Abstract
Background
Electrospun nanofiber scaffolds have been widely used in tissue engineering because they can mimic extracellular matrix-like structures and offer advantages including high porosity, large specific surface area, and customizable structure. In this study, we prepared scaffolds composed of aligned and random electrospun polycaprolactone (PCL) nanofibers capable of delivering basic fibroblast growth factor (bFGF) in a sustained manner for repairing damaged tendons.
Results
Aligned and random PCL fiber scaffolds containing bFGF-loaded bovine serum albumin (BSA) nanoparticles (BSA-bFGF NPs, diameter 146 ± 32 nm) were fabricated, respectively. To validate the viability of bFGF-loaded aligned PCL nanofiber scaffold (aPCL + bFGF group) in tendon tissue engineering, we assessed the in vitro differentiation of human amniotic mesenchymal stem cells (hAMSCs) towards a tenogenic lineage and the in vivo regeneration of tendons using a rat Achilles tendon defect model. The encapsulated bFGF could be delivered in a sustained manner in vitro. The aPCL + bFGF scaffold promoted the in vitro differentiation of human amniotic mesenchymal stem cells (hAMSCs) towards a tenogenic lineage. In the repair of a rat Achilles tendon defect model, the aPCL + bFGF group showed a better repair effect. The scaffold offers a promising substrate for the regeneration of tendon tissue.
Conclusions
The aligned and random PCL fiber scaffolds containing bFGF nanoparticles were successfully prepared, and their physical and chemical properties were characterized. The aPCL + bFGF scaffold could promote the expression of the related genes and proteins of tendon-forming, facilitating tendon differentiation. In the rat Achilles tendon defect experiments, the aPCL + bFGF exhibited excellent tendon regeneration effects.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12951-024-03022-1.
Keywords: Electrospun nanofiber, Fiber orientation, Drug release, Tendon repair, Tissue engineering
Introduction
Tendon is comprised of dense fibrous connective tissue which facilitates muscle movement and maintains bone stability, playing an indispensable role in transmitting force to achieve body movement [1]. Caused by trauma, chronic tendinitis and tendinopathy, tendon injuries are common in both courts and daily exercises [2]. Globally, there are over 30 million cases of tendon injuries each year, leading to medical expenses exceeding 156 billion dollars [3]. Due to factors like inadequate blood supply, reduced elasticity, weakened mechanical properties, and high-intensity exercise, tendons can be susceptible to rupture under intense loads, even at physiological levels, resulting in the accumulation of fatigue injuries [4]. Various strategies have been developed to promote tendon healing, such as physiotherapy, rehabilitation exercise, and tendon suture, as well as transplantation of autografts, allografts, and xenografts. These strategies often have pros and cons in clinical practice.
The development of the concept of engineered tendons in tissue engineering has offered a promising therapeutic avenue, introducing new possibilities and approaches to sports medicine [5–7]. The primary seeded cells commonly employed in tendon tissue engineering include tenocytes, tendon stem cells, mesenchymal stem cells, adipose stem cells, and so on [8, 9]. As a permanently differentiated cell, tenocytes exhibit decreased metabolic activity and loss of tension markers over the course of continued passage. Stem cells are essential components in constructing tissue-engineered tendons, capable of undergoing multi-directional differentiation under suitable conditions for the treatment of acute or chronic tendon injuries [10]. Although tendon stem cells have strong proliferation ability and tension expression, the finite applicability of autologous tendons limits their clinical application [11, 12]. Human amniotic mesenchymal stem cells (hAMSCs) are abundant sources with high differentiation potential and healing ability, which have been widely used as seed cells in tendon tissue engineering [13–15].
As an indispensable component in tissue engineering, scaffolds play a crucial role in providing a conducive living environment and nutrient transportation for the proliferation and differentiation of seeded cells [16, 17]. The ideal scaffolds should possess good mechanical properties while the composition and topography can mimic the extracellular matrix (ECM) of the native status [18]. For the past few years, nanofibrous scaffolds prepared by electrospinning technology have been widely applied in tendon tissue engineering [19]. Nanofiber scaffolds offer numerous advantages, including excellent biocompatibility, high aspect ratio, and adjustable porosity [19, 20]. The topographical features of scaffolds have fundamental impacts on cellular activities, including cell attachment, proliferation, and differentiation [21]. Notably, aligned scaffolds have been reported to induce ideal tendon differentiation, while random scaffolds are more inclined to induce osteogenic differentiation [22]. The tendon structure is formed by neatly aligned fiber bundles, with collagen fiber bundles having a diameter of 20 nm and tendon fiber bundles having a diameter of 2–3 mm [23, 24]. Therefore, the construction of nanofiber scaffolds mimicking the microstructure of tendon is key to promote tendon regeneration.
The incorporation of bioactive factors is also important to promote tendon regeneration. Currently, different drug delivery systems have been developed to maintain the biological activity and sustained release of growth factors from scaffolds [25]. Incorporating basic fibroblast growth factor (bFGF) with heparin-binding sites on the surface of fiber scaffold can maintain the sustain release and long-term activity of the growth factor [26]. Electrospun technology has been applied to prepare scaffolds with a diameter of the fibers in the range of 100 nm to 5 μm [27]. This property makes electrospun fiber scaffolds extremely valuable for loading growth factors to increase the duration period. Beyond the excellent biocompatibility, high porosity, and multi-porous structure, the morphological characteristics of the scaffold also significantly impact the biological activity of cells [28, 29].
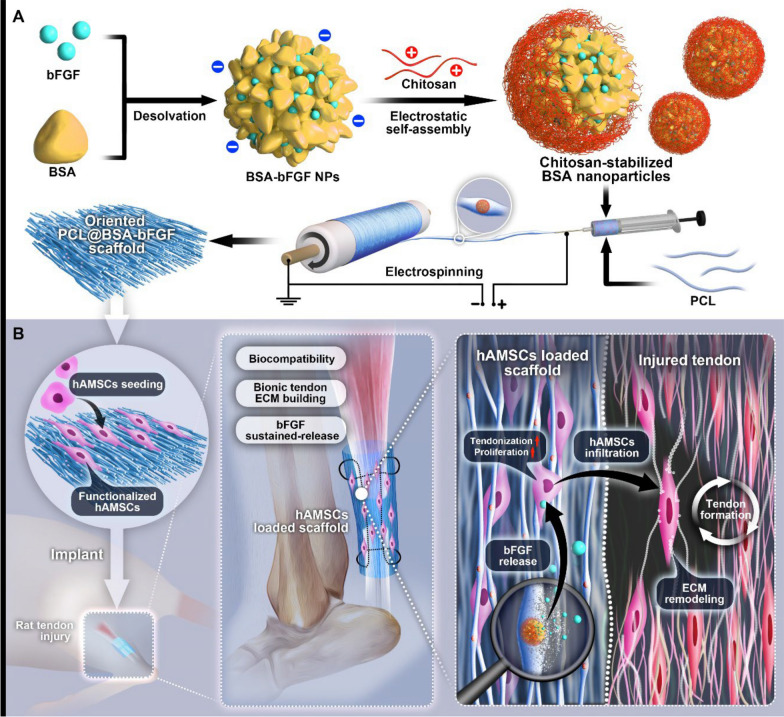
Herein, scaffolds composed of random and aligned PCL fibers were developed to regulate the delivery of bFGF for promoting tendon regeneration (Fig. 1). bFGF was loaded onto bovine serum albumin (BSA) nanoparticles and chitosan was then adsorbed on the shell of the nanoparticles by electrostatic self-assembly. Subsequently, the nanoparticles containing bFGF were loaded in the aligned and random PCL nanofibers to construct tendon repair scaffolds. Afterwards, the scaffolds were co-cultured with hAMSCs and then implanted into injured Achilles tendons to investigate the feasibility of the nanofiber scaffolds for repairing the injured tendon.
Fig. 1.
Schematic illustration indicating the fabrication of the tissue engineered electrospun nanofiber scaffold for repairing tendon injury. A Schematic illustration of the preparation of chitosan-stabilized bFGF-loaded electrospun PCL nanofiber scaffold. B Schematic illustration indicating aligned PCL nanofiber scaffold seeded with hAMSCs was transplanted to repair injured Achilles tendon
Materials and methods
Preparation of BSA nanoparticles loaded with human bFGF
BSA nanoparticles were fabricated by desolvation method as previously reported [30]. In detail, 100 mg BSA was dissolved in 10 mL distilled water and stirred continuously with a microsyringe pump, and then 40 mL ethanol was pumped into the 1 w/v% BSA solution. The mixture was continuously stirred overnight. Subsequently, 40 mL chitosan solution (1 mg/mL) dissolved in 1 w/v% acetic acid was pumped into the obtained mixture at a rate of 0.5 mL/min, and the stabilized chitosan-BSA nanoparticles were obtained after 8 h of stirring. The nanoparticles were collected by centrifugation at 10,000 rpm for 20 min, while the nanoparticles were washed three times with distilled water/ethanol mixed solution at ratio of 1:1 (v/v). Finally, the white precipitate was collected and stored for further experiments. To prepare BSA nanoparticles loaded with human bFGF (BSA-bFGF NPs), 160 μL bFGF stock solution at a concentration of 50 mg/100 mL in phosphate buffer was added to 50 mL BSA solution, and then the above steps were applied. The chitosan-BSA NPs and BSA-bFGF NPs were dispersed in ethanol at a concentration of 75%. The size of the NPs was measured by a dynamic light scattering instrument. The NPs were observed under a transmission electron microscope. In brief, chitosan-BSA NPs and BSA-bFGF NPs were dispersed in an appropriate concentration of ethanol, deposited on carbon film copper mesh, and dried under vacuum for 72 h. The samples were then examined using a field emission transmission electron microscope operating at 150 kV to obtain detailed observations of the structures and features.
