Abstract
Background
Pinus thunbergii is an economically important conifer species that plays a fundamental role in forest ecosystems. However, the population has declined dramatically in recent years as a result of the pine wilt disease outbreak. Thus, developing pine wilt-resistant P. thunbergii is an effective strategy for combating this epidemic.
Results
The somatic embryogenesis of nematode-resistant P. thunbergii was previously reported by our group. The current study looked into the potential commercialization of suspension cultures as a means of large-scale production of nematode-resistant P. thunbergii seedlings. According to our findings, P. thunbergii suspension cultures were suitable for an initial inoculum of embryogenic tissue (2 g) and a subculture inoculum (6.7% (v/v)). Suspension cultures were cultivated for 8–10 days in a 30 mL liquid medium (Gupta and Durzan medium, DCR medium) to facilitate their maturation. The suspension cultures produced a large number of high-quality somatic embryos, which were then used to regenerate the plants and move them into the field. A more accurate assessment of the quality of suspension cultures for somatic embryogenesis could come from the suspension’s dynamics. The results showed that the medium’s phosphate, ammonium, nitrate, and carbohydrates were quickly eaten from day 0 to day 10. In terms of the absorption of nitrogen sources, the ammonium (NH4+) was absorbed prior to nitrate (NO3−). Additionally, the activity of mitochondrial succinate dehydrogenase and superoxide dismutase was directly related to cell growth.
Conclusions
This study presents an approach for selecting appropriate suspension cultures for efficient somatic maturation of P. thunbergii that can also be applied to other conifers. Furthermore, it is possible to commercialize nematode-resistant P. thunbergii seedlings using bioreactors, according to the suspension culture system we describe. To the best of our knowledge, this is the first work to describe a P. thunbergii suspension culture.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-024-05938-0.
Keywords: Enzyme activity, Mass propagation, Nutrient consumption, Pinus thunbergii, Somatic embryogenesis
Introduction
Pinus thunbergii Parl. was initially introduced to China’s coastal areas, including Shandong, Fujian, and Zhejiang Provinces, as a good lumber and economic species due to its drought and infertility resistance as well as windproof properties. Since these attributes maintained the effectiveness of soil and water, it was selected as an optimal species for the reforestation of barren mountains and coastal protection [1]. Since these attributes maintained the effectiveness of soil and water, it was chosen as an ideal species for regeneration of barren highlands and coastal protection. However, many P. thunbergii trees have since died across China due to an epidemic of pine wilt disease, resulting in significant economic losses [2]. Thus, a breeding program for elite nematode-resistant Pinus species has proven to be an effective long-term strategy for controlling pine wilt disease [3, 4]. Traditional seed orchards struggle to meet the demands of mass production in a short period of time. Somatic embryogenesis is a promising method for mass propagation of conifers [5, 6]. The large-scale propagation of nematode-resistant P. thunbergii somatic plants will give technological support for the future establishment of P. thunbergii plantation forests to prevent and control pine wilt disease.
Suspension cultures are an essential large-scale cell culturing technique that allow for the uniform distribution of nutrients and oxygen [7, 8]. This approach can be applied in bioreactors [9, 10], wherein the production of somatic embryos from suspension cultures enables the large-scale mass production of conifers. Using suspension cultures, Weyerhaeuser Company successfully generated somatic Douglas fir embryos on a commercial scale. Each flask yielded almost 10,000 distinctive somatic Douglas fir embryos, considerably lowering labor expenses [11]. Although commercial-scale production of Douglas fir has been reported, mass production in bioreactors has proven challenging for the majority of conifers, particularly Pinus species. As a result, additional efforts will be required to establish a plant regeneration system based on suspension cultures for Pinus species mass propagation. Suspension cultures have been reported for several Pinus species to date (P. strobus [12], P. taeda [13], P. pinaster [14], P. pinea [15], and P. nigra [16]); but output remains low. These cultures demonstrated fast proliferation rates and high-quality mature somatic embryos in suspensions [15, 17]. The establishment of suspension cultures and the investigation of growth parameters in aqueous culture systems are crucial for the mass propagation of somatic embryos in bioreactors [17, 18]. In suspension culture systems, nutrient consumption and enzyme activity are essential growth parameters. The intake of nutrients represents the suspension cultures’ growth environment, and it is used to assess their quality [9]. Cellular enzyme activity variations represent the actual growth and metabolic state of the cells [19, 20]. The constant and precise quantification of nutrient consumption and intracellular enzyme activities of cell metabolism during the culturing process can reliably control the cell content at relatively stable levels, which is conducive to cell growth [21, 22]. Aqueous suspensions are useful for aseptic operations in large, sealed bioreactors, which aids in the automation of somatic embryo production and commercial applications in the forest industry. The establishment of embryogenic cell suspensions serves as a precursor to mass propagation.
In 2004, our group introduced several nematode-resistant P. thunbergii seeds from Japan, and the resulting seedlings were resistant to pine wilt disease [23]. Nematode-resistant family 39 showed greater resistance to three pine wood nematode (PWN) isolates, including both virulent isolates (from Japan and China) and toxic isolates (from China). Disease incidence was less than 60% in nematode-resistant family 39, but 100% in susceptible P. thunbergii trees after PWN inoculation [23]. Our group previously reported on the somatic embryogenesis and plant regeneration of nematode-resistant P. thunbergii [4, 24]. Toward the further application of somatic plants against pine wilt disease in the future, a large-scale multiplication effort using cloned nematode-resistant P. thunbergii is urgently required.
Materials and methods
Plant materials and culture conditions
The embryogenic tissue from families 34, 36, 37, and 39 was used as the primary research material for this investigation. The cell line (1536-1, 1539-1, 2234-1, 2237-1 and LYG-1) was obtained from a whole megagametophyte (at about the cleavage poly-embryo stage) from open-pollinated cones. The mother tree’s cone was collected from the Nanjing Forestry University gene pool of nematode-resistant pines (P. thunbergii and P. densiflora) in Jiangsu, China [4, 23]. Embryogenic tissue was cultivated on a Douglas-fir cotyledon revised (DCR) maintenance medium [25] for two weeks at 25 ± 1℃ in the dark (standard conditions) (Fig. S1).
The DCR maintenance medium was supplemented with 1 mg/L 2,4-dichlorophenoxyacetic acid (2,4-D); 0.5 mg/L 6-benzyladenine (BA); 3% (w/v) maltose; 1 g/L myo-inositol; 500 mg/L casein acid hydrolysate; 250 mg/L 2-(4-morpholino) ethanesulfonic acid (MES); and 500 mg/L L-glutamine (filter-sterilized). Prior to autoclaving, the pH of the medium was adjusted to 5.8, and 2 g/L phytagel (Sigma, St. Louis, U.S.A.) was added. All media were sterilized at 121℃ for 20 min.
The nematode-resistance of embryogenic cell line (1539-1) was confirmed, i.e., the regenerated plants produced by the cell line 1539-1 showed resistance by artificial inoculation with pine wood nematodes (Fig.S2). Moreover, resistance genes for the suppression of pine wood nematode infestation in regenerated plants (from cell line 1539-1) have been identified [26].
