Abstract
Introduction
Stenotrophomonas maltophilia is an opportunistic pathogen associated with various nosocomial infections and is known for its intrinsic multidrug resistance. This study aims to provide a comprehensive overview of the epidemiology and resistance patterns of S. maltophilia in China from 2014 to 2021.
Methods
Data were extracted from the China Antimicrobial Resistance Surveillance System (CARSS) and the Blood Bacterial Resistance Investigation Collaborative System (BRICS), encompassing 1412 medical institutions across 31 provinces in China. We analyzed the prevalence of S. maltophilia in clinical isolates, focusing on specific patient populations and departments, as well as resistance profiles to recommended first-line antibiotics, including sulfamethoxazole-trimethoprim, levofloxacin, and minocycline.
Results
A total of 514,768 S. maltophilia strains were analyzed. The overall prevalence of S. maltophilia among all clinical bacterial isolates remained stable at approximately 2.1%, with higher rates observed in intensive care units and elderly patients. Resistance rates to sulfamethoxazole-trimethoprim decreased from 9.8% in 2014 to 7.5% in 2021. In contrast, resistance to levofloxacin showed a slight upward trend, increasing from 8.5% in 2014 to 9.5% in 2021. Meanwhile, minocycline resistance remained low, fluctuating marginally from 2.7% in 2014 to 1.7% in 2021.
Conclusions
This study highlights the stable prevalence of S. maltophilia in clinical settings in China and the overall low resistance rates to recommended first-line antibiotics. However, alarmingly high resistance rates were observed in specific specimen types, particularly in blood cultures, suggesting that minocycline may be the only reliable therapeutic option among the six tested antibiotics for treating such infections in China. Continuous surveillance and effective infection control measures are essential to manage S. maltophilia infections, particularly in vulnerable populations. Future research should focus on measuring the true burden of these infections and monitoring the susceptibility of the newly introduced antibiotics, such as cefiderocol.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-024-01099-7.
Keywords: Antimicrobial resistance, Epidemiology, Infection control, Nosocomial infections, Stenotrophomonas maltophilia
Key Summary Points
| Why carry out this study? |
| Stenotrophomonas maltophilia has emerged as a significant pathogen of clinical concern. While its prevalence and resistance to first-line antibiotics for the treatment of known S. maltophilia infection are increasing globally, comprehensive epidemiological data and resistance profiles in China remain poorly characterized. |
| What was learned from the study? |
| The prevalence of S. maltophilia is stable in China from 2014 to 2021, at approximately 2.1% of all clinical isolates |
| Resistance to sulfamethoxazole-trimethoprim decreased from 9.8% in 2014 to 7.5% in 2021. In contrast, levofloxacin resistance showed a slight increase but remained below 9.5%. Among the first-recommended antibiotics, minocycline exhibited the lowest resistance rate, staying below 3% throughout the surveillance period. |
Introduction
Stenotrophomonas maltophilia is a non-fermenting gram-negative bacterium commonly found in water, soil, plants, and other environments [1]. It is known to cause various nosocomial infections, including respiratory and bloodstream infections, as well as skin, soft-tissue, urinary tract, and occasionally bone and joint infections [2]. S. maltophilia ranks among the top 10 pathogens responsible for hospital-acquired pneumonia in Latin America, and the fourth leading cause of intra-abdominal infections in the Asia–Pacific region [3, 4]. According to the China Antimicrobial Surveillance Network (CHINET), S. maltophilia is the ninth most frequently isolated hospital pathogen [5]. A multicenter retrospective study in the USA identified S. maltophilia as the primary carbapenem-resistant Gram-negative bacterium in bloodstream infections [6]. Beyond its widespread prevalence, S. maltophilia is associated with high mortality rates. A recent meta-analysis reported that 40.5% of patients with bloodstream infections died as a result of S. maltophilia infection [7]. In patients with hematological malignancies, the mortality can reach as high as 71.4% [8]. For patients with hospital-acquired pneumonia, the corresponding mortality is around 50% and could even be up to 75% in some particular populations [9, 10].
Effective initial empirical treatment depends on a comprehensive understanding of the local distribution of pathogens and their antimicrobial resistance pattern. S. maltophilia is intrinsically resistant to various antibiotics, including β-lactams, aminoglycosides, and macrolides. This resistance is chromosomally encoded and enables the bacterium to resist a wide range of antibiotics, including all carbapenems, without acquiring external resistance genes. For example, the chromosomally encoded L1 provides resistance to carbapenems, L2 confers broad-spectrum resistance to β-lactams, while the overexpression of multidrug efflux pumps contribute resistance against fluoroquinolones and other antibiotic classes [11]. Additionally, the continued exposure to antibiotics has increased its resistance to previously effective drugs through acquiring resistant genes like sul1, sul2, qac, and smr, presenting significant challenges for effectively treating S. maltophilia infections [12–14]. Typically, S. maltophilia infections could be treated with sulfamethoxazole-trimethoprim and levofloxacin [15]. However, increasing resistance against these antibiotics has been observed in Southeast Asia and globally [4, 16]. Despite the introduction of the newly marketed antibiotic cefiderocol, which demonstrates strong both in vitro and in vivo activity against S. maltophilia [17], its availability in Chinese hospitals remains limited. As a result, treatments for S. maltophilia infections still rely primarily on sulfamethoxazole-trimethoprim, levofloxacin, and minocycline [18].
On the basis of data from the SENTRY Antimicrobial Surveillance Program, there has been a modest overall increase in resistance to sulfamethoxazole-trimethoprim, climbing from 2.8% in 2001–2004 to 4.3% in 2013–2016; similar increasing resistance to levofloxacin has also been documented [4]. However, within the SENTRY surveillance program, as well as other global surveillance programs, data regarding S. maltophilia in China are rarely available. As one of the world’s most populous nations facing significant antimicrobial resistance challenges, it is crucial to gain a comprehensive understanding of the epidemiology and resistance profiles of S. maltophilia in this region.
