Abstract
Sepsis and septic shock are global healthcare problems associated with high mortality rates. Activation of the renin-angiotensin-aldosterone system (RAAS) is an early event in sepsis, and elevated renin may be predictive of worse outcomes. In a subset of sepsis patients enrolled in the Vitamin C, Thiamine and Steroids in Sepsis (VICTAS) trial, elevated levels of active renin (median value > 189 pg/mL or 5.1 pM) at baseline (day 0) were strongly associated with mortality; however, corresponding plasma levels of the vasopressor hormone Angiotensin II were not substantially increased nor was Angiotensin II associated with disease severity. The current study assessed RAAS components that may impact the Angiotensin II response in control subjects, normal renin sepsis (NRS, renin < 5.1 pM) and high renin sepsis (HRS, renin > 5.1 pM) patients. NRS and HRS subjects exhibited a similar reduction in ACE (40%), but increased levels of ACE2 and DPP3. The ACE to DPP3 ratio was higher in controls but this relationship was reversed in both NRS and HRS subjects. Intact angiotensinogen was 50% lower in the HRS than control or NRS subjects, whereas the intact angiotensinogen to renin ratio was <10% of control or NRS subjects. We conclude that altered expression of ACE, ACE2, DPP3 and angiotensinogen may attenuate the expected increase in Angiotensin II, particularly in sepsis subjects with high renin concentrations.
Keywords: ACE, ACE2, Angiotnesin II, Angiotnesin-(1-7), Angiotensinogen, Sepsis
Introduction
Sepsis and septic shock present significant healthcare issues associated with mortality rates of up to 40% in the several million patients affected every year [1,2]. Circulating markers of septic shock severity may constitute a clinically relevant approach to identify patients at increased risk of poor outcomes early in the course of the disease, which could facilitate early intervention to improve patient outcomes. Currently used septic shock biomarkers, including lactate, may be non-specific and have limited ability to reliably predict prognosis and/or guide disease management [3–5]. Recent studies suggest that activation of the renin-angiotensin-aldosterone system (RAAS) is an important early event in the progression to septic shock, and that elevated levels of circulating renin, the early and committed step in the overall RAAS cascade, may be a better predictor than lactate for worse outcomes, including mortality [6–13]. A post hoc analysis of the ATHOS3 trial for vasodilatory shock revealed that administration of exogenous Angiotensin II (Ang II) decreased renin and was associated with a survival benefit in patients with higher renin levels, pointing to the potential utility of this biomarker as an aid in therapeutic decision-making [7].
We recently reported that high circulating levels of active renin (median value > 189 pg/mL or 5.1 pM at day 0) were strongly associated with mortality in a subset of sepsis patients enrolled in the Vitamin C, Thiamine, and Steroids in Sepsis (VICTAS) trial with a linear response between renin levels and mortality [6]. Surprisingly, we found that the plasma Ang II levels were not associated with higher mortality at day 0 (baseline) [6]. Moreover, the Ang II response was not proportional to the increase in renin, particularly in those patients with elevated levels of renin [6,14]. In critically ill patients, elevated renin concentrations may reflect lower Ang II and attenuated Ang II-dependent negative feedback on renin release, as well as reduced blood pressure and sympathetic activation among other factors [14–18]. In lieu of the attenuated circulating levels of Ang II in sepsis, the current study builds upon our previous report by including a non-hospitalized healthy control group to evaluate the RAAS response to the original sepsis cohort, as well as assessing other RAAS components, including both total and intact forms of the precursor angiotensinogen, the mineralocorticoid aldosterone and the peptidase dipeptidyl aminopeptidase III (DAP3 or DPP3), recently shown to degrade Ang II in vivo [19–22]. Therefore, the present study undertook a more comprehensive characterization of the RAAS to address the hypothesis that alterations in the enzymatic processing pathways may influence circulating angiotensins in sepsis patients, particularly those subjects with high renin levels and increased mortality.
Methods
Patient population
Plasma and serum samples were obtained from the VICTAS biorepository (Johns Hopkins School of Medicine, Baltimore, MD, U.S.A.) and shipped overnight on dry ice to the Wake Forest University School of Medicine (Winston-Salem, NC, U.S.A.) for processing as described [6]. All RAAS measurements were performed on patient samples at day 0 (baseline) following hospitalization and within 24 hours of the onset of respiratory failure, septic shock or both [6]. Patient characteristics from this subset were previously published and are shown in Table S1[6]. Previously published RAAS values in the VICTAS cohort (active renin, ACE, ACE2, Ang II, Ang-(1-7)) at baseline (day 0) to determine the association with mortality were included in the current study; however, the sample size may vary based on the available samples for the various RAAS measurements from our original report [6].
Plasma and serum samples that constitute a control group of non-hospitalized healthy subjects were obtained from a commercial supplier (BioIVT, Hicksville, NY, U.S.A.) and shipped overnight on dry ice. The designated control group consisted of 10 males (35 to 55 years of age) and 10 females (35 to 55 years of age) with no known health issues. Comparable RAAS indices for the current control group to previously published values in control subjects are shown in Table S2.
Active renin
Active renin was directly measured in serum using an active renin enzyme-linked immunoassay (ELISA, DRG International, Fisher Scientific, Waltham MA, U.S.A.) as described with the precaution of thawing frozen samples at 37°C to prevent cryoactivation of prorenin to active renin [6]. The sensitivity of the renin assay is 1 picogram per millilitre (1 pg/mL), and renin levels were converted to molar values based on a molecular size of 37 kilodaltons (37 kDa) and expressed as picomoles per litre (pM).
ACE, ACE2 and DPP3
ACE and ACE2 activities were determined using separate fluorescent assays as previously described [6]. Specificity of the assays was assessed by the addition of the ACE inhibitor lisinopril and the ACE2 inhibitor MLN4760 (MedChem Express) as described [6]. The obtained peptidase activities were converted to protein content using human ACE and ACE2 active standards (R&D Systems, Minneapolis, MN, U.S.A.) and expressed as nanomoles per litre (nM) based on molecular sizes for ACE and ACE2 of 150 kDa and 90 kDa, respectively [6]. The ACE substrate MCA-Arg-Pro-Pro-Gly-Phe-Ser-Ala-Phe-Lys-DNP and the ACE2 substrate MCA-Ala-Pro-Lys-DNP were obtained from Enzo Life Sciences (VWR, Atlanta, GA, U.S.A.), diluted in dimethyl sulfoxide (DMSO) to 10 mM and stored at −80°C. Serum levels of DPP3 were determined by a LifeSpan Biosciences ELISA (LS-Bio, Shirley, MA, U.S.A.) with a sensitivity of 94 pg/mL and reported as nM concentration based on a molecular size of 83 kDa. The ACE to DPP3 ratio is shown in Figure S2.
