Abstract
The poultry red mite (PRM), Dermanyssus gallinae, a significant ectoparasite causing diseases in poultry, is globally prevalent and necessitates effective control strategies. There are restrictions on the use of acaricides in poultry across several nations due to worries about medication residues. Consequently, finding safe and efficient treatments for PRM is imperative. Fluralaner solution has emerged as a potential therapeutic agent, distinguished by its rapid onset, enduring efficacy, and lack of a withdrawal period for egg production. To ascertain the optimal dosage and therapeutic efficacy of fluralaner solution in PRM treatment, this study evaluated blood biochemical parameters and mite populations across various treatment groups. A cohort of 500 laying hens was randomly assigned to one of five treatment groups, each comprising 100 individuals:high-dose group (0.1 ml/kg-bw, group 4 ×, coop 1), medium-dose group (0.05 ml/kg-bw, group 2 ×, coop 5), low-dose group (0.025 ml/kg-bw, group 1 ×, coop 2), drug-control group (Intervet Productions, 0.05 ml/kg-bw recommended dose, coop 3) and blank control group (coop 4). Hens received their respective dosages of the fluralaner solution via oral administration on Days 0 (D0) and 7 (D7) of the experimental period. Over the study's ninety days, blood biochemical markers and mite counts in each coop were measured to evaluate the drug's safety, effectiveness, and ideal dosage. Data analysis was performed utilizing SPSS software. The study findings indicated that, for effective PRM infestation treatment, a dosage of 0.5 mg fluralaner per kilogram of body weight (equivalent to 0.05 ml) administered daily, followed by a subsequent dose post a 7-day interval, is recommended. Additionally, clinical observations coupled with blood biochemical assessments confirmed the safety of fluralaner across the three tested dosage levels in hens.
keywords: Poultry red mite, Fluralaner solution, Efficacy evaluation, Safety evaluation
Introduction
The poultry red mite (PRM), Dermanyssus gallinae, is an ectoparasite of significant concern within the phylum Arthropoda, class Arachnida, family Dermanyssidae, and genus Dermanyssus. This pervasive parasite adversely impacts poultry health globally. Infection rates in poultry can reach up to 94% in countries such as the Netherlands, Germany, and Belgium, and between 80% to 90% in Serbia, the UK, and Italy (Hoglund et al., 1995; Tomley and Sparagano, 2018; Baker et al., 2020, ). In China, PRM has been reported in various provinces and cities (Yin and Jia, 2010; Wang, 2015; Wang et al., 2015; Zhang et al., 2016; Jian et al., 2023). PRM is one of the worst blood-sucking ectoparasites that mostly affects breeding and laying hens.
Various acaricidal compounds, including isoxazolines, carbamates, formamidines, organophosphates, macrolides, organochlorines, and pyrethroids, have been employed to control PRM infestations (Sparagano et al., 2014). Isoxazolines, in particular, have garnered significant attention due to their broad-spectrum insecticidal activity, unique mode of action, and minimal cross-resistance. Fluralaner, a novel isoxazoline, is a γ-aminobutyric acid (GABA)-gated chloride channel blocker that targets insect nervous systems by blocking GABA-activated chloride channels, leading to hyperexcitation and convulsion in insects. As the principal inhibitory neurotransmitter in insect neural conduction, GABA is predominantly located in the central nervous system. Fluralaner exhibits a unique mode of action, offering both stomach poison and contact toxicity effects, with an extended persistence against pests(Gassel et al., 2014; Asahi et al., 2015). It has demonstrated efficacy in managing PRM. Studies by Thomas et, al. revealed that administering 0.5 mg/kg of fluralaner to hens twice, with a 7-day interval, resulted in high tolerance and efficacy (2017). Brauneis et, al. further confirmed that oral administration of fluralaner every two weeks provided long-lasting protection against PRM infestations (2017). Additional contact sensitivity tests indicated that fluralaner effectively eliminated red mite strains resistant to various commonly used pesticides (Thomas et al., 2018). Additionally, Moreover, Sleeckx et, al. reported significant improvements in production performance in flocks severely affected by PRM following fluralaner treatment (2019).
Fluralaner, a next-generation veterinary antiparasitic agent, has been extensively used for controlling external parasites in animals since Merck obtained the U.S. patent for BRAVECTO® (Susi Alteheld et al., 2019). A topical solution was introduced (FDA, 2016), followed by a combination product containing fluralaner and moxidectin (FDA, 2019). Domestic manufacturers have developed the technology for producing fluralaner chewable tablets (Liao et al., 2021) and have created fluralaner solution products for feed incorporation (Wang et al., 2020). Recently, a new injectable suspension for treating fleas and mites has been developed (Fisara and Guerino, 2023).
Following oral administration, fluralaner is rapidly absorbed, exhibits extensive tissue distribution, binds extensively to plasma proteins, and has no withdrawal period for eggs. The medication demonstrates good tolerance, low acute toxicity, and lacks genotoxic and carcinogenic properties in target animals. This study aims to evaluate the efficacy of fluralaner solution against PRM, determine the optimal dosage, reduce costs for poultry farms, and support the prevention of parasitic diseases in animals, thereby enhancing public health safety.