To address this gap, in the present study, we extracted data regarding S. maltophilia from the annual reports of the China Antimicrobial Resistance Surveillance System (CARSS), which collected data from 1412 medical institutions across 31 provinces in China from 2014 to 2021 [19]. Since S. maltophilia is often co-isolated with other pathogens, particularly in the respiratory tract and intra-abdominal infections, data from bloodstream infections provide more reliable information. However, as a result of the absence of specific data on bloodstream infections caused by S. maltophilia in the CARSS annual reports, we therefore incorporated data from the Blood Bacterial Resistance Investigation Collaborative System (BRICS), which focuses on tracking antimicrobial resistance in bloodstream infections across China [20].
The aim of this study is to integrate data from multiple sources to provide a comprehensive overview of the epidemiology and resistance patterns of S. maltophilia in China, thereby informing antibiotic stewardship and optimizing the clinical management of such infections.
Methods
Overview of Data Sources
In the present study, we extracted data from two national surveillance programs, the CARSS and BRICS. Initiated by the National Health Commission in 2012, CARSS encompasses 1412 medical institutions across 31 provinces in China, covering both tertiary and secondary hospitals (https://carss.cn). The CARSS surveillance program collects routine antimicrobial susceptibility testing data quarterly from participating healthcare institutions through the Bacterial Resistance Monitoring Information System. These data are compiled into annual reports that present resistance rates of clinically significant pathogens against various antibiotic classes, regardless of whether the pathogen represents colonization or infection. BRICS consists of 54 tertiary hospitals from 18 provinces in China, focusing on monitoring pathogens responsible for bloodstream infections and their corresponding resistant profiles [20]. Within this system, pathogens isolated from blood cultures and their corresponding antimicrobial susceptibility testing results were documented. For cases where the same pathogen was repeatedly isolated from a patient during one episode, only the first isolation was included in the analysis. To ensure the quality of the surveillance data, all participating institutions are equipped with accredited microbiology laboratories and adhere to standardized operational protocols. Pathogen identification was performed using either the VITEK system or MALDI-TOF mass spectrometry. Antibiotic susceptibility testing was conducted using microdilution, disk diffusion, or E-test methods, as determined by each institution, in accordance with the Clinical and Laboratory Standards Institute (CLSI) guidelines. The geographical distribution of the participating hospitals from CARSS and BRICS is depicted in the supplementary document (Fig. S1).
Data Collection and Statistical Analysis
All data used in the present study were sourced from publicly available reports by CARSS and BRICS. Detailed sources are cited in the supplementary document (Table S1). To illustrate the epidemiological trends of S. maltophilia, we extracted and calculated data on the number and percentage of S. maltophilia isolates from all clinical samples, bronchoalveolar lavage fluid, and blood cultures spanning 2014 to 2021. Given that elderly patients are at high risk for S. maltophilia infections, we also reported its prevalence within this demographic during the surveillance period. Moreover, we also extracted data on the percentage of S. maltophilia in specific departments for the year 2021, including the intensive care unit, emergency intensive care unit, respiratory intensive care unit, cardiac care unit, respiratory department, hematology department, oncology department, emergency department, infectious diseases department, neurology department, neurosurgery department, and outpatient department.
In terms of antibiotic resistance profiles, we collected antibiotic susceptibility testing results for six antibiotics (ceftazidime, chloramphenicol, ticarcillin-clavulanic acid, levofloxacin, sulfamethoxazole-trimethoprim, and minocycline) from all strains between 2014 and 2021, as these antibiotics might still exhibit activity against S. maltophilia, despite its intrinsic resistance. The resistance trends were further analyzed on the basis of specimen type, including blood, bronchoalveolar lavage fluid, peritoneal fluid, wound swab, and pleural fluid. To comprehensively illustrate the antibiotic resistance profiles, we also examined the resistance trends of S. maltophilia against the six tested antibiotics in different hospital levels (tertiary and secondary hospitals) and among elderly patients. Furthermore, we reported the resistance rates of S. maltophilia in specific departments in 2021. The epidemiology and resistant profiles of S. maltophilia are presented as numbers and percentages, with visualization prepared using Excel (Microsoft), R (RCoreTeam, version 4.1.2), and GraphPad (PRISM, version 9.0).
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. The study did not require approval because it utilized publicly available data.
Results
Epidemiological Trends of S. maltophilia in Clinical Isolates
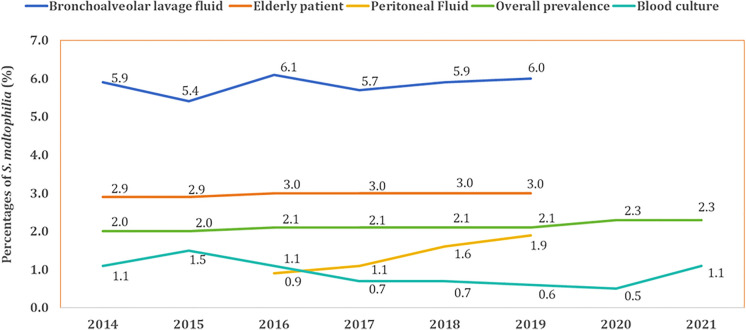
In this study, we gathered data on 514,348 strains of S. maltophilia from CARSS and 420 strains from BRICS between 2014 and 2021. Throughout the study period, the overall prevalence of S. maltophilia in all clinical isolates remained relatively stable, hovering around 2.0%. Similar stable epidemiological trends were also observed in samples obtained from blood cultures, bronchoalveolar lavage fluid cultures, and elderly patients. However, despite these stable trends, the prevalence of S. maltophilia was significantly higher in bronchoalveolar lavage fluid samples (5.4–6.1%) and among elderly patients (2.9–3.0%). Conversely, its prevalence in bloodstream infections was relatively low, ranging from 0.5% to 1.5%. Additionally, a slight upward trend was detected in peritoneal fluid cultures, with the prevalence increasing from 0.9% in 2016 to 1.9% in 2019 (Fig. 1).
