Abstract
Spontaneous adverse drug reactions (ADRs) reporting by health care professionals (HCPs) plays a vital role in pharmacovigilance (PV). However, under-reporting remain a major challenge worldwide, especially in low and middle-income countries, including Lao PDR. This cluster-randomized controlled trial evaluated the effectiveness of the modified TaWai mobile app for ADR reporting compared with the usual practice in hospitals. Two tertiary hospitals in Lao PDR (cluster units) were randomized into two groups: the intervention group (16 HCPs), which used the modified TaWai mobile app along with an educational workshop, and the control group (18 HCPs), which followed usual practice with the same educational workshop. The intervention group reported more ADR cases (28 vs. 3), and produced a higher number of high-quality reports (28 vs. 2) than the control group. The modified TaWai mobile app was highly rated by all participating HCPs. Questionnaire responses indicated that the tool is user-friendly, time-efficient, and well-suited for ADR reporting in hospitals in Lao PDR. In conclusion, these findings highlight the potential of the modified TaWai mobile app to enhance ADR reporting practices in hospitals, and its features make it a promising solution for strengthening PV in Lao PDR and similar settings.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-82474-9.
Keywords: TaWai mobile App, ADR reporting, Pharmacovigilance, Low/middle-income countries, Lao PDR
Subject terms: Health care, Medical research
Introduction
Pharmacovigilance (PV) is the science and activities related to the detection, assessment, understanding, and prevention of adverse drug reactions (ADRs) or any other drug-related problems1. It is a crucial component of public health. A well-functioning PV system is an essential public health tool that promotes the prevention, detection, and evaluation of suspected ADRs, aiming to minimize their impact on patient health and maximize the benefits of medicinal products. The spontaneous reporting of ADRs by healthcare professionals (HCPs) is crucial for PV. However, under-reporting of ADRs remains a significant challenge2–5, particularly in low- and middle-income countries (LMICs) including Lao PDR4–6. This issue hampers the effectiveness of PV systems, potentially compromising both patient safety and public health.
In Lao PDR, one national PV center is located in the capital city. Founded in 2012, this center reports all ADRs data to the World Health Organization Collaborating Center for International Drug Monitoring at the Uppsala Monitoring Centre (WHO-UMC), where it is incorporated into the international database. The Spontaneous Reporting System (SRS) is used to report ADRs and monitor medicines’ safety in Lao PDR hospitals. Physicians and pharmacists in three tertiary hospitals play a crucial role in this system. The physicians identify patients’ ADRs and assess the causality between the medicines and the suspected reactions. They then report the essential components of the ADR to a pharmacist. The pharmacist collects the data and records it on an ADR reporting form. If there are any problems or uncertainties in the case report, the pharmacist consult the physician again. Finally, the pharmacist submits the report to the PV center, where the data is collected, evaluated, and synchronized with the WHO-UMC.
Although the Lao PV Center has been established for several years, spontaneous ADR reporting by HCPs in Lao PDR remains low. According to the Lao National PV Center Database, the ADR reporting rate is very low, with only 22 reports submitted to the PV Center between 2016 and 20196,7. Like other LMICs, various factors contribute to the under-reporting of ADRs in Lao PDR, including limited resources, a lack of awareness and training among HCPs, and the absence of user-friendly reporting mechanisms. Traditional paper-based ADR reporting systems often face challenges such as time constraints, cumbersome procedures, and limited accessibility, which further exacerbate the issue of under-reporting3–5,8. Recently, several tools, including smartphones, tablets, and mobile apps, have been developed to enhance ADR reporting9–11. Current evidence confirms their effectiveness in increasing ADR reporting worldwide11–14. However, no studies have been conducted on the use of these tools to enhance ADR reporting by HCPs in Lao PDR.
The TaWai for Health tool has been used to promote ADR reporting and monitor the safety of health products in the Thai PV system. Developed and authorized by the researchers from Innovation Unit for Consumer Protection in Healthcare Products (TaWai for Health Unit) Faculty of Pharmaceutical Sciences, Prince of Songkla University, Thailand, this tool was originally designed as a web-based program, and can also be adapted into a mobile application15. It facilitates the collection of HCPs-reports outcomes in real-world settings15 and has been shown to increase both the quantity and quality of ADR reported submitted by hospitals in Thailand16. The rapid advancement of mobile technology and the advantages of mobile device applications (app) over web-based programs, has led to the development of a novel and promising mobile app to enhance ADR reporting11,12. Mobile apps offer a convenient, accessible, and efficient platform for HCPs to report ADRs in real time, potentially overcoming many of the barriers associated with traditional reporting systems11. In addition, Lao PDR and Thailand share similarities in cultures, languages, and WHO criteria for ADR reporting. Therefore, the TaWai for Health tool in Thai was translated into Lao language and modified to include specific patient information, details, and sources of suspected medications, as a mobile application was aimed at enhancing ADR reporting by HCPs in Lao PDR. However, the effectiveness of this mobile app in improving ADR reporting in Lao PDR remains undetermined.
This research, through a cluster-randomized controlled trial, aimed to evaluate whether the use of the TaWai mobile app could increase the quantity and quality of ADR reports, and to assess the satisfaction of HCPs involved in ADR reporting. The findings from this study could provide valuable insights into the potential of mobile technology to strengthen PV systems in resource-limited settings and ultimately enhance patient safety.
Methods
Overall study design
We conducted an unblinded cluster-randomized controlled trial (cluster RCT) to determine the effectiveness of the modified TaWai mobile app, combined with a face-to-face educational workshop, compared to the usual practice combined with the same workshop of intervention group, on ADR reporting in hospitals in Lao PDR. A cluster of three tertiary hospitals in Lao PDR were randomly assigned by a researcher, in a 1:1 ratio, to either the intervention group (n = 1 hospital) or the control group (n = 1 hospital). Both groups participated in two sessions of face-to-face educational workshops, each lasting 2 h, during the 4-month follow-up period from July to October 2022. This educational workshop, led by a lecturer in Lao PDR, aimed to ensure that the HCPs’ knowledge of ADRs would not interfere with their reporting. It comprised of an overview of ADRs, ADR monitoring, evaluation, and reporting; common ADRs associated with medicines used in TB and HIV patients; and the management of these ADRs. This study was conducted in accordance with Good Clinical Practice (GCP) guidelines and the Declaration of Helsinki.