Materials and methods
This study was conducted following the relevant requirements outlined in the Ministry of Agriculture and Rural Affairs Good Clinical Practice for Veterinary Drugs, the Compilation of Guidelines for Veterinary Drug Research (2006-2011), the Technical Specifications for Clinical Trials (Trial) (1992) on insecticide testing, and the European Medicines Agency's “Guidelines for the Evaluation of Antiparasitic Drug Efficacy in vitro (1994), ensuring that the trial complies with the specified standards.
This study was conducted at Wangzhong Chicken Farm, located in Zhenlai Town, Zhenlai County, Baicheng City, Jilin Province, China (referred to as the 'test site'). The test site is equipped with comprehensive facilities and adheres to standardized feeding management practices, fulfilling the requirements for the conduct of this experiment. Based on the inclusion criteria for the test subjects, 500 laying hens were randomly assigned to five groups, with 100 hens per group. The groups were designated as follows: high-dosage group (0.1 ml/kg-bw, group 4 ×, coop 1), medium-dose group (0.05 ml/kg-bw, group 2 ×, coop 5), low-dose group (0.025 ml/kg-bw, group 1 ×, coop 2), drug-control group (Intervet Productions. 0.05 ml/kg-bw recommended dose, coop 3) and blank control group (coop 4).
The test medication, fluralaner solution, was produced by Luoyang Huizhong Animal Medicine Co., Ltd., and was formulated to treat PRM in layer, broiler, and breeder hens. The reference medication, also a fluralaner solution, was manufactured by Intervet Productions at their facility in France for the management and treatment of PRM.
Before treatment(D-1) and post-treatment (D6), the 4-hour water consumption and overall body weight of the hens in each group were recorded to determine the precise dosing amounts. The high-dose, medium-dose, and low-dose groups received their respective doses of 4 ×, 2 ×, and 1 × via drinking water on the dosing days (D0 and D7). The drug control group (Intervet Productions) was administered fluralaner at the recommended dose of 0.5 mg/kg body weight (equivalent to 0.05 ml/kg body weight). During the preparation of the test and control drug solutions and their administration via drinking water, small samples of the solutions were collected for drug concentration analysis. The blank control group did not receive any drug treatment.
Throughout the trial, the hens were managed following standard rearing procedures, including the regulation of feed, water, and environmental conditions such as temperature and humidity. The hens were provided with either commercial complete feed or farm-formulated feed, with no antiparasitic drugs added. Tap water was used for drinking, and the drinking water systems in each group's henhouse were independently set up to prevent interconnection.
Collection, counting, and identification of red mites
Before treatment (D-1), ten mite traps (provided by Luoyang Huizhong Veterinary Drug Co., Ltd.) were evenly fixed onto the water pipes of the chicken roosts in each henhouse, positioned near the laying hens. Each trap was numbered and its location was recorded. The traps were collected by the primary investigator after 24 hours of placement and then frozen for 48 hours before counting the red mites.
At each subsequent time point post-grouping, ten mite traps were placed in each henhouse. The traps were positioned horizontally in locations inaccessible to the test hens and as far as possible from the ventilation system.
In the laboratory, traps were sequentially removed from the freezer. Each trap was unpacked and examined. Since some mites might adhere to the traps and plastic bags, a brush was used to carefully dislodge all mites from the traps, corrugated cardboard, and plastic bags, collecting them into a 9 cm diameter petri dish. The petri dish was divided into eight equal sections by parallel lines, which served as counting markers under the microscope to prevent double counting. Red mites (including adults and nymphs) were then counted using a microscope. During the counting process, all collected red mites were subjected to morphological identification, and a subset of samples was selected for molecular biological identification.
Determination of biochemical indicators
Blood biochemical indicators are crucial for assessing and reflecting the overall metabolic and nutritional health status of animals, and they serve as significant markers of biological function (Dou et al., 2016; Li et al., 2016; Qi et al., 2016; Wang et al., 2018; Yuan et al., 2018). In this study, blood samples were collected from five hens per group one day before treatment (D-1) and on Day 8 (D8). Biochemical indicators were measured using a BS-240VET automated biochemical analyzer (Shenzhen Mindray Bio-Medical Electronics Co., Ltd.). The primary biochemical indicators assessed included: calcium (CA), urea nitrogen (UREA), glucose (GLU), alanine aminotransferase (ALT), uric acid (UA), aspartate aminotransferase (AST), total bilirubin (TBIL).
Data analysis methods
In this study, the statistical analysis unit was defined as the mite trap. To evaluate the efficacy of the acaricide against red mites, the average reduction rate of red mites in each group was calculated using the following formula: Average Reduction Rate of Red Mites (%) = 100 × (Tb – Tp) / Tb;(Tb: mean of the average number of red mites in each group one day before treatment and Tp: mean of the average number of red mites in each group at various time points post-treatment). The average mite counts included all life stages present during the feeding phase, including both nymphs (in two stages) and adults, calculated using arithmetic means.
