Abstract
Purpose
This work investigated the effect of FBXO5 in hepatocellular carcinoma (HCC) and the mechanism of action of arbutin in its inhibition.
Methods
FBXO5 mRNA and protein expressions in the tumor were assessed using TCGA, ICGC and HPA databases. Cox regression analysis and Kaplan–Meier survival curves were employed to assess the impact of FBXO5 on the survival outcomes of patients with HCC. Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG), Gene Set Enrichment Analysis (GSEA), and Gene Set Variation Analysis (GSVA) were used to investigate the biological function associated with FBXO5-related genes. The role of FBXO5 as oncogene and the inhibitory mechanism of arbutin were confirmed through western blotting (WB), reverse transcription quantitative polymerase chain reaction (RT-qPCR), and in vitro experiments such as scratch wound-healing migration assay, plate clone formation assay, and transwell migration assay.
Results
Patients with high FBXO5 expression showed shorted overall survival (OS), progression-free survival (PFS), disease-specific survival (DSS), and disease-free survival (DFS). FBXO5 was identified as an independent prognostic risk factor associated with the cell cycle. In vitro investigations indicated that FBXO5 facilitated HCC progression by modulating the cell cycle, while arbutin suppressed FBXO5 expression and regulated cell cycle dynamics.
Conclusion
FBXO5 is a potential diagnostic and prognostic biomarker for HCC, and arbutin may exert anticancer effects through the suppression of FBXO5 expression.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12672-024-01729-z.
Keywords: Arbutin, HCC, FBXO5, Cell cycle
Introduction
Liver cancer is a prevalent and deadly malignant tumor with significant morbidity and mortality rates globally, particularly in China [1]. The primary types of liver cancer are hepatocellular carcinoma (HCC), cholangiocarcinoma, and mixed variants, with HCC being the most prevalent, representing 75–85% of all primary liver cancers worldwide and 93% in China [2]. Surgical intervention is the preferred approach for early-stage HCC, while chemotherapy and immunotherapy are used in advanced [3]. However, the efficacy of these treatments is limited, since only approximately one-third of patients show positive responses, and the development of drug resistance often hampers treatment outcomes. Consequently, it is of the utmost importance to develop innovative chemotherapeutic agents.
Natural compounds improve the beneficial effect of cancer therapy. A study performed in Europe established a correlation between the intake of fruits and vegetables and the decreased risk of cancer [4]. Certain natural compounds present in food and spices inhibit the growth of tumors, representing a promising avenue for the development of innovative chemotherapeutic agents [5]. Arbutin (p-hydroxyphenyl-β-D-glucoside) is a naturally soluble glycosylated phenol with antioxidant, antimicrobial, antitumor and anti-inflammatory properties. It is characterized by low binding affinity to plasma proteins, low lipophilicity, limited permeability, short half-life, and high oral bioavailability, thus facilitating its administration and absorption relative to certain poorly soluble natural compounds [6]. Ongoing research on arbutin revealed its expanding applications in the treatment of various diseases, especially its promising role in neuroprotection [7]. Additionally, arbutin has demonstrated potential therapeutic effects in addressing intestinal disorders. A study conducted by Di et al. indicated that arbutin enhances the maintenance of intestinal homeostasis by inhibiting the formation of neutrophil extracellular traps, preserving the integrity of the mucosal barrier, and modulating the composition of the gut microbiota, thereby alleviating colitis induced by dextran sulfate sodium [8]. Furthermore, the research of Oguzhan Birdal et al. demonstrated that arbutin is a protective agent against the cardiotoxic effects of doxorubicin in cancer patients [9]. Other investigations showed that arbutin significantly mitigates complete Freund’s adjuvant-induced arthritis by modulating anti-inflammatory cytokines, such as interleukin-10 (IL-10) and IL-4, as well as pro-inflammatory cytokine pathways, including nuclear factor kappa -B (NF-κB), tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), IL-6, prostaglandin E2 (PGE-2), 5-lipoxygenase (5-LOX), and cyclooxygenase-2 (COX-2), in addition to oxidative stress biomarkers [10]. Nevertheless, the potential role of arbutin in HCC treatment remains unexplored, thus in the need of comprehensive investigation.
Recent research demonstrated that F-box proteins (FBPs) serve as substrate receptors in the SCF ubiquitin ligase complex, comprising Skp1, CUL1, RBX1/ROC1, and various FBP components [11]. FBP is involved in determining the substrate specificity of the complex and modulating cellular growth and differentiation. FBPs are divided into three subclasses: FBXW, FBXL, and FBXO, the latter being the most diverse, encompassing 37 members including FBXO1, FBXO5, and FBXO45. FBPs are involved in the regulation of proliferation, apoptosis, drug resistance, and cancer [12, 13]. Specifically, the FBXO subclass, with its diverse members, is involved in the regulation of proliferation, apoptosis, drug resistance, cancer stem cells, epithelial–mesenchymal transition, and metastasis. Any dysregulation in FBP may disrupt the hydrolysis of protein substrates, potentially leading to the onset of malignant tumors [14].
FBXO5 is involved in various cellular processes such as cell proliferation, apoptosis, and epithelial-mesenchymal transition [15]. A study demonstrated that FBXO5 is upregulated in several types of malignant tumors, and its increased expression is closely associated to tumor invasion, metastasis, and unfavorable prognosis [16]. Recent investigations revealed that FBXO5 facilitates tumor cell proliferation by inhibiting Skp2 stability and promoting the degradation of p27 [17]. These findings underscore the significant involvement of FBXO5 in tumorigenesis and progression. Furthermore, a study suggested a potential correlation between FBXO5 mRNA expression and the impact of ribavirin on HCC, indicating that FBXO5 is an oncogene serving as a potential therapeutic target in HCC [18].