Preparation and characterization of electrospun PCL nanofiber scaffolds
BSA-bFGF NPs were embedded in PCL nanofibers by electrospinning. Firstly, 100 mg PCL pellets was dissolved in 2.5 mL of N,N-dimethylformamide (DMF), and 1.45 g PCL pellets were dissolved in 7.5 mL dichloromethane (DCM). Then, the two solutions were mixed and stirred overnight at room temperature. The mixed solution was then pushed through a micro-injection pump at a speed of 1 mL/h for electrospinning. The spinning voltage was set at 19 ± 0.5 kV, and the receiving distance was 18 cm. A roller was applied as the collector. By adjusting the rotation speed of the roller to 1800 rpm and 20 rpm, the scaffolds composed of aligned and random nanofibers were obtained, respectively. Pristine PCL nanofibers in an aligned and random-arrays were also fabricated. Four different types of PCL nanofiber scaffolds were fabricated, including aligned PCL nanofiber scaffold (aPCL), random PCL nanofiber scaffold (rPCL group), BSA-bFGF NPs-loaded aligned PCL nanofiber scaffold (aPCL + bFGF group), and BSA-bFGF NPs-loaded random PCL nanofiber scaffold (rPCL + bFGF group). The morphology of nanofibers was observed by a transmission electron microscopy (TEM) and scanning electron microscopy (SEM). The diameters and orientations of the nanofiber scaffolds were measured and calculated. The mechanical strengths of the fiber scaffolds were measured using a universal mechanical testing machine.
The loading efficiency and release of bFGF in BSA-bFGF NPs
The drug loading efficiency of BSA-bFGF NPs was calculated. The unbound BSA on the surface of the NPs was rinsed off by deionized water. After centrifugation, the supernatant was collected, and the loss of bFGF was detected by human bFGF enzyme-linked immunosorbent assay (ELISA) kit. The unbound BSA in the solution was measured at the wavelength of 562 nm to calculate the drug loading efficiency of BSA-bFGF NPs based on the following equation:
Drug loading efficiency = (The amount of bFGF—the amount of unbound BSA)/amount of microsphere × 100%).
To evaluate the release of bFGF, the scaffolds loaded with bFGF were separately placed in a centrifuge tube, with each sample weighing 6 mg, and then 2 mL of phosphate-buffered saline (PBS) solution was added to each tube. Each sample was prepared in triplicate. At 1, 2, 4, 6, 8, 10, 14, 18, 22, 26, 30, and 35 days, the cumulatively released amount of bFGF was detected using the ELISA method.
Isolation and cultivation of hAMSCs
hAMSCs were derived from the placental tissue of healthy adult parturients, and informed consent was provided by the patients before delivery. This study was approved by the Research Ethics Committee of Zunyi Medical University Affiliated Hospital (KLLY-2018–164). The hAMSCs were extracted by trypsin-type II collagenase digestion as previously reported [31]. Cells at third passage were used for subsequent experiments. The cells were cultured in LG-DMEM medium with 10% fetal bovine serum (FBS), 1.176 g NaHCO3, 1% penicillin and streptomycin, 1% L-glutamine and non-essential amino acids. The hAMSCs were incubated at 37 °C with 5% humidified CO2. Every three days, the medium was refreshed after washing the cells three times with PBS. Upon reaching 80–90% confluence, the cells were digested for 3 min with 0.125% trypsin/0.01% EDTA-2Na and then divided at a 1:2 ratio for subculture.
Cell viability and proliferation experiment in vitro
The nanofiber scaffolds were cut into a disc shape with a diameter of 1.5 cm and then placed in the wells of a 24-well cell culture plate. Afterwards, cells were seeded at a density of 2 × 104 (cells/cm2). The cell viability on the scaffolds was measured with live/dead staining according to the instruction from the manufacturer (Solarbio, Beijing). After incubation for one and four days, the medium was removed, and a mixture solution containing 2 μM calcein AM and 4 μM propidium iodide (PI) was then added. The culture plate was placed in an incubator at 37 °C and 5% CO2 for 15 min. Subsequently, the live and dead cells were observed and differentiated under a fluorescence microscope. Cell proliferation was detected by CCK-8 assay, and the harvested scaffold-cell hybrids were incubated for 2 h in 10%(v/v) CCK-8 solution after incubation for 1, 3, 5, and 7 days, respectively. Subsequently, 100 μL culture medium was transferred to a new 96-well plate, and the absorbance at 450 nm was measured using a 680-type micro-tablet reader (Bio-Rad, USA).
RNA isolation and quantitative real-time polymerase chain reaction analysis (qRT-PCR)
To investigate the effects of different fiber scaffolds on the tenogenic differentiation, hAMSCs seeded on the different scaffolds (2 × 104 cells/cm2) were collected on day 7 and 14. The total RNA was extracted in accordance with the operating specifications of Trizol kit (TAKARA, Japan), and hAMSCs cultured in the well of the culture plate under the same conditions were used as the control group. The extracted RNA samples were reversely transcribed into cDNA by Prime-Script RT kit (TAKARA, Japan), and primers were designed and synthesized after completing the PCR amplification reaction. The primer sequences are listed in Table S1, and the reaction condition was 40 cycles. β-actin was used as the reference housekeeping gene, and the relative expression level of the target gene was calculated using the 2−△△Ct method. The result was expressed as a multiple of the expression level in the control group.
Western blotting assay
The hAMSCs cultured on different scaffolds were digested by trypsin after 7 and 14 days and collected into 1.5 mL EP tubes. After washed with pre-cooled PBS, the cells were centrifuged at 1000 rpm/min for 4 min at 4 ℃. After lysate radio-immunoprecipitation assay (RIPA) was added and incubated with phenylmethanesulfonyl fluoride (PMSF) on ice for 0.5 h, the mixture was centrifuged at 8000 rpm/min for 8 min at 4 ℃. The supernatant was taken and added protein loading buffer boiled for denaturation for 5 min and stored at − 80℃. The protein concentration was detected by the BCA method followed by SDS-PAGE electrophoresis. Subsequently, the sample was transferred to PVDF membrane by wet method and added with TBST buffer of defatted milk for incubation at 37 ℃ for 2 h. The rabbit anti-human Scleraxis (SCX) and Tenomodulin (TNMD) protein antibodies were added, respectively, for incubation for 24 h at 4 ℃. The PVDF membrane was washed with TBST for 10 min and repeated for 3 times. Then, the mouse anti-rabbit fluorescent secondary antibody was added and incubated for 60 min in the dark condition at 4 ℃. The PVDF membrane was also washed with TBST for a total of 3 times at each time of 10 min.
Implantation of scaffolds in Achilles tendon defect model
Seventy-five 8-week-old adult male Sprague–Dawley rats weighing 200–230 g were randomly divided into five groups: 1) defect group, 2) aPCL group, 3) rPCL group, 4) aPCL + bFGF group, 5) rPCL + bFGF group, and 15 rats were set in each group. The density of hAMSCs was adjusted to 104/cm2 and then incubated on the surface of nanofiber scaffolds for 4 days. The nanofiber scaffold was adjusted to 8 mm × 8 mm with a square shape. Ultraviolet light was used to sterilize the scaffold overnight.
The Achilles tendon rupture model was created following a method described in previous literature [32]. Briefly, the rats were administered general anesthesia by intraperitoneal injection of sodium pentobarbital (36 mg/kg), and then the left hind limb was shaved and sterilized with 5% iodophor. A 20-mm longitudinal incision was made into the skin of left limbs, and a 2-mm defect was made in the medial gastrocnemius tendon. The different scaffolds were implanted into the defect and sutured with 4–0 suture (Vicryl, Ethicon Company, USA). The outer layer was covered with a 2 mm nanofiber scaffold for secure fixation. The rats undergo surgery without implanting hybrids used as the negative control (defect group). Then skin incision was closed with 2–0 sutures. The wound of Achilles tendon was disinfected with povidone-iodine postoperatively and put back to the cage for free feeding.
Magnetic resonance imaging (MRI) analysis
MRI scans of the rat Achilles tendon were performed at 3 day, 4 weeks, and 8 weeks after surgery, respectively. The MRI sequence using the surface coils was sagittal T2-weighted imaging (the parameter was set as follow: REARE, TR = 4000 ms, TE = 45 ms, FOV = 30 × 30 mm, matrix size = 256 × 256, layer thickness = 0.5 mm, and layer spacing = 0 mm). According to the MRI images, 20 points were randomly selected above the posterior superior horn of the calcaneus to measure the anterior and posterior diameters of the Achilles tendon. According to the T2-weighted images, the area ratio of intra-tendon lesions as the ratio of high and medium signal area (AHS) to total longitudinal area (AT) in the Achilles tendon region was scanned. The ratio of the intensity of the intra-tendon lesion to the background was also calculated.
