Abstract
Background
MYB transcription factors (TFs) play crucial roles in the response to diverse abiotic and biotic stress factors in plants. In this study, the GsMYB10 gene encoding a MYB-CC transcription factor was cloned from wild soybean BW69 line. However, there is less report on the aluminum (Al)-tolerant gene in this subfamily.
Results
The GsMYB10 gene was up-regulated by acidic aluminum stress and rich in the roots with a constitutive expression pattern in soybean. It was found that GsMYB10 protein contains the MYB and coiled-coil (CC) domains, localizes in the nucleus and holds transcriptional activity. The analysis of the transgenic phenotype revealed that the taproot length and root fresh weights of the GsMYB10-OE plants were greater than those of the wild type when subjected to AlCl3 treatments. While the accumulation of Al3+ in root tip of GsMYB10 transgenic plants (59.37 ± 3.59 µg/g) significantly reduced compared with that of wild type (80.40 ± 3.16 µg/g) which were shallowly stained by hematoxylin under the treatments of AlCl3. Physiological indexes showed that the proline content significantly increased 39–45% and the malondialdehyde content significantly reduced 37–42% in GsMYB10-OE plants compared with that of wild type. Transcriptomic analysis showed that overexpression of GsMYB10 induced a large number of differentially expressed genes (DEGs) with Al-treatment, which were related to wall modification related genes included PGs (such as Glyma.19g006200, Glyma.05g005800), XTHs (such as Glyma.12g080100, Glyma.12g101800, Glyma.08g093900 and Glyma.13g322500), NRAMPs and ABCs.
Conclusions
In summary, the data presented in this paper indicate that GsMYB10, as a new soybean MYB-CC TF, is a positive regulator and increases the adaptability of soybeans to acidic aluminum stress. The findings will contribute to the understanding of soybean response to acidic aluminum stress.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-024-06004-5.
Keywords: Soybean, Aluminum stress, MYB-CC family, Transcription factor
Background
Aluminum (Al) toxicity is a primary factor reducing crop yields on acidic soils, as much as 40–50% of the world’s arable land is acidic [1]. At low soil pH values (pH < 5.0), the phytotoxic Al3+ is released from the soil, which can rapidly inhibit root growth and damage physiological functions, thus reduce crop yield [2]. Therefore, enhancing the aluminum tolerance of crops is one of the effective ways to improve crop yield on acidic soil.
Many plant species have developed strategies to cope with Al toxic tolerance mechanism and recovery from Al-induced damages. Two main types of Al resistance mechanisms have been documented. The organic acid anions are secreted through the roots by chelating Al3+ ions and preventing them from entering the roots in Al exclusion mechanism. The Al tolerance mechanisms are classified as apoplasmic or symplasmicin mechanisms which are relative to property modification of the root cell wall, and sequestration or compartmentalization of Al once it enters the root symplast [3, 4]. In plants, the first identified aluminum resistance genes were malate and citric acid efflux transporters encoding ALMT (aluminum activated malate transporters) and MATE (multidrug and toxic compound extrusion) family membrane transporters, respectively [5]. Subsequently, more genes related to aluminum resistance have been reported. A large number of MATE members were implicated in citric acid efflux, such as AtMATE in Arabidopsis thaliana [6], TaMATE1 in wheat (Triticum aestivum) [7], OsFRDL4 in rice (Oryza sativa) [8], ZmMATE1 and ZmMATE2 in maize (Zea mays) [9]. ALMT family is control of the Al tolerance mechanism based on malate exudation. TaALMT1 is the first Al-tolerant gene cloned in plants, which encodes a transporter protein that involves the isolation of malate from the root tip and is responsible for wheat tolerance to aluminum stress [5]. Functional ALMT homologs associated with Al tolerance were also identified in Arabidopsis (AtALMT1) [10], Secale cereale (ScALMT1) [11], and Hordeum vulgare (HvALMT1) [12]. In addition, plants improve aluminum resistance by altering Al3+ binding in cell wall through cell wall components modification. The most important effect of aluminum is on cell wall enzymes among the cell wall modification, including pectin methylesterase, endo-β-1,4-glucanases, xyloglucan endotransglucosylase/hydrolases (XTHs), and expansins. For example, pectin methylation in root tip cell wall was related to aluminum resistance in Arabidopsis and rice, XTH31 affects Al sensitivity by modulating cell wall xyloglucan content and Al binding capacity in Arabidopsis [13].
Transcriptional responses of plants to environmental stresses have been extensively investigated and TFs regulate the expression of genes at the transcriptional level. However, only a few transcription factors regulating Al tolerance have been identified in several higher plant species. Two zinc-finger proteins, sensitive to proton rhizotoxicity 1 (STOP1) in Arabidopsis and Al3+ resistance transcription factor 1 (ART1) in rice were conferred hypersensitivity to Al toxicity [14, 15]. Furthermore, the expression of many Al-tolerance genes (e.g. MATE, ALS1, FRDL4, FRDL2, ALMT1) are regulated by these two transcription factors [16–18]. Two WRKY family members AtWRKY46 and OsWRKY22 also have mechanisms related to aluminum resistance. AtWRKY46 is a negative regulator of ALMT1, wrky46 leads to increased malate secretion and reduced Al accumulation in root apices, and thus confers higher Al resistance [19]. OsWRKY22 promotes Al-induced increases in OsFRDL4 expression by enhancing Al-induced citrate secretion and Al tolerance in rice [20]. GmWRKY21, a soybean WRKY transcription factor gene, enhances the tolerance to aluminum stress in Arabidopsis thaliana [21].
MYB transcription factors play a crucial role in regulating plant responses to abiotic stresses [22], including drought, salinity, cold, and aluminum toxicity. For instance, expression of the Arabidopsis AtMYB44 gene confers drought/salt-stress tolerance in transgenic soybean [23]. GhMYB36 positively regulated drought stress response both in Arabidopsis and cotton (Gossypium hirsutum) [24]. GmMYB68-overexpression lines showed enhanced resistance to salt and alkali stresses [25]. Transgenic apple calli and Arabidopsis with overexpression of MdMYB23 exhibited increased cold tolerance [26]. Furthermore, MsMYB741 enhances the accumulation of flavonoids in the root system and promotes root tip secretion by transcriptionally activating the expression of MsPAL1 and MsCHI, thereby improving alfalfa’s resistance to aluminum stress [27]. OsMYB30 negatively regulates Al resistance in rice [28]. GsMYB7 may enhance soybean tolerance to acidic aluminum stress by regulating the downstream genes [29]. MYB-CC TFs are the members of the MYB TF superfamily which are characterized by containing a conserved MYB DNA-binding domain and a coiled-coil (CC) domain [30]. The MYB-CC transcription factor has been demonstrated to play a role in the inorganic phosphate (Pi) starvation response and regulates a series of Pi starvation-inducible genes, such as, AtPHR1s [31]. The genes including PHL1, PHL2, PHL3 (UNE16) [32], OsPHR1, OsPHR3 [33], OsPHR2 [34], OsPHR4 [35], GmPHR1 [36], and GmPHR25 [37] respond to phosphorus starvation. However, there is limited information regarding aluminum (Al)-tolerant genes within this subfamily.