ACE inhibitory activity
To assess the presence of ACE inhibitors in patient samples, serum samples were incubated at 70°C for 120 mins in a water bath to abolish both N-domain and C-domain ACE activity [23]. Residual ACE activity in the heat-treated samples was compared with the non-treated samples to determine the extent of inhibition. We also determined the effect of heat treatment on the ACE inhibitor Lisinopril against the ACE standard. Heat-treated serums or Lisinopril were then added to the ACE standard, and the exogenous ACE activity was determined. The extent of ACE inhibition was expressed as the percent (%) change in ACE activity compared with the standard alone or the non-treated serum samples (see Figure S3).
Ang II, Ang-(1-7) and aldosterone
Plasma levels of Ang II and Ang-(1-7) were determined by separate radioimmunoassays (RIAs) following solid-phase extraction on Sep-Pak C18 columns as previously described [6]. Sensitivities of the Ang II and Ang-(1-7) RIAs were 2.0 and 2.5 pg/mL, respectively. Peptide content in plasma was corrected for recovery by addition of a trace amount of 125I-Ang II prior to extraction, converted to molar values (Ang II: 1.05 kDa; Ang-(1-7): 0.90 kDa) and both expressed as pM [6,14]. 125I-Ang II was iodinated by chloramine T, and the mono-iodinated form was purified as described [24]. Serum aldosterone levels were directly quantified by an Abcam ELISA (Cambridge, MA, U.S.A.) with a sensitivity of 0.4 pg/mL, converted to molar values (0.36 kDa) and expressed as pM concentration (Figure S1).
Intact and total angiotensinogen
Intact and total angiotensinogen levels were measured directly using ELISAs for both intact (Ang I-containing) and total angiotensinogen (des-Ang I and Ang I forms) from IBL America (Minneapolis, MN, U.S.A.) with sensitivities of 93 and 30 pg/mL, respectively. The renin inhibitor aliskiren (MedChem Express, Monmouth Junction, NJ, U.S.A.) was added to each sample (1 µM, final concentration) to stabilize intact angiotensinogen during the assay. Angiotensinogen levels were converted to molar values and expressed as nM using a molecular size of 54 kDa.
Statistics
Data were expressed as the population median value with minimum-maximum values in the figures or the mean ± SD in the supplemental tables. Analysis of multiple groups was by one-way ANOVA with Tukey’s post-test or Kruskal-Wallis for non-parametric data (Prism 10.2, GraphPad, San Diego, CA, U.S.A.).
Results
Active renin, Ang II and Ang-(1-7) levels
Expressing the renin concentration below or above the median value (5.1 pM) of the VICTAS cohort revealed no differences in active renin levels between the control and the normal renin sepsis groups (NRS; renin < 5.1 pM); however, the high renin sepsis group (HRS; renin > 5.1 pM) exhibited enzyme levels 11-fold and 9-fold higher than controls and the NRS group, respectively (Figure 1A, Table S3). Comparison of plasma Ang II levels revealed no differences between the control and the HRS subjects despite the markedly higher renin levels in the HRS group but lower Ang II levels in the NRS subjects (Figure 1B, Table S4). Ang II content was ~2 fold lower in the NRS as compared with HRS patients (Figure 1B). Plasma Ang-(1-7) levels were higher in the HRS patients as compared with controls but not different between NRS and HRS subjects (Figure 1C, Table S4). In comparison with controls, the plasma Ang II to Ang-(1-7) ratio was lower in the NRS group (Figure 1D). Serum aldosterone levels were not different among the three groups (Figure S1, Table S4).
Figure 1. Comparison of circulating renin, Ang II and Ang-(1-7) in controls and sepsis subjects.
A: Renin content (picomolar; pM) was higher in the high renin VICTAS group (HRS, renin > 5.1 pM) as compared with control subjects (controls); no difference between controls and the normal renin group (NRS, active renin < 5.1 pM). B: Ang II content was lower in the NRS versus both controls and the HRS group. C: Ang-(1-7) content was higher in the HRS groups versus controls. D: Peptide ratio of Ang II to Ang-(1-7) (AII:A7 ratio) was higher in controls versus the NRS group. Statistical comparisons by one-way ANOVA with Kruskal-Wallis post hoc test among all groups.
ACE, ACE2 and DPP3 levels
Circulating ACE was ~40% lower in the NRS and HRS patients as compared with controls (Figure 2A, Table S3). In contrast, serum ACE2 levels were higher in the HRS group than in controls (Figure 2B, Table S3). Overall, the ACE to ACE2 ratio was higher in the controls versus the NRS and HRS groups (Figure 2C). Serum DPP3 content was also higher in NRS and HRS patients as compared with control subjects (Figure 2D, Table S3). Moreover, the ACE to DPP3 relationship was reversed between the control (ratio of 29) versus both NRS and HRS groups (ratio < 0.1; Figure S2). As lower ACE activity in sepsis patients may be owing to an endogenous inhibitor [23], the potential inhibitory activity in heat-treated serum samples was determined in the three groups. We identified 4 patient samples in each sepsis group that inhibited standard ACE >20%, but none in the control subjects (Figure S2).
Figure 2. Comparison of circulating ACE, ACE2 and DPP3 in controls and sepsis subjects.
A: Serum ACE (nanomolar, nM) was lower in NRS and HRS groups as compared with controls. B: Serum ACE2 content (nM) was higher in the HRS group as compared with controls. C: Serum ACE:ACE2 ratios are lower in NRS and HRS groups as compared with controls. D: Serum DPP3 levels (nM) were higher in the NRS and HRS groups as compared with controls. Statistical comparisons by one-way ANOVA with Tukey’s (A,B) or Kruskal-Wallis (C,D) post hoc tests among all groups.