Macroscopic and histological evaluation of regenerated tendon
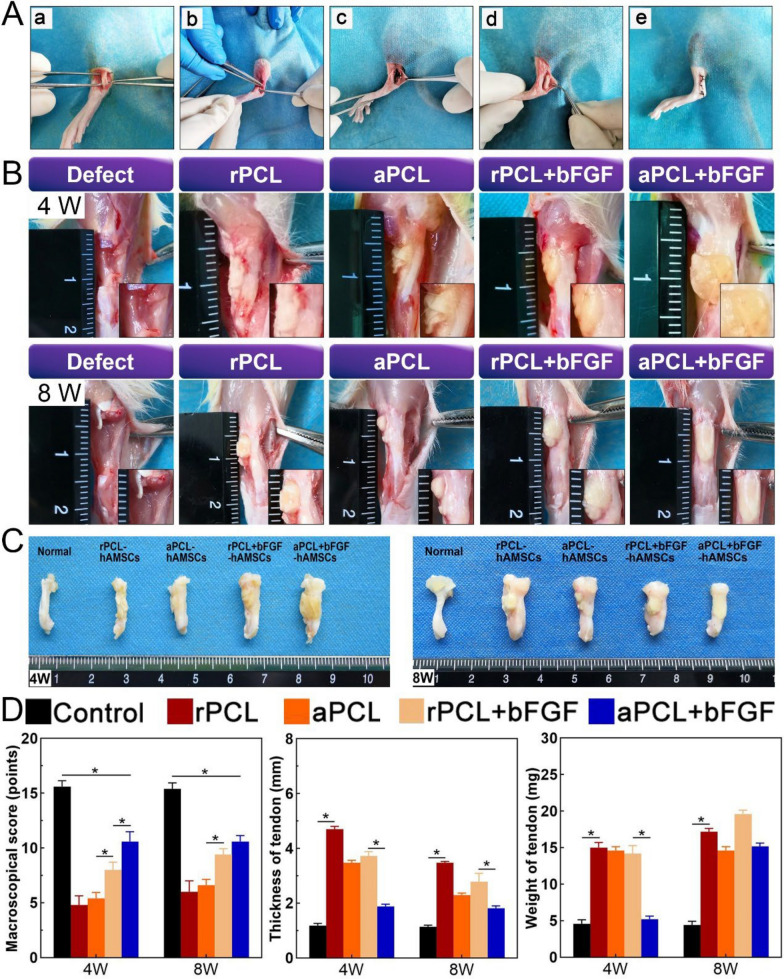
Animals were sacrificed 4 and 8 weeks after surgery, and regenerated Achilles tendons were evaluated. High-concentration sodium pentobarbital solution was prepared. After narcotized by intraperitoneal injection of excessive sodium pentobarbital, rats were sacrificed under the environment of high concentration of CO2. Tendon samples were then collected by bluntly separated skin and subcutaneous tissue. The samples of animals were scored according to the general scoring system (Table. S2) [33]. The full-length tendon complex located between gastrocnemius and calcaneus was excised to expose the Achilles tendon tissue and photographed with a camera. The tendon weight and thickness of each group were then measured and compared directly with the corresponding normal tendon of the right hind limb. The samples (n = 5) obtained at different time points were fixed in 10% formaldehyde at room temperature for 24 h, dehydrated in a gradient of ethanol, embedded in paraffin, and sectioned for HE, Masson and Sirius red staining, respectively. The quality of regenerated tissue was evaluated using a modified histological scoring system (Table. S3). Micrographs of all sections were taken in the suture zone. Three fields of view were randomly selected for examination under light microscopy.
Gene and protein analysis of tissue
Quantitative reverse transcription polymerase chain reaction (qPCR) and western blot assay were used to detect the expression of tendon-related genes and proteins. The healing quality of the regenerated tissue was analyzed. The tissue samples were ground three times in liquid nitrogen, lysed in 2 g/L Trizol reagent and 1 g/L RIPA lysis buffer, and centrifuged for 5 min at 12,000 rpm/min after tissues were homogenized, and then the supernatant was collected.
Statistical analysis
All quantitative results are expressed as the mean ± standard deviation. Statistical significance between the groups was tested by the student’s t-test. Statistical difference was considered at p < 0.05.
Results
Characterization of nanoparticles and electrospun fibers
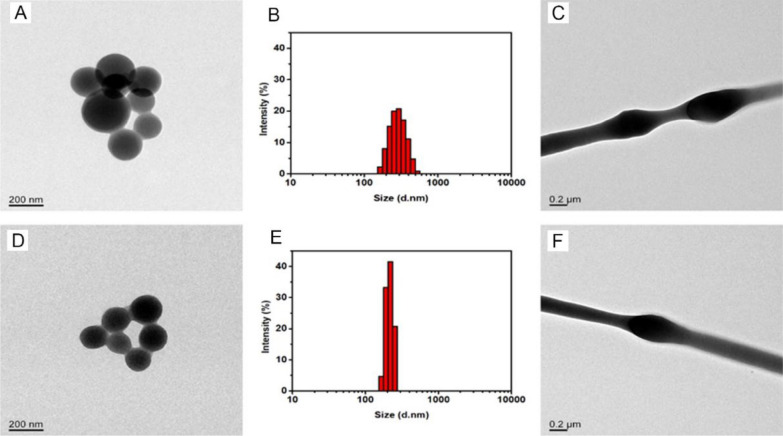
The BSA NPs and BSA-bFGF NPs were characterized using TEM and DLS, indicating that the average diameters nanoparticles were 205 ± 40 nm and 146 ± 32 nm, respectively (Fig. 2A, B, D, E). The size of nanoparticles decreased upon storage in PBS, resulting in the smaller size of BSA-bFGF NPs compared to chitosan-coated BSA NPs. This size reduction contributed to a higher drug loading rate of bFGF in BSA-bFGF NPs (90.22 ± 3.47%) compared to single NPs. The double-layer barrier in the nanoparticles could preserve the biological activity of bFGF by preventing from the contact between the growth factors and organic solvents during the electrospinning process. The surfaces of the nanoparticles showed smooth and homogeneous morphology, and the nanoparticle size exhibited a relatively uniform distribution (Fig. 2C, F). In addition, the nanoparticles were distributed and wrapped in the nanofibers.
Fig. 2.
Morphological analysis of nanoparticles and nanofibers. A and D are TEM images of BSA NPs and BSA-bFGF NPs, respectively, and B and E are the particle size distribution charts of corresponding nanoparticles measured by DLS; C and F are TEM images of nanofibers, containing BSA NPs and BSA-bFGF NPs, respectively
The orientation and characterization of electrospun nanofiber scaffolds
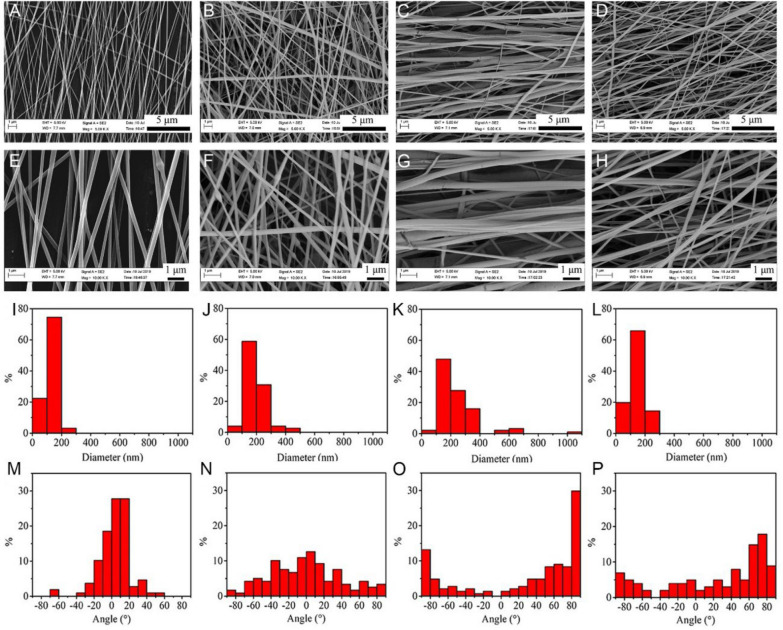
The diameter and orientation distribution of four different PCL nanofiber scaffolds were observed by SEM. The average diameters of aPCL + bFGF, rPCL + bFGF, aPCL and rPCL groups were 129 ± 33 nm, 194 ± 73 nm, 239 ± 146 nm, and 146 ± 47 nm, respectively (Fig. 3A–H). The SEM results demonstrated the loading of nanoparticles had less effect on the diameter of the fibers. The surfaces of the four types of nanofiber scaffolds appeared to be smooth. In contrast to the individual PCL nanofibers, a minor portion of the nanofiber surface exhibited the presence of protruding nanoparticles, which were not entirely enveloped by the nanofibers. The fiber orientation was affected by the rotation speed of the collector. A higher rotational speed resulted in a better fiber orientation, while a lower speed led to disordered fibers. Briefly, the above results indicated that PCL nanofiber scaffolds with different orientations containing BSA-bFGF NPs were successfully prepared (Fig. 3I–P).
Fig. 3.