Data analysis was performed utilizing SPSS software. Paired t-tests were applied for within-group comparisons to ascertain significant variations, whereas independent samples t-tests were implemented for comparisons between distinct groups. All hypothesis tests were conducted at a significance level of 0.05. P > 0.05 was considered non-significant, P < 0.05 was regarded as significant, and P < 0.01 was considered highly significant.
Efficacy and safety evaluation
The evaluation of efficacy and safety includes analysis of red mite counts, reduction rates of red mites, and blood biochemical data. Both red mite counts and blood biochemical data are presented as mean ± standard deviation(). Comparative analyses of intra-group variations at different time points, as well as inter-group variations at corresponding time points, were conducted to evaluate the statistical significance of these parameters. Additionally, the safety of the drug will be evaluated and summarized based on the clinical symptoms and adverse events observed during the trial.
During the trial, any animals requiring special care due to concurrent diseases, combined treatments, or those needing to be euthanized due to interrupted treatment will be meticulously documented. The necropsy and disposal of trial animals will be conducted following national regulations and the standard operating procedures for the disposal of animal carcasses at the farm.
Results
Efficacy evaluation
Identification of red mites
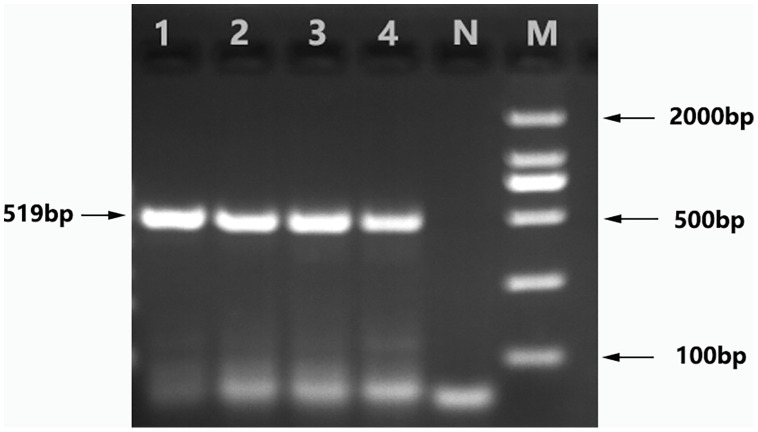
According to the key identification points outlined in Veterinary Parasitology (Song and Zhang, 2009): The body length of red mites ranges from 0.45 to 1.45 mm, with a dorsoventrally flattened body that is divided into two distinct parts: the pseudohead and the body proper. The cranium is elongated and tongue-shaped, with a pointed anterior end. The chelicerae are long, either whip-like or scissor-like, with those of the male mite having evolved into guide toes. The pincers are small in size. The shield plate typically consists of a single piece, although some mites may have two, with the posterior piece being smaller. The spiracular sulcus is slender and generally extends beyond the coxa of the third foot. Female mites have several chitin plates on their ventral surface, with the reproductive plate and web plate often fused to form the reproductive web plate. The sternum usually bears two to three pairs of setae, and the anal plate is triangular with three pairs of setae. In male mites, the chitin plate on the ventral aspect tends to fuse into a single, unified piece. The female mites' genital pore is located posterior to the sternum, whereas in males, it is situated at the anterior margin of the sternum. The life cycle of red mites includes eggs, larvae (with three pairs of legs and non-feeding), nymphs (blood-feeding), and adults. Morphological identification of the collected red mite samples was performed using a microscope, and the findings were consistent with the descriptions provided in Veterinary Parasitology, as shown in Fig. 1. Additionally, PCR methods reported in the literature (Liu et al., 2020) were used to determine the genetic sequence of the red mites, and these sequences were compared with those in the GenBank database. Gel electrophoresis of the PCR products revealed a specific band of approximately 519 bp, as illustrated in Fig. 2. The PCR products were sent to a sequencing company, and a relatively complete red mite gene sequence was obtained. This sequence was submitted to NCBI for BLAST analysis, and the results indicated that the gene sequence of the red mites showed greater than 99% similarity with the red mite gene sequences published in NCBI. This further validated the accuracy of the morphological identification results.
Fig. 1.
Morphological characteristics of red mite. (a) Dorsal view of the adult red mite.(b) Ventral view of the adult red mite.(c) Dorsal view of the red mite nymph.(d) Ventral view of the red mite nymph.
Fig. 2.
Agarose gel electrophoresis of red mite. (M: DNA marker; N: Negative control;1,2,3,4: Sample numbers).
Analysis of red mite population changes
Before treatment (D-1), the average numbers of red mites in the group 4 ×, group 2 ×, group 1 ×, drug control group, and blank control group were 810.10, 829.80, 779.60, 878.40, and 792.80, respectively. Statistical analysis indicated that these differences in red mite numbers among the groups were not significant (P > 0.05).
