Abstract
目的
通过将哮喘支气管上皮细胞与骨髓间充质干细胞共培养方式,观察miR-139-5p调控Notch1信号通路对哮喘骨髓间充质干细胞归巢的影响。
方法
将大鼠骨髓间充质干细胞和支气管上皮细胞共培养,并予miR-139-5p mimics进行干预。实验分为阴性对照组(NC组:正常大鼠骨髓间充质干细胞+支气管上皮细胞共培养)、模型对照组(MC组:正常大鼠骨髓间充质干细胞+哮喘大鼠支气管上皮细胞共培养)、miR-139-5p mimics组(miR-139-5p mimics+MC)、miR-139-5p mimics-NC组(miR-139-5p mimics-NC+MC)。检测细胞活力和细胞周期变化;免疫荧光染色检测CXCR4、SDF-1表达;经细胞转染后观察miR-139-5p表达量和BMSCs归巢水平;免疫印迹观察Notch1/RBP-J/Hes1蛋白表达量;ELISA检测Th1、Th2相关因子表达情况。
结果
与NC组比较,MC组miR-139-5p、IL-2、IL-12表达量降低(P<0.05),BMSCs归巢水平增加,CXCR4、SDF-1、IL-5、IL-9表达升高(P<0.05),Notch1、RBP-J、Hes1 mRNA、蛋白表达升高(P<0.05)。与MC组、miR-139-5p mimics-NC组比较,miR-139-5p mimics组miR-139-5p、BMSCs归巢水平升高,CXCR4、SDF-1、IL-2表达升高(P<0.05),Notch1、RBP-J、Hes1mRNA、蛋白表达降低,IL-5、IL-9表达降低(P<0.05)。相关性分析显示,BMSCs归巢水平与miR-139-5p、IL-12呈正相关,SDF-1与miR-139-5p呈正相关(P<0.05);与IL-5表达呈负相关,CXCR4与Activated Notch1表达呈负相关,SDF-1与Notch1表达呈负相关(P<0.05)。
结论
miR-139-5p可能通过靶向Notch1信号通路促进哮喘骨髓间充质干细胞归巢,作用于Th1、Th2细胞因子表达,改善哮喘气道炎症。
Keywords: 支气管哮喘, 骨髓间充质干细胞, miR-139-5p, Notch1信号通路
Abstract
Objective
To observe the role of miR-139-5p and Notch1 signaling pathway in regulation of homing of bone mesenchymal stem cells (BMSCs) of asthmatic rats.
Methods
Normal rat BMSCs were co-cultured with bronchial epithelial cells from normal or asthmatic rats, followed by transfection with miR-139-5p mimics or a negative control sequence. The changes in cell viability and cell cycle were analyzed, and the cellular expressions of CXCR4 and SDF-1 were detected using immunofluorescence staining. The changes of BMSC homing after the transfection were observed, and the expressions of Notch1, RBP-J, and Hes1 mRNAs and proteins and Th1/Th2 cytokines were detected with RT-qPCR, Western blotting or ELISA.
Results
The co-cultures of BMSCs and asthmatic bronchial epithelial cells showed significantly decreased expressions of miR-139-5p, IL-2 and IL-12 and increased expressions of CXCR4, SDF-1, IL-5, IL-9, Notch1, RBP-J, and Hes1. Transfection with miR-139-5p mimics significantly increased the expressions of miR-139-5p, IL-2, CXCR4 and SDF-1 and lowered the expression levels of IL-5, IL-9, Notch1, activated Notch1, and Hes1 in the co-cultured cells. Correlation analysis showed that BMSC homing was positively correlated with miR-139-5p and IL-12 and negatively correlated with IL-5 expression. The expression of CXCR4 was negatively correlated with activated Notch1, and SDF-1 was positively correlated with miR-139-5p but negatively correlated with Notch1 expression.
Conclusion
High expression of miR-139-5p promotes homing of BMSCs in asthma by targeting the Notch1 signaling pathway to regulate the expressions of Th1/Th2 cytokines, thereby alleviating airway inflammation.