The characterization of morphology, diameter distribution, and orientation distribution of the nanofiber scaffolds by SEM. The SEM micrographs of A, E aPCL + bFGF group, B, F rPCL + bFGF group, C, G aPCL group, and D, H rPCL group at different magnifications. I–L The fiber diameter distribution and M–P the fiber orientation distribution corresponding to (A–D), respectively
Cell proliferative activity, drug release, and mechanical property of scaffolds in vitro
The aligned nanofiber scaffolds demonstrated the ability to promote cell proliferative activity, and the continuous release of bFGF further enhanced cell proliferation. CCK-8 assay results showed no significant differences among the groups on day 1, indicating that the PCL nanofiber scaffold exhibited good biocompatibility with hAMSCs initially. As the incubation time increased, cell viability showed a positive correlation in all groups, suggesting cell proliferation over time. By day 3, the aPCL + bFGF group exhibited higher cell proliferative activity compared to the other groups, and this trend continued through days 5 and 7 (Fig. 4A). These results indicate that the combination of topographical cues provided by aligned nanofibers and biochemical cues delivered by bFGF has a synergistic effect in promoting cell proliferation. The aligned nanofibers create a conducive environment for cell alignment and growth, while the sustained release of bFGF further supports cell proliferation, resulting in enhanced overall cell activity in the aPCL + bFGF group.
Fig. 4.
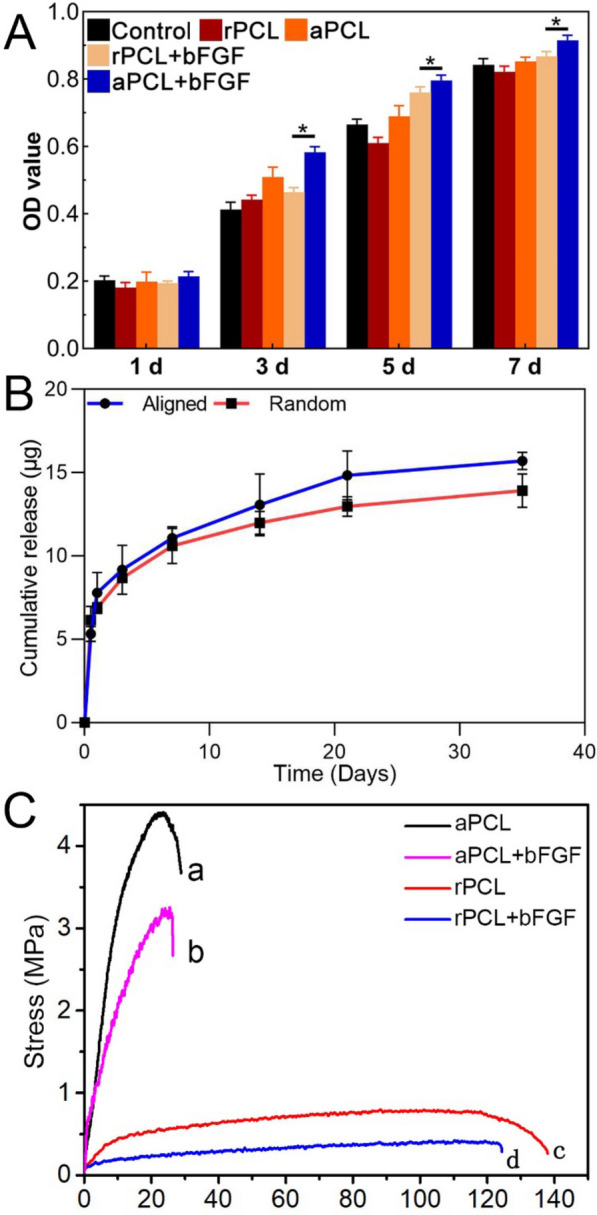
Biocompatibility, cumulative drug release profile, and mechanical properties of the electrospun nanofiber scaffolds. A Proliferation activity of hAMSCs incubated on the different types of nanofiber scaffolds detected by CCK-8 assay. B Cumulative release curves of bFGF-loaded nanofiber scaffolds. C Stress–strain curves of the different types of nanofiber scaffolds
From the in vitro release curves (Fig. 4B), bFGF were continuously released from both the oriented and non-oriented nanofiber scaffolds within 35 days, with no obviously burst release, which was because of the multi-layer barrier. Compared with the scaffolds loaded with bFGF, the nanoparticles with double protection were more effective in maintaining biological activity and sustained release, and the action time could be maintained longer.
The tensile strengths of the aPCL, aPCL + bFGF, rPCL and rPCL + bFGF groups were 4.48 ± 0.33 MPa, 3.35 ± 0.22 MPa, 0.74 ± 0.25 MPa and 0.49 ± 0.31 MPa, respectively. The stress–strain results presented the tensile strength in aPCL + bFGF group was lower than in aPCL group, while the tensile strength of in rPCL + bFGF and rPCL group was not as good as that of aligned fibers (Fig. 4C). The tensile strength of the scaffolds was high enough for the application.
Cell morphology and potential of tendon differentiation in vitro
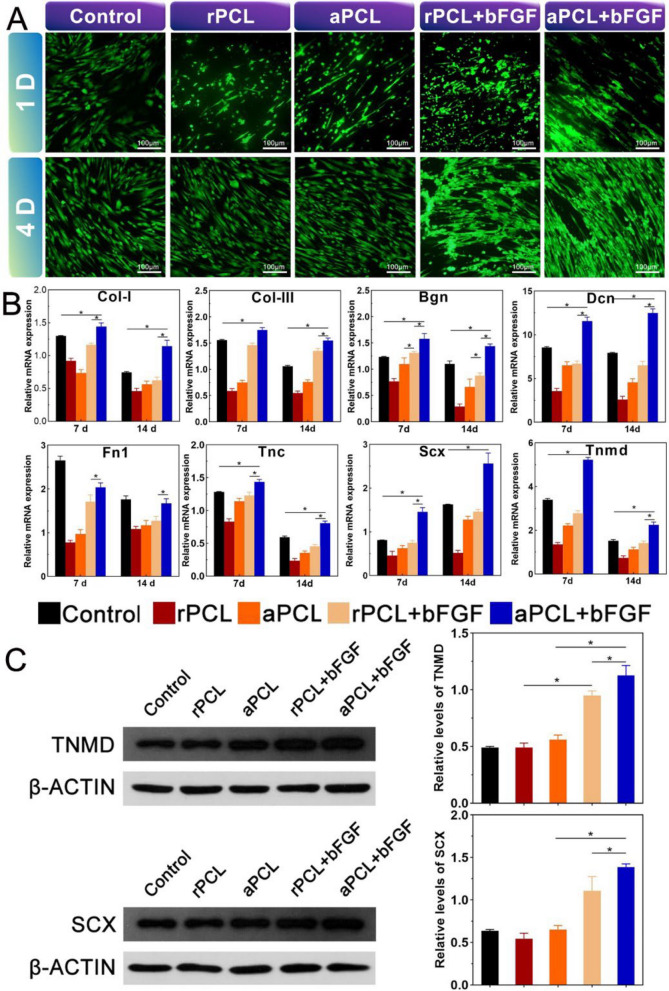
The live/dead staining showed hAMSCs grew well after incubated on nanofiber scaffolds in each group (Fig. 5A). The morphology of cells exhibited elongated and spindle-shaped, with good activity, suggesting that the prepared nanofiber scaffold could provide space for cell adhesion and proliferation. Meanwhile, hAMSCs could grow along the direction of the fiber orientation on the aligned fibers while grow disorderly on the random nanofiber scaffold. The proliferation of cells was significantly higher in aPCL + bFGF group compared with other groups. To further verify the effect of tendinous differentiation on PCL nanofiber scaffolds, the levels of tendon-related genes and proteins of hAMSCs in each group were detected. Type I and III collagen (COL-I, COL-III) play a vital role in the regeneration of tendon tissue, while Scleraxis (SCX) and tenomodulin (TNMD), as the tendon-specific transcription factors, play an essential role in tendon development. In addition, biglycan (BGN), fibronectin 1 (FN1), decorin (DCN) and tenascin-C (TNC) are intriguingly proteoglycans that regulate the function of collagen fibers in the tendon. The cells in the aPCL + bFGF group showed higher levels of mRNA expression of the tendon-related genes than in the other groups at day 7. The expression of DCN, SCX and TNMD in aPCL + bFGF group were up-regulated by about 2.5-fold, twofold and threefold, respectively, compared with the control group. A similar expression trend was observed at day 14 (Fig. 5B). At day 14, protein expressions of SCX and TNMD in aPCL + bFGF group were higher than those in aPCL and rPCL + bFGF group (Fig. 5C). The fibrous orientation in the aPCL group could regulate cell morphology and promote the elongation of hAMSCs. Additionally, the incorporation of bFGF into the aligned fibers (aPCL + bFGF group) further enhanced the oriented collagen synthesis, promoting and enhancing tendon differentiation.
Fig. 5.
