Abstract
Attention-deficit/hyperactivity disorder (ADHD) affects approximately 12% of children worldwide. With a 50% chance of persistence into adulthood and associations with impairments in various domains, including social and emotional ones, early diagnosis is crucial. The exact neural substrates of ADHD are still unclear. This study aimed to reassess the behavioral and neural metrics of executive functions and neural substrates of facial affect recognition. A total of 117 ADHD patients and 183 healthy controls were evaluated by two Go/NoGo tasks: the classic visual continuous performance test and the emotional continuous performance test, which requires facial affect encoding. Group differences between ADHD subjects and healthy controls were assessed using analysis of covariance (ANCOVA), with age and sex included as covariates. Dependent variables comprised behavioral (number of omission and commission errors, reaction time, and reaction time variability) and neurophysiological measures (event-related potentials [ERPs]). As the main result, we identified significant differences between ADHD patients and healthy controls in all behavioral metrics, one neural marker of action inhibition (P3d) and the facial processing marker (N170). The differences were moderate-to-large when expressed as effect size measures in behavioral variables and small-to-moderate for neurophysiological variables. The small-to-moderate effect sizes obtained from the neurophysiological measures suggest that ERPs are insufficient as sole markers for effectively screening emotion and face processing abnormalities in ADHD.
Keywords: attention-deficit hyperactivity disorder, VCPT, ECPT, ERP, facial affect recognition
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is widely recognized as a neurodevelopmental disorder characterized by impairments in impulse control, hyperactivity, and attention deficits.1,2 ADHD typically begins before the age of seven (according to the ICD-10) or twelve (according to DSM-5)3,4 and affects approximately 8%-12% of children worldwide, with about a 50% chance of persisting into adulthood.5–8 Recent research highlights that ADHD is associated with difficulties in various social domains, such as social skills, 9 interpreting social cues, maintaining eye contact, and developing peer relationships.10–15
Social cognition encompasses interconnected cognitive abilities required to process social information and navigate social interactions effectively. These abilities include affect recognition, pragmatic language, theory of mind (ToM), and empathy. 16 Marsh and Williams specifically note that facial emotion encoding is particularly impaired in adults with ADHD. 17 Dan further observes that individuals with ADHD require more time and make more errors than healthy controls when identifying facial emotions.18 Facial affect expressions should be encoded early in facial recognition to facilitate effective non-verbal communication, as they often convey biologically significant information. 19 For example, an angry facial expression can heighten sensitivity to potential environmental dangers.20,21 Despite extensive research on the neurobiological substrates of ADHD,22–26 the underlying mechanisms of its symptoms remain unclear, making it one of the most debated diagnoses,25,27 and research specifically focusing on social cognition deficits in ADHD is even more limited and controversial.16,28
Many studies have examined the neural basis of ADHD using electroencephalogram (EEG), structural and functional MRI techniques.29–35 Several studies suggest a link between emotion processing and social cognition impairments, frontostriatal deficits, and executive functions (EF) in ADHD.16,29,31,32,36,37 In a study by Ibanez et al on adult ADHD, the N170 Event-Related Potential (ERP) component was used as a neurophysiological marker for facial emotion processing. The researchers also assessed EF and ToM. They found reduced N170 amplitudes in the ADHD group were associated with lower performance on an emotional inference ToM task and poorer EF. 38
Several behavioral studies using standard Go/NoGo tasks have highlighted the involvement of neural structures in executive function (EF) control, revealing moderate-to-strong differences between ADHD subjects and healthy controls.39–44 ERP variants of the Go/NoGo paradigm are widely used to delineate the underlying neurophysiological processes.43,45,46 In the cueing variant of this paradigm, a Go-stimulus is preceded by a cue, allowing participants to prepare for a response. Occasionally, a NoGo-stimulus follows the cue, requiring them to withhold their response.42,46,47
Comparing the ERP responses to Go and NoGo stimuli reveals two dominant components: (a) the fronto-central N2d, associated with conflict monitoring, and (b) the fronto-central P3d, linked to action inhibition.48–51 Intracortical source modeling indicates that conflict detection is managed by the frontostriatal network, including the anterior cingulate and basal ganglia, while action inhibition involves the supplementary and pre-supplementary motor areas.52–54 As conflict detection and action inhibition are critical EFs affected in ADHD, ERP Go/NoGo tasks are frequently used to differentiate ADHD subjects from healthy controls. The N170 ERP component, generated from the fusiform gyrus and superior temporal sulcus, is a biomarker specifically linked to facial processing in many studies with normative populations and individuals with ADHD.55,56 This makes the N170 component an ideal marker for evaluating potential facial affect impairments in ADHD subjects.
This study re-assessed the prospect and extent of differences between ADHD subjects and healthy controls in standard behavioral and neurophysiological measures of EFs by two similar Go/NoGo tasks (visual continuous performance test [VCPT] and emotional continuous performance test [ECPT]). Based on existing literature,46,57–59 we hypothesized that ADHD subjects would demonstrate increased errors, reaction time (RT), ERP latencies, and attenuated ERP amplitudes. Several papers suggest a link between social cognition impairments and EFs in ADHD.16,29,31,32,36,37 Thus, we were particularly keen on the EF-related responses to the rarely used ECPT task,60,61 which, unlike VCPT, requires facial emotion recognition. We anticipated distinct performance in EF-associated measures of the ECPT task between the ADHD and control groups. Also, the study examined whether the face-sensitive N170 component differs between ADHD and control groups in the ECPT task, Go/NoGo conditions, and left/right hemispheres.
To our knowledge, no study has compared the neurophysiological and behavioral responses to these different Go/NoGo task variants in ADHD and healthy subjects.
Methods
Subjects
Data used in this research was obtained from a multicenter clinical and observational study by the Brain and Trauma Foundation Grisons, Switzerland. The data collection method has been extensively described in a study by Münger et al 62 The Brain and Trauma Foundation project, with more than 674 participants, was approved by the cantonal ethics committee of Zurich (LeitEKZH_2013-0327/EKNZ_2014_160). In this project, certified psychiatrists and clinical psychologists diagnosed ADHD subjects according to the DSM-5. Several psychometric tests were administered.
This paper assessed the psychometric intelligence test using standard pen-and-pencil tests in the German language (≤ 9 years, CFT 1-R; 9 to 16 years, CFT 20-R part I 63 ; > 16 years, WMT-2). 64 The exclusion criteria for the study were traumatic brain injury, loss of consciousness in the past, primary mental disorders other than ADHD, drug abuse, pregnancy, epilepsy, and an IQ score below 80. The participants needed to be able to follow the study instructions in German and provide informed consent. All subjects were medication-free on the assessment day. However, 139 ADHD subjects (31%) had been taking methylphenidate-comprising medication daily (eg, Ritalin, Concerta, Elvanse). One hundred ninety-two subjects (43%) had not used methylphenidate. Medication information was missing for 116 of the subjects (26%).
We selected subjects with complete demographic information from this large sample who completed both ECPT and VCPT tasks. A total of 300 subjects (183 controls, 117 ADHD), aged 6-60 (M = 26.14, SD = 17), were analyzed. The sample comprised 89 children (age range 6-12 years; ADHD n = 57, controls n = 32), 43 adolescents (age range 13-18 years; ADHD n = 18, controls n = 25), and 168 adults (age >18 years; ADHD n = 108, controls n = 60). All subjects were drawn from the same pool as the Münger et al study, which reported only VCPT task results. 62 This study compared the results obtained from both VCPT and ECPT tasks (Table 1).
Table 1.
Descriptive Information of the Participating Subjects (n = 300).
| Controls | ADHD | |
|---|---|---|
| N total | 183 | 117 |
| N (%), male | 72 (39%) | 74 (63%) |
| N (%), female | 111 (60%) | 43 (36%) |
| Age (mean ± SD) | 29.23 (20.19) | 24.22 (14.38) |
| IQ | 105 ± 14 | 99 ± 16 |
Shown are the number of male and female subjects. In addition, the mean age and IQ (and standard deviation) for both groups are shown.
EEG Recording and Processing
EEG data were recorded during the resting state (not reported here) and during the two Go/NoGo tasks controlled by a computer. EEG was recorded with a 23-channel digital EEG system (NeuroAmp® x23 amplifier) with direct current coupling and 24-bit resolution (BEE Medic GmbH, Switzerland). The input signals were bandpass filtered (0.5 and 50 Hz) with a sampling rate of 500 Hz decimated to 250 Hz. Before data processing, the montage was changed from linked earlobes to common-average reference. Based on the international 10-20 system, the electrodes were placed using a fitting electrode cap with 19 tin electrodes (Electro-cap International Inc., USA). The impedance for all electrodes was kept below five kΩ.
Raw EEGs recorded using ERPrec software (BEE Medic GmbH, Switzerland) were processed using MATLAB-based in-house software. Eye blinks and horizontal eye movements recorded on Fp1, Fp2, T3, and T4 were automatically detected using the independent component analysis (ICA) decomposition and removed from the EEGs by zeroing the activation of the respective components. To remove the remaining artifacts, the filtered EEG segments with extreme activity in the 0-3 and 20-50 Hz frequency bands and segments with amplitudes larger than100 µV (defined as a threshold of six standard deviations above the mean for each channel) were rejected. The artifact-free data were further analyzed with a custom-built EEGlab plug-in.
