Abstract
目的
探讨AURKB在促进骨肉瘤细胞恶性表型中的具体调控机制。
方法
分别转染HA-Vector和HA-AURKB于293T细胞中,采用免疫沉淀结合液相色谱-串联质谱技术筛选AURKB的潜在互作分子,免疫共沉淀和Western blotting验证其互作分子。通过RNA干扰技术,干扰骨肉瘤细胞中AURKB的表达;设置对照组:转染对照组慢病毒143B细胞,sh-AURKB组:转染干扰AURKB慢病毒143B细胞,sh-con+Flag-DHX9组:对照组转染过表达DHX9慢病毒,sh-AURKB+Flag-DHX9组:沉默AURKB组转染过表达DHX9慢病毒。采用EDU、Transwell实验分别观测细胞的增殖、迁移和侵袭活性。机制分析采用Co-IP、体内泛素化实验等检测AURKB和DHX9的相互作用、DHX9磷酸化水平、DHX9泛素化水平。Western blotting检测AURKB及DHX9对NF-κB的信号激活作用。
结果
AURKB在骨肉瘤细胞系高表达。沉默骨肉瘤143B细胞中AURKB的表达,细胞增殖、迁移和侵袭能力下降(P<0.05)。而AURKB与DHX9存在相互作用,且AURKB及DHX9在骨肉瘤组织中均高表达,沉默AURKB能够降低DHX9的蛋白表达,过表达AURKB后DHX9磷酸化水平升高(P<0.05)。沉默AURKB后不影响DHX9的转录翻译,但可以加快DHX9降解速度及增加DHX9泛素化水平。此外,过表达DHX9可以逆转沉默AURKB对IKBα蛋白及磷酸化p65的影响,促进p65入核激活NF-κB信号通路,并且增强了骨肉瘤细胞增殖、迁移和侵袭能力。
结论
AURKB通过调控DHX9激活NF-κB信号通路,促进骨肉瘤细胞恶性表型。
Keywords: 骨肉瘤, AURKB, DHX9, 磷酸化
Abstract
Objective
To investigate the regulatory mechanism of aurora kinase B (AURKB) for promoting malignant phenotype of osteosarcoma cells.
Methods
HA-Vector or HA-AURKB was transfected in 293T cells to identify the molecules interacting with AURKB using immunoprecipitation combined with liquid chromatography-tandem mass spectrometry followed by verification with co-immunoprecipitation and Western blotting. In cultured osteosarcoma cells with lentivirus-mediated RNA interference of AURKB or DHX9 or their overexpression, the changes in cell proliferation, migration, and invasion activities were observed with EDU and Transwell assays. Mechanistic analysis was performed using Co-IP and in vivo ubiquitination experiments to detect the interaction between AURKB and DHX9 and the phosphorylation and ubiquitination levels of DHX9. Western blotting was used to detect the effect of AURKB and DHX9 on activation of nuclear factor-κB (NF-κB) signaling.
Results
AURKB was highly expressed in osteosarcoma cell lines, and in osteosarcoma 143B cells, AURKB silencing significantly reduced cell proliferation, migration and invasion abilities. Interactions between AURKB and DHX9 were detected, and they were both highly expressed in osteosarcoma tissues; silencing AURKB reduced the protein expression of DHX9, and AURKB overexpression increased DHX9 phosphorylation. Silencing AURKB did not significantly affect the transcription and translation of DHX9 but accelerated its degradation and ubiquitination. Overexpression of DHX9 effectively reversed the effects of AURKB silencing on IKBα protein and phosphorylated p65, promoted nuclear translocation of p65 to activate the NF-κB signaling pathway, and enhanced the proliferation, migration, and invasion abilities of cultured osteosarcoma cells.
Conclusion
AURKB overexpression promotes the malignant phenotype of osteosarcoma cells by activating the NF-κB signaling pathway via regulating DHX9.