Keywords: asthma, bone mesenchymal stem cells, miR-139-5p, Notch1 signaling pathway
哮喘是临床常见的慢性呼吸系统疾病,以胸闷气促、喘息、咳嗽等为主要表现。其发病与多基因表达、信号通路调节有关,气道炎症反应受多种炎症介质介导[1]。既往研究表明[2],Th2细胞介导的多种细胞因子(IL-3、IL-4等)在哮喘过程中分泌异常增多,并导致肥大细胞刺激、嗜酸性粒细胞增多、白细胞增多和IgE产生,而与此同时Th1型细胞因子(IL-2、IL-12等)分泌受到抑制,这种Th1/Th2细胞失衡是诱发或加重哮喘气道炎症的重要因素[3]。
miRNAs可参与对呼吸系统疾病的调控[4]。miRNAs被认为是转录后修饰的关键分子。它们可以直接结合到目标mRNA的3'-UTR上,导致mRNA降解或翻译抑制。由于miRNAs的稳定表达和保守作用,miRNAs可以通过结合多种靶点促进或抑制哮喘发生,其作为哮喘生物标志物和治疗靶点的研究越来越多[5]。被认为具有治疗哮喘潜力,成为近年来的研究热点[6]。miR-139-5p在其他呼吸系统疾病中也被证实是抑制因子,包括哮喘[7]、慢性阻塞性肺疾病[8]等。miR-139-5p在哮喘患者样本中的表达往往低于正常者,并与临床症状显著相关[9]。miR-139-5p及其下游Notch信号通路可能参与影响Th1/Th2细胞平衡,并在炎症中发挥调节作用[10]。但在哮喘中的作用机制尚未完全阐明。骨髓间充质干细胞(BMSCs)作为来源于骨髓的多能干细胞,在炎症发生时可通过其归巢轴迁移至炎症部位,发挥其特有的免疫调节作用[11]。为证实miR-139-5p是否可以调控Notch1信号通路表达,参与BMSCs归巢,从而影响哮喘气道炎症表达,本研究通过将哮喘支气管上皮细胞与BMSCs共培养,观察miR-139-5p调控Notch1信号通路对哮喘BMSCs的影响,试图为间充质干细胞治疗哮喘从细胞层面提供新的依据。
1. 材料和方法
1.1. 动物、仪器和材料
清洁级雄性SD大鼠由安徽医科大学实验动物管理中心提供,所有实验已获得安徽中医药大学动物伦理委员会审批(伦理批号:AHCUM-rats-2021131)。荧光定量 PCR(Thermo Scientific),流式细胞仪(BD),数字切片扫描仪(3DHISTECH)。卵清蛋白、5,6-羰基荧光素二乙酸盐琥珀酰亚胺脂(CFSE, Sigma)。白介素(IL)-2、IL-12、IL-5、IL-9 ELISA 试剂盒(Invitrogen);胎牛血清白蛋白(BSA)、链酶蛋白酶(Solarbio)。LipofectamineTM 2000 (Thermo Fisher);miR-139-5p-mimics(上海吉玛制药技术有限公司);TritonX-100(ebiogo);Notch1抗体(Abcam);Activated Notch1、RBP-J、Hes1抗体(Bioss);山羊抗兔IgG-FITC、山羊抗小鼠IgG(Zsbio);细胞周期试剂盒(BD);PCR试剂盒(TaKaRa)。
1.2. 方法
1.2.1. 支气管上皮细胞的分离、培养
取4~6周雄性SD大鼠完成慢性哮喘模型建立[12],另取4~6周正常雄性SD大鼠。在无菌环境下,予3%戊巴比妥钠30 mg/kg腹腔注射麻醉,将大鼠自环状软骨下方插管,生理盐水清洗3次,左右肺门处扎紧,灌入1%链酶蛋白酶,上端结扎,4 oC过夜。剪取气管-支气管,去除周边血管及软组织,予含10%胎牛血清的Ham' s F12培养基冲洗,再用细胞刷充分刷洗,收集刷洗液。离心,洗涤,获取细胞并培养。
1.2.2. BMSCs分离培养、标记
无菌条件下,大鼠暴露骨髓腔,予含2%胎牛血清(FBS)的磷酸缓冲液(PBS)溶液反复冲洗,过滤,收集细胞,接种于25 cm2培养瓶培养。细胞融合至80%~90%,加入0.25%胰蛋白酶和0.02%的乙二胺四乙酸(EDTA)消化3~5 min,1∶2传代。避光条件下,将50 μg CFSE溶解于18 μL二甲亚砜(DMSO),配成5 mmol/L的原液,将细胞5×106悬浮于2 mL 0.1% BSA内,加入2 μL CFSE原液孵育,加4倍体积FBS终止反应,重悬1×106细胞于1 mL PBS备用。
1.2.3. 细胞共培养
将支气管上皮细胞置于培养基加补充试剂盒和100 U/mL青霉素、100 μg/mL链霉素和0.25 μg/mL真菌剂中培养。BMSCs在DMEM低糖中培养。将支气管上皮细胞以10 000/cm2的密度放置入跨孔渗透6孔板支架,每孔96 000细胞。24 h后,将BMSCs接种于相同孔的植入物中。共培养3 d后,收集支气管上皮细胞和培养基进行分析。
1.2.4. 细胞转染与分组
消化共培养后的细胞,用培养基重悬、培养。将pcDNA 3.1/mimics/NC对照冻干粉加125 μL 焦碳酸二乙酯(DEPC)水,稀释后浓度为20 μmol/L。在250 μL无血清培养基中加入浓度为20 μmol/L的miRNA mimics 5 μL,pcDNA 3.1 5 μL NC对照;取5 μL Lipofectamine 2000在250 μL无血清培养基中稀释,室温孵育5 min。将前两步所稀释的和Lipofectamine 2000混合,轻轻混匀,室温放置20 min。在每孔细胞中加入500 μL转染液,轻轻摇匀。转染4~6 h后更换培养基进行培养。转染24 h后,胰酶消化细胞,PBS清洗2遍,收集细胞沉淀,置于-80 ℃冰箱保存用于后续实验。
实验分为阴性对照组(NC组:正常大鼠骨髓间充质干细胞+支气管上皮细胞共培养)、模型对照组(MC组:正常大鼠骨髓间充质干细胞+哮喘大鼠支气管上皮细胞共培养)、miR-139-5p mimics组(miR-139-5p mimics+MC)、miR-139-5p mimics-NC组(miR-139-5p mimics-NC+MC)。
1.2.5. 细胞周期检测
预冷PBS清洗细胞,胰酶消化,2000 r/min 5 min,用预冷PBS重悬洗涤,小心地吸去上清,残留约50 μL,不要倾倒。细胞沉淀用1 mL预冷的乙醇混匀,4 ℃固定2 h以上或者过夜。然后离心,去上清。将过夜固定好的细胞用预冷PBS 洗两次,2000 r/min 5 min去除PBS。在细胞沉淀中加入0.5 mL RI/RNase Staining Buffer,室温避光孵育15 min,上机流式检测。