Soybean is one of the most important crops that serve as a crucial source of high-quality protein foods and vegetable oils. Nevertheless, acidic aluminum stress has inflicted severe consequences on soybean yields, particularly in the southern regions. In contrast, wild soybean (Glycine soja) has undergone long-term natural selection and has evolved special mechanisms to survive in aluminum stress. Previous studies have shown that the GsMYB10 gene is rapidly induced in response to aluminum stress in the wild soybean BW69 line (an aluminum tolerant line of Glycine soja) [38]. In this study, the GsMYB10 gene encoding a MYB-CC transcription factor was cloned from the BW69 line and transformed in recipient plant Huachun6. The GsMYB10 gene could improve the tolerance of transgenic plants to aluminum stress. The results revealed that GsMYB10 was involved in plant aluminum stress response, which will provide more genetic resources for soybean molecular breeding resistant to aluminum toxicity.
Results
Isolation and sequence analysis of GsMYB10
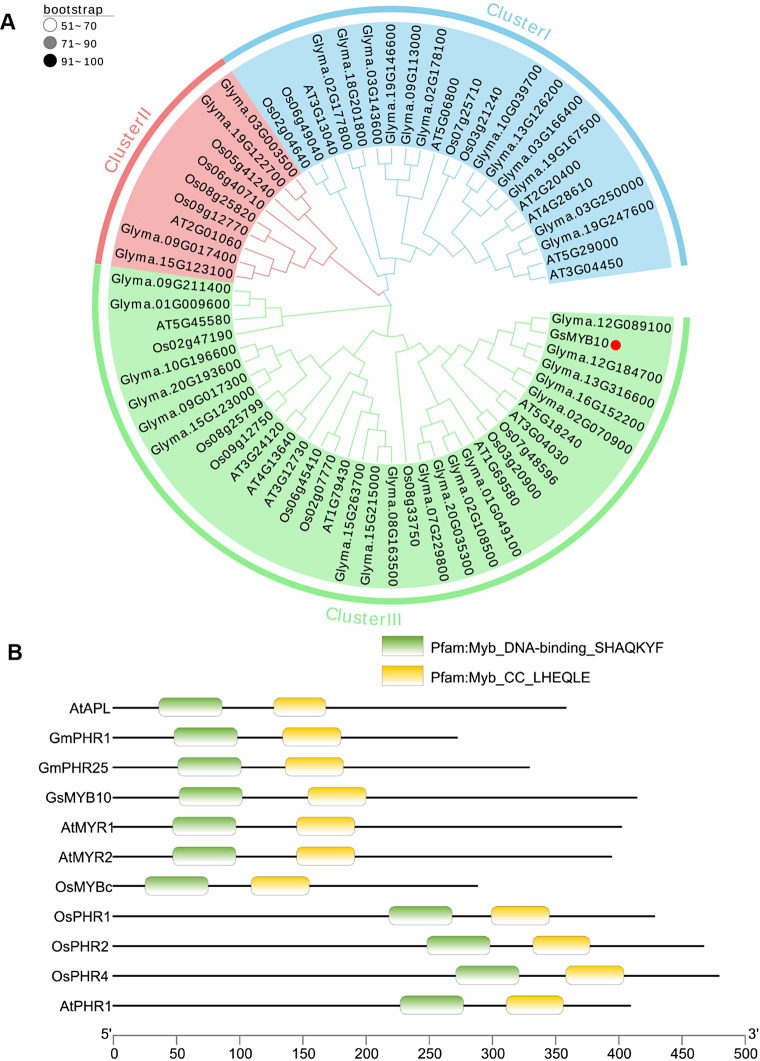
The full-length genome sequence of GsMYB10 is 3695 bp including 6 exons and 5 introns, containing an open reading frame of 1245 bp. The full length cDNA of GsMYB10 was cloned from the wild soybean BW69, which encoded the same sequence with cultivar soybean Huachun 6. To explore the phylogenetic associations among the identified genes in different MYB-CC gene families, we aligned 35 soybean, 15 Arabidopsis, and 16 rice MYB-CC proteins and constructed phylogenetic trees using the maximum likelihood method (Fig. 1A). Phylogenetic analysis showed that the MYB-CC family could be classified into three clusters (from I to III). The GsMYB10 protein belongs to class III, shares a close evolutionary relationship with MYR1 (AT5G18240) and MYR2 (AT3G04030) in Arabidopsis. In addition, the analysis of conserved motif distributions revealed that the GsMYB10 protein has a conserved MYB DNA binding domain and a coiled-coil (CC) domain with other published MYB-CC proteins (Fig. 1B).
Fig. 1.
The phylogenetic tree of MYB-CC family proteins from soybean, rice and Arabidopsis. (A) The phylogenetic tree was constructed using MEGA X with the Maximum likelihood (ML) method. The three groups corresponding to three branches are marked by numbers (I-III). Bootstrap values in percentages (1000 replicates) are indicated on the nodes. Different subgroups use different colors of clades to distinguish. B and C are comparative alignments of conserved domain sequences of known functional genes in the MYB-CC family. (B) The MYB domain and CC (coiled-coil) domain of GsMYB10 and other MYB-CC proteins. The Genbank accession numbers of proteins or genes loci for other species are as follows: AtAPL (At1g79430), GmPHR1 (Glyma.19g122700), GmPHR25 (Glyma.15g123100), AtMYR1 (At5g18240), AtMYR2 (At3g04030), OsMYBc (LOC_Os09g12770), OsPHR1 (LOC_Os03g21240), OsPHR2 (LOC_Os07g25710), OsPHR3 (LOC_Os02g04640), OsPHR4 (LOC_Os06g49040), AtPHR1 (At4g28610), AtUNE16 (At4g13640)
Expression patterns of GsMYB10 in tissues and response to Al stress
The transcript levels of GsMYB10 in BW69 and Huachun6 plants were determined by Quantitative real-time PCR (qRT-PCR) analysis of roots, stems leaves, flowers, pods, and tops. The results showed that GsMYB10 was constitutively expressed and was highly expressed in roots. In addition, GsMYB10 was also expressed in high abundance in flowers and fruit pods of wild soybean BW69 plants (Fig. 2A and Supplementary Fig. 1A). In addition, GsMYB10 responded to acid-aluminum stress at different concentrations. The transcripts of GsMYB10 were the highest in BW69 with 50 µM Al-treatment (Fig. 2B), meanwhile, were the highest in Huachun6 under 75 µM Al-treatment (Supplementary Fig. 1B). The expression of GsMYB10 was up-regulated by acidic aluminum with the treatment time in the Huchun6. However, the expression levels of GsMYB10 in BW69 showed a trend of increasing first and then slowly decreasing with the treatment time. The transcript of GsMYB10 in BW69 was up to the highest level at the AlCl3 treatment of 8 h (Fig. 2C and Supplementary Fig. 1C).
