Abstract
Contactin‐2 (CNTN2), an immunoglobulin cell adhesion molecule (IgCAM) expressed on the neural cell surface, regulates the formation of myelin sheaths, facilitates communication between neurons and axoglial cells, and coordinates the migration of neural cells. However, the assembly of full‐length CNTN2 is still not fully elucidated. Here, we found that the full‐length human CNTN2 forms a concentration‐dependent homodimer. We further determined the cryo‐EM structures of the full‐length CNTN2, revealing a novel bowknot‐shaped scaffold constituted of the Ig1‐6 repeats from two protomers, with the flexible ribbon‐like FNIII repeats extending outward in opposite directions. The Ig1‐6 domains, rather than the previously proposed Ig1‐4 domains, have an indispensable role in mediating CNTN2‐dependent cell adhesion and clustering. Moreover, structure‐guided mutagenesis analyses supported the idea that CNTN2 homodimerization observed in our structure is essential for cell adhesion. Our findings offer novel insights into the mechanism through which CNTN2 forms a homodimer to maintain cell–cell contacts in the nervous system.
Keywords: cell adhesion, contactin‐2, cryo‐EM, dimerization, full‐length
This study investigated the Cryo‐EM structures of full‐length human contactin‐2 (CNTN2). We found that full‐length human CNTN2 exhibits concentration‐dependent homodimerization. Notably, our results indicate that the Ig1‐6 domains, rather than the previously proposed Ig1‐4 domains, are crucial for facilitating CNTN2‐dependent cell adhesion and clustering. Additionally, structure‐guided mutagenesis analyses further confirmed that the homodimerization of CNTN2, as observed in our structural data, is essential for effective cell adhesion.
Abbreviations
- APP
amyloid precursor protein
- CNS
central nervous system
- CNTN
Contactin
- CNTN2
Contactin‐2
- CNTNAPs
contactin‐associated proteins
- cryo‐EM
cryogenic electron microscopy
- DAPI
4′, 6‐diamidino‐2‐phenylindole
- EM
Electron microscopy
- FNIII
fibronectin type III
- GPI
glycophosphatidylinositol
- HEPES
N‐2‐hydroxyethylpiperazine‐N′‐2‐ethanesulfonic acid
- IgCAM
immunoglobulin cell adhesion molecule
- PNS
peripheral nervous system
- PTPRG
tyrosine phosphatases gamma
- PTPRZ
tyrosine phosphatases gamma zeta
- RSMD
root mean square deviation
- SDS/‐PAGE
Sodium dodecyl‐sulfate polyacrylamide gel electrophoresis
- SV‐AUC
sedimentation velocity‐Analytical Ultracentrifugation
Introduction
The Contactin (CNTN) family consists of a group of immunoglobulin cell adhesion molecules (IgCAMs) that play crucial roles in neurodevelopment and physiological processes, such as axon guidance, neural cell migration, and the organization of myelin subdomains [1, 2, 3, 4]. This family comprises six members known as CNTN1‐6. The CNTNs are dynamically expressed in various regions of the central nervous system (CNS) and peripheral nervous system (PNS) during both embryonic development and adulthood [5]. Contactins are primarily expressed in neurons, as well as in oligodendrocytes and their precursors, showing structural and functional conservation across different species [6, 7]. Existing evidence suggests that each CNTN has distinct roles during early CNS development [5]. CNTN1 and CNTN2 (also referred to as TAG‐1) are predominantly located at the junctions of myelin and axons, facilitating communication among neurons, oligodendrocytes, and astrocytes [7, 8]. Both CNTN1 and CNTN2 are highly expressed in the adult human brain, with CNTN1 found in the temporal cortex and CNTN2 in the hippocampus of adults. CNTN3 is mainly expressed in the granule cell layers of the olfactory bulb, while CNTN4 mediates neuronal wiring in sensory neurons. CNTN5 is present in the cerebellum and plays a role in the auditory pathway, whereas CNTN6 is located in the vestibulocerebellum and is involved in regulating balance and eye movement [9].
Despite having distinct functions, CNTNs in vertebrates exhibit a highly conserved architecture [6]. As IgCAMs, CNTNs share six N‐terminal Ig repeats followed by four fibronectin type III (FNIII) repeats and a C‐terminal glycophosphatidylinositol (GPI) anchor that attaches to the neuronal cell membrane [10, 11]. Due to the lack of an intracellular domain, CNTNs rely on interacting partners within the cell for signal transduction [12]. Five out of the six CNTNs bind to the homologous receptor protein tyrosine phosphatases gamma (PTPRG) and zeta (PTPRZ), except for CNTN2. Additionally, CNTN3‐5 interacts with amyloid precursor protein (APP) and amyloid beta precursor‐like protein 1, while CNTN4 and CNTN5 bind to amyloid beta precursor‐like protein 2 [13]. Among the CNTNs, a specific subset of co‐receptors, CNTNAPs (contactin‐associated proteins or Casprs), form cis‐complexes with CNTNs, regulating the clustering of voltage‐gated ion channels involved in saltatory action potential propagation and axonal excitability [14], as observed with various pairs such as CNTNAP1 and CNTN1, CNTNAP2 and CNTN2, and CNTNAP4 with CNTN4 and 5 [5, 15, 16, 17]. The roles of complexes involving CNTN4 and 5 are still being investigated.
Throughout the developmental process, both CNTN1 and CNTN2 are present in myelinated fibers but are localized in different regions. They can temporarily modulate the expression levels of genes that regulate myelin and myelin‐related processes [7]. CNTN1 organizes the paranodal domains of axons, interacts with glial neurofascin‐155, and establishes axon‐glial contacts by cis‐binding with CNTNAP1 to position Kv1.2, contributing to proper action potential repolarization during action potential conduction [15, 18]. In contrast, CNTN2 is essential for organizing the juxtaparanodes together with CNTNAP2 to cluster the distribution of Shaker‐type Kv1.1 potassium channels, maintaining voltage‐gated potassium channels at the juxtaparanodal region [17]. Dysfunction of CNTN2 or CNTNAP2 has been associated with various neuropsychiatric disorders and autoimmune diseases of the human nervous system, including epilepsy and autism [19, 20]. Knocking out CNTNAP2 in mice leads to spontaneous seizures [21].
Clinical studies have shown that homozygous frameshift or deleterious homozygous variants in CNTN2 can result in cortical myoclonic tremor and epilepsy [20, 21, 22]. Additionally, the expression of CNTN2 is closely linked to Alzheimer's disease and Parkinson's disease [23, 24]. The absence of CNTN2 can lead to diminished branching of oligodendrocytes, hypomyelination in fiber tracts, and impaired axon conduction [7]. Notably, CNTN2 is unique within the contactin family as a self‐associating member with distinct structural features, engaging exclusively in homophilic binding [25].
CNTN2 stands out as the sole self‐associating member in the contactin family, distinguished by its distinct structural features. While Ig superfamily proteins can facilitate both homophilic and heterophilic interactions to regulate axon‐target specificity, protein‐binding assays suggest that CNTN2 exclusively engages in homophilic binding [13]. Previous structural studies on human and chicken CNTN2, which is highly conserved across these two species with 75% identities, suggested two different modes of its homophilic interactions through its horseshoe‐shaped Ig1‐4 domains: zipper mode and four or multiple molecule mode [11, 26]. The smaller number of N‐terminal extracellular domains in CNTN2 suggests that it may form the difference in architecture. Previous research showed that fibronectin domains of the CNTN2 are essential for homophilic binding [27]. Furthermore, both the immunoglobulin and fibronectin domains have been shown to promote neurite outgrowth when used as substrates [28, 29, 30]. Moreover, whether the FNIII domains also play a role in stabilizing the adhesion interfaces, although they may have trans interactions between cells based on molecular dynamics has been reported [31]. The organization of the full‐length CNTN2 dimer that promotes intracellular activity is not yet fully understood, indicating a need for further investigation into the molecular mechanisms underlying CNTN2 dimerization.
To clarify the discrepancies between these findings, we purified full‐length mature human CNTN2 and determined its structure at high resolution by cryo‐EM (cryogenic electron microscopy). We discovered a novel homophilic assembly of CNTN2 forming an asymmetric, interlocked bowknot‐like homodimer dependent on Ig3‐6 domains. Structure‐guided mutagenesis analysis confirmed the significance of interface residues for CNTN2 dimerization and cell–cell contacts mediated by CNTN2. This research provides new insights into the structural basis of CNTN2‐mediated trans neural cell adhesion.
