There were significant positive associations of hyperlipidemia with effusion-synovitis using ACLOAS method (P < 0.001), effusion-synovitis using MOAKS method (P < 0.001), size of IPFP abnormality (P < 0.001), highest signal intensity of IPFP abnormality (P < 0.001), SPS in knee (P < 0.001), and SPS in popliteal cyst (P < 0.001), as well as cartilage (P < 0.001), bone marrow edema (P < 0.001), meniscus (P < 0.001), and total WOMS scores (P < 0.001). No significant association was observed between obesity or hyperlipidemia and subchondral cyst score (P > 0.05).
Table 5.
Relationship between obesity or hyperlipidemia and synovitis or structural abnormalities of knee OA
| Obesity | Hyperlipidemia | |
|---|---|---|
| Effusion-synovitis | ||
| ACLOAS method | 0.213 (P < 0.001) | 0.243 (P < 0.001) |
| MOAKS method | 0.229 (P < 0.001) | 0.250 (P < 0.001) |
| IPFP abnormality | ||
| Size | 0.218 (P < 0.001) | 0.246 (P < 0.001) |
| Highest signal intensity | 0.213 (P < 0.001) | 0.231 (P < 0.001) |
| SPS | ||
| Knee | 0.182 (P < 0.001) | 0.209 (P < 0.001) |
| Popliteal cyst | 0.124 (P = 0.013) | 0.141 (P < 0.001) |
| WORMS score | ||
| Cartilage | 0.194 (P < 0.001) | 0.270 (P < 0.001) |
| Bone marrow edema | 0.214 (P < 0.001) | 0.247 (P < 0.001) |
| Meniscus | 0.200 (P < 0.001) | 0.213 (P < 0.001) |
| Subchondral cyst | 0.079 (P = 0.113) | 0.059 (P = 0.237) |
| Total | 0.303 (P < 0.001) | 0.367 (P < 0.001) |
OA: osteoarthritis; ACLOAS: Anterior Cruciate Ligament OsteoArthritis Score; MOAKS: MRI Osteoarthritis Knee Score; IPFP: infrapatellar fat pad; SPS: synovial proliferation score; WORMS: Whole-Organ Magnetic Resonance Imaging Score. The values were presented as Spearman’s rho (P)
Clinical outcomes
Functional outcomes including WOMAC, FJS, OKS, KSKS, and KSFS were shown in Table 6. WOMAC, FJS, OKS, KSKS, and KSFS were significantly improved in all groups at 2 years follow-up postoperatively. Group 2, Group 3, Group 4 had significantly higher WOMAC and lower FJS, OKS, KSKS, and KSFS compared with Group 1 at baseline and 2 years follow-up. Group 4 had significantly higher WOMAC and lower FJS, OKS, KSKS, and KSFS than those of Group 2 and Group 3 at baseline and 2 years follow-up. There was no significant difference in all functional outcomes between Group 2 and Group 3. SMDs showed that there were small differences in WOMAC, FJS, OKS, KSKS, and KSFS between Group 2 and Group 3 at baseline and 2 years follow-up. There was no major complication and reoperation in all groups during 2 years follow-up. The proportion of patients who were satisfied was 94.9% in Group 1, 93.0% in Group 2, 93.8% in Group 3, and 89.7% in Group 4.
Table 6.
Functional outcomes at baseline and 2 yeas follow-up
| Functional Outcomes | Group 1 | Group 2 | Group 3 | Group 4 | P Value and SMD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | 1 vs. 2 | 1 vs. 3 | 1 vs. 4 | 2 vs. 3 | 2 vs. 4 | 3 vs. 4 | |||||
| WOMAC | |||||||||||
| Baseline | 47.2 ± 8.0 | 50.2 ± 6.9 | 50.6 ± 5.9 | 53.5 ± 6.7 | < 0.001 | 0.002 | < 0.001 | < 0.001 | 0.644 | 0.001 | 0.004 |
| 0.43 | 0.58 | 0.94 | 0.07 | 0.49 | 0.43 | ||||||
| 2 years follow-up | 24.8 ± 7.5 | 30.7 ± 5.5 | 29.9 ± 6.0 | 33.1 ± 5.9 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.315 | 0.022 | 0.001 |
| 1.07 | 0.85 | 1.41 | 0.13 | 0.41 | 0.54 | ||||||
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | - | - | - | - | - | - | - |
| FJS | |||||||||||
| Baseline | 20.1 ± 7.0 | 17.5 ± 6.9 | 18.0 ± 7.6 | 14.9 ± 5.4 | < 0.001 | 0.007 | 0.023 | < 0.001 | 0.654 | 0.006 | 0.001 |
| 0.38 | 0.28 | 0.96 | 0.07 | 0.48 | 0.57 | ||||||
| 2 years follow-up | 76.6 ± 12.9 | 72.4 ± 11.1 | 72.9 ± 11.4 | 67.8 ± 12.4 | < 0.001 | 0.016 | 0.030 | < 0.001 | 0.806 | 0.008 | 0.004 |
| 0.38 | 0.32 | 0.71 | 0.04 | 0.37 | 0.71 | ||||||
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | - | - | - | - | - | - | - |
| OKS | |||||||||||
| Baseline | 20.2 ± 4.1 | 18.1 ± 5.1 | 17.6 ± 4.4 | 14.9 ± 4.6 | < 0.001 | 0.002 | < 0.001 | < 0.001 | 0.370 | < 0.001 | < 0.001 |