Establishment of cell suspensions
The ET was subsequently employed to establish the suspension cultures, and transferred to a 100 ml flask containing a 30 ml DCR liquid medium. The suspensions were then cultured using a gyratory shaker at 90 rpm under standard conditions. Cell proliferation served as the main criteria for confirming the establishment of the suspensions. Here, we explored the growth capacity of the suspension cultures as correlated with the initial and subculture inoculum, and the proliferation rate (fold) as a parameter to evaluate cell growth. For all treatments, there were three replicates per group that were repeated in triplicate. The morphology characteristics of suspension cells were monitored. Briefly, 20 µL suspensions were transferred to a slide, then a cover glass was placed on the slide and the slide washed. The samples were observed with a stereomicroscope (Stemi 508, Germany) for stereological analysis.
The DCR liquid medium was supplemented with 15 g/L maltose; 1 g/L inositol, 450 mg/L glutamine (Gln); 0.3 mg/L 2,4-D; 0.2 mg/L 6-BA; 0.2 mg/L Naphthaleneacetic acid (NAA); 500 mg-L-1 hydrolysed casein (CH); and 250 mg-L-12 -(N-morpholinyl) ethanesulfonic acid (MES). Prior to autoclaving, the pH of the medium was adjusted to 5.8, and the media were sterilized at 121℃ for 20 min.
Determination of ET biomass
For the inoculum experiments, the sedimented cell volume (SCV) was employed to assess the growth capacity of the suspension cultures, while for the dynamics analysis tests, fresh and dry weights of the suspension cultures were used to further elucidate cell proliferation and growth.
SCV procedure
referred to a preceding report [17], where (briefly) the flask contents were poured into 100 ml measuring cylinders and allowed to settle until the suspension culture volumes were stable.
ET fresh weight
Filter paper (Ø9 cm) was weighed as W1, folded into a funnel shape and placed on a 100 mL flask. The suspension cultures were then transferred into the funnel and any excess water was absorbed using dry filter paper for five hours. The suspension cultures with filter paper were then weighed as W2. The fresh weights of suspension cultures were W2 minus W1.
ET dry weight
After quantifying the fresh weight, the filter paper containing the suspension cultures were dried in an oven at 70℃ [15, 27, 28] to balance, and designated as W3. The dry weights of the suspension cultures were W3 minus W1.
Comparison of ET growth
the ET (1 g) was placed to a semisolid and liquid medium to proliferate for one growth cycle (8 days). The fresh weights of suspension and clump cells were W3 and W4, respectively, whereas the dry weights were W5 and W6. The amount of embryogenic cell growth was calculated as the weight after 8 days of culture minus the beginning weight.
Initial inoculum screening
The fresh ET(cell lines 1539-1, 1536-1 and 2237-1)was cultured on a semisolid medium to create the initial inoculum. The fresh ET (0.5, 1.0, and 2.0 g) was transferred to a 100 mL flask with 30 mL of a DCR liquid media. The flasks were shaken on a gyratory shaker (90 rpm) under standard conditions for 14 days. The suspensions were then placed to a 100 mL cylinder, and the SCV was measured every two days. Each treatment contained three duplicates, which were repeated three times. The suspension proliferation (fold) was taken as the evaluation parameter. Proliferation rate (fold) = fresh weight after seven days culture / initial inoculum (fresh weight).
Subculture inoculum screening
The cell lines 1539-1, 1536-1 and 2237-1 were employed as the experimental material. At the end of the initial culture, a certain SCV from the suspension cultures (1, 2, 3, and 5 mL) was pipetted into a 100 mL flask containing 30 mL of new liquid media for proliferation experiments. The inoculum densities were determined using a volume ratio of suspension cultures and fresh liquid medium (v/v) of 1/30 (3.3%), 1/15 (6.7%), 1/10 (10%), and 1/6 (16.7%). The suspensions were then cultured on a gyratory shaker at 90 rpm for seven days. Each treatment contained three duplicates, which were repeated three times. The conditions for the replicates were identical to the initial culture. The biomass of the suspension cultures (fresh weight) at different inoculum densities was measured after seven days of culturing.
Nutrient consumption and enzyme activities of suspension cultures
The cell lines 1539-1, 1536-1 and 2237-1 were employed as the experimental material. To track the physiological changes of the suspension cultures and the nutrient consumption of the fresh medium, 2 mL of each suspension culture was transferred into flasks (supplemented with 30 ml fresh liquid medium) for 14 days of culturing. The evaluation parameters (fresh weight, dry weight, pH, cell activities, enzyme activities, carbohydrate content, and NO3−, NH4+, and PO43− consumption) were recorded every two days. For all treatments, three replicates per group were repeated in triplicate. The ET fresh and dry weights were determined through the methods described above.
Determination of carbohydrates
The determination of carbohydrates (total soluble sugar, glucose, and fructose) proceeded according to the method described by Min et al. [29]. Briefly, the suspension cultures (5 mL) were centrifuged at 3000 rpm for 3 min to extract the carbohydrates. The carbohydrate contents were determined via high performance liquid chromatography (HPLC) using a Gilson system consisting of a plunger pump (model 307) and a refractive index detector (model 132) with Jones chromatography (Cardiff, UK). The mobile phase consisted of U.P. 2.5 M sulfuric acid in water, with arabinose (5 g/L) as the internal standard, and the samples were run through a HyperRez H + LG column (Thermo Fisher Scientific, Waltham, MA, USA) at 37℃.
Determination of NO3−, NH4+, and PO43− content
The suspensions (5 mL) were centrifuged at 3000 rpm for 3 min, after which the supernatant was diluted and passed through a 0.22 μm filter to determine the nitrate, ammonium, and phosphate ion contents. Samples were extracted every two days with three replicates. The main experimental instrument employed was an American thermoelectric ICP inductively coupled plasma emission spectrometer ICAP63004.
Determination of antioxidant enzyme activities
The antioxidant enzyme activities of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) were determined according to the method described earlier by our group [27]. Briefly, the SOD activity was assayed using 200 µL of the extract in 4 mL of reagent containing 50 mM of a phosphate buffer (pH 7.4), 0.1 mM EDTA, 10 µM nitroblue-tetrazolium (NBT), 10 mM l-methionine, and 0.001 mM riboflavin. After 0.5 min, the absorbance was measured at 560 nm for 10 min. One SOD enzyme unit was defined as the quantity of SOD required to produce a 50% inhibition in the reduction of NBT. The POD activity was assayed using 200 µL of extract added in 3 mL of the reaction solution, which contained guaiacol (50 mM), 50 mM of a phosphate buffer (pH 6.8), and H2O2 (2%). After 0.5 min, the absorbance at 470 nm was measured for 4 min. The CAT activity was assayed using the reaction mixture (3 mL), which contained 200 µL of the extract, 50 mM of phosphate buffer (pH 7.0) with 0.001 M EDTA, and H2O2 (3% w/v). After 0.5 min, the absorbance at 240 nm was recorded for 4 min to determine the amount of H2O2 reduced per min. One unit of CAT activity was defined as one µmole H2O2 destroyed per min.
Determination of cell activities
The determination of cell activities proceeded according to the method described by Liu and Mei [30]. Briefly, 1 mL of suspension cultures was transferred to a centrifuge tube (5 mL), rinsed three times with distilled water, and then centrifuged at 3000 rpm for 2 min. The supernatant was then removed to which 2.5 mL triphenyltetrazolium chloride (0.4%) and 2.5 mL of buffer (0.1 mol/L sodium phosphate, pH 7.0) was added, and incubated for 13 h at 25℃ in the dark. Next, the supernatant was removed, and the cells were rinsed three times with distilled water. The suspension cultures were placed in a 60 ℃ water bath for 30 min following the addition of 5 mL ethanol (95%) until the cells were decolored, and then centrifuged for five min (4000 rpm), after which the supernatant was collecting. The absorbance (OD) of the supernatant was measured at 485 nm using a spectrophotometer (Thermo Fisher Scientific Inc., America), and the OD indicators determined the cell viability.