Clinical setting
The target setting of this study was all departments in two tertiary hospitals in Lao PDR. However, only the TB and HIV departments of each hospital agreed to participate.
Intervention
The main intervention used in this study were the modified TaWai mobile app in addition to the aforementioned educational workshops. All HCPs in the intervention group received 30 min of training on how to use the modified TaWai mobile app before implementing this tool for ADR reporting in their hospital.
Control
Similar to the intervention group, the control group also participated in the educational workshops. However, all HCPs in this group continued to use the usual tool (paper or computer-based forms) for reporting ADR in their hospital.
Modified TaWai mobile app
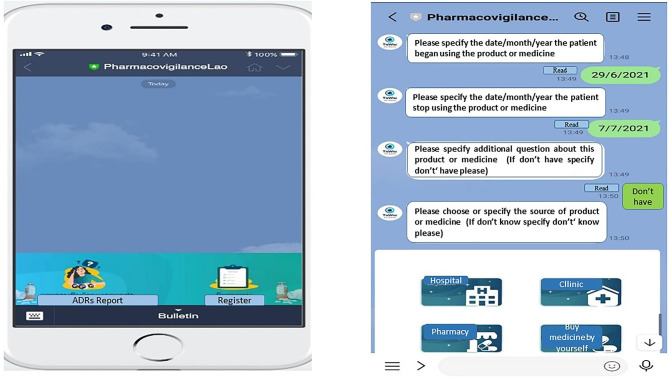
Details and required information for both ADR report tools are provided in Supplementary Table 1. The modified TaWai mobile app was developed in Lao language with permission from the TaWai for Health Unit committee in Thailand. This version was designed as a draft for a chatbot to be used on mobile phones. The items in this version were adapted from the Thai version and tailored to the context of Lao PDR, incorporating the national PV guidelines, ADR reporting regulations, and relevant laws. Important ADR reporting information required by the WHO was also included. Modified sections were items related to patient information, as well as the types and sources of suspected medicines. The TaWai mobile app consists of four dimensions of ADR reporting based on WHO criteria: (1) patient information, (2) ADR information, (3) suspected medicine information, and (4) reporter information. An example of the TaWai app features is presented in Fig. 1. Although the Thai and Lao languages are similar in spoken form, their writing systems are completely different. The researchers involved in modifying and drafting the chatbot used both Thai and Lao languages in their work and daily life, demonstrating their proficiency in both languages17. The content validity of this tool was evaluated by three experts including one Ph.D. in clinical pharmacy, one Ph.D. in social and administrative pharmacy, and the head of the PV center, who also holds a Ph.D. in social and administrative pharmacy. The content validity of all items in the modified draft of chatbot was confirmed, with an Index of Content Validity (IOC) of one prior to its implementation in the mobile app.
Fig. 1.
Example of TaWai mobile app for ADRs reporting.
Outcomes measures
The primary outcomes were the total number or rate, and quality of ADR reports collected during the 4-month study period. Additionally, satisfaction and knowledge of the reporters were evaluated as secondary outcomes. Throughout the study period, the primary outcomes were collected and evaluated every month for four months, resulting in a total of five assessments, including baseline. Knowledge of each HCP was assessed by a questionnaire administered before and after the workshop sessions in both groups, while satisfaction with the TaWai mobile app was evaluated only in the intervention group.
The quality of ADR reports in this study was assessed using WHO criteria1 by a researcher. A report was considered high quality if it included all essential items according to WHO standards. Key elements for good case reports included: (1) a description of the adverse reaction or disease experience, (2) details of suspected and concomitant medicines, (3) patient characteristics, (4) documentation of the diagnosis of the reactions, including methods used, and (5) the clinical course of the reaction and patient outcomes, such as hospitalization or death. The absence of any of these elements, even a single one, automatically classifies ADR reports as low quality1.
The knowledge questionnaire comprised of five domains including the definition of ADRs and PV process, health effects, the relationship between ADRs and mortality rates or costs associated with ADRs, the importance of ADR reporting, and common ADRs related to TB and HIV drugs. The total knowledge score was 46 points, with a higher score indicating greater knowledge. Based on the criteria for evaluating knowledge by Bloom’s Taxonomy18, the scores were categorized into three levels: high knowledge (≥ 80%, or 37–46 scores), moderate knowledge (60–79%, or 28–36 scores), and low knowledge (≤ 60%, or ≤ 28 scores).
The satisfaction questionnaire was specifically developed for this study using a 5-point Likert scale. Each item was rated from 1 (very low satisfaction) to 5 (very high satisfaction). The content validity test of both satisfaction and knowledge questionnaires were approved by three experts including one specialist physician in TB infection (Head of TB Unit), one specialist physician in HIV infection (head HIV Unit), and the head of the PV center, who holds a Ph.D. in social and administrative pharmacy. The assessor’s qualifications are the people who understand Lao language, and working related with PV process or ADR reporting in Lao PDR.
Participants
The inclusion criterion for cluster units was being a tertiary hospital in Lao PDR. After randomizing the hospitals to either the intervention group or the control group, healthcare professional (HCP) teams from all departments in each randomized hospital were recruited via a formal invitation letter sent through the head of each department. Only HCP teams from the tuberculosis (TB) and human immunodeficiency virus (HIV) departments agreed to participate. HCPs aged 18 and older were included if they had worked in the TB and HIV departments for at least six months, were involved in ADR reporting, and were willing to participate in the study. HCPs were excluded if they were not familiar with a smartphone, tablet, or mobile device capable of hosting the app, could not understand or write in Lao, or refused to participate in the study.