Cell morphology and mRNA and protein expression of tendon differentiation of hAMSCs in vitro. A Fluorescence micrographs of live and dead cells staining in each group (scale bar = 100 μm). B mRNA expression levels of tendon-related genes after incubating hAMSCs on different scaffolds for 7 and 14 days. C After culturing hAMSCs on different scaffolds for 14 days, Western blotting was used to detect the expression of SCX and TNMD, and the quantitative analysis of the expression of SCX and TNMD according to the images and normalized to β-actin. The data are expressed as mean ± SD, *p < 0.05
Macroscopic evaluation of regenerated tendon
To evaluate the effectiveness of hAMSCs-seeded nanofiber scaffolds for tendon regeneration in vivo, a rat Achilles tendon defect model was employed. The macroscopic evaluations included examining the color, surface structure, thickness, and adhesion of the regenerated tendon (Fig. 6A). The gross samples showed a healthy tendon which was bright, with a non-rough surface and clear boundary with other surrounding tissues. After surgery, tendon samples of rPCL and rPCL + bFGF groups exhibit a yellowish, rough surface and adhesion with the paratestin, fascia, and subcutaneous tissues skin. In addition, post-surgical tendon thickening could be generally observed. After 8 weeks, new tendinous tissue was observed with negligible adhesion to the surrounding tissue in aPCL and aPCL + bFGF group (Fig. 6B). According to Christiane's modified macroscopic scoring system [33], the macroscopic scores (Table S2) of the aPCL + bFGF group was significantly higher than the other operation groups (rPCL, aPCL and rPCL + bFGF group) at 4 and 8 weeks, indicating that the aPCL + bFGF group showed an improved healing effect on tendon defects (Fig. 6C).
Fig. 6.
Surgical procedures and macroscopic evaluation of regenerated tendon. A Surgical procedures of Achilles tendon defect model (a) exposed Achilles tendon, (b) defect caused by no suture, (c) Achilles tendon suture, (d) PCL-hAMSCs construct implantation, and (e) skin suture. B, C The general shape and anatomical images of the regenerated Achilles tendon at 4 and 8 weeks after surgery. D Quantitative analysis of histological results, including the macroscopical scores, thickness, and weight of tendon
To assess the postoperative healing of the Achilles tendon, the thickness and weight of the healing tendons were quantified. The defect group was excluded from the analysis due to severe tendon contracture, making it unsuitable for sampling. At 4 weeks after surgery, the tendon thickness was significantly increased compared to the native group. However, at week 8, the thickness of the tendons in the aPCL and aPCL + bFGF groups were slightly reduced, and they exhibited significantly less thickness than the other groups. This suggests that the structure of the aPCL + bFGF group resulted in weaker swelling compared to the rPCL + bFGF group, possibly due to the directional arrangement of the aPCL + bFGF fibers (Fig. 6D). The alignment of fibers in the aPCL + bFGF group contributed to a better tendon structure and reduced swelling, supporting its potential for promoting tendon healing.
Imaging evaluation of regenerated tendon
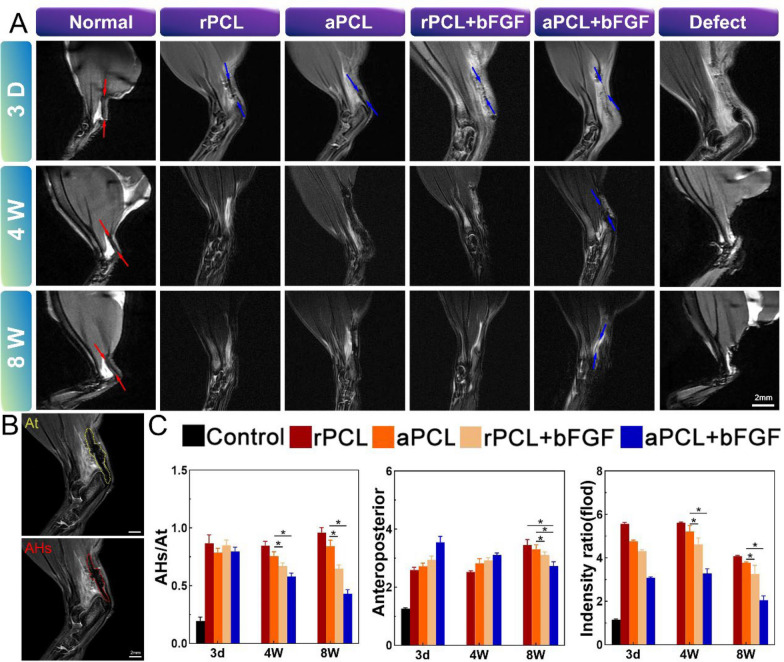
MRI is a valuable non-invasive imaging technique used for assessing soft tissue conditions, including the healing and regeneration processes of the Achilles tendon. In this study, sagittal T2-weighted MRI was employed to observe the regeneration of the rat Achilles tendon after surgery. In the healthy Achilles tendon, clear edges and low, uniform signals were observed. The major components of the Achilles tendon were distinguishable through continuous cross-sectional imaging. On postoperative day 3, the MRI images revealed an intra-tendon lesion with surrounding tissues exhibiting evident uplift, irregular profile, and uneven high or discontinuous signal. This indicated the successful establishment of the rat model with Achilles tendon defect and the presence of inflammatory reaction in the defect area (Fig. 7A). By 4 weeks after surgery, a palpable bulge was noticeable, but the high-signal area on the MRI images was gradually reduced, especially in the rPCL + bFGF and aPCL + bFGF groups. Despite having similar anteroposterior dimensions, the AHS/AT values and intensity ratios in the aPCL + bFGF group were significantly lower than those in the rPCL and rPCL + bFGF groups. It is worth noting that the anteroposterior dimensions in the rPCL group differed from the macroscopic tendon thickness detected.
Fig. 7.
Sagittal T2-weighted MRI scan results of regenerated Achilles tendon and related quantitative analysis. A MRI scan results in each group at day 3, week 4 and 8. Regenerated Achilles tendon structure (red arrow indicates tendon area and blue arrow indicates implant construct). B Quantitative measurement of high- or intermediate-intensity signal area (AHS) and total longitudinal area (AT) within the Achilles tendon region. C The AHS/AT ratio analysis and the ratio of the intensity of intra-tendon lesions based on the background and the results were normalized to the level of the control group; mean anteroposterior analysis. The data were expressed as mean ± SD, *p < 0.05
Overall, the MRI findings provided valuable insights into the regeneration process of the rat Achilles tendon and indicated the potential benefits of using aligned nanofiber scaffolds encapsulated with bFGF for promoting a better tendon healing. This difference may be attributed to the high-intensity signal expression usually caused by a variety of factors such as hemorrhage, edema, and exudation that affected the dimensional measurement. At 8 weeks after surgery, the rPCL and rPCL + bFGF group still had unclear borders, high-signal signal regions and lordosis, while the signal strength and size of the aPCL + bFGF group were significantly reduced. (Fig. 7 B, C).
Tendon-related protein expression analysis
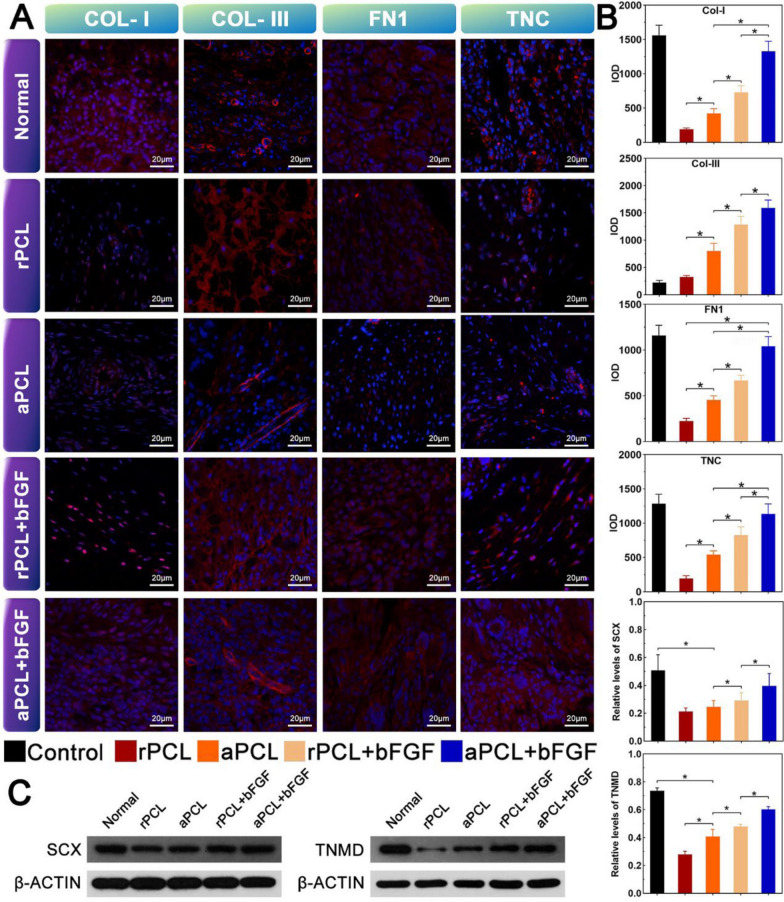
Immunofluorescence staining was performed to verify the tendon-forming ability in each group. At 8 weeks after surgery, the immunofluorescence results of the regenerated tendons in each group showed a more widespread distribution of expression for type I collagen (COL-I), type III collagen (COL-III), fibronectin (FN1), and tenascin-C (TNC). The proteins exhibited a web-like agglomerate pattern, emanating from the cell nucleus. The red color represented the protein expression, while the blue represented the cell nucleus (Fig. 8A). Quantitative immunofluorescence analysis revealed that the expression levels of COL-I, COL-III, FN1, and TNC in the aPCL + bFGF group were higher compared to the other groups (Fig. 8B). These findings indicate that the aligned nanofiber scaffolds encapsulated with bFGF promoted a more robust expression of tendon-related proteins, contributing to the enhanced tendon-forming ability and better tendon regeneration.