Maturation and germination of somatic embryos
The cell lines 1539-1, 1536-1 2234-1, 2237-1 and LYG-1 were employed as the experimental material. The maturation and germination of the P. thunbergii somatic embryos proceeded as described in our previous reports [4, 25]. Briefly, the suspension cultures (cell line 1539-1) were cultured for eight days in DCR liquid medium, after which they were spread (2 mL) onto the top of a stack of five pieces of sterilized filter paper. Once the liquid medium was absorbed, the filter paper containing the cultures was placed on the surface of the solidified medium for embryo maturation. Approximately 500 mg of ET was transferred to each Petri dish. The suspension cells were cultured on maturation medium in the dark at 25 ± 1 °C for 10 weeks (without intervals). As a control, the maturation of the cell mass proceeded in the same way. After 10 weeks of culturing, the number of mature somatic (cotyledonary) embryos was recorded, and their population per gm of fresh mass was used as a parameter for evaluating the maturation capacity of the suspension culture. There were three replicates per treatment, which were repeated in triplicate. The maturation medium consisted of basic LP medium [31], which contained 3% (w/v) maltose; 500 mg/L casein acid hydrolysate; 10 mg/L abscisic acid; 1 g/L myo-inositol; 2 g/L activated charcoal; 130 g/L polyethylene glycol 8000 (PEG 8000); 1.5 g/L glutamine (filtering sterilization); and 3.5 g/L phytagel (Sigma, St. Louis, U.S.A.).
Furthermore, the maturation of P. thunbergii (cell lines 1539-1) somatic embryos in liquid medium was investigated, with 2 mL suspensions or fresh ET (0.5 g/L) cultivated on a semisolid medium being added to the liquid maturation medium. The embryogenic cells were grown on liquid maturation media in the dark at 25 ± 1 °C (90 rpm) for one month without intervals. The liquid maturation medium is made composed of basic LP medium with 3% (w/v) maltose, 10 mg/L abscisic acid, 1 g/L myo-inositol, and 1.5 g/L glutamine (filtered sterilized). We then studied the formation and maturation of embryogenic cells in liquid media.
Mature cotyledonary embryos (from cell line 1539-1) were transferred to a PGR-free semisolid DCR basal medium with 2 g/L activated carbon (AC) for germination. The germination medium was a DCR basic media supplemented with 20 g/L sucrose, 2 g/L activated charcoal, and 8 g/L agar. The plates were incubated for the first seven days in the dark and then exposed to light (16-hour photoperiod at 120 mol·m− 2·s− 1 provided by cool white fluorescent light) for five weeks at 25 ± 1℃. After six weeks, the regenerated plantlets were transferred to a growth medium to enhance their quality. Following growth enhancement, the somatic plantlets were transplanted into disposable paper cups (9 cm high) for six months. The potting soil was a mixture of pine forest soil, perlite, and vermiculite at a 2:1:1 ratio. After six months, the plantlets were transferred into pots (Ø8 cm x 15 cm high) that contained pine forest soil. The regeneration plantlets grew in a culture room under cool white fluorescent light (16/8 h) at 25 ± 1℃, and were watered once a week. The humidity of the culture room was maintained at > 85%.
Statistical analyses
The means were compared using Duncan’s multiple range test with the significance set at P < 0.05. All analyses were done using SPSS version 19 (IBM, Armonk, NY, USA) and Prism software.
Results
Establishment of suspension cultures
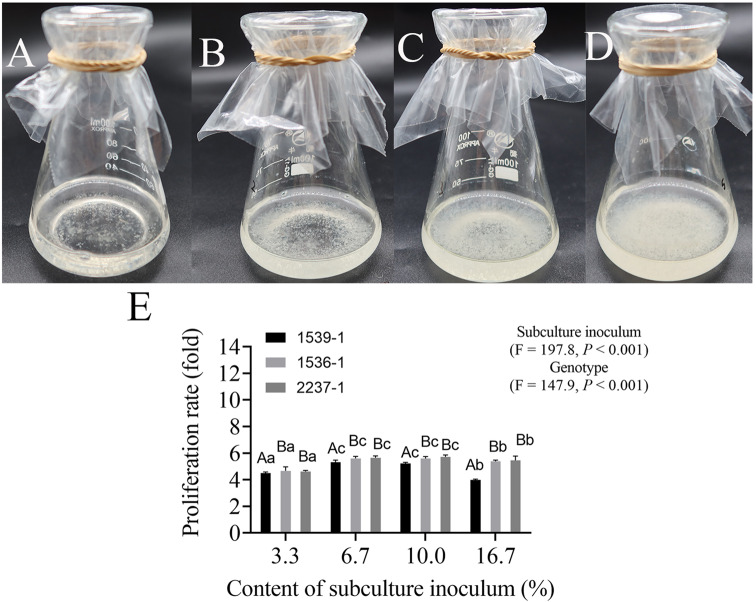
The result revealed that when the initial inoculum increased from 0.5 to 2 g, the suspension cultures in the lag phase (preparation for rapid growth) were shorter (from four to two days) and reached the stationary phase earlier. The proliferation rate of the biomass rarely changed when initial inoculum was increased from 1 to 2 g. The maximum biomass (SCV: 25.77 cm) and proliferation rate (12.89) were obtained using 2 g of initial inoculum. The genotype (P < 0.0001) and inoculum amount (P < 0.0001) significantly affected the proliferation of suspension cells. For the same genotypes, the proliferation rate of suspension cells was significantly affected (P < 0.0001) when the initial inoculum amount was increased from 0.5 g to 1 g (or 2 g) ET. However, no significant variation was observed when the initial inoculum amount was increased from 1 g to 2 g ET. Additionally, no significant differences in the proliferation rate of suspension cells were found among the genotypes when the initial inoculum amount was set at 2 g ET (Table 1). These results indicated that with a greater quantity of initial inoculum (2 g ET) the suspension cultures initiated proliferation earlier. Conversely, the proliferation rates at inoculum densities of 6.7% and 10% were higher than that at 16.7% in the subculture of the cell lines (1539-1, 1536-1 and 2237-1) (Fig. 1). The highest proliferation rate was obtained at inoculum density of 6.7% (1536-1, 1539-1) and 10% (2237-1), which decreased when the subculture inoculum content was > 10%. In the suspensions, the cells at inoculum density of 3.3% and 6.7% were evenly distributed and clear, without the brown aging cells of the other treatments (Fig. 1A, B, C and D). Thus, we determined that a suitable initial P. thunbergii suspension culture required 2 g of ET initial inoculum. This was introduced into a 100 mL conical flask, containing 30 mL of liquid medium, and cultured for eight days, after which 2 mL (6.7%) suspension cultures were transferred to a fresh liquid medium.
Table 1.