Fig. 1.
Prevalence trends of S. maltophilia among different clinical isolates and special patient group (2014–2021)
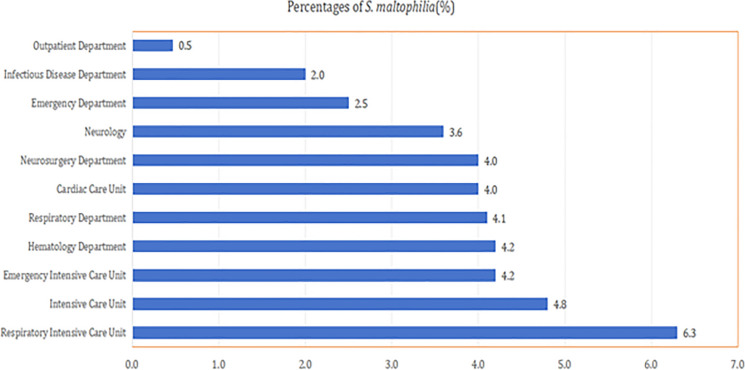
In terms of individual departments, the highest prevalence rates of S. maltophilia in 2021 were observed in critical care units. The respiratory intensive care unit led with 6.3%, followed by the general intensive care unit at 4.8% and the emergency intensive care unit at 4.2%. In contrast, lower prevalence rates were found in the emergency department (2.5%), infectious diseases department (2%), and outpatient department (0.47%). In several other specialized units, including the hematology department, respiratory department, cardiac care unit, and neurosurgery department, the prevalence rates of S. maltophilia were all around 4% (Fig. 2).
Fig. 2.
Department-specific prevalence of S. maltophilia in 2021
Antimicrobial Resistance Profiles of S. maltophilia
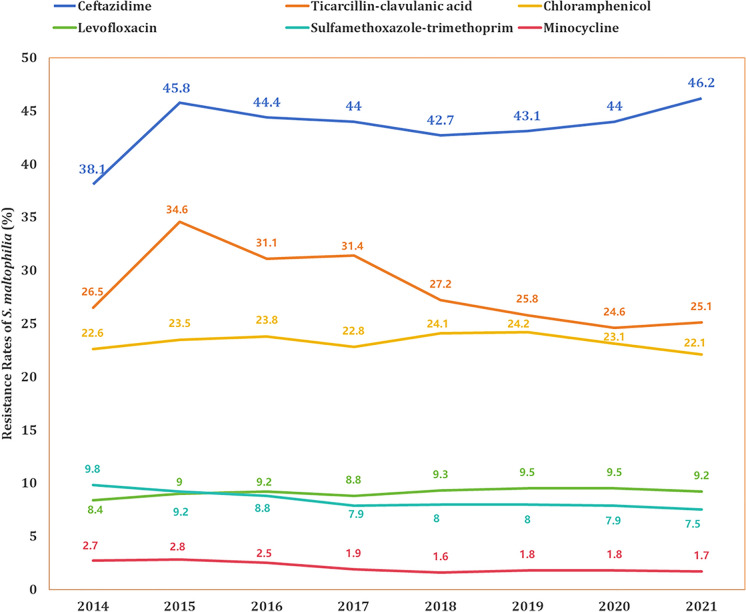
Among the 514,348 S. maltophilia strains from CARSS, we analyzed their resistance patterns and trends against six antibiotics between 2014 and 2021, as shown in Fig. 3. In 2014, 38.1% of the strains were resistant to ceftazidime, and this resistance increased to 46.2% by 2021. In contrast, resistance to ticarcillin-clavulanic acid showed a declining trend, decreasing from 34.6% in 2015 to 25.1% in 2021. Meanwhile, the resistance rate to chloramphenicol remained stable, consistently ranging between 22% and 24%.
Fig. 3.
Trends in antibiotic resistance of S. maltophilia (2014–2021)
Regarding the recommended first-line antibiotics, resistance rates were generally low. Specifically, although resistance to levofloxacin showed a slight upward trend, it stayed below 9.5% throughout the study period. Conversely, resistance to sulfamethoxazole-trimethoprim exhibited a decreasing trend, falling from 9.8% in 2014 to 7.5% in 2021. Among the recommended first-line antibiotics, minocycline demonstrated the lowest resistance rate, staying under 3% and showing a declining trend from 2.7% in 2014 to 1.7% in 2021.
