Abstract
Objective
The aim of this systematic review is to identify pain profiling parameters that are reliably different between patients with migraine and healthy controls, using Quantitative Sensory Testing (QST) including Temporal Summation (TS), Conditioned Pain Modulation (CPM), and Corneal Confocal Microscopy (CCM).
Methods
A comprehensive literature search was conducted (up to 23 May 2024). The quality of the research was assessed using the Newcastle-Ottawa Scale (NOS) for non-randomized studies.
Results
Twenty-eight studies were included after screening. The QST studies indicate that migraine patients exhibit lower pressure pain thresholds (PPT), particularly in the trigeminal region. A previous meta-analysis reported lower heat pain thresholds (HPT). CPM studies suggest a (mild) inhibitory or absent response in migraine patients, not different from controls. High-frequency and chronic migraine patients may exhibit a facilitatory CPM response. With repeated executions of CPM, migraine patients display a diminishing CPM response, a phenomenon not observed in control subjects. CCM investigations in migraine patients revealed conflicting outcomes, likely as a result of small sample sizes and limited characterization of migraine features.
Conclusion
Pain profiling migraine patients varies due to sensory modality, applied methods, anatomical sites, and migraine features. Understanding pain profiling offers insights into migraine pathophysiology, requiring careful selection of parameters and differentiation among migraine subtypes.
Keywords: Migraine, Chronic migraine (CM), Episodic migraine (EM), Pain profile, Quantitative Sensory Testing (QST), Conditioned Pain Modulation (CPM), Corneal Confocal Microscopy (CCM), Psychophysical measure, Allodynia
Introduction
Migraine is a prevalent and intricate neurological condition, marked by recurring, incapacitating headache episodes accompanied by symptoms such as nausea, occasional vomiting, and increased sensitivity to light and sound [21]. Additionally, about one-third of individuals with migraine experiences temporary neurological disturbances, often visual in nature, known as migraine auras [21]. In essence, migraine represents a multifaceted pain disorder. While the majority of patients has the episodic form of migraine (EM), approximately 3% of them convert annually from EM to chronic migraine (CM), characterized by experiencing 15 or more headache days per month, of which at least 8 are migraine days—a transition often referred to as migraine chronification [2]. This shift is significantly influenced by the repeated and prolonged use of acute migraine medications, making it a major risk factor for migraine chronification [4, 21]. Consequently, the vast majority of individuals with CM become dependent on acute medication to manage their condition. The mechanism of migraine chronification remains uncertain, and while there is substantial knowledge, not all aspects are fully understood.
The endogenous pain modulatory system consists of both inhibitory and facilitatory processes. Disruption of this modulatory system not only increases the likelihood of experiencing pain but also intensifies susceptibility to both short-term and long-term pain. This can be further explored by examining key patient-related factors that are known to influence the development of chronic pain, such as the endogenous pain inhibitory system and the facilitation system [37]. The imbalance between these inhibitory and facilitatory pain mechanisms can vary for different types of pain conditions. In many chronic pain disorders, either heightened pain facilitation (referred to as central sensitization) or a deficiency in pain inhibition is proposed as the underlying mechanism. The pain modulatory system can be quantified by experimental techniques, allowing the construction of sensory profiles or subgroups.
Central sensitization has been suggested to be an important mechanism in migraine chronification. It is presumed to occur in second and third order neurons sequentially, resulting in an analogous spatial distribution of cutaneous allodynia with cephalic and extracephalic symptoms during migraine attacks [7]. Cutaneous allodynia may have a predictive value for treatment response in chronic migraine [47]. Managing chronic migraine can be challenging and individuals suffering from it often resort to frequent use of pain-relieving medications. Overuse of acute treatments leads to complex mechanisms involving peripheral and central factors, potentially resulting in central sensitization, increased neuronal excitability, and the upregulation of pain-related pathways [4]. Therefore, it is important to understand involvement of the pain modulatory system in migraine.
Quantitative Sensory Testing (QST) is a method to assess somatosensory function by applying controlled stimuli [34]. Through this approach, it becomes possible to determine the detection and pain thresholds for various stimuli such as heat, cold, pressure, vibration and mechanical inputs [48]. Central sensitization, which involves the pain facilitatory system, can be assessed using the temporal summation (TS) test. This test evaluates the amplification of pain intensity in response to a repeated pain stimulus [48]. In patients with chronic pain syndromes, such as fibromyalgia, chronic lower back pain and neuropathic pain syndromes, an increase in TS response has been reported [29, 36, 38, 58].
Conditioned pain modulation (CPM) tests are utilized to evaluate the endogenous pain inhibitory system [61]. These tests examine the suppression of a painful stimulus (test stimulus) via the administration of a secondary painful stimulus (conditioning stimulus) at a remote area. In patients with chronic pain syndromes, such as temporomandibular disorder, fibromyalgia, complex regional pain syndrome and neuropathic pain syndromes, a decrease or complete loss in CPM response is seen [25, 37, 38, 51]. For migraine CPM response has been extensively explored.
Corneal confocal microscopy (CCM) is used to quantify the corneal nerve plexus. This subepithelial, densely intensified and highly dynamic plexus comprises small nerve fibers that are the distal ends of the ophthalmic branch of the trigeminal nerve. Abnormalities in corneal nerve parameters have been observed in patients with peripheral neuropathies [6, 46]. The corneal nerve densities has been shown to change in response to treatment for other disorders [56, 59]. Given the significant involvement of the trigeminovascular system in the pathophysiology of migraine, the quantification of this plexus may hold relevance for migraine [2]. CCM assesses the small nerve fibers, and might provide valuable insights into the role of these fibers in pain perception, making this a potentially useful addition to pain profiling.
This systematic review aims to summarize current findings on pain profiles in migraine, with a specific focus on QST, CPM, and CCM tests. It aims to encapsulate existing knowledge about sensory processing and pain modulation in migraine patients. The insights may aid researchers and healthcare professionals in selecting appropriate pain profiling parameters to distinguish migraine patients from healthy individuals and obtain more insights in pain mechanisms for migraine. Additionally, they can guide the investigation of these parameters during transitions between episodic and chronic migraine (with medication overuse) and vice versa.
Methods
Search strategy and data sources
This systematic review was performed according to the PRISMA checklist [32]. To investigate the literature we made a search on the QST, TS, CPM and CCM tests in PubMed, MEDLINE, EMBASE, Web of Science & the Cochrane Library. Previously, a systematic review for QST in patients with migraine was done by Nahman-Averbuch et al. in 2018 [34]. Their literary search was done for papers until January 2017. Therefore, for this review, we limited the search for papers on QST after January 2017 to bring a renewed perspective of more recent QST findings. For the other tests there was no time limit on the search. The total search was done up to 23 May 2024, using the key words (“migraine” OR “migraine disorders”) AND (“quantitative sensory testing” OR “temporal summation” OR “pain modulation” OR “conditioned pain modulation” OR “migraine modulation” OR “headache modulation” OR “diffuse noxious inhibitory control” OR “corneal confocal microscopy” OR “in vivo confocal microscopy”).
Studies were divided based on the pain tests used: QST, CPM or CCM. Studies were required to: (1) have full text availability; (2) be in English; (3) involve only human subjects; (4) present case–control differences; (5) enroll at least 1 migraine patient group that was not mixed with patients with other headache conditions (with an exception for medication overuse headache (MOH)); (6) use at least one psychophysical measure; (7) be published before 23 May 2024. Information specialists of the Walaeus library of Leiden University Medical Center assisted in retrieving full text manuscripts not available to the Leiden University Medical Center library. Articles were excluded if they met any of the following criteria: (1) were case reports, meeting abstracts, editorials, commentaries, or articles focusing on a pediatric population (age < 18 years), or contained incomplete information; (2) did not present original data; (3) lacked baseline data; (4) did not include a comparison with a control group for QST or CCM.
FCvW and GMT/MvV independently reviewed all abstracts and full text papers for inclusion using the above criteria. Agreement between the reviewers on study selection was 93.5%. Disagreement was resolved by discussion. Data were extracted by two reviewers (FCvW/GMT for QST and CPM, FCvW/MvV for CCM) using a form that was developed to capture information on study groups including: (1) total subjects in migraine and control groups; (2) number of males and females; (3) mean age and SD; (4) migraine type; (5) migraine characteristics if provided; (6) study design (measure, modality, and location of tests); (7) results; (8) other comments.
Risk of bias
The quality of the research was assessed using the Newcastle-Ottawa Scale (NOS) for non-randomized studies [54]. The NOS is comprised of three domains which contribute to the overall quality score: selection of groups; comparability; and outcome assessment. A numerical score (0–9) was assigned evaluating these domains. A higher NOS score signifies superior methodological quality and reduced bias risk, facilitating the assessment and comparison of research findings across various studies [54].