Keywords: osteosarcoma, aurora kinase B, DHX9, phosphorylation
骨肉瘤是最常见的骨原发性恶性肿瘤,5年生存率为60%~70%[1]。使用常规化疗和手术治疗的生存期趋势目前停滞不前[2]。此外,骨肉瘤病例对传统化疗的耐药性逐渐增多,大剂量化疗容易导致严重的副作用,如中性粒细胞减少、感染和血小板减少等[2, 3],迫切需要新颖的治疗策略作为传统治疗方式的辅助,以改善骨肉瘤患者的预后[4]。目前,大约1/3的骨肉瘤相关临床试验(如ClinicalTrials.gov ) 涉及针对细胞过程各个方面的靶向药物[5]。因此,进一步寻找骨肉瘤新的干预靶点,有助于骨肉瘤的靶向治疗[4, 5]。
AURKB是一种蛋白丝氨酸/苏氨酸激酶,属于极光激酶家族[6]。AURKB与着丝粒内蛋白等共同形成染色体乘客复合体,该复合体通过靶向有丝分裂不同阶段的特定位点来调节染色体分离和细胞分裂[7]。研究表明,AURKB在许多人类癌症中高度表达,其上调与癌症的发生和进展有关[8]。AURKB的活化与对EGFR酪氨酸激酶抑制剂的获得性耐药性有关,使其成为非小细胞肺癌患者抗EGFR治疗的潜在靶点[9]。AURKB的过表达也与白血病中DNA损伤反应的减少和p53靶蛋白细胞周期抑制蛋白p21水平的降低有关[10]。在胃癌中,AURKB在胃癌的氧化磷酸化过程和肿瘤免疫微环境之间发挥重要的连接作用[11]。在肺癌中,AURKB已被证实能直接磷酸化细胞分裂周期相关基因8(CDCA8)的关键位点,包括Ser154、Ser219、Ser275和Thr278。这种磷酸化作用似乎有助于稳定肺癌细胞中的CDCA8蛋白[12]。此外,11R-CDCA8261-280中的小肽段对CDCA8和AURKB相互作用的功能性抑制,显著降低了CDCA8的磷酸化,进而导致肺癌细胞的生长受到抑制[12]。而骨肉瘤的表型多样性可能源于其复杂的翻译后修饰过程,这一过程涉及多蛋白相互作用和高度精确的分子调控机制[13]。但是,AURKB在骨肉瘤翻译后修饰作用,目前文献报道较少。
为进一步研究AURKB在骨肉瘤发生、发展过程中的作用,我们通过免疫沉淀(IP) 结合液相色谱-串联质谱 (LC-MS/MS),发现DNA解旋酶Ⅱ和RNA解旋酶A (DHX9) 是AURKB潜在的相互作用分子。DHX9是一种NTP依赖性解旋酶蛋白,能够解旋RNA和DNA以及异常的多核苷酸结构,这表明它在DNA复制和维持基因组稳定性方面发挥作用[14-16]。DHX9被确定为非小细胞肺癌中参与抑制双链RNA和R环的病毒模拟诱导因子[17]。在骨肉瘤中,DHX9通过与MCM2和MCM3的相互作用,促进骨肉瘤细胞的生长,而DHX9的消耗则能阻碍骨肉瘤细胞的增殖[18]。本研究中,我们探讨了AURKB促进骨肉瘤细胞恶性表型的作用及其分子机制,为开发针对骨肉瘤的新型治疗策略提供潜在的分子靶点。
1. 材料和方法
1.1. 细胞培养
人骨肉瘤细胞株143B和HOS、永生化人软骨细胞hFOB1.19(中科院上海细胞库)均购自中国科学院细胞资源中心(中国上海)。上述细胞在含有10%胎牛血清(FBS,Gibco)和1%青霉素/链霉素溶液(P/S)的α-MEM(Gibco)中培养。同时,293T细胞则在含有相同比例胎牛血清和青霉素/链霉素溶液(Beyotime)的Dulbecco's 改良Eagle's培养基DMEM(Gibco)中培养。所有细胞均在37 ℃、5% CO2的条件下培养于恒温培养箱中。
1.2. 细胞转染
慢病毒质粒:pLV3-U6-shRNA-Negative-Control-CopGFP-Puro、pLV3-U6-AURKB(human)‑shRNA1-CopGFP-Puro、pLV3-U6-AURKB(human)‑shRNA2-CopGFP-Puro和pLV3-U6-AURKB(human)-shRNA3-CopGFP-Puro;pLV2-TRE3GS-DHX9(human)‑3×FLAG-TetOne-Blast及其对照质粒 (淼灵)。收获的慢病毒通过HiTransGA病毒感染试剂(REVG004GKE)进行转染。
