Abstract
The widespread occurrence of acidic soils presents a major challenge for agriculture, as it hampers productivity via a combination of mineral toxicity, nutrient deficiency, and poor water uptake. Conventional remediation methods, such as amending the soil with lime, magnesium, or calcium, are expensive and not environmentally friendly. The most effective method to mitigate soil acidity is the cultivation of acid-tolerant cultivars. The ability of plants to tolerate acidic soils varies significantly, and a key factor influencing this tolerance is aluminum (Al) toxicity. Therefore, understanding the physiological, molecular, and genetic underpinnings of Al tolerance is essential for the successful breeding of acid-tolerant crops. Different tolerance mechanisms are regulated by various genes and quantitative trait loci in various plant species, and molecular markers have been developed to facilitate gene cloning and to support marker-assisted selection for breeding Al-tolerant cultivars. This study provides a comprehensive review of the current developments in understanding the physiological and molecular mechanisms underlying Al resistance. Through the application of genome-wide association methods, it is expected that new Al-resistant genes can be identified and utilized to cultivate Al-resistant varieties through intercrossing, backcrossing, and molecular marker-assisted selection, promoting the sustainable use of acidic soils.
Keywords: Acidic soils, Aluminum toxicity, Agriculture, Remediation methods, Acid-tolerant cultivars, Soil amendment
1. Introduction
Acidic soils (pH < 5.5) pose a significant challenge to global agriculture [1]. Approximately 2.5 billion hectares of arable and potentially arable land, accounting for 40%–50% of the world's potentially arable land, are acidic in nature [2]. However, mineral nutrient deficiencies, such as phosphorus (P) deficiency, aluminum (Al), manganese (Mn), and proton (H+) toxicities, and other stress factors in acidic soils restrict crop production and hinder the full exploitation of their agricultural potential [3]. Paddy soil acidification has become an increasingly serious problem, exacerbated by the excessive application of nitrogen (N) fertilizers [4]. Long-term fertilizing experiments on red soil in Hu'nan Province have demonstrated that soil acidification results in severely reduced crop growth or even crop failure [5]. Soil acidification is likely to remain a significant problem in southern China's agricultural production in the future. Therefore, it is of utmost importance to alleviate the adverse effects of acidic soils on crop growth and to promote the sustainable utilization of acidic soils for maximum crop production potential.
1.1. Distribution of acidic soils
Acidic soil is a common phenomenon and is primarily found in two belts: the southern belt in the hot and humid tropical regions, which encompasses regions such as New Zealand, Australia, South America, and South Africa, and the northern belt in the cold and humid temperate zone, including Russia, South Asia, and North America [3]. Soil acidity affects approximately 3950 million acres of arable land, with 60% of these soils located in the subtropics and tropics, where many developing countries are located [3]. Significant portions of farmland in Sub-Saharan Africa (56%), Latin America (31%), Southeast Asia (38%), East Asia (20%), and some parts of North America are affected by soil acidity [6], [7]. In the Americas, a total of 1616 million hectares of land are affected, primarily in South America, and approximately 239 million hectare of agricultural land in New Zealand and Australia are acidic [3]. In China and India, 212 million acres of agricultural land are categorized as acidic, with China's acidic soils mainly distributed across 15 provinces (municipalities and autonomous regions) in the red soil areas of southern China, covering 2.18 million km2 and accounting for 22.7% of the country's total land [8]. Acidic soils are most commonly found in areas with high temperatures and rainy conditions, which are also suitable for agricultural production, thereby offering substantial crop production potential.
1.2. Causes of acidification in soil
The acidification of soil is caused by a range of agricultural and industrial practices. Such practices include acid rain [9], the use of acid-forming nitrogen fertilizers [10], and organic matter degradation [11], all of which contribute to increasing soil and natural acidity. The H+ ions present in acid rain interact with soil cations and displace them from their original binding sites, causing a reduction in cation exchange capacity and an increase in H+ concentrations in soil water, leading to leaching [12]. Harvest and removal of crops from the ground also leads to loss of some basic components that maintain the acidity of the soil, which contributes to an increase in soil acidity. According to Guo et al. [10], intensive farming practices and excessive use of N fertilizers are responsible for the widespread soil acidity in China.
1.3. Harmful effects of acidic soils
Heavy metal toxicity, nutrient deficiencies, and soil acidity are significant contributors to the toxicity of acidic soils. As soil pH decreases, the availability of soil nutrients is negatively affected due to high levels of H+ ions [13]; this results in a reduction of nutrient availability. Furthermore, heavy metals such as zinc (Zn), Mn, copper (Cu), Al, and iron (Fe) become more soluble at low pH levels, which can be harmful to plant growth as these heavy metals are not a significant portion of plant-growth requirements and many of them can be hazardous or toxic [14].
Acid sulfate and acidic organic soils are particularly harmful to plants because they have a pH below 4 and significant quantities of H+ [1]. In China, Al toxicity is considered to be more detrimental than H+ for two main reasons: first, Al ions are the primary elements in soil solution that reduce the ability of plants to absorb nutrients; second, most acidic soils in China have pH values above 4 [1].
1.4. Mechanisms of plant adaptation to acidic soils
Throughout the course of evolution, plants have developed various mechanisms of tolerance to adapt to acidic soils. Al toxicity is widely recognized as the main limiting factor for crop yields in acidic soils worldwide [15], particularly in regions where food security is vulnerable. Therefore, research on Al toxicity has garnered significant attention and become the most extensively studied aspect of this issue, as it constitutes a global agronomic challenge.
1.5. Sustainable use of acidic soil: breeding acidic soil tolerant plants
For a long time, increasing soil pH has been the primary focus of agronomic strategies to address the toxicity of acidic soils, with the most commonly used technique being the application of lime. The annual use of lime in Western Australia increased by 57,143 tons from 2004 to 2010 [16]. Lime is added to soil to reduce Al absorption and improve cell defenses [17], [18]. Despite its success in enhancing plant development, this approach has several disadvantages. The topsoil pH can be increased by applying lime; however, the subsoil where Al toxicity is often present remains unaffected, hindering the ability of plant roots to expand deeply and absorb nutrients and water. Additionally, in developing countries, the cost of application of significant quantities of lime to acidic soils may pose a challenge. While the toxicities of H+, Al, and Mn can be reduced by adding lime, deficits in other minerals such as N and P persist [4,19]. Therefore, caution must be exercised when using lime to address soil acidity.
The toxicity of acidic soils has been traditionally addressed through increasing soil pH by the application of lime. However, this method has limitations such as the difficulty for plant roots to expand deeply and the persistence of mineral deficits, such as N and P. An alternative solution is the application of Magnesium (Mg), which may increase the outflow of organic acids [20], although it may become toxic when present in excess [21]. Other chemicals, such as boron (B) and silicon (Si), have also been proposed to mitigate Al toxicity [22], [23], but their effectiveness may depend on the species or genotype. However, the most effective way to deal with Al toxicity remains the development of plants that are more tolerant to acidic soils. Conventional breeding techniques have been successful in breeding Al-resistant crops. For instance, cereals can be bred for acidic soil tolerance using conventional breeding techniques such as intercrossing, backcrossing, topcrossing, and single-seed descent. Breeding of acidic soil-tolerant crops is becoming more successful owing to developments in molecular methods, such as marker-assisted selection. For more than 60 years, researchers have exploited the significant genetic diversity of Al resistance among various crop varieties to successfully breed Al-resistant crops [24], [25], particularly in tropical breeding programs [26]. Owing to the agronomic importance of acidic soils, a large number of studies have been completed in the past 30 years on the genetically based physiological and molecular biology of Al resistance in crops to identify Al resistance genes and their underlying mechanisms [27], [28], [29], [30]. Therefore, the aim of this review was to complement and improve conventional breeding programs to produce Al-resistant crops through genetically based biotechnological methods and/or molecular breeding.
In depth research into Al resistance mechanisms has revealed that the regulation and triggering of cellular processes play a crucial role in a plant's response to Al toxicity. The transcription of genes responsible for Al resistance is triggered by Al, as well as the activation of post- transcription and post-translational pathways, such as regulatory pathways and/or proteins associated with Al resistance. In the present review, we focus on the recent advancements in research regarding the role of plants in Al resistance through the regulation of Al sensing and signaling.
2. Effects of Al toxicity on plant growth
Al is the third most extensive and widely distributed metal element in the Earth's crust, accounting for 7% of its mass; however, its role in biology remains largely unknown [30]. As a component of mineral soils, its harmless aluminosilicate form, gibbsite, is present in nearly all living organisms [31]. The acidification of soil below a pH of 5 leads to the dissolution of Al to a certain extent, resulting in the dissolution of monomeric Al3+, which also creates mononuclear species, including Al(OH)4−, Al(OH)3, AlOH2+, and Al(OH)2+ [32]; although Al3+is regarded as the most poisonous form [30]. In plants, the onset of Al toxicity manifests as a rapid suppression of root elongation, with root tips being the most sensitive sites [28]. Moreover, the distal transition zone of root tips, which serves as a key site for sensing and responding to environmental stimuli or endogenous phytohormones, was found to be the root's most Al-sensitive area. [33], [34].