Fig. 8.
The immunofluorescence results of regenerated tendons in each group 8 weeks after surgery. A Immunofluorescence micrographs indicating the expression levels of Col-I, COL-III, FN1, TNC and TNC in different groups. The red fluorescence showed the expression of the protein, and the blue showed the nucleus (scale bar = 100 μm). B Quantification of the expressions of the corresponding proteins in each group by measuring the integrated optical density (IOD); C Protein expression level and quantification of SCX and TNMD at 8 weeks after surgery, ∗ p < 0.05
The effect of PCL-hAMSCs hybrids structure on tendon regeneration can be assessed by the expression of various tendon-related genes and proteins. At 8 weeks after surgery, the expression levels of tendon-associated transcription factor genes (SCX, TNMD) and extracellular matrix genes (Col-I, Col-III, BGN, DCN, FN1, and TNC) in aPCL + bFGF group were significantly higher than those in rPCL + bFGF group (Fig. S2). Among them, the protein levels of SCX and TNMD in aPCL + bFGF group were significantly higher than those in the rPCL + bFGF group. It is suggested that aPCL + bFGF group enhanced the tendon differentiation and regeneration which was triggered by the collagen fibers' axial alignment (Fig. 8C).
Histological evaluation
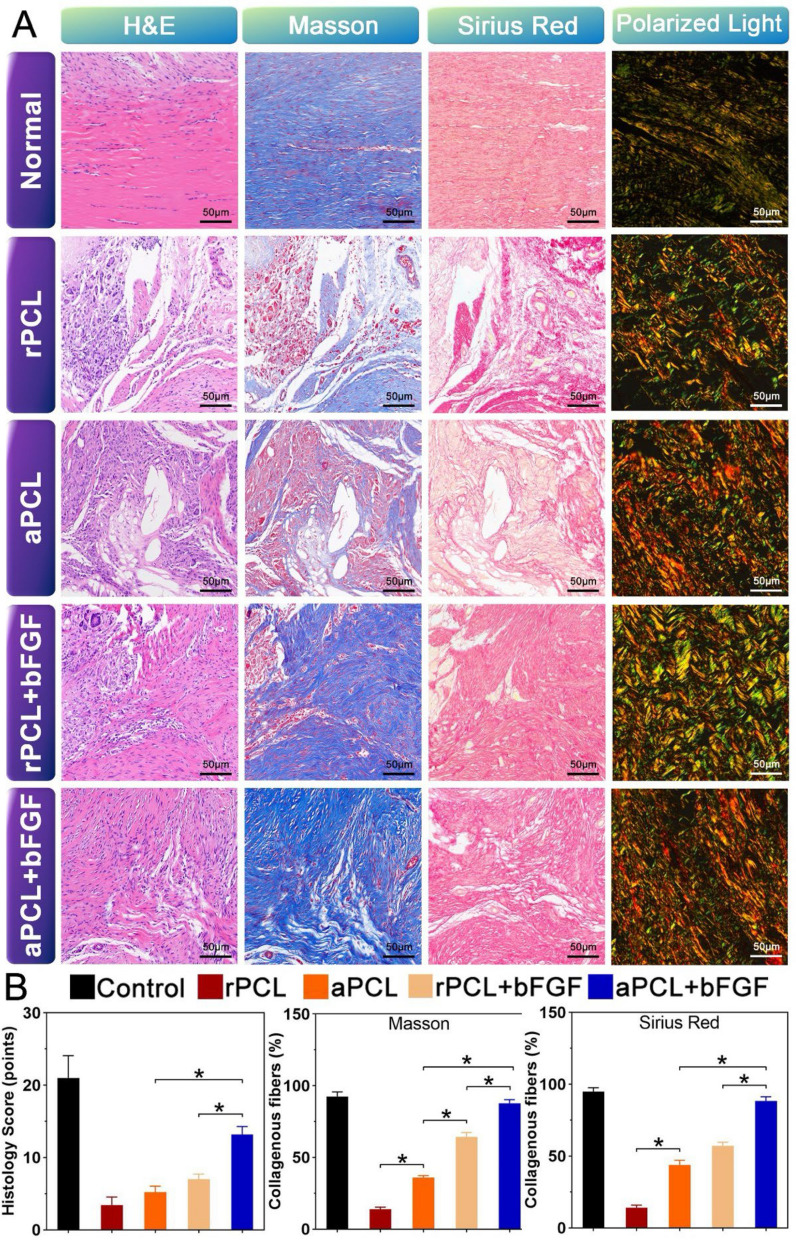
To verify the repairing effect of the nanofiber scaffolds for the Achilles tendon repairing, histological analysis was performed on the collected samples, including HE, Masson staining and Sirius red (polarized light). In the control group, corrugated and dense ECM could be observed in the Achilles tendon, with fibers arranged in parallel and rows of cells arranged along the fibers, with the characteristics of elongated nucleus. At 8 weeks after surgery, the fibers in aPCL + bFGF group were more regular than those in other groups. The ECM density of aPCL + bFGF group was higher than that of rPCL + bFGF group.
The Sirius red staining demonstrated that the expression levels of collagen type I and III in the aPCL + bFGF group were higher compared to the other groups, but still lower than those in the control group (p < 0.05). Masson staining revealed the histomorphological changes in collagen tissue recombination. The samples taken at 8 weeks exhibited two types of collagen fibers, mature collagen fibers (stained in red) and immature collagen fibers (stained in blue). The number of mature collagen fibers in the aPCL + bFGF group was significantly larger than that in the rPCL + bFGF group and equivalent to that in the control group. Additionally, the Sirius red staining combined with polarized light observation showed that the mature collagen fibers in the aPCL + bFGF group were densely arranged at a high level, resembling the structure of autologous tendon. In contrast, the rPCL + bFGF group exhibited a disordered and loose arrangement of collagen fibers (Fig. 9A, B). These results indicate that the aPCL + bFGF structure provided nearly equivalent remodeling properties to that of autologous tendon grafts, indicating its effectiveness in promoting a better tendon regeneration and tissue recombination. In conclusion, histological scores, and quantitative analysis confirm the above observations that the quality of regenerated tendon in the aPCL + bFGF group is significantly superior to other groups at 8 weeks postoperatively.
Fig. 9.
Representative histological staining of normal control tendons and regenerated tendons. A Results of hematoxylin–eosin (HE), Masson's trichrome, Sirius red staining and polarized light observation. (Scale bar = 50 μm). B Histological scores were detected by quantitative analysis of regenerated Achilles tendon 8 weeks after surgery; Quantification of collagen fibers in Masson staining and in polarized light observation of Sirius red staining, *p < 0.05
Discussion
In this study, the electrospun nanofiber scaffolds loaded with BSA-bFGF NPs were applied for the repair of Achilles tendon rupture. Different oriented nanofiber scaffolds were prepared by loading BSA-bFGF NPs into the PCL scaffold. The DLS results demonstrated that the nanoparticles were relatively homogeneous in size. The drug delivery assay confirmed that the nanofiber scaffolds exhibited sustained release properties in vitro. Furthermore, the mechanical strength of the aligned nanofiber scaffold was superior to that of the random one. Cell proliferation assays showed that the scaffolds achieved more than 80% cell viability in each group, indicating that the scaffolds were biocompatible and supported normal and highly proliferative cell growth. The live/dead staining results demonstrated that cells exhibited fine morphology and generally aligned with the orientation of the fibers. In vitro detection of tendon-related genes and proteins revealed that the bFGF-loaded aligned nanofiber scaffolds (aPCL + bFGF group) exhibited promising differentiation ability into tendon-like fibroblasts. These findings highlight the potential of the aligned nanofiber scaffolds encapsulated with bFGF as a promising approach for promoting tendon regeneration and tissue recombination in the context of Achilles tendon rupture.
BSA has tremendous advantages such as low price, biodegradable absorption, favorable biosafety, non-toxic degradation products, and immunogenicity. It has the superiority as a carrier for carrying BMP-2, VEGF, bFGF and other growth factors [34–37]. However, nanoparticles composed of BSA alone could not completely meet the requirements for tendon repair or reconstruction. The process of tendon regeneration usually took more than several months, even if it was coated with polymers such as PLGA and PLLA [38–40]. Therefore, in this experiment, we used nanofiber scaffolds loaded with nanoparticles to provide multiple guarantees for bFGF. The scaffolds not only avoided bFGF loss but also achieved the goal of sustained release. The fiber orientation and the structure of bionic tendon are a crucial point in the treatment of tendon repair. The oriented nano-fiber scaffold mimicked the microstructure of Achilles tendon, and the sustained release of bFGF functionally enhanced the ECM secretion. The synergistic effect of bFGF initially stimulated the proliferation of hAMSCs and favored the differentiation into tendon fibroblasts. In the scaffolds, the in vitro drug release results also confirmed that bFGF was released for up to 35 days, and about 80% of bFGF could still be delivered. Cell proliferation and expression of aponeurotic genes and proteins indicated that bFGF maintained its biological activity. Notably, the cell activity and proliferation on aPCL + bFGF group were significantly different from other groups, indicating the synergistic effect of bFGF and aligned fibrous scaffold was more conducive to promoting the differentiation of hAMSCs into tendon-like fibroblasts. In accordance with the stress–strain results, the maximum mechanical tensile strength of aPCL + bFGF nanofiber scaffold is decreased compared with aPCL group, while the tensile strength of rPCL + bFGF and rPCL fiber scaffolds is lower than that of oriented fibers. This result may contribute to the fiber orientation giving rise to different mechanical properties. The tenacity and mechanical characteristics of the aligned nanofiber scaffolds closely resemble the architecture and functionality of tendons.