Fig. 2.
Expression patterns analysis of GsMYB10 in wild soybean BW69 line. (A) Tissue expression pattern of GsMYB10. The samples of roots, stems, leaves, flowers, pods and tops (apical tissues) were harvested during the pod stage. (B) The three-day seedlings were cultured in 0.5 mM CaCl2 solution containing 50 µM AlCl3 (pH 4.5) for 0, 1, 2, 4, 8, 12 and 24 h. (C) Soybean seedlings were treated with 0, 25, 50, 75 and 100 µM AlCl3 in 0.5 mM CaCl2 solution (pH4.5) for 8 h. Total RNA was extracted from root apices (0–6 cm). The data were represented as the mean ± SD of three biologic replicates. Student’s t-test was used to calculate the p-values, *, P<0.05;**, P < 0.01
Transcriptional activation activity assay and subcellular localization
To verify whether the GsMYB10 protein has transcription activity, the plasmid pGBKT7 (control) and the fusion plasmid pGBKT7-GsMYB10 were transformed into yeast Y2H gold, respectively. The yeast cells were grown on control medium plates (SD/-Trp) or selective medium plates (SD/-Trp/-X-α-Gal). The Y2H strains containing pGBKT7-GsMYB10 grew and showed blue color on selective medium (Fig. 3A). The results suggested that GsMYB10 protein has transcriptional activation activity.
Fig. 3.

Transcriptional activity assays and subcellular localization of GsMYB10 protein. (A) Transcriptional activation activity of GsMYB10 protein in yeast cells. (B) Subcellular localization of the 35S::GsMYB10-GFP fusion protein in leaf epidermal cells of Nicotiana benthamiana. Leaf epidermal cells transformed with 35S::GFP were used as a control. The RFP was nuclear localization protein marker (PJIT-mCherry-Nuc). Scale bars = 10 μm
To detect the subcellular localization of the GsMYB10 protein, both 35S-GFP and 35S-GsMYB10-GFP plasmids were transfected into the young leaves of tobacco plants with the nuclear localization protein marker (PJIT-mCherry-Nuc), respectively. The analysis using laser confocal microscopy showed that 35S-GFP was distributed throughout the whole cells, whereas the 35S-GsMYB10-GFP fusion protein localizes only in the nucleus (Fig. 3B). The results indicated that GsMYB10 is a nuclear localization protein.
GsMYB10 improved the tolerance of the overexpressing lines to Al-stress
More than 8 transgenic lines were obtained by transforming GsMYB10 in Huachun 6. Three lines of T3 generations with high expression levels were selected for the phenotype identification (Supplementary Fig. 2). Hematoxylin staining is an Al indicator in assessment of Al localization and accumulation in roots tip. GsMYB10-OE plants and wild type were stained with hematoxylin after treatment in a solution consisting of 25 µM AlCl3 for 8 h to verify the aluminum resistance. The results showed that the roots of GsMYB10 transgenic lines were lightly stained compared with those of wild type (Fig. 4A). The content of aluminum ions in root tips of transgenic plants was 59.37 ± 3.59 µg/g, which was lower than that of wild type 80.40 ± 3.16 µg/g (Fig. 4B). These results indicated that GsMYB10 enhances resistance to aluminum toxicity by reducing the accumulation of aluminum.
Fig. 4.
Physiological indexes related to aluminum stress change. The hematoxylin staining (A) root Al content (B) proline concentration (C) and MDA concentration (D) in transgenic plants and wild type under Al stress. Both WT and GsMYB10 transgenic lines were exposed to 0.5 mM CaCl2 solution with 25 µM AlCl3 (pH 4.5) for 8 h (in A and B) or 24 h (in C and D). Data were represented as mean ± SD of three biological replicates. Student’s t-test was used to calculate the p-values, ns = no significant difference
The phenotype of short-term (24 h) Al treatment was first performed to identify the phenotypes as shown in Supplementary Fig. 3. The taproot relative elongation of transgenic lines was higher than that of the wild type treated with different Al concentrations. In addition, MDA is one of the most important products of membrane lipid peroxidation, and its production can also aggravate the damage of membrane. Al treatment significantly increased the accumulation of MDA. The content of MDA in root of transgenic lines was 6.57 ± 0.27 µM/g lower than that of WT 10.42 ± 0.43µM/g (Fig. 4C). Overexpression of GsMYB10 contributes to reducing MDA levels under aluminum stress and reduces Al toxicity damage in plant roots. Proline plays an important role in protecting plants against various abiotic stresses. The proline content of the transgenic plants and wild-type plants increased after aluminum treatment, but the proline content in the GsMYB10-OE plants was much higher than that in the wild-type plants (Fig. 4D).
Subsequently, we performed phenotypic identification at seedling stage (two true leaves open). The results show that the GsMYB10-OE plants (L1, L2) were phenotypically identical to wild-type (WT) plants without Al treatment. However, the growth of primary and lateral roots of the plants was significantly inhibited under 25 µM and 50 µM aluminum treatments (Fig. 5A). Under the treatments of 25 µΜ and 50 µΜ AlCl3, the taproot lengths (Fig. 5B) and the root fresh weights (Fig. 5C) of GsMYB10-OE plants were significantly higher than wild type, and the root tip aluminum ion content in GsMYB10-OE plants was significantly lower than that of wild-type plants (Fig. 5D). Taken together, these results showed that GsMYB10 overexpression enhance the soybean tolerance to aluminum stress.
Fig. 5.
Overexpression of GsMYB10 conferred enhanced aluminum tolerance in transgenic plants. (A) The phenotype of GsMYB10 and wild type soybean seedlings which were treated using the solution including 0 µM, 25 µM and 50 µM AlCl3 in 0.5 mM CaCl2 solution (pH4.5) for 7 days. The taproot length (B), the fresh weight (C) and the aluminum content (D) of the underground part of transgenic lines (L1, L2) and wild type (WT). Data were represented as mean ± SD of three biological replicates, Student’s t-test was used to calculate the p-values, ns = no significant difference
Transcriptome analysis of GsMYB10 transgenic soybeans
To investigate the role of GsMYB10 in response to aluminum stress, RNA sequencing (RNA-seq) was conducted using the GsMYB10-OE plants (L1) and wild-type plants under control conditions and aluminum treatment (25 µM AlCl3 for 8 h). The analysis of RNA-seq results showed that 629 genes were identified as differentially expressed genes (DEGs) in the GsMYB10-OE compared to the wild type (WTvsOE). Following the 8 h aluminum treatment, there were 530 DEGs identified in the comparison of WT + Al and OE + Al. Meanwhile, 1081 genes were differentially expressed in the wild type relative to the control treatment (WTvsWT + Al), with 822 upregulated genes and 259 downregulated genes (Fig. 6A and Supplementary Fig. 4). However, more transcriptomic changes in GsMYB10-OE plants (OEvsOE + Al) were due to aluminum treatment relative to the wild-type plants (WTvsWT + Al). The results suggested that overexpression GsMYB10 regulated the expression of a large number of genes under aluminum stress.