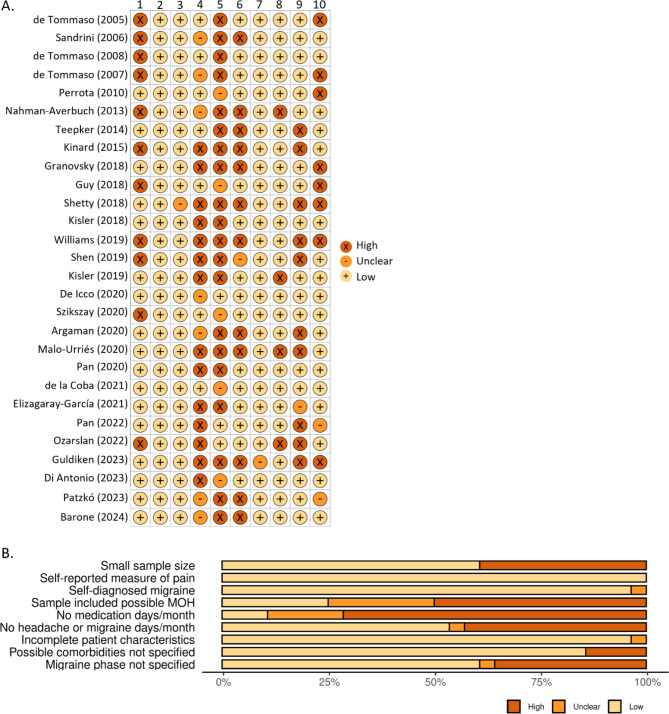
Risk of bias was assessed by evaluating the selection bias, performance bias and confounding bias in 10 subcategories. The subcategories were: (1) small sample size (n < 20); (2) used a self-reported measure of pain; (3) used self-diagnosed migraine; (4) the sample included a subset of subjects with possible medication overuse headache (MOH) (most often this is the case for CM or high-frequency migraine); (5) absence of verified acute medication days/month, headache days/month or migraine days/month; (6) patient clinical characteristics are incomplete; (7) absence of specified possible comorbidities for the tests; (8) absence of specified when the measurements were done in relation to the migraine phase; (9) difference in the sex distribution; and (10) other. The subcategories were classied as high, low or unclear risk according to the Cochrane risk of bias [22]. The Robvis tool was used to visualize the risk-of-bias assessment [31].
Data reporting
For studies presenting identical datasets we included only one publication as a representative. For studies reporting several repetitions of measurements, the baseline data and data after intervention were assessed, but only baseline data were included. Some studies reported data on several related, but distinct, outcomes in the same participants (e.g., different stimulus locations or stimulus paradigms in the same subjects). To determine whether migraine was associated with global or local differences in sensitivity, data were categorized by QST location of and/or stimuli in the trigeminal nerve region (V1, V2 and V3), neck region, head region or more distal regions (forearm, hand and lower limbs).
Results
The search provided 598 possible articles after removal of duplicates (23-May-2024). After the first screening, 46 articles were assessed for eligibility. Finally, 28 papers were included in this review: QST (n = 12), CPM (n = 13) and CCM (n = 5). The flowchart is shown in Fig. 1.
Fig. 1.
Flowchart of the systematic literary screening. The search was performed on 23 May 2024. Exclusion criteria were:1) no full text availability; 2) not in English; 3) not in human study; 4) case report, meeting abstracts, editorials, commentaries, articles with a pediatric population (age < 18) or articles with incomplete information; 5) does not present own data with a prospective design; 6) did not enroll at least 1 migraine patient group that was not mixed with patients with other headache conditions (excluding MOH); 7) does not use 1 psychophysical measure (and QST published before January 2017); and 8) no baseline data available for all, or did not include a comparison with a control group for QST or CCM. QST = Quantitative Sensory Testing; CPM = Conditioned Pain Modulation; CCM = Corneal Confocal Microscopy
Quantitative sensory testing (QST)
For QST studies before January 2017 we refer to Nahman-Averbuch et al. in 2018 [34]. Twelve studies published after January 2017 contained original QST data of migraine patients compared to a non-migraine control group, shown in Table 1.
Table 1.
Information on the studies on quantitative sensory testing (QST) in patients with migraine and controls
| Author type of study |
Groups: total n, number of females, mean age (SD). Migraine specifications: EM/CM/MOH and mean MHD/MMD/MAMD |
QST Test paradigm | Stimulus/ location | Results | Comments | Treatment | Risk of bias | NOS score |
|---|---|---|---|---|---|---|---|---|
|
Guy [20] Case-control study |
Migraine group EM (MO): - n = 12 (12 females) - age: 33.83 ± 3.11 years - 3 MHD CM with MOH: - n = 12 (12 females) - age: 35.25 ± 3.13 years - 30 MHD Control group - n = 12 (12 females) - age: 37.25 ± 3.01 years |
MPT & HPT |
MPT: electronic algometer (3x) HPT: laser stimulation Location: back of non-dominant hand |
MPT & HPT: - EM = CM = HC |
Tested < 24 h after attack. |
EM: no prophylactic treatment < 2 months. CM + MOH: withdrawal with 5 days hospitalization (ice packs +/- hydroxyzine 25 mg/day). |
2 H, 1 U | 6 |
|
De Icco [10] Single-blind controlled study |
Migraine group Low-frequency-EM (MO): - n = 28 (20 females) - age: 34.1 ± 8.5 years - 4.0 ± 0.9 MHD - 3.5 ± 0.9 MAMD High-frequency-EM (MO): - n = 19 (17 females) - age: 34.0 ± 7.5 years - 9.1 ± 2.0 MHD - 8.1 ± 1.6 MAMD Control group - n = 21 (13females) - age: 32.0 ± 6.5 years |
TS |
TS: nociceptive withdrawal reflex (1 vs. 5th stimulus) Location: right lower limb |
TS baseline: - HF-EM > LF-EM = HC TS after nitroglycerin administration: - Increase of TS for LF-EM at 60 and 120 min - No difference of TS for HF-EM and HC - Reduction of TS threshold in LF-EM at 60 and 120 min - Reduction of TS threshold in HF-EM at 30, 60, and 120 min - No change in TS threshold in HC |
TS not indicated as ratio but as VAS with 1 vs. repetitive stimuli. | TS threshold was recorded at baseline and 30, 60, and 120 min after nitroglycerin administration (0.9 mg sublingual). | 1 U | 7 |
|
Szikszay [55] Cross-sectional study |
Migraine group EM (19/26 MO): - n = 26 (21 females) - age: 41.9 ± 11.8 years - 7.5 ± 3.2 MHD - 5.5 ± 3.1 MAMD Control group - n = 26 (21 females) - age: 37.3 ± 11.4 years |
MDT, MPT, HDT, HPT & HPS |
MDT: von Frey filaments (5x) MPT: PinPrick stimulators (5x) HDT & HPT: heat stimulus (3x) HPS: heat stimulus of NRS = 60 (3x) Location: trigeminal nerve (V1) & forearm |
MDT, MPT, HDT, HPT & HPS: - EM = HC |
> 48 headache free/medication free before testing. | 1 H, 1 U | 8 | |
|
Argaman [1] Case-control study |
Migraine group EM: - n = 32 (28 females) - age: 26.0 years - 5.0 ± 2.2 attacks/month Control group - n = 23 (20 females) - age: 26.0 years |
TS |
TS: von Frey filament (10x) Location: left side of forehead & left volar forearm. |
TS: - EM = HC |
No alcohol/pain medication > 24 h before tests & no caffeine > 3 h before testing. | No prophylactic treatment for < 3 months. | 3 H, 1 U | 3 |
|
Malo-Urriés [30] Comparative study |
Migraine group - n = 52 (34 females) - age: 42.7 ± 12.7 years Control group - n = 30 (17 females) - age: 41.2 ± 12.7 years |
PPT, TDT, PDT & 2PDT |
PPT: pressure algometer (3x) TDT & PDT: Semmes-Weinstein monofilaments (5x) 2PDT: Semmes-Weinstein monofilaments & digital caliper (5x) Location: trigeminal nerve (V1 t/m V3), cranium on the parietal bone, upper trapezius muscle, articular pillar of C2-C3, suboccipital muscles & thenar eminence |
PPT: - Migraine < HC (in V2, m. trapezius, C2-C3 facet joint, suboccipital muscles) TDT. PDT & 2PDT: - Migraine = HC |
Study also included patients with Cluster Headache and Tension-type Headache. | 5 H | 8 | |
|
Pan [40] Case-control study |
Migraine group EM: - n = 64 (52 females) - age: 34.2 ± 8.7 years - 7.0 ± 3.6 MHD CM: - n = 28 (24 females) - age: 37.3 ± 11.4 years - 24.3 ± 4.1 MHD Control group - n = 32 (27 females) - age: 37.8 ± 7.0 years |
MPT, HPT & CPT |
MPT: electronic von Frey filaments HPT: heat stimulus (5x) CPT: cold stimulus (5x) Location: left supraorbital (V1) & proximal medio-ventral forearm (T1) |
MPT: - EM > CM (in V1/T1) - EM > HC (in V1) - CM = HC HPT & CPT: - EM = CM = HC For EM: headache-frequency negatively correlated with MPT (not found in CM) For EM: MPT was higher in the Interictal phase compared to the preictal/ ictal/postictal phase |
Low-frequency TTH (< 1 headache day/month) allowed. No acute abortive treatment < 3 days of testing. |
2 H | 7 | |
|
De la Coba [11] Case-control study |
Migraine group EM (MO): - n = 40 (40 females) - age: 20.95 ± 3.05 years - 6.68 ± 3.74 MMD - Regular analgesics use, 24 (60%) Control group - n = 40 (40 females) - age: 21.25 ± 3.45 years |
SREP & TS |
SREP: series of 9 suprathreshold painful pressure stimuli of 5 s & 30 s interstimulus interval (increase in pain ratings across stimuli) TS: nylon monofilament (1 vs. 10th stimulus) Location SREP: third fingernail of left hand Location TS: thenar eminence of left hand |
SREP: - EM = HC - Pain intensity ratings increased progressively in EM but not in HC TS: - EM = HC |
Tested < 48 h after attack. Low-frequency tension-type headache (< 1 headache day/month) allowed. TS not indicated as ratio but as VAS with 1 vs. repetitive stimuli. |
1 U | 6 | |
|
Elizagaray-García [16] Observational study |
Migraine group CM: - n = 38 (35 females) - age: 43.47 ± 14.07 years - 19.89 ± 10.43 MHD Control group - n = 31 (29 females) - age: 48.13 ± 11.46 years |
PPT & TS |
PPT: pressure algometer (3x) TS: von Frey filaments (10x) Location PPT: trigeminal region, neck region & distal region. Location TS: temporalis muscle & over the tibialis anterior muscle. |
PPT: - CM < HC TS: - CM > HC |
Headache free at time of tests. | 2 H, 1 U | 9 | |
|
Pan [41] Case-control study |
Migraine group CM: - n = 84 (74 females) - age: 38.3 ± 11.5 years - 21.7 ± 6.0 MHD - 3.6 ± 5.3 MAMD Responders: - n = 24 (23 females) - age: 38.2 ± 12.3 years - 20.9 ± 6.3 MHD - 3.3 ± 4.7 MAMD Non-responders: - n = 60 (55 females) - age: 38.3 ± 11.3 years - 22.0 ± 5.9 MHD - 3.8 ± 5.5 MAMD Control group - n = 50 (42 females) - age: 37.4 ± 9.2 years |