| 0.41 | 0.59 | 1.15 | 0.11 | 0.70 | 0.59 | ||||||
| 2 years follow-up | 40.4 ± 6.9 | 37.5 ± 4.8 | 37.0 ± 2.7 | 34.2 ± 4.8 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.459 | < 0.001 | < 0.001 |
| 0.60 | 1.26 | 1.29 | 0.19 | 0.69 | 0.58 | ||||||
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | - | - | - | - | - | - | - |
| KSKS | |||||||||||
| Baseline | 45.3 ± 5.6 | 43.1 ± 6.4 | 43.3 ± 3.4 | 41.1 ± 5.2 | < 0.001 | 0.004 | 0.009 | < 0.001 | 0.748 | 0.009 | 0.004 |
| 0.34 | 0.59 | 0.81 | 0.06 | 0.38 | 0.42 | ||||||
| 2 years follow-up | 88.9 ± 5.4 | 85.7 ± 5.7 | 85.1 ± 5.5 | 83.1 ± 6.2 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.499 | < 0.001 | 0.005 |
| 0.56 | 0.69 | 0.93 | 0.11 | 0.42 | 0.32 | ||||||
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | - | - | - | - | - | - | - |
| KSFS | |||||||||||
| Baseline | 46.5 ± 6.0 | 44.7 ± 3.4 | 43.9 ± 5.9 | 42.3 ± 5.8 | < 0.001 | 0.020 | 0.001 | < 0.001 | 0.275 | 0.002 | 0.037 |
| 0.53 | 0.44 | 0.72 | 0.14 | 0.41 | 0.28 | ||||||
| 2 years follow-up | 97.4 ± 3.2 | 94.9 ± 5.3 | 94.1 ± 5.4 | 92.3 ± 4.6 | < 0.001 | < 0.002 | < 0.001 | < 0.001 | 0.415 | 0.003 | 0026 |
| 0.47 | 0.61 | 1.11 | 0.15 | 0.57 | 0.39 | ||||||
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | - | - | - | - | - | - | - |
WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index; FJS: Forgotten Joint Score; OKS: Oxford Knee Score; KSKS: Knee Society Knee Score; KSFS: Knee Society Function Score; SMD: standardized mean difference. The values were presented as mean ± standard deviation otherwise indicated
Relationship between obesity or hyperlipidemia and functional outcomes
Relationship between obesity or hyperlipidemia and functional outcomes was shown in Table 7. At baseline, there were significant negative associations of obesity with FJS (P = 0.001), OKS (P < 0.001), KSKS, (P = 0.001) and KSFS (P < 0.001), of hyperlipidemia with FJS (P < 0.001), OKS (P < 0.001), KSKS, (P < 0.001) and KSFS (P = 0.009), and positive associations of obesity with WOMAC (P < 0.001), and of hyperlipidemia with WOMAC (P < 0.001). At 2 years follow-up, there were significant negative associations of obesity with FJS (P = 0.001), OKS (P = 0.013), KSKS, (P < 0.001) and KSFS (P < 0.001), of hyperlipidemia with FJS (P < 0.001), OKS (P < 0.001), KSKS, (P < 0.001) and KSFS (P < 0.001), and positive associations of obesity with WOMAC (P < 0.001), and of hyperlipidemia with WOMAC (P < 0.001).
Table 7.
Relationship between obesity or hyperlipidemia and functional outcomes
| Obesity | Hyperlipidemia | |
|---|---|---|
| WOMAC | ||
| Baseline | 0.218 (P < 0.001) | 0.194 (P < 0.001) |
| 2 years follow-up | 0.246 (P < 0.001) | 0.306 (P < 0.001) |
| FJS | ||
| Baseline | -0.171 (P = 0.001) | -0.197 (P < 0.001) |
| 2 years follow-up | -0.168 (P = 0.001) | -0.187 (P < 0.001) |
| OKS | ||
| Baseline | -0.295 (P < 0.001) | -0.253 (P < 0.001) |
| 2 years follow-up | -0.359 (P = 0.013) | -0.252 (P < 0.001) |
| KSKS | ||
| Baseline | -0.160 (P = 0.001) | 0.194 (P < 0.001) |
| 2 years follow-up | -0.249 (P < 0.001) | -0.192 (P < 0.001) |
| KSFS | ||
| Baseline | -0.213 (P < 0.001) | -0.131 (P = 0.009) |
| 2 years follow-up | -0.302 (P < 0.001) | -0.229 (P < 0.001) |
WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index; FJS: Forgotten Joint Score; OKS: Oxford Knee Score; KSKS: Knee Society Knee Score; KSFS: Knee Society Function Score. The values were presented as Spearman’s rho (P)
Reproducibility of measurements
For measurements of synovial inflammatory markers, the Cohen’s Kappa values for interobserver reproducibility ranged from 0.89 to 0.94, and values for intraobserver reproducibility ranged from 0.87 to 0.95. For measurements of WORMS, the Cohen’s Kappa values for interobserver reproducibility ranged from 0.85 to 0.93, and values for intraobserver reproducibility ranged from 0.87 to 0.96. These values demonstrated almost perfect reproducibility for all measurements of synovitis and structural abnormalities of knee OA.
