Abstract
Infectious disease surveillance systems, including wastewater surveillance, can alert communities to the threat of emerging pathogens. We need methods to infer understanding of transmission dynamics from non-detection. We estimate a sensitivity of detection of poliovirus in wastewater to inform the sensitivity of wastewater surveillance for poliovirus using both a clinical epidemiology and fecal shedding approach. We then apply freedom from disease to estimate the sensitivity of the wastewater surveillance network. Estimated sensitivity to detect a single poliovirus infection was low, <11% at most wastewater treatment plants and <3% in most counties. However, the maximum threshold for the number of infections when polio is not detected in wastewater was much lower. Prospective wastewater surveillance can confirm the absence of a polio threat and be escalated in the case of poliovirus detection. These methods can be applied to any emerging or re-emerging pathogen, and improve understanding from wastewater surveillance.
Introduction
Increasing vaccine hesitancy [1] and pandemic-disrupted childhood vaccine schedules [2] raise the potential for polio outbreaks in countries where polio had previously been eliminated. For example in 2022, London, England saw sustained transmission of polio (but no paralytic cases) [3] and New York State had a paralytic polio case and detection of poliovirus in wastewater [4, 5]. Although completely effective at preventing paralysis from a polio infection, the inactivated polio vaccine administered in the US and other wealthy countries is not completely effective at preventing onward transmission [6]. Further, county- and state-level vaccine coverages mask gaps in community-level vaccine coverages, with pockets of communities with very low vaccine uptake [7].
In the United States, the transmission of poliovirus is currently monitored through acute flaccid paralysis (AFP) surveillance and follow-up testing for poliovirus. However, among individuals not vaccinated against poliovirus AFP is estimated to occur only once in 200 wildtype polio infections and once in 2,000 infections of vaccine-derived poliovirus (VDPV) [8]. Moreover, with high polio vaccination coverage the vast majority of infections are asymptomatic [9] suggesting that poliovirus could potentially circulate without AFP surveillance providing any indication.
Testing for poliovirus in wastewater complements AFP surveillance across the globe [10], often to good effect. For example, in Pakistan, polio circulation was detected via wastewater an average of four months before AFP surveillance [11]. And in Israel, detection of poliovirus in wastewater prompted deployment of bivalent oral polio vaccine to prevent transmission among vaccinated individuals thereby reducing transmission and preventing paralytic cases of polio among unvaccinated individuals [12]. Despite the utility of wastewater surveillance for polio and its widespread implementation, its systemwide sensitivity to detect an outbreak of poliovirus is not well defined. Studies of environmental surveillance often consider sensitivity as site-specific. For example, if a community is known to have poliovirus transmission, the sensitivity of a sampling site can be estimated as the proportion of samples that return a positive (also called a detection rate). In Afghanistan and Pakistan the site-specific sensitivity of detecting polio in the wastewater of communities where the prevalence of VDPV ranged from 0.5–3% has been estimated to be 35–50% [8]. These estimates are helpful, but are only derived from a single context and depend heavily upon the prevalence in the community. Accurate estimates of the sensitivity of the system to detect an outbreak are needed for estimating the probability of not only detecting an emerging pathogen, but also confirming that the community is free from transmission upon consecutive non-detections following freedom from disease principles [13, 14].
In July of 2022 a local case of VDPV was confirmed in a patient with AFP in Rockland County, New York [4]. There was no routine environmental surveillance for poliovirus in New York State prior to this local case. Immediately in coordination with the CDC, New York State’s wastewater surveillance network (established in response to COVID-19) began testing for polio in the wastewater throughout Rockland County, surrounding counties, New York City, and Long Island [5]. Widespread detection of poliovirus in the wastewater of Rockland County and five surrounding jurisdictions led New York State to declare a disaster emergency [15]. To aid interpretation of the non-detection of poliovirus in wastewater, we estimated the sensitivity of the wastewater surveillance system in detecting circulating poliovirus within each county and individual sewersheds using two different approaches: one based on spatiotemporal coverage of a represented population and another based on mass balance and fecal shedding. Based partially on these estimates, we outline a wastewater surveillance plan considering polio vaccine coverage to ensure both the elimination of polio transmission and provide early indication of any polio re-emergence.
Methods
Wastewater sampling and testing
Throughout the New York State wastewater surveillance network, wastewater treatment plant operators pull 24-hour composite samples 1–2 times weekly and ship overnight with ice packs to participating labs for SARS-CoV-2 testing [16]. Each participating laboratory in the network has a different method of concentrating viral nucleic acid from wastewater–the laboratories servicing the counties herein used ultracentrifugation through a sucrose cushion according to Wilder et al. (Orange, Rockland, and Sullivan counties) [17] or polyethylene glycol precipitation (Kings, Nassau, and Richmond counties). Upon the confirmation of a case of AFP caused by poliovirus in July of 2022, the network shipped stored extracted nucleic acids dating back to March of 2022 from the areas suspected to have polio transmission to the US CDC for polio testing. Extracted nucleic acids were then stored at -80°C and shipped with dry ice to the United States Centers for Disease Control for a pan-poliovirus real-time PCR test and genetic sequencing to link back to the case of paralytic polio as described by Ryerson et al [5].