Results
Overall structure of the CNTN2
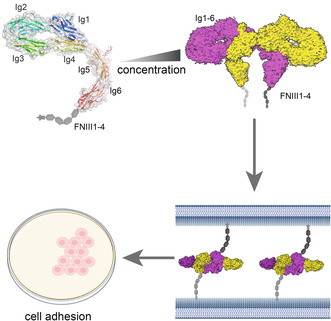
To investigate the overall architecture of CNTN2, we expressed and purified the full‐length mature CNTN2 (Fig. S1). Through 2D classes, we identified particles in an asymmetric state, and subsequent 3D reconstructions, with and without applied symmetry, yielded two cryo‐EM maps of dimeric CNTN2 (Figs S2, S3). The cryo‐EM map in C1 symmetry achieved an overall resolution of 3.73 Å, while the map with C2 symmetry exhibited a higher resolution of 3.49 Å. Following local refinement, the C2 symmetry map was further refined to 3.3 Å. By confidently docking reported models of CNTN Ig1‐4 repeats, Ig5‐6 repeats, and FNIII1‐2 repeats to the maps (PDB: 2OM5, 5I99, 5E4S), we were able to observe clear densities of most residues in Ig1/4/5 repeats involved in dimerization (Fig. 1B,C) [26, 32]. Details on data collection and refinement statistics can be found in Table 1 and Figs S2, S3.
Fig. 1.

Cryo‐EM (Cryogenic electron microscopy) analysis of human CNTN2 (Contactin‐2) Ig1‐6. (A) Schematic of CNTN2 full‐length domain organization. The yellow color represents the Ig domain, which is the structure we studied. The gray represents the FNIII domain. (B, C) Overall cryo‐EM map and structure of CNTN2 Ig1‐6 homodimer shown in two views. One monomer is colored yellow, and the other is colored magenta. (D) Distribution diagram of average sedimentation coefficient of samples at three concentrations (ls‐g (s) model). The experiment was conducted once. (E) CNTN2 mature full‐length gel filtration analysis shows a concentration‐dependent peak shift. The gel filtration analysis was conducted in duplicate. The structural figures were generated utilizing UCSF Chimera software.
Table 1.
Summary of Cryo‐EM data collection, data processing, and structure refinement.
| Data collection | ||
| EM equipment | Titan Krios | |
| Voltage (kV) | 300 | |
| Detector | Gatan K3 Summit | |
| Energy filter | Gatan GIF, 20 eV slit | |
| Pixel size (Å) | 0.92 | |
| Total Electron dose (e−/Å2) | 50 | |
| Defocus range (μm) | −1.5 to −2.5 | |
| 3D reconstruction | ||
| Software | cryoSPARC | |
| Data set | CNTN2 | CNTN2 |
| EMDB and PDB No. | 36 853, 8K3J | 36 896, 8K53 |
| Number of micrographs | 5802 | 5802 |
| Final particles | 292 684 | 294 917 |
| Symmetry | C2 | C1 |
| Final resolution (Å) | 3.3 | 3.73 |
|
Map sharpening B‐factor (Å2) |
−149 | −132 |
| Refinement | ||
| Software | Phenix | |
| Model composition | ||
| Protein residues | 1148 | 1350 |
| Ligand | 16 | 12 |
| R.M.S. deviations | ||
| Bonds length (Å) | 0.002 | 0.005 |
| Bonds Angle (°) | 0.542 | 0.666 |
| MolProbity score | 1.80 | 1.70 |
| Clash score | 8.59 | 6.63 |
| Rotamer outliers | 0 | 0 |
| Ramachandran plot statistics (%) | ||
| Preferred | 98.09 | 96.91 |
| Allowed | 1.91 | 3.09 |
| Outliers | 0 | 0 |
Both maps of CNTN2 revealed density corresponding to CNTN2 Ig1‐6 repeats. In the structure with C1 symmetry, the root mean square deviation (RSMD) of Cα atoms of the Ig1‐6 repeats from two protomers was found to be 0.3 Å, indicating that the two protomers adopt highly similar conformations. Additionally, in this map, low‐resolution density matching the first two FNIII repeats (FNIII1‐2 repeats) was observed in only one of the two protomers, while the FNIII3‐4 repeats remained invisible.
CNTN2 forms a dimer in solution
Regarding the dimerization behavior of CNTN2 in solution, our cryo‐EM structures revealed a dimeric state. To validate this finding, we utilized sedimentation velocity analytical ultracentrifugation (SV‐AUC) and gel filtration to assess the oligomerization state in the solution. We performed SV‐AUC experiments on the CNTN2 samples at three concentrations (Fig. 1D). Upon fitting the data with the ls‐g (s) model, we observed a concentration‐dependent increase in the average apparent sedimentation coefficient (from 5.55 S to 6.06 S) as the protein concentration increases (ranges from 0.04 to 3.57 mg·mL−1). Based on the observations, the molecular weight calculated was from 137 to 168 kDa (Figs 1E, S4). These results indicate that CNTN2 exhibited a reversible polymerization state in a pH 7.5 buffer. Furthermore, CNTN2 dimerized at high concentration in solution as assessed by concentration‐dependent peak shifts and molecular weight increases in size‐exclusion chromatography (Fig. 1E). We observed monomeric CNTN2 in negative staining EM (electron microscopy) images as we used very low concentration (~0.09 μm) in Fig. S5, while the dimeric CNTN2 is dominant in cryo‐EM images with higher concentration sample, supporting the concentration‐dependent dimerization of CNTN2.
Interface of CNTN2 dimer
The Ig1‐6 repeats, along with FNIII1‐2 repeats of CNTN2, collectively form a side‐by‐side, interlocked bowknot‐like homodimer structure. In this configuration, two Ig1‐4 repeats in the CNTN2 dimer shape the two “loops” of the bowknot, while the extended Ig5‐6 and FNIII repeats constitute the “ribbon” with a ~120° bend at the Ig4‐5 junction and a ~55 Å separation between Ig6 repeats (Fig. 1B). The N‐terminal symmetric Ig1‐6 repeat dimer has a size of around 195 Å × 88 Å × 55 Å, burying a surface area of ~2073 Å2 (Fig. 1C). Single Ig1‐4 repeats in CNTN2 fold to a U‐shaped structure (Fig. 2B).
Fig. 2.
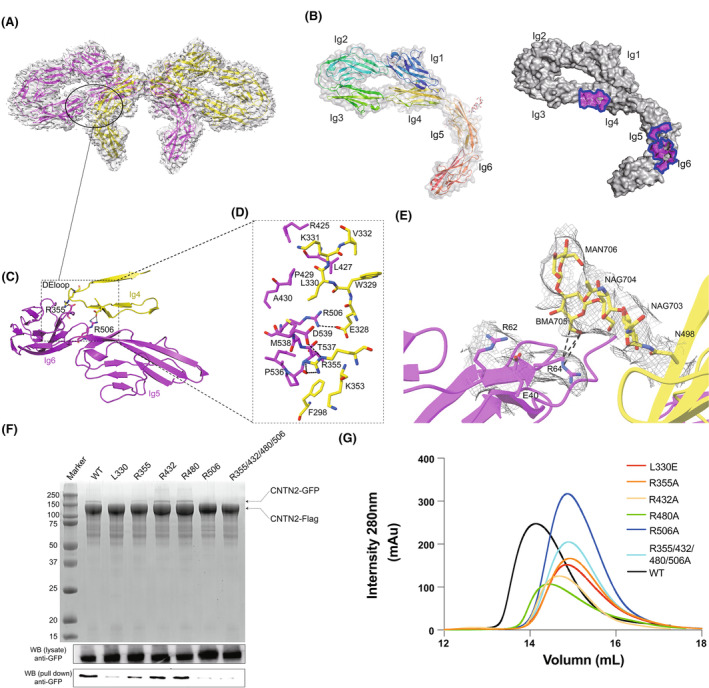
CNTN2 (contactin‐2) dimerization is mediated through two small interfaces between the Ig3‐6 interface. (A) A broad view of the CNTN2 Ig 3, 4, 5, and 6 interface interactions underlying the characteristic architecture. One monomer is colored yellow, and the other is colored magenta. (B) CNTN2 Ig1‐6 monomer (domain rainbow colored) and monomer of the contactin 2 Ig1‐6 in surface representation colored according to various properties and with dimerization surfaces outlined (homodimer surfaces purple colored). (C, D) The molecular details of CNTN2 Ig 4 with lg 5 and lg 6 interface interactions. The key residues of dimerization interface are shown as sticks. The black dotted lines represent hydrogen bonding. (E) Close‐up and overlay of cryo‐EM density (gray) of the N498‐glycan on Ig5, which is part of the extensive interface. The N498‐glycan contacts with E40, R62, R64 of Ig1, shown as sticks. The black dotted lines represent hydrogen bonding. (F) Pull‐down assay of Flag‐tagged CNTN2 wild‐type protein and GFP‐tagged CNTN2 point mutations from transgenic cell lysates, utilizing Coomassie Blue staining and western blot analysis. The western blot experiments were conducted in duplicate. (G) Gel filtration/mutagenesis studies of the dimerization interface of CNTN2, show various effects of the mutations on the apparent size. The gel filtration experiment was conducted in duplicate. The structural figures were generated utilizing UCSF Chimera software.