Discussion
The most important findings of this study were: (1) obesity and hyperlipidemia were associated with more severe synovitis and structural abnormalities of knee OA as well as inferior preoperative and postoperative functional outcomes; and (2) the negative effects of obesity and hyperlipidemia on knee OA could be mutually enhanced. The findings of this study emphasized the negative effects of obesity and hyperlipidemia on the symptoms and outcomes of knee OA, and suggested that paying attention to the blood lipids of OA patients with normal weight to reduce the adverse effect of hyperlipidemia on functional outcomes following TKA.
The role of obesity in the development and progression of OA has always been a hot research topic. As one of the important components of metabolic syndrome, obesity shares a similar ascension trend with OA under the background of complex and various risk factors and etiologies [19]. In addition, with the worrying increasing rates of obesity and sports injuries, the incidence of knee OA in young people is gradually rising, which has a profound impact on work ability and psychosocial well-being [46]. However, the association between obesity and OA is complicated and multifactorial. Traditionally, obesity was considered to induce OA by over-increasing joint mechanical load and leading to the destruction of articular cartilage, which was supported by the evidence that a 5-unit increase in BMI caused an 35% of increased risk of knee OA [47]. However, based on the fact that high fat mass rather than weight itself was more closely related to knee OA [48] and the incidence of OA of non-weight-bearing joints such as hand was also correlated to obesity [15–17], more and more attention has been paid to the role of metabolic and immune dysregulation as well as chronic low-grade systemic inflammatory state caused by obesity in OA pathogenesis [19], which challenged the classical mechanical mechanism of obesity. Therefore, obesity-related OA can be classified as an independent type of secondary OA, which has different pathogenesis from primary OA and secondary OA caused by other factors, such as trauma [49].
Emerging research has shown that there is an inflammatory component in OA accompanied by inflammatory cell infiltration in synovium, which highlights the important significance of synovitis with a relatively lower inflammatory profile in the occurrence and development of OA [50]. Synovitis has been increasingly regarded as a common symptom of knee OA both in early and late stages [5], which partially mediated the association between cartilage damage and worsening pain, and had close relationships with structural degenerative abnormalities and radiographic progression of OA [34, 51, 52]. Inflammatory synovium can promote cartilage degradation by secreting proinflammatory mediators and catabolic factors, and the degradation products of cartilage in turn aggravate synovial inflammation, thus causing a vicious circle and accelerating the progression of OA [53, 54].
Regarding the relationship between obesity and knee OA, this study showed that obesity was associated with more severe synovitis and structural abnormalities of knee OA, which is consistent with the findings of Kanthawang et al. [29]. Hung et al. [55] reported that obesity was associated with higher risks of incidence and progression of knee effusion. Bañuls-Mirete et al. [30] found that the association between BMI and progression of knee OA was mediated by worsening effusion-synovitis, especially in obese individuals. These studies emphasized the adverse effects of synovitis in obese patients with OA, which may be a potential target for therapeutic intervention to alleviate the symptoms and progression of OA.
Although the negative impact of obesity on OA has been clearly determined, the potential effect of hyperlipidemia on OA, which is closely related to obesity and often occurs in obese population, is still controversial. Zhou et al. [56] reported that hyperlipidemia was associated with increased risks of pain and knee OA, with 1-unit increase in TG predicting 9% and 5% of increases in the risks of prevalence and onset of knee OA. However, several studies demonstrated that there was no clear evidence to confirm the relationship between hyperlipidemia and knee OA [23, 24]. In addition, these studies did not rule out the potential effects of obesity, and may confuse the effects of obesity and hyperlipidemia on knee OA, thus failing to adequately reveal the role of hyperlipidemia in OA.
To investigate the correlation between hyperlipidemia and knee OA, patients with obesity or hyperlipidemia were strictly distinguished in this study to eliminate their mutual influence. It was found that compared with OA patients with normal weight and blood lipids, OA patients with hyperlipidemia had more severe synovitis and structural abnormalities of knee OA, as well as worse preoperative and postoperative functional outcomes, while there was no significant difference when compared with obese OA patients. These results showed that the negative effect of hyperlipidemia on OA was no less than that of obesity, and suggested that the blood lipid levels of OA patients with normal weight cannot be neglected.