MPT, HPT, CPT & PPT |
MPT: electronic von Frey filaments HPT: heat stimulus (5x) CPT: cold stimulus (5x) PPT: not mentioned Location: left supraorbital (V1) & medio-ventral forearm (T1) |
MPT: - CM = HC HPT: - CM < HC CPT: - CM > HC PPT: - CM < HC (in T1) After flunarizine treatment: - Responders = HC - Non-responders < HC for MPT (in V1), HPT, CPT & PPT - Responder > non-responders for HPT (in V1), MPT (in V1) and PPT (in V1) |
No acute medication < 48 h before tests. 52% had migraine during QST (not analyzed separately). |
flunarizine (5 or 10 mg/day) for 12 weeks. | 2 H, 1 U | 6 |
|
Ozarslan [39] Observational study, case-control study |
Migraine group CM (+/- MOH): - n = 22 (22 females) - age: 38.1 ± 7.2 years - 20.5 MHD - 20 MAMD Control group - n = 22 (22 females) - age: 36.6 ± 7.6 years |
HDT & CDT |
HDT: heat stimulus CDT: cold stimulus (in total 20 stimuli) Location: forehead & dorsum of the hand (left & right side) |
HDT & CDT: - HC = CM. |
No sedative or tranquilizing substances < 48 h before testing. | Onabotulinumtoxin A injections (155 units). | 4 H | 8 |
|
Di Antonio [15] Cross-sectional observation study |
Migraine group Low-frequency EM: - n = 105 (85 females) High-frequency-EM: - n = 74 (67 females) CM: - n = 32 (28 females) For details see reference Control group - n = 56 (40 females) - age: 37.2 ± 14.3 years |
PPT & TS |
PPT: pressure algometer TS: mechanical pinprick (10x) Location PPT; temporalis muscles, the upper cervical spine & lower cervical spine Location TS: temporalis muscle |
PPT: - LF-EM < HC in upper & lower cervical spine in preictal & postictal phase - LF-EM < HC in temporalis muscle & lower cervical spine in interictal phase - HF-EM < HC in preictal & postictal phase - HF-EM = CM < HC in ictal phase TS: - LF-EM = HC in all phases - HF-EM = CM > HC in ictal phase |
This study reported a secondary analysis for some subjects. |
No change in prophylactic treatment for < 3 months. No acute pharmacologic treatment < 24 h before testing or headache < 24 h after testing. |
1 H, 1 U | 8 |
|
Barone [5] Cross-sectional study |
Migraine group - n = 26 (26 females) - age: 24.0 ± 2.75 years (median ± interquartile range) - EM-MA: n = 10 - EM-MO: n = 8 - CM: n = 8 Control group - n = 26 (26 females) - age: 27.5 ± 13 years (median ± interquartile range) |
MDT, PPT & TS |
MDT: Semmes-Weistein monofilament (5x) PPT: pressure algometer (3x) TS: increasing pressure algometer (10x) Location MTP, PPT & TS: trigeminal nerve (V1), masseter muscle (MAS) & tibialis anterior muscle (TA) |
MDT: - Migraine > HC in V1 & MAS - Migraine = HC in TA PPT: - Migraine < HC in all regions TS: - Migraine = HC in all regions |
No headache or migraine during testing. | 2 H, 1 U | 7 |
CM = Chronic Migraine; EM = Episodic Migraine; MO = Migraine withOut aura; MA = Migraine with Aura; MHD = Monthly Headache Days; MMD = Monthly Migraine Days; MAMD = Monthly Acute Medication Days; MOH = Medication Overuse Headache; HC = Healthy Control; LF-EM = Low-Frequency Episodic Migraine; HF-EM = High-Frequency Episodic Migraine; CPT = Cold Pain Threshold; HDT/WDT = Heat/Warm Detection Threshold; HPS = Heat Pain Suprathreshold; HPT = Heat Pain Threshold; MPT = Mechanical Pain Threshold; PPT = Pressure Pain Threshold; PDT = Prick Detection Threshold; 2PDT = Two-Point Detection Threshold; QST = Quantitative Sensory Testing; SREP = Slowly Repeated Evoked Pain; TS = Temporal Summation; TDT = Tactile Detection Threshold; H = High risk of bias; U = Unclear risk of bias
Sensory detection thresholds
For the sensory detection thresholds, most studies found no differences for any stimulus (mechanical, heat, cold, tactile, prick and two prick determination) in any region (trigeminal nerve region, neck and distal region) in migraine patients compared to healthy controls [30, 39, 55]. Only one recent study reported a higher mechanical detection threshold (MDT) in the trigeminal nerve regions for female migraine patients compared to controls [5].
Pain detection thresholds
The pressure pain detection (PPT) was reported to be lower for migraine patients. Lower PPT was reported in CM compared to controls in trigeminal nerve regions and distal regions [16]. Similarly, lower PPT was reported for female migraine patients compared to controls in trigeminal nerve and distal regions [5]. Another study found a lower PPT in trigeminal nerve regions and neck in migraine patients compared to healthy controls, however, no differences were found in the distal region and on the head [30]. In contrast, a third study found lower PPT in CM compared to healthy controls in the distal region and no difference in PPT in the trigeminal nerve area [41]. Furthermore, a large study found a reduced PPT in the neck region for low-frequency EM in the preictal and postictal phase and a reduced PPT in the neck and trigeminal nerve region for low-frequency EM in the interictal phase compared to healthy controls. This study also reported a reduced PPT in the neck and trigeminal nerve region for high-frequency EM in the preictal and postictal phase compared to healthy controls. The PPT was also reduced in the neck and trigeminal nerve region for high-frequency EM and CM in the ictal phase compared to healthy controls [15].
Although the mechanical pain detection threshold (MPT) seemed higher in EM compared to controls in trigeminal nerve regions and MPT was reported to be higher in EM compared to CM in the trigeminal nerve area and distal region in a large study, this was not replicated in small studies [40]. These small studies found no difference in MPT in migraine patients compared to controls in the trigeminal nerve area or a distal region [20, 41, 55].
The heat pain threshold (HPT) was reported to be lower in CM compared to controls in the trigeminal nerve area and in the distal region [41]. However, this was not replicated in other studies where no difference was found in HPT between migraine patients and controls in the trigeminal area and distal region [20, 40, 55]. The cold pain detection threshold (CPT) was reported to be higher in CM compared to controls in the trigeminal nerve area and in the distal region [41], but another study reported no difference in CPT for migraine versus controls in these areas [40].
Pain suprathreshold
For the suprathreshold, the heat pain that gave a NRS of 60 out of 100 was reported as the heat pain suprathreshold (HPS). One study reported the HPS of EM patients outside a migraine attack, no difference was found compared with healthy controls [55].
Temporal summation (TS)
One study reported higher TS values in the distal region in individuals with high-frequency EM compared to those with low-frequency EM and healthy controls [10]. Another study found no difference in TS between EM and controls in the distal region [11]. No differences in TS in the trigeminal nerve area or neck region in any migraine phase of low-frequency EM patients were found compared to healthy controls, however higher TS was reported for high-frequency EM and CM in the ictal phase compared to healthy controls [15]. Furthermore, higher TS was seen in CM compared to healthy controls in trigeminal nerve region and distal region [16]. A small study with pinprick TS failed to replicate differences in TS between EM and controls in the trigeminal nerve area and distal region [1]. Another study in female migraine patients reported no difference in TS compared to controls in the trigeminal nerve area and distal region [5].
Slowly repeated evoked pain (SREP)
For SREP, the changes in pain perception were assessed in response to repeated nociceptive stimuli as a measurement for central sensitization. No overall difference between EM and healthy controls was found for SREP. Pain intensity ratings increased progressively in EM but not in healthy controls [11].
Conditioned pain modulation (CPM)
Thirteen studies were identified that described original CPM data of migraine patients, shown in Table 2. The test stimulus (ts) consisted of heat, pressure or electrical stimuli and the conditioned stimulus (cs) consisted of cold, heat, capsaicin and ischemic stimuli. One study included an attention task as conditioned stimulus [14]. No consistent differences were observed between different locations of test and conditioned stimulus, between contralateral and ipsilateral CPM protocols and the different stimuli. An inhibitory CPM response refers to increased pain inhibition, where the body reduces pain perception in response to a conditioning stimulus. In contrast, a facilitatory CPM response involves decreased pain inhibition, meaning the conditioning stimulus leads to heightened or maintained pain sensitivity instead of reducing it.
Table 2.