Overall, the specific pattern of the CNTN2 dimer structure depends on contacts restricted to Ig3‐Ig6 repeats per molecule, with the Ig4 repeat providing the dimer most of the contacts with an interface area of 1047 Å2. Notably, the Ig3 and Ig4 repeats of the one CNTN2 protomer establish significant contacts with the Ig5 and Ig6 repeats of the other protomer, with the Ig3‐Ig4 of the one hinge crosses the Ig5‐Ig6 hinge of the other. On one side of the interface between the two protomers, the acidic residue Glu328 in the Ig4 repeat of one molecule interacts with the basic residue Arg506 in the Ig5 repeat of the other molecule through ion pairs. This interaction may also involve the residues Glu299 in the Ig3 repeat, and Arg432 and Arg480 in the Ig5 repeat. On the other side, the residue Arg355 in the Ig4 repeat also participates in intermolecular electrostatic interactions with the residues Asp539 and Glu567 in the Ig6 repeat. Hydrophobic interactions further reinforce the Ig repeat dimerization. Specifically, Phe298 in Ig3 repeat packs with Pro536 in Ig6 repeat, while the residue Leu330 in Ig4 repeat of the one molecule, and Met538 and Val592 in Ig5 repeat of the other molecule form a hydrophobic core. Additionally, hydrogen bonds and van der Waals force contribute to the dimer formation, involving residues Ala351, Asp507, and Thr537 (Fig. 2). Most of the residues at the interface are located in the turns connecting β‐strands. Sequence alignment indicates that these regions are variable between CNTNs, but conserved in CNTN2 across species (Fig. S6).
Besides the contacts between Ig3‐4 repeats and Ig5‐6 repeats, the Ig1 repeat from one protomer is proximal to the Ig5 repeat from the other protomer. The residue Gln32 in Ig1 repeats at the N‐terminal end packs with residue Arg420 in the Ig5 repeat. Several extra densities were found near asparagine residues including Asn76, Asn198, Asn461, Asn477, Asn498, and Asn525, indicating these residues are N‐linked glycosylation sites (Fig. S7). Interestingly, the Asn498‐linked glycan in Ig5 from one protomer is poised to form hydrogen bonds with Glu40, Arg62, and Arg64 in Ig1 from the other protomer (Fig. 2E). However, in our cell adhesion assay, Asn498 did not affect cell adhesion (Fig. S8).
Biochemical validation of CNTN2 dimerization
To assess the significance of interactions identified from our structural analysis, we introduced point mutations on residues within the dimeric interface and evaluated their impact on dimerization using gel filtration column analysis. We mutated the residue Leu330 which is involved in hydrophobic interactions with glutamate (L330E), or positively charged residues Arg355, Arg432, Arg480, and Arg506 that form salt bridges to alanine individually (R355A, R432A, R480A, and R506A) or in combination (R355/432/480/506A). All of these mutants had longer retention time on the gel filtration column than wild‐type protein, suggesting a smaller overall apparent size and disruption of its dimeric form (Fig. 2G, Table S1).
Furthermore, we used pull‐down experiments to detect whether these mutants self‐associate or not when expressed in HEK293F cells. These mutants were well expressed with a similar level as wild‐type protein. FLAG‐tagged wild‐type CNTN2 could pull‐down GFP‐tagged CNTN2. However, the L330E mutation did not support CNTN2 self‐association. The mutations R506A and R355/432/480/506A, and to a lesser extent, R355A, but not R432A or R480A, disrupted the dimerization of CNTN2 (Fig. 2F). In the structure, Arg432 and Arg480 locate approximately in space and are relatively far away to reach Glu299 from the other protomer. These two arginine residues are not as critical as Arg506 which is close to Glu328. It is reasonable that each mutation cannot effectively disrupt the CNTN2 dimer. Therefore, in consistency with the observation in our structure, the biochemical analysis confirmed that the residues including Leu330, Arg355, and Arg506 in the dimerization interface are crucial for CNTN2 homophilic interactions.
CNTN2 forms a unique dimer and higher‐order oligomers
The structure of the CNTN2 horseshoe‐shaped Ig1‐4 repeats shares remarkable similarity with the reported structures of other CNTNs and IgSFs, including CNTN1, CNTN4, Dscam1, Neurofascin, and Sdk1 with RMSDs range from 2.9 to 6.9 Å (Fig. 3A) [33], although the sequence identities between CNTN2 and these proteins are low (around 30–50%) (Fig. S9). In the horseshoe‐shaped structure, the junction region has two rigid groups, the Ig2 and Ig3 domains are bent in an antiparallel fashion, and the Ig1 domain is in contact with the Ig4 domain.
Fig. 3.

Comparison of the ectodomain structures of CNTN (Contactin) 2 with CNTN1. (A) Structural alignment of the single protomer CNTN2 Ig1‐6 and IgSFs Ig1‐4 crystal structures (PDB ID: 1CS6, 3JXA, 3P3Y, 4X9H, 5K6U) revealing that their overall architecture is highly similar, as shown by a horseshoe arrangement of Ig1‐4. (B) Superimposition of the three Ig domains of CNTN2 (this study, colored in magenta) and CNTN1 (PDB ID: 7OL4, 7OL2, colored in blue and gray). The superimposition is based on the N‐terminal Ig1‐4 domain. The conformational difference between the two structures is evident from the large deviation between the CNTN2–Ig 5, 6 and CNTN1‐Ig 5, 6 in this superimposition. (C, D) the CNTN2 Ig1‐6 (Left) compared with CNTN1 Ig 1–6 (Right PDB ID: 7OL2) in electrostatic and hydrophobic surface representation colored according to various properties. For hydrophobic potential, the hydrophilic residues are labeled green and the hydrophobic residues are labeled yellow. For electrostatic potential, the negative charged residues are labeled red and the positive charged residues are labeled blue. The Unit of electrostatic potential is kT e−1. (E) Gel filtration studies of CNTN2 (2.0 mg·mL−1) and CNTN1 (3.0 mg·mL−1) mature full length, showing milli‐absorbance units (mAu) at different volumes. The gel filtration experiment was conducted in duplicate. (F) Sequence alignment of CNTN2 interface residues from the Ig4:Ig5, and Ig4:Ig6 generated from multiple sequence alignments of vertebrate CNTNs protein sequences. Key interfacial residues are indicated in red dots. The relative accessions for human CNTN1‐6 and Chicken CNTN2 sequences are Q12860, Q02246, Q9P232, Q8IWV2, O94779, Q9UQ52, and P28685. ESPript was used to generate the alignment result. (G) Four representative class averages of the high‐ordered CNTN2 conformation and a cartoon, which depend on the dimer for their formation (Scale bar: 2 nm). The structural figures were generated utilizing UCSF Chimera software.
Recently, the dimeric states of mouse CNTN1 Ig1‐6 have been also determined by X‐ray crystallography. Our human CNTN2 Ig1‐6 have Cα RMSD 6.83 Å and 7.79 Å to mouse unliganded and liganded CNTN1 Ig1‐6 (PDB: 7OL2 and 7OL4). Notably, in comparison to the crystal structure of CNTN1 Ig1‐6, the overall conformation of CNTN2 Ig1‐6 is more planar. The Ig5‐Ig6 domains in CNTN2 exhibit hinge angles of 20° and 30° in the unliganded and liganded states of CNTN1, respectively, as they orient towards an adjacent protomer in CNTN2, as illustrated in Fig. 3B,C. Furthermore, the Ig3‐4 interface of CNTN2 exhibits a mixed hydrophobic and hydrophilic nature, with complementary electrostatic interactions to Ig5‐6. In contrast, the region of CNTN1 is characterized by a predominance of negative charge (Fig. 3D). This might explain why CNTN1 cannot form a homodimer, which is consistent with the gel filtration result of CNTN1 full‐length (Fig. 3E; Fig. S10).