Estimating sensitivity of wastewater surveillance of poliovirus
The sensitivity of an infectious disease surveillance system to detect emerging threats such as polio is a product of the proportion of the population covered by the surveillance system (population coverage), the frequency that the surveillance system tests the population under surveillance (temporal coverage), and the probability of detecting the emerging pathogen given the components of the surveillance system and characteristics of the pathogen [18]. For wastewater surveillance, the probability of detection varies by pathogen and depends upon the prevalence of the pathogen in the population contributing to the sample, the amount of wastewater from which the sample is taken (larger communities create more wastewater), and the amount of genetic material shed into wastewater. The methods of sampling, sample processing, and laboratory testing also greatly affect the probability of detecting a pathogen in wastewater surveillance [19], however in this manuscript we hold these factors constant and focus on system-wide sensitivity of wastewater surveillance.
We used equation 1 to estimate the system-wide sensitivity of wastewater surveillance for poliovirus. We defined population coverage (Cp) as the proportion of the population connected to the wastewater surveillance network. For estimates at the treatment plant level, we assumed 100%. For estimates at the county level we use the calculated proportion of the population connected to the wastewater surveillance network from our previous work [20]. We relate temporal coverage (Ct) to the length of poliovirus fecal shedding. Polio infections on average shed virus in feces for 3–4 weeks (midpoint of 25 days) [21]. We divided 25 days by the number of days between consecutive wastewater sampling events. For sampling intervals smaller than 25 days we assigned Ct a value of 100%.
We calculated the sensitivity of detection (Sed) of poliovirus in wastewater in two ways. First, we analyzed the New York State polio outbreak of 2022 [4], and estimated the number of infections in communities where poliovirus was detected in the wastewater. Second, we used modeled estimates of viral copy detection per wastewater treatment plant flow.
| Equation 1: The sensitivity of wastewater surveillance to detect a pathogen such as polio (WWSsensitivity) is a product of three proportions: 1) the population coverage of wastewater surveillance (Cp), 2) the temporal coverage of wastewater surveillance (Ct), and 3) the sensitivity of detection (Sed) or limit of detection of the wastewater test. |
Estimating sensitivity of detection through the number of infections present during detection in wastewater
The New York State polio outbreak of 2022 was identified when a young adult presented to the hospital with AFP caused by vaccine-derived poliovirus [4]. No other paralysis cases have yet been observed (as of October 2024), despite numerous detections of poliovirus RNA in wastewater in multiple communities. Equation 2 shows our approach to estimating the sensitivity of detection of wastewater surveillance at a wastewater treatment plant from the New York State polio outbreak. We first simulated the total number of polio infections among unvaccinated individuals with 10,000 iterations (n) of a binomial distribution (B) using a probability of 1 paralytic case per 2,000 infections (p). From the literature we assumed that one paralysis case of vaccine-derived poliovirus occurs for every 2,000 infections among unvaccinated individuals [8]. From this estimated number of infections we calculated a prevalence of polio among unvaccinated individuals (prevunvaccinated). Assuming equal mixing among unvaccinated and vaccinated populations, we applied the prevalence of polio among unvaccinated individuals (prevunvaccinated) to the population of vaccinated individuals (populationvaccinated) and estimated the number of polio infections among vaccinated individuals (infectionsvaccinated). We then distributed the estimated total number of infections (infectionstotal) across the sewersheds where poliovirus was detected proportional to the population of the sewershed (w = population weight of the wwtp) to estimate the number of infections in each sewershed (infectionswwtp). Lastly, the population size required for 1 successful detection (populationdetect1) in the sewershed was divided by the wwtp population (populationwwtp) to get the sensitivity of detection (Sed).
|
n = 10,000 p = 1/2,000 |
Equation 2: Estimate of the sensitivity of detection or limit of detection of poliovirus infection at a wastewater treatment plant using the New York State polio case of 2022 |
Estimating the sensitivity of detection through studies of fecal shedding
Fecal shedding studies of poliovirus are limited with fecal shedding studies reporting duration and temporality of shedding but not quantity [21]. Still, Berchenko et al. estimated that wastewater surveillance was able to identify one poliovirus shedder per 400,000 liters of sewage following an oral polio vaccine campaign in Israel [22]. We can then estimate the probability of wastewater surveillance to identify poliovirus circulation as a function of a wastewater treatment plant’s daily flow using Berchenko et al.’s estimate (Equation 3). We applied the mean daily flow to estimates of probability for each treatment plant. For any treatment plant without mean daily flow we applied an estimated mean ratio of daily flow to permitted discharge capacity of those treatment plants with data to those treatment plants without data.
|
|
Equation 3: Estimate of lower limit of sensitivity of wastewater surveillance to detect a single poliovirus infection based upon limits of detection calculated by Berchenko et al. [22] |
Understanding non-detection of poliovirus
With the estimated sensitivity of the wastewater surveillance network to detect poliovirus from equation 1, we calculated the probability that a community was free from poliovirus circulation (freedom from disease) using equation 4. From the two estimated sensitivities of detection (Sed in equations 2 and 3) we applied whichever was lower. We estimated this probability for three consecutive non-detections of poliovirus in wastewater for all treatment plants in the state network and for each NY state county.