This study confirmed the increased FBXO5 mRNA and protein expression through the analysis of various databases. The impact of FBXO5 expression on the survival of patients with HCC was assessed using Cox regression analysis and Kaplan–Meier curves. Various analytical methods were used to investigate the biological functions of genes associated with FBXO5. Experimental validation techniques were used to confirm the oncogenic role of FBXO5 and the inhibitory mechanism of arbutin on HCC proliferation. These approaches provided insights into the involvement of FBXO5 in HCC progression and the potential therapeutic impact of arbutin targeting FBXO5 in HCC.
Materials and methods
Data collection and processing
RNA sequencing data and corresponding clinical information on HCC were obtained from The Cancer Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov/). The HCC microarray datasets GSE76427 and GSE64041 were obtained from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/). Moreover, ICGC-LIRI-JP gene expression data and its corresponding clinical information were obtained from the International Cancer Genome Consortium (ICGC) database (https://dcc.icgc.org/).
Survival analysis
The TCGA-Liver hepatocellular carcinoma (LIHC) cohort was divided into high and low expression groups according to the median FBXO5 expression. Kaplan–Meier survival analysis was used to determine the potential significant difference between the high and low expression groups in terms of overall survival (OS), progression-free survival (PFS), disease-free survival (DFS) and disease-specific survival (DSS), and the ICGC-LIRI-JP dataset was used as the validation set.
COX regression analysis and construction of a nomogram
The potential role of FBXO5 as an independent prognostic factor for OS in HCC patients was assessed by univariate and multivariate Cox regression analysis. The prognostic accuracy and predictive power were improved by the construction of a nomogram combining FBXO5 expression and clinical features to estimate the expected OS based on the TCGA-LIHC dataset. The precision, discrimination, and accuracy of the nomogram were evaluated using calibration curves, while the prognostic value of FBXO5 and other clinicopathological factors were evaluated using the ROC curves.
Functional enrichment analysis
Single-sample gene set enrichment analysis (ssGSEA) was used to calculate the enrichment score for the gene sets in the TCGA-LIHC dataset. In addition, Gene Set Variation Analysis (GSVA) score Gene Set Enrichment Analysis (GSEA) analysis was performed on 50 characterized pathways between the two groups. Differentially expressed genes (DEGs) between normal liver and HCC samples were identified using limma, and genes associated with FBXO5 in DEGs were identified using Pearson correlation coefficient. Finally, gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis were performed on FBXO5-related genes.
Molecular docking analysis
A molecular docking analysis of FBXO5 and arbutin was performed using an molecular docking module (www.dockeasy.cn) intrinsic to the website www.home-for-researchers.com. The results were subsequently visualized by PyMOL (version 2.40).
Cell culture
Hep-3B and SNU-449 HCC cell lines were obtained from Wuhan Purcell Life Sciences Co. and our laboratory, respectively. They were cultured in Gibco 1640 and DMEM mixed medium respectively, with 10% South American calf serum and antibiotics at 37 °C under 5% CO2. Arbutin analytical standard was dissolved in the medium according to the instructions to obtain a 122.42 mmol arbutin stock solution.
Transfection and construction of stable cell lines
siFBXO5-1 (5′-CCGGACUAAUACGUAAATT-3′) and siFBXO5-2 (5′-GCCCUAGGAUUUGUACAACUTT-3′) were purchased from Gemma Genetics (Shanghai, China), and transfection was performed at a concentration of 50 nM for 8 h. The FBXO5 overexpression stably transfected cell line was obtained by the construction of a lentivirus using an infection method (Hanhen), which was encoding the target gene or negative control lentivirus according to the 1/2 small volume infection method described in the instruction manual.
Real-time PCR
Cells were first treated with the Vazyme RNA Extraction Kit, total RNA was extracted, and reverse transcribed using a one-step reverse transcription system containing 5 × All-in-one qRT SuperMix, enzyme mix, template RNA, and RNase-free ddH2O. The reverse transcription reaction program was set to 50 °C for 15 min and 85 °C for 5 s. Finally, the PCR reaction system was set up according to the instructions of the TranStart Green qPCR Supermix kit. The primer sequences are shown in Table S1.
Western blot
Treated cells were collected and placed on ice for lysis, and then vortexed to extract total proteins. Next, they were quantified using a BCA kit (Biyun Tian, China) and denatured in a metal bath at 100 °C. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was performed using a 10% concentration gel at 70 V for 90 min and then at 110 V for 30 min. A standard western blot protocol was performed using Proteintech’s FBXO5 antibody (catalogue no. 10872-1-AP), ABclonal’s β-actin antibody (catalogue no. AC026) as a loading control, and Unitech Biotech’s goat anti-rabbit secondary antibody (catalogue no. GAR007).
Cell proliferation and migration assay
The number of surviving cells was measured using Cell Counting Kit-8 (CCK-8). CCK-8 solution was added to the cells into a 96-well plate and incubated for 2 h at 37 °C. Then, the absorbance of each well was measured at 450 nm. As regards the colony formation assay, 1000 cells were seeded in each well of a 6-well plate, and the medium was changed every 72 h. Cells were stained and fixed with crystal violet and 4% paraformaldehyde respectively, and clonal colonies were observed under a microscope. The migration ability of the cells was assessed using the wound healing assay and Transwell assay according to a standard protocol.
Flow cytometry
After 24 h of incubation with arbutin, the cells were digested with 0.25% trypsin without EDTA, centrifuged, resuspended in 70% pre-cooled ethanol, and incubated for 12–24 h at 4 °C. These cells were then resuspended in 500 μL PI premix and incubated at 37 °C for 30 min in the dark. The cell cycle was determined by flow cytometry and the relevant data were analyzed with ModFit LT5.0.
Statistical analysis
Statistical analysis was performed using SPSS 26.0 and R software 4.3.1. Statistical graphs were generated using GraphPadPrism 9.0. Results were expressed as mean ± standard deviation/standard error of the mean of at least three independent experiments. A value of P < 0.05 was considered statistically significant.
