Abstract
Background:
NXT629, a PPAR-alpha antagonist, exerts widespread effects in many diseases; however, its function and relevant mechanism in cholesterol gallstones (CG) remain largely unknown.
Methods:
Male C57BL/6 J mice were fed a regular diet or lithogenic diet (LD), followed by treatment with intraperitoneal injection of NXT629. H&E staining was performed to analyze hepatic pathological changes, and Oil red O staining was conducted to detect lipid accumulation. Concentrations of total cholesterol (TC), triglyceride (TG), phospholipids (PL), total bile acids (TBA), and cholesterol saturation index (CSI) in both bile and serum were analyzed using commercially available kits. The mRNA expressions of ABCG5/8, CYP7A1, CYP7B1, PPAR-α, and ABCB11 in mouse liver tissues were measured by qRT-PCR assay. Overexpression of glycerol-3-phosphate acyltransferase mitochondrial (GPAM) was constructed to investigate the molecular mechanism of NXT629 in CG.
Results:
NXT629 could prevent the formation of cholesterol gallstones (CG) and improve lipid metabolic disorders in mice fed a lithogenic diet (LD). Treatment with NXT629 significantly reduced the levels of ABCG5, ABCG8, and ABCB11, while increasing the levels of CYP7A1 and CYP7B1 in the LD group. Additionally, NXT629 treatment downregulated GPAM expression in hepatic tissue from LD-fed mice. Overexpression of GPAM partially counteracted the beneficial effects of NXT629 on CG formation, lipid metabolic disorders, and lipid-related gene expressions.
Conclusion:
NXT629 can inhibit CG formation, improve lipid metabolism disorders and cholesterol homeostasis by inhibiting GPAM expression, suggesting that NXT629 may serve as a potential therapeutic strategy for cholesterol stones prevention and treatment.
Keywords: NXT629, PPAR-α, Cholesterol gallstones, GPAM, Lipid metabolism disorder
Introduction
Gallstone disease is a common digestive disorder, and the most frequent type is cholesterol gallstone (CG) disease [1, 2]. Approximately 75% of patients with gallstones are asymptomatic in the early stages. As gallstones advance in formation, they can induce symptoms like nausea, epigastric colic, diarrhea, anorexia, and so forth. Ultimately, obstruction caused by gallstones can result in critical conditions including acute cholangitis, acute cholecystitis, and biliary pancreatitis, resulting in situations that are potentially fatal [3]. Presently, cholecystectomy is the predominant therapy for CG; however, this approach carries a high risk of recurrence and complications such as acute cholecystitis and cholecystic carcinoma [4, 5]. And CG can also be managed with ursodeoxycholic acid and hydrophilic bile acids, but the clinical efficacy was constrained by challenges in dose optimization, the risk of stone recurrence, and high costs [6, 7]. Therefore, it is urgent to develop effective new therapeutic strategies to prevent CG formation.
The formation of CG is associated with genetic predisposition, cholesterol supersaturation caused by in balance of cholesterol and bile acids (BA) in the bile, and impaired gallbladder motility [8]. Cholesterol supersaturation is mainly caused by hepatic cholesterol overproduction and/or BA deficiency, leading to cholesterol crystal precipitation, cholesterol crystal accumulation and CG formation [9, 10]. And cholesterol secretion is facilitated by the adenosine triphosphate binding cassette (ABC) transport proteins ABCG5 and ABCG8 [11]. The synthesis of BA is a major route in the catabolism of cholesterol. Enhancing hepatic BA synthesis can prevent the production of CG[12]. Cholesterol 7alpha-hydroxylase (Cyp7a1) and oxysterol 7alpha-hydroxylase (Cyp7b1) serve as rate-limiting enzymes that control the classical and alternative pathways of BA synthesis [13], which play important roles in BA synthesis.