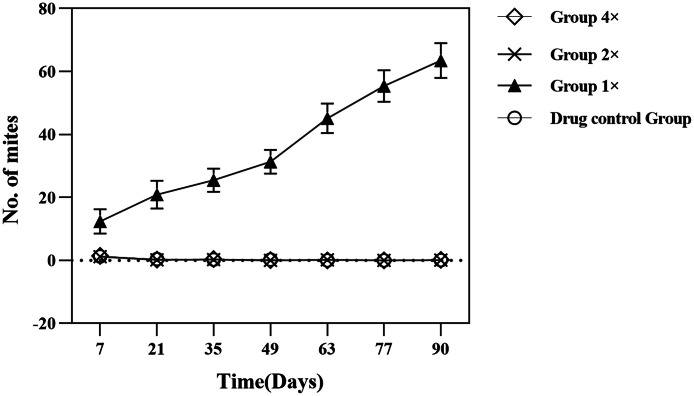
For the group 4 ×, group 2 ×, group 1 × and drug control group, the average numbers of red mites at various time points post-treatment (D7, D21, D35, D49, D63, D77, D90) showed a highly significant decrease compared to D-1 (P < 0.01). However, in group 1 ×, we observed an increasing trend in the average number of red mites at each time point after treatment, as illustrated in Fig. 3(Group4 ×:Fluralaner, 1 mg/kg bw, Group2 ×:Fluralaner, 0.5 mg/kg bw, Group1 ×:Fluralaner, 0.25 mg/kg bw, Drug control group: Intervet Productions Fluralaner, 0.05 ml/kg bw recommended dose).
Fig. 3.
Changes in mite numbers in each treatment group after administration.
Regarding the blank control group, the average numbers of red mites at time points D7, D21, D35, D49, and D63 were 780.20, 782.70, 796.80, 1172.00, and 1302.90, respectively, and these changes were not significant compared to D-1 (P > 0.05). However, it is noteworthy that at time points D77 and D90, the average number of red mites in the blank control group showed a significant increase compared to D-1 (P < 0.05), reaching 1530.70 and 1594.90, respectively.
Analysis of red mite reduction rates
At different time points (D7, D21, D35, D49, D63, D77, D90), the average reduction rates of red mites in the group 4 ×, group 2 ×, group 1 ×, and drug control group were as shown in Table 1, statistical analysis revealed that the average reduction rates of red mites in the group 4 ×, group 2 ×, and drug control group at D7, D21, D35, D49, D63, D77, and D90 were significantly higher than group 1 × (P < 0.01). Comparisons among the groups(4 ×, 2 ×, drug control group) showed no significant differences in the average reduction rates of red mites (P > 0.05).
Table 1.
The average reduction rates of red mites in each group on different evaluation days.
| Day |
Group 4 × |
Group 2 × |
Group 1 × |
Drug control group |
||||
|---|---|---|---|---|---|---|---|---|
| Number | Rate(%) | Number | Rate(%) | Number | Rate(%) | Number | Rate(%) | |
| D-1 | 810.10±555.89 | / | 829.80±572.24 | / | 779.60±527.51 | / | 878.40±554.57 | / |
| D7 | 1.40±2.37 | 99.83 | 1.20±2.30 | 99.86 | 12.40±12.36 | 98.41 | 1.30±2.41 | 99.85 |
| D21 | 0.30±0.67 | 99.96 | 0.20±0.63 | 99.98 | 20.90±14.08 | 97.32* | 0.40±0.84 | 99.95 |
| D35 | 0.40±0.70 | 99.95 | 0.30±0.67 | 99.96 | 25.50±11.69 | 96.73* | 0.30±0.67 | 99.97 |
| D49 | 0.10±0.32 | 99.99 | 0.00±0.00 | 100.00 | 31.30±11.94 | 95.99* | 0.30±0.67 | 99.97 |
| D63 | 0.00±0.00 | 100.00 | 0.20±0.63 | 99.98 | 45.10±14.93 | 94.21* | 0.00±0.00 | 100.00 |
| D77 | 0.00±0.00 | 100.00 | 0.00±0.00 | 100.00 | 55.40±15.85 | 92.89* | 0.00±0.00 | 100.00 |
| D90 | 0.20±0.63 | 99.98 | 0.10±0.32 | 99.99 | 63.50±17.46 | 91.85 | 0.10±0.32 | 99.99 |
Group4 × (Fluralenar, 1 mg/kg bw)
Group2 × (Fluralenar, 0.5 mg/kg bw)
Group1 × (Fluralenar, 0.25 mg/kg bw)
Drug control group (Intervet Productions Fluralenar, 0.05 ml/kg bw recommended dose)
D-1(One day before treatment),D7(Seven day post-treatment)
indicates a statistically significant difference in the average reduction rate of red mites among the groups (P < 0.001).
This analysis indicates that group 4 ×, group 2 × and the drug control group demonstrated highly significant efficacy in controlling red mites, whereas the effect of group 1 × was relatively limited.