3. Al-resistant mechanisms in plants
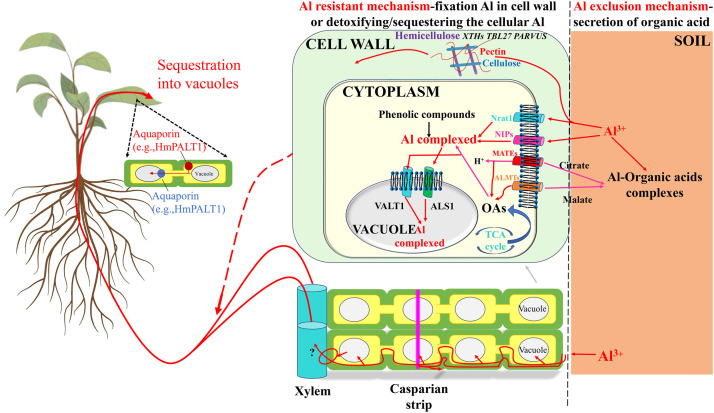
Studies on Al-resistance mechanisms in plants initially focused on physiological aspects, leading to the identification of two main Al resistance mechanisms (Fig. 1). The first mechanism, known as the Al exclusion mechanism, involves the prevention of Al entry into the roots, both symplasm and apoplasm, through the secretion of organic acids. The second mechanism, referred to as the Al-resistant mechanism, involves the fixation of Al to the cell wall or detoxification/sequestration of cellular Al. In this review, we focused on the previously identified and/or proposed physiological and molecular Al resistance mechanisms outlining the different mechanisms through which Al3+ enters the roots.
Fig. 1.
Model generalizing the plants’ Al resistant mechanisms of exclusion and internal detoxification with some modifications according to Kochian et al. [2]. Upon the absorption of Al3+ by roots from the soil solution, a portion of the Al is subsequently transferred and accumulated in the shoots. The chelation of Al3+ by organic acids largely occurs in the upper root, where Al exclusion and internal Al tolerance processes are established. Root exudation of organic acid anions through multidrug and toxic compound extrusion (MATEs) or Al-activated malate transporters (ALMTs) localized in the plasma membrane are key components of root Al exclusion mechanisms. The influx of Al3+ into the cytosol occurs through nodulin 26-like intrinsic protein (NIP) Al transporters and/or natural resistance-associated macrophage proteins (Nramps). Finally, Al (either an Al-chelate complex or free Al3+ ion) is transported into the vacuole, possibly through the action of a vacuolar Al transporter (VALT) aquaporin transporter or an Al-sensitive 1-type ATP-binding cassette (ALS1-type ABC) transporter. Red arrows indicate Al influx into the cells, whereas green arrows indicate organic acid anion effluxes. The capacity of the cell wall to fix Al3+ is modulated by plant-mediated changes in cell-wall components, including hemicellulose rather than pectin, which constitutes an additional mechanism of Al tolerance. The translocation of Al organic acid compounds to the shoot occurs through an unidentified mechanism, in which, either Al or Al-chelate complexes are loaded to the xylem. This translocation, largely stored in leaf vacuoles, occurs in the Al accumulating plant species like Hydrangea macrophylla, through transport procedures involving permeases such as HmVALT, Hydrangea macrophylla plasma membrane Al transporter 1 (HmPALT1), and aquaporins. Abbreviations: Nrat1, Nramp Al transporter 1; OA, organic acid; TCA, tricarboxylic acid.
3.1. Al exclusion mechanism
To date, the most well-documented exclusion mechanism of Al resistance in plants is the secretion of organic acids from roots into the rhizosphere. This mechanism is Al-dependent and operates by chelating Al3+ ions and forming nontoxic compounds that do not penetrate the roots. Studies dating back to the 1990s have shown a correlation between increased Al resistance in wheat (Triticum aestivum) and snap beans (Phaseolus vulgaris) with their secretion of malate and citrate [35], [36], [37]. Since then, numerous studies have shown a strong connection between plant resistance to Al toxicity and the exudation of organic acids, including malate, oxalate, and citrate [38]. For example, Lespedeza bicolor's primary defense against Al toxicity involves the secretion of malate and citrate during Al stress to protect the root apex from Al [39].
Furthermore, the Aluminum Tolerance Organic Acid transporter (TaALMT1) gene, first discovered in wheat, encodes a malate efflux transporter that contributes significantly to Al resistance [40]. The TaALMT1 gene has been found to belong to a new transporter family and regulates the selective efflux of malate. For instance, TaALMT1 in tobacco (Nicotiana tabacum) and Xenopus oocytes indicated that TaALMT1 regulates the selective efflux of malate rather than other anions [41], [42]. Orthologs of TaALMT1 have been identified in various other plant species, including BnALMT1 and BnALMT2 in rape [43], AtALMT1 in Arabidopsis [44], HvALMT1 in barley [45], ZmALMT2 in maize [46]. Another Al-resistance gene, MATE, which encodes citrate transporters, was identified in barley [47] and sorghum [48], and its homologue were then identified as OsFRDL4 (a MATE family citrate transporter) in rice [49], VuMATE1 in rice bean [50], AtMATE1 in Arabidopsis [51], and ZmMATE1 in maize [52]. The expression of VuMATE1, SbMATE or ZmMATE1 in Xenopus oocytes has been shown to alter the efflux of citrate through a Na+ or citrate/H+ antiport mechanism [48,50,52]. Of note, the structures and functions of ALMT and MATE are quite different, with the transport characteristics of MATE, such as SbMATE, remaining elusive compared to those of ALMT.
3.2. Al resistant mechanism
Al toxicity is a major abiotic stress that affects plant growth and productivity, particularly in acidic soils. Notably, Al-resistant plant species, such as rice (Oryza sativa), do not secrete organic acids [53]. The Al-resistance mechanisms in plants vary and involve detoxification mechanisms associated with cell wall fixation, which accounts for 85% and/or 99.9% of root Al [54], the incorporation of Al into the plant and its subsequent sequestration or translocation, and modification of cell wall properties. The root cell wall, which is composed of cellulose, pectin, and hemicellulose, is the first site of Al interaction with the plants [55]. Al3+ can not only adsorb to uncharged hemicellulose polymers but also electrostatically interact with the negatively charged carboxyl groups of pectin [56]. This interaction could poison cell wall enzymes, including expansins, alter cell wall properties such as plasticity and/or elasticity [56], and change cell wall composition and/or structure, which is modified by xyloglucan endotransglucosylase/hydrolases (XTHs), pectin methylesterases (PMEs), expansins, and endo-β−1,4-glucanases [57]. The primary Al-binding site of the cell wall has been identified as pectin due to the presence of negatively charged carboxylic groups obtained through the action of PME in pectin. The higher the PME activity, the higher the negative charge in pectin, which in turn increases Al resistance in rice [58], [59], maize [60], and potato (Solanum tuberosum) [61]. However, recent studies have demonstrated that hemicellulose, particularly xyloglucan, acts as the primary Al-binding site in the cell wall [57,62]. Any strategy that could affect the content and structure of xyloglucan can affect the Al-binding capacity of the cell wall and ultimately influence the Al resistance of plants.
Several genes and proteins have been found to play a role in Al resistance in plants. For example, the loss function of either AtXTH31 or AtXTH17 affects the Al resistance of Arabidopsis by influencing the activity of the xyloglucan endotransglucosylase (XET) enzyme, which causes hemicellulose polymers to split and rejoin during cell expansion [63], while the loss function of TRICHOME BIREFRINGENCE-LIKE27 (AtTBL27) decreases the O-acetylation degree of xyloglucan to alter the cell wall's Al binding capacity [64]. Modification of α-d-glucuronic acid (GlcA) side chains in glucuronoxylan, which is controlled by the GALACTURONOSYLTRANSFERASE-LIKE1 (AtGATL1)/ AtPARVUS that belongs to the GAUT1-related gene family, is involved in Arabidopsis' Al resistance through regulating its cell wall's Al binding capacity [65]. Sensitive to Al rhizotoxicity 1/2 (OsSTAR1/2, encodes an ABC transporter protein that forms a complex to regulate the efflux of UDP-glucose into the cell wall and is associated with the Al-binding capacity of the cell wall in rice [66].