However, the reasons underlying the relationship between hyperlipidemia and OA are still not sufficiently clear, which may be associated with multiple factors. First, as a sign of lipid metabolism disorder, hyperlipidemia may interrupt the balance between lipid metabolism and osteochondral metabolism, considering that adipocytes share the same mesenchymal stem cell precursors with osteoblasts and chondrocytes [57]. Second, inflammatory mediators such as free fatty acids increased in the presence of hyperlipidemia, which increased levels of reactive oxygen species and expressions of proinflammatory cytokines, thus inducing mitochondrial dysfunction and chondrocytes apoptosis [58]. Third, vascular pathology may play a potential role in linking hyperlipidemia with knee OA. Serum TC and TG levels were associated with the incidence of bone marrow lesions over 2 years, which may be due to the reduced blood flow and subchondral ischemia caused by lipid emboli and thrombi, ultimately leading to bone necrosis [59].
The findings of this study showed that patients with obesity alone or hyperlipidemia alone had comparable synovitis and structural abnormalities of knee OA as well as functional outcomes, which may be due to possible shared mechanisms affecting OA between obesity and hyperlipidemia. First, infiltrating synovial macrophages with a proinflammatory phenotype may be the main mediator of the inflammation during OA process, and both obesity and hyperlipidemia could enhance the proinflammatory responses of macrophages [19, 60]. Second, increased oxidative stress and overproduced reactive oxygen species during OA, which can be promoted by both obesity and hyperlipidemia, contribute to synovial inflammation and cartilage degradation through regulating intracellular signaling processes, such as mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR), and nuclear factor-kappa B (NFκB) pathways [61, 62]. Third, both obesity and hyperlipidemia could significantly increase the release of proinflammatory cytokines, leading to the destruction of synovium and cartilage [19, 63]. However, the possible mechanism is not definite at present, which still needs further exploration.
It is worth noting that the OA patients with both obesity and hyperlipidemia had the most serious synovitis and structural abnormalities of knee OA, and the worst preoperative and postoperative functional outcomes compared with the OA patients with only one of them. In addition to the mutual superposition and promotion of the respective effects of obesity and hyperlipidemia, several metabolic and inflammatory factors may play an important role in enhancing their negative effects. First, insulin resistance is involved in OA under the background of obesity and hyperlipidemia, which may elevate the level of free fatty acids and increase the sensitivity of the synovium and articular cartilage to the activity of proinflammatory mediators [64]. Second, various adipokines from adipose tissues are implicated in OA, which regulate cartilage homeostasis through complex interactions, such as synthesis and decomposition of extracellular matrix, and release and inhibition of proinflammatory cytokines [19]. Third, age-related muscle dysfunction is a major factor in the development of OA. Sarcopenic obesity describes the cases in which the weight gain caused by obesity is offset by the loss of muscle mass [64]. The pathogenesis linking knee OA and sarcopenia could lie in the arthrogenous muscle inhibition caused by the impaired afferent input from the OA knee and subsequent altered efferent motor neuron stimulation on the quadriceps, leading to quadriceps weakness [65].
The findings of this study are of great significance to public health, because the relatively high prevalence of knee OA seriously affects health and quality of life for middle-aged and elderly people. It is meaningful to pay attention to patients’ body weight and blood lipids to improve the treatment and prognosis of knee OA. Future research should focus on whether controlling obesity and hyperlipidemia could have a positive effect on the progression and prognosis of knee OA. Obesity and hyperlipidemia can be prevented by diet and physical exercise. In addition, for OA patients with normal weight, the evaluation of blood lipids cannot be neglected, because the influence of hyperlipidemia on OA is probably no less than that of obesity. Besides, this study emphasized the importance of synovitis in OA patients with obesity and hyperlipidemia, and provided meaningful evidence for controlling inflammation to alleviate the symptoms and progression of OA.
Younger patients tend to have more functional exercises and daily activities, while older patients usually have less activities. To avoid the influence of these two extreme cases on the functional evaluation, the age of the patients included was defined as 40–80 years old. This study has several limitations. First, semi-quantitative assessment of synovitis was performed, which has relatively low sensitivity and accuracy than quantitative methods. Second, due to the lack of MRI images at the final follow-up, synovitis cannot be evaluated at the final follow-up, and thus the correlation between synovitis progression and postoperative functional outcomes cannot be determined. Third, because of the high prevalence rate of knee OA in middle-aged and elderly people, subjects without knee OA were not included. Fourth, the lower prevalence rate of knee OA in men led to sex imbalance of included patients, which may affect the accuracy of the results. Fifth, although patients who took drugs that may affect their weight or blood lipids at baseline were excluded, the patients’ medication was not well monitored during the follow-up. Changes in body weight or blood lipid levels during the follow-up may affect functional outcomes. Sixth, information regarding dietary patterns was not collected, and it is impossible to evaluate the possible confounding effect of this factor. Finally, BMI was not monitored during the final follow-up, leading to uncertainty whether the functional improvement was attributed to the type of grouping or weight loss during the follow-up.