| Equation 4: Probable confidence in the community being free from poliovirus circulation following a non-detection of poliovirus in wastewater. WWSsensitivity is estimated from equation 1; n is the number of consecutive non-detections. |
We also estimate an upper limit to the number of polio infections given 3 consecutive non-detections and an estimated system-wide sensitivity for wastewater surveillance derived from equations 1–3. Using equation 4, a WWSsensitivity of 63% is required to obtain 95% confidence in zero infections with three consecutive non-detections. We estimate an upper limit of the number of poliovirus infections present with three consecutive non-detections of poliovirus in wastewater for all treatment plants in the state network and for each NY state county using equation 5.
| Equation 5: Reversing equation 4, a WWSsensitivity of 63% is required to ensure 95% confidence in zero infections with three consecutive non-detections. The upper limit of poliovirus infections in a community can be estimated as 0.63 divided by the actual estimated WWSsensitivity. |
Spatial comparison of vaccination rates
We compared zip code level polio vaccination rates provided by New York State Department of Health to municipal wastewater treatment plant (WWTP) catchment areas in NY with permitted discharge capacity of at least 1 million gallons per day (mgd). Zip codes with vaccination rates of less than 70% that intersected with sewersheds were flagged as being areas that were both extra vulnerable to polio transmission and also within the wastewater surveillance network. Sewersheds that intersected with these zip codes were then classified for whether they would be routine participants in the state’s wastewater surveillance network (i.e., permitted discharge capacity above 1 mgd) or if they would not be routinely tested but could be enrolled for testing under special circumstances. Zip codes that did not intersect with any sewersheds were also noted as potential blind spots for the use of wastewater to test for polio presence.
We also explored other potential risk factors for polio circulation including social vulnerability. We estimated the Centers for Disease Control’s (CDC) Social Vulnerability Index (SVI) [23] for each sewershed. The SVI uses U.S. Census data to identify if a community might have greater vulnerability to external stressors that might make the area at higher risk for negative outcomes of natural disasters or disease outbreaks. SVI values range from 0 to 1 with higher indexes being more vulnerable. We calculate the mean SVI for each sewershed from the NY census tracts that intersected with the sewershed. We then assessed correlation between the SVI and polio vaccine coverage using a Pearson correlation test. All spatial analyses were conducted in R programming software [24] using the package “sf” [25].
Results
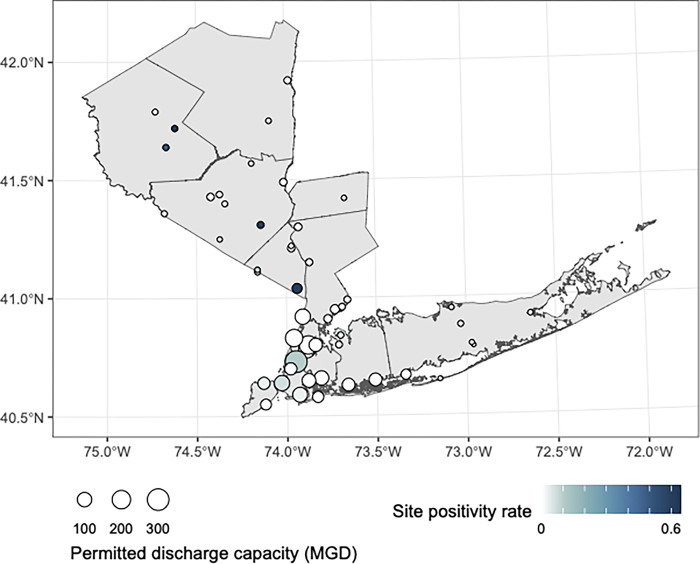
Wastewater testing for poliovirus began in New York State in July of 2022 and is ongoing, with stored nucleic acids from samples taken in March of 2022 the earliest tested for poliovirus. Polio was detected in 12 (23.5)% of the 51 wastewater treatment plants in downstate New York participating in wastewater surveillance of polio from March through October of 2022, with polio detection rates ranging from 0–60% at wastewater treatment plants across the surveilled counties (Fig 1).
Fig 1. The site positivity of wastewater treatment plants for poliovirus from March through October of 2022.
MGD refers to millions of gallons per day that the wastewater treatment plant is permitted to discharge (larger numbers mean larger populations are represented). This map was created in R version 4.2.3 [24] using county boundaries from the US Census TIGER/Line shapefiles.