Go/NoGo Tasks
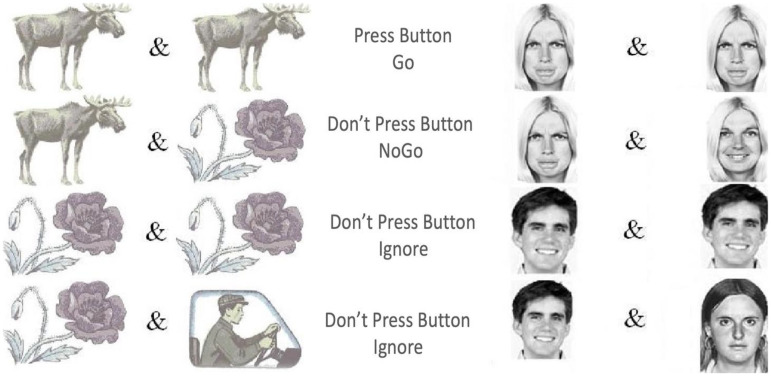
This study used two established Go/NoGo tasks: VCPT and ECPT.60,61,65 The VCPT task presents images of animals, plants, and humans as stimuli. A beep tone is presented simultaneously with the human images (Figure 1). In Go conditions, subjects were instructed to immediately click on the computer mouse when an image of an animal was followed by another image of an animal (animal-animal). In NoGo conditions, the subjects were instructed not to click on the mouse when an image of an animal was followed by an image of a plant (animal-plant). No action was required in the Ignore-conditions (images of plant-plant/human as stimuli). 66
Figure 1.
VCPT and ECPT conditions, including Go, NoGo, and distractor/ignore.
The ECPT task has a similar structure to the VCPT task, except for the content of the presented stimuli.60,61 In the ECPT task, the stimuli consist of images of female and male actors, 67 with angry, happy, and neutral facial expressions. In Go-conditions, subjects were instructed to immediately click on the computer mouse when an image of an angry face was followed by another angry face image (angry-angry). In NoGo conditions, the subjects were instructed not to respond when an image of an angry face was followed by an image of a happy face (angry-happy). The Ignore-conditions required no action (images of happy-happy/neutral faces with a beep tone). Each condition consisted of 100 trials for 100 ms in both tasks, with an inter-trial interval of 3000 ms. The first stimulus was presented at 300 ms and the second at 1400. Each task lasted about 22 min (Figure 1).
In the behavioral measures, RT variability was calculated for Go conditions, defined as the coefficient of variance for RT. Omission errors (response failure in Go conditions) and commission errors (response failure in NoGo conditions) obtained from both CPT tasks were computed separately for each subject.
In the neurophysiological measures, three ERP components were assessed: the N2d as a marker of conflict detection, the P3d as the action inhibition marker,49,68,69 and the N170 as the face processing marker.56,70–72 The N2d and P3d are ERPs obtained during the Go trials minus the ERP obtained during the NoGo trials. The ERP latencies and amplitudes obtained during both tasks were set according to the maximum-peak method, 73 using a MATLAB-based custom-built EEGLAB plug-in. After baseline correction using the 100 ms pre-stimulus period, the peak-detection method reliably detected the components of interest. The amplitudes were set at the highest peak on the ERP waveform in a time window whose size was adjusted to 80% of the time interval between our targeted component peak and the previous peak. For instance, if N2 is at 200 ms and P1 at 100 ms, the window for N2 was set from 160 to 240 ms. Self-modeling warping functions were applied to control the inter-individual temporal variability. 74 All ERP components were measured where the waves are known to be most prominent. The N2d and P3d components were measured at the frontocentral (Cz), and the N170 was assessed in parieto-occipital scalp areas (T5-T6).61,66,75–77
Statistical Analyses
For the behavioral measures, N2d and P3d ERP components, 2 × 2 ANCOVAs were applied with group (ADHD, controls) as grouping factor and task (VCPT, ECPT) as repeated measurements factor. For the N170 component, a 2 × 2 × 2 ANCOVA was performed on the ECPT task with “group” (ADHD, controls) as the grouping factor, “condition” (Go, NoGo), and “hemisphere” (left, right) as repeated measurement factors. Dependent variables were the four behavioral measures (omission errors, commission errors, RT, and RT variability) and the six ERP amplitude and latency measures (N2d, P3d, and N170). We used sex and age as covariates in the ANCOVA analysis to control for their potential confounding effects and enhance our findings’ accuracy. By adjusting for these variables, we aimed to isolate the effects of the primary independent variables. The analysis results of sex and age variables are included in the ANCOVA results tables (Tables 2, 3, 4).
Table 2.
Summary of the ANCOVA Results Separately for all Behavioral Variables Used as Dependent Variables. Age and Sex are Used as Covariates.
| Group | Task | Age | Sex | Group × Task | Age × Task | Sex × Task | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p | η2G | P | η2G | P | η2G | P | η2G | P | η2G | P | η2G | P | η2G | df | |
| VarRT | ≤ .001 | .15 | ≤ .001 | .02 | ≤ .001 | .03 | .06 | ≤ .001 | ≤ .001 | .001 | .64 | ≤ .001 | .21 | ≤ .001 | 294 |
| RT | ≤ .001 | .05 | ≤ .001 | .03 | .06 | ≤ .001 | .94 | ≤ .001 | ≤ .001 | ≤ .001 | .18 | ≤ .001 | ≤ .001 | ≤ .001 | 294 |
| Om | ≤ .001 | .11 | ≤ .001 | .07 | ≤ .05 | .01 | .13 | ≤ .001 | ≤ .001 | .02 | ≤ .02 | ≤ .001 | ≤ .001 | ≤ .001 | 294 |
| Com | ≤ .001 | .02 | ≤ .001 | .03 | ≤ .05 | .01 | .91 | ≤ .001 | ≤ .001 | .002 | .90 | ≤ .001 | ≤ .001 | ≤ .001 | 294 |
Shown are the ANCOVA results for the main effects group (ADHD and controls), task (VCPT and ECPT), and the interaction between both main effects (group × task). Depicted are the p-values, the effect sizes as generalized eta square values (η2G), and degrees of freedom (df).
VarRT: variability of reaction time; RT: reaction time; Om: Omission errors; Com: commission errors.
Table 3.
Summary of the ANCOVAs Separately for the N2d and P3d ERP Component Measures (Amplitudes And Latencies).
| N2d | P3d | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| μV | ms | μV | ms | df | |||||
| P | η2G | P | η2G | P | η2G | P | η2G | 294 | |
| Group | . 45 | ≤ .001 | ≤ .001 | .02 | ≤ .001 | .03 | ≤ .001 | .02 | 294 |
| Task | ≤ .001 | .06 | ≤ .001 | .06 | ≤ .05 | ≤ .01 | ≤ .05 | ≤ .01 | 294 |
| Age | ≤ .001 | .03 | .06 | .006 | ≤ .04 | .01 | ≤ .03 | .01 | 294 |
| Sex | .06 | .001 | .94 | ≤ .001 | .13 | .006 | .91 | ≤ .001 | 294 |
| Group × Task | .61 | ≤ .001 | ≤ .05 | ≤ .001 | .07 | ≤ .01 | .14 | ≤ .001 | 294 |
| Age × Task | ≤ .001 | .01 | ≤ .04 | .005 | .80 | ≤ .001 | .76 | ≤ .001 | 294 |
| Sex × Task | .94 | ≤ .001 | .40 | ≤ .001 | .29 | ≤ .001 | ≤ .05 | ≤ .003 | 294 |
Shown are the ANCOVA results for the main effects group (ADHD and control) and task (VCPT and ECPT) and the interaction between both main effects (group × task). The Age and Sex effects are also presented. Depicted are the P-values, the effect sizes as generalized eta square values (η2G) and the degrees of freedom (df).
Table 4.
Summary of the ANCOVAs Separately for N170 ERP Amplitudes (in μV) and Latencies (in ms).
| N170 μV | N170 ms | df | |||
|---|---|---|---|---|---|
| P | (η2G) | P | (η2G) | ||
| Group | ≤ .001 | .03 | ≤ .001 | .01 | 294 |
| Task | ≤ .001 | .05 | ≤ .001 | .01 | 294 |
| Age | ≤ .001 | .05 | ≤ .001 | .03 | 294 |
| Sex | ≤ .05 | ≤ .001 | ≤ .001 | ≤ .001 | 294 |
| Group × Task | ≤ .001 | ≤ .001 | ≤ .001 | .01 | 294 |
| Age × Task | ≤ .001 | .01 | ≤ .001 | .01 | 294 |
| Sex × Task | .76 | ≤ .001 | .24 | ≤ .001 | 294 |
| Condition | ≤ .003 | .001 | .07 | ≤ .001 | 294 |
| Group × Condition | .13 | ≤ .001 | .18 | ≤ .001 | 294 |
| Group × Condition × Task | .35 | ≤ .001 | .07 | ≤ .001 | 294 |
| Age × Condition | .22 | ≤ .001 | ≤ .001 | ≤ .001 | 294 |
| Sex × Condition | .12 | ≤ .001 | .12 | ≤ .001 | 294 |
| Hemisphere | .38 | ≤ .001 | .34 | ≤ .001 | 294 |
| Group × Hemisphere | ≤ .05 | ≤ .001 | .93 | ≤ .001 | 294 |
| Group × Task × Hemisphere | .91 | ≤ .001 | .34 | ≤ .001 | 294 |
| Group × Condition × Hemisphere | .09 | ≤ .001 | .93 | ≤ .001 | 294 |
| Age × Hemisphere | ≤ .01 | ≤ .002 | ≤ .01 | ≤ .001 | 294 |
| Sex × Hemisphere | .30 | ≤ .001 | .34 | ≤ .001 | 294 |
Shown are the ANCOVA results (corrected for age and sex) for the main effects. Group (ADHD and control), task (VCPT and ECPT), condition (Go and NoGo), hemisphere (left and right), and the interactions between the main effects. Sex and Age effects are also represented. Depicted are the P-values and the effect sizes as generalized eta square values (η2G) and the degrees of freedom (df).