Randomization
Since this study was designed to enhance the ADR reporting by HCPs across all ward teams in each hospital, the unit of randomization was the hospital unit in order to minimize data contamination. Based on a researcher survey conducted in 2022, there are ten government hospitals in Lao PDR. However, we selected only tertiary hospitals as the clusters for the targeted setting because this type of hospital plays a key role in ADR reporting for the PV center in Lao PDR. Therefore, three tertiary hospitals were chosen as the population clusters for this study.
Two steps of randomization were performed by a researcher who was not involved in the recruitment and data collection. Initially, two of the three tertiary hospitals were selected as sample clusters using a simple random technique with a computer randomizer. Then, these two hospitals were randomized into the intervention and control groups using the same technique. Random allocation codes were placed in sealed envelopes by a researcher who was not involved in enrollment or data collection. Two hospitals were informed of the randomization on the day of the trial initiation visit. Based on the concept of cluster randomization, all departments in each cluster hospital were assigned into the intervention and the control group following the randomization code for each hospital. Therefore, HCPs in each included tertiary hospital who met inclusion exclusion criteria mentioned above were recruited for this study.
Statistical analysis
Data analyses were performed using STATA 15.0. The intention-to-treat approach was employed for all analyses. Descriptive statistics were used to summarize the characteristics, satisfaction and knowledge of included HCPs. For between-group comparison, categorical variables such as sex, occupation, education level, the number of overall ADR reports, and high-quality reports were analyzed using the Chi-square or Fisher’s exact test. Continuous variables such as age, experience and duration of work, and knowledge scores were analyzed using the Independent t-test or Mann–Whitney U test. Two-sided p-values were calculated, with values less than 0.05 considered statistically significant. Due to the limited number of target population (HCPs involved in ADR reporting), the sample size was not estimated. Instead, we included all HCPs in each cluster who met the inclusion and exclusion criteria. Based on this criterion, approximately 65 HCPs were eligible; however, only 34 HCPs were willing to participate in the study and served as the final sample size. Subgroup analysis was performed based on department type.
Ethics statement and trial registration
The study protocol was approved by Mahasarakham University (ID: 115-074/2022), and the Lao National Ethics Committee for Health Research (ID: 065/NECHR). The study protocol was registered in the Thai Clinical Trials Registry (TCTR20220607002 available at https://www.thaiclinicaltrials.org/show/TCTR20220607002) on 07 June 2022. However, some discrepancies between the initial study protocol and the final report are provided in Supplementary Table 2. In summary, we adjusted the frequency of the workshop and the duration of follow-up due to the COVID-19 pandemic. After completing the workshop session, we had further discussions with the team and decided to reprioritize the outcomes by moving the rate and quality of ADR reports from secondary to primary outcomes, as the quality of the reports is just as important as the total number of ADR reports which is the original primary outcome. In addition, the rate of ADR reports is equivalent to the total number of reports, but they reflect different aspects of reporting, making it reasonable to move them to a primary outcome. The knowledge of HCPs was initially not predefined as a study outcome, as the goal of the pre- and post-workshop measurements was to ensure that this factor did not affect the quantity or quality of the reports. However, we decided to include it as a secondary outcome to provide greater clarity about what we measured in the study. This study was reported following CONSORT recommendations extended for cluster randomized controlled trials (Supplementary Table 3–4)19,20. Written informed consent was obtained from all participants before the enrollment procedure.
Results
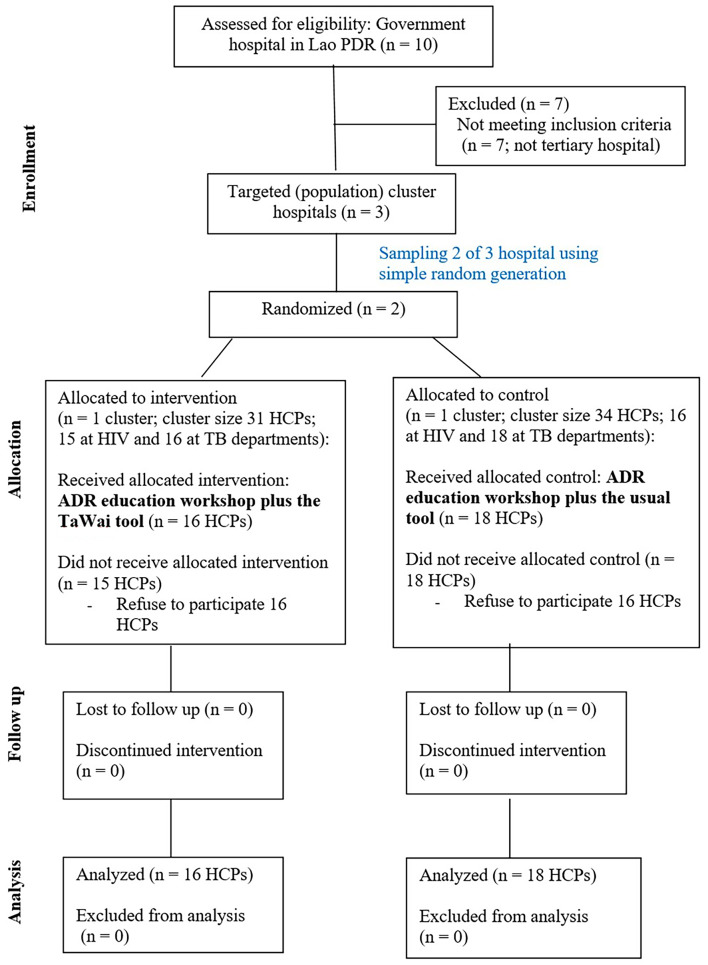
A cluster-RCT was performed to evaluate the effects of the modified TaWai mobile app in Lao PDR. Two tertiary hospitals in Lao PDR were randomized into the intervention and control groups. Eighteen HCPs in the control group received an ADR education workshop plus the usual tool, while sixteen HCPs in the intervention group received an ADR education workshop plus the TaWai tool for ADR reporting in their hospital (Fig. 2). The characteristics of the included hospitals, including the size of the hospital, service units, types and numbers of patients, and HCPs, were comparable between the groups. Overall, 34 HCPs with an average age of 38 (SD, 8.8) years were enrolled and participated in the ADR education workshop of this study. Characteristics of all included HCPs in this study were reported in Table 1. Most of them were females (65%). Work experience and duration of work in the hospital of all HCPs was approximately 13 and 12 years, respectively. All characteristics, including gender, age, occupation, education, marital status, duration of work, and work experience, were comparable between the two groups (Table 1).