Estimating sensitivity of wastewater surveillance of poliovirus
From the New York State polio outbreak we estimated 1,401 unvaccinated individuals infected with polio (95% CI = 1,359–1,439) in the counties with polio found in the wastewater. This represents a prevalence of 0.13% (95% CI = 0.12–0.13%) among the 1,115,881 unvaccinated individuals across the study area. Assuming the same prevalence among the 4,329,977 vaccinated individuals across the study area we estimated 5,436 vaccinated individuals infected with polio (95% CI = 5,273–5,584). After distributing all 6,837 estimated polio infections (95% CI = 6,632–7,023) across wastewater treatment plants proportional to the population served by the wastewater treatment plant we estimate that wastewater surveillance was consistently able to detect one polio infection among 472 people connected to the sewer system (95% CI = 460–487). This resulted in a sensitivity of detection of a single polio infection ranging from 0.04% in the largest treatment plant in the study area to 12.2% in the smallest (Table 1).
Table 1. Estimates of the probability of having at least one paralytic case among unvaccinated infections and lower limits of sensitivity of detection of poliovirus in wastewater based upon a single paralytic polio case in Rockland County, NY and subsequent detections of poliovirus in wastewater in surrounding counties using the New York State polio outbreak.
| County | Treatment plant | WWTP capacity (millions of liters) | Population served | Paralytic polio cases | Number of wastewater samples (positive for polio) | Polio vaccine coverage | Estimated polio infections (95% CI) | Approach #1 estimated sensitivity of detection (95% CI) | Approach #2 estimated sensitivity of detection (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Kings (Brooklyn) | Coney Island | 416 | 711,000 | 0 | 123 (1) | 85% | 1505 (1459–1545) | 0.07% (0.06–0.07%) | 0.13% (0.07–0.22%) |
| Kings (Brooklyn) | Newtown Creek | 1,173 | 1,197,000 | 0 | 111 (4) | 87% | 2533 (2457–2602) | 0.04% (0.04–0.04%) | 0.05% (0.03–0.09%) |
| Kings (Brooklyn) | Owls Head | 454 | 825,000 | 0 | 119 (2) | 86% | 1746 (1693–1793) | 0.06% (0.06–0.06%) | 0.11% (0.06–0.18%) |
| Nassau | Port Washington | 15 | 21,000 | 0 | 123 (1) | 66% | 44 (43–46) | 2.3% (2.2–2.3%) | 3.87% (2.29–6.52%) |
| Orange | Harriman | 23 | 43,000 | 0 | 147 (29) | 53% | 91 (88–93) | 1.1% (1.1–1.1%) | 2.24% (1.32–3.76%) |
| Richmond (Staten Island) | Port Richmond | 227 | 211,000 | 0 | 124 (1) | 86% | 447 (433–459) | 0.2% (0.2–0.2%) | 0.38% (0.22–0.64%) |
| Rockland | Rockland County #1 | 109 | 207,000 | 1 | 109 (16) | 76% | 438 (425–450) | 0.2% (0.2–0.2%) | 0.48% (0.29–0.81%) |
| Rockland | Western Ramapo | 5.7 | 4,000 | 0 | 64 (2) | 66% | 9 (8–9) | 11.8% (11.5–12.2%) | 8.50% (5.04–14.32%) |
| Sullivan | Monticello | 11.7 | 7,000 | 0 | 59 (4) | 73% | 15 (14–15) | 6.8% (6.6–7.0%) | 4.92% (2.92–8.30%) |
| Sullivan | South Fallsburg | 10 | 5,000 | 0 | 61 (9) | 57% | 11 (10–11) | 9.5% (9.2–9.7%) | 4.83% (2.86–8.13%) |
Using Berchenko et al’s estimate of wastewater surveillance being able to detect one polio infection per 400,000 liters of flow (95% CI: 231,000–656,000) [22], we find the sensitivity of detection to range from 0.05% in the largest treatment plants in the study area to 8.5% in the smallest (Table 1).
Understanding non-detection of poliovirus
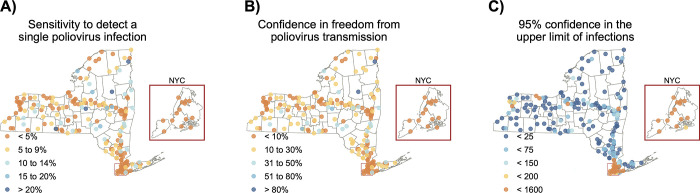
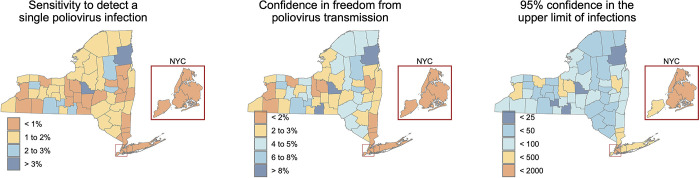
Fig 2A shows estimates of sensitivity to detect a single poliovirus infection at the wastewater treatment plant level across New York State, using whichever estimated sensitivity of detection (Sed) is higher from the two different approaches. Three consecutive non-detections of poliovirus provide confidence in zero poliovirus infections in the community ranging from 0.2% to 99% (Fig 2B) and 95% confidence that the number of infections within a community range from 0 to fewer than 1,079 (Fig 2C).
Fig 2.
The sensitivity of wastewater treatment plants in New York State to a) detect a single poliovirus infection, b) provide confidence in the freedom from poliovirus transmission with three consecutive non-detections, and c) provide 95% confidence in the upper limit of the number of poliovirus infections with three consecutive non-detections. These maps were created in R version 4.2.3 [24] using county boundaries from the US Census TIGER/Line shapefiles.