Peroxisome-proliferator activated receptors (PPAR)-α is a member of the nuclear hormone receptor superfamily. It is a key "metabolic" nuclear receptor that functions as a sensor for fatty acids and other metabolites, allowing the organism to swiftly adapt to environmental alterations by activating or suppressing relevant metabolic genes and pathways [14]. As a key regulator of lipid homeostasis, PPAR-α is widely expressed in various normal tissues that are metabolically active, such as the liver, heart, kidney, skeletal muscle [15], and its expression is increased in diseased tissues, including melanoma, chronic lymphocytic leukemia, ovarian cancer, et al. [16]. As a novel and selective PPAR-a antagonist, NXT629 may perform a myriad of roles in various diseases affecting different organisms. In diseases associated with skin tissue, the suppression of PPAR-α by NXT629 reduced the incidence of lung metastasis in B16F10 cells in a mouse model [16]. In disorders related to hematologic system, PPAR-α is highly expressed in chronic lymphocytic leukemia (CLL) cells and plays a role in protecting them from challenging microenvironmental conditions. However, NXT629 treatment effectively induces apoptosis and inhibits the proliferation of CLL cells both in vitro and in vivo [17]. And studies also reported that the NXT629 could suppress tumor growth in ovarian [16]. Besides, in a mouse model of middle cerebral artery occlusion, PPAR-α inhibitor NXT629 abolished the neuroprotective effects of Qilong Quyu (a Chinese traditional medicine). Previous studies have suggested that PPAR-α functions as a hepatic lipid regulatory transcription factor and is a candidate gene for gallstone formation [18, 19]. Based on this, we hypothesized that NXT629 may have an impact on cholesterol gallstones, and its role and mechanism need to be further explored.
In this research, we constructed CG mouse models by giving mice with LD for 6 weeks and then intraperitoneally injecting NXT629 for 4 weeks to explore the effects of NXT629 on CG progressions, and the relative mechanism was detected as well.
Materials and Methods
Animals and Treatment
All animal experiments were conducted according to the ARRIVE guidelines and approved by the Ethics Committee of Animal Experiments of Guangzhou Jennio Biotech Co., Ltd (Approval No. JENNIO-IACUC-2023-A056). 24 Male C57BL/6 J mice (6 weeks old, 18–22 g) were obtained from Laboratory Animal Center of Southern Medical University and kept in temperature-controlled room (20–24 °C), with a light–dark cycle of 12:12 h and free access to water and food. They were randomly divided into four groups (n = 6): (1) Control group: mice were fed a regular diet (Xietong Biotech Co., Ltd, Jiangsu, China) for 6 weeks; (2) LD group: mice was fed a diet (Xietong Biotech Co., Ltd) consisting of 15% fat, 1.25% cholesterol, and 0.5% cholic acid for 6 weeks; (3) LD + NXT629 group: after finishing the LD feeding, mice were received daily intraperitoneal injection of 30 mg/kg of NXT629 (Abmole, Shanghai, China) for 4 weeks; (4) LD + NXT629 + Ad-glycerol-3-phosphate acyltransferase mitochondrial (GPAM) group: Adenovirus-mediated GPAM overexpression (6 × 108 PFU/mouse) was injected through the tail vein. After 10 weeks, the body weight of each mouse was measured. The gallbladder and liver were dissected and photographed to record the stones.
Hematoxylin and Eosin (H&E) Staining
Hepatic specimens were fixed with 4% paraformaldehyde and paraffin-embedded. Paraffin blocks were divided into 5 μm-thick slices, followed by dewaxing and hydrating. Slices were stained with hematoxylin and eosin (H&E) solution (Sigma Aldrich, St. Louis, MO, the USA) for 10 min. Five different fields of the sections were observed to determine pathological changes in the liver.
Oil Red O staining
Hepatic specimens were taken for frozen Sects. (6 μm) and then stained with Oil Red O solution (Solarbio Science & Technology Co., Ltd, Beijing, China) according to the manufacture’s instructions, and the percentage of steatotic hepatocytes was assessed in terms of relative lipid content and quantified by ImageJ.
Analysis of Mouse Gallbladder Bile and Serum
The levels of total cholesterol (TC), triglycerides (TG) in mouse bile and in serum were determined using kits purchased from Jiancheng Biotech Co., Ltd (Nanjing, China). The phospholipids (PL) and total bile acid (TBA) levels in bile were measured using kits obtained from Abcam. The levels of low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) in mouse serum were detected with kits from Jiancheng Biotech Co., Ltd based on the manufacturer’s instructions.
Cholesterol Saturation Index (CSI) Calculation
Calculation of gallbladder bile CSI was performed on the basis of the Carey table [20]. That is, actual molar percentage of TC in bile/highest concentration of soluble TC for a given molar concentration of bile.
Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction
Total RNA from hepatic specimens was extracted using TRIzol (Invitrogen, CA, USA). The RNA was converted into complementary DNA using the Reverse Transcription Kit (Takara, Dalian, China), Quantitative real-time reverse transcription-polymerase chain reaction (qRT-PCR) was executed using the SYBR Select Master Mix (Yeasen, Shanghai, China). All reactions were performed using ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA, the USA). Fold changes were calculated using the cycle threshold (2−ΔΔCT) method. The primers used in this research were listed in Table 1.
Table 1.