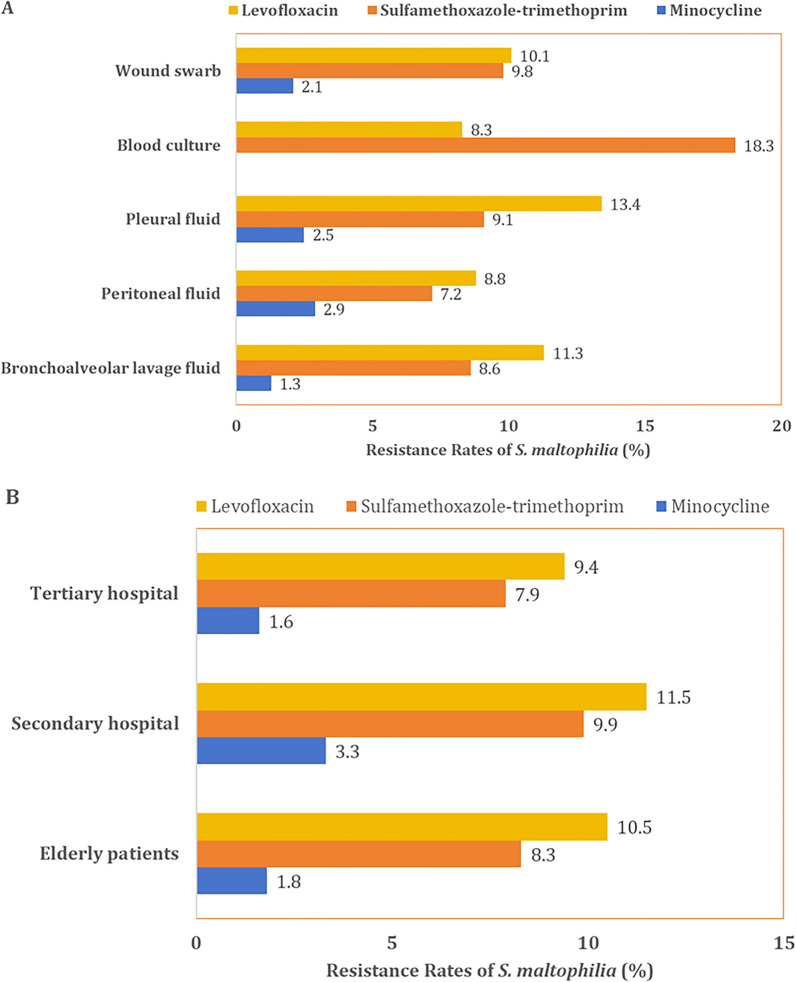
In terms of specimen sources, strains isolated from blood cultures exhibited the highest resistance rate to sulfamethoxazole-trimethoprim at 18.3%, followed by wound swabs (9.8%) and pleural fluid cultures (9.1%). For levofloxacin, the highest resistance rates were observed in patients with respiratory and thoracic infections, with 13.4% in pleural fluid cultures and 11.3% in bronchoalveolar lavage fluid cultures. Resistance rates to minocycline were consistently low across all specimen sources, with isolates from bronchoalveolar lavage fluid cultures showing the lowest resistance rate at 1.3% (Fig. 4a). We also compared the resistance rates of the recommended first-line antibiotics across different hospital levels and among the elderly patients. The results indicated that secondary hospitals exhibited the highest resistance rates to these antibiotics, followed by elderly patients, while tertiary hospitals had the lowest resistance rates (Fig. 4b). Detailed resistance trends of these antibiotics across different sources and hospital levels from 2014 to 2019 are provided in the supplementary document (Fig. S2).
Fig. 4.
Antibiotic resistance patterns of S. maltophilia across a specimen sources and b hospital levels and elderly patients in 2021
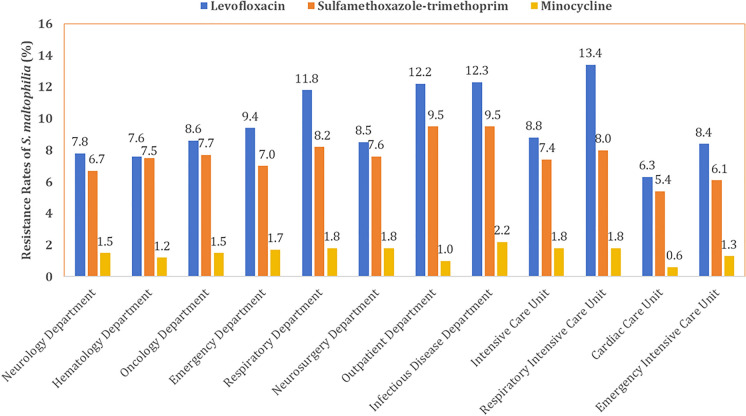
The resistance profiles of S. maltophilia exhibited variations across different departments (Fig. 5). For sulfamethoxazole-trimethoprim, the highest resistance rates were observed in the infectious disease and outpatient departments, both at 9.5%, followed by the respiratory department (8.2%) and respiratory intensive care unit (8.0%). The lowest resistance rates were found in the cardiac care unit (5.4%), neurology department (6.1%), and emergency intensive care unit (6.7%). Regarding levofloxacin, isolates from the respiratory intensive care unit, infectious disease department, outpatient department, and respiratory department showed resistance rates exceeding 11%, specifically 13.4%, 12.3%, 12.2%, and 11.8%, respectively. In contrast, resistance rates in other departments were all lower than 10%, with the lowest reported in the cardiac care unit (6.3%), followed by hematology department (7.6%) and neurology department (7.8%). Resistance rates against minocycline were generally low across all departments. The cardiac care unit (0.6%), outpatient department (1.0%), and hematology department (1.2%) demonstrated the lowest resistance rates, while the infectious diseases department reported the highest resistance at 2.2%.
Fig. 5.
Departmental antibiotic resistance profiles of S. maltophilia in 2021
Discussion
In the present study, we synthesized data from the CARSS and BRICS surveillance systems to depict the epidemiology and resistance profile of S. maltophilia in China. The analysis included data on 514,768 S. maltophilia strains collected from 2014 to 2021. The overall prevalence rate of S. maltophilia among all bacterial pathogens, including both Gram-negative and Gram-positive bacteria, was 2.1%, which is comparable to other countries [21, 22]. However, it is noteworthy that the prevalence rates were higher in certain departments, such as intensive care units, including specific care units, the respiratory department, and the hematology department. Such high prevalence aligns with findings from other studies [8, 23–26]. Since S. maltophilia is an opportunistic pathogen, it often colonizes medical devices, indwelling catheters, and respiratory epithelial cells [23]. Moreover, patients in these departments are typically at high risk of S. maltophilia infections [27, 28]. Given these two facilitators, it is unsurprising to observe the high prevalence of S. maltophilia in these departments. However, in the present study, apart from blood culture isolates which could provide reliable pathogenic evidence, the high recovery rates from other specimen types should be interpreted cautiously as they may not necessarily represent the actual burden of S. maltophilia infections [29]. Therefore, future epidemiological studies should focus on accurately measuring the true infection burden of this pathogen in infectious syndromes beyond bloodstream infections to provide a more precise understanding of its clinical impact.