1.2.6. 免疫荧光染色检测CXCR4、SDF-1表达
将培养获得的支气管上皮细胞予4%多聚甲醛固定20 min,预温PBS洗涤3遍,5 min/次;经0.5% Triton X-100通透处理5 min,PBS洗涤3遍;封闭液(1%牛血清白蛋白)室温封闭30 min;4 ℃下,分别予兔抗CXCR4、SDF-1一抗(1∶100),培养细胞过夜;加FITC荧光标记的二抗(1∶500),避光环境下37 ℃孵育30 min,PBS洗涤3遍;DAPI核复染2 min,封片,荧光显微镜下拍照;采用Image J软件,半定量分析用平均荧光强度(Mean):Mean=该区域荧光强度总和(IntDen)/该区域面积(Area),测定每组图片中CXCR4、SDF-1的Mean值,代表CXCR4、SDF-1的相对表达量。
1.2.7. ELISA法检测细胞因子表达
各组干预结束后,收集上清液,支气管上皮细胞计数,重悬细胞2×105 /mL,按ELISA操作步骤(具体步骤同体内实验部分)。在酶标仪上测A 490 nm IFN-γ、IL-5、IL-13值。
1.2.8. RT-qPCR检测miR-139-5p及Notch通路相关基因表达
细胞总RNA 提取,Total RNA质检,逆转录:按比例及反应条件进行操作,完成cDNA的合成。荧光定量PCR反应。使用在初步运行中显示最低CT值的cDNA样品创建每个靶点扩增的阈值周期与总RNA的对数浓度的标准曲线,并对每个样品进行相对定量。所有结果定量值均为miR-139-5p及Notch1通路相关基因(Notch1、Jagged1、Hes1)的定量值/内参的定量值,以2-△△CT计算相对表达量。引物序列由安徽中抗生物技术有限公司合成,引物序列(表1)。
表1.
引物序列
Tab.1 Primer sequences for RT-qPCR
| Primer | Sequence (5' to 3') | Length (bp) |
|---|---|---|
| Notch1-F | CCAGGAAAGAGGGCAGC | 198 |
| Notch1-R | GGAGCATCTCAAGCCTCTT | |
| RBP-J-F | CAGGCATTCTACAGGAAGTTTG | 99 |
| RBP-J-R | TACTGTTTGATCCCCTCGTTC | |
| Hes1-F | AAACCAAAGACAGCCTCTGA | 184 |
| Hes1-R | GGTGCTTCACTGTCATTTCC | |
| miR139-5p-F | ACACTCCAGCTGGGTCTACAGTGCACGTGT | 66 |
| miR139-5p-R | TGGTGTCGTGGAGTCG | |
| β-actin-F | CCCATCTATGAGGGTTACGC | 150 |
| β-actin-R | TTTAATGTCACGCACGATTTC |
1.2.9. Western blotting检测Notch1通路相关蛋白表达
提取各组支气管上皮细胞总蛋白,按抗体孵育步骤加相应指标的一抗(稀释浓度分别为Notch1 1∶1000、Activated Notch1 1∶500、RBP-J 1∶1000、Hes1 1∶500)和二抗(山羊抗兔IgG稀释浓度1∶5000),4 ℃下孵育2 h。TBST洗涤3次(8 min/次),AB液等体积混合,均匀涂于PVDF膜上,曝光胶片,显影再定影,Image J分析条带灰度值。
1.3. 统计学方法
采用统计软件SPSS 26.0进行数据分析,计量资料数据以均数±标准差表示,组间比较采用独立样本t检验,相关性分析采用Spearman检验,以P<0.05为差异具有统计学意义。
2. 结果
2.1. 共培养细胞细胞活力、细胞周期的变化
CCK-8检测结果显示,与NC组比较,MC组细胞活力降低(P<0.05)。与MC组、miR-139-5p mimics-NC组比较,miR-139-5p mimics组细胞活力升高(P<0.05)。
细胞周期观察发现,与NC组相比,MC组细胞S、G2期比例降低,G1期比例升高。与MC组、miR-139-5p mimics-NC组相比,miR-139-5p mimics组S、G2期比例升高,G1期比例降低(P<0.01,表2)。
表2.
各组细胞活力、细胞周期的比较
Tab.2 Comparison of cell viability and cell cycle among the groups (Mean±SD, n=6)
| Group | Cell viability | G1(%) | S (%) | G2(%) |
|---|---|---|---|---|
| NC | 1.000±0.014 | 56.74±2.15 | 8.80±0.72 | 34.46±0.77 |
| MC | 0.558±0.033Δ | 73.99±2.48 ΔΔ | 4.24±0.63ΔΔ | 21.78±0.66ΔΔ |
| miR-139-5p mimics | 0.892±0.046*▲ | 62.61±1.89*▲ | 8.26±0.49*▲ | 29.13±0.72*▲ |
| miR-139-5p mimics-NC | 0.544±0.122Δ | 72.94±2.49 | 5.27±0.62 | 21.79±0.94 |
NC: Co-culture of normal rat BMSCs and normal rat bronchial epithelial cells group; MC: Co-culture of normal rat BMSCs and bronchial epithelial cells from asthmatic rats; miR-139-5p mimics-NC: miR-139-5p mimics-NC+MC group. Δ P<0.01, ΔΔ P<0.01 vs NC group; *P<0.05 vs MC group; ▲ P<0.05 vs miR-139-5p mimics-NC group.