1.3. 实验分组及处理
实验分为4组:sh-con+Flag-Vector组(转染敲低AURKB阴性对照及过表达DHX9阴性对照慢病毒);sh-AURKB+Flag-Vector组(转染敲低AURKB及过表达DHX9阴性对照慢病毒);sh-con+Flag-DHX9组(转染阴性对照及过表达DHX9慢病毒);sh-AURKB+Flag-DHX9组(转染敲低AURKB及过表达DHX9慢病毒)。随后,使用1.0 μg/mL的嘌呤霉素(Beyotime)进行筛选,持续1周以确保细胞系的稳定性。
1.4. 降解实验
环己酰亚胺(CHX)时间梯度(0、6、12、24 h)同时处理沉默AURKB及对照组的143B细胞,取得细胞提取细胞裂解液进行Western blotting实验。用蛋白酶体抑制剂MG132(20 μmol/L)和自噬抑制剂氯喹(CQ,20 μmol/L)处理敲低AURKB组143B细胞12h后,提取细胞裂解液进行Western blotting实验。
1.5. Western blotting实验
通过SDS-聚丙烯酰胺凝胶电泳 (SDS-PAGE) 对蛋白质进行分离,随后将分离的蛋白质转移到聚偏氟乙烯(PVDF)膜上。使用5%脱脂奶粉在TBST(Tris-Buffered Saline with Tween-20)中稀释后,将膜进行封闭以减少非特异性结合。在4℃下,将膜与以下一抗孵育过夜:抗AURKB抗体(Abcam,1∶2000)、抗DHX9抗体(CST,1∶1000),抗Flag-tag抗体、抗HA-tag抗体、抗GAPDH抗体,抗Histone H3抗体(Proteintech,稀释比例均为1∶2000)。第2天,使用TBST冲洗膜3次以去除未结合的一抗,加入山羊抗小鼠/兔二抗(CST,1∶3000),在室温下孵育1 h。孵育完成后,再次使用TBST进行3次快速振动洗涤以去除未结合的二抗。最后,我们使用Bio-Rad ChemiDoc MP成像系统对膜上的蛋白质条带进行检测和成像分析。
1.6. EDU检测
将细胞种植在96孔板或者培养皿中,并使其贴壁生长至50%~60%的密度。根据EDU试剂盒(翌圣生物)所介绍方法加入EDU试剂孵育,在EDU孵育后,用PBS清洗细胞,然后4%多聚甲醛固定细胞。使用0.5% Triton X-100对固定后的细胞进行渗透处理,根据试剂盒进行染色,最后使用荧光显微镜观察和计数EDU阳性细胞,每组随机取3张图像统计分析以评估细胞的增殖情况。
1.7. 细胞迁移、侵袭实验
Transwell法检测细胞迁移和侵袭。为了使细胞迁移,将指数期骨肉瘤细胞接种于Transwell板 (CORNING) 的小室中,接种200 μl无血清培养基,细胞总数为3×104。为评估细胞侵袭情况,将基质胶 (CORNING) 和无血清基础培养基按1∶10的比例加入Transwell板的上腔,37 ℃处理1 h,下腔中加入700 μL含20% FBS的DMEM培养基,37 ℃培养24 h,取出Transwell板,下腔细胞在4%多聚甲醛中固定20 min。然后用1%结晶紫溶液 (Beyotime)染色30 min。PBS洗涤后,在显微镜下随机取3张图像。
1.8. 免疫共沉淀实验
用IP特异性RIPA缓冲液(Beyotime)提取蛋白质。将同一种的一抗或IgG(1 μg抗体∶1 mg细胞蛋白)加入蛋白悬液中,4 ℃孵育过夜。次日,取20 μL蛋白A/G磁珠(HY-K0202MCEUSA)结合120 min,用RIPA缓冲液洗涤3次,加入2X上样缓冲液变性。最后进行SDS-PAGE Western blotting。