Conclusion
Obesity and hyperlipidemia were associated with more severe synovitis and structural abnormalities of knee OA as well as inferior preoperative and postoperative functional outcomes. The negative effects of obesity and hyperlipidemia on knee OA could be mutually enhanced. The findings of this study emphasized the negative effects of obesity and hyperlipidemia on the symptoms and outcomes of knee OA, and highlighted the association of obesity and hyperlipidemia with synovitis.
Acknowledgements
The authors would like to acknowledge the MRI Department of our hospital for their technical supports.
Abbreviations
- OA
osteoarthritis
- TKA
total knee arthroplasty
- BMI
body mass index
- TC
total cholesterol
- TG
triglycerides
- LDL-C
Low-density lipoprotein cholesterol
- HDL-C
High-density lipoprotein cholesterol
- MRI
magnetic resonance imaging
- IW
intermediate-weighted
- TSE
turbo spin-echo
- DESS
dual-echo steady-state
- ACLOAS
Anterior Cruciate Ligament Osteo Arthritis Score
- MOAKS
MRI Osteoarthritis Knee Score
- IPFP
infrapatellar fat pad
- SPS
synovial proliferation score
- WORMS
Whole-Organ MRI Score
- WOMAC
Western Ontario and McMaster Universities Osteoarthritis Index
- FJS
Forgotten Joint Score
- OKS
Oxford Knee Score
- KSS
Knee Society Score
- KSKS
Knee Society Knee Score
- KSFS
Knee Society Function Score
- SMD
standardized mean difference
Author contributions
FW contributed to the conceptualization and project administration of the study. KH contributed to the study design, patient selection, data collection and statistical analysis. JW performed patient selection and parameter measurements. YN performed parameter measurements and data analysis. The first draft of the manuscript was written by KH. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
No funding was received.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by Institutional Review Board of Hebei medical university third hospital and followed the Declaration of Helsinki. Informed consent was obtained from all patients.
Consent for publication
Written informed consent was obtained from all patients to authorize the publication of their data.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hunter DJ, Bierma-Zeinstra S, Osteoarthritis. Lancet. 2019;393(10182):1745–59. [DOI] [PubMed] [Google Scholar]
- 2.Chen D, Shen J, Zhao W, Wang T, Han L, Hamilton JL, Im HJ. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. 2017;5:16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palazzo C, Nguyen C, Lefevre-Colau MM, Rannou F, Poiraudeau S. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. 2016;59(3):134–8. [DOI] [PubMed] [Google Scholar]
- 4.Greene MA, Loeser RF. Aging-related inflammation in osteoarthritis. Osteoarthritis Cartilage. 2015;23(11):1966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nedunchezhiyan U, Varughese I, Sun AR, Wu X, Crawford R, Prasadam I. Obesity, inflammation, and Immune System in Osteoarthritis. Front Immunol. 2022;13:907750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sowers MR, Karvonen-Gutierrez CA. The evolving role of obesity in knee osteoarthritis. Curr Opin Rheumatol. 2010;22(5):533–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobs CA, Vranceanu AM, Thompson KL, Lattermann C. Rapid Progression of knee Pain and Osteoarthritis biomarkers Greatest for patients with combined obesity and depression: data from the Osteoarthritis Initiative. Cartilage. 2020;11(1):38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raud B, Gay C, Guiguet-Auclair C, Bonnin A, Gerbaud L, Pereira B, Duclos M, Boirie Y, Coudeyre E. Level of obesity is directly associated with the clinical and functional consequences of knee osteoarthritis. Sci Rep. 2020;10(1):3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gersing AS, Schwaiger BJ, Nevitt MC, Zarnowski J, Joseph GB, Feuerriegel G, Jungmann PM, Guimaraes JB, Facchetti L, McCulloch CE, Link TM. Weight loss regimen in obese and overweight individuals is associated with reduced cartilage degeneration: 96-month data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2019;27(6):863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gelber AC, Hochberg MC, Mead LA, Wang NY, Wigley FM, Klag MJ. Body mass index in young men and the risk of subsequent knee and hip osteoarthritis. Am J Med. 1999;107(6):542–8. [DOI] [PubMed] [Google Scholar]