2.2. miR-139-5p mimics下共培养细胞miR-139-5p变化
与NC组比较,MC组miR-139-5p表达量降低(P<0.05)。与MC组、miR-139-5p mimics-NC组比较,miR-139-5p mimics组miR-139-5p表达量升高(P<0.05,表3)。
表3.
各组miR-139的相对表达量
Tab.3 Relative expression levels of miR-139 in each group (Mean±SD, n=6)
| Group | Ave CT | 2-△△CT |
|---|---|---|
| NC | 18.58 | 1.00±0.04 |
| MC | 19.08 | 0.54±0.05Δ |
| miR-139-5p mimics | 18.77 | 0.82±0.04*▲ |
| miR-139-5p mimics-NC | 19.11 | 0.55±0.02Δ |
Δ P<0.05 vs NC group; *P<0.05 vs MC group; ▲ P<0.05 vs miR-139-5p mimics-NC group.
2.3. miR-139-5p mimics下BMSCs归巢水平变化
与NC组比较,MC组BMSCs归巢水平增加 (P<0.05)。与MC组、miR-139-5p mimics-NC组比较,miR-139-5p mimics组BMSCs归巢水平进一步升高(P <0.05,表4)。
表4.
各组BMSCs归巢水平变化
Tab.4 Comparison of homing levels of BMSCs among the groups (Mean±SD, n=3)
| Group | The homing level of BMSCs (%) |
|---|---|
| NC | 2.42±0.06 |
| MC | 8.26±0.15Δ |
| miR-139-5p mimics | 14.23±0.39*▲ |
| miR-139-5p mimics-NC | 8.55±0.13Δ |
Δ P<0.05 vs NC group; *P<0.05 vs MC group; ▲ P<0.05 vs miR-139-5p mimics-NC group.
2.4. BMSCs归巢标志物CXCR4、SDF-1表达
免疫荧光检测结果显示,与NC组比较,MC组CXCR4、SDF-1表达升高(P<0.05)。与MC组、miR-139-5p mimics-NC组比较,miR-139-5p mimics组CXCR4、SDF-1表达进一步升高(P<0.05,表5,图1)。
表5.
各组CXCR4、SDF-1表达量
Tab.5 Expressions of CXCR4 and SDF-1 in each group (Mean±SD, n=3)
| Group | CXCR4 | SDF-1 |
|---|---|---|
| NC | 10.254±2.692 | 12.471±2.752 |
| MC | 17.345±3.219ΔΔ | 16.126±2.978Δ |
| miR-139-5p mimics | 20.026±3.753*▲ | 18.955±3.583*▲ |
| miR-139-5p mimics-NC | 16.429±2.916ΔΔ | 17.056±2.746Δ |
Δ P<0.05, ΔΔ P<0.01 vs NC group; *P<0.05 vs MC group; ▲ P<0.05 vs miR-139-5p mimics-NC group.
图1.
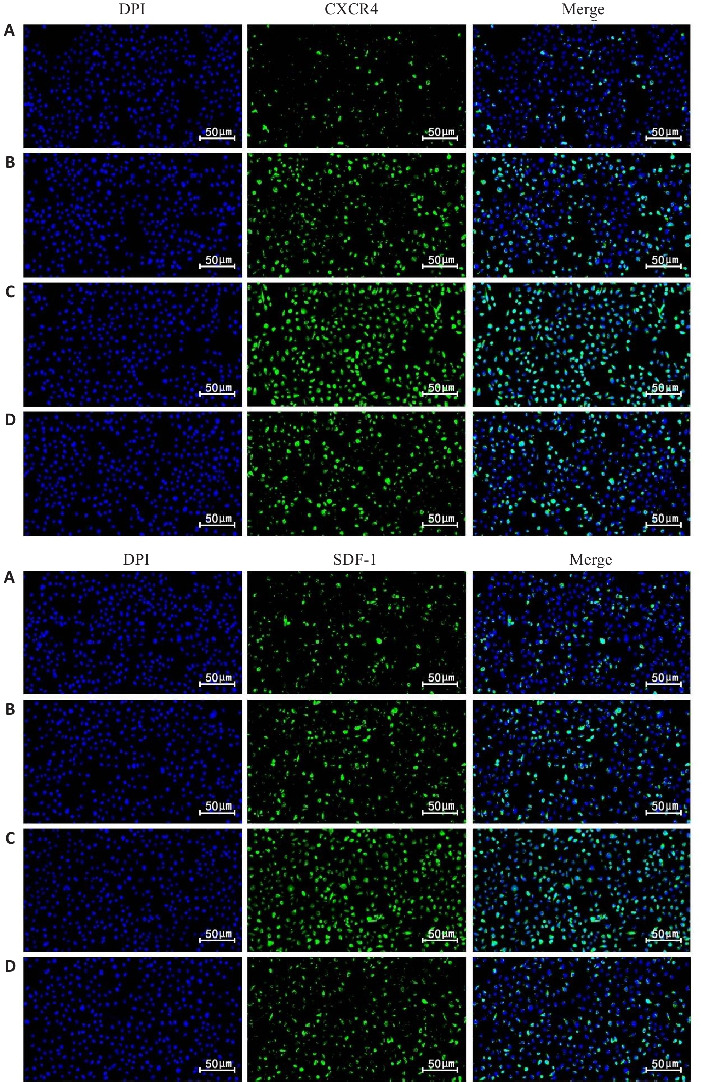
各组BMSCs归巢标志物CXCR4、SDF-1的表达
Fig.1 Expressions of homing markers CXCR4 and SDF-1 in BMSCs in each group. A: NC group. B: MC group. C: miR-139-5p mimics group. D: miR-139-5p mimics-NC group.