Safety evaluation
Analysis of blood biochemical results
The results of blood biochemical tests showed that there were no significant differences in ALT, AST, UREA, CA, GLU, TBIL, and UA levels within the groups (4 ×,2 ×,1 ×, drug control group, and blank control group) when comparing D-1 and D8 (P > 0.05). Furthermore, no systematic changes or adverse effects on the liver function indicators (ALT, AST, UA, TBIL) and kidney function indicators (UREA) were observed in the test hens after treatment.
These findings indicate that after receiving high, medium, and low doses of the experimental drug, the test hens population did not exhibit any significant adverse reactions. As shown in Table 2, these results further confirm the safety of the experimental drug for hens at different dosage levels.
Table 2.
Before and post-treatment blood biochemical results for each experimental group.
| Group | Index |
ALT (U/L) |
AST (U/L) |
UREA (mmol/L) | CA (mmol/L) | GLU (mmol/L) | TBIL (umol/L) | UA (umol/L) |
|---|---|---|---|---|---|---|---|---|
| 4 × | D-1 | 4.46±1.56 | 196.30±43.35 | 0.19±0.08 | 3.05±0.87 | 14.20±0.87 | 6.39±2.92 | 152.62±72.43 |
| D8 | 2.52±0.97 | 192.96±18.79 | 0.61±1.15 | 3.08±0.63 | 14.00±1.92 | 6.33±0.86 | 168.02±35.38 | |
| P | 0.074 | 0.824 | 0.429 | 0.952 | 0.831 | 0.972 | 0.721 | |
| 2 × | D-1 | 5.46±2.20 | 209.22±51.20 | 0.53±0.66 | 2.55±1.06 | 14.33±2.46 | 5.59±2.65 | 109.34±59.27 |
| D8 | 3.06±0.96 | 218.40±39.29 | 0.11±0.09 | 3.28±0.97 | 13.60±0.98 | 6.17±2.28 | 161.02±40.39 | |
| P | 0.109 | 0.783 | 0.236 | 0.337 | 0.415 | 0.729 | 0.076 | |
| 1 × | D-1 | 2.92±0.79 | 190.70±49.55 | 0.22±0.11 | 2.54±0.52 | 13.84±1.37 | 5.94±1.56 | 107.00±42.62 |
| D8 | 2.46±0.29 | 159.96±18.66 | 0.12±0.08 | 2.22±0.46 | 15.72±1.45 | 5.20±3.07 | 116.64±27.06 | |
| P | 0.303 | 0.335 | 0.15 | 0.297 | 0.143 | 0.683 | 0.633 | |
| Drug control | D-1 | 8.68±5.55 | 221.60±37.04 | 1.19±1.28 | 3.13±0.47 | 14.14±1.65 | 8.67±2.35 | 178.00±76.91 |
| D8 | 2.92±0.39 | 183.34±37.92 | 0.15±0.12 | 2.71±0.54 | 13.17±0.88 | 6.98±2.81 | 177.10±82.30 | |
| P | 0.075 | 0.1 | 0.125 | 0.184 | 0.219 | 0.355 | 0.99 | |
|
Blank control |
D-1 | 4.82±1.72 | 179.54±70.94 | 0.24±0.14 | 2.49±0.38 | 13.52±1.54 | 7.50±3.85 | 173.72±76.75 |
| D8 | 2.60±0.75 | 191.42±22.43 | 0.14±0.02 | 2.88±0.97 | 12.72±1.40 | 6.19±3.66 | 163.16±20.73 | |
| P | 0.068 | 0.741 | 0.226 | 0.345 | 0.447 | 0.551 | 0.819 |
Group4 × (Fluralenar, 1 mg/kg bw)
Group2 × (Fluralenar, 0.5 mg/kg bw)
Group1 × (Fluralenar, 0.25 mg/kg bw)
Drug control group (Intervet Productions Fluralenar, 0.05 ml/kg bw recommended dose)
D-1(One day before treatment),D8 (Eight days post-treatment)
P values compare liver and kidney function indices between D-1 and D8 within each group; P < 0.05 denotes a significant difference.
Observation of clinical symptoms
The observation of clinical symptoms indicated that throughout the study period, hens in all experimental groups exhibited normal drinking, feeding, and general behavior before and after treatment, with no adverse reactions observed. This suggests that the experimental drug, whether administered at high, medium, or low doses, demonstrated good safety for hens.
Discussion
PRM poses a significant threat to the poultry industry and has become a major factor affecting the health and productivity of hen flocks worldwide. Statistical data indicate that, 64.1% of poultry farms in China report infestations of red mites(Wang et al., 2010). Mite infestations not only lead to a marked decrease in egg production but also result in substantial economic losses(Bartlow et al., 2019). In this context, developing effective acaricides has become a crucial task for the poultry industry.
Currently, there are relatively few acaricides available in the poultry market, with fluralaner emerging as a new and effective option for controlling red mites in hens (Gassel et al., 2014; Huyghe et al., 2017; Mullens et al., 2017; Hinkle et al., 2018). Fluralaner, a broad-spectrum insecticide, exerts its action by binding to the nervous system of red mites, thereby inhibiting nerve impulse transmission and inducing a lethal effect. Recent studies have demonstrated that the application of fluralaner in commercial laying hens can effectively control red mites and significantly enhance the production efficiency of poultry flocks.