Information on the studies on conditioned Pain Modulation (CPM) in patients with migraine and controls
| Author Type of study |
Groups: total n, number of females, mean age (SD). Migraine specifications: EM/CM/MOH and mean MHD/MMD/MAMD/mean attacks per month |
CPM Test paradigm | Results | Comments | Treatment | Risk of bias | NOS score |
|---|---|---|---|---|---|---|---|
|
De Tommaso [12] Case-control study |
Migraine group Migraine (MO) - n = 8 (6 females) - age: 32.3 ± 4.4 years - 5.3 ± 3.1 MHD - No MOH (> 2 months) Control group - n = 10 (7 females) - age: 34.1 ± 3.5 years |
ts Heat pain to the right supraorbital zone. Laser-evoked potentials and pain ratings were assessed. cs Capsaicin to the right supraorbital zone. |
Migraine: reduction during capsaicin. Controls: reduction during capsaicin and slightly reduced after capsaicin compared to before application. Overall: CPM response was reduced in migraine patient compared to control. |
Migraine; measured at least 72 h before and after attack. ts and cs on same location. |
No study treatment and no prophylactic treatment. | 3 H | 5 |
|
Sandrini [49] Case-control study |
Migraine group Migraine (MO) - n = 24 (12 females) - age: 36 ± 12 years - 3.2 ± 1.1 attacks/ month Control group - n = 20 (14 females) - age: 32 ± 7 years |
ts Electrical stimulus to the sural nerve. Nociceptive flexion reflex (RIII area) and pain ratings were assessed. cs CPT of contralateral hand immersion in water (5–6 °C). |
Migraine: facilitatory CPM response for NFR area and pain rating. Controls: inhibitory CPM response for NFR area and pain ratings. |
Migraine; measured at least 48 h after an attack and 24 h before an attack. Females were patients and controls were matched for menstrual period. Study also included chronic TTH. |
No prophylactic treatment < 2 months and drug-free > 24 h before testing. | 3 H, 1 U | 7 |
|
De Tommaso [13] Case-control study |
Migraine group EM (MO): - n = 11 (8 females) - age: 40.5 ± 7.4 years - 5.25 ± 4.2 MHD CM: - n = 9 (5 females) - age: 47.2 ± 9.6 years - 22.8 ± 1.5 MHD - no MOH (at least 2 months) Control group - n = 14 (10 females) - age: 44.1 ± 3.5 years |
ts Electrical stimuli to the right supraorbital zone and the blink reflex parameters were assessed. cs Capsaicin to the right hand dorsum. |
CM: facilitatory CPM response. EM: no CPM response. Controls: inhibitory CPM response. |
BR recording 72 h pain-free state. For CM; 58.3 ± 2.8 h. No headache 24 h after testing. |
No prophylactic treatment. | 2 H | 6 |
|
De Tommaso [14] Case-control study |
Migraine group EM (MO): - n = 8 (6 females) - age: 35.3 ± 6.5 years - 3.2 ± 1.2 MHD Control group - n = 8 (6 females) - age: 32.5 ± 7.8 years |
ts Heat pain to the right supraorbital zone. Laser-evoked potentials and pain ratings were assessed. cs Attention spatial discrimination tasks. |
Migraine: no CPM response. Controls: inhibitory CPM response. |
Pain and medication free state 72 h before testing and 48 h after. CPM measured with non-painful cs. |
No prophylactic treatment for < 2 months. | 3 H, 1 U | 5 |
|
Perrotta [45] Case-control study |
Migraine group EM (MO): - n = 28 (16 females) - age: 38.6 ± 9.6 years - 4.1 ± 1.9 MHD - No MOH CM with MOH (MO): - n = 31 (18 females) - age: 43.2 ± 13.2 years - 21.6 ± 6.6 MHD - 24.3 ± 4.7 days/months medication intake Control group - n = 23 (13 females) - age: 40.4 ± 4.6 years |
ts Electrical stimuli to the sural nerve to the right lateral malleolus. cs CPT of hand immersion in water (2–4 °C). |
CM + MOH before withdrawal: no CPM response. Successful withdrawal: normalization of CPM response (inhibitory) that was similar to the EM and controls. EM: inhibitory CPM response. Controls: inhibitory CPM response. |
Only CM/MOH that reverted to EM after withdrawal. Female patients and controls were aligned for menstrual period (but not for LH surge). CM + MOH measured before and 8–10 days after start of withdrawal. EM measured 3 days before and after attack. |
CM/MOH group; excluded MOH patients after withdrawal that did not improve, only successful withdrawal group was analyzed. Not a repeated measurements analysis. |
1 H, 1 U | 7 |
|
Nahman-Averbuch [33] Case-control study |
Migraine group Migraine: - n = 26 (26 females) - age: 35.3 ± 11.6 years Control group - n = 35 (35 females) - age: 29.3 ± 9.3 years |
ts Noxious heat stimuli to the lower left leg (47.5 °C for 30 s). cs Immersion of right foot (10–12 °C). |
Overall: CPM response significantly different in migraine patients, not in healthy controls, waning of CPM when repeated. |
Test > 24 h after attack. Possible comorbidities only exclusion criteria for healthy controls (not specified for migraine patients). |
No preventative or analgesic medication < 24 h before testing. | 4 H, 1 U | 6 |
|
Teepker [57] Case-control study |
Migraine group EM (MA n = 24) - n = 32 (32 females) - age: 28.9 ± 8.8 years - 2.28 ± 0.99 attacks/month Control group - n = 20 (20 females) - age: 27.1 ± 6.6 years |
ts Electrical threshold on right forearm. cs Painful stimulus; 1 °C above HPT. Non painful stimulus; 0.3 °C below HPT to left forearm. |
Migraine: inhibitory CPM response. Controls: inhibitory CPM response. Overall: no difference between migraine and controls. |
Examined on days 1, 4, 14 and 22 of menstrual cycle (alignment menstruation period, not LH surge). CPM did not vary over the menstrual cycle in migraine or controls. Examined during & between attacks (analyzed as one). |
No prophylactic treatment. Some participants used the oral contraceptive pill. Analgesics permitted if a migraine attack began shortly before or during the tests. |
3 H | 5 |
|
Granovsky [18] Cross-sectional study |
Migraine group MA - n = 30 (23 females) - age: 39.8 ± 12.4 years - 8.5 ± 3.2 mean attacks/month MO - n = 23 (23 females) - age: 40.9 ± 11.9 years - 6.5 ± 3.1 attacks/month No Control group |
ts Heat stimulus of 6 NRS. cs Immersion of non-dominant hand in water (46.5 °C) for 1 min. |
Overall: No significant difference between migraine with and without aura. |
Possible selection bias (snowball sampling). Tests > 48 h after attack and > 12 h analgesics free. Aura selection based on 1 question. MA had higher attack frequency than MO. |
No prophylactic treatment < 3 months. | 4 H | 4 |
|
Guy [20] Case-control study |
Migraine group EM (MO): - n = 12 (12 females) - age: 33.83 ± 3.11 years - 3 MHD CM with MOH: - n = 12 (12 females) - age: 35.25 ± 3.13 years - 30 MHD Control group - n = 12 (12 females) - age: 37.25 ± 3.01 years |
ts Heat (laser evoked) temporal summation to the back of the non-dominant hand. cs CPT of contralateral foot immersion in water (8 °C). |
MOH before withdrawal: inhibitory CPM response during and after cs (higher CPM-effect compared to EM and similar to control). MOH after withdrawal: no CPM response during cs & facilitation CPM response after cs. EM: inhibitory CPM response during cs and slightly inhibited after cs (lower CPM-effect compared to controls/MOH before withdrawal). Controls: inhibitory CPM response during and after cs. CM + MOH = HC > EM > 3 wks after withdrawal. |
Only females included. Measurements during the menstrual cycle, follicular phase. Testing > 24 h before and after attack. MOH measured before and 3 weeks after withdrawal. |
EM; no prophylactic treatment < 2 months. MOH withdrawal with 5 days hospitalization, afterwards triptans allowed. |
2 H, 1 U | 6 |
|
Kisler [26] Case-control study |
Migraine group Migraine: - n = 39 (32 females) - age: 29.48 ± 6.96 years - 5.1 ± 2.1 MHD - 6.37 ± 2.42 MMD - 4–15 attacks/month Control group - n = 35 (30 females) - age: 27.07 ± 4.64 years |
ts Heat (47 °C for 30s) stimulus to the left volar forearm. cs Immersion of right foot in water (9–12 °C) for 76 s. |
Migraine: no CPM response. Controls: inhibitory CPM response. Overall: no significant difference between migraine and control. |
No alcohol and analgesics < 24 h before testing and no caffeine < 3 h before. Testing > 24 h after last attack. A later study reported on partially the same results [1]. |
No prophylactic treatment < 3 months. | 2 H | 4 |
|
Williams [60] Case-control study |
Migraine group Migraine - n = 23 (17 females) - age: 24.32 ± 7.94 years Control group - n = 32 (23 females) - age: 21.75 ± 8.71 - n = 27 occasional TTH |
ts Electrical stimulus to the left supraorbital nerve. Pain rating and nerve blink reflex (nBR) were assessed. cs Non-dominant forearm ischemia. |
Migraine: inhibitory CPM response for pain ratings and no CPM response for nBR. Controls: inhibitory CPM response for pain ratings and nBR. |
Pain threshold (≥ 50 on a 0-100 NRS scale). Stimulus of 150% of the pain threshold was used. |
6 H | 4 | |
|
Kisler [27] Randomized, double-blinded, controlled trial* |
Migraine group Migraine patients: - n = 55 (46 females) - 27 duloxetine, 28 placebo - age: 31.2 ± 7.8 and 32.1 ± 8.0 - 5.3 ± 2.2 and 4.7 ± 2.0 mean attacks/month - 6.9 ± 2.2 and 6.1 ± 2.0 MMD No Control group |
ts Heat 30-Sect. 47 °C to nondominant volar forearm. cs CPT of contralateral foot immersion in water (9–12 °C for 78 s). |
Migraine: inhibitory CPM response. After treatment: more efficient CPM in the duloxetine group compared to placebo. |
No pain-relieving drugs or alcohol < 24 h of testing and no caffeine < 3 h prior. Testing > 24 h after attack. |
Treatment; duloxetine or placebo. No prophylactic treatment < 3 months. |
3 H | 7 |
|
Barone [5] Cross-sectional study |
Migraine group - n = 26 (26 females) - age: 24.0 ± 2.75 years (median ± interquartile range) - EM-MA: n = 10 - EM-MO: n = 8 - CM: n = 8 Control group - n = 26 (26 females) age: 27.5 ± 13 years (median ± interquartile range) |
ts Painful stimulus with pressure algometer in trigeminal nerve (V1), masseter muscle (MAS) & tibialis anterior muscle (TA) (before, 30 s and 90 s after cs). cs Occlusion cuff on opposite arm (5 NRS). |
Migraine: inhibitory CPM response. Controls: inhibitory CPM response. Overall: CPM response was less efficient in migraine patients compared to control in the V1 region after 30s. The other regions and timepoint showed no difference. |
No headache or migraine during testing. | 2 H, 1 U | 7 |
CM = Chronic Migraine; EM = Episodic Migraine; MO = Migraine withOut aura; MA = Migraine with Aura; MHD = Monthly Headache Days; MMD = Monthly Migraine Days; MAMD = Monthly Acute Medication Days; MOH = Medication Overuse Headache; TTH = Tension-Type Headache; ts = test stimulus; cs = conditioned stimulus; NRS = Numerical Rating Scale; nBR = nerve blink reflex; CPM = Conditioned Pain Modulation; H = High risk of bias; U = Unclear risk of bias
* From this randomized-controlled trail, only baseline measurements were included and thus the NOS-score was assessed
The majority of studies with adequate numbers of participants suggested a (mild) inhibitory (and some absence of response) in migraine patients, with no difference between migraine subtypes (MA and MO) [18], and no difference compared to the inhibitory response found in controls [26, 33, 57]. However, when CPM was executed multiple times, migraine patients showed a waning of CPM response, which was not found in controls [33]. One study in female migraine patient reported a less efficient CPM response compared to control in the V1 region 30 s after cs application, however, no differences were found after 90 s or in the V3 area or distal region [5].