In addition, Al transporters have been found to play a role in Al resistance in plants, including the plasma membrane and tonoplasts. Despite the widespread idea that symplastic Al is extremely toxic to plants, the crop species rice can tolerate high Al in the cell wall, a place where a specific protein, the natural resistance-associated macrophage protein OsNRAT1, transports Al to the cytoplasm [67], [68], where it is subsequently sequestered to the vacuole through the action of the vacuolar ABC transporter OsALS1 in rice [69] and HmVALT in hydrangea [70]. Buckwheat (Fagopyrum esculentum Moench.), which is a common crop in China's red soils and accumulates Al, detoxifies Al through oxalate complexation and vacuole sequestration [71]. Through this novel mechanism, NRAT1 may confer Al resistance by reducing toxic Al in the cell wall, and both NRAT1 and its orthologs could be useful tools to improve Al resistance in plants. Furthermore, the plasma membrane-localized Mg uptake transporters Oryza sativa MAGNESIUM TRANSPORTER1 (OsMGT1) and OsCDT3 (a small cysteine-rich peptide) are required for Al resistance in rice [72], [73]. These findings highlight the complexity of Al resistance mechanisms in plants and suggest that a combination of different strategies may be necessary to effectively improve Al resistance in crops. Further research is needed to better understand the molecular and biochemical mechanisms underlying Al resistance in plants.
4. Al-induced signaling in plant cells to regulate al resistant proteins/genes
The regulation of gene expression and protein function is a crucial aspect of Al resistance in plants, which has been the subject of extensive research in earlier studies. Numerous studies have shown that Al exposure can induce the transcription levels of several genes in various plant species including OsFRDL2/4 [49,74], OsSTAR1/2 [66], OsCDT3 [73], OsALS1 [69], OsNRAT1 [68], OsMGT1 [72], OsEXPA10 (an expansin gene) [75], ABSCISIC ACID, STRESS AND RIPENING (OsASR5) [29], and OsART2 in rice [76], ZmMATE1 in maize [52], AtMATE/AtALMT1 in Arabidopsis [51], and VuMATE in rice bean [50], among others. Al also plays a role in post-transcriptional enhancement and/or activation of Al-resistant proteins activity; for example, Al has been shown to enhance the efflux of malate that was regulated by TaALMT1 [77]. Although the mechanism by which Al triggers this enhancement remains unknown, researchers have proposed two possible explanations. One is that Al interacts directly with the TaALMT1 protein, while the other is that Al may bind to an unknown Al sensor in the plasma membrane, which may directly regulate SENSITIVE TO PROTON RHIZOTOXICITY 1 (AtSTOP1) and OsART1 (Fig. 2) [56].
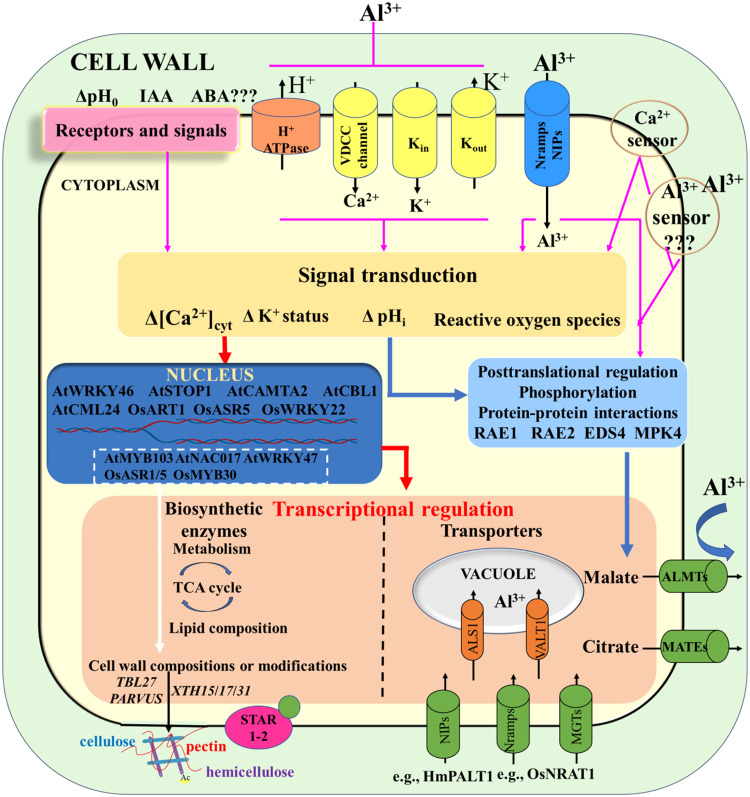
Fig. 2.
Illustration of the involvement of a general signaling pathway in perception of Al stress in plant roots with some modifications according to Kochian et al. [2]. Perception of Al stress (as indicated by purple arrows) can occur through various pathways, including the direct interaction of Al3+ ions with one or more putative plasma membrane Al receptors (upper right corner of the model), or indirectly through the increased cytoplasmic Al levels caused by Al3+ influx via plasma membrane Al transporters, such as NIPs and NRAMPs, and indirectly through plasma membrane signal transducers such as Ca2+ sensors (upper right) and hormones (upper left). Inhibition of membrane transport by Al may also play a role. These modifications potentially trigger signal transduction cascades leading to the activation of mechanisms resulting in Al resistance through two main processes. One is an Al-induced transcriptional activation of membrane transporters and biosynthetic enzymes, which underlie Al exclusion and internal Al resistance mechanisms (red arrows). This process is influenced by transcription factors such as AtSTOP1, AtWRKY46 (a WRKY domain-containing transcription factor), AtCAMTA2, AtCBL1, and AtCML24 in Arabidopsis, and OsART1, OsWRKY22, OsASR1/5, and OsMYB30 (an R2R3-MYB family transcription factor) in rice, which are involved in the relationship between Al perception and the control of their downstream genes. The other mechanism involves posttranslational Al activation and/or regulation at the protein functional level (blue arrows). This involves the activation of organic acid efflux transporters by Al, through direct binding to the transport protein, or indirectly, possibly through procedures such as protein phosphorylation and dephosphorylation and/or other protein-protein interactions that modify the activity of the transporter. Abbreviations: IAA, indole-3-acetic acid; VDCC, voltage-dependent calcium channel.
Furthermore, the entry of Al into the plant cytosol requires specific transporters located in the plasma membrane, including HmPALT1 in hydrangea, which belongs to the NIP protein subfamily [70], OsNRAT1 in rice [68], and voltage-dependent cation channels generally responsible for Ca2+ influx, which can activate regulatory pathways or signaling. Al disrupts or alters a series of cytosolic homeostatic processes, including K+, pH, and Ca2+, which in turn can trigger downstream events in the Al-signaling process [26]. Several studies have uncovered the key involvement of cytoplasmic Ca2+ in Al stress [78], [79], [80]. In root cells, Al exposure rapidly induces fluctuations in free cytosolic calcium ([Ca2+]cyt) and activates [Ca2+]cyt transients with biphasic and monophasic characteristics, mainly in the transition zone of the root apexs [79,81]. This rapid increase in endogenous Ca2+ levels is thought to accelerate the binding and activation of calmodulin (CaM), which in turn binds and activates intracellular glutamine synthetase (GAD) to form gamma-aminobutyric acid (GABA), thereby mediating ALMT1-regulated malate exudation in wheat [82]. Additionally, the transcriptional regulation of ALMT1, CALMODULIN BINDING TRANSCRIPTION ACTIVATOR2 (AtCAMTA2), CALCINEURIN B-LKE PROTEIN 1 (AtCBL1), and CALMODULIN-LIKE24 (AtCML24) were found to be involved in Arabidopsis' Al resistance [80,83]. Nevertheless, the plant mechanisms of Al signals reception and transmission through the cytosolic Ca2+, particularly at the molecular level the regulatory pathways of the cytosolic Ca2+ signaling in Al resistance, remain unclear.
Phytohormones and reactive oxygen species (ROS) are two additional potential mechanisms for detecting and transmitting Al signals [84], [85], [86]. The production of ROS has been linked to plant signaling responses and cellular damage [87]. For instance, prolonged exposure of sorghum roots to Al (4–6 days) is necessary to induce SbMATE transcription, citrate secretion, and even Al resistance [48]. This delayed response of gene expression to Al might be attributed to the accumulation of cellular signals produced by Al, including ROS to a certain extent. Al-sensitive and Al-resistant sorghum near-isogenic lines were treated with Al for 4 days, and the Al-induced accumulation of callose, Al, cell damage, and ROS production were observed in their root tips through microscopic analysis [88]. The expression of SbMATE and its protein was analyzed by Sivaguru et al. [88] through immunolocalization and laser capture microdissection, demonstrating that the root distal transition zone, where the expression of SbMATE was highest, was also the place where most cell damage and ROS production occurred. This suggests that plants expressed citrate transporters where Al toxicity is maximum, indicating that the production of ROS may be related to Al signaling pathway. Therefore, in this region of the root, high ROS levels may act as a warning signal to induce events that increase SbMATE abundance and protect the cells from Al toxicity.