Within this study, the fluralaner solution achieved an average reduction rate exceeding 99% in the high and medium dosage groups (4 × and 2 ×), exhibiting enduring efficacy for a minimum of 90 days. This indicates that the fluralaner is capable of significantly suppressing the number of red mites at high and medium concentrations and produces a long-lasting effect. This result is consistent with the findings of Soares et al., who documented fluralaner's superior efficacy in treating red mite infestations and its effectiveness in mitigating mite-induced dermatitis. The study also showed that the application of fluralaner can significantly improve the health and production performance of the hen flock(Soares et al., 2022).
Thomas further confirmed the efficacy of fluralaner against different strains of Dermanyssus gallinae (Thomas et al., 2018). Their study found that fluralaner exhibited high sensitivity towards D. gallinae strains from Europe and Brazil, with efficacy lasting up to 8 months during field trials. This result underscores the broad applicability of fluralaner across various geographic regions and environmental conditions.
In alignment with these findings, Prohaczik also supported the safety and effectiveness of fluralaner (Prohaczik et al., 2017). Their study, using the recommended treatment dose (0.5 mg/kg body weight, administered twice weekly via drinking water), assessed the fluralaner's safety in laying hens. The results indicated that fluralaner did not affect egg production or egg quality, consistent with the findings of this study. Additionally, a field study in Europe demonstrated that treatment with fluralaner significantly improved animal welfare.
Despite the excellent performance of the fluralaner in controlling red mites in hens, several potential challenges should be considered in practical applications. Firstly, the high efficacy of fluralaner may lead to the development of resistance in red mites (Shah et al., 2024). Therefore, it is recommended to integrate other control measures to mitigate the risk of resistance development. Economic analyses have also shown that using the fluralaner for red mite control is cost-effective. Secondly, varying environmental conditions may influence the effectiveness of the fluralaner. Factors such as climate, stocking density, and the health status of the flock could impact the efficacy of the drug.
Future research should focus on evaluating the long-term effects and safety of fluralaner under different environmental conditions. For example, whether the acaricidal effect of fluralaner varies with different climates and stocking densities. Additionally, studies should examine the combined use of fluralaner with other control measures, such as integrating biological control methods, to achieve better-integrated pest management outcomes (Baker et al., 2020). By employing a comprehensive approach to various control strategies, we can more effectively manage red mites in poultry and enhance overall production efficiency and animal welfare.
Conclusion
The experimental results demonstrate that varying doses of fluralaner effectively treated PRM. Specifically, the 4 × group (0.1 ml of fluralaner per kg body weight, equivalent to 1 mg/kg·bw) and the 2 × group (0.05 ml of fluralaner per kg body weight, equivalent to 0.5 mg/kg·bw) exhibited significantly superior outcomes compared to the 1 × group (0.025 ml of fluralaner per kg body weight, equivalent to 0.25 mg/kg·bw). Additionally, the efficacy of the 4 × and 2 × groups was comparable to that of the control drug, with no significant differences observed. These findings suggest that higher doses of fluralaner offer more effective and pronounced control of PRM.
Detailed data analysis revealed that both the group 4 × and the group 2 × achieved an average reduction rate of over 99% in the number of red mites post-treatment, with efficacy maintained for at least 90 days. This demonstrates that fluralaner provides a long-lasting effect in controlling red mites in hens, presenting a viable long-term solution for poultry farms. The recommended dosage is 0.5 mg of fluralaner per kg body weight (equivalent to 0.05 ml of the product), administered once daily, with a repeat dose after 7 days. This dosage and administration regimen effectively achieves optimal therapeutic outcomes in practical settings.
Further safety assessments showed that, based on blood biochemical analysis and observations of clinical symptoms during the trial, no adverse reactions were detected in the test hens. This indicates that fluralaner, when used at the recommended dosage, is safe for hens and does not have significant negative effects on flock health. The trial results support the application of fluralaner in controlling PRM, providing a safe and effective treatment option.
In summary, fluralaner is an effective red mite control agent with significant therapeutic effects and good safety profiles, offering a valuable solution for managing red mites in poultry. Future research should continue to address its long-term effects, resistance issues, and combined use with other control measures to further optimize red mite control strategies and enhance overall production efficiency and animal welfare in the poultry industry.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported by the National Key Research and Development Program of China (2023YFD1801204) and the Agricultural Science and Technology Innovation Program (ASTIP, No. IFR-06).