In contrast, a facilitatory CPM response was suggested in high-frequency EM patients (n = 24) versus an inhibitory CPM response in controls (n = 20) [49], which was also suggested in a small study for CM (n = 9) versus absence of response for EM (n = 11) and an inhibitory CPM for healthy controls (n = 14) [13]. This group also conducted the CPM paradigm with an attention task as a conditioned stimulus, and found an absence of CPM response in migraine patients [14]. They also performed the CPM test with the test and conditioned stimulus on the same location in the trigeminal nerve region which resulted in an inhibitory CPM response for migraine patients [12]. A study that investigated the CPM response in CM patients with MOH (n = 31) that underwent two months of withdrawal of acute pain medication and found these patients showed an absent of CPM response before withdrawal. Shortly (8–10 days) after the withdrawal, an inhibitory response was seen, similar to the EM patients (n = 28) and healthy controls (n = 23). Notably, this study excluded all CM patients that did not improve to episodic migraine, so no responder versus non-responder analysis was performed [45]. In contrast to the above, another short “medication overuse withdrawal” study investigated CM with MOH patients and suggested an inhibitory response in CM with MOH before and an absence of CPM response after withdrawal. However, the design of this study applied a protocol that consisted of a short 5 day hospitalization (ice packs and hydroxyzine 25 mg/day as rescue treatment) and after discharge the patients could start using triptans again [20]. This protocol does not align with the recommended MOH withdrawal treatment [4]. The CPM response was also measured at a short term with three weeks after hospitalization [20].
We want to separately mention a CPM study on the blink reflex: patients with a migraine history (n = 23) showed an inhibitory CPM response for a painful test stimulus, however for the blink reflex test stimulus migraine patients showed an absence of CPM response. Healthy controls (n = 32) showed an inhibitory CPM response for both [60].
A randomized, double-blinded, placebo-controlled trial on duloxetine found an inhibitory CPM response for migraine patients (n = 55) at baseline, but no control group was included [27].
Corneal confocal microscopy (CCM)
Only five studies, most of them with small sample sizes, could be included and described original CCM data of migraine patients, as shown in Table 3. All studies reported the output measurements corneal nerve fiber density (CNFD), corneal nerve fiber length (CNFL), and corneal nerve branch density (CNBD). Two small studies reported the nerve tortuosity in CM [24] and EM patients [52]. Three studies reported the total branch density (CTBD), total nerve fiber area (CNFA) and corneal nerve fiber width (CNFW), where one reported on CM and EM compared to controls [19], one reported EM patients compared to controls [42] and another reported CM with and without ictal photophobia [53]. This latter study found no differences between CM without photophobia compared to controls [53]. Some differences were found for those with photophobia but it was not specified to which group these results were compared. Therefore, the results for migraine with photophobia were not considered [53].
Table 3.
Information on the studies on Corneal Confocal Microscopy (CCM) in patients with migraine and controls
| Author type of study |
Groups: total n, number of females, mean age (SD). Migraine specifications: EM/CM/MOH and mean MHD/MMD/MAMD/mean attacks per month |
CCM output | Results | Comments | Risk of bias | NOS score |
|---|---|---|---|---|---|---|
|
Kinard [24] case-control study |
Migraine group CM: - n = 19 (14 females) - age: 38.6 years Control group - n = 30 (18 females) - age: 44.7 years |
1. CNFD 2. CNFL 3. CNBD 4. tortuosity coefficient |
• CNFD are lower in migraineurs compared to controls. • Significant for CNFD. • Not significant for CNFL, CNBD & tortuosity. |
Non-categorized (numerical stats), small n. | 5 H | 4 |
|
Shetty [53] case-control study |
Migraine group CM/MO with photophobia (MP): - n = 36 (20 females) - age: 32.4 ± 4.8 years CM/MO without photophobia (MNP): - n = 24 (14 females) - age: 31.6 ± 3.5 year Control group - n = 24 (14 females) - age: 33.7 ± 5.5 year |
1. CNFD 2. CNFL 3. CNBD 4. CTBD 5. CNFA 6. CNFW |
• No difference between migraine without photophobia and controls. • Migraine photophobia is compared to controls or migraine without photophobia (not specified which). |
Anova between 3 groups. | 5 H, 1 U | 3 |
|
Shen [52] cross-sectional observation study |
Migraine group EM: - n = 10 (9 females) - age: 38.9 ± 6.31 years - 4.11 ± 2.57 attacks/month - 10% MA, 80% photophobia Control group - n = 10 (8 females) - age: 37.3 ± 5.54 years |
1. CNFD 2. CNFL 3. CNBD 4. tortuosity (0–4) |
• CCM measurements are higher in migraineurs compared to controls. • Significant for CNFL, CNBD & tortuosity. • Not significant for CNFD. |
Small n. | 4 H, 1 U | 5 |
|
Guldiken [19] cross-sectional observation study |
Migraine group 25 EM/MA, 7 EM/MO, 28 CM; total: - n = 60 (49 females) - age: 34.67 ± 1.12 years - 56 (93.3%) photophobia Control group - n = 20 (9 females) - age: 35.10 ± 2.21 years |
Automated analyses 1. CNFD 2. CNFL 3. CNBD 4. CTBD 5. CNFA 6. CNFW |
• CCM measurements are higher in migraine patients (EM/MA, EM/MO and CM) compared to controls. • Significant for CNFD, CNFL, CNBD, CTBD and CNFA. • Not significant for CNFW. |
More woman in migraine group compared to control group. | 5 H, 1 U | 5 |
|
Patzkó [42] case-control study |
Migraine group EM: - n = 44 (37 females) - age: 33.23 ± 11.41 years - 4.37 ± 0.86 attacks/month Control group - n = 25 (19 females) age: 30.16 ± 12.59 years |
1. CNFD 2. CNFL 3. CNBD 4. CTBD 5. CNFA 6. CNFW 7. fractal dimension 8. corneal dendritic cell density 9. corneal dendritic cell area |
• No differences between EM an controls for CCM measurements. • EM showed lower corneal dendritic cell density and corneal dendritic cell area compared to controls. |
Recruitment and requirements for healthy controls is not mentioned. | 2 H, 2 U | 3 |
CM = Chronic Migraine; EM = Episodic Migraine; MO = Migraine withOut aura; MA = Migraine with Aura; MHD = Monthly Headache Days; MMD = Monthly Migraine Days; MAMD = Monthly Acute Medication Days; MOH = Medication Overuse Headache; CNFD = Corneal Nerve Fiber Density; CNFL = Corneal Nerve Fiber Length; CNBD = Corneal Nerve Branch Density; CTBD = Corneal Total Branch Density; CNFA = Corneal Fiber Area; CNFW = Corneal Nerve Fiber Width; H = High risk of bias; U = Unclear risk of bias
Contrasting results were found for the CNFD in migraine compared to controls. A study in CM and a large study in EM (MA and MO) and CM both reported lower CNFD in migraine patients compared to controls [19, 24]. No differences were found for EM or CM without photophobia compared to controls [42, 52, 53]. The CNFL was found to be shorter in EM (MA and MO) and CM patients compared to controls in one study [19]. In contrast, a small study suggested CNFL to be larger in EM compared to controls [52], and other studies did not find a significant difference [24, 42, 53]. Similarly, conflicting results were reported for the CNBD, with lower density in the EM (MA and MO) and CM study [19], higher density in a small EM study [52] and the other studies reported no difference [24, 42, 53].
The tortuosity was reported to be increased in EM patients compared to controls, but no significant difference was found for CM patients [24, 52]. The CTBD and CNFA was reported to be lower in EM (MO and MA) and CM patients compared to controls by one study [19]. However, this was not replicated in CM without photophobia or EM by other studies [42, 53]. The total nerve width (CTFW) was not found to be different from controls in any study [19, 42, 53]. For EM patients the fractal dimension was not found to be different between EM and controls [42]. In one study lower CNFD and CNFA was found in EM patients compared to controls and it was suggested that a presence of neuroinflammation in the cornea of migraine patients might affect the peripheral trigeminal system [42].