Angiotensinogen levels
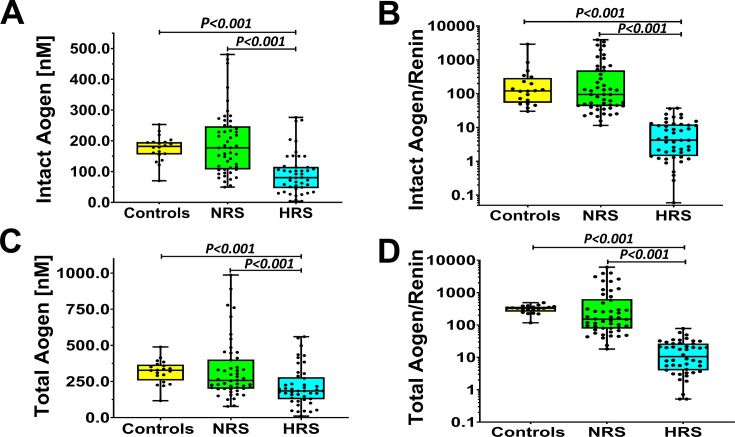
Finally, we assessed circulating levels of both intact angiotensinogen that contains the Ang I domain released by renin and total angiotensinogen (both intact and des-Ang I forms). Serum levels of intact angiotensinogen were ~50% lower in the HRS group as compared with both the control and NRS subjects (Figure 3A, Table S4). The intact angiotensinogen to renin ratio was markedly lower (<10%) in the HRS group than in control or NRS subjects (Figure 3B). Both total angiotensinogen and the total angiotensinogen/renin ratio were also lower in the HRS subjects as compared with the control and NRS groups (Figure 3C and D, respectively; Table S4).
Figure 3. Circulating levels of intact and total angiotensinogen in control and sepsis groups.
A: Serum levels (nanomolar, nM) of intact angiotensinogen (Aogen) were lower in HRS versus the controls or NRS group. B: The intact Aogen to renin ratio was also lower in the HRS compared with the controls or NRS patients. C: Total Aogen levels were also lower in the HRS versus controls or NRS groups. D: Total Aogen to renin ratio was reduced in the HRS versus controls or NRS groups. Statistical comparisons by one-way ANOVA with Tukey’s (A,C) or Kruskal-Wallis (B,D) post hoc tests among all groups.
Discussion
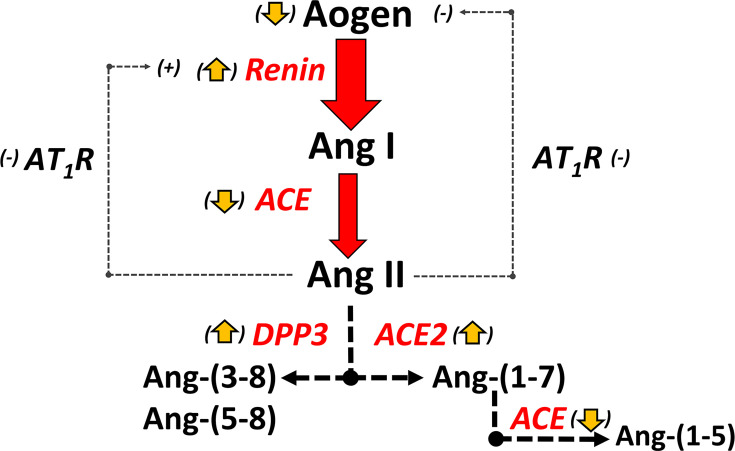
Accumulating clinical and experimental evidence suggests that the classical axis of the RAAS is dysfunctional in sepsis and various types of shock [6,7,14–18]. An attenuated increase in circulating Ang II despite high levels of active renin may contribute to increased disease severity in those patients with elevated renins [6–9,11–18]. Indeed, high renin levels are proposed as a clinically relevant indicator for exogenous Ang II treatment to acutely increase blood pressure and tissue perfusion in critically ill patients [6,7,11,13,14,17]. The current study reveals lower circulating ACE but higher concentrations of DPP3 and ACE2 that may contribute to lower Ang II levels in the NRS group. Moreover, the hyperreninemic sepsis patients exhibited lower levels of intact angiotensinogen in addition to the differential regulation of RAAS processing enzymes. The current results suggest that a reduced capacity to convert Ang I to Ang II, increased Ang II metabolism and depleted levels of intact angiotensinogen may together contribute to an attenuated Ang II response in the HRS patients, possibly accounting for the higher mortality in this cohort (Figure 4) [6].
Figure 4. Potential mechanisms that contribute to an attenuated Ang II response in hyperreninemic sepsis.
In sepsis patients with high renins, inappropriate low levels of Ang II would stimulate renin release owing to loss of negative feedback (attenuated short feedback loop), as well as reduced blood pressure and increased sympathetic tone. Lower ACE but higher ACE2 and DPP3 may reduce circulating levels of Ang II. Higher Ang-(1-7) levels may reflect increased ACE2 but lower ACE that reduces metabolism of Ang-(1-7) to Ang-(1-5). Lower Ang II may also contribute to the reduced release of Aogen from the liver that may further suppress the Ang II response. Reduced expression of the AT1R and/or reduced signalling (-) in the kidney and liver of sepsis patients may also contribute to high renin levels (attenuated negative feedback) but reduced Aogen release into the circulation.
Lower circulating ACE (~40%) in both sepsis groups supports previous studies of reduced ACE content or activity in critically ill patients [16–18,25–27]. Orfanos and colleagues [26] demonstrated reduced ACE activity across the lung in critically ill patients, suggesting lower expression of the peptidase in the pulmonary endothelium. Moreover, LPS exposure of human pulmonary arterial endothelial cells reduced tissue ACE activity that reflected lower mRNA levels of ACE, as well as increased shedding of the enzyme [27]. In contrast, Pode-Shakked et al. [25] found that 70% of their paediatric subjects with septic shock had no detectable serum ACE activity despite higher circulating levels of the ACE protein. This discrepancy between ACE activity and protein levels could portend an endogenous inhibitor blocking the catalytic sites of ACE in sepsis patients. However, our assessment of an inhibitor revealed that only a few samples (<10%) inhibited the human ACE standard, which suggests the absence of ACE inhibitors (endogenous or pharmacologic) and reduced ACE expression in sepsis patients.