All statistical analyses were conducted in R (version 3.3.2, http://www.R-project.org). The “afex-package” in R was used to calculate the ANCOVAs. 78 Since ten ANCOVAs were conducted, multiple testing was controlled using the Bonferroni-Holm procedure. (starting with .05/10 = .005). 79 Moreover, since P-values depend on sample size, effect sizes were calculated for more accuracy. 80 Effect sizes in the context of the ANCOVAs were given using the generalized η2 recommended for a repeated-measures design. 80 A generalized η2 of more than .02 is considered a “small effect,” more than .06 a “moderate effect,” and more than .14 is considered a “large effect size.”80,81 Cohen's effect size was calculated for subsequent post-hoc tests. A d = .2 is considered a small effect, a d = .5 medium, and d = .8 represents a large effect size. 82
Results
Behavioral Measures
The results of the ANCOVAs for the behavioral measures are shown in Table 2. We found significant group × task interactions for RT (η2g = .001) and omission errors (η2g = .02) with a small effect size. We conducted subsequent post-hoc tests using the Bonferroni-Holm correction to control for multiple comparisons. We confirmed that the ADHD group exhibited longer RTs and more omission errors than healthy subjects in the ECPT task, relative to the VCPT, with medium-to-large effect sizes as indicated by Cohen's d. (RT: t (294), P ≤ .001, d = .5; omission error: t (294), P ≤ .001, d = .8).
Since no group × task interaction effects were found for RT variability and commission errors, we examined the main group and task effects separately. There were significant group effects for both variables with strong effect sizes for RT variability (η2g = .14) and small effects for commission errors (η2g = .02). The obtained interactions were further tested for multiple comparison corrections. The results confirmed higher RT variability and commission errors for ADHD subjects. The adjusted P-values were very small, and effect sizes ranged from small to large (RT variability t (294), P ≤ .001, d = 1.01; commission errors t (294), P ≤ .001, d = .4). Task interactions had significant RT variability and commission errors effects (P ≤ .001), with effect sizes ranging from small to medium. (RT: η2g = .03, commission error: η2g = .03). The post-hoc tests characterized these effects by increased RT variability and commission errors in the ECPT task compared to the VCPT task across all subjects, with large effect sizes (RT variability t (294), P ≤ .001, d = 1.5; commission errors t (294), P ≤ .001, d = 1.2).
ERP Measures
The results for the ANCOVAs for the N2d and P3d components are shown in Table 3. In the group × task interaction, we discovered a significant difference for N2d latencies with negligible power of effects (η2g = ≤ .001). The subsequent post-hoc tests confirmed longer N2d-latencies in the ADHD group during the ECPT task, in comparison to controls and to ADHD during the VCPT task with small-to-medium effect size (t (294) = 3.59, P ≤ .001, d = .4).
For the N2d-P3d amplitudes and P3d latencies variables with no significant group × task interactions, we checked for the group and task interactions separately. In group effects, we found significant differences in P3d amplitudes and latencies with a small power of effect (Table 3). In the subsequent post-hoc tests in P3d amplitudes, ADHD was confirmed to demonstrate lower amplitudes (t (294), P ≤ .001, d = .4) and longer latencies than healthy controls with small-to-medium effect sizes (t (294), P ≤ .001, d = .3). Figures 2 and 3 present the N2d and P3d ERP waveforms and topography plots of both groups in both tasks. In task interactions, we found significant effects in all N2d-P3d variables with small-to-medium effect sizes (Table 3), confirmed after multiple comparison corrections. In both N2d and P3d, all subjects demonstrated lower amplitudes in the ECPT task compared to the VCPT task with large and medium effect sizes (N2d t (294), P ≤ .001, d = 1.2; P3d t (294), P ≤ .001, d = .5). In P3d latencies all subjects presented longer latencies in the ECPT task than the VCPT with large magnitude of effects (t (294), P ≤ .001, d = .8).
Figure 2.
The ERP difference curves (N2d, P3d) for the ADHD and control groups. The ERPs after the second stimulus (at 1400 ms) in ECPT and VCPT are displayed.
Figure 3.
The N2d and P3d ERP topography plots for ADHD and control groups in ECPT and VCPT tasks are presented.
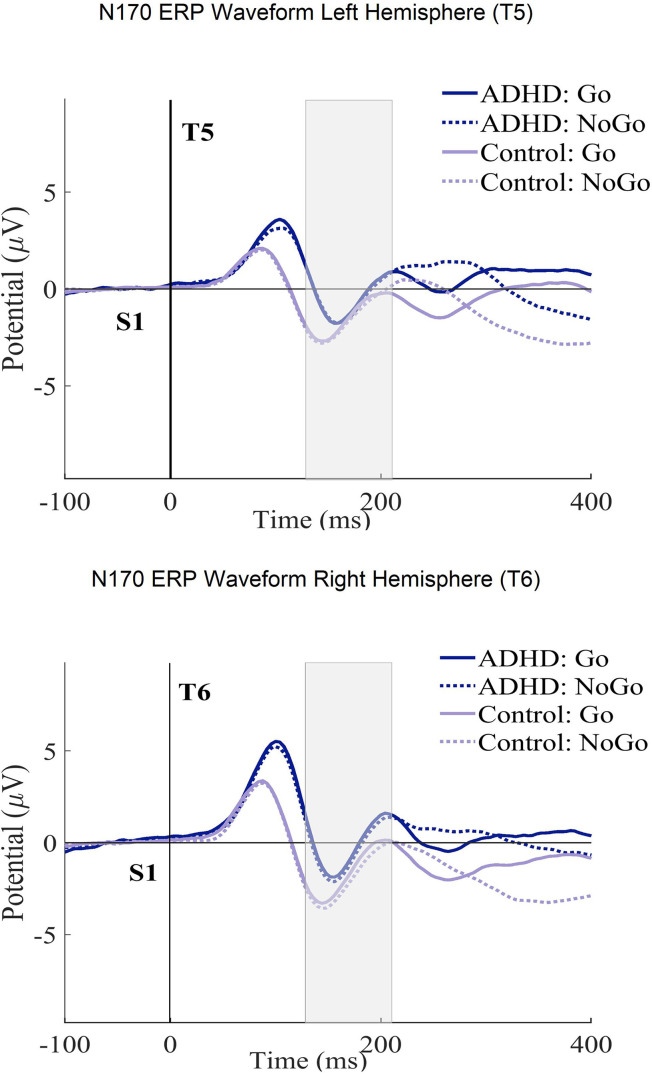
Table 4 shows the ANCOVA results with the N170 amplitudes and latencies in the ECPT task. For the group interactions, only the N170 amplitudes met the significance threshold (P ≤ .04) with a small effect size (η2g ≤ .01). The consecutive post-hoc tests could successfully confirm these results qualified by the ADHD presenting significantly lower amplitudes than healthy controls with a small Cohen's magnitude of effects (t (294), P ≤ .01, d = .02). No significant differences were found between subjects for the condition and hemisphere interactions. For a better overview, the N170 ERP waveforms and topography plots are presented in both groups for each condition and hemisphere in Figures 4 and 5.
Figure 4.
The N170 ERP curves in ECPT and VCPT after the second stimulus (at 1400 ms) for both T5-T6 (left-right hemispheres) electrode sites in groups (ADHD and control) and conditions (Go and NoGo) are presented.
Figure 5.
N170 ERP topography plot in the ECPT and VCPT tasks between groups (ADHD and control) and conditions (Go and NoGo).
Discussion
The present study combined behavioral and brain function measures to re-assess behavioral and neurophysiological EF parameter differences between ADHD patients and healthy controls. It also compared the findings of facial emotion processing in ADHD and healthy controls.
For behavioral measures, group × task interactions, which reflect the group differences across tasks, confirmed that ADHD subjects demonstrate significant deficiencies in RT and omission errors in the ECPT task (Table 2). Our findings were in line with several ADHD studies, indicating that ADHD individuals have impaired basic cognitive processing, as seen by their higher level of distractibility and inefficient processing speed.83–89 The RT is linked with the decision-making speed and reflects the interval between the stimulus presentation and the individual's response. 90 Omission error is linked with lapses of attention and distractibility.43,91,92 RT and performance accuracy combined are suggested to represent poor basic information processing rather than separate impacts of executive dysfunctions in children with ADHD, as demonstrated by Metin et al 93 and Salum et al 94 The slow rate of information accumulation suggests that distinguishing signals from noise may be challenging, which could account for the delayed RTs and higher frequency of errors in ADHD performance. Ultimately, the RT lag and higher omission errors in ADHD compared to controls during the ECPT task may suggest that these processes are more impaired during the higher cognitive demand of face processing combined with EF and could contribute to a more in-depth understanding of the facial emotion processing impairments in ADHD. Additionally, in the group effects, ADHD showed higher reaction-time variability with a large effect size (η2g = .14) and higher commission errors with a small magnitude of effects (η2g = .2). Our findings align with the literature suggesting RT variability as one of the most reliable markers in ADHD diagnosis, not only distinguishing ADHD from healthy individuals but also from other psychiatric disorders.42,95–98 Commission error is associated with one of the core ADHD symptoms: response control and inhibition deficit.47,85,89,92,99–104 Identifying significant group interactions in RT variability and commission errors, as opposed to group × task interactions, suggests that these impairments are independent of face processing in individuals with ADHD.