NOS quality
The mean NOS quality score of the included studies was 5.82 ± 1.70 (mean ± SD). The QST papers had a mean score of 6.92 ± 1.56, the CPM papers scored a 5.62 ± 1.19 on average and the CCM papers had an NOS score of 4.00 ± 1.00.
Risk of bias assessment
The risk of bias assessment is shown in Fig. 2. Eleven studies (39.3%) included a small sample size. The reporting of acute medication days/month (MAMD) was missing or unclear for 25 studies (89.3%). This value is necessary for the diagnosis of MOH. The specification whether the study population included patients with MOH was missing or unclear in 21 studies (75.0%). The reporting of headache or migraine days/month (MHD/MMD) was missing or unclear (because it was defined as attacks per month) for 13 studies (46.4%). Eleven studies (39.3%) did not report when the measurements were performed in relation to the migraine phase. Four studies (14.3%) did not specify the handling or inclusion of possible comorbidities. Moreover, the studies containing CCM data had very limited description of the migraine population and the control group and, therefore, have a higher risk of bias. The studies report all results including when no differences were found. We assessed the certainty of the body of evidence for these studies moderate, varying from weak to good between studies. The risk of bias was taken into account in the interpretation of the reported results.
Fig. 2.
Risk of Bias assessment from included studies. A) Traffic light plot per study with 1 = small sample size, 2 = self-reported measure of pain, 3 = self-diagnosed migraine, 4 = sample included possible medication overuse headache (MOH), 5 = no medication days/month reported, 6 = no headache or migraine days/month reported, 7 = incomplete patient characteristics, 8 = possible comorbidities not specified, 9 = migraine phase not specified and 10 = other bias. (B) Summary plot of risk of bias per subcategory. The Robvis tool was used to visualize the risk of bias assessment [31].
Discussion
Although pain profiling tests have been widely used by investigators in patients with migraine for many years, there is a wide methodological variety in these tests leading to conflicting results and difficulty in understanding underlying mechanisms. Understanding the current state of pain profiling research in migraine is essential to identify informative tests to distinguish between migraine patients and healthy individuals. This review highlights findings and knowledge gaps in our understanding of pain processing in migraine.
Quantitative sensory testing (QST)
The quantitative sensory testing (QST) studies indicate that migraine patients exhibit lower pressure pain threshold (PPT) in the trigeminal region or in distal areas. The mechanical pain detection (MPT) and heat and cold pain threshold (HPT and CPT) showed conflicting results. The previous systematic review and metanalysis conducted in 2018 reported similar results regarding the PPT. Importantly, the meta-analysis reported a lowered HPT and higher pain ratings to electrical and cold suprathreshold stimulations for migraine patients and no differences for other QST paradigms such as electrical detection thresholds, cold and mechanical [34].
The temporal summation (TS) test has been suggested to be useful to assess central sensitization in chronic pain syndromes, where an increased TS response has been observed as a result of an increased ascending pain facilitation pathway. However, migraine studies reported discordant findings on TS differences between migraine patients and controls, with some indicating higher TS in HF-EM and CM patients compared to controls, while others showed no significant differences. In addition, the SREP, another measure for central sensitization, has only been conducted by the same research group and has yet to be replicated elsewhere. Therefore, further studies are needed to validate these findings and draw definitive conclusions.
It has been suggested to use cut-off values for the application of QST for a definition of heightened and lowered sensitivity. These cut-off values can be based on previous literature and receptor characteristics [3]. This methodology may be helpful in the standardization of QST measurements keeping in mind that alterations in nociceptive processing of patients with migraine seem to be modality, measure, and location specific, and may differ between EM and CM patients.
Conditioned pain modulation (CPM)
The majority of studies with adequate numbers of participants suggested a (mild) inhibitory or absence of response in (episodic) migraine patients, with no difference between those with and without aura, and no difference with the response compared to controls. However, migraine patients showed a waning of CPM response, which was not found in controls. In contrast, a facilitatory CPM response was suggested in HF-EM patients and those with CM. One study found an absence of CPM response in CM patients with MOH that reverted to an inhibitory response after short withdrawal time, but unfortunately no comparison between responders and non-responders after withdrawal therapy was made.
CPM is well-suited for assessing inhibitory pain pathways because it focuses on detecting the absence of inhibitory responses, which indicates dysfunction in pain inhibitory modulation [23]. For migraine, it seems that this dysfunction of the pain system is correlated to the frequency of migraine attacks. However, when it comes to CM, the available data are limited [45]. Furthermore, most studies performed the CPM tests with both test and conditioned stimulus in a distal region. Only three studies performed the conditioned stimulus in a distal area and the test stimulus in the trigeminal region, which is a special region of interests for migraine pathophysiology. Two of these studies had either low number of participants or high risk of bias [13, 60]. The other study reported conflicting results for the V1 and V3 area [5]. We recommend further research to determine CPM in the trigeminal region and whether CPM functions differently for CM (+/- MOH) compared to EM, and to investigate the transition from CM to EM.
Corneal confocal microscopy (CCM)
The CCM studies showed conflicting results probably due to small sample size and limited phenotyping of migraine characteristics. Therefore, more studies are needed to be able to confirm any conclusions with larger sample sizes and clear migraine characteristics (e.g. monthly migraine/headache/medication days). One study aimed to asses ictal photophobia based on the first 7 questions of an 8-part photophobia questionnaire [8, 53]. One might speculate that the Leiden Visual Sensitivity Scale (L-VISS), a validated 9-item questionnaire can provide more insight into visual hypersensitivity as the L-VISS scores differ not only between migraine patients versus controls, but also between MO versus MA patients, CM versus EM and ictal versus interictal period [44]. Furthermore, the L-VISS questionnaire employs a linear scale, enabling straightforward comparisons across various groups, in contrast to other instruments that utilize binary or qualitative scales. Therefore, future research in CCM in relation to hypersensitivity might utilize the L-VISS questionnaire within the migraine population.
Treatment effect
Psychophysical tests can be employed to evaluate treatment effects in migraine. By recording baseline and post-treatment measurements, the treatment’s impact on the pain system can be assessed. Furthermore, these tests might be useful in predicting treatment response; however, large study populations and replication of results are needed before this can be applied in clinical settings.
Studies have predominantly utilized QST tests to study treatment effects. Multiple studies reported no changes in QST measurement after treatments [17, 27, 28]. A large study in CM patients receiving flunarizine showed that responders were comparable to healthy controls whereas non-responders reported significant pain hypersensitivity [41]. A study in HF-EM/CM patients treated with galcanezumab reported a more allodynic HPT, CPT and MPT in non-responders [3]. Another galcanezumab study including EM and CM patients reported only HPT in distal regions to be associated with a clinical response [43]. An erenumab treatment study in CM showed increased TS threshold for the responders versus non-responders; however, there was no difference in VAS scores for stimulus 1 versus stimulus 5 between responders and non-responders suggesting no difference in central sensitization [9]. Pre-treatment QST measurements could not predict treatment outcome [9]. Since conflicting evidence exist, further research is necessary to ascertain the predictive value of QST measurements to identify treatment responders.
Additionally, the CPM paradigm and CCM measurements may possibly be useful tools to assess and predict treatment response. Two studies reported an improvement of CPM response after treatment [27, 35]. For CCM, no studies were conducted that included treatment intervention. Previous studies in neuropathy showed that corneal nerve densities can change in response to treatment [56, 59]. Currently, no clear CCM profile for migraine patients has been established, however, it might possible be a useful tool to evaluate or even predict treatment response. More research is needed to determine the potential role of CPM and explore the role of CCM in the evaluation of treatment effect in migraine.
Migraine phases
Many psychophysical studies have conducted QST or CPM assessments during the interictal phase, with several failing to specify the assessment’s timing relative to the headache phase, while only a few considered the migraine phase and aimed to explore the interaction of (inter)ictal phase and psychophysical outcome parameters.
A large study comprehensively examined QST measurements across migraine phases, finding lower PPT values for HF-EM compared to controls in preictal and postictal phases, while HF-EM and CM exhibited similar values to controls in the ictal phase; LF-EM showed inconclusive results for PPT assessment, and TS values were similar between LF-EM and controls across phases, but higher for HF-EM and CM compared to controls in the ictal phase [15]. In another study involving EM and CM patients, QST measurements analyzed across migraine phases revealed a negative correlation between headache frequency and MPT for EM patients, with no such correlation observed for CM patients, additionally reporting higher MPT values in the interictal phase compared to the preictal, ictal, and postictal phases for EM patients [40]. Moreover, a study administering nitroglycerin (as migraine-like attack provocation) to EM patients revealed increased TS for LF-EM and no differences for HF-EM and controls post-administration [10]. In contrast, other studies employing phase-matched categories found no influence on CPM response [60] or QST measurement [43] or showed an increased mechanical sensitivity in all phases compared to the interictal phase [50]. Overall, inconsistent results are reported regarding alterations in QST or CPM measurements in relation to the migraine phases.
A strength of our systematic review is the thorough literature search through multiple databases, ensuring a comprehensive inclusion of all relevant papers. We were mindful of potential publication bias and therefore took measures to minimize its impact by applying well-defined criteria for study inclusion and conducting a thorough evaluation of the methodological quality of the included studies, contributing to the quality of our review. A limitation of our review is that we included only papers on human studies. Secondly, it is important to acknowledge the limitations of the current evidence based method, as some studies did not primarily focus comparison of migraine patients versus controls but focused for instance on treatment effect. We tried to include those studies in our discussion section.
Conclusion
This systematic review aimed to summarize current findings on pain profiles in migraine, with a specific focus on QST, CPM, and CCM tests, encapsulating existing knowledge about sensory processing and pain modulation in migraine patients. In summary, pain processing measurements in migraine sufferers varies based on factors like sensory modality and measurement approach, and migraine features.
Take home messages
The Quantitative sensory testing (QST) studies indicate that migraine patients exhibit lower pressure pain threshold (PPT) in the trigeminal region or in distal areas.