- 11.Giordano L, Maffulli N, Morenghi E, Quaglia A, Prospero E, Rosa F, Volpi P. A BMI above 30 results in satisfying outcomes in patients undergoing fixed-bearing lateral unicompartmental knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2023;31(3):1106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Berardinis L, Piovan G, Screpis D, Senarighi M, Baldini M, Povegliano L, Gigante AP, Zorzi C. Mid-term outcomes of medial metal backed and all-polyethylene unicompartmental knee arthroplasty in obese patients: a retrospective propensity-matched analysis. J Orthop Surg Res. 2024;19(1):651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hakim J, Volpin G, Amashah M, Alkeesh F, Khamaisy S, Cohen M, Ownallah J. Long-term outcome of total knee arthroplasty in patients with morbid obesity. Int Orthop. 2020;44(1):95–104. [DOI] [PubMed] [Google Scholar]
- 14.Uvodich ME, Dugdale EM, Pagnano MW, Berry DJ, Abdel MP, Bedard NA. Outcomes of obese patients undergoing primary total knee arthroplasty: Trends over 30 years. J Bone Joint Surg Am. 2024;106(21):1963–70. [DOI] [PubMed] [Google Scholar]
- 15.Carman WJ, Sowers M, Hawthorne VM, Weissfeld LA. Obesity as a risk factor for osteoarthritis of the hand and wrist: a prospective study. Am J Epidemiol. 1994;139(2):119–29. [DOI] [PubMed] [Google Scholar]
- 16.Grotle M, Hagen KB, Natvig B, Dahl FA, Kvien TK. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet Disord. 2008;9:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reyes C, Leyland KM, Peat G, Cooper C, Arden NK, Prieto-Alhambra D. Association between Overweight and Obesity and risk of clinically diagnosed knee, hip, and Hand Osteoarthritis: a Population-based Cohort Study. Arthritis Rheumatol. 2016;68(8):1869–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sobieh BH, El-Mesallamy HO, Kassem DH. Beyond mechanical loading: the metabolic contribution of obesity in osteoarthritis unveils novel therapeutic targets. Heliyon. 2023;9(5):e15700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mocanu V, Timofte DV, Zară-Dănceanu CM, Labusca L, Obesity. Metabolic syndrome, and Osteoarthritis Require Integrative understanding and management. Biomedicines. 2024;12(6):1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joint committee issued Chinese guideline for the management of dyslipidemia in adults. [2016 Chinese guideline for the management of dyslipidemia in adults]. Zhonghua Xin xue guan bing za zhi. 2016;44(10):833–53. [DOI] [PubMed] [Google Scholar]
- 21.Andersson M, Haglund E, Aili K, Bremander A, Bergman S. Associations between metabolic factors and radiographic knee osteoarthritis in early disease - a cross-sectional study of individuals with knee pain. BMC Musculoskelet Disord. 2022;23(1):938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan F, Tian J, Cicuttini F, Jones G. Metabolic syndrome and trajectory of knee pain in older adults. Osteoarthritis Cartilage. 2020;28(1):45–52. [DOI] [PubMed] [Google Scholar]
- 23.Xiong J, Long J, Chen X, Li Y, Song H. Dyslipidemia might be Associated with an increased risk of Osteoarthritis. Biomed Res Int. 2020;2020:3105248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie Y, Zhou W, Zhong Z, Zhao Z, Yu H, Huang Y, Zhang P. Metabolic syndrome, hypertension, and hyperglycemia were positively associated with knee osteoarthritis, while dyslipidemia showed no association with knee osteoarthritis. Clin Rheumatol. 2021;40(2):711–24. [DOI] [PubMed] [Google Scholar]
- 25.Zheng C, Liu Y, Xu C, Zeng S, Wang Q, Guo Y, Li J, Li S, Dong M, Luo X, Wu Q. Association between obesity and the prevalence of dyslipidemia in middle-aged and older people: an observational study. Sci Rep. 2024;14(1):11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Agostino MA, Conaghan P, Le Bars M, Baron G, Grassi W, Martin-Mola E, Wakefield R, Brasseur JL, So A, Backhaus M, Malaise M, Burmester G, Schmidely N, Ravaud P, Dougados M, Emery P. EULAR report on the use of ultrasonography in painful knee osteoarthritis. Part 1: prevalence of inflammation in osteoarthritis. Ann Rheum Dis. 2005;64(12):1703–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez-Lopez E, Coras R, Torres A, Lane NE, Guma M. Synovial inflammation in osteoarthritis progression. Nat Rev Rheumatol. 2022;18(5):258–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarmanova A, Hall M, Fernandes GS, Bhattacharya A, Valdes AM, Walsh DA, Doherty M, Zhang W. Association between ultrasound-detected synovitis and knee pain: a population-based case-control study with both cross-sectional and follow-up data. Arthritis Res Ther. 2017;19(1):281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanthawang T, Bodden J, Joseph GB, Lane NE, Nevitt M, McCulloch CE, Link TM. Obese and overweight individuals have greater knee synovial inflammation and associated structural and cartilage compositional degeneration: data from the osteoarthritis initiative. Skeletal Radiol. 