The sequences of primers and oligonucleotides used in this study
| Primer names | Sequences: 5’–3’ |
|---|---|
| GAPDH-mus-QPCR-F | 5' AGGCCAACCGTGAAAAGATG 3' |
| GAPDH–mus-QPCR-R | 5' ATGCCAGTGGTACGACCAGA 3' |
| ABCG5-mus-QPCR-F | 5' CGTCCAGAACAACACGCTAA 3' |
| ABCG5-mus-QPCR-R | 5' GCTCTCAGCATGGGAAACAG 3' |
| ABCG8-mus-QPCR-F | 5' CTTCTGCAATGCCCTCTACAA 3' |
| ABCG8-mus-QPCR-R | 5' TGGAGATCCATGCAGGCACTA 3' |
| CYP7A1-mus-QPCR-F | 5' ATGTCCTGGAAGACTATTTGCTG 3' |
| CYP7A1-mus-QPCR-R | 5' GGACTGATCTAGAGGGGGACACT 3' |
| PPAR-α-mus-QPCR-F | 5' TGAACAAAGACGGGATGCTGA 3' |
| PPAR-α-mus-QPCR-R | 5' AGGCATTGAACTTCATAGCGA 3' |
| CYP7B1-mus-QPCR-F | 5' GGAGAAACAACGCATTGCTATT 3' |
| CYP7B1-mus-QPCR-R | 5' GCAATGACAATACAGGTCCGAC 3' |
| ABCB11-mus-QPCR-F | 5' TCCATCCGTCAACGATCTAAGTC 3' |
| ABCB11-mus-QPCR-R | 5' GGCAGGTTCAACTTCTTCCAC 3' |
| GPAM-mus-QPCR-F | 5' TTATCACCAGGACGGAAAGGA 3' |
| GPAM-mus-QPCR-R | 5' CGGTTGCACTGAGGTAGGAA 3' |
Western Blotting
Total protein from hepatic specimens were extracted using radioimmunoprecipitation assay buffer (Beyotime, Shanghai, China). And total protein concentration was quantified with BCA Protein Assay Kit (Beyotime). Separation of protein samples was carried out by sodium dodecyl sulfate polyacrylamide gel electrophoresis, followed by transferring onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). Following blocking with 5% skimmed milk for 1 h, the membranes were incubated with primary antibody against GPAM (1:500, Kanglang Biotechnology Co., Ltd, Shanghai, China) and GAPDH (1:3000; Affinity Biosciences, Cincinnati, OH, USA) at 4 °C overnight. After incubation with a horseradish peroxidase-conjugated secondary antibody (1:5000; Affinity Biosciences), the bands were detected with enhanced chemiluminescence detection reagent (Beyotime) and analyzed using ImageJ software (NIH, Bethesda, MD, USA).
Statistical Analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences software version 21.0 (SPSS Inc., Chicago, IL, the USA). Data were expressed as mean ± standard deviation (SD). Differences between two groups were determined by Student’s t-test, whereas differences among multiple groups were analyzed using one-way or two-way analysis of variance. Differences were considered significant at P < 0.05.
Results
NXT629 Prevented Lithogenic Diet-Induced Cholesterol Gallstone Formation in Mice Model
Mice in the control group were observed to have clear gallbladders without CG formation, with a stone formation rate of 0%. However, the majority of mice in the LD group were observed to harbour yellow granular CG in the gallbladder, with a stone formation rate of 90%, which was significantly decreased following treatment with NXT629, with a stone formation rate around 30%, (Fig. 1a–b). Moreover, the liver weight was significantly increased in LD group compared to that in control group, which was then reversed by NXT629 treatment (Fig. 1c), and there is no significant differences in body weight gain among the three groups (Fig. 1d), the liver weight/body weight ratio increased by LD diet was also reversed by NXT629 therapy (Fig. 1e). H&E staining showed a large number of fat vacuoles, disturbed hepatic cords and twisted hepatic sinusoids in mice livers from LD group, suggesting the presence of steatotic lesions in the liver, but NXT629 treatment significantly improved these pathological changes (Fig. 1f). Oil red O staining indicates that there was increasing lipid droplets in the hepatic tissues in LD group compared to control group, which was significantly reduced after NXT629 treatment (Fig. 1f). Collectively, these results manifested that LD-induced CG formation in mice liver could be prevented by NXT629 administration.
Fig. 1.