Conditioned pain modulation (CPM) studies in migraine patients suggest a normal inhibitory response, but with a decline in CPM response compared to controls. High-frequency and chronic migraine patients may exhibit a facilitatory CPM response.
Corneal confocal microscopy (CCM) studies in migraine show conflicting results, likely due to small sample sizes and limited phenotyping of migraine characteristics.
We recommend further research to determine pain profiling for chronic migraine (with/without medication overuse headache) compared to episodic migraine, and to investigate the transition from chronic to episodic migraine as this will provide more insight in the pain pathophysiology underlying migraine.
Acknowledgements
No acknowledgements.
Abbreviations
- CM
Chronic Migraine
- CCM
Corneal Confocal Microscopy
- CGRP
Calcitonin Gene-Related Peptide
- CNFD
Corneal Nerve Fiber Density
- CNFL
Corneal Nerve Fiber Length
- CNBD
Corneal Nerve Branch Density
- CTBD
Corneal Total Branch Density
- CNFA
Corneal Fiber Area
- CNFW
Corneal Nerve Fiber Width
- CPM
Conditioned Pain Modulation
- CPT
Cold Pain Threshold
- cs
conditioned stimulus
- EM
Episodic Migraine
- HDT/WDT
Heat/Warm Detection Threshold
- HF-EM
High-Frequency Episodic Migraine
- HPS
Heat Pain Suprathreshold
- HPT
Heat Pain Threshold
- ICHD-3
International Classification of Headache Disorders 3rd edition
- LF-EM
Low-Frequency Episodic Migraine
- L-VISS
Leiden Visual Sensitivity Scale
- MA
Migraine with Aura
- MO
Migraine withOut aura
- MAMD
Monthly Acute Medication Days
- MDT
Mechanical Detection Threshold
- MHD
Monthly Headache Days
- MMD
Monthly Migraine Days
- MOH
Medication Overuse Headache
- MPT
Mechanical Pain Threshold
- nBR
Nerve blink reflex
- NRS
Numerical Rating Scale
- PPT
Pressure Pain Threshold
- PDT
Prick Detection Threshold
- 2PDT
Two-Point Detection Threshold
- QST
Quantitative Sensory Testing
- SREP
Slowly Repeated Evoked Pain
- ts
Test stimulus
- TS
Temporal Summation
- TDT
Tactile Detection Threshold
- TTH
Tension-Type Headache
Author contributions
FvW substantial contributed to the conception, design of the work, acquisition, analysis and interpretation of data and drafted the manuscript. GMT substantial contributed to the conception, design of the work, acquisition and interpretation of data and revised the manuscript. MvV substantial contributed to the conception, design of the work, acquisition and interpretation of data and revised the manuscript. AD substantively revised the manuscript. All authors reviewed and approved the manuscript.
Funding
No funding.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Argaman Y, Kisler LB, Granovsky Y, Coghill RC, Sprecher E, Manor D, Weissman-Fogel I (2020) The endogenous analgesia signature in the resting brain of healthy adults and migraineurs. J Pain 21(7–8):905–918 [DOI] [PubMed] [Google Scholar]
- 2.Ashina M, Terwindt GM, Al-Karagholi MA, de Boer I, Lee MJ, Hay DL, Schulte LH, Hadjikhani N, Sinclair AJ, Ashina H, Schwedt TJ, Goadsby PJ (2021) Migraine: disease characterisation, biomarkers, and precision medicine. Lancet 397(10283):1496–1504 [DOI] [PubMed] [Google Scholar]
- 3.Ashina S, Melo-Carrillo A, Szabo E, Borsook D, Burstein R (2023) Pre-treatment non-ictal cephalic allodynia identifies responders to prophylactic treatment of chronic and episodic migraine patients with galcanezumab: a prospective quantitative sensory testing study (NCT04271202). Cephalalgia 43(3):3331024221147881 [DOI] [PubMed] [Google Scholar]
- 4.Ashina S, Terwindt GM, Steiner TJ, Lee MJ, Porreca F, Tassorelli C, Schwedt TJ, Jensen RH, Diener HC, Lipton RB (2023) Medication overuse headache. Nat Rev Dis Primers 9(1):5 [DOI] [PubMed] [Google Scholar]
- 5.Barone M, Imaz F, De la Torre Canales G, Venosta M, Dri J, Intelangelo L (2024) Somatosensory and psychosocial profile of migraine patients: a cross-sectional study. Musculoskelet Sci Pract 70:102924 [DOI] [PubMed] [Google Scholar]
- 6.Brines M, Culver DA, Ferdousi M, Tannemaat MR, van Velzen M, Dahan A, Malik RA (2018) Corneal nerve fiber size adds utility to the diagnosis and assessment of therapeutic response in patients with small fiber neuropathy. Sci Rep 8(1):4734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burstein R, Jakubowski M, Garcia-Nicas E, Kainz V, Bajwa Z, Hargreaves R, Becerra L, Borsook D (2010) Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol 68(1):81–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi JY, Oh K, Kim BJ, Chung CS, Koh SB, Park KW (2009) Usefulness of a photophobia questionnaire in patients with migraine. Cephalalgia 29(9):953–959 [DOI] [PubMed] [Google Scholar]
- 9.De Icco R, Fiamingo G, Greco R, Bottiroli S, Demartini C, Zanaboni AM, Allena M, Guaschino E, Martinelli D, Putortì A, Grillo V, Sances G, Tassorelli C (2020) Neurophysiological and biomolecular effects of erenumab in chronic migraine: an open label study. Cephalalgia 40(12):1336–1345 [DOI] [PubMed] [Google Scholar]
- 10.De Icco R, Perrotta A, Grillo V, Cosentino G, Sances G, Sandrini G, Tassorelli C (2020) Experimentally induced spinal nociceptive sensitization increases with migraine frequency: a single-blind controlled study. Pain 161(2):429–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de la Coba P, Bruehl S, Del Paso GAR (2021) Slowly repeated evoked pain (SREP) as a central sensitization marker in episodic migraine patients. Sci Rep 11(1):4582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Tommaso M, Losito L, Difruscolo O, Sardaro M, Libro G, Guido M, Lamberti P, Livrea P (2005) Capsaicin failed in suppressing cortical processing of CO2 laser pain in migraine patients. Neurosci Lett 384(1–2):150–155 [DOI] [PubMed] [Google Scholar]
- 13.de Tommaso M, Sardaro M, Pecoraro C, Di Fruscolo O, Serpino C, Lamberti P, Livrea P (2007) Effects of the remote C fibres stimulation induced by capsaicin on the blink reflex in chronic migraine. Cephalalgia 27(8):881–890 [DOI] [PubMed] [Google Scholar]
- 14.de Tommaso M, Baumgartner U, Sardaro M, Difruscolo O, Serpino C, Treede RD (2008) Effects of distraction versus spatial discrimination on laser-evoked potentials in migraine. Headache 48(3):408–416 [DOI] [PubMed] [Google Scholar]
- 15.Di Antonio S, Arendt-Nielsen L, Ponzano M, Bovis F, Torelli P, Finocchi C, Castaldo M Trigeminocervical pain sensitivity during the migraine cycle depends on headache frequency. Neurol Sci 2023:1–12 [DOI] [PMC free article] [PubMed]
- 16.Elizagaray-Garcia I, Carvalho GF, Szikszay TM, Adamczyk WM, Navarro-Fernandez G, Alvarez-Testillano P, Diaz-de-Teran J, Luedtke K, Gil-Martinez A (2022) Psychophysical testing in chronic migraine and chronic tension type headache: an observational study. Cephalalgia 42(7):618–630 [DOI] [PubMed] [Google Scholar]
- 17.Gazerani P, Fuglsang R, Pedersen JG, Sørensen J, Kjeldsen JL, Yassin H, Nedergaard BS (2019) A randomized, double-blinded, placebo-controlled, parallel trial of vitamin D(3) supplementation in adult patients with migraine. Curr Med Res Opin 35(4):715–723 [DOI] [PubMed] [Google Scholar]
- 18.Granovsky Y, Shor M, Shifrin A, Sprecher E, Yarnitsky D, Bar-Shalita T (2018) Assessment of responsiveness to Everyday Non-noxious Stimuli in Pain-Free Migraineurs with Versus without Aura. J Pain 19(8):943–951 [DOI] [PubMed] [Google Scholar]
- 19.Guldiken YC, Petropoulos IN, Malik A, Malik RA, Yüksel R, Budak F, Selekler HM (2023) Corneal confocal microscopy identifies corneal nerve fiber loss in patients with migraine. Cephalalgia 43(5):3331024231170810 [DOI] [PubMed] [Google Scholar]
- 20.Guy N, Voisin D, Mulliez A, Clavelou P, Dallel R (2018) Medication overuse reinstates conditioned pain modulation in women with migraine. Cephalalgia 38(6):1148–1158 [DOI] [PubMed] [Google Scholar]
- 21.Headache_Classification_Committee_of_the_International_Headache_Society (2018); 38(1):1-211
- 22.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane 2023
- 23.Kennedy DL, Kemp HI, Ridout D, Yarnitsky D, Rice ASC (2016) Reliability of conditioned pain modulation: a systematic review. Pain 157(11):2410–2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinard KI, Smith AG, Singleton JR, Lessard MK, Katz BJ, Warner JE, Crum AV, Mifflin MD, Brennan KC, Digre KB (2015) Chronic migraine is associated with reduced corneal nerve fiber density and symptoms of dry eye. Headache 55(4):543–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King CD, Wong F, Currie T, Mauderli AP, Fillingim RB, Riley JL 3 (2009) Deficiency in endogenous modulation of prolonged heat pain in patients with irritable bowel syndrome and temporomandibular disorder. Pain 143(3):172–178 [DOI] [PMC free article] [PubMed]