Fig. 2.
Study flow chart for randomization HCPs.
Table 1.
Characteristics of included healthcare professionals from each cluster.
| Characteristics | All participants (N = 34) | Intervention group (N = 16) | Control group (N = 18) | P-value |
|---|---|---|---|---|
| Gender, n (%) | ||||
| Male | 12 (35.3) | 4 (25.0) | 8 (44.4) | 0.23a |
| Female | 22 (64.7) | 12 (75.0) | 10 (55.6) | |
| Age (year), Mean ± SD | 37.9 ± 8.8 | 37.7 ± 9.0 | 38.1 ± 8.8 | 0.53b |
| Range | 26–59 | 28–58 | 26–59 | |
| Occupation, N (%) | ||||
| Doctor | 27 (79.4) | 12 (68.8) | 15 (83.3) | 0.54a |
| Pharmacist | 7 (20.6) | 4 (31.2) | 3 (16.7) | |
| Education, N (%) | ||||
| Bachelor degree | 23 (64.7) | 12 (68.8) | 11 (61.1) | 0.61a |
| Master degree | 11 (32.4) | 4 (31.2) | 6 (33.3) | |
| PhD degree | 1 (2.9) | 0 (0.0) | 1 (5.6) | |
| Marital status, N (%) | ||||
| Single | 8 (3.5) | 4 (25.0) | 4 (22.2) | 0.53a |
| Married | 25 (73.5) | 11 (68.8) | 14 (77.8) | |
| Widow | 1 (2.9) | 1 (6.2) | 0 (0.0) | |
| Duration of work (year) | ||||
| Mean ± SD | 12.6 ± 7.9 | 12.1 ± 7.4 | 13.1 ± 8.1 | 0.81b |
| Range | 2–32 | 3–32 | 2–32 | |
| Experience of work at | ||||
| Hospital (year), Mean ± SD | 12.4 ± 7.7 | 11.6 ± 7.01 | 13.0 ± 8.5 | 0.64b |
| Range | 2–32 | 3–32 | 2–32 | |
| Knowledge scores of participants (total score = 46) | ||||
| Before participated educating workshop | N/A | 36.3 ± 3.2 | 36.2 ± 1.7 | 0.97b |
| After participated educating workshop | N/A | 37.4 ± 3.2 | 40.6 ± 2.1 | 0.45b |
a, Fisher’s exact test; b, independent t-test; SD, Standard deviation; N, number of health care professional; N/A, Data not available.
Knowledge of health care professionals (HCPs)
Differences in knowledge or lack thereof among HCPs may influence both the quantity and quality of reports, which are the main outcomes of this study. To ensure comparable knowledge of ADR and the PV process between the intervention and control groups, all participating HCPs were required to attend an ADR face-to-face education workshop. Their knowledge was assessed before and after the two workshop sessions using a validated questionnaire. The results indicated that the pre-and post-workshop knowledge of HCPs on ADR and the PV process was comparable between the intervention and control groups (Table 1). Their scores indicated high levels of knowledge (at least 37 out of 46 points) after participating in the education workshop. These findings suggest that knowledge variation among HCPs between the two groups was effectively controlled, reducing potential bias.
Effects of modified TaWai mobile app
The modified TaWai mobile app increased both the number and quality of ADR reports. The number, quality, and types of ADR reports submitted by the intervention and control groups are summarized in Table 2. During the four month follow-up period, the number of ADR reports in the intervention group was higher than that in the control group (28 vs. 3 reports; p-value = 0.0002). The overall incidence of ADR reporting in both groups was 0.23 case/person-month, while this rate was 0.44 and 0.04 case/person-month in the intervention, and control groups, respectively. The incidence ratio of the overall reports in the intervention group was 10.5 times higher than that in the control group. Most reporters in the intervention group were physicians (79%), while those in the control group were pharmacists (67%). Most of the ADRs reported in the intervention group came from the HIV department (68%), while those in the control group came from the TB department (100%). Most ADRs reported were non-serious ADRs of oral dosage forms of medicine approved in Lao PDR. However, six of the 28 cases (21%) reported in the intervention group were defined as serious ADRs (Table 2). The subgroup analysis by department type is provided in Supplementary Table 5. In summary, the results were consistent with the main findings, showing that the total number of ADR reports in the intervention group for both the TB and HIV departments was higher than in the control group (TB department: 9 vs. 3 reports, HIV department: 19 vs. 0 reports; p-value < 0.02 for both).
Table 2.
Number, quality and types of ADR reports.