NXT629 prevented lithogenic diet-induced cholesterol gallstone formation in mice model. a Appearance of gallbladder in mice from control, LD, and LD + NXT629 groups. b The formation rate of gallstones. c Liver weight. d Body weight gain. e The ratio of liver weight/body weight. f Representative images of H&E staining and Oil red O staining of hepatic tissues from different groups. * P < 0.05, **P < 0. 01, ***P < 0. 001
NXT629 Ameliorated Lithogenic Diet-Induced Lipid Metabolic Disorders in Mice Model
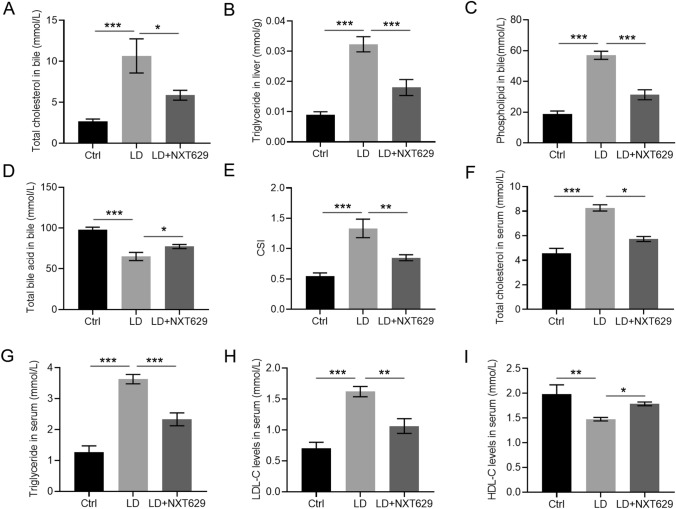
Enhanced levels of TC, TG, PL while reduced levels of TBA in mice bile were observed in the LD group, but NXT629 treatment significantly reversed these changes (Fig. 2a–d). Moreover, mice in LD group presented higher CSI in comparison to those from the control group, but NXT629 addition led to a remarkable CSI decrease (Fig. 2e). Equivalent findings were acquired through the examination of TC, TG, LDL-C and HDL-C in mice serum (Fig. 2f–i). These findings manifested that NXT629 could alleviate lipid metabolism disorders resulted from LD diet.
Fig. 2.
NXT629 ameliorated lithogenic diet-induced lipid metabolic disorders in mice model. a–d The levels of TC, TG, PL and TBA in mice bile from different groups. e The value of CSI in mice bile was assessed based on the Carey table. f–i The levels of TC, TG, LDL-C, and HDL-C in mice serum. * P < 0.05, **P < 0. 01, ***P < 0. 001
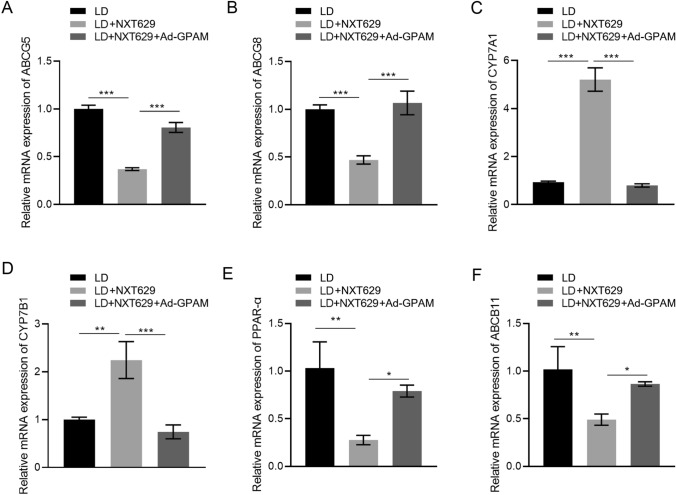
NXT629 Altered Levels of Lipid-Related Genes in Hepatic Tissues of Lithogenic Diet-Fed Mice
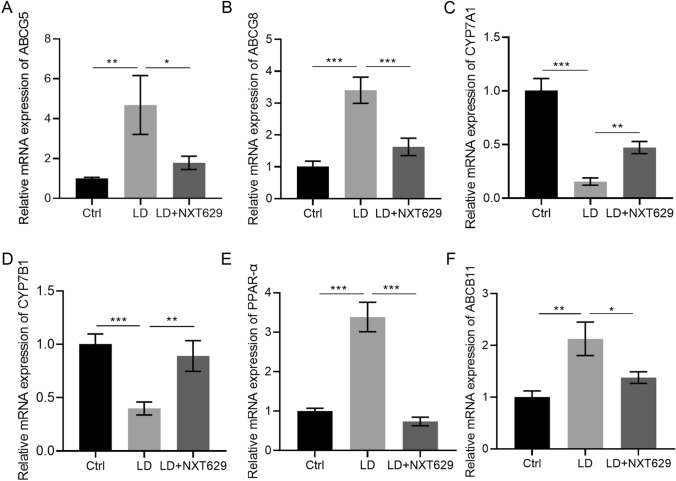
Next, we analyzed the effects of NXT629 on lipid-related gene expression in LD-fed mice. qRT-PCR results showed higher mRNA levels of cholesterol transporter proteins ABCG5 and ABCG8 in the hepatic tissues of LD-fed mice versus mice in the control group, which were effectively suppressed by NXT629 treatment (Fig. 3a–b). And the mRNA levels of CYP7A1 and CYP7B1 were significantly downregulated in LD group compared to those in control group, but NXT629 adminstration partially overturned the changes (Fig. 3c–d). In addition, increased mRNA levels of PPAR-α and ABCB11 were observed in LD group, but these changes were impaired following NXT629 treatment (Fig. 3e–f). To sum up, NXT629 mediated LD-induced lipid metabolic disorders by regulating lipid-related genes expressions.