Results
High FBXO5 expression in HCC
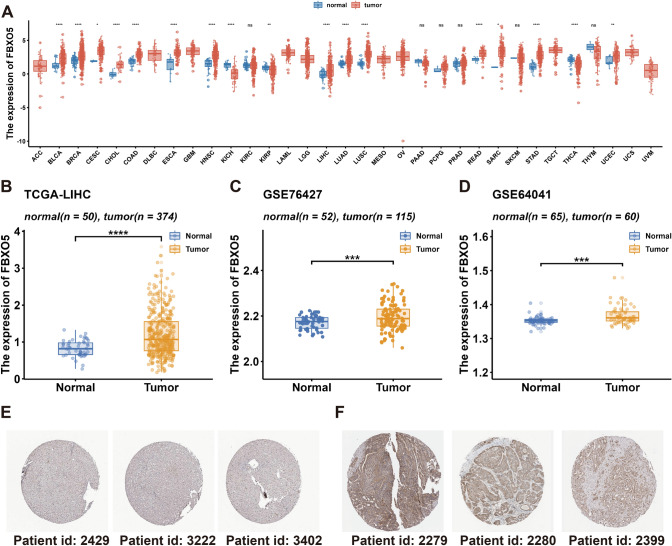
The expression of FBXO5 in cancer was assessed using TCGA pan-cancer data and various liver cancer datasets. The results shown in Fig. 1a indicated a significant upregulation of FBXO5 in bladder cancer, breast cancer, bile duct cell carcinoma, colon adenocarcinoma, head and neck squamous cell carcinoma, HCC, lung adenocarcinoma, and lung squamous cell carcinoma across pan-cancer datasets. Conversely, low FBXO5 expression was found in renal medullary kidney carcinoma, papillary renal cell carcinoma, and thyroid carcinoma. This differential expression profile underscored the potential pivotal role of FBXO5 in cancer pathogenesis. Furthermore, Fig. 1b (TCGA-LIHC), 1C (GSE76427), and 1D (GSE64041) show high FBXO5 expression in HCC tissues compared to normal controls. Immunohistochemistry results from the HPA database corroborated these findings by revealing higher FBXO5 protein expression in HCC tumor tissues than in normal tissues, as illustrated in Fig. 1e and f.
Fig. 1.
FBXO5 expression. a Pan-cancer expression of FBXO5. b Differential expression of FBXO5 in the tumor versus normal tissue in the TCGA-LIHC cohort. c Differential expression of FBXO5 in the tumor versus normal tissue in the GSE76427 cohort. d Differential expression of FBXO5 in the tumor versus normal tissue in the GSE64041 cohort. e Immunohistochemical staining of FBXO5 in normal liver tissue, as revealed by the HPA database. f Immunohistochemical staining of FBXO5 in HCC tissue, as revealed by the HPA database. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
High FBXO5 expression is associated with prognosis in HCC patients
The clinical implications of FBXO5 expression were evaluated by categorizing HCC patients based on age, stage, and T stage. Data analysis revealed a different FBXO5 expression on each of these patient subgroups. Patients younger than 65 years showed high FBXO5 expression (Fig. 2a). Stratification by cancer stage demonstrated distinct FBXO5 expression patterns in relation to both stage and T stage (Fig. 2b and c), suggesting a potential pivotal role of FBXO5 in the tumorigenesis process. Furthermore, the investigation into the impact of FBXO5 on the survival outcome of HCC patients with high FBXO5 expression revealed a potential association with poorer OS, PFS, DFS, and DSS prognosis in TCGA-LIHC and ICGC-LIRI-JP cohorts than patients with low FBXO5 expression (Fig. 2d–h).
Fig. 2.
Prognostic analysis of FBXO5. a Differential expression of FBXO5 across various age subgroups in the TCGA-LIHC cohort. b Differential expression of FBXO5 across different stages of HCC in the TCGA-LIHC cohort. c Differential expression of FBXO5 across T-stages of HCC in the TCGA- LIHC cohort. d-g Survival curves of OS, PFS, DFS, DSS in patients with high and low FBXO5 expression in the TCGA-LIHC cohort; h Correlation between FBXO5 expression and OS in the ICGC-LIRI-JP cohort. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
FBXO5 is an independent risk factor for HCC
The prognostic characteristics of FBXO5 were investigated by Cox regression modeling. FBXO5 was a risk factor in both the TCGA-LIHC dataset (HR: 1.62, 95% CI 1.33–1.96, P < 0.001) and the ICGC-LIRI-JP dataset (HR: 2.11, 95% CI 1.46–3.05, P < 0.001) (Fig. S1A, B) according to the univariate Cox regression analysis. FBXO5 was also an independent risk factor for overall survival (OS) in the TCGA-LIHC dataset (HR: 1.58, 95% CI 1.46–3.05, P < 0.001) and the ICGC-LIRI-JP dataset (HR: 2.23, 95% CI 1.49–3.34, P < 0.001) (Fig. S1C and D) according to the multivariate Cox regression analysis. To enhance the prediction of survival in patients with HCC, a nomogram was constructed based on FBXO5 and other independent clinicopathological factors (Fig. S1E). A score was assigned to each variable derived from multifactor Cox regression results, and these scores were aggregated to obtain a total score. The prognostic probability at 1, 3, and 5 years for each HCC patient was determined by correlating the total score with the corresponding outcome line. The nomogram’s performance was evaluated by calibration curves, illustrating its accurate prediction of mortality at 1, 3, and 5 years in HCC patients (Fig. S1F). Additionally, the prognostic value of FBXO5 and other clinicopathological factors were compared using receiver operating characteristic (ROC) curves, indicating that FBXO5 exhibited superior prognostic value relative to other independent clinicopathological factors (Fig. S1G-I).