Once applying county-level population coverage, we find the sensitivity of wastewater surveillance to detect a single poliovirus infection to range from 0.5% in New York County (Manhattan) to 10.7% in Clinton County (Fig 3A). Three consecutive non-detections of poliovirus provide confidence in zero poliovirus infections in the county ranging from 0.1% to 28.7% (Fig 3B) and 95% confidence that the number of infections within a county range from fewer than 6 to fewer than 1,570 (Fig 3C).
Fig 3.
The sensitivity of New York State’s wastewater surveillance to identify at the county level a) a single poliovirus infection, b) confidence in the freedom from poliovirus transmission with three consecutive non-detections, and c) an upper limit of the number of poliovirus infections given three consecutive non-detections. These maps were created in R version 4.2.3 [24] using county boundaries from the US Census TIGER/Line shapefiles.
Spatial comparison of vaccination rates
Sewersheds with higher social vulnerability had lower vaccination rates (Pearson correlation coefficient of -0.2298, p value < 0.01). The majority of sewersheds in New York (n = 459, 76.6%) have vaccination rates above 70% and lower vaccination coverage sewersheds (n = 140, 23.4%) ranged in vulnerability between 0.1 and 0.85 with a median SVI of 0.51. Sewersheds with an average vaccination rate greater than 70% ranged between an SVI of 0 and 0.8 with a median SVI of 0.45.
An estimated 1.76 million New Yorkers reside in zip codes with poliovirus vaccination coverage < 70%. The majority of these New Yorkers (81%) are covered by the wastewater surveillance network (Table 2). A further 11% of these New Yorkers live in communities connected to sewer, but their wastewater treatment plants are not currently enrolled. (The threshold for inclusion in the state’s wastewater surveillance network is a treatment plant permitted to discharge at least 1 million gallons per day). Eight percent of New Yorkers living in zip codes with poliovirus vaccination coverage < 70% are not connected to any public sewer system.
Table 2. Number of zip codes across NY with vaccination rates below 70% and whether they are within a sewer system that can be monitored using wastewater surveillance.
| Status of zip code | Number of zip codes with vaccination rates <70% | Total population of the zip codes | Percent of total |
|---|---|---|---|
| Zip code included in network and eligible for ongoing surveillance | 87 | 1,420,000 | 81 |
| Zip code intersects with a small sewershed and could be included in potential scale-up of surveillance | 67 | 190,000 | 11 |
| Zip code not in a sewershed | 115 | 150,000 | 8 |
| Total | 269 | 1,760,000 | 100 |
Discussion
We find that the sensitivity of wastewater surveillance to detect any circulating poliovirus is highly dependent upon the size of the population contributing to the wastewater sample and unique to each sewershed. In general, when using the input parameters herein the sensitivity of a single test of wastewater collected from a wastewater treatment plant to detect poliovirus is low, < 10% throughout much of New York State. This issue is compounded by gaps in population coverage of wastewater surveillance when estimating county-level sensitivity, < 2% sensitivity throughout much of New York State. Three consecutive non-detections of poliovirus are slightly better at treatment plants throughout the state but still < 5% sensitivity at the county level. Despite the low sensitivities observed, non-detection of poliovirus in wastewater can still provide maximum thresholds of the number of poliovirus infections within the sewer catchment or the county.
These estimates of sensitivity are primarily driven by the sensitivity of detection of poliovirus in wastewater, which we consider in these analyses to be highly conservative. For example, we estimate a limit of detection of one shedder per 472 population (0.2% prevalence), much lower than the one shedder per 10,000 population that Hovi et al. estimated following flush experiments of oral polio vaccine [26]. If using Hovi et al’s one shedder per 10,000 population the confidence in no transmission is greatly increased, above 50% throughout much of New York State. We elected not to include results using Hovi et al’s estimated sensitivity of detection due to the huge difference between that method and the methods estimated from Berchenko et al. and our modeling. Our estimates of the sensitivity of detection are also more in line with Kroiss et al. who found detection rates of 35–50% when polio prevalence was estimated to be 0.5–3% [8]. The low sensitivity in these results further align with those by O’Reilly et al., who also found < 10% sensitivity in the surveillance system to identify polio transmission when the number of shedders is minimal [27].