2021;50(1):217–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bañuls-Mirete M, Lombardi AF, Posis AIB, Shadyab AH, Chang EY, Lane NE, Guma M. Effusion-synovitis worsening mediates the association between body mass index and Kellgren-Lawrence progression in obese individuals: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2022;30(9):1278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, Roemer FW. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI osteoarthritis knee score). Osteoarthritis Cartilage. 2011;19(8):990–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roemer FW, Frobell R, Lohmander LS, Niu J, Guermazi A. Anterior cruciate ligament OsteoArthritis score (ACLOAS): longitudinal MRI-based whole joint assessment of anterior cruciate ligament injury. Osteoarthritis Cartilage. 2014;22(5):668–82. [DOI] [PubMed] [Google Scholar]
- 34.Ramezanpour S, Kanthawang T, Lynch J, McCulloch CE, Nevitt MC, Link TM, Joseph GB. Impact of sustained synovitis on knee joint structural degeneration: 4-Year MRI Data from the Osteoarthritis Initiative. J Magn Reson Imaging. 2023;57(1):153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, Foreman SC, Joseph GB, Neumann J, Tien PC, Li X, Lane NE, Nevitt MC, McCulloch CE, Link TM. Is treated HIV infection associated with knee cartilage degeneration and structural changes? A longitudinal study using data from the osteoarthritis initiative. BMC Musculoskelet Disord. 2019;20(1):190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heilmeier U, Mamoto K, Amano K, Eck B, Tanaka M, Bullen JA, Schwaiger BJ, Huebner JL, Stabler TV, Kraus VB, Ma CB, Link TM, Li X. Infrapatellar fat pad abnormalities are associated with a higher inflammatory synovial fluid cytokine profile in young adults following ACL tear. Osteoarthr Cartil. 2020;28(1):82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, Kothari M, Lu Y, Fye K, Zhao S, Genant HK. Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12(3):177–90. [DOI] [PubMed] [Google Scholar]
- 38.Bucknor MD, Nardo L, Joseph GB, Alizai H, Srikhum W, Nevitt MC, Lynch JA, McCulloch CE, Link TM. Association of cartilage degeneration with four year weight gain–3T MRI data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2015;23(4):525–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gersing AS, Schwaiger BJ, Nevitt MC, Joseph GB, Chanchek N, Guimaraes JB, Mbapte Wamba J, Facchetti L, McCulloch CE, Link TM. Is weight loss Associated with less progression of changes in knee articular cartilage among obese and overweight patients as assessed with MR Imaging over 48 months? Data from the Osteoarthritis Initiative. Radiology. 2017;284(2):508–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joseph GB, Baum T, Alizai H, Carballido-Gamio J, Nardo L, Virayavanich W, Lynch JA, Nevitt MC, McCulloch CE, Majumdar S, Link TM. Baseline mean and heterogeneity of MR cartilage T2 are associated with morphologic degeneration of cartilage, meniscus, and bone marrow over 3 years–data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2012l;20(7):727–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McConnell S, Kolopack P, Davis AM. The Western Ontario and McMaster universities osteoarthritis Index (WOMAC): a review of its utility and measurement properties. Arthritis Rheum. 2001;45(5):453–61. [DOI] [PubMed] [Google Scholar]
- 42.Behrend H, Giesinger K, Giesinger JM, Kuster MS. The forgotten joint as the ultimate goal in joint arthroplasty. J Arthroplasty. 2012;27(3):430–e4361. [DOI] [PubMed] [Google Scholar]
- 43.Dawson J, Fitzpatrick R, Murray D, Carr A. Questionnaire on the perceptions of patients about total knee replacement. J Bone Joint Surg Br. 1998;80(1):63–9. [DOI] [PubMed] [Google Scholar]
- 44.Noble PC, Scuderi GR, Brekke AC, Sikorskii A, Benjamin JB, Lonner JH, Chadha P, Daylamani DA, Scott WN, Bourne RB. Development of a new Knee Society scoring system. Clin Orthop Relat Res. 2012;470(1):20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74. [PubMed] [Google Scholar]
- 46.Ackerman IN, Kemp JL, Crossley KM, Culvenor AG, Hinman RS. Hip and knee osteoarthritis affects younger people, too. J Orthop Sports Phys Ther. 2017;47(2):67–79. [DOI] [PubMed] [Google Scholar]
- 47.Jiang L, Tian W, Wang Y, Rong J, Bao C, Liu Y, Zhao Y, Wang C. Body mass index and susceptibility to knee osteoarthritis: a systematic review and meta-analysis. Joint Bone Spine. 2012;79(3):291–7. [DOI] [PubMed] [Google Scholar]