In contrast to ACE, higher circulating levels of ACE2 were evident in the HRS subjects. ACE2 exhibits the highest specificity constant (kcat/Km = 2.2) among peptidases that metabolize Ang II; a higher value of the ratio of catalytic function (kcat) to substrate preference (Km) implies greater enzyme specificity for a particular substrate [24]. In comparison, the kcat/Km of ACE (0.58) for Ang-(1-7) metabolism is 3-fold higher than the hydrolysis of Ang I to Ang II (0.18) [24]. In this regard, higher ACE2 and lower ACE concentrations may contribute to a shift in the balance of the RAAS in sepsis towards the Ang-(1-7) axis (Figure 4). The higher circulating levels of ACE2 likely reflect shedding of the membrane-bound to the soluble, catalytically active form of ACE2 by ADAM17, a protease stimulated by inflammatory conditions, such as SARS-CoV-2, that also activates TNF-α, TGFβR1, and matrix metalloproteinases among others [28,29]. Although plasma Ang-(1-7) levels were higher in the HRS patients, the apparent shift to increase Ang-(1-7) may not completely account for the disproportionate Ang II response to elevated renins. Circulating DPP3 was also higher in both NRS and HRS groups than in controls. Interestingly, the ACE to DPP3 ratio was reversed between controls and both sepsis groups. DPP3 initially hydrolyzes the dipeptide Asp1-Arg2 from the N-terminus of Ang II to generate Ang-(3-8) and then immediately converts Ang-(3-8) to Ang-(5-8), which reflects the enzyme’s preference for shorter length peptides (Figure 4) [19,20]. The Ang II assay does not detect Ang-(5-8) (Chappell, unpublished data); thus, we were unable to quantify changes in the DPP3-dependent processing of Ang II. Preclinical studies find that male DPP3-/- transgenic mice exhibit an exaggerated blood pressure response to exogenous Ang II, suggesting a reduced capacity to metabolize Ang II into metabolites that fail to adequately stimulate the AT1R [20]. Treatment with recombinant DPP3 also reduced blood pressure in Ang II-infused male mice, which further supports a functional role for DPP3 in RAAS regulation by promoting the metabolism of Ang II [22]. Moreover, administration of a DPP3 monoclonal antibody that blocks the catalytic site improved cardiac function in a mouse model of sepsis, although assessment of cardiac or plasma Ang II was not determined [22]. DPP3 is primarily a cytosolic peptidase, and it is currently unclear what the tissue source or the mechanism for the elevated levels of DPP3 in critically ill patients is [19,30].
Angiotensinogen is typically considered to be at saturating levels in relation to renin; however, its concentration in humans is at or below renin’s Km value (~1000 nM), and changes in the precursor has an impact on the generation of Ang I and subsequently Ang II [24]. Circulating levels of intact angiotensinogen that can be converted to Ang I by renin were 50% lower in the HRS subjects. Reduced intact angiotensinogen in the HRS subjects may reflect high renin levels that hydrolyse the precursor to the des-Ang I form, which is not recognized by the intact angiotensinogen ELISA. Collins and Hamilton [31] reported reduced circulating levels of intact angiotensinogen (then termed the renin substrate hypertensinogen) in experimental haemorrhagic shock owing to excessive renin activity over 80 years ago. However, our study also finds that total angiotensinogen, comprising both intact and des-Ang I isoforms, was lower in the HRS group. This could reflect a more rapid clearance of des-Ang I angiotensinogen, although earlier studies on potential differences in the clearance rates of intact and des-Ang I isoforms in normal animals were equivocal [32–34]. Bouhnik et al. [32] and Lewicki et al. [33] found no difference in the half-lives of intact and des-Ang I isoforms in rat and rabbit, respectively, whereas Hilgenfeldt [34] reported a shorter half-life of des-Ang I angiotensinogen in rat, although no mechanism for the more rapid clearance of the des-Ang I form was identified. Alternatively, the lower levels of angiotensinogen may reflect an attenuated ability of the liver, the primary source of circulating angiotensinogen, to maintain sufficient levels of the precursor (Figure 4). Preclinical studies in rodent models of sepsis demonstrate reduced AT1R expression and/or attenuated receptor signalling [15,35,36]. APACHE and SOFA scores were similar among the VICTAS groups, which suggests liver injury does not explain differences in angiotensinogen (Table S1), but reduced Ang II-AT1R signalling in hepatocytes could contribute to lower angiotensinogen as Ang II stimulates the precursor protein (Figure 4) [37,38]. The liver also expresses a functional ACE2-Ang-(1-7)-MasR axis [39,40]. As Ang-(1-7) typically antagonizes the actions of the Ang II-AT1R axis, increased Ang-(1-7) levels or a reduced ratio of Ang II to Ang-(1-7) may attenuate the release of angiotensinogen in hepatocytes. Other circulating factors, such as increased levels of microRNAs (miR133, miR433), may potentially inhibit angiotensinogen expression or release in sepsis patients [41–43]. Angiotensinogen may also homodimerize or heterodimerize with other proteins to form high molecular weight oligomers in conditions of elevated oxidative stress [44–46]. These oligomeric forms are poor substrates for renin and, if present in sepsis patients, could contribute to a reduced Ang II response as well [44]. Additional studies are necessary to examine the regulation of angiotensinogen and the potential presence of angiotensinogen oligomers in critically ill patients.
We note several clinical implications drawn from the current study regarding the RAAS response in this cohort of sepsis patients, particularly in the HRS group with lower levels of intact angiotensinogen. First, the commonly used plasma renin activity (PRA) assay, which utilizes intact angiotensinogen as the assay substrate that is converted to Ang I, may underestimate renin levels in the HRS patients with lower angiotensinogen [47,48]. In sepsis patients, the use of the active renin ELISA or the plasma renin concentration (PRC) assay, in which exogenous angiotensinogen is the added substrate source rather than endogenous angiotensinogen, may be the preferred methods to assess renin activity. However, the active renin ELISA is a simpler assay with excellent sensitivity, whereas both PRA and PRC assays require multiple steps, including the measurement of Ang I generation and an additional blank activity sample, as well as exogenous human angiotensinogen for PRC [14,24,47,48]. Second, simultaneous assessment of both active renin and intact angiotensinogen may facilitate the treatment approaches for critically ill patients regarding the use of pressor agents to maintain blood pressure and tissue perfusion. A high level of renin combined with a reduced concentration of intact angiotensinogen in sepsis patients may be particularly informative of an inability to generate or maintain adequate Ang II (Figure 4). Indeed, an attenuated ability to increase Ang II levels coupled with potentially lower AT1R expression may exacerbate conditions that could require exogenous Ang II treatment in sepsis or septic shock patients. In this regard, both active renin and intact angiotensinogen ELISAs should be suitable for the rapid and automated analysis in critically ill patients, as well as exhibit better specificity than current Ang II ELISAs [49,50]. We also found comparable levels of aldosterone among the control, NRS and HRS subjects, which may reflect attenuated Ang II-AT1R stimulation of the mineralocorticoid [17,18,51–53]. Previous studies have suggested a decoupling between elevated renin and inappropriately low levels of aldosterone, termed hyperreninemic hypoaldosteronism, in critically ill patients that may further contribute to an inability to support blood pressure [16,17,51–53].