In the ANCOVA analysis for the neurophysiological metrics of EF (N2d-P3d), only the N2d latencies were proven to be significantly different in the group × task interactions (Table 3). These differences were qualified by the ADHD showing longer latencies than controls in the ECPT task compared to VCPT. These results indicate that facial emotion processing is slower in ADHD. However, the small magnitude of effects suggests these impairments to be mild (η2g ≤ .001). The N2d reflects conflict detection and monitoring23,105 and is considered a neurophysiological marker of EF.106–108 EFs are essential in the higher-order mental processes that monitor human thoughts, emotions, and behavior. 109 It is also suggested that the development of EF is closely linked to ToM development.110–112 These psychological functions share some common neural underpinnings, specifically involving prefrontal cortical regions. 113 ToM is the ability to understand the beliefs, intentions, and emotions of others.114–116 The link between the EF and ToM might explain why ADHD subjects demonstrate more difficulties with tasks that require emotion encoding. Another explanation might be that the more diverse images presented as stimuli in the VCPT task (animals, humans, plants) compared to the ECPT task (facial expressions) can potentially cause more conflict monitoring in the brain, which nonetheless is an indicator of executive dysfunction in ADHD. In group effects, we found significantly lower amplitudes and longer latencies in P3d as a neurophysiological marker of response inhibition.59,66,75,76,106,117–119 The attenuated P3d amplitudes and longer latencies observed in individuals with ADHD across both tasks confirm previous findings on ADHD and executive dysfunction, suggesting that these impairments are independent of deficits in emotion recognition.59,66,75,76,106,117–119
In the facial-encoding-sensitive N170 component, the ADHD subjects demonstrated significantly lower amplitudes than healthy subjects (Table 4). This finding points to a particular deficit in facial affect processing and confirms the previous findings.70–72,120–122 The fusiform gyrus is considered one of the main origins of N170. 55 Several studies assume that the fusiform gyrus is connected to the amygdala, a fundamental neural structure for processing negative emotions and social cognition.123–125 These two structures contribute to an emotion-processing network.126–128 Vuilleumier et al129,130 have demonstrated increased fusiform gyrus activation for emotional faces stemming from direct amygdala inputs. The amygdala of individuals with ADHD has appeared to have abnormalities.22,123,127,128,131,132 N170 does not directly measure amygdala activity. However, the reduced amplitudes in ADHD subjects during the ECPT task could be linked to amygdala–fusiform connection impairments, leading to social problems in ADHD. The small effect size suggests the mentioned impairments are mild. We found no significant condition and hemisphere interactions. Our findings differed from some studies on facial processing using the ECPT task.15,72,133 However, they were consistent with another similar study with a smaller sample size. 61 These inconsistencies can be explained by differences in the task designs and electrode placements used to examine facial affect processing. Since the N170 is associated with sensitivity to the long-term familiarity of faces,115–119 we controlled for this effect by comparing the first and last 50 trials for each condition but found no significant effects (μV (P = .31), ms (P = .16)).
Our findings confirm the differences between ADHD subjects and healthy controls in behavioral markers of EF. This study found that individuals with ADHD show deficiencies in facial affect recognition. However, the differences obtained from the neuropsychological markers have small-to-moderate effect sizes, suggesting the processes behind these markers are mildly impaired in ADHD.
Conclusion and Limitations
The data for this project were obtained in a clinical setting. We did our best to control the data acquisition as precisely as possible. However, clinical settings are often associated with greater variance during data acquisition than strictly controlled laboratory experiments. Another identified issue, also present in other studies, was that the diagnosis of ADHD is entirely based on subjective criteria with low reliability and validity. 134 A misdiagnosis of nonverbal learning disorder and ADHD is sometimes made on this basis.135–137 Comparing ADHD and control subjects based on ERP components strongly suffers from this issue.
In addition, our clinical and control samples were heterogeneous regarding age and gender.
This study analyzed a relatively large clinical dataset with a wide age range. The adjusted group differences were assessed using two CPT tasks involving different neurophysiological processes. In behavioral metrics of EF, significant group differences were found with a moderate-to-large magnitude of effects (Table 2) and small-to-moderate effects in the N2d-latency component (Table 3). Our findings on the N170 component imply that ADHD subjects have deficiencies in facial emotion recognition (Table 4). Overall, the lower values demonstrated by the ADHD group in the N170 component and in measures associated with EF while processing facial emotions add evidence to the premise of a link between emotion processing and EF impairments in ADHD.16,29,31,32,36,37 However, the small-to-moderate effect sizes in neuropsychological measures suggest that ERPs are insufficient as exclusive markers for effective screening of emotion processing and EF deficits in ADHD without comprehensive data, such as behavioral measurements. This is especially true for clinical studies such as ours. In tightly controlled experimental settings, the findings may be different.
While our study did not differentiate the ADHD subtypes, some studies suggest that different subtypes (inattentive, hyperactive, and combined) 3 have different EEG profiles.132,133 One major limitation is the wide age range (6-60 years) used to calculate the grand ERP averages in Figures 2 to 5. ERPs and their components are known to undergo substantial changes across this age span, especially between children and adults.138–140 These developmental changes in brain function can potentially introduce variability that may obscure or confound the effects observed in our study.138,140,141 Thus, the ERP figures should be considered carefully. Although analyzing a wide age range offers benefits, such as a broad overview of ERP patterns and identifying age-independent interactions, future research could focus on age-specific analyses or subdivide the age range. This would allow for a clearer understanding of ERP development in ADHD and healthy controls during face processing, potentially revealing age-specific patterns missed in the current analysis
Ultimately, previous attempts at finding neurophysiological markers of ADHD suggest a cautious approach to their application.125–133 However, this does not mean the search for easily measurable biomarkers is obsolete. Simple neurophysiological characteristics may need to be evaluated by new mathematical methods to detect even minor neurophysiological abnormalities. Initial approaches to detecting neurophysiological problems that may suggest the basis of psychological problems have already been presented.142–144 The application of these similar findings in ADHD research remains to be explored.
Acknowledgments
We would like to thank Maryam Rostami for her invaluable input in this project.
Additionally, we would like to extend our sincere thanks to the participants in this study and the clinical staff involved in the data collection.
We acknowledge the use of OpenAI for assistance with language refinement, including rephrasing and synonym suggestions, in the preparation of this manuscript.
Footnotes
Author Contributions: SV processed the experimental data, performed the analysis, and drafted the manuscript. GC collected data, processed the experimental data, and reviewed the manuscript. JK and HAR collected data. DE and AM designed the project. LJ supervised the project and contributed to writing the manuscript and interpreting the results. All authors revised the manuscript.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Five private clinics from different parts of Switzerland participated in data collection. The study was approved by the cantonal ethics committee of Zurich (LeitEKZH_2013-0327/EKNZ_2014_160).
Funding: The authors disclose the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Uniscientia Foundation and the Brain and Trauma Foundation Grison Switzerland.