Although colonization does not equate to true infection, the clinical impact of S. maltophilia colonization in certain populations should not be overlooked. In intensive care unit patients, colonization by S. maltophilia is associated with an increased risk of infection [26]. McDaniel et al. have shown that being colonized with S. maltophilia could lead to notable weight loss, immune cell infiltration, and elevated inflammatory responses in the lungs [30]. Furthermore, this colonization could increase the density of Pseudomonas aeruginosa in the lungs, potentially resulting in co-infection of these two problematic pathogens, and in turn leads to increased mortality and longer hospital stays [30]. In patients with cystic fibrosis, S. maltophilia colonization can exacerbate lung function deterioration more significantly than those without colonization [31]. Therefore, it is important to monitor and manage S. maltophilia colonization to mitigate its potential adverse effects on the vulnerable patient populations.
Regarding infection sources, the respiratory system is the primary infection site for S. maltophilia. In our study, the highest prevalence rate of S. maltophilia was observed in bronchoalveolar lavage fluid cultures, aligning with findings from the European Antimicrobial Resistance Surveillance Network (EARS-Net) annual report, which identifies S. maltophilia as a predominant cause of hospital-acquired pneumonia [32]. In contrast, the prevalence of S. maltophilia in bloodstream infections is comparatively low, with an average rate of 0.93% reported in our study. Despite this low prevalence, it remains the most common carbapenem-resistant Gram-negative bacterium causing bloodstream infections in the USA [33]. Moreover, the mortality associated with S. maltophilia in bloodstream infections is strikingly high, particularly among patients with underlying conditions. In China, a retrospective study of 56 patients with acute myeloid leukemia and bloodstream infections from 2019 to 2021 found a 30-day mortality rate of 71.4% for those infected with S. maltophilia [8]. Similarly, a study conducted in South Korea reported a comparable mortality rate, with an overall mortality of 64.5% [33]. Given the high mortality and nosocomial infection nature of S. maltophilia, effective infection prevention and control measures, along with effective therapy regimens, are urgently needed.
The intrinsic resistance of S. maltophilia to various classes of antibiotics, including carbapenems, poses great challenges to clinical treatment. As indicated in our study, the resistance rates to ceftazidime, ticarcillin-clavulanic acid, and chloramphenicol were significantly high, with 46.2%, 25.1%, and 22.1% reported in 2021, severely restricting their clinical use. Similar high resistance rates of these antibiotics have also been reported globally [34, 35]. Therefore, therapies for S. maltophilia infections primarily rely on sulfamethoxazole-trimethoprim, fluoroquinolones, and tetracyclines (tigecycline and minocycline) owing to their comparatively lower resistance rates [15]. However, alarming increases in resistance to these recommended antibiotics have been reported in other countries [34–36]. A recent retrospective study of 4466 S. maltophilia strains revealed that resistance rates to levofloxacin reached 27.7% in 2019–2022, while resistance to sulfamethoxazole-trimethoprim increased from 6.2% in 2004–2008 to 20.0% in 2019–2022 [36]. In contrast, our findings demonstrated that the overall resistance rates to these antibiotics remained stable at below 10%, with no significant increasing trends. However, we observed concerningly high resistance rates to levofloxacin and sulfamethoxazole-trimethoprim in specific specimen types, particularly in blood cultures. This highlights the need for cautious consideration when selecting these antibiotics for treating bloodstream infections caused by S. maltophilia.
Two factors may explain this discrepancy. Firstly, the higher resistance rates in other studies might be attributed to the predominance of S. maltophilia isolates originating from intensive care units, where the use of broad-spectrum antibiotics and invasive procedures could facilitate the selection of resistant strains [37]. In contrast, our study included data from the entire country across different provinces, hospitals, and departments, along with a large sample size, therefore providing a more comprehensive analysis. Secondly, the limited availability of these tested antibiotics, coupled with stringent antimicrobial stewardship programs in Chinese hospitals, likely contributes to lower resistance rates in clinical settings [18]. This hypothesis is further supported by a study of S. maltophilia isolates in China between 2005 and 2019 [38]. The study observed increased resistance to sulfamethoxazole-trimethoprim and minocycline from 2005 to 2014, followed by a decreasing trend from 2014 to 2019. This shift coincides with the enhancement of national action plans against antimicrobial resistance in 2012 [39]. Despite geographical variations in the resistance profiles of S. maltophilia, sulfamethoxazole-trimethoprim, tetracyclines (tigecycline and minocycline), and levofloxacin remain the most effective antibiotics against S. maltophilia in vitro on a global scale [34].
In addition to the aforementioned antibiotics, newly marketed antibiotics, like cefiderocol and ceftazidime-avibactam, also present good in vitro activity against S. maltophilia [40, 41]. Several in vitro studies indicate that combining two effective agents would enhance the bactericidal effect on S. maltophilia compared to monotherapy [42, 43]. Moreover, a multicenter observational study involving 307 patients with S. maltophilia pneumonia found that combination therapy was linked to reduced mortality rates among immunocompromised and critically ill patients [44]. Although combination therapy was not associated with reduced mortality in the overall patient cohort, the 2024 Infectious Diseases Society of America (IDSA) guidelines panel still recommends either using at least two active agents or the specific combination of ceftazidime-avibactam and aztreonam for S. maltophilia infections until clinical improvement is achieved, after which monotherapy may be considered [45]. However, given the relatively limited strength of the current evidence, further clinical studies evaluating various treatment regimens for S. maltophilia infections are strongly encouraged. At the current stage, it is better to follow these latest guidelines, even though it is not always the practice in many Chinese hospitals because of local constraints [18].