5. Transcriptional regulation of Al resistant genes induced by Al
The identification of plant Al-resistance genes into distinct categories based on their expression characteristics is crucial for understanding the regulation of gene expression under Al stress. Currently, Al resistant genes can be divided into three categories according to their expression characteristics [2]. First, the transcription level of resistant genes were higher in the resistant genotypes, and their expression was confined to the root apex, which is the main site of Al toxicity. Second, the transcription level of resistant genes were elevated by Al, such as in the case of BnALMT1, ScALMT, SbMATE, AtALMT, ZmMATE1, ScFRDL2,VuMATE1, OsFRDL4, ABA-STRESS AND RIPENING 1/5 (OsASR1/5) [29,43,[48], [49], [50], [51], [52],[89], [90]]. Third, the transcription level of the resistant genes were constitutively expressed, and Al had no effect on their expression, as observed in TaMATE1B, HvAACT1 and TaALMT1 [40,47,91,92]. These gene expression patterns were closely related to the physiological traits of root citrate and malate secretion. In the case of MATE and ALMT constitutively expressed plants, there is no discernible lag between organic acid release and root Al exposure. However, for Al-inducible MATE and ALMT, there is a time hysteresis between the maximum release of organic acids and root Al exposure, with a lag of 4–6 days for sorghum and 2–6 h for Arabidopsis and maize [48,52,93].
Numerous studies have been conducted to understand the biochemical and molecular components of Al-induced gene expression pathways, resulting in the classification of several cis-elements and trans-factors [94]. In terms of transcription factors that participate in inducing the expression of Al-resistant genes, two relevant members of the Cys2his2-type zinc finger transcription factor clan, AtSTOP1 and OsART1, were identified (Fig. 2) [95], [96]. Iuchi et al. [95] identified AtSTOP1 through the positional cloning of a low-pH hypersensitive Arabidopsis mutant, and its loss-of-function resulted in a phenotype sensitive to Al rather than to other toxic metals by regulating the transcription levels of AtALS3, AtMATE1, and AtALMT1 [51,[97], [98]]. While the expression of AtSTOP1 does not respond to Al, the quantity, stability, and activity of STOP1 can be mediated by complex mechanisms under Al stress, such as posttranslational modification [99]. A series of proteins involved in the modification of AtSTOP1 were identified through the use of rae (Regulation of AtALMT1 expression) mutants, which alter the transcription level of AtATALMT1 in Arabidopsis. For instance, an F-box protein component of the SCF-type E3 ligase complex, AtRAE1, and RAE1 HOMOLOG 1 (AtRAH1) can degrade AtSTOP1 through ubiquitination, thus altering the transcription level of AtALMT1 [99], while the SUMO E3 ligase SIZ1 and SUMO protease RAE5/EARLY IN SHORT DAYS 4 (AtESD4) can alter the transcription level of AtALMT1 through SUMOylation and deSMOylation of STOP1, respectively [100], [101], [102]. AtRAE2 (AtTEX1) and AtRAE3 (AtHPR1), two components of the THO/TREX complex, are responsible for regulating Al resistance, although unlike AtRAE3, AtRAE2 does not mediate the mRNA export of AtSTOP1 [103], [104]. Recently, Zhou et al. [105] demonstrated that AtMPK4 could phosphorylate AtSTOP1, thus decreasing its interaction with AtRAE1and enhancing the stability of AtSTOP1 by regulating its degradation.
A Genome-Wide Association (GWAS) method was employed to examine the role of POLYGARACTRONASE INHIBITOR PROTEIN 1 (AtPGIP1) in Arabidopsis Al endurance [106]. The study found that the promoter harbors the cis-elements that are recognized by AtSTOP1 and identified two pathways that regulate the transcription of AtPGIP1 under Al toxicity, a phosphatidylinositol (PI) signaling pathway that is dependent on AtSTOP1, and an NO signaling pathway that is independent of AtSTOP1 and participates in regulating PGIP1 transcription under Al toxicity. The former contains an R-R-type MYB transcription factor, NAM, ATAF1/2, and cup-shaped cotyledon 27 (AtNAC27), whereas the latter contains TRX superfamily proteins [106]. Further analysis indicated that PI signaling might also be involved in the regulation of ALUMINUM SENSITIVE3 (AtALS3) by activating AtSTOP1, although it is the Ca signaling pathway rather than the STOP1 pathway that is engaged in this Al-enhanced transcriptional level of AtALS3 [98].
Several partners have been identified to work with AtSTOP1 in regulating the transcription of Al-resistant genes. For example, AtWRKY46, a member of the WRKY domain, has been shown to participate in Al resistance by negatively mediating the transcription levels of AtALMT1 [107]. Additionally, AtWRKY47 has been shown to contribute to Al resistance in Arabidopsis by directly regulating the expression of AtXTH17 and EXTENSIN-LIKE PROTEIN (AtELP), two genes responsible for cell wall modification, which ultimately contributed to Arabidopsis's Al resistance [108]. Our recent research has provided new insights into the role of two types of transcription factors in regulating cell wall composition and modification when Arabidopisis thaliana responds to Al stress [109], [110]. One such factor is AtNAC017, which determines Arabidopsis Al resistance by directly regulating the expression of AtXTH31 [109]. Another is an R2R3-type transcription factor, AtMYB103, which participates in Arabidopsis Al resistance by directly binding to the promoter of TRICHOME BIREFRINGENCE-LIKE27 (AtTBL27), a gene that controls the level of xyloglucan's O-acetylation [110].
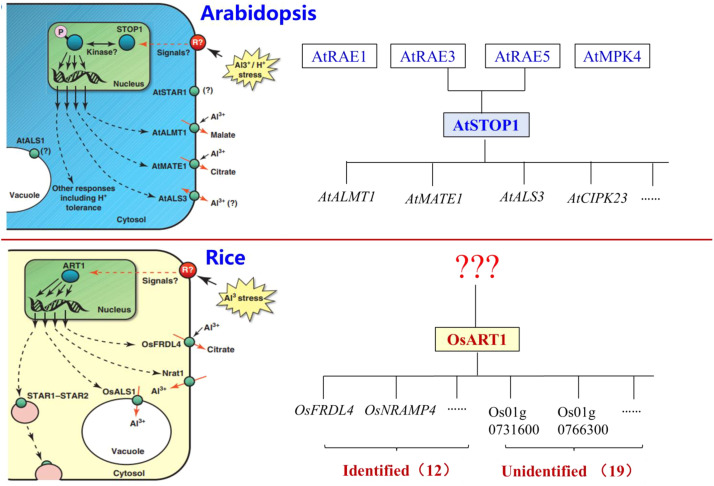
In a study on Al-tolerance in rice, a map-based method was employed to clone an Al-sensitive rice mutant, leading to the identification of AtSTOP1 homologous gene OsART1 in Arabidopsis thaliana's [96]. Although OsART1 is constitutively expressed in rice, similar to AtSTOP1, its involvement in low pH resistance has not been observed [2,96]. Comparative gene expression profiling between mutants that lost the function of OsART1 and wild-type rice, 31 genes were demonstrated to act downstream of OsART1, including OsSTAR1/2 [66], OsNRAT1 [68], OsFRDL4 [49], OsMGT1 [72], and OsCDT3 [73]. In addition, the contribution of OsART2, an ortholog gene of OsART1, to Al tolerance was lower compared to that of OsART1, despite its transcription was induced by Al [76]. However, unlike AtSTOP1, whose post-translational and post-transcriptional regulation has been extensively studied in Arabidopsis, relatively little is known about the regulation of OsART1 (Fig. 3). Therefore, further investigation into the upstream regulatory mechanisms of ART1 is necessary for an improved understanding of the regulatory network involved in Al tolerance in rice.
Fig. 3.
General model illustrating the post-transcriptional and translational regulation of AtSTOP1 and OsART1. Despite the extensive studies on the post-transcriptional and translational regulation of AtSTOP1, a classical Al resistant transcription factor in Arabidopsis, little is known about the upstream regulatory mechanism of OsART1, a counterpart of classical Al resistant transcription factor in rice. Therefore, there is a need for further research to shed light on the post-transcriptional and translational regulation of OsART1.
In addition to AtWRKY46 and AtWRKY47, which were previously discussed, OsWRKY22, a member of the WRKY family, promotes Al tolerance in rice by working in conjunction with the core transcription factor, OsART1, to positively regulate OsFRDL4 expression and citrate secretion [111]. Moreover, OsASR1 and OsASR5 interact with each other to regulate gene expression in response to Al stress [29]. Furthermore, OsMYB30 has been found to be regulated through a novel mechanism that controls cell-wall modification and Al resistance [112].
A detailed gel mobility shift-based mapping of the OsSTAR1 promoter was then conducted using the OsART1 protein and OsSTAR1 promoter, and the results revealed the presence of a cis-acting element, (GGN(T/g/a/C)V(C/A/g)S(C/G)), as a canonical OsART1 binding motif. Subsequent analysis of 31 genes downstream of OsART1 revealed that 29 of these genes harbored this motif, although the mechanism by which the signal of Al stress is transmitted to activate OsART1remains unclear. Moreover, previous studies on cis-elements in plant Al-resistance gene promoters have focused on promoter motifs and/or structures that affect local apical expression or high expression. For instance, high expression of TaALMT1 in the root tips of wheat has been found to significantly contribute to Al resistance and was attributed to enhancer-triplicated and duplicated tandem repeats in TaALMT1 allele promoters [113], which was confirmed by the investigation of transgenic lines expressing the TaALMT1 promoter: GUS reporter. In addition, sequence repeats have been shown to elevate the transcript levels of SbMATE in sorghum, as demonstrated by Magalhaes et al. [48] through the analysis of 12 sorghum varieties with different Al resistance levels. They found that the transcription level of SbMATE was closely related to Al resistance and further analysis revealed the existence of a polymorphic tourist-like miniature inverted repeat transposable element (MITE) in their promoters, the size of which was related to the expression of SbMATE's. Through the analysis of MITE insertions in various sizes, they found that as the number of these replicates increased, the size of the MITE increased, and the MITEs had a repetitive sequence structure.