1.9. 银染实验
用IP特异性RIPA缓冲液(Beyotime)提取蛋白质。将同一种的一抗或IgG(1 μg抗体∶1 mg细胞蛋白)加入蛋白悬液中,4 ℃孵育过夜。次日,取20 μL蛋白A/G磁珠(HY-K0202MCEUSA)结合120 min,用RIPA缓冲液洗涤3次,加入2×上样缓冲液变性。最后进行电泳。电泳结束后,取目的凝胶通过快速银染试剂盒(Beyotime)进行银染鉴定。
1.10. 生物信息学分析
通过从TCGA数据库 下载并整理TCGA-SARC项目STAR流程的RNAseq数据,并提取TPM格式的数据以及临床数据。运用R4.2.1版本对基因AURKB及DHX9进行预后生存分析并绘制生存曲线。生存数据选择总体生存期(OS),通过Kaplan-Meier生存曲线评估AURKB及DHX9在肿瘤中的预后价值。
1. .11 组织标本收集
收集南昌大学第一附属医院8对骨肉瘤患者的组织标本,通过伦理审查会的批准[(2024)CDYFYYLK(05-007)]。
1.12. 统计分析
采用GraphPadPrism 8.0进行统计分析,计量资料以均数±标准差表示。统计学分析采用t检验和单因素方差分析。统计图中斜线代表配对分析,表明来自同一患者的癌和癌旁组织。P<0.05时为差异有统计学意义。
2. 结果
2.1. AURKB促进骨肉瘤细胞增殖、迁移和侵袭
通过TCGA数据库对肉瘤患者的AURKB表达水平进行预后分析显示,与AURKB低表达的肉瘤患者相比,AURKB高表达的患者预后差(P=0.002,图1A)。此外,与hFOB1.19相比,AURKB在5个骨肉瘤细胞系中均呈高表达(图1B)。EDU实验检测143B细胞的增殖活性结果显示:相比于阴性对照组(51.67%±7.64%),沉默AURKB组(22.97%±6.66%)143B细胞的增殖能力减弱(P<0.05,图1C)。基质胶侵袭实验检测143B细胞侵袭能力:相比于阴性对照组(900.7±24.2),沉默AURKB组(554.7±29.7)穿过Transwell小室的细胞更少(P<0.01,图1D);Migration实验检测143B细胞迁移能力:相比于阴性对照组(424.3±11.6),沉默AURKB组(238.3±16.7)穿过Transwell小室的细胞更少(图P<0.01,1D)。
图1.
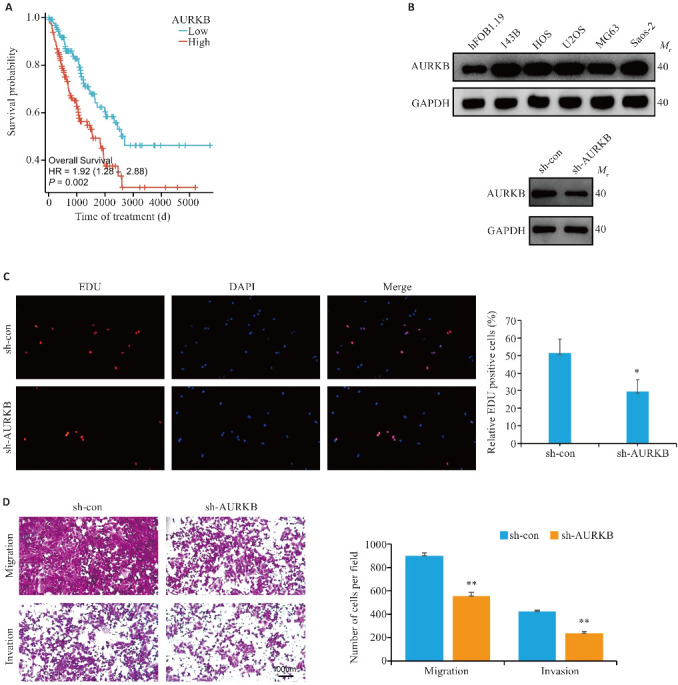
沉默AURKB抑制骨肉瘤143B细胞的增殖、迁移和侵袭能力
Fig.1 Silencing AURKB inhibits proliferation, migration and invasion of osteosarcoma 143B cells. A: Survival curves of sarcoma patients with different AURKB expression levels. B: Western blotting for detecting AURKB expression levels in osteosarcoma cell lines and for verifying the efficiency of AURKB knockdown in 143B cells. C: EDU assay for detecting the effect of AURKB silencing on proliferation of osteosarcoma cells (Original magnification: ×100). D: Migration and invasion assays of osteosarcoma cells after silencing AURKB (×100). *P<0.05,**P<0.01 vs sh-con.