Fig. 3.
NXT629 altered levels of lipid-related genes in hepatic tissues of lithogenic diet-fed mice. a–f The mRNA levels of ABCG5, ABCG8, CYP7A1, CYP7B1, PPAR-α, and ABCB11 in hepatic tissues from different groups were detected by qRT-PCR. * P < 0.05, **P < 0. 01, ***P < 0. 001
NXT629 Prevented Cholesterol Gallstone Formation by Mediating GPAM Pathway in Mice Model
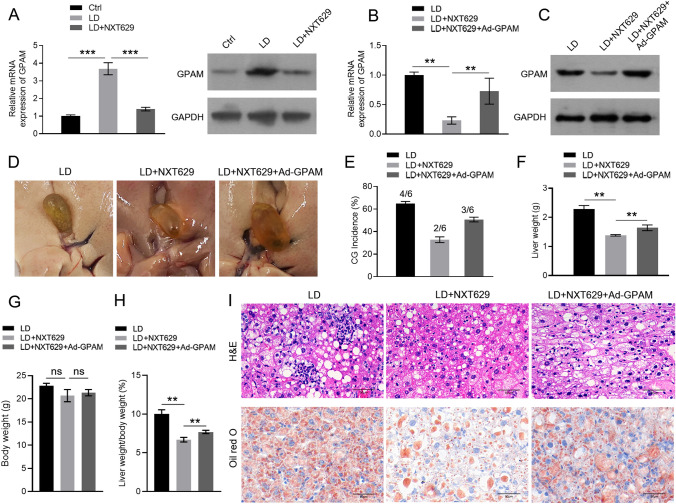
Previous researches suggested that the GPAM pathway is associated with lipid metabolism [21, 22]. Therefore, we further investigated whether NXT629 regulates CG formation by mediating GPAM expression. As expected, the mRNA and protein levels of GPAM were highly expressed in LD group, which was attenuated by NXT629 treatment (Fig. 4a). Subsequently, GPAM overexpressing adenovirus was injected into the tail vein of LD-fed mice. The mRNA and protein levels of GPAM restrained by NXT629 were sharply reversed by GPAM overexpression (Fig. 4b–c). Moreover, NXT629-mediated reduction in the rate of CG formation was also reversed by GPAM overexpression (Fig. 4d–e). Consistent findings were noted in the analysis of liver weight, as well as liver wight/body weight ratio, but with no discrepancies observed in body weight ratio (Fig. 4f–h). In addition, the effects of NXT629 treatment on the improvement of pathological changes and the reduction in lipid droplets in liver tissue were overturned by GPAM overexpression (Fig. 4i). These findings suggested that NXT629 improve LD-induced CG formation in mice model by mediating the GPAM expressions.
Fig. 4.