Fig. 6.
Transcriptome analysis of differentially expressed genes regulated by GsMYB10. (A) Upset diagram showing number of differentially expressed genes (DEGs) in different groups. (B) GO terms which were statistically enriched in GsMYB10 regulated genes involved in aluminum treatment which were identified using the DEGs in A. (C) Heatmap of DEGs in the GO terms of xyloglucan metabolic process, extracellular region, plant-type vacuole, cellulose catabolic process, polygalacturonase activity. (D) Expression levels of the Al-responsive genes in GsMYB10 overexpression transgenic soybean plants. Data were represented as mean ± SD of three biological replicates. Student’s t-test was used to calculate the p-values, *, P < 0.05; **, P < 0.01
To investigate the biological processes in which GsMYB10 participates under aluminum stress, Gene Ontology (GO) enrichment analysis was performed using the 988 DEGs in comparisons (OEvsOE + Al) and the 133 DEGs (only shared by OEvsOE + Al and WT + AlvsOE + Al) (Fig. 6A). GO terms were especially enriched in the processes including oxidation-reduction process, hydrogen peroxide catabolic process, cell wall organization, cellular glucan metabolic process, xyloglucan metabolic process (Fig. 6B). The cell wall has an extremely important role in resisting acid-aluminum stress, acting as a fixation function for Al3+ and improving the aluminum tolerance of plants. Intriguingly, the transcript levels of cell wall related genes were dramatically changed in GsMYB10-OE plants, especially upon aluminum treatment (Fig. 6C). Furthermore, the qRT-PCR was carried out to confirm the results of transcriptome analysis. The tested DEGs were regulated under aluminum treatment, which was consistent with those of transcriptome analysis (Fig. 6D and Supplementary Fig. 5). The results suggested that cell wall related genes may contribute to enhancing aluminum resistance in GsMYB10-OE plants.
Discussion
Aluminum toxicity in acidic soil is one of the major constraints for crop production worldwide. Searching for novel genes involved in Al-tolerance provides an important guide to breed [39]. MYB-CC TFs are the members of the MYB TF superfamily which are characterized by containing a conserved MYB DNA-binding domain and a coiled-coil (CC) domain. In this study, GsMYB10 cloned from wild soybean belongs to the MYB-CC subfamily [30]. Many functions of the MYB-CC family have been reported about Pi availability. Overexpressing GmPHR25 increased Pi concentration in transgenic soybean hairy roots under normal conditions, accompanied with a significant decrease in hairy root growth, GmPHR25 is a vital regulator in the P signaling network, and controls Pi homeostasis in soybean [37]. The deficiency of phosphorus (P) and the toxicity of aluminum (Al) are recognized as two major nutritional stress factors that significantly hinder plant growth in acidic soils [40, 41]. Tang et al discovered that the exogenous addition of phosphorus could mitigate aluminum toxicity, indicating that phosphorus has the potential to enhance root morphological development and nutrient uptake in plants under aluminum stress [42]. A total of 35 MYB-CC family members were identified in soybean, and the GsMYB10 gene (named GmPH19) was significantly up-regulated in soybean roots during phosphorus starvation treatment. In this study, the expression of GsMYB10 was up-regulated under aluminum treatment. The relative root elongation and fresh weight of GsMYB10 overexpressed plants was significantly greater than that of wild type, meanwhile the Al content was significantly decreased (Fig. 4). Overexpression of wheat TaALMT1 in barley can improve P absorption and grain yield of acidic soil crops, which is largely attributed to the TaALMT1-mediated Al resistance that maintains normal root growth in acidic soil [43]. Whether GsMYB10 plays the same role in regulating soybean phosphorus homeostasis and the P absorption of the GsMYB10-OE plants with Al-treatment remains to be explored.
Plants exposed to aluminum stress will produce toxic effects in roots in a short time. The root growth inhibition is certainly the most easily recognizable trait of Al toxicity which can widely be marked as a measure of Al toxicity in plants. In this study, treatment with AlCl3 significantly inhibited the growth of both GsMYB10-OE plants and wild-type plants. However, the root growth of the GsMYB10 overexpression lines was less affected compared to that of the WT plants (Fig. 4 and Supplementary Fig. 3). Hematoxylin-stained root tips were used as indicators of Al tolerance [44]. The shallower hematoxylin staining indicates less apical bound aluminum. As can be seen in Fig. 4A, the overexpression plants of GsMYB10 were significantly less colored than wild type. In addition, the accumulation of Al3+ in the root tips of transgenic plants was significantly lower than that of wild type (Fig. 4B and Fig. 5D). These results indicate that overexpression of GsMYB10 may help increase the aluminum tolerance of transgenic plants by reducing the accumulation of aluminum ions in roots.
The RNA-seq is an effective method to elucidate the molecular mechanism of gene functions in plant. In present study, transcriptome analysis showed that aluminum treatment induced a large number of DEGs in GsMYB10-OE plants compared with those of wild-type plants (Fig. 6A). GO enrichment analysis showed that genes related to plant cell wall modification were significantly enriched. As the first physical barrier of aluminum, cell wall plays an increasingly important role in aluminum tolerance [45]. It is generally believed that the main binding site of aluminum in cell wall is pectin polysaccharide [41]. In this study, the results of transcriptome analysis and qRT-PCR showed that the expression levels of Glyma.19g006200 and Glyma.05g005800 in GsMYB10-OE plants were significantly increased under aluminum treatment compared with those of wild type (Fig. 6C and D). We found Glyma.19g006200 and Glyma.05g005800 encode a cell wall localized polygalacturonase (PG), one of the hydrolases responsible for cell wall pectin degradation [46]. The results suggest that the increased expression of Glyma.19g006200 and Glyma.05g005800 may change the composition and structure of pectin and activate cell wall defense against aluminum stress in GsMYB10-OE plants. GO enrichment analysis also show that Glyma.12g080100, Glyma.12g101800, Glyma.08g093900 and Glyma.13g322500 belong to the GO:0010411 (xyloglucan metabolic process) were enriched. Recent studies have found that hemicellulose in Arabidopsis is as important as pectin in binding Al, and affecting Al tolerance [13]. A subclass of XTH genes encoding xyloglucan endotransglucosylases (XET) responsible for cleaving and rejoining hemicellulosic xyloglucan polymers during cell expansion were revealed to respond to and function in Al stress. XTH31 transcript levels were selectively reduced by Al stress. XTH31 is involved in cell wall modification and cell elongation lead to increased aluminum tolerance, and XTH17 could physically interact with XTH31 to regulate the Al binding ability of cell wall, thus affecting Al sensitivity [13, 47] (Fig. 6C). Recent study shows that XTH17 and ELP are the potential downstream genes of WRKY47, XTH17 and ELP mediate the Al tolerance conferred by WRKY47 in Arabidopsis. ANAC017 regulates aluminum resistance in Arabidopsis by acting upstream of XTH31 [48, 49]. Glyma.12g101800 and Glyma.13g322500 was the homolog of AtXTH31, we found that the expression of Glyma.12g101800 and Glyma.13g322500 were increased significantly in GsMYB10 overexpressing plants. We speculated that GsMYB10 may also act as the upstream regulator of them. Therefore, GsMYB10 may enhance resistance to aluminum by regulating the expression of cell wall-related genes.