Sequence alignment of CNTNs, CNTN2 dimer interface Ig4‐6, and sequence variation of Ig4‐5 may lead to structural differences with other CNTNs in Fig. 3F, manifested as self‐associated modules. Thus, the dimerization interface appears to lack conserved features in other contact proteins but is conserved between human and chicken (Fig. 3F; Fig. S6, repeated experiment in Fig. S11).
CNTNs potentially assemble into high‐order oligomers to mediate cell–cell adhesion. Surprisingly, from the 2D particle sorting cryo‐EM results, higher‐order oligomers including dimer and trimer of the CNTN2 dimer constituting tetramers and hexamers were observed (Fig. 3G). Each dimeric CNTN2 appears to form higher‐order oligomers via the N‐terminal Ig1‐4 repeats. The angle between dimeric planes in the CNTN2 tetramer ranges from 20° to 180°, while a hexamer composed of three dimers contacts head‐to‐head at nearly 120° (Fig. 3). In our analysis, we conducted multimer examination through western blotting using a Myc antibody on isoforms in samples treated with DTT and boiled, as well as samples without DTT and unboiled. DTT was used to stabilize proteins with free sulfhydryl groups and can restore activity that has been lost due to the oxidation of these groups. The CNTN2 in the boiled samples migrated as a singular entity with a molecular mass of approximately 130 kDa, aligning with the monomer's size (Fig. S12). Conversely, in the unboiled samples without DTT, two substantial bands migrated following the monomer (Fig. S12). These findings are consistent with several different crystallographical homophilic dimer conformations for other IgSF proteins by the horseshoe Ig1‐4 domain in Fig. S13. These results suggest that multiple modes of homophilic interaction among IgSF members contribute to the geometry of CNTN2 molecules on the cell surface.
CNTN2 contains an extended Ig5‐6–FNIII1‐2 domains
In the asymmetric state, the density for FNIII1‐2 repeats was resolved in only one of two CNTN2 protomers. The Ig5‐FNIII1‐2 repeats adopt a ribbon‐like extended conformation. The resolution for FNIII1‐2 repeats is relatively low, while the remaining FNIII repeats are invisible, indicating conformational heterogeneity and flexibility of entire FNIII repeats. This flexibility is supported by 3D Variability analysis in this region and aligns with multiple conformations seen in reported CNTN FNIII repeat structures (Fig. 4). In the structure, the Ig5 repeat is bent from Ig4 repeat with an angle of 120°, a configuration maintained in the symmetric state. The angle between adjacent two domains in the Ig5‐6‐FNIII1‐2 repeats range is a variable range. The overall conformation of CNTN2 Ig5‐6‐FNIII1‐2 resembles that of CNTN3 except for the Ig5 repeat. The angle of the Ig5‐6 repeats in CNTN2 is much smaller than that of CNTN3, which has a nearly straighter shape. Similar to CNTN3 Ig5‐FNIII1‐2 (PDB:5I99), the RMSD between them was 6.09 Å with ~30° deflection between the Ig and FNIII junctions (Fig. 4D). The remainder of the FNIII region was not visible in our asymmetric reconstruction, likely because of the flexible connector between FNIII 2 and 3. In all reported crystal structures of CNTNs, FNIII1‐3 adopts a L‐shaped conformation with the longer FNIII1‐2 and shorter FNIII3 sides. To gain further insight into the conformational flexibility of the FNIII domain, we superimposed mouse CNTN 2 FNIII1‐3 (PDB:5E7L) with our structure with an RMSD of 7.51 Å between them (Fig. 4E). The hinge between FNIII2 and FNIII3 appears to be twisted by 90° in the side elevation of the Ig1‐6 domains, consistent with 2D results observed from different viewing angles, as shown in Fig. 4E,F. In conclusion, the importance of flexible regions (FNIII2 and FNIII3) allows CNTN2 to tilt within a wide range and form diverse complexes, facilitating movement on various cell surfaces.
Fig. 4.

The FNIII (fibronectin type III) domain of CNTN2 (contactin‐2) reveals a flexible conformation and does not contribute to the dimerization of CNTN2. (A, B) Three views of the CNTN2 Ig‐FNIII1‐2 cryo‐EM structure. (C) Superimposition of the two protomers of the asymmetric CNTN2 dimer reveals a highly similar Ig domain structure. The black lines and circles represent the rotation direction and angle. (D, E) Superimposition of CNTN2 Ig5‐FNIII1‐2 (colored in magnate) with CNTN5 (colored in orange, PDB ID:5I99) and CNTN2 FNIII1‐2 (colored in yellow) with mouse CNTN2 FNIII1‐3 (colored in blue, PDB ID:5E7L). (F) Representative 2D class averages of the CNTN2 full‐length conformation showing the flexibility of FNIII domain in solution (Scale bar: 2 nm). The structural figures were generated utilizing UCSF Chimera software.
CNTN2 mediates cell adhesion through the N‐terminal Ig domains, dependent on the homodimerization
To assess the structural features of CNTN2‐mediated cell adhesion, we generated a series of CNTN2 constructs with GFP inserted at the C‐terminal between the FNIII4 domain and GPI site (GPI‐anchor amidated asparagine), transfected HEK293F cells to measure the cell–cell cluster by confocal fluorescence microscopy (Fig. 5). The results showed that cell adhesion could be observed by transfection of full‐length CNTN2 and CNTN2 Ig1‐6 domains composed of a dimer interface (Fig. 5). In contrast, CNTN2 Ig1‐4 domains and CNTN2 C‐terminal FNIII domains failed to form an adhesive interface between cells (Fig. 5), confirming the importance of Ig‐like domains in CNTN2 homophilic adhesion. Moreover, the wild‐type CNTN2 and its Ig1‐6 domains exhibited localization at cell–cell junctions, with Ig1‐6 displaying a more distinct presence on the cell membrane compared to the wild type. Conversely, the CNTN2 Ig1‐4 mutant showed a diffuse distribution across the cell surface, while CNTN2 FNIII was located within the nucleus. This implies that, in the absence of Ig domains, even with its signal peptides, FNIII struggles to exit the nucleus and operate on the cell surface, indicating it doesn't contribute to cell adhesion independently. This observation aligns with findings from the cryo‐EM dimer structure. Furthermore, we investigated the impact of disrupting key residues at the CNTN2 dimerization interface on cell–cell contacts. Mutations, such as L330E, R355A, R506A, or R355/506A at key homodimer contact sites, resulted in a noticeable decrease in cell clustering, highlighting the crucial role of CNTN2 dimerization in CNTN2‐mediated cell adhesion. Notably, cells expressing the CNTN2 R355/506A mutant exhibited significantly reduced aggregation compared to those expressing wild‐type CNTN2 (Fig. 6). Moreover, CNTN2 mutants (L330E, R355A, and R506A) demonstrated a notable decrease in cell adhesion. Importantly, disrupting dimers did not impact the localization of CNTN2 cells on the cell membrane compared to the wild type, as shown in Fig. 6. These results suggest that the cryo‐EM dimer illustrates the adhesive interaction formed between molecules from opposing cells, indicating a trans interaction.
Fig. 5.

Cell adhesion is mediated by CNTN2 (contactin‐2) in HEK293F cells. (A) A diagram of the CNTN2 constructs with GFP (green) generated for cell adhesion assays. (B) Confocal images of subcellular localization of CNTN2 full length, CNTN2 lg1‐4, CNTN2 lg1‐6, and CNTN2 FNIII domains. The confocal experiments were conducted in duplicate (Scale bar: 10 μm). (C) Cell–cell adhesion was monitored by microscopy. HEK293F cells were transfected with CNTN2 full length, CNTN2 lg1‐4, CNTN2 lg1‐6, CNTN2 FNIII domains to form cell clusters (Scale bar: 100 μm). (D) Aggregation percent; the relative percentage of the 4 cells, 10 cells were classified compared to control as a cluster (Average ± SEM, n = 10). The experiments were conducted in duplicate, with a minimum of 500 cells counted from each data point. One‐way ANOVA was employed to compare the wild‐type (WT) group and the mutant groups for the counts of 4 cells and 10 cells, respectively. The structural figures were generated utilizing UCSF Chimera software.