2.5. 各组Notch1/RBP-J/Hes1mRNA表达比较
RT-PCR检测结果显示,与NC组比较,MC组Notch1、RBP-J、Hes1mRNA表达升高(P<0.05)。与MC组、miR-139-5p mimics-NC组比较,miR-139-5p mimics组Notch1、RBP-J、Hes1mRNA表达降低(P<0.05)。Western blotting检测显示,与NC组比较,MC组Notch1、Activated Notch1、RBP-J、Hes1蛋白表达升高(P<0.05)。与MC组、miR-139-5p mimics-NC组比较,miR-139-5p mimics组Notch1、Activated Notch1、Hes1蛋白表达降低(P<0.05,表6、7,图2)。
表6.
各组Notch1、RBP-J、Hes1 mRNA表达
Tab.6 Expressions of Notch1, RBP-J, and Hes1 mRNA in each Group (Mean±SD, n=6)
| Group | Notch1 | RBP-J | Hes1 |
|---|---|---|---|
| NC | 1.00±0.13 | 1.00±0.16 | 1 .00±0.07 |
| MC | 2.62±0.51ΔΔ | 2.76±0.40ΔΔ | 1.94±0.17Δ |
| miR-139-5p mimics | 1.81±0.10*▲ | 1.82±0.06*▲ | 1.35±0.06*▲ |
| miR-139-5p mimics-NC | 2.68±0.15ΔΔ | 2.72±0.34ΔΔ | 1.88±0.07ΔΔ |
Δ P<0.05, ΔΔ P<0.01 vs NC group; *P<0.05 vs MC group; ▲ P<0.05 vs miR-139-5p mimics-NC group.
表7.
各组Notch1、Activated Notch1、RBP-J、Hes1 蛋白表达
Tab.7 Expression of Notch1, activated Notch1, RBP-J, and Hes1 proteins in each group (Mean±SD, n=3)
| Group | Notch1 | Activated Notch1 | RBP-J | Hes1 |
|---|---|---|---|---|
| NC | 0.231±0.107 | 0.409±0.033 | 0.293±0.108 | 0.158±0.024 |
| MC | 0.631±0.058Δ | 0.811±0.089Δ | 0.664±0.115Δ | 0.470±0.059ΔΔ |
| miR-139-5p mimics | 0.452±0.034*▲ | 0.560±0.108*▲ | 0.531±0.025 | 0.314±0.048*▲ |
| miR-139-5p mimics-NC | 0.645±0.030Δ | 0.822±0.053Δ | 0.680±0.083 | 0.471±0.047ΔΔ |
Δ P<0.05, ΔΔ P<0.01 vs NC group; *P<0.05 vs MC group; ▲ P<0.05 vs miR-139-5p mimics-NC group.
图2.

各组Notch1、RBP-J、Hes1 蛋白表达
Fig.2 Expression of Notch1, RBP-J, and Hes1 proteins in each group detected by Western blotting. A: NC group. B: MC group. C: miR-139-5p mimics group. D: miR-139-5p mimics-NC group.
2.6. 各组Th1、Th2相关细胞因子表达比较
ELISA检测结果显示,与NC组比较,MC组Th1细胞分泌的细胞因子(IL-2、IL-12)表达降低(P<0.05),Th2细胞分泌的细胞因子(IL-5、IL-9)表达升高(P<0.05)。与MC组、miR-139-5p mimics-NC组比较,miR-139-5p mimics组IL-2表达升高(P<0.05),IL-5、IL-9表达降低(P<0.05,表8)。
表8.
各组Th1、Th2细胞因子表达量
Tab.8 Expression levels of Th1 and Th2 cytokines in each group (ng/mL, Mean±SD, n=10)
| Group | IL-2 | IL-12 | IL-5 | IL-9 |
|---|---|---|---|---|
| NC | 48.365±8.896 | 30.222±4.214 | 42.549±4.651 | 15.644±2.492 |
| MC | 42.316±4.736Δ | 20.374±2.392Δ | 49.626±3.794Δ | 29.435±3.264ΔΔ |
| miR-139-5p mimics | 45.263±6.587*▲ | 21.463±3.442 | 45.623±4.532*▲ | 20.486±2.785**▲▲ |
| miR-139-5p mimics-NC | 43.542±5.093Δ | 19.587±2.518Δ | 48.042±5.758Δ | 28.929±2.552ΔΔ |
Δ P<0.05, ΔΔ P<0.01 vs NC group; *P<0.05, **P<0.05 vs MC group; ▲ P<0.05,▲▲ P<0.01 vs miR-139-5p mimics-NC group.