The two independent methods we used to estimate the sensitivy of detecting poliovirus RNA in wastewater produced similar results. Still, they have a number of limitations. The first method we used, which required estimating the number of poliovirus infections across the study area, is particularly driven by a number of key assumptions. We use a probability distribution of 1 paralytic case per 2,000 infections in unvaccinated individuals–any fluctuation in that and the number of infections would be quite different. We also presume equal mixing and equal onward polio transmission of vaccinated and unvaccinated populations. Polio vaccines are extremely effective at eliminating the risk of paralysis, however only oral polio vaccine is considered a transmission-interrupting vaccine. The inactivated polio vaccine that is administered throughout New York does not produce sterilizing immunity–vaccinated individuals can still contract and transmit polio albeit to a lesser degree than unvaccinated individuals [28]. It is unlikely that poliovirus is widespread and circulating everywhere among individuals vaccinated with inactivated polio vaccine [8], otherwise we would see much more paralytic polio among unvaccinated individuals. To date (April 2024) from the Rockland County outbreak we have only seen a single paralytic case of polio, and this is the first polio case detected in New York since 2013. The exclusion of the effect of vaccines from estimates on the number of infected individuals likely overestimates the number of polio infections throughout New York State in Table 1, thereby reducing the estimates of sensitivity we calculated. Others have also estimated a similar number of polio infections during the New York State outbreak [29]. For the second method of estimating the sensitivity of detection we rely heavily on Berchanko et al. [22] in the absence of adequate fecal shedding studies. Fecal shedding studies that estimate the number of viral copies per gram of feces excreted among polio-infected individuals would allow for a more robust approach, however this approach is labor intensive and not practical for the current state of global polio transmission. The two independent methods produced similar results.
Throughout these analyses we did not account for differences in sampling or site-specific factors that might affect the sensitivity of detection, but rather applied a constant sensitivity of detection across sampling sites. Site-specific factors including biological and nonbiological contaminants that inhibit PCR reactions can have significant impact on the recovery of pathogen nucleic acids from wastewater [19]. We also used 24-hour composite samples, and the sampling approach may affect recovery. For polio, site-specific sensitivity is often assessed by the detection of oral polio vaccine, with confidence in a sampling site growing over time. However, the US does not distribute oral polio vaccine and the inactivated polio vaccine that is distributed across the US is not shed in feces. Further work needs to examine how site-specific factors affect the sensitivity of detection to maximize understanding from of non-detections and increase confidence in the freedom from disease.
In New York State the most vulnerable communities to polio outbreaks (zip codes with < 70% vaccine coverage) are largely connected to the state’s wastewater surveillance network that we have established. In these communities 81% of residents are wholly encompassed within the network, and a further 11% of residents intersect in some way with smaller treatment plant catchments. It is likely that any polio outbreak in low vaccination communities would first be identified in wastewater, as has been observed elsewhere [11, 12].
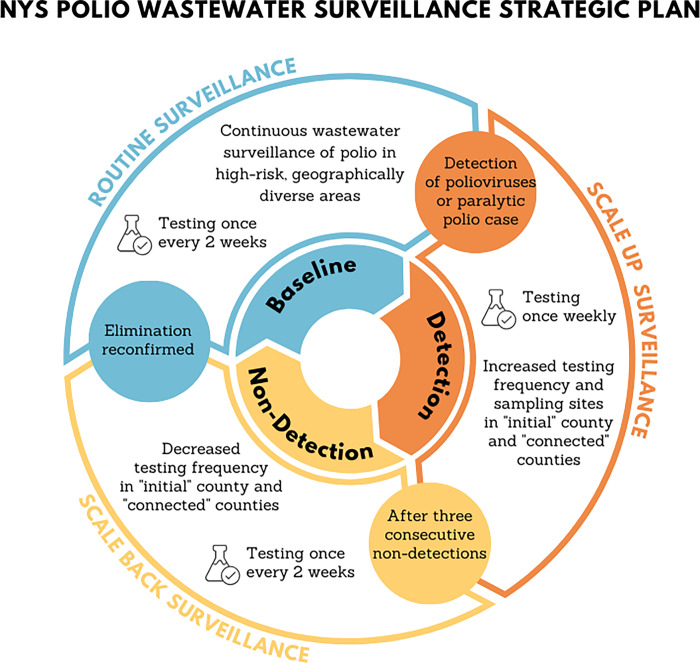
We can apply these results to inform testing frequency in a statewide wastewater surveillance network (Fig 4). The cost of wastewater surveillance is primarily driven by the number of samples (the spatial scale) and frequency of sampling. The system should more often test communities vulnerable to outbreak including communities with lower vaccine coverage, larger cities, and globally connected communities. We suggest a baseline prospective surveillance sampling frequency of once every two weeks (or monthly if resources are limited) in a select number of vulnerable communities. If poliovirus is detected in wastewater, we recommend scaling up the sampling frequency to weekly and expanding the spatial coverage in the county with the detection and connected communities. Three consecutive non-detections prompt a reduction in temporal frequency, reducing to once every two weeks (scaling back surveillance). Once elimination is confirmed again (zero poliovirus found in consecutive samples in any area) then baseline prospective surveillance can resume. This is just one way of operationalizing wastewater surveillance system with guidance on where and how often to test wastewater.
Fig 4. Conceptual framework to guide the frequency and scale of testing wastewater for poliovirus.
Baseline prospective surveillance regularly tests wastewater from communities vulnerable to polio outbreaks. Detection prompts a scale-up in surveillance, increasing both the spatial coverage and temporal frequency. Consecutive non-detections prompt scaling back in surveillance, reducing the temporal frequency. Once elimination is confirmed the system returns to baseline prospective surveillance.