Fig. 6.

Cell adhesion mediated by CNTN2 (contactin‐2) Ig4‐6 domains. (A) A diagram of the CNTN2 mutant constructs with GFP (green) generated for cell adhesion assays. (B) A diagram of the CNTN2 point mutation constructs with GFP (green) generated for cell adhesion assays (Scale bar: 10 μm). The red channel was modified to magenta to enhance accessibility for individuals with color vision deficiencies. The confocal experiments were conducted in duplicate. (C) Cell–cell adhesion monitored by fluorescence microscopy. HEK293F cell transfected with L330E, R355A, R432A, R480A, R506A, or R355/432/480/506A mutations (Scale bar: 100 μm). (D) Clustering index; the proportion of the total segmented cell area classified as a cluster (Average ± SEM, n = 10). The experiments were conducted in duplicate, with a minimum of 500 cells counted from each data point. One‐way ANOVA was employed to compare the wild‐type (WT) group and the mutant groups for the counts of 4 cells and 10 cells, respectively.
Discussion
The IgSF neural recognition proteins play pivotal roles in the formation and maintenance of the nervous system, including axon pathfinding, neuronal migration, target recognition, synapse formation, and myelination. These proteins consist of varying numbers of Ig and FNIII repeats and many of them form homophilic or heterophilic assemblies [8, 34, 35, 36]. Understanding the specific conformations of these proteins for self‐assembly or interaction with their partners is crucial for unraveling their cellular functions. While numerous structures of IgSF proteins have been reported, including Drosophila Dscam1, mouse sidekick1/2, human CNTN2, mouse neurexin155, and human L1 families, many studies have utilized truncated forms of these proteins with different combinations of Ig and FNIII repeats. The overall structures and assemblies in full‐length form remain largely unexplored.
In our study, we conducted biochemical and structural analyses of full‐length human CNTN2, revealing an interlocked bowknot‐like homodimer structure not previously unveiled by crystal structures. The horseshoe‐shaped Ig1‐4 repeats were traditionally considered crucial for CNTN2 dimer or polymer formation over the past two decades. However, the specific homophilic interaction model of CNTN2 has remained contentious. Crystallographic research only focused on Ig1‐4 repeats of human and chicken. CNTN2 found two distinct dimeric conformations and accordingly proposed either a zipper mode or a four/multiple molecule mode. In the zipper mode, CNTN2 forms a zipper‐shape polymer involving the interactions between the Ig2 FG loop of one molecule and the extended Ig3 CE loop of the next molecule. In the four or multiple molecule models, the symmetric dimer of Ig1‐4 repeats relying on the intermolecular β sheet formed by their Ig1 and Ig2 repeats is the assembly unit of CNTN2, and further oligomerization of CNTN2 requires the cis‐association of FNIII domains that have not been verified. However, the Ig1‐4 repeats of chicken and human CNTN2 exhibited monomeric behavior in solution, and thus, it is unclear whether the dimeric states of Ig1‐4 repeats captured by crystallographic analysis represent the physiological state of CNTN2. Our structure of the full‐length CNTN2 indicated that the interlocked Ig3‐6 repeats but not Ig1‐4 repeats that were chosen for crystallographic studies mediate the formation of CNTN2 homodimer. During the revision of our manuscript, Chataigner et al. reported the crystal structure of mouse CNTN2 Ig1‐6 repeats [37]. In the crystal, besides the intermolecular contact mediated by Ig1 and Ig2 repeats as found in the crystal structure of human CNTN2 Ig1‐4, mouse CNTN2 Ig1‐6 repeats also adopt a dimerization mode involving the Ig3‐6 repeats, which is consistent with our observation. Using a cell adhesion system, we found that CNTN2 Ig1‐6 repeats but not Ig1‐4 repeats can support cell–cell adhesion and cell clustering. Furthermore, mutations in the interface of Ig3‐6 repeats that would not disrupt Ig1‐4 oligomerization significantly attenuated cell clustering, suggesting the Ig5‐6 repeats is essential for the physiological functions of CNTN2. Besides, our 2D classification analysis indicated the presence of higher‐order oligomeric states, such as dimers or trimers of CNTN2 dimers. This observation may explain the existence of multiple modes in CNTN2 homophilic interactions, and the Ig1‐6‐mediated dimer resolved in our structure is the basic unit for its high‐order assembly. When superimposing the crystal structures of Ig1‐4 repeats on our structure, we found the human CNTN2 Ig1‐4 dimer [26], but not chicken Ig1‐4 dimer [11], is compatible with our CNTN2 dimer, suggesting the two types of homophilic interaction employed by human CNTN2 may constitute a linear CNTN2 oligomer between cell surfaces to mediate cell–cell contacts.
The homophilic Interaction mode of CNTN2 is different from those used by other IgCAM proteins. While the intermolecular β sheet constituted by Ig1/2 repeats is adopted by Neurofascin homodimers and by the CNTN1‐Neurofascin heterodimer, side‐by‐side stacking is employed by Dscam1 and SDK1. Notably, in a recent structure of CNTN1 Ig1‐6, the Ig6 repeat also participates in the homophilic interaction and contacts Ig3/4 repeats in the other protomer as in CNTN2 dimer, although two proteins display distinct dimeric structures. Further determination of other CNTN’ structures based on full‐length proteins or at least Ig1‐6 repeats will prove whether the Ig6‐Ig3/4 contact is a conserved manner for homodimer formation.
In contrast to the Ig repeats, our understanding of the FNIII domains’' contributions to the biological functions of CNTNs remains limited. Some studies have proposed that FNIII repeats may assist in organizing or clustering CNTNs on the cell surface when located in lipid rafts [38]. A study on the chicken CNTN2 demonstrated monoclonal antibodies targeting the FNIII4 repeat impaired homophilic cis‐binding properties [39]. However, the cryo‐EM structure of CNTN2 reveals the FNIII domain does not participate in dimerization. The flexible FNIII repeats may impart a unique unknown function. Negative staining EM images have demonstrated that the outer diameter distribution of human CNTN2 monomers varies from 210 to 315 Å [35, 40]. However, at interstitial axis‐rubber junctions, CNTN2, in conjunction with Caspr 2, is observed to span a range of 74 Å (paranodal width) to 150 Å (intermodal domain width) [35, 40]. The reduced super‐dimensions observed in CNTN2 dimers between paranodes and internodes are contingent on the flexibility of FNIII repeats preceding the GPI anchor.
Our study reveals the range of orientations observed for the C‐terminal FNIII domain and provides important information about a highly flexible region that induces trans patterning of the CNTN2 molecule, allowing it to switch between dimer and monomer states at the plasma membrane, While the dimer interaction interface is not directly influenced (Figs 6, 7), prior studies revealed S2 cell–cell aggregation through human CNTN2 and its FNIII domains [41]. Antibody mapping experiments, coupled with myeloma cell–cell interaction studies, pinpointed the significance of the FNIII domains, particularly the fourth domain, in CNTN2 induced cis cell–cell interaction [39]. Our cell experiments showed that the FNIII domain cannot autonomously mediate cell adhesion, which indicates that CNTN2 molecules trans interact forming a homodimer without in cis via its FNIII domains with other CNTN2 molecules. Furthermore, CNTN2 exhibits a reversible polymerization status in solution, which may play an important role in the dynamic behaviors of neural cell adhesion. The flexibility structure enables CNTN2 the ability to rapidly switch between dimer and monomer states, facilitating the recruitment or dissociation of partners within limited intercellular space, thus enabling fast assembly and disassembly during cell migration and mediating the function of voltage‐gated potassium ion channels. The intricate nature of full‐length IgSF protein structural analyses poses a challenge in comprehending the coupling mechanisms of their extracellular and intracellular regions. The few reported structures of full‐length IgSF proteins lack high‐resolution resolution of hetero‐ or homodimerization. Additionally, cryo‐EM structures of IgSF have never been reported. To enhance our comprehension of this vital protein family, additional structural investigations of the highly ordered CNTN2 full‐length protein anchored on lipid membranes and its complex with heterologous partners are necessary.
Fig. 7.