Tendons usually contain two major extracellular matrix proteins, collagen type I and collagen type III are essential for the repair and regeneration of fibrous tissue [41, 42]. In this study, the expressions of type I and III collagen in nanofiber scaffolds of different groups were up-regulated at day 7 and 14. In addition, among non-collagen ECM proteins, fibronectin (FN1), DCN, TNC, biglycan (BGN), SCX and TNMD were up-regulated within 2 weeks. FN1 is essential for regulating the initial cell adhesion and survival, while BGN, DCN and TNC are proteoglycans that mainly function to regulate the activity of growth factors in the process of tendon repair and the assembly of collagen fibers [43–45]. SCX and TNMD are tendon-specific transcription factors that are crucial for tendon development [10, 46–50]. After 14 days of cell incubation, both SCX and TNMD proteins of hAMSCs were expressed on different scaffolds, and ECM gene expression of hAMSCs on different scaffolds was up-regulated, indicating that the released bFGF maintained its active tendinous differentiation activity at early stage. The arrangement of the fibers played a positive feedback role in the tensile differentiation of MSCs. The results showed that compared with the aPCL group, the aPCL + bFGF group could promote the tendinous differentiation of hAMSCs. This further verified the synergistic effect of bFGF and aligned nanofiber scaffolds in promoting tendinous differentiation.
Previous studies have shown cell differentiation or even the subsequent function of cells [51, 52]. In this study, the nanofiber scaffolds with slow-release growth factors in different orientations were obtained by adjusting the parameters in the electrospinning process. The aPCL + bFGF-hAMSCs hybrids showed higher expression of tendon-related genes and proteins, providing the appropriate microenvironment for promoting differentiation. In addition, the high-tension differentiation of aPCL + bFGF-hAMSCs hybrids structurally exhibited a more mature and aligned collagen matrix. The well-arranged fibrous scaffolds mimic the morphological structure of physiological ECMs of tendons, making them an ideal choice for engineered tendons [53, 54]. This study verified the feasibility of preparing bFGF-loaded bionic scaffolds for tendon repair by electrospinning and provided a promising pathway for the effective regeneration of tendon defects.
Conclusions
Combining the electrospinning technology with the specific requirements of tendon tissue engineering, we have successfully prepared electrospun nanofiber scaffolds capable of delivering bFGF in a sustained manner. The physicochemical characteristics and biocompatibility of the nanofiber scaffolds were characterized. Through in vitro tendon differentiation assays and in vivo tendon regeneration studies, the bFGF-loaded aligned nanofiber scaffolds (aPCL + bFGF group) exhibited capability of promoting the differentiation of hAMSCs into tendon cells and demonstrated a more pronounced therapeutic effect in promoting tendon repair. Moreover, the aPCL + bFGF scaffold showed strong tendon-forming ability, indicating that the aligned nanofiber scaffolds with sustained release could propose a new biological approach for promoting tendon repair.
Supplementary Information
Acknowledgements
Not applicable.
Author contributions
Yuwan Li, Zhen Ge and Ziming Liu conceptualized, carried out and interpreted the main experiments, writing-original draft. Longfei Li, Jian Song, and Honde Wang were the main contributors to the investigation and formal analysis of the work. Pengfei Lei, Long Li, and Jiajia Xue supervised and administrated the project. All authors reviewed and edited the original draft and approved the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 52221006, 52073014 and 82302853), National Key R&D Program of China (2022YFC2408200), and Fundamental Research Funds for the Central Universities (buctrc202020).
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This project was under the approval of the institutional review board at the Ethics Committee of the First Affiliated Hospital of Zunyi Medical University.
Consent for publication
All participating patients signed the informed consents before enrolled in the trial.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuwan Li, Zhen Ge and Ziming Liu contributed equally to this work.
Contributor Information
Pengfei Lei, Email: leipengfei@zju.edu.cn.
Long Li, Email: lilong@gzu.edu.cn.
Jiajia Xue, Email: jiajiaxue@mail.buct.edu.cn.
References
- 1.Sheean AJ, Arner JW, Bradley JP. Proximal hamstring tendon injuries: diagnosis and management. Arthroscopy. 2021;37(2):435–7. [DOI] [PubMed] [Google Scholar]
- 2.Skinner S, Isaacs J. Extensor tendon injuries in the athlete. Clin Sports Med. 2020;39(2):259–77. [DOI] [PubMed] [Google Scholar]
- 3.Ross RK, Kinlaw AC, Herzog MM, Jonsson Funk M, Gerber JS. Fluoroquinolone antibiotics and tendon injury in adolescents. Pediatrics. 2021. 10.1542/peds.2020-033316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kane SF, Olewinski LH, Tamminga KS. Management of chronic tendon injuries. Am Fam Physician. 2019;100(3):147–57. [PubMed] [Google Scholar]
- 5.Ruiz-Alonso S, Lafuente-Merchan M, Ciriza J, Saenz-Del-Burgo L, Pedraz JL. Tendon tissue engineering: cells, growth factors, scaffolds and production techniques. J Control Release. 2021;333:448–86. [DOI] [PubMed] [Google Scholar]
- 6.Shiroud Heidari B, Ruan R, De-Juan-Pardo EM, Zheng M, Doyle B. Biofabrication and signaling strategies for tendon/ligament interfacial tissue engineering. ACS Biomater Sci Eng. 2021;7(2):383–99. [DOI] [PubMed] [Google Scholar]
- 7.Liu Q, Zhu Y, Zhu W, Zhang G, Yang YP, Zhao C. The role of MicroRNAs in tendon injury, repair, and related tissue engineering. Biomaterials. 2021;277: 121083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shengnan Q, Bennett S, Wen W, Aiguo L, Jiake X. The role of tendon derived stem/progenitor cells and extracellular matrix components in the bone tendon junction repair. Bone. 2021;153: 116172. [DOI] [PubMed] [Google Scholar]
- 9.Migliorini F, Tingart M, Maffulli N. Progress with stem cell therapies for tendon tissue regeneration. Expert Opin Biol Ther. 2020;20(11):1373–9. [DOI] [PubMed] [Google Scholar]
- 10.Yu H, Cheng J, Shi W, Ren B, Zhao F, Shi Y, et al. Bone marrow mesenchymal stem cell-derived exosomes promote tendon regeneration by facilitating the proliferation and migration of endogenous tendon stem/progenitor cells. Acta Biomater. 2020;106:328–41. [DOI] [PubMed] [Google Scholar]
- 11.Chen CH, Li DL, Chuang AD, Dash BS, Chen JP. Tension stimulation of tenocytes in aligned hyaluronic acid/platelet-rich plasma-polycaprolactone core-sheath nanofiber membrane scaffold for tendon tissue engineering. Int J Mol Sci. 2021;22(20):11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donderwinkel I, Tuan RS, Cameron NR, Frith JE. Tendon tissue engineering: Current progress towards an optimized tenogenic differentiation protocol for human stem cells. Acta Biomater. 2022;145:25–42. [DOI] [PubMed] [Google Scholar]
- 13.Jafari A, Rezaei-Tavirani M, Farhadihosseinabadi B, Zali H, Niknejad H. Human amniotic mesenchymal stem cells to promote/suppress cancer: two sides of the same coin. Stem Cell Res Ther. 2021;12(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu QW, Huang QM, Wu HY, Zuo GS, Gu HC, Deng KY, et al. Characteristics and therapeutic potential of human amnion-derived stem cells. Int J Mol Sci. 2021;22(2):970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao S, Xiao C, Miao Y, Wang J, Chen R, Fan Z, et al. Human acellular amniotic membrane incorporating exosomes from adipose-derived mesenchymal stem cells promotes diabetic wound healing. Stem Cell Res Ther. 2021;12(1):255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Pei Z, Wang C, Li M, Zhang H, Qu J. Electrohydrodynamic 3D printing scaffolds for repair of achilles tendon defect in rats. Tissue Eng Part A. 2021;27(19–20):1343–54. [DOI] [PubMed] [Google Scholar]
- 17.Zhu C, Qiu J, Thomopoulos S, Xia Y. Augmenting tendon-to-bone repair with functionally graded scaffolds. Adv Healthc Mater. 2021;10(9): e2002269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang JN, Pelletier MR, Chylack LT Jr. Front surface fluorometric study of lens insoluble proteins. Curr Eye Res. 1988;7(1):61–7. [DOI] [PubMed] [Google Scholar]
- 19.Wu S, Liu J, Qi Y, Cai J, Zhao J, Duan B, et al. Tendon-bioinspired wavy nanofibrous scaffolds provide tunable anisotropy and promote tenogenesis for tendon tissue engineering. Mater Sci Eng C Mater Biol Appl. 2021;126: 112181. [DOI] [PubMed] [Google Scholar]