NXT629 prevented cholesterol gallstone formation by mediating GPAM pathway in mice model. a–c The mRNA and protein levels of GPAM in hepatic tissues from different groups were determined by qRT-PCR and western blot. d Appearance of gallbladder in mice from different groups. e The formation rate of gallstones. f Liver weight. g Body weight gain. h The value of liver weight/body weight. i Representative images of H&E staining and Oil red O staining of hepatic tissues. * P < 0.05, **P < 0. 01, ***P < 0. 001
NXT629 Weakened Lithogenic Diet-Induced Lipid Metabolic Disorders by Repressing GPAM Pathway
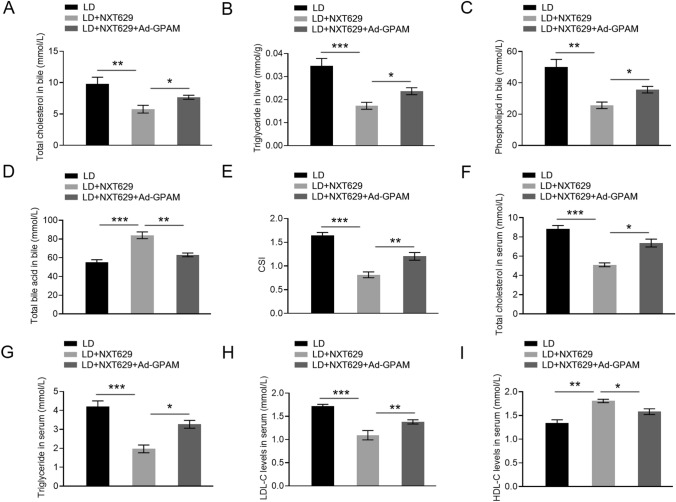
The inhibitory effects of NXT629 on TC, TG and PL levels and its stimulatory effects on TBA levels in mice bile were abolished by GPAM overexpression (Fig. 5a–d). Moreover, decreased CSI in the bile of LD-fed mice were relieved after GPAM upregulation (Fig. 5e). Consistent changes were observed in the analysis of TC, TG, LDL-C and HDL-C levels in serum (Fig. 5f–i). Together, these outcomes suggested that NXT629 could lighten lipid metabolism disorders by repressing GPAM expression.
Fig. 5.
NXT629 weakened lithogenic diet-induced lipid metabolic disorders by repressing GPAM pathway. a–d The levels of TC, TG, PL and TBA in mice bile. e The value of CSI in mice bile was assessed based on the Carey table. f–i) The levels of TC, TG, LDL-C and HDL-C in mice serum. * P < 0.05, **P < 0. 01, ***P < 0. 001
NXT629 Mediated Lipid-Related Gene Expression by GPAM Pathway
Further experiments were conducted to investigate whether NXT629 regulates the expression of lipid-related genes in LD-fed mice through GPAM pathway. It was observed that GPAM overexpression could revise the expressions of ABCG5, ABCG8 in LD + NXT629 group restrained by NXT629 treatment (Fig. 6a–b). Moreover, elevated GPAM mitigated the upregulation of CYP7A1 and CYP7B1 in NXT629—treated mice tissue (Fig. 6c–d). And GPAM overexpression also overturned the low levels of PPAR-α and ABCB11 in LD + NXT629 group. In summary, above findings indicates that NXT629 may mediate the lipid-related gene expressions by regulating GPAM pathway.
Fig. 6.
NXT629 mediated lipid-related gene expression by GPAM pathway. a–f Relative mRNA levels of ABCG5, ABCG8, CYP7A1, CYP7B1, PPAR-α, and ABCB11 in hepatic tissues from different groups. * P < 0.05, **P < 0. 01, ***P < 0. 001
Discussion
CG disease is the most common type of Gallstone disease, with an incidence rate of approximately 10–20% among adults, resulting in huge social and economic burden [2, 9]. Thus, in this research, we applied the LD-induced CG mouse models to explore the potential novel therapies for CG diseases.
Hepatic lipid metabolism modulates the composition of the biliary system and affects the development of cholesterol gallstones [23]. Fan et al. treated C57BL/6 J mice with LD diet, a sharp increasing of bile lipid indicators including TC, PL and CSI was found in CG mice, indicating the promoting role of lipid metabolism disorder in CG formation [18]. PPAR-α is a nuclear receptor of clinical interest for medication targeting in a range of metabolic illnesses [24], its activation is involved in hepatic lipid metabolism [25]. In this context, we surmised that NXT629, a selective PPAR-a antagonist, may have an impact on CG formation. In our research, mice fed with LD diet for 6 weeks induces obvious CGs in gallbladder, and the imbalance of lipid metabolism was progressively disturbed, indicated by the high levels of the TC, TG,PL, LDL-C in mice bile and /or in serum, and low levels of TBA in bile and HDL-C in serum are also observed, suggesting the lipid metabolism disorder in CG mice, the NXT629 treatment significantly reversed the levels of these lipid indicators, showing a protecting effects on lipid metabolism disorder in CG formation. Besides, cholesterol hyper-saturation in bile is another key factor for CG formation, the surplus cholesterol in oversaturated bile cannot be dissolved by bile acids and phospholipids, leading to cholesterol crystal accumulation and CG formation [26]. The synthesis of BAs is a key pathway for cholesterol catabolism and essential for regulating overall cholesterol homeostasis in the body [12], with ABCG5 and ABCG8 being the major regulators for cholesterol secretion [27], and CYP7A1, CYP7B1 being the key enzymes in the BA synthesis pathway [28]. In this study, qRT-PCR analysis showed that LD treatment resulted in an elevation of ABCG5 and ABCG8 expressions as well as a decrease of CYP7A1 and CYP7B1 expressions, suggesting the inhibition of cholesterol metabolism in LD fed mice, whereas, NXT629 intervention effectively reversed the harmful effects of LD administration, and protected the cholesterol homeostasis.