References
- Asahi M., Kobayashi M., Matsui H., Nakahira K. Differential mechanisms of action of the novel gamma-aminobutyric acid receptor antagonist ectoparasiticides fluralaner (A1443) and fipronil. Pest. Manage Sci. 2015;71:91–95. doi: 10.1002/ps.3768. [DOI] [PubMed] [Google Scholar]
- Baker B.P., Green T.A., Loker A.J. Biological control and integrated pest management in organic and conventional systems. Biol. Control. 2020;140 [Google Scholar]
- Bartlow A.W., Manore C., Xu C., Kaufeld K.A., Del Valle S., Ziemann A., Fairchild G., Fair J.M. Forecasting zoonotic infectious disease response to climate change: mosquito vectors and a changing environment. Vet. Sci. 2019;6:40. doi: 10.3390/vetsci6020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauneis M.D., Zoller H., Williams H., Zschiesche E., Heckeroth A.R. The acaricidal speed of kill of orally administered fluralaner against poultry red mites (Dermanyssus gallinae) on laying hens and its impact on mite reproduction. Parasit. Vectors. 2017;10:594. doi: 10.1186/s13071-017-2534-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou T.F.W.Y.W.u, Tong H.Q., Liu L.X., Gu D.H., Li Q.H., Ge C.R., Jia J.J., Xu Z.Q. Determination of blood physiological and biochemical indexes of silky fowl in Yanjin. China Poultry. 2016;38:58–60. [Google Scholar]
- FDA, 2016. NADA 141-459 . BRAVECTO® . Accessed Oct. 2024. https://animaldrugsatfda.fda.gov/adafda/app/search/public/document/downloadFoi/945.
- FDA, 2019. NADA 141-518. BRAVECTO® PLUS. Accessed Oct. 2024. https://animaldrugsatfda.fda.gov/adafda/app/search/public/document/downloadFoi/7947.
- Fisara P. Year-round efficacy of a single treatment of fluralaner injectable suspension (Bravecto QuantumTM) against repeated infestations with Ixodes holocyclus in dogs. Parasit. Vectors. 2023;16(1) doi: 10.1186/s13071-023-05951-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassel M., Wolf C., Noack S., Williams H., Ilg T. The novel isoxazoline ectoparasiticide fluralaner: selective inhibition of arthropod gamma-aminobutyric acid- and L-glutamate-gated chloride channels and insecticidal/acaricidal activity. Insect Biochem. Mol. Biol. 2014;45:111–124. doi: 10.1016/j.ibmb.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Hinkle N.C., Jirjis F., Szewczyk E., Sun F., Flochlay-Sigognault A. Efficacy and safety assessment of a water-soluble formulation of fluralaner for treatment of natural Ornithonyssus sylviarum infestations in laying hens. Parasit. Vectors. 2018;11:99. doi: 10.1186/s13071-018-2678-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoglund J., Nordenfors H., Uggla A. Prevalence of the poultry red mite, dermanyssus gallinae, in different types of production systems for egg layers in Sweden. Poult. Sci. 1995;74:1793–1798. doi: 10.3382/ps.0741793. [DOI] [PubMed] [Google Scholar]
- Huyghe B., Le Traon G., Flochlay-Sigognault A. Safety of fluralaner oral solution, a novel systemic poultry red mite treatment, for chicken breeders’ reproductive performances. Parasit. Vectors. 2017;10 doi: 10.1186/s13071-017-2480-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian Y.C.Q.G., Li S.H., Li J., Liu S.Q., Xu H.Y., Jian F.C. Species identification of mites in layer farms in some areas of Henan Province. China Poultry. 2023;45:27–32. [Google Scholar]
- Liao, H., T. Long, H. Wu, and H. Q. Ni (2021). Fluralaner chewable tablet for pet and preparation method thereof: China, CN113476419A.
- Li S.M.K.H.W., Qu L., Dou T.C., Shen M.M. Analysis of blood physiological and biochemical indexes in adult Xuhai chickens. China Poultry. 2016;38:65–67. [Google Scholar]
- Liu C.Q.H.L.i, Gao J., Yao H.J., Yi J.Z., Sun F.P. PCR diagnosis of chicken dermanyssus gallinae and the sequence analysis of 18S rRNA, ITS and 28S rRNA genes. J. Anhui Agric. Sci. 2020;48:104–106. [Google Scholar]
- Mullens B.A., Murillo A.C., Zoller H., Heckeroth A.R., Jirjis F., Flochlay-Sigognault A. Comparative in vitro evaluation of contact activity of fluralaner, spinosad, phoxim, propoxur, permethrin and deltamethrin against the northern fowl mite, Ornithonyssus sylviarum. Parasit. Vectors. 2017;10:358. doi: 10.1186/s13071-017-2289-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prohaczik A., Menge M., Huyghe B., Flochlay-Sigognault A., Traon G.L. Safety of fluralaner oral solution, a novel systemic antiparasitic treatment for chickens, in laying hens after oral administration via drinking water. Parasit. Vectors. 2017;10:363. doi: 10.1186/s13071-017-2291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi C.Y.Z.Q.J., Rong H., Teng F., Liu J.Y., Li Q.H., Yang X.D., Li J.N., Ge C.R., Jia J.J., Liu L.X. Determination of the blood biochemical indices of Zhenyuan Piao chickens from Yunnan. Heilongjiang Anim. Sci. Vet. Med. 2016:51–53. [Google Scholar]