In addition, GmNRAMP7 (Glyma.06g115800) belongs to the Nramp (Natural resistance-associated macrophage protein) family which were induced in GsMYB10-OE plant under Al treatment. OsNrat1 belonging to the Nramp family and encoding a plasma membrane-localized transporter plays an important role in rice aluminum tolerance by operating in concert with a vacuolar ABC transporter. Nrat1 can also increase aluminum tolerance in Arabidopsis by the same way [50, 51]. Phylogenetic relationship showed that GmNRAMP7 is the only soybean NRAMP protein in a small branch with several monocot NRAMPs [52]. Moreover, the expression of some vacuolar ABC transporter genes such as Glyma.13g118800, Glyma.20g242000, Glyma.08g270300 and Glyma.08g194600 were increased by Al treatment in GsMYB10-OE plant. The results indicate that the GsMYB10 gene may mitigate the toxic effects of aluminum ions in the roots by regulating the expression of the aforementioned differential genes, thereby enhancing the resistance of GsMYB10-OE plants.
In summary, the data presented in this paper indicate that GsMYB10, as a new soybean MYB-CC TF, is a positive regulator and increases the adaptability of soybeans to acidic aluminum stress. These findings will contribute to the understanding of soybean response to acidic aluminum stress.
Conclusions
The GsMYB10 gene could positively regulate soybean tolerance to acidic aluminum stress. The results suggested that GsMYB10 may regulate the aluminum resistance of soybean by regulating differential genes related to cell wall components, modification, and aluminum ion transport.
Methods
Plant materials and stress treatments
The seeds of wild soybean BW69 line and soybean cultivar HC6 were obtained from the Guangdong Subcenter of the National Center for Soybean Improvement (Guangzhou, China). For hydroponics, seeds were sterilized with 1% (v/v) NaClO for 5 min, subsequently washed three times with deionized water. The seeds were then germinated in vermiculite pots for 4 d in a growth chamber with a 14-h light (28 °C)/10-h dark (24 °C) photoperiod with approximately 100 µM m− 2s− 1 photon density and 70% relative humidity. The resultant seedlings were then grown in full strength nutrient solution for 24 h before various treatments. For the time-course experiment, soybean seedlings were transplanted to Al (50 µM AlCl3, 0.5 mM CaCl2, pH4.5) treatments for 0, 1, 2, 4, 8, 12, and 24 h, respectively. For the Al dose experiment, soybean seedlings were treated with 0, 25, 50, 75, and 100 µM AlCl3 in 0.5 mM CaCl2 solution (pH4.5) for 8 h, respectively [53]. The samples of root tips (0–6 cm) were separately harvested for gene expression assays for the concentration response experiment and time-course experiment. In addition, wild soybean (BW69) and soybean cultivar HC6 was sown in the field of the agricultural trial station of South China Agricultural University. The samples of roots, stems, leaves, flowers, pods and apical tissues were harvested and immediately dipped into liquid nitrogen during the pod stage. The samples were then stored at -80℃. All the experiments had three biological replicates.
RNA extraction and quantitative real-time PCR analysis
Total RNA was extracted using a Trizol reagent (TIANGEN, China) and treated with DNase I (Takara) to remove genomic DNA. Synthesis of cDNA was conducted using Revert Aid First Strand cDNA synthesis Kit (Takara). Triplicate quantitative assays were performed on cDNA with the SYBR Green Master mix and the SsoFast EvaGreen Supermix Kit (BIO-RAD) on an ABI 7900 sequence detection system according to the manufacturers’ instructions. The data were normalized using the reference gene Actin3. The quantitative variation between the examined replicates was evaluated by the 2–∆∆Ct method [54]. The primers used for qRT-PCR were listed in Supplementary Table S1.
Cloning and sequence analysis
The sequence information of GsMYB10 was obtained from the database of the National Center for Biotechnology Information (NCBI) with the accession number LOC100781878. To obtain the full-length ORF of GsMYB10, the primers (5´-TGGCTTTGCAGGTTGA-3´and 5´-AACTCATATTTGGCTAG-3´) were designed to amplify the GsMYB10 gene on the basis of soybean genome sequence information. The PCR fragments were cloned into the pLB vector (Zero Background Quick Cloning Kit, TIANGEN, China).
Phylogenetic tree analysis was conducted by a Clustal X multiple-sequence alignment and the Maximum likelihood (ML) method from MEGAX [55]. Phylogenetic analysis of MYB-CC family members in soybean [37], Arabidopsis [32], and rice [35], as previously reported. Multiple sequence alignments were performed using Clustal X software based on the two conserved domains (MYB and coiled-coil) of GsMYB10 and other published MYB-CC family genes. All the sequences of the GsMYB10 gene were obtained using BLAST in the NCBI and Phytozome database.
Subcellular localization analysis
To determine the subcellular localization of GsMYB10 protein, the complete open reading frame (ORF) without the stop codon was amplified and inserted into the NcoI and SpeI sites of pCAMBIA1302 containing the GFP reporter gene under the control of cauliflower mosaic virus (CaMV) 35S promoter to form a construct 35S::GsMYB10-GFP. The fusion construct (35S::GsMYB10-GFP) and control vector (35S::GFP) were integrated into Agrobacterium tumefaciens GV3101. Tobacco (Nicotiana benthamiana) leaves were agroinfiltrated with GV3101 carrying either the fusion construct or the control using the method described previously. The infiltrated plants were grown for an additional two days prior to fluorescence signal detection using a laser scanning confocal microscope (Leica TCS SP8).
Transcriptional activation activity assay
To detect transcriptional activity of GsMYB10 protein, the full-length coding region of GsMYB10 was amplified with special primers (Supplementary Table S1) and inserted into the sites of NcoI and BamHI of pGBKT7 vector to form the GsMYB10-pGBKT7 construct (Clontech). The fusion construct and empty vector were separately transformed in yeast strain Y2H. The yeast cells were plated on synthetic dropout SD/-Trp medium. Activity of α-galactosidase was examined by plating the transformants on SD/-Trp medium containing X-α-Gal. The above experimental methods are analyzed according to previous studies [56].
Vector construction and plant transformation
The coding sequence of GsMYB10 was cloned into pZY101 vector to generate overexpression construct. The GsMYB10-pZY101 construct was transferred into soybean cultivar Huachun 6 using the transformation method of Agrobacterium-mediated cotyledon node described previously [57]. The transgenic positive plants were identified with specific primers (Supplementary Table 1). Expression levels of GsMYB10 in transgenic plants were examined by qRT-PCR. The transgenic lines of T3 generation were used for the subsequent experiments.