Transcriptome profiling of HCC patients with different FBXO5 expression
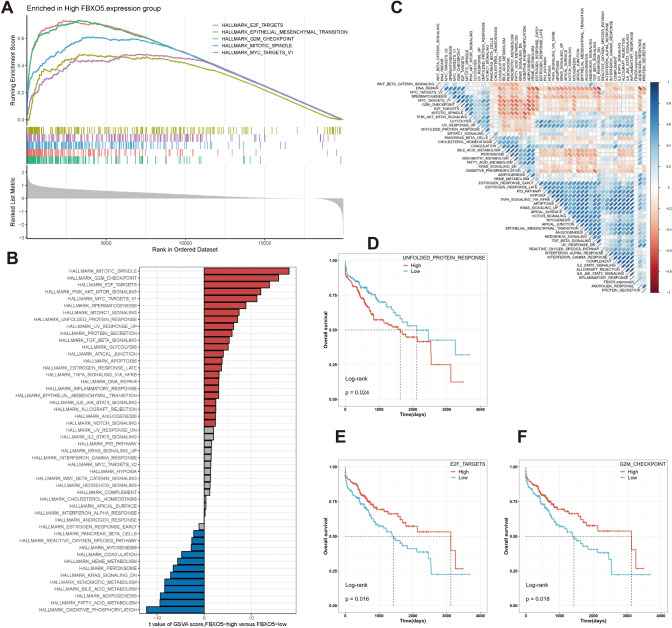
The molecular mechanism of FBXO5 expression and HCC prognosis was investigated by functional enrichment analysis. GSEA analysis using the Hallmark gene set in the Molecular Signatures Database showed that pathways associated with the cell cycle, cell proliferation, and epithelial-mesenchymal transition, including E2F targets, epithelial-mesenchymal transition, G2M checkpoints, mitotic spindle, and MYC target v1 were enriched in the FBXO5 high-expression group (Fig. 3a). In addition, GSVA analysis (Fig. 3b) showed that the FBXO5 high-expression group exhibited higher activity in pathways closely related to tumorigenesis, such as cell proliferation and regulation, energy metabolism, immune response, and inflammatory response, while the FBXO5 low-expression group exhibited stronger activity in cell metabolism and oxidative stress-related pathways. Correlation analysis further confirmed the association between FBXO5 expression and marker pathway scores (Fig. 3c), indicating that its expression is closely associated with cancer-related biological processes and metabolic pathways.
Fig. 3.
FBXO5-related functional enrichment analysis. a GSEA analysis revealing the enriched GO terms in the group with high FBXO5 expression. b GSVA scores indicating differences in hallmark pathway activity between the groups with high and low FBXO5 expression. c GSVA scores demonstrate a correlation between FBXO5 expression and hallmark pathway activities. d–f Kaplan–Meier survival plots illustrating a significant correlation between OS and GSVA scores of G2M_CHECKPOINT (d), E2F_TARGETS (e), and UNFOLDED_PROTEIN_RESPONSE (f)
The relationship between the marker pathways and HCC prognosis was investigated by the Kaplan–Meier curve analysis. The results showed that the pathways G2M_CHECKPOINT, E2F_TARGETS and UNFOLDED_PROTEIN_RESPONSE were positively correlated with FBXO5 expression (Fig. 3d–f). Activation or inhibition of these pathways might be responsible for the different prognoses of patients with high FBXO5 expression.
The differences in gene expression between tumors and normal tissues revealed that 3618 DEGs were identified based on the TCGA-LIHC cohort using the limma software package (|logFC|> 0.585 and FDR < 0.05) with the results visualized by heatmap (Fig. S2A). The Pearson correlation coefficient between DEGs and FBXO5 was subsequently calculated (|correlation|> 0.8 and P < 0.05), revealing that 63 genes were significantly associated with FBXO5 (Fig. S2B). An enrichment analysis using these 63 genes associated with FBXO5 was performed to gain insight into the biological processes associated with the expression of FBXO5. The KEGG enrichment results indicated that FBXO5-related genes were mainly involved in cell cycle-related pathways (Fig. S2C). GO results also showed that FBXO5-related genes were mainly involved in cell division and cell cycle processes in Biological Processes (BP), chromosomes, spindle, and microtubule-containing matrices in Cell Components (CC), as well as microtubule-binding, kinetochore-binding, and microtubule-protein-binding in Molecular Functions (MF) (Fig. S2D).
FBXO5 affects the proliferation and migration of HCC cells
Transient transfection using the interfering fragments FBXO5-siRNA1 and FBXO5-siRNA2 reduced FBXO5 mRNA expression in Hep-3B and SNU-449 cells, as confirmed by RT-qPCR (Fig. 4a). Stable cell lines overexpressing FBXO5, termed SNU449-FBXO5-overexpression (449-F5-OE), were generated using lentiviral infection of SNU-449 cells. Fluorescence imaging showed the effectiveness of viral packaging in HCC cells, and RT-qPCR confirmed the significant overexpression of FBXO5 mRNA (Fig. 4b and c). Scratch wound-healing migration assay showed that FBXO5 gene knockdown using interfering fragments significantly impaired cell healing and prolonged the healing time (Fig. 4d–e). Colony formation and transwell migration assays performed on SNU-449 cells, and revealed that the proliferation and migration abilities of the FBXO5 knockdown group were reduced compared to the control group (Fig. 4f–i). These results suggested that the down-regulation of the FBXO5 gene could inhibit the proliferation and migration of HCC cells.
Fig. 4.
FBXO5 affects HCC cell proliferation and migration. a Impact of various interference fragments on FBXO5 mRNA expression in Hep-3B and SNU-449 cells by RT-qPCR. b Spontaneous fluorescent imaging of virus encapsulation. c FBXO5 mRNA expression in SNU449-FBXO5-OE cell lines by RT-qPCR. d Scratch wound-healing migration assay performed on Hep-3B and SNU-449 cells pre and post-interference. e Healing rate derived from the wound healing assays. f–g Plate clone formation assays and transwell migration assay performed on SNU-449 cells. h–i Quantification of the plate clone formation and transwell migration assay on SNU-449 cells. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
Arbutin inhibits the proliferation and migration of HCC cells
High concentrations of arbutin significantly reduced the viability of Hep-3B and SNU-449 HCC cells (Fig. 5a), with IC50s of 43 mM and 48 mM, respectively. Ten mM arbutin had a slight promoting effect on Hep-3B cells, but no effect on SNU-449 cells. Arbutin significantly inhibited the expression of FBXO5 mRNA at both 30 mM and 40 mM, with the latter inducing a stronger inhibitory effect (Fig. 5b). Therefore, 40 mM was chosen as the working concentration of arbutin in the subsequent functional experiments. FBXO5 mRNA expression in 449-F5-OE was significantly reduced by the addition of arbutin (Fig. 5c). The results of the scratch wound-healing migration assay showed that wound repair was significantly reduced by the addition of 30 mM and 40 mM arbutin to SNU-449 and Hep-3B cells, respectively, and the wound recovery time was significantly prolonged by the addition of arbutin compared with the control group (Fig. 5d). In addition, 40 mM arbutin significantly inhibited the proliferation of Hep-3B and SNU-449 HCC cells, as revealed by colony formation assay (Fig. 5e and g). Arbutin effectively inhibited the migration ability of Hep-3B and SNU-449 HCC cells, as revealed by transwell migration assay (Fig. 5f and h).