ORCID iD: Saghar Vosough https://orcid.org/0000-0003-1917-0352
References
- 1.Murphy K. Psychosocial treatments for ADHD in teens and adults: A practice-friendly review. J Clin Psychol. 2005;61(5):607–619. doi: 10.1002/JCLP.20123 [DOI] [PubMed] [Google Scholar]
- 2.Swanson J, Deutsch C, Cantwell D, et al. Genes and attention-deficit hyperactivity disorder. Clin Neurosci Res. 2001;1(3):207–216. doi: 10.1016/S1566-2772(01)00007-X [DOI] [Google Scholar]
- 3.Association DAP. Diagnostic and statistical manual of mental disorders: DSM-5. 2013. Accessed May 21, 2022. https://www.amberton.edu/media/Syllabi/Spring%202022/Graduate/CSL6798_E1.pdf
- 4.Organization WH. International statistical classification of diseases and related health problems: Alphabetical index. 2004. Accessed May 21, 2022. https://books.google.com/books?hl=en&lr=&id=Tw5eAtsatiUC&oi=fnd&pg=PA1&dq=World+Health+Organization.+(2004).+International+Statistical+Classification+of+Diseases+and+Related+Health+Problems:+Alphabetical+index.+World+Health+Organization.+https://books.google.com/books/about/International_Statistical_Classification.html%3Fhl%3D%26id%3DHD&ots=o4c_gYuIqM&sig=Yjru9sqpvcei6WHO9iHYp1PMkLU
- 5.Barkley RA, Fischer M, Smallish L, Fletcher K. The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. J Abnorm Psychol. 2002;111(2):279–289. doi: 10.1037/0021-843X.111.2.279 [DOI] [PubMed] [Google Scholar]
- 6.Faraone SV, Sergeant J, Gillberg C, Biederman J. The worldwide prevalence of ADHD: Is it an American condition? World Psychiatry. 2003;2(2):104. Accessed May 21, 2022. /pmc/articles/PMC1525089/. [PMC free article] [PubMed] [Google Scholar]
- 7.Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: A systematic review of longitudinal studies. Lancet Psychiatry. 2016;3(12):1157–1165. doi: 10.1016/S2215-0366(16)30190-0 [DOI] [PubMed] [Google Scholar]
- 8.Sibley MH, Swanson JM, Arnold LE, et al. Defining ADHD symptom persistence in adulthood: Optimizing sensitivity and specificity. J Child Psychol Psychiatry. 2017;58(6):655–662. doi: 10.1111/JCPP.12620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfiffner LJ, McBurnett K. Social skills training with parent generalization: Treatment effects for children with attention deficit disorder. J Consult Clin Psychol. 1997;65(5):749–757. doi: 10.1037/0022-006X.65.5.749 [DOI] [PubMed] [Google Scholar]
- 10.Hoza B. Peer functioning in children with ADHD. J Pediatr Psychol. 2007;32(6):655–663. doi: 10.1093/JPEPSY/JSM024 [DOI] [PubMed] [Google Scholar]
- 11.Schindler S, Bublatzky F. Attention and emotion: An integrative review of emotional face processing as a function of attention. Cortex. 2020;130:362–386. doi: 10.1016/J.CORTEX.2020.06.010 [DOI] [PubMed] [Google Scholar]
- 12.Staff AI, Luman M, van der Oord S, Bergwerff CE, van den Hoofdakker BJ, Oosterlaan J. Facial emotion recognition impairment predicts social and emotional problems in children with (subthreshold) ADHD. Eur Child Adolesc Psychiatry. 2022;31(5):715–727. doi: 10.1007/S00787-020-01709-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Da Fonseca D, Seguier V, Santos A, Poinso F, Deruelle C. Emotion understanding in children with ADHD. Child Psychiatry Hum Dev. 2008;40(1):111–121. doi: 10.1007/S10578-008-0114-9 [DOI] [PubMed] [Google Scholar]
- 14.Psychology PLJ of S and E. Emotion and motivation: Attention, perception, and action. journals.humankinetics.comPJ LangJournal of Sport and Exercise Psychology, 2000•journals.humankinetics.com. 2000. Accessed May 11, 2024. https://journals.humankinetics.com/view/journals/jsep/22/s1/article-pS122.xml
- 15.Demirci E, Erdogan A. Correction to: Is emotion recognition the only problem in ADHD? Effects of pharmacotherapy on face and emotion recognition in children with ADHD. ADHD Attention Deficit Hyperactivity Disord. 2017;10(2):161–161. doi: 10.1007/S12402-017-0247-4 [DOI] [PubMed] [Google Scholar]
- 16.Uekermann J, Kraemer M, Abdel-Hamid M, et al. Social cognition in attention-deficit hyperactivity disorder (ADHD). Neurosci Biobehav Rev. 2010;34(5):734–743. doi: 10.1016/J.NEUBIOREV.2009.10.009 [DOI] [PubMed] [Google Scholar]
- 17.Marsh PJ, Williams LM. ADHD And schizophrenia phenomenology: Visual scanpaths to emotional faces as a potential psychophysiological marker? Neurosci Biobehav Rev. 2006;30(5):651–665. doi: 10.1016/j.neubiorev.2005.11.004 [DOI] [PubMed] [Google Scholar]
- 18.Dan O. Recognition of emotional facial expressions in adolescents with attention deficit/hyperactivity disorder. J Adolesc. 2020;82(1):1–10. doi: 10.1016/J.ADOLESCENCE.2020.04.010 [DOI] [PubMed] [Google Scholar]
- 19.Batty M, Taylor MJ. Early processing of the six basic facial emotional expressions. Cognit Brain Res. 2003;17(3):613–620. doi: 10.1016/S0926-6410(03)00174-5 [DOI] [PubMed] [Google Scholar]
- 20.Izard C. Facial expression, emotion, and motivation. 1979. Accessed May 21, 2022. https://books.google.com/books?hl=en&lr=&id=S-xFBQAAQBAJ&oi=fnd&pg=PA31&dq=FACIAL+EXPRESSION,+EMOTION,+AND+MOTIVATION.+&ots=0FTvLCXtsR&sig=pup3Tk7WDlgfRSA_C3mxjbOXBBk
- 21.Kolassa IT, Miltner WHR. Psychophysiological correlates of face processing in social phobia. Brain Res. 2006;1118(1):130–141. doi: 10.1016/J.BRAINRES.2006.08.019 [DOI] [PubMed] [Google Scholar]
- 22.Frodl T, Stauber J, Schaaff N, et al. Amygdala reduction in patients with ADHD compared with major depression and healthy volunteers. Acta Psychiatr Scand. 2010;121(2):111–118. doi: 10.1111/J.1600-0447.2009.01489.X [DOI] [PubMed] [Google Scholar]
- 23.Randall WM, Smith JL. Conflict and inhibition in the cued-Go/NoGo task. Clin Neurophysiol. 2011;122(12):2400–2407. doi: 10.1016/J.CLINPH.2011.05.012 [DOI] [PubMed] [Google Scholar]
- 24.Cadesky EB, Mota VL, Schachar RJ. Beyond words: How do children with ADHD and/or conduct problems process nonverbal information about affect? J Am Acad Child Adolesc Psychiatry. 2000;39(9):1160–1167. doi: 10.1097/00004583-200009000-00016 [DOI] [PubMed] [Google Scholar]
- 25.Da Fonseca D, Valérie AE, Ae S, et al. Emotion understanding in children with ADHD. SpringerPaperpile. 2009;40(1):111–121. doi: 10.1007/s10578-008-0114-9 [DOI] [PubMed] [Google Scholar]
- 26.Yuill N, Lyon J. Selective difficulty in recognising facial expressions of emotion in boys with ADHD: General performance impairments or specific problems in social cognition? Eur Child Adolesc Psychiatry. 2007;16(6):398–404. doi: 10.1007/S00787-007-0612-5 [DOI] [PubMed] [Google Scholar]
- 27.Luo Y, Weibman D, Halperin JM, Li X. A review of heterogeneity in attention deficit/hyperactivity disorder (ADHD). Front Hum Neurosci. 2019;13. doi: 10.3389/FNHUM.2019.00042/FULL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin I, McDonald S. Weak coherence, no theory of mind, or executive dysfunction? Solving the puzzle of pragmatic language disorders. Brain Lang. 2003;85(3):451–466. doi: 10.1016/S0093-934X(03)00070-1 [DOI] [PubMed] [Google Scholar]
- 29.Arns M, Conners CK, Kraemer HC. A decade of EEG theta/Beta ratio research in ADHD: A meta-analysis. J Atten Disord. 2013;17(5):374–383. doi: 10.1177/1087054712460087 [DOI] [PubMed] [Google Scholar]
- 30.Arns M, Vollebregt MA, Palmer D, et al. Electroencephalographic biomarkers as predictors of methylphenidate response in attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol. 2018;28(8):881–891. doi: 10.1016/J.EURONEURO.2018.06.002 [DOI] [PubMed] [Google Scholar]
- 31.De La Fuente A, Xia S, Branch C, Li X. A review of attention-deficit/hyperactivity disorder from the perspective of brain networks. Front Hum Neurosci. 2013;7(MAY):192. doi: 10.3389/FNHUM.2013.00192/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoogman M, Muetzel R, Guimaraes JP, et al. Brain imaging of the cortex in ADHD: A coordinated analysis of large-scale clinical and population-based samples. Am J Psychiatry. 2019;176(7):531–542. doi: 10.1176/APPI.AJP.2019.18091033/ASSET/IMAGES/LARGE/APPI.AJP.2019.18091033F2.JPEG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng X, Lin P, Zhang T, Wang J. Extreme learning machine-based classification of ADHD using brain structural MRI data. PLoS One. 2013;8(11). doi: 10.1371/JOURNAL.PONE.0079476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Doren J, Arns M, Heinrich H, Vollebregt MA, Strehl U, Loo S K. Sustained effects of neurofeedback in ADHD: A systematic review and meta-analysis. Eur Child Adolesc Psychiatry. 2019;28(3):293–305. doi: 10.1007/S00787-018-1121-4/FIGURES/3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wen D, Wei Z, Zhou Y, Li G, Zhang X, Han W. Deep learning methods to process fmri data and their application in the diagnosis of cognitive impairment: A brief overview and our opinion. Front Neuroinform. 2018;12. doi: 10.3389/FNINF.2018.00023/FULL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Owen AM. Cognitive planning in humans: Neuropsychological, neuroanatomical and neuropharmacological perspectives. Prog Neurobiol. 1997;53(4):431–450. doi: 10.1016/S0301-0082(97)00042-7 [DOI] [PubMed] [Google Scholar]
- 37.Sonuga-Barke EJS. The dual pathway model of AD/HD: An elaboration of neuro-developmental characteristics. Neurosci Biobehav Rev. 2003;27(7):593–604. doi: 10.1016/J.NEUBIOREV.2003.08.005 [DOI] [PubMed] [Google Scholar]