- 26.Kisler LB, Granovsky Y, Coghill RC, Sprecher E, Manor D, Yarnitsky D, Weissman-Fogel I (2018) Do patients with interictal migraine modulate pain differently from healthy controls? A psychophysical and brain imaging study. Pain 159(12):2667–2677 [DOI] [PubMed] [Google Scholar]
- 27.Kisler LB, Weissman-Fogel I, Coghill RC, Sprecher E, Yarnitsky D, Granovsky Y (2019) Individualization of Migraine Prevention: a randomized controlled trial of psychophysical-based prediction of Duloxetine Efficacy. Clin J Pain 35(9):753–765 [DOI] [PubMed] [Google Scholar]
- 28.Kroll LS, Hammarlund CS, Gard G, Jensen RH, Bendtsen L (2018) Has aerobic exercise effect on pain perception in persons with migraine and coexisting tension-type headache and neck pain? A randomized, controlled, clinical trial. Eur J Pain 22(8):1399–1408 [DOI] [PubMed] [Google Scholar]
- 29.Lutolf R, Rosner J, Curt A, Hubli M (2022) Indicators of central sensitization in chronic neuropathic pain after spinal cord injury. Eur J Pain 26(10):2162–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malo-Urriés M, Estébanez-de-Miguel E, Bueno-Gracia E, Tricás-Moreno JM, Santos-Lasaosa S, Hidalgo-García C (2020) Sensory function in headache: a comparative study among patients with cluster headache, migraine, tension-type headache, and asymptomatic subjects. Neurol Sci 41(10):2801–2810 [DOI] [PubMed] [Google Scholar]
- 31.McGuinness LA, Higgins JPT (2021) Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods 12(1):55–61 [DOI] [PubMed] [Google Scholar]
- 32.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8(5):336–341 [DOI] [PubMed] [Google Scholar]
- 33.Nahman-Averbuch H, Granovsky Y, Coghill RC, Yarnitsky D, Sprecher E, Weissman-Fogel I (2013) Waning of conditioned pain modulation: a novel expression of subtle pronociception in migraine. Headache 53(7):1104–1115 [DOI] [PubMed] [Google Scholar]
- 34.Nahman-Averbuch H, Shefi T, Schneider VJ 2nd, Li D, Ding L, King CD, Coghill RC (2018) Quantitative sensory testing in patients with migraine: a systematic review and meta-analysis. Pain 159(7):1202–1223 [DOI] [PubMed]
- 35.Nahman-Averbuch H, Schneider VJ 2nd, Chamberlin LA, Van Kroon AM, Peugh JL, Lee GR, Radhakrishnan R, Hershey AD, Powers SW, Coghill RC, King CD (2021) Identification of neural and psychophysical predictors of headache reduction after cognitive behavioral therapy in adolescents with migraine. Pain 162(2):372–381 [DOI] [PMC free article] [PubMed]
- 36.Neziri AY, Haesler S, Petersen-Felix S, Muller M, Arendt-Nielsen L, Manresa JB, Andersen OK, Curatolo M (2010) Generalized expansion of nociceptive reflex receptive fields in chronic pain patients. Pain 151(3):798–805 [DOI] [PubMed] [Google Scholar]
- 37.Niesters M, Proto PL, Aarts L, Sarton EY, Drewes AM, Dahan A (2014) Tapentadol potentiates descending pain inhibition in chronic pain patients with diabetic polyneuropathy. Br J Anaesth 113(1):148–156 [DOI] [PubMed] [Google Scholar]
- 38.O’Brien AT, Deitos A, Trinanes Pego Y, Fregni F, Carrillo-de-la-Pena MT (2018) Defective Endogenous Pain Modulation in Fibromyalgia: a Meta-analysis of temporal summation and conditioned Pain Modulation paradigms. J Pain 19(8):819–836 [DOI] [PubMed] [Google Scholar]
- 39.Ozarslan M, Matur Z, Tuzun E, Oge AE (2022) Cutaneous allodynia and thermal thresholds in chronic migraine: the effect of onabotulinumtoxinA. Clin Neurol Neurosurg 220:107357 [DOI] [PubMed] [Google Scholar]
- 40.Pan LH, Wang YF, Lai KL, Chen WT, Chen SP, Ling YH, Chou LW, Treede RD, Wang SJ (2020) Mechanical punctate pain threshold is associated with headache frequency and phase in patients with migraine. Cephalalgia 40(9):990–997 [DOI] [PubMed] [Google Scholar]
- 41.Pan LH, Wang YF, Ling YH, Lai KL, Chen SP, Chen WT, Treede RD, Wang SJ (2022) Pain sensitivities predict prophylactic treatment outcomes of flunarizine in chronic migraine patients: a prospective study. Cephalalgia 42(9):899–909 [DOI] [PubMed] [Google Scholar]
- 42.Patzko A, Csutak A, Toth N, Kolkedi Z, Pfund Z, Kis-Jakab G, Bosnyak E, Rozgonyi R, Szalai E (2024) Analysis of the ocular surface functional unit in episodic migraine. Graefes Arch Clin Exp Ophthalmol 262(5):1591–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng KP, Basedau H, Oppermann T, May A (2022) Trigeminal sensory modulatory effects of galcanezumab and clinical response prediction. Pain 163(11):2194–2199 [DOI] [PubMed] [Google Scholar]
- 44.Perenboom MJL, Zamanipoor Najafabadi AH, Zielman R, Carpay JA, Ferrari MD (2018) Quantifying visual allodynia across migraine subtypes: the Leiden Visual Sensitivity Scale. Pain 159(11):2375–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perrotta A, Serrao M, Sandrini G, Burstein R, Sances G, Rossi P, Bartolo M, Pierelli F, Nappi G (2010) Sensitisation of spinal cord pain processing in medication overuse headache involves supraspinal pain control. Cephalalgia 30(3):272–284 [DOI] [PubMed] [Google Scholar]
- 46.Petropoulos IN, Alam U, Fadavi H, Asghar O, Green P, Ponirakis G, Marshall A, Boulton AJ, Tavakoli M, Malik RA (2013) Corneal nerve loss detected with corneal confocal microscopy is symmetrical and related to the severity of diabetic polyneuropathy. Diabetes Care 36(11):3646–3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pijpers JA, Kies DA, van Zwet EW, de Boer I, Terwindt GM (2023) Cutaneous allodynia as predictor for treatment response in chronic migraine: a cohort study. J Headache Pain 24(1):118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rolke R, Baron R, Maier C, Tolle TR, Treede DR, Beyer A, Binder A, Birbaumer N, Birklein F, Botefur IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihofner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B (2006) Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain 123(3):231–243 [DOI] [PubMed] [Google Scholar]
- 49.Sandrini G, Rossi P, Milanov I, Serrao M, Cecchini AP, Nappi G (2006) Abnormal modulatory influence of diffuse noxious inhibitory controls in migraine and chronic tension-type headache patients. Cephalalgia 26(7):782–789 [DOI] [PubMed] [Google Scholar]
- 50.Scholten-Peeters GGM, Coppieters MW, Durge TSC, Castien RF (2020) Fluctuations in local and widespread mechanical sensitivity throughout the migraine cycle: a prospective longitudinal study. J Headache Pain 21(1):16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seifert F, Kiefer G, DeCol R, Schmelz M, Maihofner C (2009) Differential endogenous pain modulation in complex-regional pain syndrome. Brain 132(Pt 3):788–800 [DOI] [PubMed] [Google Scholar]
- 52.Shen F, Dong X, Zhou X, Yan L, Wan Q (2019) Corneal subbasal nerve plexus changes in patients with episodic migraine: an in vivo confocal microscopy study. J Pain Res 12:1489–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shetty R, Deshmukh R, Shroff R, Dedhiya C, Jayadev C (2018) Subbasal nerve plexus changes in chronic migraine. Cornea 37(1):72–75 [DOI] [PubMed] [Google Scholar]
- 54.Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605 [DOI] [PubMed] [Google Scholar]
- 55.Szikszay TM, Adamczyk WM, Carvalho GF, May A, Luedtke K (2020) Offset analgesia: somatotopic endogenous pain modulation in migraine. Pain 161(3):557–564 [DOI] [PubMed] [Google Scholar]
- 56.Tavakoli M, Mitu-Pretorian M, Petropoulos IN, Fadavi H, Asghar O, Alam U, Ponirakis G, Jeziorska M, Marshall A, Efron N, Boulton AJ, Augustine T, Malik RA (2013) Corneal confocal microscopy detects early nerve regeneration in diabetic neuropathy after simultaneous pancreas and kidney transplantation. Diabetes 62(1):254–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teepker M, Kunz M, Peters M, Kundermann B, Schepelmann K, Lautenbacher S (2014) Endogenous pain inhibition during menstrual cycle in migraine. Eur J Pain 18(7):989–998 [DOI] [PubMed] [Google Scholar]
- 58.van de Donk T, van Cosburgh J, van Dasselaar T, van Velzen M, Drewes AM, Dahan A, Niesters M (2020) Tapentadol treatment results in long-term pain relief in patients with chronic low back pain and associates with reduced segmental sensitization. Pain Rep 5(6):e877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Velzen M, Heij L, Niesters M, Cerami A, Dunne A, Dahan A, Brines M (2014) ARA 290 for treatment of small fiber neuropathy in sarcoidosis. Expert Opin Investig Drugs 23(4):541–550 [DOI] [PubMed] [Google Scholar]
- 60.Williams AE, Miller MM, Bartley EJ, McCabe KM, Kerr KL, Rhudy JL (2019) Impairment of inhibition of Trigeminal Nociception via Conditioned Pain Modulation in persons with Migraine headaches. Pain Med 20(8):1600–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yarnitsky D, Granot M, Granovsky Y (2014) Pain modulation profile and pain therapy: between pro- and antinociception. Pain 155(4):663–665 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.