In the case of HvAACT1, a MATE transporter barley responsible for citrate secretion and Al resistance, changes in physiological function and expression have been observed as a result of the insertion of transposable elements into its promoter. Initially, the role of HvAACT1 was to secrete citrate from xylem parenchymal cells to the xylem, where citrate complexes with Fe and is then transported to the shoot [114]. The discovery of a second allele of HvAACT1, which was identified based on the insertion of a 1 kb transposable element in its promoter, revealed interesting insights into the impact of such changes. This insertion not only elevated the transcription level of HvAACT1 in the root apex but also changed its localization from the xylem parenchyma to the root tip cortex and epidermis. Therefore, the insertion of this transposable element into the HvAACT1 promoter resulted in alteration of its tissue and cellular localization, ultimately altering Al resistance in barley.
6. How to adapt to acidic soils via improvement of varieties to realize the sustainable utilization of the acid soils
The utilization of acid soils in a sustainable manner can be improved through the development of plant varieties that are adapted to such conditions. One effective approach involves creating plants that are acclimated to acidic soils to promote their growth and development. This strategy not only holds promise for sustainability but also for cost-effectiveness as it potentially reduces the need for lime and fertilizer application requirements. Biotechnological and/or marker-assisted breeding methods can be employed to produce such plants. The recent identification and cloning of genes governing Al tolerance in plants has facilitated the ability to increase Al tolerance in plants through molecular and genetic methods. Currently, the main commercial, genetically modified crops are easily altered by adding or changing genes with specific genetic characteristics [115].
A variety of advantageous polygenic features should be focused on when considering to genetically alter target crops for growth in acidic soils, as several pressures often coexist in these soils. Various species have the capability to tolerate unfavorable aspects of acidic soils. As previously mentioned, the plant genome has at least three features (NH4+ preference, P uptake effectiveness, and Al tolerance). Plants that grow in acidic soils can be bred or engineered to tolerate multiple pressures. A transgenic barley strain expressing a wheat Al-resistance gene serves as an effective example, exhibiting improved P nutrition and grain output when grown in acidic soil [116].
7. Conclusion and perspective
In recent years, significant progress has been made in the sustainable use of acidic soils and the understanding of Al resistance mechanisms in plants. Al, one of the most prevalent metals in the crust of the planet, is found in large quantities in acidic soils. The increasing global population has amplified the need to reduce Al toxicity in acidic soils for improved plant output. Although various methods for introducing exogenous drugs have been successful, breeding acidic soil-tolerant plants holds the most potential.
Significant advancements in the understanding of genetic pathways, using molecular methods, have been made in recent decades. Despite the wide variation in Al tolerance among plants, they may share common tolerance mechanisms. Numerous studies have demonstrated the significance of external mechanisms, particularly the secretion of organic acids, in Al detoxification. SNP, RFLP, and SSR markers, among others, have been developed to track relevant genes and offer useful resources for molecular marker-assisted selection. Marker-assisted selection has evolved from being linked to gene-specific molecular markers to direct gene tracing. HvAACT1 and TaALMT1 have been used to produce gene-specific indicators that are effective for marker-assisted selection [117], [118].
Recent developments in marker development are expected to result in a stronger effect of marker-assisted selection in breeding. Currently, markers are distant from Al-tolerant genes, despite the efficacy of marker-assisted selection for Al tolerance [16]; nevertheless, closer markers, gene-specific markers, and recently developed GWAS analysis can increase the efficacy of selection for Al tolerance. The identification of new genes will continue to be a primary focus for increasing Al tolerance in crop plants, as multiple tolerance mechanisms can be combined to provide superior tolerance. In the future, new Al-resistant genes may be identified through GWAS analysis and Al-resistant varieties that can grow well in acidic soils might be obtained through intercrossing and molecular marker-assisted selection. Therefore, to achieve yield and a sustainable utilization of acid soils, it is not imperative to improve the acidic soil itself.
Declaration of competing interest
The authors declare that they have no conflicts of interest in this work.
Acknowledgments
We would like to thank Editage (www.editage.cn) for English language editing. This work was financially supported by the Key project of National Natural Science Foundation of China (42230711).
Biographies

Zhu Xiaofang is a professor of the Institute of Soil Science, Chinese Academy of Sciences. Her main research interests are elucidating the mechanisms of plants in response to Al toxicity and Cd toxicity. She has systematically studied the role of hemicellulose in the cell wall on Al and Cd toxicity. She found that Al was bound to xyloglucan. She also revealed that the modification of the xyloglucan and xylan affected the Al binding capacity of the cell wall.

Shen Renfang(BRID: 09016.00.98827) is a professor of the Institute of Soil Science, Chinese Academy of Sciences. His main research interests are sustainable use of acidic soils, including elucidating the physiological and molecular mechanisms of plant adaptation to acidic soils. He has systematically studied the role of nutrient supply such as nitrogen (N) and phosphorus (P) in acidic soils. He also identified two new woody Al-tolerant species; Camellia oleifera and Lespedeza. Furthermore, he revealed that the internal detoxification of Al is achieved by sequestering Al into the vacuoles in Al-accumulating buckwheat. He also found that the roots of Lespedeza bicolor secreted citrate in response to Al stress.
References
- 1.Che J., Zhao X.Q., Shen R.F. Molecular mechanisms of plant adaptation to acid soils. Pedosphere. 2022;33:14–22. [Google Scholar]
- 2.Kochian L.V., Pineros M.A., Liu J.P., Magalhaes J.V. Plant adaptation to acid soils: The molecular basis for crop aluminum resistance. Annu. Rev. Plant Biol. 2015;66:571–598. doi: 10.1146/annurev-arplant-043014-114822. [DOI] [PubMed] [Google Scholar]
- 3.Vonuexküll H.R., Mutert E. Global extent, development and economic-impact of acid soils. Plant Soil. 1995;171:1–15. [Google Scholar]
- 4.Zhao X.Q., Chen R.F., Shen R.F. Coadaptation of plants to multiple stresses in acidic soils. Soil Sci. 2014;179:503–513. [Google Scholar]
- 5.Wang C., Zheng M.M., Song W.F., et al. Impact of 25 years of inorganic fertilization on diazotrophic abundance and community structure in an acidic soil in southern China. Soil Biol. Biochem. 2017;113:240–249. [Google Scholar]
- 6.Wood S., Sebastian K., Scherr S.J. Pilot analysis of global ecosystems: Agroecosystems. Tech. Rep., Int. Food Policy Res. Inst. and World Resour. Inst., Washington, DC. 2000:45–54. [Google Scholar]
- 7.Hoekenga O.A., Maron L.G., Pineros M.A., et al. AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2006;103:9738–9743. doi: 10.1073/pnas.0602868103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen R.F. Science Press; Beijing: 2008. The Behavior of Aluminum in Soil-Plant Systems and Its Adaptive Mechanisms For Plants (in Chinese) [Google Scholar]
- 9.Krug E.C., Frink C.R. Acid rain on acid soil-a new perspective. Science. 1983;221:520–525. doi: 10.1126/science.221.4610.520. [DOI] [PubMed] [Google Scholar]
- 10.Guo J.H., Liu X.J., Zhang Y., et al. Significant acidification in major Chinese croplands. Science. 2010;327:1008–1010. doi: 10.1126/science.1182570. [DOI] [PubMed] [Google Scholar]
- 11.Ma J.F., Ryan P.R. Understanding how plants cope with acid soils. Funct. Plant Biol. 2010;37 III-VI. [Google Scholar]