The strengths of the current study were the inclusion of a non-hospitalized control group with blood samples obtained in a similar manner to the VICTAS cohort and that all samples were assessed by identical assays. We utilized a well-characterized, multisite cohort of sepsis patients to distinguish both intact and total isoforms of angiotensinogen, as well as the specific assessment of active renin. We acknowledge this is a post hoc retrospective study that was not designed to assess the RAAS response per se, and we cannot account for various confounders. We also acknowledge that a single time point (day 0) was assessed prior to treatment and the absence of a validation cohort for the sepsis patients. As previously noted, we lack data on premorbid use of antihypertensive treatments in the VICTAS group, particularly use of ARBs and ACEIs that may increase renin as well as Ang II (ARBs), although it is likely that a minority of the VICTAS cohort (<15%) were treated with RAAS blockers, which was evident by our ACE inhibitor results [6,54,55]. All plasma samples were collected in EDTA-only collection tubes that inhibit various metallopeptidases (ACE, ACE2, DPP3) involved in the generation or metabolism of Ang II and Ang-(1-7); however, renin is not inhibited by EDTA, which obviates the accurate measurement of Ang I [24]. Finally, our study did not assess serum levels of other peptidases that metabolize Ang II. Itskovitz et al. [56] originally reported that severe endotoxic or haemorrhagic shock in dogs was associated with increased metabolism of exogenous Ang II and [Ala1]-Ang II in plasma ex vivo, owing, in part, to higher aminopeptidase (AP) activity. APs that prefer acidic aspartyl/glutamyl residues (APA) to generate Ang-(2-8) (Ang III) or prefer basic residues (APB) such as arginine to form Ang-(3-8) (Ang IV) comprise a group of Ang II peptidases classically termed angiotensinases [24]. The current Ang II RIA also detects Ang III and Ang IV and greater Ang II metabolism to Ang III or Ang IV in sepsis patients could reflect an overall increase in total Ang II levels (Ang II, Ang III and Ang IV). Apart from ACE2, circulating levels of the post-proline cleaving enzyme prolyl oligopeptidase, capable of converting both Ang I and Ang II directly to Ang-(1-7), were reportedly higher in septic shock patients as well [57].
We conclude that the ACE-Ang II-AT1R axis of the RAAS may be functionally attenuated and contribute to higher mortality in hyperreninemic sepsis patients. This may be accompanied by an imbalance in the RAAS favouring the ACE2-Ang-(1-7)-MasR pathway [14,15]. The rationale for the therapeutic use of exogenous Ang II in sepsis is compelling, particularly during the acute phase, given the numerous pathways stimulated by Ang II to elevate blood pressure, as well as augment immune, cardiac and renal function [7,58–67]. Further investigation is required to identify biomarkers that most accurately reflect the health and function of the RAAS to optimally guide the use of therapeutic Ang II in critical illness.
Clinical Relevance.
Sepsis and septic shock are global healthcare problems associated with high mortality rates and limited treatment options. Activation of the renin-angiotensin-aldosterone system (RAAS) is an early event in sepsis and elevated renin may be predictive of worse outcomes; however, the Angiotensin II response may be attenuated, limiting adequate blood pressure and tissue perfusion.
The current study revealed the differential regulation of the RAAS, including lower levels of ACE and angiotensinogen that may attenuate Angiotensin II generation, coupled with elevated ACE2 and DPP3 that may degrade Ang II in high renin sepsis patients. Moreover, higher ACE2 and Angiotensin-(1-7) levels may constitute an inappropriate response in sepsis patients.
The early assessment of both active renin and intact angiotensinogen may facilitate more precise treatments, including the use of synthetic Angiotensin II to reduce mortality in critically ill patients.
Supplementary material
Abbreviations
- ACE2
Angiotensin converting enzyme 2
- ACE
Angiotensin converting enzyme
- ADAM
A disintegrin and metalloproteinase
- ALDO
Aldosterone
- ANG
Angiotensin
- AOGEN
Angiotensinogen
- AP
Aminopeptidase
- APA
Aspartyl/glutamyl aminopeptidase
- APACHE
Acute physiology and chronic health evaluation 2
- APB
Arginyl aminopeptidase
- DPP3
Dipeptidyl aminopeptidase III (3)
- HRS
High renin sepsis
- KCAT
Enzyme catalytic constant
- KM
Michaelis-Menten constant
- Kcat/Km
Specificity constant
- LPS
Lipopolysaccharide
- NRS
Normal renin sepsis
- RAAS
Renin-angiotensin-aldosterone system
- SOFA
Sequential organ failure assessment
- TGFβ1R
Transforming growth factor beta 1 receptor
- TNF-α
Tumour necrosis factor alpha
- VICTAS
Vitamin C, Thiamine, and Steroids in Sepsis clinical trial
- kDA
Kilodaltons
Data Availability
The data in the manuscript regarding the RAAS measurements is available in the online repository figshare (https://info.figshare.com).
Conflicts of Interest
The Authors declare that there are no competing interests associated with this manuscript.
Funding
This work was supported in part by grants from the Wake Forest University Health Cardiovascular Sciences Center (AKK, MCC). Additional funding from grants R01-HL146818 (MCC) and K01AG073581 (CLS) from NIH, and the Groskert Heart Fund, the Wake Forest Venture Fund, the Farley-Hudson Foundation (Jacksonville, NC, USA).
Ethics Approval
The current study was approved by Wake Forest University School of Medicine IRB 00073908 and all procedures were followed by in accordance with local ethics standards and Helsinki Declaration of 1976. All patients provided their written informed consent.