Phenotype and statistical analyses of transgenic plants treated with aluminum
The soybean seeds of T3 generation were sown in pots filled with vermiculite for 3 days. Then the seedlings were transplanted into 0.5 mM CaCl2 (pH = 4.5) solution and cultured for 24 h. Pre-cultured seedlings were exposed to 0.5 mM CaCl2 solution containing 0 or 25 µM AlCl3 (pH4.5). The root length was measured by taking pictures with a Nikon camera and then using the software Image J. The relative root elongation (RRE) was calculated to evaluate Al sensitivity [58]. The fresh weights were measured using a Sartorius BSA224S-CW 1/1000 analytical balance.
Root tips were stained with the hematoxylin which was used as an indicator of Al tolerance [59, 60]. After 8 h of treatment, the root tips of 2 cm length were washed with distilled water for half an hour, stained with hematoxylin for half an hour, and then washed again with distilled water for half an hour. The cleaned root tips were imaged with a Stereomicroscope Leica S8AP0.
For determination of root Al content, the last 0–2 cm root tips from the roots with similar length were washed three times with distilled water. 12 roots were extracted by 2 M HCl for 48 h with occasional shaking. Content of Al3+ was determined by inductively coupled plasma-atomic emission spectrometry (ICP-AES, IRIS-Advantage, 710-ES, VARIAN, USA).
Measurements of MDA and Proline content
For physiological parameter measurements, the leaves of aluminum treated WT and GsMYB10-OE plants were harvested for the measurements of MDA content and proline content. MDA content is determined by thiobarbituric acid (TBA) reaction [61]. Proline content is determined by sulfosalicylic acid method [62].
Transcriptome analysis of transgenic soybean plants
Total RNA was extracted from mock-treated (0 µM AlCl3) and Al-treated (25 µM AlCl3) for 8 h root of the seedlings (HuaChun6 and GsMYB10-OE) using Trizol reagent (Invitrogen, CA, USA). RNA-sequencing (RNA-seq) was performed by LC-BIO (Hangzhou, China). There were three biological replicates for each sample. GO term enrichment analysis of gene sets was performed to identify the enriched GO terms. The calculated P-value was subjected to Bonferroni correction, taking a corrected p-value ≦ 0.05 as a threshold for significance. GO terms fulfilling this condition are defined as significantly enriched GO terms in DEGs [63].
Statistical analyses
All statistical analyses were performed by using IBM SPSS Statistics for Windows, Version 22.0 and Graphpad Prism 8.0.1. Data were represented as mean ± SD (standard deviation) of three biological replicates. Comparison between different groups was tested by one-way ANOVA, followed by t-test. A p-value less than 0.05 was considered significantly different (*, P < 0.05; **, P < 0.01).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Prof. Zhanyuan Zhang (University of Missouri, USA) for kindly providing us with the pZY101 vector.
Abbreviations
- CC
Coiled-coil
- TFs
Transcription factors
- DEGs
Differentially expressed genes
- HC6
The cultivar HuaChun 6
- WT
Wild type
- MDA
Malondialdehyde
- RNA-seq
RNA-sequencing
- GO
Gene ontology
Author contributions
QM and HN designed and conceived the work. CY performed all experiments. XL, ZL involved in soybean transformation. HW, DD and YC participated in the breeding of materials and identification of transgenic materials. TL involved in regulatory gene analysis and data analysis. CL and KH participated in the expression of GsMYB10 in cultivar soybean. CY, XL and QM performed data as well as statistical analysis. CY prepared the manuscript which was edited by QM and HN. All authors discussed the results and commented on the manuscript.
Funding
This work was supported by the grants from the projects of the National Natural Science Foundation of China (31771816, 31971965 and 31371642), the New Varieties Cultivation of Genetically Modified Organisms (2016ZX08004002-007), the China Agricultural Research System (CARS-04-PS09), Ministry of Agriculture and Rural Affairs agricultural products quality and safety supervision special (4100-C17106, 21301091702101), the Projects of Key Area Research and Development Program of Guangdong Province (2020B020220008), the project of the Guangdong Provincial Laboratory of Lingnan Modern Agricultural Science and Technology (NZ2021012), the Research Project of the State Key Laboratory of Agricultural and Biological Resources Protection and Utilization in Subtropics (4100–31331), the National Key R&D Program of China (2017YFD0101500), and the Science and Technology of Guangzhou (201804020015).
Data Availability
All data generated in this study are included in this published article (additional files). The RNA-seq data has been uploaded to NCBI database, with accession number PRJNA833532
Declarations
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ce Yang and Xiang Lu contributed equally to this work.
Contributor Information
Hai Nian, Email: hnian@scau.edu.cn.
Qibin Ma, Email: maqibin@scau.edu.cn.
References
- 1.von Uexküll HR, Mutert E. Global extent, development and economic impact of acid soils. Plant Soil. 1995;171(1):1–15. [Google Scholar]
- 2.Kochian LV. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol. 1995;46(1):237–60. [Google Scholar]
- 3.Chauhan DK, Yadav V, Vaculík M, Gassmann W, Pike S, Arif N, Singh VP, Deshmukh R, Sahi S, Tripathi DK. Aluminum toxicity and aluminum stress-induced physiological tolerance responses in higher plants. Crit Rev Biotechnol. 2021;41(5):715–30. [DOI] [PubMed] [Google Scholar]
- 4.Kochian LV, Pineros MA, Liu J, Magalhaes JV. Plant adaptation to acid soils: the molecular basis for crop aluminum resistance. Annu Rev Plant Biol. 2015;66:571–98. [DOI] [PubMed] [Google Scholar]
- 5.Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, Ryan PR, Delhaize E, Matsumoto H. A wheat gene encoding an aluminum-activated malate transporter. Plant J. 2004;37(5):645–53. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Magalhaes JV, Shaff J, Kochian LV. Aluminum-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. Plant J. 2009;57(3):389–99. [DOI] [PubMed] [Google Scholar]
- 7.Ryan PR, Raman H, Gupta S, Horst WJ, Delhaize E, Kellogg E, Buell CR. A second mechanism for aluminum resistance in wheat relies on the constitutive efflux of citrate from roots. Plant Physiol. 2009;149(1):340–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokosho K, Yamaji N, Ma JF. An Al-inducible MATE gene is involved in external detoxification of Al in rice. Plant J. 2011;68(6):1061–69. [DOI] [PubMed] [Google Scholar]