(GPAM) is an enzyme that catalyzes the first and most important step of glycerolipid production in animal lipid metabolic pathways [29]. Genetic variation in GPAM is associated with susceptibility to liver injury [30]. Upregulation of GPAM-mediated lipid biosynthesis promotes hepatocellular carcinoma growth and metastasis [31]. Therefore, we analyzed whether NXT629 regulates lipid metabolism disorders and CG symptom in LD-fed mouse models by mediating GPAM pathway. Our research found significant high levels of GPAM in hepatic samples of LD-fed mice, indicating the activation of GPAM in CG progress. NXT629 treatment effectively reversed the GPAM overexpression in LD group. Moreover, GPAM overexpression partly counteracted the improvements of NXT629 on CG formation, lipid metabolic disorders, and cholesterol supersaturation.
In conclusion, our research indicated that NXT629 may inhibited CG formation by improving lipid metabolism disorder and cholesterol homeostasis, which was achieved by downregulating GPAM expression, suggesting NXT629 may serve as the potential therapeutic approach for CG treatment.
Acknowledgments
We are thankful for Guangzhou Jennio Biotech Co., Ltd for providing assistance in animal experiments.
Author’s contributions
Conceptualization, Methodology, Writing—Original Draft and Project administration: Hao Chen Writing-Review & Editing, Supervision and Funding acquisition: Hao Chen, Liang Lin Software and Validation: Laibin Zou Formal analysis and Investigation: Shenglan Guo Resources and Data Curation: Xubin Qiu Visualization: Keqiang Ma.
Funding Statement
This study was jointly funded by Guangzhou Medical Key Subject Construction Project of China (2025-2027) and Intramural Fund Project of Huadu District People's Hospital of Guangzhou (No. 2021B02).
Data availability
The datasets generated and/or analysed during the current study are not publicly available as the data is confidential but are available from the corresponding author on reasonable request.
Declaration
Conflict of interest
The authors declare no competing interests.
Ethical considerations
All animal experiments were conducted according to the ARRIVE guidelines and approved by the Ethics Committee of Animal Experiments of Guangzhou Jennio Biotech Co., Ltd (Approval No. JENNIO-IACUC-2023-A056).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang C et al. Resistance to Cholesterol Gallstone Disease: Hepatic Cholesterol Metabolism. J Clin Endocrinol Metab 2024;109:912–923. [DOI] [PubMed] [Google Scholar]
- 2.Di Ciaula A, Wang DQ, Portincasa P. An update on the pathogenesis of cholesterol gallstone disease. Curr Opin Gastroenterol 2018;34:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka H et al. Claudin-3 regulates bile canalicular paracellular barrier and cholesterol gallstone core formation in mice. J Hepatol 2018;69:1308–1316. [DOI] [PubMed] [Google Scholar]
- 4.Kozyk M et al. Recurrence of common bile duct stones after endoscopic clearance and its predictors: A systematic review. DEN Open 2024;4:e294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papageorge MV et al. The Evaluation of Gallstone Disease in the Year Before Pancreatic Cancer Diagnosis. J Surg Res 2023;291:282–288. [DOI] [PubMed] [Google Scholar]
- 6.Guarino MP et al. Ursodeoxycholic acid improves muscle contractility and inflammation in symptomatic gallbladders with cholesterol gallstones. Gut 2007;56:815–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang HH et al. Prevention of cholesterol gallstones by inhibiting hepatic biosynthesis and intestinal absorption of cholesterol. Eur J Clin Invest 2013;43:413–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gründel D et al. Relation of gallbladder motility to viscosity and composition of gallbladder bile in patients with cholesterol gallstones. Digestion 2009;79:229–234. [DOI] [PubMed] [Google Scholar]
- 9.Sun, H., et al., Factors Influencing Gallstone Formation: A Review of the Literature. Biomolecules, 2022. 12(4). [DOI] [PMC free article] [PubMed]
- 10.Rudling M, Laskar A, Straniero S. Gallbladder bile supersaturated with cholesterol in gallstone patients preferentially develops from shortage of bile acids. J Lipid Res 2019;60:498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castro-Torres IG et al. Future therapeutic targets for the treatment and prevention of cholesterol gallstones. Eur J Pharmacol 2015;765:366–374. [DOI] [PubMed] [Google Scholar]