2.7. 相关性分析
相关性分析显示,BMSCs归巢水平与miR-139-5p、IL-12呈正相关,与IL-5表达呈负相关(P<0.05)。CXCR4与Activated Notch1表达呈负相关(P<0.05)。SDF-1与miR-139-5p呈正相关,SDF-1与Notch1表达呈负相关(P<0.05,表9)。
表9.
miR139-5p、Th1/Th2因子与Notch通路相关性分析(r)
Tab.9 Correlation analysis of miR139-5p, Th1/Th2 factors and the Notch pathway (correlation coefficient)
| Index | miR-139-5p | Notch1 | Activated Notch1 | RBP-J | Hes1 | IL-2 | IL-12 | IL-5 | IL-9 |
|---|---|---|---|---|---|---|---|---|---|
|
The homing level of BMSCs |
0.626* | 0.353 | 0.142 | 0.259 | 0.316 | 0.213 | 0.634* | -0.598* | 0.186 |
| CXCR4 | 0.317 | 0.033 | -0.605* | 0.363 | -0.034 | -0.167 | 0.069 | 0.104 | 0.199 |
| SDF-1 | 0.678* | -0.547* | 0.336 | 0.260 | 0.143 | -0.158 | -0.368 | 0.179 | 0.167 |
*P<0.05, **P<0.01 for correlations between the horizontal and vertical coordinates.
3. 讨论
哮喘是一种复杂的免疫性呼吸系统疾病,以可变气流阻塞、支气管高反应性和气道炎症为特征[13]。支气管上皮细胞作为关键的免疫调节子,在正常的免疫稳态平衡过程中发挥重要作用,而哮喘状态下,受到变态反应损害后,不仅可诱导炎症反应,还可调节随后的固有免疫和适应性免疫反应[14]。
BMSCs是组织再生研究中常用的种子细胞,但如何诱导其定向归巢分化一直是研究的重点和难点[15]。间充质干细胞在目标组织的脉管系统里被捕获,随后跨越血管内皮细胞迁移至目标组织的过程[16]。当机体缺血、缺氧、损伤时,机体内或者外源性间充质干细胞具有向损伤部位优势分布的特质[17]。归巢是间充质干细胞安全有效的应用于临床的关键。间充质干细胞归巢的免疫调节作用已被证实在哮喘的免疫炎症过程中具有调节作用,这其中就包括其对于Th1/Th2细胞失衡的调节[18]。
本研究通过BMSCs与哮喘支气管上皮细胞共培养方式体外模拟哮喘发病环境。在共培养体系中,支气管上皮细胞与BMSCs共同接种于同一培养环境中,通过细胞间的直接接触、细胞因子的分泌与接收,及细胞外基质共享等方式进行相互作用[19]。BMSCs与哮喘支气管上皮细胞共培养可促进细胞间的信号传导,影响细胞的增殖、分化及功能表达。本研究发现,CCK-8检测结果显示,BMSCs与哮喘支气管上皮细胞共培养细胞活力明显降低。同时共培养体系下细胞间的相互作用通过可溶性因子介导[20],这些因子在细胞间的传递和接收过程中,可能触发一系列的生物学效应,BMSCs可通过分泌生长因子、细胞因子等信号分子来影响支气管上皮细胞的功能[21]。本研究显示,共培养体系下Th1细胞分泌的细胞因子IL-2、IL-12表达降低,Th2细胞分泌的细胞因子IL-5、IL-9表达升高。说明BMSCs与哮喘支气管上皮细胞共培养可调节细胞因子,特别是Th1、Th2细胞的分化,从而发挥其生理功能。而BMSCs具有强大的免疫调节能力,能够抑制免疫细胞的活化,减轻呼吸道炎症[22]。本研究显示,BMSCs归巢水平与IL-5表达呈负相关,与IL-12呈正相关。提示通过抑制Th2细胞因子异常分泌,纠正Th1/Th2细胞平衡向Th2倾斜,促进BMSCs归巢抑制免疫炎症。Notch1信号通路参与哮喘气道炎症,该通路的持续激活可导致Th1/Th2失衡及炎症细胞在肺组织的聚集[23]。Notch信号通路是细胞存活和细胞增殖的关键启动子,故而也被认为可以决定细胞命运,并可通过促进干细胞的自我更新和细胞分化[24]。Notch1信号通路参与了对BMSCs的调控[25]。本研究中通过与正常组比较,哮喘模型组Notch1、RBP-J、Hes1表达升高。Notch1通路在BMSCs的归巢过程中起重要作用[26],通过阻断Notch1信号传导或敲除BMSCs中Notch1信号的转录因子Hes1、RBP-J,可导致CXCR4上调,并促进BMSCs在体外对SDF-1的反应。本研究发现,CXCR4与Activated Notch1表达呈负相关,SDF-1与Notch1表达呈负相关,说明阻断Notch1可能增强BMSCs归巢。
miRNA与多种生理病理过程有关[27]。miR-139-5p参与各种细胞增殖、侵袭、分化、凋亡和自噬[28]。本研究通过将BMSCs与哮喘支气管上皮细胞共培养,观察到miR-139-5p mimics干扰下,共培养细胞miR-139-5p表达量降低,同时与MC组、miR-139-5p mimics-NC组比较,miR-139-5p mimics组miR-139-5p表达量升高。说明增加miR-139-5p表达,哮喘支气管上皮细胞和BMSCs共培养中细胞活力降低,S、G2期比例升高,同时细胞周期G1期比例降低,而BMSCs归巢水平进一步升高,提示miR-139-5p可能参与哮喘进程和BMSCs的归巢。miR-139-5p mimics组CXCR4、SDF-1表达进一步升高,提示高表达miR-139-5p后增加了哮喘BMSCs的归巢水平。miR-139-5p与Notch1信号通路存在关联。其通过靶向于下游靶基因Notch1,抑制Notch信号通路调节生物学功能[29]。Notch1作为miR-139-5p的靶基因,在miR-139-5p诱导的细胞迁移、侵袭、细胞凋亡和药物再致敏中发挥关键作用[30]。本研究发现升高miR-139-5p表达,细胞Notch1、RBP-J、Hes1基因和蛋白表达降低,进一步证实了Notch1是细胞中miR-139-5p的直接靶点,表明miR-139-5p通过下调Notch1的表达抑制哮喘发生。本研究显示,miR-139-5p的过表达抑制了Notch1蛋白表达,诱导了BMSCs中CXCR4蛋白的表达。这些结果表明,Notch1在miR-139-5p对BMSCs归巢的影响中起关键作用。本研究结果显示,增加miR-139-5p后Notch1信号通路表达降低同时,IL-2表达升高,而Th细胞因子IL-5、IL-9表达出现降低,表明在哮喘细胞模型中miR-139-5p参与调节Th1/Th2平衡。本研究发现miR-139-5p直接靶向Notch1,Notch1可以调控诱导BMSCs归巢,表明哮喘细胞中升高miR-139-5p可下调Notch1信号通路蛋白表达。不仅如此,在哮喘支气管上皮细胞中增加miR-139-5p下调Notch1蛋白并调控Th1、Th2细胞表达,诱导BMSCs归巢。说明miR-139-5p通过调控下游Notch通路参与哮喘Th1/Th2细胞的平衡调节,这与miR-139-5p通过靶向Notch1具有抑制气道炎症的潜在保护作用有一定的关联。
综上所述,miR-139-5p通过靶向Notch1的信号通路促进BMSCs归巢,从而影响Th1、Th2细胞因子表达。miR-139-5p靶向于Notch/RBP-J/Hes1轴可能是哮喘的潜在治疗靶点,并且miR-139-5p可能在BMSCs归巢中具有潜在的临床应用价值。
基金资助
国家自然科学基金(82205053);安徽省高等学校自然科学研究项目(2022AH050531);新安医学教育部重点实验室开放课题项目(2022xayx03);安徽中医药大学第一批人才支持计划项目(2022rcyb015)
Supported by National Natural Science Foundation of China (82205053).