Fig. 5.
Arbutin reduces HCC cell proliferation and migration. a Cytotoxic effect of arbutin on Hep-3B and SNU-449 HCC cells by CCK8 assay. b Effect of different concentrations of arbutin on FBXO5 mRNA expression in Hep-3B and SNU-449 HCC cells by RT-qPCR. c Effect of different concentrations of arbutin Hep-3B and SNU-449 HCC cell migration by Scratch wound-healing migration assay. d Wound healing rate comparison before and after treatment. e Colony formation assay performed on Hep-3B and SNU-449 HCC cells after the treatment with arbutin. f Cell migration assay performed on Hep-3B and SNU-449 HCC cells. g Number of colonies before and after treatment. h Cell migration before and after treatment. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
Arbutin affects HCC by inhibiting FBXO5 expression
The potential relationship between arbutin and FBXO5 was assessed to elucidate the mechanism used by arbutin to inhibit the proliferation and migration of HCC cells. Molecular docking experiments identified potential binding sites for arbutin and FBXO5, revealing that this gene might be a potential target of arbutin (Fig. 6a). Forty mM arbutin significantly inhibited the expression of FBXO5 mRNA in SNU449-FBXO5-OE cells (Fig. 6b), and FBXO5 protein expression was increased in the cell line compared with that in the control group. Arbutin treatment significantly reduced this expression (Fig. 6c). Arbutin 30 mM and 40 mM treatment reduced FBXO5 protein expression in Hep-3B and SNU-449 cells, respectively (Fig. 6d). SNU449-FBXO5-OE cells migrated better than the control, while arbutin affected this migration (Fig. 6e and f). FBXO5 knockdown resulted in SNU-449 cells aggregating in the G0/G1 phase, whereas arbutin led to S-phase blockage in SNU-449 cells (Fig. 6g). FBXO5 gene overexpression resulted in an increase in cells entering the G2/M phase (Fig. 6h), whereas arbutin inhibited this increase. These findings suggested that FBXO5 might interact with cell cycle regulatory proteins to promote the transition of cells from the G0/G1 phase to the G2/M phase; thus arbutin not only exerted an effect on the cell cycle, but also blocked FBXO5 overexpressing cells in the S phase.
Fig. 6.
Arbutin affects HCC by inhibiting FBXO5 expression. a Molecular docking of FBXO5 with arbutin. b FBXO5 mRNA expression in different treatment groups. c-d FBXO5 protein expression in different treatment groups. e–f Cell migration in different treatment groups, and their quantification. g-h Cell cycle results in different treatment groups. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
Discussion
HCC is one of the most malignant tumors, usually originating from hepatocellular or intrahepatic cholangiocarcinoma cells. A serious feature of HCC is the rapid metastasis to multiple organs, including the lungs, bones, and brain, leading to multi-organ dysfunction [19]. Although surgical treatment provides good outcomes for early-stage patients, the survival prospects for advanced-stage patients remain limited [20]. Therefore, it is crucial to identify new biomarkers for HCC diagnosis and treatment options.
FBXO5 is a target gene of the E2F transcription factor and it is a cell cycle regulatory protein [21]. FBXO5 is also involved in replication fidelity by inhibiting the mitotic E3 ligase APC/C, which results in a reduction in FBXO5 expression, leading to impaired DNA replication [22, 23]. In addition, FBXO5 promotes tumor angiogenesis [24, 25]. An increasing number of studies showed a significant association between FBXO5 expression and tumor progression. Our research indicated that FBXO5 expression was significantly upregulated in HCC tissues, being involved in the regulation of HCC proliferation, differentiation and cell cycle progression.
Further studies by clinical correlation analysis that FBXO5 was highly expressed in late and T stages. In addition, prognostic analysis showed that FBXO5 was associated with poor OS, PFS, DFS, and DSS prognostic outcomes. The impact of FBXO5 on prognosis was investigated by integrating FBXO5 and conventional clinicopathological variables into univariate and multivariate Cox regression analyses. The results showed that FBXO5 served as an independent prognostic factor for poor OS in HCC patients. Subsequently, the plotted FBXO5-associated nomograms combining FBXO5 expression and other clinical factors (including age, gender, stage, T stage, N stage, and M stage) showed that the predicted FBXO5-related nomograms closely matched the actual observed 1, 3 and 5-year OS probabilities. In addition, the ROC curves showed that FBXO5 predicted 1, 3 and 5-year OS more accurately in HCC patients compared with other conventional clinicopathological factors. In conclusion, FBXO5 was important in the prognosis of HCC patients.
GSEA, GSVA, GO, and KEGG were used to investigate the role of FBXO5 in HCC. GSEA and GSVA enrichment analyses showed that the high FBXO5 expression was associated with the mitotic spindle, and the G2M checkpoint and the E2F targeting pathways were significantly correlated. In addition, GO and KEGG enrichment results indicated that FBXO5-related genes were mainly enriched in pathways related to cell division and cell cycle. Previous studies emphasized the key role of the G2M checkpoint in the development and progression of HCC [26, 27]. In addition, E2F is the downstream effector of the cell cycle signaling pathway with significant effects on cell proliferation, differentiation and apoptosis [28–30]. Moreover, the mitotic spindle increases chromosomal instability and accelerates the progression of HCC. Therefore, FBXO5 might play a key role in the occurrence and development of HCC by regulating the cell cycle, but its molecular mechanism remains unclear.