Effects of initial inoculum on proliferation of suspension cultures
| Genotypes | Initial inoculum (g) | Lag phase (d) | Stationary phase (d) |
Suspension cells biomass (SCV, ml) |
Proliferation rate (fold) |
|---|---|---|---|---|---|
| 1539-1 | 0.5 | 4 | 10 | 2.43 ± 0.07Aa | 4.85 ± 0.14Aa |
| 1 | 2 | 10 | 11.58 ± 0.14Ab | 11.58 ± 0.14Ab | |
| 2 | 2 | 8 | 23.85 ± 0.42Ac | 11.92 ± 0.21Ab | |
| 1536-1 | 0.5 | 4 | 9 | 2.65 ± 0.15Aa | 5.30 ± 0.30Aa |
| 1 | 2 | 9 | 11.86 ± 0.30Ab | 11.86 ± 0.23Ab | |
| 2 | 2 | 7 | 24.55 ± 0.58Ac | 12.27 ± 0.29Ab | |
| 2237-1 | 0.5 | 3 | 9 | 3.00 ± 0.06Ba | 6.00 ± 0.12Ba |
| 1 | 2 | 9 | 14.78 ± 0.51Bb | 14.78 ± 0.51Bb | |
| 2 | 2 | 7 | 25.77 ± 0.46Bc | 12.89 ± 0.23Bb |
Two-way ANOVA analysis (P values) of suspension cells biomass
Initial inoculum (N = 6), P < 0.0001; Genotype (N = 6), P = 0.0001; Initial inoculum x Genotype, P = 0.003; Two-way ANOVA analysis (P values) of suspension cells proliferation rate (fold) Initial inoculum (N = 6), P < 0.0001; Genotype (N = 6), P < 0.0001;
Initial inoculum x Genotype, P = 0.016
Note Data represent mean ± standard error
The means followed by different capital letters indicate significant differences among genotypes with the same initial inoculation amount (P < 0.05) by Duncan’s test, while the lowercase letters indicate significant differences between different cell inoculation amounts of the same genotype (P < 0.05)
The biomass was determined by the SCV of suspension cultures after 14 days of culturing. The proliferation rate was calculated according to the SCV ratio
Fig. 1.
Suspension cells proliferation of P. thunbergii. The performance of suspension cells (1539-1) cultured for seven days with an inoculum content of 3.3% (A), 6.7% (B), 10% (C) and 16.7% (D). The performance of proliferation rate in genotypes (1539-1, 1536-1 and 2237-1) and subculture inoculum (E). Data represent the mean ± SD of nine replicates. Capital and lowercase letters represent the significant differences (P < 0.05) of genotype and inoculum amount on the proliferation rate by Duncan’s test, respectively
Proliferation of suspension cultures
During the subsequent 14-day growth cycle, the further growth of the suspension cultures was observed in accordance with the method described above. The increased (fresh weight and dry weight) suspension cultures exhibited an S-shape curve under continuous culturing for 14 days (Fig. 2A). Similarly, four stages were observed in our experiment (lag phase (0-two days), exponential phase (two-eight days), stationary phase (eight-12 days), and aging phase (12–14 days)). During the lag phase, the suspension culture biomass rarely increased. However, during the exponential phase the suspension cultures underwent rapid growth and the fresh weight continuously increased from two to 12 days, albeit the dry weight tended to stably proliferate from 8 to 12 days. The cell viability values were also maximized on tenth day, after which the cell viability values decreased (Fig. 2B). Specifically, the cell growth and material accumulation were not synchronized from the exponential phase. During the aging phase, the dry weight began to drop but the fresh weight did not decrease. Meanwhile, highly vacuolated and elongated suspensor cells were obviously visible during both the lag and aging phases (Fig. 3A, C). The structure of the embryogenic suspensor mass was primarily observed during the exponential phase (Fig. 3B). In addition, we examined cell growth in solid and liquid media. The results revealed that the fresh weight of cells cultivated in liquid media was about three times that of solid medium, and the dry weight was approximately seven times that of solid medium (Fig. S3).
Fig. 2.
Proliferation (A) and activities (B) of P. thunbergii suspension cultures (cell line 1539-1). Various uppercase and lowercase letters indicate significant differences (P < 0.05) between treatments over time based on Duncan’s test. Data represent the mean ± SD of three replicates. FW indicates fresh weight. DW indicates dry weight
Fig. 3.
Structures of suspension cultures in lag phase (A) exponential phase (B), and aging phase (C) of P. thunbergii. Bars A-C 200 μm
Nutrient consumption of suspensions
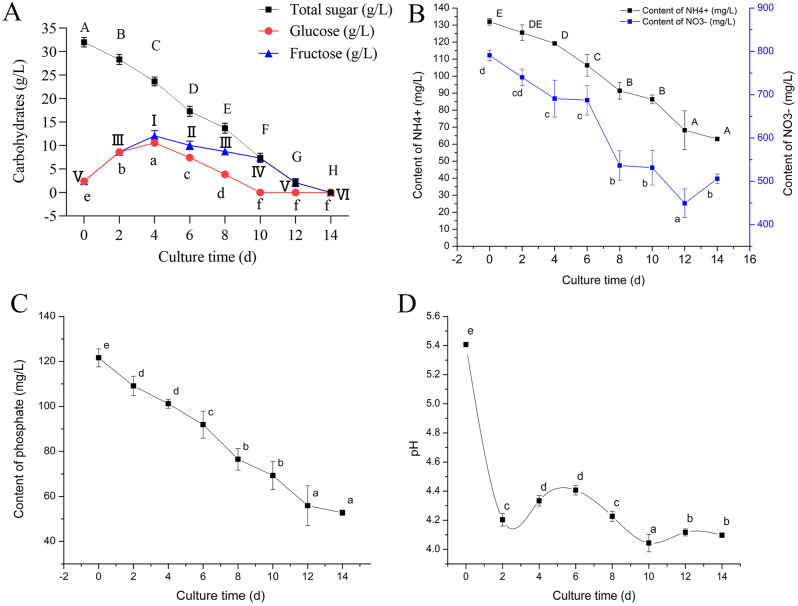
Changes in the carbohydrates of the liquid medium revealed that the total sugars were depleted on the twelfth day. The monosaccharides (glucose and fructose) showed an increasing trend from day 0 to day four, and began to be rapidly consumed from the fourth day. In terms of monosaccharide absorption, glucose was absorbed prior to fructose, and both were depleted on the tenth and twelfth days, respectively (Fig. 4A). The initial concentrations (following sterilization) of nitrate, ammonium, and phosphate were 802 mg/L, 130 mg/L, and 120 mg/L, respectively. Over the 14-day growth cycle, the phosphate and ammonium continuously diminished over time; however, the nitrate decreased from 0 to 12 days but then increased from 12 to 14 days (Fig. 4B, C). During the exponential phase (six-eight days), the absorption of nitrate ions was significantly higher than that of ammonium ions, which indicated that the demand for nitrate ions was intense during the rapid proliferation of the suspension cultures. Moreover, both nitrate and ammonium ions were substantially absorbed (10–12 days) before the cells entered the aging phase (12–14 days). These results showed that both nitrate and ammonium ions were essential nutrients for cell proliferation, and the aging of suspension cultures might be correlated with the reduction of these elements. Further, the pH of the DCR liquid medium decreased significantly from 0-two days (lag phase), and then increased slowly with the nutrient consumption and proliferation of suspension cultures. With the rapid consumption of nutrients the pH decreased significantly, while the biomass increased rapidly during this stage (exponential phase). Once the pH slowly increased again the nitrate showed an abnormal increase, after which the suspension cultures entered the aging phase (Fig. 4D).