- 48.Suh DH, Han KD, Hong JY, Park JH, Bae JH, Moon YW, Kim JG. Body composition is more closely related to the development of knee osteoarthritis in women than men: a cross-sectional study using the Fifth Korea National Health and Nutrition Examination Survey (KNHANES V-1, 2). Osteoarthritis Cartilage. 2016;24(4):605–11. [DOI] [PubMed] [Google Scholar]
- 49.Shumnalieva R, Kotov G, Ermencheva P, Monov S. Pathogenic mechanisms and therapeutic approaches in obesity-related knee osteoarthritis. Biomedicines. 2023;12(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saito I, Koshino T, Nakashima K, Uesugi M, Saito T. Increased Cellular Infiltrate in Inflammatory Synovia of Osteoarthritic Knees. Osteoarthritis Cartilage. 2002;10(2):156–62. [DOI] [PubMed] [Google Scholar]
- 51.Roemer FW, Guermazi A, Felson DT, Niu J, Nevitt MC, Crema MD, Lynch JA, Lewis CE, Torner J, Zhang Y. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann Rheum Dis. 2011;70(10):1804–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bacon K, LaValley MP, Jafarzadeh SR, Felson D. Does cartilage loss cause pain in osteoarthritis and if so, how much? Ann Rheum Dis. 2020;79(8):1105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Lange-Brokaar BJ, Ioan-Facsinay A, van Osch GJ, Zuurmond AM, Schoones J, Toes RE, Huizinga TW, Kloppenburg M. Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis Cartilage. 2012;20(12):1484–99. [DOI] [PubMed] [Google Scholar]
- 54.Wang X, Hunter DJ, Jin X, Ding C. The importance of synovial inflammation in osteoarthritis: current evidence from imaging assessments and clinical trials. Osteoarthritis Cartilage. 2018;26(2):165–74. [DOI] [PubMed] [Google Scholar]
- 55.Hung A, Sayre EC, Guermazi A, Esdaile JM, Kopec JA, Thorne A, Singer J, Wong H, Nicolaou S, Cibere J. Association of Body Mass Index with incidence and progression of knee effusion on magnetic resonance imaging and on knee examination. Arthritis Care Res (Hoboken). 2016;68(4):511–6. [DOI] [PubMed] [Google Scholar]
- 56.Zhou M, Guo Y, Wang D, Shi D, Li W, Liu Y, Yuan J, He M, Zhang X, Guo H, Wu T, Chen W. The cross-sectional and longitudinal effect of hyperlipidemia on knee osteoarthritis: results from the Dongfeng-Tongji cohort in China. Sci Rep. 2017;7(1):9739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. [DOI] [PubMed] [Google Scholar]
- 58.Medina-Luna D, Santamaría-Olmedo MG, Zamudio-Cuevas Y, Martínez-Flores K, Fernández-Torres J, Martínez-Nava GA, Clavijo-Cornejo D, Hernández-Díaz C, Olivos-Meza A, Gomez-Quiroz LE, Gutiérrez-Ruiz MC, Pineda C, Blanco F, Reginato AM, López-Reyes A. Hyperlipidemic microenvironment conditionates damage mechanisms in human chondrocytes by oxidative stress. Lipids Health Dis. 2017;16(1):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davies-Tuck ML, Hanna F, Davis SR, Bell RJ, Davison SL, Wluka AE, Adams J, Cicuttini FM. Total cholesterol and triglycerides are associated with the development of new bone marrow lesions in asymptomatic middle-aged women - a prospective cohort study. Arthritis Res Ther. 2009;11(6):R181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Tits LJ, Stienstra R, van Lent PL, Netea MG, Joosten LA, Stalenhoef AF. Oxidized LDL enhances pro-inflammatory responses of alternatively activated M2 macrophages: a crucial role for Krüppel-like factor 2. Atherosclerosis. 2011;214(2):345–9. [DOI] [PubMed] [Google Scholar]
- 61.Hashimoto K, Akagi M. The role of oxidation of low-density lipids in pathogenesis of osteoarthritis: a narrative review. J Int Med Res. 2020;48(6):300060520931609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Senol O, Gundogdu G, Gundogdu K, Miloglu FD. Investigation of the relationships between knee osteoarthritis and obesity via untargeted metabolomics analysis. Clin Rheumatol. 2019;38(5):1351–60. [DOI] [PubMed] [Google Scholar]
- 63.Thijssen E, van Caam A, van der Kraan PM. Obesity and osteoarthritis, more than just wear and tear: pivotal roles for inflamed adipose tissue and dyslipidaemia in obesity-induced osteoarthritis. Rheumatology (Oxford). 2015;54(4):588–600. [DOI] [PubMed] [Google Scholar]
- 64.Shumnalieva R, Kotov G, Monov S. Obesity-related knee osteoarthritis-current concepts. Life (Basel). 2023;13(8):1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pegreffi F, Balestra A, De Lucia O, Smith L, Barbagallo M, Veronese N. Prevalence of Sarcopenia in knee osteoarthritis: a systematic review and Meta-analysis. J Clin Med. 2023;12(4):1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.