The key role of FBXO5 as an oncogene in the pathogenesis of HCC was identified by bioinformatics analysis. Our in vitro experiments revealed that FBXO5 overexpression enhanced the proliferation and migration of HCC cells, while FBXO5 knockdown inhibited these processes. Our findings were consistent with those of Zhang et al. [31] and Jiang et al. [32] who investigated the effects of FBXO5 on tumor cell growth in gastric and cervical cancer. These loads of evidence strengthened the oncogenic role of FBXO5 and suggested that it could be a potential new target for cancer therapy.
Arbutin is a biologically active polyphenol consisting of two isomers: α-arbutin and β-arbutin [33]. It is widely used by the cosmetic industry for its ability to inhibit tyrosinase and hinder melanogenesis. Recent studies revealed the anti-inflammatory and anticancer properties of arbutin. For example, arbutin effectively induced apoptosis in glioblastoma cells with an IC50 of 30 mM [34], which is similar to what was found in Hep-3B and SNU-449 HCC cells in our experiments. This finding implies that high concentrations of arbutin might be effective. Further experiments showed that arbutin at concentrations of 30 mM and 40 mM inhibited the proliferation and migration of Hep-3B and SNU-449 HCC cells.
The study of the mechanism used by arbutin to suppress the proliferation and migratory ability of HCC cells, revealed that arbutin reduced the expression of both FBXO5 mRNA and protein. Molecular docking analyses further suggested the presence of potential binding sites between arbutin and FBXO5. These findings implied that arbutin might modulate the transcription of FBXO5 by affecting its promoter or associated transcription factors. Additionally, although RT-qPCR results indicated a reduction in mRNA expression, it remains essential to evaluate whether arbutin exerts its effects on FBXO5 through pathways related to protein degradation. Furthermore, arbutin exhibits broad anticancer potential through antioxidant, anti-inflammatory, and pro-apoptotic mechanisms. It reduces ROS, induces apoptosis, and suppresses inflammatory cytokines in prostate cancer [35], while inhibiting proliferation and migration in osteosarcoma via the miR-338-3p/MTHFD1L axis and AKT/mTOR signaling pathway [36]. In breast cancer, β-arbutin induces apoptosis through the p53-Caspase 3 pathway and inhibits estrogen receptor-α but may cause inflammation at higher doses [37]. In liver cancer, arbutin mitigates DEN-induced carcinogenesis by enhancing antioxidant activity and reducing inflammatory markers [38]. Additionally, acetylated arbutin promotes apoptosis and G1 arrest in melanoma by disrupting mitochondria and downregulating Bcl-xL and Bcl-2 [39]. These findings support the therapeutic potential of arbutin against multiple cancers.
In addition, a higher proportion of cells in the FBXO5 knockout group were arrested in the G0/G1 phase compared to the control group, while more cells in the FBXO5 overexpression group entered the G2/M phase. This result suggested that FBXO5 promoted cell cycle progression from G0/G1 to G2/M. These observations were consistent with those of other studies [40] and supported the hypothesis that FBXO5 is involved in cell cycle regulation in HCC. Nonetheless, the underlying mechanisms remain to be further investigated. Notably, arbutin significantly affected the cell cycle of the HCC cell line SNU449, resulting in most cells being arrested in S phase, which was different from the results of FBXO5 knockdown. Our hypothesis was that in addition to the down-regulation of FBXO5 expression, arbutin might target other cell cycle-related proteins, leading to cell arrest in S phase. More detailed exploration and studies are needed to elucidate these effects.
In conclusion, our bioinformatics analyses and experiments confirmed the high expression of FBXO5 in HCC tissues. FBXO5 resulted involved in cell cycle progression, proliferation and migration of HCC cells. Moreover, our investigation revealed that arbutin inhibited both the proliferation and migration of HCC cells. Preliminary findings suggested that this inhibitory effect was associated with the ability of arbutin to downregulate FBXO5 mRNA and protein expression. However, our study is not without limitations. The specific downstream signaling pathways regulated by FBXO5 were not investigated, nor the effects of arbutin on HCC in vivo through animal models. Addressing these limitations will be the primary focus of our future research. Thus, as an oncogene, FBXO5 contributed to tumor development and might be a potential therapeutic target for HCC. Arbutin may hold potential for treating HCC by inhibiting the expression of FBXO5, suggesting that it could serve as a novel targeted therapeutic agent.
Supplementary Information
Author contributions
SZ took the lead in designing and executing the experiment, as well as drafting the primary content of the manuscript. KY conducted certain bioinformatics analyses and contributed to the manuscript. YP, SY, and ZH were involved in executing a portion of the experiment. XP and TL were responsible for manuscript revisions and providing guidance throughout the experiment.
Funding
This study was supported by the Scientific Research Fund of Anhui Provincial Department of Education (KJ2021A0711), the Anhui Provincial College Students’ Innovation Training Programme Project (S202310367124) and the Natural Science Key Project of Bengbu Medical University (2022byzd023).
Data availability
RNA sequencing data for HCC were obtained from TCGA (https://portal.gdc.cancer.gov/), LIHC microarray data were obtained from GEO (https://www.ncbi.nlm.nih.gov/geo/), ICGC-LIRI- JP and its corresponding clinical information were obtained from ICGC (https://dcc.icgc.org/).
Declarations
Competing interests
The authors declare that they do not have any competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xueshan Pan, Email: panxues2011@163.com.
Tonggang Li, Email: ltg2020@bbmc.edu.cn.