- 12.Zhuang Q et al. Gypenosides Prevent and Dissolve Cholesterol Gallstones by Modulating the Homeostasis of Cholesterol and Bile Acids. Front Med (Lausanne) 2022;9:818144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi YJ et al. Intratracheal exposure to polyhexamethylene guanidine phosphate disrupts coordinate regulation of FXR-SHP-mediated cholesterol and bile acid homeostasis in mouse liver. Ecotoxicol Environ Saf 2022;247:114213. [DOI] [PubMed] [Google Scholar]
- 14.Francis GA, Annicotte JS, Auwerx J. PPAR-alpha effects on the heart and other vascular tissues. Am J Physiol Heart Circ Physiol 2003;285:H1-9. [DOI] [PubMed] [Google Scholar]
- 15.Francis GA et al. Nuclear receptors and the control of metabolism. Annu Rev Physiol 2003;65:261–311. [DOI] [PubMed] [Google Scholar]
- 16.Stebbins KJ et al. In vitro and in vivo pharmacology of NXT629, a novel and selective PPARα antagonist. Eur J Pharmacol 2017;809:130–140. [DOI] [PubMed] [Google Scholar]
- 17.Messmer D et al. A Selective Novel Peroxisome Proliferator-Activated Receptor (PPAR)-α Antagonist Induces Apoptosis and Inhibits Proliferation of CLL Cells In Vitro and In Vivo. Mol Med 2015;21:410–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan N et al. The effect of ursodeoxycholic acid on the relative expression of the lipid metabolism genes in mouse cholesterol gallstone models. Lipids Health Dis 2020;19:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie C et al. Hepatocyte peroxisome proliferator-activated receptor α regulates bile acid synthesis and transport. Biochim Biophys Acta Mol Cell Biol Lipids 2019;1864:1396–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carey MC. Critical tables for calculating the cholesterol saturation of native bile. J Lipid Res 1978;19:945–955. [PubMed] [Google Scholar]
- 21.Moyes KM et al. Gene network and pathway analysis of bovine mammary tissue challenged with Streptococcus uberis reveals induction of cell proliferation and inhibition of PPARgamma signaling as potential mechanism for the negative relationships between immune response and lipid metabolism. BMC Genomics 2009;10:542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu, L., et al., High-Dose Dexamethasone Manipulates the Tumor Microenvironment and Internal Metabolic Pathways in Anti-Tumor Progression. Int J Mol Sci, 2020. 21(5). [DOI] [PMC free article] [PubMed]
- 23.He C et al. Circadian Rhythm Disruption Influenced Hepatic Lipid Metabolism, Gut Microbiota and Promoted Cholesterol Gallstone Formation in Mice. Front Endocrinol (Lausanne) 2021;12:723918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bougarne N et al. Molecular Actions of PPARα in Lipid Metabolism and Inflammation. Endocr Rev 2018;39:760–802. [DOI] [PubMed] [Google Scholar]
- 25.Yang W et al. PPARα/ACOX1 as a novel target for hepatic lipid metabolism disorders induced by per- and polyfluoroalkyl substances: An integrated approach. Environ Int 2023;178:108138. [DOI] [PubMed] [Google Scholar]
- 26.Lammert F et al. Gallstones. Nat Rev Dis Primers 2016;2:16024. [DOI] [PubMed] [Google Scholar]
- 27.Bydlowski SP, Levy D. Association of ABCG5 and ABCG8 Transporters with Sitosterolemia. Adv Exp Med Biol 2024;1440:31–42. [DOI] [PubMed] [Google Scholar]
- 28.Yang N et al. Alternative pathway of bile acid biosynthesis contributes to ameliorate NASH after induction of NAMPT/NAD(+)/SIRT1 axis. Biomed Pharmacother 2023;164:114987. [DOI] [PubMed] [Google Scholar]
- 29.Yu H et al. Transcriptome analysis of CRISPR/Cas9-mediated GPAM(-/-) in bovine mammary epithelial cell-line unravelled the effects of GPAM gene on lipid metabolism. Gene 2022;834:146574. [DOI] [PubMed] [Google Scholar]
- 30.Jamialahmadi O et al. Exome-Wide Association Study on Alanine Aminotransferase Identifies Sequence Variants in the GPAM and APOE Associated With Fatty Liver Disease. Gastroenterology 2021;160:1634-1646.e7. [DOI] [PubMed] [Google Scholar]
- 31.Yang Y et al. Down-regulation of BMAL1 by MiR-494-3p Promotes Hepatocellular Carcinoma Growth and Metastasis by Increasing GPAM-mediated Lipid Biosynthesis. Int J Biol Sci 2022;18:6129–6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available as the data is confidential but are available from the corresponding author on reasonable request.