Schematic diagram of how CNTN2 (contactin‐2) regulates the myelin sheath distance based on concentration. The dimerization of CNTN2 exhibits a concentration‐dependent behavior. At relatively low concentrations of CNTN2, the protein predominantly exists in a monomeric form. However, as the concentration increases, there is a tendency for CNTN2 to form homodimers.
Materials and methods
Protein expression and purification
The cDNAs of human CNTN2 (residues 29‐1012, Gene ID: NM_005076.2) were subcloned into the mammalian secretory expression plasmid pSecTag2B vector with 6 × His‐Myc tag at the C‐terminus. CNTN2 mutations were generated by the Quikchange method using Q5 DNA polymerase (New England Biolabs, Ipswich, MA, USA).
HEK293F cells (Catalog No. R79007, RRID:CVCL_D603) have undergone authentication within the last 3 years, as all experiments were conducted within this timeframe following their acquisition from Thermo Fisher (Waltham, MA, USA). Furthermore, all experiments utilized mycoplasma‐free cells.
Plasmids encoding CNTN2 were transiently transfected into FreeStyle HEK293‐F cells with mediated by polyethyleneimine and SMM medium. After 5 days, the cell medium was collected by centrifugation and then passed through a 0.22 μm filter. The secreted proteins were bound to Ni‐NTA beads and eluted with 20 mm HEPES (N‐2‐hydroxyethylpiperazine‐N′‐2‐ethanesulfonic acid), pH 7.5, 150 mm NaCl, and 300 mm imidazole buffer. The target proteins were further purified by size‐exclusion chromatography using a Superose 6 increase 10/300 column (GE Healthcare, Chicago, IL, USA) in buffer 20 mm HEPES, pH 7.5, 150 mm NaCl. The proteins were initially concentrated to a concentration of 10 mg·mL−1. Subsequently, a series of dilutions were performed to obtain different low concentrations ranging from 0.04 to 3.57 mg·mL−1. These diluted samples were then injected into a Superose 6 increase 10/300 column for gel filtration experiments.
Cryo‐EM sample preparation and data collection
Purified CNTN2 was diluted to a concentration of 1 mg·mL−1 in 20 mm HEPES, pH 7.5, 150 mm NaCl, 0.01% LMNG, and applied to glow discharged holey carbon Quantifoil CF‐400 1.2/1.3 grids before being blotted for 3 s in an FEI Vitrobot Mark IV (Thermo‐Fisher Scientific, Waltham, MA, USA) and plunge frozen into liquid ethane. 5802 movies were collected from a single grid using a 300 kV FEI Titan Krios G3i (Thermo‐Fisher Scientific) equipped with a K3 detector (Gatan, Pleasanton, CA, USA). Data were collected at a magnification of 165 000× using seriaiem software [42], corresponding to a calibrated pixel size of 0.92 Å/pix, with a total electron dose of 50 e/Å2 for 32 frames and a defocus range from −1.0 to −2.5 μm. A full description of the data collection parameters can be found in Table 1.
Cryo‐EM data processing and atomic model building
The motion correction was performed using the MotionCor2 program [43]. The dose‐weighted and motion‐corrected movies were then imported into RELION‐3.125 to determine their defocus values and CTF parameters via gctf v.1.18. The particle picking was performed in Gautomatch developed by Kai Zhang (http://www.mrc‐lmb.cam.ac.uk/kzhang/) [44]. Particles were then imported into cryoSPARC v3.3.1 for 2D classification, ab initio 3D reconstruction, heterogeneous 3D refinement, non‐uniform homogeneous refinement, and local refinement. The two molecular reconstructions were sharpened in cryoSPARC [45]. The initial monomer model of CNTN2 was generated using the reported models of CNTN Ig1‐4 repeats, Ig5‐6 repeats, and FNIII1‐2 repeats (PDB:2OM5, 5I99, 5E4S), Iterative model building and refinement were performed with Chimera, Coot, and Phenix [46, 47, 48].
Analytical ultracentrifugation (AUC)
Purified CNTN2 was performed ultracentrifugation (Optima AUC‐A/I, Beckman Coulter, Indianapolis, IN, USA) with three different concentrations (3.8, 1.5, 0.5 mg·mL−1) at 20 °C, 280 nm/250 nm, 128 822 g , AN 60 Ti rotor, absorbance detector. The sample buffer: 20 mm HEPES, pH 7.5, 150 mm NaCl. A full description of the data processing parameters can be found in Table 2.
Table 2.
Data processing parameter.
| Parameter | |
| Resolution | 200 |
| s min | 0 S |
| s max | 15 S |
| Partial specific volume | 0.7300 |
| Buffer density | 1.0068 g·cm−3 |
| Buffer viscosity | 1.038 mPa·s |
| Confidence level (F‐ratio) | 0.68 |
| The parameter of c(s) model settings | |
| Parameter | |
| Resolution | 100 |
| s min | 0.5 S |
| s max | 15 S |
| Confidence level (F‐ratio) | 0.683 |
Cell adhesion assay
For cell adhesion assays, full‐length human CNTN2 with C‐terminal GFPSpark tag (Cat: HG10457‐ACG) was ordered from Sino Biological Company (Beijing, China). The removal of the GFP tag between the FNIII4 domain and GPI region in the CNTN2 construct, as well as the truncation and point mutant preparations, were performed using the Q5 Site‐Directed Mutagenesis Kit (NEB). For cell adhesion assays, HEK293F cells (2 × 107) were cultured in SMM medium (Bino Biological Inc.) at a density of 106 cells·mL−1. The cells were maintained at 37 °C, 5% CO2, and 120 rpm. After 48 h of transfection, approximately 1 × 106 cells were harvested, washed once with 1 × PBS buffer, and treated with trypsin for 30 s. The cells were then diluted to a concentration of 5 × 105 cells·mL−1 in SMM medium and incubated at room temperature with shaking for 1 h. Cell aggregation images were acquired using a ZEISS LSM 980 confocal microscope with a 10× objective lens, after confirmed the GFP expression of cells (Fig. S14). The quantification of cell aggregations follows the method previously reported by Beat Kunz et al. [39]. Briefly, cell aggregation was assessed by counting individual cells (1–4 cells), small aggregates (5–10 cells), and large aggregates (more than 10 cells). For each measurement, 10 randomly selected images were captured at a size of 900 × 675 μm. Subsequently, the “Analyze Particles” function of Fiji ImageJ was utilized after setting the appropriate threshold and particle size range. Approximately 200–500 particles per image were analyzed, and the percentage of aggregation for each image was normalized to the average of wild‐type groups and then plotted in the figures. The aggregation percentage of the 4 cells, 10 cells clusters were calculated as described. The data were presented as Average ± SEM [39].
In another part of the experiment, cells (5 × 104) were dispersed and incubated at 120 rpm for 2 h. Subsequently, the cells were plated on a 30 mm culture dish coated with 0.1 mg·mL−1 poly‐L‐Lysine in Borax buffer (pH 8.5) and incubated overnight. The cells were fixed with 4% (w/v) PFA, washed with PBS, stained with 1,1′‐Dioctadecyl‐3,3,3′,3′‐Tetramethylindodicarbocyanine, 4‐Chlorobenzenesulfonate Salt (Did) for cell plasma membrane (C1995S, Beyotime, Shanghhai, China), and stained with DAPI (4′, 6‐diamidino‐2‐phenylindole) for nuclear staining (Invitrogen™, D1306, Carlsbad, CA, USA). The samples were then mounted. All images were acquired using a ZEISS LSM 980 confocal microscope with a 63× objective lens with appropriate lasers for excitation (405, 488, and 638 nm) [32].
Pull‐down assay
Flag‐tagged secreted CNTN2 protein‐coupled on Anti‐DYKDDDDK G1 Affinity resin at 4 °C for 1 h in 20 mm Hepes pH 7.5, 150 mm NaCl, was mixed with cell lysis transfected with GFP‐CNTN2 mutations at 4 °C for overnight in buffer (20 mm Hepes pH 7.5, 150 mm NaCl, 5 mm PMSF), and washed three times with binding buffer, then eluted with 1 mg·mL−1 3× FLAG peptide. The input and output samples were boiled and loaded on 10% SDS‐PAGE (Sodium dodecyl‐sulfate polyacrylamide gel electrophoresis) followed by Coomassie blue (Absbio, Yancheng, Jiangsu, China) staining.
Conflict of interest
The authors affirm that the research was conducted without any commercial or financial relationships that might be perceived as a potential conflict of interest.