References
- 1.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–14. 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 2.Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 2020;1873: 188314. 10.1016/j.bbcan.2019.188314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao L, Wu ZX, Assaraf YG, Chen ZS, Wang L. Overcoming anti-cancer drug resistance via restoration of tumor suppressor gene function. Drug Resist Updat. 2021;57: 100770. 10.1016/j.drup.2021.100770. [DOI] [PubMed] [Google Scholar]
- 4.Soerjomataram I, Oomen D, Lemmens V, Oenema A, Benetou V, Trichopoulou A, Coebergh JW, Barendregt J, de Vries E. Increased consumption of fruit and vegetables and future cancer incidence in selected European countries. Eur J Cancer. 2010;46:2563–80. 10.1016/j.ejca.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 5.Garg P, Garg R, Horne D, Awasthi S, Salgia R, Singhal SS. Prognostic significance of natural products against multidrug tumor resistance. Cancer Lett. 2023;557: 216079. 10.1016/j.canlet.2023.216079. [DOI] [PubMed] [Google Scholar]
- 6.Wang QL, Zhang PX, Shen R, Xu M, Han L, Shi X, et al. Determination of arbutin in vitro and in vivo by LC-MS/MS: Pre-clinical evaluation of natural product arbutin for its early medicinal properties. J Ethnopharmacol. 2024;330: 118232. 10.1016/j.jep.2024.118232. [DOI] [PubMed] [Google Scholar]
- 7.Sharma A, Kakkar A, Khanna M, Devi S. Arbutin’s potential in neuroprotection: a promising role in mitigating neurodegenerative diseases. Curr Drug Res Rev. 2024. 10.2174/0125899775298987240528050110. [DOI] [PubMed] [Google Scholar]
- 8.Qin D, Liu J, Guo W, Ju T, Fu S, Liu D, et al. Arbutin alleviates intestinal colitis by regulating neutrophil extracellular traps formation and microbiota composition. Phytomedicine. 2024;130: 155741. 10.1016/j.phymed.2024.155741. [DOI] [PubMed] [Google Scholar]
- 9.Birdal O, Ferah Okkay I, Okkay U, Bayram C, Mokthare B, Ertugrul MS, et al. Protective effects of arbutin against doxorubicin-induced cardiac damage. Mol Biol Rep. 2024;51:532. 10.1007/s11033-024-09488-4. [DOI] [PubMed] [Google Scholar]
- 10.Sial NT, Malik A, Iqbal U, Rehman M. Arbutin attenuates CFA-induced arthritis by modulating expression levels of 5-LOX, NF-κB, IL-17, PGE-2 and TNF-α. Inflammopharmacology. 2024;32:2377–94. 10.1007/s10787-024-01480-5. [DOI] [PubMed] [Google Scholar]
- 11.Skowyra D, Craig KL, Tyers M, Elledge SJ, Harper JW. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell. 1997;91:209–19. 10.1016/s0092-8674(00)80403-1. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, Liu P, Inuzuka H, Wei W. Roles of F-box proteins in cancer. Nat Rev Cancer. 2014;14:233–47. 10.1038/nrc3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uddin S, Bhat AA, Krishnankutty R, Mir F, Kulinski M, Mohammad RM. Involvement of F-BOX proteins in progression and development of human malignancies. Semin Cancer Biol. 2016;36:18–32. 10.1016/j.semcancer.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Yan L, Lin M, Pan S, Assaraf YG, Wang ZW, Zhu X. Emerging roles of F-box proteins in cancer drug resistance. Drug Resist Updat. 2020;49: 100673. 10.1016/j.drup.2019.100673. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Y, Tang Q, Ni R, Huang X, Wang Y, Lu C, Shen A, Wang Y, Li C, Yuan Q, Chen H, Cheng C, He S. Early mitotic inhibitor-1, an anaphase-promoting complex/cyclosome inhibitor, can control tumor cell proliferation in hepatocellular carcinoma: correlation with Skp2 stability and degradation of p27(Kip1). Hum Pathol. 2013;44:365–73. 10.1016/j.humpath.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 16.Liu P, Wang X, Pan L, Han B, He Z. Prognostic significance and immunological role of FBXO5 in human cancers: a systematic pan-cancer analysis. Front Immunol. 2022;13: 901784. 10.3389/fimmu.2022.901784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heo J, Eki R, Abbas T. Deregulation of F-box proteins and its consequence on cancer development, progression and metastasis. Semin Cancer Biol. 2016;36:33–51. 10.1016/j.semcancer.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu C, Luo L, Yu Y, Zhang Z, Zhang Y, Li H, Cheng Y, Qin H, Zhang X, Ma H, Li Y. Screening therapeutic targets of ribavirin in hepatocellular carcinoma. Oncol Lett. 2018;15:9625–32. 10.3892/ol.2018.8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nathan H, Hyder O, Mayo SC, Hirose K, Wolfgang CL, Choti MA, Pawlik TM. Surgical therapy for early hepatocellular carcinoma in the modern era: a 10-year SEER-medicare analysis. Ann Surg. 2013;258:1022–7. 10.1097/SLA.0b013e31827da749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang C, Zhang H, Zhang L, Zhu AX, Bernards R, Qin W, Wang C. Evolving therapeutic landscape of advanced hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2023;20:203–22. 10.1038/s41575-022-00704-9. [DOI] [PubMed] [Google Scholar]
- 21.Kent LN, Leone G. The broken cycle: E2F dysfunction in cancer. Nat Rev Cancer. 2019;19:326–38. 10.1038/s41568-019-0143-7. [DOI] [PubMed] [Google Scholar]