| Items | Intervention group (N = 16) | Control group (N = 18) | p-Value |
|---|---|---|---|
| Total number of overall ADR reports in 4 months, n | 28 | 3 | 0.0002 |
| Incidence rate of overall reports, rate/ person-month | 0.44 | 0.04 | < 0.001 |
| Incidence rate difference of overall reports, RD (95%CI) | 0.40 (0.23, 0.56) | < 0.001 | |
| Incidence rate ratio of overall reports, RR(95%CI) | 10.50 (3.24, 53.97) | < 0.001 | |
| Quality of ADR reports by WHO criteria | < 0.001 | ||
| High quality | 28 (100) | 2 (66.7) | |
| Low quality | 0 (0.0) | 1 (33.3) | |
| Incidence rate of high quality reports | 0.44 | 0.03 | < 0.001 |
| Incidence rate difference of high quality reports | 0.41 (0.24, 0.58) | < 0.001 | |
| Incidence rate ratio of high quality reports | 15.75 (3.97, 136.44) | < 0.001 | |
| Number of ADR reports classified by reporters | |||
| Doctor | 22 (78.6) | 0 (0.0) | < 0.001a |
| Pharmacist | 6 (21.4) | 2 (66.7) | |
| Unidentified | 0 (0.0) | 1 (33.3) | |
| Number of ADR reports classified by department | |||
| TB | 9 (32.2) | 3 (100) | |
| HIV | 19 (67.8) | 0 (0.00) | < 0.001a |
| Number of ADR reports classified suspected medications | |||
| Bactrim | 9 (32.2) | 0 (0.0) | 0.006a |
| Anti-TB drug | 6 (21.4) | 3 (100) | |
| Anti-viral | 3 (10.7) | 0 (0.0) | |
| Analgesic | 3 (10.7) | 0 (0.0) | |
| Fixed combination of Tenofovir/ Lamivudine/ | |||
| Dolutegravir | 3 (10.7) | 0 (0.0) | |
| NSAIDs | 1 (3.6) | 0 (0.0) | |
| Combination of linezolid, bedaquidine, levofloxacin, and clofazamine | 1 (3.6) | 0 (0.0) | |
| Other: amphotericin B, azithromycin | 2 (7.1) | 0 (0.0) | |
| Number of ADR reports classified by dosage | |||
| Tablet (oral) | 28 (100) | 2 (66.7) | 0.0001 |
| Unidentified | 0 (0.0) | 1 (33.3) | |
| Number of ADR reports classified by medication approved in Lao PDR | 0.0004 | ||
| No | 6 (21.4) | 0 (0.0) | |
| Yes | 22 (78.6) | 2 (66.7) | |
| Unidentified | 0 (0.0) | 1 (33.3) | |
| Number of ADR reports classified by medication source | 0.0005 | ||
| Tertiary hospital | 25 (89.3) | 3 (100) | |
| Community Pharmacy | 3 (10.7) | 0 (0.0) | |
| Types of ADR reports classified by severity | 0.024a | ||
| Serious | 6 (21.4) | 0 (0.0) | |
| Non serious | 22 (78.6) | 2 (66.7) | |
| Unidentified | 0 (0.0) | 1 (33.3) | |
| Type of ADR report classified by mechanism | 0.0005 | ||
| (1) Type B ADR (Drug allergy) | |||
| Rash | 10 (35.7) | 1 (33.3) | |
| Rash with itchy | 9 (32.2) | 2 (66.7) | |
| Rash + SOB with or without itchy | 3 (10.7) | 0 (0.0) | |
| Rash + fever with or without itchy/ SOB | 2 (7.1) | 0 (0.0) | |
| Edema | 1 (3.6) | 0 (0.0) | |
| (2) Type A ADR (true ADR) | |||
| Renal toxicity | 2 (7.1) | 0 (0.0) | |
| Hepatotoxicity | 1 (3.6) | 0 (0.0) | |
| Types of ADR reports classified by Naranjo algorithm | 0.0001 | ||
| Definite | 2 (7.1) | 0 (0.0) | |
| Probable | 26 (92.9) | 2 (66.7) | |
| Unidentified | 0 (0.0) | 1 (33.3) | |
aFisher’s exact test; SD = Standard deviation; N = number of HCPs; n = number of reports; 4FDC = Rifampicin 150 mg, Isoniazid 75 mg, Pyrazinamide 400 mg and Ethambutol 275 mg; Bactrim = trimethroprim/sulfamethoxazole; SOB = Shortness of breath; NSAIDs = Non-Steroidal Anti-inflammatory Drugs; HIV = Human Immunodeficiency Virus; TB = Tuberculosis; MDR-TB = Multidrug resistant tuberculosis.
Significant values are in bold.
The quality of ADR reports is provided in Table 2. Among all ADR reports, 30 (96.77%) were rated as high quality. The number of high-quality reports was higher in the intervention group than in the control group (28 vs. 2 reports; p-value < 0.001). One ADR report submitted from the control group was rated as a low-quality report because there were missing several items of WHO criteria including the seriousness of the reaction, date to start and stop of the drug, date the reaction started, stopped, comorbidity, dose of use, frequency, dosage form, route of administration, and a detail of the reporter. The overall incidence rate of high-quality reports in the two clusters was 0.22 cases per person-month. This rate was 0.44 cases per person-month in the treatment group and 0.03 cases per person-month in the control group. The incidence rate ratio of high-quality reports in the treatment group was 15.75 times higher compared to the control group.
The characteristics of patients and medicines reported in ADR reports are presented in Table 3. The average age of patients who presented ADRs was approximately 44 (SD = 10.0) years old in the intervention group and 34 (SD = 8.5) years old in the control group. In both groups, most of them were male with no history of drug allergy, but they were smokers or drinkers. Most patients in the intervention group used medicine for the treatment of HIV (39%), while in the control group, patients used medicine for relief of fever (100%).
Table 3.
Characteristics of patients reported in ADRs reports.