The methods we outline here can be used to estimate the sensitivity of wastewater surveillance for any pathogen [30]. Required inputs are fecal/urinary shedding rates and/or case and population data. In the absence of incident polio cases without paralysis, we used an upper limit of the number of infections thought to occur before a paralytic case is observed. This approach was not needed for our estimates of the sensitivity of wastewater surveillance to detect COVID-19 infections, where we used reported case data [13], nor would it be needed for any infectious disease where case data more accurately reflect the number of infected individuals. As wastewater surveillance becomes more routine and established [31], the methods outlined here can inform public health understanding from non-detections. We expect these methods to be most valuable in the context of emerging and re-emerging diseases, or disease elimination situations.
Data Availability
Wastewater data in the analyses are included in Table 1. The Zip-code level polio vaccination rates can be retrieved from the New York State Department of Health website at https://health.ny.gov/diseases/communicable/polio/data.htm.
Funding Statement
This work was supported by the CDC’s ELC Program, NYS Unique Federal Award Number NU50CK000516 (NYS Epidemiology and Laboratory Capacity for Prevention and Control of Emerging Infectious Diseases). Additional support was made provided by the Environmental Public Health Tracking (EPHT) grant. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.McClure CC, Cataldi JR, O’Leary ST. Vaccine Hesitancy: Where We Are and Where We Are Going. Clinical Therapeutics. 2017;39: 1550–1562. doi: 10.1016/j.clinthera.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 2.Bramer CA, Kimmins LM, Swanson R, Kuo J, Vranesich P, Jacques-Carroll LA, et al. Decline in child vaccination coverage during the COVID-19 pandemic—Michigan Care Improvement Registry, May 2016-May 2020. American Journal of Transplantation. 2020;20: 1930–1931. doi: 10.1111/ajt.16112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klapsa D, Wilton T, Zealand A, Bujaki E, Saxentoff E, Troman C, et al. Sustained detection of type 2 poliovirus in London sewage between February and July, 2022, by enhanced environmental surveillance. The Lancet. [cited 17 Oct 2022]. doi: 10.1016/S0140-6736(22)01804-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Link-Gelles R, Lutterloh E, Ruppert PS, Backenson PB, St. George K, Rosenberg ES, et al. Public Health Response to a Case of Paralytic Poliomyelitis in an Unvaccinated Person and Detection of Poliovirus in Wastewater—New York, June–August 2022. MMWR Morb Mortal Wkly Rep. 2022;71. doi: 10.15585/mmwr.mm7133e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryerson AB. Wastewater Testing and Detection of Poliovirus Type 2 Genetically Linked to Virus Isolated from a Paralytic Polio Case—New York, March 9–October 11, 2022. MMWR Morb Mortal Wkly Rep. 2022;71. doi: 10.15585/mmwr.mm7144e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grassly NC. Immunogenicity and Effectiveness of Routine Immunization With 1 or 2 Doses of Inactivated Poliovirus Vaccine: Systematic Review and Meta-analysis. The Journal of Infectious Diseases. 2014;210: S439–S446. doi: 10.1093/infdis/jit601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masters NB, Eisenberg MC, Delamater PL, Kay M, Boulton ML, Zelner J. Fine-scale spatial clustering of measles nonvaccination that increases outbreak potential is obscured by aggregated reporting data. Proc Natl Acad Sci USA. 2020;117: 28506–28514. doi: 10.1073/pnas.2011529117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kroiss SJ, Ahmadzai M, Ahmed J, Alam MM, Chabot-Couture G, Famulare M, et al. Assessing the sensitivity of the polio environmental surveillance system. Iturriza-Gómara M, editor. PLoS ONE. 2018;13: e0208336. doi: 10.1371/journal.pone.0208336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melnick J L. Current status of poliovirus infections. Clinical Microbiology Reviews. 1996;9: 293–300. doi: 10.1128/CMR.9.3.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asghar H, Diop OM, Weldegebriel G, Malik F, Shetty S, Bassioni LE, et al. Environmental surveillance for polioviruses in the global polio eradication initiative. Journal of Infectious Diseases. 2014;210: S294–S303. doi: 10.1093/infdis/jiu384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowger TL, Burns CC, Sharif S, Gary HE, Iber J, Henderson E, et al. The role of supplementary environmental surveillance to complement acute flaccid paralysis surveillance for wild poliovirus in Pakistan—2011–2013. PLoS One. 2017;12: e0180608. doi: 10.1371/journal.pone.0180608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brouwer AF, Eisenberg JNS, Pomeroy CD, Shulman LM, Hindiyeh M, Manor Y, et al. Epidemiology of the silent polio outbreak in Rahat, Israel, based on modeling of environmental surveillance data. Proceedings of the National Academy of Sciences of the United States of America. 2018;115: E10625–E10633. doi: 10.1073/pnas.1808798115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsen DA, Collins MB, Du Q, Hill D, Insaf TZ, Kilaru P, et al. Coupling freedom from disease principles and early warning from wastewater surveillance to improve health security. PNAS Nexus. 2022;1: pgac001. doi: 10.1093/pnasnexus/pgac001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cameron AR, Baldock FC. A new probability formula for surveys to substantiate freedom from disease. Preventive Veterinary Medicine. 1998;34: 1–17. doi: 10.1016/s0167-5877(97)00081-0 [DOI] [PubMed] [Google Scholar]