2.2. AURKB与DHX9存在相互作用
通过LC-MS/MS得到AURKB的质谱:DHX9的肽段(图2A),对骨肉瘤细胞中AURKB的互作蛋白进行富集,结果显示:IP AURKB组比IgG组显现出更多蛋白质(图2B)。Western blotting检测结果显示,在293T细胞中,外源性AURKB与DHX9结合形成复合物,发生相互作用,而这一现象在空白对照组中未观察到(图2C);在143B细胞中,AURKB与DHX9均能互相富集形成复合物,IgG组中未观察到(图2D)。AURKB在骨肉瘤组织高表达(P=0.0022,图2E),DHX9在骨肉瘤组织中高表达(P=0.0012,图2E)。
图2.

AURKB与DHX9存在相互作用
Fig2 AURKB interacts with DHX9. A: DHX9 peptide identified by mass spectrometry. B: Silver-staining assay reveals AURKB-bound proteins. C: CO-IP detection of AURKB and DHX9 interaction in HEK-293 cells. D: CO-IP detection of AURKB and DHX9 interaction in 143B cells. E: Protein expression levels of AURKB and DHX9 in 8 pairs of osteosarcoma tissues and adjacent tissues (T: Tumor; N: Normal). **P<0.01.
2.3. AURKB磷酸化DHX9抑制其泛素化降解
与DHX9低表达患者相比,DHX9高表达患者预后差(P=0.035,图3A)。Western blotting检测结果显示,与hFOB1.19相比,DHX9在4个骨肉瘤细胞系中高表达(图3B)。在骨肉瘤细胞中,与阴性对照组相比,沉默AURKB的蛋白表达,DHX9蛋白水平降低(P<0.05,图3C)。在293T细胞中,过表达AURKB组磷酸化的DHX9蛋白多于对照组(P=0.0042,图3D)。实时荧光定量PCR实验提示:沉默143B细胞中AURKB的表达并不影响DHX9的mRNA水平(图3E)。与对照组相比,沉默AURKB组DHX9蛋白半衰期缩短(P<0.05,图3F)。MG132处理沉默AURKB组,DHX9蛋白表达量上升,CQ处理组未观察到该现象(图3G)。沉默AURKB后可以观察到DHX9泛素化水平升高(图3H)。
图3.
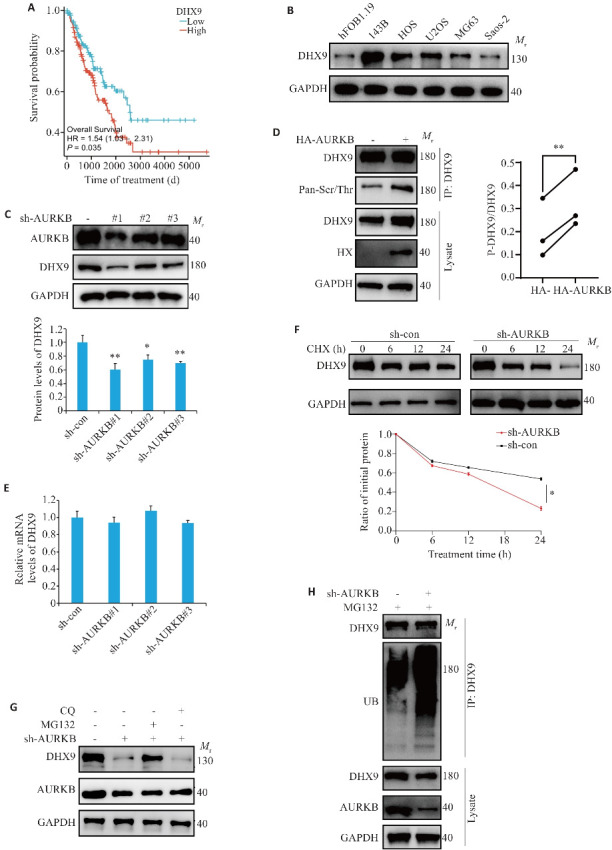
AURKB磷酸化DHX9抑制其泛素化降解
Fig.3 AURKB phosphorylates DHX9 to inhibit its ubiquitinated degradation. A: Survival curves of osteosarcoma patients with low and high AURKB expression. B: Expression of DHX9 in osteosarcoma cell lines. C: Impact of AURKB knockdown on DHX9 protein expression. D: Co-IP analysis of DHX9 protein phosphorylation levels following AURKB overexpression (The diagonal lines represent paired analyses from the same set of experiments). E: DHX9 mRNA expression levels in 143B cells with AURKB silencing. F: Time-dependent analysis of DHX9 protein expression in AURKB-silenced 143B cells treated with cycloheximide. G: Levels of DHX9 and AURKB in AURKB-knockdown 143B cell treated with MG132 (20 μmol/L) or chloroquine (CQ, 20 μmol/L) for 12 h detected by Western blotting. H: Ubiquitination levels of DHX9 in AURKB-knockdown 143B cells. *P<0.05, **P<0.01 vs sh-con.