Fig. 4.
Dynamics of P. thunbergii suspensions (cell line 1539-1). Consumption of carbohydrate (A) ammonium and nitrate (B) and phosphate (C). pH changes (D). Various uppercase and lowercase letters indicate significant differences (P < 0.05) between treatments over time based on Duncan’s test. Data represent the mean ± SD of three replicates. I-VI indicates significant differences (P < 0.05) between treatments (fructose) over time based on Duncan’s test
Enzyme activities of suspension cultures
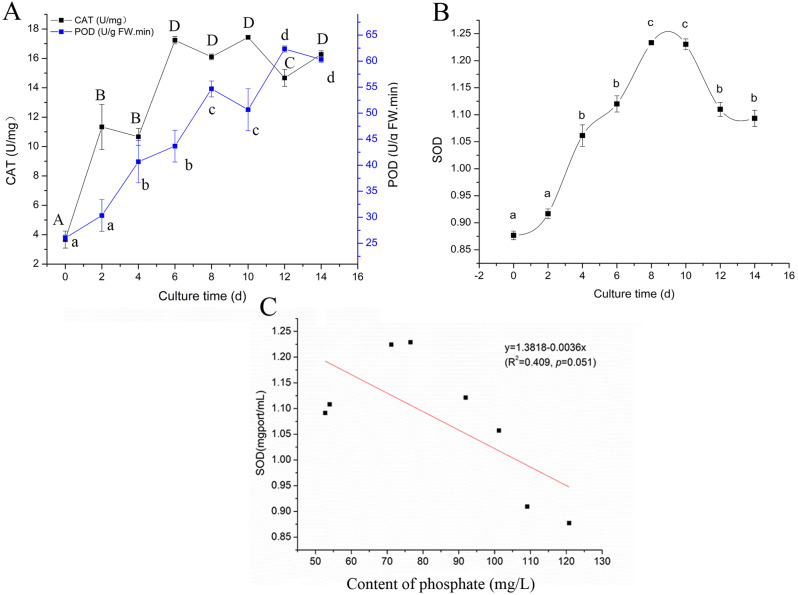
The intracellular enzyme activities of CAT and POD increased rapidly during the lag phase in response to the introduction of fresh medium and exhibited an upward trend over time (Fig. 5A). In contrast, the SOD activity was not significantly altered from 0-two day (lag phase) but increased greatly during exponential phase to reach a maximum on the eighth day. Later, the SOD activity decreased significantly and then stabilized during the aging phase (Fig. 5B). Moreover, it was found that there was no significant correlation between the SOD and phosphate (P = 0.051) (Fig. 5C). To ensure experimental rigor, we incorporated these evaluation indicators for additional genotypes. The results reveal that suspension cells in the novel genotype (cell lines 1536-1 and 2237-1) followed a similar development pattern in liquid media (Fig. 6). Based on the growth curves and suspension dynamics, the optimal time to harvest the suspension cultures in the maturation experiment was determined as the eighth day. Thus, a suitable transfer interval for the suspension system of nematode resistant P. thunbergii was established at eight days, using an initial inoculum of 2 g ET and subcultured inoculum 6.7% (v/v) of suspension cultures in 30 mL of aqueous medium.
Fig. 5.
Changes of intracellular enzyme activities in cell line 1539-1. (A) Activities of CAT and POD. (B) Activities of SOD. (C) The correlation analysis between SOD and phosphate. Various uppercase and lowercase letters indicate significant differences (P < 0.05) between treatments over time based on Duncan’s test. Data represent the mean ± SD of three replicates
Fig. 6.
The evaluation indications for other genotypes in the established suspension culture system. A-H indicate cell line1536-1; I-P indicate cell line 2237-1. (A, I) The proliferation or growth of embryogenic cells in the suspension culture system. FW represents fresh weight. The acronym DW stands for dry weight. (B, J) Cell activity varies in a suspension culture system. (C, K) Variations in the intracellular CAT and POD enzyme activity. (D, L) Variations in intracellular SOD enzyme activity. (E, M) Ammonium and nitrate consumption. (F, N) The consumption of phosphate. (G, O) Consumption of carbohydrates. And (H, P) pH varies in liquid medium. The data show the mean ± SD
Maturation and regeneration of somatic embryos
The suspension cultures (1539-1) generated an average of 1072 somatic embryos/g fresh weight (FW), which was ~ 5 fold higher than the cell mass (block cells) (Fig. 7A). The mature cotyledonary embryos developed from both the suspension cultures and cell mass exhibited characteristics that included an opening cotyledon, contracted and elongated hypocotyl, and a milky white or light-yellow color (Fig. 7B, C). The cotyledonary embryos generated by the suspension cultures appeared to be better synchronized than those produced by the cell mass. Following six weeks of incubation on the germination medium, the cotyledonary embryos sprouted roots and buds and were transformed to emblings (Fig. 7D, E). Over 80% of the cotyledonary embryos were converted to emblings in the germination medium, which effectively expedited the breeding time. The quality of the plantlets was enhanced, and a portion thereof sprouted needle leaves once the emblings were transferred to the growth medium for one month (Fig. 7F). Finally, the mature plantlets were transplanted to the field for further monitoring following two domestications (eight months) (Fig. 7G, H, I). To ensure experimental rigor, we incorporated these evaluation indicators for additional genotypes and reset numerous genotypes to test the yield of mature somatic embryos in the established somatic embryogenesis system. The results reveal that suspension cells in the novel genotypes followed a similar development pattern in liquid media. And the suspension cells from nubermous genotypes still maintained a high level of somatic embryo maturity in the created somatic embryogenesis system (Fig. 7A). Furthermore, the nematode-resistant P. thunbergii somatic plants from the suspensions were transplanted in the field and showed an excellent growth trend in China. (Fig. 8). Furthermore, the maturity of P. thunbergii somatic embryos in liquid media was investigated. In liquid maturation medium, we noticed that embryonic cells (of the same genotype) occasionally produced embryo-like structures similar to mature somatic embryos (Fig. S4). More research was still needed on this topic.
Fig. 7.
Somatic embryos (SE) maturation and plant regeneration of nematode-resistant P. thunbergii. Variations in the genotype of mature somatic embryos produced by cell mass and suspension cells (A). Maturation of somatic embryos from suspension cells (B) and cell mass (C). Germinated somatic embryos on semisolid medium (D and E). Enhancement of regenerated plants in sterile culture chamber (F). The regenerated plants were transplanted to the field (G) and the tolerance of individuals were screened for PWN (H, I). Data represent the mean ± SD. * indicates P < 0.05, *** indicates P < 0.001, **** indicates P < 0.0001
Fig. 8.