- 9.Maron LG, Piñeros MA, Guimarães CT, Magalhaes JV, Pleiman JK, Mao CZ, Shaff J, Belicuas SNJ, Kochian LV. Two functionally distinct members of the MATE (multi-drug and toxic compound extrusion) family of transporters potentially underlie two major aluminum tolerance QTLs in maize. Plant J. 2010;61(5):728–40. [DOI] [PubMed] [Google Scholar]
- 10.Hoekenga OA, Maron LG, Piñeros MA, Cançado GMA, Shaff J, Kobayashi Y, et al. AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc Natl Acad Sci. 2006;103(25):9738–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins N, Shirley N, Saeed M, Pallotta M, Gustafson J. An ALMT1 gene cluster controlling aluminum tolerance at the Alt4 locus of rye (Secale cereale L). Genetics. 2008;179(1):669–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gruber BD, Ryan PR, Richardson AE, Tyerman SD, Ramesh S, Hebb DM, Howitt SM, Delhaize E. HvALMT1 from barley is involved in the transport of organic anions. J Exp Bot. 2010;61(5):1455–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu X, Shi Y, Lei G, Fry S, Zhang B, Zhou Y, et al. XTH31, encoding an in vitro XEH/XET-active enzyme, regulates aluminum sensitivity by modulating in vivo XET action, cell wall xyloglucan content, and aluminum binding capacity in Arabidopsis. Plant Cell. 2012;24(11):4731–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamaji N, Chao F, Nagao S, Yano M, Sato Y, Nagamura Y, Jian F. A zinc finger transcription factor ART1 regulates multiple genes implicated in aluminum tolerance in rice. Plant Cell. 2009;21(10):3339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iuchi S, Koyama H, Iuchi A, Kobayashi Y, Kitabayashi S, Kobayashi Y, Ikka T, Hirayama T, Shinozaki K, Kobayashi M. Zinc finger protein STOP1 is critical for proton tolerance in Arabidopsis and coregulates a key gene in aluminum tolerance. Proc Natl Acad Sci U S A. 2007;104(23):9900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang C, Yamaji N, Chen Z, Ma J. A tonoplast-localized half-size ABC transporter is required for internal detoxification of aluminum in rice. Plant J. 2012;69(5):857–67. [DOI] [PubMed] [Google Scholar]
- 17.Sawaki Y, Iuchi S, Kobayashi Y, Kobayashi Y, Ikka T, Sakurai N, Fujita M, Shinozaki K, Shibata D, Kobayashi M. STOP1 regulates multiple genes that protect ArabidopsisProtonproton and aluminum toxicities. Plant Physiol. 2009;150(1):281–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yokosho K, Yamaji N, Fujii-Kashino M, Ma JF. Functional analysis of a mate gene OSFRDL2 revealed its involvement in Al-induced secretion of citrate, but a lower contribution to Al tolerance in rice. Plant Cell Physiol. 2016;57(5):976–85. [DOI] [PubMed] [Google Scholar]
- 19.Ding Z, Yan J, Xu X, Li G, Zheng S. WRKY46 functions as a transcriptional repressor of ALMT1, regulating aluminum-induced malate secretion in Arabidopsis. Plant J. 2013;76(5):825–35. [DOI] [PubMed] [Google Scholar]
- 20.Li G, Wang Z, Yokosho, Ding B, Fan W, Gong Q, et al. Transcription factor WRKY22 promotes aluminum tolerance via activation of OsFRDL4 expression and enhancement of citrate secretion in rice (Oryza sativa). New Phytol. 2018;219(1):149–62. [DOI] [PubMed] [Google Scholar]
- 21.Han Z, Wang J, Wang X, Zhang X, Cheng Y, Cai Z, Nian H, Ma Q. GmWRKY21, a soybean wrky transcription factor gene, enhances the tolerance to aluminum stress in Arabidopsis thaliana. Front Plant Sci. 2022;13:833326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Niu Y, Zheng Y. Multiple functions of MYB transcription factors in abiotic stress responses. Int J Mol Sci. 2021;22(11):6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seo JS, Sohn HB, Noh K, Jung C, An JH, Donovan CM, Somers DA, Kim DI, Jeong S, Kim C, et al. Expression of the Arabidopsis AtMYB44 gene confers drought/salt-stress tolerance in transgenic soybean. Mol Breed. 2012;29(3):601–8. [Google Scholar]
- 24.Liu T, Chen T, Kan J, Yao Y, Guo D, Yang Y, Ling X, Wang J, Zhang B. The GhMYB36 transcription factor confers resistance to biotic and abiotic stress by enhancing PR1 gene expression in plants. Plant Biotechnol J. 2022;20(4):722–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He Y, Dong Y, Yang X, Guo D, Qian X, Yan F, Wang Y, Li J, Wang Q. Functional activation of a novel R2R3-MYB protein gene, GmMYB68, confers salt-alkali resistance in soybean (Glycine max L). Genome. 2020;63(1):13–26. [DOI] [PubMed] [Google Scholar]
- 26.An J, Li R, Qu F, You C, Wang X, Hao Y. R2R3-MYB transcription factor MdMYB23 is involved in the cold tolerance and proanthocyanidin accumulation in apple. Plant J. 2018;96(3):562–77. [DOI] [PubMed] [Google Scholar]
- 27.Su L, Lv A, Wen W, Fan N, Li J, Gao L, Zhou P, An Y. MsMYB741 is involved in alfalfa resistance to aluminum stress by regulating flavonoid biosynthesis. Plant J. 2022;112(3):756–71. [DOI] [PubMed] [Google Scholar]
- 28.Gao L, Liu X, Gao K, Cui M, Zhu H, Li G, Yan J, Wu Y, Ding Z, Chen XW, Ma J, Harberd NP. ART1 and putrescine contribute to rice aluminum resistance via OsMYB30 in cell wall modification. J Integr Plant Biol. 2023;65(4):934–49. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Yin X, Du D, Liang Z, Han Z, Nian H, Ma Q. GsMYB7 encoding a R2R3-type MYB transcription factor enhances the tolerance to aluminum stress in soybean (Glycine max L). BMC Genomics. 2022;23(1):529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubio V, Linhares F, Solano R, Martin AC, Iglesias J, Leyva A, Paz-Ares J. A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev. 2001;15(16):2122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nilsson L, Muller R, Nielsen TH. Increased expression of the MYB-related transcription factor, PHR1, leads to enhanced phosphate uptake in Arabidopsis thaliana. Plant Cell Environ. 2007;30(12):1499–512. [DOI] [PubMed] [Google Scholar]
- 32.Sun L, Song L, Zhang Y, Zheng Z, Liu D. Arabidopsis PHL2 and PHR1 act redundantly as the key components of the central regulatory system controlling transcriptional responses to phosphate starvation. Plant Physiol. 2016;170(1):499–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo M, Ruan W, Li C, Huang F, Zeng M, Liu Y, Yu Y, Ding X, Wu Y, Wu Z, Mao C, Yi K, Wu P, Mo X. Integrative comparison of the role of the phosphate response1 subfamily in phosphate signaling and homeostasis in rice. Plant Physiol. 2015;168(4):1762–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou J, Jiao F, Wu Z, Li Y, Wang X, He X, Zhong W, Wu P. OsPHR2 is involved in phosphate-starvation signaling and excessive phosphate accumulation in shoots of plants. Plant Physiol. 2008;146(4):1673–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruan W, Guo M, Wu P, Yi K. Phosphate starvation induced OsPHR4 mediates Pi-signaling and homeostasis in rice. Plant Mol Biol. 2017;93(3):327–40. [DOI] [PubMed] [Google Scholar]