- 38.Ibáñez A, Petroni A, Urquina H, et al. Cortical deficits of emotional face processing in adults with ADHD: Its relation to social cognition and executive function. Soc Neurosci. 2011;6(5-6):464–481. doi: 10.1080/17470919.2011.620769 [DOI] [PubMed] [Google Scholar]
- 39.Barkley RA. Attention-Deficit/Hyperactivity disorder, self-regulation, and time. J Dev Behav Pediatr. 1997;18(4):271–279. doi: 10.1097/00004703-199708000-00009 [DOI] [PubMed] [Google Scholar]
- 40.Corbett BA, Constantine LJ. Autism and attention deficit hyperactivity disorder: Assessing attention and response control with the integrated visual and auditory continuous performance test. Child Neuropsychol. 2007;12(4-5):335–348. doi: 10.1080/09297040500350938 [DOI] [PubMed] [Google Scholar]
- 41.Fasmer OB, Mjeldheim K, Førland W, et al. Linear and non-linear analyses of Conner’s Continuous Performance Test-II discriminate adult patients with attention deficit hyperactivity disorder from patients with mood and anxiety disorders. BMC Psychiatry. 2016;16(1). doi: 10.1186/S12888-016-0993-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karalunas SL, Geurts HM, Konrad K, Bender S, Nigg JT. Annual research review: Reaction time variability in ADHD and autism spectrum disorders: Measurement and mechanisms of a proposed trans-diagnostic phenotype. Wiley Online LibraryPaperpile. 2014;55(6):685–710. doi: 10.1111/jcpp.12217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Metin B, Roeyers H, Wiersema JR, Van Der Meere J, Sonuga-Barke E. A meta-analytic study of event rate effects on go/No-go performance in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;72(12):990–996. doi: 10.1016/J.BIOPSYCH.2012.08.023 [DOI] [PubMed] [Google Scholar]
- 44.Willcutt EG, Doyle AE, Nigg JT, Faraone S V, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biol Psychiatry. 2005;57(11):1336–1346. doi: 10.1016/J.BIOPSYCH.2005.02.006 [DOI] [PubMed] [Google Scholar]
- 45.Barry RJ, Clarke AR, Johnstone SJ. A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clin Neurophysiol. 2003;114(2):171–183. doi: 10.1016/S1388-2457(02)00362-0 [DOI] [PubMed] [Google Scholar]
- 46.Johnstone SJ, Galletta D. Event-rate effects in the flanker task: ERPs and task performance in children with and without AD/HD. Int J Psychophysiol. 2013;87(3):340–348. doi: 10.1016/J.IJPSYCHO.2012.07.170 [DOI] [PubMed] [Google Scholar]
- 47.Losier BJ, McGrath PJ, Klein RM. Error patterns on the continuous performance test in non-medicated and medicated samples of children with and without ADHD: A meta-analytic review. J Child Psychol Psychiatry. 1996;37(8):971–987. doi: 10.1111/J.1469-7610.1996.TB01494.X [DOI] [PubMed] [Google Scholar]
- 48.Alahmadi NA. Cognitive control in children with learning disabilities: Neuromarker for deficient executive functions. Neuroreport. 2017;28(11):638–644. doi: 10.1097/WNR.0000000000000805 [DOI] [PubMed] [Google Scholar]
- 49.Burkhard A, Elmer S, Kara D, Brauchli C, Jäncke L. The effect of background music on inhibitory functions: An ERP study. Front Hum Neurosci. 2018;12:293. doi: 10.3389/FNHUM.2018.00293/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Falkenstein M, Hoormann J, Hohnsbein J. ERP Components in Go/Nogo tasks and their relation to inhibition. Acta Psychol (Amst. 1999;101(2-3):267–291. doi: 10.1016/S0001-6918(99)00008-6 [DOI] [PubMed] [Google Scholar]
- 51.Falkenstein M, Hoormann J, Hohnsbein J. Inhibition-related ERP components: Variation with modality, age, and time-on-task. J Psychophysiol. . 2002;16(3):167–175. 10.1027/0269-8803.16.3.167 [DOI] [Google Scholar]
- 52.Diesburg D, Tatz JR. Unexpected events activate a frontal-basal-ganglia inhibitory network: What is the role of the pre-supplementary motor area? J Neurosci. 2021;41(24). doi: 10.1523/JNEUROSCI.0565-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dutra IC, Waller DA, Wessel JR. Perceptual surprise improves action stopping by nonselectively suppressing motor activity via a neural mechanism for motor inhibition. J Neurosci. 2018;38(6):1482–1492. doi: 10.1523/JNEUROSCI.3091-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elchlepp H, Lavric A, Chambers CD, Verbruggen F. Proactive inhibitory control: A general biasing account. Cogn Psychol. 2016;86:27–61. doi: 10.1016/J.COGPSYCH.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deffke I, Sander T, Heidenreich J, et al. MEG/EEG sources of the 170-ms response to faces are co-localized in the fusiform gyrus. Neuroimage. 2007;35(4):1495–1501. doi: 10.1016/J.NEUROIMAGE.2007.01.034 [DOI] [PubMed] [Google Scholar]
- 56.Sadeh B, Zhdanov A, Podlipsky I, Hendler T, Yovel G. The validity of the face-selective ERP N170 component during simultaneous recording with functional MRI. Neuroimage. 2008;42(2):778–786. doi: 10.1016/J.NEUROIMAGE.2008.04.168 [DOI] [PubMed] [Google Scholar]
- 57.Gamma A, Kara O. Event-Related potentials for diagnosing children and adults with ADHD. J Atten Disord. 2020;24(11):1581–1587. doi: 10.1177/1087054716631821 [DOI] [PubMed] [Google Scholar]
- 58.Kaiser A, Aggensteiner PM, Baumeister S, Holz NE, Banaschewski T, Brandeis D. Earlier versus later cognitive event-related potentials (ERPs) in attention-deficit/hyperactivity disorder (ADHD): A meta-analysis. Neurosci Biobehav Rev. 2020;112:117–134. doi: 10.1016/J.NEUBIOREV.2020.01.019 [DOI] [PubMed] [Google Scholar]
- 59.Kropotov JD. Functional neuromarkers for neuropsychology. Acta Neuropsychol. 2018;16(1):1–7. doi: 10.5604/01.3001.0011.6504 [DOI] [Google Scholar]
- 60.Meier NM, Perrig W, Koenig T. Neurophysiological correlates of delinquent behaviour in adult subjects with ADHD. Int J Psychophysiol. 2012;84(1):1–16. doi: 10.1016/J.IJPSYCHO.2011.12.011 [DOI] [PubMed] [Google Scholar]
- 61.Rinke L, Candrian G, Loher S, Blunck A, Mueller A, Jäncke L. Facial emotion recognition deficits in children with and without attention deficit hyperactivity disorder: A behavioral and neurophysiological approach. Neuroreport. 2017;28(14):917–921. doi: 10.1097/WNR.0000000000000858 [DOI] [PubMed] [Google Scholar]
- 62.Münger M, Candrian G, Kasper J, et al. Behavioral and neurophysiological markers of ADHD in children, adolescents, and adults: A large-scale clinical study. Clin EEG Neurosci. 2021;52(5):311–320. doi: 10.1177/1550059421993340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wei RH. Grundintelligenztest Skala 2-Revisio Google Scholar. 2007. Accessed May 21, 2022. https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Wei%C3%9F%2C+R.+H.+%282007%29.+Grundintelligenztest+Skala+2-Revision+%28CFT+20-R%29%3A+mit+Wortschatztest+und+Zahlenfolgentest-Revision+%28WS%2FZF-R%29.+Hogrefe.&btnG=
- 64.Formann AK, Piswanger K, Waldherr K. Google Scholar. 2011. Accessed May 21, 2022. https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Formann%2C+A.+K.%2C+Piswanger%2C+K.%2C+%26+Waldherr%2C+K.+%282011%29.+Wiener+Matrizen-Test+2%3A+Ein+Rasch-skaldierter+sprachfreier+Kurztest+zu+Erfassung+der+Intelligenz.+Hogrefe.&btnG=
- 65.Markovska-Simoska S, Pop-Jordanova N. Comparison of visual and emotional continuous performance test related to sequence of presentation, gender and age. Prilozi. 2009;30(1):167–178. https://www.academia.edu/download/45271391/Comparison_of_visual_and_emotional_conti20160502-26973-1ex8pw0.pdf [PubMed] [Google Scholar]
- 66.Ponomarev VA, Pronina M V, Kropotov YD. Latent components of event-related potentials in a visual cued go/NoGo task. Hum Physiol. 2019;45(5):474–482. doi: 10.1134/S0362119719050141/TABLES/2 [DOI] [Google Scholar]
- 67.Ekman P, Friesen W V. Measuring facial movement. Environ Psychol Nonverbal Behav. 1976;1(1):56–75. doi: 10.1007/BF01115465 [DOI] [Google Scholar]
- 68.Fallgatter AJ, Bartsch AJ, Herrmann MJ. Electrophysiological measurements of anterior cingulate function. J Neural Transm. 2002;109(5):977–988. doi: 10.1007/S007020200080 [DOI] [PubMed] [Google Scholar]
- 69.Kropotov JD, Ponomarev VA, Pronina M, et al. Functional indexes of reactive cognitive control: ERPs in cued go/no-go tasks. Psychophysiology. 2017;54(12):1899–1915. doi: 10.1111/PSYP.12960 [DOI] [PubMed] [Google Scholar]
- 70.Itier RJ, Taylor MJ. Source analysis of the N170 to faces and objects. Neuroreport. 2004;15(8):1261–1265. doi: 10.1097/01.WNR.0000127827.73576.D8 [DOI] [PubMed] [Google Scholar]
- 71.Eimer M. The face-sensitivity of the N170 component. Front Hum Neurosci. 2011;5. doi: 10.3389/FNHUM.2011.00119/FULL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blau VC, Maurer U, Tottenham N, McCandliss BD. The face-specific N170 component is modulated by emotional facial expression. Behav Brain Funct. 2007;3(1):1–13. doi: 10.1186/1744-9081-3-7/TABLES/1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kappenman ES, Farrens JL, Zhang W, Stewart AX, Luck SJ. ERP CORE: An open resource for human event-related potential research. Neuroimage. 2021;225:117465. doi: 10.1016/J.NEUROIMAGE.2020.117465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gervini D, Gasser T. Self-modelling warping functions. J R Stat Soc Series B Stat Methodol. 2004;66(4):959–971. doi: 10.1111/J.1467-9868.2004.B5582.X [DOI] [Google Scholar]