References
- 1.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., et al. The third lnternational consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans L., Rhodes A., Alhazzani W., Antonelli M., Coopersmith C.M., French C., et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181–1247. doi: 10.1007/s00134-021-06506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinberger J., Klompas M., Rhee C. What is the utility of measuring lactate Levels in patients with sepsis and septic shock? Semin. Respir. Crit. Care Med. 2021;42:650–661. doi: 10.1055/s-0041-1733915. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez G., Bellomo R., Bakker J. The ten pitfalls of lactate clearance in sepsis. Intensive Care Med. 2019;45:82–85. doi: 10.1007/s00134-018-5213-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tapia P., Soto D., Bruhn A., Alegría L., Jarufe N., Luengo C., et al. Impairment of exogenous lactate clearance in experimental hyperdynamic septic shock is not related to total liver hypoperfusion. Crit. Care. 2015;19:188. doi: 10.1186/s13054-015-0928-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Busse L.W., Schaich C.L., Chappell M.C., McCurdy M.T., Staples E.M., Ten Lohuis C.C., et al. Association of active renin content with mortality in critically ill patients: A post hoc analysis of the Vitamin C, Thiamine, and Steroids in Sepsis (VICTAS) trial. Crit. Care Med. 2024;52:441–451. doi: 10.1097/CCM.0000000000006095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellomo R., Forni L.G., Busse L.W., McCurdy M.T., Ham K.R., Boldt D.W., et al. Renin and survival in patients given angiotensin II for catecholamine-resistant vasodilatory shock: A clinical trial. Am. J. Respir. Crit. Care Med. 2020;202:1253–1261. doi: 10.1164/rccm.201911-2172OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flannery A.H., Ortiz-Soriano V., Li X., Gianella F.G., Toto R.D., Moe O.W., et al. Serum renin and major adverse kidney events in critically ill patients: a multicenter prospective study. Crit. Care. 2021;25:294. doi: 10.1186/s13054-021-03725-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gleeson P.J., Crippa I.A., Mongkolpun W., Cavicchi F.Z., Van Meerhaeghe T., Brimioulle S., et al. Renin as a marker of tissue-perfusion and prognosis in critically Ill patients. Crit. Care Med. 2019;47:152–158. doi: 10.1097/CCM.0000000000003544. [DOI] [PubMed] [Google Scholar]
- 10.Jeyaraju M., McCurdy M.T., Levine A.R., Devarajan P., Mazzeffi M.A., Mullins K.E., et al. Renin kinetics are superior to lactate kinetics for predicting in-hospital mortality in hypotensive critically ill patients. Crit. Care Med. 2022;50:50–60. doi: 10.1097/CCM.0000000000005143. [DOI] [PubMed] [Google Scholar]
- 11.Leśnik P., Łysenko L., Krzystek-Korpacka M., Woźnica-Niesobska E., Mierzchała-Pasierb M., Janc J. Renin as a marker of tissue perfusion, septic shock, and mortality in septic patients: A prospective observational study. Int. J. Mol. Sci. 23:9133. doi: 10.3390/ijms23169133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen M., Denimal D., Dargent A., Guinot P.G., Duvillard L., Quenot J.P., et al. Plasma renin concentration is associated with hemodynamic deficiency and adverse renal outcome in septic shock. Shock. 2019;52:e22–e30. doi: 10.1097/SHK.0000000000001285. [DOI] [PubMed] [Google Scholar]
- 13.Chakradhar A., Baron R.M., Vera M.P., Devarajan P., Chawla L., Hou PC. Plasma renin as a novel prognostic biomarker of sepsis-associated acute respiratory distress syndrome. Sci. Rep. 2024;14:6667. doi: 10.1038/s41598-024-56994-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaich C.L., Leisman D.E., Goldberg M.B., Filbin M.R., Khanna A.K., Chappell M.C. Dysfunction of the renin-angiotensin-aldosterone system in human septic shock. Peptides. 2024;176:S0196-9781(24)00054-8. doi: 10.1016/j.peptides.2024.171201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Picod A., Garcia B., Van Lier D., Pickkers P., Herpain A., Mebazaa A., et al. Impaired angiotensin II signaling in septic shock. Ann. Intensive Care. 2024;14:89.:89. doi: 10.1186/s13613-024-01325-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bitker L., Burrell L.M. Classic and nonclassic renin-angiotensin systems in the critically ill. Crit. Care Clin. 2019;35:213–227. doi: 10.1016/j.ccc.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung K.S., Song J.H., Jung W.J., Kim Y.S., Kim S.K., Chang J., et al. Implications of plasma renin activity and plasma aldosterone concentration in critically ill patients with septic shock. Korean J. Crit. Care Med. 2017;32:142–153. doi: 10.4266/kjccm.2017.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W., Chen X., Huang L., Lu N., Zhou L., Wu G., et al. Severe sepsis: Low expression of the renin-angiotensin system is associated with poor prognosis. Exp. Ther. Med. 2014;7:1342–1348. doi: 10.3892/etm.2014.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jha S., Taschler U., Domenig O., Poglitsch M., Bourgeois B., Pollheimer M., et al. Dipeptidyl peptidase 3 modulates the renin-angiotensin system in mice. J. Biol. Chem. 2020;295:13711–13723.:S0021-9258(17)49823-1. doi: 10.1074/jbc.RA120.014183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deniau B, Picod A., Van Lier D., Vaittinada Ayar P., Santos K., Hartmann O., et al. High plasma dipeptidyl peptidase 3 levels are associated with mortality and organ failure in shock: results from the international, prospective and observational FROG-ICU cohort. Br. J. Anaesth. 2022;128:e54–e57.:S0007-0912(21)00750-9. doi: 10.1016/j.bja.2021.11.021. [DOI] [PubMed] [Google Scholar]
- 21.Malovan G., Hierzberger B., Suraci S., Schaefer M., Santos K., Jha S, et al. The emerging role of dipeptidyl peptidase 3 in pathophysiology. FEBS J. 2023;290:2246–2262. doi: 10.111/febs.16429. [DOI] [PubMed] [Google Scholar]
- 22.Pang X., Shimizu A., Kurita S., Zankov D.P., Takeuchi K., Yasuda-Yamahara M., et al. Novel therapeutic role for dipeptidyl peptidase III in the treatment of hypertension. Hypertension. 2016;68:630–641. doi: 10.1161/HYPERTENSIONAHA.116.07357. [DOI] [PubMed] [Google Scholar]