Author contributions
ZZZ, DPW, and FP designed and supervised the whole project. ZZZ performed biochemical preparations, ZZZ and WC performed cell adhesion studies and analysis, ZZZ prepared the protein samples, ZZZ and ZBS processed the cryo‐EM data, built the model, and performed the structural analysis of CNTN2. ZZZ collected the cryo‐EM data. ZZZ, WC, and ZBS prepared the manuscript. ZZZ, DPW, and FP have the final approval of the manuscript. All authors proofread the manuscript. Correspondence and requests for materials should be addressed to ZZZ.
Peer review
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer‐review/10.1111/febs.17364.
Supporting information
Fig. S1. Purification of CNTN2 and CNTN2 mutation.
Fig. S2. Data processing of the CNTN2 homodimer.
Fig. S3. Overall and local resolutions of maps.
Fig. S4. Analytical ultracentrifugation (AUC) fits for sedimentation velocity data of contactin 2 variants at different concentrations.
Fig. S5. The Negative staining image of CNTN2.
Fig. S6. Sequence alignment of CNTN2.
Fig. S7. Representative density maps of various parts of the structure.
Fig. S8. Cell–cell adhesion and Aggregation assays for N498A mutant.
Fig. S9. Sequence alignment of the CNTN Ig1‐4 domains with other IgSF proteins.
Fig. S10. Gel filtration analysis of CNTN1 at different concentrations.
Fig. S11. Repeated experiment of pull‐down assay of Flag‐tagged CNTN2 wild‐type protein and GFP‐tagged CNTN2 point mutations from transgenic cell lysates.
Fig. S12. CNTN2 expressed in 293F cells formed multimers.
Fig. S13. IgSF proteins homophilic interaction modes with the horseshoe domains.
Fig. S14. A set of images of CNTN2‐GFP transfected cells used for cell aggregation assays.
Table S1. CNTN2 full‐length analytical gel filtration data summary.
Acknowledgements
This work was supported in part by grants from Young Scientists Fund of the National Natural Science Foundation of China (No. 82201315), and the Shenzhen Science and Technology Program (No. KQTD20210811090115019). We thank Dr Kaige Yan, Dr Huawei Zhang, and Lei Wang (Southern University of Science and Technology) for their advises on sample preparation and data collection. We would like to express our gratitude to the Cryo‐EM Center at Southern University of Science and Technology for their assistance with data collection, as well as to the HPC‐Service Station.
Zhenzhen Zhang and Wei Chen contributed equally to this work.
Contributor Information
Zhenzhen Zhang, Email: zz.zhang@siat.ac.cn.
Fan Pan, Email: fan.pan@siat.ac.cn.
Daping Wang, Email: wangdap@mail.sustech.edu.cn.
Data availability statement
The atomic coordinates and cryo‐EM map have been deposited into the RCSB (entry ID: 8K3J, 8K53) and EMD database (entry ID: EMD‐36853, 36896), respectively. All the relevant data are available from the authors. Source data are provided with this paper.
References
- 1. Dodd J & Jessell TM (1988) Axon guidance and the patterning of neuronal projections in vertebrates. Science 242, 692–699. [DOI] [PubMed] [Google Scholar]
- 2. Shimoda Y & Watanabe K (2009) Contactins: emerging key roles in the development and function of the nervous system. Cell Adh Migr 3, 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Colakoglu G, Bergstrom‐Tyrberg U, Berglund EO & Ranscht B (2014) Contactin‐1 regulates myelination and nodal/paranodal domain organization in the central nervous system. Proc Natl Acad Sci USA 111, E394–E403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen YA, Lu IL & Tsai JW (2018) Contactin‐1/F3 regulates neuronal migration and morphogenesis through modulating RhoA activity. Front Mol Neurosci 11, 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chatterjee M, Schild D & Teunissen CE (2019) Contactins in the central nervous system: role in health and disease. Neural Regen Res 14, 206–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mohebiany AN, Harroch S & Bouyain S (2014) New insights into the roles of the contactin cell adhesion molecules in neural development. Adv Neurobiol 8, 165–194. [DOI] [PubMed] [Google Scholar]
- 7. Zoupi L, Savvaki M, Kalemaki K, Kalafatakis I, Sidiropoulou K & Karagogeos D (2018) The function of contactin‐2/TAG‐1 in oligodendrocytes in health and demyelinating pathology. Glia 66, 576–591. [DOI] [PubMed] [Google Scholar]
- 8. Grumet M & Edelman GM (1988) Neuron‐glia cell adhesion molecule interacts with neurons and astroglia via different binding mechanisms. J Cell Biol 106, 487–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tassano E, Uccella S, Giacomini T, Severino M, Fiorio P, Gimelli G & Ronchetto P (2018) Clinical and molecular characterization of two patients with CNTN6 copy number variations. Cytogenet Genome Res 156, 144–149. [DOI] [PubMed] [Google Scholar]
- 10. Bencharit S, Cui CB, Siddiqui A, Howard‐Williams EL, Sondek J, Zuobi‐Hasona K & Aukhil I (2007) Structural insights into fibronectin type III domain‐mediated signaling. J Mol Biol 367, 303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Freigang J, Proba K, Leder L, Diederichs K, Sonderegger P & Welte W (2000) The crystal structure of the ligand binding module of axonin‐1/TAG‐1 suggests a zipper mechanism for neural cell adhesion. Cell 101, 425–433. [DOI] [PubMed] [Google Scholar]
- 12. Dalva MB, McClelland AC & Kayser MS (2007) Cell adhesion molecules: signalling functions at the synapse. Nat Rev Neurosci 8, 206–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karuppan SJ, Vogt A, Fischer Z, Ladutska A, Swiastyn J, McGraw HF & Bouyain S (2022) Members of the vertebrate contactin and amyloid precursor protein families interact through a conserved interface. J Biol Chem 298, 101541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peles E & Salzer JL (2000) Molecular domains of myelinated axons. Curr Opin Neurobiol 10, 558–565. [DOI] [PubMed] [Google Scholar]
- 15. Chataigner LMP, Gogou C, den Boer MA, Frias CP, Thies‐Weesie DME, Granneman JCM, Heck AJR, Meijer DH & Janssen BJC (2022) Structural insights into the contactin 1 ‐ neurofascin 155 adhesion complex. Nat Commun 13, 6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lu Z, Reddy MV, Liu J, Kalichava A, Liu J, Zhang L, Chen F, Wang Y, Holthauzen LM, White MA et al. (2016) Molecular architecture of Contactin‐associated protein‐like 2 (CNTNAP2) and its interaction with Contactin 2 (CNTN2). J Biol Chem 291, 24133–24147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Traka M, Goutebroze L, Denisenko N, Bessa M, Nifli A, Havaki S, Iwakura Y, Fukamauchi F, Watanabe K, Soliven B et al. (2003) Association of TAG‐1 with Caspr2 is essential for the molecular organization of juxtaparanodal regions of myelinated fibers. J Cell Biol 162, 1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pinatel D, Hivert B, Saint‐Martin M, Noraz N, Savvaki M, Karagogeos D & Faivre‐Sarrailh C (2017) The Kv1‐associated molecules TAG‐1 and Caspr2 are selectively targeted to the axon initial segment in hippocampal neurons. J Cell Sci 130, 2209–2220. [DOI] [PubMed] [Google Scholar]
- 19. Fukamauchi F, Aihara O, Wang YJ, Akasaka K, Takeda Y, Horie M, Kawano H, Sudo K, Asano M, Watanabe K et al. (2001) TAG‐1‐deficient mice have marked elevation of adenosine A1 receptors in the hippocampus. Biochem Biophys Res Commun 281, 220–226. [DOI] [PubMed] [Google Scholar]