- 75.Kropotov J, Ponomarev V, Tereshchenko EP, Müller A, Jäncke L. Effect of aging on ERP components of cognitive control. Front Aging Neurosci. 2016;8(APR):69. doi: 10.3389/FNAGI.2016.00069/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kropotov JD, Ponomarev VA, Hollup S, Mueller A. Dissociating action inhibition, conflict monitoring and sensory mismatch into independent components of event related potentials in GO/NOGO task. Neuroimage. 2011;57(2):565–575. doi: 10.1016/J.NEUROIMAGE.2011.04.060 [DOI] [PubMed] [Google Scholar]
- 77.Rossion B, Jacques C. Does physical interstimulus variance account for early electrophysiological face sensitive responses in the human brain? Ten lessons on the N170. Neuroimage. 2008;39(4):1959–1979. doi: 10.1016/J.NEUROIMAGE.2007.10.011 [DOI] [PubMed] [Google Scholar]
- 78.Singmann H, Bolker B, Westfall J, FAR package version 0. 13. afex: Analysis of factorial experiments. baselr.orgPaperpile. 2015. Accessed May 21, 2022. http://www.baselr.org/wp-content/uploads/sites/4/presentations/BaselR_-_afex_An_R_Package_-_Henrik_Singmann_-_20130529.pdf
- 79.Holland BS, Copenhaver MD. An improved sequentially rejective Bonferroni test procedure. Biometrics. 1987;43(2):417. doi: 10.2307/2531823 [DOI] [Google Scholar]
- 80.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front Psychol. 2013;4(NOV). doi: 10.3389/FPSYG.2013.00863/FULL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cohen J. Statistical power analysis for the behavioral sciences. In Statistical power analysis for the behavioral sciences. American Psychological Association; 2013. doi: 10.4324/9780203771587 [DOI] [Google Scholar]
- 82.Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. doi: 10.1037/0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- 83.Goth-Owens TL, Martinez-Torteya C, Martel MM, Nigg JT. Processing speed weakness in children and adolescents with non-hyperactive but inattentive ADHD (ADD). Child Neuropsychol. 2010;16(6):577–591. doi: 10.1080/09297049.2010.485126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Adams ZW, Roberts WM, Milich R, Fillmore MT. Does response variability predict distractibility among adults with attention-deficit/hyperactivity disorder? Psychol Assess. 2011;23(2):427–436. doi: 10.1037/A0022112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Butzbach M, Fuermaier ABM, Aschenbrenner S, Weisbrod M, Tucha L, Tucha O. Basic processes as foundations of cognitive impairment in adult ADHD. J Neural Transm. 2019;126(10):1347–1362. doi: 10.1007/S00702-019-02049-1/TABLES/5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thorsen AL, Meza J, Hinshaw S, Lundervold AJ. Processing speed mediates the longitudinal association between ADHD symptoms and preadolescent peer problems. Front Psychol. 2018;8(FEB):309387. doi: 10.3389/FPSYG.2017.02154/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shanahan MA, Pennington BF, Yerys BE, et al. Processing speed deficits in attention deficit/hyperactivity disorder and Reading disability. J Abnorm Child Psychol. 2006;34(5):585–602. doi: 10.1007/S10802-006-9037-8/METRICS [DOI] [PubMed] [Google Scholar]
- 88.Nigg JT, Gustafsson HC, Karalunas SL, et al. Working memory and vigilance as multivariate endophenotypes related to common genetic risk for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2018;57(3):175–182. doi: 10.1016/J.JAAC.2017.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mohamed SMH, Butzbach M, Fuermaier ABM, et al. Basic and complex cognitive functions in adult ADHD. PLoS One. 2021;16(9):e0256228. doi: 10.1371/JOURNAL.PONE.0256228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kline P. A handbook of test construction (psychology revivals): Introduction to psychometric design. In A handbook of test construction (psychology revivals). Routledge; 2015. doi: 10.4324/9781315695990. [DOI] [Google Scholar]
- 91.Hasson R, Fine JG. Gender differences among children with ADHD on continuous performance tests: A meta-analytic review. J Atten Disord. 2012;16(3):190–198. doi: 10.1177/1087054711427398 [DOI] [PubMed] [Google Scholar]
- 92.Acosta-López JE, Suárez I, Pineda DA, et al. Impulsive and omission errors: Potential temporal processing endophenotypes in ADHD. Brain Sci. 2021;11(9):1218. doi: 10.3390/BRAINSCI11091218/S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Metin B, Roeyers H, Wiersema JR, van der Meere JJ, Thompson M, Sonuga-Barke E. ADHD Performance reflects inefficient but not impulsive information processing: A diffusion model analysis. Neuropsychology. 2013;27(2):193–200. doi: 10.1037/a0031533 [DOI] [PubMed] [Google Scholar]
- 94.Salum GA, Sergeant J, Sonuga-Barke E, et al. Specificity of basic information processing and inhibitory control in attention deficit hyperactivity disorder. Psychol Med. 2014;44(3):617–631. doi: 10.1017/S0033291713000639 [DOI] [PubMed] [Google Scholar]
- 95.Tamm L, Narad ME, Antonini TN, O’Brien KM, Hawk LW, Epstein JN. Reaction time variability in ADHD: A review. Neurotherapeutics. 2012;9(3):500–508. doi: 10.1007/S13311-012-0138-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kofler MJ, Rapport MD, Sarver DE, et al. Reaction time variability in ADHD: A meta-analytic review of 319 studies. Clin Psychol Rev. 2013;33(6):795–811. doi: 10.1016/J.CPR.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 97.Torkamani-Azar M, Member S, Demir Kanik S, Aydin S, Cetin M. Demir Kanik are with S. Prediction of reaction time and vigilance variability from spatio-spectral features of resting-state eeg in a long sustained attention task; prediction of reaction time and vigilance variability from spatio-spectral features of resting-state EEG in a long sustained attention task. IEEE J Biomed Health Inform. 2020;24(9). [DOI] [PubMed] [Google Scholar]
- 98.Kuntsi J. Commentary: From noise to insight? Reaction time variability in ADHD and autism spectrum disorders – a commentary on Karalunas, et al. (2014). J Child Psychol Psychiatry. 2014;55(6):711–713. doi: 10.1111/JCPP.12262 [DOI] [PubMed] [Google Scholar]
- 99.Faraone S, Bonvicini C, Scassellati C. Biomarkers in the diagnosis of ADHD–promising directions. Curr Psychiatry Rep. 2014;16(11). doi: 10.1007/s11920-014-0497-1 [DOI] [PubMed] [Google Scholar]
- 100.Sergeant JA, Geurts H, Huijbregts S, Scheres A, Oosterlaan J. The top and the bottom of ADHD: A neuropsychological perspective. Neurosci Biobehav Rev. 2003;27(7):583–592. doi: 10.1016/J.NEUBIOREV.2003.08.004 [DOI] [PubMed] [Google Scholar]
- 101.Pineda-Alhucema W, Aristizabal E, Escudero-Cabarcas J, Acosta-López JE, Vélez JI. Executive function and theory of mind in children with ADHD: A systematic review. Neuropsychol Rev. 2018;28(3):341–358. doi: 10.1007/S11065-018-9381-9 [DOI] [PubMed] [Google Scholar]
- 102.Milich R, Balentine AC, Lynam DR. ADHD Combined type and ADHD predominantly inattentive type are distinct and unrelated disorders. Clin Psychol Sci Practice. 2001;8(4):463–488. 10.1093/clipsy.8.4.463 [DOI] [Google Scholar]
- 103.Psychiatry JSJ of C. Role of executive function in ADHD. psychiatrist.comJM SwansonJournal of Clinical Psychiatry, 2003•psychiatrist.com. 2003. Accessed April 28, 2024. https://www.psychiatrist.com/wp-content/uploads/2021/02/25676_role-executive-function-adhd.pdf
- 104.Epstein JN, Erkanli A, Conners CK, Klaric J, Costello JE, Angold A. Relations between continuous performance test performance measures and ADHD behaviors. J Abnorm Child Psychol. 2003;31(5):543–554. doi: 10.1023/a:1025405216339 [DOI] [PubMed] [Google Scholar]
- 105.Zhou DD, Zhao L, Ma LL, et al. Altered neural reactivity in adolescents with nonsuicidal self-injury during exposure to self-injury related cues: Electrophysiological evidence from a two-choice oddball paradigm. Front Psychiatry. 2022;13:827480. doi: 10.3389/FPSYT.2022.827480/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Albert J, López-Martín S, Hinojosa JA, Carretié L. Spatiotemporal characterization of response inhibition. Neuroimage. 2013;76:272–281. doi: 10.1016/J.NEUROIMAGE.2013.03.011 [DOI] [PubMed] [Google Scholar]
- 107.Enriquez-Geppert S, Konrad C, Pantev C, Huster RJ. Conflict and inhibition differentially affect the N200/P300 complex in a combined go/nogo and stop-signal task. Neuroimage. 2010;51(2):877–887. doi: 10.1016/J.NEUROIMAGE.2010.02.043 [DOI] [PubMed] [Google Scholar]
- 108.Smith NA, Schmuckler MA. Dial A440 for absolute pitch: Absolute pitch memory by non-absolute pitch possessors. J Acoust Soc Am. 2008;123(4):EL77–EL84. doi: 10.1121/1.2896106 [DOI] [PubMed] [Google Scholar]
- 109.Karr JE, Areshenkoff CN, Rast P, Hofer SM, Iverson GL, Garcia-Barrera MA. The unity and diversity of executive functions: A systematic review and re-analysis of latent variable studies. Psychol Bull. 2018;144(11):1147–1185. doi: 10.1037/BUL0000160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Baez S, Marengo J, Perez A, et al. Theory of mind and its relationship with executive functions and emotion recognition in borderline personality disorder. Wiley Online LibraryPaperpile. 2015;9(2):203–218. doi: 10.1111/jnp.12046 [DOI] [PubMed] [Google Scholar]
- 111.Hughes C, Dunn J, White A. Trick or treat?: Uneven understanding of mind and emotion and executive dysfunction in “hard-to-manage”. Preschoolers. J Child Psychol Psychiatry. 1998;39(7):981–994. doi: 10.1111/1469-7610.00401 [DOI] [PubMed] [Google Scholar]
- 112. Theory of mind and executive function: Is there a developmental relationship? - PsycNET. Accessed May 22, 2022. https://psycnet.apa.org/record/2007-01999-007.