- 20.Alimohammadi M, Aghli Y, Fakhraei O, Moradi A, Passandideh-Fard M, Ebrahimzadeh MH, et al. Electrospun nanofibrous membranes for preventing tendon adhesion. ACS Biomater Sci Eng. 2020;6(8):4356–76. [DOI] [PubMed] [Google Scholar]
- 21.Sarikaya B, Gumusderelioglu M. Aligned silk fibroin/poly-3-hydroxybutyrate nanofibrous scaffolds seeded with adipose-derived stem cells for tendon tissue engineering. Int J Biol Macromol. 2021;193(Pt A):276–86. [DOI] [PubMed] [Google Scholar]
- 22.Sensini A, Gualandi C, Zucchelli A, Boyle LA, Kao AP, Reilly GC, et al. Tendon fascicle-inspired nanofibrous scaffold of polylactic acid/collagen with enhanced 3D-structure and biomechanical properties. Sci Rep. 2018;8(1):17167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uehlin AF, Vines JB, Feldman DS, Nyairo E, Dean DR, Thomas V. Uni-directionally oriented fibro-porous PLLA/fibrin bio-hybrid scaffold: mechano-morphological and cell studies. Pharmaceutics. 2022;14(2):277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leonardi L, Strocchi R, Castellani PP, Guizzardi S, Ottani V. Ultrastructural findings on collagen fibers of tendon sheaths from the rat tail. Boll Soc Ital Biol Sper. 1982;58(22):1478–84. [PubMed] [Google Scholar]
- 25.Kim HS, Sun X, Lee JH, Kim HW, Fu X, Leong KW. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv Drug Deliv Rev. 2019;146:209–39. [DOI] [PubMed] [Google Scholar]
- 26.Li L, Li Q, Gui L, Deng Y, Wang L, Jiao J, et al. Sequential gastrodin release PU/n-HA composite scaffolds reprogram macrophages for improved osteogenesis and angiogenesis. Bioact Mater. 2023;19:24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stapelfeldt K, Stamboroski S, Mednikova P, Bruggemann D. Fabrication of 3D-nanofibrous fibrinogen scaffolds using salt-induced self assembly. Biofabrication. 2019;11(2): 025010. [DOI] [PubMed] [Google Scholar]
- 28.Dong L, Li L, Song Y, Fang Y, Liu J, Chen P, et al. MSC-derived immunomodulatory extracellular matrix functionalized electrospun fibers for mitigating foreign-body reaction and tendon adhesion. Acta Biomater. 2021;133:280–96. [DOI] [PubMed] [Google Scholar]
- 29.Xue Y, Kim HJ, Lee J, Liu Y, Hoffman T, Chen Y, et al. Co-electrospun silk fibroin and gelatin methacryloyl sheet seeded with mesenchymal stem cells for tendon regeneration. Small. 2022;18(21): e2107714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li L, Zhou G, Wang Y, Yang G, Ding S, Zhou S. Controlled dual delivery of BMP-2 and dexamethasone by nanoparticle-embedded electrospun nanofibers for the efficient repair of critical-sized rat calvarial defect. Biomaterials. 2015;37:218–29. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Liu Z, Jin Y, Zhu X, Wang S, Yang J, et al. Differentiation of human amniotic mesenchymal stem cells into human anterior cruciate ligament fibroblast cells by in vitro coculture. Biomed Res Int. 2017;2017:7360354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller SA, Durselen L, Heisterbach P, Evans C, Majewski M. Effect of a simple collagen type i sponge for achilles tendon repair in a rat model. Am J Sports Med. 2016;44(8):1998–2004. [DOI] [PubMed] [Google Scholar]
- 33.Stoll C, John T, Conrad C, Lohan A, Hondke S, Ertel W, et al. Healing parameters in a rabbit partial tendon defect following tenocyte/biomaterial implantation. Biomaterials. 2011;32(21):4806–15. [DOI] [PubMed] [Google Scholar]
- 34.Khanna S, Singh AK, Behera SP, Gupta S. Thermoresponsive BSA hydrogels with phase tunability. Mater Sci Eng C Mater Biol Appl. 2021;119: 111590. [DOI] [PubMed] [Google Scholar]
- 35.Yilgor P, Sousa RA, Reis RL, Hasirci N, Hasirci V. Effect of scaffold architecture and BMP-2/BMP-7 delivery on in vitro bone regeneration. J Mater Sci Mater Med. 2010;21(11):2999–3008. [DOI] [PubMed] [Google Scholar]
- 36.Seyednejad H, Ji W, Yang F, van Nostrum CF, Vermonden T, van den Beucken JJ, et al. Coaxially electrospun scaffolds based on hydroxyl-functionalized poly(epsilon-caprolactone) and loaded with VEGF for tissue engineering applications. Biomacromol. 2012;13(11):3650–60. [DOI] [PubMed] [Google Scholar]
- 37.Zhang S, Wang G, Lin X, Chatzinikolaidou M, Jennissen HP, Laub M, et al. Polyethylenimine-coated albumin nanoparticles for BMP-2 delivery. Biotechnol Prog. 2008;24(4):945–56. [DOI] [PubMed] [Google Scholar]
- 38.Kolluru PV, Lipner J, Liu W, Xia Y, Thomopoulos S, Genin GM, et al. Strong and tough mineralized PLGA nanofibers for tendon-to-bone scaffolds. Acta Biomater. 2013;9(12):9442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu S, Peng H, Li X, Streubel PN, Liu Y, Duan B. Effect of scaffold morphology and cell co-culture on tenogenic differentiation of HADMSC on centrifugal melt electrospun poly (L-lactic acid) fibrous meshes. Biofabrication. 2017;9(4): 044106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sensini A, Gualandi C, Cristofolini L, Tozzi G, Dicarlo M, Teti G, et al. Biofabrication of bundles of poly(lactic acid)-collagen blends mimicking the fascicles of the human Achille tendon. Biofabrication. 2017;9(1): 015025. [DOI] [PubMed] [Google Scholar]
- 41.Naomi R, Ridzuan PM, Bahari H. Current insights into collagen type I. Polymers (Basel). 2021;13(16):2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Magnusson SP, Langberg H, Kjaer M. The pathogenesis of tendinopathy: balancing the response to loading. Nat Rev Rheumatol. 2010;6(5):262–8. [DOI] [PubMed] [Google Scholar]
- 43.Halper J, Kjaer M. Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv Exp Med Biol. 2014;802:31–47. [DOI] [PubMed] [Google Scholar]
- 44.Stadler BM, Maier KP. Hepatocellular carcinoma: interdisciplinary treatment concept. Praxis. 1998;87(44):1475–7. [PubMed] [Google Scholar]
- 45.Xu K, Shao Y, Xia Y, Qian Y, Jiang N, Liu X, et al. Tenascin-C regulates migration of SOX10 tendon stem cells via integrin-alpha9 for promoting patellar tendon remodeling. BioFactors. 2021;47(5):768–77. [DOI] [PubMed] [Google Scholar]
- 46.Feng W, Jin Q, Ming-Yu Y, Yang H, Xu T, You-Xing S, et al. MiR-6924-5p-rich exosomes derived from genetically modified Scleraxis-overexpressing PDGFRalpha(+) BMMSCs as novel nanotherapeutics for treating osteolysis during tendon-bone healing and improving healing strength. Biomaterials. 2021;279: 121242. [DOI] [PubMed] [Google Scholar]
- 47.Gumucio JP, Schonk MM, Kharaz YA, Comerford E, Mendias CL. Scleraxis is required for the growth of adult tendons in response to mechanical loading. JCI Insight. 2020. 10.1172/jci.insight.138295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ackerman JE, Best KT, Muscat SN, Pritchett EM, Nichols AEC, Wu CL, et al. Defining the spatial-molecular map of fibrotic tendon healing and the drivers of Scleraxis-lineage cell fate and function. Cell Rep. 2022;41(8): 111706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Delgado Caceres M, Angerpointner K, Galler M, Lin D, Michel PA, Brochhausen C, et al. Tenomodulin knockout mice exhibit worse late healing outcomes with augmented trauma-induced heterotopic ossification of Achilles tendon. Cell Death Dis. 2021;12(11):1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dex S, Alberton P, Willkomm L, Sollradl T, Bago S, Milz S, et al. Tenomodulin is required for tendon endurance running and collagen i fibril adaptation to mechanical load. EBioMedicine. 2017;20:240–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang W, Li L, Zhang D, Huang S, Jing Z, Wu Y, et al. Incorporation of aligned PCL-PEG nanofibers into porous chitosan scaffolds improved the orientation of collagen fibers in regenerated periodontium. Acta Biomater. 2015;25:240–52. [DOI] [PubMed] [Google Scholar]
- 52.Gluais M, Clouet J, Fusellier M, Decante C, Moraru C, Dutilleul M, et al. In vitro and in vivo evaluation of an electrospun-aligned microfibrous implant for Annulus fibrosus repair. Biomaterials. 2019;205:81–93. [DOI] [PubMed] [Google Scholar]
- 53.Li W, Midgley AC, Bai Y, Zhu M, Chang H, Zhu W, et al. Subcutaneously engineered autologous extracellular matrix scaffolds with aligned microchannels for enhanced tendon regeneration: aligned microchannel scaffolds for tendon repair. Biomaterials. 2019;224: 119488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goncalves AI, Rodrigues MT, Carvalho PP, Banobre-Lopez M, Paz E, Freitas P, et al. Exploring the potential of starch/polycaprolactone aligned magnetic responsive scaffolds for tendon regeneration. Adv Healthc Mater. 2016;5(2):213–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.