- 20. Shiota Y, Hirosawa T, Yoshimura Y, Tanaka S, Hasegawa C, Iwasaki S, An KM, Soma D, Sano M, Yokoyama S et al. (2021) A common variant of CNTNAP2 is associated with sub‐threshold autistic traits and intellectual disability. PLoS One 16, e0260548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Penagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, Sonnenblick LI, Gruver R, Almajano J, Bragin A et al. (2011) Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism‐related deficits. Cell 147, 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Penagarikano O & Geschwind DH (2012) What does CNTNAP2 reveal about autism spectrum disorder? Trends Mol Med 18, 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fathi A, Mirzaei M, Dolatyar B, Sharifitabar M, Bayat M, Shahbazi E, Lee J, Javan M, Zhang SC, Gupta V et al. (2018) Discovery of novel cell surface markers for purification of embryonic dopamine progenitors for transplantation in Parkinson's disease animal models. Mol Cell Proteomics 17, 1670–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meng N, Pan P, Hu S, Miao C, Hu Y, Wang F, Zhang J & An L (2023) The molecular mechanism of gamma‐aminobutyric acid against AD: the role of CEBPalpha/circAPLP2/miR‐671‐5p in regulating CNTN1/2 expression. Food Funct 14, 2082–2095. [DOI] [PubMed] [Google Scholar]
- 25. Poliak S, Salomon D, Elhanany H, Sabanay H, Kiernan B, Pevny L, Stewart CL, Xu X, Chiu SY, Shrager P et al. (2003) Juxtaparanodal clustering of shaker‐like K+ channels in myelinated axons depends on Caspr2 and TAG‐1. J Cell Biol 162, 1149–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mortl M, Sonderegger P, Diederichs K & Welte W (2007) The crystal structure of the ligand‐binding module of human TAG‐1 suggests a new mode of homophilic interaction. Protein Sci 16, 2174–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsiotra PC, Theodorakis K, Papamatheakis J & Karagogeos D (1996) The fibronectin domains of the neural adhesion molecule TAX‐1 are necessary and sufficient for homophilic binding. J Biol Chem 271, 29216–29222. [DOI] [PubMed] [Google Scholar]
- 28. Furley AJ, Morton SB, Manalo D, Karagogeos D, Dodd J & Jessell TM (1990) The axonal glycoprotein TAG‐1 is an immunoglobulin superfamily member with neurite outgrowth‐promoting activity. Cell 61, 157–170. [DOI] [PubMed] [Google Scholar]
- 29. Lieberoth A, Splittstoesser F, Katagihallimath N, Jakovcevski I, Loers G, Ranscht B, Karagogeos D, Schachner M & Kleene R (2009) Lewis(x) and alpha2,3‐sialyl glycans and their receptors TAG‐1, Contactin, and L1 mediate CD24‐dependent neurite outgrowth. J Neurosci 29, 6677–6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yoshihara Y, Kawasaki M, Tani A, Tamada A, Nagata S, Kagamiyama H & Mori K (1994) BIG‐1: a new TAG‐1/F3‐related member of the immunoglobulin superfamily with neurite outgrowth‐promoting activity. Neuron 13, 415–426. [DOI] [PubMed] [Google Scholar]
- 31. Tang H, Chang H, Dong Y, Guo L, Shi X, Wu Y, Huang Y & He Y (2018) Architecture of cell‐cell adhesion mediated by sidekicks. Proc Natl Acad Sci USA 115, 9246–9251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nikolaienko RM, Hammel M, Dubreuil V, Zalmai R, Hall DR, Mehzabeen N, Karuppan SJ, Harroch S, Stella SL & Bouyain S (2016) Structural basis for interactions between Contactin family members and protein‐tyrosine phosphatase receptor type G in neural tissues. J Biol Chem 291, 21335–21349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goodman KM, Yamagata M, Jin X, Mannepalli S, Katsamba PS, Ahlsen G, Sergeeva AP, Honig B, Sanes JR & Shapiro L (2016) Molecular basis of sidekick‐mediated cell‐cell adhesion and specificity. eLife 5, e19058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Faivre‐Sarrailh C & Devaux JJ (2013) Neuro‐glial interactions at the nodes of Ranvier: implication in health and diseases. Front Cell Neurosci 7, 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nans A, Einheber S, Salzer JL & Stokes DL (2011) Electron tomography of paranodal septate‐like junctions and the associated axonal and glial cytoskeletons in the central nervous system. J Neurosci Res 89, 310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pedraza L, Huang JK & Colman DR (2001) Organizing principles of the axoglial apparatus. Neuron 30, 335–344. [DOI] [PubMed] [Google Scholar]
- 37. Chataigner LMP, Tharichen L, Beugelink JW, Granneman JCM, Mokiem NJ, Snijder J, Forster F & Janssen BJC (2024) Contactin 2 homophilic adhesion structure and conformational plasticity. Structure 32, 60–73. [DOI] [PubMed] [Google Scholar]
- 38. Harris TJ, Ravandi A, Awrey DE & Siu CH (2003) Cytoskeleton interactions involved in the assembly and function of glycoprotein‐80 adhesion complexes in dictyostelium. J Biol Chem 278, 2614–2623. [DOI] [PubMed] [Google Scholar]
- 39. Kunz B, Lierheimer R, Rader C, Spirig M, Ziegler U & Sonderegger P (2002) Axonin‐1/TAG‐1 mediates cell‐cell adhesion by a cis‐assisted trans‐interaction. J Biol Chem 277, 4551–4557. [DOI] [PubMed] [Google Scholar]
- 40. Lu Z, Lei D, Seshadrinathan S, Szwed A, Liu J, Liu J, Rudenko G & Ren G (2018) 3D images of neuronal adhesion molecule Contactin‐2 reveal an unanticipated two‐state architecture. bioRxiv 386102. [Google Scholar]
- 41. Yazaki T, Han CX & Uemura K (1996) Cell adhesion proteins in the nervous system: immunoglobulin superfamily – structure, function and involvement in neurological diseases. No To Hattatsu 28, 475–483. [PubMed] [Google Scholar]
- 42. Mastronarde DN (2005) Automated electron microscope tomography using robust prediction of specimen movements. J Struct Biol 152, 36–51. [DOI] [PubMed] [Google Scholar]
- 43. Zheng SQ, Palovcak E, Armache JP, Verba KA, Cheng Y & Agard DA (2017) MotionCor2: anisotropic correction of beam‐induced motion for improved cryo‐electron microscopy. Nat Methods 14, 331–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Walter RL, Thiel DJ, Barna SL, Tate MW, Wall ME, Eikenberry EF, Gruner SM & Ealick SE (1995) High‐resolution macromolecular structure determination using CCD detectors and synchrotron radiation. Structure 3, 835–844. [DOI] [PubMed] [Google Scholar]
- 45. Punjani A, Rubinstein JL, Fleet DJ & Brubaker MA (2017) cryoSPARC: algorithms for rapid unsupervised cryo‐EM structure determination. Nat Methods 14, 290–296. [DOI] [PubMed] [Google Scholar]
- 46. Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC & Ferrin TE (2004) UCSF chimera – a visualization system for exploratory research and analysis. J Comput Chem 25, 1605–1612. [DOI] [PubMed] [Google Scholar]
- 47. Emsley P, Lohkamp B, Scott WG & Cowtan K (2010) Features and development of coot. Acta Crystallogr D Biol Crystallogr 66, 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liebschner D, Afonine PV, Baker ML, Bunkoczi G, Chen VB, Croll TI, Hintze B, Hung LW, Jain S, McCoy AJ et al. (2019) Macromolecular structure determination using X‐rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr D Struct Biol 75, 861–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Purification of CNTN2 and CNTN2 mutation.
Fig. S2. Data processing of the CNTN2 homodimer.
Fig. S3. Overall and local resolutions of maps.
Fig. S4. Analytical ultracentrifugation (AUC) fits for sedimentation velocity data of contactin 2 variants at different concentrations.
Fig. S5. The Negative staining image of CNTN2.
Fig. S6. Sequence alignment of CNTN2.
Fig. S7. Representative density maps of various parts of the structure.
Fig. S8. Cell–cell adhesion and Aggregation assays for N498A mutant.
Fig. S9. Sequence alignment of the CNTN Ig1‐4 domains with other IgSF proteins.
Fig. S10. Gel filtration analysis of CNTN1 at different concentrations.
Fig. S11. Repeated experiment of pull‐down assay of Flag‐tagged CNTN2 wild‐type protein and GFP‐tagged CNTN2 point mutations from transgenic cell lysates.
Fig. S12. CNTN2 expressed in 293F cells formed multimers.
Fig. S13. IgSF proteins homophilic interaction modes with the horseshoe domains.
Fig. S14. A set of images of CNTN2‐GFP transfected cells used for cell aggregation assays.
Table S1. CNTN2 full‐length analytical gel filtration data summary.
Data Availability Statement
The atomic coordinates and cryo‐EM map have been deposited into the RCSB (entry ID: 8K3J, 8K53) and EMD database (entry ID: EMD‐36853, 36896), respectively. All the relevant data are available from the authors. Source data are provided with this paper.