- 12.Zhang J.E., Ouyang Y., Ling D.J. Impacts of simulated acid rain on cation leaching from the Latosol in South China. Chemosphere. 2007;67:2131–2137. doi: 10.1016/j.chemosphere.2006.12.095. [DOI] [PubMed] [Google Scholar]
- 13.Wang X., Ai S., Liao H. Deciphering interactions between phosphorus status and toxic metal exposure in plants and rhizospheres to improve crops reared on acid soil. Cells. 2023;12:441. doi: 10.3390/cells12030441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginocchio R., de la Fuente L., Sanchez P., et al. Soil acidification as a confounding factor on metal phytotoxicity in soils spiked with copper-rich mine wastes. Environ. Toxicol. Chem. 2009;28:2069–2081. doi: 10.1897/08-617.1. [DOI] [PubMed] [Google Scholar]
- 15.Ma J.F., Taketa S., Yang Z.M. Aluminum tolerance genes on the short arm of chromosome 3R are linked to organic acid release in triticale. Plant Physiol. 2000;122:687–694. doi: 10.1104/pp.122.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bian M., Zhou M., Sun D., et al. Molecular approaches unravel the mechanism of acid soil tolerance in plants. Crop J. 2013;1:91–104. [Google Scholar]
- 17.Guo T.R., Chen Y., Zhang Y.H., et al. Alleviation of Al toxicity in barley by addition of calcium. Agric Sci. China. 2006;5:828–833. [Google Scholar]
- 18.Kinraide T.B. Three mechanisms for the calcium alleviation of mineral toxicities. Plant Physiol. 1998;118:513–520. doi: 10.1104/pp.118.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao I.M., Zeigler R.S., Vera R., et al. Selection and breeding for acid-soil tolerance in crops. Bioscience. 1993;43:454–465. [Google Scholar]
- 20.Bose J., Babourina O., Rengel Z. Role of magnesium in alleviation of aluminium toxicity in plants. J. Exp. Bot. 2011;62:2251–2264. doi: 10.1093/jxb/erq456. [DOI] [PubMed] [Google Scholar]
- 21.Venkatesan S., Jayaganesh S. Characterisation of magnesium toxicity, its influence on amino acid synthesis pathway and biochemical parameters of tea. Res. J. Phytochem. 2010;4:67–77. [Google Scholar]
- 22.Hammond K.E., Evans D.E., Hodson M.J. Aluminium/silicon interactions in barley (Hordeum vulgare L.) seedlings. Plant Soil. 1995;173:89–95. [Google Scholar]
- 23.Yu M., Shen R., Xiao H., et al. Boron alleviates aluminum toxicity in pea (Pisum sativum) Plant Soil. 2009;314:87–98. [Google Scholar]
- 24.Marschner H. Academic Press; New York: 1995. Mineral Nutrition of Higher Plants; p. 889. [Google Scholar]
- 25.Gupta N., Gaurav S.S., Kumar A. Molecular basis of aluminium toxicity in plants: A review. Am. J. Plant Sci. 2013;4:21–37. [Google Scholar]
- 26.Liu J., Piñeros M.A., Kochian L.V. The role of aluminum sensing and signaling in plant aluminum resistance. J. Integr. Plant Biol. 2014;56:221–230. doi: 10.1111/jipb.12162. [DOI] [PubMed] [Google Scholar]
- 27.Silva S. Aluminium toxicity targets in plants. J. Bot. 2012;219462:1–8. [Google Scholar]
- 28.Delhaize E., Ryan P.R. Aluminum toxicity and tolerance in plants. Plant Physiol. 1995;107:315–321. doi: 10.1104/pp.107.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arenhart R.A., Chunemann M., Neto L.B., et al. Rice ASR1 and ASR5 are complementary transcription factors regulating aluminium responsive genes. Plant Cell Environ. 2016;3:645. doi: 10.1111/pce.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh S., Tripathi D.K., Singh S., et al. Toxicity of aluminium on various levels of plant cells and organism: A review. Environ. Exp. Bot. 2017;137:177–193. [Google Scholar]
- 31.Poschenrieder C. A glance into aluminum toxicity and resistance in plants. Sci. Total Environ. 2008;400:356–368. doi: 10.1016/j.scitotenv.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Panda S.K., Matsumoto H. Molecular physiology of aluminum toxicity and tolerance in plants. Bot. Rev. 2007;73:326–347. [Google Scholar]
- 33.Baluska F., Lev-Yadun S., Mancuso S. Swarm intelligence in plant roots response. Trends Ecol. Evol. 2010;25:682–683. doi: 10.1016/j.tree.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Yang Z.B., Geng X., He C., et al. TAA1-regulated local auxin biosynthesis in the root-apex transition zone mediates the aluminum-induced inhibition of root growth in Arabidopsis. Plant Cell. 2014;26:2889–2904. doi: 10.1105/tpc.114.127993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delhaize E., Craig S., Beaton C.D., et al. Aluminum tolerance in wheat (Triticum asetivum L.): I. Uptake and distribution of aluminum in root apices. Plant Physiol. 1993;103:685–693. doi: 10.1104/pp.103.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delhaize E., Ryan P.R., Randall P.J. Aluminum tolerance in wheat (Triticum aestivum L.): II. Aluminum-stimulated excretion of malic acid from root apices. Plant Physiol. 1993;103:695–702. doi: 10.1104/pp.103.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyasaka S.C., Buta J.G., Howell R.K., et al. Mechanism of aluminum tolerance in snapbeans: Root exudation of citric acid. Plant Physiol. 1991;96:737–743. doi: 10.1104/pp.96.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kochian L.V., Hoekenga O.A., Pineros M.A. How do crop plants tolerate acid soils? - Mechanisms of aluminum tolerance and phosphorous efficiency. Annu. Rev. Plant Biol. 2004;55:459–493. doi: 10.1146/annurev.arplant.55.031903.141655. [DOI] [PubMed] [Google Scholar]
- 39.Dong X.Y., Shen R.F., Chen R.F., et al. Secretion of malate and citrate from roots is related to high Al-resistance in Lespedeza bicolor. Plant Soil. 2008;306:139–147. [Google Scholar]
- 40.Sasaki T., Yamamoto Y., Ezaki B., et al. A wheat gene encoding an aluminum-activated malate transporter. Plant J. 2004;37:645–653. doi: 10.1111/j.1365-313x.2003.01991.x. [DOI] [PubMed] [Google Scholar]
- 41.Zhang W.H., Ryan P.R., Tyerman S.D. Malate-permeable channels and cation channels activated by aluminum in the apical cells of wheat roots. Plant Physiol. 2001;125:1459–1472. doi: 10.1104/pp.125.3.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piñeros M.A., Cancado G.M.A., Kochian L.V. Novel properties of the wheat aluminum tolerance organic acid transporter (TaALMT1) revealed by electrophysiological characterization in Xenopus oocytes: Functional and structural implications. Plant Physiol. 2008;147:2131–2146. doi: 10.1104/pp.108.119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ligaba A., Katsuhara M., Ryan P.R., et al. The BnALMT1 and BnALMT2 genes from rape encode aluminum-activated malate transporters that enhance the aluminum resistance of plant cells. Plant Physiol. 2006;142:1294–1303. doi: 10.1104/pp.106.085233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoekenga O.A., Maron L.G., Pineros M.A., et al. AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2006;103:9738–9743. doi: 10.1073/pnas.0602868103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gruber B.D., Delhaize E., Richardson A.E., et al. Characterisation of HvALMT1 function in transgenic barley plants. Funct. Plant Biol. 2011;38:163–175. doi: 10.1071/FP10140. [DOI] [PubMed] [Google Scholar]
- 46.Ligaba A., Maron L., Shaff J., et al. Maize ZmALMT2 is a root anion transporter that mediates constitutive root malate efflux. Plant Cell Environ. 2012;35:1185–1200. doi: 10.1111/j.1365-3040.2011.02479.x. [DOI] [PubMed] [Google Scholar]
- 47.Furukawa J., Yamaji N., Wang H., et al. An aluminum-activated citrate transporter in barley. Plant Cell Physiol. 2007;48:1081–1091. doi: 10.1093/pcp/pcm091. [DOI] [PubMed] [Google Scholar]
- 48.Magalhaes J.V., Liu J., Guimaraes C.T., et al. A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat. Genet. 2007;39:1156–1161. doi: 10.1038/ng2074. [DOI] [PubMed] [Google Scholar]
- 49.Yokosho K., Yamaji N., Ma J.F. An Al-inducible MATE gene is involved in external detoxification of Al in rice. Plant J. 2011;68:1061–1069. doi: 10.1111/j.1365-313X.2011.04757.x. [DOI] [PubMed] [Google Scholar]
- 50.Yang X.Y., Yang J.L., Zhou Y., et al. A de novo synthesis citrate transporter, Vigna umbellata multidrug and toxic compound extrusion, implicates in Al-activated citrate efflux in rice bean (Vigna umbellata) root apex. Plant Cell Environ. 2011;34:2138–2148. doi: 10.1111/j.1365-3040.2011.02410.x. [DOI] [PubMed] [Google Scholar]
- 51.Liu J., Magalhaes J.V., Shaff J., et al. Aluminum-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. Plant J. 2009;57:389–399. doi: 10.1111/j.1365-313X.2008.03696.x. [DOI] [PubMed] [Google Scholar]