- 23.Danilov S.M., Balyasnikova I.V., Albrecht R.F., Kost O.A. Simultaneous determination of ACE activity with two substrates provides information on the status of somatic ACE and allows detection of inhibitors in human blood. J. Cardiovasc. Pharmacol. 2008;52:90–103. doi: 10.1097/FJC.0b013e31817fd3bc. [DOI] [PubMed] [Google Scholar]
- 24.Chappell MC. Biochemical evaluation of the renin-angiotensin system: the good, bad, and absolute? Am. J. Physiol. Heart Circ. Physiol. 2016;310:H137–52. doi: 10.1152/ajpheart.00618.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pode-Shakked N., Ceschia G., Rose J.E., Goldstein S.L., Stanski N.L., and Genomics of Pediatric Septic Shock Investigators Increasing angiotensin-converting enzyme concentrations and absent angiotensin-converting enzyme activity are associated with adverse kidney outcomes in pediatric septic shock. Crit. Care. 2023;27:230. doi: 10.1186/s13054-023-04518-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orfanos S.E., Armaganidis A., Glynos C., Psevdi E., Kaltsas P., Sarafidou P., et al. Pulmonary capillary endothelium-bound angiotensin-converting enzyme activity in acute lung injury. Circulation. 2000;102:2011–2018. doi: 10.1161/01.cir.102.16.2011. [DOI] [PubMed] [Google Scholar]
- 27.Hermanns M.I., Müller A.M., Tsokos M., Kirkpatrick C.J. LPS-induced effects on angiotensin I-converting enzyme expression and shedding in human pulmonary microvascular endothelial cells. In Vitro Cell.Dev.Biol.-Animal. 2014;50:287–295. doi: 10.1007/s11626-013-9707-0. [DOI] [PubMed] [Google Scholar]
- 28.Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.-C., Turner A.J., et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: Celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawai T., Elliott K.J., Scalia R., Eguchi S. Contribution of ADAM17 and related ADAMs in cardiovascular diseases. Cell. Mol. Life Sci. 2021;78:4161–4187. doi: 10.1007/s00018-021-03779-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deniau B., Blet A., Santos K., Vaittinada Ayar P., Genest M., Kästorf M., et al. Inhibition of circulating dipeptidyl-peptidase 3 restores cardiac function in A sepsis-induced model in rats: A proof of concept study. PLOS ONE. 2020;15:e0238039. doi: 10.1371/journal.pone.0238039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Collins DA, Hamilton AS. Changes in the renin-angiotonin system in hemorrhagic shock. Am J Physiol. 1944;149(4):499–512. doi: 10.1152/ajplegacy.1944.140.4.499. [DOI] [Google Scholar]
- 32.Bouhnik J., Campbell D.J., Corvol P., Menard J. Clearance of angiotensinogen and des-Angiotensin I angiotensinogen in rats in different physiological states. Endocrinology: Proceeding of the 7th International Congress of Endocrinology. 1984;274:27. vol. p. [Google Scholar]
- 33.Lewicki JA, Printz JM, Printz MP. Clearance of rabbit plasma angiotensinogen and relationship to CSF angiotensinogen. Am J Physiol. 1983;244(4):H577–85. doi: 10.1152/ajpheart.1983.244.4.H577. [DOI] [PubMed] [Google Scholar]
- 34.Hilgenfeldt U. Half-life of rat angiotensinogen: influence of nephrectomy and lipopolysaccharide stimulation. Mol Cell Endocrinol. 1988;56(1-2):91–98. doi: 10.1016/0303-7207(88)90012-3. [DOI] [PubMed] [Google Scholar]
- 35.Bucher M, Ittner KP, Hobbhahn J, Taeger K, Kurtz A. Downregulation of angiotensin II type 1 receptors during sepsis. Hypertension. 2001;38:177–182. doi: 10.1161/01.hyp.38.2.177. [DOI] [PubMed] [Google Scholar]
- 36.Leisman D.E., Fernandes T.D., Bijol V., Abraham M.N., Lehman J.R., Taylor M.D., et al. Impaired angiotensin II type 1 receptor signaling contributes to sepsis-induced acute kidney injury. Kidney Int. 2021;99:148–160. doi: 10.1016/j.kint.2020.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klett C., Nobiling R., Gierschik P., Hackenthal E. Angiotensin II stimulates the synthesis of angiotensinogen in hepatocytes by inhibiting adenylylcyclase activity and stabilizing angiotensinogen mRNA. J. Biol. Chem. 1993;268:25095–25107. [PubMed] [Google Scholar]
- 38.Li J., Brasier AR. Angiotensinogen gene activation by angiotensin II is mediated by the rel A (nuclear factor-kappaB p65) transcription factor: one mechanism for the renin angiotensin system positive feedback loop in hepatocytes. Mol. Endocrinol. 1996;10:252–264. doi: 10.1210/mend.10.3.8833654. [DOI] [PubMed] [Google Scholar]
- 39.Rajapaksha I.G., Gunarathne L.S., Angus P.W., Herath CB. Update on new aspects of the renin-angiotensin system in hepatic fibrosis and portal hypertension: Implications for novel therapeutic options. J. Clin. Med. 2021;10:702–715.:702. doi: 10.3390/jcm10040702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cai S.-M., Yang R.-Q., Li Y., Ning Z.-W., Zhang L.-L., Zhou G.-S., et al. Angiotensin-(1-7) improves liver fibrosis by regulating the NLRP3 inflammasome via redox balance modulation. Antioxid. Redox Signal. 2016;24:795–812. doi: 10.1089/ars.2015.6498. [DOI] [PubMed] [Google Scholar]
- 41.Kemp J.R., Unal H., Desnoyer R., Yue H., Bhatnagar A., Karnik S.S. Angiotensin II-regulated microRNA 483-3p directly targets multiple components of the renin–angiotensin system. J. Mol. Cell. Cardiol. 2014;75:25–39. doi: 10.1016/j.yjmcc.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin B., Feng D.G., Xu J. microRNA-665 silencing improves cardiac function in rats with heart failure through activation of the cAMP signaling pathway. J. Cell. Physiol. 2019;234:13169–13181. doi: 10.1002/jcp.27987. [DOI] [PubMed] [Google Scholar]
- 43.Chen Y., Wang X. miRDB: an online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020;48:D127–D131. doi: 10.1093/nar/gkz757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stanley P., Serpell L.C., Stein P.E. Polymerization of human angiotensinogen: insights into its structural mechanism and functional significance. Biochem. J. 2006;400:169–178. doi: 10.1042/BJ20060444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kløverpris S., Skov L.L., Glerup S., Pihl K., Christiansen M., Oxvig C. Formation of high-molecular-weight angiotensinogen during pregnancy is a result of competing redox reactions with the proform of eosinophil major basic protein. Biochem. J. 2013;449:209–217. doi: 10.1042/BJ20120801. [DOI] [PubMed] [Google Scholar]