Mass propagation of nematode-resistant P. thunbergii plantlets by suspension culture
Discussion
Establishment of P. Thunbergii cell suspensions
An efficient suspension system was the basis for the mass propagation of nematode resistant P. thunbergii somatic plants. For this study, the culture system was comprised of 100 ml conical flasks containing 30 ml of liquid medium. The initial inoculum included 2 g of fresh weight ET and subculture inoculum of 6.7% (v/v) suspension cultures. A culture period of from eight-ten days was favorable for the establishment of P. thunbergii suspension cultures, while an appropriate inoculum enabled the rapid initiation of proliferation. A lower inoculum (0.5 g ET and 3.3% cell density) resulted in poor growth and low biomass. Similarly, poor growth and even degeneration were observed under a low initial inoculum (ET 0.5 g, fresh weight) of P. nigra suspensions [17]. The suspension cultures are required to attain a suitable minimum cell density to accumulate the “condition factors” that initiate proliferation [32]. Chen et al. [33] suggested that a minimum critical density was required before suspension cell multiplication, possibly because the initiation of cell division required a certain level of an intracellular substance called conditioning factor. And this can be accomplished by increasing the suspension cell inoculum and fresh medium. Higher inoculum allowed for far greater intercellular communication, where the messaging between suspension cultures is likely beneficial for the initiation of proliferation. It was speculated that the constrained proliferation due to low inoculum might be related to the adaptation to the culture environment in the early stages. Thus, we surmised that a lower inoculum was unfavorable for the establishment of the suspension cultures. For P. thunbergii, the most suitable initial cell density was 6.7% (v/v); a concentration that was the lowest for conifer suspension cultures according to earlier reports (P. elliottii, 50% [34], P. massoniana, 16.7% [35], and P. pinaster, 14.3% [14]). The initial inoculum was associated with the critical ET concentrations of different tree species [36]. Consequently, it was necessary to investigate the optimal inoculation quantity of P. thunbergii for the establishment of suspensions.
Suspension cell culture dynamics analysis
The timely collection of high-quality suspension cultures during continuous proliferation was key for maintaining the embryogenic potential [37, 38]. The growth curve of cells reflected the physiological states of the suspension cultures to a certain degree [35, 39]. However, effective somatic embryogenesis required a more accurate assessment of the physiological states of the suspension cultures [40], the improved elucidation of which were provided by dynamics analysis [28, 41, 42]. This study revealed that the suspension cultures were not actively proliferating during the lag phase (0-two days). However, the pH and major nutrients of the media were rapidly reduced, while the CAT and POD activities increased. This indicated that although the proliferation of suspension cultures was stagnant, the internal metabolism of the cells was active. These metabolic activities might be a prerequisite for the initiation of cell proliferation. Guo et al. [43] suggested that the rapid decline of nutrients in the liquid media during the lag phase might have been due to the inoculated cells being at the late stage of a previous culture cycle, which therefore contained inadequate intracellular nutrients. However, Li et al. [36] proposed that the slow growth of P. elliottii × P. caribaea suspension cultures might have been related to a rapid decline in the pH, programmed cell death (PCD), and other physiological/biochemical changes. In a study of Larix leptolepis, the intracellular hydrogen peroxide (H2O2) content increased with changes in the exogenous environment [44], which was consistent with the increased POD and CAT activities in this study. Therefore, we preferred the latter explanation. Overall, the arrested growth of cell suspensions was the result of their adapting to fresh media via self-regulation toward the initiation of proliferation. The abilities of the cells might have been modified during the initial adjustment phase. Further, we found that the growth trends of dry and fresh weights were not synchronized in the exponential phase. The fresh weight was delayed into the stationary phase, in contrast to the dry weight. During the aging phase (12–14 days), the cell dry weight decreased significantly; however, the fresh weight considerably increased. By observing the cell structures, we found that the nuclear material was decreased, while the suspensor elongation and vacuolar cells increased, which might account for the greater fresh weight. The OD values showed a degree of consistency with the dry weight from 0 to 10 ds. After the tenth day, the OD value decreased significantly and the dry weight also began to decrease; however, the fresh weight continued to increase. Thus, it was more accurate to combine the OD and dry weight curve to assess the cell status, where changes in nutrient and intracellular oxidase activities supported this assertion. The dry weight of the suspension cultures did not increase once the glucose was depleted on the tenth day, but the fresh weight still increased. Phosphate, ammonium, and nitrate showed a significant decrease prior to the cells entering the aging phase (10–12 ds), after which they did not change significantly at 12–14 days (aging phase). Furthermore, there was a significant decrease in phosphate at six-eight days (exponential phase), and when the suspension cultures entered the aging phase (12–14 days) the phosphate content tended to stabilize. This suggested that the nutrient uptake of the suspension cultures began to decline during the aging phase. It is worthy of note that an abnormal increase of nitrate ions occurred as the suspension cultures transitioned to the aging phase. Nitrate and ammonium nitrogen are critical influencing factors for cell morphology, totipotency, and their proliferation rates, as they can modify the synthesis of proteins and enzymes [14]. An increase in cellular vacuoles and suspensors was observed during the aging phase, which was obviously distinct from the exponential phase. The abnormal increase in nitrate may have been related to the cell recession. Phosphate regulated cell morphology and proliferation by participating in the formation of nucleotides, proteins, and the phosphorylation of sugar [45, 46]. In earlier research, phosphate was observed to affect SOD synthesis by regulating the formation of nucleic acids [47]. Conversely, our results revealed that the concentration of phosphate in the DCR liquid medium had no significant (P = 0.51) impact on the accumulation of SOD. The trend in POD changes was unique; thus, we speculated that this might be related to cells maintaining the stability of their internal environment, which will require further study. Overall, it was found to be suitable to collect the high-quality suspension cultures of P. thunbergii on the eighth day according to the nutrient dynamics, intracellular enzyme activities, pH, and OD values.
Maturation and regeneration of suspension cultures
In this study the suspension cultures produced ~ 5 fold more somatic embryos (1072 somatic embryos/g FW) than did the cell mass (block cells). Further, the cotyledonary embryos generated by the suspension cultures appeared to be better synchronized than those of the cell mass. Furthermore, the maturation of somatic embryos in liquid media was tested, and certain embryo-like structures similar to mature somatic embryos were developed in liquid maturation medium. However, the majority of cotyledonary somatic embryos developed in semisolid maturation media. More extensive investigation was still required on this topic. These findings revealed that no universal and repeatable approach for Pinus species somatic embryo development in liquid conditions has been discovered thus far. Although an embryogenic cell line was employed to demonstrate the feasibility of somatic embryogenesis in liquid medium, we were able to effectively generate mature somatic embryos from various P. thunbergii genotypes using the suspension culture procedure described in the study [4, 24]. And the regenerated plants were transplanted in the field [48]. In the future, we will test more genotypes to ensure that this suspension culture approach is applicable to a wider range of Pinus species. The rapid and uniform growth of somatic embryogenesis in suspensions allow for the development of an automated mass production process for the large-scale generation of conifer somatic embryos [49, 50]. In previous reports on P. thunbergii, Taniguchi [51] obtained 32 somatic embryos per plate, while Li et al. [52] obtained 72 somatic embryos per gram of ET (block cells). Moreover, Maruyama et al. [5] obtained > 900 somatic embryos per plate from suspension cultures. These studies demonstrated the suitability of suspension cultures for the mass production of somatic embryos in P. thunbergii. However, high quality suspension cultures were a prerequisite, as those without quality screening produced somatic embryos at a low level. For example, a maximum of 59 (/2 ml SCV) cotyledonary embryos were obtained from suspension cultures in the six selected cell lines of P. nigra [17]. In a P. koraiensis study, the maximum yield of 160 cotyledonary embryos/g FW was obtained from suspension cultures [53]. In our experiments, high quality suspension cultures were obtained by monitoring the corresponding temporal dynamics of nutrients and pH in the liquid medium, as well as the viability of antioxidant enzyme activities. An abundance of cotyledon embryos and successfully regenerated plantlets were obtained from the collection of high-quality suspension cultures using the above parameters. High-quality suspension cultures were the main influencing factor for high yields of somatic embryos in this experiment. Although the maturation of suspension cultures occurred for several conifers, the survival of regenerated plants in the field was less frequent [54, 55]. In this study, > 300 regenerated plantlets were transplanted in the field for four years [48]. Overall, we established an effective system for the mass production of P. thunbergii somatic plants using suspension cultures. These data provide a reference for future research and the development of bioreactors for the large-scale production somatic embryos of nematode-resistant P. thunbergii.