- 15.Tanne JH. Polio emergency declared in New York State over virus found in wastewater. BMJ. 2022;378: o2211. doi: 10.1136/bmj.o2211 [DOI] [PubMed] [Google Scholar]
- 16.Neyra M, Hill DT, Bennett LJ, Dunham CN, Larsen DA, New York State Wastewater Surveillance Network Team*. Establishing a Statewide Wastewater Surveillance System in Response to the COVID-19 Pandemic: A Reliable Model for Continuous and Emerging Public Health Threats. Journal of Public Health Management and Practice. 2023;29. Available: https://journals.lww.com/jphmp/fulltext/2023/11000/establishing_a_statewide_wastewater_surveillance.14.aspx [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilder ML, Middleton F, Larsen DA, Du Q, Fenty A, Zeng T, et al. Co-quantification of crAssphage increases confidence in wastewater-based epidemiology for SARS-CoV-2 in low prevalence areas. Water Research X. 2021; 100100. doi: 10.1016/j.wroa.2021.100100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cameron AR, Meyer A, Faverjon C, Mackenzie C. Quantification of the sensitivity of early detection surveillance. Transboundary and Emerging Diseases. 2020;67: 2532–2543. doi: 10.1111/tbed.13598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamisu AW, Blake IM, Sume G, Braka F, Jimoh A, Dahiru H, et al. Characterizing Environmental Surveillance Sites in Nigeria and Their Sensitivity to Detect Poliovirus and Other Enteroviruses. The Journal of Infectious Diseases. 2022;225: 1377–1386. doi: 10.1093/infdis/jiaa175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill DT, Larsen DA. Using geographic information systems to link population estimates to wastewater surveillance data in New York State, USA. PLOS Global Public Health. 2023;3: e0001062. doi: 10.1371/journal.pgph.0001062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alexander JP Jr, Gary HE Jr, Pallansch MA. Duration of Poliovirus Excretion and Its Implications for Acute Flaccid Paralysis Surveillance: A Review of the Literature. The Journal of Infectious Diseases. 1997;175: S176–S182. doi: 10.1093/infdis/175.supplement_1.s176 [DOI] [PubMed] [Google Scholar]
- 22.Berchenko Y, Manor Y, Freedman LS, Kaliner E, Grotto I, Mendelson E, et al. Estimation of polio infection prevalence from environmental surveillance data. Sci Transl Med. 2017;9. doi: 10.1126/scitranslmed.aaf6786 [DOI] [PubMed] [Google Scholar]
- 23.CDC. CDC/ATSDR Social Vulnerability Index (SVI). 13 Jun 2022. [cited 31 Aug 2022]. Available: https://www.atsdr.cdc.gov/placeandhealth/svi/index.html [Google Scholar]
- 24.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2021. Available: https://www.R-project.org/ [Google Scholar]
- 25.Pebesma E. Simple features for R: Standardized support for spatial vector data. The R Journal. 2018;10: 439–446. 10.32614/RJ-2018-009 [DOI] [Google Scholar]
- 26.Hovi T, Stenvik M, Partanen H, Kangas A. Poliovirus Surveillance by Examining Sewage Specimens. Quantitative Recovery of Virus after Introduction into Sewerage at Remote Upstream Location. Epidemiology and Infection. 2001;127: 101–106. doi: 10.1017/s0950268801005787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Reilly KM, Grassly NC, Allen DJ, Bannister-Tyrrell M, Cameron A, Carrion Martin AI, et al. Surveillance optimisation to detect poliovirus in the pre-eradication era: a modelling study of England and Wales. Epidemiol Infect. 148: e157. doi: 10.1017/S0950268820001004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parker EP, Molodecky NA, Pons-Salort M, O’Reilly KM, Grassly NC. Impact of inactivated poliovirus vaccine on mucosal immunity: implications for the polio eradication endgame. Expert Review of Vaccines. 2015;14: 1113–1123. doi: 10.1586/14760584.2015.1052800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson KM, Kalkowska DA, Routh JA, Brenner IR, Rosenberg ES, Zucker JR, et al. Modeling Poliovirus Transmission and Responses in New York State. The Journal of Infectious Diseases. 2024;229: 1097–1106. doi: 10.1093/infdis/jiad355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilaru P, Hill D, Anderson K, Collins MB, Green H, Kmush BL, et al. Wastewater Surveillance for Infectious Disease: A Systematic Review. American Journal of Epidemiology. 2022; kwac175. doi: 10.1093/aje/kwac175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirby AE. Using Wastewater Surveillance Data to Support the COVID-19 Response—United States, 2020–2021. MMWR Morb Mortal Wkly Rep. 2021;70. doi: 10.15585/mmwr.mm7036a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Wastewater data in the analyses are included in Table 1. The Zip-code level polio vaccination rates can be retrieved from the New York State Department of Health website at https://health.ny.gov/diseases/communicable/polio/data.htm.