There are several limitations to this study. First, while we collected data on S. maltophilia strains isolated from various medical institutions across China on a large scale, we could only measure its prevalence among all isolated pathogens. Although blood culture isolates provide reliable pathogenic evidence, the high recovery rates from other specimen types should be interpreted cautiously as they may not necessarily represent the actual burden of S. maltophilia infections. Second, in the present study, we were only able to collect data specific to the pathogen itself, including the time of isolation, the department of isolation, the specimen type, and antimicrobial susceptibility testing results. This limitation restricts us to provide insights into its role in mixed infections, which is a common clinical scenario. Moreover, in the present study, we report only resistance rates rather than exact MIC values, which could provide more detailed information for clinicians. Third, although we have provided comprehensive resistance profiles across different departments, specimen types, hospital levels, and elderly patient populations, other key demographic factors and clinical outcomes were still absent. Fourth, our analysis focused primarily on susceptibility to conventional antibiotics, and information regarding the newly introduced antibiotic cefiderocol was absent. As cefiderocol is widely used in clinical settings globally, incorporating such data in future studies would aid in selecting appropriate regimens for treating S. maltophilia infections. Fifth, data sourced from the CARSS and BRICS surveillance systems may not fully represent the entire spectrum of S. maltophilia infections across all healthcare settings in China. The participating hospitals may have varying levels of resources, patient populations, and infection control practices, which could influence the resistance profiles and prevalence rates observed. However, despite these limitations, this study represents the largest analysis to date reporting the epidemiology and resistance profile of S. maltophilia in China. The insights gained from this research could significantly inform clinical practice, guiding antibiotic stewardship and optimizing treatment strategies for infections caused by this opportunistic pathogen.
Conclusions
This study provides a comprehensive overview of the epidemiology and antimicrobial resistance profiles of S. maltophilia in China from 2014 to 2021. Our findings indicate that while the overall prevalence of S. maltophilia among clinical isolates remains stable, higher rates are observed in specific patient populations, particularly in intensive care units and among elderly patients. Notably, while the overall resistance rates to the recommended first-line therapies such as sulfamethoxazole-trimethoprim and levofloxacin remain below 10%, we observed alarmingly high resistance rates in specific specimen types, particularly in blood cultures. This concerning finding suggests that minocycline might be the only reliable therapeutic option among the six tested antibiotics for treating such infections in China. Given the high mortality associated with S. maltophilia, particularly in vulnerable populations, effective infection prevention and control measures are essential. Future research should focus on accurately assessing the true burden of S. maltophilia infections and exploring the efficacy of cefiderocol, and combination therapies to improve patient outcomes. Overall, this study serves as a critical resource for healthcare professionals and policymakers in addressing the challenges posed by S. maltophilia in clinical settings across China.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge all the participating hospitals from CARSS and BRICS and all contributors to the cited sources.
Author Contributions
Design of Study and Conceptualization: Lei Zha, Qinghai You, and Yusheng Cheng. Data Collection: Lei Zha, Shirong Li, Hanli Wang, Susheng Zhou and Zhiwei Lu. Data analysis: Lei Zha, Hanli Wang, Shirong Li, Xingwu Chen, Haoyu Ji and Yixin Hu. Original Draft Construction: Lei Zha, Hanli Wang and Shirong Li. Draft Review and Scientific Revisions: Lei Zha, Qinghai You, and Yusheng Cheng. Final draft approval: all authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
The study was supported by the Special Research Start-up Fund for Recruited Talent of the First Affiliated Hospital of Wannan Medical College (YR202408), which included the journal’s publication fees, and the Key Research Project of Anhui Provincial Health Commission (AHWJ2023A10132).
Data Availability
The data utilized in this study were sourced from published literature and publicly accessible datasets. The synthesized data used in this study can be requested from the corresponding authors.
Declarations
Conflict of Interest
The authors (Hanli Wang, Shirong Li, Haoyu Ji, Yixin Hu, Susheng Zhou, Xingwu Chen, Zhiwei Lu, Qinghai You, Yusheng Cheng and Lei Zha) have no conflicts of interest to declare.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. The study did not require approval because it utilized publicly available data.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hanli Wang and Shirong Li contributed equally to this work.
Contributor Information
Yusheng Cheng, Email: chengyusheng@yjsyy.com.
Lei Zha, Email: zhalei@yjsyy.com.
References
- 1.Brooke JS. Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin Microbiol Rev. 2012;25(1):2–41. 10.1128/cmr.00019-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arthur C, Tang X, Romero JR, Gossett JG, Harik N, Prodhan P. Stenotrophomonas maltophilia infection among young children in a cardiac intensive care unit: a single institution experience. Pediatr Cardiol. 2015;36(3):509–15. 10.1007/s00246-014-1041-0. [DOI] [PubMed] [Google Scholar]