| Characteristics of patients | Intervention group, N (%) | Control group, N (%) |
|---|---|---|
| Age (year), Mean ± SD | 44.2 ± 10.0 | 33.6 ± 8.5 |
| Range | 24–70 | 25–42 |
| Sex | ||
| Male | 18 (64.3) | 2 (66.7) |
| Female | 10 (35.7) | 1 (33.3) |
| Nationality | ||
| Lao | 27 (96.4) | 3 (100) |
| Foreigner | 1 (3.6) | 0 (0.0) |
| History of drug allergy | ||
| No | 24 (85.7) | 2 (66.7) |
| Yes: Occurring of rash | 4 (14.3) | 0 (0.0) |
| Pyrazinamide | 2 (7.1) | 0 (0.0) |
| 4FDC | 1 (3.6) | 0 (0.0) |
| Bactrim | 1 (3.6) | 0 (0.0) |
| Unidentified | 0 (0.0) | 1 (33.3) |
| Comorbidity | ||
| No | 15 (53.6) | 2 (66.7) |
| Yes | 13 (46.4) | 0 (0.0) |
| Hypertension | 6 (21.4) | 0 (0.0) |
| Gout | 6 (21.4) | 0 (0.0) |
| Diabetic | 1 (3.6) | 0 (0.0) |
| Unidentified | 0 (0.0) | (33.3) |
| Smoke or alcohol drinking, N (%) | ||
| No | 13(46.4) | 0 (0.0) |
| Yes | 15 (53.6) | 2 (66.7) |
| Unidentified | 0 (0.0) | 1 (33.3) |
| Indication of medicine, N (%) | ||
| HIV | 11 (39.3) | 0 (0.0) |
| TB/MDR-TB | 7 (25.0) | 0 (0.0) |
| Fever | 3 (10.7) | 3 (100) |
| Pneumonia | 3 (10.7) | 0 (0.0) |
| Other (back pain, meningitis, prophylaxis OI, toxoplasma) | 4 (14.3) | 0 (0.0) |
4FDC = Rifampicin 150 mg, Isoniazid 75 mg, Pyrazinamide 400 mg and Ethambutol 275 mg; Bactrim = trimethroprim/sulfamethoxazole; SD = Standard deviation; N = number of patients; HIV = Human Immunodeficiency Virus; TB = Tuberculosis; MDR-TB = Multidrug resistant tuberculosis; OI = Opportunistic Infection.
Satisfaction of HCP on TaWai mobile app
Sixteen HCPs in the intervention group used the modified TaWai app in Lao to report ADR in their hospital. Their satisfaction with this tool was assessed at the end of the study. The results indicated that overall satisfaction with the modified TaWai app among all HCPs (16/16) was high to very high. Additionally, they were highly to very highly satisfied with other aspects, such as ease of use and access, usefulness for patients, HCPs, and the hospital, appropriateness for the context of Laos, and reduction of time and difficulty for ADR reporting (Table 4).
Table 4.
Satisfaction of HCPs on using of the modified TaWai tool application.
| Items of assessment | Numbers of HCPs (%) |
|---|---|
| (1) Overall satisfaction with tool, n (%) | |
| Very low | 0 (0.0) |
| Low | 0 (0.0) |
| Moderate | 0 (0.0) |
| High | 11 (68.8) |
| Very high | 5 (31.2) |
| (2) Questionnaire on TaWai tool are consist with real work practice, n (%) | |
| Very low | 0 (0.0) |
| Low | 0 (0.0) |
| Moderate | 0 (0.0) |
| High | 10 (62.5) |
| Very high | 6 (37.5) |
| (3) Tools are very useful for patients, n (%) | |
| Very low | 0 (0.0) |
| Low | 0 (0.0) |
| Moderate | 0 (0.0) |
| High | 7 (43.8) |
| Very high | 9 (56.2) |
| (4) Tools are very useful for HCPs, n (%) | |
| Very low | 0 (0.0) |
| Low | 0 (0.0) |
| Moderate | 0 (0.0) |
| High | 9 (56.2) |
| Very high | 7 (43.8) |
| (5) Tools are appropriate for the hospital, n (%) | |
| Very low | 0 (0.0) |
| Low | 0 (0.0) |
| Moderate | 0 (0.0) |
| High | 6 (37.5) |
| Very high | 10 (62.5) |
| (6) Tool are appropriate for the context of Laos, n (%) | |
| Very low | 0 (0.0) |
| Low | 0 (0.0) |
| Moderate | 0 (0.0) |
| High | 10 (62.5) |
| Very high | 6 (37.5) |
| (7) Suitability of characteristic and letters, n (%) | |
| Very low | 0 (0.0) |
| Low | 0 (0.0) |
| Moderate | 0 (0.0) |
| High | 13 (81.2) |
| Very high | 3 (18.8) |
| (8) Easy access to work | |
| Very low | 0 (0.0) |
| Low | 0 (0.0) |
| Moderate | 0 (0.0) |
| High | 10 (62.5) |
| Very high | 6 (37.5) |
| (9) TaWai tool easy to access | |
| Very low | 0 (0.0) |
| Low | 0 (0.0) |
| Moderate | 1 (6.3) |
| High | 9 (56.2) |
| Very high | (37.5) |
| (10) Easy to use | |
| Very low | 0 (0.0) |
| Low | 0 (0.0) |
| Moderate | 1 (6.3) |
| High | 9 (56.2) |
| Very high | 6 (37.5) |
| (11) Can access anytime and anywhere | |
| Very low | 0 (0.0) |
| Low | 0 (0.0) |
| Moderate | 1 (6.3) |
| High | 8 (50.0) |
| Very high | 7 (43.7) |
| (12) Characteristic and size of letters is beautiful | |
| Very low | 0 (0.00) |
| Low | 0 (0.00) |
| Moderate | 1 (6.2) |
| High | 12 (75.0) |
| Very high | 3 (18.8) |
| (13) Convenient to use | |
| Very low | 0 (0.0) |
| Low | 0 (0.0) |
| Moderate | 1 (6.3) |
| High | 5 (31.2) |
| Very high | 10 (62.5) |
| (14) Reduce the difficulty for ADR reporting | |
| Very low | 0 (0.0) |
| Low | 0 (0.0) |
| Moderate | 1 (6.3) |
| High | 10 (62.5) |
| Very high | 5 (31.2) |
| (15) Times to access not so long | |
| Very low | 0 (0.0) |
| Low | 0 (0.0) |
| Moderate | 2 (12.6) |
| High | 9 (56.2) |
| Very high | 5 (31.2) |
| (16) Reduce time for report ADRs | |
| Very low | 0 (0.0) |
| Low | 0 (0.0) |
| Moderate | 2 (12.6) |
| High | 9 (56.2) |
| Very high | 5 (31.2) |
Discussions
During the four months of the study, the use of the modified TaWai mobile app considerably improved the reporting rates and quality of ADR reports compared to the control group. In addition, ADR reporting rates in both groups increased more than those of the general practice in Lao PDR, where spontaneous ADR reporting is very low. The national PV center of Lao PDR received only 25 reports from all hospitals over four years (2017–2020)6. In the tertiary hospitals included in this study, only six ADR reports were submitted from the hospital assigned to the intervention group and five from the hospital assigned to the control group during 20216. No reports were submitted in the first four months of 2022 before the study began7.