- 52.Maron L., Piñeros M.A., Guimarães C., et al. Two functionally distinct members of the MATE multidrug and toxic compound extrusion family of transporters potentially underlie two major Al tolerance QTL in maize. Plant J. 2010;61:728–740. doi: 10.1111/j.1365-313X.2009.04103.x. [DOI] [PubMed] [Google Scholar]
- 53.Ma J.F., Shen R., Zhao Z., et al. Response of rice to Al stress and identification of quantitative trait loci for Al tolerance. Plant Cell Physiol. 2008;43:652–659. doi: 10.1093/pcp/pcf081. [DOI] [PubMed] [Google Scholar]
- 54.Ma J.F. Syndrome of aluminum toxicity and diversity of aluminum resistance in higher plants. Int. Rev. Cytol. 2007;264:225–252. doi: 10.1016/S0074-7696(07)64005-4. [DOI] [PubMed] [Google Scholar]
- 55.Sasidharan R., Voesenek L.A.C.J., Pierik R. Cell wall modifying proteins mediate plant acclimatization to biotic and abiotic stresses. Crit. Rev. Plant Sci. 2011;30:548–562. [Google Scholar]
- 56.Kochian L.V. Cellular mechanisms of aluminum toxicity and resistance in plants. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1995;46:237–260. [Google Scholar]
- 57.Zhu X.F., Shi Y.Z., Lei G.J., et al. XTH31, encoding an in vitro XEH/XET-active enzyme, regulates aluminum sensitivity by modulating in vivo XET action, cell wall xyloglucan content, and aluminum binding capacity in Arabidopsis. Plant Cell. 2012;24:4731–4747. doi: 10.1105/tpc.112.106039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang J.L., Li Y.Y., Zhang Y.J., et al. Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiol. 2008;146:323–324. doi: 10.1104/pp.107.111989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang X.Y., Zeng Z.H., Yan J.Y., et al. Association of specific pectin methylesterases with Al-induced root elongation inhibition in rice. Physiol. Plant. 2013;148:502–511. doi: 10.1111/ppl.12005. [DOI] [PubMed] [Google Scholar]
- 60.Eticha D., Stass A., Horst W.J. Cell-wall pectin and its degree of methylation in the maize root-apex: Significance for genotypic differences in aluminium resistance. Plant Cell Environ. 2005;28:1410–1420. [Google Scholar]
- 61.Schmohl N., Pilling J., Fisahn J., et al. Pectin methylesterase modulates aluminium sensitivity in Zea mays and Solanum tuberosum. Physiol. Plant. 2000;109:419–427. [Google Scholar]
- 62.Yang J.L., Zhu X.F., Peng Y.X., et al. Cell wall hemicellulose contributes significantly to aluminum adsorption and root growth in Arabidopsis. Plant Physiol. 2011;155:1885–1892. doi: 10.1104/pp.111.172221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu X.F., Wan J.X., Sun Y., et al. Xyloglucan endotransglucosylase-hydrolase17 interacts with xyloglucan endotransglucosylase-hydrolase31 to confer xyloglucan endotransglucosylase action and affect aluminum sensitivity in Arabidopsis. Plant Physiol. 2014;165:1566–1574. doi: 10.1104/pp.114.243790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu X.F., Sun Y., Zhang B.C., et al. Trichome birefringence-like27 affects aluminum sensitivity by modulating the O-acetylation of xyloglucan and aluminum-binding capacity in Arabidopsis. Plant Physiol. 2014;166:181–189. doi: 10.1104/pp.114.243808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu X.F., Wan J.X., Wu Q., et al. PARVUS affects aluminum sensitivity by modulating the structure of glucuronoxylan in Arabidopsis thaliana. Plant Cell Environ. 2017;40:1916–1925. doi: 10.1111/pce.12999. 2017. [DOI] [PubMed] [Google Scholar]
- 66.Huang C.F., Yamaji N., Mitani N., et al. A bacterial-type ABC transporter is involved in aluminum tolerance in rice. Plant Cell. 2009;21:655–667. doi: 10.1105/tpc.108.064543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xia J.X., Yamaji N., Kasai T., et al. Plasma membrane-localized transporter for aluminum in rice. Proc. Natl. Acad. Sci. USA. 2010;107:18381–18385. doi: 10.1073/pnas.1004949107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li J.Y., Liu J., Dong D., et al. Natural variation underlies alterations in Nramp aluminum transporter (NRAT1) expression and function that play a key role in rice aluminum tolerance. Proc. Natl. Acad. Sci. USA. 2014;111:6503–6508. doi: 10.1073/pnas.1318975111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang C.F., Yamaji N., Chen Z., et al. A tonoplast-localized half-size ABC transporter is required for internal detoxification of aluminum in rice. Plant J. 2012;69:857–867. doi: 10.1111/j.1365-313X.2011.04837.x. [DOI] [PubMed] [Google Scholar]
- 70.Negishi T., Oshima K., Hattori M., et al. Tonoplast- and plasma membrane-localized aquaporin-family transporters in blue hydrangea sepals of aluminum hyperaccumulating plant. PLoS One. 2012;7:e43189. doi: 10.1371/journal.pone.0043189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shen R.F., Ma J.F., Kyo M., et al. Compartmentation of aluminium in leaves of an Al-accumulator, Fagopyrum esculentum Moench. Planta. 2002;215:394–398. doi: 10.1007/s00425-002-0763-z. [DOI] [PubMed] [Google Scholar]
- 72.Chen Z.C., Yamaji N., Motoyama R., et al. Up-regulation of a magnesium transporter gene OSMGT1 is required for conferring aluminum tolerance in rice. Plant Physiol. 2012;159:1624–1633. doi: 10.1104/pp.112.199778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xia J.X., Yamaji N., Ma J.F. A plasma membrane-localized small peptide is involved in rice aluminum tolerance. Plant J. 2013;76:345–355. doi: 10.1111/tpj.12296. [DOI] [PubMed] [Google Scholar]
- 74.Yokosho K., Yamaji N., Fujii-Kashino M., et al. Functional analysis of a MATE gene OsFRDL2 revealed its involvement in Al-induced secretion of citrate, but a lower contribution to Al tolerance in rice. Plant Cell Physiol. 2016;57:976–985. doi: 10.1093/pcp/pcw026. [DOI] [PubMed] [Google Scholar]
- 75.Che J., Yamaji N., Shen R.F., et al. An Al-inducible expansin gene, OsEXPA10, is involved in root cell elongation of rice. Plant J. 2016;88:132–142. doi: 10.1111/tpj.13237. [DOI] [PubMed] [Google Scholar]
- 76.Che J., Tsutsui T., Yokosho K., et al. Functional characterization of an aluminum (Al)-inducible transcription factor, ART2, revealed a different pathway for Al tolerance in rice. New Phytol. 2018;220:209–218. doi: 10.1111/nph.15252. [DOI] [PubMed] [Google Scholar]
- 77.Ligaba A., Dreyer I., Margaryan A., et al. Functional, structural and phylogenetic analysis of domains underlying the Al-sensitivity of the aluminium-activated malate/anion transporter, TaALMT1. Plant J. 2013;76:766–780. doi: 10.1111/tpj.12332. [DOI] [PubMed] [Google Scholar]
- 78.Rengel Z., Zhang W.H. Role of dynamics of intracellular calcium in aluminium-toxicity syndrome. New Phytol. 2003;159:295–314. doi: 10.1046/j.1469-8137.2003.00821.x. [DOI] [PubMed] [Google Scholar]
- 79.Matsumoto H., Riechers D.E., Lygin A.V., et al. In: Panda S.K., Baluška F., editors. vol. 24. Springer International Publishing; Cham, Switzerland: 2015. Aluminum signaling and potential links with safener-induced detoxification in plants; pp. 1–35. (Aluminum Stress Adaptation in Plants, Signaling and Communication in Plants). [Google Scholar]
- 80.Ligaba-Osena A., Fei Z., Liu J., et al. Loss-of-function mutation of the calcium sensor CBL1 increases aluminum sensitivity in Arabidopsis. New Phytol. 2017;214:830–841. doi: 10.1111/nph.14420. [DOI] [PubMed] [Google Scholar]
- 81.Rincón-Zachary M., Teaster N.D., Sparks J.A., et al. Fluorescence resonance energy transfer-sensitized emission of yellow cameleon 3.60 reveals root zone-specific calcium signatures in Arabidopsis in response to aluminum and other trivalent cations. Plant Physiol. 2010;152:1442–1458. doi: 10.1104/pp.109.147256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ramesh S.A., Tyerman S.D., Xu B., et al. GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat. Commun. 2015:6. doi: 10.1038/ncomms8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu X., Wang P., Bai Z.M., et al. Calmodulin-like protein CML24 interacts with CAMTA2 and WRKY46 to regulate ALMT1-dependent Al resistance in Arabidopsis thaliana. New Phytol. 2022;233:2471–2487. doi: 10.1111/nph.17812. [DOI] [PubMed] [Google Scholar]
- 84.Jones D.L., Blancaflor E.B., Kochian L.V., et al. Spatial coordination of aluminium uptake, production of reactive oxygen species, callose production and wall rigidification in maize roots. Plant Cell Environ. 2006;29:1309–1318. doi: 10.1111/j.1365-3040.2006.01509.x. [DOI] [PubMed] [Google Scholar]