- 3.Gales AC, Castanheira M, Jones RN, Sader HS. Antimicrobial resistance among gram-negative bacilli isolated from Latin America: results from SENTRY antimicrobial surveillance program (Latin America, 2008–2010). Diagn Microbiol Infect Dis. 2012;73(4):354–60. 10.1016/j.diagmicrobio.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Gales AC, Seifert H, Gur D, Castanheira M, Jones RN, Sader HS. Antimicrobial susceptibility of acinetobacter calcoaceticus-Acinetobacterbaumannii COMPLEX and Stenotrophomonas maltophilia clinical isolates: results from the SENTRY antimicrobial surveillance program (1997–2016). Open Forum Infect Dis. 2019;6(Suppl 1):S34-s46. 10.1093/ofid/ofy293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu F, Guo Y, Zhu DM. CHINET surveillance of bacterial resistance: results of 2020. Chin J Infect Chemother. 2021;21(04):377–87. 10.16718/j.1009-7708.2021.04.001. [Google Scholar]
- 6.Cai B, Tillotson G, Benjumea D, Callahan P, Echols R. The burden of bloodstream infections due to the burden of bloodstream infections due to Stenotrophomonas maltophilia in the United States: a large, retrospective database study in the United States: a large, retrospective database study. Open Forum Infect Dis. 2020;7(5):ofaa141. 10.1093/ofid/ofaa141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C, Lin L, Kuo S. Risk factors for mortality in Stenotrophomonas maltophilia bacteremia - a meta-analysis. Infect Dis (Lond). 2024;56(5):335–47. 10.1080/23744235.2024.2324365. [DOI] [PubMed] [Google Scholar]
- 8.Wu H, Li M, Shou C, et al. Pathogenic spectrum and drug resistance of bloodstream infection in patients with acute myeloid leukaemia: a single centre retrospective study. Front Cell Infect Microbiol. 2024;14:1390053. 10.3389/fcimb.2024.1390053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guerci P, Bellut H, Mokhtari M, et al. Outcomes of Stenotrophomonas maltophilia hospital-acquired pneumonia in intensive care unit: a nationwide retrospective study. Crit Care. 2019;23(1):371. 10.1186/s13054-019-2649-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paez JI, Tengan FM, Barone AA, Levin AS, Costa SF. Factors associated with mortality in patients with bloodstream infection and pneumonia due to Stenotrophomonas maltophilia. Eur J Clin Microbiol Infect Dis. 2008;27(10):901–6. 10.1007/s10096-008-0518-2. [DOI] [PubMed] [Google Scholar]
- 11.Brooke JS. New strategies against Stenotrophomonas maltophilia: a serious worldwide intrinsically drug-resistant opportunistic pathogen. Expert Rev Anti Infect Ther. 2014;12(1):1–4. 10.1586/14787210.2014.864553. [DOI] [PubMed] [Google Scholar]
- 12.Hase R, Sakurai A, Suzuki M, et al. Clinical characteristics and genome epidemiology of Stenotrophomonas maltophilia in Japan. J Antimicrob Chemother. 2024;79(8):1843–55. 10.1093/jac/dkae168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gil-Gil T, Martínez JL, Blanco P. Mechanisms of antimicrobial resistance in Stenotrophomonas maltophilia: a review of current knowledge. Expert Rev Anti Infect Ther. 2020;18(4):335–47. 10.1080/14787210.2020.1730178. [DOI] [PubMed] [Google Scholar]
- 14.Kaur P, Gautam V, Tewari R. Distribution of class 1 integrons, sul1 and sul2 genes among clinical isolates of Stenotrophomonas maltophilia from a tertiary care hospital in North India. Microb Drug Resist. 2015;21(4):380–5. 10.1089/mdr.2014.0176. [DOI] [PubMed] [Google Scholar]
- 15.Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America guidance on the treatment of AmpC β-lactamase-producing enterobacterales, carbapenem-resistant Acinetobacterbaumannii, and Stenotrophomonas maltophilia infections. Clin Infect Dis. 2022;74(12):2089–114. 10.1093/cid/ciab1013. [DOI] [PubMed] [Google Scholar]
- 16.Sethi S, Sharma M, Kumar S, Singhal L, Gautam V, Ray P. Antimicrobial susceptibility pattern of Burkholderiacepacia complex & Stenotrophomonas maltophilia from North India: Trend over a decade (2007–2016). Indian J Med Res. 2020;152(6):656–61. 10.4103/ijmr.IJMR_9_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petraitis V, Petraitiene R, Kavaliauskas P, et al. Efficacy of cefiderocol in experimental Stenotrophomonas maltophilia pneumonia in persistently neutropenic rabbits. Antimicrob Agents Chemother. 2022;66(10):e0061822. 10.1128/aac.00618-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zha L, Li S, Ren Z, et al. Clinical management of infections caused by carbapenem-resistant Klebsiella pneumoniae in critically ill patients: a nationwide survey of tertiary hospitals in mainland China. J Infect. 2022;84(6):e108–10. 10.1016/j.jinf.2022.03.023. [DOI] [PubMed] [Google Scholar]
- 19.CARSS. http://www.carss.cn/. Accessed 22 June 2024
- 20.Chen Y, Ji J, Ying C, et al. Blood bacterial resistant investigation collaborative system (BRICS) report: a national surveillance in China from 2014 to 2019. Antimicrob Resist Infect Control. 2022;11(1):17. 10.1186/s13756-022-01055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Velázquez-Acosta C, Zarco-Márquez S, Jiménez-Andrade MC, Volkow-Fernández P, Cornejo-Juárez P. Stenotrophomonas maltophilia bacteremia and pneumonia at a tertiary-care oncology center: a review of 16 years. Support Care Cancer. 2018;26(6):1953–60. 10.1007/s00520-017-4032-x. [DOI] [PubMed] [Google Scholar]
- 22.Karlowsky JA, Adam HJ, Baxter MR, et al. In vitro activity of ceftaroline-avibactam against gram-negative and gram-positive pathogens isolated from patients in Canadian hospitals from 2010 to 2012: results from the CANWARD surveillance study. Antimicrob Agents Chemother. 2013;57(11):5600–11. 10.1128/aac.01485-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Looney WJ, Narita M, Mühlemann K. Stenotrophomonas maltophilia: an emerging opportunist human pathogen. Lancet Infect Dis. 2009;9(5):312–23. 10.1016/s1473-3099(09)70083-0. [DOI] [PubMed] [Google Scholar]
- 24.Lai CH, Wong WW, Chin C, et al. Central venous catheter-related Stenotrophomonas maltophilia bacteraemia and associated relapsing bacteraemia in haematology and oncology patients. Clin Microbiol Infect. 2006;12(10):986–91. 10.1111/j.1469-0691.2006.01511.x. [DOI] [PubMed] [Google Scholar]