The increase in ADR reporting in both groups may have resulted from the face-to-face HCPs educational workshops provided for HCPs in this study. Face-to-face educational workshops have proven to be an effective strategy to enhance ADR reporting21–23. However, the rates of overall ADR reporting and high-quality reporting in the intervention group in this study were higher than those reported in previous studies21–23 and greater than those in the control group. This finding implies that the modified TaWai mobile app enhances the effectiveness ADR reporting during face-to-face educational workshops.
Differences in HCPs’ knowledge or lack thereof may influence both the quantity and quality of ADR reports, which were the main outcomes of this study. Inthis study, we conducted the same educational workshops for all participants to enhance HCPs’ knowledge of the ADR reporting process and minimize bias resulting from variations in knowledge between groups. Our findings indicate that HCPs’ knowledge of ADR reporting was good and comparable between the groups, suggesting that bias due to knowledge variation was effectively controlled. Considering the knowledge scores after the workshop, there was a slight increase for each group. This may be due to the high baseline knowledge of HCPs in this study, which limited their ability to detect substancial differences between pre-and post-workshop scores. Focusing on the characteristics of the intervention, a previous study indicated that the rate of ADR reporting decreased over time after an educational workshop was discontinued3,8,24. Therefore, when educational workshop are implemented, ongoing interventions are required to maintain a high number of reports. In this study, although the educational workshop was conducted only twice a week, the rate of ADR reporting in the intervention group remained high. This suggests that adding an ADR reporting application, such as the modified TaWai app, to an educational workshop enhance and maintain a high reporting rate.
Utilization of the modified TaWai mobile app resulted in a markedly higher number and percentage of ADR cases reported in this study. This substantial increase highlights the potential of mobile technology to facilitate more efficient and accessible reporting processes for HCPs. Enhanced reporting can be attributed to the ease of use, convenience, ability to shorten the time required to report ADRs, and real-time accessibility of the mobile app, which addresses some of the barriers associated with traditional reporting methods25. This result was further supported by the high satisfaction rate among HCPs using this app. Moreover, the quality of the ADR reports was substantially higher when the modified TaWai mobile app was used. This finding suggests that the structured and user-friendly interface of the modified TaWai mobile app supports HCPs in providing comprehensive and accurate information, which is crucial for effective PV. However, this is the first study to evaluate the effectiveness of this system in a tertiary hospital. More studies in other settings in Lao PDR are needed to confirm this effect before it can be established at the national level.
In terms of implementation, the modified TaWai mobile app may be suitable for use in Lao PDR for several reasons. First, this application was substantially effective in increasing the number and quality of ADR reports submitted to the Lao PDR PV center. Second, the application was adapted from the TaWai app in Thailand, where the culture is similar to that of Lao PDR. Moreover, during the development process, we considered and included specific information relevant to the culture of Lao PDR, such as language, laws, and situations related to ADR reporting. Third, there was a high satisfaction rate among HCPs regarding the overall use and various features of the app. Highly rated features included user-friendliness, ease of use and access (available anytime and anywhere via mobile devices or smartphones), and reduced reporting time. This implies that the modified TaWai mobile app is well received HCPs and may have the potential to be widely adopted if integrated into the routine practice of Lao PDR. Additionally, the app is inexpensive compared to web-based programs, making it a feasible option for resource-limited settings, such as Lao PDR and similar setting.
To the best our knowledge, this is the first RCT to evaluate the effectiveness of the modified TaWai mobile app as a technological aid for enhancing spontaneous ADR reporting in Lao PDR and Asia. Although originally developed in Thailand, no RCTs have been conducted to assess its efficacy there. Only one pre-post study conducted in southern Thailand indicated that using the TaWai mobile app increased the number and quality of ADR reports15. Our findings in Lao PDR were consistent with those results from Thailand. Supporting the effectiveness of the modified TaWai mobile app. Conducting RCTs to evaluate interventions is the standard method for reducing selection bias. This design increases confidence in the results compared to non-RCT designs and suggests that the results are due to the intervention. In addition to improving the rate and quality of ADR reports for the policy system, this study provided direct benefits to patients. Since medicine allergy cards are not issued to patients in Lao PDR, we provided these cards when data were obtained from ADR reports. The aim of this process is to reduce the recurrence of drug allergies which may require further consideration and systematic implementation.
However, some limitations should be considered, including the short duration of the study, open-label design, small number of clusters and participants, and COVID-19 pandemic, which limited the number of patients visiting the hospital. These limitations may have affected the findings and resulted in a lower rate of ADR reporting than the actual occurrences. Therefore, further large-scale RCTs with long-term follow-up should be conducted to confirm our findings.
Conclusions
This study demonstrates the substantial benefits of the modified TaWai mobile app in improving the number and quality of ADR reporting in hospitals. The high satisfaction levels among HCPs regarding the app’s ease of use, cost-effectiveness, and efficiency make it a promising solution for strengthening PV systems in Lao PDR and similar setting. However, a long-term study with a larger and more diverse sample size needed to generalize these findings to broader populations and settings.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research project is financially supported by Mahasarakham University, Thailand and Fondation Pierre Fabre, France. We would like to thank my friends, my colleagues, my professors in the Faculty of Pharmacy, Lao PDR, and all healthcare providers in Setthathilad and Mahosot hospitals and providers of Pharmacovigilance center in Lao PDR who cordially helped me during the data collection. We would like to thank Editage (www.editage.com) for English language editing.
Author contributions
NM, RS, PSO, and PP conceived and designed the study, reviewed the quality of data. NM and RS performed the experiments, collect and analysis the data. All authors (NM, RS, PSO, PP and SK) wrote the article and approved the final manuscript.
Funding
This research project is financially supported by Mahasarakham University (Contract number. 6501003/2565) and Fondation Pierre Fabre, France.