参考文献
- 1. Maldonado-Puebla M, Cardet JC. The international variation in asthma phenotypes[J]. Allergy Asthma Immunol Res, 2024, 16(4): 317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xu YD, Cheng M, Mao JX, et al. Clara cell 10 (CC10) protein attenuates allergic airway inflammation by modulating lung dendritic cell functions[J]. Cell Mol Life Sci, 2024, 81(1): 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hu Y, Wang MQ, Xie J, et al. Exposure to ephedrine attenuates Th1/Th2 imbalance underlying OVA-induced asthma through airway epithelial cell-derived exosomal lnc-TRPM2-AS[J]. Chin J Nat Med, 2024, 22(6): 530-40. [DOI] [PubMed] [Google Scholar]
- 4. Hong XN, Jiang MY, Kho AT, et al. Circulating miRNAs associate with historical childhood asthma hospitalization in different serum vitamin D groups[J]. Respir Res, 2024, 25(1): 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang M, Han Y. MicroRNAs in chronic pediatric diseases (Review)[J]. Exp Ther Med, 2024, 27(3): 100. 27(3): 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hernández-Díazcouder A, Romero-Nava R, Del-Río-Navarro BE, et al. The roles of microRNAs in asthma and emerging insights into the effects of vitamin D3 supplementation[J]. Nutrients, 2024, 16(3): 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhu MX, Huang LH, Zhu YK, et al. LncRNA NEAT1 promotes airway smooth muscle cell inflammation by activating the JAK3/STAT5 pathway through targeting of miR-139[J]. Exp Lung Res, 2021, 47(4): 161-72. [DOI] [PubMed] [Google Scholar]
- 8. Musri MM, Coll-Bonfill N, Maron BA, et al. MicroRNA dysregulation in pulmonary arteries from chronic obstructive pulmonary disease. relationships with vascular remodeling[J]. Am J Respir Cell Mol Biol, 2018, 59(4): 490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang JJ, Hu Y, Wang YX, et al. Activation of Notch1-GATA3 pathway in asthma bronchial epithelial cells induced by acute PM2.5 exposure and the potential protective role of microRNA-139-5p[J]. J Asthma, 2024, 61(9): 959-69. [DOI] [PubMed] [Google Scholar]
- 10. 王 坤, 朱慧志, 杨 磊, 等. MiR-139下调Notch1/Hes1通路促进骨髓间充质干细胞归巢于哮喘大鼠肺组织抑制Th2细胞炎症反应[J]. 细胞与分子免疫学杂志, 2021, 37(2): 97-104. [PubMed] [Google Scholar]
- 11. Meng J, Gao X, Liu XJ, et al. Effects of xenogeneic transplantation of umbilical cord-derived mesenchymal stem cells combined with irbesartan on renal podocyte damage in diabetic rats[J]. Stem Cell Res Ther, 2024, 15(1): 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Palmans E, Kips JC, Pauwels RA. Prolonged allergen exposure induces structural airway changes in sensitized rats[J]. Am J Respir Crit Care Med, 2000, 161(2 Pt 1): 627-35. [DOI] [PubMed] [Google Scholar]
- 13. Chen XD, Yong SB, Yii CY, et al. Intestinal microbiota and probiotic intervention in children with bronchial asthma[J]. Heliyon, 2024, 10(15): e34916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Duraisamy SK, Sundar IK. REV-ERBα agonist SR10067 attenuates Th2 cytokine-mediated barrier dysfunction in human bronchial epithelial cells[J]. Clin Sci, 2024, 138(19): 1209-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang K, Liu T, Zhang Y, et al. Combined Placental Mesenchymal Stem Cells with Guided Nanoparticles Effective Against Diabetic Nephropathy in Mouse Model [J]. Int J Nanomedicine, 2024, 19: 901-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen QH, Zhang Y, Gu X, et al. Microvesicles derived from mesenchymal