2.4. AURKB通过调控DHX9激活NF-κB信号通路促进骨肉瘤细胞增殖、迁移和侵袭
在沉默AURKB条件下过表达DHX9,可以观察到骨肉瘤细胞增殖、侵袭、迁移增多(P<0.05,图4A、B)。通过Western blotting实验可以观察到沉默AURKB后,总体的p65蛋白表达不受到影响,IKBα蛋白表达升高,磷酸化p65及磷酸化IKBα蛋白表达减少。在沉默AURKB的基础上过表达DHX9后,p65表达不受影响,与对照组相比磷酸化p65及IKBα蛋白表达升高(P<0.05,图4C)。沉默AURKB,可以观察到p65蛋白入核减少(P=0.0049),在此基础上过表达DHX9,可以观察到p65入核增多(P=0.0039,图4D)。
图4.

AURKB通过调控DHX9激活NF-κB信号通路促进骨肉瘤细胞恶性表型
Fig.4 High expression of AURKB enhances malignant phenotype of osteosarcoma cells by activating NF-κB signaling via regulating DHX9. A: EDU experiment for assessing the effects of AURKB silencing and DHX9 overexpression on proliferation of osteosarcoma cells (×100). B: Migration and invasion experiments for assessing the effects of AURKB silencing and DHX9 overexpression on migration and invasion of osteosarcoma cells (×100). C: Western blotting for detecting protein levels of IKBα, p65, phosphorylated IKBα, and phosphorylated p65 in osteosarcoma cells with AURKB silencing and DHX9 overexpression. D: Western blotting for assessing the impact of AURKB silencing and DHX9 overexpression on nuclear translocation of p65. *P<0.05, **P<0.01, ***P<0.001 vs sh-con+Flag-Vector. # P<0.05, ## P<0.01 vs sh-AURKB+Flag-Vector.
3. 讨论
AURKB作为一种丝氨酸/苏氨酸激酶,在多种人类肿瘤中过度表达,这种过度表达可能导致纺锤体组装检查点功能异常,引发染色体错误分离和中心体扩增,最终导致染色体不稳定和肿瘤的发生[19]。AURKB与MAD2L2相互作用并调节其表达从而抑制膀胱癌细胞中的p53DNA损伤反应通路[20]。肺癌中AURKB通过对CDCA8的磷酸化作用抑制肺癌细胞的生长[12]。我们在前期的研究中也发现,AURKB在骨肉瘤中高表达并促进骨肉瘤的侵袭、转移,其可能是通过激活PI3K/AKT/NF-κB信号通路,上调MMP2、MMP9的表达有关[21, 22]。本研究结果也证实,AURKB在5个骨瘤细胞系中均呈高表达,沉默AURKB可抑制骨肉瘤细胞增殖、迁移和侵袭能力,提示AURKB参与骨肉瘤恶性表型进展。
我们通过Co-IP实验筛选出AURKB的互作分子DHX9;并且过表达AURKB后,DHX9分子的磷酸化水平升高。沉默AURKB后,在不影响DHX9分子转录的情况下缩短DHX9蛋白的降解周期,且DHX9泛素化水平升高。因此,我们推测AURKB对DHX9的磷酸化作用有助于稳定DHX9蛋白,但两者的结合区域及DHX9蛋白的磷酸化位点尚未明确。另一方面,AURKB可增强MDM2介导的p53泛素化[23]。AURKB通过触发组蛋白 H3在丝氨酸10位点的磷酸化以激活细胞周期蛋白E1表达来促进结直肠癌进展[24]。有丝分裂激酶 polo 样激酶1与AURKB差异磷酸化存活素以抑制三阴性乳腺癌细胞增殖[25]。所以,AURKB介导DHX9的泛素化改变是否依赖于其对DHX9的磷酸化作用,还需要进一步探讨。
DHX9的异常表达可能参与多种疾病[26],DHX9能够通过激活NF-κB信号通路,参与结直肠癌的恶性表型[27]。在骨肉瘤中,DHX9的表达降低会导致骨肉瘤细胞和骨髓瘤细胞的细胞周期停滞[28]。在食管鳞状癌中,SNORA42通过促进DHX9与p-p65相互作用来激活NF-κB信号,从而诱导NF-κB下游基因表达[29]。RAC-α丝氨酸/苏氨酸蛋白激酶(AKT1)与DHX9相互作用以减轻卵巢癌中R环诱导的复制应激[30]。本研究结果显示,在沉默AURKB、过表达DHX9回补实验中,过表达DHX9能够一定程度上逆转沉默AURKB对骨肉瘤细胞恶性表型的抑制作用。同时,过表达DHX9可以拯救沉默AURKB导致的p-p65、p-IKBα蛋白水平降低影响,过表达DHX9也能够促进p65入核。这表明,DHX9参与AURKB激活NF-κB信号通路的过程。
综上所述,本研究利用IP-LC-MS/MS技术,揭示了AURKB在骨肉瘤发展中的潜在作用机制。我们发现,AURKB和DHX9在骨肉瘤组织和细胞系中均高表达。AURKB促进骨肉瘤细胞的增殖、迁移和侵袭能力;并且AURKB可能通过磷酸化作用增强DHX9蛋白的稳定性,从而调控其蛋白表达。此外,在骨肉瘤细胞中,DHX9的过表达能够逆转AURKB下调对细胞增殖的抑制效果,并且能够恢复沉默AURKB对NF-κB信号通路的抑制作用。深入研究AURKB和DHX9在骨肉瘤发展及NF-κB信号通路激活中的作用,有望为开发针对骨肉瘤的新型治疗方案提供新的视角和策略。
基金资助
江西省“双千计划”首批培养类项目;江西省自然科学基金(20192ACBL21041,20232ACB206043)
参考文献
- 1. Friebele JC, Peck J, Pan XL, et al. Osteosarcoma: a meta-analysis and review of the literature[J]. Am J Orthop, 2015, 44(12): 547-53. [PubMed] [Google Scholar]
- 2. Gill J, Ahluwalia MK, Geller D, et al. New targets and approaches in osteosarcoma[J]. Pharmacol Ther, 2013, 137(1): 89-99. [DOI] [PubMed] [Google Scholar]