- 22.Di Fiore B, Pines J. Emi1 is needed to couple DNA replication with mitosis but does not regulate activation of the mitotic APC/C. J Cell Biol. 2007;177:425–37. 10.1083/jcb.200611166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Machida YJ, Dutta A. The APC/C inhibitor, Emi1, is essential for prevention of rereplication. Genes Dev. 2007;21:184–94. 10.1101/gad.1495007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gütgemann I, Lehman NL, Jackson PK, Longacre TA. Emi1 protein accumulation implicates misregulation of the anaphase promoting complex/cyclosome pathway in ovarian clear cell carcinoma. Mod Pathol. 2008;21:445–54. 10.1038/modpathol.3801022. [DOI] [PubMed] [Google Scholar]
- 25.Min KW, Park MH, Hong SR, Lee H, Kwon SY, Hong SH, Joo HJ, Park IA, An HJ, Suh KS, Oh HK, Yoo CW, Kim MJ, Chang HK, Jun SY, Yoon HK, Chang ED, Kim DW, Kim I, Gynecologic Pathology Study Group of the Korean Society of Pathologists. Clear cell carcinomas of the ovary: a multi-institutional study of 129 cases in Korea with prognostic significance of Emi1 and Galectin-3. Int J Gynecol Pathol. 2013;32:3-14. 10.1097/PGP.0b013e31825554e9 [DOI] [PubMed]
- 26.Lehman NL, Verschuren EW, Hsu JY, Cherry AM, Jackson PK. Overexpression of the anaphase promoting complex/cyclosome inhibitor Emi1 leads to tetraploidy and genomic instability of p53-deficient cells. Cell Cycle. 2006;5:1569–73. 10.4161/cc.5.14.2925. [DOI] [PubMed] [Google Scholar]
- 27.Chen JY, Wang MC, Hung WC. Bcr-Abl-induced tyrosine phosphorylation of Emi1 to stabilize Skp2 protein via inhibition of ubiquitination in chronic myeloid leukemia cells. J Cell Physiol. 2011;226:407–13. 10.1002/jcp.22346. [DOI] [PubMed] [Google Scholar]
- 28.Xie H, Kang Y, Wang S, Zheng P, Chen Z, Roy S, Zhao C. E2f5 is a versatile transcriptional activator required for spermatogenesis and multiciliated cell differentiation in zebrafish. PLoS Genet. 2020;16: e1008655. 10.1371/journal.pgen.1008655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao S, Zhang Y, Lu X, Ding H, Han B, Song X, Miao H, Cui X, Wei S, Liu W, Chen S, Wang J. CDC20 regulates the cell proliferation and radiosensitivity of P53 mutant HCC cells through the Bcl-2/Bax pathway. Int J Biol Sci. 2021;17:3608–21. 10.7150/ijbs.64003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kassab A, Gupta I, Moustafa AA. Role of E2F transcription factor in oral cancer: recent insight and advancements. Semin Cancer Biol. 2023;92:28–41. 10.1016/j.semcancer.2023.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Zhang G, Wang K, Cui F, Yang H, Jiao Z. Exploring the role of FBXO5 in gastric cancer. Mol Cell Probes. 2023;69: 101915. 10.1016/j.mcp.2023.101915. [DOI] [PubMed] [Google Scholar]
- 32.Jiang S, Zheng J, Cui Z, Li Y, Wu Q, Cai X, Zheng C, Sun Y. FBXO5 acts as a novel prognostic biomarker for patients with cervical cancer. Front Cell Dev Biol. 2023;11:1200197. 10.3389/fcell.2023.1200197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seo DH, Jung JH, Lee JE, Jeon EJ, Kim W, Park CS. Biotechnological production of arbutins (α- and β-arbutins), skin-lightening agents, and their derivatives. Appl Microbiol Biotechnol. 2012;95:1417–25. 10.1007/s00253-012-4297-4. [DOI] [PubMed] [Google Scholar]
- 34.Yang Z, Shi H, Chinnathambi A, Salmen SH, Alharbi SA, Veeraraghavan VP, Surapaneni KM, Arulselvan P. Arbutin exerts anticancer activity against rat C6 glioma cells by inducing apoptosis and inhibiting the inflammatory markers and P13/Akt/mTOR cascade. J Biochem Mol Toxicol. 2021;35: e22857. 10.1002/jbt.22857. [DOI] [PubMed] [Google Scholar]
- 35.Safari H, Zabihi E, Pouramir M, Morakabati P, Abedian Z, Karkhah A, et al. Decrease of intracellular ROS by arbutin is associated with apoptosis induction and downregulation of IL-1β and TNF-α in LNCaP; prostate cancer. J Food Biochem. 2020;44: e13360. 10.1111/jfbc.13360. [DOI] [PubMed] [Google Scholar]
- 36.Wang CQ, Wang XM, Li BL, Zhang YM, Wang L. Arbutin suppresses osteosarcoma progression via miR-338-3p/MTHFD1L and inactivation of the AKT/mTOR pathway. FEBS Open Bio. 2021;11:289–99. 10.1002/2211-5463.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hazman Ö, Sarıova A, Bozkurt MF, Ciğerci İH. The anticarcinogen activity of β-arbutin on MCF-7 cells: stimulation of apoptosis through estrogen receptor-α signal pathway, inflammation and genotoxicity. Mol Cell Biochem. 2021;476:349–60. 10.1007/s11010-020-03911-7. [DOI] [PubMed] [Google Scholar]
- 38.Zeng X, Liu H, Huang Z, Dong P, Chen X. Anticancer Effect of Arbutin on Diethylnitrosamine-Induced Liver Carcinoma in Rats via the GRP and GADD Pathway. J Environ Pathol Toxicol Oncol. 2022;41:15–26. 10.1615/JEnvironPatholToxicolOncol.2021039772. [DOI] [PubMed] [Google Scholar]
- 39.Jiang L, Wang D, Zhang Y, Li J, Wu Z, Wang Z, et al. Investigation of the pro-apoptotic effects of arbutin and its acetylated derivative on murine melanoma cells. Int J Mol Med. 2018;41:1048–54. 10.3892/ijmm.2017.3256. [DOI] [PubMed] [Google Scholar]
- 40.Bolhuis DL, Martinez-Chacin RC, Welsh KA, Bodrug T, Cui L, Emanuele MJ, Brown NG. Examining the mechanistic relationship of APC/C(CDH1) and its interphase inhibitor EMI1. Protein Sci. 2022;31: e4324. 10.1002/pro.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA sequencing data for HCC were obtained from TCGA (https://portal.gdc.cancer.gov/), LIHC microarray data were obtained from GEO (https://www.ncbi.nlm.nih.gov/geo/), ICGC-LIRI- JP and its corresponding clinical information were obtained from ICGC (https://dcc.icgc.org/).