stem cells inhibit acute respiratory distress syndrome-related pulmonary fibrosis in mouse partly through hepatocyte growth factor[J]. World J Stem Cells, 2024, 16(8): 811-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jiang ZW, Chen LL, Huang L, et al. Bioactive materials that promote the homing of endogenous mesenchymal stem cells to improve wound healing[J]. Int J Nanomedicine, 2024, 19: 7751-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yuan Y, Tan SF, Wang HH, et al. Mesenchymal stem cell-derived exosomal miRNA-222-3p increases Th1/Th2 ratio and promotes apoptosis of acute myeloid leukemia cells[J]. Anal Cell Pathol, 2023, 2023: 4024887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schmelzer E, Miceli V, Chinnici CM, et al. Effects of mesenchymal stem cell coculture on human lung small airway epithelial cells[J]. Biomed Res Int, 2020, 2020: 9847579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin Q, Yu TX, Li XH, et al. Umbilical cord mesenchymal stem cells inhibited inflammation of bronchial epithelial cells by regulating Hedgehog pathway[J]. Eur J Histochem, 2023, 67(4): 3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang J, Xue J, Hu W, et al. Human embryonic stem cell-derived mesenchymal stem cell secretome reverts silica-induced airway epithelial cell injury by regulating Bmi1 signaling[J]. ToxicolEnviron, 2023, 38(9): 2084-99. [DOI] [PubMed] [Google Scholar]
- 22. Wang Y, Fang JK, Liu BM, et al. Reciprocal regulation of mesenchymal stem cells and immune responses[J]. Cell Stem Cell, 2022, 29(11): 1515-30. [DOI] [PubMed] [Google Scholar]
- 23. Nie Y, Yang BK, Hu JF, et al. Bruceine D ameliorates the balance of Th1/Th2 in a mouse model of ovalbumin-induced allergic asthma via inhibiting the NOTCH pathway[J]. Allergol Immunopathol, 2021, 49(6): 73-9. [DOI] [PubMed] [Google Scholar]
- 24. Gioftsidi S, Relaix F, Mourikis P. The Notch signaling network in muscle stem cells during development, homeostasis, and disease[J]. Skelet Muscle, 2022, 12(1): 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yu MX, Zhou M, Li JH, et al. Notch-activated mesenchymal stromal/stem cells enhance the protective effect against acetaminophen-induced acute liver injury by activating AMPK/SIRT1 pathway[J]. Stem Cell Res Ther, 2022, 13(1): 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang YZ, Yao B, Duan XL, et al. Notch1 down-regulation in lineage-restricted niches is involved in the development of mouse eccrine sweat glands[J]. J Mol Histol, 2022, 53(5): 857-67. [DOI] [PubMed] [Google Scholar]
- 27. Ho PTB, Clark IM, Le LTT. MicroRNA-based diagnosis and therapy[J]. Int J Mol Sci, 2022, 23(13): 7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang K, Jiang XL, Jiang Y, et al. EZH2-H3K27me3-mediated silencing of mir-139-5p inhibits cellular senescence in hepatocellular carcinoma by activating TOP2A[J]. J Exp Clin Cancer Res, 2023, 42(1): 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li SZ, Ren KX, Zhao J, et al. MiR-139/PDE2A-Notch1 feedback circuit represses stemness of gliomas by inhibiting Wnt/β‑catenin signaling[J]. Int J Biol Sci, 2021, 17(13): 3508-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen HB, Gong XY, Shen WH, et al. Exosomal AC068768.1 enhances the proliferation, migration, and invasion of laryngeal squamous cell carcinoma through miR-139-5p/NOTCH1 axis[J]. Heliyon, 2024, 10(16): e36358. [DOI] [PMC free article] [PubMed] [Google Scholar]