- Shah H.K., Srinivasan V., Venkatesan S., Balakrishnan V., Candasamy S., Mathew N., Kumar A., Kuttiatt V.S. Evaluation of the mosquitocidal efficacy of fluralaner, a potential candidate for drug based vector control. Sci. Rep. 2024;14:5628. doi: 10.1038/s41598-024-56053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleeckx N., Van Gorp S., Koopman R., Kempen I., Van Hoye K., De Baere K., Zoons J., De Herdt P. Production losses in laying hens during infestation with the poultry red mite dermanyssus gallinae. Avian Pathol.: J. W.V.P.A. 2019;48:S17–S21. doi: 10.1080/03079457.2019.1641179. [DOI] [PubMed] [Google Scholar]
- Soares N.M., Tucci E.C., Perdoncini G., Junior R. Efficacy of fluralaner (Exzolt) for the treatment of natural Allopsoroptoides galli infestations in laying hens. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M.X., Zhang L.X. Science Press; 2009. Veterinary Parasitology. [Google Scholar]
- Sparagano O.A., George D.R., Harrington D.W., Giangaspero A. Significance and control of the poultry red mite, Dermanyssus gallinae. Annu. Rev. Entomol. 2014;59:447–466. doi: 10.1146/annurev-ento-011613-162101. [DOI] [PubMed] [Google Scholar]
- Susi Alteheld, B. K., E. Stefan Fuchs, G. Carina Hang, and W. Jürgen Lutz, 2019. SOFT CHEWABLE PHARMACEUTICAL PRODUCTS. United States Patent. Accessed Oct. 2024. https://ppubs.uspto.gov/dirsearch-public/print/downloadBasicPdf/11285101?requestToken=eyJzdWIiOiI0ZGY2Mzc2Mi1mYzEyLTQ2ODItYWJiNS1mMjZiZWE4MjA4YzAiLCJ2ZXIiOiJkZThlNzRmYy0yNDI3LTQ3MDMtOWU4Zi1hZjVhZTE5OTI2ZTgiLCJleHAiOjB9.
- Thomas E., Chiquet M., Sander B., Zschiesche E., Flochlay A.S. Field efficacy and safety of fluralaner solution for administration in drinking water for the treatment of poultry red mite (Dermanyssus gallinae) infestations in commercial flocks in Europe. Parasit. Vectors. 2017;10:457. doi: 10.1186/s13071-017-2390-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E., Zoller H., Liebisch G., Alves L., Vettorato L., Chiummo R.M., Sigognault-Flochlay A. In vitro activity of fluralaner and commonly used acaricides against Dermanyssus gallinae isolates from Europe and Brazil. Parasit. Vectors. 2018;11:361. doi: 10.1186/s13071-018-2956-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomley F.M., Sparagano O. Spotlight on avian pathology: red mite, a serious emergent problem in layer hens. Avian Pathol.: J. W.V.P.A. 2018;47:533–535. doi: 10.1080/03079457.2018.1490493. [DOI] [PubMed] [Google Scholar]
- Wang F.F., Wang M., Xu F.R., Liang D.M., Pan B.L. Survey of prevalence and control of ectoparasites in caged poultry in China. Vet. Rec. 2010;167:934–937. doi: 10.1136/vr.c6212. [DOI] [PubMed] [Google Scholar]
- Wang, M., X. J. Cheng, W. Zhang, N. Zhang, J. Wang, P. P. Zhen, X. T. Yu, Z. G. Cui, and L. Zhang (2020). A compound flurellana suspension and its preparation and application.
- Wang Q. Diagnosis and treatment of a case of dermatophagoides gallinarum. Chin. J. Anim. Husbandry Vet. Med. 2015:34. [Google Scholar]
- Wang Y.T.R.F.Z., Liu Q., Zhang J.W., Pan B.L., Liu X.D., He H.X., Ma Z.J., Guo F. Diagnosis and prevention of dermatophagoides gallinae. Poultry Husbandry Dis. Control. 2015:17–18. [Google Scholar]
- Wang Z.M.Y.O.X.u, Yang L., Xu L.Y., Wang Y.M. A study on the physiological and biochemical indicators of Tibetan chickens in different altitude areas. Anim. Husbandry Feed Sci. 2018;39:1–7. [Google Scholar]
- Yin F.Q., Jia Z.J. Diagnosis and prevention of dermatophagoides gallinae. Anim. Sci. Abroad(Pigs Poultry) 2010;30:70–71. [Google Scholar]
- Yuan J.Q.H.W.u, Xu M.C., Qiu T.W., Liu W., Zhu C.B., Tian Y.G., Zhang J.N., Gu W.W. Analysis and measurement of blood physiological and biochemical parameters in Tibetan chickens bred in Guangzhou. Chin. J. Comparat. Med. 2018;28:69–72. [Google Scholar]
- Zhang X.X.Q.L.M., Qiao J., Liu T.L., Gon S.S., Cai X.P., Chen C.F. Morphology identification and comparison of cox1 geneofDer manyssus gallinae in Shihezi, Xinjiang. J. Shihezi Univ.: Nat. Sci. 2016;34:30–35. [Google Scholar]