- 25.Victor MA, Arpi M, Bruun B, Jønsson V, Hansen MM. Xanthomonas maltophilia bacteremia in immunocompromised hematological patients. Scand J Infect Dis. 1994;26(2):163–70. 10.3109/00365549409011780. [DOI] [PubMed] [Google Scholar]
- 26.Ibn Saied W, Merceron S, Schwebel C, et al. Ventilator-associated pneumonia due to Stenotrophomonas maltophilia: risk factors and outcome. J Infect. 2020;80(3):279–85. 10.1016/j.jinf.2019.10.021. [DOI] [PubMed] [Google Scholar]
- 27.Fihman V, Le Monnier A, Corvec S, et al. Stenotrophomonas maltophilia–the most worrisome threat among unusual non-fermentative gram-negative bacilli from hospitalized patients: a prospective multicenter study. J Infect. 2012;64(4):391–8. 10.1016/j.jinf.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Herrera-Heredia SA, Pezina-Cantú C, Garza-González E, et al. Risk factors and molecular mechanisms associated with trimethoprim-sulfamethoxazole resistance in Stenotrophomonas maltophilia in Mexico. J Med Microbiol. 2017;66(8):1102–9. 10.1099/jmm.0.000550. [DOI] [PubMed] [Google Scholar]
- 29.Torres I, Huntley D, Tormo M, et al. Multi-body-site colonization screening cultures for predicting multi-drug resistant gram-negative and Gram-positive bacteremia in hematological patients. BMC Infect Dis. 2022;22(1):172. 10.1186/s12879-022-07154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDaniel MS, Schoeb T, Swords WE. Cooperativity between Stenotrophomonas maltophilia and Pseudomonas aeruginosa during Polymicrobial Airway Infections. Infect Immun. 2020;88(4):e00855-19. 10.1128/iai.00855-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Junge S, Görlich D, den Reijer M, et al. Factors associated with worse lung function in cystic fibrosis patients with persistent Staphylococcusaureus. PLoS ONE. 2016;11(11):e0166220. 10.1371/journal.pone.0166220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.European Centre for Disease Prevention and Control. Healthcare-associated infections acquired in intensive care units—Annual Epidemiological Report for 2019. Stockholm: ECDC; 2023.
- 33.Cho SY, Lee DG, Choi SM, et al. Stenotrophomonas maltophilia bloodstream infection in patients with hematologic malignancies: a retrospective study and in vitro activities of antimicrobial combinations. BMC Infect Dis. 2015;15:69. 10.1186/s12879-015-0801-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bostanghadiri N, Sholeh M, Navidifar T, et al. Global mapping of antibiotic resistance rates among clinical isolates of Stenotrophomonas maltophilia: a systematic review and meta-analysis. Ann Clin Microbiol Antimicrob. 2024;23(1):26. 10.1186/s12941-024-00685-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mendes ET, Paez JIG, Ferraz JR, et al. Clinical and microbiological characteristics of patients colonized or infected by Stenotrophomonas maltophilia: is resistance to sulfamethoxazole/trimethoprim a problem? Rev Inst Med Trop Sao Paulo. 2020;62:e96. 10.1590/s1678-9946202062096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.AlFonaisan MK, Mubaraki MA, Althawadi SI, et al. Temporal analysis of prevalence and antibiotic-resistance patterns in Stenotrophomonas maltophilia clinical isolates in a 19-year retrospective study. Sci Rep. 2024;14(1):14459. 10.1038/s41598-024-65509-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dimopoulos G, Garnacho-Montero J, Paramythiotou E, et al. Upraising Stenotrophomonas maltophilia in critically ill patients: a new enemy? Diagnostics (Basel). 2023. 10.3390/diagnostics13061106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu LF, Xu XH, Li HR, et al. Surveillance of antimicrobial susceptibility patterns among Stenotrophomonas maltophilia isolated in China during the 10-year period of 2005–2014. J Chemother. 2018;30(1):25–30. 10.1080/1120009x.2017.1378834. [DOI] [PubMed] [Google Scholar]
- 39.Chen S, Zhang J, Wu Y. National action plan in antimicrobial resistance using framework analysis for China. China CDC Wkly. 2023;5(22):492–8. 10.46234/ccdcw2023.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakamura R, Oota M, Matsumoto S, Sato T, Yamano Y. In vitro activity and in vivo efficacy of cefiderocol against Stenotrophomonas maltophilia. Antimicrob Agents Chemother. 2021. 10.1128/aac.01436-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moriceau C, Eveillard M, Lemarié C, Chenouard R, Pailhoriès H, Kempf M. Stenotrophomonas maltophilia susceptibility to ceftazidime-avibactam combination versus ceftazidime alone. Med Mal Infect. 2020;50(3):305–7. 10.1016/j.medmal.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Biagi M, Vialichka A, Jurkovic M, et al. Activity of cefiderocol alone and in combination with levofloxacin, minocycline, polymyxin B, or trimethoprim-sulfamethoxazole against multidrug-resistant Stenotrophomonas maltophilia. Antimicrob Agents Chemother. 2020. 10.1128/aac.00559-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei C, Ni W, Cai X, Zhao J, Cui J. Evaluation of trimethoprim/sulfamethoxazole (SXT), minocycline, tigecycline, moxifloxacin, and ceftazidime alone and in combinations for SXT-susceptible and SXT-resistant Stenotrophomonas maltophilia by in vitro time-kill experiments. PLoS ONE. 2016;11(3):e0152132. 10.1371/journal.pone.0152132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen L, Hua J, Hong S, et al. Assessment of the relative benefits of monotherapy and combination therapy approaches to the treatment of hospital-acquired Stenotrophomonas maltophilia pneumonia: a multicenter, observational, real-world study. Ann Intensiv Care. 2023;13(1):47. 10.1186/s13613-023-01144-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tamma PD, Heil EL, Justo JA, Mathers AJ, Satlin MJ, Bonomo RA. Infectious Diseases Society of America 2024 guidance on the treatment of antimicrobial-resistant gram-negative infections. Clin Infect Dis. 2024. 10.1093/cid/ciae403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data utilized in this study were sourced from published literature and publicly accessible datasets. The synthesized data used in this study can be requested from the corresponding authors.