Conclusion
This study describes the development of an effective suspension cell culture system for the mass propagation of P. thunbergii. The suspension cell culture system included 100 ml conical flasks with 30 ml of liquid medium. The initial inoculum was comprised of fresh weight ET (2 g) and a subculture inoculum (6.7% (v/v)) suspension cultures. A culture period of eight-ten days was favorable for the establishment of the P. thunbergii suspension cultures, and an abundance of somatic embryos were obtained. Moreover, the germinated somatic seedlings were successfully transplanted in the field. This study demonstrated that it is possible to create an efficient suspension culture system for the mass proliferation of P. thunbergii somatic plants. In-depth dynamics analyses of the suspension cultures facilitated a more accurate elucidation of their physiological and biological attributes. This investigation provides a technical reference for the establishment of large commercial-scale P. thunbergii suspension cell culture system using bioreactors. Mass propagation of nematode-resistant P. thunbergii somatic plants to replace susceptible pines will greatly facilitate the establishment and management of high-quality pine forests in the future. This will better utilise the multiple functions of pine forests. In addition, the breeding program of nematode-resistant P. thunbergii provides a long-term control strategy for future control of pine wilt disease.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful for the support given by Key Laboratory of National Forestry and Grassland Administration on Prevention and Control Technology of Pine Wilt Disease (202402).
Author contributions
Ye Jianren and Wu Xiaoqin guided the research. Tingyu Sun conducted the experiments and wrote the manuscript.
Funding
This work was financially supported by Natural Science Foundation of Jiangsu Province of China (BK20230393), the National Key Research and Development Programe of China (2021YFD1400900), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Data availability
The data supporting the results are concluded in the article and supplementary information files.
Declarations
Ethics approval and consent to participate
We declare that the plant materials for the experiments were collected and studied in accordance with the relevant institutional, national, and international guidelines and legislation.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ding XL, Lin SX, Zhao R, et al. First report of needle blight on Pinus thunbergii Parl. Caused by Fusarium proliferatum in China. Plant Dis; 2022.
- 2.Li M, Li H, Ding XL, et al. The detection of pine wilt disease: a literature review. Int J Mol Sci. 2022;23(18):10797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maruyama TE, Hosoi Y. Post-maturation treatment improves and synchronizes somatic embryo germination of three species of Japanese pines. Plant Cell Tiss Org. 2012;110(1):45–52. [Google Scholar]
- 4.Sun T, Wang Y, Zhu L, et al. Plant regeneration by somatic embryogenesis in Pinus thunbergii resistant to the pine wood nematode. Can J for Res. 2019;49(12):1604–12. [Google Scholar]
- 5.Maruyama E, Hosoi Y, Ishii K. Somatic embryo production and plant regeneration of Japanese black pine (Pinus thunbergii). J Res. 2005;10(5):403–7. [Google Scholar]
- 6.Pullman GS, Bucalo K. Pine somatic embryogenesis: analyses of seed tissue and medium to improve protocol development. New for. 2014;45:353–77. [Google Scholar]
- 7.Ramírez-Mosqueda MA, Iglesias-Andreu LG. Vanilla (Vanilla planifolia jacks.) Cell suspension cultures: establishment, characterization, and applications. 3 Biotech. 2017;7:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Genichiro F, Yuta K, Yusuke T, et al. Suspension culture in a T-flask with acoustic flow induced by ultrasonic irradiation. Ultrason Sonochem. 2021;73:105488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belfiore L, Volpato F, Paulino A, et al. Dynamic shear-stress-enhanced rates of nutrient consumption in gasliquid semi-continuous-flow suspensions. J Non-Equil Thermody. 2011;36(4):337–71. [Google Scholar]
- 10.Motolinía-Alcántara EA, Castillo-Araiza CO, Rodríguez-Monroy M, et al. Engineering considerations to produce bioactive compounds from plant cell suspension culture in bioreactors. Plants. 2021;10:2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta PK, Timmis R. Mass propagation of conifer trees in liquid cultures progress towards commercialization. Plant Cell Tiss Org. 2005;81:339–46. [Google Scholar]
- 12.Finer JJ, Kriebel HB, Becwar MR. Initiation of embryogenic callus and suspension cultures of eastern white pine (Pinus strobus L). Plant Cell Rep. 1989;8:203–6. [DOI] [PubMed] [Google Scholar]
- 13.Tang W, Newton RJ. Glucocorticoid-inducible transgene expression in loblolly pine (Pinus taeda L.) cell suspension cultures. Plant Sci. 2004;166(5):1351–8. [Google Scholar]
- 14.Azevedo H, Dias A, Rui MT. Establishment and characterization of Pinus pinaster suspension cell cultures. Plant Cell Tiss Org. 2008;93:115–21. [Google Scholar]
- 15.González-Cabrero N, Ruiz-Galea M, Alegre J, et al. Growth, morphology and maturation ability of Pinus pinea embryogenic suspension cultures. Plant Cell Tiss Organ Cult. 2018;135:331–46. [Google Scholar]
- 16.Pernis M, Salaj T, Bellová J, et al. Secretome analysis revealed that cell wall remodeling and starch catabolism underlie the early stages of somatic embryogenesis in Pinus nigra. Front Plant Sci. 2023;14:1225424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salaj T, Blehova A, Salaj J. Embryogenic suspension cultures of Pinus nigra Arn.: growth parameters and maturation ability. Acta Physiol Plant. 2007;29(3):225–31. [Google Scholar]
- 18.Li SF, Ye TW, Xu X, et al. Callus induction, suspension culture and protoplast isolation in Camellia Oleifera. Sci Hort. 2021;286:110193. [Google Scholar]
- 19.Truelsen TA, Laird J, Fry SC. Xyloglucan metabolizing enzyme activities in suspension cultured tobacco cells. J Plant Physiol. 1999;154(1):95–101. [Google Scholar]
- 20.Zotter A, Bäuerle F, Dey D, et al. Quantifying enzyme activity in living cells. J Biol Chem. 2017;292(38):15838–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Gao WY, Zhang J, et al. Dynamic change of metabolites and nutrients in suspension cells of Panax Quinquefolium L. in bioreactor. Acta Physiol Plant. 2010;32:463–7. [Google Scholar]
- 22.Singh M, Chaturvedi R. Evaluation of nutrient uptake and physical parameters on cell biomass growth and production of spilanthol in suspension cultures of Spilanthes acmella Murr. Bioproc Biosyst Eng. 2012;35(6):943–51. [DOI] [PubMed] [Google Scholar]
- 23.Wu XQ, Zhang Y, Chen WS, et al. Resistance and histopathological observation of wilt -resistant Pinus thunbergii families from Japan to Bursaphelenchus Xylophilus. Acta Phytopathologica Sinica. 2008;38(1):44–50. [Google Scholar]