- 46.Tewksbury D.A., Goodman J.R., Kaiser S.J., Burrill R.E., Brown H.L. Quantitation of the five forms of plasma high molecular weight angiotensinogen in women with pregnancy-induced hypertension. Am. J. Hypertens. 2000;13:221–225. doi: 10.1016/S0895-7061(99)00191-0. [DOI] [PubMed] [Google Scholar]
- 47.Arnal J.-F., Cudek P., Plouin P.-F., Guyenne T.-T., Michel J.-B., Corvol P. Low angiotensinogen levels are related to the severity and liver dysfunction of congestive heart failure: implications for renin measurements. Am. J. Med. 1991;90:17–22. doi: 10.1016/0002-9343(91)90501-N. [DOI] [PubMed] [Google Scholar]
- 48.Campbell D.J., Nussberger J., Stowasser M., Danser A.H.J., Morganti A., Frandsen E., et al. Activity assays and immunoassays for plasma Renin and prorenin: information provided and precautions necessary for accurate measurement. Clin. Chem. 2009;55:867–877. doi: 10.1373/clinchem.2008.118000. [DOI] [PubMed] [Google Scholar]
- 49.Chappell M.C., Pirro N.T., South A.M., Gwathmey TM. Concerns on the Specificity of Commercial ELISAs for the Measurement of Angiotensin (1-7) and Angiotensin II in Human Plasma. Hypertension. 2021;77:e29–e31. doi: 10.1161/HYPERTENSIONAHA.120.16724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Łebek-Szatańska A., Papierska L., Glinicki P., Zgliczyński W. Poor performance of angiotensin II enzyme-linked immuno-sorbent assays in a mostly hypertensive cohort routinely screened for primary aldosteronism. Diagnostics (Basel). 2022;12:1124. doi: 10.3390/diagnostics12051124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nethathe G.D., Cohen J., Lipman J., Anderson R., Feldman C. Mineralocorticoid dysfunction during critical illness: A review of the evidence. Anesthesiology. 2020;133:439–457.:doi. doi: 10.1097/ALN.0000000000003365. [DOI] [PubMed] [Google Scholar]
- 52.Cheyron A, Lesage A., Daubin C., Ramakers M., Charbonneau P. Hyperreninemic hypoaldosteronism: a possible etiological factor of septic shock-induced acute renal failure. Intensive Care Med. 2003;29:1703–1709. doi: 10.1007/s00134-003-1986-6. [DOI] [PubMed] [Google Scholar]
- 53.Davenport M.W., Zipser RD. Association of hypotension with hyperreninemic hypoaldosteronism in the critically ill patient. Arch. Intern. Med. (Chic). 1983;143:735–737. [PubMed] [Google Scholar]
- 54.Johansen M.E., Niforatos J.D., Sussman JB. The ecology of antihypertensives in the United States, 1997-2017. J. Gen. Intern. Med. 2021;36:699–704. doi: 10.1007/s11606-020-06214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leisman D.E., Handisides D.R., Busse L.W., Chappell M.C., Chawla L.S., Filbin M.R., et al. ACE inhibitors and angiotensin receptor blockers differentially alter the response to angiotensin II treatment in vasodilatory shock. Crit. Care. 2024;28:130.:130. doi: 10.1186/s13054-024-04910-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Itskovitz H.D., Miller L., Ural W., Zapp J., White R. Inactivation of angiotensin in shock. Am. J. Physiol. 1969;216:5–10. doi: 10.1152/ajplegacy.1969.216.1.5. [DOI] [PubMed] [Google Scholar]
- 57.Vliegen G., Kehoe K., Bracke A., Hert E., Verkerk R., Fransen E, et al. Dysregulated activities of proline-specific enzymes in septic shock patients (Sepsis-2) PLoS ONE. 2020;15 doi: 10.1371/journal.pone.0231555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leisman D.E., Privratsky J.R., Lehman J.R., Abraham M.N., Yaipan O.Y., Brewer M.R., et al. Angiotensin II enhances bacterial clearance via myeloid signaling in a murine sepsis model. Proc. Natl. Acad. Sci. U.S.A. 2022;119:e2211370119. doi: 10.1073/pnas.2211370119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leisman D.E., Handisides D.R., Chawla L.S., Albertson T.E., Busse L.W., Boldt D.W., et al. Angiotensin II treatment is associated with improved oxygenation in ARDS patients with refractory vasodilatory shock. Ann. Intensive Care. 2023;13:128. doi: 10.1186/s13613-023-01227-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang J., Patel M.B., Song Y.-S., Griffiths R., Burchette J., Ruiz P., et al. A novel role for type 1 angiotensin receptors on T lymphocytes to limit target organ damage in hypertension. Circ. Res. 2012;110:1604–1617. doi: 10.1161/CIRCRESAHA.111.261768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cao D.-Y., Saito S., Veiras L.C., Okwan-Duodu D., Bernstein E.A., Giani J.F., et al. Role of angiotensin-converting enzyme in myeloid cell immune responses. Cell. Mol. Biol. Lett. 2020;25:31.:31. doi: 10.1186/s11658-020-00225-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Flannery A.H., Kiser A.S., Behal M.L., Li X., Neyra J.A. RAS inhibition and sepsis-associated acute kidney injury. J. Crit. Care. 2022;69:153986.:S0883-9441(22)00004-1. doi: 10.1016/j.jcrc.2022.153986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wieruszewski P.M., Khanna AK. Vasopressor choice and timing in vasodilatory shock. Crit. Care. 2022;26:76.:76. doi: 10.1186/s13054-022-03911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khanna A., English S.W., Wang X.S., Ham K., Tumlin J., Szerlip H, et al. ATHOS-3 Investigators (2017) Angiotensin II for the treatment of vasodilatory shock. N. Engl. J. Med. 2017;377:419–430. doi: 10.1056/NEJMoa1704154. [DOI] [PubMed] [Google Scholar]
- 65.Tumlin J.A., Murugan R., Deane A.M., Ostermann M., Busse L.W., Ham K.R, et al. Angiotensin II for the Treatment of High-Output Shock 3 (ATHOS-3) Investigators. Crit. Care Med. 2018;46:949–957. doi: 10.1097/CCM.0000000000003092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.See E.J., Clapham C., Liu J., Khasin M., Liskaser G., Chan J.W., et al. A pilot study of angiotensin II as primary vasopressor in critically ill adults with vasodilatory hypotension: The ARAMIS study. Shock. 2023;59:691–696. doi: 10.1097/SHK.0000000000002109. [DOI] [PubMed] [Google Scholar]
- 67.Alamami A., Rahhal A., Alqudah B., Shebani A., Alammora A., Mohammad H., et al. Clinical outcomes of angiotensin II therapy in vasoplegic shock: A systematic review and meta-analysis. Life (Basel). 2024;14:1085. doi: 10.3390/life14091085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data in the manuscript regarding the RAAS measurements is available in the online repository figshare (https://info.figshare.com).