Data availability
The data underlying this article cannot be shared publicly due to the privacy of individuals who participated in the study. The dataset is available upon request from the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. The importance of pharmacovigilance. Safety Monitoring of Medicinal Products. https://iris.who.int/handle/10665/42493 (2002).
- 2.Gahr, M., Eller, J., Connemann, B. J. & Schönfeldt-Lecuona, C. Underreporting of adverse drug reactions: Results from a survey among physicians. Eur. Psychiatry41, S369–S369. 10.1016/j.eurpsy.2017.02.377 (2020). [Google Scholar]
- 3.Katusiime, B., Semakula, D. & Lubinga, S. J. Adverse drug reaction reporting among health care workers at Mulago National Referral and Teaching hospital in Uganda. Afr. Health Sci.15, 1308–1317. 10.4314/ahs.v15i4.34 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khalili, M. et al. Evaluation of pharmacovigilance system in Iran. Int. J. Health Policy Manag.11, 990–1000. 10.34172/ijhpm.2020.243 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tandon, V. R., Mahajan, V., Khajuria, V. & Gillani, Z. Under-reporting of adverse drug reactions: A challenge for pharmacovigilance in India. Indian J. Pharmacol.47, 65–71. 10.4103/0253-7613.150344 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lao PV Center. Report of ADR reporting in Lao based on data from pharmacovigilance center in Lao PDR (2021).
- 7.Lao PV Center. Report of ADR reporting in Lao based on data from pharmacovigilance center in Lao PDR (2022).
- 8.Adisa, R. & Omitogun, T. I. Awareness, knowledge, attitude and practice of adverse drug reaction reporting among health workers and patients in selected primary healthcare centres in Ibadan, southwestern Nigeria. BMC Health Serv. Res.19, 926. 10.1186/s12913-019-4775-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abadie, D., Chebane, L., Bert, M., Durrieu, G. & Montastruc, J. L. Online reporting of adverse drug reactions: A study from a French regional pharmacovigilance center. Therapie69, 395–400. 10.2515/therapie/2014035 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Goldstein, L. H., Berlin, M., Saliba, W., Elias, M. & Berkovitch, M. Founding an adverse drug reaction (ADR) network: A method for improving doctors spontaneous ADR reporting in a general hospital. J. Clin. Pharmacol.53, 1220–1225. 10.1002/jcph.149 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Fukushima, A., Iessa, N., Balakrishnan, M. R. & Pal, S. N. Smartphone-based mobile applications for adverse drug reactions reporting: Global status and country experience. BMC Med. Inf. Decis. Making22, 118. 10.1186/s12911-022-01832-7 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berrewaerts, J., Delbecque, L., Orban, P. & Desseilles, M. Patient participation and the use of ehealth tools for pharmacoviligance. Front. Pharmacol.7, 90. 10.3389/fphar.2016.00090 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Defer, G. et al. Adverse drug reaction reporting using a mobile device application by persons with multiple sclerosis: A cluster randomized controlled trial. Drug Saf.44, 223–323. 10.1007/s40264-020-01009-z (2021). [DOI] [PubMed] [Google Scholar]
- 14.Leskur, D. et al. Adverse drug reaction reporting via mobile applications: A narrative review. Int. J. Med. Inf.168, 104895. 10.1016/j.ijmedinf.2022.104895 (2022). [DOI] [PubMed] [Google Scholar]
- 15.Kongwong, R., Yoongthong, W., Ruangying, J. & Puttarak, P. Optimization a guideline for consumer protection using TaWai for health application: A case study of Warin Chamrap, Ubon Ratchathani. Southern College Netw. J. Nurs. Public Health. 7(3), 142–154. https://www.tci-thaijo.org/index.php/scnet/index (2020).
- 16.Databases statistic TaWai for health (2020).
- 17.Im, G.-H., Shin, D. & Cheng, L. Critical review of validation models and practices in language testing: Their limitations and future directions for validation research. Lang. Test. Asia9, 14. 10.1186/s40468-019-0089-4 (2019). [Google Scholar]
- 18.Bloom, B. S. In Mastery learning: Theory and practice (ed J.H. Block) (New York: Holt, Rinehart & Winston, 1971). https://www.sciepub.com/reference/105839 (1971).
- 19.Campbell, M. K., Elbourne, D. R. & Altman, D. G. CONSORT statement: Extension to cluster randomised trials. BMJ (Clin. Res. Ed.)328, 702–708. 10.1136/bmj.328.7441.702 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campbell, M. K., Piaggio, G., Elbourne, D. R. & Altman, D. G. Consort 2010 statement: Extension to cluster randomised trials. BMJ (Clin. Res. Ed.)345(e5661), 2012. 10.1136/bmj.e5661 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Gonzalez, E., Herdeiro, M. T., Piñeiro-Lamas, M. & Figueiras, A. Effect of an educational intervention to improve adverse drug reaction reporting in physicians: A cluster randomized controlled trial. Drug Saf.38, 189–196. 10.1007/s40264-014-0256-6 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Figueiras, A., Herdeiro, M. T., Polónia, J. & Gestal-Otero, J. J. An educational intervention to improve physician reporting of adverse drug reactions: A cluster-randomized controlled trial. JAMA296, 1086–1093. 10.1001/jama.296.9.1086 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Herdeiro, M. T., Polónia, J., Gestal-Otero, J. J. & Figueiras, A. Improving the reporting of adverse drug reactions: A cluster-randomized trial among pharmacists in Portugal. Drug Saf.31, 335–344. 10.2165/00002018-200831040-00007 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Potlog Shchory, M. et al. Increasing adverse drug reaction reporting—How can we do better?. PloS One15, e0235591. 10.1371/journal.pone.0235591 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.García-Abeijon, P. et al. Factors associated with underreporting of adverse drug reactions by health care professionals: A systematic review update. Drug Saf.46, 625–636. 10.1007/s40264-023-01302-7 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to the privacy of individuals who participated in the study. The dataset is available upon request from the corresponding author.