- 85.Yang Z.B., He C., Ma Y., et al. Jasmonic acid enhances Al-induced root growth inhibition. Plant Physiol. 2017;173:1420–1433. doi: 10.1104/pp.16.01756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang Z.B., Liu G., Liu J., et al. Synergistic action of auxin and cytokinin mediates aluminum-induced root growth inhibition in Arabidopsis. EMBO Rep. 2017;18:1213–1230. doi: 10.15252/embr.201643806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Apel K., Hirt H. Reactive oxygen species: Metabolism, oxidative stress and signal transduction. Ann. Rev. Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 88.Sivaguru M., Liu J.L., Kochian L.V. Targeted expression of SbMATE in the root distal transition zone is responsible for sorghum aluminum resistance. Plant J. 2013;76:297–307. doi: 10.1111/tpj.12290. [DOI] [PubMed] [Google Scholar]
- 89.Collins N.C., Shirley N.J., Saeed M., et al. An ALMT1 gene cluster controlling aluminium tolerance at the Alt4 locus of rye (Secale cereale L.) Genetics. 2008;179:669–682. doi: 10.1534/genetics.107.083451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yokosho K., Yamaji N., Ma J.F. Isolation and characterisation of two MATE genes in rye. Funct. Plant Bio. 2010;37:296–303. [Google Scholar]
- 91.Ryan P.R., Raman H., Gupta S., et al. A second mechanism for aluminum resistance in wheat relies on the constitutive efflux of citrate from roots. Plant Physiol. 2009;149:340–351. doi: 10.1104/pp.108.129155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tovkach A., Ryan P.R., Richardson A.E., et al. Transposon-mediated alteration of TaMATE1B expression in wheat confers constitutive citrate efflux from root apices. Plant Physiol. 2013;161:880–892. doi: 10.1104/pp.112.207142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kobayashi Y., Hoekenga O.A., Itoh H., et al. Characterization of AtALMT1 expression in aluminum inducible malate release and its role for rhizotoxic stress tolerance in Arabidopsis thaliana. Plant Physiol. 2007;145:843–852. doi: 10.1104/pp.107.102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Delhaize E., Ma J.F., Ryan P.R. Transcriptional regulation of aluminium tolerance genes. Trends Plant Sci. 2012;17:341–348. doi: 10.1016/j.tplants.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 95.Iuchi S., Koyama H., Iuchi A., et al. Zinc finger protein STOP1 is critical for proton tolerance in Arabidopsis and coregulates a key gene in aluminum tolerance. Proc. Natl. Acad. Sci. USA. 2007;104:9900–9905. doi: 10.1073/pnas.0700117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yamaji N., Huang C.F., Nagao S., et al. A zinc finger transcription factor ART1 regulates multiple genes implicated in aluminum tolerance in rice. Plant Cell. 2009;21:3339–3349. doi: 10.1105/tpc.109.070771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sawaki Y., Iuchi S., Kobayashi Y., et al. STOP1 regulates multiple genes that protect Arabidopsis from proton and aluminum toxicities. Plant Physiol. 2009;150:281–294. doi: 10.1104/pp.108.134700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sadhukhan A., Agrahari R.K., Wu L.J., et al. Expression genome-wide association study identifies that phosphatidylinositol-derived signalling regulates ALUMINIUM SENSITIVE3 expression under aluminium stress in the shoots of Arabidopsis thaliana. Plant Sci. 2021 doi: 10.1016/j.plantsci.2020.110711. [DOI] [PubMed] [Google Scholar]
- 99.Zhang Y., Zhang J., Guo J.L., et al. F-box protein RAE1 regulates the stability of the aluminum-resistance transcription factor STOP1 in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2019;116:319–327. doi: 10.1073/pnas.1814426116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fang Q., Zhang J., Zhang Y., et al. Regulation of aluminum resistance in Arabidopsis involves the SUMOylation of the zinc finger transcription factor STOP1. Plant Cell. 2020;32:3921–3938. doi: 10.1105/tpc.20.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fang Q., Zhang J., Yang D., et al. The SUMO E3 ligase SIZ1 partially regulates STOP1 SUMOylation and stability in Arabidopsis thaliana. Plant Signal Behav. 2021;16 doi: 10.1080/15592324.2021.1899487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu J.M., Zhu J.Y., Liu J.J., et al. SIZ1 negatively regulates aluminum resistance by mediating the STOP1-ALMT1 pathway in Arabidopsis. J. Integr. Plant Biol. 2021;63:1147–1160. doi: 10.1111/jipb.13091. [DOI] [PubMed] [Google Scholar]
- 103.Guo J., Zhang Y., Gao H., et al. Mutation of HPR1 encoding a component of the THO/TREX complex reduces STOP1 accumulation and aluminium resistance in Arabidopsis thaliana. New Phytol. 2020;228:179–193. doi: 10.1111/nph.16658. [DOI] [PubMed] [Google Scholar]
- 104.Zhu Y.F., Guo J., Zhang Y., et al. The THO/TREX complex component RAE2/TEX1 is involved in the regulation of aluminum resistance and low phosphate response in Arabidopsis. Front. Plant Sci. 2021;12 doi: 10.3389/fpls.2021.698443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhou F.L., Singh S., Zhang J., et al. The MEKK1-MKK1/2-MPK4 cascade phosphorylates and stabilizes STOP1 to confer aluminum resistance in Arabidopsis. Mol. Plant. 2022 doi: 10.1016/j.molp.2022.11.010. [DOI] [PubMed] [Google Scholar]
- 106.Agrahari R.K., Enomoto T., Ito H., et al. Expression GWAS of PGIP1 identifies STOP1-dependent and STOP1-independent regulation of PGIP1 in aluminum stress signaling in Arabidopsis. Front. Plant Sci. 2021:12. doi: 10.3389/fpls.2021.774687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ding Z.J., Yan J.Y., Xu X.Y., et al. WRKY46 functions as a transcriptional repressor of ALMT1, regulating aluminum-induced malate secretion in Arabidopsis. Plant J. 2013;76:825–835. doi: 10.1111/tpj.12337. [DOI] [PubMed] [Google Scholar]
- 108.Li C.X., Yan J.Y., Ren J.Y., et al. A WRKY transcription factor confers aluminum tolerance via regulation of cell wall modifying genes. J. Integr. Plant Biol. 2020;62:1176–1192. doi: 10.1111/jipb.12888. [DOI] [PubMed] [Google Scholar]
- 109.Tao Y., Wan J.X., Liu Y.S., et al. The NAC transcription factor ANAC017 regulates aluminum tolerance by regulating the cell wall-modifying genes. Plant Physiol. 2022;189:2517–2534. doi: 10.1093/plphys/kiac197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wu Q., Tao Y., Huang J., et al. The MYB transcription factor MYB103 acts upstream of TRICHOME BIREFRINGENCE-LIKE27 in regulating aluminum sensitivity by modulating the O-acetylation level of cell wall xyloglucan in Arabidopsis thaliana. Plant J. 2022;111:529–545. doi: 10.1111/tpj.15837. [DOI] [PubMed] [Google Scholar]
- 111.Li G.Z., Wang Z.Q., Yokosho K., et al. Transcription factor WRKY22 promotes aluminum tolerance via activation of OsFRDL4 expression and enhancement of citrate secretion in rice (Oryza sativa) New Phytol. 2018;219:149–162. doi: 10.1111/nph.15143. [DOI] [PubMed] [Google Scholar]
- 112.Gao L.J., Liu X.P., Gao K.K., et al. ART1 and putrescine contribute to rice aluminum resistance via OsMYB30 in cell wall modification. J. Integr. Plant Biol. 2022:13429. doi: 10.1111/jipb.13429. [DOI] [PubMed] [Google Scholar]
- 113.Ryan P.R., Raman H., Gupta S., et al. The multiple origins of aluminium resistance in hexaploid wheat include Aegilops tauschii and more recent cis mutations to TaALMT1. Plant J. 2010;64:446–455. doi: 10.1111/j.1365-313X.2010.04338.x. [DOI] [PubMed] [Google Scholar]
- 114.Fujii M., Yokosho K., Yamaji N., et al. Acquisition of aluminium tolerance by modification of a single gene in barley. Nat. Commun. 2012;3:713. doi: 10.1038/ncomms1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Godfray H.C.J., Beddington J.R., Crute I.R., et al. Food security: The challenge of feeding 9 billion people. Science. 2010;327:812–818. doi: 10.1126/science.1185383. [DOI] [PubMed] [Google Scholar]
- 116.Delhaize E., TaylorP Hocking PJ, Simpson R.J., et al. Transgenic barley (Hordeum vulgare L.) expressing the wheat aluminium resistance gene (TaALMT1) shows enhanced phosphorus nutrition and grain production when grown on an acid soil. Plant Biotechnol. J. 2009;7:391–400. doi: 10.1111/j.1467-7652.2009.00403.x. [DOI] [PubMed] [Google Scholar]
- 117.Bian M., Waters I., Broughton S., et al. Development of gene-specific markers for acid soil/aluminium tolerance in barley (Hordeum vulgare L.) Mol. Breed. 2013:1–10. [Google Scholar]
- 118.Raman H., Raman R., Wood R., et al. Repetitive inDel markers within the ALMT1 gene conditioning aluminium tolerance in wheat (Triticum aestivum L.) Mol. Breed. 2006;18:171–183. [Google Scholar]