- 3. Whelan JS, Bielack SS, Marina N, et al. EURAMOS-1, an international randomised study for osteosarcoma: results from pre-randomisation treatment[J]. Ann Oncol, 2015, 26(2): 407-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu Y, Huang NG, Liao SJ, et al. Current research progress in targeted anti-angiogenesis therapy for osteosarcoma[J]. Cell Prolif, 2021, 54(9): e13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen YQ, Liu RZ, Wang W, et al. Advances in targeted therapy for osteosarcoma based on molecular classification[J]. Pharmacol Res, 2021, 169: 105684. [DOI] [PubMed] [Google Scholar]
- 6. Nigg EA. Mitotic kinases as regulators of cell division and its checkpoints[J]. Nat Rev Mol Cell Biol, 2001, 2(1): 21-32. [DOI] [PubMed] [Google Scholar]
- 7. Carmena M, Wheelock M, Funabiki H, et al. The chromosomal passenger complex (CPC): from easy rider to the godfather of mitosis[J]. Nat Rev Mol Cell Biol, 2012, 13(12): 789-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Borah NA, Reddy MM. Aurora kinase B inhibition: a potential therapeutic strategy for cancer[J]. Molecules, 2021, 26(7): 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bertran-Alamillo J, Cattan V, Schoumacher M, et al. AURKB as a target in non-small cell lung cancer with acquired resistance to anti-EGFR therapy[J]. Nat Commun, 2019, 10(1): 1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. González-Loyola A, Fernández-Miranda G, Trakala M, et al. Aurora B overexpression causes aneuploidy and p21Cip1 repression during tumor development[J]. Mol Cell Biol, 2015, 35(20): 3566-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen S, Wang Y, Cai X, et al. AURKB is a key to connect oxidative phosphorylation and immune microenvironment in Gastric Cancer [J]. Res Square, 2023. DOI/10.21203/rs.3.rs-3316505/vi. [Google Scholar]
- 12. Hayama S, Daigo Y, Yamabuki T, et al. Phosphorylation and activation of cell division cycle associated 8 by aurora kinase B plays a significant role in human lung carcinogenesis[J]. Cancer Res, 2007, 67(9): 4113-22. [DOI] [PubMed] [Google Scholar]
- 13. Wang D, Bu F, Zhang WW. The role of ubiquitination in regulating embryonic stem cell maintenance and cancer development[J]. Int J Mol Sci, 2019, 20(11): 2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jain A, Bacolla A, Chakraborty P, et al. Human DHX9 helicase unwinds triple-helical DNA structures[J]. Biochemistry, 2010, 49(33): 6992-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang S, Grosse F. Nuclear DNA helicase II unwinds both DNA and RNA[J]. Biochemistry, 1994, 33(13): 3906-12. [DOI] [PubMed] [Google Scholar]