- 24.Sun T, Wang Y, Zhu L, et al. Evaluation of somatic embryo production during embryogenic tissue proliferation stage using morphology, maternal genotype, proliferation rate and tissue age of Pinus thunbergii Parl. J Forestry Res. 2022;33:445–54. [Google Scholar]
- 25.Gupta PK, Durzan DJ. Shoot multiplication from mature trees of Douglas fir (Pseudotsuga menziesii) and sugar pine (Pinus lambertiana). Plant Cell Rep. 1985;4:177–9. [DOI] [PubMed] [Google Scholar]
- 26.Sun T, Rahman M, Wu X, et al. Resistant and susceptible Pinus thunbergii ParL. Show highly divergent patterns of differentially expressed genes during the infection process by Bursaphelenchus Xylophilus. Int J Mol Sci. 2023;24:14376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong DZ, Dunstan DI. Growth parameters, protein and DNA synthesis of a embryogenic suspension culture of white spruce. J Plant Physiol. 1994;144:201–8. [Google Scholar]
- 28.Sathish S, Venkatesh R, Safia N, et al. Studies on growth dynamics of embryogenic cell suspension cultures of commercially important Indica rice cultivars ASD16 and Pusa basmati. 3 Biotech. 2018;8:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Min X, Xu H, Huang F, et al. GC-MS-based metabolite profiling of key differential metabolites between superior and inferior spikelets of rice during the grain filling stage. BMC Plant Biol. 2021;21:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu H, Mei XG. The determination of cell viability of Taxus chinensis by TTC. Plant Physiol J. 2001;37(6):537–9. [Google Scholar]
- 31.Quoirin MP, Lepoivre P. Etudes De Milieux adaptes aux cultures in vitro de Prunus. Acta Horticulturae Sinica. 1977;78:437–42. [Google Scholar]
- 32.Wang GD, Xu CY, Yan S, et al. An efficient somatic embryo liquid culture system for potential use in large-scale and synchronic production of Anthurium andraeanum seedligns. Front Plant Sci. 2019;10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen JG, Shangguan XC, Yin ZP, et al. Establishment of the cell suspension culture system of Cyclocarya paliurus and matrix consumption laws (in Chinese). Mod Food Sci Technol. 2014;30(1):44–9. [Google Scholar]
- 34.Fang L, Wu XQ. Suspension culture of somatic cells of Pinus elliottii against brown spot needle blight of pine. Biotechnol Bull. 2019;35(3):13–8. [Google Scholar]
- 35.Shi K, Yang MH, Li ZH, et al. Establishment of embryogenic suspension culture in somatic embryogenesis of Pinus massoniana Lamb. J Cent South Univ T. 2014;34:64–8. [Google Scholar]
- 36.Li F, Yao J, Hu L, et al. Multiple methods synergistically promote the synchronization of somatic embryogenesis through suspension culture in the new hybrid between Pinus elliottii and Pinus caribaea. Front Plant Sci. 2022;13:857972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Z, Chen JH, Bian L, et al. Establishment of embryogenic callus suspension culture system in Liriodendron Hybrids. Mol Plant Breed. 2007;5:137–40. [Google Scholar]
- 38.Jie EU, Atong NS, Ahn WS, et al. High-frequency plant regeneration from embryogenic cell suspension cultures of Gynura procumbens. Plant Biotechnol Rep. 2019;13:27–33. [Google Scholar]
- 39.Lin TY, Zhou R, Wu YG, et al. The relationship between morphology changes and antioxidant enzymes activity during somatic embryogenesis of long-term-maintained callus of Zoysia matrella Merr. Can J Plant Sci. 2021;101:1–24. [Google Scholar]
- 40.Shomali A, Mashayekhi K, Pahlevani M, et al. Effects of various nitrogen sources on synchronization of tomato somatic embryogenesis during induction and realization phases. J Plant Physiol Breed. 2018;8:53–8. [Google Scholar]
- 41.Naill MC, Roberts SC. Flow cytometric analysis of protein content in Taxus protoplasts and single cells as compared to aggregated suspension cultures. Plant Cell Rep. 2005;23:528–33. [DOI] [PubMed] [Google Scholar]
- 42.Ricco MV, Bari ML, Catalano AV, et al. Dynamics of polyphenol biosynthesis by calli cultures, suspension cultures and wild specimens of the medicinal plant Ligaria Cuneifolia (Ruiz & Pav.) Tiegh. (Loranthaceae). Analysis of their biological activity. Plants. 2021;10(8):1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo Z, Du J, Liu R. Kinetic investigation of Taxus cell growth and nutrient consumption. J Tsinghua Univ (Sci &Tech). 2002;42(5):599–602. [Google Scholar]
- 44.Zhang LF, Li WF, Han SY, et al. cDNA cloning, genomic organization and expression analysis during somatic embryogenesis of the translationally controlled tumor protein (TCTP) gene from Japanese larch (Larix leptolepis). Gene. 2013;529:150–8. [DOI] [PubMed] [Google Scholar]
- 45.Maor BP, Malcolm AON. Plant nucleotide sugar formation, interconversion, and salvage by sugar recycling. Annu Rev Plant Biol. 2011;62:127–55. [DOI] [PubMed] [Google Scholar]
- 46.Chen PH, Lun CP, Tsai JC, et al. Grafting of 2-hydroxyethyl methacrylate onto polyacrylonitrile using supercritical carbon dioxide. J Supercrit Fluids. 2022;186:105589. [Google Scholar]
- 47.Spohn M, Diakova K, Aburto F, et al. Sorption and desorption of organic matter in soils as affected by phosphate. Geoderma. 2021;405:115377. [Google Scholar]
- 48.Sun T, Wang Y, Wu X, et al. Promoting the application of Pinus thunbergii Parl. To enhance the growth and survival rates of post-germination somatic plantlets. BMC Plant Biol. 2023;23:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruffoni B, Savona M. The temporary immersion system (T.I.S.) for the improvement of micropropagation of ornamental plants. Acta Horticulturae Sinica. 2005;683:445–53. [Google Scholar]
- 50.Pourianezhad F, Rahnama H, Mousavi A, et al. Parthenolide production in cell suspension culture of feverfew. Bioresour Bioprocess. 2019;6:23. [Google Scholar]
- 51.Taniguchi T. (2001). Plant regeneration from somatic embryos in Pinus thunbergii (Japanese black pine) and Pinus densiflora (Japanese red pine). In Molecular Breeding of Woody Plants, Proceedings of the International Wood Biotechnology Symposium (IWBS). pp. 319–324.
- 52.Li QQ, Ye JR, Zhu LH, et al. Somatic embryogenesis and plantlet regeneration from immature zygotic embryos of Pinus thunbergii. Sci Silvae Sinicae. 2012;48(12):42–7. [Google Scholar]
- 53.Peng C, Gao F, Wang H, et al. Optimization of maturation process for somatic embryo production and cryopreservation of embryogenic tissue in Pinus koraiensis. Plant Cell Tiss Organ Cult. 2021;144:185–94. [Google Scholar]
- 54.Schwarzerová K, Vondráková Z, Fischer L, et al. The role of actin isoforms in somatic embryogenesis in Norway spruce. BMC Plant Biol. 2010;10:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodrigues AS, De Vega JJ, Miguel CM. Comprehensive assembly and analysis of the transcriptome of maritime pine developing embryos. BMC Plant Biol. 2018;18:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the results are concluded in the article and supplementary information files.