- 113.Wade M, Prime H, Jenkins JM, Yeates KO, Williams T, Lee K. On the relation between theory of mind and executive functioning: A developmental cognitive neuroscience perspective. Psychon Bull Rev. 2018;25(6):2119–2140. doi: 10.3758/S13423-018-1459-0 [DOI] [PubMed] [Google Scholar]
- 114.Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a “theory of mind”? Cognition. 1985;21(1):37–46. https://www.sciencedirect.com/science/article/pii/0010027785900228 [DOI] [PubMed] [Google Scholar]
- 115.Frith CD. The cognitive neuropsychology of schizophrenia: classic edition. Psychology Press; 2015:1–139. doi: 10.4324/9781315749174 [DOI] [Google Scholar]
- 116.Tatar ZB, Cansız A. Executive function deficits contribute to poor theory of mind abilities in adults with ADHD. Appl Neuropsychol Adult. 2020;29(2):244–251. doi: 10.1080/23279095.2020.1736074 [DOI] [PubMed] [Google Scholar]
- 117.Ponomarev VA, Kropotov YD. Improving source localization of event-related potentials in the GO/NOGO task by modeling their cross-covariance structure. Hum Physiol. 2013;39(1):27–39. doi: 10.1134/S036211971301012X [DOI] [PubMed] [Google Scholar]
- 118.Inoue Y, Inagaki M, Gunji Aet al. Altered effect of preceding response execution on inhibitory processing in children with AD/HD: An ERP study. Int J Psychophysiol. 2010;77(2):118–125. https://www.sciencedirect.com/science/article/pii/S0167876010001236 . . [DOI] [PubMed] [Google Scholar]
- 119.Kuntsi J, Oosterlaan J, Stevenson J. Psychological mechanisms in hyperactivity: I response inhibition deficit, working memory impairment, delay aversion, or something else? J Child Psychol Psychiatry. 2001;42(2):199–210. doi: 10.1017/S0021963001006709 [DOI] [PubMed] [Google Scholar]
- 120.Mercure E, Kadosh KC, Johnson MH. The N170 shows differential repetition effects for faces, objects, and orthographic stimuli. Front Hum Neurosci. 2011;5(JANUARY):1–10. doi: 10.3389/fnhum.2011.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Face processing in ADHD: A review of the N170 event-related potential. Accessed May 14, 2024. https://psycnet.apa.org/record/2024-30097-001.
- 122.Roberts B, Trossman R. Face processing in ADHD: A review of the N170 event-related potential. Can J Exp Psychol / Revue Canadienne de Psychologie Expérimentale. 2024;78(1):36–49. 10.1037/cep0000321 [DOI] [PubMed] [Google Scholar]
- 123.Frank DW, Costa VD, Averbeck BB, Sabatinelli D. Higher neural functions and behavior: Directional interconnectivity of the human amygdala, fusiform gyrus, and orbitofrontal cortex in emotional scene perception. J Neurophysiol. 2019;122(4):1530. doi: 10.1152/JN.00780.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Viering T, Naaijen J, van Rooij D, et al. Amygdala reactivity and ventromedial prefrontal cortex coupling in the processing of emotional face stimuli in attention-deficit/hyperactivity disorder. Eur Child Adolesc Psychiatry. 2021;1:1–13. doi: 10.1007/S00787-021-01809-3/FIGURES/4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Amaral DG. The primate amygdala and the neurobiology of social behavior: Implications for understanding social anxiety. Biol Psychiatry. 2002;51(1):11–17. doi: 10.1016/S0006-3223(01)01307-5 [DOI] [PubMed] [Google Scholar]
- 126.LeDoux J. Rethinking the emotional brain. Neuron. 2012;73(4):653–676. doi: 10.1016/J.NEURON.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Phelps EA. Emotion and cognition: Insights from studies of the human amygdala. Annu Rev Psychol. 2005;57:27–53. doi: 10.1146/ANNUREV.PSYCH.56.091103.070234 [DOI] [PubMed] [Google Scholar]
- 128.Davis M, Whalen PJ. The amygdala: Vigilance and emotion. Mol Psychiatry. 2000;6(1):13–34. doi: 10.1038/sj.mp.4000812 [DOI] [PubMed] [Google Scholar]
- 129.Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: An event-related fMRI study. Neuron. 2001;30(3):829–841. doi: 10.1016/S0896-6273(01)00328-2 [DOI] [PubMed] [Google Scholar]
- 130.Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. nature. ComPaperpile. 2004;7(11):1271–1278. doi: 10.1038/nn1341 [DOI] [PubMed] [Google Scholar]
- 131.Flegenheimer C, Lugo-Candelas C, Harvey E, McDermott JM. Neural processing of threat cues in young children with attention-deficit/hyperactivity symptoms. J Clin Child Adolesc Psychol. 2018;47(2):336–344. doi: 10.1080/15374416.2017.1286593 [DOI] [PubMed] [Google Scholar]
- 132.Posner J, Nagel BJ, Maia T V, et al. Abnormal amygdalar activation and connectivity in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2011;50(8):828–837.e3. doi: 10.1016/J.JAAC.2011.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pelc K, Kornreich C, Foisy ML, Dan B. Recognition of emotional facial expressions in attention-deficit hyperactivity disorder. Pediatr Neurol. 2006;35(2):93–97. doi: 10.1016/J.PEDIATRNEUROL.2006.01.014 [DOI] [PubMed] [Google Scholar]
- 134.Mościcki EK, Clarke DE, Kuramoto SJ, et al. Testing DSM-5 in routine clinical practice settings: Feasibility and clinical utility. Psychiatr Serv. 2013;64(10):952–960. doi: 10.1176/APPI.PS.201300098/ASSET/IMAGES/LARGE/952F5.JPEG [DOI] [PubMed] [Google Scholar]
- 135.Mammarella IC, Cardillo R, Broitman J. Understanding nonverbal learning disability: a guide to symptoms, management and treatment. In Understanding nonverbal learning disability: a guide to symptoms management and treatment. Sage Publications; 2021;22:1–104. doi: 10.4324/9780429399008/UNDERSTANDING-NONVERBAL-LEARNING-DISABILITY-IRENE-MAMMARELLA-RAMONA-CARDILLO-JESSICA-BROITMAN [DOI] [Google Scholar]
- 136.Rourke BP, Young GC, Leenaars AA. A childhood learning disability that predisposes those afflicted to adolescent and adult depression and suicide risk. J Learn Disabil. 2016;22(3):169–175. doi: 10.1177/002221948902200305 [DOI] [PubMed] [Google Scholar]
- 137.Broitman J, Davis JM. Overview of NVLD. In Treating NVLD in children. Taylor & Francis; 2013:9–27. doi: 10.1007/978-1-4614-6179-1_2 [DOI] [Google Scholar]
- 138.Friedman D. Cognition and aging: A highly selective overview of event-related potential (ERP) data. J Clin Exp Neuropsychol. 2003;25(5):702–720. doi: 10.1076/JCEN.25.5.702.14578 [DOI] [PubMed] [Google Scholar]
- 139.DeFrance JF, Sands S, Schweitzer FC, Ginsberg L, Sharma V JC. Age-related changes in cognitive ERPs of attenuation. Brain Topogr. 1997;9(4):283–293. doi: 10.1007/BF01464483/METRICS [DOI] [PubMed] [Google Scholar]
- 140.Boutet I, Shah DK, Collin CA, Berti S, Persike M, Meinhardt-Injac B. Age-related changes in amplitude, latency and specialization of ERP responses to faces and watches. Aging Neuropsychol Cognit. 2021;28(1):37–64. doi: 10.1080/13825585.2019.1708253 [DOI] [PubMed] [Google Scholar]
- 141.Yasumura A, Omori M, Fukuda A, et al. Age-related differences in frontal lobe function in children with ADHD. Brain Dev. 2019;41(7):577–586. doi: 10.1016/J.BRAINDEV.2019.03.006 [DOI] [PubMed] [Google Scholar]
- 142.Kohler M, Strauß S, Horn U, et al. P54. Differences in neuronal representation of mental rotation in CRPS patients and healthy controls. Clin Neurophysiol. 2018;129(8):e89–e90. doi: 10.1016/J.CLINPH.2018.04.691 [DOI] [PubMed] [Google Scholar]
- 143.Strauß M, Ulke C, Paucke M, et al. Brain arousal regulation in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. 2018;261:102–108. doi: 10.1016/J.PSYCHRES.2017.12.043 [DOI] [PubMed] [Google Scholar]
- 144.Wu W, Zhang Y, Jiang J, et al. An electroencephalographic signature predicts antidepressant response in major depression. Nat Biotechnol. 2020;38(4):439–447. doi: 10.1038/s41587-019-0397-3 [DOI] [PMC free article] [PubMed] [Google Scholar]