- 36.Li X, Wang Y, WU B, Kong Y, LI W, Chang W, Zhang C. GmPHR1, a novel homolog of the AtPHR1 transcription factor, plays a role in plant tolerance to phosphate starvation. J Integr Agric. 2014;13(12):2584–93. [Google Scholar]
- 37.Xue Y, Xiao B, Zhu S, Mo X, Liang C, Tian J, et al. GmPHR25, a GmPHR member up-regulated by phosphate starvation, controls phosphate homeostasis in soybean. J Exp Bot. 2017;68(17):4951–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeng Q, Yang C, Ma Q, Li X, Dong W, Nian H. Identification of wild soybean miRNAs and their target genes responsive to aluminum stress. BMC Plant Biol. 2012;12:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryan PR, Kochian LV. Interaction between aluminum toxicity and calcium uptake at the root apex in near-isogenic lines of wheat (Triticum Aestivum L.) differing in aluminum tolerance. Plant Physiol. 1993;102(3):975–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng SJ. Crop production on acidic soils: overcoming aluminium toxicity and phosphorus deficiency. Ann Bot. 2010;106(1):183–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kochian LV, Hoekenga OA, Pineros MA. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu Rev Plant Biol. 2004;55:459–93. [DOI] [PubMed] [Google Scholar]
- 42.Tan K, Keltjens WG. Interaction between aluminium and phosphorus in sorghum plants. Plant Soil. 1990;124(1):25–32. [Google Scholar]
- 43.Delhaize E, Taylor P, Hocking PJ, Simpson RJ, Ryan PR, Richardson AE. Transgenic barley (Hordeum vulgare L.) expressing the wheat aluminium resistance gene (TaALMT1) shows enhanced phosphorus nutrition and grain production when grown on an acid soil. Plant Biotechnol J. 2009;7(5):391–400. [DOI] [PubMed] [Google Scholar]
- 44.Polle E, Konzak CF, Kattrick JA. Visual detection of aluminum tolerance levels in wheat by hematoxylin staining of seedling roots. Crop Sci. 1978;18(5):823–7. [Google Scholar]
- 45.Yang J, Zhu X, Peng Y, Zheng C, Li G, Liu Y, Shi Z, Zheng S. Cell wall hemicellulose contributes significantly to aluminum adsorption and root growth in Arabidopsis. Plant Physiol. 2011;155(4):1885–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao Y, Zhang Y, Chen Y, Yu N, Liaqat S, Wu W, et al. OsPG1 encodes a polygalacturonase that determines cell wall architecture and affects resistance to bacterial blight pathogen in rice. Rice. 2021;14(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu X, Wan J, Sun Y, Shi Y, Braam J, Li G, Zheng S. Xyloglucan endotransglucosylase-hydrolase17 interacts with xyloglucan endotransglucosylase-hydrolase31 to confer xyloglucan endotransglucosylase action and affect aluminum sensitivity in Arabidopsis. Plant Physiol. 2014;165(4):1566–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li C, Yan J, Ren J, Sun L, Xu C, Li G, Ding Z, Zheng S. A WRKY transcription factor confers aluminum tolerance via regulation of cell wall modifying genes. J Integr Plant Biol. 2020;62(8):1176–92. [DOI] [PubMed] [Google Scholar]
- 49.Tao Y, Wan J, Liu Y, Yang X, Shen R, Zhu X. The NAC transcription factor ANAC017 regulates aluminum tolerance by regulating the cell wall-modifying genes. Plant Physiol 2022:c197. [DOI] [PMC free article] [PubMed]
- 50.Li J, Liu J, Dong D, Jia X, McCouch SR, Kochian LV. Natural variation underlies alterations in Nramp aluminum transporter (NRAT1) expression and function that play a key role in rice aluminum tolerance. Proc Natl Acad Sci. 2014;111(17):6503–6508. [DOI] [PMC free article] [PubMed]
- 51.Xia J, Yamaji N, Kasai T, Ma J. Plasma membrane-localized transporter for aluminum in rice. Proc Natl Acad Sci. 2010;107(43):18381–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qin L, Han P, Chen L, Walk TC, Li Y, Hu X, Xie L, Liao H, Liao X. Genome-wide identification and expression analysis of NRAMP family genes in soybean (Glycine Max L). Front Plant Sci. 2017;8:1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X, Cheng Y, Yang C, Yang C, Mu Y, Xia Q, Ma Q. QTL mapping for aluminum tolerance in RIL population of soybean (Glycine max L.) by RAD sequencing. PLoS ONE. 2019;14(10):e223674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Livak KJ. TD Schmittgen 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods.2001; 25(4): 402–8. [DOI] [PubMed] [Google Scholar]
- 55.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma Q, Xia Z, Cai Z, Li L, Cheng Y, Liu J, Nian H. GmWRKY16 enhances drought and salt tolerance through an aba-mediated pathway in Arabidopsis thaliana. Front Plant Sci 2018, 9:1979. [DOI] [PMC free article] [PubMed]
- 57.Zeng P, Vadnais DA, Zhang Z, Polacco JC. Refined glufosinate selection in Agrobacterium-mediated transformation of soybean [Glycine max (L.) Merrill]. Plant Cell Rep. 2004;22(7):478–82. [DOI] [PubMed] [Google Scholar]
- 58.Ryan PR, Delhaize E, Randall PJ. Malate efflux from root apices and tolerance to aluminium are highly correlated in wheat. Funct Plant Biol. 1995;22(22):531–6. [Google Scholar]
- 59.Delhaize E, Craig S, Beaton CD, Bennet RJ, Jagadish VC, Randall PJ. Aluminum tolerance in wheat (Triticum aestivum L.) (I. uptake and distribution of aluminum in root apices). Plant Physiol. 1993;103(3):685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liang C, Piñeros MA, Tian J, Yao Z, Sun L, Liu J, Shaff J, Coluccio A, Kochian LV, Liao H. Low pH, aluminum, and phosphorus coordinately regulate malate exudation through GmALMT1 to improve soybean adaptation to acid soils. Plant Physiol. 2013;161(3):1347–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmedes A, Hølmer G. A new thiobarbituric acid (TBA) method for determining free malondialdehyde (MDA) and hydroperoxides selectively as a measure of lipid peroxidation. J Am Oil Chem Soc. 1989;66(6):813–7. [Google Scholar]
- 62.Abraham E, Hourton-Cabassa C, Erdei L, Szabados L. Methods for determination of proline in plants. Methods Mol Biol. 2010;639:317–31. [DOI] [PubMed] [Google Scholar]
- 63.Tian T, Liu Y, Yan H, You Q, Yi X, Du Z, Xu W, Su Z. agriGO v2.0: a GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Res. 2017;45(W1):W122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated in this study are included in this published article (additional files). The RNA-seq data has been uploaded to NCBI database, with accession number PRJNA833532