- 16. Lee T, Pelletier J. The biology of DHX9 and its potential as a therapeutic target[J]. Oncotarget, 2016, 7(27): 42716-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Murayama T, Nakayama J, Jiang XP, et al. Targeting DHX9 triggers tumor-intrinsic interferon response and replication stress in small cell lung cancer[J]. Cancer Discov, 2024, 14(3): 468-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cheng DD, Zhang HZ, Yuan JQ, et al. Minichromosome maintenance protein 2 and 3 promote osteosarcoma progression via DHX9 and predict poor patient prognosis[J]. Oncotarget, 2017, 8(16): 26380-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hochegger H, Hégarat N, Pereira-Leal JB. Aurora at the pole and equator: overlapping functions of Aurora kinases in the mitotic spindle[J]. Open Biol, 2013, 3(3): 120185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li LZ, Jiang PC, Hu WM, et al. AURKB promotes bladder cancer progression by deregulating the p53 DNA damage response pathway via MAD2L2[J]. J Transl Med, 2024, 22(1): 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. 吴 昕, 刘家明, 宋宏海, 等. 抑制Aurora激酶B的表达可促进骨肉瘤143B细胞凋亡[J]. 南方医科大学学报, 2020, 40(9): 1273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yu JJ, Pi WS, Cao Y, et al. Let-7a inhibits osteosarcoma cell growth and lung metastasis by targeting Aurora-B[J]. Cancer Manag Res, 2018, 10: 6305-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gully CP, Velazquez-Torres G, Shin JH, et al. Aurora B kinase phosphorylates and instigates degradation of p53[J]. Proc Natl Acad Sci U S A, 2012, 109(24): E1513-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li L, Xie K, Xie HH, et al. AURKB promotes colorectal cancer progression by triggering the phosphorylation of histone H3 at serine 10 to activate CCNE1 expression[J]. Aging, 2024, 16(9): 8019-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garlapati C, Joshi S, Bhattarai S, et al. PLK1 and AURKB phosphorylate survivin differentially to affect proliferation in racially distinct triple-negative breast cancer[J]. Cell Death Dis, 2023, 14(1): 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shen BC, Chen YM, Hu J, et al. Hepatitis B virus X protein modulates upregulation of DHX9 to promote viral DNA replication[J]. Cell Microbiol, 2020, 22(3): e13148. [DOI] [PubMed] [Google Scholar]
- 27. Liu SL, He LM, Wu JH, et al. DHX9 contributes to the malignant phenotypes of colorectal cancer via activating NF‑κB signaling pathway[J]. Cell Mol Life Sci, 2021, 78(24): 8261-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee T, Paquet M, Larsson O, et al. Tumor cell survival dependence on the DHX9 DExH-box helicase[J]. Oncogene, 2016, 35(39): 5093-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shan YN, Wei SS, Xiang XH, et al. SNORA42 promotes oesophageal squamous cell carcinoma development through triggering the DHX9/p65 axis[J]. Genomics, 2021, 113(5): 3015-29. [DOI] [PubMed] [Google Scholar]
- 30. Huang TT, Chiang CY, Nair JR, et al. AKT1 interacts with DHX9 to mitigate R loop-induced replication stress in ovarian cancer[J]. Cancer Res, 2024, 84(6): 887-904. [DOI] [PMC free article] [PubMed] [Google Scholar]


